Abstract
Heterocycles containing the pyranopyrimidine motif have attracted the interest of researchers in recent decades due to their ability to synthesize and explore at a large scale to explore the biological diversity. Therefore, this review highlights the biological characteristics and synthetic approaches adopted to prepare pyranopyrimidine analogs in the last five years. Several novel preparation procedures have been summarized to synthesize these compounds using ionic, basic, or nanocatalysts or catalyst-free conditions to obtain these compounds in good yields. Pyranopyrimidines could also be used as ligands in the preparation of metal complexes with increased biological potency. The different sections include the antimicrobial, antitubercular, antimalarial, antiviral “SARS-CoV-2 inhibitors”, antidiabetic, antitumor, cytotoxic, antiinflammatory, antioxidant, anticoagulant, urease inhibitory activities, and tyrosine inhibitors. The results are discussed based on the structure–activity relationships (SARs) and the mechanism of action.
The current review highlights the importance of pyranopyrimidines as privileged biologically active molecules. It also discusses recent synthetic strategies for the synthesis of these compounds, the mechanism of action, and SARs.
1. Introduction
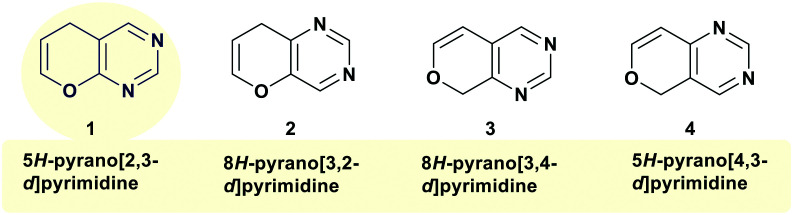
The critical importance of heterocyclic compounds in medical research lies in the possibility of synthesizing drug-like chemical compounds and initiating more effective drug discovery projects. Medicinal chemistry provides pharmaceutical chemists with detailed sympathetic drug mechanisms of action, structure–activity relationships (SARs), acid–base and physicochemical characters, absorption, distribution, metabolism, excretion, and toxicity profiles. On the other hand, nitrogen-, oxygen-, and sulfur-based heterocycles hold a distinctive position as an appreciated source of therapeutic agents in medicinal chemistry.1–4 Therefore, pyranopyrimidines are compounds of biological significance and distinguished medical and pharmacological properties, which are briefly listed below and in detail under different topics of research. In particular, pyranopyrimidines are 6 + 6 bicyclic heterocyclic systems consisting of a pyran ring with an oxygen atom annulated with a pyrimidine ring with 1,3-nitrogen atoms. Therefore, four potential isomers are proposed for these compounds (Fig. 1), in which the nitrogen atoms of the pyrimidine ring cannot be found at the ring junction centers.
Fig. 1. The conceivable structural isomers of pyranopyrimidines.
The synthesis of this category of compounds involves the synthesis of bicyclic systems at a large scale, predominately by multicomponent one-pot reactions that prefer to be applied through green methods, under solvent-free,5 catalyst-free,6,7 or catalytic conditions, e.g., nanocatalysts,8,9 in a basic medium,10 polyethylene glycol 400,11 under microwave irradiation,12 or conventional conditions.13 The synthesis of annulated bicyclic pyranopyrimidines was extended for the four-component synthesis,14 under catalyst-free conditions. The mechanism of bicyclic ring constructions was proposed in general through Knoevenagel condensation, Michael addition, and intramolecular ring cyclization. The tricyclic systems were synthesized by four-component reactions under catalytic, solvent-free, and sonication or heating conditions.15–18 Other methods involving three-component reactions for the synthesis of tricyclic pyranopyrimidines were reported19 under microwave irradiation conditions or by multi-step synthesis.20 Polycyclic systems were efficiently synthesized from multicomponent reactions utilizing 2-naphthol as a reactant,21 or thiazolopyrimidine-dione.22 Spirocyclic systems were also synthesized at a large scale by multicomponent reactions that involved the use of isatins as reactants.23,24 The pyranodipyrimidines tended to form metal complexes with improved biological characteristics.25
Amirnejat et al.26 evaluated the antibacterial potency of a series of synthesized 6-cyano-7-amino-2,4-dioxo-5-aryl-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidines “prepared by multicomponent reactions using catalytic BaFe12O19 nanoparticles”. Also, Khatavi and Kamanna prepared pyrano[2,3-d]pyrimidine-trione analogs under catalytic conditions using Citrus sinensis and assessed their antimicrobial potency.27 In addition, various analogs of heterocycle-incorporated pyranopyrimidine motifs demonstrated privileged antimicrobial activity.28 Furthermore, Chate et al.29 have synthesized pyranopyrimidinone analogs under catalytic conditions of β-cyclodextrin and investigated their antimicrobial activities with higher potency than ciprofloxacin. Other research deals with the synthesis of bicyclic and fused cyclic compounds with pyranopyrimidine skeleton as the biological characters of these compounds with successful results as antitubercular, antimicrobial, and antimalarial agents.30–35
The biological significance of these compounds was undertaken for the investigation of 6-aryl-7-amino-pyrido[2,3-d]pyrimidines as antihypertensive agents,36 pyranopyrimidine-based curcumin as an antioxidant, and antidiabetic agents for α-amylase and α-glucosidase,37 polycyclic heterocycles with pyranopyrimidine core, and bicyclic pyranopyrimidines linked with heterocycles as antiproliferative and anticancer agents,38,39 inotropic agents “increase muscular contraction strength”,40 antiviral agents,41–44 sirtuin inhibitors,45 and hedgehog signaling pathway inhibitors.46
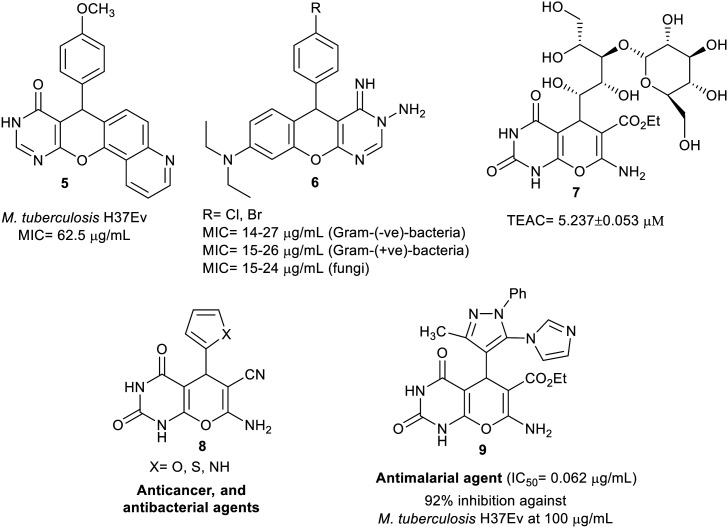
A pattern of biologically active molecules with pyranopyrimidine basic skeleton is presented in Fig. 1. Consequently, Kamdar et al.47 designed tetracyclic system 5 as a potent antimalarial agent against M. tuberculosis H37Ev. Correspondingly, Sabry et al.48 specified that the tricyclic, chromenopyrimidines 6 displayed remarkable antimicrobial activities against diverse bacterial and fungal species; MIC ranges in μg mL−1 are shown in Fig. 2. Compound 7 displayed significant antioxidant characteristics with a TEAC value of 5.237 μM.49 Paliwal et al.50 detected the capacity of bicyclic systems 8 as noteworthy anticancer and antibacterial agents. Also, Kalaria et al.51 designed and appraised the considerable antimalarial activity of compound 9 with the lowest IC50 value and potent percentage of inhibition.
Fig. 2. Patterns of compounds with pyranopyrimidine motif with privileged biologically active characters.
Thus, this study has been undertaken as a persistence of our previous programs and studies on highlighting the chemistry of heterocyclic compounds with miscellaneous biological activities and their significance in the fields of medicinal and pharmaceutical chemistry.52–59 Our program deals with highlighting and exploring the prosperous medicinal chemistry of pyranopyrimidine compounds, particularly pyrano[2,3-d]pyrimidines, in the last five years. The topic of this research is an extension of our previous study that is interesting to highlight the recent methods and progress in the multicomponent synthesis of heterocycle-incorporated pyrano[2,3-d]pyrimidine motif under different conditions.60
2. Biological features
2.1. Antimicrobial activity
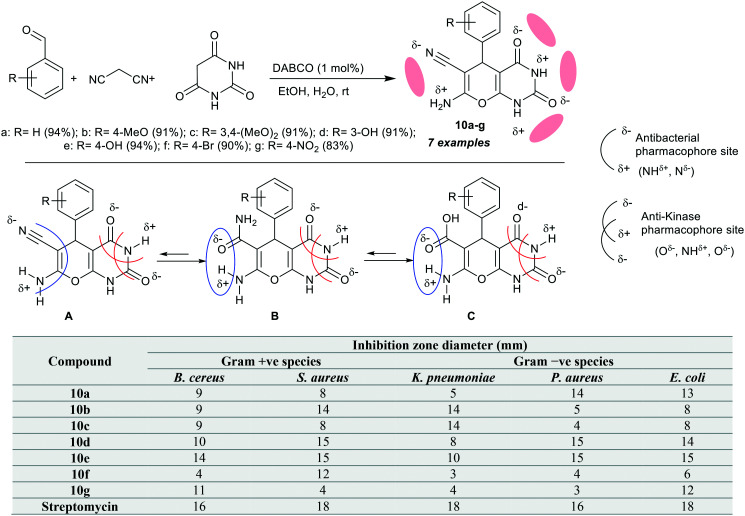
The antimicrobial activity could be well-defined as a cooperative expression for all active agents that causes inhibition of bacterial growth, prohibit microbial colonies formation, and may kill microorganisms.61 Bhat et al.62 employed an identical procedure for the multicomponent one-pot synthesis of pyranopyrimidine bicyclic systems 10a–g in 83–97% yields under DABCO catalytic conditions in water/ethanol. Subsequently, this series of compounds was assessed as antibacterial agents versus B. cereus (ATCC-14579), S. aureus (NCTC-7447), K. pneumoniae (UC57), P. aureus (ATCC 27853), and E. coli species. Therefore, all these compounds exhibited antibacterial potency against the verified bacterial species. In accordance, compounds 10d (inhibition zone diameter = 10 mm), 10e (14 mm), and 10g (11 mm) are the broadest spectrum in comparison to Streptomycin (16 mm) against B. cereus strains. The results also indicated a broad spectrum for compound 10a against P. aureus and E. coli species, compound 10b established broad spectrum activity against S. aureus and K. pneumoniae species, compound 10c is more effective against K. pneumoniae species. Furthermore, compound 10d is also effective for inhibiting the growth of S. aureus, P. aureus, and E. coli species, compound 10e is an effective antibacterial agent against all the bacterial species with low potency against K. pneumoniae strains, compound 10f is the lowest potent compound in this series but with effective potency against S. aureus species, and compound 10g displayed the same potency as compound 10f but with improved potency toward B. cereus, and E. coli species. Besides, compound 10e is the most potent agent for B. cereus species, compounds 10b and 10c are the most potent antibacterial agents on K. pneumoniae species, and compounds 10d and 10e are the most potent agents for S. aureus, P. aureus, and E. coli species. Thus, the SARs were investigated through the introduction of electron-donating groups such as methoxy and hydroxy substituents at the phenyl ring linked at the C4 position of the pyran ring, enhancing the antibacterial characters of these compounds. An impeccable correlation exists between experimental results and the observed POM/DFT/docking calculations. The nature of substituents on the phenyl ring involved special effects on the antimicrobial results. The basic skeleton of pyranopyrimidine-based enaminonitrile and substituents at the phenyl ring are vital for potent antibacterial results with increased penetration effect on the bacterial cell wall. The hydrolysis of nitrile group into amide permitted intramolecular interaction, causing a precarious pattern for antibacterial –NH, HO-pharmacophore sites (Scheme 1). Besides, –O C–NH–C O as an antiKinase pharmacophore site was theoretically appraised by POM combinatorial analysis. The hydrolysis of a nitrile group to the corresponding acid led to the accomplishment of an N,O-bidentate site benefit for the future design of therapeutic materials.62
Scheme 1. Synthesis and antibacterial results of tetrahydro-2H-pyranopyrimidines.
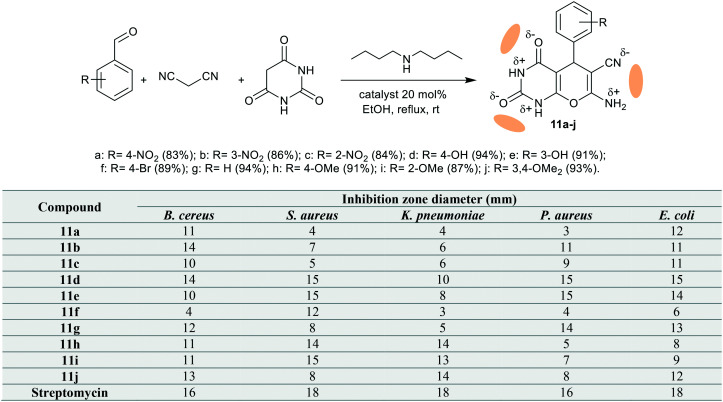
Bhat et al.63 have also prepared a series of bicyclic pyranopyrimidines 11a–j (Scheme 2) under catalytic conditions using dibutyl amine in refluxing ethanol. The antibacterial results demonstrated that compounds 11b, 11d (14 mm), 11g (12 mm), 11h, 11i (11 mm), and 11j (13 mm) revealed a broad spectrum with very strong potency against B. cereus (ATCC-14579) species. Also, compounds 11d, 11e (15 mm), 11f (12 mm), 11h (14 mm), and 11i (15 mm) presented strong potency against S. aureus (NCTC-7447) species. In addition, the broadest antibacterial activities were recorded for compounds 11h, 11i, and 11j (13–14 mm) against K. pneumoniae (UC57), and compounds 11d, 11e, and 11g (14–15 mm) against P. aureus (ATCC 27853) and E. coli (ATCC 14169) species relative to the results of Streptomycin (16–18 mm). The introduction of a bicyclic pyranopyrimidine-based enaminonitrile system is favored for potent antibacterial activities since these atoms have NHδ+ and Nδ− pharmacophores, leading to established activities. The theoretical studies revealed that the introduction of (O C–NH–C O) as an antikinase pharmacophore is recommended for future research.
Scheme 2. Synthesis of bicyclic pyranopyrimidines.
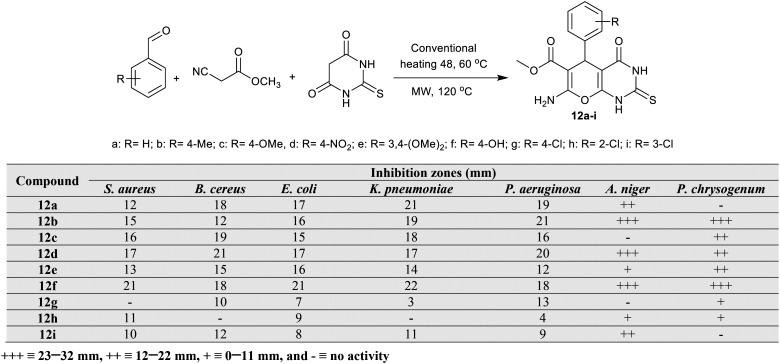
A series of methyl tetrahydropyranopyrimidine-carboxylates 12a–i were synthesized by Bhat et al.64via multicomponent reactions as shown in Scheme 3 under either conventional heating or microwave irradiation conditions. These compounds have been formerly synthesized under assorted conditions.65–68 The method for the preparation of methyl carboxylates 12a–i controls the produced yields based on the reaction temperature and time, in which the reactions under conventional conditions produced products with 69–86% yields at 48 °C, while the yields slightly increased (71–87%) at 60 °C and 77–94% under microwave irradiation conditions that involved reduced reaction time. Compounds 12a–i were in vitro estimated as antimicrobial agents by the disc diffusion method toward the variety of microbial species, in which the compounds, in general, indicated relatively respectable antimicrobial potency. Thus, compounds 12a–f displayed supreme antimicrobial potency toward S. aureus, B. cereus, E. coli, K. pneumoniae, and P. aeruginosa species. Analogous tetrahydropyranopyrimidine 12f (R = 4-OH) demonstrated superior antifungal activity toward A. niger and P. chrysogenum species. In particular, compounds 12d (inhibition zone diameter = 17 mm) and 12f (21 mm) are the most potent against S. aureus species, while compounds 12c (19 mm) and 12d (21 mm) presented the highest potencies against B. cereus species relative to the antibacterial standard Streptomycin. In addition, compound 12f (21 mm) displayed potent antibacterial influence on E. coli species with potency activities of compounds 12a (21 mm) and 12f (22 mm) against K. pneumoniae species, and compounds 12b (21 mm) and 12d (20 mm) against P. aeruginosa species. Furthermore, compounds 12b, 12d, and 12f are the most effective antifungal agents against A. niger species, accompanied by the most effective potency recorded by compounds 12b and 12f against P. chrysogenum species (23–32 mm) relative to the standard antifungal Mycostatin. The SARs postulated the high activities of the compounds of these series for inhibiting the microbial cell growth, whether the type of substituents and any position of linkage at the phenyl ring at C4 are not directly affected by the compound potency. As the results have nearly similar potency, it is difficult to incorporate the substituents' effect to detect the structure–activity for the potency results. It was preferred to introduce the hydroxy substituent at the para position of the phenyl ring for effective antimicrobial results.64
Scheme 3. Synthesis of methyl tetrahydropyranopyrimidine-carboxylates.
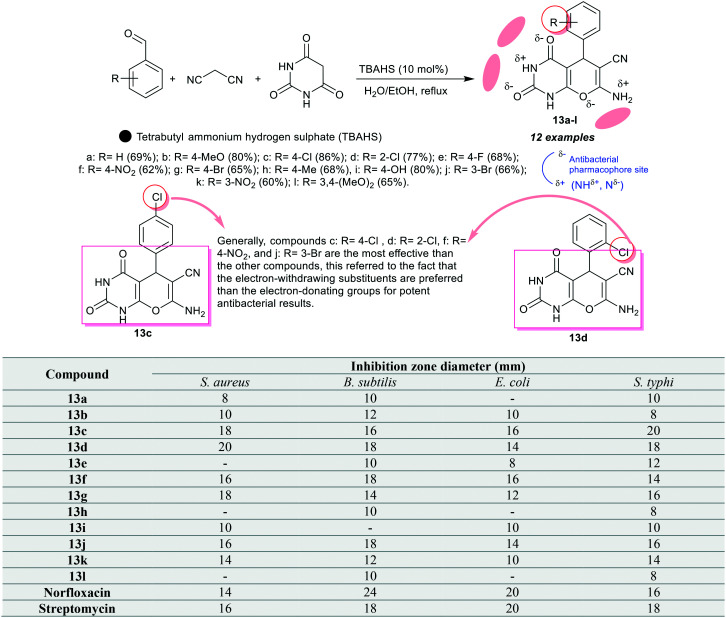
Angulwar et al.69 also designed a series of pyranopyrimidines bearing enaminonitrile moiety 13a–l through a green protocol involving a multicomponent one-pot synthetic route under TBAHS (10 mol%) catalytic conditions. The results of the antibacterial activity estimated for this series of compounds 13a–l revealed that compounds 13c (inhibition zone diameter = 18 mm), 13d (20 mm), 13f (16 mm), 13g (18 mm), and 13j (16 mm) displayed equal or improved results than the antibiotics Norfloxacin (14 mm) and streptomycin (16 mm) against S. aureus (Gram +ve) species. In addition, compounds 13d, 13f, and 13j showed broad spectrum activity against B. subtilis (Gram +ve) strains with inhibition zones at 18 mm; these results match with that of streptomycin (18 mm). Moreover, compounds 13c and 13f displayed the broadest spectrum activity against E. coli (Gram –ve) strains with inhibition zone diameters of 16 mm, while compounds 13c (20 mm) and 13d (18 mm) are the most potent antibacterial agents against S. typhi (Gram –ve) strains with potency stronger than Norfloxacin (16 mm) and equivalent or higher than that of Streptomycin (18 mm). The SARs as mentioned in Scheme 4 demonstrated the value of the bicyclic ring along with the substituents' nature, while the type of these compounds are considered as active compounds against the bacteria of the type of Gram-positive species.
Scheme 4. Synthesis, antibacterial results, and SARs of pyranopyrimidines.
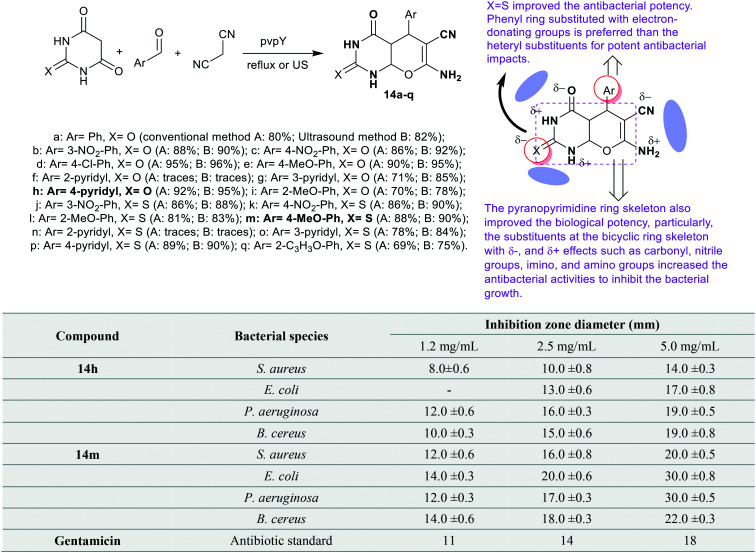
Mokhtari et al.70 have applied the synthesis of bicyclic pyranopyrimidine analogs 14a–q by one-pot multicomponent reactions under catalytic conditions using poly(4-vinyl pyridine) in a water and ethanol mixture. The reactions gave the target products 14a–q with improved yields using ultrasound irradiation in a conventional method. Besides, the product yield depended on the nature of aryl substituents linked to the aldehyde, and the reactivity of barbituric or thiobarbituric acids (Scheme 5). The antibacterial activity was estimated for bicyclic pyranopyrimidines 14h and 14m against a variety of bacterial species, particularly, “S. aureus, B. cereus, P. aeruginosa, and E. coli”. Thus, both compounds displayed a broad spectrum of inhibition of bacterial growth except for compound 14h against E. coli strains. At different concentrations (1.2, 2.5, and 5.0 mg mL−1), compound 14m is the most potent against E. coli and B. cereus species with potency recorded at (14 ± 0.3, 20 ± 0.6, and 30 ± 0.8 mm), and (14 ± 0.6, 18 ± 0.3, and 22 ± 0.3 mm), respectively, better than the standard Gentamicin (11, 14, and 18 mm). At the higher concentration (5.0 mg mL−1), the potent activity of compound 14m was noticed at 30 ± 0.5 mm against P. aeruginosa species. Also, compound 14h is more commonly effective than the standard antibiotic against P. aeruginosa 12 ± 0.6, 16 ± 0.3 and 19 ± 0.5 mm) than its activity against other species. The results also indicated high efficiency of compounds 14h (19 ± 0.8 mm) against B. cereus species at the higher concentration (5.0 mg mL−1). Generally, the inhibition efficiency of different bacterial growth was more efficaciously affected by compound 14m than the influences of compound 14h, although there was increased activity in some cases of compound 14h than the standard, Gentamicin. Thus, the incorporation of the thione group “X=S” improved the antibacterial potency of the carbonyl group. Consistently, the phenyl ring substituted with electron-donating groups is preferred over the heteroaryl substituents for potent antibacterial impacts.
Scheme 5. Synthesis and SARs of bicyclic pyranopyrimidines.
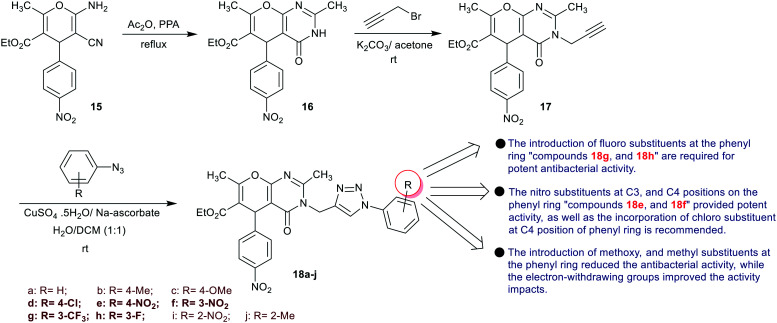
Maddila et al.30 designed the synthesis of triazole-based pyranopyrimidine analogs 18a–j through multi-step synthesis. Therefore, the reaction of enaminonitrile 15 with acetic anhydride, followed by cyclization in the presence of PPA, giving pyranopyrimidine 16. Further, N-alkylation of 16 with alkyne bromide yielded the desired propynyl-pyrano-pyrimidine 17. The triazole ring cyclization step gave a series of triazole-based pyranopyrimidine derivatives 18via reactions of the aryl azides with compound 17 (Scheme 6). The antibacterial activity of triazole-based pyrano-pyrimidines 18a–j was in vitro assessed by the disc diffusion technique against four bacterial species at two concentrations (100 and 50 μg mL−1). The results verified that compounds 18d (13.0 mm), 18e (12.5 mm), and 18g (12.0 mm) are the most potent agents against B. subtilis species at higher concentration (Amoxicillin, 15.5 mm). Compounds 18d (15.0 mm), 18e (14.0 mm), 18f (16.0 mm), and 18h (14.5 mm) displayed the broadest activity against S. aureus species (Amoxicillin, 17.5 mm). Also, compounds 18d (16.5 mm), 18e (14.0 mm), 18g (15.5 mm), and 18h (14.5 mm) presented broad activities against K. pneumoniae species (Amoxicillin, 18.0 mm). Furthermore, compounds 18d (17 mm), 18e (14.5 mm), 18f (15.0 mm), 18g (14.0 mm), and 18h (15.5 mm) gave broad activities against Escherichia coli species (Amoxicillin, 19.5 mm). The antifungal activity by agar diffusion technique was estimated against C. albicans and A. flavus species for triazole-based pyranopyrimidines 18a–j. The compounds revealed potent activities against both fungal species, in which compounds 18d (17.5 mm) and 18f (16.5 mm) have the broadest potential against C. albicans species at the higher concentration (100 μg mL−1) relative to Ketoconazole (20.5 mm). In addition, compounds 18d (16.0 mm) and 18h (14.5 mm) revealed the most potent activities against A. flavus species (Ketoconazole, 18.5 mm). It was a significant statement that the pyranopyrimidine core bearing the triazole ring provided potent antifungal results. Also, the substituents at the phenyl ring are preferred to be in the type of electron-withdrawing characteristics for potent antifungal results.30
Scheme 6. Synthesis of triazole-based pyranopyrimidine series.
