Abstract
In the last decades, obesity has reached epidemic proportions worldwide. Obesity is a chronic disease associated with a wide range of comorbidities, including insulin resistance and type 2 diabetes mellitus (T2D), which results in significant burden of disease and major consequences on health care systems. Of note, intricate interactions, including different signaling pathways, are necessary for the establishment and progression of these two closely related conditions. Altered cell-to-cell communication among the different players implicated in this equation leads to the perpetuation of a vicious circle associated with an increased risk for the development of obesity-related complications, such as T2D, which in turn contributes to the development of cardiovascular disease. In this regard, the dialogue between the adipocyte and pancreatic beta cells has been extensively studied, although some connections are yet to be fully elucidated. In this review, we explore the potential pathological mechanisms linking adipocyte dysfunction and pancreatic beta cell impairment/insulin resistance. In addition, we evaluate the role of emerging actors, such as the gut microbiome, in this complex crosstalk.
Keywords: adipose tissue, beta cell, insulin resistance, diabetes, adipokines, gut microbiota, inflammation
1 Introduction
The global prevalence of overweight and obesity has dramatically increased in the last few decades with a major impact on health and significant socioeconomic burden (1, 2). Overweight and obesity are often associated with a cluster of metabolic abnormalities, such as dyslipidemia, hypertension, and type 2 diabetes mellitus (T2D), which may lead to the development of metabolic syndrome syndrome (MetS) (3). In parallel with the growing obesity pandemic, the prevalence of T2D is also increasing worldwide, and it is expected to continue to rise in the coming years, resulting in devastating consequences (4). It is noteworthy that pancreatic beta cells are key players in the pathophysiology of T2D (5). Therefore, the central event in this condition consists of a relative insulin deficiency due to beta cell dysfunction, which often coexists with insulin resistance (5). In this regard, metabolic stress leads to beta cell apoptosis, which results in progressive loss of functional beta cell mass (5). Importantly, reciprocal interactions may occur among clustering components of MetS, leading to an increased risk for the development of cardiovascular disease (3). In line with this, central fat distribution related to MetS has been demonstrated to play a vital role in the pathophysiology of T2D, whereas disrupted glucose homeostasis and beta cell dysfunction may also promote visceral fat accumulation (6). However, some of the intricate connections and metabolic pathways involved in the crosstalk between adipose tissue and pancreatic beta cells remain poorly understood.
In recent years, the gut microbiome has emerged as a central player in the development, progression, and therapeutics of obesity and T2D (7). The human gut microbiota is composed of trillions of microorganisms located in the gastrointestinal tract that have a close symbiotic relationship with the host (8). Notably, bacterial metabolites, such as short-chain fatty acids (SCFAs), vitamins, amino acids, and bile acids (BAs), are also involved in essential bacteria and host cell-to-cell interactions (9). Therefore, when the fragile equilibrium between intestinal microbiota and host metabolism is disrupted, several disorders may develop, including overweight/obesity, ectopic fat accumulation, hyperlipidemia, insulin resistance, and hyperglycemia (10). Taken together, disturbed homeostasis between adipose tissue and pancreatic beta cells may be driven, in part, by pathological shifts in the gut microbiome and derived metabolites.
In this review, we discuss the main mechanisms involved in the interplay between adipose tissue and pancreatic beta cells, with special attention to the bidirectional influences leading to beta cell dysfunction/insulin resistance and adipocyte dysfunction. In addition, we summarize the novel insights into the role of the gut microbiome and related metabolites in the mediation of this complex crosstalk, including an integrative view of the relationship between adipose tissue-derived bacteria and beta cell/adipose tissue dysfunction.
2 Adipose Tissue and Pancreatic Beta Cell Communication: A Complex Dialogue
2.1 What Is the Role of Adipose Tissue in Beta Cell Dysfunction?
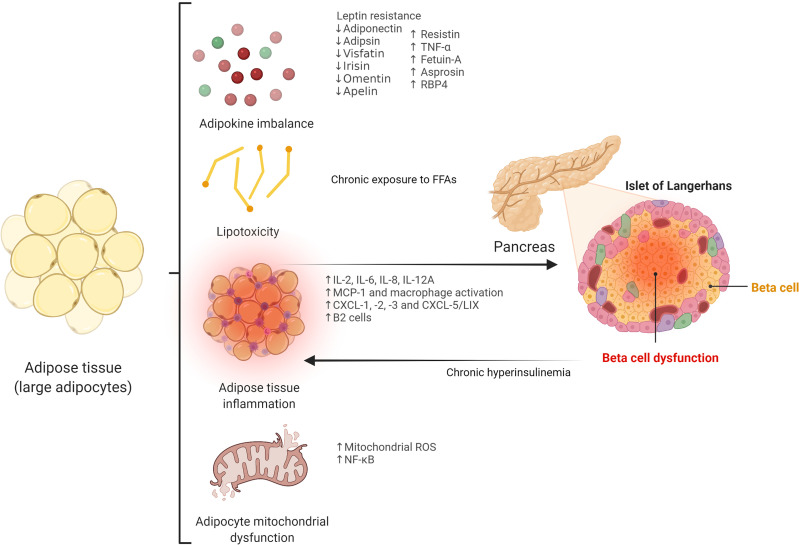
Central distribution of adipose tissue, as opposed to peripheral locations (i.e., femoro-gluteal adipose tissue) is a well-known risk factor for the development of insulin resistance and T2D (11). Importantly, impaired subcutaneous adipose tissue expandability, determined by environmental and genetic factors, has been postulated as the main mechanism leading to visceral fat accumulation (12–14). Thus, when the adipose tissue storage capacity limit is reached, excess fat may accumulate in ectopic deposits, including key organs such as skeletal muscle, liver, and pancreas, constituting an important cause of insulin resistance and beta cell dysfunction (15). Beyond its storage function, adipose tissue is a metabolically active organ with a major role in beta cell dysfunction via different mechanisms, including adipokine production, lipotoxicity, and increased inflammatory response ( Figure 1 ).
Figure 1.
Potential adipose tissue-related mechanisms leading to beta cell dysfunction. TNF-α, tumor necrosis factor α; RBP4, retinol-binding protein 4; FFAs, free fatty acids; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; CXCL, chemokine (C-X-C motif) ligand; CXCL-5/LIX, chemokine (C-X-C motif) ligand-5/lipopolysaccharide-induced CXC chemokine; ROS, reactive oxygen species; NF-κB, nuclear factor-kappa B.
2.1.1 Adipokines
Adipose tissue constitutes an important source of bioactive hormones, which are key factors in beta cell function and impairment. Among them, leptin and adiponectin have been extensively studied. Leptin exerts direct effects on pancreatic beta cells through the activation of the leptin receptor, which in turn stimulates the Janus-kinase (JAK)/signal transducer of activation (STAT) - mitogen-activated protein kinase (MAPK) signaling pathway (16). Leptin inhibits ectopic fat deposition in beta cells and reduces triglyceride accumulation in islets, preventing apoptosis and beta cell dysfunction, although its role in insulin secretion remains controversial (17, 18). Other mechanisms involved in apoptosis prevention by leptin include the inhibition of inducible nitric oxide synthase (iNOS) expression (19) and the regulation of B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax) (17). However, leptin may also exert harmful effects on beta cells. Thus, leptin increases the release of interleukin-1b (IL-1b) from beta cells and decreases the expression of the IL-1 receptor antagonist, leading to impaired beta cell function and apoptosis (20). Also, leptin has been reported to induce beta cell apoptosis and impairment of glucose-stimulated insulin secretion via c-Jun N-terminal kinase (JNK) activation (21). On the other hand, adiponectin has protective and anti-apoptotic effects on beta cells, and low levels of this adipokine have been associated with insulin resistance and beta cell dysfunction (22). Adipsin has also been reported to improve beta cell function, and its deficiency triggers beta cell failure and insulinopenia (23). Visfatin stimulates insulin secretion and inhibits beta cell apoptosis through the MAPK and phosphatidylinositol 3-kinase pathway (PI3K)/protein kinase B (AKT) pathway (24), whereas irisin improves glucolipotoxicity associated with beta cell dysfunction through adenosine monophosphate- activated protein kinase (AMPK) signaling and reduces the inflammatory response (25, 26). Decreased omentin levels may also be related to the development of T2D, since this adipokine has been demonstrated to have an influence on beta cell survival (27). Apelin significantly increased beta cell mass in preclinical models (28), although high concentrations of this adipokine were previously reported to inhibit insulin response to glucose (29). On the other hand, increased levels of some adipokines have been related to a negative impact on pancreatic beta cells. Thus, resistin induces insulin resistance and impairs insulin secretion in pancreatic beta cells via the increased expression of suppressor of cytokine signaling 3 (SOCS-3) and reduced AKT phosphorylation (30). In addition, tumor necrosis factor α (TNF- α), a pro-inflammatory cytokine and adipokine, induces beta cell apoptosis (31). Fetuin-A, a hepato-adipokine, leads to beta cell failure and apoptosis via the toll-like receptor-4 (TLR4)- JNK- nuclear factor-kappa B (NF-κB) signaling pathway (32). Recently, the novel adipokines asprosin and retinol-binding protein 4 (RBP4) have been reported as important players in the pathophysiology of T2D and beta cell dysfunction in preclinical studies. Thus, asprosin contributed to beta cell apoptosis by the inhibition of protective autophagy in beta cells through the AMPK-mammalian target of rapamycin (mTOR) pathway in in vitro models (33), whereas RBP4 has been shown to be stimulated by retinoic acid 6 (STRA6), which provoked pancreatic beta cell failure and T2D progression in rodent models (34).
