Abstract
BACKGROUND
Pneumopericardium, presence of air in pericardial cavity, is rare entity with potentially severe complications and mortality. In the neonatal period, it is associated with prematurity, low birth weight, and assisted ventilation, but in full term neonates its occurrence after resuscitation is exceedingly rare.
CASE REPORT
Our patient was a 2-day old full term neonate who developed respiratory distress following active resuscitation which was carried out at the time of birth in lieu of perinatal asphyxia. He was immediately put on mechanical ventilatory support. Chest x-ray showed a "halo sign"- rim of air completely surrounding the heart, and echocardiography confirmed pneumopericardium with cardiac tamponade. Pericardiocentesis was performed as a life saving measure.
CONCLUSION
He was successfully discharged on the tenth day following sheath removal, and is doing fine on follow up.
Keywords: Pneumopericardium, Pericardiocentesis, Cardiac Tamponade
Introduction
Pneumopericardium (PPC) is defined as the collection of air in pericardial sac. In term infants, it is often associated with active resuscitation, meconium aspiration syndrome, and rarely mechanical ventilation, whereas chest trauma and thoracotomy are few of the causes among adults. Neonatal PPC may be either isolated (in the absence of underlying lung pathology) or secondary to other air leak syndromes like pneumomediastinum, pneumothorax, pneumoperitonium, subcutaneous emphysema, and interstitial emphysema. It carries significant morbidity and mortality which may be complicated by cardiac tamponade. It can readily be diagnosed with the help of chest X-ray and echocardiography, and pericardiocentesis is a lifesaving procedure if promptly diagnosed.1,2
Case Report
The male infant weighing 2.980 Kg was born trans-vaginally to a 27-year-old, primigravida, primipara mother (G1+P1) following at 40-week gestation. It was uncomplicated delivery except the prolonged second stage and third stage of labour. His Apgar score was 4 in the first minute after birth. Following the birth, the infant developed perinatal asphyxia for which he was actively resuscitated using the non-invasive ventilation, but did not improved. As his oxygen saturation was 80%, he was immediately intubated. His Apgar score improved to 7 and 10 in the fifth and tenth minutes, respectively. He was transferred to the neonatal intensive care unit (NICU). His blood pressure was 70/50 mm Hg in the left arm as measured by the neonatal cuff. His heart rate and breathing rate were 144 beats/min and 66/min, respectively.
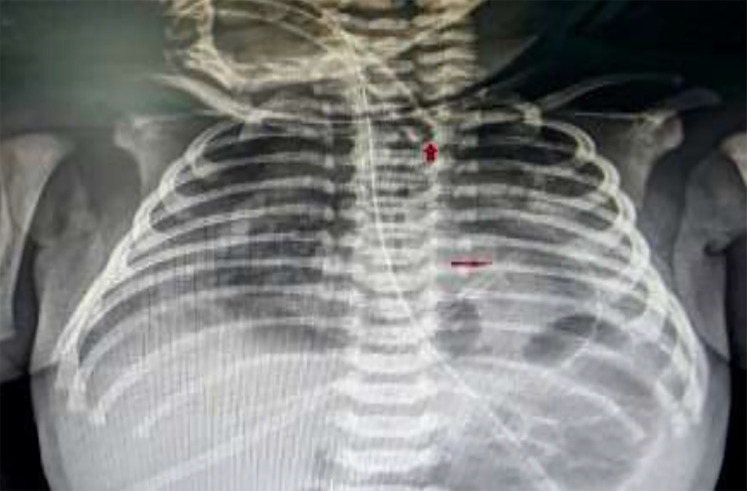
On the second day, he developed respiratory distress. He was afebrile and tachypnic. There was no history of aspiration, lethargy, and rash. His blood pressure was 52/38 mm Hg and his pulse rate was 186/min and regular. S1 and S2 were muffled. Both lungs were clear on auscultation. The electrocardiogram (ECG) showed low voltage QRS complex with non-specific ST-T wave changes. The haematological parameters were normal except mild leucocytosis (13800/mm3). The cardiac troponin (I and T) and myocardial enzymes - creatine kinase [CK], and its MB isoenzymes (CK-MB) were normal. Immediate bed side chest X-ray revealed continuous radiolucent band of air that was conformed to cardiac silhouette and not extending beyond the level of great vessels which is known as halo sign, a pathognomonic of PPC (Figure 1). The distal end of the endotracheal tube was lying deep in the right main bronchus (Figure 1).
Figure 1.
Chest X-ray showing a halo signcontinuous radiolucent band of air confined to cardiac silhouette extending beyond the level of great vessels; The distal end of the endotracheal tube (red arrow) was lying deep in carina near the right main bronchus.