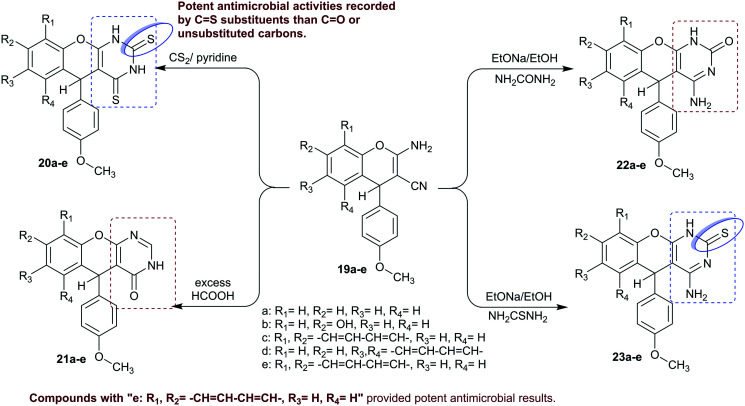
The reactivity of the synthetic precursors, 4H-chromene with enaminonitrile moiety, “compounds 19” were utilized for the synthesis of chromenopyrimidin-one(thione) derivatives 20–23a–e (Scheme 7) as reported by Kamdar et al.47 thus, the reactions of compounds 19 with each of carbon disulfide, formic acid, urea, and thiourea yielded the corresponding chromenopyrimidines 20–23a–e in commendable yields. The antimicrobial activity of the investigated chromenopyrimidines 20–23a–e was in vitro evaluated against four bacterial and one fungal species by the estimation of the MIC values in μg mL−1. The results specified good potency of compounds 20e and 21e against the bacterial species, e.g., S. aureus [ATCC-25923], S. pyogenes [MTCC-443], E. coli [ATCC-25922], and P. aeruginosa [MTCC-441] species (MIC = 62.5–125 μg mL−1) relative to ampicillin (MIC = 100–250 μg mL−1). Also, the most effective antimicrobial potency was estimated for compound 21e (MIC = 62.5 μg mL−1) against E. coli, 20e, 21d, 21e, 22b, 22e, and 23e (MIC = 62.5 μg mL−1) against P. aeruginosa; compounds 20b, 20e, 23b, and 23e (MIC = 62.5 μg mL−1) against S. pyogenes; compounds 20b, 20e, 22b, 23b, and 23e (MIC = 62.5 μg mL−1) toward S. aureus; and compounds 20c and 21e (MIC = 125 μg mL−1) against A. niger [MTCC-282] species.
Scheme 7. Synthesis of tricyclic pyranopyrimidines.
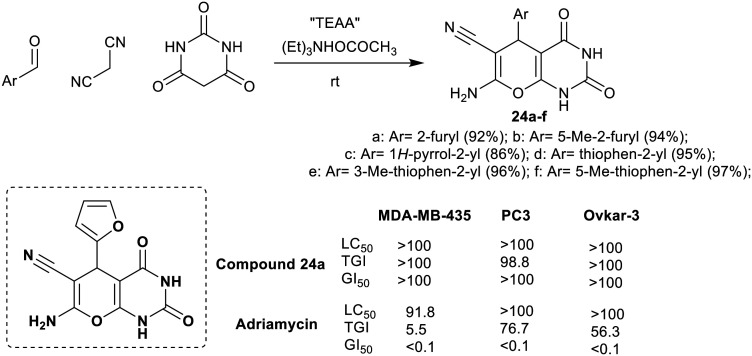
The method of Paliwal et al.50 was designed to synthesize a series of pyranopyrimidines 24a–f under catalytic conditions. Thus, the method was proceeded using triethylammonium acetate “a volatile buffering agent” as an efficient catalyst. One-pot three-component reactions of aryl aldehydes with malononitrile and barbituric acid yielded the target products with tremendous yields (86–97%) although the aryl aldehydes are heteroaryl type (Scheme 8).
Scheme 8. Synthesis of bicyclic pyranopyrimidines.
The antibacterial potency of pyranopyrimidines 24a–f was assessed using the disc diffusion assay. The results indicated potent activities against all bacterial species, in particular, compounds 24a–f (Scheme 8) demonstrated the most effective potency against the growth of K. pneumoniae (16–22 mm) and S. typhi (18–22 mm) species. Besides, compounds 24c–f are the most potent (12 mm) against C. freundii ATCC 8090 and K. pneumoniae ATCC 15380 (20–22 mm) species. Compounds 24a and 24b (14 mm) presented the most potent activities against B. megaterium ATCC 12872 species. In addition, compounds 24c–f displayed the broadest activities against E. coli ATCC 25922 (14–16 mm), S. typhi ATCC 19430 (20–22 mm), and P. aeruginosa ATCC 27853 species (18–22 mm).50 It was noteworthy that the integration of aryl substituents at the C4 position of pyranopyrimidine skeleton delivered potent antibacterial results. The pyrrole and thiophene substituents are required for more potent results than the furan substituents. The furyl substituents are effective only in the case of their improved activity against B. megaterium species; thus, the type of the bacterial species also controls the estimated mechanism of the inhibition of bacterial growth.50
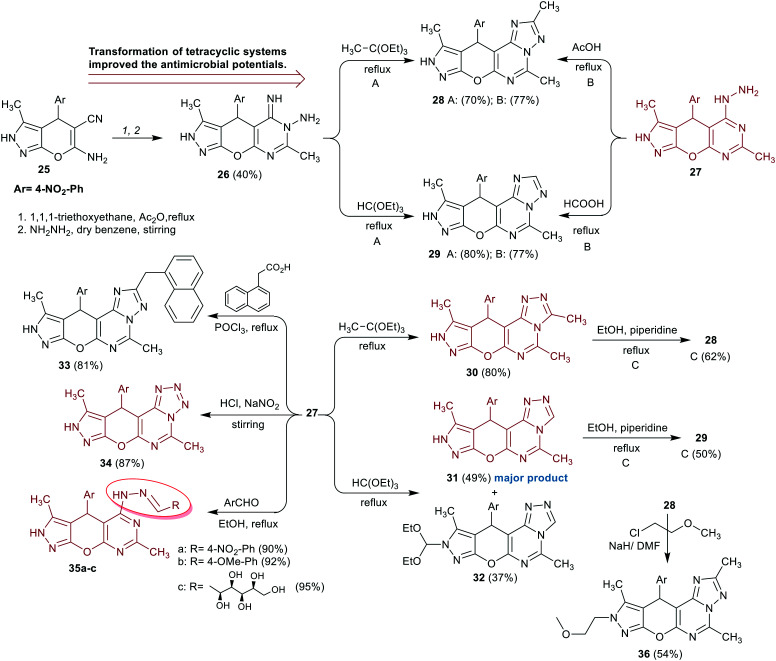
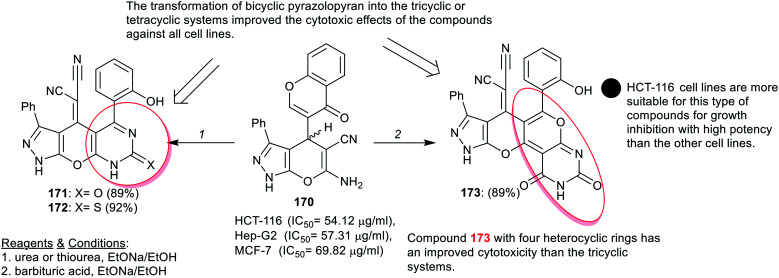
Shamroukh et al.71 titled magnificent synthetic routes for the preparation of tricyclic pyranopyrimidine “compound 26” based on the reactivity of bicyclic pyrazolopyran with enaminonitrile moiety (compound 25), and pyrazolopyranopyrimidine linked to hydrazide moiety in the synthesis of polycyclic systems “compounds 28–36”. Compounds 28 and 29 were efficiently synthesized from either compound 26 or 27 by reactions of 26 with 1,1,1-triethoxyethane or triethyl orthoformate or reactions of compound 27 with acetic or formic acids under heating conditions. Another route was investigated for the synthesis of compounds 28 and 29 from compounds 30 and 31, respectively, by heating in ethanol containing basic piperidine. The reactivity of hydrazide 27 was investigated in the reactions with 1,1,1-triethoxyethane, triethyl orthoformate, 2-(naphthalen-1-yl)acetic acid, diazotization, and reactions with aldehydes to yield compounds 30–35, respectively. Also, the reactivity of compound 28 in the reaction with 1-chloro-2-methoxyethane led to N-methoxyethyl analog 36 in moderate yield (Scheme 9).
Scheme 9. Synthesis of tri- and polycyclic pyranopyrimidines.
The antimicrobial activity of tri- and polycyclic pyranopyrimidines 26–35 was assessed by the disc diffusion method. The results demonstrated that compounds 27, 30, 31, 35c, and 34 displayed distinct slight activity against both types of bacterial species, namely, E. coli and B. subtilis. In addition, compounds 30 and 31 displayed the broadest activities against Candida albicans yeast species, while compounds 27, 28, 30, 31, 34, and 35c estimated moderate to strong sensitivity to the growth of Aspergillus niger fungal species. Generally, compound 30 revealed the broadest antifungal activity relative to the tested compounds and the standard antibiotic “Streptomycin”. All the tested compounds are active for growth inhibition of E. coli species and only compounds 27, 30, 31, 35c, and 34 have a slight effect on the bacterial growth of B. subtilis species. Compound 30 displayed potent activity against the growth of the yeast “C. albicans” and A. niger species. The scaffold [1,2,4]triazolo[4,3-c]pyrimidine “30” presented enhanced antibacterial results compared to [1,2,4]triazolo[1,5-c]pyrimidine “28 and 29” or tetrazolo[1,5-c]pyrimidines “34”. The conversion of compounds 26 and 27 into compounds 28 and 29 slightly affected the antibacterial potency, nevertheless enhancing the antifungal potency with surprising degrees (Scheme 9).71
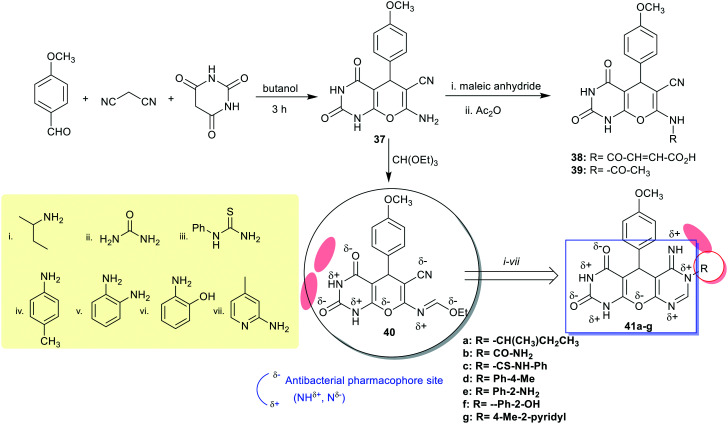
Recently, Abd El-Sattar72 has designed the synthesis of pyranodipyrimidine-diones 41a–g through multi-step synthesis. Thus, multicomponent one-pot reactions of the aldehyde with malononitrile and barbituric acid gave the predicted bicyclic pyranopyrimidine 37, which upon reaction with triethyl orthoformate and subsequent reactions with alkyl and aryl amines afforded the final products pyranodipyrimidine-diones 41a–g (Scheme 10).
Scheme 10. Synthetic routes for the multi-step synthesis of pyranodipyrimidine-diones.
The antimicrobial activity of pyranopyrimidines 37–40 and pyranodipyrimidine-diones 41a–g was assessed against the diverse four bacterial and two fungal species. The results specified a broad spectrum for most of the compounds in these series. Thus, compounds 41a, 41b revealed the most potent activities against B. subtilis species with inhibition zone diameters at 26.0 ± 0.32 μm (MIC = 0.097 μg mL−1) relative to Ampicillin (32.4 ± 0.58 μm, MIC = 0.45 μg mL−1). Also, these compounds revealed broad spectrum activity against S. aureus species (20.8 ± 0.53 and 21.7 ± 0.77 μm, MIC = 1.562 and 0.781 μg mL−1, respectively). Compound 41e (R = Ph-2-NH2) is the most potent antibacterial agent against E. coli species (18.1 ± 0.33 μm, MIC = 0.781 μg mL−1), similar to the result of Gentamicin (21.3 ± 0.58, MIC = 3.68 μg mL−1), and compound 41g revealed the highest potency against P. aeruginosa species with inhibition zone diameter of 17.3 ± 0.91 μm (MIC = 3.125 μg mL−1); this potency precisely matched with that recorded for the antibiotic standard (Gentamicin = 17.3 ± 0.63 μm, MIC = 15.36 μg mL−1). Moreover, compound 41g (R = 4-Me-2-pyridyl) is the most active antifungal agent against A. niger species (17.2 ± 0.56 μm, MIC = 25.0 μg mL−1) but still with less potency than the standard, Amphotericin B (26.3 ± 0.3 μm, MIC = 0.781 μg mL−1). Thus, the transformation of enaminonitrile “pyranopyrimidines” into the tricyclic systems (compounds 41a–g) “pyranodipyrimidine” is beneficial for enhanced antimicrobial results since this transformation introduced antibacterial pharmacophore sites that are responsible for improved biological profiles.72
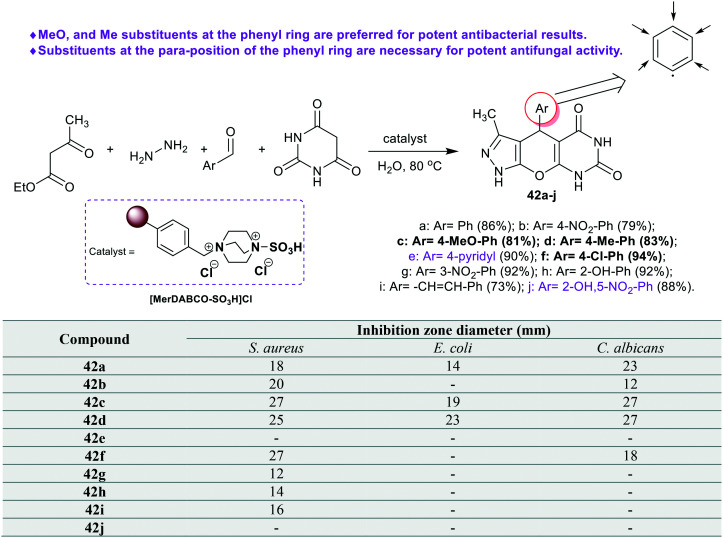
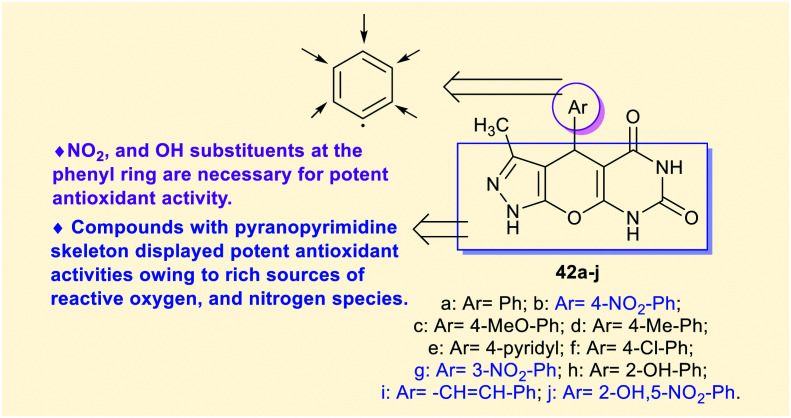
Patil et al.73 developed the synthesis of tricyclic pyrazolo-pyranopyrimidine-diones 42a–j (Scheme 11) by the action of the [MerDABCO-SO3H]Cl catalyst “prepared from Merrifield resin, 1,4-diazabicyclo[2.2.2]octane, and sulfonic acid”. The reactions were achieved in a one-pot procedure to improve the product yields using a green procedure. Agar well diffusion techniques were used to in vitro estimate the antimicrobial activity of pyrazolo-pyranopyrimidine-diones 42a–j toward S. aureus, E. coli, and C. albicans bacterial, and fungal species. Compounds 42a (18 mm), 42b (20 mm), 42c (27 mm), 42d (25 mm), 42f (27 mm), 42g (12 mm), 42h (14 mm), and 42i (16 mm) displayed good activity, and compounds 42e and 42j are inactive agents against S. aureus species. Thus, the methoxy, methyl, and chloro-phenyl substituents are necessary for potent antibacterial results against S. aureus species. The results against E. coli indicated broad activity for compounds 42a (14 mm), 42c (19 mm), and 42d (23 mm), along with inactive influences for the rest of the compounds. In general, the introduction of methoxy and methyl substituents at the phenyl ring is beneficial for potential antibacterial results of both the bacterial species. Also, compounds 42a (23 mm), 42b (12 mm), 42c (27 mm), 42d (27 mm), and 42f (18 mm) presented good antifungal activities against C. albicans species along with no activity for the other compounds. The substituents at the para position of the phenyl ring are preferred for potent antifungal activity than the ortho- or meta-substituents, or the heteroaryl ring at the C4 position of the bicyclic system.
Scheme 11. Synthesis of pyrazolo-pyranopyrimidine-diones.
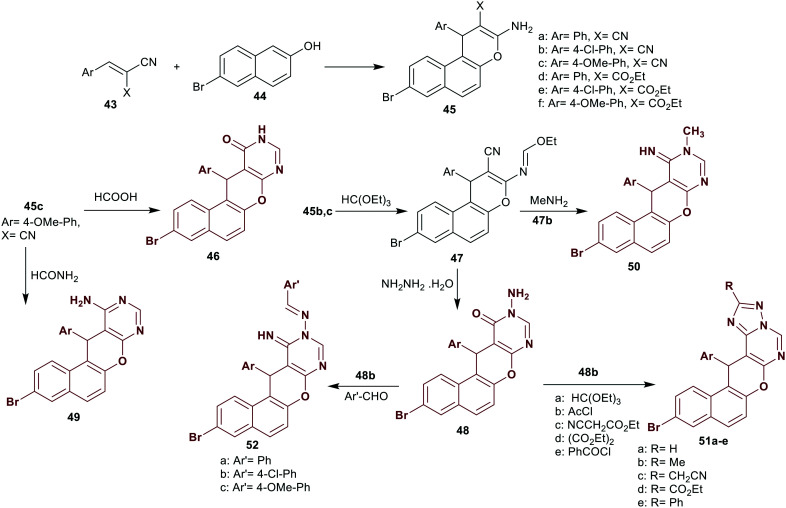
Bedair et al.28 have designed a proficient procedure for the synthesis of polycyclic benzochromeno-pyrimidines 46–52 starting from enaminoesters and enaminonitriles of 1-aryl-8-bromo-1H-benzochromenes 45b, c as reactive precursors. Therefore, compound 45c reacted with formic acid and formamide to yield the respective benzochromeno-pyrimidines 46 and 49, respectively. The reaction of compounds 45b, c with triethyl orthoformate, followed by a reaction with methylamine, gave the desired benzochromeno-pyrimidine 50. Compound 47 reacted with hydrazine hydrate to give the corresponding N-substituted amine 48, which reacted with aryl aldehydes to give Schiff bases 52. Also, compounds 48 reacted with each of triethyl orthoformate, acetyl chloride, ethyl cyanoacetate, diethyl oxalate, and benzoyl chloride to give compounds 51a–e, respectively (Scheme 12).
Scheme 12. Synthetic routes for the preparation of polycyclic benzochromenopyrimidines.
In particular, compounds 46, 48b, 49, 50, 51a–e, and 52b, c were assessed in vitro as antimicrobial agents by the disc diffusion assay. The results distinctively specified that most of the compounds displayed broad spectrum of antifungal activities with inhibition zone diameters at 17–20 mm against Aspergillus ochraceus species relative to the standard Mycostatine (22 mm). Compounds 51a, 51d, 52b, and 52c are the most potent compounds against A. ochraceus species, while compound 51d is the most potent against P. chrysogenum species (22 mm) with broad-spectrum activity to the other compounds with potency ranging from 13 to 20 mm. The results are in comparison to that of the standard Mycostatine with an inhibition zone diameter of 24 mm. Also, the antibacterial activities were estimated against four bacterial species, namely, S. aureus (NCTC-7447), B. cereus (ATCC-14579), S. marcescens (IMRU-70), and P. mirabilis (NTCC-289). The results declared that all the compounds displayed broad-spectrum activities relative to the standard antibiotic, ampicillin. In particular, compound 51c is the most active against S. aureus species (25 mm), compounds 48b and 51d (23 mm) are the most potent against B. cereus species, compounds 51c and 51d (25 mm) are the most potent against S. marcescens species, and compounds 51b (23 mm), 51c (23 mm), 51d (24 mm), and 52b (23 mm) are the most potent against S. marcescens species relative to Ampicillin (25–26 mm) for inhibiting the growth of the bacterial species. The electron-withdrawing character of the chlorine atom at the phenyl ring of Schiff base 52b is preferred over the electron-donating substituent “compound 52c”. The pyranopyrimidine skeleton provided potent antibacterial potency for the tested compounds against all the bacterial and fungal species. The incorporation of the triazole ring improved the antifungal and antibacterial potency since the substituents at the triazole ring have an impact on the activity with the preferred effectiveness of the ester group on the growth inhibition of the microbial species (Scheme 12).28
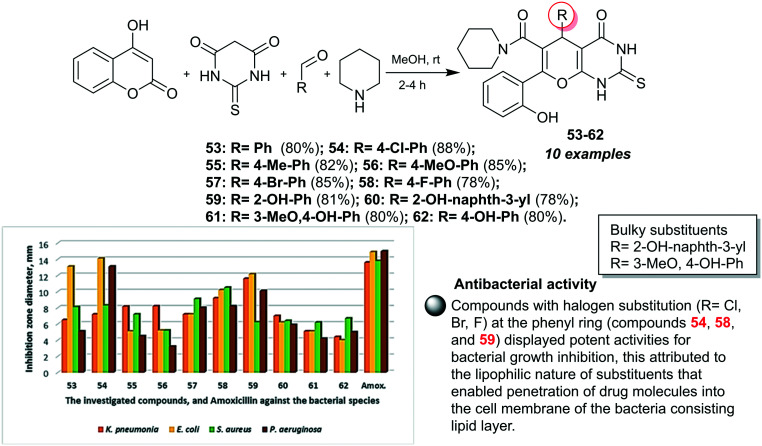
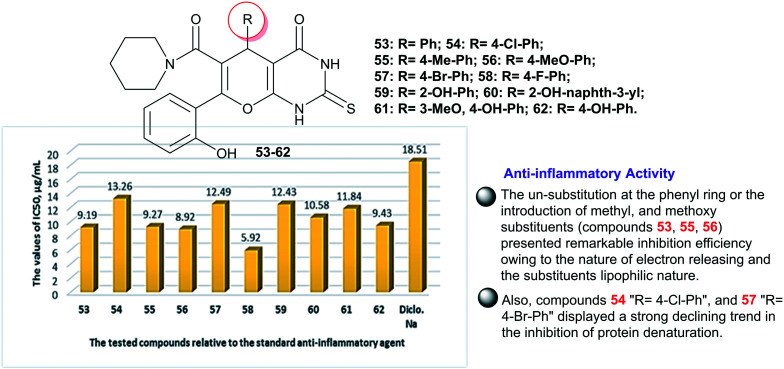
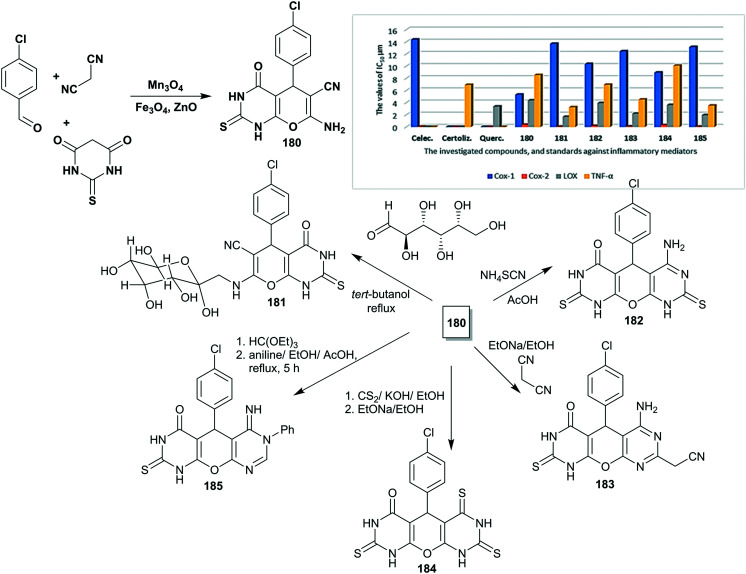
Naik et al.14 designed an eco-friendly procedure for the preparation of 2-thioxo-tetrahydropyrano-pyrimidinones 53–62via four-component one-pot reactions under non-catalytic conditions with predominately good yields (78–88%) (Fig. 3). The antibacterial activity of compounds 53–62 was in vitro assessed by the agar well diffusion performance at three concentrations of the tested samples (0.1, 0.05, and 0.025 mg mL−1).14 A notable antibacterial activity for these compounds was recorded against four diverse bacterial species, namely, K. pneumoniae, E. coli, P. aeruginosa, and S. aureus species. In this course, the broadest spectrum activity was recorded for compound 54 (R = 4-Cl-Ph) against E. coli (14.1 ± 0.36 mm) and P. aeruginosa (13.1 ± 0.28 mm) relative to Amoxicillin (14.9 ± 0.36 mm and 15.0 ± 0.11 mm, respectively). Also, compound 53 (R = Ph) displayed good activity against E. coli species (13.1 ± 0.28 mm). In addition, compound 59 (R = 2-OH-Ph) is the most active agent against K. pneumoniae species (11.6 ± 0.32 mm) with excellent affinity relative to Amoxicillin (13.6 ± 0.57 mm). Compound 58 (R = 4-F-Ph) presented good activity against S. aureus species (10.5 ± 0.5 mm) with the most active impact relative to Amoxicillin (13.8 ± 0.28 mm). It was noticed that compounds 55 (R = 4-Me-Ph) and 56 (R = 4-MeO-Ph) presented diminished activities, while the most reduced activities were recorded for compounds 61 (R = 3-MeO,4-OH-Ph) and 62 (R = 4-OH-Ph). In detail, the results at 0.1 mg mL−1 demonstrated that compounds 58 (9.2 ± 0.25 mm) and 59 (11.6 ± 0.32 mm) are the most potent against K. pneumoniae species, accompanied by lower potency for compounds 61 and 62. Compounds 1 (13.1 ± 0.28 mm) and 59 (12.16 ± 0.15 mm) are the most potent against E. coli species in line with lower potency for compounds 55, 56, 61, and 62. Also, the superior potent antibacterial agent toward S. aureus species was recorded for compounds 57 (9.1 ± 0.15 mm) and 58 (10.5 ± 0.5 mm), while the remnant compounds exhibited moderate to good activities. Compounds 54 (13.1 ± 0.28 mm) and 59 (10.1 ± 0.15 mm) revealed the most potent activity against P. aeruginosa species, which was followed by lower activity for compounds 56, 60, 61, and 62. The SARs demonstrated that reduced antibacterial activities were recorded for compounds with electron-donating groups (R = OMe and OH), which are responsible for this impact; however, compounds with bulky substituents showed diminished activity owing to the steric hindrance effects that affect the binding site of the biological targets.14
Fig. 3. Synthesis and SARs of tetrahydropyranopyrimidinones as antibacterial agents.
