Abstract
Neuroinflammation is a cause of neurodevelopmental disorders such as autism spectrum disorders, fetal alcohol syndrome, and cerebral palsy. Converging lines of evidence from basic and clinical sciences suggest that dysregulation of the epigenetic landscape, including DNA methylation and miRNA expression, is associated with neuroinflammation. Genetic and environmental factors can affect the interaction between epigenetics and neuroinflammation, which may cause neurodevelopmental disorders. In this minireview, we focus on neuroinflammation that might be mediated by epigenetic dysregulation in microglia, and compare studies using mammals and zebrafish.
Keywords: microglia, microRNA, DNA methylation, zebrafish, rodents, fetal alcohol syndrome, autism spectrum disorders, Rett syndrome
Introduction
Neurodevelopmental disorders (NDDs) are characterized by developmental abnormalities in cognition, language, communication, learning, and motor skills (Wilfert et al., 2017; Wamsley and Geschwind, 2020; Savatt and Myers, 2021). NDDs include intellectual disability, learning disorders, autism spectrum disorder (ASD), cerebral palsy, and fetal alcohol syndrome (FAS) (Wozniak et al., 2019; Savatt and Myers, 2021). NDDs are important from both basic and clinical research perspectives (Wilfert et al., 2017; Wamsley and Geschwind, 2020; Díaz-Caneja et al., 2021; Savatt and Myers, 2021). For example, the worldwide prevalence of FAS, ASD, and cerebral palsy is estimated to be 0.75%, 1–2%, and 2%, respectively (Baxter et al., 2015; Stavsky et al., 2017; May et al., 2018). This high prevalence highlights the need to decipher their etiologies and develop early diagnosis and effective treatment strategies. NDDs are considered to be multifactorial (Thapar and Cooper, 2016; Lovely et al., 2017; Wilfert et al., 2017; Díaz-Caneja et al., 2021), but several studies have suggested that convergent pathways exist (Geschwind and State, 2015; de la Torre-Ubieta et al., 2016; Sullivan et al., 2019; Shohat et al., 2021), and dysregulation of neuroinflammation has been reported as a convergent pathway in NDDs (Voineagu et al., 2011; Mottahedin et al., 2017; Cattane et al., 2020; Martino et al., 2020; Panisi et al., 2021).
Microglia, resident macrophages of the central nervous system (Ginhoux and Prinz, 2015), play fundamental roles in neuroinflammation (Paolicelli and Ferretti, 2017; Lenz and Nelson, 2018). Microglia can be classified into pro-inflammatory (M1) and anti-inflammatory or alternative activation (M2) phenotypes, although it is now recognized that they exhibit a diverse range of phenotypes (Orihuela et al., 2016; Cheray and Joseph, 2018; Prinz et al., 2019; Stratoulias et al., 2019; Thion and Garel, 2020). The M1 phenotype produces various pro-inflammatory molecules, such as tumor necrosis factor α (TNF), interleukin-1β, -6, and -12, and reactive oxygen species, whereas the M2 phenotype produces anti-inflammatory molecules, such as interleukin-4, -10, and -13 (Orihuela et al., 2016) and neuro-protective and trophic factors, such as insulin-like growth factor 1 and brain-derived neurotrophic factor (Wang et al., 2015). The polarization of microglia into the M1/M2 phenotype is regulated by various epigenetic mechanisms, including DNA methylation, histone modification, and microRNA (miRNA) expression (Kaminska et al., 2016; Cheray and Joseph, 2018). For example, sirtuin 1, a member of the histone deacetylase (HDAC) family, deacetylates various epigenetic regulators, such as E1A binding protein p300, a histone acetyltransferase, and DNA methyltransferase 1 (DNMT1), and promotes M2 polarization (Cheray and Joseph, 2018; van Heesbeen and Smidt, 2019; Wu et al., 2020). In addition, microglia play an important role in brain development through their involvement in neuronal proliferation, survival, neurogenesis, neuronal migration, neural projections, and synaptic plasticity (Li and Barres, 2018). Microglia function is regulated by multiple mechanisms that can be affected by environmental, genetic, and epigenetic factors (Kaminska et al., 2016; Paolicelli and Ferretti, 2017; Cheray and Joseph, 2018). Accumulating evidence suggests that the interaction between epigenetics and neuroinflammation is involved in the etiology of NDDs (Nardone and Elliott, 2016; Boda et al., 2020; Vogel Ciernia et al., 2020).
Rodents have been successfully used to analyze the role of microglia in neuroinflammation associated with NDDs (Johnson and Kaffman, 2018). In mice, primitive microglia derived from yolk sac progenitors (erythromyeloid precursors) migrate into the brain around embryonic day (E) 9.5, where they differentiate into microglia, colonize various brain regions, and regulate neurodevelopment (Prinz et al., 2019; Stratoulias et al., 2019; Thion and Garel, 2020; Sharma et al., 2021). The entry of primitive macrophages and colonization of the brain are also conserved in zebrafish, an alternative animal model for various diseases, including NDDs (Xu et al., 2015; Réu et al., 2017; Ferrero et al., 2018; Bian et al., 2020; Neely and Lyons, 2021). When the second wave of hematopoiesis occurs in mice, microglia progenitors expressing homeobox B8 are generated in the yolk sac, are present in the aorta-gonad-mesonephros (AGM) and fetal liver, and seed into the brain around E12.5 (De et al., 2018). In zebrafish, definitive hematopoiesis begins 15 days post-fertilization in the ventral wall of the dorsal aorta, which is the analogous region of AGM in mammals, leading to the formation of adult microglia in the brain (Xu et al., 2015; Ferrero et al., 2018). Embryonic microglia derived from primitive macrophages gradually disappear in zebrafish (Xu et al., 2015; Ferrero et al., 2018; Sharma et al., 2021). In mice, microglia derived from both primitive and definitive hematopoiesis coexist in the adult brain (Sharma et al., 2021). Despite these differences, the core microglial gene expression signature and microglial functions, such as immune surveillance, cellular debris cleaning, response to injury, and integration with neural circuits, are conserved between mammals and zebrafish (Mazzolini et al., 2020; Neely and Lyons, 2021).
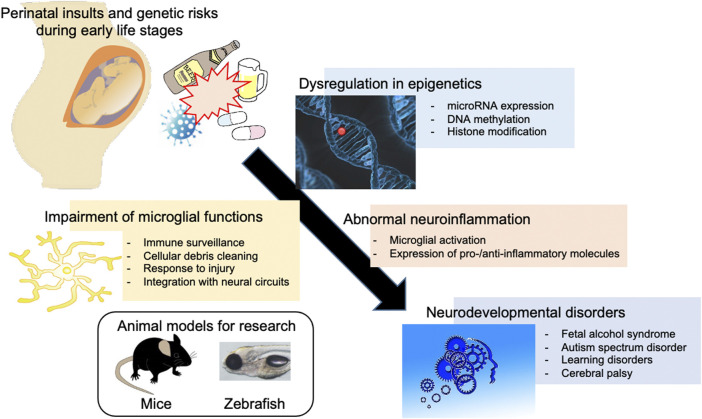
In this minireview, we describe our current understanding of the interaction between epigenetics and neuroinflammation, focusing on microglia in relation to miRNA-124 and 153 (miR124 and miR153), methyl-CpG binding protein 2 (MECP2), and ubiquitin-like with PHD and ring finger domains 1 (UHRF1) (Figure 1). We also compare studies using mammals and zebrafish to provide a future direction for zebrafish-based research on the epigenetic regulation of microglia and neuroinflammation in NDDs (Table 1).
FIGURE 1.
Involvement of environmental and genetic factors in the epigenetic dysregulation and neuroinflammation associated with neurodevelopmental disorders. Perinatal environmental insults such as alcohol and cocaine exposure during development and/or genetic risks such as mutation of MECP2 and UHRF1 can cause epigenetic dysregulation in microglia, including the expression of microRNAs important for the development and function of microglia, leading to neuroinflammation and neurodevelopmental disorders. Zebrafish, as well as rodents, can be used to analyze the effect of such factors.
