Highlights
-
•
22 edible oils can be discriminated based on tocopherol and phytosterol contents.
-
•
In vitro antioxidant activity is correlated to polyphenol, tocopherol, and squalene.
-
•
Oxidative and heat stress resistance is correlated to tocopherol and phytosterol.
-
•
In vivo antioxidant activity is correlated to polyphenol, squalene, MUFA and PUFA.
Keywords: Fatty acid, Phytochemical, Antioxidant capacity, Stress resistance, Chemometrics
Abstract
In the last decade, with a growing emphasis on healthy diets, functional edible oils with high nutritional quality are becoming increasingly popular around the world. This study systematically compared the chemical composition and protective effect of 22 vegetable oils using multivariate chemometric tools. The results showed that the fatty acid composition and minor compounds were extremely variable among tested oils. Hierarchical cluster and principal component analysis discriminated these oils according to the tocopherol and phytosterol contents. The Pearson’s correlation analysis indicated that in vitro radical scavenging capacity was significantly correlated to polyphenol, tocopherol, and squalene. Additionally, the ameliorate effects on the heat and oxidative stress, ROS contents, and antioxidant enzyme activities were measured in Caenorhabditis elegans. The results showed that the antioxidant activity and stress resistance were positively correlated to polyphenol, tocopherol, phytosterol, MUFA, and PUFA, respectively. This study may offer an insight into oil discrimination and functional oil exploitation.
1. Introduction
Aging is defined as a universal and irreversible natural deterioration of functions in cells, tissues, organs, and organisms, which significantly raises the risk of developing many aging-related diseases, including type-2 diabetes mellitus, neurodegenerative disease, cancer, cardiovascular diseases, and other chronic diseases. Exploring potential strategies to delay aging and ameliorate the pathogenesis of aging has attracted tremendous attention. In particular, research concentrated on dietary and nutritional interventions to improve the healthspan has become a hot topic in this field. Many studies have shown that supplementation of dietary bioactive substances significantly extended the lifespan and healthspan across different animal models (Yang et al., 2018).
Edible plant oils (EPOs) are dietary fatty acids derived from seeds, pulps, fruits, and plumules of oil crops. As an indispensable nutritional resource for human health, EPOs is primarily consumed for edible purposes and applied in the cosmetics and nutraceutical industry. In the market, the conventional EPOs included soybean oil, sunflower oil, rapeseed oil, corn oil, and peanut oil. Recently, with the increasing emphasis on healthy diets, consuming novel EPOs with high nutritional value has been encouraged by the World Health Organization (Mensink, 2016). The standpoint of using EPOs with high unsaturated fatty acids instead of animal oil for cooking to reduce the mortality of cardiovascular and cerebrovascular diseases is increasingly recognized (Przykaza et al., 2021).
The adherence dietary habit to EPOs derived from some woody crops, such as olive oil, walnut oil and oil-tea seed (Camellia oleifera Abel.) oil, contributes to numerous health benefits, like reducing the risk of aging-associated diseases, extending longevity, and promoting healthspan (Yang et al., 2018). The beneficial effects between these EPOs are mainly related to unsaturated fatty acids and various functional phytochemicals, including polyphenol, tocopherol, squalene, and phytosterol. In addition, adjusting the ratio of certain unsaturated fatty acids, low level of n-6/n-3 Polyunsaturated fatty acids (PUFA) ratio in the diet, for instance, improves lipid metabolism, inflammation, and oxidative stress in rats (Zou et al., 2018).
For decades, dozens of new oils crops have been approved to be cultivated in China, which leads to a growing number of novel EPOs emerging in the market. These novel lipid products are reported to exert many unique nutritional characteristics, such as anti-obesity, anti-hyperlipidemia, anti-arthritic activities, anti-inflammation, and anti-oxidative stress (Yang et al., 2018). In order to screen high-nutritional value edible oils, comprehensive composition determination and bioactivities evaluation coupling with chemometrics tools had been applied based on nutrition, composition, and functional properties among EPOs. Several studies utilized hierarchical cluster analysis (HCA) and principal component analysis (PCA) to discriminate different EPOs according to the lipid composition and minor constituent profile (Deng et al., 2018, Shi et al., 2018). The Pearson's correlation analysis was applied to demonstrate that the phenolic content in extra virgin olive oils was positively correlated to the antioxidant activity of protecting cells from oxidative stress (Presti et al., 2017). Multiple linear regression analysis of different walnut oils indicated unsaturated fatty acid, certain kinds of saturated fatty acid, like C16:0 and C20:0, tocopherols, squalene, and phytosterols, disparately contribute to the free radical scavenging capacity (Gao et al., 2019). Many efforts went into assessing the associations between oils composition and ROS scavenging effects based on in vitro assay. However, the correlation to in vivo antioxidant properties, such as enhancing stress resistance and triggering the intrinsic antioxidant defense system in living organisms, remains unclear (Shi et al., 2018).
The soil-dwelling nematode Caenorhabditis elegans has emerged as a conventional in vivo animal model to study aging and interventions affecting the aging process. It is not only because of their short lifespan and ease of pharmacological manipulations but also the highly conserved genome sequences. For example, the core lipid metabolic pathways, including fatty acid β-oxidation, transporter, synthesis, elongation, and desaturation, are well conserved from worms to mammals. Studies on lipid metabolism using C. elegans have attracted extensive attention due to human obesity and diabetes epidemics. Since only 20% of fatty acid can be synthesized de novo from acetyl-CoA in worms, the roles of dietary fatty acids for worms are emerging as a hot spot in this field. Recently, numerous studies have concentrated on the connection between lipids and lifespan in C. elegans (Chen et al., 2019). Some dietary and endogenous synthesized MUFAs were reported to regulate the longevity and healthspan of worms (Han et al., 2017, Watts and Ristow, 2017). In addition, minor compounds in EPOs, like polyphenol, tocopherols, and phytosterols, were also proved to enhance the stress resistance in worms (Chang et al., 2021). Although a large number of studies revealed these beneficial effects from the perspective of single vegetable oil or oil-derived substance. The comparison studies among different EPOs are still needed to clarify the preferable features of chemical composition contributing to the healthspan promoting effects.
In the present study, the fatty acid composition of 22 EPOs was characterized. The content of major bioactive compounds in EPOs, including polyphenol, tocopherols, squalene, and phytosterols, were measured. In vitro free radical scavenging capacities were assessed by DPPH and FRAP. In addition, the in vivo stress resistance activities were tested in C. elegans. The similarities/differences and the correlation between chemical composition and healthspan promoting effects of 22 EPOs were analyzed by various chemometrics tools.
2. Materials and methods
2.1. Materials and reagents
Vegetable oils, namely, peanut oil (PNO), soybean oil (SBO), corn oil (CO), flaxseed oil (FO), rapeseed oil (RSO), sunflower seed oil (SUSO), sesame seed oil (SESO), cotton seed oil (CSO), rice bran oil (RBO), pumpkin seed oil (PKSO), hemp seed oil (HSO), perilla seed oil (PLSO), peony seed oil (PNSO), safflower seed oil (SASO), grape seed oil (GSO), and seven woody oil, namely, walnut oil (WO), palm oil (PO), coconut oil (CNO), olive oil (OO), oil-tea seed oil (OSO), yellowhorn seed oil (YSO), sacha inchi oil (SIO) were obtained from local markets.