In addition, the agarose gel electrophoresis performance was employed for compounds 53–62, in which DNA cleavage is particularly dependent on the separation of compounds based on their structures under the applied electric field. The results indicated that the compounds in this series can cleave the helical strand of the DNA molecule. The utility of a lower concentration (nearly 100 μg mL−1) of these compounds 53–62 led to total cleavages of the DNA molecule based on the ability of pyrimidine ring interaction with DNA with progressive responsiveness concerning the DNA molecule. The mechanism of this potency was proposed as these ligands inhibited the growth of diverse pathogenic bacterial species through DNA cleavage.14
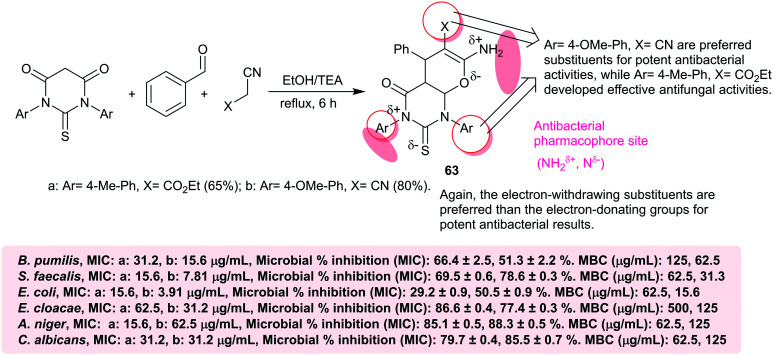
The identical approach for the multicomponent synthesis of bicyclic triaryl-substituted-pyrano-pyrimidines 63a, b was designed by Abd-Elaziz et al.10 by applying a simple green procedure under noncatalytic conditions. These compounds were evaluated as antimicrobial agents by the agar well diffusion method against B. pumilis, E. faecalis, E. coli, and E. cloacae as pathogenic bacterial species, and multicellular fungal species such as A. niger and unicellular fungal species, i.e., C. albicans. The results specified that both compounds are active against all bacterial and fungal species; in particular, compound 63b is more potent than compound 63a against B. pumilis, S. faecalis, and E. coli species with inhibition zone diameters at 16.3 ± 0.6, 21.7 ± 0.6, and 20.3 ± 0.6 mm, respectively. Contrariwise, compound 63a is more potent than compound 63b against E. cloacae, A. niger, and C. albicans species with zone diameters of 21.7 ± 0.6, 21.7 ± 0.6, and 12.3 ± 0.6 mm, respectively (c.f. Scheme 13). Principally, compound 63b (21.7 ± 0.6 mm) is more potent than the standard penicillin G (17.3 ± 0.6 mm) against S. faecalis species, and has equal activity as that of ciprofloxacin against E. cloacae species (20.7 ± 0.6 mm). The research was intended to study the antimicrobial activities of this class of heterocycles including MIC and MBC/MFC, which were assessed by the microdilution approach using a 96-well microplate.
Scheme 13. Synthesis and SARs of bioactive hexahydropyranopyrimidines.
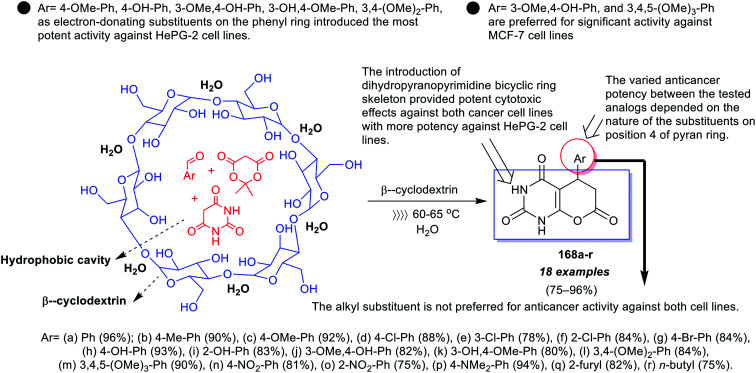
A series of pyranopyrimidinones 64a–q was efficiently synthesized under catalytic conditions of cyclodextrins (CDs) by one-pot three-component reactions (Scheme 14).29 The antibacterial activity of this series was in vitro assessed toward E. coli, P. aeruginosa, B. subtilis, and S. aureus species. The antifungal potency was also assessed toward C. albicans, A. flavus, and A. niger species. The results specified that compounds 64c, 64h, 64i, 64k, 64m, and 64p inhibited the bacterial growth. In particular, compound 64i demonstrated worthy activities against the entirely tested fungal strains. The most effective results against E. coli and P. aeruginosa strains were recorded for compounds 64h (MIC = 25 ± 0.25, 87.5 ± 0.47 μg mL−1), 64m (MIC = 75 ± 0.69, 25 ± 0.49 μg mL−1), and 64p (MIC = 25 ± 0.43, 25 ± 0.88 μg mL−1) with lower MIC values. Furthermore, compounds 64m (MIC = 25 ± 0.77 μg mL−1) and 64p (MIC = 25 ± 0.75 μg mL−1) displayed exceptional antibacterial activities against S. aureus species relative to ciprofloxacin (MIC = 50 ± 1.44 μg mL−1). The results of the activity against S. aureus verified that compounds 64c, 64i, 64m, and 64p displayed broad spectrum activity with maximum potency relative to ampicillin (MIC = 250 ± 2.99 μg mL−1). Also, a broad spectrum of activities was recorded for compounds 64i and 64p against B. subtilis species relative to ciprofloxacin (MIC = 50 ± 0.96 μg mL−1). The compounds revealed weak antifungal effects with MIC values ranging from 125 ± 0.28 to 237.5 ± 0.11 μg mL−1 against C. albicans species, 162.5 ± 0.40 to 225 ± 1.02 μg mL−1 against A. niger, and 150 ± 0.11 to 225 ± 0.69 μg mL−1 against A. flavus species.
Scheme 14. Multicomponent synthesis and SARs of pyranopyrimidines.
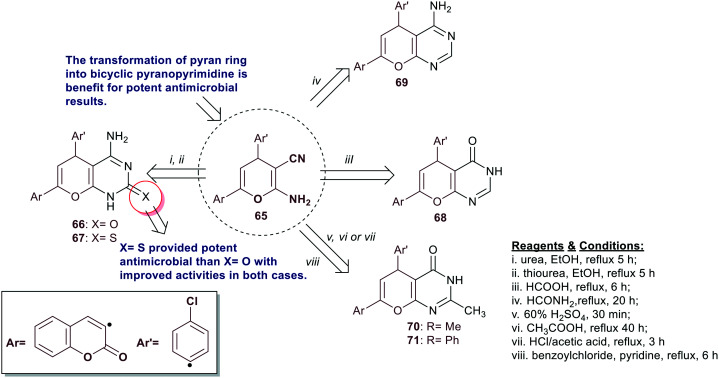
Fayed et al.74 reported the stepwise synthesis of bicyclic pyranopyrimidines 66–71 utilizing enaminonitrile 2 as a reactive synthetic precursor. Thus, reactions of pyran-based enaminonitrile 65 with each of urea, thiourea, formic acid, formamide, acetic acid, or benzoyl chloride yielded the targeted bicyclic products 66–71, respectively (Scheme 15). The antibacterial activity was in vitro assessed against S. pneumoniae, S. epidermidis, S. aureus, E. coli, K. pneumoniae, and S. paratyphi species. Compounds 66 (10 mm) and 70 (14 mm) displayed broad spectrum activity against S. pneumoniae, while the other compounds are inactive. Also, the compounds are inactive against S. epidermidis species. Accordingly, only compounds 66, 68, and 70 displayed moderate to potent activity toward S. aureus strains with inhibition zone diameters of 18 ± 0.30, 10 ± 0.15, and 10 ± 0.11 mm, respectively. In addition, compounds 67–69 and 71 presented moderate influences for bacterial growth inhibition against E. coli species with inhibition zone diameters ranging from 10 ± 0.19 to 13 ± 1.05 mm relative to the standard Amoxicillin (24 ± 0.05 mm). Compounds 67 (12 ± 0.15 mm), 69 (14 ± 1.11 mm), and 70 (20 ± 0.15 mm) are the most effective against K. pneumoniae species than the impact of the compounds on the other bacterial species relative to the standard Amoxicillin (15 ± 0.18 mm). The antifungal activity was also assessed against P. italicum and A. fumigatus species. The results indicated broad spectrum activity for compound 67 (16 ± 0.28 mm) against P. italicum species relative to the standard Miconazole nitrate (17 ± 0.25 mm), along with inactive influences on the other compounds. All the compounds displayed potent activity against A. fumigatus species except compound 71 with antifungal activities ranging from 10 ± 0.23 mm to 18 ± 0.01 mm (Miconazole nitrate, 19 ± 1.2 mm). The MIC results specified that compound 66 (MIC = 7.8 ± 0.15 μg mL−1) acts with the same efficiency as that of the standard Amoxicillin (MIC = 7.8 ± 0.25 μg mL−1) against S. pneumoniae along with MIC values at 31.3 ± 0.21, and 15.6 ± 0.27 μg mL−1 against S. aureus and A. fumigatus species, respectively. The most effective MIC values were recorded by compound 67 at 0.40 ± 15.3 and 0.46 ± 15.5 μg mL−1 against S. epidermidis and A. fumigatus species, respectively. The DAN gyrase inhibitory assay against E. coli revealed a comparative value for compound 67 at IC50 = 4.91 ± 0.20 nM to the result of Novobiocin (IC50 = 4.52 ± 0.10 nM) against DNA gyrase B inhibition, along with weak activities for compounds 66 and 67 ranging from IC50 = 237 ± 1.02 nM to 341 ± 1.45 nM for inhibiting Topoisomerase IV.74
Scheme 15. Synthesis of bicyclic pyranopyrimidine derivatives.
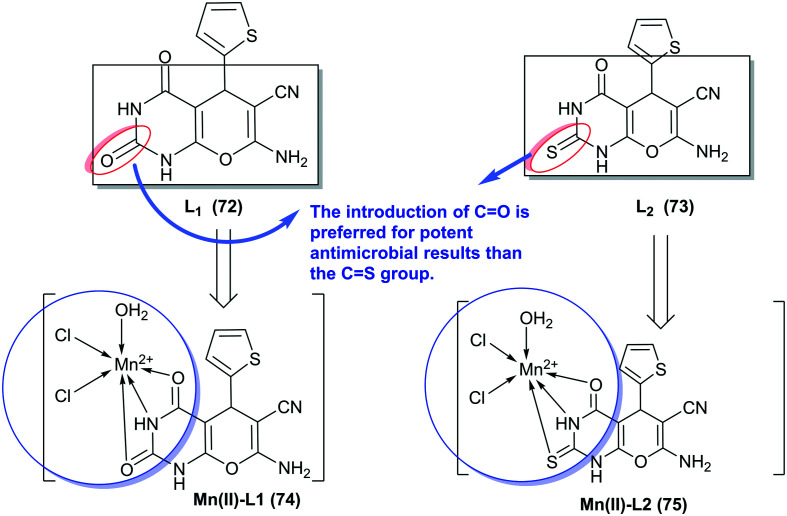
Shehab and El-Shwiniy75 have prepared the two ligands “bicyclic pyranopyrimidines bearing enaminonitrile moiety” 72 and 73 by multicomponent one-pot reactions of thiophene-2-carbaldehyde with malononitrile and (thio)barbituric acids under catalytic conditions of manganese(iii) oxide. These ligands were utilized for the synthesis of Mn(ii)-L1 (74) and Mn(ii)-L2 (75) complexes by stirring with an aqueous solution of manganese chloride in acetone (Scheme 16). The antimicrobial activity was in vitro assessed against fungal “A. fumigatus and G. candidum”, and bacterial species “S. aureus and E. coli”. The results, in general, demonstrated improved antimicrobial activities for the metal complexes than their free ligands. Predominantly, Mn(ii)-L1 (74) complex displayed the broadest activities with MIC values ranging from 1.25 to 2.5 mg mL−1, and MIC values from 1.25 to 5.0 mg mL−1 for Mn(ii)-L2 (75) complex against all the tested species. It can be settled that the introduction of the ketone group at the C2 position is more privileged than the thione group for potent results of antimicrobial activities.
Scheme 16. Synthesis of Mn(ii)-L1 and Mn(ii)-L2 complexes.
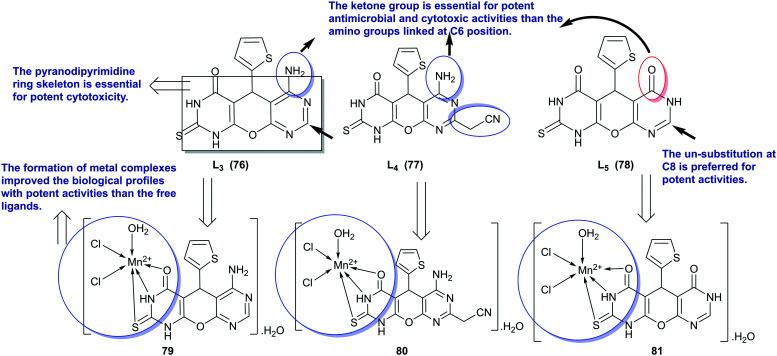
Mn(ii)-complexes 79–81 were synthesized by the treatment of pyranodipyrimidine ligands L3–L5 “76–78”25 in acetone with a solution of manganese(ii) chloride tetrahydrate in water at room temperature (Scheme 17). Therefore, pyranodipyrimidine ligands L3–L5 “76–78” and Mn(ii)-complexes 79–81 were in vitro assessed as antimicrobial agents against A. fumigatus and G. candidum fungal species, as well as S. aureus and E. coli bacterial species. The metal complexes demonstrated improved antimicrobial activities than its free ligand with high potency. In accordance, the transformation of L3–76 (3.1 mm) into [Mn(L3)Cl2(H2O)].H2O complex (6.2 mm) enabled to obtain double antifungal efficiency against A. fumigatus species. The most effective antifungal agent was recorded for the [Mn(L5)Cl2(H2O)]·H2O complex (12 ± 0.91 and 16 ± 0.63 mm) against A. fumigatus and G. candidum species, respectively. L4–77 is inactive for inhibiting the growth of A. fumigatus species, while its metal complex displayed potency with an inhibition zone diameter of 5.5 ± 0.2 mm. Also, [Mn(L5)Cl2(H2O)]·H2O complex (22.4 ± 0.08 and 9.1 ± 0.07 mm) and its ligand L5–78 (14 ± 0.3 and 4.0 ± 0.4 mm) displayed the broadest antibacterial activities against S. aureus and E. coli species, respectively. Generally, compounds L3–L5 “76–78” and their metal complexes have high efficiencies to inhibit the growth of bacterial cells of type S. aureus more than their efficiencies in inhibiting the growth of E. coli species. The order of potency against all microbial species was found for L3 and its metal complex exhibited broad spectrum activity than L4–77 and its metal complex, or L3–76 and its metal complex.25
Scheme 17. The SARs and synthesis of Mn(ii) complexes of pyranodipyrimidine as antimicrobial agents.
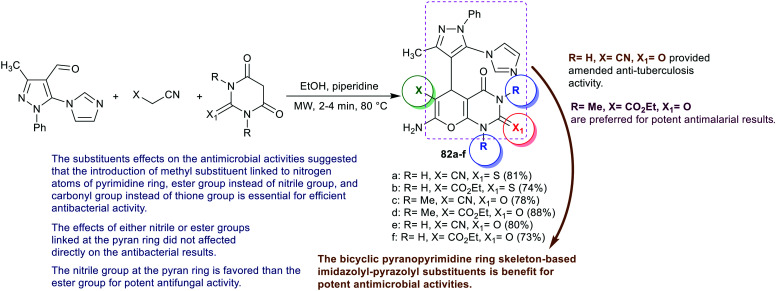
An efficient route for the preparation of binary pyrazolyl-pyranopyrimidines 82a–f was estimated by Kalaria et al.51 under microwave irradiation and heating conditions. Therefore, the one-pot three-component reactions of the heteroaryl aldehyde with active nitriles and substituted-unsubstituted/(thio)barbituric acids in a basic medium gave the anticipated products 82a–f in respectable yields (Scheme 18). The antimicrobial results indicated comparative MIC values for compound 82d with 100 μg mL−1 identical to that of Ampicillin against S. pneumoniae species. Also, compounds 82c–f revealed the most effective MIC values against B. subtilis species with values at 250, 100, 200, and 250 μg mL−1, relative to 250, 100, and 50 μg mL−1 for the standards, Ampicillin, Norfloxacin, and Ciprofloxacin, respectively. Compounds 82a–e are the most potent antibacterial agents (MIC = 100–250 μg mL−1) toward C. tetani species, while compounds 82d and 82f are the broadest agents against E. coli species (MIC = 62.5 and 100 μg mL−1, respectively). Compounds 82c and 82d (MIC = 100 μg mL−1) displayed comparative performance similar to the influence of ampicillin (MIC = 100 μg mL−1) and lower activity than norfloxacin (MIC = 10 μg mL−1), and ciprofloxacin (MIC = 25 μg mL−1) against S. typhi species. In addition, compound 82e is the most effective antibacterial agent against V. cholera species (MIC = 62.5 μg mL−1) than ampicillin (MIC = 100 μg mL−1). On the other hand, compounds 82a and 82c displayed good antifungal activities against C. albicans strains with MIC = 500 μg mL−1, equal to that of griseofulvin (MIC = 500 μg mL−1), while all compounds are weak antifungal agents against A. fumigatus species (MIC = 200–500 μg mL−1).51
Scheme 18. Synthesis of binary pyrazoles-based bicyclic pyranopyrimidines.
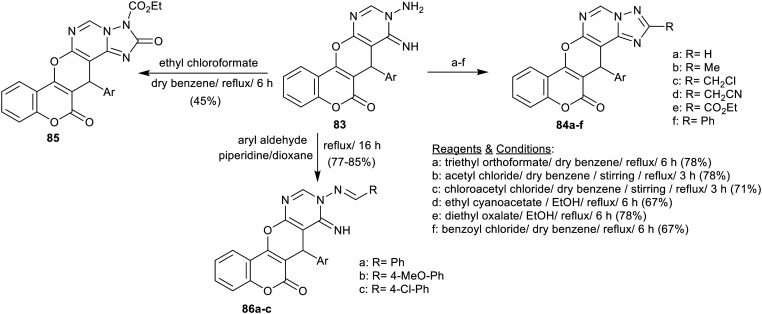
A series of tetracyclic and pentacyclic heterocycles with pyranopyrimidine skeleton 84a–f, 85, and 86a–c was efficiently synthesized through the high reactivity of chromenopyrano[2,3-d]pyrimidinone 83 toward a variety of reagents (Scheme 19). Consequently, the reactions of compound 83 with each of triethyl orthoformate, acetyl chloride, chloroacetyl chloride, ethyl cyanoacetate, diethyl oxalate, and benzoyl chloride yielded the individual pentacyclic heterocycles 84a–f, respectively. The reaction of compound 83 with two equivalents of ethyl chloroformate gave compound 85, while its condensation with aryl aldehydes yielded the Schiff bases 86a–c.76 The antimicrobial activity was evaluated for compounds 84c, 84d, 84e, 85, and 86e against four types of bacterial species, and two types of fungal species by the disc diffusion assay method. The results demonstrated potent and broad-spectrum against all bacterial species, particularly 18–24 mm against S. aureus (NCTC-7447), 19–22 mm against B. cereus (ATCC-14579), 21–25 mm against S. marcesens (IMRU-70), and 21–23 mm against P. merabitis (NTCC-289) (Ampicillin: 25–27 mm). Also, a broad spectrum of antifungal activities was recorded against A. ochraceus Wilhelm (AUCC-230) (inhibition zone diameter = 15–19 mm) and P. chrysogenum Thom (AUCC-530) (13–20 mm) (Mycostatin: 22 and 24 mm). The best results were identified for compounds 84e and 85 against all microbial species but with a slightly improved degree than the other compounds in these series. Thus, the pentacyclic systems with chromenopyrano[1,2,4]-triazolo-pyrimidine core improved the activity of these compounds.76
Scheme 19. Synthesis of chromenopyrano[1,2,4]triazolopyrimidines, and chromenopyranopyrimidinones.
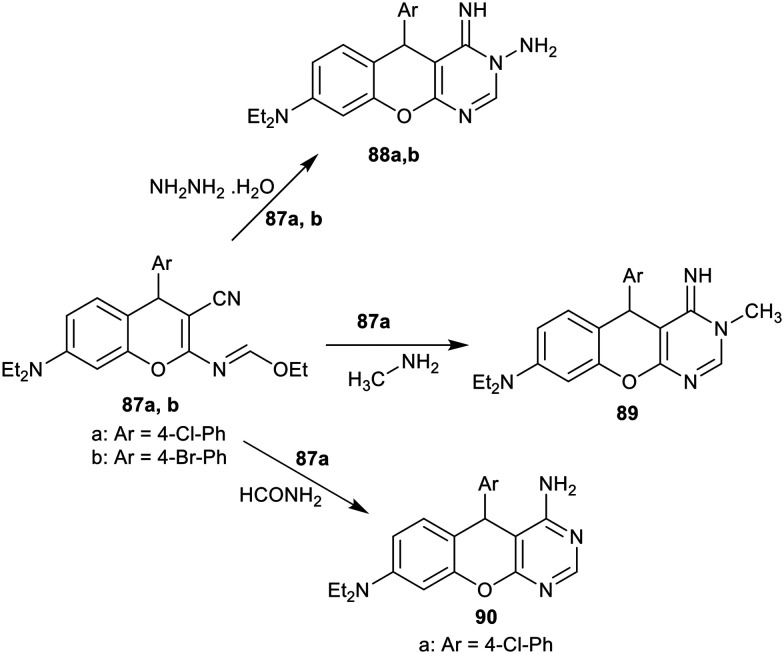
The reactions of compounds 87a,b with hydrazine hydrate yielded the desired chromenopyrimidines 88a,b, respectively. Also, compound 87a reacted with each of methylamine and formamide to give the corresponding chromenopyrimidines 89 and 90, respectively (Scheme 20).48 Compounds 88, 89, and 90 were evaluated in vitro as antibacterial agents against B. bronchiseptica, E. coli, B. pumilus, B. subtilis, S. aureus, and S. epidermidis bacterial species, and as antifungal agents against C. albicans and S. cerevisa fungal species. The results identified that the compounds revealed exceptional performance toward all the microbial species. In particular, their activity against B. bronchiseptica species was found near to that of Ampicillin (MIC = 24 μg mL−1) with MIC ranging from 20 to 22 μg mL−1. Also, potent antibacterial results for these compounds were recorded against E. coli (MIC = 16–21 μg mL−1), B. pumilus (MIC = 25–27 μg mL−1), B. subtilis (MIC = 15–21 μg mL−1), S. aureus (MIC = 24–26 μg mL−1), and S. epidermidis (MIC = 22–25 μg mL−1) species. The MIC values for these compounds also against C. albicans and S. Cerevisa fungal species were found at MIC = 14–16 and 20–24 μg mL−1. By comparing the results of these compounds with each other, it was found that compounds 88b and 89 are the most effective but they remain with a slight capacity difference from the other compounds. Compound 88b displayed more potent activity than compound 88a. Thus, the bromo substituent at the phenyl ring is preferred over the chloro substituent for potent antimicrobial results. Compounds 87 are inactive antimicrobial agents against all microbial species; thus, the transformation of these compounds into the tricyclic chromenopyrimidine systems improved the antimicrobial activities against all species.48
Scheme 20. Synthesis of chromenopyrimidines.