TABLE 1.
Reviewed studies on epigenetics and neuroinflammation associated with neurodevelopmental disorders.
| Gene | Findings in Mammals | Findings in Zebrafish | References |
|---|---|---|---|
| miR124 | PCE causes the promoter hypermethylation and decreases the expression of miR124 in mouse MG. | The inhibition of miR124 activates zebrafish MG. | Guo et al. (2016) |
| The decrease of miR124 caused by cocaine exposure post-transcriptionally increases the expression of TLR4 and STAT3 in rat MG. | Periyasamy et al. (2018) | ||
| Chivero et al. (2020) | |||
| Svahn et al. (2016) | |||
| miR153 | The addition of miR153 mimic suppress the expression of Tnf in mouse MG. | The expression of miR153c is decreased in zebrafish embryo exposed to ethanol | Qiu et al. (2021b) |
| Knockdown of miR153c causes the phenotype similar to those of zebrafish exposed to ethanol | Tal et al. (2012) | ||
| MECP2 | Genes associated with the differentially-methylated regions in the brains of Rett syndrome patients show significant enrichment in genes regulated during MG development | The total numbers of mpx-positive neutrophils, but not mpeg-positive MG/macrophages, in the body is increased in the zebrafish model of Rett syndrome | Vogel Ciernia et al. (2020) |
| Mecp2 deficiency causes dysregulation of inflammatory response in mouse microglia | Cronk et al. (2015) | ||
| Mecp2-null microglia show the rise of mitochondrial reactive oxygen and the decrease of mitochondrial ATP production in mice | Zhao et al. (2017) | ||
| Jin et al. (2015) | |||
| Van der Vaart et al. (2017) | |||
| UHRF1 | Knockout of Uhrf1 causes Tnf promoter hypomethylation and increases the expression of Tnf in macrophages, leading to colitis in mice | Knockout of uhrf1 causes tnf promoter hypomethylation and increases the expression of tnf in intestinal epithelial cells, leading to intestinal damage in zebrafish | Qi et al. (2019) |
| Intestinal damage activates peripheral immune cells, leading to the breakdown of the blood-brain barrier and dysfunction of MG. | Marjoram et al. (2015) | ||
| Fung et al. (2017) | |||
| CSF1R | Knockout of Csf1r causes a lack of MG in rat brain | Knockout of csf1r cause a lack of MG in zebrafish brains | Patkar et al. (2021) |
| Oosterhof et al. (2018) | |||
| RNASET2 | Knockout of Rnaset2 causes abnormal activation of MG and increases the expression of interferon-stimulated genes in mouse brain | Knockout of rnaset2 causes abnormal activation of MG and increases the expression of interferon-stimulated genes in zebrafish brain | Kettwig et al. (2021) |
| Hamilton et al. (2020) |
MG, microglia; PCE, prenatal cocaine exposure; TLR4, Toll-like receptor 4; STAT3, signal transducer and activator of transcription 3; TNF, tumor necrosis factor α; MECP2, methyl-CpG binding protein 2; ASD, autism spectrum disorder; UHRF1, ubiquitin-like with PHD and ring finger domains 1; CSF1R, colony-stimulating factor 1 receptor; RNASET2, ribonuclease T2.
miR124 and miR153
miRNAs play important roles in the regulation of neurodevelopment by modulating the expression of target genes via binding to the 3′-untranslated regions (Thomas et al., 2018). miRNAs are also involved in microglial function (Butovsky and Weiner, 2018; Cheray and Joseph, 2018; Guo et al., 2019; Qiu M. et al., 2021; Zhao et al., 2021). The expression of these miRNAs is epigenetically regulated by prenatal exposure (Knopik et al., 2019; Sushma et al., 2021). Prenatal cocaine exposure (PCE) dysregulates DNA methylation and the expression of miRNAs that are important for the neurodevelopment of offspring (Lambert and Bauer, 2012; Richardson et al., 2015; Vaillancourt et al., 2017). For example, PCE in mice can cause hypermethylation of insulin growth factor II (Igf2), leading to decreased expression of Igf2 in the hippocampus of offspring and impairment of cognitive function (Zhao et al., 2015). PCE also downregulates miR124 in microglia through promoter hypermethylation in mice (Guo et al., 2016). The cocaine-mediated downregulation of miR124 in rat primary microglia leads to increased expression of target genes, including Toll-like receptor 4 (TLR4) and signal transducer and activator of transcription 3, and aberrant activation of microglia (Periyasamy et al., 2018; Chivero et al., 2020). Inhibition of miR124 also activates microglia in zebrafish (Svahn et al., 2016). These studies suggest that the anti-inflammatory role of miR124 in microglia is conserved between mammals and zebrafish.
Exposure to ethanol during development can have deleterious effects on various cell types, including neurons, oligodendrocytes, astrocytes, and microglia, depending on the dose and timing of exposure and the brain region (Wilhelm and Guizzetti, 2016; Wong et al., 2017; Stratoulias et al., 2019; Almeida et al., 2020; Kane and Drew, 2021; Lussier et al., 2021). The expression of miR153 is decreased in mouse fetal cerebral cortical-derived neural progenitor cells exposed to ethanol (Balaraman et al., 2012). In microglia located in the hypothalamus of a rat FAS model, the expression is increased (Chastain et al., 2019). TNF secreted from microglia exposed to ethanol can cause neuronal apoptosis and neuroinflammation (Boyadjieva and Sarkar, 2010; Shrivastava et al., 2017). The addition of an miR153 mimic to mouse microglia suppresses the production of TNF (Qiu T. et al., 2021). In zebrafish exposed to ethanol from 4 to 24 h post-fertilization (hpf), the expression of miR153c, a zebrafish homolog of miR153, was decreased (Tal et al., 2012). Knockdown of miR153c causes phenotypes similar to those of zebrafish exposed to ethanol from 4 to 24 hpf (Tal et al., 2012). Supplementation with folic acid rescued developmental defects in zebrafish FAS models (Muralidharan et al., 2015; Jiang et al., 2020) and ameliorated the dysregulation of miRNA in a mouse FAS model (Wang et al., 2009). Folic acid also affects DNA methylation (Crider et al., 2012). Cocaine exposure decreases the expression of miR153 in a human neuroblastoma cell line (Cabana-Domínguez et al., 2018). These studies suggest that prenatal substance exposure may affect promoter methylation of miR153 and decrease its expression in both mammalian and zebrafish microglia, leading to neuroinflammation.
MECP2
Mutation in MECP2 is the most prevalent cause of Rett syndrome, a progressive NDD with ASD-like features (Amir et al., 1999; Fagiolini et al., 2020). MECP2 is a DNA methylation reader with two major domains: a methyl-binding domain and a transcriptional repressor domain (Fagiolini et al., 2020). MECP2 has a high affinity for methylated CpG (mCG), methylated CpA (mCA), and hydroxymethylated CpA (hmCA), but not for hydroxymethylated CpG (hmCG) (Ip et al., 2018; Connolly and Zhou, 2019; Lavery and Zoghbi, 2019; Tillotson and Bird, 2019). An integrative genome-wide analysis of the methylome and transcriptome using brains from patients with Rett syndrome, idiopathic ASD, and controls revealed that genes associated with the differentially-methylated regions in these NDDs compared with the controls showed significant enrichment in genes regulated during microglial development (Vogel Ciernia et al., 2020). Transcriptome analyses using mouse models have revealed that Mecp2 deficiency causes dysregulation of the microglial inflammatory response (Cronk et al., 2015; Zhao et al., 2017). Mecp2-null microglia also show increased uptake of glutamate, leading to an increase in mitochondrial reactive oxygen species and a decrease in mitochondrial ATP production in mice (Jin et al., 2015). These findings are consistent with other studies demonstrating the dysregulation of neuroinflammation and microglial/macrophage functions in Rett syndrome and ASD (Voineagu et al., 2011; Gupta et al., 2014; O'Driscoll et al., 2015; Parikshak et al., 2016; Schafer et al., 2016; Nance et al., 2017; Kahanovitch et al., 2019; Pecorelli et al., 2020; Marballi and Macdonald, 2021; Wittrahm et al., 2021). Furthermore, these findings suggest that dysregulation of microglia and peripheral immune cells may play pathogenic roles in NDDs and serve as therapeutic targets (Garay and Mcallister, 2010; Reemst et al., 2016; Kaur et al., 2017; Komada et al., 2017; Coomey et al., 2020).
