Abstract
Objective
To provide an overview and critical appraisal of prediction models for bronchopulmonary dysplasia (BPD) in preterm infants.
Methods
We searched PubMed, Embase, and the Cochrane Library to identify relevant studies (up to November 2021). We included studies that reported prediction model development and/or validation of BPD in preterm infants born at ≤32 weeks and/or ≤1,500 g birth weight. We extracted the data independently based on the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS). We assessed risk of bias and applicability independently using the Prediction model Risk Of Bias ASsessment Tool (PROBAST).
Results
Twenty-one prediction models from 13 studies reporting on model development and 21 models from 10 studies reporting on external validation were included. Oxygen dependency at 36 weeks’ postmenstrual age was the most frequently reported outcome in both development studies (71%) and validation studies (81%). The most frequently used predictors in the models were birth weight (67%), gestational age (62%), and sex (52%). Nearly all included studies had high risk of bias, most often due to inadequate analysis. Small sample sizes and insufficient event patients were common in both study types. Missing data were often not reported or were discarded. Most studies reported on the models’ discrimination, while calibration was seldom assessed (development, 19%; validation, 10%). Internal validation was lacking in 69% of development studies.
Conclusion
The included studies had many methodological shortcomings. Future work should focus on following the recommended approaches for developing and validating BPD prediction models.
Keywords: prediction, model, bronchopulmonary dysplasia, preterm infants, systematic review
Introduction
Preterm infant survival has increased in the last three decades (1–3), while bronchopulmonary dysplasia (BPD) remains the most prevalent serious complication of prematurity, affecting 10.8–37.1% of preterm neonates born at 240/7 to 316/7 weeks’ gestational age and birth weight <1,500 g (4). As survivors with BPD have high risk of poor long-term pulmonary and neurodevelopmental outcomes in childhood and even adulthood (5–8), it is imperative to optimize BPD prevention and treatment strategies. Early identification of infants at risk of developing BPD would benefit preventive interventions when airway injury is still functional and reversible. To aid health care providers in estimating the probability of BPD occurrence in the future and to inform decision-making, many models for predicting BPD have been established in recent years. Nevertheless, such models are often of variable quality and yield inconsistent findings, leading to confusion or uncertainty among health care providers regarding which model to use or recommend.
In a 2013 systematic review, Onland et al. reported 26 prediction models for assessing the probability of BPD or death in all preterm infants born at <37 weeks’ gestation, where most existing clinical prediction models were poor to moderate BPD predictors (9). Furthermore, during that review, no guides for systematic reviews of prediction modeling studies or standardization tools for assessing the prediction models’ risk of bias (ROB) were available. Since then, more BPD prediction modeling studies have been published, whereas systematic reviews of such studies have not yet been updated in the last 9 years. The guideline CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS) has been available since 2014 (10), and the Prediction model Risk Of Bias ASsessment Tool (PROBAST) for assessing the ROB and applicability of prediction model studies has been available since 2019 (11).
Accordingly, the present systematic review was aimed at updating the systematic review of BPD prediction models and critically evaluating the methods and reporting of studies that developed or externally validated prediction models for BPD in preterm infants born at ≤32 weeks and/or ≤1,500 g birth weight based on the CHARMS checklist and PROBAST.
Methods
This systematic review of all studies on prediction models for BPD in preterm infants is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12).
Search Strategy
PubMed (MEDLINE), Embase, and the Cochrane Library were systematically searched from inception through to 12 November 2021, for studies reporting prediction models of BPD in preterm infants. We identified relevant studies and maximized search accuracy using the following terms: BPD, chronic lung disease, preterm infants, and prediction. The online Supplementary Material 1 shows the electronic search strategies. The search was not limited by language.
Eligibility Criteria
Articles were included if: (1) the target population was preterm infants born at ≤32 weeks and/or ≤1,500 g birth weight; (2) the study detailed prediction model development and/or external validation; (3) the main prediction outcome was BPD, defined as oxygen requirement at 28 days of life (BPD28) and/or oxygen requirement at 36 weeks’ postmenstrual age (PMA) (BPD36); (4) the model was constructed with at least two predictors; and (5) the purpose of the model was for predicting BPD in preterm infants from the first 2 weeks of life. Articles were ineligible when the studies used the data of infants born before 1990, as surfactant was not routinely used before this year (pre-surfactant era); if the outcome to be predicted was the composite outcome “BPD or death”; when the prognostic use of lung ultrasound scores (LUS) was investigated; when the study was conducted at high altitudes; when it was only a methodological study; when the article was not published in English; or when the article was a conference abstract, review, or letter.
Study Selection and Data Extraction
Two reviewers independently screened the titles, abstracts, and full texts in duplicate for eligibility. In case of discrepancies, a third reviewer was involved to establish consensus. The reviewers used a standardized data extraction form based on the CHARMS checklist (10). The following items were extracted from the studies on prediction model development: study design, study population, predicted outcome and time horizon, intended moment of model use, number of candidate predictors, sample size, number of events, missing data approach, variables selection method, modeling method, model presentation, predictors included in the final model, internal validation method, and assessment of model performance (i.e., discrimination and calibration). The following items were extracted from the prediction model external validation studies: study design, study population, predicted outcome and time horizon, intended moment of model use, sample size, number of events, missing data approach, and assessment of model performance (i.e., discrimination and calibration). The events per variable (EPV) was defined as the number of events divided by the number of candidate predictor variables used. The outcome BPD28 was defined as oxygen dependency at 28 days of life; BPD36 was defined as oxygen dependency at 36 weeks PMA.
Assessment of Bias
We assessed the ROB and applicability of each article with PROBAST. PROBAST consists of 20 signaling questions across four domains (participants, predictors, outcome, and analysis). The ROB and applicability of original studies were classified as high, low, or unclear for each domain via comprehensive evaluation. Only if each domain had low ROB would a study be classified as overall low ROB.
Model Performance
The results of the development and external validation studies were summarized by using descriptive statistics. If an article described the development or external validation of multiple (existing) models, separate data extraction for each model was conducted. Each model’s predictive performance, including model discrimination and calibration measures, was extracted. Discrimination is often quantified by the C statistic. The C statistic is the most commonly used measure for determining the discriminative performance for binary outcomes. Generally, a C statistic < 0.6 is considered poor, a C statistic between 0.6 and 0.75 is possibly helpful, a C statistic > 0.75 is clearly useful (13). Calibration is often quantified by the calibration intercept and calibration slope.