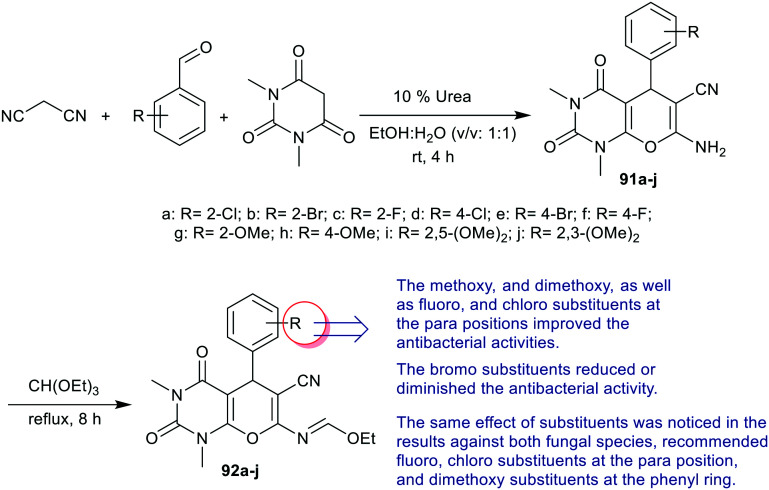
Yalagala et al.77 designed the synthesis of bicyclic pyranopyrimidines 91a–j by multicomponent one-pot reactions of aryl aldehydes with di-substituted barbituric acid, and malononitrile under catalytic conditions using urea. Subsequent reactions of 91a–j with triethyl orthoformate yielded the respective ethyl formimidate derivatives 92a–j (Scheme 21).
Scheme 21. Synthesis of tetrahydropyranopyrimidines and their ethyl formimidate derivatives.
The antimicrobial activity of these compounds “ethyl formimidate derivatives 92a–j” (Scheme 21) was assessed against four bacterial and two fungal species. The results identified potent activates against S. aureus, E. coli, K. pneumoniae, and P. aeruginosa bacterial species for compounds 92d, 92f, 92g, 92i, and 92j (MIC = 6.25 μg mL−1) relative to the standard Ciprofloxacin (MIC = 3.12 μg mL−1). Also, compounds 92a, 92c, and 92h (MIC = 6.25–50 μg mL−1) displayed good activities with less potency in comparison to the impacts of the other compounds. Compounds 92b and 5e (MIC = 100 μg mL−1 → non-active) are inactive antibacterial and antifungal agents against all the tested species. On the other hand, compounds 92c (MIC = 6.25 μg mL−1), 92d, 92f (MIC = 3.12 μg mL−1), 92i (MIC = 3.12 μg mL−1), and 92j (MIC = 3.12 and 6.25 μg mL−1) displayed the broadest antifungal activities against A. flavus and C. albicans species relative to fluconazole (MIC = 1.56 μg mL−1).77
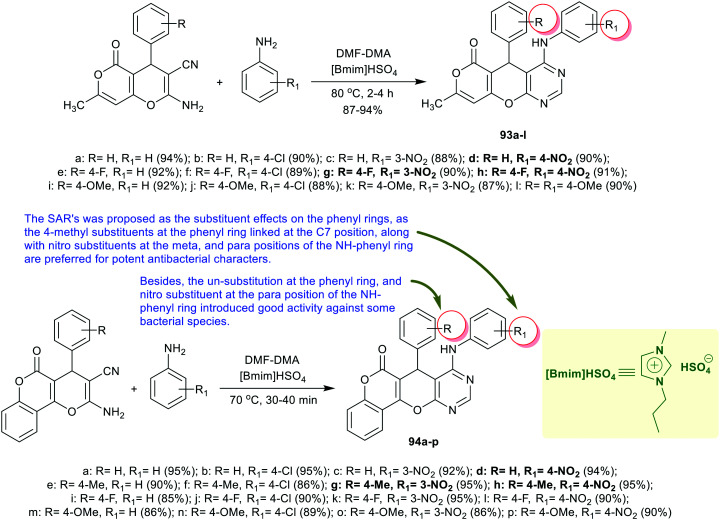
An efficient protocol was applied in an eco-friendly manner for the synthesis of a series of pyranopyrano[2,3-d]pyrimidinones 93a–l by Suresh et al.78 through multicomponent one-pot reactions of 2-amino-7-methyl-5-oxo-4-aryl-4H,5H-pyrano[4,3-b]pyran-3-carbonitriles with aryl amines, and DMF-DMA under [Bmim]HSO4 ionic liquid catalyst. The antimicrobial activities of compounds 93a–l (Scheme 22) were in vitro assessed.78 Subsequently, compound 93l (R = R1 = OMe) presented good potency toward M. luteus MTCC 2470 and S. aureus MTCC 96 (MIC = 15.6 μg mL−1, MBC = 7.8–31.2 μg mL−1, IC50 = 11.5 ± 0.26–2.5 ± 0.18 μM), S. aureus MLS-16 MTCC 2940 (MIC = 3.9 μg mL−1), and B. subtilis MTCC 121, E. coli MTCC 739, and K. planticola MTCC 530 (MIC = 7.8 μg mL−1). Also, compounds 93a (R = R1 = H), 93b (R = H, R1 = 4-Cl), 93c (R = H, R1 = 3-NO2), 93d (R = H, R1 = 4-NO2), and 93h (R = 4-F, R1 = 4-NO2) revealed good potency against S. aureus MLS-16 MTCC 2940 strains (MIC = 15.6–31.2 μg mL−1). Compounds 93c (R = H, R1 = 3-NO2), 93d (R = H, R1 = 4-NO2), 93e (R = 4-F, R1 = H), 93h (R = 4-F, R1 = 4-NO2), and 93i (R = 4-MeO, R1 = H) presented worthy activities toward S. aureus MTCC 96 strains (MIC = 7.8–31.2 μg mL−1). On the other hand, compound 93l (R = R1 = OMe) presented worthy antifungal potency against thirteen fungal species, e.g., C. albicans MTCC 183, C. albicans MTCC 227, C. albicans MTCC 854, C. albicans MTCC 1637, C. albicans MTCC 3018, C. parapsilosis MTCC 1744, C. glabrata MTCC 3019, C. krusei MTCC 3020, and I. hanoiensis MTCC 4755 species (MIC = 7.8–62.5 μg mL−1). Briefly, compound 93l displayed the most potent antimicrobial and antibiofilm activities.78
Scheme 22. Synthesis of pyranopyrano[2,3-d]pyrimidinones and chromenopyrano[2,3-d]pyrimidinones.
Suresh et al.79 extended their previous protocol to synthesize a series of chromenopyrano[2,3-d]pyrimidinones 94a–p through multicomponent reactions in one-pot procedures. Therefore, the reactions of pyrano-chromene-carbonitrile 93 with DMF-DMA and aryl amines catalyzed by [Bmim]HSO4 as an ionic liquid gave the corresponding chromeno-pyrano-pyrimidinones 94a–p in exceptional yields (86–95%) (Scheme 22).79 The antibacterial activity was in vitro estimated for these compounds against a variety of bacterial species. In general, compounds 94g and 94h displayed the most potent activities with the lowest MIC values against most of the tested species but with much lower potency as compared with ciprofloxacin (MIC = 0.9 μg mL−1). In particular, compound 94g presented broad spectrum activity against all bacterial species with MIC values at 15.6 μg mL−1 except its activity against Bacillus subtilis MTCC 121 species with MIC value at 31.2 μg mL−1. In addition, compound 94h demonstrated potent activities against M. luteus MTCC 2470, S. aureus MLS-16 MTCC 2940, B. subtilis MTCC 121, E. coli MTCC 739, and K. planticola MTCC 530 species with MIC values at 15.6 μg mL−1, while its activity against S. aureus MLS-16 MTCC 2940 showed its MIC value of 31.2 μg mL−1 along with reduced activity against P. aeruginosa MTCC 2453 species (MIC => 125 μg mL−1). Also, compound 94d displayed good activity with MIC values of 31.2 μg mL−1 against all tested species except for its activity against S. aureus, E. coli, and P. aeruginosa (MIC => 125 μg mL−1). The MBC results of compounds 94g and 94h revealed also good potency against S. aureus, B. subtilis, and S. aureus species with values ranging from 15.6 to 31.2 μg mL−1.79
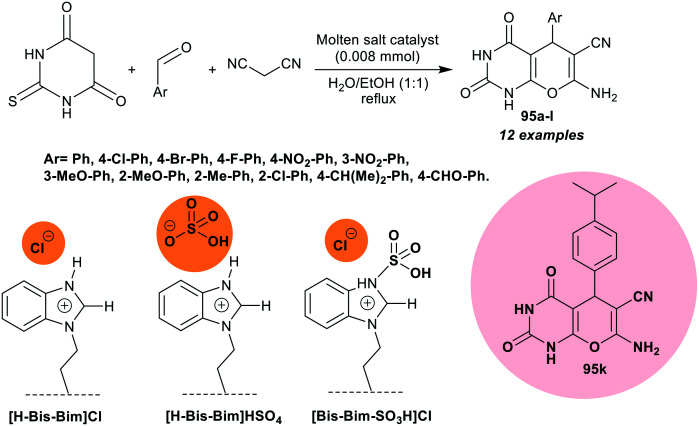
A series of pyrano[2,3-d]pyrimidinone analogs 95a–l was synthesized by Khakiani et al.80 under catalytic conditions following a green approach. A novel compound in this document is compound 95k (Ar = 4-CH(Me)2-Ph) (Scheme 23) was obtained in 65% using [H-bis-Bim]Cl, 92% using [H-Bis-Bim]HSO4, and 79% using [Bis-Bim-SO3H]Cl. The antibacterial properties of compound 95k were evaluated against B. subtilis (Gram-positive) and E. coli (Gram-negative) by the agar well diffusion technique. Moderate activity was recorded for this compound against B. subtilis (inhibition zone diameter = 13 mm) and E. coli species with an inhibition zone diameter of 10.80 mm; these results are comparable to that of the standard Levofloxacin (26 and 29.35 mm, respectively).
Scheme 23. The synthetic route of pyranopyrimidines under catalytic conditions.
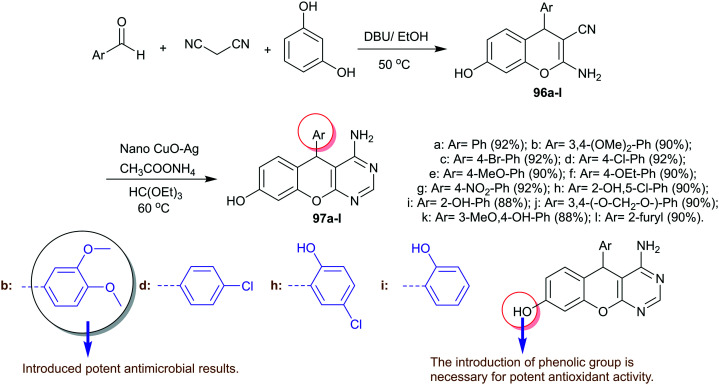
Hese et al.81 designed the synthesis of 5H-chromenopyrimidinol derivatives 97a–l by two step-synthesis involving multicomponent one-pot reactions of aryl aldehydes with malononitrile and resorcinol in the first step. The reactions were progressed using DBU as a nucleophilic catalyst in ethanol. Accordingly, the reactions of the produced 4H-chromene-carbonitriles 96 with triethyl orthoformate and cyclization with ammonium acetate under nano CuO–Ag catalytic conditions yielded the desired chromenopyrimidinol compounds 97a–l (Scheme 24). The antimicrobial activity by the agar diffusion method was estimated for compounds 97a–l against B. subtilis, S. aureus, P. aeruginosa, and E. coli bacterial species and on C. globrata and C. albicans fungal species. The results of antibacterial activity demonstrated good to moderate activities of these compounds relative to tetracycline, as well as the results of antifungal activity compared to griseofulvin. The broadest activities were evaluated using compounds 97b, 97d, and 97h on Gram-positive species and compounds 97b, 97d, 97h, and 97i on Gram-negative bacterial species. In addition, compounds 97b, 97j, and 97l revealed a broad spectrum of activities than the standard antibiotic for both fungal species.81
Scheme 24. Multi-step synthesis of 5H-chromenopyrimidinol derivatives.
2.2. Antitubercular activity
Antitubercular medicines are rifampin, isoniazid, pyrazinamide, and ethambutol are FDA-appropriate to cure infections of Mycobacterium tuberculosis.82 Antitubercular medicines are a set of drugs applied to treat tuberculosis. The antitubercular activity was in vitro assessed for chromenopyrimidines 20–23a–e (Scheme 7) by agar microdilution assay against M. tuberculosis H37Rv (ATCC-27294). The results of the activity were expressed as MIC values in μg mL−1. Thus, compounds 21e and 23e revealed the most potent activities with MIC = 62.5 μg mL−1 relative to the result of the standard drug rifampicin (MIC = 40 μg mL−1). The conversion of the 4H-chromene derivatives 19 into the tricyclic chromenopyrimidines is necessary for potent antitubercular activity. The second order of potency was recorded for compounds 20b, 20e, 21c, 21d, and 22b with MIC values at MIC = 125 μg mL−1. The compounds of the series 21a–e are the most effective antitubercular agents with the most lower MIC values in comparison to the other series. The introduction of the hydroxy group at the chromene ring is beneficial for the potent activity of “compounds 20–23e”. The substituents' effects on the pyrimidine ring are preferred to be pyrimidinones than pyrimidinthiones or aminopyrimidinones or aminopyrimidinthions.47
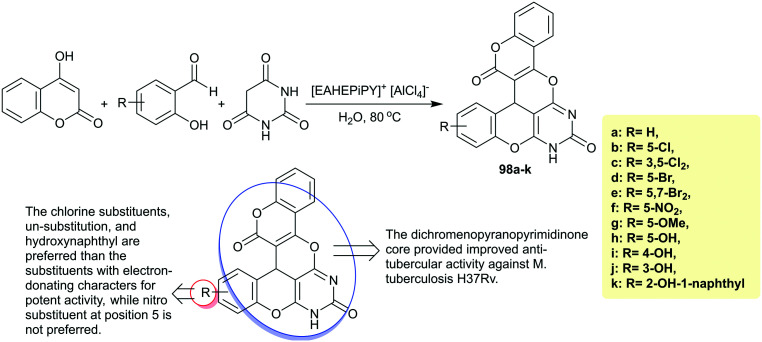
Dige et al.34 reported the synthesis of polycyclic diazanaphtho-tetraphene-diones 98a–k (Scheme 25) with pyranopyrimidine core via a green technique involving three-component one-pot reactions of 4-hydroxy-2H-chromen-2-one with aryl aldehydes and barbituric acid under catalytic conditions. The antitubercular activity was in vitro assessed for these compounds against M. tuberculosis H37Rv by the MABA method. Pyrazinamide (MIC = 3.125 μg mL−1), streptomycin (MIC = 6.25 μg mL−1), and ciprofloxacin (MIC = 3.125 μg mL−1) were used as standards for comparison scales with the results of the investigated compounds. It was noted that compounds 98d and 98e revealed the lowest minimum inhibitory concentrations “MIC” at 1.6 μg mL−1; thus, the incorporation of bromine substituents at the phenyl ring enhanced the activity in comparison to the results of the tested antibiotics. The second order of the reactivity of the compounds was noted by compound 98b with MIC at 6.25 μg mL−1 with equal potency with streptomycin. Also, compounds 98a (MIC = 12.5 μg mL−1) and 98k (MIC = 25 μg mL−1) displayed noticeable activity, while compounds 98c, 98g, 98h, 98i, and 98j showed reduced efficiency with higher MIC values at 50–100 μg mL−1 along with no activity recorded for compound 98f against M. tuberculosis. The introduction of chlorine substituents, unsubstitution, and hydroxynaphthyl are preferred for improved potent activities. The introduction of substituents with electron-donating characteristics such as OMe and OH groups reduced the antitubercular activity. The introduction of the nitro substituent at position 5 is not preferred for potent results owing to the loss of antitubercular activity.34
Scheme 25. Multicomponent synthesis and SARs of polycyclic diazanaphtho-tetraphene-diones.
The antituberculosis activity was in vitro assessed for binary pyrazoles based bicyclic pyranopyrimidines 82a–f (Scheme 18) as percentages of inhibition toward M. tuberculosis H37Rv at different concentrations (250 μg mL−1 and 100 μg mL−1). The results indicated that compounds 82d and 82e are the most effective antituberculosis agents at 250 μg mL−1 with% inhibition of 84% and 95% relative to the standards Rifampicin (98%) and Isoniazid (99%). In addition, compound 6e revealed the most effective activity at 100 μg mL−1 with% inhibition of 92%, in comparison with the antibiotic standards Rifampicin (98%) and Isoniazid (99%). The rest of the compounds in this series displayed moderate to good activities with inhibition ranges of 37–77% at 250 μg mL−1 and 30–71% at 100 μg mL−1.51
2.3. Antimalarial activity
Antimalarial drugs are applied for the inhibition and treatment of infections caused by malaria. The target of most antimalarial medicines is the erythrocytic phase, in which malarial infections in this phase cause symptomatic illness.83 The antimalarial activity was assessed for binary pyrazoles-based bicyclic pyranopyrimidines 82a–f (Scheme 18) against P. falciparum strains. The results specified effective activities for compounds 82c (IC50 = 0.082 μg mL−1) and 82d (IC50 = 0.049 μg mL−1) relative to the standards Chloroquine (IC50 = 0.02 μg mL−1) and Quinine (IC50 = 0.268 μg mL−1). The other compounds displayed good antimalarial results (IC50 = 0.97–1.88 μg mL−1) but with reduced activities compared to the reference standards. It was found that the introduction of N,N-dimethyl-substituents at the pyrimidine ring and two carbonyl groups of pyrimidin-dione ring are essential for potent antimalarial results. The ester group is preferred over the nitrile group for potent antimalarial results.51
2.4. Antiviral activity
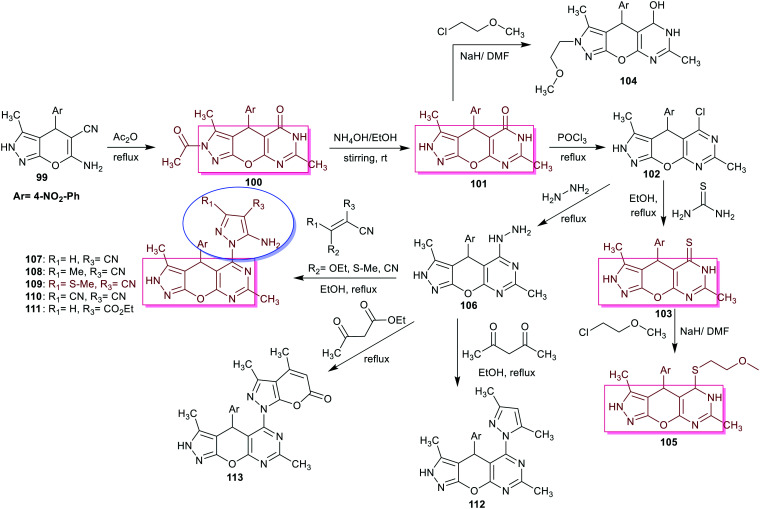
Antiviral activity was expressed by inhibitory possessions of viral replication in cell culture, which has frequently been used to in vitro assess the pharmacologic influence of interferons.84 In more recent years, pyrimidine derivatives were designed and estimated as antiviral drugs against COVID-19.85 The scope of the research was extended for in silico studies to predict the potency of pyranopyrimidinone-derived isoxazole conjugates as potential antiSARS-CoV-2 agents.86 Shamroukh et al.42 designed the synthesis of a series including binary and tricyclic pyranopyrimidines 100–113, as shown in Scheme 26. The protocol involved the utility of 5-cyano-6-amino-4-aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole 99 to synthesize the desired tricyclic pyrazolopyranopyrimidines 100–102 through multi-step synthesis involving the reaction with acetic anhydride, treatment with ammonium hydroxide, and next treatment with phosphorus oxychloride. The N-Alkylation of compound 101 gave product 104, while treatment of 102 with thiourea gave 103, which reacted with 1-chloro-2-methoxyethane to give S-alkylated product 105. The nucleophilic substitution reaction of 102 with hydrazine gave hydrazide 106, which was transformed into the binary pyrazoles 107–113 by reactions with either arylidenes or β-diketones under heating conditions (Scheme 26). The antiviral activity of these compounds (100, 101, 103, 105, 107–110, and 113) was assessed to estimate viricidal influences, virus adsorption, and impact on virus replication for HSV-1. The relatively potent toxic effect on the viral growth was recorded by compound 109, while compounds 100, 101, 103, and 105 are the most potent antiHSV-1 agents compared to the control, acyclovir. Consequently, the compounds revealed reduced potency at different concentrations than acyclovir. The results also indicated that compound 101 has the most potent activity on HSV-1 relative to the other assessed compounds with increased antiviral activity from 63% to 95% by increasing its concentration from 20 μg/105 cells to 40 μg/105 cells. The SARs demonstrated that the introduction of tricyclic systems such as pyrazolopyranopyrimidine scaffold “compounds 100, 101, 103, and 105” is recommended for improved antiviral results. Also, potent activity was recorded for compound 101 against HSV-1; thus, the transformation of compound 100 with acetyl group removal into compound 101 is preferred, which was supported by the influence of two free imino groups. The transformation of compound 101 into 103 decreased the activity due to the sulfur atom effect. The formation of binary pyrazoles “compounds 107–110, and 113” reduced the antiviral activities against the reduction of HSV-1.42
Scheme 26. Synthesis of binary and tricyclic pyranopyrimidines.
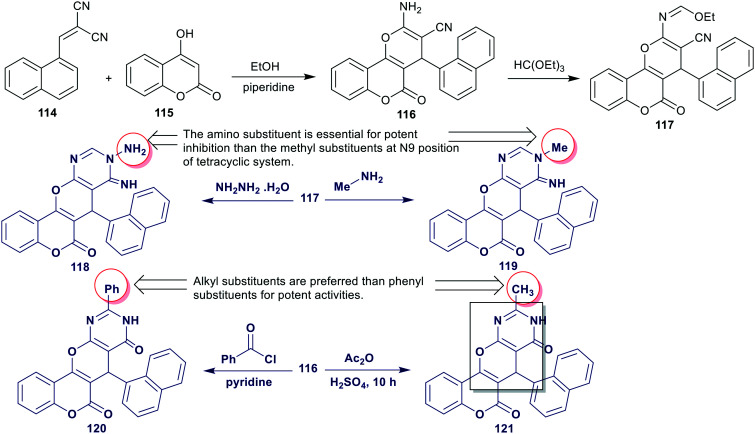
A series of chromenopyrano[2,3-d]pyrimidine-diones 118–121 were synthesized by efficient methods as reported by Al-Masoudi et al.87 Thus, reactions of ethyl formimidate 117 with each hydrazine hydrate and methylamine yielded the respective tetracyclic systems 118 and 119, respectively. Besides, the reactions of enaminonitrile 116 with each benzoyl chloride and acetic anhydride/sulfuric acid gave the desired products 120 and 121, respectively (Scheme 27). The inhibitory activity of the investigated chromenopyranopyrimidine-diones 118–121 were in vitro assessed against HIV-1 and HIV-2 in human MT-4 cells by MTT assay using azidothymidine (DDN/AZT) as the standards. Compounds 118 and 121 inhibited HIV-1 (EC50 => 4.84 and > 3.63 μg mL−1) and HIV-2 (EC50 => 4.84 and > 3.63 μg mL−1) replication in cell cultures, respectively, with selectivity index (CC50 = EC50) < 1. The standards, nevirapine and azidothymidine displayed inhibitions for HIV-1 with EC50 values at 0.050 and 0.0022 μg mL−1 and HIV-2 with EC50 values at > 4.0 and 0.00094 μg mL−1. The introduction of the pyranopyrimidine skeleton is necessary for potent results for the inhibition of HIV-1 and HIV-2.