Transrthoracic echocardiography (VIVID 7TM, GE, Germany) was performed through sub-costal route, which revealed air bubbles in the pericardial sac and diastolic collapse of the right atrium and right ventricular outflow tract PPC with cardiac tamponade (Figure 2). His bi-ventricular functions were normal. Infection (viral, bacterial, tubercular) and sepsis were ruled out on the basis of age, absence of fever, normal blood count, and temporal course of events. Similarly, acute respiratory distress syndrome (ARDS) was ruled out on the basis of the clear lung fields on auscultation and chest x-ray. Normal cardiac enzymes, ECG, and normal biventricular functions also ruled out myocarditis. In view of hemodynamic instability and tamponade physiology, pericardiocentesis was planned under echocardiographic guidance after obtaining informed consent from his parents.
Figure 2.
Echocardiography (ECG) through sub-costal route showing air bubbles (horizontal red arrow) visible in pericardial sac which confirmed pneumopericardium. Vertical red arrow represents margin of visceral (down arrows) and parietal pericardium (up arrows).
Intravenous fluid (normal saline) was started. Ceftriaxone- 250 mg was administered intravenously. The sub-costal area was aseptically prepared and the skin was infiltrated with 2% xylocaine. A 21-G needle on a 2 ml syringe was carefully entered into the pericardial cavity. A successful entry was confirmed by the presence of air bubbles in the syringe when it was little sucked. 0.021" guide wires (Cordis, USA) were inserted into the cavity through the needle. 4F radial angiographic sheath (Cook Medical, USA) was inserted over the wire with its side arm connected to the underwater seal. The wire and dilator were removed subsequently, thus leaving sheath in situ (Figure 3). 40-50 ml of air was aspirated with the help of a 5-ml syringe from the underwater sealto relieve him from haemodynamic instability.
Figure 3.
Chest X-ray after endotracheal tube was repositioned by pulling it little up to bring its distal tip (vertical arrow) into the main bronchus. The tip of sheath (horizontal arrow) is visible into pericardial sac.
It restored hemodynamic stability as the blood pressure rose to 66/42 mm Hg. The endotracheal tube was repositioned by pulling it to bring its distal tip into the main bronchus. Repeated ECG revealed minimal amount of air on anterior cardiac surface (Figure 4A). On the next day, the pericardial sheath was removed as the repeated ECG showed further resolution of air from the cavity. Repeated chest x-ray on the next day showed complete resolution of PPC (Figure 4B). He was gradually weaned off from the mechanical ventilation over one week and discharged on the tenth day with a stable condition.
Figure 4.
Repeated echocardiogram (ECG) revealed minimal amount of air (red arrows) on anterior and posterior cardiac surface (A); Repeated chest x-ray on the next day showed complete resolution of pneumopericardium (PPC); Horizontal arrows indicate repositioned tip of the endotracheal (ET) tube (B).
Discussion
During 60s-90s in the last century, the incidence of PPC was high as the respiratory distress syndrome was mainly used to be managed with the ventilatory support. With the early recognition of the risk factors and better understanding of the pathophysiology of hyaline membrane disease (HMD), availability of steroids, exogenous surfactant, and extra corporeal membrane oxygen (ECMO), the incidence of various air leak syndromes has come down.
It was noted in 50 cases among 3841 babies as reported by Burt and Lester3 In their series, 66% of cases had associated pneumomediastinum and overall mortality was 70%. Isolated cases were reported only in 3 babies. Prematurity, low birth weight with HMD, active ventilation, continous positive airway pressure, meconium aspiration, and improper placement of endotracheal tube (in the right main stem bronchus) were other contributory factors.3 In a retrospective study, its incidence was 2% among 2389 very low birth weight and 3.5% among 1349 ventilated infants with 17% and 60% survival, respectively. Associated factors were pneumothorax (49%), pulmonary interstitial emphysema (46%), pneumomediastinum (39%), subcutaneous emphysema (17%), pneumoperitoneum (10%), and systemic vascular air embolus (5%).4
It needs to be promptly diagnosed as other air leak syndrome, particularly pneumomediastinum which is a close mimicker. Chest X-ray showing telltale features which include halo sign, presence of infra cardiac air shadow, and medial placement of concavity of pericardium (Figure 1) along with demonstration of air bubbles in pericardial space on echocardiogram are sufficient to clinch the diagnosis of PPC.5 The exact mechanism of the isolated PPC is not known. However, a plausible mechanism is the microscopic dissection caused by high intra alveolar pressure at the junction of parietal pericardium and pulmonary vein ostia as they share reflection of parietal and visceral pericardium, leading to the entry of air into the pericardial cavity.6,7 Reduced number and size of the Kohn pores in newborns also adds to this as there is an inability for air to equilibrate between aerated and non-aerated alveoli.4 Sometimes, first few breaths taken by the newborn after birth may suddenly lead to an increase in the intrathoracic pressures creating minute air leaks, which are further aggravated by continuous ventilation.8 The symptoms will depend on rapidity of air accumulation, which may compromise venous return to heart, and by compressing pulmonary veins further, may lead to circulatory collapse.9
The clinical manifestation varies from an asymptomatic stage to cardiac tamponade including tachycardia, bradycardia, hypotension, cyanosis, and circulatory collapse, depending on the volume and rapidity with which free air accumulates into pericardial cavity. In long term, few of patients may also show a neuro-developmental delay.8 In our case, deep placement of the endotracheal tube (more into right main stem bronchus) and continuous ventilation, thus by increasing peak inspiratory pressure causing volutrauma, may have been possible as the neonate was stable and initial x-ray was normal. The patient became hemodynamically unstable as a result of tamponade after 48 hours of receiving continous ventilation. Mostly, PPC does not translates into cardiac tamponade as intra-pericardial pressure rarely exceeds atrial filling pressure by 20-25cm of water, but it is more likely to develop in patients receiving positive pressure ventilation as in our case.
