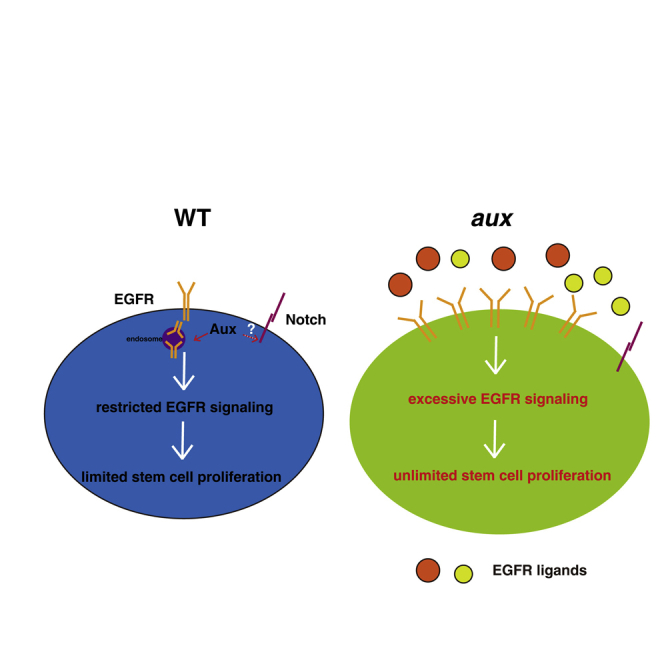
Summary
Adult tissue homeostasis is maintained by residential stem cells. The proliferation and differentiation of adult stem cells must be tightly balanced to avoid excessive proliferation or premature differentiation. However, how stem cell proliferation is properly controlled remains elusive. Here, we find that auxilin (Aux) restricts intestinal stem cell (ISC) proliferation mainly through EGFR signaling. aux depletion leads to excessive ISC proliferation and midgut homeostasis disruption, which is unlikely caused by defective Notch signaling. Aux is expressed in multiple types of intestinal cells. Interestingly, aux depletion causes a dramatic increase in EGFR signaling, with a strong accumulation of EGFR at the plasma membrane and an increased expression of EGFR ligands in response to tissue stress. Furthermore, Aux co-localizes and associates with EGFR. Finally, blocking EGFR signaling completely suppresses the defects caused by aux depletion. Together, these data demonstrate that Aux mainly safeguards EGFR activation to keep a proper ISC proliferation rate to maintain midgut homeostasis.
Keywords: auxilin, intestinal stem cell, Drosophila, tissue homeostasis, EGFR, endocytosis, Notch signaling, plasma membrane
Graphical abstract
Highlights
-
•
Aux safeguards ISCs from rapid proliferation to maintain intestinal homeostasis
-
•
Notch signaling is unaffected in the absence of aux
-
•
EGFR signaling is ectopically activated upon aux depletion
-
•
Aux associates with EGFR and mediates its clearance from the plasma membrane
In this article, Zhou and colleagues show that auxilin restricts intestinal stem cell (ISC) proliferation mainly through EGFR signaling. aux depletion in progenitors disrupts intestinal homeostasis by increased EGFR signaling and increased expression of EGFR ligands. Aux associates with EGFR and mediates its clearance from the plasma membrane to restrain ISC proliferation, thereby maintaining intestinal homeostasis under physiological conditions.
Introduction
Adult stem cells constantly divide and produce differentiated cells to replenish the frequently lost cells in the tissue in which they reside, thereby maintaining tissue homeostasis. Thus, the proliferation and differentiation of adult stem cells must be tightly balanced. Disruption of this balance leads to either excessive stem cells or stem cell exhaustion, eventually resulting in various diseases such as cancer and precocious aging (Lin, 2008; Morrison and Spradling, 2008; Radtke and Clevers, 2005; Xie and Spradling, 1998). Therefore, understanding of the underlying mechanisms controlling adult stem cell proliferation is important for the development of therapeutics to treat human diseases.
The adult Drosophila posterior midgut is an excellent system to study stem cell regulation. Mammalian and Drosophila intestines are similar in terms of development, cellular makeup, and genetic control (Casali and Batlle, 2009; Edgar, 2012; Stainier, 2005; Wang and Hou, 2010). Intestinal stem cells (ISCs) are distributed along the basement membrane of the adult Drosophila midgut (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Previous studies have shown that ISCs asymmetrically divide and produce differentiating enteroblasts (EBs) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Recent studies have shown that in response to differentiation and subsequent loss of a neighboring ISC (or vice versa), a significant proportion of ISCs divides symmetrically (de Navascués et al., 2012; Goulas et al., 2012; O'Brien et al., 2011). Delta (Dl), one of the Notch ligands, is specifically expressed in ISCs, while Notch (N) is expressed in both ISCs and EBs (progenitors). Previous studies showed that Notch signaling is activated in EBs, which differentiate into either absorptive enterocytes (ECs) or secretory enteroendocrine cells (EEs), depending on their signaling environments (Beebe et al., 2010; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2007; Perdigoto et al., 2011; Yeung et al., 2011). Recent studies have found that EEs may not be differentiated from EBs, but directly from ISCs or EE progenitors (EEPs) (Biteau and Jasper, 2014; Chen et al., 2018; Zeng et al., 2015).
ISCs constantly adjust their proliferation rate to sense tissue needs to maintain tissue homeostasis. The proliferation and differentiation of ISCs under physiological and stressed conditions are regulated by multiple signaling pathways such as Notch and epidermal growth factor receptor (EGFR) (see reviews by Colombani and Andersen, 2020; Gervais and Bardin, 2017; Jiang et al., 2016; and Joly and Rousset, 2020 and references therein). Notch signaling acts in a bidirectional and context-dependent manner to regulate ISC maintenance and progeny differentiation, and the inactivation of Notch signaling results in rapid ISC proliferation and defective progeny differentiation (Guo and Ohlstein, 2015; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006, 2007; Shi et al., 2021). EGFR signaling integrates multiple signals to regulate ISC proliferation and differentiation, defective EGFR signaling inhibits ISC proliferation and tissue regeneration, while ectopic EGFR activation promotes ISC proliferation and tumorigenesis (Biteau and Jasper, 2011; Jiang et al., 2011; Ma et al., 2016; Patel et al., 2015; Xu et al., 2011). However, it remains unclear how EGFR activity is properly controlled to maintain intestinal homeostasis under physiological conditions.
Endocytosis is at the hub of cellular processes such as receptor downregulation, signaling, and development. Auxilin (Aux), a DnaJ domain-containing protein, is recruited to clathrin-coated endocytic vesicles (CCVs) to disassemble the clathrin lattice together with the ATPase Hsc70 (Eisenberg and Greene, 2007; Lemmon, 2001; Ungewickell et al., 1995). Aux also participates in other steps of the CCV cycle (Eisenberg and Greene, 2007; Newmyer et al., 2003). Recent studies revealed that Aux facilitates membrane traffic in the early secretory pathway and is required for the formation of Golgi-derived CCVs during Drosophila spermatogenesis (Ding et al., 2016; Zhou et al., 2011). Two isoforms of Aux (the brain-specific Aux 1 and the ubiquitous Aux 2 or cyclin G-associated kinase [GAK]) exist in vertebrates, while Drosophila has only one Aux ortholog, which is more structurally similar to GAK (Eisenberg and Greene, 2007; Umeda et al., 2000; Ungewickell et al., 1995). Previous studies found that GAK regulates EGFR signaling in mammalian cells and Aux functions as an integral regulator of the Notch signaling pathway in the signal-sending cells in several Notch-dependent processes in Drosophila (Eun et al., 2008; Hagedorn et al., 2006; Kandachar et al., 2008; Zhang et al., 2004). However, it remains unknown what roles it plays in ISC proliferation and differentiation.
In this study, we provide evidence that Aux restricts ISC proliferation under normal homeostasis. Importantly, we demonstrate that Aux interacts with EGFR and safeguards the activation of EGFR to maintain a proper stem cell proliferation rate. Thus, our data uncover the underlying mechanism of Aux in stem cell proliferation control and midgut homeostasis maintenance.
Results
aux negatively regulates ISC proliferation
To identify additional factors regulating ISC proliferation and differentiation, we performed a genome-wide RNAi screen in the posterior midgut using the esgGal4, UAS-GFP, tubGal80ts (esgts) driver (Liu et al., 2022; Ma et al., 2016; Zhao et al., 2022). The screen identified, Aux, the sole Drosophila ortholog of mammalian GAK, as a candidate. In the control midguts, small esg+ cells were observed in isolation or in clusters containing fewer than three cells; in contrast, the number of esg+ cells was significantly increased when aux was depleted (Figures S1A and S1B) (see the supplemental experimental procedures for the names of the aux RNAi lines). These esg+ cells formed large clusters with varied cell sizes, many of which were polyploid, and the percentage of esg+ cells was dramatically increased (Figures S1B, S1I, and S1J). To exclude the possibility of an off-target effect, we induced four other independent aux RNAi constructs (auxv20, auxv22, auxR−1, and auxR−2) and they showed a similar but stronger phenotype as that of auxv10 (Figures S1C–S1J). Consistent with the phenotypic severity, quantitative RT-PCR (qRT-PCR) confirmed that these RNAi constructs have higher knockdown efficiency than auxv10 (Figure S1K). Meanwhile, a significant increase in the number of mitotic cells was observed in aux-depleted intestines, suggesting that the dramatic increase in esg+ cells was caused by excessive ISC proliferation (Figures 1A–1G). Furthermore, the size of auxRNAi ISC MARCM clones was significantly increased compared to that of control clones (Figures S1L and S1M). Taken together, these data indicate that Aux negatively regulates ISC proliferation under physiological conditions.