In a zebrafish model of Rett syndrome, a premature stop codon has been introduced before the methyl-binding domain of mecp2 (Pietri et al., 2013). This zebrafish Rett syndrome model shows increased expression of inflammatory cytokines, impaired locomotion, and decreased anxiety-like behavior, which may be associated with the phenotypes observed in patients with and rodent models of Rett syndrome (Pietri et al., 2013; Van der Vaart et al., 2017). Proteomic analysis using the zebrafish Rett model found that proteins associated with ATP generation and skeletal muscle are dysregulated, which may be associated with impaired motor behaviors in the model (Pietri et al., 2013; Cortelazzo et al., 2017). These findings suggest that MECP2 function is well conserved between zebrafish and mammals. It should be noted, however, that the total number of mpx-positive neutrophils, but not mpeg-positive microglia/macrophages in the body, is increased in the zebrafish model of Rett syndrome (Van der Vaart et al., 2017). Thus, the role of mecp2 in zebrafish microglia remains unclear.
UHRF1
The microbiome is involved in the development and maintenance of microglia (Stilling et al., 2014; Erny et al., 2015; Thion et al., 2018; Wang et al., 2018; Erny and Prinz, 2020; Davoli-Ferreira et al., 2021). The densities of microglia in the somatosensory cortex and striatum of germ-free (GF) mice were significantly higher than those of specific-pathogen-free (SPF) mice at E14.5 and E16.5 (Thion et al., 2018). In adults, microglia of GF mice show deficits in the signaling of type I interferon receptors and polarization towards specific phenotypes (Erny et al., 2015). The impairment of microglial maturation is also caused by temporal eradication of the host microbiota or limited microbiota complexity in SPF mice, whereas recolonization with a complex microbiota or supplementation with short-chain fatty acids (SCFA) restores microglial function in GF mice (Erny et al., 2015). SCFA, such as butyrate, propionate, and pyruvate, show inhibitory effects on HDAC activity, suggesting that the function of microglia may be epigenetically regulated by SCFA-producing microbes through the modulation of histone acetylation (Stilling et al., 2014; Fung et al., 2017). Consistent with this idea, genome-wide analysis of chromatin accessibility revealed that there are differentially accessible regions between microglia in GF and SPF mice (Thion et al., 2018). Dysregulation of maternal microbiota caused by maternal infection and exposure to environmental factors during pregnancy can disrupt microglial function and fetal brain development, leading to NDDs (Davoli-Ferreira et al., 2021). The innate immunity regulated by commensal microbiota is conserved in zebrafish (Murdoch and Rawls, 2019).
UHRF1 is a RING E3 ubiquitin ligase that interacts with DNMT1 to copy pre-existing mCG to newly synthesized daughter strands during replication (Li et al., 2021). In mice, knockout of Uhrf1 decreases mCG at the Tnf promoter and increases the expression of Tnf in macrophages, which causes colitis, a type of inflammatory bowel disease (IBD) (Qi et al., 2019). In zebrafish, knockout of uhrf1 decreases mCG at the tnf promoter and increases the expression of tnf in intestinal epithelial cells, leading to IBD-like intestinal damage (Marjoram et al., 2015). Knockout of dnmt1 also increases the expression of tnf in intestinal epithelial cells (Marjoram et al., 2015). In both models, blocking TNF ameliorates IBD-like phenotypes (Marjoram et al., 2015; Qi et al., 2019). Intestinal damage activates peripheral immune cells, including TH17 cells and macrophages, leading to breakdown of the blood-brain barrier and dysfunction of microglia (Fung et al., 2017; Wang et al., 2018; Abdel-Haq et al., 2019; Davoli-Ferreira et al., 2021). These findings suggest that zebrafish is a useful tool for analyzing the gut-microglia connection associated with epigenetics and NDDs.
Discussion
In addition to the examples discussed above, several studies have demonstrated conserved functions of microglia associated with NDDs in mammals and zebrafish. Leukodystrophies are a group of NDDs characterized by white matter abnormalities (Van der Knaap and Bugiani, 2017). The clinical symptoms include cerebral palsy and cognitive decline (Van der Knaap and Bugiani, 2017). Microglial dysfunction plays an important role in the etiology of leukodystrophy (Garcia et al., 2020; Berdowski et al., 2021). Homozygous mutations in colony-stimulating factor 1 receptor (CSF1R) cause pediatric onset leukoencephalopathy (Oosterhof et al., 2019). Homozygous knockout of CSF1R homologs in rats and zebrafish causes a lack of microglia in the brain, which is consistent with the findings in humans (Oosterhof et al., 2018; Oosterhof et al., 2019; Patkar et al., 2021). Loss of function mutations in ribonuclease T2 (RNASET2) cause early onset leukoencephalopathy resembling congenital cytomegalovirus brain infection in humans (Henneke et al., 2009). Homozygous knockout of RNASET2 homologs in mice and zebrafish causes abnormal activation of microglia and increased expression of interferon-stimulated genes in the brains (Hamilton et al., 2020; Kettwig et al., 2021; Rutherford et al., 2021). These results warrant further examination to reveal the epigenetic mechanisms underlying leukoencephalopathies using zebrafish models.
Microglia can acquire a specific phenotype depending on the context (Stratoulias et al., 2019), and epigenetics play an important role in the plasticity of microglia (Cheray and Joseph, 2018; Martins-Ferreira et al., 2021). For example, upon stimulation with lipopolysaccharide (LPS), the enhancer of zeste homolog 2, a component of polycomb repressive complex 2 (Prc2), which has histone methyltransferase activity, is increased in mouse microglia, leading to an increase in tri-methylation of histone H3 lysine 27 (H3K27) and pro-inflammatory gene expression through toll-like receptor-induced activation of nuclear factor κB (Nfkb1) (Arifuzzaman et al., 2017; Zhang et al., 2018). The Nfkb1 activation by LPS-TLR4 signaling increases the expression of tet methylcytosine dioxygenase 2 (TET2) and stimulates the expression of LPS-mediated pro-inflammatory cytokines in mouse microglia (Carrillo-Jimenez et al., 2019). TET2 catalyzes the oxidation of 5-methylcytosine (5mC) to 5-hydroxymetylcytosine (5 hmC) (Macarthur and Dawlaty, 2021). 5mC is established de novo by two DNA methyltransferases, DNMT3A/B and maintained by DNMT1 (Wu and Zhang, 2014; Lavery and Zoghbi, 2019). Activation of TET2 and/or inhibition of DNMT3A/B decreases mCG levels, resulting in the detachment of MECP2 from genomic mCG sequences (Ip et al., 2018). DNMT3A haploinsufficiency in mice causes behavioral abnormalities and epigenomic dysregulation that overlap with Rett syndrome and ASD (Christian et al., 2020). Notably, the expression of Mecp2, Uhrf1, Tet2, Dnmt1, and Dnmt3a is also dysregulated in various rodent FAS models (Chen et al., 2013; Kim et al., 2013; Nagre et al., 2015; Varadinova and Boyadjieva, 2015; Veazey et al., 2017; Boschen et al., 2018; Alberry et al., 2021; Lussier et al., 2021). Epigenetic dysregulation caused by exposure to environmental chemicals during development may cause neuroinflammation and NDDs through polarization of microglia into pro-inflammatory phenotypes. Zebrafish are well-suited for analyzing the epigenetic effects of developmental chemical exposure (Aluru, 2017; Cavalieri and Spinelli, 2017).