Scheme 27. Synthesis and SARs of chromenopyrano[2,3-d]pyrimidine-diones as antiHIV-1 and HIV-2.
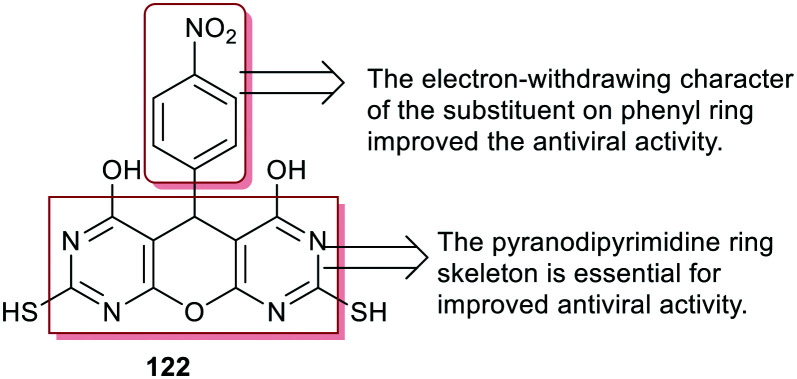
On the other hand, pyranodipyrimidine-4,6-diol 122 (Fig. 4) was evaluated as an antiviral agent by measuring its effect on viral entry and integration by quantitative PCR as well as studying the antiviral resistance. PDP-resistant HIV-1 strains were selected through virus growing at a high concentration of V-165. Frequent mutations were perceived in the reverse transcriptase (RT), integrase (IN), and env genes “encode the protein to form the viral envelope” of the selected virus in the presence of V-165. The results for V-165 indicated the multimodal mechanism of action through the inhibition of the viral entry at 19 μM, and in vivo integration. V-165 was crudely recovered from the human hepatic microsomal matrix and 1% BSA.88
Fig. 4. The structure of pyranodipyrimidine-4,6-diol as an antiviral agent.
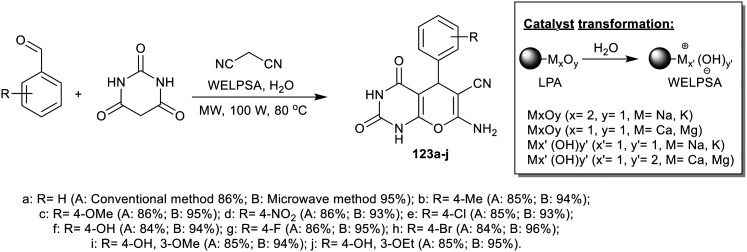
Nesaragi et al.12 efficiently synthesized a series of pyranopyrimidines bearing enaminnitrile fragments 123a–j through multicomponent synthesis using catalytic WELPSA “lemon (Citrus limon)” (Scheme 28). The method involved the preparation of this series of compounds under either microwave irradiation or conventional conditions. The formation of the products was projected by the tandem Knoevenagel condensation, Michael addition, and intramolecular cyclization of the pyran ring. Virus inhibition was assumed based on the inhibition of the RNA transcript and translation by the antiviral drug that acts on the viral nucleoplasmid protein. The prepared compounds were investigated as SARS-CoV-2 inhibitors by molecular docking studies. Thus, the molecular docking of the compounds on the SARS-CoV-2 nucleocapsid protein N-terminal RNA binding domain was studied using (PDB ID 6M3M, 2.70 Å X-ray diffraction) using the Surflex-Dock program of the sybyl-X 2.0 software. There are four possible hydrogen bond interactions for compound 123j at the active sites of the enzyme, in which two of these interactions involved the hydrogen bond interaction between hydrogen atoms of the amino group at the C7 position with oxygen atoms of ARG89 and ARG90. The oxygen atom at C2 formed a hydrogen bond with the hydrogen atom of SER52 along with the additional hydrogen bond involving the hydrogen atom of NH with the oxygen atom of TYR112. The results, in general, identified a favorable binding arising between the pyranopyrimidine compounds and protein, hence presenting inhibition.
Scheme 28. Synthesis of tetrahydro-pyranopyrimidines.
2.5. Antidiabetic activity
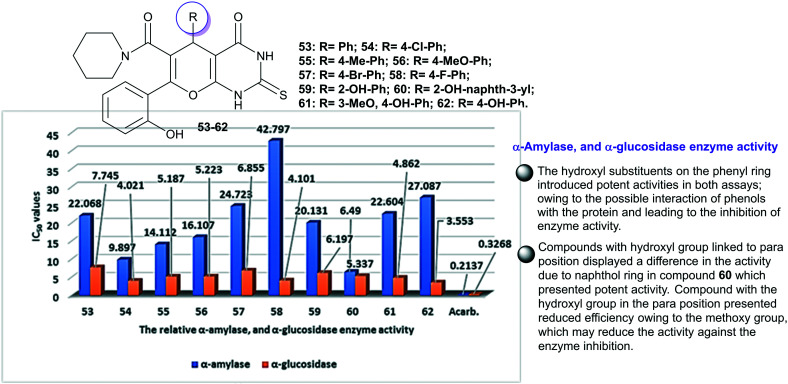
Diabetes mellitus is a disease affecting about 200 million people around the world and the number is predicted to hit 350 million by year 2025.89 The desired tetrahydropyranopyrimidinones 53–62 (Fig. 5) were evaluated in vitro as probable enzymes for α-amylase and α-glucosidase inhibitors. Consequently, the potency of the compounds is amplified with apparent enzymatic potency for α-glucosidases than for α-amylases due to the lower concentrations essential to inhibit enzymatic activity in the case of α-glucosidases than for α-amylases. The results identified that compounds 54 “R = 4-Cl-Ph” (IC50 = 4.021 Mm), 56 “R = 4-MeO-Ph” (IC50 = 4.101 Mm), 61 “R = 3-MeO,4-OH-Ph” (IC50 = 4.862 Mm), and 62 “R = 4-OH-Ph” (IC50 = 3.553 Mm) demonstrated the most potent activities for α-glucosidase; nevertheless, they still have lower potency than the standard acarbose (IC50 = 0.3268 Mm). Besides, compounds with 60 “R = 2-OH-naphth-3-yl” presented the greatest effective activity for α-amylase with IC50 = 6.490 Mm.90
Fig. 5. SARs of pyranopyrimidinones as α-amylase and α-glucosidase inhibitors.
A series of chromenopyranopyrimidine-triones 124a–j was efficiently prepared, as reported by Hese et al.91 through multicomponent one-pot synthesis. Thus, the reactions of barbituric acid with aryl- or heteroaryl aldehydes, and 4-hydroxy-2H-chromen-2-one under catalytic conditions produced the anticipated chromenopyranopyrimidine-triones 124a–j (Scheme 29). The tetracyclic chromenopyranopyrimidine-triones 124a–j were in vitro evaluated for AR inhibitory activity. The results demonstrated that compounds 124a (47 ± 0.3%), 124b (49 ± 0.4%), 124c (59 ± 0.4%); 124e (46 ± 0.7%), 124g (44 ± 0.8%), and 124i (60 ± 0.5%) displayed the most potent ARI inhibitory potentials, these results are analogous to that of quercetin (92 ± 0.3%). The other compounds displayed moderate inhibition capacity. An interesting activity was recorded for compound 124i (Ar = 5-Cl-3-Me-1-Ph-1H-pyrazol-4-yl) with 60 ± 0.5% inhibition with a value of binding free energy at −10.42 kcal mol−1. Therefore, the pyrazole ring substituted with chloro, methyl, and phenyl moieties is required for potent activity. Also, the direct halogen substitution such as chloro and fluoro atoms at the phenyl ring reduced the activity, while the nitro substituents at the phenyl ring improved the AR inhibitory activity.91
Scheme 29. Synthesis of chromenopyranopyrimidine-triones.
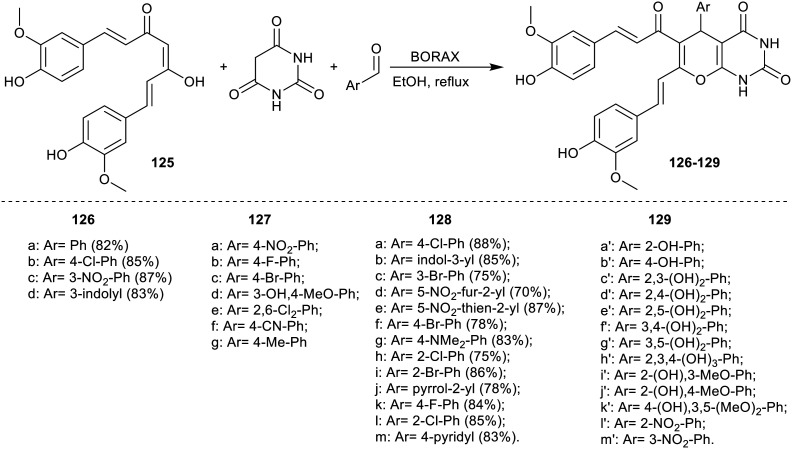
Ghaffarian et al.92 developed the multicomponent synthesis of pyranopyrimidines bearing curcumin fragments (Scheme 30) (10 examples: “Ar = 4-Cl-Ph, 4-NO2-Ph, 3-NO2-Ph, 4-Br-Ph, 2,4-Cl2-Ph, 4-CN-Ph, 3-OH-Ph, 4-SMe-Ph, Ph, 3-NH-C8H6”) using CoFe2O4@OCMC@Cu (BDC) recyclable nanocatalyst with improved yields (81–94%) without additional biological applications. In 2015, Yousefi et al.37 reported the multicomponent synthesis of pyranopyrimidines-based curcumin fragment “series 126” “4 examples” using p-toluene sulfonic acid in refluxing ethanol. In 2020, Hasaninezhad et al.93 reported also the synthesis of these series of pyranopyrimidines incorporated curcumin moiety 127 using catalytic SAA-MNPs (8 mol%). In 2021, Mehrabi et al.13 and Esmaeili et al.94 synthesized these series of compounds 128 and 129 with different substituents by multicomponent one-pot synthesis using borax with 10% in ethanol at reflux temperature. All these studies investigated the antidiabetic activity of these compounds for α-amylase and α-glucosidase “carbohydrate-hydrolyzing enzymes”, which are significant molecular targets for the decrease of postprandial hyperglycemia.
Scheme 30. Synthesis of dihydropyrano[2,3-d]pyrimidine-diones.
The results of Yousefi et al.37 toward both mouse and yeast α-Gls for compounds 126a–d (Scheme 30) indicated that these compounds displayed an inexpensive mode of inhibition toward mouse α-Gls. The results against the yeast enzyme revealed that curcumin (IC50 = 44.8 ± 1.4 μM) and compound 126a verified inhibitory mode of action, while the other compounds 126b–d displayed a mixed-type of inhibition. Also, potent inhibitory activity for compounds 126c “Ar = 3-NO2-Ph” (IC50 = 9.7 ± 0.3 μM) and 126d “Ar = 3-indolyl” (IC50 = 35.3 ± 1.3 μM) toward yeast α-Gls. The compounds revealed reduced activity toward mouse α-Gls. Compound 2d also provided reduced inhibitory activity toward mouse enzyme with selective inhibitory characters of these ligands “126c and 126d” toward yeast α-Gls.
On the other hand, pyranopyrimidine compounds “series127a–g” were evaluated as antidiabetic agents, and found as potential inhibitors for α-amylase and α-glucosidase, as reported by Hasaninezhad et al.93 The results indicated that compounds 127 “Ar = 4-NO2-Ph” (Yeast: IC50 = 12.41 ± 1.10 μM; Mouse: IC50 = 106.80 ± 2.10 μM) and 127 “Ar = 2,6-Cl2-Ph” (Yeast: IC50 = 27.60 ± 1.30 μM; Mouse: IC50 = 46.04 ± 2.10 μM) accessible rational inhibitory activity on α-glucosidase presented by reduced inhibitory activity on α-amylase. Thus, the compounds showed significant antioxidant characteristics as vital antidiabetic agents.93
The antidiabetic activity of pyranopyrimidine-diones “series128a–m” (Scheme 30) was estimated by Mehrabi et al.13 through the evaluation of their antidiabetic activity by Rat α-Glu inhibition and pancreatic α-Amy assays and their antioxidant potentials. Accordingly, the compounds demonstrated potent α-Glu and α-Amy enzyme activities along with relevant antioxidant influence. The in vitro tests specified that compounds 128d: Ar = 5-NO2-fur-2-yl, 128e: Ar = 5-NO2-thien-2-yl, 128h: Ar = 2-Cl-Ph, 128i: Ar = 2-Br-Ph, 128j: Ar = pyrrol-2-yl, 128k: Ar = 4-F-Ph, xxl: Ar = 2-Cl-Ph, 128m: Ar = 4-pyridyl revealed the most potent inhibition for α-Glu enzyme than curcumin, while compounds 128h: Ar = 2-Cl-Ph, 128i: Ar = 2-Br-Ph, 128j: Ar = pyrrol-2-yl, 128k: Ar = 4-F-Ph, 128l: Ar = 2-Cl-Ph, 128 m: Ar = 4-pyridyl presented stronger inhibition for the α-Amy enzyme. The lowest IC50 value was verified for compound 128l “Ar = 2-Cl-Ph” as the most effective inhibitor for both enzymes.
Also, compounds (series 129a′–m′) (Scheme 30) were assessed as antioxidant and glycohydrolase inhibitors as evaluated by Khodarahmi's group.94 The results identified that compounds 129h′ (Ar = 2,3,4-(OH)3-Ph), 129k′ (Ar = 3,5-(OMe)2,4-OH-Ph), and 129g′ (Ar = 3,5-(OH)2-Ph) exhibited potent activities toward α-Glu enzyme, and compounds 129h′ (Ar = 2,3,4-(OH)3-Ph), 129g′ (Ar = 3,5-(OH)2-Ph), 129k′ (Ar = 3,5-(OMe)2,4-OH-Ph), 129f′ (Ar = 3,4-(OH)2-Ph), and 129e′ (Ar = 2,5-(OH)2-Ph) demonstrated the most noteworthy activities toward the α-Amy enzyme. Alternatively, compounds 129g′ (Ar = 3,5-(OH)2-Ph), 129h′ (Ar = 2,3,4-(OH)3-Ph), and 129k′ (Ar = 3,5-(OMe)2,4-OH-Ph) inhibited α-Glu than α-Amy along with applicable results of characteristic antioxidant (by the DPPH˙ assay).
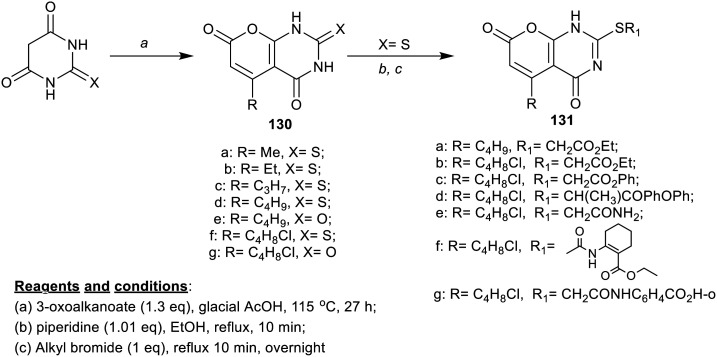
Bisenieks et al.95 prepared a series of pyranopyrimidine-diones 130a–g and their S-alkyl derivatives 131a–g from barbituric and thiobarbituric acids in multi-step synthetic routes (Scheme 31). The action of these compounds on the short-chain fatty acid receptors FFA2/GPR43 and FFA3/GPR41 was studied, in addition to evaluating their activity against hydroxy-carboxylic acid receptor HCA2/GPR109A. The compounds worked as HCA2/GPR109A ligand receptors and motivated the FFA3/GPR41 receptor in low concentration ranges (micro-molar or sub-micro-molar) as well as these compounds motivated the FFA2/GPR43 receptor with poorer potency. It was noted that chlorine atom introduction in the alkyl chain diminished the potency of thioxo derivatives 130d (EC50 = 0.74 ± 0.08 μM) and 130f (EC50 = 2.5 ± 0.4 μM) on the FFA2/GPR43 receptor, along with increased potency of the oxo derivatives 130e (not active) and 130g (EC50 = 0.38 ± 0.06 μM). The long chain of alkyl from n-propyl to n-butyl is effective for potent potency against all the receptors. Also, improved potency was recorded for S-alkyl analogs against FFA3/GPR41 receptor (EC50 = 0.22–4.8 μM) than HCA2/GPR109A receptor (EC50 = 0.051–7.1 μM). Compounds 131c (Emax = 85 ± 10%) and 131e (Emax = 87 ± 6%) have increased potency against the HCA2/GPR109A receptor. The substituent effects capable of altering the gene expression of these tested receptors decreased the LPS-mediated pro-inflammatory cytokine gene expression, showing the effect of the HCA2/GPR109A ligand niacin, and FFA2/GPR43 and FFA3/GPR41 endogenous ligand propionate. These compounds have a definite selectivity against the investigated receptors with improved pleiotropic activity.
Scheme 31. Synthesis of pyranopyrimidines and their S-alkyl analogs.
2.6. Antitumor and cytotoxic activities
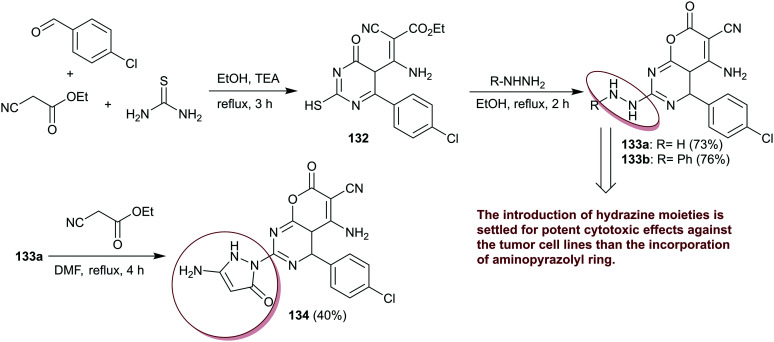
Anticancer or antineoplastic drugs are effective compounds for the treatment of malignant or tumorous diseases.96 The major categories of anticancer drugs include alkylating agents, antimetabolites, natural products, and hormones. Consequently, Abdo97 reported the multi-step synthesis of 5-amino-6-cyano-pyranopyrimidines 133–134 (Scheme 32) by multicomponent one-pot reaction of thiourea with ethyl cyanoacetate and aryl aldehyde in basic medium, followed by reactions with either hydrazine or phenylhydrazine. Subsequently, pyrazolyl-pyranopyrimidine 134 was obtained with a low yield by reaction of 133a with ethyl cyanoacetate. The results showed good potency of compound 133b (IC50 = 55 nM) against NUGC relative to CHS 828 (IC50 = 25 nM) along with weak activities of compounds 133a (IC50 = 328 nM) and 134 (IC50 = 2208 nM). The compounds 133a, b and 134 (IC50 = 608, 146, and 2128 nM) displayed good activities compared to the result of CHS 828 (IC50 = 2315 nM) against DLD1 cells. Contrariwise, compound 133a (IC50 = 282 nM) is the most potent with high effectiveness than the standard, CHS 828 (IC50 = 2067 nM) against HA22T, this potency was decreased to IC50 = 523 nM against HePG2 cells, but is still higher than that of CHS 828 (IC50 = 1245 nM). The potency of compound 133a (IC50 = 263 nM) is the most effective against HepG2 cells than the standard anticancer agent. The altered potency of compounds 133a (IC50 = 201 nM) and 133b (IC50 = 536 nM) against HONE1 cells but with intensive reduced potency relative to CHS 828 (IC50 = 15 nM). Compounds 133a (IC50 = 348 nM) and 133b (IC50 = 285 nM) are the most effective agents against MCF cells but also with neglected activities than CHS 828 (IC50 = 18 nM). The compounds revealed non-cytotoxic effects against the WI38, except for compound 133b (IC50 = 222 nM), which is a very toxic agent with LC50 of 18.38 μg mL−1.97
Scheme 32. Synthesis and SARs of 5-amino-6-cyano-pyranopyrimidines.
The SRB assessment was utilized to estimate the anticancer activity of compound 24a (Scheme 8) against MDA-MB-435, PC3, and Ovkar-3 cell lines. This research studied the GI50, TGI, and LC50 parameters using a sulforhodamine B assay.50 The results showed very weak cytotoxic effects against all cells at different concentrations (10−7, 10−6, 10−5, and 10−4 M) relative to the result of Adriamycin.
The cytotoxic activity of the pyranodipyrimidine ligands L3–L5 “76–78” and Mn(ii)-complexes 74–76 (Scheme 17)25 was assessed by the MTT assay on MCF-7 and HCT-116 human tumor cells. In general, the results indicated improved cytotoxic effects by the metal complexes against both the cells than the impact of the free ligand. As a result, the most potent activity was recorded by the [Mn(L5)Cl2(H2O)]·H2O complex (IC50 = 5.58 mg mL−1) against the MCF-7 cell line, followed by the high potency of [Mn(L3)Cl2(H2O)]·H2O complex (IC50 = 7.54 mg mL−1), [Mn(L4)Cl2(H2O)]·H2O complex (IC50 = 8.21 mg mL−1), and L5–78 (IC50 = 11.5 mg mL−1). Also, the ligand L3–76 displayed good potency on MCF-7 and HCT-116 tumor cells with IC50 values at 17.2 and 17.8 mg mL−1, respectively. The rule that with respect to the impacts of the prepared metal complexes than the free ligands was not found in the result of the L4–77 ligand against the HCT-116 cell line. Conversely, it was found that L4–77 displayed cytotoxicity than its Mn(ii) complexes. The order of cytotoxic potency on the HCT-116 cell line was recorded by the L5–78 complex with the highest impact (IC50 = 5.13 mg mL−1), followed by Mn(ii)-L1 (IC50 = 8.58 mg mL−1).25
A series of ethyl carboxylates of triazolylpyranopyrimidines 137a–m was prepared through an efficient method reported by Boda et al.98 Therefore, one-pot multicomponent reactions of aldehyde, malononitrile, and ethyl acetoacetate gave the monocyclic pyran derivatives, which reacted with acetic anhydride and cyclized in sulfuric acid to give the desired pyranopyrimidines 135. The alkylation of compounds 135 with propargyl bromide at the N3 atom of the pyrimidine ring gave products 136, which reacted with aryl or alkyl azides under catalytic conditions to afford the desired triazolylpyranopyrimidine analogs 137a–m (Scheme 33). Compounds 137a–m were in vitro assessed as cytotoxic agents by the MTT assay against A549, HepG2, MCF-7, and SKOV3 tumor cell lines. The results specified that compounds 137a (0.69 ± 0.02 μM), 137b (6.74 ± 0.11 μM), 137f (5.76 ± 0.01 μM), 7l (1.85 ± 0.01 μM), and 137m (3.62 ± 0.02 μM) established potent activities relative to the standard anticancer drug, doxorubicin (0.14 ± 0.0002 μM) on A549 tumor cell lines. The compounds presented weak to non-cytotoxic effects on the other tumor cell lines, particularly compound 137m (69.85 ± 0.02 μM) against HepG2 cell lines, compound 137a (71.41 ± 0.02 μM) against MCF-7 cell lines, and compound 137a (96.46 ± 0.79 μM) against SKOV3 cell lines are the most slightly weak active cytotoxic agents in this route. The potency of the active compounds that inhibited the growth of the A549 tumor cell lines was extended to inhibit human DNA topoisomerase-II, as estimated by molecular docking studies agreeing with the stranded factors of drug reproduction. Correspondingly, compounds 137a and 137l revealed the potential to cleave pBR322 plasmid DNA deprived of any external agents.98
Scheme 33. Multi-step synthesis of ethyl-1,2,3-triazolyl-pyrano-pyrimidine-carboxylates.
Abd El-Sattar et al.99 designed an example of the bicyclic enaminonitrile 138 for the preparation of heterocycles with a pyranopyrimidine skeleton. Thus, a green procedure was investigated for the synthesis of compound 138 in 95% yield by the one-pot three-component reaction of thiophen-2-carbaldehyde with malononitrile, and barbituric acid in water and ethanol mixture under heating and non-catalytic conditions. Subsequently, the reactivity of compound 138 was utilized toward a variety of chemical reagents for the synthesis of bicyclic and tricyclic systems with pyranopyrimidine nucleus “compounds 139–148” in comparatively good yields (70–90%) (Scheme 34). The PARP-1 inhibitory activities of “antitumor agents” such as pyranopyrimidines 138–147 were in vitro assessed by PARP-1 inhibitory assessment. The results indicated admirable potencies of these compounds (IC50 = 3.61 ± 0.15–114.95 ± 7.22 nM) relative to that of the standard Olaparib (IC50 = 5.77 ± 0.26 nM). In particular, compounds 139 (IC50 = 4.06 ± 0.18 nM) and 144 (IC50 = 3.61 ± 0.15 nM) displayed potent inhibitory than the standard Olaparib, while compounds 141 (IC50 = 14.94 ± 1.03 nM), 142 (IC50 = 11.07 ± 0.76 nM), 145 (IC50 = 15.79 ± 0.86 nM), and 147 (IC50 = 16.16 ± 0.88 nM) revealed good potency but with slight efficiency than Olaparib. The less PARP-1 inhibitory activities were documented by compounds 138 (IC50 = 49.06 ± 2.32 nM), 143 (IC50 = 114.95 ± 7.22 nM), and 146 (IC50 = 48.55 ± 3.25 nM), demonstrating that the introduction of an additional heterocyclic ring fused to the pyranopyrimidine skeleton developed the antitumor activity. The cytotoxicity of compounds 138–147 was estimated by the MTT assay on MCF-7 and HCT-116 tumor cells. The results specified the capacity of the compounds to inhibit the growth of the cancer cells with high efficiency. Consequently, compounds 139 (IC50 = 2.65 ± 0.05 μM), 140 (IC50 = 3.17 ± 1.04 μM), 141 (IC50 = 0.87 ± 0.07 μM), 142 (IC50 = 4.95 ± 0.07 μM), 143 (IC50 = 3.12 ± 0.11 μM), 144 (IC50 = 1.28 ± 1.12 μM), 145 (IC50 = 0.66 ± 0.05 μM), and 147 (IC50 = 4.48 ± 0.43 μM) demonstrated more potent cytotoxicity than the standard anticancer agent staurosporine (IC50 = 7.25 ± 0.13 μM) on the MCF-7 cell. The compounds also presented potent cytotoxicity against HCT-116 cells with IC50 values ranging from 2.76 ± 0.06 to 46.86 ± 1.82 μM investigated with the highest potency for compounds 139–143 and 145–147, and the lowest potency for compounds 138 and 144. Therefore, compounds 140 (IC50 = 2.80 ± 0.07 μM), 141 (IC50 = 7.25 ± 0.13 μM), 143 (IC50 = 6.38 ± 0.11 μM), 145 (IC50 = 2.76 ± 0.06 μM), and 146 (IC50 = 2.90 ± 0.04 μM) displayed cytotoxic effects with magnificent potencies than the standard anticancer agent staurosporine (IC50 = 6.94 ± 0.21 μM) against HCT-116 tumor cells.99
Scheme 34. The reactivity of enaminonitrile toward the variety of reagents for the synthesis of pyranopyrimidine heterocycles.