The Standards for fatty acid methyl esters, α-tocopherol, squalene, campesterol, stigmasterol, and β-sitosterol were from Sigma-Aldrich (St. Louis, MO, USA). The solvents used for the HPLC analysis are chromatography grade from Merck (Darmstadt, Germany). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and 2′,7′-dichlorofluorescein diacetate (H2DCF-DA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals and solvents, like Folin-Ciocalteu, sodium carbonate, and potassium persulfate, were analytical grade purchased from Aladdin (Shanghai, China). Superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.2. Animals
Wild-type N2 strains were obtained from Caenorhabditis Genetics Centre (CGC) and maintained at 20 °C on nematode growth medium (NGM). 0.25 mg/mL of EPO was selected for all worm assays based on concentration screens (Supplementary Data Fig. S4).
2.3. Analysis of peroxide value and acidity
The peroxide value and acidity of different EPOs were measured by the method of American Oil Chemists' Society (American Oil Chemists' Society, 1997, American Oil Chemists' Society, 2009).
2.4. Analysis of fatty acid composition
Methyl esterification of EPOs samples was prepared following a previous study with some modification (Gao et al., 2019). The obtained fatty acid methyl esters (FAME) was analyzed by an Agilent 7890B gas chromatograph (GC) (Agilent Technologies, Santa Clara, CA, USA) equipped with an HP-5 MS capillary column (0.25 µm, 30.0 m × 0.22 mm) and a 5977A mass detector (MSD) (Agilent Technologies, Santa Clara, CA, USA). The temperature of the injector was 250 °C, and the injection volume was 1.0 µL. The split ratio was set at 50:1. The oven temperature started at 80 °C for 1 min, then raised to 160 °C for 1 min, and finally programmed to 200 °C for 1 min. Helium was used as the carrier gas. The conditions for mass detector were as follows Electron impact (EI) ion source, scan range from 50 to 500 m/z, and 3 mins of solvent delay. The peaks were identified by comparing their retention index (RI) with standards and mass spectra with a computerized MS-database using NIST data.
2.5. Analysis of tocopherols and squalene
The contents of tocopherols and squalene were measured by an Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a ZORBAX SB-C18 column (4.6 mm, 150 mm, 5.0 μm) and a Diode Array Detector (DAD) following a previous study with some modification (Gao et al., 2019). In brief, phase A was methanol: acetonitrile (70:30) and phase B was methanol: isopropanol: n-hexane (15:65:20). Accurately weighed 1.5 g of oil, and dissolved it in phase B and fixed the volume in a 25 mL volumetric flask. Similarly, squalene and tocopherol standards were dissolved in phase B and mixed standard curve was prepared so that the standard curve range of squalene is 5–300 µg/mL and the standard curve concentration of tocopherol is 10–700 µg/mL. The gradient elution profile was programmed as follows: 0–25 min, 100% phase A; 25–27 min, 100% − 30% phase A; 27–37 min, 30% phase A; 37–39 min, 30–100% phase A; 39–50 min, 100% phase A. The measurement conditions for tocopherols and squalene were as follows: 10.0 µL of injection volume, mobile phase with a flow rate of 0.8 mL/min, 35 °C of column temperature, and 210 nm of determining wavelength.
2.6. Analysis of phytosterols
The extraction of phytosterols from EPOs was based on the previous method (Gao et al., 2019). To saponify EPOs, 500 mg of oils were mixed with 5 mL ethanolic (95%)/hydroxide (2 M) and heated at 85 °C for 1 h. The unsaponifiable matter in oils was extracted with 5 mL n-hexane, dried with nitrogen, then dissolved in 5 mL toluene. The phytosterols content was measured by Agilent 7890 GC–MS (Agilent Technologies, Santa Clara, CA, USA) equipped with an HP-5 MS capillary column 30 mm × 0.25 mm × 0.25 μm). Helium with a flow rate of 1.2 mL/min was used as the carrier gas. The temperature rise procedure was set at 200 °C for 1 min, then raised to 300 °C at a rate of 10 °C/min, and maintained for 15 min. The injection temperature was 290 °C. The injection volume was 5 μL. The split ratio was set to 30:1. The conditions for mass detector were as follows: EI ion source, scan range from 50 to 500 m/z, and 3 mins of solvent delay. The peaks were identified by comparing their RI with standards and mass spectra with a computerized MS-database using NIST data. The concentration range of the standard curve was 15.625 μg/mL to 500 μg/mL. According to the standard curve, the peak areas of three phytosterols were used to quantify the absolute content.
2.7. Analysis of total polyphenol
The total polyphenol content in samples was measured using the Folin–Ciocalteu reagent described previously with some modification (Gao et al., 2019). Briefly, the total polyphenol in 1 g oil samples was extracted with 5.0 mL of methanol for three times. 0.2 mL combined supernatants were mixed with 0.8 mL methanol and 1 mL Folin-Ciocalteu reagent. After 5 mins incubation, 1 mL sodium carbonate solution (7% w/v) was added to the reaction mixture. After 2 h of incubation in the dark, the absorbance at 760 nm was measured by a micro-plate reader (Spectra Max M2, Molecular Devices, Sunnyvale, CA, USA). The total phenols content (TPC) was expressed as gallic acid equivalent (GAE mg/100 g).
2.8. Analysis of in vitro antioxidant capacity
The DPPH radical scavenging assay was performed as described previously with some modification (Cheng, et al., 2013). In brief, 1 mL of samples was incubated with 3 mL of 0.2 mM ethanol DPPH for 30 mins in the dark. The absorbance of the mixture was measured at 517 nm (Beckman UV–Vis spectrophotometer, California, USA). FRAP was performed as described previously with some modifications (Cheng et al., 2013). In brief, 2.5 g of oil was extracted with 3 mL of methanol, stirred in the dark for 10 min, then centrifuged at 4500 rpm for 5 min. The extraction steps were repeated three times, and the methanol phase was combined and collected as oil extract sample for subsequent measurement. Fe3+-TPTZ reagent was prepared by mixing 10 mM TPTZ (in 40 mM HCl), 20 mM FeCl3, and 300 mM acetate buffer (pH 3.6) in a ratio of 1:1:10 (v/v/v). 100 μL sample was incubated with 500 μL FRAP reagent for 10 mins in the dark. The absorbance of the mixture was measured at 593 nm (Beckman UV–Vis spectrophotometer, California, USA). The in vitro antioxidant capacity of oil samples was expressed as Trolox equivalent (TE µmol/100g).
2.9. Analysis of stress resistance
The oxidative stress resistance assay in wild-type worms was carried out as described previously (Feng et al., 2018). In brief, synchronized L1 larvae were maintained in liquid culture supplemented with sample oils. After 48 h of treatment, the worms were exposed to 50 mM paraquat to induce lethal oxidative stress. The number of alive, escaped, and dead worms were checked every hour for 12 h. The results for oxidative stress resistance were shown as the mean lifespan calculated by Kaplan-Meier analysis and log-rank test.