2.1.2 Lipotoxicity
Free fatty acids (FFAs) are released into the circulation from adipose tissue lipolysis, constituting an important energy source during starvation (35). Also, they are crucial signal transducing molecules in several pathways, including those involved in glucose metabolism, insulin resistance, and beta cell function (36). Despite the fact that the acute release of FFAs increases beta cell mass and insulin secretion (37), chronically elevated levels of FFAs inhibit glucose-stimulated insulin secretion and lead to beta cell dysfunction via cytotoxic mechanisms that result in beta cell apoptosis (38, 39). Thus, chronic exposure to FFAs is associated with ceramide synthesis, mitochondrial dysfunction, and overexpression of apoptotic genes in beta cells (40). Besides, FFAs trigger intracellular triglyceride accumulation in pancreatic beta cells promoted by the activation of sterol regulatory element-binding proteins (SREBPs) (41).
2.1.3 Adipose Tissue Inflammation and Release of Pro-Inflammatory Factors
Visceral adipose tissue is able to secrete several pro-inflammatory factors, such as IL-2, IL-6, IL-8, IL-12A, or monocyte chemoattractant protein-1 (MCP-1), which may have a role in beta cell dysfunction (42, 43). Interestingly, recent data show that peripancreatic adipose tissue may have a strong influence on beta cell function, since close contact is established between this ectopic fat accumulation and islets of Langerhans, facilitating adipocyte-beta cell paracrine communication. Thus, increased expression of peripancreatic adipose tissue-derived factors, such as chemokine (C-X-C motif) ligand (CXCL)-1, -2, -3, and CXCL-5/lipopolysaccharide-induced CXC chemokine (LIX) acting on CXC receptor-2, as well as macrophage activation, have been shown to be implicated in the impairment of beta cell function (44). Moreover, additional organs may play a role in this equation: increased levels of hepatokine fetuin-A in non-alcoholic fatty liver disease induce impaired insulin secretion and islet cell death via the stimulation of peripancreatic adipocytes, which produce IL-6, IL-8, and MCP-1 through TLR4-dependent mechanisms (45).
Importantly, activated macrophages infiltrating adipose tissue are essential players in the development and maintenance of the pro-inflammatory state associated with harmful effects on pancreatic beta cells (46). Intriguingly, recent research has revealed that macrophages may also have an impact on beta cells independently of inflammatory mechanisms (i.e., via the release of miRNA-containing extracellular vesicles) (47, 48). Extracellular vesicles released by inflamed adipocytes can also cause beta cell death (49). Other adipose tissue-resident immune cells, such as B2 lymphocytes, may promote insulin resistance via the chemokine leukotriene B4 (LTB4) and its receptor, LTB4 receptor-1 (50).
Specific adipose tissue proteomic and transcriptomic profiles associated with inflammatory pathways may also be involved in beta cell dysfunction (51). Recently, the transcriptional coregulator GPS2 in white adipose tissue has been associated with beta cell insulin secretion (52).
Finally, adipocyte mitochondrial dysfunction and reactive oxygen species (ROS) overload may contribute to beta cell impairment. Thus, mitochondrial ROS pathway and NF-κB signaling have been associated with mitophagy-mediated adipose inflammation that promotes pancreatic beta cell damage (53).
2.2 What Is the Role of Beta Cells in Adipose Tissue Dysfunction?
Beta cells are key regulators of adipose tissue metabolism. Insulin exerts important anabolic effects on adipose tissue, including those involved in adipocyte function, growth, and differentiation (54). Insulin resistance and beta cell dysfunction are the two main mechanisms implicated in the pathogenesis of T2D, constituting a vicious cycle in which adaptive insulin hypersecretion to meet elevated metabolic demand is followed by the progressive loss of beta cell mass and function (55), and both conditions act synergistically in adipocyte dysfunction. In this line, chronic hyperinsulinemia has been reported to enhance adipose tissue inflammation and drive adipose tissue dysfunction in obese mice, and lowering circulating insulin levels was demonstrated to decrease macrophage content in adipose tissue (56). Hyperinsulinemia can also contribute to the pro-inflammatory M1:M2 macrophage imbalance in adipose tissue, which promotes iNOS, ultimately resulting in extracellular matrix deposition and adipose tissue fibrosis (57). Previous studies conducted in human subjects have revealed similar results. In this regard, Krogh-Madsen et al. found that hyperinsulinemia prompts IL-6 and TNF- α gene expression in adipose tissue (58). Of note, a recent study showed that chronic hyperinsulinemia leads to premature adipocyte senescence and a pro-inflammatory secretory profile in vitro and in vivo (59).
3 Gut Microbiome and Derived Metabolites, Additional Players in Beta Cell-Adipose Tissue Crosstalk
3.1 The Gut Microbiome Regulates Adipocyte and Beta Cell Function
Mounting evidence suggests that altered gut microbiome composition, known as gut dysbiosis, is involved in the development of adipose tissue dysfunction and insulin resistance/T2D (60). In line with this, gut barrier dysfunction and increased gut permeability, which results in the impairment of biological homeostasis by the translocation of bacterial toxins inducing systemic inflammation, may be a major factor related to these conditions (61). Thus, gut dysbiosis can affect the intestinal epithelial barrier by the modulation of the immune system, including TLR signaling, which regulates the integrity of tight junction complexes (61). Remarkably, some modulators of intracellular tight junctions and gut permeability, such as zonulin, may also play a crucial role (62). Accordingly, increased circulating levels of zonulin, an important marker of tight junction disassembly and increased gut permeability, have been correlated with gut dysbiosis and the development of metabolic disturbances (63–65). Apart from gut dysbiosis, additional factors, such as diet, should be taken into consideration in the pathogenesis of gut permeability and pro-inflammatory response in obesity and T2D (60).
With regard to the influence of the gut microbiome on adipose tissue, Bäckhed et al. reported for the first time that the gut microbiota was a key environmental factor in the predisposition towards adiposity, since it can regulate body fat storage and adipocyte metabolism (66). Indeed, the causative role of gut microbiota in the development of obesity is supported by mice models, which showed that an obese phenotype could be transferred through fecal microbiota transplantation (67, 68). Notably, a number of studies have revealed that some gut microbial patterns have a strong influence on adipose tissue inflammation, which constitutes one of the essential features in adipocyte dysfunction and may also lead to beta cell impairment, as previously described. In animal models, specific gut microbiota profiles have been demonstrated to drive Western-type diet-induced adipose tissue inflammation via myeloid differentiation primary response 88 (Myd88) and TLR signaling (69). Besides, increased intestinal permeability due to dysbiosis triggers the translocation of bacterial endotoxins that may have deleterious effects on adipose tissue. In line with this, intestinal permeability has been associated with increased visceral lipid deposition in healthy women (70). Also, elevated serum levels of lipopolysaccharide (LPS) from the Gram-negative bacterial membrane promote the inflammatory reaction in adipose tissue in obesity, including the pro-inflammatory activation of macrophages and adipocyte death by pyroptosis (71). Gut dysbiosis leads to the release of zonulin, which modulates immune response and increases gut permeability in distinct metabolic disorders, including obesity (64, 65). Of note, low serum levels of zonulin have been associated with high alpha diversity in pregnant women with obesity (72). Importantly, disruptions in the microbiome-immune-metabolic axis in early life, including gut barrier alterations and secondary immune-mediated inflammatory chronic activation related to childhood obesity, could impact adult overweight and obesity (73).