Results
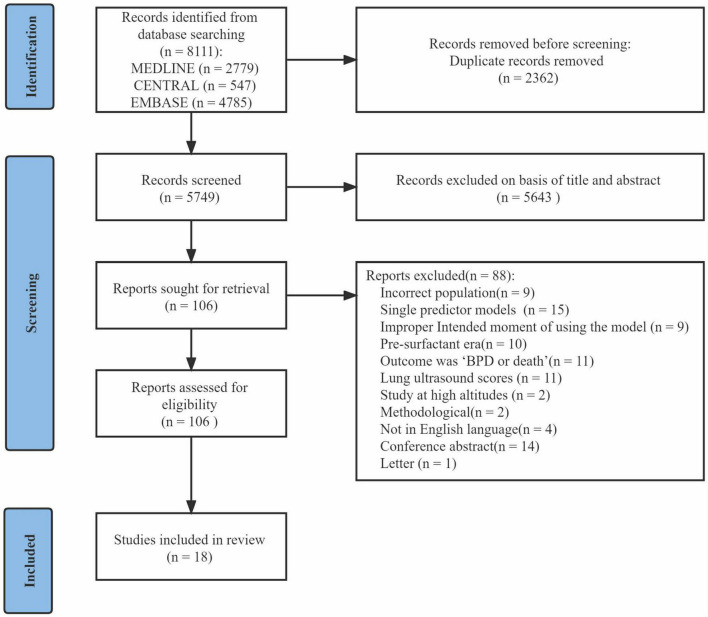
After excluding duplicates, the initial search returned 5,749 articles. After title and abstract screening, 106 articles were provisionally selected for full-text screening. Subsequently, 88 articles were excluded, among which 11 articles used the composite outcome “BPD or death.” In total, 18 studies (14–31) were included in this systematic review (Figure 1). Eight studies (14, 16, 19, 21, 22, 25–27) described model development without external validation, five studies (15, 17, 24, 29, 30) described model development with external validation in independent data, and five studies (18, 20, 23, 28, 31) described external validation with or without model updating.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Characteristics of Studies Describing Bronchopulmonary Dysplasia Prediction Model Development
Thirteen studies described BPD prediction model development, in which 21 models were developed. Table 1 shows the key characteristics of study design, study population, outcome, and intended moment of model use in the included model development studies. Table 2 shows the study and performance characteristics of the developed models.
TABLE 1.
Design characteristics of the 13 studies describing the development of BPD prediction models.
| Study | Country | Study design | Years of data | Study population | Timing of BPD | Intended moment of model use | Models, n | Differences between models caused by differences in the following: |
| El Faleh et al. (17) | Switzerland | Registry | 2009–2010 | BW < 1,501 g and/or GA between 23 0/7 and 31 6/7 weeks | DOL28 and PMA36 | 1 day | 2 | Timing of BPD |
| Shim et al. (15) | Korea | Registry | 2013–2016 | BW < 1,500 g and GA ≥ 22 weeks | PMA36 | 1 h | 3 | Severity of BPD |
| Ushida et al. (14) | Japan | Registry | 2006–2015 | GA < 32 weeks and BW ≤ 1,500 g | PMA36 | At birth | 1 | NA |
| Verder et al. (16) | Denmark | Prospective cohort | 2019 | GA 24–31 weeks | DOL28 | At birth | 1 | NA |
| Valenzuela-Stutman et al. (19) | Argentina, Chile, Paraguay, Peru, Uruguay | Registry | 2001–2015 | BW 500–1,500 g | PMA36 | At birth, 3 day, 7 day, 14 day | 4 | Intended moment of using the model |
| Sullivan et al. (21) | United States | Prospective cohort | 2009–2015 | BW < 1,500 g | PMA36 | 7 day | 3 | Predictors |
| Bentsen et al. (22) | United States | Prospective cohort | 2014–2016 | GA < 28 weeks | PMA36 | 2 day | 1 | NA |
| Gursoy et al. (24) | Turkey | Retrospective cohort | 2006–2009 | GA ≤ 32 weeks and BW ≤ 1,500 g | DOL28 | 3 day | 1 | NA |
| Tian et al. (26) | China | Prospective cohort | 2010–2011 | BW ≤ 1,500 g and GA ≤ 32 weeks | DOL28 | At birth | 1 | NA |
| Wang et al. (25) | China | Prospective cohort | 2011–2013 | BW ≤ 1,500 g and GA ≤ 32 weeks | DOL28 | 14 day | 1 | NA |
| May et al. (27) | United Kingdom | Prospective cohort | 2004–2007 | GA 24–32 weeks | DOL28 | 14 day | 1 | NA |
| Henderson-Smart et al. (29) | Australia, New Zealand | Registry | 1998–1999 | GA 22–31 weeks | PMA36 | At birth | 1 | NA |
| Kim et al. (30) | Korea | Retrospective cohort | 1997–1999 | BW < 1,500 g | PMA36 | 4 day, 7 day, 10 day | 1 | NA |
BPD, bronchopulmonary dysplasia; BW, birth weight; DOL, days of life; GA, gestational age; PMA, postmenstrual age; NA, not applicable.
TABLE 2.
Study and performance characteristics of the developed prediction models.