The cytotoxic potentials for pyranopyrimidines 37–40 and pyranodipyrimidine-diones 41a–g were investigated against HepG2, HCT-116, MCF-7, and VERO cell lines.71 The data indicated that all the compounds displayed a very strong cytotoxic effect against HepG2 cell lines with IC50 ranging from 2.09 ± 0.3 to 8.87 ± 0.6 μM, referring to the highest potency for compound 41a in comparison with the result of doxorubicin (IC50 = 7.94 ± 0.6 μM). Also, the compounds revealed very strong cytotoxicity against HCT-116 and MCF-7 cells with potency range at IC50 = 2.61 ± 0.2 to 7.99 ± 0.6 μM and IC50 = 2.43 ± 0.2 to 10.65 ± 0.9 μM, respectively. Consistent with these results, it was found that the compounds displayed moderate cytotoxicity against VERO cell lines. The SARs of this series of compounds was investigated based on the obtained results, as shown in Scheme 10. Therefore, the tricyclic systems with pyranodipyrimidine skeleton are proficient for potent results against all the tested cell lines. The substituent effects on the nitrogen atom at position 7 indicated that the introduction of a 2-butyl substituent (compound 41a) is preferred for potent cytotoxicity against all tumor cell lines. In addition, a potent cytotoxic effect was recorded for compound 5c (R = –CS–NH–Ph) against HCT-116 cell lines and compound 41f (R = –Ph-2-OH) against MCF-7 cell lines, reflecting the effect of these substituents on the improved cytotoxic characters against these cell lines. In some cases, the transformation of the bicyclic system (compound 40) into the tricyclic systems (compounds 41) slightly decreases the potency of the compounds against definite cell lines based on the cell line nature and substituents effect. The DNA-binding affinity of the most potent antiproliferative derivatives 41a (R = –CH(CH3)CH2CH3) (IC50 = 26.96 ± 1.7 μM), 41c (R = –CS–NH–Ph) (IC50 = 27.13 ± 1.7 μM), and 5f (R = –Ph-2-OH) (IC50 = 29.86 ± 1.7 μM) showed their abilities to intercalate DNA. The potent cytotoxic agents compounds 41a (IC50 = 0.752 ± 0.07 μM), 41c (IC50 = 0.791 ± 0.07 μM), and 41f (IC50 = 0.776 ± 0.07 μM) displayed significant activities for Topoisomerase II inhibitory with remarkable activities than the standard doxorubicin (IC50 = 0.94 ± 0.4 μM).71
Hekal et al.100 reported the synthesis of polycyclic benzochromenopyrimidines and benzo-chromeno-[1,2,4]triazolopyrimidines 151–161via the reactions of ethyl formimidate 150 with various reagents, as shown in Scheme 35. The reactions of compound 150 with hydrazide derivatives gave the pentacyclic benzochromeno-[1,2,4]-triazolopyrimidines 152–154, while its reaction with hydrazine hydrate yielded the tetracyclic system 155. The reaction of compound 150 with amines gave the desired tetracyclic systems 151, 155, 156, and 57. Compounds 158 and 159 were prepared from reactions of compound 155 with each of 2-(ethoxymethylene)malononitrile and benzoyl chloride, respectively. The reaction of compound 155 with ethyl cyanoacetate, followed by the reaction with each of 2-hydroxybenzaldehyde and carbon disulfide/methyl iodide yielded compounds 160 and 161, respectively. Compounds 151–161 were in vitro evaluated as cytotoxic agents against three tumor cells and a normal cell line. The results specified that compounds 151 (IC50 = 10.03 ± 1.8 μM), 157 (IC50 = 3.41 ± 0.4 μM), 158 (IC50 = 9.04 ± 2.1 μM), and 159 (IC50 = 9.26 ± 1.0 μM) displayed very strong potency against HepG2 cells. Moreover, compounds 151 (IC50 = 9.29 ± 1.7 μM), 157 (IC50 = 2.67 ± 0.3 μM), 158 (IC50 = 10.02 ± 2.3 μM), and 159 (IC50 = 9.89 ± 0.8 μM) showed potent cytotoxicity against MCF-7 cells. The same potency of these compounds was recorded against HCT-29 cells for compounds 151, 157–159 with IC50 = 4.9–20.21 μM. The compounds displayed moderate to weak cytotoxicity against the normal cells WI-38. The conversion of tricyclic systems 149 and 150 into the polycyclic systems with pyranopyrimidine motif improved the cytotoxic potency against all the cell lines. The tetracyclic systems seem to be a benefit for potent cytotoxic results than the pentacyclic systems except for compound 158, which may be attributed to the solubility factor. The introduction of alkyl substituents “compound 156” reduces the cytotoxic activity. Also, the unsubstitution on the triazole ring is preferred for potent activity, and the other substituents are preferred to be cyanomethyl (CH2CN) than the ketone (C O) and amine (NH2) groups.100
Scheme 35. Synthesis of polycyclic heterocycles with pyranopyrimidines.
A series of 12H-spiro[indoloquinazoline-pyranopyrimidine] derivatives 162a–f101 were efficiently synthesized with 84–93% yields by multicomponent one-pot reactions of (thio)barbituric acids with malononitrile and indolo-quinazoline-dione (Scheme 36). The reactions were achieved in methylene chloride/TEA at their reflux temperature. The mechanism for the spirocyclic system construction was projected through Knoevenagel condensation, Michael-type addition, and pyran ring cyclization through the intramolecular nucleophilic attack of the generated intermediates.
Scheme 36. Synthesis of 12H-spiro[indoloquinazoline-pyranopyrimidine] derivatives.
The cytotoxic activities of the spirocyclic compounds 162a–f (Scheme 36) were in vitro estimated by MTT assessment against HT-29, Panc1, MDAMB-231 tumor cells, and HDF normal cells. The results verified that compound 162a is a very strong cytotoxic agent against the HT-29 cell line (IC50 = 23.67 ± 0.011 μM) than the positive control Etoposide (IC50 = 25.63 ± 0.01 μM). Besides, compound 162e displayed strong activities against Panc1 and MDA-MB-231 cancer cells (IC50 = 14.15 ± 0.012, and 10.89 ± 0.001 μM) with potent cytotoxic effects than Etoposide (IC50 = 24.35 ± 0.001 and 30.63 ± 0.014 μM), respectively. Also, the compounds are non-cytotoxic agents against HDF normal cell lines (IC50 => 100 μM). Thus, compounds 162a and 162e seem to be reliable for further cancer drug design.101
Haggam et al.102 designed a series of bicyclic pyranopyrimidines 164–167 from the readily available arylidene barbituric acid 163. Subsequently, the reactions of compound 163 with each acetylacetone, 2-cyanoacetamide, malononitrile, and ethyl cyanoacetate yielded the desired bicyclic pyranopyrimidines 164–167, respectively (Scheme 37). The cytotoxic activity of these compounds was in vitro investigated against HCT-116, HepG2, and PC-3 cell lines. The results have shown that the compounds possess very strong cytotoxic effects relative to the anticancer standard doxorubicin (IC50 = 0.467–0.73 μg mL−1). Specifically, compound 167 is more effective than the other compounds with IC50 = 0.25–0.53 μg mL−1 against the three tested cell lines. In addition, compound 164 displayed moderate activities against HCT-116 (IC50 = 7.65 μg mL−1), HepG2 (IC50 = 5.21 μg mL−1), and PC-3 (IC50 = 4.80 μg mL−1) relative to the results of the other compounds and the anticancer drug. The order of the cytotoxic potency of the compounds against all cell lines was found as follows: compound 167 has the most potent cytotoxicity than compound 165, compound 166, and finally, compound 164 has the lowest cytotoxic effects. The ester group of compound 167 at the C6 position improved the cytotoxic potential more than the amide of compound 165, the nitrile group of compound 166, and the acetyl group of 5. Also, the amine group (NH2) at the C7 position improved the cytotoxicity more than the methyl substituent.102
Scheme 37. Synthesis of bicyclic pyranopyrimidines.
Bhosle et al.103 developed the multicomponent synthesis of dihydropyranopyrimidine-triones 168a–r under β-cyclodextrin catalytic conditions by applying a proficient green protocol (Scheme 38). The anticancer activity of dihydropyranopyrimidine-triones 168a–r was investigated by the MTT assay against well-known HepG2 and MCF-7 cell lines using 5-Fu as an anticancer standard. Compounds 168c (Ar = 4-OMe-Ph, IC50= 10.6 μM), 168h (Ar = 4-OH-Ph, IC50 = 8.2 μM), 168j (Ar = 3-OMe,4-OH-Ph, IC50 = 10.6 μM), 168k (Ar = 3-OH,4-OMe-Ph, IC50 = 8.8 μM), and 168l (Ar = 3,4-(OMe)2-Ph, IC50 = 8.9 μM) show substantial potency for growth inhibition against HepG2 cell lines. In addition, compounds 168j (Ar = 3-OMe,4-OH-Ph, IC50 = 8.9 μM) and 168m (3,4,5-(OMe)3-Ph, IC50 = 8.9 μM) revealed the most significant activities against MCF-7 cell lines relative to the result of 5-Fu (IC50 = 5.4 μM). Through these results, it was found that these compounds showed more distinct activity toward HepG2 cells than their activity in inhibiting the growth of MCF-7 cell lines. The SARs for the obtained anticancer results is dependent on the substituents' nature and the cancer cell type, as described in Scheme 37.103
Scheme 38. Synthesis of dihydropyranopyrimidine-triones.
β-Cyclodextrin supported the multicomponent synthesis of 2H-chromenopyrimidine-diones 169a–h under gentle heating in water, as indicated by Bhosle et al.104 The reactions, in this case, progressed in one-pot and under a green procedure to synthesize the target products in relatively moderate to excellent yields with the simplicity of product separation (Scheme 39). The cytotoxic activity of compounds 169a–h was in vitro evaluated against HeLa and MCF-7 cancer cell lines. The results specified weak to non-cytotoxic effects against both cancer cells. In particular, compound 169b is the most effective cytotoxic agent against both cancer cells with IC50 values of 46 and 33 μM, respectively, in comparison with the results of the other tested compounds (IC50 = 38 to > 100 μM). Compounds 169b and 169c displayed the most effective cytotoxic potency against both tumor cell lines (IC50 = 46 and 59 μM against HeLa, and IC50 = 33 and 38 μM against MCF-7 cells) with moderate influences relative to the standard anticancer drug adriamycin (IC50 = <10 μM). Also, non-cytotoxic effects were recorded by compounds 169a and 169f against both cancer cells, compound 169e against MCF-7 cells, and very weak activity against HeLa (IC50 = 90 μM). The SARs of the obtained results indicated that the introduction of electron-withdrawing groups at the para position of the phenyl ring improved the cytotoxic effects against both tumor cell lines. The unsubstituted phenyl ring, hydroxy substituents at the ortho- and meta-positions of the phenyl ring linked to the C4 position of the tricyclic systems are not preferred for potent cytotoxic effects. Improved cytotoxic effects with a very insignificant amount of the hydroxy substituent in the para-position at the phenyl ring is greater than the effect produced by either the hydroxy group at the ortho- and meta-positions.104
Scheme 39. Multicomponent synthesis of 2H-chromenopyrimidine-diones.
Bakhotmah et al.105 have also explored the reactivity of 4-oxo-4H-chromenyl-dihydropyranopyrazole 170 in the reactions with urea, thiourea, and barbituric acid to synthesize polycyclic pyranopyrimidine systems 171–173, respectively (Scheme 40). Compounds 171–173 were evaluated as cytotoxic agents using MTT colorimetric assay against HCT-116, HepG2, and MCF-7 cell lines. The compounds, in general, presented moderate cytotoxicity against all cell lines relative to doxorubicin. Compound 173 is revealed to have good cytotoxic potential on HCT-116 (IC50 = 18.46 μg mL−1), HepG2 (IC50 = 21.92 μg mL−1), and MCF-7 (IC50 = 22.61 μg mL−1) cell lines but still lower cytotoxicity than the standard anticancer agent doxorubicin (IC50 = 0.467–0.493 μg mL−1). Also, compound 172 (IC50 = 22.62–34.19 μg mL−1) displayed moderate cytotoxicity with good potency than compound 171 (IC50 = 30.12–46.71 μg mL−1).105
Scheme 40. Synthesis of polycyclic pyranopyrimidines.
Compound 63b was in vitro assessed as a cytotoxic agent against Vero (normal kidney) and Caco-2 cancerous (colorectal adenocarcinoma) cell lines using the MTT assay.10 The results indicated moderate cytotoxicity against Caco-2 cancerous cell lines (IC50 = 65.94 ± 2.36 μg mL−1) alongside the lowest cell viability percentage at the higher sample concentration. Also, the compound is a weak cytotoxic agent against Vero (normal kidney) cell lines with IC50 = 138.07 ± 8.21 μg mL−1 (c.f. Scheme 13).10
2.7. Sirtuin inhibitors
Sirtuins are protein enzymes that control several cellular functions, comprising aging, inflammation, detoxification, stress resistance, circadian rhythms, fat and glucose metabolism, and mitochondrial biogenesis.106 Accordingly, many researchers have been interested in synthesizing heterocyclic compounds to assess their activities as sirtuin inhibitors. Thus, the reactions of N-phenyl(thio)barbituric acids with 2-hydroxy-1-naphthaldehyde in ethanol produced a mixture of arylidenes 174 and 175, and benzochromeno-pyrimidinones 176 and 177 (Scheme 41).45 Tricyclic and tetracyclic pyranopyrimidinones 176, 178, and 179 were evaluated as sirtuin inhibitors toward human recombinant SIRT1 and SIRT2 (hr) produced in E. coli species (at 50 μM) using Fluor de Lys-SIRT1/2 (Biomol) substrate in the presence of NAD+, pursued by Developer II addition to generate the fluorescent signal using cambinol as the reference standard drug. Compounds 178 and 179 were synthesized as analogous structures to compound 176 with better solubility as these compounds are tricyclic systems that allow greater solubility. The results identified that compounds 176 (IC50 = 191.2 ± 25.6 μM) and 179 (IC50 = 243.6 ± 30.3 μM) revealed weak to inactive influence against SIRT2 inhibition with great selectivity against SIRT1. Compounds 176 (IC50 = 8.4 ± 0.2 μM), 7 (IC50 = 4.2 ± 0.9 μM), and 179 (IC50 = 5.3 ± 2.3 μM) revealed significant selective inhibition activities against SIRT1. Western blot analysis was implemented in MCF7 cells to identify the influences of these compounds on the acetylation of two substrates of SIRT1 and SIRT2, in particular, p53 and α-tubulin. Compounds 176 and 179 increase the level of acetylation in the p53 assay for the generation of acetyl-p53, confirming the inhibition against SIRT1. In the second α-tubulin assay, only the standard drug showed a weak level of protein acetylation. In addition, compound 176 at a concentration of 50 μM revealed 28.4% cell cycle apoptosis against U937 leukemia cells and showed remarkable potency of antiproliferation effects against Raji, DLD1, and HeLa cancer cells. Therefore, this compound is suggested for drug design in cancer treatment as a favorable candidate.
Scheme 41. Synthesis of benzochromenopyrimidinones.
2.8. Antiinflammatory activity
Antiinflammatory activity is significant for wound healing procedures, in which chemicals from white blood cells enter the blood or tissues to defend the body from invaders, increasing blood flow to the area of injury. Some chemicals seep into the tissues and cause swelling.107,108 In this section, the antiinflammatory activities of 2-thioxo-tetrahydropyranopyrimidinones 53–62 (Fig. 6) were estimated by the protein (bovine serum albumin) denaturation assay using different concentrations (3.12–50 μg mL−1).14 The results specified that the most potent activity was recorded for compound 58 (R = 4-F-Ph) (IC50 = 5.92 μg mL−1). In addition, compounds 53 (R = Ph, IC50 = 9.19 μg mL−1), 55 (4-Me-Ph, IC50 = 9.27 μg mL−1), 56 (R = 4-MeO-Ph, IC50 = 8.92 μg mL−1), and 62 (R = 4-OH-Ph, IC50 = 9.43 μg mL−1) show significant activities. The high potency of compound 6 is perhaps associated with the high electronegativity character of fluorine atom, non-covalent intermolecular interactions of C–F, and the potential hydrogen bond affinity. The results are on a respectable scale with potent activity compared to that of the standard diclofenac sodium (IC50 = 18.51 μg mL−1).14
Fig. 6. SARs of tetrahydropyranopyrimidinones.
AbdEl-Azim et al.109 established the synthesis of tetrahydro-2H-pyranopyrimidine 180 (88%) (Scheme 42) under heating and the catalytic conditions of Mn3O4 nanoparticles. Therefore, the reactivity of this compound was investigated through reactions with glucose, ammonium thiocyanate, malononitrile in sodium ethoxide, carbon disulfide, and triethyl orthoformate. The antiinflammatory activity for compounds 180–185 were in vitro assessed using 5-LOX, TNF-α, and NF-κB assays. The results demonstrated that most of the compounds revealed potent activity for inhibiting COX-2, LOX, and tumor necrosis TNF-α. The results specifically verified that compound 181 inhibited COX-2 to 0.04 ± 0.09 μm relative to celecoxib (0.04 ± 0.01 μm), inhibited LOX to 1.66 ± 0.01 μm relative to quercetin (3.34 ± 0.12 μm), and inhibited TNF-α to 3.23 ± 0.15 μm relative to certolizumab (6.90 ± 0.26 μm). Also, compound 185 inhibited COX-2 with IC50 = 0.04 ± 0.02 μm, LOX with IC50 = 1.93 ± 0.02 μm, and TNF-α with IC50 = 3.50 ± 0.10 μm. These results reflect the potency of compounds 181 and 185 as potent antiinflammatory agents for the inhibition of COX-2, LOX, and TNF-α.
Scheme 42. Schematic synthesis of substituted bicyclic pyranopyrimidines and tricyclic pyranodipyrimidines.
2.9. Antioxidant activity
The antioxidant characteristics are well-defined as an inhibition limitation of nutrient oxidation, in particular, proteins and lipids, by the termination of the radical chain reactions.110 The antioxidant activity of the tricyclic pyrazolo-pyranopyrimidine-diones 42a–j (Fig. 7 and Scheme 11) was estimated by the DPPH˙ assay.73 The results verified the potent activities recorded by compounds 42b (Ar = 4-NO2-Ph, 98.38%), 42g (Ar = 3-NO2-Ph, 90.32%), 42i (Ar = –CH CH–Ph, 90.32%), and 42j (Ar = 2-OH,5-NO2-Ph, 89.08%) as compared with the result of ascorbic acid (94.62%). Also, compounds 42a (65.83%), 42d (71.91%), 42e (59.03%), and 42f (58.76%) displayed good antioxidant activities. The rest of the compounds displayed moderate activities lower than 50% inhibition. The SARs of the effect of the substituent at the C4 position control the mechanism of inhibition as the increased ability of the compound to trap the free radicals of DPPH˙ is the scale of antioxidant potency. All the compounds have a pyranopyrimidine skeleton that is preferred for potent activity with reactive oxygen, and nitrogen species and compounds substituted phenyl are preferred over the heteroaryl substituents. The phenolic groups “hydroxyl groups linked at the phenyl ring” are preferred for potency results as the formed radicals are stable by the resonance at the phenyl ring. Also, the nitro group as electron-withdrawing groups is “stabilized by resonance effect”, leading to high potency.
Fig. 7. The structures of the tricyclic pyrazolopyrano-pyrimidine-diones as antioxidizing agents.
On the other hand, other examples for the tricyclic systems such as 5H-chromenopyrimidinol derivatives 97a–l (Fig. 8, Scheme 24) were evaluated as antioxidizing agents by three methods, e.g., DPPH, H2O2, and NO scavenging assessments.81 The results were compared to that of BHT as an antioxidant standard. The DPPH assay indicated that compounds 97d (IC50 = 36.82 ± 1.38 μg mL−1), 97g (IC50 = 34.42 ± 1.21 μg mL−1), 97i (IC50 = 36.82 ± 1.76 μg mL−1), and 97j (IC50 = 39.46 ± 1.24 μg mL−1) displayed the most potent antioxidant activities relative to the result of BHT (IC50 = 37.52 ± 1.26 μg mL−1). In addition, the results of these compounds using the H2O2 radical scavenging assay demonstrated potent activities of these compounds with IC50 ranging from 53.54 ± 1.32 to 86.83 ± 1.67 μg mL−1 relative to that of the standard BHT (IC50 = 65.33 ± 1.19 μg mL−1) with the highest strengths recorded for compounds 97b and 97d. The results of the NO radical scavenging test identified the good activities of these compounds with IC50 ranging from 50.32 ± 1.09 to 84.30 ± 1.08 μg mL−1 comparable to the result of BHT (IC50 = 56.90 ± 1.18 μg mL−1). The most potent antioxidant agents from this method are compounds 97b–97d, 97h, and 97i with activity ranging from 50.32 ± 1.09 to 59.45 ± 1.13 μg mL−1. Therefore, the introduction of the hydroxy group at the C8 position of the tricyclic systems improved the activity by radical trapping with stabilized free phenolic radicals. Also, the effect of substituents linked to the phenyl ring at the C5 position is preferred to be hydroxy groups or electron-withdrawing groups such as the nitro group linked at the phenyl ring.81
Fig. 8. The structures of 5H-chromenopyrimidinol derivatives as antioxidant agents.
Ali et al.111 reported the schematic synthesis of the tricyclic systems linked to heterocyclic systems, e.g., pyrazolo-pyranopyrimidinyl-chromenone analogs 188–193, starting from the reactive precursor, dihydro-pyranopyrazole 186 with enaminonitrile moiety. Compound 188 was synthesized from compound 186 by reaction with formic acid or from compound 187 by reaction with DMF-DMA in dioxane under heating conditions. Also, the reactions of compound 186 with formamide or carbon disulfide in pyridine gave the corresponding tricyclic systems 189 and 192, respectively. Compound 191 was synthesized from 186 through a multi-step synthetic procedure, involving reaction with triethyl orthoformate, hydrazine hydrate, and cyclization by heating in DMF/piperidine system. The acetylation of compound 187 with acetic anhydride, followed by reflux in acetic acid/acetic anhydride, yielded the desired N-acetylated tricyclic system 190. The reactivity of compound 186 toward phenyl isothiocyanate in refluxing pyridine led to the tricyclic thione 193 (Scheme 43). The antioxidant activity of these tricyclic systems (compounds 188–193) in addition to the starting compounds 186 and 187 was investigated by the DPPH assay at four concentrations (25–100 μg mL−1) in comparison with ascorbic acid. The results verified that compounds 187 (62.45 ± 0.28%) and 193 (61.59 ± 0.24%) are the most potent at a lower concentration (25 μg mL−1); nevertheless, compounds 192 (66.53 ± 0.28%) and 193 (66.36 ± 0.2%) are the most potent at 50 μg mL−1. Also, at the concentration of 75 μg mL−1, compounds 191 (82.52 ± 0.29%) and 192 (84.28 ± 0.26%) showed a high potency to trap or scavenge the DPPH free radicals with respectable potency relative to the result of ascorbic acid (89.42 ± 0.34%). Compounds 188 (87.46 ± 0.27%) and 192 (86.39 ± 0.31%) are the most potent antioxidants at the higher concentration but still have lower potency than ascorbic acid (97.56 ± 0.29%).111 Thus, the antioxidant capacity is dependent on the nature of substituents attached to the heterocyclic systems along with the sample concentration but this factor is not a major one that directly affects the antioxidant aptitude.
Scheme 43. Multi-step synthesis of pyrazolopyranopyrimidinyl-chromenone and SARs of these compounds as potential antioxidant agents.