On the other hand, a growing body of evidence shows that the gut microbiome has a major role in the pathophysiology of T2D (74). Thus, bacterial genera such as Ruminococcus, Fusobacterium, and Blautia have been positively associated with this condition, whereas Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia, and Roseburia are inversely related to T2D (74). Moreover, increased gut permeability derived from gut dysbiosis may be related to the pathogenesis of T2D, as shown in preclinical studies (75). In this regard, higher zonulin levels have been reported in patients with a recent diagnosis of T2D, and may play a role in the pathophysiology of this disease, although further research is needed (76). Insulin sensitivity/resistance is also mediated by the gut microbiota (77). Interestingly, preclinical studies show that the loss of some beneficial bacteria, such as Akkermansia muciniphila, causes impaired intestinal integrity and systemic inflammation, leading to insulin resistance, while the increased abundance of this bacterium restores normal insulin response (78). Also, circulating levels of zonulin have been shown to be closely related to insulin resistance in clinical studies (64, 76). On the other side, clinical studies have revealed that calorie restriction may ameliorate insulin sensitivity through positive changes in the gut microbiota (79). Further research in humans has also corroborated that gut microbiota composition is closely linked to insulin resistance (80, 81). In addition, animal models have shown that gut microbiota is required for early beta cell development and proliferation (82), and gut microbiota signals (e.g., nucleotide-binding oligomerization domain-containing protein 1--NOD1-ligands derived from gut microbes) are needed for normal insulin biogenesis (83). In animal models showing that an obese phenotype can be transferred by fecal microbiota transplantation, mild glucose intolerance was an early manifestation in the host, a fact that suggests that the gut microbiome may affect both adipose tissue and beta cell function (68). Importantly, beta cell hyperactivity and subsequent hyperinsulinemia, which has a strong influence on adipose tissue dysfunction, can be transmitted early to recipient mice of obese microbiota despite only a minor increase in weight gain and adiposity (84). Also, hyperglycemia may increase gut permeability, which could aggravate metabolic inflammation and lead to the development of adipose tissue dysfunction and obesity (60).
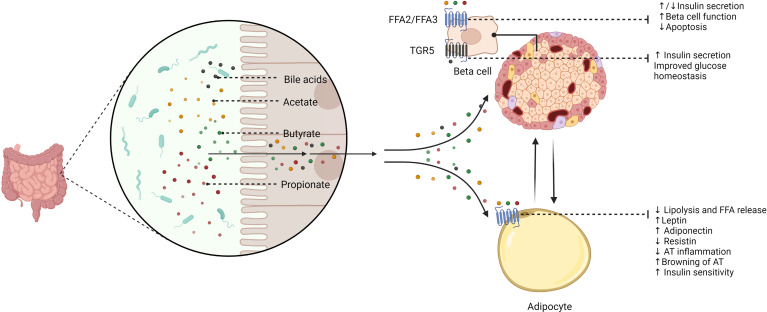
Remarkably, gut microbiota-related metabolites have direct effects on adipocyte and beta cell function ( Figure 2 ). The gut microbiota secretes several molecules that reach key cells through specific receptors. By the fermentation of non-digestible dietary fibers, gut microbes produce SCFAs, including propionate, acetate, and butyrate, which exert direct actions through cell-surface G-protein-coupled receptors (GPCRs) (85). Additional bacterial products, such as amino acids, triglyceride metabolites, and BAs can also target these receptors (85). Pancreatic beta cells express SCFAs receptors-2 and 3 (FFA2/GPR43 and FFA3/GPR41), which have direct effects on insulin secretion; however, mixed results have been reported in this regard. On the one hand, acetate was proven to inhibit glucose-stimulated insulin secretion via FFA2 and FFA3 in mouse and human beta cells (86). Conversely, another study showed that acetate enhances glucose-stimulated insulin secretion through the activation of the parasympathetic nervous system, although these effects appear to be related to hyperphagia, ectopic lipid deposition, and insulin resistance (87). Further studies have confirmed that acetate stimulates insulin secretion (88, 89). Butyrate may prevent pro-inflammatory cytokine-beta cell dysfunction and induce insulin secretion (90, 91), whereas propionate improved beta cell function and insulin release in humans (92), although contrary results have also been described (93). Besides, transmembrane bile acid receptor Takeda G-protein coupled receptor 5 (TGR5) can enhance insulin secretion and improve glucose homeostasis (94, 95). FFA2 and FFA3 are also expressed by adipocytes and are mainly associated with the regulation of adipokine release and adipose tissue metabolism (96, 97). SCFAs may also induce the browning of adipose tissue (98). Interestingly, butyrate can modulate adipocyte expansion and favor adipogenesis and adiponectin production through the upregulation of peroxisome proliferator-activated receptor gamma (PPAR-γ) (99) and suppresses adipocyte inflammation via the inhibition of the NOD-like receptor family pyrin domain containing 3 (NLRP3) pathway (100). Similarly, propionate ameliorates adipose tissue inflammation (101), whilst acetate could lead to adipose tissue dysfunction by TNF-α-induced MCP-1 production (102).
Figure 2.
The potential role of gut microbiota-derived metabolites in beta cell and adipocyte function. The gut microbiome secretes several signaling molecules with direct effects on beta cell and adipocyte function. Short-chain fatty acids (SCFAs), including acetate, butyrate, and propionate, exert different effects on beta cells via binding short-chain fatty acid receptor-2 (FFA2) and FFA3. Thus, SCFAs inhibit apoptosis, improve beta cell function, and enhance insulin secretion. However, it has been reported that some SCFAs (i.e., acetate and propionate) could also inhibit insulin secretion. Bile acids may stimulate insulin secretion and improve glucose homeostasis through Takeda G-protein coupled receptor 5 (TGR5). SCFAs also have a role in adipocyte function via FFA2 and FFA3. Therefore, acetate, butyrate, and propionate regulate adipocyte metabolism and adipokine balance. These effects may result in reciprocal influences between beta cells and the adipocyte. FFA2/FFA3, short-chain fatty acid receptor 2/3; TGR5, Takeda G-protein coupled receptor 5; FFA, free fatty acids; AT, adipose tissue.
3.2 Gut Microbiota: A Potential Link Between Adipose Tissue and Beta Cell Communication
In previous sections, we have discussed the role of lipotoxicity, adipose tissue inflammation, and altered adipokine expression in the development of beta cell dysfunction and insulin resistance. Since pathological shifts in gut microbiota composition and related metabolites may lead to adipose tissue dysfunction via the aforementioned mechanisms, derived consequences are expected in beta cell survival and function. Thus, Faecalibacterium prausnitzii decreases adipocyte inflammation and increases adiponectin expression in visceral adipose tissue, which is related to insulin-sensitizing effects (103). Similarly, A. muciniphila reverses adipose tissue inflammation and restores insulin sensitivity in T2D (104). In addition, Akkermansia has been shown to be an important predictor of serum levels of FFAs, which are involved in lipotoxicity and beta cell impairment, presenting an inverse relationship with them and the pro-inflammatory cytokine IL-6 (105). Notably, in a study evaluating the role of angiopoietin-like 4 (ANGPTL4) in metabolic dysfunction, the loss of the expression of this adipokine uncoupled visceral fat accumulation from glucose intolerance via the gut microbiota (106).
Gut microbiome-derived metabolites are also important intermediates of the adipose tissue-beta cell crosstalk. Tryptophan-derived compounds produced by the gut microbiota regulate miRNA-181 expression in white adipose tissue, involved in glucose tolerance and insulin sensitivity (107). Thus, a decrease in tryptophan-derived metabolites is associated with the overexpression of miRNA-181, which favors the development of adipose tissue inflammation, impaired glucose tolerance, and insulin resistance (107). It is also known that butyrate stimulates adipocyte differentiation and adiponectin expression, favoring insulin sensitivity (108), whereas propionate enhances leptin expression and reduces resistin expression, which are closely involved in beta cell function (109). On the other hand, gut microbiota metabolites modulate insulin sensitivity/resistance in the host, which in turn affects adipocyte function. Thus, elevated circulating levels of LPS in individuals with T2D activate TLR-2 expression and trigger immune response and inflammation in adipose tissue (110). Metabolic endotoxemia induced by LPS triggers insulin resistance and the subsequent expression of inflammatory markers in adipose tissue to a similar extent as a high-fat diet (111).
In light of the above, gut dysbiosis and impaired metabolite secretion appear to drive an altered adipokine balance and induce adipose tissue inflammation, a fact that ultimately results in insulin resistance and beta cell dysfunction, which can also aggravate adipocyte inflammation via the gut microbiota, perpetuating the vicious cycle. However, further mechanisms, such as the direct bacterial presence in adipose tissue, constituting a specific-tissue microbiota, have been postulated in this intricate relationship.
3.3 The Role of Adipose Tissue-Derived Bacteria in Adipocyte/Beta Cell Dysfunction
It is noteworthy that bacterial translocation from the intestine to adipose tissue due to increased gut permeability, as proposed by the “tissue microbiota hypothesis” (112), could have an impact on adipose tissue-beta cell crosstalk (113–117) ( Table 1 ). Accordingly, in animal models, the presence of bacteria in adipose tissue was previously reported (118). In mice, a high-fat diet induced the translocation of Gram-negative bacteria through intestinal mucosa to circulation and mesenteric adipose tissue via pathogen-associated molecular patterns (PAMPs) recognition, Myd88 signaling, and leptin regulation, resulting in low-grade inflammation, linked to the early stages of T2D (113). Increased metabolic inflammation and insulin resistance have been associated with bacterial translocation from the intestine into adipose tissue in NOD2-/- mice (114). Conversely, the identification of bacterial DNA in human adipose tissue has been a challenging task (119). Recently, the presence of specific microbial signatures in three different adipose tissues (omental, mesenteric, and subcutaneous adipose tissue) has been identified in subjects with morbid obesity, varying between individuals with and without T2D, with more evident signatures in mesenteric adipose tissue, including a decrease of health-promoting bacteria, such as Faecalibacterium and increased abundance of pathogens (e.g., Enterobacteriaceae) in subjects with T2D (115). In addition, Massier et al. also detected bacterial DNA in omental, mesenteric, and subcutaneous adipose tissue from 75 participants with obesity with or without T2D (116). Once more, mesenteric adipose tissue presented the highest bacterial quantity, which was associated with adipose tissue inflammation, and adipose tissue microbiota composition was different between subjects with and without diabetes (116). However, devoted clinical studies are needed to confirm these results.