| Study | Outcome | Intended moment of model use | Sample size | Events | EPV | Missing data | Univariable analysis | Modeling method | Model presentation | Predictors, n | Discrimination (C statistic) | Calibration | Internal validation |
| El Faleh et al. (17) | BPD28 | 1 day | 1,232 | 266 | 22.2 | Complete case study | Yes | Logistic regression | Formula; web calculator | 7 | 0.88 | HL test | NR |
| BPD36 | 1 day | 1,225 | 138 | 11.5 | Complete case study | Yes | Logistic regression | Formula | 5 | 0.84 | HL test | NR | |
| Shim et al. (15) | All BPD36 | 1 h | 4,600 | 2,583 | 184.5 | Complete case study | No | Logistic regression | Formula | 4 | 0.908 (0.899–0.916) | NR | NR |
| Moderate to severe BPD36 | 1 h | 4,600 | 1,370 | 97.9 | Complete case study | No | Logistic regression | Formula | 7 | 0.815 (0.802–0.828) | NR | NR | |
| Severe BPD36 | 1 h | 4,600 | 818 | 58.4 | Complete case study | No | Logistic regression | Formula | 8 | 0.815 (0.800–0.831) | NR | NR | |
| Ushida et al. (14) | BPD36 | At birth | 18,858 | 4,986 | 415.5 | Complete case study | No | Logistic regression | Formula | 8 | 0.80 (0.79–0.81) | Calibration plots | Split-sample validation |
| Verder et al. (16) | BPD28 | At birth | 61 | 26 | 2 | NR | No | SVM | NR | 4 | NR | NR | Cross validation |
| Valenzuela-Stutman et al. (19) | BPD36 | At birth | 16,407 | 2,580 | 215 | NR | No | Logistic regression | Web calculator | 5 | 0.788 | NR | Split-sample validation |
| BPD36 | 3 day | 16,407 | 2,580 | 151.8 | NR | No | Logistic regression | Web calculator | 5 | 0.818 | NR | Split-sample validation | |
| BPD36 | 7 day | 16,407 | 2,580 | 143.3 | NR | No | Logistic regression | Web calculator | 5 | 0.827 | NR | Split-sample validation | |
| BPD36 | 14 day | 16,407 | 2,580 | 127.5 | NR | No | Logistic regression | Web calculator | 5 | 0.894 | NR | Split-sample validation | |
| Sullivan et al. (21) | BPD36 | 7 day | 443 | 159 | NA | Complete case study | No | Logistic regression | NR | 6 | 0.921 (0.897–0.945) | NR | Bootstrapping |
| BPD36 | 7 day | 443 | 159 | NA | Complete case study | No | Logistic regression | NR | 5 | 0.886 (0.854–0.913) | NR | Bootstrapping | |
| BPD36 | 7 day | 443 | 159 | NA | Complete case study | No | Logistic regression | NR | 11 | 0.935 (0.920–0.951) | NR | Bootstrapping | |
| Bentsen et al. (22) | BPD36 | 2 day | 37 | 18 | 1.1 | NR | Yes | Logistic regression | NR | 3 | 0.893 (0.735–0.973) | NR | NR |
| Gursoy et al. (24) | BPD28 | 3 day | 652 | 150 | 4.8 | NR | Yes | Logistic regression | Scoring system | 7 | 0.93 | NR | NR |
| Tian et al. (26) | BPD28 | 14 day | 73 | 24 | NA | Complete case study | Yes | Logistic regression | NR | 2 | 0.974 | NR | NR |
| Wang et al. (25) | BPD28 | At birth | 134 | 35 | NA | NR | No | Logistic regression | NR | 2 | 0.849 | NR | NR |
| May et al. (27) | BPD28 | 14 day | 78 | 39 | 2.6 | NR | No | Logistic regression | NR | 2 | 0.97 | NR | NR |
| Henderson-Smart et al. (29) | BPD36 | At birth | 5,599 | 1,235 | 58.8 | Complete case study | Yes | Logistic regression | NR | 3 | 0.84 | HL test | NR |
| Kim et al. (30) | BPD36 | 4 day | 161 | 30 | 1.6 | NR | No | Logistic regression | Scoring system | 8 | 0.76 | NR | NR |
BPD, bronchopulmonary dysplasia; DOL, days of life; EPV, events per variable; HL test, Hosmer–Lemeshow test; NA, not available; NR, not reported; PMA, postmenstrual age; SVM, support vector machine.
Study Design
Eleven included studies (85%) originated from registry or prospective cohorts; two studies (15%) were derived from retrospective cohorts. The data used for developing the models were collected between 1997 and 2019. Of all 13 model development studies, four (31%) used only gestational age as the inclusion criterion, three studies (23%) used only birth weight as the inclusion criterion, and six studies (46%) used both gestational age and birth weight as inclusion criteria. All studies were developed based on statistical methods. Twelve studies (92%) used logistic regression as the prediction modeling approach; one study (8%) used machine learning.
Outcome to Be Predicted
The outcome to be predicted in all included studies was BPD, yet the definitions of BPD varied across the models. Six models (29%) used BPD28 as the primary outcome; the median incidence was 29% (range, 22–50%). Fifteen models (71%) used BPD36 as the primary outcome, with values of 11–56% (median, 22%). Eighteen models (86%) were developed to predict the risk of developing BPD within 7 days of life, and three models (14%) were developed to be used between 7 and 14 days of life.
Predictors
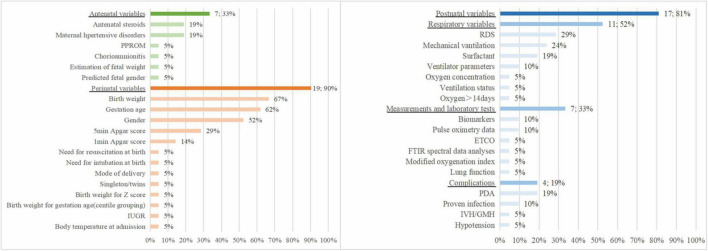
Ten of the 13 studies reported the number of candidate predictors considered for inclusion in the BPD prediction models, with 12–31 candidate predictors (median, 15). Two to 11 predictors were included in the final model (median, 5). Five studies (38%) used univariable analysis to select predictors in the multivariable analyses.
Figure 2 shows the predictors included in the final prediction models. Nineteen models (90%) used perinatal variables, 7 studies (33%) used antenatal variables, and 17 models (81%) used postnatal variables. The most frequently included predictor in the 21 prediction models was birth weight (n = 14, 67%), followed by gestational age (n = 13, 62%), sex (n = 11, 52%), 5-min Apgar score (n = 6, 29%), respiratory distress syndrome (n = 6, 29%), mechanical ventilation (n = 5, 24%), antenatal steroids (n = 4, 19%), maternal hypertensive disorders (n = 4, 19%), surfactant (n = 4, 19%), and patent ductus arteriosus (n = 4, 19%).
FIGURE 2.
Predictors included in the final development models.
Sample Size
The models were developed with 37–18,858 participants (median, 1,225), and there were 18–4,986 events (median, 159). The EPV could be calculated in 16 models (76%) with a median of 59 and a range of 1–416. The EPV was <10 in 31% of the models in which it was calculated.
