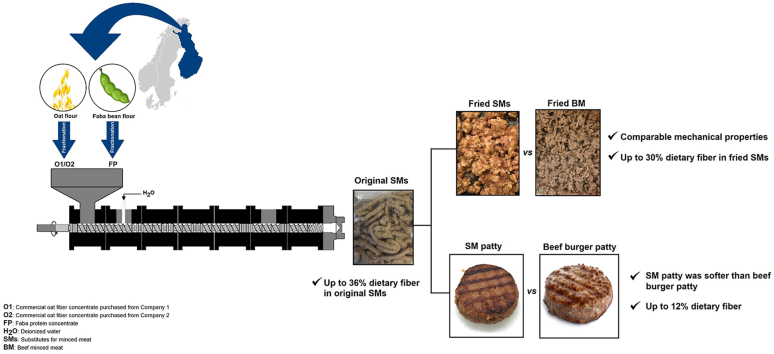
Abstract
Oat has been recognized for its health-promoting fiber, β-glucan, while protein-rich faba bean has remained underutilized in Nordic countries despite its good nutritional quality. This research investigated the functionality of oat fiber concentrate and faba bean protein concentrate in plant-based substitutes for minced meat (SMs). The resulting product aimed at mimicking the mechanical and physicochemical characteristics of beef minced meat (BM) and its applications (i.e., fried and burger patty). In this regard, the mechanical properties (e.g., chewiness, Young's modulus) of original/fried SMs were comparable to or higher than those of original/fried BM. SM patties (45% SMs) were structurally weaker than beef burger patties (100% BM). The rheological analysis showed that the presence of oat fiber concentrate increased the gel-like properties of the blend, which correlated with the overall strength of original SMs (e.g., Young's modulus). The results suggested that SMs could be used as BM for the preparation of vegetarian meat-like products.
Keywords: Faba bean, β-glucan, Protein concentrate, Extrusion, Meat substitute
Graphical abstract
Highlights
-
•
SMs containing up to 36% dietary fiber—the half being β-glucan—were obtained.
-
•
Oat-fiber- and faba-bean-protein-concentrate were successfully combined into SMs.
-
•
SMs were mechanically comparable to beef minced meat.
-
•
Patties containing SMs were softer than beef patties.
-
•
Oat fiber concentrate increased the gel-like properties of the blend.
Abbreviation
- SMs
Substitutes for minced meat
- BM
Beef minced meat
- O1
Commercial oat fiber concentrate purchased from Company 1
- O2
Commercial oat fiber concentrate purchased from Company 2
- FP
Faba bean protein concentrate
Storage modulus
Loss modulus
Calculated storage modulus
Calculated loss modulus
Exponent of relaxation corresponding to
Exponent of relaxation corresponding to
Exponent of relaxation corresponding to the polymer blending law
1. Introduction
Plant-based protein concentrates/isolates have become essential ingredients for the development of meat substitutes (burger patty, shredded meat, sausages etc.) when aiming at decreasing the consumption of animal-based foods. In 2017, the European market share for meat substitutes was estimated to be the world's largest (39%; Mordor Intelligence, 2018). However, there are concerns about the sustainability of some raw materials. For instance, most of the production of meat substitutes revolves around popular soybean. The growth of soybean harvesting area has serious implications in biodiversity loss, particularly, in the Amazon rainforest (Baletti, 2012; FAOSTAT). Contrary to this, oat, and faba bean—which can be cultivated in the Northern hemisphere—have been insufficiently exploited for the development of meat substitutes.
Interest in oat is linked to the water-soluble mixed linkage (1 → 3)(1 → 4)-β-D-glucan, which upon consumption has been proven to reduce the incidence of cardiovascular diseases via the reduction of plasma cholesterol levels (Othman et al., 2011). Additionally, the viscosity of β-glucan (in the upper digestive track) has been associated with the suppression of postprandial rise in blood glucose (Regand et al., 2011). As previously reported (Mälkki and Virtanen, 2001; Wood, 2010; Tosh, 2013), the viscosity of β-glucan (associated with the degree of solubility/extractability in water) seems to impede digestive enzymes from hydrolyzing substrates thereby regulating the absorption of nutrients. These health benefits have led to the approval of various health claims in the USA (FDA, 2012) and the EU (EFSA Panel on Dietetic Products, Nutrition and Allergies, 2009, 2010, 2011). Another nutritious yet poorly consumed grain legume is faba bean. Despite its nitrogen-fixating characteristics and high protein content/quality (Mayer-Labba et al., 2021), faba bean has been more widely used for animal feed than for human consumption in various places including the Northern hemisphere. Based on the comparative study conducted by Stone et al. (2019), native faba bean flour had substantially higher protein content (33.2%) than lentils (var. red, 29%), peas (var. green, 25%; var. yellow, 25%) and chickpeas (var. desi, 23%; var. kabuli, 17%), but lower content than soybean (39%). Regarding anti-nutritional factors, wider availability of low/zero-vicine and -convicine cultivars has further improved the faba bean's properties and suitability for food use (Khazaei et al., 2019; Mayer-Labba et al., 2021).
Faba bean is mostly consumed as whole grain, and has experimentally been used for (i) the production of pasta/bread (Farooq and Boye, 2011), (ii) development of extruded snacks (Smith and Hardacre, 2011) and (iii) nutritionally enrichment of wheat bread (Coda et al., 2017). Oat, on the other hand, has already gained popularity in applications like oat drink and bread. Given its content of water-binding soluble fiber (e.g., β-glucan), the incorporation of oat could contribute to the production of meat substitutes. In fact, soluble fiber could boost the hydration capacity of protein-based polymeric systems. Even though there are some studies dealing with extrusion of oat and faba bean for snack production (Liu et al., 2000; Farooq and Boye, 2011), no study—to the best of our knowledge—combines oat and faba bean (as oat fiber concentrate and faba bean protein concentrate) for the development of extruded meat substitutes unless plant protein isolate is involved. It should be noted that faba bean in combination with oat results in excellent amino acid composition.
Wet extrusion (>50% water content of blend) is the most popular technology for the production of plant-based meat substitutes. During extrusion, mechanical shearing at high temperatures and pressures modifies the physicochemical state of the ingredients. Thus, novel structures could be obtained using extrusion, if e.g., traditional ingredients are processed under optimal and controlled conditions. The behavior of oat (containing β-glucan) and faba bean (containing protein) ingredients during extrusion could reveal changes in melt viscosity of large polymeric components (Choi et al., 2003; Philipp et al., 2018).
The aim of the present study was to investigate the functionality of oat fiber concentrate on the mechanical, physicochemical, and rheological characteristics of faba-bean-protein-concentrate-containing substitutes for minced meat—through wet extrusion—and presented as original and fried. Additionally, the use of these substitutes in a popular food application, patty, was studied. The substantial ratios of β-glucan (present in oat fiber concentrate) should allow health claims to be carried, thereby providing not only a sustainable but also a truly healthy substitute for minced meat.
2. Materials and methods
2.1. Materials
Two commercial oat fiber concentrates (O1 and O2) and one commercial faba bean protein concentrate (FP) were purchased from companies in Finland. The manufacturing companies used locally grown oat (Avena sativa) and faba bean (Vicia faba) as native ingredients. Oat fiber concentrate and faba bean protein concentrate were manufactured by a dry fractionation process. Beef minced meat [BM; 10% fat (as is basis), Snellman Ltd., Finland] was used as a control sample.
Total dietary fiber (soluble and insoluble fiber) was determined with the AOAC 991.43 method, while soluble dietary fiber in oat ingredients was determined as β-glucan with the AOAC 995.16 method. Moisture content of concentrates was measured according to AACC 44–15.02 method. Protein content was determined with the Dumas combustion method (Vario MAX CN, Germany) using a nitrogen-to-protein conversion factor of 6.25. Fat content was determined as the sum of fatty acid methyl esters. Fat was extracted with acetone using an ASE instrument (Dionex ASE-200, Dionex Corporation, Sunnyvale, CA) (Lampi et al., 2015). The extracted lipids were methylated, and subsequently analyzed with a gas chromatographic method according to Liu et al. (2018). Chemical compositions of concentrates are shown in Table 1.
Table 1.