The phenotypes of microglia are also dependent on the region in which they colonize (Stratoulias et al., 2019; Thion and Garel, 2020). In mice, microglia located in the cerebellum show higher clearance activity than those located in the cerebral cortex or striatum (Ayata et al., 2018). In microglia located in the cerebellar cortex or striatum, PRC2 causes trimethylation of H3K27, resulting in the suppression of gene expression related to clearance activity (Ayata et al., 2018). Regional differences in microglial phenotypes have also been observed in zebrafish (Silva et al., 2021). Epigenetic regulation during development, as well as the ontogeny and function of microglia, is relatively well conserved between zebrafish and mammals (Balasubramanian et al., 2019), making zebrafish a suitable model for analyzing the association between epigenetics, neuroinflammation, and NDDs.
Acknowledgments
We thank Nami Nagaya for drawing illustrations and Rie Ikeyama for her secretarial assistance.
Author Contributions
MK and YN planned and wrote the manuscript.
Funding
This work was supported in part by the Japan Society for the Promotion of Science KAKENHI (17K08500 to MK and 19K07318 to YN) and the Long-Range Research Initiative of the Japan Chemical Industrial Association (20-3-08 to YN).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdel-Haq R., Schlachetzki J. C. M., Glass C. K., Mazmanian S. K. (2019). Microbiome-microglia Connections via the Gut-Brain axis. J. Exp. Med. 216, 41–59. 10.1084/jem.20180794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberry B., Laufer B. I., Chater-Diehl E., Singh S. M. (2021). Epigenetic Impacts of Early Life Stress in Fetal Alcohol Spectrum Disorders Shape the Neurodevelopmental Continuum. Front. Mol. Neurosci. 14, 671891. 10.3389/fnmol.2021.671891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida L., Andreu-Fernández V., Navarro-Tapia E., Aras-López R., Serra-Delgado M., Martínez L., et al. (2020). Murine Models for the Study of Fetal Alcohol Spectrum Disorders: An Overview. Front. Pediatr. 8, 359. 10.3389/fped.2020.00359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluru N. (2017). Epigenetic Effects of Environmental Chemicals: Insights from Zebrafish. Curr. Opin. Toxicol. 6, 26–33. 10.1016/j.cotox.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R. E., Van Den Veyver I. B., Wan M., Tran C. Q., Francke U., Zoghbi H. Y. (1999). Rett Syndrome Is Caused by Mutations in X-Linked MECP2, Encoding Methyl-CpG-Binding Protein 2. Nat. Genet. 23, 185–188. 10.1038/13810 [DOI] [PubMed] [Google Scholar]
- Arifuzzaman S., Das A., Kim S. H., Yoon T., Lee Y. S., Jung K. H., et al. (2017). Selective Inhibition of EZH2 by a Small Molecule Inhibitor Regulates Microglial Gene Expression Essential for Inflammation. Biochem. Pharmacol. 137, 61–80. 10.1016/j.bcp.2017.04.016 [DOI] [PubMed] [Google Scholar]
- Ayata P., Badimon A., Strasburger H. J., Duff M. K., Montgomery S. E., Loh Y.-H. E., et al. (2018). Epigenetic Regulation of Brain Region-specific Microglia Clearance Activity. Nat. Neurosci. 21, 1049–1060. 10.1038/s41593-018-0192-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaraman S., Winzer-Serhan U. H., Miranda R. C. (2012). Opposing Actions of Ethanol and Nicotine on microRNAs Are Mediated by Nicotinic Acetylcholine Receptors in Fetal Cerebral Cortical-Derived Neural Progenitor Cells. Alcohol Clin. Exp. Res. 36, 1669–1677. 10.1111/j.1530-0277.2012.01793.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S., Raghunath A., Perumal E. (2019). Role of Epigenetics in Zebrafish Development. Gene 718, 144049. 10.1016/j.gene.2019.144049 [DOI] [PubMed] [Google Scholar]
- Baxter A. J., Brugha T. S., Erskine H. E., Scheurer R. W., Vos T., Scott J. G. (2015). The Epidemiology and Global Burden of Autism Spectrum Disorders. Psychol. Med. 45, 601–613. 10.1017/s003329171400172x [DOI] [PubMed] [Google Scholar]
- Berdowski W. M., Sanderson L. E., Van Ham T. J. (2021). The Multicellular Interplay of Microglia in Health and Disease: Lessons from Leukodystrophy. Dis. Model Mech. 14. 10.1242/dmm.048925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Z., Gong Y., Huang T., Lee C. Z. W., Bian L., Bai Z., et al. (2020). Deciphering Human Macrophage Development at Single-Cell Resolution. Nature 582, 571–576. 10.1038/s41586-020-2316-7 [DOI] [PubMed] [Google Scholar]
- Boda E., Rigamonti A. E., Bollati V. (2020). Understanding the Effects of Air Pollution on Neurogenesis and Gliogenesis in the Growing and Adult Brain. Curr. Opin. Pharmacol. 50, 61–66. 10.1016/j.coph.2019.12.003 [DOI] [PubMed] [Google Scholar]
- Boschen K. E., Keller S. M., Roth T. L., Klintsova A. Y. (2018). Epigenetic Mechanisms in Alcohol- and Adversity-Induced Developmental Origins of Neurobehavioral Functioning. Neurotoxicology Teratol. 66, 63–79. 10.1016/j.ntt.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjieva N. I., Sarkar D. K. (2010). Role of Microglia in Ethanol's Apoptotic Action on Hypothalamic Neuronal Cells in Primary Cultures. Alcohol Clin. Exp. Res. 34, 1835–1842. 10.1111/j.1530-0277.2010.01271.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O., Weiner H. L. (2018). Microglial Signatures and Their Role in Health and Disease. Nat. Rev. Neurosci. 19, 622–635. 10.1038/s41583-018-0057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabana-Domínguez J., Arenas C., Cormand B., Fernàndez-Castillo N. (2018). MiR-9, miR-153 and miR-124 Are Down-Regulated by Acute Exposure to Cocaine in a Dopaminergic Cell Model and May Contribute to Cocaine Dependence. Transl. Psychiatry 8, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Jimenez A., Deniz Ö., Niklison-Chirou M. V., Ruiz R., Bezerra-Salomão K., Stratoulias V., et al. (2019). TET2 Regulates the Neuroinflammatory Response in Microglia. Cell Rep. 29, 697–713. e698. 10.1016/j.celrep.2019.09.013 [DOI] [PubMed] [Google Scholar]
- Cattane N., Richetto J., Cattaneo A. (2020). Prenatal Exposure to Environmental Insults and Enhanced Risk of Developing Schizophrenia and Autism Spectrum Disorder: Focus on Biological Pathways and Epigenetic Mechanisms. Neurosci. Biobehav. Rev. 117, 253–278. 10.1016/j.neubiorev.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Cavalieri V., Spinelli G. (2017). Environmental Epigenetics in Zebrafish. Epigenetics Chromatin 10, 46. 10.1186/s13072-017-0154-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain L. G., Franklin T., Gangisetty O., Cabrera M. A., Mukherjee S., Shrivastava P., et al. (2019). Early Life Alcohol Exposure Primes Hypothalamic Microglia to Later-Life Hypersensitivity to Immune Stress: Possible Epigenetic Mechanism. Neuropsychopharmacol. 44, 1579–1588. 10.1038/s41386-019-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Ozturk N. C., Zhou F. C. (2013). DNA Methylation Program in Developing hippocampus and its Alteration by Alcohol. PLoS One 8, e60503. 10.1371/journal.pone.0060503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheray M., Joseph B. (2018). Epigenetics Control Microglia Plasticity. Front. Cell. Neurosci. 12, 243. 10.3389/fncel.2018.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivero E. T., Liao K., Niu F., Tripathi A., Tian C., Buch S., et al. (2020). Engineered Extracellular Vesicles Loaded with miR-124 Attenuate Cocaine-Mediated Activation of Microglia. Front. Cell Dev. Biol. 8, 573. 10.3389/fcell.2020.