Table 1.
Animal models and clinical studies assessing the potential association between adipose tissue-derived bacteria and adipose tissue function/glucose homeostasis.
| Study | Animals/Participants | Adipose tissue bacteria | Adipose tissue-related findings | Glucose homeostasis-related findings |
|---|---|---|---|---|
| Amar et al. (113) | NC/HFD-fed mice | Gram-negative bacteria (experimental translocation model). | Increased TNF-α and IFN-γ in MAT, correlating with bacterial DNA concentration. | Increasing MAT bacterial DNA concentration in the progression of prediabetes to diabetes. Probiotic treatment reduced mucosal dysbiosis, bacterial translocation, and improved glucose metabolism. |
| Denou et al. (114) | NOD2-/- mice | Commensal bacteria (experimental translocation model). | Increased inflammation (IL-6, TNF-α) in visceral adipose tissue. | Increased insulin resistance. |
| Ahnê et al. (115) | Subjects with morbid obesity with T2D (n-20) and without T2D (n-20) | Different compartmentalization according to specific tissue (MAT, OAT, SAT). | Not assessed. | More evident T2D signatures in MAT: reduced bacterial diversity and Gram-positive bacteria (i.e., Faecalibacterium) and increased Gram-negative Enterobacteriaceae. |
| Massier et al. (116) | Subjects with obesity with T2D (n-33) and without T2D (n-42) | Proteobacteria and Firmicutes were the predominant phyla in adipose tissue (MAT, OAT, SAT). Higher bacterial quantity and diversity in MAT. | Bacterial DNA correlated with macrophage infiltration in OAT (especially in T2D), TNF-α in SAT, and IL-1B in MAT; bacterial DNA induced adipokine secretion. | Eighteen genera were shown to present different abundance between subjects with T2D and subjects without T2D. |
| Bakker et al. (117) | Subjects with obesity and metabolic syndrome receiving lean donor FMT (n-8); BMI- matched controls not receiving FMT (n-16) | Very low quantity of bacterial DNA in visceral adipose tissue. | FMT did not alter bacterial translocation to adipose tissue. No differences in visceral bacterial DNA content/macrophage infiltration between groups. | Not assessed. |
NC, normal chow; HFD, high-fat diet; MAT, mesenteric adipose tissue; OAT, omental adipose tissue; SAT, subcutaneous adipose tissue; TNF- α, tumor necrosis factor α; IFN- γ, interferon γ; NOD2, oligomerization domain-2; IL-6, interleukin 6; IL-1B, interleukin-1B; TD2, type 2 diabetes mellitus; FMT, fecal microbiota transplantation; BMI, body mass index.
3.4 Impact of Gut Microbiome Modulation on Adipose Tissue-Beta Cell Crosstalk
The gut microbiome may be targeted to modulate the metabolic dialogue between adipose tissue and pancreatic beta cells. Hence, prebiotic approaches [i.e., non-digestible food components that benefit the host by the selective stimulation of the growth/activity of specific bacterial strains (120)] have emerged as promising interventions. Oligofructose supplementation in high-fat diet-fed mice increased gut Bifidobacterium spp. and prevented the elevation of adipose tissue inflammatory markers, which was linked to the improvement of glucose tolerance and the restoration of glucose-induced insulin secretion (121). Moreover, an oligofructose-enriched diet decreased Firmicutes and increased Bacteroidetes abundance, reducing adipose lipid peroxidation and ameliorating leptin sensitivity and glucose tolerance (122). The combination of the dietary flavonoid isoquercetin with soluble fiber (inulin) attenuated weight gain, improved glucose tolerance/insulin sensitivity, reduced adipocyte hypertrophy/ectopic fat accumulation, and restored adipokine balance in high fat diet-fed mice (123). On the other hand, the direct administration of health-promoting live microorganisms (probiotics) could confer several benefits. Lactic acid bacteria strains were demonstrated to modulate the adipokine profile in in vitro models (124). Besides, probiotic interventions targeting key gut microbes in the protection against adipocyte/beta cell dysfunction, such as A.muciniphila and F.prausnitzii, may constitute an attractive approach (103, 104, 125). Postbiotics, defined as bioactive substances produced by microorganisms with positive effects on the host (126), can also modulate adipocyte and beta cell function. The previously discussed SCFAs are relevant postbiotics in this regard (85, 108, 109). The combination of inulin and SCFAs reduced adipocyte size and prevented diet-induced obesity and insulin resistance in animal models (127). Interestingly, the administration of the natural metabolite 4-cresol reduced adiposity and enhanced insulin secretion and beta cell proliferation in mouse islets (128). Fecal microbiota transplantation from lean donors to patients with obesity and metabolic syndrome transiently improved insulin sensitivity (129), and animal models have revealed that this therapy may reverse beta cell dysfunction (130). However, further research is needed to confirm these results.
4 Concluding Remarks
Obesity and T2D are increasing in prevalence, resulting in major health and socioeconomic consequences. The relationships between these two disorders are well established; however, some of the underlying mechanisms involved in their pathophysiology and bidirectional links are not fully understood. Pancreatic beta cells and adipose tissue are closely interconnected through the presence of a number of bioactive hormones and intricate signaling pathways. Also, the gut microbiome may play a key role in the mediation of the complex dialogue between the adipocyte and beta cell, with derived potential therapeutic strategies in this field. However, important issues are yet to be elucidated. Cells do not live in isolation, and multiple interactions are expected to occur beyond the dialogue among the gut microbiome, adipose tissue, and pancreatic beta cells. Therefore, additional players, such as the skeletal muscle and the liver, may be included in this metabolic crosstalk. Future perspectives in this area should also focus on the development of therapeutic approaches (e.g., nutritional therapy) targeting the gut microbiota and the distinct dysfunctional metabolic pathways. Finally, dedicated clinical studies are warranted to fully unravel the role of the gut microbiome and related metabolites in the crosstalk between pancreatic beta cells and adipose tissue.
Author Contributions
Conceptualization, JM-M and FT. Investigation, JM-M, MD-F, and JF-G. Original draft preparation, JM-M and MD-F. Writing- review and editing, JM-M, JF-G, and FT. Supervision, FT. All authors contributed to the article and approved the submitted version.
Funding
MD-F was supported by Rio Hortega from the Spanish Ministry of Economy and Competitiveness (ISCIII) and co-funded by Fondo Europeo de Desarrollo Regional-FEDER (CM20/00183). JF-G was supported by an intensification research program (INT21/00078, ISCIII, Spain; co-funded by the Fondo Europeo de Desarrollo Regional-FEDER). This study was supported by the “Centros de Investigación Biomédica en Red” (CIBER) of the Institute of Health Carlos III (ISCIII) (CB06/03/0018), and research grants from the ISCIII (PI18/01160), and co-financed by the European Regional Development Fund (ERDF).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Figures were created with BioRender.com.