Measured composition of raw materials (O1 and O2, oat fiber concentrates; FP, faba bean protein concentrate), and calculated composition of original substitutes for minced meat (SMs), fried SMs and SM patties.
|
Content based on 100 g d.m. |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture content, % | Protein, g | Fat, g | Dietary fiber, g | Insoluble fiber, g | Soluble fiber, g | Starch1 | |||
| O1 | 100 | 8.6 ± 0.2 | 24.8 ± 0.1 | 4.4* | 43.9* | 23.1* | 20.82* | 26.9 | |
| O2 | 100 | 7.7 ± 0.3 | 23.1 ± 0.0 | 7.2* | 35.5* | 19.6* | 15.92* | 34.2 | |
| FP | 100 | 6.5 ± 0.2 | 53.6 ± 0.3 | 2.4* | 11.2* | 11.2* | 1.3* | 32.8 | |
| Original | O1:FP | 25 : 75 | 58.8 ± 0.9 | 46.4 | 2.9 | 19.4 | 14.2 | 6.2 | 31.3 |
| 50 : 50 | 59.5 ± 0.8 | 39.2 | 3.4 | 27.6 | 17.2 | 11.1 | 29.8 | ||
| 75 : 25 | 62.8 ± 0.3 | 32.0 | 3.9 | 35.7 | 20.1 | 15.9 | 28.4 | ||
| O2:FP | 25 : 75 | 64.6 ± 0.5 | 46.0 | 3.6 | 17.3 | 13.3 | 5.0 | 33.2 | |
| 50 : 50 | 64.6 ± 0.7 | 38.4 | 4.8 | 23.4 | 15.4 | 8.6 | 33.5 | ||
| 75 : 25 | 65.8 ± 1.5 | 30.7 | 6.0 | 29.4 | 17.5 | 12.3 | 33.8 | ||
| Control3 | 68.6 ± 0.3 | 60.5** | 54.1** | – | – | – | – | ||
| Fried | O1:FP | 25 : 75 | 32.4 ± 5.1 | 41.2 | 15.5 | 17.2 | 11.7 | 5.5 | 26.2 |
| 50 : 50 | 31.6 ± 4.0 | 34.9 | 15.9 | 24.5 | 14.7 | 9.8 | 24.8 | ||
| 75 : 25 | 41.1 ± 0.9 | 28.0 | 17.9 | 31.2 | 17.3 | 13.9 | 23.0 | ||
| O2:FP | 25 : 75 | 35.0 ± 3.0 | 40.6 | 16.8 | 15.3 | 10.9 | 4.4 | 27.3 | |
| 50 : 50 | 39.3 ± 3.6 | 33.6 | 18.9 | 20.5 | 12.9 | 7.5 | 27.0 | ||
| 75 : 25 | 42.0 ± 1.4 | 26.8 | 20.8 | 25.6 | 15.0 | 10.7 | 26.7 | ||
| Control4 | 37.0 ± 1.8 | 44.3** | 46.4** | – | – | – | – | ||
| Patty | O1:FP | 25 : 75 | 29.0* | 19.9 | 29.2 | 8.2 | 6.6 | 1.6 | 42.7 |
| 50 : 50 | 29.3* | 17.8 | 29.3 | 10.2 | 7.4 | 2.8 | 42.6 | ||
| 75 : 25 | 30.8* | 15.3 | 29.3 | 11.6 | 7.9 | 3.7 | 43.7 | ||
| O2:FP | 25 : 75 | 31.6* | 18.1 | 29.2 | 7.1 | 6.0 | 1.1 | 45.6 | |
| 50 : 50 | 31.6* | 16.4 | 29.5 | 8.4 | 6.5 | 1.9 | 45.7 | ||
| 75 : 25 | 32.1* | 14.5 | 29.7 | 9.6 | 6.9 | 2.6 | 46.3 | ||
| Control5 | 66.1 ± 2.3 | 56.4** | 57.2** | – | – | – | – | ||
Average value ± standard deviation.
1Starch content calculated as 100 – (protein + fat + dietary fiber contents); 2Determine as β-glucan content in O1 and O2, and as total soluble fiber in FP; 3Beef minced meat, 4Pan-fried beef minced meat; 5Beef burger patty.
*Test conducted in duplicate; **Based on information provided by the producer.
The capacity of O1 and FP to absorb or dissolve in Milli-Q water (WAI/WSI) was measured by following the method developed by Anderson et al. (1970) (Appendix section).
2.2. Extrusion process
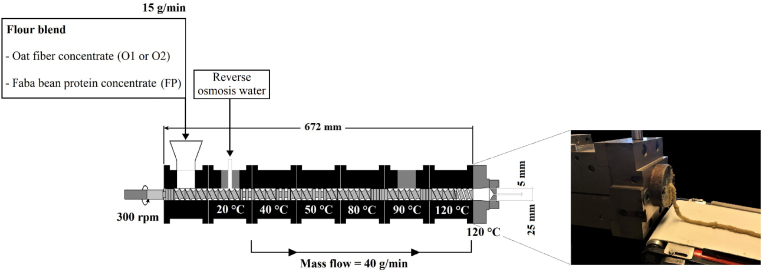
Six substitutes for minced meat (SMs) varying in type of oat fiber concentrate (O1 and O2) and oat-fiber-concentrate:faba-bean-protein-concentrate (O1:FP or O2:FP) ratios (25:75, 50:50 and 75:25) were prepared under the same extrusion conditions; a factorial design of 2 (replica) 2 (type) 3 (ratios) was followed. SMs were prepared using a twin-screw laboratory extruder (Thermo Prism PTW24, Thermo Haake, Polylab System, Germany) with a short concentric die (diameter, 5 mm). The extruder barrel consisted of seven sections—from which the temperature of six sections could be controlled (heating and cooling system setup)—and a heatable die; processing conditions are specified in Fig. 1. Reverse osmosis water was the only liquid ingredient fed into the extruder (65% water content). After extrusion, the original SMs were cooled down for 5 min at room temperature, and subsequently stored at 20 °C in polyethylene zip-lock bags. Cold thawing (5 °C for 72 h) in a confined space was applied prior to the analyses. The moisture content of SMs and BM was measured according to the AOAC 950.46 method.
Fig. 1.
Technical description of the twin-screw extruder used for the production of substitutes for minced meat (SMs) containing oat fiber concentrate and faba bean protein concentrate.
2.3. Determination of viscoelastic properties of selected oat fiber concentrate and faba bean protein concentrate blends
Viscoelasticity of blends (def. dough resulting from the combination of oat fiber concentrate and faba protein concentrate with 65% water) was assessed using temperature conditions and shear forces that simulated (to some extent) extrusion processing. Five blends were prepared using different O1:FP ratios (100:0, 75:25, 50:50, 25:75, 0:100) at 65% water content. The storage () and loss modulus () of blends were determined by an Advanced Polymer Analyzer APA2000 (Alpha Technologies, Germany). Parallel conical plates with a diameter of 75 mm were utilized. The experiment was conducted in two consecutive phases. Temperature-sweep test (1) was performed as a function of increasing (from 80 to 160 °C) and decreasing (from 160 to 70 °C) temperature. Frequency-sweep test (2) was performed as a function of frequency ranging from 0.1 to 10 Hz at 70 °C. The experimental values of G′ and G″ were numerically examined for structural traits using Equations (1), (2), defined as
| (1) |
| (2) |
where and represent the deformation of and , and the gap between and relates to the strength of intermolecular interactions of gel networks. Besides, n’ and n’’ (relaxation exponents) indicate the rate of change of and with increasing frequency, . According to Campo and Tovar (2008), a stable gel should exhibit the same proportional change in and with frequency over a wide range, thereby showing almost identical n’ and n’‘. Additionally, the rheological response of blends was described using the polymer blending law (Morris, 1992, Equation (3)) defined as
| (3) |
where GX, GY and GXY are the dynamic moduli [G* = f(T)] corresponding to FP, O1 and the blend, and φX and φY are the fractions of FP and O1. The calculated exponent of relaxation, , indicates whether the blend consists of a strong continuous phase ( = 1), a weak discontinuous/dispersed phase ( = −1), or a bi-continuous phase ( = 0.2).
2.4. Food applications
Fried SMs: Frozen original SMs were stored in a cold room (5 °C) for at least 12 h prior to frying. A frying pan was heated up for 1 min, and then 8 ml of rapeseed oil was added and allowed to heat up for 30 s. SMs (85 g) were then added and stirred constantly for 3 min. The product was removed and placed on a paper towel for 30 s. Fried SMs were cooled to room temperature prior to analysis. Fried BM (following the described protocol) acted as control sample. SM Patty (vegetarian patty containing SMs and other ingredients): The formulated patties, prepared by a professional cook, were meant to resemble beef patties externally (crust) and internally (structure). A culinary formulation was applied for the development of patties containing (in all cases) around 45% original SMs. Among the main ingredients, there were cooked and mashed quinoa grains (16%; Lantmännen Cerealia Ltd., Finland), chick pea flour (7%; Risenta Ltd., Sweden), corn starch (7%; Unilever Finland Ltd., Finland), fresh eggs (7%; Kieku Ltd., Finland), coconut oil (7%; Haugen-Gruppen Ltd., Denmark) and cocoa butter (7%; Foodin – Rawmance Ltd., Finland). Minor ingredients included agar (1%; NewCakes Ltd., The Netherlands), maltodextrin-based thickener (1%; El Bulli Ltd., Spain), yeast (1%; Valioravinto Ltd., Finland) and beetroot extract [0.5–1%; dry ground beetroot (58 °C/14 h) was mixed with water (1:10), and the supernatant subsequently added]. Each formulated SM patty was fried in 5 ml of rapeseed oil for 3 min. For the control, 100% BM was used with no additional ingredients.
