Abstract
The microbial degradation of low-molecular-weight polydimethylsiloxanes was investigated through laboratory experiments. Octamethylcyclotetrasiloxane was found to be biodegraded under anaerobic conditions in composted sewage sludge, as monitored by the occurrence of the main polydimethylsiloxane degradation product, dimethylsilanediol, compared to that found in experiments with sterilized control samples.
High quantities of polydimethylsiloxane (PDMS) are found in a wide variety of industrial and consumer products. Most of the PDMSs that enter the environment do so when they are used in down-the-drain applications (2). Low-molecular-weight methylsiloxanes are volatile and involved in the polymerization process, or they represent constituents in cosmetics and other personal-care products (7). Due to its widespread use in consumer applications, octamethylcyclotetrasiloxane (D4) especially has been subjected to a number of environmental-fate and toxicological studies (5, 8, 12, 14).
The fate and the degradation of PDMS in various natural and anthropogenic environments have been discussed controversially in the literature. Although PDMSs were previously thought to be inert, more recent studies have indicated that they are degraded and that the main degradation product is dimethylsilanediol (DMSD), which is found in soils (1, 11) and in composted sludges mixed with wood chips and ash (15). The degradation is thought to occur via chemical processes, e.g., clay-catalyzed hydrolysis (1, 11).
Reports on the biodegradability of PDMS, however, are rather scarce. Based on studies using pure and mixed cultures of Pseudomonas fluorescens and Pseudomonas putida, biodegradation of silicone oils (high- and low-molecular-weight PDMSs) under aerobic conditions was reported (16), but no biodegradation under anaerobic conditions could be shown yet. A previous report stated that high-molecular-weight [14C]PDMSs are not degraded to labeled CO2 by anaerobic sewage bacteria (6).
In the present study we demonstrated that the low-molecular-weight siloxane D4 is susceptible to anaerobic degradation by sewage microorganisms under anaerobic conditions.
Materials.
Reagent-grade D4 was obtained from Gelest, Inc. (Karlsruhe, Germany), and a DMSD standard (about 79%) was provided by Wacker Chemie (Burghausen, Germany). The sewage sludge originated from a municipal wastewater treatment plant that is fed mainly with domestic sewage. Sludge was stored in plastic bottles at 4°C prior to use (1 week). Sterilization and incubation experiments were carried out in 100-ml bottles made of a chemically resistant and biocompatible tetrafluorethylene-hexafluorpropylene-copolymer obtained from Reichelt Chemietechnik (Heidelberg, Germany). Since silanols are rather unstable compounds and may react with alkalies of the glass surface, we avoided using glass materials.
Spiking, sterilization, and incubation of sewage sludge samples.
Fifty milliliters of sewage sludge was transferred into the 100-ml tetrafluorethylene-hexafluorpropylene-copolymer bottles and was spiked with D4 (100 mg liter−1). Subsequently, the gas phase was exchanged for a mixture of CO2 and H2 (20:80 [vol/vol]) and was pressurized at 1,000 hPa. The bottles were incubated in an anaerobic container (gas phase, CO2/H2; 20:80 [vol/vol]) at 37°C in the dark and were moderately shaken (150 rpm). The samples that did not have D4 added were treated in the same way. For sterilization, sewage sludge samples were autoclaved at 121°C for 20 min prior to spiking and incubation.
Analytical methods.
Before measurement, the sewage sludge samples were filtered through 0.45-μm-pore-size sterile syringe filters. DMSD was analyzed by coupling a high-pressure liquid chromatography (HPLC) unit with an inductively coupled plasma-optical emission spectrometer (ICP-OES). A detailed description of this method for silanol detection in environmental water samples is given elsewhere (3). For DMSD standards in a sewage sludge matrix, a recovery of 56% and a standard deviation of 10% (n = 6) could be established.
Biodegradation of high-molecular-weight PDMS.
Anaerobic incubation of original sludge samples for 3 months at 37°C without the addition of D4 did not result in any detectable degradation of PDMS enriched under such conditions (2, 9). Thus, this study confirms reports on the resistance of high-molecular-weight PDMS against degradation by anaerobic microorganisms.
Biodegradation of D4.
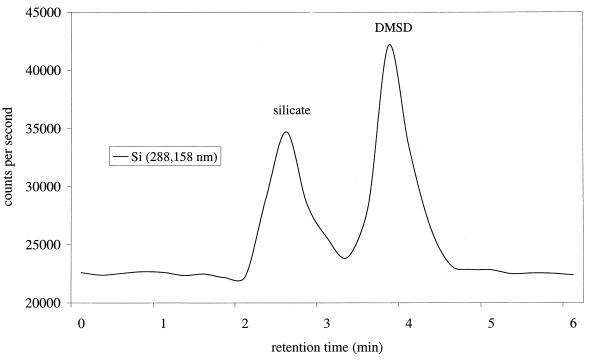
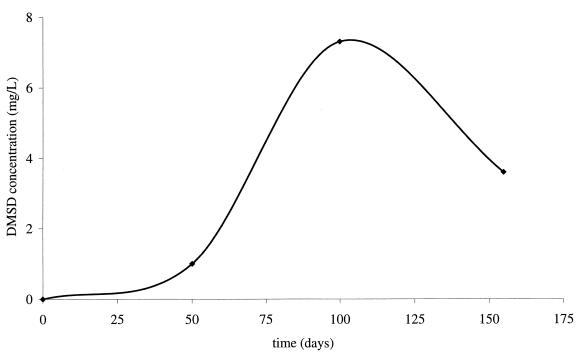
In all incubated samples spiked with D4, production of DMSD was detected. A typical chromatogram of DMSD separation on an HPLC–ICP-OES is presented in Fig. 1. In a representative experiment, the DMSD concentration reached a maximum of 7.3 mg kg−1, corresponding to approximately 3% of the added D4 after 100 days of incubation (Fig. 2). Prolonged incubation resulted in a decrease of DMSD concentration after another 2 months. The loss of DMSD may be due to biodegradation (13) or sorption processes (10).