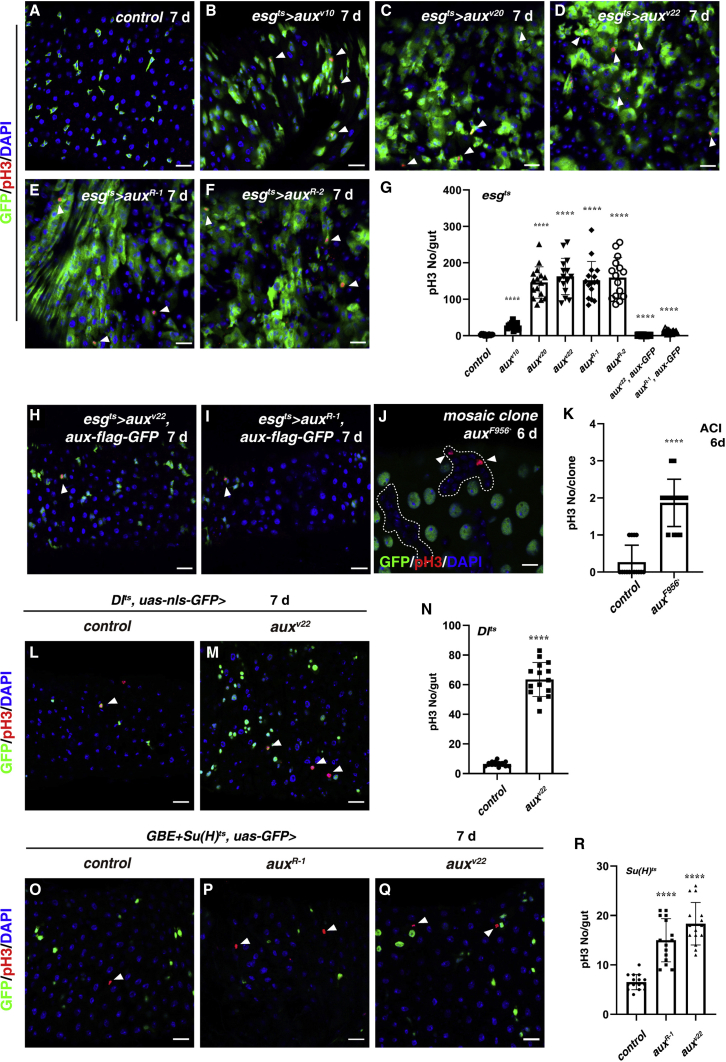
Figure 1.
Aux functions in progenitors to restrict ISC proliferation
(A) pH3 (red) in control flies.
(B–F) pH3 in esgts>auxRNAi flies (white arrowheads).
(G) Quantification of pH3 number/gut in different genotypes indicated. Means ± SDs are shown. n ≥ 15. ∗∗∗∗p < 0.0001. Please note that the esgts>auxRNAi intestines are highly deformed, preventing the accurate quantification of pH3 numbers.
(H and I) pH3 in esgts>auxRNAi, UAS-aux-flag-GFP flies (white arrowheads).
(J) pH3 (red) in auxF596X clones (white arrowheads, 6 days after clone induction [ACI]). The auxF596X clones are outlined with dotted lines.
(K) Quantification of pH3 number/clone in control and auxF596X clones. Means ± SDs are shown. n = 15. ∗∗∗∗p < 0.0001.
(L) pH3 in control flies (Dlts>wRNAi).
(M) pH3 in Dlts>auxv22 flies (white arrowheads).
(N) Quantification of pH3 number/gut in control and Dlts>auxv22 intestines. Means ± SDs are shown. n = 15. ∗∗∗∗p < 0.0001.
(O) pH3 in control flies (GBE + Su(H)ts>wRNAi).
(P and Q) pH3 in GBE + Su(H)ts>auxRNAi flies (white arrowheads).
(R) Quantification of pH3 number/gut in control and GBE + Su(H)ts>auxRNAi intestines. Means ± SDs are shown. n = 15. ∗∗∗∗p < 0.0001.
Scale bars, 20 μm (J, 10 μm).
Moreover, the co-expression of an UAS-aux-flag-GFP transgene with auxRNAi constructs completely restored the midgut homeostasis, further confirming that the defects are specific to aux depletion (Figures 1G–1I and S1G–S1J). Finally, we found that FLP-induced clones of auxF956X, a strong loss-of-function or null allele (Kandachar et al., 2008), were dramatically increased in size and contained more mitotic cells (Figures 1J, 1K, S1O, and S1P). Collectively, these data demonstrate that Aux negatively regulates ISC proliferation in a cell-autonomous manner to maintain intestinal homeostasis under normal conditions.
Aux is required in progenitors for ISC proliferation
To further pinpoint the cellular requirement of aux function in ISC proliferation control, we selectively depleted aux in ISCs or EBs using ISC-specific driver DlGal4 and EB-specific driver GBE+Su(H)Gal4, respectively (Lu and Li, 2015; Zeng et al., 2010). The depletion of aux in ISCs resulted in a significant increase in ISCs, albeit at a lower percentage of Dl+ cells compared to those of esgts>auxRNAi intestines; interestingly, some cells expressing low or no GFP are also Dl positive, indicating that these cells are the differentiating descendants of ISCs (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 72A–S2D). Consistently, the number of mitotic ISCs in Dlts>auxRNAi intestines was significantly increased compared to that of controls, suggesting that Aux is required in ISCs to control ISC proliferation (Figures 1L–1N). The number of EBs (GBE+Su(H)>GFP+) and mitotic cells per intestine was significantly increased when aux was selectively depleted in EBs, similar to that of signal transducer and activator of transcription (STAT) depletion in EBs as previously reported (Figures 1O–1R and S2E–S2H) (Zhai et al., 2017). Together, these data indicate that Aux functions in both ISCs and EBs to regulate ISC proliferation under physiological conditions.
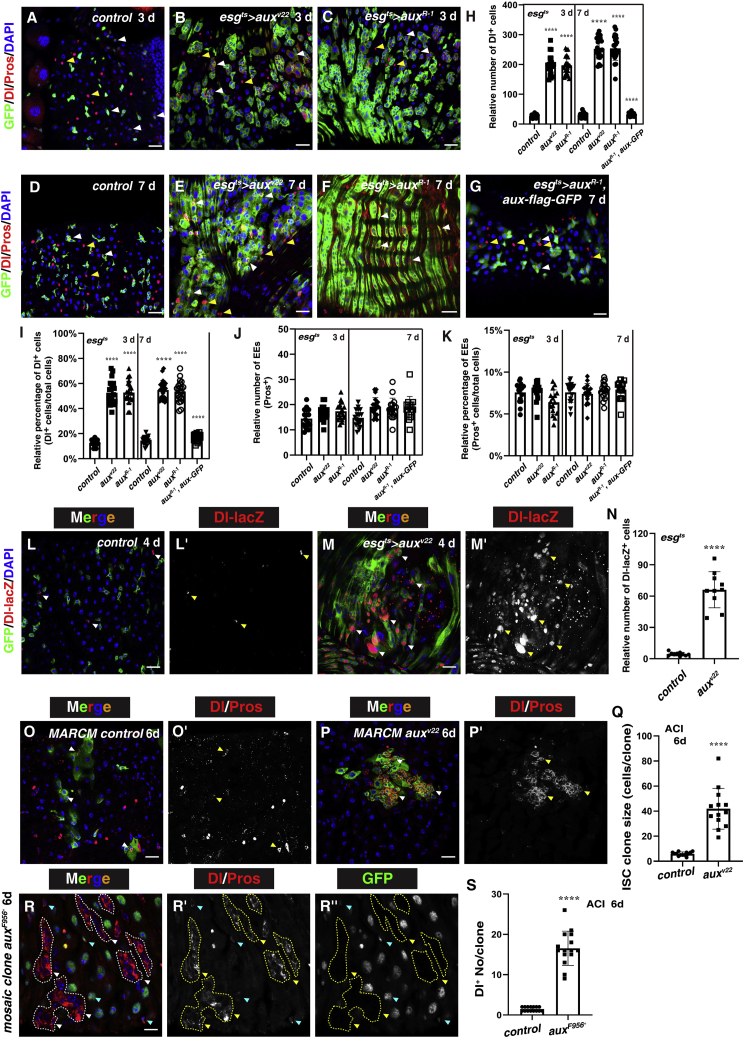
Figure 2.
Intestinal homeostasis is disrupted in the absence of Aux
(A) Dl and Pros in control intestines (3 days).
(B and C) Dl and Pros in esgts>auxRNAi intestines (white and yellow arrowheads, respectively).
(D) Dl and Pros in control intestines (white and yellow arrowheads, 7 days).
(E and F) Dl and Pros in esgts>auxRNAi intestines.
(G) Dl and Pros in esgts>auxRNAi, aux-flag-GFP intestines.
(H) Quantification of Dl+ cells in control and esgts>auxRNAi intestines. Means ± SDs are shown. n ≥ 15. ∗∗∗∗p < 0.0001.
(I) Quantification of the percentage of Dl+ cells in control and esgts>auxRNAi intestines. Means ± SDs are shown. n ≥ 15. ∗∗∗∗p < 0.0001.
(J) Quantification of Pros+ cells in control and esgts>auxRNAi intestines. Means ± SDs are shown. n ≥ 15. ∗∗∗∗p < 0.0001.