The antioxidant activity of tetracyclic systems, e.g., chromenopyranopyrimidine-triones 124a–j (Scheme 29), was evaluated by DPPH and OH radical scavenging assays. The compounds revealed remarkable results with potent scavenging capacity relative to ascorbic acid (DPPH: 85.42%; OH: 84.34%). Consequently, the most potent results were recorded for compounds 124a (DPPH: 71.55%; OH: 68.31%), 124d (DPPH: 70.13%; OH: 62.06%), 124e (DPPH: 72.42%; OH: 67.24%), and 124j (DPPH: 71.21%; OH: 65.36%). The other compounds revealed good antioxidant inhibitions with percentages of radical scavenging in the ranges of 59.14–68.84% in the DPPH test and 54.30–63.22% in the OH assay. The aryl substituents such as “Ar = 4-Cl-Ph; 4-F-Ph; 2-Cl-Ph; and 2,6-Cl2-Ph” presented the most effective capacities for radical scavenging in the antioxidant tests. It was worth stating that the substituents with a source of free radical “preferred stable free radical” can stabilize the free radical of DPPH, or OH in the solution improved the antioxidant activity.91
Dangolani et al.49 synthesized a series of bicyclic pyranopyrimidine-based carbohydrates 194a–k by multicomponent one-pot reactions under catalytic conditions of p-toluenesulfonic acid (p-TSA). Thus, the reactions of carbohydrates, e.g., glucose, arabinose, lactose, maltose, or galactose with barbituric acid and active methylene compounds yielded the desired carbohydrates-linked pyranopyrimidin-diones 194a–k (Scheme 44). The antioxidant activity identified as TEAC was estimated for these compounds 194a–k. The results specified that the strongest activities were recorded for compounds 194c, 194j, and 194k with TEAC values at 4.914 ± 0.196, 5.237 ± 0.053, and 4.978 ± 0.018 μM, respectively. Also, compounds 194a and 194d–h displayed good activities with TEAC values ranging from 3.070 ± 0.070 to 3.995 ± 0.036 μM but with reduced capacities than compounds 194c, 194j, and 194k. The SARs postulated for these compounds based on the significant activities is due to the pyranopyrimidine skeleton, the effects of the introduction of sugar type, and moieties linked to the bicyclic system, e.g., enaminonitrile, enaminoester, and β-ketoester fragments. Therefore, it was worth mentioning that the introduction of arabinose sugar type along with enaminonitrile improved the antioxidant characteristics. The galactose substituents reduced the activity, while significant activity was recorded for compounds with the β-ketoester moiety.49
Scheme 44. Synthesis of pyranopyrimidine-based carbohydrates.
2.10. Anticoagulant activity
Anticoagulants are drugs or medicines that inhibit the blood from clotting as efficiently as that normally.112 Debbabi et al.113 developed the synthesis of two series of chromeno-pyrimidinones 197a–f and chromeno-triazolo-pyrimidinones 198a–d through multi-step synthesis to evaluate their anticoagulant activity. Thus, a multicomponent reaction of malononitrile with benzaldehyde and dimedone in a one-pot method gave the anticipated pyranopyrimidine 195. The reaction of 195 with triethyl-orthoformate in acetic anhydride yielded the corresponding ethyl formimidate 196. Compound 196 reacted with each of the arylamines and hydrazide derivatives to yield the target products 197a–f and 198a–d, respectively (Scheme 45). The chromeno-pyrimidinones 197 and chromeno-triazolo-pyrimidinones 198 were in vitro evaluated as anticoagulant agents through the measurement of the activated aPTT in the normal human plasma. The results identified respectable potency of the compounds stated by the remarkable prolongation of aPTT in a concentration-dependent method ranging from 53.7 to 72.8 s relative to the negative control, plasma (aPTT = 33.0 s). The results also stated the highest potency of compounds 197a (57.1 s), 197b (53.7 s), 197c (61.8 s), 197d (54.8 s), 197e (57.0 s), 197f (63.2 s), 198a (59.4 s), 198b (60.1 s), 198c (63.4 s), and 198d (55.1 s) relative to the result of the negative control “plasma” (33.5 s). Heparin (10 μg mL−1, 125.2 s) was tested as a positive control for comparing its results with the tested compounds. The most effective anticoagulant agent was recorded for compound 198c (63.4 s) relative to the compounds in both series, while compound 197f (63.2 s) is the most potent anticoagulant agent between the chromenopyrimidinone derivatives. Thus, the introduction of a naphthyl group “compound 197f” into the chromenopyrimidinone skeleton delivered potent activity for the tricyclic systems. Consistently, the 4-Et-Ph “compound 197c” is necessary for potent activity, while the introduction of 3-Me-Ph resulted in reduced activity. The incorporation of 4-OMe-Ph is also preferred for the incorporation of “3-Cl-Ph” aryl substituents at the N3 position of the pyrimidine ring, which could be influenced by the nature of substituents that are affected by the electronic and mesomeric effects of the substituents. Moreover, compound 198c (R = CH2CN) presented the maximum activity, followed by compound 198b (R = Me). The differences in the activity of the chromeno-triazolo-pyrimidinone series could be related to the structured nature but there are no great differences were noticed between the compounds of this series except for compound 198d, reflecting that the introduction of the coumarin ring is not preferred for the potent activity. The molecule size and polarity factors also control the anticoagulant potency of these compounds.113
Scheme 45. Synthesis of chromenopyrimidinones and chromeno[1,2,4]triazolopyrimidinones.
2.11. Tyrosine inhibitors
Tyrosine kinase inhibitors are pharmaceutical drugs that inhibited tyrosine kinases.114 Tyrosine kinases are enzymes liable for the activation of several proteins by indication transduction cascades. The proteins are activated by the phosphorylation of the protein, in which tyrosine kinase inhibitors were inhibited in this step. In this section, numerous studies have been focused on to prepare a series of pyranopyrimidines to be assessed as tyrosine kinase inhibitors. The tyrosinase enzyme is presented in the skin that is responsible for the transformation of tyrosine “amino-acid” into melanin “dark-colored pigment” as its gene mutation or absence led to pigmentation halting. The two series of chromeno-pyrimidinones 197a–f and chromeno-triazolo-pyrimidinones 198a–d (Scheme 45 and Fig. 9)113 were in vitro assessed as antityrosinase agents. Consequently, remarkable antityrosinase activities were recorded with percentages of inhibition from 73.77 ± 1.10 to 98.84 ± 1.81% ranges with comparative potency to that recorded for kojic acid as the positive control (85.50 ± 1.00%). Therefore, compounds 197a (91.35 ± 1.00%), 197c (84.14 ± 2.00%), and 197d (94.23 ± 1.45%) presented antityrosinase abilities, demonstrating that the substitution of the phenyl ring with chlorine atom is essential for potent activity produced by compound 197d. The introduction of a methyl group at the position C3 of the phenyl ring “197b”, methoxy substituent at the C4 position of the phenyl ring “197e”, and naphthyl substituent “197f” resulted in the loss of the antityrosinase activity. Alternatively, the introduction of the methyl substituent at the triazole ring of “compound 198b” yields no activity. Also, the introduction of cyanomethyl substituent “198c: 93.94 ± 1.58%” is beneficial for potent activity, as well as the introduction of coumarin substituent at the triazole ring “198d: 90.12 ± 2.1%”, and un-substitution “198a: 78.09 ± 0.94%”. The performance of compounds 198c and 198d was found to be higher than that of the standard kojic acid (85.50 ± 1.0%).113
Fig. 9. The SARs of polycyclic pyranopyrimidines as efficient antityrosinase agents.
Thanh et al.32 have designed a series of pyranopyrimidines-based [1,2,3]triazole motifs 202a–yvia a multi-step route. Therefore, three-component reactions of ethyl acetoacetate with malononitrile and aryl aldehydes under ambient conditions gave the desired pyran derivatives 199. The acetylation of 199 with acetic anhydride lead to cyclization with sulfuric acid to give the bicyclic systems 200. The N-alkylation of 200 with propargyl bromide yielded the respective alkynes 201. The reactions of glucose acetate-derived azide with compounds 201 led to the triazole ring closure with the generation of bicyclic bearing triazole cores 202. The inspected triazolyl-pyranopyrimidines 202a–y (Scheme 46) were in vitro evaluated as inhibitors for Mycobacterium tuberculosis protein tyrosine phosphatase B (MtbPtpB). The results demonstrated that compounds 202g (IC50 = 9.52 ± 1.13 μM), 203t (IC50 = 7.94 ± 0.23 μM), 202u (IC50 = 7.51 ± 0.33 μM), 202v (IC50 = 2.22 ± 0.23 μM), 202x (IC50 = 3.53 ± 0.19 μM), and 202y (IC50 = 1.56 ± 0.21 μM) presented the most potent inhibition on MtbPtpB. The theoretical considerations, e.g., kinetic and in silico studies, recommended that the strong potency of compounds 202v, 202x, and 202y (IC50 = 1.56–3.53 μM) arise from the possible interaction of the amino acids of Arg63 with the substituents of anion characters, for instance, the hydroxy group in the para position of the phenyl ring through the formation of the salt bridge of the iminium cation. Thus, the introduction of substituents such as hydroxy, methoxy, and ethoxy groups in the meta- and para-positions of the phenyl ring is necessary for potent inhibitory activity. Also, the substitution of the phenyl ring with a variety of groups or atoms such as nitro, alkyl, chloro, and bromo substituents or unsubstituted are not preferred for potent activity, although the mono-chloro substituent at the meta position and nitro at the meta position revealed good inhibitory activity. Thus, the introduction of substituents at the para and meta positions at the same time is preferred for improved activities. The introduction of methoxy and nitro substituents at the ortho- or para-positions of the phenyl ring decreased the activity. The inhibition constants for the most potent compounds 202x, 202y, and 202v were found to be 2.16, 1.10, and 1.80, respectively, identifying competitive inhibition mechanisms.32
Scheme 46. Schematic routes for the multi-step synthesis of pyranopyrimidines bearing triazole ring.
2.12. Urease inhibitory activity
Urease inhibitors are applied to temporarily decrease the activity of the enzyme and slow the rate at which urea is hydrolyzed.115,116 Solvent-free multicomponent one-pot reactions of barbituric acid or its N,N-dimethyl analog with aryl aldehydes and malononitrile gave the desired bicyclic pyranopyrimidines 203a–l.117 The yields of the products prepared by this method have poor to good yields (30–90%) under SBA-Pr-SO3H nanocatalyst conditions (Scheme 47). The urease inhibitory activities of the investigated bicyclic pyranopyrimidines 203a–l were assessed against Jack bean urease using the phenol red technique. A slight inhibitory activity was documented by compound 203a (IC50 = 98.77 μM), while compounds 203d (IC50 = 98.93 μM) and 203l (IC50= 107.62 μM) are inactive agents. Besides, compounds 203b (IC50 = 19.45 μM), 203k (IC50 = 41.0 μM), 203f (IC50 = 41.13 μM), 203e (IC50 = 49.65 μM), 203j (IC50 = 51.31 μM), 203g (IC50 = 62.92 μM), and 203c (IC50 = 71.92 μM) revealed respectable to moderate potency, relative to the results of thiourea (IC50 = 21.0 μM). Thus, the introduction of substituents with hydrophobic characters is necessary for potent activity, as well as substituents with electron-donating characters, which allowed the interaction with the enzyme active sites, leading to hydrolysis inhibition of the substrate. Reduction in the activity was perceived by the introduction of substituents with electron-withdrawing characteristics, besides the methoxy substituent at the meta position of the phenyl ring. The methyl substituent at both the nitrogen atoms of the pyrimidine ring (R1, R2 = Me) are not preferable for potent urease inhibitory activities.117
Scheme 47. Multicomponent synthesis and SARs of bicyclic pyranopyrimidines as urease inhibitors.
3. Concluding remarks
The current review highlighted the various approaches to implement for the synthesize of different compounds, incorporating the pyranopyrimidine core with distinct and privileged biological activity. Many compounds have been evaluated as antimicrobial agents, especially bicyclic compounds and their derivatives, and experiments have shown that these compounds have remarkable properties against microbial activity depending on the type of microbial species and the nature of the chemical structure. Tricyclic pyranopyrimidines and diazanaphtho-tetraphenediones verified potent antituberculous activities against M. tuberculosis H37Rv; this type should apply for bicyclic pyranopyrinidine compounds. Bicyclic pyranopyrimidines-based pyrazoles have also shown antimalarial activity with significant potency. The antiviral activity of tricyclic systems, specifically, pyrazolopyranopyrimidines and pyranodipyrimidine against HSV-1, HIV-1, and HIV-2, was estimated, in addition to the activity of bicyclic systems as inhibitors of SARS-CoV-2. In addition, the antidiabetic activity of pyranopyrimidinones and dihydropyranopyrimidinediones-based curcumin with potent α-glucosidase activities was evaluated. The bicyclic, polycyclic, spirocyclic, and binary systems, e.g., triazolyl-pyranopyrimidine, pyrazolyl-pyranopyrimidine, and thienyl-pyrazolopyranopyrimidine systems, were found to have cytotoxic potential against a variety of tumor cell lines. Furthermore, these compounds showed remarkable potency as antiinflammatory, antioxidant, and anticoagulant agents, inhibitors of urease, tyrosine, and sirtuin.
4. Future perspective
Heterocycles are cyclic compounds with a ring containing carbon and other elements such as oxygen, nitrogen, or sulfur. In this document, pyranopyrimidines are related to bicyclic 6 + 6 systems with the pyran ring fused to pyrimidine ring. In addition to the four possible isomeric structures of the pyranopyrimidines, this class of heterocycles is analogous to the class of pyridopyrimidines. Therefore, the same biological evaluations of the pyridopyrimidines should be applied to pyranopyrimidines in future research owing to the valuable biological significance of these heterocycles. In addition, a lack of interest from scientists for the biological evaluation of pyranopyrimidines was noted, in which recent works have reported the preparation of the target compounds with improved yields using green protocols under catalyst-free conditions or nanocatalysts. Subsequently, future studies should include the evaluation of bicyclic, tricyclic, binary, and spirocyclic pyranopyrimidines in all aspects.
Abbreviations
- DABCO
1,4-diazabicyclo[2.2.2]octane;
- MIC
Minimum inhibitory concentration
- MBC
Minimum bactericidal concentration
- MFC
Minimum fungicidal concentration
Microbial species
- B. cereus
Bacillus cereus
- S. aureus
Staphylococcus aureus
- S. epidermidis
Staphylococcus epidermidis
- K. pneumoniae
Klebsiella pneumoniae
- P. aureus
Pseudomonas aureus
- B. cereus
Bacillus cereus
- E. coli
Escherichia coli
- P. aeruginosa
Pseudomonas aeruginosa
- A. Niger
Aspergillus Niger
- P. chrysogenum
Penicillium chrysogenum
- B. subtilis
Bacillus subtilis
- S. pyogenes
Streptococcus pyogenes
- B. megaterium
Bacillus megaterium
- C. freundii
Citrobacter freundii
- S. typhi
Salmonella typhi
- S. paratyphi
Salmonella paratyphi
- V. cholera
Vibrio cholera
- S. marcesens
Serratia marcesens
- P. merabitis
Proteus merabitis
- B. bronchiseptica
Bordetella bronchiseptica
- B. pumilus
Bacillus pumilus
- M. luteus
Micrococcus luteus
- K. planticola
Klebsiella planticola
- K. planticola
Klebsiella planticola
- C. albicans
Candida albicans
- A. flavus
Aspergillus flavus
- A. niger
Aspergillus niger
- A. ochraceus
Aspergillus ochraceus
- S. marcescens
Serratia marcescens
- P. mirabilis
Proteus mirabilis
- B. pumilis
Bacillus pumilus
- E. faecalis
Enterococcus faecalis
- G. candidum
Geotrichum candidum
- A. fumigatus
Aspergillus fumigatus
- C. tetani
Clostridium tetani
- P. chrysogenum Thom
Penicillium chrysogenum Thom
- S. cerevisiae
Saccharomyces cerevisiae
- C. parapsilosis
Candida parapsilosis
- C. glabrata
Candida glabrata
- C. krusei
Candida krusei
- I. hanoiensis
Issatchenkia hanoiensis
- M. tuberculosis
Mycobacterium tuberculosis
- IC50
Half-maximal inhibitory concentration
- HSV-1
Herpes simplex virus type 1
- EC50
Half maximal effective concentration
- HIV “HIV-1, HIV-2”
Human immunodeficiency virus
- PCR
Polymerase chain reaction
- PDP
Prescription Drug Plan
- BSA
Bovine Serum Albumin
- SARS-COV-2
Coronavirus
- AR
Cells that have a protein that binds to androgens (male hormones)
Tumor cells
- NUGC
Human gastric cancer
- DLD1
Human colon cancer
- HA22T
Human liver cancer
- HONE1
Nasopharyngeal carcinoma
- WI38
Normal fibroblast cells
- MDA-MB-435
Human breast cancer cell line
- PC3
Human prostate cancer cell line
- Ovkar-3
Human ovarian cancer cell line
- A549
Human lung adenocarcinoma cell line
- HepG2
Liver Carcinoma
- MCF-7
Human breast adenocarcinoma
- SKOV3
Human ovarian carcinoma cell line
- HCT-116
Colon Carcinoma
- HCT-29
Colon
- HT-29
Human Colorectal Adenocarcinoma Cell Line
- Panc1
Human pancreatic cancer cell
- MDAMB-231
Epithelial, human breast cancer cell line
- HDF
Normal human adult dermal fibroblast cell line
- HeLa
Cervical
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- DNA
Deoxyribonucleic Acid
- TNF-alpha
Tumor Necrosis Factor-alpha
- COX-2 inhibitors
Cyclooxygenase-2
- LOX
Lipoxygenase
- TNF-α
Pro-inflammatory mediator “necrosis factor-alpha”
- NF-κB
Nuclear factor kappa B
- DMF-DMA
Dimethylformamide Dimethylacetal
- DPPH
1,1-Diphenyl-2-picrylhydrazyl
- TEAC
Trolox equivalent antioxidant capacity
- BHT
Butylated hydroxytoluene
- aPTT
Partial Thromboplastin Time
- [Bmim]HSO4
1-Butyl-3-methylimidazolium hydrogen sulfate
- DBU
2,3,4,6,7,8,9,10-Octahydropyrimido[1,2-a]azepine
Conflicts of interest
The authors state no conflict of interest.
Supplementary Material
Biographies
Biography
Khaled M. Elattar.

Dr. Khaled M. Elattar was born in Menyet Sammanoud, Aga, El-dakahlia, Egypt (1979). He received his B.Sc. in 2002 from the Faculty of Science, Mansoura University, Egypt, and M.Sc. in 2006 from the Faculty of Science, Benha University, Benha, Egypt. He obtained his Ph.D. in organic chemistry in 2011 from the Faculty of Science, Mansoura University, Egypt (Ph.D. supervisors: Prof. A. A. Fadda and Prof. A. S. Fouda). He is a Lecturer in Organic Chemistry, at the Faculty of Education of the Sert University, Sert, Libya, from 2012 to 2015. He is a member of the Egyptian Chemical Society. He is a reviewer for some scientific international journals. His main research interests are in the field of organic synthesis, the synthesis of heterocyclic compounds of the pharmaceutical interest, and medicinal chemistry. He joins the editorial board of OA Journal Pharmaceutics (2018). https://oa.enpress-publisher.com/index.php/Pha/about/editorialTeam.
Biography
Ayman Y. El-Khateeb.

Prof. Dr. Ayman Y. El-Khateeb was born in Bani Souwaif, Egypt (1978). He received his B.Sc. Agricultural Sciences in 1999 from the Faculty of Agriculture, Mansoura University, Egypt. In 2005, he received his master’s degree in Agricultural Chemistry from the Faculty of Agriculture, Mansoura University. He obtained his Ph.D. in Agricultural Chemistry in 2010 from the Faculty of Agriculture, Mansoura University, Egypt. Ayman El-Khateeb was appointed as an academic staff member in the Chemistry department, Faculty of Agriculture, Mansoura University, Egypt in 1999. He is currently a Professor Doctor and serves as the Chairman of the Agricultural Chemistry Department, Faculty of Agriculture, Mansoura University, Egypt. He is a reviewer of several international journals. His research interests focus on the biotechnology field and using nanotechnology as a tool including extraction, isolation, identification, and characterization of natural compounds from medicinal plants or by-products and investigating their biological activities. He attended about 100 conferences, symposiums, training, and workshops. He is highly recognized for his endless contributions to the chemistry department and serves as a committee member for graduate students in the same field. His applicable academic scientific researches stand out for its novelty, originality, and credibility.
Biography
Sahar E. Hamed.

Prof. Dr. Sahar El-Sayed Hamed was born in Damietta, Egypt (1986). She received her B.Sc. Agricultural Sciences in 2007 from the Faculty of Agriculture, Mansoura University, Egypt. In 2012, she received her master’s degree in Agricultural Chemistry from the Faculty of Agriculture, Mansoura University. She obtained her Ph.D. in Agricultural Chemistry in 2017 from the Faculty of Agriculture, Damietta University, Egypt. She received the award for Best Ph.D. thesis in agricultural sciences for the academic year 2018. Sahar Hamed was appointed as an academic staff member in the Chemistry department, Faculty of Agriculture, Damietta University, Egypt in 2008. She is currently a lecturer in the Department of Agricultural Chemistry, Faculty of Agriculture, Damietta University, Egypt. Sahar Hamed's research interests focus on the biotechnology field and using nanotechnology as a tool including extraction, isolation, identification, and characterization of natural compounds from medicinal plants or by-products and investigating their biological activities such as antioxidant, antifungal, anti-inflammatory, antibacterial, antidiabetic, and anticancer. She is a member of two scientific projects focused on nanotechnology and human health. She has supervised about 7 thesis and 4 graduation projects. She teaches about 10 academic courses in the field of chemistry and biotechnology at bachelor's and postgraduate levels. She has attended about 20 conferences, symposiums, training, and workshops.