Missing Data
Seven studies (54%) did not mention missing data. Six studies (46%) mentioned the methods for addressing missing data, where they all used complete case analysis.
Model Presentation
Presentation was available for 12 models (57%). Five models were presented as regression formulae, two models were presented as scoring systems, four models were presented as web calculators, and one model was presented as both a regression formula and web calculator.
Apparent Predictive Performance
Twelve studies (95%) assessed discrimination with the C statistic, with values of 0.76–0.97. Calibration was assessed for four models (19%), two models used the Hosmer–Lemeshow goodness-of-fit test, and one model used calibration plots.
Internal Validation
Nine studies (69%) did not report internal validation of the developed models. Nine models developed in four studies were internally validated. Validation was performed for five models (56%) with split sampling, in one model (11%) with cross-validation, and in three models (33%) with bootstrapping.
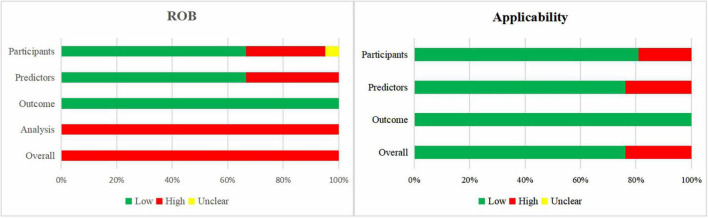
Risk of Bias and Applicability Assessment of the Included Model Development Studies
Figure 3 shows a summary of the ROB and applicability for all developed models. For the domain outcome, the ROB of all models was considered low, as a broad definition of BPD was accepted. There was high participants’ domain-related ROB in 29% of the models. For the domain predictors, 33 and 67% of the models had high and low ROB, respectively. The domain analysis was assessed as having high ROB in all prediction models. No study handled missing data appropriately, as information on missing data was rarely reported or participants with missing data were omitted. Prediction model calibration was insufficient, as only one study reported calibration plots, while the other studies did not report calibration or only used the Hosmer–Lemeshow test. In summary, the overall ROB was high across all models.
FIGURE 3.
Risk of bias and applicability assessment of developed models using Prediction model Risk Of Bias ASsessment Tool (PROBAST).
When the 21 models were assessed according to applicability concerns, 24% of the models were assessed as high concern due to the inclusion of participants different from those in our research question (n = 4) or inconsistency between predictors and the review question (n = 5).
Characteristics of Studies Describing External Validation of the Bronchopulmonary Dysplasia Prediction Models
We included 10 studies that externally validated 21 BPD prediction models (Table 3). Five of these studies also described prediction model development. Table 4 shows the study and performance characteristics of the validated models.
TABLE 3.
Design characteristics of the 10 studies describing external validation of BPD prediction models.
| Study | Country | Study design | Years of data | Study population | Timing of BPD | Intended moment of model use | Model validated |
| El Faleh et al. (17) | Switzerland | Registry | 2014–2015 | BW < 1,501 g and/or GA between 23 0/7 and 31 6/7 weeks | DOL28 or PMA36 | 1 day | El Faleh et al. (17) |
| Shim et al. (15) | Korea | Registry | 2017 | BW < 1,500 g and GA ≥ 22 weeks | PMA36 | 1 h | Shim et al. (15) |
| Bhattacharjee et al. (18) | United States | Retrospective cohort | 2012–2013 | BW < 1,500 g | PMA36 | 3 day | RSS |
| Lee et al. (20) | Korea | Registry | 2013–2016 | BW < 1,500 g and GA 22–32 weeks | PMA36 | 1 h | CRIB II; CRIB II-BE |
| Sullivan et al. (23) | United States | Retrospective cohort | 2004–2014 | BW < 1,500 g | PMA36 | 12 h, 1 day, 7 day | aHRC-24h, aHRC-7d, SNAP-II, CRIB-II |
| Gursoy et al. (24) | Turkey | Prospective cohort | 2012 | GA ≤ 32 weeks and BW ≤ 1,500 g | DOL28 | 3 day | BPD-TM score |
| May et al. (28) | United Kingdom | Retrospective cohort | 1995–1998 | BW < 1,500 g and GA < 33 weeks | DOL28 or PMA36 | 2 day | Simple pulmonary score |
| Henderson-Smart et al. (29) | Australia, New Zealand | Registry | 2000–2001 | GA 22–31 weeks | PMA36 | At birth | Henderson-Smart et al. (29) |
| Kim et al. (30) | Korea | Prospective cohort | 2000–2001 | BW < 1,500 g | PMA36 | 4 day, 7 day, 10 day | SMUMRV; Yoder model (55) |
| Chien et al. (31) | Canada | Registry | 1996–1997 | GA ≤ 32 weeks | PMA36 | 12 h | SNAP-II + GA, SGA, sex, low Apgar score, and outborn status |
BPD, bronchopulmonary dysplasia; BPD-TM, bronchopulmonary dysplasia test measure; BW, birth weight; CRIB, Clinical Risk Indicator fores; CRIB II-BE, Clinical Risk Indicator fores omitting base excess; DOL, days of life; GA, gestational age; HRC, heart rate characteristics; aHRC-24h, average first day HRC index; aHRC-7d, average first week HRC index within 7 days of birth; PMA, postmenstrual age; RSS, respiratory severity score; SGA, small for gestational age; SMUMRV, modified respiratory variables; SNAP-II, Score for Neonatal Acute Physiology-II.
TABLE 4.