The chemical composition of fried SMs and patties was calculated from (i) the analyzed composition of oat fiber concentrate (O1 and O2) and FP (Table 1), and (ii) the producer's specifications. Water loss and fat absorption were taken into account during the calculations.
2.5. Determination of the mechanical properties and color of original SMs, fried SMs, and SM patties
Mechanical properties were measured using a Texture Analyzer TAXT2i (50-kg load cell; Stable Micro Systems, Godalming, Surrey, England). For texture profile analysis (TPA), a platform-fixed aluminum cubical sample holder (inner volume, 26 mm 26 mm 25 mm) was filled with original SMs or fried SMs, while a squared flat probe (25 mm 25 mm 6 mm; fixed to the Texture Analyzer's mechanical arm; miniature Ottawa cell set-up) performed two sequential vertical descents. The settings used were: pre-test speed, 1 mm/s; test speed, 1 mm/s; post-test speed, 5 mm/s; deformation distance, 5 mm; resting time, 5 s; trigger force, 5 g. A sample holder was not needed for the analysis of SM patties due to their uniformity upon cutting (26 mm 26 mm 1.5 mm). Given the substantial structural changes of SMs—upon processing—to become fried SMs and SM patties, descriptors usually attributed to semisolid or solid foods were measured. Each measurement resulted in a two-cycle force-time curve from which gumminess (def'n: energy required to disintegrate a food product prior to swallowing; Szczesniak, 1963), springiness, chewiness (def'n: energy required to masticate a food product prior to swallowing; Szczesniak, 1963) and Young's modulus were calculated (Equations (4), (5), (6), (7))):
| (4) |
| (5) |
| (6) |
| (7) |
where A1 or A2 is the area-to-peak force corresponding to cycle 1 or cycle 2, respectively; is the maximum force corresponding to area A1; surface L1 or L2 is the length of time corresponding to A1 or A2, respectively; surface is the sample area in direct contact with the probe; strain is the amount of deformation in the direction of the applied force divided by the initial height of the material. For shear test, an aluminum cubical sample holder (inner volume, 26 mm 26 mm 25 mm) fixed to a slot-perforated platform was filled with original SMs, fried SMs or SM patty, while a 5-bladed head (overall volume, 44 mm 25 mm 24.5 mm; blade width, 2.5 mm; gap, 3 mm; fixed to the Texture Analyzer's mechanical arm; Kramer shear cell set-up) performed one vertical descent. The settings used were: test speed, 1 mm/s; penetration distance, 30 mm. The measured force-time curve was used to calculate the energy caused by adhesive and frictional forces (AFF; negative area), shear energy (positive area) and hardness (maximum force).
For color analysis, the original or fried SMs (15–20 g) were placed on a Petri dish and pressed into a planar surface, while SM patties were diced into cubes (23 mm 23 mm 1.5 mm), thereby making the crust and inner side observable. The color space parameters L*, a*, and b* were measured (L*: lightness; a*: redness; b*: yellowness) using a Minolta CR-400 chromometer (Konica Minolta Sensing, Inc., Osaka, Japan). Redness (+a*) and yellowness (+b*) are jointly referred to as ‘brightness’ in the present study. The color difference between the extrudates and control samples (ΔE) was determined according to Equation (8):
| (8) |
2.6. Statistical analysis
Measurements were mostly conducted in triplicate (except for the determination of fat, dietary fiber, β-glucan and G’/G″) and the results were expressed as means and standard deviation/error. Statistical comparison between tested and control samples (beef minced meat presented as raw, fried or patty) was conducted via one-way ANOVA in SPSS (SPSS 18.0, PASW Statistics, Chicago, IL, USA) (Table 2, Table 3). The main and interaction effects of three factors (replica [two]; type [O1 or O2]; content of oat fiber concentrate [25:75, 50:50 and 75:25]) on the properties of original SMs, fried SMs and SM patties were statistically analyzed by using a two-way repeated-measures ANOVA in SPSS. The tables of analyses corresponding to original SMs, fried SMs and SM patties are presented in Appendix C, D and E, respectively.
Table 2.
Mechanical and physicochemical properties of substitutes for minced meat (SM) tested as original SMs, fried SMs and SM patty.
|
Texture profile analysis (TPA) |
Shear test |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gumminess, N | Springiness | Chewiness, N | Young's modulus, kPa | AFF, N.s | Shear energy, N.s | Hardness, N | |||
| Original | O1:FP | 25 : 75 | 2.2cd ± 0.1 | 0.82a±0.02 | 1.8cd ± 0.1 | 24.8b ± 1.0 | 22.4ab ± 2.2 | 1445.7b ± 65.5 | 147.0b ± 5.1 |
| 50 : 50 | 2.9ab ± 0.2 | 0.87a±0.02 | 2.5a±0.2 | 30.5a±1.9 | 16.2cd ± 1.8 | 1106.6cd ± 87.8 | 100.6cde ± 9.2 | ||
| 75 : 25 | 3.9a±0.6 | 0.83a±0.03 | 3.2a±0.5 | 34.1a±3.9 | 27.2a±1.1 | 1466.1b ± 58.3 | 139.0b ± 6.1 | ||
| O2:FP | 25 : 75 | 2.7b ± 0.2 | 0.83a±0.02 | 2.3ab ± 0.2 | 29.8a±1.8 | 22.3b ± 1.3 | 1274.3c±42.5 | 101.8c±3.0 | |
| 50 : 50 | 2.4bc±0.1 | 0.86a±0.02 | 2.1bc±0.1 | 25.1b ± 0.8 | 22.3bc±1.8 | 1175.5c±35.1 | 93.7d ± 1.4 | ||
| 75 : 25 | 2.0d ± 0.1 | 0.84a±0.01 | 1.7d ± 0.1 | 21.2c±0.9 | 15.7d ± 1.1 | 974.3d ± 57.0 | 83.4e±5.0 | ||
| Control1 | 1.1e±0.1 | 0.6b ± 0.01 | 0.7e±0.0 | 16.3d ± 1.4 | 19.6c±1.4 | 1795.9a±81.4 | 268.2a±10.4 | ||
| Fried | O1:FP | 25 : 75 | 3.4a±0.2 | 0.84ab ± 0.03 | 2.9a±0.3 | 68.1a±3.9 | n.d. | n.d. | n.d. |
| 50 : 50 | 3.1a±0.2 | 0.80bc±0.02 | 2.5abc±0.2 | 69.0a±4.7 | n.d. | n.d. | n.d. | ||
| 75 : 25 | 1.6c±0.2 | 0.79c±0.01 | 1.3e±0.2 | 53.9b ± 4.2 | n.d. | n.d. | n.d. | ||
| O2:FP | 25 : 75 | 3.5a±0.2 | 0.80bc±0.02 | 2.8a±0.1 | 39.1c±2.0 | n.d. | n.d. | n.d. | |
| 50 : 50 | 3.5a±0.2 | 0.83b ± 0.02 | 2.9a±0.2 | 36.9c±2.4 | n.d. | n.d. | n.d. | ||
| 75 : 25 | 2.3b ± 0.2 | 0.82b ± 0.02 | 1.9cd ± 0.2 | 25.6d ± 2.1 | n.d. | n.d. | n.d. | ||
| Control2 | 2.0bc±0.1 | 0.9a±0.02 | 1.7d ± 0.1 | 20.0e±0.8 | n.d. | n.d. | n.d. | ||
| Patty | O1:FP | 25 : 75 | 1.3b ± 0.04 | 0.34c±0.01 | 0.4c±0.02 | 23.1a±0.6 | 8.3c±1.2 | 881.9c±78.9 | 76.1c±8.6 |
| 50 : 50 | 1.0cd ± 0.09 | 0.32d ± 0.01 | 0.3d ± 0.03 | 17.4bc±1.5 | 9.4b ± 1.4 | 797.0c±58.4 | 76.2c±8.3 | ||
| 75 : 25 | 1.4b ± 0.08 | 0.39b ± 0.01 | 0.6b ± 0.04 | 23.4a±1.2 | 9.8b ± 1.2 | 1016.7bc±126.3 | 90.7bc±16.1 | ||
| O2:FP | 25 : 75 | 1.0c±0.06 | 0.37bc±0.01 | 0.3d ± 0.03 | 18.1b ± 1.2 | 7.3cd ± 1.4 | 643.1d ± 86.7 | 64.3c±10.3 | |
| 50 : 50 | 0.8d ± 0.05 | 0.38b ± 0.01 | 0.3d ± 0.03 | 14.0e±0.6 | 5.7d ± 0.7 | 665.2d ± 61.3 | 71.2c±4.3 | ||
| 75 : 25 | 1.5b ± 0.20 | 0.57a±0.07 | 1.0b ± 0.21 | 14.6dce±0.9 | 12.8b ± 2.1 | 1199.2b ± 123.9 | 120.8b ± 17.9 | ||
| Control3 | 2.8a±0.5 | 0.69a±0.02 | 1.9a±0.35 | 20.4abd±2.8 | 31.2a±3.3 | 5094.4a±286.6 | 390.3a±36.1 |
Average value ± standard error.