References
- 1. GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health Effects of Overweight and Obesity in 195 Countries Over 25 Years. N Engl J Med (2017) 377(1):13–27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tremmel M, Gerdtham U-G, Nilsson PM, Saha S. Economic Burden of Obesity: A Systematic Literature Review. Int J Environ Res Public Health (2017) 14(4):435. doi: 10.3390/ijerph14040435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The Metabolic Syndrome. Endocr Rev (2008) 29(7):777–822. doi: 10.1210/er.2008-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results From the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 5. Eizirik DL, Pasquali L, Cnop M. Pancreatic β-Cells in Type 1 and Type 2 Diabetes Mellitus: Different Pathways to Failure. Nat Rev Endocrinol (2020) 16(7):349–62. doi: 10.1038/s41574-020-0355-7 [DOI] [PubMed] [Google Scholar]
- 6. Gastaldelli A, Miyazaki Y, Pettiti M, Matsuda M, Mahankali S, Santini E, et al. Metabolic Effects of Visceral Fat Accumulation in Type 2 Diabetes. J Clin Endocrinol Metab (2002) 87(11):5098–103. doi: 10.1210/jc.2002-020696 [DOI] [PubMed] [Google Scholar]
- 7. Fan Y, Pedersen O. Gut Microbiota in Human Metabolic Health and Disease. Nat Rev Microbiol (2021) 19(1):55–71. doi: 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- 8. Lloyd-Price J, Abu-Ali G, Huttenhower C. The Healthy Human Microbiome. Genome Med (2016) 8(1):51. doi: 10.1186/s13073-016-0307-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur J Nutr (2018) 57(1):1–24. doi: 10.1007/s00394-017-1445-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stephens RW, Arhire L, Covasa M. Gut Microbiota: From Microorganisms to Metabolic Organ Influencing Obesity. Obesity (2018) 26(5):801–9. doi: 10.1002/oby.22179 [DOI] [PubMed] [Google Scholar]
- 11. Livingston EH. Lower Body Subcutaneous Fat Accumulation and Diabetes Mellitus Risk. Surg Obes Relat Dis (2006) 2(3):362–8. doi: 10.1016/j.soard.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 12. Pereira MJ, Vranic M, Kamble PG, Jernow H, Kristófi R, Holbikova E, et al. CDKN2C Expression in Adipose Tissue Is Reduced in Type II Diabetes and Central Obesity: Impact on Adipocyte Differentiation and Lipid Storage? Transl Res (2021)242:105-21. doi: 10.1016/j.trsl.2021.12.003 [DOI] [PubMed] [Google Scholar]
- 13. Lotta LA, Gulati P, Day FR, Payne F, Ongen H, van de Bunt M, et al. Integrative Genomic Analysis Implicates Limited Peripheral Adipose Storage Capacity in the Pathogenesis of Human Insulin Resistance. Nat Genet (2017) 49(1):17–26. doi: 10.1038/ng.3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Virtue S, Vidal-Puig A. Adipose Tissue Expandability, Lipotoxicity and the Metabolic Syndrome–An Allostatic Perspective. Biochim Biophys Acta (2010) 1801(3):338–49. doi: 10.1016/j.bbalip.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 15. Sattar N, Gill JM. Type 2 Diabetes as a Disease of Ectopic Fat? BMC Med (2014) 12(1):123. doi: 10.1186/s12916-014-0123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frühbeck G. Intracellular Signalling Pathways Activated by Leptin. Biochem J (2006) 393(Pt 1):7–20. doi: 10.1042/BJ20051578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown JEP, Dunmore SJ. Leptin Decreases Apoptosis and Alters BCL-2: Bax Ratio in Clonal Rodent Pancreatic Beta-Cells. Diabetes Metab Res Rev (2007) 23(6):497–502. doi: 10.1002/dmrr.726 [DOI] [PubMed] [Google Scholar]
- 18. Lee Y, Magkos F, Mantzoros CS, Kang ES. Effects of Leptin and Adiponectin on Pancreatic β-Cell Function. Metabolism (2011) 60(12):1664–72. doi: 10.1016/j.metabol.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 19. Okuya S, Tanabe K, Tanizawa Y, Oka Y. Leptin Increases the Viability of Isolated Rat Pancreatic Islets by Suppressing Apoptosis. Endocrinology (2001) 142(11):4827–30. doi: 10.1210/endo.142.11.8494 [DOI] [PubMed] [Google Scholar]
- 20. Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, et al. Leptin Modulates Beta Cell Expression of IL-1 Receptor Antagonist and Release of IL-1beta in Human Islets. Proc Natl Acad Sci USA (2004) 101(21):8138–43. doi: 10.1073/pnas.0305683101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maedler K, Schulthess FT, Bielman C, Berney T, Bonny C, Prentki M, et al. Glucose and Leptin Induce Apoptosis in Human β- Cells and Impair Glucose-Stimulated Insulin Secretion Through Activation of C-Jun N-Terminal Kinases. FASEB J (2008) 22(6):1905–13. doi: 10.1096/fj.07-101824 [DOI] [PubMed] [Google Scholar]
- 22. Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in Youth: Relationship to Visceral Adiposity, Insulin Sensitivity, and Beta-Cell Function. Diabetes Care (2004) 27(2):547–52. doi: 10.2337/diacare.27.2.547 [DOI] [PubMed] [Google Scholar]
- 23. Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, et al. Adipsin Is an Adipokine That Improves β Cell Function in Diabetes. Cell (2014) 158(1):41–53. doi: 10.1016/j.cell.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng Q, Dong W, Qian L, Wu J, Peng Y. Visfatin Inhibits Apoptosis of Pancreatic β-Cell Line, MIN6, via the Mitogen-Activated Protein Kinase/Phosphoinositide 3-Kinase Pathway. J Mol Endocrinol (2011) 47(1):13–21. doi: 10.1530/JME-10-0106 [DOI] [PubMed] [Google Scholar]
- 25. Zhang D, Xie T, Leung PS. Irisin Ameliorates Glucolipotoxicity-Associated β-Cell Dysfunction and Apoptosis via AMPK Signaling and Anti-Inflammatory Actions. Cell Physiol Biochem (2018) 51(2):924–37. doi: 10.1159/000495395 [DOI] [PubMed] [Google Scholar]
- 26. Liu S, Du F, Li X, Wang M, Duan R, Zhang J, et al. Effects and Underlying Mechanisms of Irisin on the Proliferation and Apoptosis of Pancreatic β Cells. PloS One (2017) 12(4):e0175498. doi: 10.1371/journal.pone.0175498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan X, Kaminga AC, Wen SW, Acheampong K, Liu A. Omentin-1 in Diabetes Mellitus: A Systematic Review and Meta-Analysis. PloS One (2019) 14(12):e0226292. doi: 10.1371/journal.pone.0226292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng J, Zhao H, Du M, Wu X. The Effect of Apelin-13 on Pancreatic Islet Beta Cell Mass and Myocardial Fatty Acid and Glucose Metabolism of Experimental Type 2 Diabetic Rats. Peptides (2019) 114:1–7. doi: 10.1016/j.peptides.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 29. Guo L, Li Q, Wang W, Yu P, Pan H, Li P, et al. Apelin Inhibits Insulin Secretion in Pancreatic β-Cells by Activation of PI3-Kinase-Phosphodiesterase 3b. Endocr Res (2009) 34(4):142–54. doi: 10.3109/07435800903287079 [DOI] [PubMed] [Google Scholar]
- 30. Nakata M, Okada T, Ozawa K, Yada T. Resistin Induces Insulin Resistance in Pancreatic Islets to Impair Glucose-Induced Insulin Release. Biochem Biophys Res Commun (2007) 353(4):1046–51. doi: 10.1016/j.bbrc.2006.12.134 [DOI] [PubMed] [Google Scholar]
- 31. Parkash J, Chaudhry MA, Rhoten WB. Tumor Necrosis Factor-Alpha-Induced Changes in Insulin-Producing Beta-Cells. Anat Rec A Discov Mol Cell Evol Biol (2005) 286(2):982–93. doi: 10.1002/ar.a.20229 [DOI] [PubMed] [Google Scholar]
- 32. Shen X, Yang L, Yan S, Zheng H, Liang L, Cai X, et al. Fetuin A Promotes Lipotoxicity in β Cells Through the TLR4 Signaling Pathway and the Role of Pioglitazone in Anti-Lipotoxicity. Mol Cell Endocrinol (2015) 412:1–11. doi: 10.1016/j.mce.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 33. Wang R, Hu W. Asprosin Promotes β-Cell Apoptosis by Inhibiting the Autophagy of β-Cell via AMPK-mTOR Pathway. J Cell Physiol (2021) 236(1):215–21. doi: 10.1002/jcp.29835 [DOI] [PubMed] [Google Scholar]
- 34. Huang R, Bai X, Li X, Wang X, Zhao L. Retinol-Binding Protein 4 Activates STRA6, Provoking Pancreatic β-Cell Dysfunction in Type 2 Diabetes. Diabetes (2021) 70(2):449–63. doi: 10.2337/db19-1241 [DOI] [PubMed] [Google Scholar]
- 35. Lafontan M, Langin D. Lipolysis and Lipid Mobilization in Human Adipose Tissue. Prog Lipid Res (2009) 48(5):275–97. doi: 10.1016/j.plipres.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 36. Boden G. Effects of Free Fatty Acids (FFA) on Glucose Metabolism: Significance for Insulin Resistance and Type 2 Diabetes. Exp Clin Endocrinol Diabetes (2003) 111(3):121–4. doi: 10.1055/s-2003-39781 [DOI] [PubMed] [Google Scholar]