The heat stress resistance assay in wild-type worms was evaluated as described previously (Feng et al., 2018). In brief, synchronized L1 larvae were maintained in liquid culture supplemented with sample oils. After 48 h of treatment, the worms were transferred to 35 °C to induce lethal heat stress. The number of alive, escaped, and dead worms were checked every hour for a total of 12 h. The results for heat stress resistance were shown as the mean lifespan calculated by Kaplan-Meier analysis and log-rank test.
2.10. Analysis of intracellular ROS
The intracellular reactive oxygen species (ROS) contents in worms after incubation with EPOs were evaluated by H2DCF-DA assay according to the method described previously (Feng et al., 2018). In brief, synchronized L1 larvae were cultured with sample oils. After 48 h treatment, worms were washed three times with S-basal. In order to induce ROS generation, the worms were exposed to 10 mM paraquat for 24 h. Then the worms were incubated with 20 μM H2DCF-DA, a ROS indicator, for half-hour. The fluorescent oxidized products were measured by a fluorescence microscope (BX53, Olympus Corporation, Tokyo, Japan). The level of intracellular ROS was quantified by Image J software (National Institutes of Health, Bethesda, MD, USA). At least 20 worms were tested in each group with three replications. Results were expressed as intracellular ROS inhibition abilities calculated by comparing to the non-oil treated control group.
2.11. Analysis of in vivo antioxidant enzyme activity
Superoxide dismutases (SODs) and catalases (CATs) activities in worms were determined as previously described (Feng et al., 2018). Briefly, synchronized L1 worms were treated with samples for 48 h. Then, the worms were moved to 35 °C to induce the activities of SODs and CATs. After 3 h of treatment, worms were washed with M9 buffer and crushed by an ultrasonic wave. The supernatants were collected for total protein content and enzyme activities test following the guide from corresponding kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The total protein content was used to normalize the enzyme activities among different groups.
2.12. Analysis of malondialdehyde
The level of malondialdehyde (MDA) was determined as previously described with slight modifications (Feng et al., 2018). In brief, age-synchronized wild-type nematodes were treated with oil samples at 25 °C. After 48 h, worms were washed with M9 buffer and crushed by an ultrasonic wave. The supernatants were collected for total protein content and MDA content test following the guide from corresponding kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The total protein content was used to normalize the MDA level among different groups. Results were expressed as MDA inhibition abilities calculated by comparing to the non-oil treated control group.
2.13. Statistical analysis
All data were expressed as the mean ± standard deviation (S.D.) from three replications. Survival analysis was performed by GraphPad Prism 8.0 (GraphPad Prism Software Inc., San Diego, CA, USA). The significant differences (P ≤ 0.05) were calculated using one-way analysis of variance (ANOVA) with post-hoc contrasts by Bonferroni's test. HCA was used to discriminate different oils, and PCA and bivariate correlations analysis (Pearson’s Correlation) were employed to identify the interrelationships among EPOs with SIMCA 14.1 software (Umetrics, Umea, Sweden).
3. Results and discussion
3.1. Physicochemical properties
The physicochemical properties of different EPOs are listed in Table 1. Acid values represent the rancidity of oil samples, which is mainly attributed to the decomposition of triacylglycerol and the increase of free fatty acids. Edible oil with a low acid value is regarded as a high-quality product. In the present study, the acid values of all tested EPOs ranging from 0.08 to 1.06 mg KOH/g were within the permitted levels according to the Hygienic Standard of Edible Vegetable Oil in China (<3 mg KOH/g). Hemp seed oil (HSO) had the highest acid value, while walnut oil (WO) had the lowest.
Table 1.
Acid value (mg KOH/g), peroxide value (mmol/kg), and fatty acid compositions (g/100 g) in 22 EPOs.
| Component | Conventional oil crops |
Novel oilseed crops |
Woody oil crops |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNO | SBO | CO | FO | RSO | SUSO | SESO | CSO | RBO | PKSO | HSO | PLSO | PNSO | SASO | GSO | WO | PO | CNO | OO | OSO | YSO | SIO | |
| Acid value | 0.70 ± 0.01 | 0.14 ± 0.03 | 0.12 ± 0.01 | 0.65 ± 0.01 | 0.47 ± 0.01 | 0.57 ± 0.03 | 0.22 ± 0.01 | 0.61 ± 0.01 | 0.30 ± 0.02 | 0.29 ± 0.01 | 1.06 ± 0.25 | 0.55 ± 0.04 | 0.51 ± 0.03 | 0.34 ± 0.01 | 0.60 ± 0.02 | 0.08 ± 0.01 | 0.37 ± 0.01 | 0.16 ± 0.01 | 0.32 ± 0.01 | 0.58 ± 0.02 | 0.65 ± 0.01 | 0.36 ± 0.23 |
| Peroxidevalue | 1.89 ± 0.60 | 2.72 ± 0.18 | 2.94 ± 0.08 | 0.75 ± 0.06 | 5.40 ± 0.08 | 0.74 ± 0.11 | 3.74 ± 0.11 | 6.81 ± 0.13 | 1.39 ± 0.20 | 2.03 ± 0.15 | 3.72 ± 0.19 | 1.67 ± 0.27 | 1.26 ± 0.04 | 0.31 ± 0.01 | 0.21 ± 0.03 | 1.76 ± 0.09 | 0.53 ± 0.03 | 2.48 ± 0.09 | 1.11 ± 0.06 | 0.85 ± 0.11 | 2.50 ± 0.03 | 3.62 ± 0.16 |
| C16:0 | 3.77 ± 0.58 | 4.68 ± 0.25 | 5.39 ± 0.54 | 2.21 ± 0.34 | 0.88 ± 0.59 | 2.24 ± 0.45 | 2.68 ± 0.76 | 2.13 ± 0.76 | 2.60 ± 0.45 | 2.39 ± 0.67 | 3.16 ± 0.49 | 3.19 ± 0.31 | 2.07 ± 0.25 | 1.82 ± 0.06 | 2.26 ± 0.61 | 2.79 ± 0.10 | 16.62 ± 0.13 | 2.86 ± 0.73 | 3.35 ± 0.19 | 3.28 ± 0.48 | 1.41 ± 0.43 | 3.69 ± 0.82 |
| C18:0 | 12.57 ± 0.13 | 3.68 ± 0.01 | 1.36 ± 0.11 | 4.28 ± 0.12 | 0.80 ± 0.01 | 2.83 ± 0.15 | 3.93 ± 0.07 | 0.98 ± 0.09 | 0.89 ± 0.08 | 5.65 ± 0.06 | 3.07 ± 0.04 | 2.58 ± 0.00 | 1.38 ± 0.00 | 1.60 ± 0.09 | 1.40 ± 0.01 | 2.55 ± 0.13 | 3.46 ± 0.09 | 2.13 ± 0.01 | 0.86 ± 0.03 | 1.79 ± 0.16 | 1.20 ± 0.05 | 3.18 ± 1.31 |
| C18:1 | 28.19 ± 0.91 | 27.30 ± 1.58 | 27.18 ± 1.68 | nd | 23.94 ± 0.70 | 21.08 ± 1.71 | 29.35 ± 1.31 | 14.43 ± 0.21 | 36.64 ± 0.35 | 30.22 ± 1.18 | 29.53 ± 0.84 | nd | nd | 8.04 ± 1.24 | 11.88 ± 0.61 | 32.38 ± 0.23 | 56.26 ± 0.58 | 2.74 ± 0.30 | 50.24 ± 1.69 | 64.53 ± 0.89 | 21.03 ± 1.09 | 7.61 ± 2.81 |