Study and performance characteristics of externally validated models.
| Study | Model | Outcome | Sample size | Outcome events | Missing data | Discrimination (C-statistic) | Calibration |
| El Faleh et al. (17) | El Faleh et al. (17) | BPD28 | 1,733 | 437 | Complete case study | 0.92 | NR |
| El Faleh et al. (17) | BPD36 | 1,724 | 191 | Complete case study | 0.88 | NR | |
| Shim et al. (15) | Shim et al. (15) | BPD36 (all grade) | 1,740 | 1,003 | Complete case study | NR | NR |
| Shim et al. (15) | BPD36 (moderate to severe) | 1,740 | 563 | Complete case study | NR | NR | |
| Shim et al. (15) | BPD36 (severe) | 1,740 | 388 | Complete case study | NR | NR | |
| Bhattacharjee et al. (18) | RSS | BPD36 | 69 | 31 | NR | 0.61 | NR |
| Lee et al. (20) | CRIB II | BPD36 | 4,694 | 1,443 | Complete case study | 0.77 (0.76–0.79) | NR |
| CRIB II-BE | BPD36 | 6,038 | 1,916 | Complete case study | 0.77 (0.76–0.78) | NR | |
| Sullivan et al. (23) | aHRC-24h | BPD36 | 566 | 98 | NR | 0.827 | NR |
| aHRC-7d | BPD36 | 566 | 98 | NR | 0.827 | NR | |
| SNAP-II | BPD36 | 566 | 98 | NR | 0.839 | NR | |
| CRIB-II | BPD36 | 566 | 98 | NR | 0.840 | NR | |
| Gursoy et al. (24) | BPD-TM score | BPD28 | 172 | 54 | NR | 0.903 | NR |
| May et al. (28) | Simple pulmonary score-day 2 | BPD28 | 75 | 32 | NR | 0.79 (cohort 1), 0.84 (cohort 2) | NR |
| Simple pulmonary score-day 2 | BPD36 | 75 | 22 | NR | 0.86 (cohort 1), 0.76 (cohort 2) | NR | |
| Simple pulmonary score-day 7 | BPD28 | 75 | 32 | NR | 0.75 (cohort 1), 0.97 (cohort 2) | NR | |
| Simple pulmonary score-day 7 | BPD36 | 75 | 22 | NR | 0.83 (cohort 1), 0.88 (cohort 2) | NR | |
| Henderson-Smart et al. (29) | Henderson-Smart et al. (29) | BPD36 | 5,854 | 1,475 | Complete case study | 0.84 | HL test |
| Kim et al. (30) | SMUMRV | BPD36 | 96 | 9 | NR | 0.90–0.94 | NR |
| Yoder model (55, 57) | BPD36 | 96 | 9 | NR | 0.92–0.96 | NR | |
| Chien et al. (31) | SNAP-II + GA, SGA, sex, low Apgar, and outborn status | BPD36 | 4226 | NR | Complete case study | 0.83 | HL test |
BPD, bronchopulmonary dysplasia; BPD-TM, bronchopulmonary dysplasia test measure; BW, birth weight; CRIB, Clinical Risk Indicator fores; CRIB II-BE, Clinical Risk Indicator fores omitting base excess; DOL, days of life; GA, gestational age; HL test, Hosmer–Lemeshow test; HRC, heart rate characteristics; NR, not reported; PMA, postmenstrual age; RSS, respiratory severity score; SGA, small for gestational age; SMUMRV, modified respiratory variables; SNAP-II, Score for Neonatal Acute Physiology-II.
Models Validated
The most frequently validated models were CRIB-II (Clinical Risk Indicator fores-II) and SNAP-II (Score for Neonatal Acute Physiology-II); both were externally validated twice. The other models were externally validated once.
Study Design
Eight validation studies (80%) used existing data to externally validate a BPD prediction model. Two studies (20%) collected prospective data for external validation. The data used for validating the BPD prediction models were all collected between 1995 and 2017.
Outcome
Four models (19%) used BPD28 as the outcome. The incidence of BPD28 was 25–43% (median, 37%). Seventeen models (81%) used BPD36 as the outcome. The incidence of BPD36 was 9–58% (median, 24%).
Sample Size
All studies reported the number of patients. The number of event patients could be identified in nine studies (90%). The validation articles included 69–6,038 patients (median, 566). The median number of event patients was 98 (range, 9–1,916). Twelve models (60%) had <100 event patients.
Missing Data
Five studies mentioned missing data (50%). These studies all used complete case analysis to address the missing data.
Predictive Performance
Nine of the 10 validation studies (90%) assessed model discrimination with the C statistic (range, 0.61–0.97). Two models (10%) reported model calibration using the Hosmer–Lemeshow test.
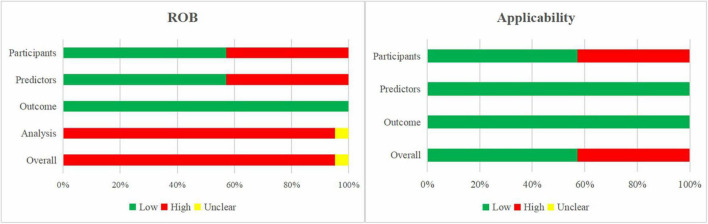
Risk of Bias and Applicability Assessment of the Included External Validation Studies
Figure 4 presents the summary of the ROB and applicability by domain. Outcome-related ROB was low across all models. For the domain analysis, 20 models (96%) were assessed as high ROB due to inappropriate handling of missing data and inefficient presentation of calibration, while one model was assessed as unclear. This resulted in an overall high ROB for the validation of 20 models (95%) and overall unclear ROB for one model (5%).
FIGURE 4.
Risk of bias and applicability assessment of externally validated models using Prediction model Risk Of Bias ASsessment Tool (PROBAST).
The models’ applicability to our research question was high concern in 43% of the models, mainly due to the inclusion of participants different from those in our research question.
Discussion
In the present systematic review, we summarize all prognostic models for developing BPD in preterm infants born at ≤32 weeks and/or ≤1,500 g birth weight. In total, 13 studies describing model development and 10 studies describing external validation were included. High ROB was observed across nearly all models, mostly due to inappropriate analysis, particularly for the handling of missing data, presenting insufficient performance statistics, and small sample size. Furthermore, several studies did not report full models, making external validation and implementation in clinical practice difficult. Meta-analysis was not possible because external validation studies of the same model were insufficient.
Prediction models are developed to support medical decision-making. Therefore, it is vital to identify a target population in which predictions serve a clinical need. Then, a representative dataset on which the prediction model is based can be developed and validated (32). In the present review, studies involving preterm infants born at ≤32 weeks and/or ≤1,500 g birth weight were included, while those that included more mature preterm infants were excluded. We excluded such studies because BPD incidence is very uncommon in infants born with birth weights of >1,500 g and after 32 weeks’ gestation (33). Accordingly, there is little clinical need for predicting BPD in such infants. Therefore, we recommend that future studies of BPD prediction models involve very low-birth weight infants or very preterm infants rather than all preterm infants.