- 37. Cen J, Sargsyan E, Bergsten P. Fatty Acids Stimulate Insulin Secretion From Human Pancreatic Islets at Fasting Glucose Concentrations via Mitochondria-Dependent and -Independent Mechanisms. Nutr Metab (Lond) (2016) 13(1):59. doi: 10.1186/s12986-016-0119-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, Brendel MD, et al. Protein Kinase C Delta Activation and Translocation to the Nucleus Are Required for Fatty Acid-Induced Apoptosis of Insulin-Secreting Cells. Diabetes (2003) 52(4):991–7. doi: 10.2337/diabetes.52.4.991 [DOI] [PubMed] [Google Scholar]
- 39. El-Assaad W, Buteau J, Peyot M-L, Nolan C, Roduit R, Hardy S, et al. Saturated Fatty Acids Synergize With Elevated Glucose to Cause Pancreatic β-Cell Death. Endocrinology (2003) 144(9):4154–63. doi: 10.1210/en.2003-0410 [DOI] [PubMed] [Google Scholar]
- 40. Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty Acid-Induced Beta Cell Apoptosis: A Link Between Obesity and Diabetes. Proc Natl Acad Sci USA (1998) 95(5):2498–502. doi: 10.1073/pnas.95.5.2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamashita T, Eto K, Okazaki Y, Yamashita S, Yamauchi T, Sekine N, et al. Role of Uncoupling Protein-2 Up-Regulation and Triglyceride Accumulation in Impaired Glucose-Stimulated Insulin Secretion in a Beta-Cell Lipotoxicity Model Overexpressing Sterol Regulatory Element-Binding Protein-1c. Endocrinology (2004) 145(8):3566–77. doi: 10.1210/en.2003-1602 [DOI] [PubMed] [Google Scholar]
- 42. Kochumon S, Al Madhoun A, Al-Rashed F, Thomas R, Sindhu S, Al-Ozairi E, et al. Elevated Adipose Tissue Associated IL-2 Expression in Obesity Correlates With Metabolic Inflammation and Insulin Resistance. Sci Rep (2020) 10(1):16364. doi: 10.1038/s41598-020-73347-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daniele G, Guardado Mendoza R, Winnier D, Fiorentino TV, Pengou Z, Cornell J, et al. The Inflammatory Status Score Including IL-6, TNF-α, Osteopontin, Fractalkine, MCP-1 and Adiponectin Underlies Whole-Body Insulin Resistance and Hyperglycemia in Type 2 Diabetes Mellitus. Acta Diabetol (2014) 51(1):123–31. doi: 10.1007/s00592-013-0543-1 [DOI] [PubMed] [Google Scholar]
- 44. Rebuffat SA, Sidot E, Guzman C, Azay-Milhau J, Jover B, Lajoix A-D, et al. Adipose Tissue Derived-Factors Impaired Pancreatic β-Cell Function in Diabetes. Biochim Biophys Acta - Mol Basis Dis (2018) 1864(10):3378–87. doi: 10.1016/j.bbadis.2018.07.024 [DOI] [PubMed] [Google Scholar]
- 45. Gerst F, Wagner R, Kaiser G, Panse M, Heni M, Machann J, et al. Metabolic Crosstalk Between Fatty Pancreas and Fatty Liver: Effects on Local Inflammation and Insulin Secretion. Diabetologia (2017) 60(11):2240–51. doi: 10.1007/s00125-017-4385-1 [DOI] [PubMed] [Google Scholar]
- 46. Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annu Rev Physiol (2010) 72:219–46. doi: 10.1146/annurev-physiol-021909-135846 [DOI] [PubMed] [Google Scholar]
- 47. Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell (2017) 171(2):72–384.e12. doi: 10.1016/j.cell.2017.08.035 [DOI] [PubMed] [Google Scholar]
- 48. Gao H, Luo Z, Jin Z, Ji Y, Ying W. Adipose Tissue Macrophages Modulate Obesity-Associated β Cell Adaptations Through Secreted miRNA-Containing Extracellular Vesicles. Cells (2021) 10(9):2451. doi: 10.3390/cells10092451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gesmundo I, Pardini B, Gargantini E, Gamba G, Birolo G, Fanciulli A, et al. Adipocyte-Derived Extracellular Vesicles Regulate Survival and Function of Pancreatic β Cells. JCI Insight (2021) 6(5):e141962. doi: 10.1172/jci.insight.141962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, et al. Adipose Tissue B2 Cells Promote Insulin Resistance Through Leukotriene LTB4/LTB4R1 Signaling. J Clin Invest (2017) 127(3):1019–30. doi: 10.1172/JCI90350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carruthers NJ, Strieder-Barboza C, Caruso JA, Flesher CG, Baker NA, Kerk SA, et al. The Human Type 2 Diabetes-Specific Visceral Adipose Tissue Proteome and Transcriptome in Obesity. Sci Rep (2021) 11(1):17394. doi: 10.1038/s41598-021-96995-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Drareni K, Ballaire R, Alzaid F, Goncalves A, Chollet C, Barilla S, et al. Adipocyte Reprogramming by the Transcriptional Coregulator GPS2 Impacts Beta Cell Insulin Secretion. Cell Rep (2020) 32(11):108141. doi: 10.1016/j.celrep.2020.108141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. He F, Huang Y, Song Z, Zhou HJ, Zhang H, Perry RJ, et al. Mitophagy-Mediated Adipose Inflammation Contributes to Type 2 Diabetes With Hepatic Insulin Resistance. J Exp Med (2021) 218(3):e20201416 doi: 10.1084/jem.20201416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cignarelli A, Genchi VA, Perrini S, Natalicchio A, Laviola L, Giorgino F. Insulin and Insulin Receptors in Adipose Tissue Development. Int J Mol Sci (2019) 20(3):759. doi: 10.3390/ijms20030759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hudish LI, Reusch JE, Sussel L. β Cell Dysfunction During Progression of Metabolic Syndrome to Type 2 Diabetes. J Clin Invest (2019) 129(10):4001–8. doi: 10.1172/JCI129188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pedersen DJ, Guilherme A, Danai LV, Heyda L, Matevossian A, Cohen J, et al. A Major Role of Insulin in Promoting Obesity-Associated Adipose Tissue Inflammation. Mol Metab (2015) 4(7):507–18. doi: 10.1016/j.molmet.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kumar D, Shankar K, Patel S, Gupta A, Varshney S, Gupta S, et al. Chronic Hyperinsulinemia Promotes Meta-Inflammation and Extracellular Matrix Deposition in Adipose Tissue: Implications of Nitric Oxide. Mol Cell Endocrinol (2018) 477:15–28. doi: 10.1016/j.mce.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 58. Krogh-Madsen R, Plomgaard P, Keller P, Keller C, Pedersen BK. Insulin Stimulates Interleukin-6 and Tumor Necrosis Factor-α Gene Expression in Human Subcutaneous Adipose Tissue. Am J Physiol Metab (2004) 286(2):E234–8. doi: 10.1152/ajpendo.00274.2003 [DOI] [PubMed] [Google Scholar]
- 59. Li Q, Hagberg CE, Silva Cascales H, Lang S, Hyvönen MT, Salehzadeh F, et al. Obesity and Hyperinsulinemia Drive Adipocytes to Activate a Cell Cycle Program and Senesce. Nat Med (2021) 27(11):1941–53. doi: 10.1038/s41591-021-01501-8 [DOI] [PubMed] [Google Scholar]
- 60. Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, et al. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front Immunol (2020) 11:571731. doi: 10.3389/fimmu.2020.571731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kinashi Y, Hase K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front Immunol (2021) 12:673708. doi: 10.3389/fimmu.2021.673708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fasano A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol Rev (2011) 91(1):151–75. doi: 10.1152/physrev.00003.2008 [DOI] [PubMed] [Google Scholar]
- 63. Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, et al. Increased Circulatory Levels of Lipopolysaccharide (LPS) and Zonulin Signify Novel Biomarkers of Proinflammation in Patients With Type 2 Diabetes. Mol Cell Biochem (2014) 388(1–2):203–10. doi: 10.1007/s11010-013-1911-4 [DOI] [PubMed] [Google Scholar]
- 64. Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernández-Real JM. Circulating Zonulin, a Marker of Intestinal Permeability, Is Increased in Association With Obesity-Associated Insulin Resistance. PloS One (2012) 7(5):e37160. doi: 10.1371/journal.pone.0037160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sturgeon C, Fasano A. Zonulin, a Regulator of Epithelial and Endothelial Barrier Functions, and Its Involvement in Chronic Inflammatory Diseases. Tissue Barriers (2016) 4(4):e1251384. doi: 10.1080/21688370.2016.1251384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc Natl Acad Sci USA (2004) 101(44):15718–23. doi: 10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An Obesity-Associated Gut Microbiome With Increased Capacity for Energy Harvest. Nature (2006) 444(7122):1027–31. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 68. Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut Microbiota From Twins Discordant for Obesity Modulate Metabolism in Mice. Science (2013) 341(6150):1241214. doi: 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tran HQ, Bretin A, Adeshirlarijaney A, Yeoh BS, Vijay-Kumar M, Zou J, et al. “Western Diet”-Induced Adipose Inflammation Requires a Complex Gut Microbiota. Cell Mol Gastroenterol Hepatol (2020) 9(2):313–33. doi: 10.1016/j.jcmgh.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gummesson A, Carlsson LMS, Storlien LH, Bäckhed F, Lundin P, Löfgren L, et al. Intestinal Permeability Is Associated With Visceral Adiposity in Healthy Women. Obesity (2011) 19(11):2280–2. doi: 10.1038/oby.2011.251 [DOI] [PubMed] [Google Scholar]