| C18:2 | 23.60 ± 0.99 | 48.30 ± 0.71 | 50.05 ± 0.67 | 8.62 ± 0.65 | 7.59 ± 0.54 | 54.09 ± 1.72 | 31.05 ± 0.66 | 40.23 ± 0.36 | 31.50 ± 1.05 | 54.00 ± 1.09 | 57.85 ± 0.47 | 28.30 ± 1.31 | 22.76 ± 0.50 | 58.65 ± 1.40 | 63.45 ± 1.52 | 60.63 ± 0.30 | 14.31 ± 0.59 | nd | 2.82 ± 0.25 | 6.61 ± 0.25 | 28.83 ± 0.56 | 37.72 ± 4.81 |
| C18:3 | nd | nd | nd | 77.34 ± 1.19 | nd | nd | nd | nd | nd | nd | nd | 53.56 ± 1.02 | 66.60 ± 0.74 | nd | nd | nd | nd | nd | nd | nd | nd | 45.41 ± 7.26 |
| SFA | 16.94 ± 0.45 | 8.35 ± 0.24 | 6.75 ± 0.43 | 6.48 ± 0.21 | 17.30 ± 0.59 | 5.08 ± 0.31 | 6.60 ± 0.69 | 7.11 ± 0.67 | 7.49 ± 0.37 | 8.03 ± 0.61 | 6.23 ± 0.45 | 5.77 ± 0.23 | 3.45 ± 0.10 | 3.43 ± 0.02 | 3.65 ± 0.60 | 5.34 ± 0.03 | 20.82 ± 0.04 | 57.49 ± 0.72 | 4.21 ± 0.16 | 5.07 ± 0.33 | 6.29 ± 0.38 | 6.87 ± 1.06 |
| MUFA | 28.19 ± 0.91 | 27.30 ± 1.58 | 27.18 ± 1.68 | nd | 39.11 ± 0.70 | 21.08 ± 1.71 | 29.35 ± 1.31 | 14.43 ± 0.21 | 36.64 ± 0.35 | 30.22 ± 1.18 | 29.53 ± 0.84 | nd | nd | 8.04 ± 1.24 | 11.88 ± 0.61 | 32.38 ± 0.23 | 56.26 ± 0.58 | 2.74 ± 0.30 | 50.24 ± 1.69 | 64.53 ± 0.89 | 24.71 ± 1.09 | 7.61 ± 2.81 |
| PUFA | 23.60 ± 0.99 | 48.30 ± 0.72 | 50.05 ± 0.68 | 85.96 ± 1.84 | 7.59 ± 0.54 | 54.09 ± 1.78 | 31.05 ± 0.71 | 40.23 ± 0.36 | 31.50 ± 1.09 | 54.00 ± 1.14 | 57.85 ± 0.48 | 81.86 ± 2.33 | 89.35 ± 1.24 | 58.65 ± 1.42 | 63.45 ± 1.52 | 60.63 ± 0.35 | 14.31 ± 0.61 | nd | 2.82 ± 0.28 | 6.61 ± 0.27 | 28.83 ± 0.63 | 83.13 ± 8.73 |
Peanut oil (PNO); soybean oil (SBO); corn oil (CO); flaxseed oil (FO); rapeseed oil (RSO); sunflower seed oil (SUSO); sesame seed oil (SESO); cotton seed oil (CSO); rice bran oil (RBO); pumpkin seed oil (PKSO); hemp seed oil (HSO); perilla seed oil (PLSO); peony seed oil (PNSO); safflower seed oil (SASO); grape seed oil (GSO); walnut oil (WO); palm oil (PO); coconut oil (CNO); olive oil (OO); oil-tea seed oil (OSO); yellowhorn seed oil (YSO); sacha inchi oil (SIO). C16:0 palmitic acid; C18:0 stearic acid; C18:1 oleic acid; C18:2 linoleic acid; C18:3 linolenic acid. Values are mean ± S.D.; nd means no detected.
Peroxide values were used to estimate the oxidation degree of oil samples. EPO with a low peroxide value (<7.5 mmol/kg) is considered as a high-quality product. In tested samples, cotton seed oil (CSO) had the highest peroxide value of 6.81 mmol/kg, followed by rapeseed oil (RSO) with the peroxide value of 5.40 mmol/kg. In contrast, grape seed oil (GSO) and safflower seed oil (SASO) had relatively low peroxide values, 0.21 and 0.31 mmol/kg, respectively. Overall, the acid and peroxide values of all EPOs used in the present study conformed to the edible vegetable oil standard.
3.2. Fatty acid composition
As one of the essential nutrients, dietary fatty acids are divided into saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), and polyunsaturated fatty acid (PUFA) according to the number of double carbon–carbon bonds. The composition of dietary fatty acids has dramatic effects on the health of mammals. Some SFAs were reported to exhibit detrimental effects, whereas intake of MUFAs and PUFAs reduced the risks of cardiovascular and other metabolic diseases, subsequently improving the healthspan (Lee et al., 2015). Therefore, the lipid composition is crucial for evaluating the quality of edible oils. The dominating fatty acid components in 22 EPOs, including palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3), were listed in Table 1. The total SFA content was also calculated from the amalgamation of saturated fatty acids from C8:0 to C22:0. The total MUFA content was obtained by combining C18:1, C20:1, and C22:1. Furthermore, the total PUFA content was amalgamated by C18:2 and C18:3. Coconut oil (CNO) had the highest SFA content which mainly consisted of medium-chain SFAs, like octanoic acid (C8:0), decanoic acid (C10:0), lauric acid (C12:0), and myristic acid (C14:0). The medium-chain SFAs accounted for 81.42% of total lipid content in CNO. This distinctive composition pattern was in agreement with other reported coconut oils (Narayanankutty, Illam, & Raghavamenon, 2018). In conventional oil crops, flaxseed oil (FO) had the highest unsaturated fatty acids (85.96 g/100 g) which was basically in line with a previous report (Varas Condori et al., 2020). However, in another traditional vegetable oil, the content of sesame oil (SESO) was slightly lower than that of other results, which might be attributed to different sesame cultivars (Rodríguez et al., 2020). Compared with conventional oil crops, most of novel oilseed crops were abundant with unsaturated fatty acids, and the unsaturated fatty acids content of PKSO, HSO, PLSO and PNSO exceeded 81 g/100 g, similar to former reports (Tura et al., 2022, X. Wang et al., 2021, Z. Wang et al., 2021). The relatively higher MUFA contents were demonstrated from three woody oils, oil-tea oil (OSO), palm oil (PO), and olive oil (OO). The levels of oleic acid, the major MUFA in OSO and OO, were 64.53 and 50.24 g/100 g, lower than former reports, 76.18% and 72.77%, respectively (Guo et al., 2017, Liu et al., 2021, Z. Wang et al., 2021, Suealek et al., 2021). The oleic acid in PO was 56.26 g/100 g which was in agreement with a crude palm oil study ranging from 47.4% to 53.5% (Morcillo et al., 2021). For PUFA, peony seed oil (PNSO), perilla seed oil (PLSO), and flaxseed oil (FO) had comparative higher contents ranging from 81.86 to 89.35 g/100 g. Similar to previous reports, linolenic acid comprised the majority of PUFA in FO, PNSO, and PLSO (Yang et al., 2018, Varas Condori et al., 2020), while grape seed oil (GSO), walnut oil (WO), safflower oil (SASO), and hemp seed oil (HSO) contained high amounts of linoleic acid, ranged from 57.85 to 60.64 g/100 g (Yang et al., 2018). The linoleic acid in sacha inchi oil was 37.72 g/100 g, which was slightly higher than 33.4 g/100 g from the report of Rodríguez et al, but the linolenic acid content (45.41 g/100 g) was lower than the reported results (53.8 g/100 g), which might due to different the cultivar, location, and growing condition (Rodríguez et al. 2021). Overall, our data are basically in line with previous reports (Calzolari et al., 2021, Gu et al., 2019, Liu et al., 2021, Sun et al., 2021).