Same letter means no difference at a significance level of 5%. Comparison conducted within separate groups: original, fried and patty.
1Beef minced meat, 2Pan-fried beef minced meat; 3Beef burger patty.
Table 3.
L*a*b* color space and ΔE corresponding to substitutes for minced meat (SM) tested as original substitutes for minced meat (SMs), fried SMs and SM patty.
| L* | a* | b* | ΔE | |||
|---|---|---|---|---|---|---|
| Original | O1:FP | 25 : 75 | 62.8b ± 0.2 | 1.3d ± 0.1 | 16.7a±0.15 | 21.6b ± 0.62 |
| 50 : 50 | 63.9a±0.2 | 1.8bc±0.1 | 16.9a±0.11 | 22.6a±0.66 | ||
| 75 : 25 | 64.5a±0.2 | 2.1b ± 0.1 | 16.3a±0.15 | 22.8a±0.67 | ||
| O2:FP | 25 : 75 | 59.6d ± 0.2 | 1.0e±0.03 | 14.7c±0.14 | 18.9d ± 0.63 | |
| 50 : 50 | 61.4c±0.1 | 1.4d ± 0.02 | 15.1b ± 0.08 | 20.1c±0.64 | ||
| 75 : 25 | 62.7b ± 0.2 | 1.7c±0.0 | 15.3b ± 0.12 | 21.2b ± 0.64 | ||
| Control1 | 45.1e±0.8 | 12.7a±0.6 | 12.5d ± 0.40 | |||
| Fried | O1:FP | 25 : 75 | 55.3b ± 0.5 | 2.8de ± 0.15 | 16.9c±0.19 | 14.5c±0.95 |
| 50 : 50 | 56.5b ± 0.3 | 3.1bc±0.10 | 17.4b ± 0.13 | 16.0b ± 0.95 | ||
| 75 : 25 | 57.8a±0.3 | 3.4b ± 0.13 | 17.9a±0.13 | 17.1a±0.74 | ||
| O2:FP | 25 : 75 | 53.4c±0.5 | 2.6e±0.14 | 15.6e±0.24 | 12.2d ± 0.74 | |
| 50 : 50 | 53.9c±0.3 | 3.0c±0.14 | 16.4d ± 0.20 | 13.1d ± 0.63 | ||
| 75 : 25 | 56.0b ± 0.4 | 2.9cd ± 0.13 | 17.0c±0.19 | 15.4bc±0.76 | ||
| Control2 | 42.8d ± 0.9 | 5.0a±0.1 | 10.0f±0.53 | |||
| Patty (crust) | O1:FP | 25 : 75 | 37.5a±0.8 | 10.8ab ± 0.3 | 18.3a±0.7 | 12.8b ± 1.3 |
| 50 : 50 | 37.2a±1.0 | 11.7a±0.3 | 18.2a±0.8 | 13.6ab ± 1.2 | ||
| 75 : 25 | 37.8a±1.1 | 11.4ab ± 0.5 | 18.8a±1.0 | 12.4b ± 1.3 | ||
| O2:FP | 25 : 75 | 39.8a±1.3 | 10.8ab ± 0.3 | 20.4a±1.2 | 15.3a±1.5 | |
| 50 : 50 | 40.1a±1.4 | 11.5ab ± 0.5 | 20.8a±1.2 | 15.8a±1.7 | ||
| 75 : 25 | 39.8a±1.3 | 10.7b ± 0.3 | 20.7a±1.2 | 15.7a±1.6 | ||
| Control3 | 30.894b ± 2.0 | 6.2c±0.2 | 11.3b ± 1.1 | |||
| Patty (Inner side) | O1:FP | 25 : 75 | 49.0b ± 0.9 | 11.3ab ± 0.4 | 24.3bc±0.3 | 10.5c±0.5 |
| 50 : 50 | 50.3ab ± 1.1 | 12.0a±0.4 | 23.6c±0.3 | 11.5bc±0.8 | ||
| 75 : 25 | 52.7a±0.7 | 11.6a±0.4 | 24.4b ± 0.2 | 11.7b ± 0.6 | ||
| O2:FP | 25 : 75 | 53.0a±1.0 | 10.3b ± 0.4 | 24.6ab ± 0.4 | 10.9c±0.7 | |
| 50 : 50 | 53.3a±1.3 | 10.5ab ± 0.6 | 25.2a±0.2 | 12.5ab ± 0.6 | ||
| 75 : 25 | 53.7a±1.4 | 10.3b ± 0.5 | 24.8ab ± 0.4 | 13.0a±0.9 | ||
| Control3 | 48.3b ± 0.7 | 6.6c±0.2 | 16.2d ± 0.3 |
Same letter means no difference at a significant level of 5%. Comparison conducted within separate groups: original, fried, patty crust and patty inside.
1Beef minced meat, 2Pan-fried beef minced meat; 3Beef burger patty.
Principal component analyses (PCA) of mechanical and physicochemical measurements enabled sample characterization and identification of correlating patterns (SIMCA 15.0.2, MKS Umetrics, Malmö, Sweden). The root mean square error (RMSE), corresponding to the fitting of parameter n (exponent of relaxation), was minimized using the Solver function of Excel (2016, 64-Bit Edition, Microsoft Corp., Washington, USA).
3. Result and discussion
3.1. Role of composition in properties of original SMs
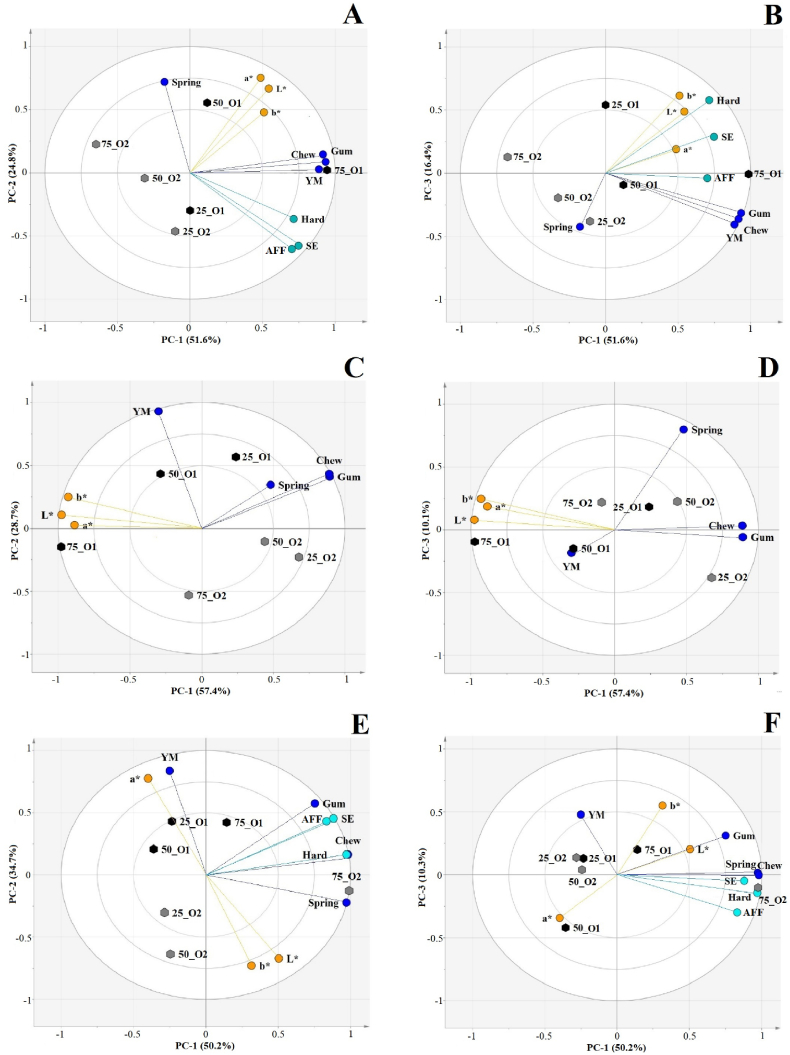
In general, oat fiber concentrate—in terms of content (25, 50 and 75%), type (O1 or O2) and potential interactions (content*type)—had noticeable effects on the structure of original SMs. Gumminess was the most sensitive characteristic in view of the type [F (1,4) = 10.6, p = 0.031] and content [F (2,8) = 5.0, p = 0.039] of oat fiber concentrate (Table 2). While higher incorporation of O1 increased gumminess by almost 80%, O2 had the opposite effect, reducing gumminess by around 25% [interaction, F (2,8) = 41.2, p = 0.0001]. A less dramatic yet similar trend was observed with chewiness [interaction, F (2,8) = 37.6, p = 0.0001] and Young's modulus [interaction, F(2,8) = 32.5, p = 0.0001], where higher incorporation of O1 and O2 had opposite effects. Although the type and content of oat fiber concentrate had considerable effects on the shear energy [type, F (1,4) = 42.5, p = 0.003; content, F (2,8) = 9.5, p = 0.008] and hardness [type, F (1,4) = 192.8, p = 0.0001; content, F (2,8) = 18.2, p = 0.001] of original SMs, the direction of the effects varied substantially [shear energy, interaction, F (2,8) = 6.9, p = 0.018; hardness, interaction, F (2,8) = 10.851, p = 0.005]. For instance, the addition of 50% O1 weakened the structure of the original SMs by roughly 25%, followed by an equivalent recovery (or rebound). By contrast, original SMs went through a steady loss of structural strength upon the addition of O2. Despite the rebound, a higher incorporation of O1 seemed to increase AFF in the original SMs, whereas O2 decreased it [interaction, F(2,8) = 17.090, p = 0.001]. To sum up, original SMs containing O1 were more associated with structural strength (high gumminess, chewiness, Young's modulus, hardness, shear energy, AFF) than those containing O2 (Fig. 2A and B). Apparently, original SMs containing 25% or 50% O1 showed minimal textural differences, except for springiness (higher in 50% O1, Fig. 2A). Upon the addition of 75% O1, the structural strength of the original SMs increased while their flexibility decreased.