- 71. Hersoug L-G, Møller P, Loft S. Role of Microbiota-Derived Lipopolysaccharide in Adipose Tissue Inflammation, Adipocyte Size and Pyroptosis During Obesity. Nutr Res Rev (2018) 31(2):153–63. doi: 10.1017/S0954422417000269 [DOI] [PubMed] [Google Scholar]
- 72. Mokkala K, Röytiö H, Munukka E, Pietilä S, Ekblad U, Rönnemaa T, et al. Gut Microbiota Richness and Composition and Dietary Intake of Overweight Pregnant Women Are Related to Serum Zonulin Concentration, a Marker for Intestinal Permeability. J Nutr (2016) 146(9):1694–700. doi: 10.3945/jn.116.235358 [DOI] [PubMed] [Google Scholar]
- 73. Kincaid HJ, Nagpal R, Yadav H. Microbiome-Immune-Metabolic Axis in the Epidemic of Childhood Obesity: Evidence and Opportunities. Obes Rev (2020) 21(2):e12963. doi: 10.1111/obr.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine (2020) 51:102590. doi: 10.1016/j.ebiom.2019.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xu J, Liang R, Zhang W, Tian K, Li J, Chen X, et al. Faecalibacterium Prausnitzii-Derived Microbial Anti-Inflammatory Molecule Regulates Intestinal Integrity in Diabetes Mellitus Mice via Modulating Tight Junction Protein Expression. J Diabetes (2020) 12(3):224–36. doi: 10.1111/1753-0407.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang D, Zhang L, Zheng Y, Yue F, Russell RD, Zeng Y. Circulating Zonulin Levels in Newly Diagnosed Chinese Type 2 Diabetes Patients. Diabetes Res Clin Pract (2014) 106(2):312–8. doi: 10.1016/j.diabres.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 77. Hermes GDA, Reijnders D, Kootte RS, Goossens GH, Smidt H, Nieuwdorp M, et al. Individual and Cohort-Specific Gut Microbiota Patterns Associated With Tissue-Specific Insulin Sensitivity in Overweight and Obese Males. Sci Rep (2020) 10(1):7523. doi: 10.1038/s41598-020-64574-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bodogai M, O’Connell J, Kim K, Kim Y, Moritoh K, Chen C, et al. Commensal Bacteria Contribute to Insulin Resistance in Aging by Activating Innate B1a Cells. Sci Transl Med (2018) 10(467):eaat4271. doi: 10.1126/scitranslmed.aat4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dao MC, Sokolovska N, Brazeilles R, Affeldt S, Pelloux V, Prifti E, et al. A Data Integration Multi-Omics Approach to Study Calorie Restriction-Induced Changes in Insulin Sensitivity. Front Physiol (2018) 9:1958. doi: 10.3389/fphys.2018.01958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology (2012) 143(4):913-6.e7. doi: 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 81. Chen Z, Radjabzadeh D, Chen L, Kurilshikov A, Kavousi M, Ahmadizar F, et al. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw Open (2021) 4(7):e2118811. doi: 10.1001/jamanetworkopen.2021.18811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hill JH, Franzosa EA, Huttenhower C, Guillemin K. A Conserved Bacterial Protein Induces Pancreatic Beta Cell Expansion During Zebrafish Development. Elife (2016) 5:e20145. doi: 10.7554/eLife.20145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang Q, Pan Y, Zeng B, Zheng X, Wang H, Shen X, et al. Intestinal Lysozyme Liberates Nod1 Ligands From Microbes to Direct Insulin Trafficking in Pancreatic Beta Cells. Cell Res (2019) 29(7):516–32. doi: 10.1038/s41422-019-0190-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ellekilde M, Selfjord E, Larsen CS, Jakesevic M, Rune I, Tranberg B, et al. Transfer of Gut Microbiota From Lean and Obese Mice to Antibiotic-Treated Mice. Sci Rep (2015) 4(1):5922. doi: 10.1038/srep05922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW. GPCR-Mediated Signaling of Metabolites. Cell Metab (2017) 25(4):777–96. doi: 10.1016/j.cmet.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 86. Tang C, Ahmed K, Gille A, Lu S, Gröne H-J, Tunaru S, et al. Loss of FFA2 and FFA3 Increases Insulin Secretion and Improves Glucose Tolerance in Type 2 Diabetes. Nat Med (2015) 21(2):173–7. doi: 10.1038/nm.3779 [DOI] [PubMed] [Google Scholar]
- 87. Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate Mediates a Microbiome–Brain–β-Cell Axis to Promote Metabolic Syndrome. Nature (2016) 534(7606):213–7. doi: 10.1038/nature18309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fuller M, Priyadarshini M, Gibbons SM, Angueira AR, Brodsky M, Hayes MG, et al. The Short-Chain Fatty Acid Receptor, FFA2, Contributes to Gestational Glucose Homeostasis. Am J Physiol Endocrinol Metab (2015) 309(10):E840–51. doi: 10.1152/ajpendo.00171.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pingitore A, Gonzalez-Abuin N, Ruz-Maldonado I, Huang GC, Frost G, Persaud SJ. Short Chain Fatty Acids Stimulate Insulin Secretion and Reduce Apoptosis in Mouse and Human Islets In Vitro: Role of Free Fatty Acid Receptor 2. Diabetes Obes Metab (2019) 21(2):330–9. doi: 10.1111/dom.13529 [DOI] [PubMed] [Google Scholar]
- 90. Prause M, Pedersen SS, Tsonkova V, Qiao M, Billestrup N. Butyrate Protects Pancreatic Beta Cells From Cytokine-Induced Dysfunction. Int J Mol Sci (2021) 22(19):10427. doi: 10.3390/ijms221910427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang S, Yuan M, Zhang L, Zhu K, Sheng C, Zhou F, et al. Sodium Butyrate Potentiates Insulin Secretion From Rat Islets at the Expense of Compromised Expression of β Cell Identity Genes. Cell Death Dis (2022) 13(1):67. doi: 10.1038/s41419-022-04517-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pingitore A, Chambers ES, Hill T, Maldonado IR, Liu B, Bewick G, et al. The Diet-Derived Short Chain Fatty Acid Propionate Improves Beta-Cell Function in Humans and Stimulates Insulin Secretion From Human Islets In Vitro . Diabetes Obes Metab (2017) 19(2):257–65. doi: 10.1111/dom.12811 [DOI] [PubMed] [Google Scholar]
- 93. Ximenes HMA, Hirata AE, Rocha MS, Curi R, Carpinelli AR. Propionate Inhibits Glucose-Induced Insulin Secretion in Isolated Rat Pancreatic Islets. Cell Biochem Funct (2007) 25(2):173–8. doi: 10.1002/cbf.1297 [DOI] [PubMed] [Google Scholar]
- 94. Kumar DP, Asgharpour A, Mirshahi F, Park SH, Liu S, Imai Y, et al. Activation of Transmembrane Bile Acid Receptor TGR5 Modulates Pancreatic Islet α Cells to Promote Glucose Homeostasis. J Biol Chem (2016) 291(13):6626–40. doi: 10.1074/jbc.M115.699504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Maczewsky J, Kaiser J, Gresch A, Gerst F, Düfer M, Krippeit-Drews P, et al. TGR5 Activation Promotes Stimulus-Secretion Coupling of Pancreatic β-Cells via a PKA-Dependent Pathway. Diabetes (2019) 68(2):324–36. doi: 10.2337/db18-0315 [DOI] [PubMed] [Google Scholar]
- 96. Zaibi MS, Stocker CJ, O’Dowd J, Davies A, Bellahcene M, Cawthorne MA, et al. Roles of GPR41 and GPR43 in Leptin Secretory Responses of Murine Adipocytes to Short Chain Fatty Acids. FEBS Lett (2010) 584(11):2381–6. doi: 10.1016/j.febslet.2010.04.027 [DOI] [PubMed] [Google Scholar]
- 97. Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen J-L, et al. Activation of G Protein-Coupled Receptor 43 in Adipocytes Leads to Inhibition of Lipolysis and Suppression of Plasma Free Fatty Acids. Endocrinology (2008) 149(9):4519–26. doi: 10.1210/en.2008-0059 [DOI] [PubMed] [Google Scholar]
- 98. Moreno-Navarrete JM, Serino M, Blasco-Baque V, Azalbert V, Barton RH, Cardellini M, et al. Gut Microbiota Interacts With Markers of Adipose Tissue Browning, Insulin Action and Plasma Acetate in Morbid Obesity. Mol Nutr Food Res (2018) 62(3). doi: 10.1002/mnfr.201700721 [DOI] [PubMed] [Google Scholar]