In the present review, the outcome to be predicted was BPD. The included studies used different definitions of BPD. Most of the included studies used BPD36 as the outcome to be predicted while a smaller proportion used BPD28. Even when the scope of BPD was the same, the definitions of BPD could still differ based on the mode of respiratory support. The lack of a uniform definition of BPD in the included studies reflects the changing BPD definition of these years (34–37). Among the included studies, seven studies used the outcome BPD28, four of which used the definition proposed by the NIH in 2001 (36), and the other three only stated BPD28 as oxygen was still required at 28 days of life, and no further details were elaborated. Death is a competing outcome of BPD, some studies used the composite outcome “BPD or death” when developing prediction models for BPD. This composite outcome avoided exclusion of deceased patients who might developed BPD if they survived. Nonetheless, not all patients with early death will develop BPD. When models developed for prediction of “BPD or death” are used to predict BPD risk only, the predictive power will be lower (9), leading to a reduction in the accuracy of the prediction results. Besides, many models for prediction of death have been developed and most of them show good predictive performance (38). Utilize different models to predict BPD and death in clinical practice will probably result in higher accuracy. Therefore, BPD was selected as the outcome in our review, rather than “BPD or death.”
Most prediction models used clinical indicators including prenatal, perinatal, and postnatal factors to develop BPD prediction models. Though a large number of studies tried to explore the correlation between biomarkers and BPD, few biomarkers were included in prediction models. Of the studies included in this systematic review, only two studies constructed prediction models with biomarkers, including interleukin-6, clara cell protein-16, and Krebs von den Lungen-6 (25, 26). Genome-wide association studies and candidate gene studies investigating the correlation between genetic predisposition and BPD have been reported, but the results of different population studies are inconsistent (39). The genes specific for BPD remain to be further investigated before they could be applied to predict risk of BPD.
Similar to other systematic reviews of prediction models (38, 40, 41), we too observed several methodological shortcomings in most of the included studies.
First, although many of the studies used a large sample acquired from registries, around half of them used a sample that was too small. For example, six models were developed with samples of EPV <10, and 12 models were validated with samples with <100 events. When developing prediction models for binary outcomes, an EPV of at least 10 has been widely adopted as a criterion to minimize overfitting (42). For external validation studies, a minimum of 100 event patients is recommended (43). Recently, Riley et al. proposed formulae for calculating the minimum sample size required for developing regression-based prediction models (44), and Pavlou et al. have proposed equations for estimating the required sample size for external validation of risk models for binary outcomes (45), rendering sample size calculation more precise and efficient. Therefore, small samples should be avoided for prediction model development and validation, and it would be better to calculate the sample size with these recently reported formulae.
Second, none of the included studies handled missing data appropriately. Most did not report missing data or only included complete cases for analysis. Missing data are a common but easily underappreciated problem in prediction studies; complete case analysis can lead to biased predictor–outcome associations and biased model performance (46–48). To avoid biased model performance as a result of the deletion or single imputation of participants’ missing data, multiple imputation is recommended (46, 49–51).
Third, around 30% of the included studies describing prediction model development selected predictors via univariable analysis. However, univariable analysis can lead to the omission of important predictors, as selection is based on their statistical significance as a single predictor rather than in context with other predictors (52). Therefore, univariable analysis should be avoided in predictor selection. Alternative approaches include listing a limited number of candidate predictors to consider for the prediction model, and some statistical selection methods, including backward elimination and forward selection (53).
Fourth, most studies did not present discrimination and calibration simultaneously. Discrimination refers to the ability of the prediction model to separate individuals with and without the outcome event while calibration reflects the level of agreement between the observed outcomes and predictions (53). Both model discrimination and calibration must be evaluated to fully assess the predictive performance of a model. Most models in our review had a C statistic of >0.75. However, these models can still perform poorly in a new population because they could have been overfitted to the development data. Calibration was reported in around 20% of models. Nevertheless, only one study used the recommended method calibration plot, while most of the studies used only the Hosmer–Lemeshow test, which has been considered insufficient (11). Therefore, both discrimination and calibration should be reported for a model and a calibration plot is recommended for assessing calibration.
Finally, over half of the developed models were not validated internally. Internal validation is important for quantifying overfitting of the developed model and optimism in its predictive performance, except when the sample size and EPV are extremely large (11). In the present review, the most frequently used method of internal validation was split sampling, followed by bootstrapping. However, split sampling is not recommended, as it is statistically inefficient because not all available data are used for producing the prediction model (54). Bootstrapping is preferred especially when the development sample is relatively small and/or a high number of candidate predictors is studied (55).
No systematic review has been published since the systematic review of BPD prediction models in 2013 by Onland et al. (9). Compared with their review, ours has several improvements. We have followed the CHARMS checklist, and extracted and assessed most key items within 11 domains. Furthermore, we assessed the ROB and applicability of the included models with a standard tool, PROBAST.
The limitations of this review are: the exclusion of LUS-related studies. However, a meta-analysis in press has revealed that the LUS is accurate for early prediction of BPD and moderate-to-severe BPD in an average population of preterm infants of <32 weeks’ gestation (56). Second, we excluded studies that included preterm infants born at >32 weeks and >1,500 g birth weight. Therefore, studies that included very low-birth weight or very preterm infants were also excluded. Third, we excluded studies intended at predicting “BPD or death.” Therefore, we were unable to assess models for that composite outcome.
Recommendations for future development studies include collecting data by conducting prospective longitudinal cohort studies, selecting preterm infants born at ≤32 weeks and/or ≤1,500 g birth weight as participants, using the outcome definition proposed by Jensen et al. (34), choosing appropriate clinical indicators and biomarkers as predictors, using sufficiently large sample size (EPV ≥ 20) and handling missing data with multiple imputation.