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian D. L., Wu D. Y., Martin J. R., Moore J. R., Liu Y. R., Clemens A. W., et al. (2020). DNMT3A Haploinsufficiency Results in Behavioral Deficits and Global Epigenomic Dysregulation Shared across Neurodevelopmental Disorders. Cell Rep. 33, 108416. 10.1016/j.celrep.2020.108416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. R., Zhou Z. (2019). Genomic Insights into MeCP2 Function: A Role for the Maintenance of Chromatin Architecture. Curr. Opin. Neurobiol. 59, 174–179. 10.1016/j.conb.2019.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomey R., Stowell R., Majewska A., Tropea D. (2020). The Role of Microglia in Neurodevelopmental Disorders and Their Therapeutics. Ctmc 20, 272–276. 10.2174/1568026620666200221172619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortelazzo A., Pietri T., De Felice C., Leoncini S., Guerranti R., Signorini C., et al. (2017). Proteomic Analysis of the Rett Syndrome Experimental Model mecp2Q63X Mutant Zebrafish. J. Proteomics 154, 128–133. 10.1016/j.jprot.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Crider K. S., Yang T. P., Berry R. J., Bailey L. B. (2012). Folate and DNA Methylation: A Review of Molecular Mechanisms and the Evidence for Folate's Role. Adv. Nutr. 3, 21–38. 10.3945/an.111.000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk J. C., Derecki N. C., Ji E., Xu Y., Lampano A. E., Smirnov I., et al. (2015). Methyl-CpG Binding Protein 2 Regulates Microglia and Macrophage Gene Expression in Response to Inflammatory Stimuli. Immunity 42, 679–691. 10.1016/j.immuni.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli-Ferreira M., Thomson C. A., Mccoy K. D. (2021). Microbiota and Microglia Interactions in ASD. Front. Immunol. 12, 676255. 10.3389/fimmu.2021.676255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Torre-Ubieta L., Won H., Stein J. L., Geschwind D. H. (2016). Advancing the Understanding of Autism Disease Mechanisms through Genetics. Nat. Med. 22, 345–361. 10.1038/nm.4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S., Van Deren D., Peden E., Hockin M., Boulet A., Titen S., et al. (2018). Two Distinct Ontogenies Confer Heterogeneity to Mouse Brain Microglia. Development 145. 10.1242/dev.152306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Caneja C. M., State M. W., Hagerman R. J., Jacquemont S., Marín O., Bagni C., et al. (2021). A White Paper on a Neurodevelopmental Framework for Drug Discovery in Autism and Other Neurodevelopmental Disorders. Eur. Neuropsychopharmacol. 48, 49–88. 10.1016/j.euroneuro.2021.02.020 [DOI] [PubMed] [Google Scholar]
- Erny D., Hrabě de Angelis A. L., Jaitin D., Wieghofer P., Staszewski O., David E., et al. (2015). Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 18, 965–977. 10.1038/nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D., Prinz M. (2020). How Microbiota Shape Microglial Phenotypes and Epigenetics. Glia 68, 1655–1672. 10.1002/glia.23822 [DOI] [PubMed] [Google Scholar]
- Fagiolini M., Patrizi A., Leblanc J., Jin L.-W., Maezawa I., Sinnett S., et al. (2020). Intellectual and Developmental Disabilities Research Centers: A Multidisciplinary Approach to Understand the Pathogenesis of Methyl-CpG Binding Protein 2-related Disorders. Neuroscience 445, 190–206. 10.1016/j.neuroscience.2020.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero G., Mahony C. B., Dupuis E., Yvernogeau L., Di Ruggiero E., Miserocchi M., et al. (2018). Embryonic Microglia Derive from Primitive Macrophages and Are Replaced by Cmyb-dependent Definitive Microglia in Zebrafish. Cell Rep. 24, 130–141. 10.1016/j.celrep.2018.05.066 [DOI] [PubMed] [Google Scholar]
- Fung T. C., Olson C. A., Hsiao E. Y. (2017). Interactions between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat. Neurosci. 20, 145–155. 10.1038/nn.4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay P., Mcallister A. K. (2010). Novel Roles for Immune Molecules in Neural Development: Implications for Neurodevelopmental Disorders. Front. Syn. Neurosci. 2, 136. 10.3389/fnsyn.2010.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. M., Hacker J. L., Sase S., Adang L., Almad A. (2020). Glial Cells in the Driver Seat of Leukodystrophy Pathogenesis. Neurobiol. Dis. 146, 105087. 10.1016/j.nbd.2020.105087 [DOI] [PubMed] [Google Scholar]
- Geschwind D. H., State M. W. (2015). Gene Hunting in Autism Spectrum Disorder: on the Path to Precision Medicine. Lancet Neurol. 10.1016/s1474-4422(15)00044-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Prinz M. (2015). Origin of Microglia: Current Concepts and Past Controversies. Cold Spring Harb. Perspect. Biol. 7, a020537. 10.1101/cshperspect.a020537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M.-L., Periyasamy P., Liao K., Kook Y. H., Niu F., Callen S. E., et al. (2016). Cocaine-mediated Downregulation of Microglial miR-124 Expression Involves Promoter DNA Methylation. Epigenetics 11, 819–830. 10.1080/15592294.2016.1232233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Hong W., Wang X., Zhang P., Körner H., Tu J., et al. (2019). MicroRNAs in Microglia: How Do MicroRNAs Affect Activation, Inflammation, Polarization of Microglia and Mediate the Interaction between Microglia and Glioma? Front. Mol. Neurosci. 12, 125. 10.3389/fnmol.2019.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Ellis S. E., Ashar F. N., Moes A., Bader J. S., Zhan J., et al. (2014). Transcriptome Analysis Reveals Dysregulation of Innate Immune Response Genes and Neuronal Activity-dependent Genes in Autism. Nat. Commun. 5, 5748. 10.1038/ncomms6748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N., Rutherford H. A., Petts J. J., Isles H. M., Weber T., Henneke M., et al. (2020). The Failure of Microglia to Digest Developmental Apoptotic Cells Contributes to the Pathology of RNASET2‐deficient Leukoencephalopathy. Glia 68, 1531–1545. 10.1002/glia.23829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneke M., Diekmann S., Ohlenbusch A., Kaiser J., Engelbrecht V., Kohlschütter A., et al. (2009). RNASET2-deficient Cystic Leukoencephalopathy Resembles Congenital Cytomegalovirus Brain Infection. Nat. Genet. 41, 773–775. 10.1038/ng.398 [DOI] [PubMed] [Google Scholar]
- Ip J. P. K., Mellios N., Sur M. (2018). Rett Syndrome: Insights into Genetic, Molecular and Circuit Mechanisms. Nat. Rev. Neurosci. 19, 368–382. 10.1038/s41583-018-0006-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Lu D., Wang F., Zhang Y., Cao L., Gui Y., et al. (2020). Folic Acid Supplement Rescues Ethanol-Induced Developmental Defects in the Zebrafish Embryos. Acta Biochim. Biophys. Sin. (Shanghai) 52, 536–545. 10.1093/abbs/gmaa030 [DOI] [PubMed] [Google Scholar]
- Jin L.-W., Horiuchi M., Wulff H., Liu X.-B., Cortopassi G. A., Erickson J. D., et al. (2015). Dysregulation of Glutamine Transporter SNAT1 in Rett Syndrome Microglia: a Mechanism for Mitochondrial Dysfunction and Neurotoxicity. J. Neurosci. 35, 2516–2529. 10.1523/jneurosci.2778-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. K., Kaffman A. (2018). Early Life Stress Perturbs the Function of Microglia in the Developing Rodent Brain: New Insights and Future Challenges. Brain, Behav. Immun. 69, 18–27. 10.1016/j.bbi.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahanovitch U., Patterson K. C., Hernandez R., Olsen M. L. (2019). Glial Dysfunction in MeCP2 Deficiency Models: Implications for Rett Syndrome. Int. J. Mol. Sci. 20. 10.3390/ijms20153813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska B., Mota M., Pizzi M. (2016). Signal Transduction and Epigenetic Mechanisms in the Control of Microglia Activation during Neuroinflammation. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1862, 339–351. 10.1016/j.bbadis.2015.10.026 [DOI] [PubMed] [Google Scholar]