(K) Quantification of the percentage of Pros+ cells in control and esgts>auxRNAi intestines. Means ± SDs are shown. n ≥ 15. ∗∗∗∗p < 0.0001.
(L) Dl-lacZ (white arrowheads) in control intestines. Dl-lacZ channel is shown separately in black and white.
(M) Dl-lacZ + cells in esgts>auxv22 intestines (white arrowheads).
(N) Quantification of Dl-lacZ+ cells in control and esgts>auxRNAi intestines. Means ± SDs are shown. n = 10. ∗∗∗∗p < 0.0001.
(O) Dl and Pros in FRT MARCM clones 6 days ACI (white arrowheads). Dl/Pros channel is shown separately in black and white.
(P) Dl and Pros in auxv22 ISC MARCM clones (6 days ACI, white arrowheads).
(Q) Quantification of the size of control and auxv22 ISC MARCM clones. Means ± SDs are shown. n = 15. ∗∗∗∗p < 0.0001.
(R) Dl and Pros in auxF596X mosaic clones (6 days ACI, white arrowheads and circles). GFP+ and Dl+ cells outside the mosaic clones are indicated by blue arrowheads. Dl/Pros and GFP channels are shown separately in black and white and the clones are outlined with yellow dotted lines.
(S) Quantification of the number of Dl+ cells per clone in control and auxF596X clones. Means ± SDs are shown. n = 15. ∗∗∗∗p < 0.0001.
Scale bars, 20 μm (O, 10 μm).
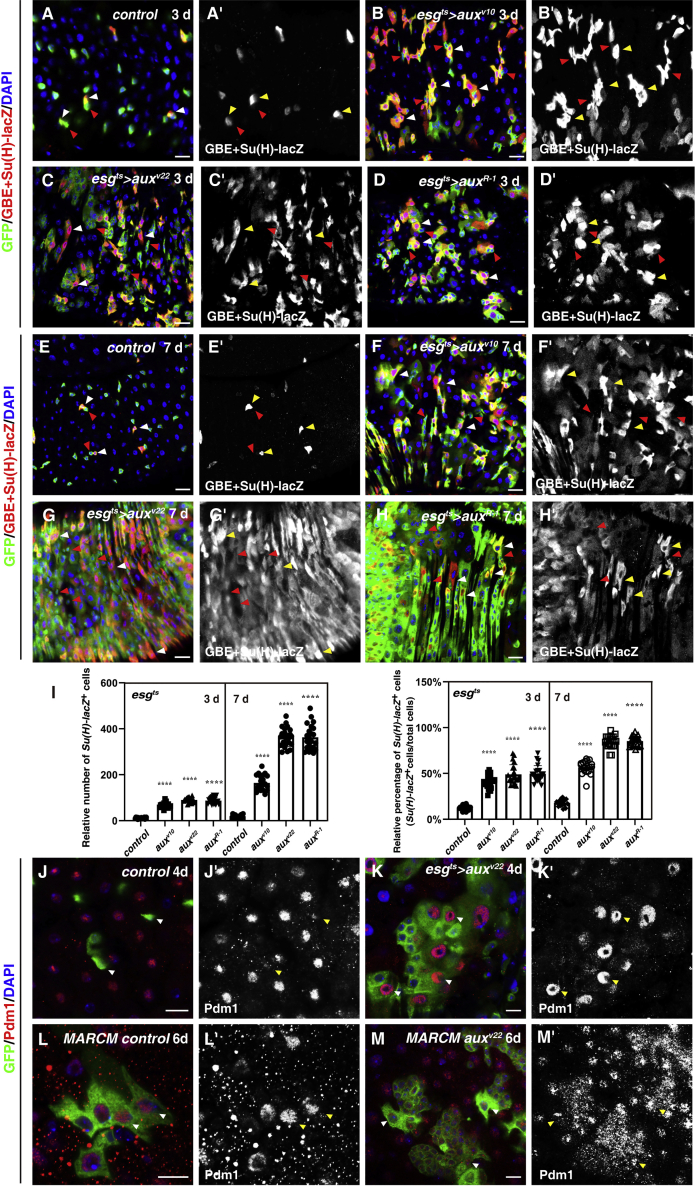
Figure 3.
Notch signaling is largely unaffected upon aux depletion
(A) Notch activation (by GBE + Su(H)-lacZ, white arrowheads) in control intestines (3 days). ISCs are GBE + Su(H)-lacZ- cells (red arrowheads, the same as follows). GBE + Su(H)-lacZ channel is shown separately in black and white.
(B–D) GBE + Su(H)-lacZ+ cells in esgts>auxRNAi intestines (white arrowheads, 3 days).
(E) Notch activation in control intestines (white arrowheads, 7 days).
(F–H) GBE + Su(H)-lacZ+ cells (white arrowheads) in esgts>auxRNAi intestines.
(I) Quantification of the number and percentage of GBE + Su(H)-lacZ+ cells in control and esgts>auxRNAi intestines, respectively. Means ± SDs are shown. n ≥ 15. ∗∗∗∗p < 0.0001.
(J) Pdm1 in control intestines. Pdm1 channel is shown separately in black and white.
(K) Pdm1 in esgts>auxv22 intestines (white arrowheads).
(L) Pdm1 in control MARCM clones (6 days ACI) (white arrowheads).
(M) Pdm1 in auxv22 ISC MARCM clones (6 days ACI) (white arrowheads).
Scale bars, 20 μm (K–N, 10 μm).
Figure 4.
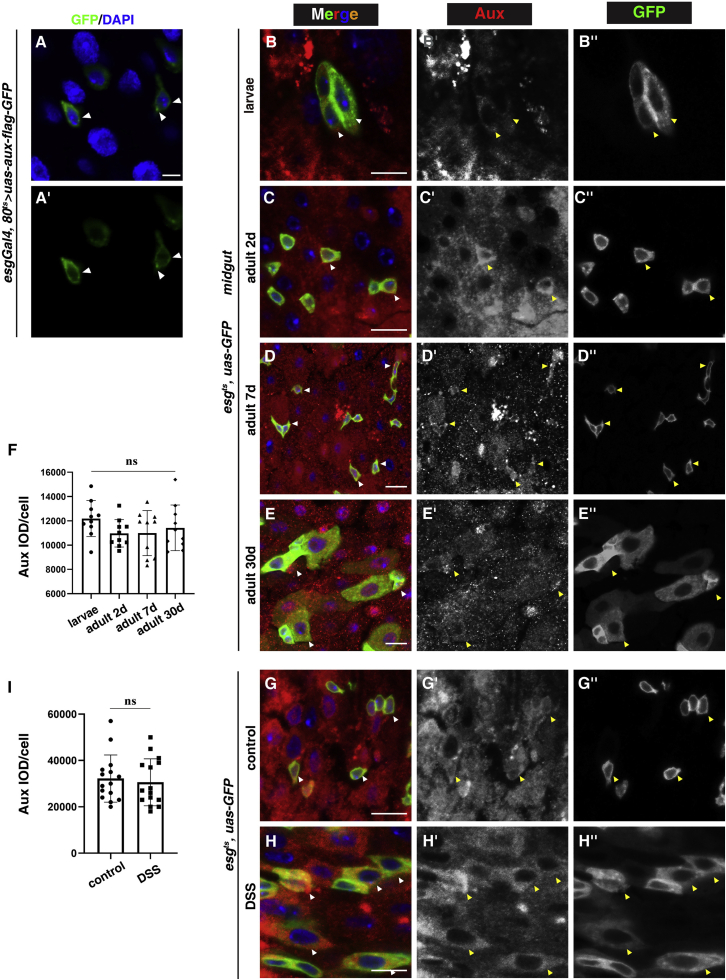
Aux expression in the midgut during aging and tissue regeneration
(A) Transiently expressed Aux-FLAG-GFP localizes to the plasma membrane and cytosol (white arrowheads). The Aux-FLAG-GFP channel is shown separately.
(B–E) Endogenous Aux (red) in midguts at different developmental stages (white arrowheads). Aux and GFP channels are shown separately in black and white.
(F) Quantification of Aux integrated optical density (IOD) in progenitors at different developmental stages. Means ± SDs are shown. n = 10. nsp > 0.05.
(G–H″) The expression of Aux in progenitors under normal and stress conditions (white arrowheads).
(I) Quantification of Aux IOD in progenitors under normal and stress conditions. Means ± SDs are shown. n = 15. nsp > 0.05.
Scale bars, 10 μm (A, 5 μm).