FIG. 1.
HPLC–ICP-OES determination of DMSD levels in a sewage sludge sample spiked with 100 mg of D4 liter−1 and incubated for 100 days.
FIG. 2.
Biodegradation of D4 in sewage sludge samples under anaerobic conditions.
After 100 days of incubation, no detectable amounts of DMSD were found in the control experiments with autoclaved sludge samples spiked with 100 mg of D4 liter−1. This result indicates that viable cells are necessary for degrading D4.
Our studies showed that low-molecular-weight PDMSs are biodegraded in sewage sludge under anaerobic conditions. After 100 days of incubation, at least 3% of the spiked D4 was converted into DMSD.
As a seeming contradiction, we were not yet able to detect PDMS degradation products in sewage gases of municipal plants (4). Possibly, the main cause for the absence of degradation products resides in the lack of volatile methylsiloxanes, which were stripped off during wastewater treatment (4, 7). As an additional cause, the retention times of sludge treatment plants (approximately 20 days) may be too short for a detectable degradation of PDMS.
REFERENCES
- 1.Carpenter J C, Cella J A, Dorn S B. Study of the degradation of polydimethylsiloxanes on soil. Environ Sci Technol. 1995;29:864–868. doi: 10.1021/es00004a005. [DOI] [PubMed] [Google Scholar]
- 2.Fendinger N J, Lehmann R G, Mihaich E M. Polydimethylsiloxane. In: Chandra G, editor. Organosilicon materials. Berlin, Germany: Springer-Verlag; 1997. pp. 181–223. [Google Scholar]
- 3.Grümping R, Hirner A V. HPLC/ICP-OES determination of water-soluble silicone (PDMS) degradation products in leachates. Fresenius’ J Anal Chem. 1999;363:347–352. [Google Scholar]
- 4.Grümping R, Mikolajczak D, Hirner A V. Determination of trimethylsilanol in the environment by LT-GC/ICP-OES and GC-MS. Fresenius’ J Anal Chem. 1998;361:133–139. [Google Scholar]
- 5.Hamelink J L, Simon P B, Silberhorn E M. Henry’s law constant, volatilization rate, and aquatic half-life of octamethylcyclotetrasiloxane. Environ Sci Technol. 1996;30:1946–1952. [Google Scholar]
- 6.Hobbs E J, Keplinger M L, Calandra J C. Toxicity of polydimethylsiloxanes in certain environmental systems. Environ Res. 1975;10:397–406. doi: 10.1016/0013-9351(75)90035-3. [DOI] [PubMed] [Google Scholar]
- 7.Hobson J F, Atkinson R, Carter W P L. Volatile methylsiloxanes. In: Chandra G, editor. Organosilicon materials. Berlin, Germany: Springer-Verlag; 1997. pp. 137–180. [Google Scholar]
- 8.Kent D, Fackler P, Hartley D, Hobson J F. Interpretation of data from nonstandard studies: the fate of octamethylcyclotetrasiloxane in a sediment/water microcosm system. Environ Toxicol Water Qual. 1996;11:145–149. [Google Scholar]
- 9.Lehmann R G, Frye C L, Tolle D A, Zwick T C. Fate of sludge-applied silicones in agricultural soil microcosms. Water Air Soil Pollut. 1996;87:231–243. [Google Scholar]
- 10.Lehmann R G, Miller J R. Volatilization and sorption of dimethylsilanediol in soil. Environ Toxicol Chem. 1996;15:1455–1460. [Google Scholar]
- 11.Lehmann R G, Varaprath S, Annelin R B, Arndt J L. Degradation of silicone polymer in a variety of soils. Environ Toxicol Chem. 1995;14:1299–1305. [Google Scholar]
- 12.Mueller J A, Di Toro D M, Maiello J A. Fate of octamethylcyclotetrasiloxane (OMCTS) in the atmosphere and in sewage treatment plants as an estimation of aquatic exposure. Environ Toxicol Chem. 1995;14:1657–1666. [Google Scholar]
- 13.Sabourin C L, Carpenter J C, Leib T K, Spivack J L. Biodegradation of dimethylsilanediol in soils. Appl Environ Microbiol. 1996;62:4352–4360. doi: 10.1128/aem.62.12.4352-4360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sousa J V, McNamara P C, Putt A E, Machado M W, Surprenant D C, Hamelink J L, Kent D J, Silberhorn E M, Hobson J F. Effects of octamethylcyclotetrasiloxane (OMCTS) on freshwater and marine organisms. Environ Toxicol Chem. 1995;14:1639–1647. [Google Scholar]
- 15.Spivack J L, Dorn S B. Hydrolysis of oligomethysiloxane-α,ω-diols and the position of hydrolytic equilibrium. Environ Sci Technol. 1994;28:2345–2352. doi: 10.1021/es00062a019. [DOI] [PubMed] [Google Scholar]
- 16.Wasserbauer R, Zadak Z. Growth of Pseudomonas putida and P. fluorescens on silicone oils. Folia Microbiol. 1990;35:384–393. [Google Scholar]