Fig. 2.
Principal component analysis bi-plots for physicochemical and mechanical properties of original substitutes for minced meat (SMs) (A and B; total variance, 92.8%), fried SMs (C and D; total variance, 96.2%) and SM patties (E and F, total variance, 95.2%). Factor scores include oat-fiber-concentrate (O1 or O2):faba-bean-protein-concentrate (FP) ratios: 25:75, 50:50 and 75:25. Factor loadings: gumminess (Gum), springiness (Spring), chewiness (Chew), Young's modulus (YM), energy caused by adhesive and frictional forces (AFF), shear energy (SE), hardness (Hard), lightness (L*; patty's inner side), redness (a*; patty's inner side) and yellowness (b*; patty's inner side).
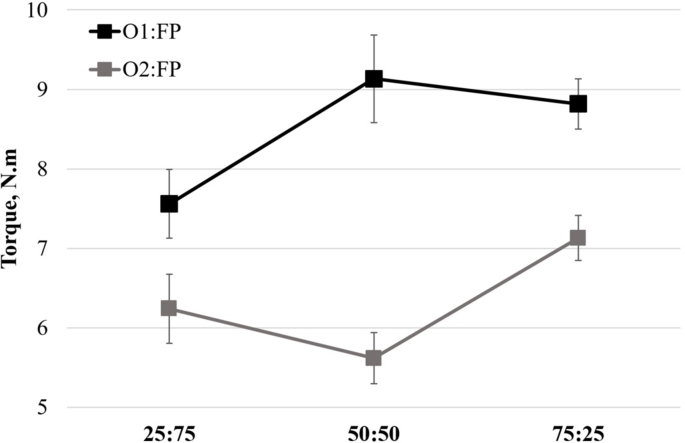
Textural disparities associated with O1 and O2 could arise from their contents of macrocomponents, like soluble fiber (β-glucan: O1, 20.8%; O2, 15.9%; Table 1), insoluble fiber, starch and fat. In the present study, mechanical torque—generally correlated with the viscosity of the mass—was larger in blends containing O1 than in those containing O2 (see Appendix A). In line with this, Lazaridou and Biliaderis (2007) observed that, depending on the molecular weight and structure of β-glucan, the higher the content of oat β-glucan (native flour), the higher the viscosity levels. On the other hand, the protein content in O1 and O2 was similar and, in both cases, nearly half of that of FP (Table 1). The largest protein fraction in either faba bean or oat is reported to be globulins (Liu et al., 2017; Boukid, 2021). Various studies (Arogundale et al., 2006; Jiang et al., 2015; Martinez-Velasco et al., 2018; Boukid, 2021) have shown that faba bean protein could present attractive functional properties (e.g., foaming ability, emulsifying properties), even at native state, while oat protein's techno-functionality remains challenging unless functionalization occurs. Compared to β-glucan, globulins have a comparatively low water absorption capacity. In the present study, faba bean proteins were associated with foaming and emulsifying activities while oat β-glucan provided structural stability in the original SMs.
The analyzed contents of insoluble fiber in the original SMs were consistently higher than those of soluble fiber (Table 1). Further, O1 had a moderately higher content of insoluble fiber than O2 (around 4% difference as raw flours; Table 1). According to Zhang et al. (2019), the incorporation of insoluble fiber (ground soybean residue of tofu production) into tofu weakened the gel structure, but it did not seem to negatively impact the water holding capacity. In the present study, structural changes following an increase in insoluble fiber in SMs could not be assessed. This could be linked to the fact that insoluble fiber increased hand-in-hand with soluble fiber (β-glucan). Extrusion can boost molecular changes (e.g., thermal degradation) in insoluble fiber thereby increasing its water-holding capacity (Artz et al., 1990). However, dry and high shearing conditions might be needed to expose water-binding chemical groups, thereby increasing the gel-formation capacity.
There is certainty that high temperature (up to 120 °C) and intense mechanical shear (Appendix A) led to gelatinization and breakdown of oat/faba starch granules. There is a possibility that amylopectin and, in particular, amylose retrogradation had some influence on the water-holding capacity of the original SMs upon cooling. Even though O2-containing original SMs had a slightly higher content of starch than those containing O1 (Table 1), this may not sufficiently explain the remarkable difference in their mechanical properties.
Clearly, the original SMs responded differently depending on whether the TPA or shear test was conducted. While TPA involved a mild sample pressing in a confined volume, the shear test broke down the samples by forcing them through a perforated bottom. With TPA, the increasing content of O1 had a linear relation to textural properties, while with the shear test, a low point was consistently noticed in the original SMs with 50% O1. At 25 (O1):75 (FP) ratio, the original SMs behaved like a stable protein-based system. However, transition into a protein-fiber hybrid [50 (O1):50 (FP) ratio] seemed to cause structural disruption, as evidenced by the shear test measurements (Table 2). By contrast, a fiber-based structure [75 (O1):25 (FP) ratio] showed remarkable structural recovery (Table 2).
Compared to original SMs, BM was less gummy, chewy, and hard. According to producer's standards, BM was considered extra lean (around 10% hard fat), which may have had some influence on its mechanical properties. Yilmaz et al. (2012) reported that low hard fat content was related to structural weakness and high volume recovery (i.e., springiness) in meat. Probably, elasticity is the property that BM possessed that original SMs clearly lacked. Original SMs were more prone to fracture (particularly those with 50% O1) than BM, which might be attributed to weak polymeric cohesiveness (lack of myofibrils) and the type/content of fat (<7% soft fat in original SMs).
Original SMs containing O1 were lighter [type, F (1,9) = 410.7, p = 0.0001] and redder [type, F(1,9) = 30.3, p = 0.0001] than those containing O2. Additionally, higher contents of oat fiber concentrate increased the lightness [content, F (2,18) = 64.5, p = 0.0001] and redness [content, F(2,18) = 59.2, p = 0.0001] of original SMs. The addition of O1 seemed to confer a more yellowish tone to the original SMs compared to O2 [type, F (1,9) = 243.3, p = 0.0001]. Unlike O2, changes in yellowness were noticeable at different O1 contents [interaction, F(2,18) = 15.4, p = 0.0001]. Overall, original SMs containing O1 were linked to lighter (higher L*) and brighter colors (higher a* and b*) than those containing O2 (Fig. 2A and B). In this regard, non-enzymatic browning might have occurred during extrusion cooking of blends. β-glucan, sugars and amino acid/peptides were most likely involved in the colorimetric differences between O1 and O2. Compared to BM (Table 3), original SMs were substantially lighter and less red (ΔE around 20), better mimicking the color of chicken meat.