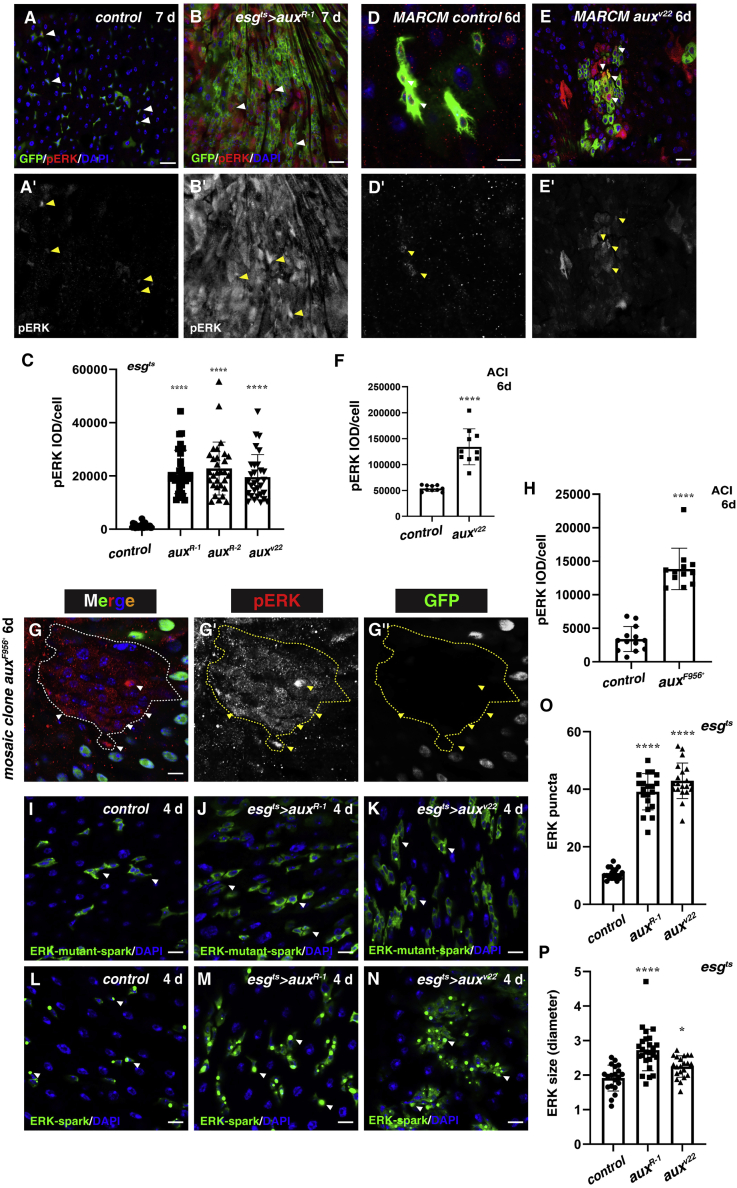
Figure 5.
EGFR signaling is dramatically activated in aux-defective intestines
(A) EGFR signaling (by pERK, red) in control flies (white arrowheads). pERK channel is shown separately in black and white (yellow arrowheads) (the same as follows).
(B) EGFR signaling is dramatically activated in esgts>auxRNAi flies (white arrowheads).
(C) Quantification of pERK IOD/cell in control and esgts>auxRNAi intestines. Means ± SDs are shown. n ≥ 15. ∗∗∗∗p < 0.0001.
(D) EGFR signaling (by pERK, red) in FRT control ISC MARCM clones (6 days ACI) (white arrowheads).
(E) The levels of pERK (red) and the number of pERK+ cells are dramatically increased in auxv22 ISC MARCM clones (6 days ACI) (white arrowheads). Note that EGFR signaling is also activated in neighboring cells surrounding the clones.
(F) Quantification of the level of pERK in control and auxv22 ISC MARCM clones. Means ± SDs are shown. n = 10. ∗∗∗∗p < 0.0001.
(G) EGFR signaling is dramatically activated in auxF596X mosaic clones (dotted lines, 6 days ACI, and white arrowheads). The pERK and GFP channels are shown separately in black and white (yellow arrowheads).
(H) Quantification of pERK IOD/cell in control and auxF596X mosaic clones. Means ± SDs are shown. n = 15. ∗∗∗∗p < 0.0001.
((I) ERK-mutant-SPARK (green) in control flies (white arrowheads).
(J and K) ERK-mutant-SPARK (green) in esgts>auxRNAi flies (white arrowheads).
(L) ERK-SPARK puncta in control flies (white arrowheads).
(M and N) ERK-SPARK puncta in esgts>auxRNAi flies (white arrowheads).
(O and P) Quantification of the number and size of ERK-SPARK puncta in control and esgts>auxRNAi intestines. Means ± SDs are shown. n = 20. ∗p < 0.05; ∗∗∗∗p < 0.0001.
Scale bars, 20 μm (F and H–M, 10 μm).
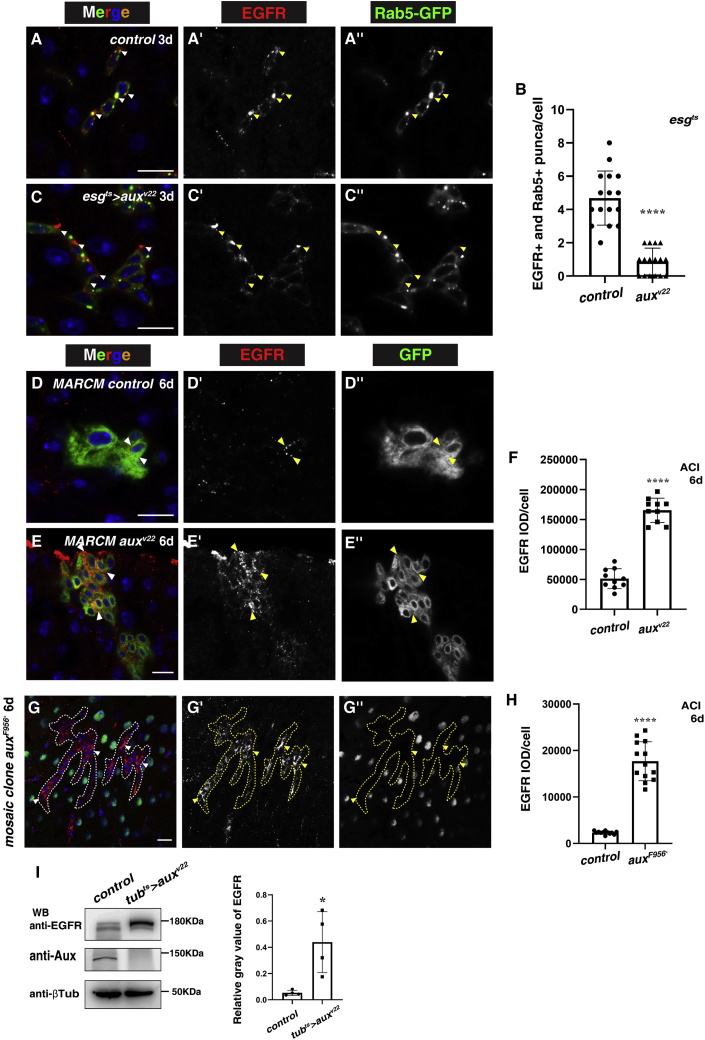
Figure 6.
EGFR is highly accumulated in the absence of aux
(A) EGFR (red) often localizes within transiently expressed Rab5-GFP puncta (green) in control intestines (white arrowheads). EGFR and Rab5-GFP channels are shown separately in black and white.
(B) Quantification of the number of Rab5-GFP+ EGFR+ puncta in control and esgts>auxRNAi intestines. Means ± SDs are shown. n = 16. ∗∗∗∗p < 0.0001.
(C) The number of Rab5-GFP+ EGFR puncta is dramatically decreased in esgts>auxv22 (white arrowheads).
(D) EGFR (red) in control MARCM clones (6 days ACI, white arrowheads). EGFR and GFP channels are shown separately in black and white.
(E) EGFR in auxv22 MARCM clones (white arrowheads).
(F) Quantification of EGFR IOD in control and auxv22 MARCM clones. Means ± SDs are shown. n = 10. ∗∗∗∗p < 0.0001.
(G) EGFR in auxF596X mosaic clones (outlined with dotted lines, white arrowheads). EGFR and GFP channels are shown separately in black and white.
(H) Quantification of EGFR IOD/cell in control and auxF596X mosaic clones. Means ± SDs are shown. n = 15. ∗∗∗∗p < 0.0001.
(I) Western blot and quantification of EGFR in control and tubts>auxv22 flies. Means ± SDs are shown. n = 4. ∗p < 0.05.
Scale bars, 10 μm.
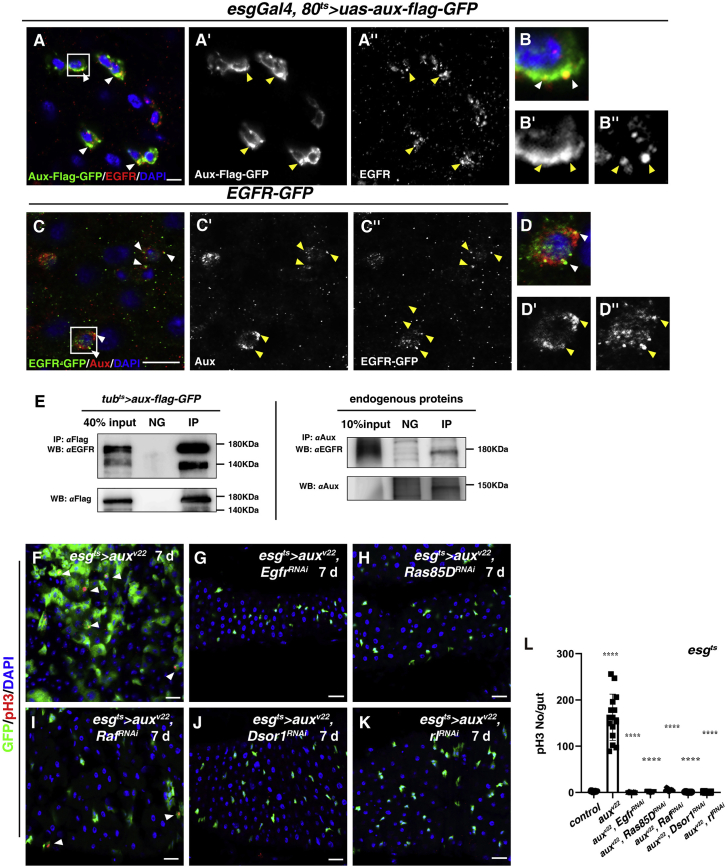
Figure 7.