3.3. Oil-derived bioactive compounds
Total polyphenol represents a large class of potential natural antioxidants consisting of phenolic acids and other phenolic compounds in edible plant oils. Clinical trials and epidemiological studies have revealed that dietary phenolic compounds exhibit broad therapeutic health effects for various chronic diseases, including cancer, neurodegenerative diseases, diabetes, and cardiovascular diseases. In recent decades, their roles in intervening in aging and aging-related diseases have been intensively studied. Dietary phenolic compounds, including EGCG, epicatechin, resveratrol, and quercetin, have been demonstrated to improve lifespan and healthspan in different animal models (Y. Wang et al., 2021). In the present study, the total polyphenol content TPC in tested EPOs ranging from 12.09 to 151.70 GAE mg/kg was listed in Table 2. Grape seed oil (GSO) had the highest polyphenol levels of 151.70 GAE mg/kg, followed by cotton seed oil (CSO) and sesame seed oil (SESO) with the polyphenol content of 98.56 and 96.21 GAE mg/kg. These results were slightly different from previous studies. Wen et al. demonstrated that the total phenol content in grape seed oil was 75.81 GAE mg/kg (Wen et al., 2016). The phenolic contents in sesame seed oil samples varied from 10.75 to 250.14 GAE mg/kg (Shi et al., 2018). Compared to the TPC of other EPOs with literature, oil-tea seed oil (OSO) and corn oil (CO) have relatively higher polyphenol contents of 74.56 and 41.72 GAE mg/kg. Nevertheless, TPC in pumpkin seed oil (PKSO), safflower seed oil (SASO), and rice bran oil (RBO) were comparatively low, which were 41.67, 30.39, and 19.93 GAE mg/kg, compared to the previous results of 128.84, 231.40, and 56.32 GAE mg/kg, respectively (Jiao et al., 2014, Yang et al., 2018). Among tested oils, The TPC of PNSO was 49.41 GAE mg/kg which was higher than a previous result of 4.78 GAE mg/kg (L. Wang et al., 2021). Furthermore, sunflower seed oil (SUSO) had the lowest polyphenol levels of 12.09 GAE mg/kg, higher than a former study reporting 3.30 GAE mg/kg of TPC (Janu et al., 2014).
Table 2.
Total polyphenol contents (GAE mg/kg) and phytochemical contents (mg/100 g) in 22 EPOs.
| Component | Conventional oil crops |
Novel oilseed crops |
Woody oil crops |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNO | SBO | CO | FO | RSO | SUSO | SESO | CSO | RBO | PKSO | HSO | PLSO | PNSO | SASO | GSO | WO | PO | CNO | OO | OSO | YSO | SIO | |
| Polyphenol | 37.47 ± 5.61 | 38.05 ± 5.68 | 41.72 ± 3.67 | 29.08 ± 3.75 | 24.05 ± 5.06 | 12.09 ± 3.10 | 96.21 ± 11.60 | 98.56 ± 13.69 | 19.93 ± 1.96 | 41.67 ± 9.12 | 45.69 ± 3.00 | 38.63 ± 8.93 | 49.41 ± 5.80 | 30.39 ± 5.55 | 151.70 ± 9.32 | 43.57 ± 5.13 | 49.87 ± 7.86 | 45.59 ± 7.33 | 62.96 ± 7.40 | 74.56 ± 8.00 | 31.57 ± 4.74 | 8.15 ± 3.12 |
| Tocopherol | 57.69 ± 1.09 | 122.35 ± 2.30 | 216.98 ± 3.23 | 235.57 ± 2.85 | 92.98 ± 1.64 | 120.58 ± 2.90 | 154.11 ± 1.90 | 498.40 ± 7.30 | 448.64 ± 8.38 | 602.50 ± 12.54 | 232.10 ± 4.53 | 121.21 ± 0.84 | 369.47 ± 0.46 | 193.23 ± 4.64 | 328.16 ± 29.77 | 245.08 ± 5.53 | 67.58 ± 1.57 | 51.86 ± 0.70 | 38.81 ± 0.37 | 171.52 ± 0.63 | 78.60 ± 1.99 | 110.12 ± 8.13 |
| Squalene | 14.65 ± 0.38 | 13.74 ± 1.12 | 8.13 ± 0.62 | 82.98 ± 1.78 | 5.70 ± 0.01 | 18.68 ± 0.44 | 8.76 ± 0.08 | 15.78 ± 0.04 | 75.79 ± 4.38 | 66.69 ± 1.18 | 13.91 ± 0.33 | 27.90 ± 0.72 | 18.69 ± 0.29 | 5.61 ± 0.16 | 11.82 ± 3.31 | 9.04 ± 3.58 | 48.31 ± 0.67 | 51.47 ± 4.71 | 234.75 ± 2.98 | 14.52 ± 0.58 | 11.26 ± 0.25 | 21.25 ± 4.28 |
| Campesterol | 3.00 ± 0.60 | 10.64 ± 1.95 | 43.65 ± 10.84 | 13.11 ± 2.48 | 48.22 ± 3.99 | 3.15 ± 0.49 | 6.52 ± 2.80 | 8.54 ± 0.63 | 132.13 ± 5.66 | 45.53 ± 0.19 | 7.20 ± 0.62 | 5.34 ± 1.12 | 1.20 ± 0.05 | 3.03 ± 0.45 | 1.87 ± 1.37 | nd | 0.17 ± 0.02 | 0.47 ± 0.07 | 3.54 ± 0.82 | nd | nd | 1.54 ± 0.61 |
| Stigmasterol | 3.11 ± 0.44 | 12.73 ± 1.76 | 9.59 ± 5.43 | 3.10 ± 0.34 | 3.32 ± 0.14 | 4.20 ± 0.44 | 5.44 ± 4.20 | nd | 90.42 ± 2.30 | 3.52 ± 0.14 | 0.55 ± 0.78 | 8.37 ± 0.05 | nd | 1.81 ± 0.55 | 4.94 ± 1.36 | nd | 0.88 ± 0.22 | 3.10 ± 0.35 | 9.24 ± 0.69 | 2.60 ± 1.20 | nd | 24.31 ± 5.21 |
| β-sitosterol | 35.23 ± 1.57 | 39.54 ± 1.46 | 201.57 ± 5.31 | 23.01 ± 0.61 | 92.51 ± 15.63 | 41.67 ± 12.90 | 43.43 ± 20.01 | 181.62 ± 10.44 | 394.59 ± 11.45 | 107.49 ± 8.54 | 59.94 ± 2.01 | 62.25 ± 6.61 | 78.87 ± 1.58 | 33.17 ± 3.99 | 59.50 ± 10.90 | 11.56 ± 3.80 | 8.09 ± 1.10 | 4.98 ± 1.01 | 11.65 ± 5.49 | 4.19 ± 2.10 | 11.22 ± 0.59 | 44.13 ± 8.61 |
| Phytosterol | 41.33 ± 2.61 | 62.91 ± 1.65 | 254.81 ± 21.58 | 39.22 ± 1.53 | 144.05 ± 19.48 | 49.02 ± 13.83 | 55.40 ± 21.41 | 190.16 ± 11.07 | 617.13 ± 14.81 | 156.54 ± 8.87 | 67.69 ± 3.41 | 75.96 ± 7.67 | 80.07 ± 1.53 | 38.00 ± 4.99 | 66.30 ± 13.63 | 11.56 ± 3.80 | 9.13 ± 1.30 | 8.55 ± 0.73 | 24.44 ± 5.36 | 6.79 ± 3.30 | 11.22 ± 0.59 | 70.16 ± 11.49 |
Values are mean ± S.D.