- Kane C. J. M., Drew P. D. (2021). Neuroinflammatory Contribution of Microglia and Astrocytes in Fetal Alcohol Spectrum Disorders. J. Neurosci. Res. 99, 1973–1985. 10.1002/jnr.24735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C., Rathnasamy G., Ling E.-A. (2017). Biology of Microglia in the Developing Brain. J. Neuropathology Exp. Neurology 76, 736–753. 10.1093/jnen/nlx056 [DOI] [PubMed] [Google Scholar]
- Kettwig M., Ternka K., Wendland K., Krüger D. M., Zampar S., Schob C., et al. (2021). Interferon-driven Brain Phenotype in a Mouse Model of RNaseT2 Deficient Leukoencephalopathy. Nat. Commun. 12, 6530. 10.1038/s41467-021-26880-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Park J. H., Choi C. S., Choi I., Joo S. H., Kim M. K., et al. (2013). Effects of Ethanol Exposure during Early Pregnancy in Hyperactive, Inattentive and Impulsive Behaviors and MeCP2 Expression in Rodent Offspring. Neurochem. Res. 38, 620–631. 10.1007/s11064-012-0960-5 [DOI] [PubMed] [Google Scholar]
- Knopik V. S., Marceau K., Bidwell L. C., Rolan E. (2019). Prenatal Substance Exposure and Offspring Development: Does DNA Methylation Play a Role? Neurotoxicology Teratol. 71, 50–63. 10.1016/j.ntt.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M., Hara N., Kawachi S., Kawachi K., Kagawa N., Nagao T., et al. (2017). Mechanisms Underlying Neuro-Inflammation and Neurodevelopmental Toxicity in the Mouse Neocortex Following Prenatal Exposure to Ethanol. Sci. Rep. 7, 4934. 10.1038/s41598-017-04289-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert B. L., Bauer C. R. (2012). Developmental and Behavioral Consequences of Prenatal Cocaine Exposure: a Review. J. Perinatol. 32, 819–828. 10.1038/jp.2012.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery L. A., Zoghbi H. Y. (2019). The Distinct Methylation Landscape of Maturing Neurons and its Role in Rett Syndrome Pathogenesis. Curr. Opin. Neurobiol. 59, 180–188. 10.1016/j.conb.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz K. M., Nelson L. H. (2018). Microglia and beyond: Innate Immune Cells as Regulators of Brain Development and Behavioral Function. Front. Immunol. 9, 698. 10.3389/fimmu.2018.00698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Barres B. A. (2018). Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 18, 225–242. 10.1038/nri.2017.125 [DOI] [PubMed] [Google Scholar]
- Li Y., Chen X., Lu C. (2021). The Interplay between DNA and Histone Methylation: Molecular Mechanisms and Disease Implications. EMBO Rep. 22, e51803. 10.15252/embr.202051803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovely C., Rampersad M., Fernandes Y., Eberhart J. (2017). Gene-environment Interactions in Development and Disease. Wiley Interdiscip. Rev. Dev. Biol. 6. 10.1002/wdev.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier A. A., Bodnar T. S., Weinberg J. (2021). Intersection of Epigenetic and Immune Alterations: Implications for Fetal Alcohol Spectrum Disorder and Mental Health. Front. Neurosci. 15, 788630. 10.3389/fnins.2021.788630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarthur I. C., Dawlaty M. M. (2021). TET Enzymes and 5-Hydroxymethylcytosine in Neural Progenitor Cell Biology and Neurodevelopment. Front. Cell Dev. Biol. 9, 645335. 10.3389/fcell.2021.645335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marballi K., Macdonald J. L. (2021). Proteomic and Transcriptional Changes Associated with MeCP2 Dysfunction Reveal Nodes for Therapeutic Intervention in Rett Syndrome. Neurochem. Int. 148, 105076. 10.1016/j.neuint.2021.105076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjoram L., Alvers A., Deerhake M. E., Bagwell J., Mankiewicz J., Cocchiaro J. L., et al. (2015). Epigenetic Control of Intestinal Barrier Function and Inflammation in Zebrafish. Proc. Natl. Acad. Sci. U.S.A. 112, 2770–2775. 10.1073/pnas.1424089112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino D., Johnson I., Leckman J. F. (2020). What Does Immunology Have to Do with Normal Brain Development and the Pathophysiology Underlying Tourette Syndrome and Related Neuropsychiatric Disorders? Front. Neurol. 11, 567407. 10.3389/fneur.2020.567407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Ferreira R., Leal B., Costa P. P., Ballestar E. (2021). Microglial Innate Memory and Epigenetic Reprogramming in Neurological Disorders. Prog. Neurobiol. 200, 101971. 10.1016/j.pneurobio.2020.101971 [DOI] [PubMed] [Google Scholar]
- May P. A., Chambers C. D., Kalberg W. O., Zellner J., Feldman H., Buckley D., et al. (2018). Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA 319, 474–482. 10.1001/jama.2017.21896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini J., Le Clerc S., Morisse G., Coulonges C., Kuil L. E., Ham T. J., et al. (2020). Gene Expression Profiling Reveals a Conserved Microglia Signature in Larval Zebrafish. Glia 68, 298–315. 10.1002/glia.23717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottahedin A., Ardalan M., Chumak T., Riebe I., Ek J., Mallard C. (2017). Effect of Neuroinflammation on Synaptic Organization and Function in the Developing Brain: Implications for Neurodevelopmental and Neurodegenerative Disorders. Front. Cell Neurosci. 11, 190. 10.3389/fncel.2017.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan P., Sarmah S., Marrs J. A. (2015). Zebrafish Retinal Defects Induced by Ethanol Exposure Are Rescued by Retinoic Acid and Folic Acid Supplement. Alcohol 49, 149–163. 10.1016/j.alcohol.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C. C., Rawls J. F. (2019). Commensal Microbiota Regulate Vertebrate Innate Immunity-Insights from the Zebrafish. Front. Immunol. 10, 2100. 10.3389/fimmu.2019.02100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagre N. N., Subbanna S., Shivakumar M., Psychoyos D., Basavarajappa B. S. (2015). CB1-receptor Knockout Neonatal Mice Are Protected against Ethanol-Induced Impairments of DNMT1, DNMT3A, and DNA Methylation. J. Neurochem. 132, 429–442. 10.1111/jnc.13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance E., Kambhampati S. P., Smith E. S., Zhang Z., Zhang F., Singh S., et al. (2017). Dendrimer-mediated Delivery of N-Acetyl Cysteine to Microglia in a Mouse Model of Rett Syndrome. J. Neuroinflammation 14, 252. 10.1186/s12974-017-1004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone S., Elliott E. (2016). The Interaction between the Immune System and Epigenetics in the Etiology of Autism Spectrum Disorders. Front. Neurosci. 10, 329. 10.3389/fnins.2016.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely S. A., Lyons D. A. (2021). Insights into Central Nervous System Glial Cell Formation and Function from Zebrafish. Front. Cell Dev. Biol. 9, 754606. 10.3389/fcell.2021.754606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'driscoll C. M., Lima M. P., Kaufmann W. E., Bressler J. P. (2015). Methyl CpG Binding Protein 2 Deficiency Enhances Expression of Inflammatory Cytokines by Sustaining NF-Κb Signaling in Myeloid Derived Cells. J. Neuroimmunol. 283, 23–29. 10.1016/j.jneuroim.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Oosterhof N., Chang I. J., Karimiani E. G., Kuil L. E., Jensen D. M., Daza R., et al. (2019). Homozygous Mutations in CSF1R Cause a Pediatric-Onset Leukoencephalopathy and Can Result in Congenital Absence of Microglia. Am. J. Hum. Genet. 104, 936–947. 10.1016/j.ajhg.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhof N., Kuil L. E., Van Der Linde H. C., Burm S. M., Berdowski W., Van Ijcken W. F. J., et al. (2018). Colony-stimulating Factor 1 Receptor (CSF1R) Regulates Microglia Density and Distribution, but Not Microglia Differentiation In Vivo . Cell Rep. 24, 1203–1217. 10.1016/j.celrep.2018.06.113 [DOI] [PubMed] [Google Scholar]