Ectopic EGFR signaling is required for homeostasis disruption in aux-deficient intestines
(A and B) Co-localization of transiently expressed Aux-FLAG-GFP (green) and endogenous EGFR (red) (white arrowheads).
(C and D) Co-localization of endogenous Aux (red) and endogenous EGFR-GFP (green) (white arrowheads).
(E) CoIP results of endogenous EGFR by transiently expressed Aux-FLAG-GFP (left) and endogenous Aux (right). NG, negative control.
(F) pH3 in esgts>auxv22 flies.
(G–K) Blocking EGFR/MAPK signaling completely suppresses ISC proliferation observed in esgts>auxv22 intestines (white arrowheads).
(L) Quantification of pH3 number/gut in different genotypes indicated. Means ± SDs are shown. n ≥ 15. ∗∗∗∗p < 0.0001.
Scale bars, 10 μm (A and C) and 20 μm (F–K).
Intestinal homeostasis is disrupted in aux-defective intestines
We then characterized the identity of these extra esg+ cells in esgts>auxRNAi intestines. We stained the esgts>auxRNAi intestines with antibodies against Dl (an ISC marker) and Pros (an EE marker) at 2 time points after RNAi induction (3 and 7 days, respectively). The number and percentage of Dl+ cells in esgts>auxRNAi intestines were significantly increased at both time points compared to those in control flies (Figures 2A–2I). In contrast, the number and the percentage of EEs were unaffected in esgts>auxRNAi intestines (Figures 2A–2K). This increase in Dl+ cells is specific to Aux depletion as the co-expression of UAS-aux-flag-GFP rescued the defects (Figures 2G–2I). The increase in Dl+ cells in esgts>auxRNAi intestines was independently verified with a Dl-lacZ reporter line (Figures 2L–2N). This increase in Dl+ cells was also seen in auxRNAi ISC MARCM clones and auxF956X mosaic clones (Figures 2O–2S). The sizes of these Dl+ cells were heterogeneous in size, indicating that they were ISCs, ISC-like, and/or EBs (Figures 2A–2F and 2L–2M′). Indeed, the number and percentage of EBs (by GBE+Su(H)-lacZ) were dramatically increased in esgts>auxRNAi intestines compared to those in control flies at both time points (Figures 3A–3I, see below for a detailed description). The number and percentage of ECs were not determined. Together, these data suggest that ISCs are overproliferative in aux-defective intestines, disrupting intestinal homeostasis.
Notch signaling is largely unaffected in the absence of aux
Previous studies show that Aux facilitates Dl endocytosis in the signal-sending cells, an event that is crucial for Notch activation (Eun et al., 2008; Hagedorn et al., 2006; Kandachar et al., 2008). As Notch signaling plays important roles in ISC proliferation and differentiation (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006, 2007), we asked whether the defects observed in aux-depleted intestines were Notch signaling dependent using the widely used Notch signaling reporter GBE+Su(H)-lacZ, which is normally expressed in EBs (Furriols and Bray, 2001). Compared to the control at both time points examined (3 and 7 days), the number and the percentage of GBE+Su(H)-lacZ+ cells was dramatically increased in esgts>auxRNAi intestines (Figures 3A–3I). Upon closer examination of ISC clusters formed after aux depletion (3 days), we found that most cells within the clusters were GBE+Su(H)-lacZ+, with only 1–4 cells were GBE+Su(H)-lacZ-, indicative of ISCs (Figures 3A–3D). In contrast, the knockdown of presenilin (Psn), a component of the γ-secretase required for generating the intracellular domain of Notch (NICD) from the plasma membrane, resulted in very low or no GBE+Su(H)-lacZ signal in most cells of the psn-depleted clusters, which is indicative of defective Notch signaling (data not shown). These data indicate that Notch signaling is not inhibited in the absence of aux.
Ectopic Notch signaling activation causes direct differentiation of ISCs into ECs (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006, 2007; Shi et al., 2021). To test whether aux depletion disrupts Notch signaling, we stained the intestines of various genotypes with Pdm1, a mature EC marker (Jiang et al., 2009). While no esg+ cells in control flies were Pdm1+, many esg+ cells in esgts>auxRNAi intestines expressed Pdm1 (Figures 3J and 3K). These results suggest that intestinal homeostasis was lost in esgts>auxRNAi intestines and aux depletion did not inhibit Notch-dependent differentiation. Similar results were observed in auxRNAi ISC MARCM clones (Figures 3L and 3M). Moreover, while no Dl>GFP+Pdm1+ cells were observed in control intestines, the specific depletion of aux in ISCs resulted in several Dl>GFP+ cells expressing Pdm1 (Figures S2I and S2J). Together, these results are consistent with a scenario in which ISCs undergo rapid proliferation and differentiation in the absence of aux, thereby disrupting intestinal homeostasis.
Finally, we carefully compared the ISC clusters formed in Dlts>auxRNAi and Dlts>NotchRNAi intestines. We found that only one type of ISC cluster was observed in the absence of aux (Figures S5A–S5C). In these Dlts>auxRNAi ISC clusters, some cells expressed higher levels of GFP (GFPhigh), while other cells expressed low levels of or no GFP (GFPlow/no). The ratio of GFPhigh/GFPlow/no cells varied between different clusters. Interestingly, almost all of the cells in the same ISC cluster expressed Dl, indicating that the GFPlow/no cells are not true ISCs but undergo differentiation (Figures S3A–S3C). Three types of ISC clusters were observed in the absence of N (Figures S3D–S3F): (1) ISC tumor cluster, all of the cells within the cluster expressed GFP and Dl, indicating that these cells are ISC and/or ISC-like cells (Figure S3D); (2) EE tumor cluster, almost all of the cells in the cluster, except a few cells (GFP+Dl+Pros−), expressed GFP and Pros, indicating that these cells are EE and/or EE-like cells (Figure S3E); and (3) differentiated EE cluster, only a few GFPlow/noPros+ cells existed in the cluster, while the other cells were GFP−Pros+, indicating that these ISCs cannot maintain their stem cell fate and will finally differentiate into EE cells (Figure S3F). Furthermore, no obvious changes in the number of EEs were observed in aux-depleted intestines, while the number of EEs was significantly increased in N-depleted intestines (Figure S3). Collectively, these data indicate that Aux has little or no effect on Notch signaling in ISC proliferation regulation, although we cannot fully exclude the possibility that Aux may affect Notch signaling at a marginal level.
Aux is involved in endocytosis for ISC proliferation
In clathrin-mediated transport, Aux recruits Hsc70, the “uncoating ATPase,” to CCVs to facilitate their disassembly (Barouch et al., 1997; Holstein et al., 1996; Xing et al., 2010; Young et al., 2013). Consistent with the role of Aux as a co-factor for Hsc70, the depletion of Hsc70-4, the Drosophila homolog of Hsc70, resulted in more progenitors and enhanced the defects of aux depletion (data not shown). To further demonstrate that endocytosis plays an important role in ISC proliferation regulation, we asked whether depletion of the other endocytic components would result in defects similar to those of aux depletion. Indeed, compromising Rab5 function, the monomeric GTPase controlling entry of endocytosed cargo into the early endosome resulted in the same defects as those of aux depletion (Figures S4A–S4C). Meanwhile, the number of GBE + Su(H)-lacZ+ cells (EBs) was also significantly increased in esgts>Rab5DN intestines, which is same as that in esgts>auxRNAi intestines, further indicating that endocytosis plays an important role in ISC proliferation, and the defects resulted from endocytosis blockage are unlikely due to defective Notch signaling (Figures S4A–S4C). Together, these data show that Aux negatively regulates ISC proliferation, and Notch signaling plays no rolea or only marginal role in this process (see discussion).
Subcellular localization and expression pattern of Aux during aging and tissue regeneration
As aux depletion affects ISC proliferation and intestinal homeostasis, we asked whether endogenous Aux protein levels are influenced by aging or tissue regeneration. To address this, we generated an Aux-specific polyclonal antibody and showed that it detected Aux expression in ISCs and other intestinal cell types (Figures S5A–S5G). Consistent with its known role in CCV trafficking, Aux localized to the plasma membrane and cytosol and with some intracellular puncta, similar to the previously reported subcellular localization in eye discs (Figures 4A–4C) (Eun et al., 2008). The subcellular distribution of Aux protein in the progenitors was similar to those transiently expressed FLAG-tagged Aux (Figures 4A–4E and S5H). Staining with this antibody revealed that Aux expression levels in progenitors were largely unchanged at different developmental stages, from AMPs in the third-instar larvae to different ages of adults (Figures 4B–4F). Similarly, Aux expression levels in progenitors in intestines recovering from dextran sodium sulfate (DSS) treatment were comparable to those in the control (Figures 4G–4I). These data suggest that Aux expression levels are not influenced by aging and tissue regeneration.
EGFR signaling is ectopically activated in the absence of Aux
Next, we determined the mechanisms by which Aux regulates ISC proliferation. We investigated the effects of aux depletion on Janus kinase (JAK)/STAT, bone morphogenetic protein (BMP), and Hippo (Hpo) signaling, which are known to regulate ISC proliferation (Jiang et al., 2009; Jin et al., 2015; Karpowicz et al., 2010; Ma et al., 2019; Ren et al., 2010; Shaw et al., 2010; Tian and Jiang, 2014). However, our results suggest that the defects observed upon aux depletion were not likely caused by defective JAK/STAT, BMP, and Hpo signaling (data not shown).