Tocopherols are a series of methylated phenols resembling vitamin E. α-tocopherol is regarded as the dominant form because it is the primary target of tocopherol transfer protein in the liver. These fat-soluble antioxidants can prevent the peroxidation of unsaturated lipids via chain-breaking reactions, which improve the oxidation stability of edible oils. Recently, their antioxidant capacities have been reported to be linked with potential therapeutic effects in preventing aging-associated diseases, like cardiovascular and Alzheimer's disease (Yang et al., 2018). As shown in Table 2, the contents of α-tocopherols varied from 38.81 mg/kg to 602.51 mg/kg. PKSO, CSO, and RBO had relatively high α-tocopherol, which were 602.51, 498.40, and 448.64 mg/100 g, respectively. These results differed slightly from the previously reported values, 772.31 mg/100 g in PKSO, 410.4 mg/100 g in CSO, and 316 mg/kg in RBO (El-Mallah and El-Shami, 2011, Jiao et al., 2014, Xu et al., 2021). In addition, the tocopherols content in CO, FO, HSO, PNSO, GSO and WO also exceeded 200 mg/100 g. Among them, the contents of HSO and FO were higher than previous results (Tura et al., 2022, Varas Condori et al., 2020).
Squalene is a highly unsaturated triterpene which firstly isolated from shark liver. It also presents in the unsaponifiable fraction of plant-derived oils, like olive and flaxseed oil. This deep-sea-originated product is exploited as nutraceutical compounds to reduce serum cholesterol levels, enhance immune responses, and ameliorate the pathogenesis of aging-related diseases (Yang et al., 2018). As shown in Table 2, olive oil possessed the highest squalenes level of 234.75 mg/100 g among 22 EPOs, which was in accordance with former reports (Bondioli et al., 1993). Followed by flaxseed oil, the content was 82.98 mg/100 g. The squalene content in RBO (75.79 mg/100 g) was markedly lower than former reports, which might attribute to the different agronomical factors and oil process methods (Zhang et al., 2021).
Phytosterols are the major unsaponifiable components in vegetable oils. These plant-derived sterols consist of more than 100 different members with similar physiological functions and structures to cholesterol. Recently, phytosterols were reported to exhibit numerous health benefits, like reducing blood cholesterol levels, preventing cardiovascular disease, anti-inflammatory, and immunomodulatory effects (Yang et al., 2018). In the present study, three phytosterols, campesterol, stigmasterol, and β-sitosterol, were quantified. Among these three, β-sitosterol was the majority component ranging from 4.19 to 394.59 mg/100 g in tested oils. Followed by campesterol, the highest amount was 132.13 mg/100 g in RBO. The contents of stigmasterol were comparatively lower, reaching 90.42 mg/100 g in RBO. RBO possessed the highest phytosterol content of 617.13 mg/100 g. Followed by CO, it contained 201.57 mg/100 g of β-sitosterol, 43.65 mg/100 g of campesterol, and 9.59 mg/100 g of stigmasterol. The phytosterol content in RBO and CO was lower than previously reported content which were 775.2 and 490.6 mg/100 g, respectively (Yang et al., 2018). Wang et al found that the content of phytosterols increased along with the temperature when peony seeds were pretreated at different temperature, which might be the reason for the low phytosterols content of plant oil extracted by cold pressing (Z. Wang et al., 2021).