3.2. Gel stability of selected blends under extrusion-simulated conditions
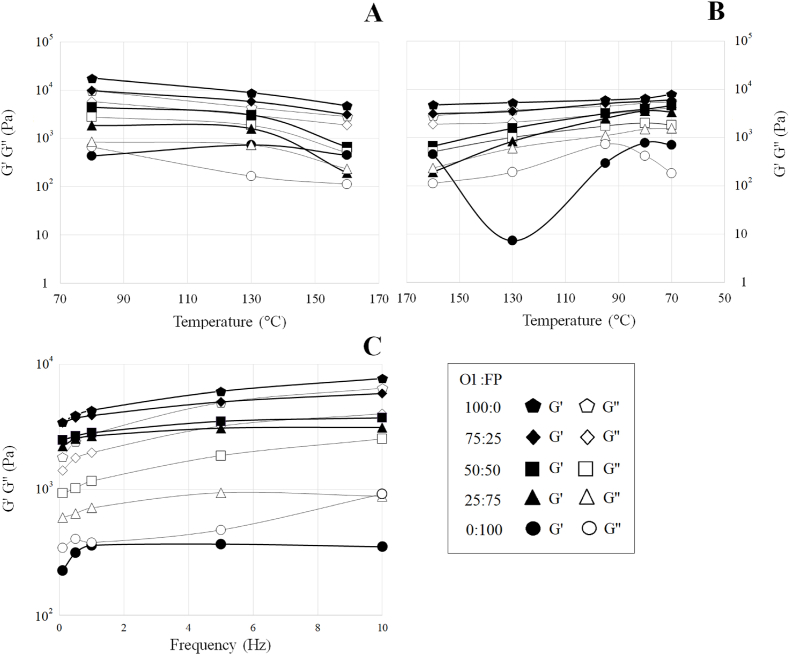
The addition of O1 increased the structural stability of the blend, and maintained a gel-like characteristic ( > ) along temperature-sweep shearing. The distance between and was relatively stable at 75% O1 compared to 25% O1. As suspected, increasing the temperature (from 80 °C to 160 °C; Fig. 3A) reduced the structural stability of the blend, which moderately reversed at decreasing temperature (from 160 °C to 70 °C; Fig. 3B). According to Sun et al. (2018, 2019), the formation of oat β-glucan-amino acid/peptide conjugates—resulting from the Maillard reaction—could increase structural sturdiness. Further, oat starch and lipid may form complexes thereby contributing to the stability of the structure upon retrogradation (Doublier et al., 1987). By contrast, FP proved incapability of absorbing water compared to O1 and, conversely, half of its mass solubilized (Appendix B). Only at high temperatures (equal to or above 130 °C) was FP able to form a gel structure ( > ). Otherwise, FP presented liquid-like characteristics ( > ).
Fig. 3.
Temperature and frequency dependency of storage (G′) and loss (G″) modulus for blends containing various oat-fiber-concentrate (O1):faba-bean-protein-concentrate (FP) ratios: 100:0 (pentagon); 75:25 (rhombus); 50:50 (square); 25:75 (triangle); 0:100 (circle).
Although the O1-containing blends presented gel characteristics along with frequency-sweep shearing (Fig. 3C, from 0.1 to 10 Hz), the stability of the structure ( is interpreted as the highest gel stability; Appendix B) varied widely. Unexpectedly, 100% O1 did not form the most stable gel-like structure ( = 0.13) but the blend with 25% O1 did ( = 0.06). By contrast, the blend containing 50% O1 appeared to be the least stable ( = 0.2), followed by 75% O1 ( = 0.15). As observed in temperature-sweep, faba bean protein concentrate (100% FP) also behaved like a liquid along the frequency-sweep ( = 0.35). These results seem to indicate that FP alone is unable to form gel structures at 70 °C during frequency-sweep test.
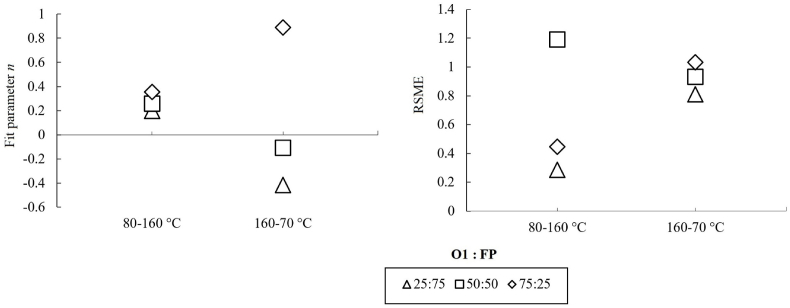
Based on the fit parameter, , of the polymer blending law, it seemed that all O1-FP blends showed a comparable bi-continuous water-protein/fiber phase ( = 0.2–0.36) during the rising temperature-sweep (from 80 to 160 °C; Fig. 4A). During the decreasing temperature-sweep, a shift toward a strong continuous fiber-dominated phase (suggesting the existence of an isostrain system in which the deformation of a weak polymer is dictated by the surrounding strong polymer; Morris, 1992) was observed in the blend with 75% O1 ( = 0.89; Fig. 4A), while a shift towards a weaker dispersed protein/fiber phase (referring to the isostress system in which a strong polymer deforms less than the surrounding weak polymer; Morris, 1992) was observed in blends with 25 and 50% O1 ( = −0.42 and −0.11, respectively; Fig. 4A). Schreuders et al. (2020) reported that blends containing soy protein isolate and wheat gluten presented bi-continuous characteristics ( = 0–0.5) that are comparable to those in the present study, particularly at rising temperature-sweep. The substantial capacity of β-glucan (O1) to absorb water was most probably involved in the formation of a continuous phase upon cooling. Rather than competing for dominance, the faba bean protein seemed to disperse in an aqueous medium. Regarding the reliability of the fitting, the RSMEs were around or below one in the present study (Fig. 4B), which was satisfactory and apparently lower than those reported by Schreuders et al. (2020).
Fig. 4.
Fit parameter, n, of a polymer blending law for blends of oat-fiber-concentrate (O1) and faba-bean-protein-concentrate (FP) (A) with their corresponding RSMEs (B). n values were calculated for rising and decreasing temperature-sweep [G* = f(T)].
In summary, these results suggest that blends (O1:FP) with high fiber content [75 (O1): 25 (FP)] were more likely to form a strong deformable polymeric system during extrusion, while blends with low fiber content [25(O1): 75 (FP)] presumably formed a weak and structurally heterogeneous system.
3.3. Physicochemical changes in SMs upon frying
The increasing ratio of oat fiber concentrate relative to faba bean protein concentrate reduced the structural strength of fried SMs expressed as gumminess [content, F(2,8) = 92.818, p = 0.0001], chewiness [content, F(2,8) = 59.031, p = 0.0001], and Young's modulus [content, F(2,8) = 28.987, p = 0.0001]. Interestingly, changes in the type of oat fiber concentrate seemed to have a distinct effect on Young's modulus. Fried SMs containing O2 were structurally weaker than those containing O1 (Fig. 2C). Springiness—regarded as the degree of structural tolerance upon compression—was a common characteristic of fried SMs with a low fiber-to-protein ratio (Fig. 2C and D).
Upon extrusion, the blend became a semisolid matrix holding together water, fiber (insoluble and soluble) and protein. The exposure of such a matrix to temperatures between 170 and 190 °C certainly altered its mechanical and physicochemical properties. Given the matrix composition and processing conditions, changes could be linked to the Maillard reaction (Sun et al., 2018). Sun et al. (2018, 2019) observed that the formation of β-glucan-amino acid/peptides conjugates resulting from the Maillard reaction was apparently linked to a distinct increase in molecular weight, fat binding capacity and viscosity. Higher structural strength of low fiber-to-protein ratio SMs [25(O1; O2):75(FP)] is probably linked to excessive loss of water upon frying, and potential structural changes associated, possibly, with the Maillard reaction (Sun et al., 2019). By contrast, the frying of high fiber-to-protein ratio SMs [75(O1; O2):25(FP)] mostly resulted in weaker structures. It is possible that these SMs were approaching collapse, given the downward trend in mechanical strength (Table 2).
Compared to fried BM, fried SMs were gummier, chewer, and harder (expressed in Young's modulus). Mechanically speaking, high fiber-to-protein ratio SMs were the closest to BM after frying. Despite this, the BM was notably more tolerant of compression (high springiness). In this case, a higher springiness may roughly indicate a reconfiguration of covalent crosslinks and myofibrillar structures resulting from the denaturation of meat proteins (Palka and Daun, 1999). By contrast, weaker polymeric crosslinks (e.g., hydrogen bonds or van der Waals interactions) might have been established upon the frying of SMs.