- 99. Aguilar EC, da Silva JF, Navia-Pelaez JM, Leonel AJ, Lopes LG, Menezes-Garcia Z, et al. Sodium Butyrate Modulates Adipocyte Expansion, Adipogenesis, and Insulin Receptor Signaling by Upregulation of PPAR-γ in Obese Apo E Knockout Mice. Nutrition (2018) 47:75–82. doi: 10.1016/j.nut.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 100. Wang X, He G, Peng Y, Zhong W, Wang Y, Zhang B. Sodium Butyrate Alleviates Adipocyte Inflammation by Inhibiting NLRP3 Pathway. Sci Rep (2015) 5(1):12676. doi: 10.1038/srep12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Al-Lahham S, Roelofsen H, Rezaee F, Weening D, Hoek A, Vonk R, et al. Propionic Acid Affects Immune Status and Metabolism in Adipose Tissue From Overweight Subjects. Eur J Clin Invest (2012) 42(4):357–64. doi: 10.1111/j.1365-2362.2011.02590.x [DOI] [PubMed] [Google Scholar]
- 102. Al-Roub A, Akhter N, Al-Sayyar A, Wilson A, Thomas R, Kochumon S, et al. Short Chain Fatty Acid Acetate Increases Tnfα-Induced MCP-1 Production in Monocytic Cells via ACSL1/MAPK/NF-κb Axis. Int J Mol Sci (2021) 22(14):7683. doi: 10.3390/ijms22147683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Munukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, et al. Faecalibacterium Prausnitzii Treatment Improves Hepatic Health and Reduces Adipose Tissue Inflammation in High-Fat Fed Mice. ISME J (2017) 11(7):1667–79. doi: 10.1038/ismej.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-Talk Between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc Natl Acad Sci (2013) 110(22):9066–71. doi: 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rodríguez-Carrio J, Salazar N, Margolles A, González S, Gueimonde M, de Los Reyes-Gavilán CG, et al. Free Fatty Acids Profiles Are Related to Gut Microbiota Signatures and Short-Chain Fatty Acids. Front Immunol (2017) 8:823. doi: 10.3389/fimmu.2017.00823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Janssen AWF, Katiraei S, Bartosinska B, Eberhard D, Willems van Dijk K, Kersten S. Loss of Angiopoietin-Like 4 (ANGPTL4) in Mice With Diet-Induced Obesity Uncouples Visceral Obesity From Glucose Intolerance Partly via the Gut Microbiota. Diabetologia (2018) 61(6):1447–58. doi: 10.1007/s00125-018-4583-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, et al. The Gut Microbiota Regulates White Adipose Tissue Inflammation and Obesity via a Family of microRNAs. Sci Transl Med (2019) 11(496):eaav1892. doi: 10.1126/scitranslmed.aav1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yan H, Ajuwon KM. Mechanism of Butyrate Stimulation of Triglyceride Storage and Adipokine Expression During Adipogenic Differentiation of Porcine Stromovascular Cells. PloS One (2015) 10(12):e0145940. Nie D, editor. doi: 10.1371/journal.pone.0145940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Al-Lahham SH, Roelofsen H, Priebe M, Weening D, Dijkstra M, Hoek A, et al. Regulation of Adipokine Production in Human Adipose Tissue by Propionic Acid. Eur J Clin Invest (2010) 40(5):401–7. doi: 10.1111/j.1365-2362.2010.02278.x [DOI] [PubMed] [Google Scholar]
- 110. Creely SJ, McTernan PG, Kusminski CM, Fisher ff M, Da Silva NF, Khanolkar M, et al. Lipopolysaccharide Activates an Innate Immune System Response in Human Adipose Tissue in Obesity and Type 2 Diabetes. Am J Physiol Endocrinol Metab (2007) 292(3):E740–7. doi: 10.1152/ajpendo.00302.2006 [DOI] [PubMed] [Google Scholar]
- 111. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes (2007) 56(7):1761–72. doi: 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 112. Burcelin R, Serino M, Chabo C, Garidou L, Pomié C, Courtney M, et al. Metagenome and Metabolism: The Tissue Microbiota Hypothesis. Diabetes Obes Metab (2013) 15(s3):61–70. doi: 10.1111/dom.12157 [DOI] [PubMed] [Google Scholar]
- 113. Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, et al. Intestinal Mucosal Adherence and Translocation of Commensal Bacteria at the Early Onset of Type 2 Diabetes: Molecular Mechanisms and Probiotic Treatment. EMBO Mol Med (2011) 3(9):559–72. doi: 10.1002/emmm.201100159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Denou E, Lolmède K, Garidou L, Pomie C, Chabo C, Lau TC, et al. Defective NOD 2 Peptidoglycan Sensing Promotes Diet-Induced Inflammation, Dysbiosis, and Insulin Resistance. EMBO Mol Med (2015) 7(3):259–74. doi: 10.15252/emmm.201404169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Anhê FF, Jensen BAH, Varin TV, Servant F, Van Blerk S, Richard D, et al. Type 2 Diabetes Influences Bacterial Tissue Compartmentalisation in Human Obesity. Nat Metab (2020) 2(3):233–42. doi: 10.1038/s42255-020-0178-9 [DOI] [PubMed] [Google Scholar]
- 116. Massier L, Chakaroun R, Tabei S, Crane A, Didt KD, Fallmann J, et al. Adipose Tissue Derived Bacteria Are Associated With Inflammation in Obesity and Type 2 Diabetes. Gut (2020) 69(10):1796–806. doi: 10.1136/gutjnl-2019-320118 [DOI] [PubMed] [Google Scholar]
- 117. Bakker GJ, Meijnikman AS, Scheithauer TP, Davids M, Aydin Ö, Boerlage TCC, et al. Fecal Microbiota Transplantation Does Not Alter Bacterial Translocation and Visceral Adipose Tissue Inflammation in Individuals With Obesity. Obes Sci Pract (2021)8(1):56-65. doi: 10.1002/osp4.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lluch J, Servant F, Païssé S, Valle C, Valière S, Kuchly C, et al. The Characterization of Novel Tissue Microbiota Using an Optimized 16s Metagenomic Sequencing Pipeline. PloS One (2015) 10(11):e0142334. Heimesaat MM, editor. doi: 10.1371/journal.pone.0142334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zulian A, Cancello R, Cesana E, Rizzi E, Consolandi C, Severgnini M, et al. Adipose Tissue Microbiota in Humans: An Open Issue. Int J Obes (2016) 40(11):1643–8. doi: 10.1038/ijo.2016.111 [DOI] [PubMed] [Google Scholar]
- 120. Gibson GR, Roberfroid MB. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J Nutr (1995) 125(6):1401–12. doi: 10.1093/jn/125.6.1401 [DOI] [PubMed] [Google Scholar]
- 121. Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective Increases of Bifidobacteria in Gut Microflora Improve High-Fat-Diet-Induced Diabetes in Mice Through a Mechanism Associated With Endotoxaemia. Diabetologia (2007) 50(11):2374–83. doi: 10.1007/s00125-007-0791-0 [DOI] [PubMed] [Google Scholar]
- 122. Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes (2011) 60(11):2775–86. doi: 10.2337/db11-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tan S, Caparros-Martin JA, Matthews VB, Koch H, O’Gara F, Croft KD, et al. Isoquercetin and Inulin Synergistically Modulate the Gut Microbiome to Prevent Development of the Metabolic Syndrome in Mice Fed a High Fat Diet. Sci Rep (2018) 8(1):10100. doi: 10.1038/s41598-018-28521-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fabersani E, Abeijon-Mukdsi MC, Ross R, Medina R, González S, Gauffin-Cano P. Specific Strains of Lactic Acid Bacteria Differentially Modulate the Profile of Adipokines In Vitro . Front Immunol (2017) 8:266. doi: 10.3389/fimmu.2017.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ashrafian F, Shahriary A, Behrouzi A, Moradi HR, Keshavarz Azizi Raftar S, Lari A, et al. Akkermansia Muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front Microbiol (2019) 10:2155. doi: 10.3389/fmicb.2019.02155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Klemashevich C, Wu C, Howsmon D, Alaniz RC, Lee K, Jayaraman A. Rational Identification of Diet-Derived Postbiotics for Improving Intestinal Microbiota Function. Curr Opin Biotechnol (2014) 26:85–90. doi: 10.1016/j.copbio.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 127. Weitkunat K, Stuhlmann C, Postel A, Rumberger S, Fankhänel M, Woting A, et al. Short-Chain Fatty Acids and Inulin, But Not Guar Gum, Prevent Diet-Induced Obesity and Insulin Resistance Through Differential Mechanisms in Mice. Sci Rep (2017) 7(1):6109. doi: 10.1038/s41598-017-06447-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Brial F, Alzaid F, Sonomura K, Kamatani Y, Meneyrol K, Le Lay A, et al. The Natural Metabolite 4-Cresol Improves Glucose Homeostasis and Enhances β-Cell Function. Cell Rep (2020) 30(7):2306–20.e5. doi: 10.1016/j.celrep.2020.01.066 [DOI] [PubMed] [Google Scholar]
- 129. Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of Insulin Sensitivity After Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab (2017) 26(4):611–619.e6. doi: 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 130. Wang H, Lu Y, Yan Y, Tian S, Zheng D, Leng D, et al. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Front Cell Infect Microbiol (2019) 9:455. doi: 10.3389/fcimb.2019.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]