3.4. In vitro antioxidant activity
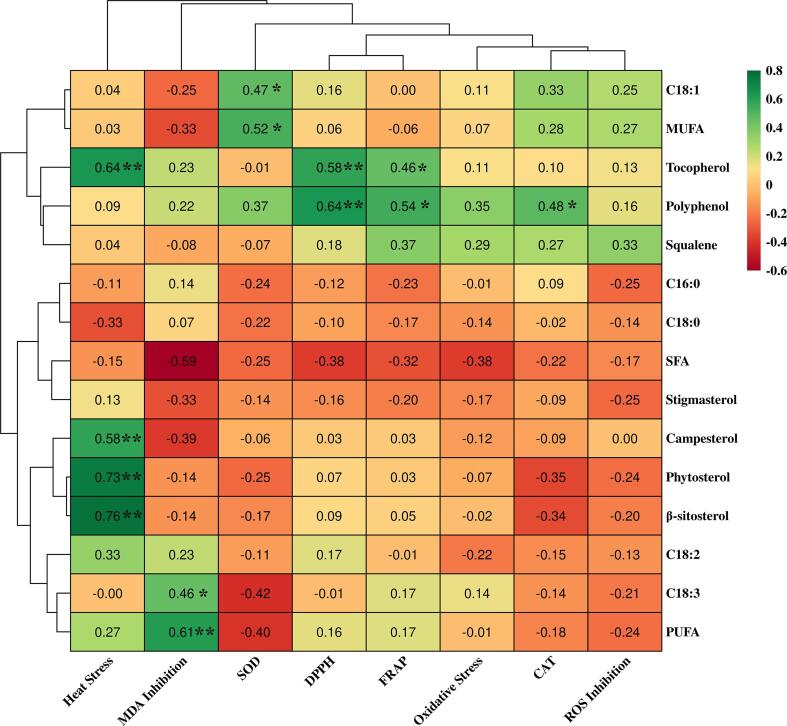
The in vitro antioxidant activity assay, DPPH and FRAP, was based on single electron transfer (SET) reaction or hydrogen atom abstraction (HAT) reaction. Potential antioxidants with lower ionization potential (IP) or lower bond dissociation enthalpy (BDE) of the H-donating group exhibit stronger ROS scavenging capacity. As shown in Table 3, the DPPH radical scavenging capacity varied from 215.26 μmol TE/100 g in RSO to 530.20 μmol TE/100 g in CSO. The FRAP data varied from 24.98 μmol TE/100 g in peanut oil (PNO) to 206.44 μmol TE/100 g in CSO. Results from these two in vitro antioxidant assays exhibited a similar tendency that CSO, GSO, and OO exhibited comparatively higher free radical scavenging capacity. Particularly, cotton seed oil exhibited the highest in vitro antioxidant activity in both assays. Followed by grape seed oil, olive oil, and flaxseed oil, the DPPH radical scavenging capacity ranged from 464.52 to 508.88 μmoL TE/100 g, and the ferric ion reducing antioxidant power ranged from 174.61 to 194.82 μmoL TE/100 g. These different results might attribute to the content of natural antioxidants in oils, like phenolic compounds, unsaturated fatty acids, squalene, and phytosterols. To further clarify the relationship between in vitro antioxidant capacity and chemical compositions, bivariate correlations analysis was utilized to investigate the correlation coefficient. As shown in Fig. 1, the DPPH showed a significant positive correlation with the polyphenols (r = 0.64, p < 0.01) and tocopherol content (r = 0.58, p < 0.01). Meanwhile, there was a positive correlation between FRAP and polyphenols (r = 0.54, p < 0.05) and tocopherol content (r = 0.46, p < 0.05). The slight differential in the regression coefficient of DPPH and FRAP might attribute to that FRAP mainly acted via SET, while DPPH acted via both SET and HAT. A similar correlation relationship between in vitro free radical scavenging capacity and polyphenols, tocopherol, and squalene contents was reported in walnut oil (Gao et al., 2019).
Table 3.
In vitro antioxidant capacity (TE μmol/100 g) and In vivo protective effects of different EPOs.
| Activity | Conventional oil crops |
Novel oilseed crops |
Woody oil crops |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNO | SBO | CO | FO | RSO | SUSO | SESO | CSO | RBO | PKSO | HSO | PLSO | PNSO | SASO | GSO | WO | PO | CNO | OO | OSO | YSO | SIO | |
| DPPH | 258.92 ± 4.22 | 357.20 ± 2.48 | 380.20 ± 0.20 | 464.52 ± 5.27 | 215.26 ± 7.26 | 356.83 ± 6.55 | 442.23 ± 4.68 | 530.20 ± 0.20 | 363.81 ± 2.52 | 472.21 ± 3.01 | 354.54 ± 5.69 | 246.89 ± 13.63 | 473.57 ± 3.10 | 366.27 ± 18.52 | 505.88 ± 2.94 | 425.65 ± 28.29 | 371.74 ± 4.19 | 353.43 ± 7.19 | 491.17 ± 10.91 | 413.94 ± 29.97 | 317.01 ± 40.29 | 338.0 ± 5.59 |
| FRAP | 24.98 ± 1.98 | 55.00 ± 8.69 | 66.29 ± 7.71 | 191.99 ± 4.68 | 41.94 ± 2.89 | 50.60 ± 10.25 | 130.74 ± 3.90 | 206.44 ± 13.03 | 120.64 ± 8.55 | 110.29 ± 4.35 | 105.24 ± 2.54 | 81.95 ± 11.37 | 174.61 ± 128.2 | 112.04 ± 5.40 | 177.04 ± 8.85 | 109.44 ± 4.37 | 115.99 ± 2.60 | 98.05 ± 12.82 | 194.82 ± 5.43 | 96.06 ± 8.23 | 75.91 ± 5.18 | 89.48 ± 9.26 |
| Oxidative Stress (h) | 3.71 ± 0.22 | 3.54 ± 0.19 | 4.71 ± 0.22** | 4.48 ± 0.11 | 4.31 ± 0.19 | 5.18 ± 0.21** | 4.73 ± 0.18** | 4.64 ± 0.58 | 4.78 ± 0.16** | 6.76 ± 0.20** | 6.48 ± 0.23** | 7.83 ± 0.31** | 8.02 ± 0.15** | 7.55 ± 0.16** | 4.50 ± 0.21* | 4.25 ± 0.21 | 7.74 ± 0.3** | 5.34 ± 0.3** | 7.02 ± 0.14** | 7.77 ± 0.26** | 4.03 ± 0.24 | 3.92 ± 0.18 |
| Heat Stress (h) | 8.77 ± 0.37* | 9.76 ± 7.27** | 10.95 ± 0.27** | 9.41 ± 0.57** | 9.95 ± 0.32** | 9.67 ± 0.38** | 8.62 ± 0.45* | 10.35 ± 0.34** | 11.39 ± 0.49** | 10.67 ± 0.38** | 10.40 ± 0.52** | 10.21 ± 0.57** | 10.87 ± 0.28** | 10.03 ± 0.30** | 10.11 ± 0.41** | 9.13 ± 0.17** | 9.62 ± 0.65** | 8.71 ± 0.66* | 9.66 ± 0.31** | 9.51 ± 0.34** | 8.12 ± 0.33 | 8.55 ± 0.41 |
| ROS Inhibition (%) | 19.03 ± 2.73** | 2.47 ± 3.45 | 13.15 ± 2.71** | 28.47 ± 2.15** | 26.96 ± 1.66** | 32.22 ± 2.78** | 21.76 ± 1.90** | 28.82 ± 2.25** | 26.27 ± 1.93** | 37.67 ± 1.45** | 26.94 ± 2.30** | 23.13 ± 2.63** | 25.08 ± 2.68** | 32.16 ± 1.29** | 24.13 ± 1.54** | 29.70 ± 1.44** | 25.81 ± 1.36** | 34.60 ± 2.41** | 35.73 ± 2.38** | 33.62 ± 1.60** | 5.88 ± 1.58 | 10.2 ± 0.9** |
| SOD (U/mgprot) | 37.05 ± 1.46 | 27.24 ± 6.41 | 48.23 ± 0.77** | 50.23 ± 3.38** | 77.25 ± 2.45** | 63.07 ± 1.16** | 91.34 ± 0.57** | 56.46 ± 2.11** | 63.97 ± 5.11** | 78.96 ± 0.21** | 71.61 ± 5.13** | 51.09 ± 1.38** | 55.59 ± 3.26** | 90.1 ± 11.63** | 64.78 ± 0.79** | 50.57 ± 2.69** | 79.01 ± 0.73** | 57.04 ± 3.63** | 85.25 ± 0.80** | 145.7 ± 1.25** | 58.71 ± 1.16** | 39.57 ± 1.29** |
| CAT (U/mgprot) | 1.03 ± 0.15 | 8.67 ± 5.46** | 3.05 ± 0.25 | 10.23 ± 1.75** | 2.10 ± 0.21 | 1.39 ± 0.08 | 2.64 ± 0.22 | 1.82 ± 0.47 | 3.27 ± 0.08 | 16.27 ± 1.16** | 9.90 ± 1.30** | 2.15 ± 0.18 | 6.96 ± 0.87** | 7.91 ± 0.05** | 19.96 ± 0.58** | 2.15 ± 0.40 | 10.48 ± 0.39** | 8.90 ± 0.71** | 20.67 ± 1.47** | 19.39 ± 1.16** | 3.72 ± 0.29* | 0.87 ± 0.13 |
| MDA Inhibition (%) | 69.95 ± 6.55** | 59.95 ± 8.66** | 76.06 ± 2.72** | 85.31 ± 2.56** | 16.38 ± 6.36 | 47.44 ± 2.49** | 36.28 ± 2.55** | 52.40 ± 4.44** | 46.77 ± 7.45** | 45.08 ± 9.40** | 90.03 ± 4.51** | 86.06 ± 2.80** | 90.75 ± 3.63** | 80.50 ± 7.35** | 87.51 ± 2.37** | 88.64 ± 8.21** | 69.90 ± 2.90** | 48.69 ± 7.20** | 62.51 ± 5.20** | 78.99 ± 1.60** | 56.94 ± 4.32** | 39.0 ± 8.53** |
Values are mean ± S.D. One-way ANOVA was used to compare the oxidative stress, heat stress, ROS inhibition, SOD, CAT, and MDA inhibition with the solvent control group. * P < 0.05 and ** P < 0.01 suggest a significant difference of values in the same row.