Isolated asymptomatic PPC in non-ventilated neonate requires close monitoring for signs of cardiac tamponade as most of them are resolved with conservative measures. Supplemental oxygen, acting as a “nitrogen washout”, has also been demonstrated to be useful in certain cases.10 Once the patient develops tamponade, urgent pericardiocentesis is warranted. A pigtail catheter may be left into pericardial sac in case of extensive or recurrent effusion. An early echocardiography following resuscitation might have detected small air in pericardial sac, had it been there which might have been missed on conventional chest x-ray as in our case. Before the introduction of the pre-surfactant era, prognosis was grave among premature neonates, but the outcome from pneumopericardium has much improved with its usage.
Pneumopericardium is a rare and potentially life-threatening condition that is usually associated with assisted ventilation, especially among preterm neonates. The position of the endotracheal tube must be checked and placed in the main stem bronchus. A conservative approach of close monitoring without aspiration may be considered if the patient is hemodynamically stable and urgent pericardiocentesis should be performed once the patient develops a sign of tamponade.
Acknowledgments
None.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Mansfield PB, Graham CB, Beckwith JB, Hall DG, Sauvage LR. Pneumopericardium and pneumomediastinum in infants and children. J Pediatr Surg. 1973;8(5):691–9. doi: 10.1016/0022-3468(73)90408-9. [DOI] [PubMed] [Google Scholar]
- 2.Suryawanshi P, Klimek J. Preterm neonate with spontaneous pneumopericardium without any other associated air leaks. J Clin Diagn Res. 2014;8(1):181–2. doi: 10.7860/JCDR/2014/5620.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burt TB, Lester PD. Neonatal pneumopericardium. Radiology. 1982;142(1):81–4. doi: 10.1148/radiology.142.1.7053553. [DOI] [PubMed] [Google Scholar]
- 4.Junghaenel S, Sreeram N, Demant A, Vierzig A, Kribs A, Roth B. Pneumopericardium as a rare complication of continuous positive airway pressure in spontaneously breathing neonates. Klin Padiatr. 2012;224(1):34–5. doi: 10.1055/s-0031-1298025. [DOI] [PubMed] [Google Scholar]
- 5.Hook B, Hack M, Morrison S, Borawski-Clark E, Newman NS, Fanaroff A. Pneumopericardium in very low birth weight infants. J Perinatol. 1995;15(1):27–31. [PubMed] [Google Scholar]
- 6.Trujillo MH, Fragachan CF, Tortoledo F. Cardiac tamponade due to pneumopericardium. Cardiology. 2006;105(1):34–6. doi: 10.1159/000088450. [DOI] [PubMed] [Google Scholar]
- 7.Narasimhan R, Krishnamurthy S. A review of non-invasive ventilation support in neonates. Paediatr Child Health. 2014;24(1):7–11. [Google Scholar]
- 8.Suresh P, Tagare A, Kadam S, Vaidya U, Pandit A. Spontaneous pneumopericardium in a healthy full-term neonate. Indian J Pediatr. 2011;78(11):1410–1. doi: 10.1007/s12098-011-0477-y. [DOI] [PubMed] [Google Scholar]
- 9.Roychoudhury S, Kaur S, Soraisham AS. Neonatal pneumopericardium in a nonventilated term infant: A case report and review of the literature. Case Rep Pediatr. 2017;2017:3149370. doi: 10.1155/2017/3149370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawson EE, Gould JB, Taeusch HW. Neonatal pneumopericardium: Current management. J Pediatr Surg. 1980;15(2):181–5. doi: 10.1016/s0022-3468(80)80013-3. [DOI] [PubMed] [Google Scholar]