As EGFR signaling is pivotal for ISC proliferation (Jiang et al., 2011; Xu et al., 2011), we then examined whether EGFR signaling was altered in aux-depleted intestines. Compared to the low levels of phosphorylated extracellular signal-regulated kinase (pERK) signals observed in the control intestines, the levels of pERK were dramatically increased in the esgts>auxRNAi intestines and in auxRNAi ISC MARCM clones (Figures 5A–5F, S6A, and S6B). This elevated pERK level was apparent after the induction of aux RNAi for just 1 day, suggesting that it is a primary effect of Aux depletion (Figures S6C–S6E). pERK signals continued to increase after 4 days of RNAi induction, suggesting that the defect is cumulative (Figures S6F–S6H). Similar to RNAi knockdown, pERK signals were significantly increased in aux mutant mosaic clones (Figures 5G and 5H). To independently monitor EGFR signaling in vivo, we used the recently developed ERK-separation of phases-based activity reporter of kinase (SPARK) reporter (Zhang et al., 2018) and found that the pattern of ERK-mutant-SPARK reporter (a negative control) was unaffected by aux depletion; in contrast, the number and the size of the ERK-SPARK condensates/puncta were significantly increased in esgts>auxRNAi intestines (Figures 5I–5P). Taken together, these data suggest that excessive EGFR activation is responsible for the defects observed in the absence of aux.
Aux is required for EGFR internalization
As EGFR is activated upon binding to its ligands at the plasma membrane, we asked whether Aux affects EGFR internalization and subcellular localization using an EGFR-specific antibody (Figures S6I–S6M). In wild-type intestines, EGFR is mainly localized in cytosolic puncta/vesicles in progenitors (Figure 6A). Most of these EGFR puncta were Rab5+, indicating that EGFR associates with the early endosomes under normal conditions (Figures 6A and 6B). While EGFR proteins accumulated on the plasma membrane and were no longer observed in Rab5+ puncta (Figures 6B and 6C), the levels of EGFR were significantly increased and EGFR proteins were accumulated at/near the plasma membrane in esgts>auxRNAi intestines and in auxRNAi ISC MARCM clones (Figures 6D–6F and S6N–S6P). The levels of EGFR protein were also dramatically increased and these EGFR proteins were accumulated at/near the plasma membrane in aux mutant mosaic clones (Figures 6G and 6H). Western blotting results show that the levels of EGFR protein were significantly increased when aux was systematically depleted (Figure 6I). Meanwhile, the levels of Egfr transcripts were not changed in the absence of aux, indicating that the elevated levels of EGFR are likely a result of defective internalization or degradation, but not increased transcription upon aux depletion (Figure S6Q). These results suggest that the internalization of EGFR is blocked upon aux depletion, which is consistent with a previous report in which EGFR is accumulated, but is not responsible for the defects caused in aux mutant clones in eye discs (Kandachar et al., 2008). Consistent with this notion, the levels of EGFR were also dramatically increased, and these EGFR proteins were also accumulated at/near the plasma membrane when endocytosis was blocked by compromising either Rab5 or dynamin in progenitors (Figures S4D–S4I). Altogether, these data indicate that Aux is required for EGFR internalization to safeguard excessive EGFR activation under physiological conditions, and ectopic activation of EGFR signaling from accumulated EGFR on the plasma membrane upon Aux depletion results in intestinal homeostasis disruption.
Aux associates with EGFR in vivo
Next, we investigated whether Aux and EGFR were co-localized in vivo. EGFR is mainly located in puncta/vesicles as well as at the plasma membrane in wild-type progenitors (Figure 6, Figure 7A and 7A). Consistent with its role in endocytosis, transiently expressed Aux-FLAG-GFP located at the plasma membrane and puncta as well (Figures 7A–7B″ and data not shown). Interestingly, EGFR co-localized with transiently expressed Aux-FLAG-GFP in puncta, indicating that Aux may associate with and regulate EGFR internalization (Figures 7A–7B″ and data not shown). Furthermore, endogenous Aux puncta often co-localized with endogenous EGFR puncta in progenitors (Figures 7C–7D″). We further performed coimmunoprecipitation (coIP) experiments to examine whether Aux associates with EGFR in vivo. The coIP results showed that endogenous EGFR could be easily precipitated by transiently expressed Aux-FLAG-GFP (Figure 7E). Moreover, endogenous EGFR also co-immunoprecipitated with endogenous Aux (Figure 7E). Together, these data show that Aux associates with EGFR directly or indirectly and is required for its clearance from the plasma membrane to ensure proper EGFR signaling under physiological conditions.
Ectopic EGFR signaling is required for the defects observed upon aux depletion
The above-mentioned results indicate that aux depletion significantly increases EGFR signaling via accumulating EGFR at/near the cell surface. Interestingly, ectopic expression of either wild type or constitutively active EGFR could cause a significant increase in progenitors; however, their ectopic expression could not mimic the strong defects observed upon aux depletion (data not shown). This suggests that in the absence of Aux, additional mechanism(s)/signaling pathway(s) contribute to the excessive activation of EGFR signaling. Previous studies showed that excessive ISC proliferation stresses intestines and EGFR ligands are induced in stressed intestines to activate EGFR signaling in ISCs (Jiang et al., 2011; Zhao et al., 2021). Interestingly, we found that the expression of EGFR ligands such as Spi and Vein was significantly increased in both esg>GFP+ cells and esg>GFP− cells in esgts>auxRNAi intestines, suggesting that esgts>auxRNAi intestines are stressed and that increased expression of EGFR ligands further activates EGFR accumulated at the cell surface (Figures S7A–S7H). Consistent with these observations, while co-depleting Spi could not significantly suppress the defects observed in esgts>auxRNAi intestines, the defects observed in esgts>auxRNAi intestines were significantly suppressed upon the depletion of Rhomboid, the protease processing EGFR ligands (Figures S7I–S7N).
To functionally determine whether ectopic EGFR signaling is required for the defects associated with aux depletion, we asked whether esgts>auxRNAi phenotypes could be suppressed by the reduction of various components in the EGFR/mitogen-activated protein kinase (MAPK) signaling pathway (Lu et al., 2019). RNAi-mediated knockdown of EGFR, Ras, Raf, MEK, and MAPK completely suppressed the aberrant number and percentage of esg+ cells in esgts>auxRNAi intestines (Figures S7O–S7V). Moreover, the significantly increased mitotic ISCs were also completely suppressed (Figures 7F–7L). These genetic interactions show that ectopic EGFR signaling is required for the defects resulting from aux depletion.
Discussion
To maintain tissue homeostasis, the activation of signaling pathways required for adult stem cell proliferation needs to be precisely controlled. Here, we demonstrate that Aux negatively affects ISC proliferation. Immunostaining and genetic data implicate EGFR as the relevant target, suggesting clearance of EGFR from the plasma membrane is important for regulating ISC proliferation. In addition to accumulative EGFR at the cell surface, depletion of aux function stresses the tissue, resulting in elevated production of EGFR ligands, which likely further activates EGFR at the cell surface. Together, our results suggest that clathrin-mediated endocytosis removes EGFR from the plasma membrane of progenitors to constrain stem cell proliferation and maintain midgut homeostasis.
Aux and endocytosis have minor roles in Notch signaling in ISC proliferation regulation
Clathrin-mediated transport, in addition being a major endocytic route, participates in vesicular trafficking from various organelles (Ding et al., 2016; Robinson, 2015; Zhou et al., 2011). The clathrin cage of a nascent CCV needs be removed before its fusion with target organelles (Lee et al., 2006; Lemmon, 2001; Massol et al., 2006). Aux functions as a co-factor for this disassembly event by recruiting Hsc70 (Eisenberg and Greene, 2007; Lemmon, 2001). While Aux also participates in other steps of the CCV cycle and in the secretory pathway in different cellular contexts (Ding et al., 2016; Eisenberg and Greene, 2007; Zhou et al., 2011), its role in EGFR trafficking (see below) in ISC appears to be endocytic. In aux-depleted cells, EGFR accumulates at the cell surface, a phenotype that is consistent with endocytosis blockage.
Aux is required for Notch activation, facilitating ligand internalization in the signal-sending cells, in multiple Notch-dependent processes, such as photoreceptor specification, lateral inhibition, and the formation of the dorsal-ventral wing boundary (Eun et al., 2008; Hagedorn et al., 2006; Kandachar et al., 2008). Given the importance of Notch signaling in ISC self-renewal and differentiation (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006, 2007), it seems reasonable that Aux depletion perturbs intestinal homeostasis by disrupting the Notch cascade. Inhibition of the Notch pathway in intestines with mutations in Dl or Neutralized, an ubiquitin E3 ligase required for Dl internalization, causes two defects: (1) tumors with increased number of EEs and (2) ISC-like tumors without EEs. If Aux is required for Dl internalization in the signal-sending cells (ISCs) to activate Notch in the signal-receiving cells (EBs), then depletion of aux in progenitors should exhibit these two expected defects. However, no increase in EEs was observed in aux-depleted intestines and many aux-defective cells continued to differentiate into ECs, which is indicative of functional Notch signaling upon aux depletion. Thus, our data indicate Aux has a crucial role in maintaining intestinal homeostasis, although its impact is unlikely to be mediated through Notch signaling. We cannot fully exclude the possibility that Aux may affect Notch signaling at a marginal level.