- Orihuela R., Mcpherson C. A., Harry G. J. (2016). Microglial M1/M2 Polarization and Metabolic States. Br. J. Pharmacol. 173, 649–665. 10.1111/bph.13139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panisi C., Guerini F. R., Abruzzo P. M., Balzola F., Biava P. M., Bolotta A., et al. (2021). Autism Spectrum Disorder from the Womb to Adulthood: Suggestions for a Paradigm Shift. J. Pers. Med. 11. 10.3390/jpm11020070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli R. C., Ferretti M. T. (2017). Function and Dysfunction of Microglia during Brain Development: Consequences for Synapses and Neural Circuits. Front. Synaptic Neurosci. 9, 9. 10.3389/fnsyn.2017.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikshak N. N., Swarup V., Belgard T. G., Irimia M., Ramaswami G., Gandal M. J., et al. (2016). Genome-wide Changes in lncRNA, Splicing, and Regional Gene Expression Patterns in Autism. Nature 540, 423–427. 10.1038/nature20612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar O. L., Caruso M., Teakle N., Keshvari S., Bush S. J., Pridans C., et al. (2021). Analysis of Homozygous and Heterozygous Csf1r Knockout in the Rat as a Model for Understanding Microglial Function in Brain Development and the Impacts of Human CSF1R Mutations. Neurobiol. Dis. 151, 105268. 10.1016/j.nbd.2021.105268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecorelli A., Cervellati C., Cordone V., Hayek J., Valacchi G. (2020). Compromised Immune/inflammatory Responses in Rett Syndrome. Free Radic. Biol. Med. 152, 100–106. 10.1016/j.freeradbiomed.2020.02.023 [DOI] [PubMed] [Google Scholar]
- Periyasamy P., Liao K., Kook Y. H., Niu F., Callen S. E., Guo M.-L., et al. (2018). Cocaine-Mediated Downregulation of miR-124 Activates Microglia by Targeting KLF4 and TLR4 Signaling. Mol. Neurobiol. 55, 3196–3210. 10.1007/s12035-017-0584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri T., Roman A. C., Guyon N., Romano S. A., Washbourne P., Moens C. B., et al. (2013). The First Mecp2-Null Zebrafish Model Shows Altered Motor Behaviors. Front. Neural Circuits 7, 118. 10.3389/fncir.2013.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M., Jung S., Priller J. (2019). Microglia Biology: One Century of Evolving Concepts. Cell 179, 292–311. 10.1016/j.cell.2019.08.053 [DOI] [PubMed] [Google Scholar]
- Qi S., Li Y., Dai Z., Xiang M., Wang G., Wang L., et al. (2019). Uhrf1-Mediated Tnf-α Gene Methylation Controls Proinflammatory Macrophages in Experimental Colitis Resembling Inflammatory Bowel Disease. J. I. 203, 3045–3053. 10.4049/jimmunol.1900467 [DOI] [PubMed] [Google Scholar]
- Qiu M., Xu E., Zhan L. (2021a). Epigenetic Regulations of Microglia/Macrophage Polarization in Ischemic Stroke. Front. Mol. Neurosci. 14, 697416. 10.3389/fnmol.2021.697416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu T., Yin H., Wang Y., Zhao C., Cai D. (2021b). miR-153 Attenuates the Inflammatory Response and Oxidative Stress Induced by Spinal Cord Injury by Targeting of NEUROD2. Am. J. Transl. Res. 13, 7968–7975. [PMC free article] [PubMed] [Google Scholar]
- Reemst K., Noctor S. C., Lucassen P. J., Hol E. M. (2016). The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front. Hum. Neurosci. 10, 566. 10.3389/fnhum.2016.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réu P., Khosravi A., Bernard S., Mold J. E., Salehpour M., Alkass K., et al. (2017). The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 20, 779–784. 10.1016/j.celrep.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson G. A., Goldschmidt L., Larkby C., Day N. L. (2015). Effects of Prenatal Cocaine Exposure on Adolescent Development. Neurotoxicology Teratol. 49, 41–48. 10.1016/j.ntt.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford H. A., Kasher P. R., Hamilton N. (2020). Dirty Fish versus Squeaky Clean Mice: Dissecting Interspecies Differences between Animal Models of Interferonopathy. Front. Immunol. 11, 623650. 10.3389/fimmu.2020.623650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatt J. M., Myers S. M. (2021). Genetic Testing in Neurodevelopmental Disorders. Front. Pediatr. 9, 526779. 10.3389/fped.2021.526779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. P., Heller C. T., Gunner G., Heller M., Gordon C., Hammond T., et al. (2016). Microglia Contribute to Circuit Defects in Mecp2 Null Mice Independent of Microglia-specific Loss of Mecp2 Expression. Elife 5. 10.7554/eLife.15224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K., Bisht K., Eyo U. B. (2021). A Comparative Biology of Microglia across Species. Front. Cell Dev. Biol. 9, 652748. 10.3389/fcell.2021.652748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat S., Amelan A., Shifman S. (2021). Convergence and Divergence in the Genetics of Psychiatric Disorders from Pathways to Developmental Stages. Biol. Psychiatry 89, 32–40. 10.1016/j.biopsych.2020.05.019 [DOI] [PubMed] [Google Scholar]
- Shrivastava P., Cabrera M. A., Chastain L. G., Boyadjieva N. I., Jabbar S., Franklin T., et al. (2017). Mu-opioid Receptor and Delta-Opioid Receptor Differentially Regulate Microglial Inflammatory Response to Control Proopiomelanocortin Neuronal Apoptosis in the Hypothalamus: Effects of Neonatal Alcohol. J. Neuroinflammation 14, 83. 10.1186/s12974-017-0844-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva N. J., Dorman L. C., Vainchtein I. D., Horneck N. C., Molofsky A. V. (2021). In Situ and Transcriptomic Identification of Microglia in Synapse-Rich Regions of the Developing Zebrafish Brain. Nat. Commun. 12, 5916. 10.1038/s41467-021-26206-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavsky M., Mor O., Mastrolia S. A., Greenbaum S., Than N. G., Erez O. (2017). Cerebral Palsy-Trends in Epidemiology and Recent Development in Prenatal Mechanisms of Disease, Treatment, and Prevention. Front. Pediatr. 5, 21. 10.3389/fped.2017.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling R. M., Dinan T. G., Cryan J. F. (2014). Microbial Genes, Brain & Behaviour - Epigenetic Regulation of the Gut-Brain axis. Genes, Brain Behav. 13, 69–86. 10.1111/gbb.12109 [DOI] [PubMed] [Google Scholar]
- Stratoulias V., Venero J. L., Tremblay M. È., Joseph B. (2019). Microglial Subtypes: Diversity within the Microglial Community. Embo J. 38, e101997. 10.15252/embj.2019101997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. M., De Rubeis S., Schaefer A. (2019). Convergence of Spectrums: Neuronal Gene Network States in Autism Spectrum Disorder. Curr. Opin. Neurobiol. 59, 102–111. 10.1016/j.conb.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sushma A., Divakar A., Kanchan S., Jha G., Mishra S., Sharma D., et al. (2021). Alcohol Induced Impairment/abnormalities in Brain: Role of MicroRNAs. Neurotoxicology 87, 11–23. 10.1016/j.neuro.2021.08.013 [DOI] [PubMed] [Google Scholar]
- Svahn A. J., Giacomotto J., Graeber M. B., Rinkwitz S., Becker T. S. (2016). miR-124Contributes to the Functional Maturity of Microglia. Devel Neurobio 76, 507–518. 10.1002/dneu.22328 [DOI] [PubMed] [Google Scholar]