Conclusion
In this review, we included 18 studies that developed or externally validated BPD prediction models. The included studies were assessed thoroughly using the CHARMS checklist (10) and PROBAST. There were many reporting or methodological shortcomings in the included studies. For better reporting of BPD prediction models, we recommend using sufficiently large samples for developing or validating a model, using multiple imputation to address missing data, avoiding univariable analysis for selecting predictors, assessing a model’s predictive performance with both discrimination and calibration, and using internal validation for newly developed models.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
H-BP, Y-LZ, and Z-BY designed the work. H-BP, Y-LZ, and YC extracted the data. Y-LZ, Z-CJ, FL, BW, and Z-BY analyzed the data. H-BP wrote the manuscript. YC and Z-BY supervised the work. All authors critically revised and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.856159/full#supplementary-material
References
- 1.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. (2015) 314:1039–51. 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santhakumaran S, Statnikov Y, Gray D, Battersby C, Ashby D, Modi N. Survival of very preterm infants admitted to neonatal care in England 2008-2014: time trends and regional variation. Arch Dis Child Fetal Neonatal Ed. (2018) 103:F208–15. 10.1136/archdischild-2017-312748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Yuan L, Wang J, Li Q, Yang C, Gao X, et al. Mortality and morbidity of infants born extremely preterm at tertiary medical centers in China from 2010 to 2019. JAMA Netw Open. (2021) 4:e219382. 10.1001/jamanetworkopen.2021.9382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lui K, Lee SK, Kusuda S, Adams M, Vento M, Reichman B, et al. Trends in outcomes for neonates born very preterm and very low birth weight in 11 high-income countries. J Pediatr. (2019) 215:32–40.e14. 10.1016/j.jpeds.2019.08.020 [DOI] [PubMed] [Google Scholar]
- 5.Praprotnik M, Stucin GI, Lučovnik M, Avčin T, Krivec U. Respiratory morbidity, lung function and fitness assessment after bronchopulmonary dysplasia. J Perinatol. (2015) 35:1037–42. 10.1038/jp.2015.124 [DOI] [PubMed] [Google Scholar]
- 6.Sriram S, Schreiber MD, Msall ME, Kuban K, Joseph RM, O’ ST, et al. Cognitive development and quality of life associated with BPD in 10-year-olds born preterm. Pediatrics. (2018) 141:e20172719. 10.1542/peds.2017-2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vom HM, Prenzel F, Uhlig HH, Robel-Tillig E. Pulmonary outcome in former preterm, very low birth weight children with bronchopulmonary dysplasia: a case-control follow-up at school age. J Pediatr. (2014) 164:40–5.e4. 10.1016/j.jpeds.2013.07.045 [DOI] [PubMed] [Google Scholar]
- 8.Gibson AM, Reddington C, McBride L, Callanan C, Robertson C, Doyle LW. Lung function in adult survivors of very low birth weight, with and without bronchopulmonary dysplasia. Pediatr Pulmonol. (2015) 50:987–94. 10.1002/ppul.23093 [DOI] [PubMed] [Google Scholar]
- 9.Onland W, Debray TP, Laughon MM, Miedema M, Cools F, Askie LM, et al. Clinical prediction models for bronchopulmonary dysplasia: a systematic review and external validation study. BMC Pediatr. (2013) 13:207. 10.1186/1471-2431-13-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. (2014) 11:e1001744. 10.1371/journal.pmed.1001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moons K, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. (2019) 170:W1–33. 10.7326/M18-1377 [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA. (2017) 318:1377–84. 10.1001/jama.2017.12126 [DOI] [PubMed] [Google Scholar]
- 14.Ushida T, Moriyama Y, Nakatochi M, Kobayashi Y, Imai K, Nakano-Kobayashi T, et al. Antenatal prediction models for short- and medium-term outcomes in preterm infants. Acta Obstet Gynecol Scand. (2021) 100:1089–96. 10.1111/aogs.14136 [DOI] [PubMed] [Google Scholar]
- 15.Shim SY, Yun JY, Cho SJ, Kim MH, Park EA. The prediction of bronchopulmonary dysplasia in very low birth weight infants through clinical indicators within 1 hour of delivery. J Korean Med Sci. (2021) 36:e81. 10.3346/jkms.2021.36.e81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verder H, Heiring C, Ramanathan R, Scoutaris N, Verder P, Jessen TE, et al. Bronchopulmonary dysplasia predicted at birth by artificial intelligence. Acta Paediatr. (2021) 110:503–9. 10.1111/apa.15438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Faleh I, Faouzi M, Adams M, Gerull R, Chnayna J, Giannoni E, et al. Bronchopulmonary dysplasia: a predictive scoring system for very low birth weight infants. A diagnostic accuracy study with prospective data collection. Eur J Pediatr. (2021) 180:2453–61. 10.1007/s00431-021-04045-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharjee I, Das A, Collin M, Aly H. Predicting outcomes of mechanically ventilated premature infants using respiratory severity score. J Matern Fetal Neonatal Med. (2020). 10.1080/14767058.2020.1858277 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Valenzuela-Stutman D, Marshall G, Tapia JL, Mariani G, Bancalari A, Gonzalez Á. Bronchopulmonary dysplasia: risk prediction models for very-low- birth-weight infants. J Perinatol. (2019) 39:1275–81. 10.1038/s41372-019-0430-x [DOI] [PubMed] [Google Scholar]
- 20.Lee SM, Lee MH, Chang YS. The clinical risk index fores II for Prediction of time-dependent mortality and short-term morbidities in very low birth weight infants. Neonatology. (2019) 116:244–51. 10.1159/000500270 [DOI] [PubMed] [Google Scholar]
- 21.Sullivan BA, Wallman-Stokes A, Isler J, Sahni R, Moorman JR, Fairchild KD, et al. Early pulse oximetry data improves prediction of death and adverse outcomes in a two-center cohort of very low birth weight infants. Am J Perinatol. (2018) 35:1331–8. 10.1055/s-0038-1654712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bentsen MH, Markestad T, Halvorsen T. Ventilator flow data predict bronchopulmonary dysplasia in extremely premature neonates. ERJ Open Res. (2018) 4:00099–2017. 10.1183/23120541.00099-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan BA, McClure C, Hicks J, Lake DE, Moorman JR, Fairchild KD. Early heart rate characteristics predict death and morbidities in preterm infants. J Pediatr. (2016) 174:57–62. 10.1016/j.jpeds.2016.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gursoy T, Hayran M, Derin H, Ovali F. A clinical scoring system to predict the development of bronchopulmonary dysplasia. Am J Perinatol. (2015) 32:659–66. 10.1055/s-0034-1393935 [DOI] [PubMed] [Google Scholar]