While the involvement of Dynamin, Neutralized, Mind Bomb, Epsin, and Aux in Notch signaling seems clear (Eun et al., 2008; Hagedorn et al., 2006; Wang and Struhl, 2005), there are Notch-dependent processes in which endocytosis seems dispensable (Wang and Struhl, 2004; Windler and Bilder, 2010). As there are multiple endocytic routes, it is possible that in certain cell types (e.g., ISCs), other endocytic routes can compensate for the loss of aux function. Alternatively, as ligand internalization is thought to generate mechanical stress on Notch to facilitate cleavage, it may be that in certain cell types, other mechanisms can induce Notch processing, bypassing the need for ligand endocytosis. Alternatively, ectopic EGFR signaling resulting from aux depletion is much more potent than that of Notch loss of function in promoting ISC proliferation, thereby dominating the defects caused by defective Notch signaling. In any case, we provide clear evidence that aux depletion has minimal, if any, impact on Notch signaling in the control of ISC proliferation rate for tissue homeostasis under physiological conditions.
Ectopic EGFR signaling is required for the defects observed upon aux depletion
Consistent with its role in CCV uncoating, it is not unexpected that CCVs containing cargo such as EGFR is accumulated upon aux depletion (Kandachar et al., 2008). Indeed, we present evidence that excessive EGFR signaling is the underlying cause for the hyperproliferation of aux-depleted progenitors. The subcellular localization of EGFR was altered by aux depletion, as EGFR becomes accumulated at/near the plasma membrane. These results are consistent with previous reports that endocytosis of EGFR is regulated (Caldieri et al., 2018; Du et al., 2020; Kandachar et al., 2008; Zhang et al., 2004, 2019). Our results support a model in which aux depletion disrupts EGFR endocytosis, resulting in a prolonged and excessive EGFR activity that leads to the increased proliferation of the progenitors.
Interestingly, ectopic expression of either wild type or constitutively active EGFR failed to produce the strong defects observed in aux-defective intestines, suggesting that additional mechanisms/signaling pathways contribute to ISC proliferation upon Aux depletion. Consistent with this notion, the expression of EGFR ligands is significantly induced in both aux-defective cells and neighboring wild-type cells, and the aux depletion hyperproliferation phenotype is sensitive to the reduction of ligand-processing protease. Thus, the hyperproliferation of aux-defective ISCs may be the combined consequences of three possible events: (1) a cell-autonomous accumulation of EGFR at the cell surface caused by endocytic disruption, (2) a non-cell-autonomous production of elevated EGFR ligands caused by intestinal stress, and (3) altered signaling downstream of the EGFR as previously described (Zhang et al., 2004). These events are expected to synergistically increase EGFR signaling in ISCs and drive ISC hyperproliferation.
Experimental procedures
Fly lines and cultures
Flies were maintained on standard media at 25°C. Crosses were raised at 18 °C in humidity-controlled incubators, or as otherwise noted. Information for alleles and transgenes used in this study can be found either in FlyBase or as noted.
Immunostaining and fluorescence microscopy
The standard immunostaining of intestines was followed as previously described (Zhao et al., 2021). The following primary antibodies were used: mouse monoclonal antibody (mAb) anti-Dl (C594.9B, 1:50, developed by S. Artavanis-Tsakonas, Developmental Studies Hybridoma Bank [DSHB]), mouse mAb anti-Prospero (MR1A, 1:100, developed by C.Q. Doe, DSHB), rabbit anti-β-galactosidase (1:5,000, Cappel, catalog number [cat. no.] 55,978), mouse anti-β-galactosidase (14B7, 1:1,000, Cell Signaling, cat. no. 2372S), rabbit anti-pH3 (pSer10, 1:2,000, Millipore, cat. no. H0412), rabbit anti-pERK (p-p44/42, 1:200, Cell Signaling, cat. no. 4370S), rabbit anti-Pdm1 (1:400, generous gifts from Drs. Xiaohang Yang and Xiaolin Bi) (Dai et al., 2020; Yeo et al., 1995), mouse anti-EGFR (1:100, Sigma-Aldrich, cat. no. E2906), rabbit anti-GFP (1:200, Abcam, cat. no. ab290), and guinea pig anti-Aux (1:1,000, the present study). All of the images were captured by a Zeiss LSM780 inverted confocal microscope and were processed in Adobe Photoshop and Illustrator.
Author contributions
Conceptualization, Zhouhua Li and H.Z.; investigation, H.Z., X.R., R.K., L.S., Zhengran Li, R.M., H.Z., F.L., and Zhouhua Li; formal analysis, H.Z., X.R., and Zhengran Li; methodology, R.M., H.C.C., and C.-H.C.; validation, H.Z., X.R., and Zhouhua Li; writing—original draft preparation, H.Z. and Zhouhua Li; writing—review and editing, H.Z., H.C.C., C.-H.C., and Zhouhua Li; supervision, Zhouhua Li; project administration, Zhouhua Li; funding acquisition, Zhouhua Li. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
We are grateful to Norbert Perrimon, Sarah Bray, Steven Hou, Rongwen Xi, Jose Pastor, Xiaohang Yang, Xiaolin Bi, Hai Huang, and Yu Cai for reagents; the Bloomington Stock Center; the VDRC, the NIG-FLY Center, TRiP at Harvard Medical School, and the Tsinghua Fly Center for fly stocks, and DSHB for antibodies. This work is supported by grants from the National Natural Science Foundation of China (nos. 92054109, 31972893, and 31471384), and the Beijing Municipal Commission of Education (no. KZ201910028040).
Published: April 14, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.03.010
Supplemental information
References
- Barouch W., Prasad K., Greene L., Eisenberg E. Auxilin-induced interaction of the molecular chaperone Hsc70 with clathrin baskets. Biochemistry. 1997;36:4303–4308. doi: 10.1021/bi962727z. [DOI] [PubMed] [Google Scholar]
- Beebe K., Lee W.C., Micchelli C.A. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev. Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Biteau B., Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138:1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B., Jasper H. Slit/robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 2014;7:1867–1875. doi: 10.1016/j.celrep.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldieri G., Malabarba M.G., Di Fiore P.P., Sigismund S. EGFR trafficking in physiology and cancer. Prog. Mol. Subcell Biol. 2018;57:235–272. doi: 10.1007/978-3-319-96704-2_9. [DOI] [PubMed] [Google Scholar]
- Casali A., Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4:124–127. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Chen J., Xu N., Wang C., Huang P., Huang H., Jin Z., Yu Z., Cai T., Jiao R., Xi R. Transient Scute activation via a self-stimulatory loop directs enteroendocrine cell pair specification from self-renewing intestinal stem cells. Nat. Cell Biol. 2018;20:152–161. doi: 10.1038/s41556-017-0020-0. [DOI] [PubMed] [Google Scholar]
- Colombani J., Andersen D.S. The Drosophila gut: a gatekeeper and coordinator of organism fitness and physiology. Wires Dev. Biol. 2020;9:e378. doi: 10.1002/wdev.378. [DOI] [PubMed] [Google Scholar]
- Dai Z., Li D., Du X., Ge Y., Hursh D.A., Bi X. Drosophila Caliban preserves intestinal homeostasis and lifespan through regulating mitochondrial dynamics and redox state in enterocytes. PLoS Genet. 2020;16:e1009140. doi: 10.1371/journal.pgen.1009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Navascués J., Perdigoto C.N., Bian Y., Schneider M.H., Bardin A.J., Martínez-Arias A., Simons B.D. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J. 2012;31:2473–2485. doi: 10.1038/emboj.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Segarra V.A., Chen S., Cai H., Lemmon S.K., Ferro-Novick S. Auxilin facilitates membrane traffic in the early secretory pathway. Mol. Biol. Cell. 2016;27:127–136. doi: 10.1091/mbc.E15-09-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Qiao Y., Zhuo Z., Zhou J., Li X., Liu Z., Li Y., Chen H. Lipoic acid rejuvenates aged intestinal stem cells by preventing age-associated endosome reduction. EMBO Rep. 2020;21:9. doi: 10.15252/embr.201949583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B.A. Intestinal stem cells: no longer immortal but ever so clever. EMBO J. 2012;31:2441–2443. doi: 10.1038/emboj.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Greene L.E. Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis. Traffic. 2007;8:640–646. doi: 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Eun S.H., Banks S.M., Fischer J.A. Auxilin is essential for Delta signaling. Development. 2008;135:1089–1095. doi: 10.1242/dev.009530. [DOI] [PubMed] [Google Scholar]
- Furriols M., Bray S. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr. Biol. 2001;11:60–64. doi: 10.1016/s0960-9822(00)00044-0. [DOI] [PubMed] [Google Scholar]