Even though frying increased the browning of SMs, color changes were still associated with the content and type of oat fiber concentrate. For instance, the content of oat fiber concentrate was linked to lighter [content, F (2,18) = 32.0, p = 0.0001], redder [content, F (2,18) = 10.2, p = 0.001] and yellower [content, F (2,18) = 24.0, p = 0.0001] SMs. Additionally, O1-containing SMs were brighter than those containing O2 [L*, type, F (1,9) = 38.6, p = 0.0001; a*, type, F (1,9) = 17.9, p = 0.002; b*, type, F (1,9) = 32.8, p = 0.0001] (Fig. 2C and D). Fried BM was comparatively redder and darker than any of the tested SMs (Table 3). ΔE between fried SMs and BM, although still large, was lower than that from original SMs and BM.
It is likely that gelatinized oat and faba bean starch were dextrinized during frying, resulting in the formation of disaccharides (e.g., maltose) and subsequent Maillard reaction. Morin et al. (2002) suggested that β-glucan's reducing ends are likely to form conjugates with available amino acid/peptides, thereby increasing the reddish-brown color of β-glucan-containing meat products (i.e., breakfast sausage).
3.4. Influence of oat fiber concentrate on SM patties
The use of various ingredients in the formation of SM patties increased the variability of results involving gumminess, springiness, and chewiness [replica, F (1,4) > 40, p < 0.003]. However, some structural patterns associated with Young's modulus were still observed. SM patties containing O2 were comparatively softer than those containing O1 [type, F (1,4) = 30.744, p = 0.005]. The AFF and shear energy of SM patties increased at a higher content of oat fiber concentrate [content, F (2,8) = 7.552, p = 0.014]. Upon the addition of O2, the AFF and shear energy of SM patties increased by 75% and 86%, respectively, while O1 showed a more modest reinforcing capacity (AFF, 18%; shear energy, 15%). Despite the minor contradictions, O2-containing SM patties seemed to exhibit sturdy characteristics (i.e., gumminess, chewiness, springiness, AFF, hardness) (Table 2; Fig. 2E).
Fat was the second largest fraction in SM patties, resulting from the incorporation of vegetable fat (soft and hard). These (and other ingredients) may have undermined the targeted effect of oat fiber concentrate on SM patties. Even though it was difficult to establish a cause-effect relationship between oat fiber concentrate and some mechanical characteristics (i.e., TPA), shearing tests revealed the effect of oat fiber concentrate on the strengthening of SM patties (Table 2). Ronda et al. (2015) reported that the incorporation of β-glucan in bread-making dough led to studier structures and, therefore, lesser capacity for volume development, a quality characteristic in bread. In the present study, sturdiness was a favorable feature, and may even encourage greater use of oat fiber concentrate into SM patties.
According to the estimates (Table 1), around 45% of the SM patty consisted of starch. Thus, starch gelatinization, formation of amylose-lipid complexes, and agar/maltodextrin water-binding capacity seemingly contributed to the development of the SM patty's structure.
Frying of the SM patty led to substantial variation in the crust color [replica, F (1,9) > 19, p < 0.001]. However, it was possible to identify some color traits associated with lightness and yellowness. The crust of the O2-containing SM patties seemed to be lighter and yellower than the crust of those containing O1. Despite the SM patty's inner side presenting a remarkable color variation [replica, F (1,19) > 39, p < 0.0001], the type of oat fiber concentrate showed appreciable effects (Fig. 2E and F). Consistent with the crust, the inner side of the O2-containing SM patties was lighter [type, F(1,9) = 214.1, p = 0.002] and yellower [type, F (1,9) = 12.6, P = 0.006] than the inner side of those containing O1. On average, the crust and inner side of the SM patties were lighter and redder than those of control (100% BM), which can be attributed to beetroot. Based on ΔE, the color of the inner side of the SM patties was closer to that of the control compared to the crust.
4. Conclusions
The present study proved that it was possible to obtain substitutes for minced meat by wet extrusion from oat fiber concentrate (two oat fiber concentrates with different chemical compositions: O1 and O2) and faba bean protein concentrate using a short concentric die. The results, however, indicated that there were considerable differences in the mechanical properties (i.e., chewiness, Young's modulus) of original SMs depending on the oat fiber concentrate used and the ingredient ratio. Original and fried SMs were observed to be considerably stiffer than—and yet as versatile as—raw and fried BM. SM patties containing oat fiber concentrate exhibited remarkable structural strength in shear testing.
The role of β-glucan-containing oat fiber concentrate as a binder and polymeric stabilizer was examined in this study, thus paving the way for further investigation of novel nutritionally-ambitious meat substitutes. It was feasible to incorporate up to 36% (d.m.) dietary fiber—from which half was β-glucan—into original SMs, thus providing adequate content of β-glucan in foods that promote healthy diet.
Based on the findings of this study, SMs not only present consumers with high-quality plant protein but also with a health claim linked to β-glucan, unprecedented characteristics for a meat substitute.
CRediT authorship contribution statement
J.M. Ramos-Diaz: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft, preparation. K. Kantanen: Investigation, Formal analysis. J.M. Edelmann: Investigation, Formal analysis. K. Jouppila: Conceptualization, Methodology, Supervision, Writing – review & editing. T. Sontag-Strohm: Conceptualization, Methodology, Supervision, Writing – review & editing. V. Piironen: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was part of 3TexVegS+H project, funded by the European Institute of Innovation and Technology (EIT Food), and Leg4Life project, funded by the Strategic Research Council at the Academy of Finland (grant number 327698).
Editor name: Xing Chen
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.04.010.
Appendix A. Supplementary data
The following are the supplementary data to this article:
figs1.
References
- Anderson R.A., Conway H.F., Peplinski A.J. Gelatinization of corn grits by roll cooking, extrusion cooking and steaming. Stärke. 1970;22:130–135. doi: 10.1002/star.19700220408. [DOI] [Google Scholar]
- AOAC . AOAC International, Gaithersburg, Maryland, USA, Official Method 950.46, 991.43 and 995.16. 18th ed. 2007. Official Methods of Analysis of AOAC International. [Google Scholar]
- AACC . Method 44-15.02. Moisture—Air-Oven Methods. Approved November 3, 1999. 11th ed. Cereals & Grains Association; St. Paul, Minnesota: 1999. Approved Methods of Analysis.https://www.cerealsgrains.org/resources/Methods/Methods/44-15.pdf [Google Scholar]
- Arogundale L.A., Tshay M., Shumey D., Manazie S. Effect of ionic strength and/or pH on extractability and physico-functional characterization of broad bean (Vicia faba L.) protein concentrate. Food Hydrocolloids. 2006;20:1124–1134. doi: 10.1016/j.foodhyd.2005.12.010. [DOI] [Google Scholar]
- Artz W.E., Warren C., Villota R. Twin-screw extrusion modification of a corn fiber and corn starch extruded blend. J. Food Sci. 1990;55(3):746–750. doi: 10.1111/j.1365-2621.1990.tb05220.x. [DOI] [Google Scholar]
- Baletti B. Odenamendo territorial: neo-developmentalism and the struggle for territory in the lower Brazilian Amazon. J. Peasant Stud. 2012;39:573–598. doi: 10.1080/03066150.2012.664139. [DOI] [Google Scholar]
- Boukid F. Oat proteins as emerging ingredients for food formulation: where we stand? Eur. Food Res. Technol. 2021;247:535–544. doi: 10.1007/s00217-020-03661-2. [DOI] [Google Scholar]
- Campo L., Tovar C. Influence of the starch content in the viscoelastic properties of surimi gels. J. Food Eng. 2008;84:140–147. doi: 10.1016/j.jfoodeng.2007.05.011. [DOI] [Google Scholar]
- Choi H.-D., Seog H.-M., Kim S.-R., Park Y.-K., Lee C.-H. Effect of β-Glucan on gelatinization of barley starch. Kor. J. Food Sci. Technol. 2003;35:545–550. https://www.koreascience.or.kr/article/JAKO200304637331158 [Google Scholar]