- Tal T. L., Franzosa J. A., Tilton S. C., Philbrick K. A., Iwaniec U. T., Turner R. T., et al. (2012). MicroRNAs Control Neurobehavioral Development and Function in Zebrafish. FASEB J. 26, 1452–1461. 10.1096/fj.11-194464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A., Cooper M. (2016). Attention Deficit Hyperactivity Disorder. Lancet 387, 1240–1250. 10.1016/s0140-6736(15)00238-x [DOI] [PubMed] [Google Scholar]
- Thion M. S., Garel S. (2020). Microglial Ontogeny, Diversity and Neurodevelopmental Functions. Curr. Opin. Genet. Dev. 65, 186–194. 10.1016/j.gde.2020.06.013 [DOI] [PubMed] [Google Scholar]
- Thion M. S., Low D., Silvin A., Chen J., Grisel P., Schulte-Schrepping J., et al. (2018). Microbiome Influences Prenatal and Adult Microglia in a Sex-specific Manner. Cell 172, 500–516. e516. 10.1016/j.cell.2017.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. T., Gross C., Bassell G. J. (2018). microRNAs Sculpt Neuronal Communication in a Tight Balance that Is Lost in Neurological Disease. Front. Mol. Neurosci. 11, 455. 10.3389/fnmol.2018.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson R., Bird A. (2019). The Molecular Basis of MeCP2 Function in the Brain. J. Mol. Biol. [DOI] [PubMed] [Google Scholar]
- Vaillancourt K., Ernst C., Mash D., Turecki G. (2017). DNA Methylation Dynamics and Cocaine in the Brain: Progress and Prospects. Genes (Basel) 8. 10.3390/genes8050138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Knaap M. S., Bugiani M. (2017). Leukodystrophies: a Proposed Classification System Based on Pathological Changes and Pathogenetic Mechanisms. Acta Neuropathol. 134, 351–382. 10.1007/s00401-017-1739-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Vaart M., Svoboda O., Weijts B. G., Espín-Palazón R., Sapp V., Pietri T., et al. (2017). Mecp2 Regulates Tnfa during Zebrafish Embryonic Development and Acute Inflammation. Dis. Model Mech. 10, 1439–1451. 10.1242/dmm.026922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heesbeen H. J., Smidt M. P. (2019). Entanglement of Genetics and Epigenetics in Parkinson's Disease. Front. Neurosci. 13, 277. 10.3389/fnins.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadinova M., Boyadjieva N. (2015). Epigenetic Mechanisms: A Possible Link between Autism Spectrum Disorders and Fetal Alcohol Spectrum Disorders. Pharmacol. Res. 102, 71–80. 10.1016/j.phrs.2015.09.011 [DOI] [PubMed] [Google Scholar]
- Veazey K. J., Wang H., Bedi Y. S., Skiles W. M., Chang R. C.-A., Golding M. C. (2017). Disconnect between Alcohol-Induced Alterations in Chromatin Structure and Gene Transcription in a Mouse Embryonic Stem Cell Model of Exposure. Alcohol 60, 121–133. 10.1016/j.alcohol.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Ciernia A., Laufer B. I., Hwang H., Dunaway K. W., Mordaunt C. E., Coulson R. L., et al. (2020). Epigenomic Convergence of Neural-Immune Risk Factors in Neurodevelopmental Disorder Cortex. Cereb. Cortex 30, 640–655. 10.1093/cercor/bhz115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineagu I., Wang X., Johnston P., Lowe J. K., Tian Y., Horvath S., et al. (2011). Transcriptomic Analysis of Autistic Brain Reveals Convergent Molecular Pathology. Nature 474, 380–384. 10.1038/nature10110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley B., Geschwind D. H. (2020). Functional Genomics Links Genetic Origins to Pathophysiology in Neurodegenerative and Neuropsychiatric Disease. Curr. Opin. Genet. Dev. 65, 117–125. 10.1016/j.gde.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. L., Zhang Z., Li Q., Yang R., Pei X., Xu Y., et al. (2009). Ethanol Exposure Induces Differential microRNA and Target Gene Expression and Teratogenic Effects Which Can Be Suppressed by Folic Acid Supplementation. Hum. Reprod. 24, 562–579. 10.1093/humrep/den439 [DOI] [PubMed] [Google Scholar]
- Wang W. Y., Tan M. S., Yu J. T., Tan L. (2015). Role of Pro-inflammatory Cytokines Released from Microglia in Alzheimer's Disease. Ann. Transl. Med. 3, 136. 10.3978/j.issn.2305-5839.2015.03.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Z., Wang Y., Li F., Jia J., Song X., et al. (2018). The Gut-Microglia Connection: Implications for Central Nervous System Diseases. Front. Immunol. 9, 2325. 10.3389/fimmu.2018.02325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfert A. B., Sulovari A., Turner T. N., Coe B. P., Eichler E. E. (2017). Recurrent De Novo Mutations in Neurodevelopmental Disorders: Properties and Clinical Implications. Genome Med. 9, 101. 10.1186/s13073-017-0498-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm C. J., Guizzetti M. (2016). Fetal Alcohol Spectrum Disorders: An Overview from the Glia Perspective. Front. Integr. Neurosci. 9, 65. 10.3389/fnint.2015.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittrahm R., Takalo M., Marttinen M., Kuulasmaa T., Mäkinen P., Kemppainen S., et al. (2021). MECP2 Increases the Pro-inflammatory Response of Microglial Cells and Phosphorylation at Serine 423 Regulates Neuronal Gene Expression upon Neuroinflammation. Cells 10. 10.3390/cells10040860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. L., Stowell R. D., Majewska A. K. (2017). What the Spectrum of Microglial Functions Can Teach Us about Fetal Alcohol Spectrum Disorder. Front. Synaptic Neurosci. 9, 11. 10.3389/fnsyn.2017.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J. R., Riley E. P., Charness M. E. (2019). Clinical Presentation, Diagnosis, and Management of Fetal Alcohol Spectrum Disorder. Lancet Neurology 18, 760–770. 10.1016/s1474-4422(19)30150-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Zhang Y. (2014). Reversing DNA Methylation: Mechanisms, Genomics, and Biological Functions. Cell 156, 45–68. 10.1016/j.cell.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.-H., Huang B.-R., Lai S.-W., Lin C., Lin H.-Y., Yang L.-Y., et al. (2020). SIRT1 Activation by Minocycline on Regulation of Microglial Polarization Homeostasis. Aging 12, 17990–18007. 10.18632/aging.103542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhu L., He S., Wu Y., Jin W., Yu T., et al. (2015). Temporal-Spatial Resolution Fate Mapping Reveals Distinct Origins for Embryonic and Adult Microglia in Zebrafish. Dev. Cell 34, 632–641. 10.1016/j.devcel.2015.08.018 [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang Y., Yuan J., Li N., Pei S., Xu J., et al. (2018). Macrophage/microglial Ezh2 Facilitates Autoimmune Inflammation through Inhibition of Socs3. J. Exp. Med. 215, 1365–1382. 10.1084/jem.20171417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Mokhtari R., Pedrosa E., Birnbaum R., Zheng D., Lachman H. M. (2017). Transcriptome Analysis of Microglia in a Mouse Model of Rett Syndrome: Differential Expression of Genes Associated with Microglia/macrophage Activation and Cellular Stress. Mol. Autism 8, 17. 10.1186/s13229-017-0134-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., He Z., Wang J. (2021). MicroRNA-124: A Key Player in Microglia-Mediated Inflammation in Neurological Diseases. Front. Cell Neurosci. 15, 771898. 10.3389/fncel.2021.771898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Hou J., Chen B., Shao X., Zhu R., Bu Q., et al. (2015). Prenatal Cocaine Exposure Impairs Cognitive Function of Progeny via Insulin Growth Factor II Epigenetic Regulation. Neurobiol. Dis. 82, 54–65. 10.1016/j.nbd.2015.05.014 [DOI] [PubMed] [Google Scholar]