References
- Kumari A. Singh R. K. Bioorg. Chem. 2020;96:103578. doi: 10.1016/j.bioorg.2020.103578. doi: 10.1016/j.bioorg.2020.103578. [DOI] [PubMed] [Google Scholar]; , PMID: 31978684
- Kumari A. Singh R. K. Bioorg. Chem. 2019;89:103021. doi: 10.1016/j.bioorg.2019.103021. doi: 10.1016/j.bioorg.2019.103021. [DOI] [PubMed] [Google Scholar]; , PMID: 31176854
- Kumar S. Singh R. K. Patial B. Goyal S. Bhardwaj T. R. J. Enzyme Inhib. Med. Chem. 2016;31(2):173–186. doi: 10.3109/14756366.2015.1016513. doi: 10.3109/14756366.2015.1016513. [DOI] [PubMed] [Google Scholar]
- Sethi N. S. Prasad D. N. Singh R. K. Mini-Rev. Med. Chem. 2020;20(4):308–330. doi: 10.2174/1389557519666191029102838. doi: 10.2174/1389557519666191029102838. [DOI] [PubMed] [Google Scholar]; , PMID: 31660809
- Zaharani L. Khaligh N. G. Gorjian H. Johan M. R. Turk. J. Chem. 2021;45(1):261–268. doi: 10.3906/kim-2010-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaabani A. Samadi S. Rahmati A. Synth. Commun. 2007;37(3):491–499. doi: 10.1080/00397910601039242. [DOI] [Google Scholar]
- Maleki N. Shakarami Z. Jamshidian S. Nazari M. Acta Chem. Iasi. 2016;24(1):20–28. doi: 10.1515/achi-2016-0002. [DOI] [Google Scholar]
- Oftadeh T. Sadeghi B. Zavar S. Iran J. Org. Chem. 2020;12(2):2831–2837. [Google Scholar]
- Sabbaghnasab E. Sheikhhosseini E. Res. Sq. 2021 doi: 10.21203/rs.3.rs-194775/v1. [DOI] [Google Scholar]
- Abd-Elaziz A. M. Aly H. M. Saleh N. M. Fouad S. A. Ismail A. A. Fouda A. J. Iran. Chem. Soc. 2021:1–18. doi: 10.1007/s13738-021-02448-w. [DOI] [Google Scholar]
- Mohamadpour F. Org. Prep. Proced. Int. 2020;52(6):503–509. doi: 10.1080/00304948.2020.1796158. doi: 10.1080/00304948.2020.1796158. [DOI] [Google Scholar]
- Nesaragi A. R. Kamble R. R. Hoolageri S. R. Mavazzan A. Madar S. F. Anand A. Joshi S. D. Appl. Organomet. Chem. 2021;36:e6469. doi: 10.1002/aoc.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabi M. Esmaeili S. Ezati M. Abassi M. Rasouli H. Nazari D. Adibi H. Khodarahmi R. Bioorg. Chem. 2021;110:104720. doi: 10.1016/j.bioorg.2021.104720. doi: 10.1016/j.bioorg.2021.104720. [DOI] [PubMed] [Google Scholar]
- Naik M. D. Bodke Y. D. Prashantha J. Naik J. K. J. Chem. Sci. 2021;133(1):1–13. doi: 10.1007/s12039-020-01875-1. doi: 10.1007/s12039-020-01875-1. [DOI] [Google Scholar]
- Avudaiappan G. Unnimaya T. J. Asha P. Unnikrishnan V. Sreekumar K. J. Heterocycl. Chem. 2020;57(1):197–209. doi: 10.1002/jhet.3765. doi: 10.1002/jhet.3765. [DOI] [Google Scholar]
- Yadav M. B. Lim K. T. Kim J. S. Jeong Y. T. Tetrahedron Lett. 2021;65:152754. doi: 10.1016/j.tetlet.2020.152754. doi: 10.1016/j.tetlet.2020.152754. [DOI] [Google Scholar]
- Wei J. Gui W. Cui Y. Zhang Z. Yousif Q. A. Pol. J. Chem. Technol. 2020;22(2):20–33. doi: 10.2478/pjct-2020-0013. doi: 10.2478/pjct-2020-0013. [DOI] [Google Scholar]
- Keshavarz M. Mamaghani M. Dekamin M. G. Nikpassand M. J. Iran. Chem. Soc. 2021;18(6):1419–1431. doi: 10.1007/s13738-020-02123-6. doi: 10.1007/s13738-020-02123-6. [DOI] [Google Scholar]
- Malathi V. Nagaraju P. Padmaja P. Reddy P. N. Chem. Data Collect. 2021;35:100749. doi: 10.1016/j.cdc.2021.100749. [DOI] [Google Scholar]
- Ali T. E. Bakhotmah D. A. Assiri M. A. Yahia I. S. Zahran H. Y. Russ. J. Org. Chem. 2021;57(3):469–475. doi: 10.1134/S1070428021030209. doi: 10.1134/S1070428021030209. [DOI] [Google Scholar]
- Karad A. R. Jadhav A. G. Wadwale N. B. Khansole G. S. Choudhare S. S. Tiwade S. S. Nawhate S. V. Bhosale V. N. J. Mol. Liq. 2021;334:115754. doi: 10.1016/j.molliq.2021.115754. doi: 10.1016/j.molliq.2021.115754. [DOI] [Google Scholar]
- Esmaeili A. A. Mesbah F. Moradi A. Khojastehnezhad A. Khalili M. Phosphorus Sulfur Silicon Relat Elem. 2021;196(9):819–825. doi: 10.1080/10426507.2021.1921775. doi: 10.1080/10426507.2021.1921775. [DOI] [Google Scholar]
- Zangouei M. Esmaeili A. A. J. Chem. Res. 2020;44(11–12):646–652. doi: 10.1177/1747519820916926. doi: 10.1177/1747519820916926. [DOI] [Google Scholar]
- Hosseini S. Esmaeili A. A. Khojastehnezhad A. Notash B. J. Sulfur Chem. 2021:1–17. doi: 10.1080/17415993.2021.1944144. doi: 10.1080/17415993.2021.1944144. [DOI] [Google Scholar]
- El-Shwiniy W. H. Shehab W. S. Zordok W. A. J. Mol. Struct. 2020;1199:126993. doi: 10.1016/j.molstruc.2019.126993. doi: 10.1016/j.molstruc.2019.126993. [DOI] [Google Scholar]
- Amirnejat S. Nosrati A. Peymanfar R. et al. . Res. Chem. Intermed. 2020;46:3683–3701. doi: 10.1007/s11164-020-04168-x. doi: 10.1007/s11164-020-04168-x. [DOI] [Google Scholar]
- Khatavi S. Y. Kamanna K. J. Mol. Struct. 2022;1250(2):131708. doi: 10.1016/j.molstruc.2021.131708. doi: 10.1016/j.molstruc.2021.131708. [DOI] [Google Scholar]
- Bedair A. H. Emam H. A. El-Hady N. A. Ahmed K. A. El-Agrody A. M. Il Farmaco. 2001;56(12):965–973. doi: 10.1016/S0014-827X(01)01168-5. [DOI] [PubMed] [Google Scholar]
- Chate A. V. Dongre R. M. Khaire M. K. Bondle G. M. Sangshetti J. N. Damale M. Res. Chem. Intermed. 2018;44(10):6119–6136. doi: 10.1007/s11164-018-3479-9. doi: 10.1007/s11164-018-3479-9. [DOI] [Google Scholar]
- Maddila S. Nagaraju K. Jonnalagadda S. B. Chem. Data Collect. 2020;28:100486. doi: 10.1016/j.cdc.2020.100486. [DOI] [Google Scholar]
- Ajmal R. B. Rajendra S. D. Rupali S. S. Int. J. Pharm. Biol. Sci. 2014;5(1):422–430. [Google Scholar]
- Thanh N. D. Ha N. T. T. Le C. T. Van H. T. K. Toan V. N. Toan D. N. Bioorg. Med. Chem. Lett. 2019;29(2):164–171. doi: 10.1016/j.bmcl.2018.12.009. doi: 10.1016/j.bmcl.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Vekariya R. H. Patel K. D. Vekariya M. K. et al. . Indian J. Chem. 2018;57B:997–1005. [Google Scholar]; , https://nopr.niscair.res.in/handle/123456789/44744
- Dige N. C. Mahajan P. G. Pore D. M. Med. Chem. Res. 2018;27(1):224–233. doi: 10.1007/s00044-017-2062-z. doi: 10.1007/s00044-017-2062-z. [DOI] [Google Scholar]
- Prashant T. M. Nimesh R. K. Dhaval D. H. Saurabh K. P. Lett. Drug Des. Discovery. 2011;8(8):750–757. doi: 10.2174/157018011796576060. doi: 10.2174/157018011796576060. [DOI] [Google Scholar]
- Bennett L. R. Blankely C. J. Fleming R. W. Smith R. D. Tessonam D. K. J. Med. Chem. 1981;24:382–389. doi: 10.1021/jm00136a006. doi: 10.1021/jm00136a006. [DOI] [PubMed] [Google Scholar]
- Yousefi A. Yousefi R. Panahi F. Sarikhani S. Zolghadr A. R. Bahaoddini A. Khalafi-Nezhad A. Int. J. Biol. Macromol. 2015;78:46–55. doi: 10.1016/j.ijbiomac.2015.03.060. doi: 10.1016/j.ijbiomac.2015.03.060. [DOI] [PubMed] [Google Scholar]
- Nagaraju P. Reddy P. N. Padmaja P. Ugale Vi. G. Lett. Org. Chem. 2020;17(12):951–958. doi: 10.2174/1570178617666200319114611. doi: 10.2174/1570178617666200319114611. [DOI] [Google Scholar]
- Ali T. E. Assiri M. A. Alzahrani A. Y. Salem M. A. Shati A. A. Alfaifi M. Y. Elbehairi S. E. I. Synth. Commun. 2021;51(21):3267–3276. doi: 10.1080/00397911.2021.1966804. [DOI] [Google Scholar]
- Heber D. Heers C. Ravens U. Pharmazie. 1993;48:537–541. [PubMed] [Google Scholar]
- Witvrouw M. Maele B. Vercammen J. Hantson A. Engelborghs Y. Clercq E. D. Pannecouque C. Debyser Z. Curr. Drug Metab. 2004;5:291–304. doi: 10.2174/1389200043335487. doi: 10.2174/1389200043335487. [DOI] [PubMed] [Google Scholar]
- Shamroukh A. H. Zaki M. A. Morsy E. M. H. Abdel-Motti F. M. Abdel-Megeid F. M. E. Arch. Pharm. 2007;340:236–243. doi: 10.1002/ardp.200700005. [DOI] [PubMed] [Google Scholar]
- Song D. W. Liu G. L. Xue M. Y. Qiu T. X. Wang H. Shan L. P. Liu L. Chen J. Virus Res. 2021;291:198221. doi: 10.1016/j.virusres.2020.198221. [DOI] [PubMed] [Google Scholar]
- Song D. W. Liu L. Fu X. Y. Liu G. L. Hu Y. Chen J. Aquaculture. 2021;540:736694. doi: 10.1016/j.aquaculture.2021.736694. [DOI] [Google Scholar]
- Rotili D. Tarantino D. Carafa V. Lara E. Meade S. Botta G. Nebbioso A. Schemies J. Jung M. Kazantsev A. G. Esteller M. ChemMedChem. 2010;5(5):674–677. doi: 10.1002/cmdc.201000030. doi: 10.1002/cmdc.201000030. [DOI] [PubMed] [Google Scholar]
- Xin M. Zhang L. Shen H. Wen J. Tu C. Liu Z. Cheng L. Zhao X. Med. Chem. Res. 2014;23(8):3784–3792. doi: 10.1007/s00044-014-0959-3. doi: 10.1007/s00044-014-0959-3. [DOI] [Google Scholar]
- Kamdar N. R. Haveliwala D. D. Mistry P. T. Patel S. K. Eur. J. Med. Chem. 2010;45(11):5056–5063. doi: 10.1016/j.ejmech.2010.08.014. doi: 10.1016/j.ejmech.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Sabry N. M. Mohamed H. M. Khattab E. S. A. E. H. Motlaq S. S. El-Agrody A. M. Eur. J. Med. Chem. 2011;46(2):765–772. doi: 10.1016/j.ejmech.2010.12.015. doi: 10.1016/j.ejmech.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Dangolani S. K. Panahi F. Tavaf Z. Nourisefat M. Yousefi R. Khalafi-Nezhad A. ACS Omega. 2018;3(8):10341–10350. doi: 10.1021/acsomega.8b01124. doi: 10.1021/acsomega.8b01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal P. K. Jetti S. R. Jain S. Med. Chem. Res. 2013;22(6):2984–2990. doi: 10.1007/s00044-012-0288-3. doi: 10.1007/s00044-012-0288-3. [DOI] [Google Scholar]
- Kalaria P. N. Satasia S. P. Raval D. K. New J. Chem. 2014;38(4):1512–1521. doi: 10.1039/C3NJ01327H. doi: 10.1039/C3NJ01327H. [DOI] [Google Scholar]
- Monier M. Abdel-Latif D. El-Mekabaty A. Elattar K. M. RSC Adv. 2019;9(53):30835–30867. doi: 10.1039/C9RA05687D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier M. Abdel-Latif D. El-Mekabaty A. Elattar K. M. Mini-Rev. Org. Chem. 2020;17(6):717–739. doi: 10.2174/1389557519666190925161145. [DOI] [Google Scholar]
- Elattar K. M. Rabie R. Hammouda M. M. Monatsh. Chem. 2017;148(4):601–627. doi: 10.1007/s00706-016-1852-1. [DOI] [Google Scholar]
- Elattar K. M. Rabie R. Hammouda M. M. Synth. Commun. 2016;46(18):1477–1498. doi: 10.1080/00397911.2016.1211702. [DOI] [Google Scholar]
- Elattar K. M. Mert B. D. RSC Adv. 2016;6(76):71827–71851. doi: 10.1039/C6RA12364C. [DOI] [Google Scholar]
- Monier M. Abdel-Latif D. El-Mekabaty A. Mert B. D. Elattar K. M. Curr. Org. Synth. 2019;16(6):812–854. doi: 10.2174/1570179416666190704113647. [DOI] [PubMed] [Google Scholar]
- Elattar K. M. El-Mekabaty A. J. Heterocycl. Chem. 2021;58(2):389–414. doi: 10.1002/jhet.4174. [DOI] [Google Scholar]
- Elattar K. M. Mert B. D. Monier M. El-Mekabaty A. RSC Adv. 2020;10(26):15461–15492. doi: 10.1039/D0RA00411A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khateeb A. Y. Hamed S. E. Elattar K. M. RSC Adv. 2022;12(19):11808–11842. doi: 10.1039/D2RA00927G. doi: 10.1039/D2RA00927G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki T. M. Gazdzik T. S. Arendt J. Szczepanski T. Krol W. Wielkoszynski T. J. Bone Joint Surg. Br. 2007;89(3):417–420. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- Bhat A. R. Dongre R. S. Almalki F. A. Berredjem M. Aissaoui M. Touzani R. Hadda T. B. Akhter M. S. Bioorg. Chem. 2021;106:104480. doi: 10.1016/j.bioorg.2020.104480. doi: 10.1016/j.bioorg.2020.104480. [DOI] [PubMed] [Google Scholar]
- Bhat A. R. Dongre R. S. Shalla A. H. Naikoo G. A. Hassan I. U. Arab J. Basic Appl. Sci. 2016;20:19–25. doi: 10.1016/j.jaubas.2015.12.004. [DOI] [Google Scholar]
- Bhat A. R. Shalla A. H. Dongre R. S. J. Adv. Res. 2015;6(6):941–948. doi: 10.1016/j.jare.2014.10.007. doi: 10.1016/j.jare.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar M. Naser F. Mohammad A. B. F. Synth. React. Inorg., Met.-Org., Nano-Met. Chem. 2010;40:179–185. doi: 10.1080/15533171003629121. [DOI] [Google Scholar]
- Heravi M. M. Ghods A. Bakhtiari K. Derikvand F. Synth. Commun. 2010;40(13):1927–1931. doi: 10.1080/00397910903174390. doi: 10.1080/00397910903174390. [DOI] [Google Scholar]
- Yu J. Wang H. Synth. Commun. 2005;35(24):3133–3140. doi: 10.1080/00397910500282661. doi: 10.1080/00397910500282661. [DOI] [Google Scholar]
- Shubha J. Pradeep K. P. Neelaiah B. G. Anjna B. J. Saudi Chem. Soc. 2014;18(5):535–540. doi: 10.1016/j.jscs.2011.10.023. [DOI] [Google Scholar]
- Angulwar J. A. Khansole G. S. Bhosale V. N. Int. J. Chem. Phys. Sci. 2018;7:1–9. [Google Scholar]
- Mokhtari T. S. Amrollahi M. A. Sheikhhosseini E. Sheibani H. Nezhad S. S. Curr. Bioact. Compd. 2018;14(1):54–59. doi: 10.2174/1573407213666170104153128. doi: 10.2174/1573407213666170104153128. [DOI] [Google Scholar]
- Shamroukh A. H. Zaki M. E. A. Morsy E. M. H. Abdel-Motti F. M. Abdel-Megeid F. M. E. Arch. Pharm. 2007;340(7):345–351. doi: 10.1002/ardp.200700007. doi: 10.1002/ardp.200700007. [DOI] [PubMed] [Google Scholar]
- Abd El-Sattar N. E. El-Adl K. El-Hashash M. A. Salama S. A. Elhady M. M. Bioorg. Chem. 2021;115:105186. doi: 10.1016/j.bioorg.2021.105186. doi: 10.1016/j.bioorg.2021.105186. [DOI] [PubMed] [Google Scholar]
- Patil P. Yadav A. Bavkar L. Nippu B. N. Satyanarayan N. D. Mane A. Gurav A. Hangirgekar S. Sankpal S. J. Mol. Struct. 2021;1242:130672. doi: 10.1016/j.molstruc.2021.130672. doi: 10.1016/j.molstruc.2021.130672. [DOI] [Google Scholar]
- Fayed E. A. Nosseir E. S. Atef A. et al. . Mol. Diversity. 2022;26:341–363. doi: 10.1007/s11030-021-10224-4. doi: 10.1007/s11030-021-10224-4. [DOI] [PubMed] [Google Scholar]
- Shehab W. S. El-Shwiniy W. H. J. Iran. Chem. Soc. 2018;15(2):431–443. doi: 10.1007/s13738-017-1244-4. doi: 10.1007/s13738-017-1244-4. [DOI] [Google Scholar]
- El-Agrody A. M. El-Latif A. El-Hady N. A. Fakery A. H. Bedair A. H. Molecules. 2001;6(6):519–527. doi: 10.3390/60600519. doi: 10.3390/60600519. [DOI] [Google Scholar]
- Yalagala K. Maddila S. Rana S. Maddila S. N. Kalva S. Skelton A. A. Jonnalagadda S. B. Res. Chem. Intermed. 2016;42(4):3763–3774. doi: 10.1007/s11164-015-2243-7. doi: 10.1007/s11164-015-2243-7. [DOI] [Google Scholar]
- Suresh L. Poornachandra Y. Kanakaraju S. Kumar C. G. Chandramouli G. V. P. Org. Biomol. Chem. 2015;13(26):7294–7306. doi: 10.1039/c5ob00693g. doi: 10.1039/c5ob00693g. [DOI] [PubMed] [Google Scholar]
- Suresh L. Kumar P. S. V. Chandramouli G. V. P. J. Mol. Struct. 2017;1134:51–58. doi: 10.1016/j.molstruc.2016.12.030. doi: 10.1016/j.molstruc.2016.12.030. [DOI] [Google Scholar]
- Khakiani B. A. Shirini F. Tajik H. Nahzomi H. T. Daneshvar N. J. Mol. Liq. 2021;342:117104. doi: 10.1016/j.molliq.2021.117104. doi: 10.1016/j.molliq.2021.117104. [DOI] [Google Scholar]
- Poola S. Shaik M. S. Sudileti M. Yakkate S. Nalluri V. Chippada A. Cirandur S. R. J. Chin. Chem. Soc. 2020;67(5):805–820. doi: 10.1002/jccs.201900256. [DOI] [Google Scholar]
- Bahuguna A. Rawat D. S. Med. Res. Rev. 2020;40(1):263–292. doi: 10.1002/med.21602. [DOI] [PubMed] [Google Scholar]
- Kumar S. Bhardwaj T. R. Prasad D. N. Singh R. K. Biomed. Pharmacother. 2018;104:8–27. doi: 10.1016/j.biopha.2018.05.009. doi: 10.1016/j.biopha.2018.05.009. [DOI] [PubMed] [Google Scholar]; , PMID: 29758416
- Choi H. J. Song J. H. Park K. S. Kwon D. H. Eur. J. Pharm. Sci. 2009;37(3–4):329–333. doi: 10.1016/j.ejps.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Rane J. S. Pandey P. Chatterjee A. Khan R. Kumar A. Prakash A. Ray S. J. Biomol. Struct. Dyn. 2021;39(15):5768–5778. doi: 10.1080/07391102.2020.1794969. doi: 10.1080/07391102.2020.1794969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algethami F. K. Cherif M. Jlizi S. Ben Hamadi N. Romdhane A. Elamin M. R. Alghamdi M. A. Ben Jannet H. Molecules. 2021;26(20):6103. doi: 10.3390/molecules26206103. doi: 10.3390/molecules26206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Masoudi N. A. Mohammed H. H. Hamdy A. M. Akrawi O. A. Eleya N. Spannenberg A. Pannecouque C. Langer P. Z. Naturforsch. B. 2013;68(3):229–238. doi: 10.5560/ZNB.2013-2297. doi: 10.5560/ZNB.2013-2297. [DOI] [Google Scholar]
- Hombrouck A. Hantson A. van Remoortel B. Michiels M. Vercammen J. Rhodes D. Tetz V. Engelborghs Y. Christ F. Debyser Z. Witvrouw M. J. Antimicrob. Chemother. 2007;59:1084–1095. doi: 10.1093/jac/dkm101. doi: 10.1093/jac/dkm101. [DOI] [PubMed] [Google Scholar]
- Wild S. Roglic G. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Naik M. D. Bodke Y. D. Prashantha J. Naik J. K. J. Chem. Res. 2021;2021:228–236. doi: 10.1177/1747519820964048. doi: 10.1177/1747519820964048. [DOI] [Google Scholar]
- Hese S. V. Meshram R. J. Kamble R. D. Mogle P. P. Patil K. K. Kamble S. S. Gacche R. N. Dawane B. S. Med. Chem. Res. 2017;26(4):805–818. doi: 10.1007/s00044-017-1794-0. [DOI] [Google Scholar]
- Ghaffarian F. Ghasemzadeh M. A. Aghaei S. S. J. Mol. Struct. 2019;1186:204–211. doi: 10.1016/j.molstruc.2019.03.029. doi: 10.1016/j.molstruc.2019.03.029. [DOI] [Google Scholar]
- Hasaninezhad F. Tavaf Z. Panahi F. Nourisefat M. Khalafi-Nezhad A. Yousefi R. J. Diabetes Metab. Disord. 2020;19:1505–1515. doi: 10.1007/s40200-020-00685-z. doi: 10.1007/s40200-020-00685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili S. Ghobadi N. Nazari D. Pourhossein A. Rasouli H. Adibi H. Khodarahmi R. Med. Chem. 2021;17(7):677–698. doi: 10.2174/1573406416666200506083718. [DOI] [PubMed] [Google Scholar]
- Bisenieks E. Vigante B. Petrovska R. Turovska B. Muhamadejev R. Soloduns V. Velena A. Pajuste K. Saso L. Klovins J. Duburs G. Pharmaceuticals. 2021;14(10):987. doi: 10.3390/ph14100987. doi: 10.3390/ph14100987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. K. Kumar S. Prasad D. N. Bhardwaj T. R. Eur. J. Med. Chem. 2018;151:401–433. doi: 10.1016/j.ejmech.2018.04.001. doi: 10.1016/j.ejmech.2018.04.001. [DOI] [PubMed] [Google Scholar]; , PMID: 29649739
- Abdo N. Y. M. Acta Chim. Slov. 2015;62(1):168–180. doi: 10.17344/acsi.2014.867. [DOI] [PubMed] [Google Scholar]
- Boda S. K. Pishka V. Lakshmi P. V. A. Chinde S. Grover P. Chem. Biodiversity. 2018;15(6):e18000101. doi: 10.1002/cbdv.201800101. doi: 10.1002/cbdv.201800101. [DOI] [PubMed] [Google Scholar]
- Abd El-Sattar N. E. Badawy E. H. Elrazaz E. Z. Ismail N. S. RSC Adv. 2021;11(8):4454–4464. doi: 10.1039/d0ra10321g. doi: 10.1039/d0ra10321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekal M. H. Samir S. S. Ali Y. M. El-Sayed W. M. Polycyclic Aromat. Compd. 2021:1–17. doi: 10.1080/10406638.2021.2006247. doi: 10.1080/10406638.2021.2006247. [DOI] [Google Scholar]
- Sadeghian Z. Bayat M. Safari F. Mol. Diversity. 2022:1–12. doi: 10.1007/s11030-022-10378-9. [DOI] [PubMed] [Google Scholar]
- Haggam R. A. Assy M. G. Mohamed E. K. Mohamed A. S. J. Heterocycl. Chem. 2020;57(2):842–850. doi: 10.1002/jhet.3830. [DOI] [Google Scholar]
- Bhosle M. R. Andil P. Wahul D. Bondle G. M. Sarkate A. Tiwari S. V. J. Iran. Chem. Soc. 2019;16(7):1553–1561. doi: 10.1007/s13738-019-01633-2. doi: 10.1007/s13738-019-01633-2. [DOI] [Google Scholar]
- Bhosle M. R. Wahul D. B. Bondle G. M. Sarkate A. Tiwari S. V. Synth. Commun. 2018;48(16):2046–2060. doi: 10.1080/00397911.2018.1480042. doi: 10.1080/00397911.2018.1480042. [DOI] [Google Scholar]
- Bakhotmah D. A. Ali T. E. Assiri M. A. Yahia I. S. Polycyclic Aromat. Compd. 2020 doi: 10.1080/10406638.2020.1827445. [DOI] [Google Scholar]
- Seifert T. Malo M. Kokkola T. Stéen E. J. L. Meinander K. Wallén E. A. Jarho E. M. Luthman K. Bioorg. Med. Chem. 2020;28(2):115231. doi: 10.1016/j.bmc.2019.115231. doi: 10.1016/j.bmc.2019.115231. [DOI] [PubMed] [Google Scholar]
- Bansal A. Bali A. Balaini A. Lett. Drug Des. Discovery. 2020;17(12):1566–1578. doi: 10.2174/1570180817999200706005247. [DOI] [Google Scholar]
- Sehajpal S. Prasad D. N. Singh R. K. Mini-Rev. Med. Chem. 2018;18(14):1199–1219. doi: 10.2174/1389557518666180330112416. doi: 10.2174/1389557518666180330112416. [DOI] [PubMed] [Google Scholar]; , PMID: 29600762
- AbdEl-Azim M. H. Aziz M. A. Mouneir S. M. EL-Farargy A. F. Shehab W. S. Arch. Pharm. 2020;353(9):e2000084. doi: 10.1002/ardp.202000084. doi: 10.1002/ardp.202000084. [DOI] [PubMed] [Google Scholar]
- Kücükakin B. Gögenur I. Reiter R. J. Rosenberg J. J. Surg. Res. 2009;152(2):338–347. doi: 10.1016/j.jss.2007.12.753. [DOI] [PubMed] [Google Scholar]
- Ali T. E. Bakhotmah D. A. Assiri M. A. Synth. Commun. 2020;50(21):3314–3325. doi: 10.1080/00397911.2020.1800744. doi: 10.1080/00397911.2020.1800744. [DOI] [Google Scholar]
- Dahlbäck B. J. Intern. Med. 2005;257(3):209–223. doi: 10.1111/j.1365-2796.2004.01444.x. [DOI] [PubMed] [Google Scholar]
- Debbabi M. Nimbarte V. D. Chekir S. Chortani S. Romdhane A. Bioorg. Chem. 2019;82:129–138. doi: 10.1016/j.bioorg.2018.10.004. doi: 10.1016/j.bioorg.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Traxler P. Bold G. Buchdunger E. Caravatti G. Furet P. Manley P. O'Reilly T. Wood J. Zimmermann J. Med. Res. Rev. 2001;21(6):499–512. doi: 10.1002/med.1022. [DOI] [PubMed] [Google Scholar]
- Zaman M. Nguyen M. L. Blennerhassett J. D. Quin B. F. Biol. Fertil. Soils. 2008;44(5):693–705. doi: 10.1007/s00374-007-0252-4. [DOI] [Google Scholar]
- Cantarella H. Otto R. Soares J. R. de Brito Silva A. G. J. Adv. Res. 2018;13:19–27. doi: 10.1016/j.jare.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziarani G. M. Faramarzi S. Asadi S. Badiei A. Bazl R. Amanlou M. Daru, J. Pharm. Sci. 2013;21(1):1–13. doi: 10.1186/2008-2231-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; , https://www.darujps.com/content/21/1/3