- Coda R., Varis J., Verni M., Rizzello C.G., Katina K. Improvement of the protein quality of wheat bread through faba bean sourdough addition. LWT-Food Sci. Technol. 2017;87:296–302. doi: 10.1016/j.lwt.2017.04.062. [DOI] [Google Scholar]
- Doublier J.-L., Paton D., Llamas G. A rheological investigation of oat starch pastes. Cereal Chem. 1987;64:21–26. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of health claims related to beta glucans and maintenance of normal blood cholesterol concentrations (ID 754, 755, 757, 801, 1465, 2934) and maintenance or achievement of a normal body weight (ID 820, 823) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009;7:1254. doi: 10.2903/j.efsa.2009.1254. [DOI] [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of a health claim related to oat beta-glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010;8:1885. https://doi:10.2903/j.efsa.2010.1885 [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of health claims related to oat and barley grain fibre and increase in faecal bulk (ID 819, 822) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011;9:2249. https://doi:10.2903/j.efsa.2011.2249 [Google Scholar]
- FAOSTAT . The Food and Agriculture Organization Corporate Statistical Database. FAO; 2020. Crops and livestock products.http://www.fao.org/faostat/en/#data/QC [Google Scholar]
- Farooq Z., Boye J.I. In: Pulse Foods Processing, Quality and Nutraceutical Applications. Tiwari B.K., Gowen A., McKenna B., editors. Academic Press; London, UK: 2011. Novel food and industrial applications of pulse flours and fractions; pp. 283–324. [Google Scholar]
- FDA Code of federal regulations. Title. 2012;21:81. Section 101. [Google Scholar]
- Jiang Zhong-qing, Sontag-Strohm T., Salovaara H., Sibakov J., Kanerva P., Loponen J. Oat protein solubility and emulsion properties improved by enzymatic deamidation. J. Cereal. Sci. 2015;64:126–132. doi: 10.1016/j.jcs.2015.04.010. [DOI] [Google Scholar]
- Khazaei H., Purves R.W., Hughes J., Link W., O'Sullivan D.M., Schulman A.H., Björnsdotter E., Geu-Flores F., Nadzieja M., Andersen S.U., Stougaard J., Vandenberg A., Stoddard Eliminating vicine and convicine, the main anti-nutritional factors restricting faba bean usage. Trends Food Sci. Technol. 2019;91:549–556. doi: 10.1016/j.tifs.2019.07.051. [DOI] [Google Scholar]
- Lampi A.M., Damerau A., Li J., Moisio T., Partanen R., Forssell P., Piironen V. Changes in lipids and volatile compounds of oat flours and extrudates during processing and storage. J. Cereal. Sci. 2015;62:102–109. doi: 10.1016/j.jcs.2014.12.011. [DOI] [Google Scholar]
- Lazaridou A., Biliaderis C.G. Molecular aspects of cereal β-glucan functionality: physical properties, technological applications and physiological effects. J. Cereal. Sci. 2007;46:101–108. doi: 10.1016/j.jcs.2007.05.003. [DOI] [Google Scholar]
- Liu Y., Hsieh F., Heymann H., Huff H.E. Effect of process conditions on the physical and sensory properties of extruded oat-corn puff. J. Food Sci. 2000;65:1253–1259. doi: 10.1111/j.1365-2621.2000.tb10274.x. [DOI] [Google Scholar]
- Liu M., Lampi A.M., Ertbjerg P. Unsaturated fat fraction from lard increases the oxidative stability of minced pork. Meat Sci. 2018;143:87–92. doi: 10.1016/j.meatsci.2018.04.028. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wu X., Hou W., Li P., Sha W., Tian Y. Structure and function of seed storage proteins in faba bean (Vicia faba L.) Biotech. 2017;7:74. doi: 10.1007/s13205-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Velasco A., Lobato-Calleros C., Hernandez-Rodriguez B.E., Roman-Guerrero A., Alvarez-Ramirez J., Vernon-Carter E.J. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: effect on surface properties, foaming ability and structural changes. Ultrason. Sonochem. 2018;44:97–105. doi: 10.1016/j.ultsonch.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Mayer-Labba I.-C., Frøkiær H., Sandberg A.-S. Nutritional and antinutritional composition of fava bean (Vicia faba L., var. minor) cultivars. Food Res. Int. 2021;140 doi: 10.1016/j.foodres.2020.110038. [DOI] [PubMed] [Google Scholar]
- Mälkki Y., Virtanen E. Gastrointestinal effects of oat bran and oat gum, a review. LWT-Food Sci. Technol. 2001;34:337–347. doi: 10.1006/fstl.2001.0795. [DOI] [Google Scholar]
- Mordor Intelligence . 2018. Meat Substitute Market – Growth, Trends and Forecasts (2019–2024)https://www.mordorintelligence.com/industry-reports/meat-substitutes-market Retrieved from. [Google Scholar]
- Morin L.A., Temelli F., McMullen L. Physical and sensory characteristics of reduced-fat breakfast sausages formulated with barley β-glucan. J. Food Sci. 2002;67:2391–2396. doi: 10.1111/j.1365-2621.2002.tb09559.x. [DOI] [Google Scholar]
- Morris R.E. The effect of solvent partition on the mechanical properties of biphasic biopolymer gels: an approximate theoretical treatment. Carbohydr. Polym. 1992;17:65–70. doi: 10.1016/0144-8617(92)90024-K. [DOI] [Google Scholar]
- Othman R.A., Moghadasian M.H., Jones P.J. Cholesterol-lowering effects of oat β-glucan. Nutr. Rev. 2011;69:299–309. doi: 10.1111/j.1753-4887.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- Palka K., Daun H. Changes in texture, cooking losses, and myofibrillar structure of bovine M. semitendinosus during heating. Meat Sci. 1999;51:237–243. doi: 10.1016/S0309-1740(98)00119-3. [DOI] [PubMed] [Google Scholar]
- Philipp C., Emin M.A., Buckow R., Silcock P., Oey I. Pea protein-fortified extruded snacks: linking melt viscosity and glass transition temperature with expansion behavior. J. Food Eng. 2018;217:93–100. doi: 10.1016/j.jfoodeng.2017.08.022. [DOI] [Google Scholar]
- Regand A., Chowdhury Z., Tosh S.M., Wolever T.M.S., Wood P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011;129:297–304. doi: 10.1016/j.foodchem.2011.04.053. [DOI] [PubMed] [Google Scholar]
- Ronda F., Perez-Quirce S., Lazaridou A., Biliaderis C.G. Effect of barley and oat β-glucan concentrates on gluten-free rice-based doughs and bread characteristics. Food Hydrocolloids. 2015;48:197–207. doi: 10.1016/j.foodhyd.2015.02.031. [DOI] [Google Scholar]
- Schreuders F.K.G., Bodnar I., Erni P., Boom R.M., van der Goot A.J. Water redistribution determined by time domain NMR explains rheological properties of dense fibrous protein blends at high temperature. Food Hydrocolloids. 2020;101:1–11. doi: 10.1016/j.foodhyd.2019.105562. [DOI] [Google Scholar]
- Smith J., Hardacre Development of an extruded snack product from the legume Vacia faba minor. Proc. Food Sci. 2011;1:1573–1580. doi: 10.1016/j.profoo.2011.09.233. [DOI] [Google Scholar]
- Stone A.K., Nosworthy M.G., Chiremba C., House J.D., Nickerson M.T. A comparative study of the functionality and protein quality of a variety of legume and cereal flours. Cereal Chem. 2019;96:1159–1169. doi: 10.1002/cche.10226. [DOI] [Google Scholar]
- Sun T., Qin Y., Xie J., Xu H., Gan J., Wu J., Bian X., Li X., Xiong Z., Xue B. Effect of Maillard reaction on rheological, physicochemical and functional properties of oat β-glucan. Food Hydrocolloids. 2019;89:90–94. doi: 10.1016/j.foodhyd.2018.10.029. [DOI] [Google Scholar]
- Sun T., Xu H., Zhang H., Ding H., Cui S., Xie J., Xue B., Hua X. Maillard reaction of oat β-glucan and the rheological property of its amino acid/peptide conjugates. Food Hydrocolloids. 2018;76:30–34. doi: 10.1016/j.foodhyd.2017.07.025. [DOI] [Google Scholar]
- Szczesniak A.S. Classification of textural characteristics. J. Food Sci. 1963;28:385–389. doi: 10.1111/j.1365-2621.1963.tb00215.x. [DOI] [Google Scholar]
- Tosh S. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur. J. Clin. Nutr. 2013;67:310–317. doi: 10.1038/ejcn.2013.25. [DOI] [PubMed] [Google Scholar]
- Wood P.J. Oat and rye β-glucan: properties and function(review) Cereal Chem. 2010:315–330. doi: 10.1094/CCHEM-87-4-0315. [DOI] [Google Scholar]
- Yilmaz M.T., Karaman S., Dogan M., Yetim H., Kayacier A. Characterization of O/W model system meat emulsions using shear creep and creep recovery tests based on mechanical simulation models and their correlation with texture profile analysis (TPA) parameters. J. Food Eng. 2012;108:327–336. doi: 10.1016/j.jfoodeng.2011.08.005. [DOI] [Google Scholar]
- Zhang M., Wang P., Zou M., Yang R., Tian M., Gu Z. Microbial transglutaminase-modified protein network and its importance in enhancing the quality of high-fiber tofu with okara. Food Chem. 2019;289:169–176. doi: 10.1016/j.foodchem.2019.03.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.