- Gervais L., Bardin A.J. Tissue homeostasis and aging: new insight from the fly intestine. Curr. Opin. Cell Biol. 2017;48:97–105. doi: 10.1016/j.ceb.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Goulas S., Conder R., Knoblich Juergen A. The par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell. 2012;11:529–540. doi: 10.1016/j.stem.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Ohlstein B. Stem cell regulation. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science. 2015;350:aab0988. doi: 10.1126/science.aab0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn E.J., Bayraktar J.L., Kandachar V.R., Bai T., Englert D.M., Chang H.C. Drosophila melanogaster auxilin regulates the internalization of Delta to control activity of the Notch signaling pathway. J. Cell Biol. 2006;173:443–452. doi: 10.1083/jcb.200602054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein S.E., Ungewickell H., Ungewickell E. Mechanism of clathrin basket dissociation: separate functions of protein domains of the DnaJ homologue auxilin. J. Cell Biol. 1996;135:925–937. doi: 10.1083/jcb.135.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Grenley M.O., Bravo M.-J., Blumhagen R.Z., Edgar B.A. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Patel P.H., Kohlmaier A., Grenley M.O., McEwen D.G., Edgar B.A. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Tian A., Jiang J. Intestinal stem cell response to injury: lessons from Drosophila. Cell. Mol. Life Sci. 2016;73:3337–3349. doi: 10.1007/s00018-016-2235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Ha N., Forés M., Xiang J., Gläßer C., Maldera J., Jiménez G., Edgar B.A. EGFR/Ras signaling controls Drosophila intestinal stem cell proliferation via capicua-regulated genes. Plos Genet. 2015;11:e1005634. doi: 10.1371/journal.pgen.1005634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly A., Rousset R. Tissue adaptation to environmental cues by symmetric and asymmetric division modes of intestinal stem cells. Int. J. Mol. Sci. 2020;21:6362. doi: 10.3390/ijms21176362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandachar V., Bai T., Chang H.C. The clathrin-binding motif and the J-domain of Drosophila Auxilin are essential for facilitating Notch ligand endocytosis. BMC Dev. Biol. 2008;8:50. doi: 10.1186/1471-213X-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P., Perez J., Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.W., Wu X., Eisenberg E., Greene L.E. Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J. Cell Sci. 2006;119:3502–3512. doi: 10.1242/jcs.03092. [DOI] [PubMed] [Google Scholar]
- Lemmon S.K. Clathrin uncoating: auxilin comes to life. Curr. Biol. 2001;11:R49–R52. doi: 10.1016/s0960-9822(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Lin H. Cell biology of stem cells: an enigma of asymmetry and self-renewal. J. Cell Biol. 2008;180:257–260. doi: 10.1083/jcb.200712159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Zhao H., Kong R., Shi L., Li Z., Ma R. Derlin-1 and TER94/VCP/p97 are required for intestinal homeostasis. J. Genet. Genomics. 2022;49:00303–00309. doi: 10.1016/j.jgg.2021.08.017. [DOI] [PubMed] [Google Scholar]
- Lu Y., Li Z. Notch signaling downstream target E(spl)mbeta is dispensable for adult midgut homeostasis in Drosophila. Gene. 2015;560:89–95. doi: 10.1016/j.gene.2015.01.053. [DOI] [PubMed] [Google Scholar]
- Lu Y., Yao Y., Li Z. Ectopic Dpp signaling promotes stem cell competition through EGFR signaling in the Drosophila testis. Sci. Rep. 2019;9:6118. doi: 10.1038/s41598-019-42630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Zhao H., Liu F., Kong R., Shi L., Wei M., Li Z. Heparan sulfate negatively regulates intestinal stem cell proliferation in Drosophila adult midgut. Biol. Open. 2019;8:047126. doi: 10.1242/bio.047126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Zhao H., Binari R., Perrimon N., Li Z. Wildtype adult stem cells, unlike tumor cells, are resistant to cellular damages in Drosophila. Dev. Biol. 2016;411:207–216. doi: 10.1016/j.ydbio.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol R.H., Boll W., Griffin A.M., Kirchhausen T. A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc. Natl. Acad. Sci. U S A. 2006;103:10265–10270. doi: 10.1073/pnas.0603369103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli C.A., Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Spradling A.C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmyer S.L., Christensen A., Sever S. Auxilin-dynamin interactions link the uncoating ATPase chaperone machinery with vesicle formation. Dev. Cell. 2003;4:929–940. doi: 10.1016/s1534-5807(03)00157-6. [DOI] [PubMed] [Google Scholar]
- O'Brien L.M., Lucy E., Soliman Sarah S., Li X., Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Patel P.H., Dutta D., Edgar B.A. Niche appropriation by Drosophila intestinal stem cell tumours. Nat. Cell Biol. 2015;17:1182–1192. doi: 10.1038/ncb3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigoto C.N., Schweisguth F., Bardin A.J. Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development. 2011;138:4585–4595. doi: 10.1242/dev.065292. [DOI] [PubMed] [Google Scholar]
- Radtke F., Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- Ren F., Wang B., Yue T., Yun E.Y., Ip Y.T., Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc. Natl. Acad. Sci. U S A. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.S. Forty years of clathrin-coated vesicles. Traffic. 2015;16:1210–1238. doi: 10.1111/tra.12335. [DOI] [PubMed] [Google Scholar]
- Shaw R.L., Kohlmaier A., Polesello C., Veelken C., Edgar B.A., Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Kong R., Li Z., Zhao H., Ma R., Bai G., Li J. Identification of a new allele of O-fucosyltransferase 1 involved in Drosophila intestinal stem cell regulation. Biol. Open. 2021;10:3. doi: 10.1242/bio.058910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier D.Y.R. No organ left behind: tales of gut development and evolution. Science. 2005;307:1902–1904. doi: 10.1126/science.1108709. [DOI] [PubMed] [Google Scholar]
- Tian A., Jiang J. Intestinal epithelium-derived BMP controls stem cell self-renewal in Drosophila adult midgut. eLife. 2014;3:e01857. doi: 10.7554/eLife.01857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda A., Meyerholz A., Ungewickell E. Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur. J. Cell Biol. 2000;79:336–342. doi: 10.1078/S0171-9335(04)70037-0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Ungewickell H., Holstein S.E., Lindner R., Prasad K., Barouch W., Martin B., Greene L.E., Eisenberg E. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- Wang P., Hou S.X. Regulation of intestinal stem cells in mammals and. Drosophila. 2010;222:33–37. doi: 10.1002/jcp.21928. [DOI] [PubMed] [Google Scholar]
- Wang W., Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- Wang W., Struhl G. Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development. 2005;132:2883–2894. doi: 10.1242/dev.01860. [DOI] [PubMed] [Google Scholar]
- Windler S.L., Bilder D. Endocytic internalization routes required for delta/notch signaling. Curr. Biol. 2010;20:538–543. doi: 10.1016/j.cub.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., Spradling A.C. Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Xing Y., Bocking T., Wolf M., Grigorieff N., Kirchhausen T., Harrison S.C. Structure of clathrin coat with bound Hsc70 and auxilin: mechanism of Hsc70-facilitated disassembly. EMBO J. 2010;29:655–665. doi: 10.1038/emboj.2009.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Wang S.Q., Tan D., Gao Y., Lin G., Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev. Biol. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Yeo S.L., Lloyd A., Kozak K., Dinh A., Dick T., Yang X., Sakonju S., Chia W. On the functional overlap between two Drosophila POU homeo domain genes and the cell fate specification of a CNS neural precursor. Genes Dev. 1995;9:1223–1236. doi: 10.1101/gad.9.10.1223. [DOI] [PubMed] [Google Scholar]
- Yeung T., Chia L., Kosinski C., Kuo C. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol. Life Sci. 2011;68:2513–2523. doi: 10.1007/s00018-011-0687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A., Stoilova-McPhie S., Rothnie A., Vallis Y., Harvey-Smith P., Ranson N., Kent H., Brodsky F.M., Pearse B.M., Roseman A., et al. Hsc70-induced changes in clathrin-auxilin cage structure suggest a role for clathrin light chains in cage disassembly. Traffic. 2013;14:987–996. doi: 10.1111/tra.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Chauhan C., Hou S.X. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in Drosophila. Genesis. 2010;48:607–611. doi: 10.1002/dvg.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Han L., Singh S.R., Liu H., Neumüller R.A., Yan D., Hu Y., Liu Y., Liu W., Lin X., et al. Genome-wide RNAi screen identifies networks involved in intestinal stem cell regulation in Drosophila. Cell Rep. 2015;10:1226–1238. doi: 10.1016/j.celrep.2015.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z., Boquete J.P., Lemaitre B. A genetic framework controlling the differentiation of intestinal stem cells during regeneration in Drosophila. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Gjoerup O., Roberts T.M. The serine/threonine kinase cyclin G-associated kinase regulates epidermal growth factor receptor signaling. Proc. Natl. Acad. Sci. U S A. 2004;101:10296–10301. doi: 10.1073/pnas.0403175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Holowatyj A.N., Roy T., Pronovost S.M., Marchetti M., Liu H., Ulrich C.M., Edgar B.A. An SH3PX1-dependent endocytosis-autophagy network restrains intestinal stem cell proliferation by counteracting EGFR-ERK signaling. Dev. Cell. 2019;49:574–589. doi: 10.1016/j.devcel.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Huang H., Zhang L., Wu R., Chung C.I., Zhang S.Q., Torra J., Schepis A., Coughlin S.R., Kornberg T.B., et al. Visualizing dynamics of cell signaling in vivo with a phase separation-based kinase reporter. Mol. Cell. 2018;69:334–346. doi: 10.1016/j.molcel.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Shi L., Kong R., Li Z., Liu F., Zhao H., Li Z. Autophagy induction in tumor surrounding cells promotes tumor growth in adult Drosophila intestines. Dev. Biol. 2021;476:294–307. doi: 10.1016/j.ydbio.2021.04.008. [DOI] [PubMed] [Google Scholar]
- Zhao H., Shi L., Li Z., Kong R., Ren X., Ma R., Jia L., Ma M., Lu S., Xu R., et al. The Yun/Prohibitin complex regulates adult Drosophila intestinal stem cell proliferation through the transcription factor E2F1. Proc. Natl. Acad. Sci. U S A. 2022;119 doi: 10.1073/pnas.2111711119. e2111711119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Fabian L., Bayraktar J.L., Ding H.M., Brill J.A., Chang H.C. Auxilin is required for formation of Golgi-derived clathrin-coated vesicles during Drosophila spermatogenesis. Development. 2011;138:1111–1120. doi: 10.1242/dev.057422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.