- 25.Wang K, Huang X, Lu H, Zhang Z. A comparison of KL-6 and Clara cell protein as markers for predicting bronchopulmonary dysplasia in preterm infants. Dis Markers. (2014) 2014:736536. 10.1155/2014/736536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian XY, Zhang XD, Li QL, Shen Y, Zheng J. Biological markers in cord blood for prediction of bronchopulmonary dysplasia in premature infants. Clin Exp Obstet Gynecol. (2014) 41:313–8. 10.12891/ceog16292014 [DOI] [PubMed] [Google Scholar]
- 27.May C, Patel S, Kennedy C, Pollina E, Rafferty GF, Peacock JL, et al. Prediction of bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. (2011) 96:F410–6. [DOI] [PubMed] [Google Scholar]
- 28.May C, Kavvadia V, Dimitriou G, Greenough A. A scoring system to predict chronic oxygen dependency. Eur J Pediatr. (2007) 166:235–40. 10.1007/s00431-006-0235-8 [DOI] [PubMed] [Google Scholar]
- 29.Henderson-Smart DJ, Hutchinson JL, Donoghue DA, Evans NJ, Simpson JM, Wright I. Prenatal predictors of chronic lung disease in very preterm infants. Arch Dis Child Fetal Neonatal Ed. (2006) 91:F40–5. 10.1136/adc.2005.072264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YD, Kim EA, Kim KS, Pi SY, Kang W. Scoring method for early prediction of neonatal chronic lung disease using modified respiratory parameters. J Korean Med Sci. (2005) 20:397–401. 10.3346/jkms.2005.20.3.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chien LY, Whyte R, Thiessen P, Walker R, Brabyn D, Lee SK. Snap-II predicts severe intraventricular hemorrhage and chronic lung disease in the neonatal intensive care unit. J Perinatol. (2002) 22:26–30. 10.1038/sj.jp.7210585 [DOI] [PubMed] [Google Scholar]
- 32.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. (2020) 369:m1328. 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol. (2003) 8:63–71. 10.1016/s1084-2756(02)00192-6 [DOI] [PubMed] [Google Scholar]
- 34.Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. (2019) 200:751–9. 10.1164/rccm.201812-2348OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. (2018) 197:300–8. 10.1016/j.jpeds.2018.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163:1723–9. [DOI] [PubMed] [Google Scholar]
- 37.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. (1988) 82:527–32. 10.1542/peds.82.4.527 [DOI] [PubMed] [Google Scholar]
- 38.van Beek PE, Andriessen P, Onland W, Schuit E. Prognostic models predicting mortality in preterm infants: systematic review and meta-analysis. Pediatrics. (2021) 147:e2020020461. 10.1542/peds.2020-020461 [DOI] [PubMed] [Google Scholar]
- 39.Leong M. Genetic approaches to bronchopulmonary dysplasia. Neoreviews. (2019) 20:e272–9. 10.1542/neo.20-5-e272 [DOI] [PubMed] [Google Scholar]
- 40.Gerry S, Bonnici T, Birks J, Kirtley S, Virdee PS, Watkinson PJ, et al. Early warning scores for detecting deterioration in adult hospital patients: systematic review and critical appraisal of methodology. BMJ. (2020) 369:m1501. 10.1136/bmj.m1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon-Pimmel J, Foucher Y, Leger M, Feuillet F, Bodet-Contentin L, Cinotti R, et al. Methodological quality of multivariate prognostic models for intracranial haemorrhages in intensive care units: a systematic review. BMJ Open. (2021) 11:e047279. 10.1136/bmjopen-2020-047279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. (1996) 49:1373–9. 10.1016/s0895-4356(96)00236-3 [DOI] [PubMed] [Google Scholar]
- 43.Collins GS, Ogundimu EO, Altman DG. Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med. (2016) 35:214–26. 10.1002/sim.6787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riley RD, Ensor J, Snell K, Harrell FJ, Martin GP, Reitsma JB, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. (2020) 368:m441. 10.1136/bmj.m441 [DOI] [PubMed] [Google Scholar]
- 45.Pavlou M, Qu C, Omar RZ, Seaman SR, Steyerberg EW, White IR, et al. Estimation of required sample size for external validation of risk models for binary outcomes. Stat Methods Med Res. (2021) 30:2187–206. 10.1177/09622802211007522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. (2009) 338:b2393. 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janssen KJ, Donders AR, Harrell FJ, Vergouwe Y, Chen Q, Grobbee DE, et al. Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol. (2010) 63:721–7. 10.1016/j.jclinepi.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 48.Groenwold RH, White IR, Donders AR, Carpenter JR, Altman DG, Moons KG. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ. (2012) 184:1265–9. 10.1503/cmaj.110977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moons KG, Donders RA, Stijnen T, Harrell FJ. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. (2006) 59:1092–101. 10.1016/j.jclinepi.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 50.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. (2006) 59:1087–91. 10.1016/j.jclinepi.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 51.de Groot JA, Janssen KJ, Zwinderman AH, Moons KG, Reitsma JB. Multiple imputation to correct for partial verification bias revisited. Stat Med. (2008) 27:5880–9. 10.1002/sim.3410 [DOI] [PubMed] [Google Scholar]
- 52.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. (1996) 49:907–16. 10.1016/0895-4356(96)00025-x [DOI] [PubMed] [Google Scholar]
- 53.Moons KG, Kengne AP, Woodward M, Royston P, Vergouwe Y, Altman DG, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. (2012) 98:683–90. 10.1136/heartjnl-2011-301246 [DOI] [PubMed] [Google Scholar]
- 54.Austin PC, Steyerberg EW. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Stat Methods Med Res. (2017) 26:796–808. 10.1177/0962280214558972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. (2009) 338:b604. 10.1136/bmj.b604 [DOI] [PubMed] [Google Scholar]
- 56.Pezza L, Alonso-Ojembarrena A, Elsayed Y, Yousef N, Vedovelli L, Raimondi F, et al. Meta-analysis of lung ultrasound scores for early prediction of bronchopulmonary dysplasia. Ann Am Thorac Soc. (2022) 19:659–67. 10.1513/AnnalsATS.202107-822OC [DOI] [PubMed] [Google Scholar]
- 57.Yoder BA, Anwar MU, Clark RH. Early prediction of neonatal chronic lung disease: a comparison of three scoring methods. Pediatr Pulmonol. (1999) 27:388–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.