Fig. 1.
The correlation heatmap between chemical compositions and biological activities.
3.5. Stress resistance and in vivo antioxidant activity
Reactive oxygen species (ROS), including ·, H2O2, and ∙OH, are naturally generated during mitochondrial oxidative metabolism. The generating rate and eliminating rate of ROS are maintained in a dynamic balance by antioxidant defense systems. However, exogenous stressors or intrinsic aging-related degeneration induce the loss of ROS homeostasis resulting in chronic oxidative stress. The aggregation of oxidative damage during chronic oxidative stress is considered as an important contributor to the pathogenesis of aging and aging-related diseases. Therefore, the supplementation of dietary antioxidants to alleviate oxidative stress provides a potential strategy to improve healthspan. The free radical scavenging capacity of dietary antioxidants can be evaluated by numerous in vitro assays. However, the corresponding in vivo antioxidant activity may differ from ROS scavenging capacity, which cannot be simply extrapolated from in vitro results. In this study, the protective effect of EPOs against oxidative stress was assessed in an in vivo nematode model. As shown in Table 3, 13 of 22 EPOs significantly ameliorated the paraquat-induced acute oxidative stress, increasing the mean lifespan from 4.48 h in FO to 8.02 h in peony seed oil (PNSO), respectively. Polyphenol-riched EPOs, CSO exhibiting remarkable free radical scavenging properties in vitro only slightly prolonged the mean survival time in worms. These results were presumably ascribed to the wide variety of phenolic composition in tested EPOs. Different phenolic compounds exhibited distinctive in vivo antioxidant properties according to their structure and relative content. Oleuropein derivatives exhibit a higher positive correlation to oxygen radical absorbance capacity (ORAC) compared to tyrosol, hydroxytyrosol, and ligstroside aglycone in olive oil (Presti et al., 2017). However, specific phenolic compounds, like gossypol in cotton seed oil, were reported to possess toxicity to a certain extent (Zhu et al., 2019). Moreover, excess polyphenol supplementation in terms of worms might intensify the oxidative stress by pro-oxidant effect rather than antioxidant effect. From the correlation heatmap in Fig. 1, a positive correlation but not statistically significant was observed between oxidative stress tolerance and palmitic acid, linolenic acid, and squalene content.
Extensive studies suggested that enhancing abiotic stress tolerance, like thermal stress, is positively coupled with healthspan improvement. As shown in Table 3, CO, RBO, and PNSO considerably improved heat stress resistance in worms, which significantly increased the mean survival time against lethal heat stress from 10.87 to 11.39 h, respectively. In accordance with former results, EPOs exerting significant improvement in oxidative stress resistance also had similar beneficial effects in heat stress resistance. PLSO and SASO, which substantially enhanced the oxidative stress resistance in worms, also significantly extended the survival time of 10.21 and 10.03 h under heat stress. However, PNO, SBO, FO, RSO, CSO, and WO, which failed to enhance the oxidative stress tolerance, significantly increased the mean survival time from 8.77 to 9.95 h against heat stress. It might attribute to the similar but not identical stress response mechanism. In the nematode, DAF-16/FOXO transcription factor plays a vital role in regulating both oxidative and heat stress response, while a heat-shock transcription factor, HSF-1, parallelly mediates heat stress response as well (Lapierre & Hansen, 2012). The correlation heatmap Fig. 1 revealed that heat stress resistance was separated from oxidative stress by a large cluster distance, indicating a different contribution pattern of oil ingredients. The Pearson’s Correlation analysis suggested that the thermotolerance improved by EPOs was positively correlated to fat-soluble phytosterols and vitamin E ingredients, including α-tocopherol, β-sitosterol, and campesterol. Although, due to the potential pro-oxidant effect of phytosterol, it might act antagonistically to α-tocopherol in scavenging DPPH radicals (Liu et al., 2021). Phytosterol was reported to play a vital role in responding to various abiotic stress by maintaining the membrane homeostasis and triggering the stress response signaling pathway in both plant and animal (Möller et al., 2020). Supplementation of phytosterol-enriched beverages remarkably increased the lifespan and stress resistance in C. elegans (López-García et al., 2020).
To further validate the in vivo antioxidant effect of EPOs, the intracellular ROS content in the worms was revealed by a membrane permeable fluorescein-based dye, H2DCF-DA. The results were expressed as the mean ROS inhibition ± S.D.(%) normalized by the untreated control group (Table 3). A total of 20 oil samples significantly eliminated intracellular ROS content compared to the solvent control group. Pumpkin seed oil exhibited the highest ROS inhibition rate of 37.67%, followed by olive oil, coconut oil, oil-tea seed oil, sunflower seed oil, and safflower seed oil, which decreased the intracellular ROS content from 32.16% to 37.67%, respectively. Several studies reported similar in vivo antioxidant activity and heat stress resistance of coconut oil, which might be attributed to the specific median-chain fatty acid components (Narayanankutty et al., 2018). In the present, a potential positive correlation with squalene (r = 0.33, p = 0.10) was demonstrated in Fig. 1.
A comprehensive intrinsic antioxidant defense system consists of numerous enzymatic antioxidants (superoxide dismutase, catalase, and glutathione reductase) and nonenzymatic antioxidants (glutathione and protein -SH groups) to maintain the redox homeostasis in organisms. Against imbalanced ROS production, superoxide dismutases catalyze the decomposition of highly reactive superoxide anion to a more stable form, hydrogen peroxide, which is subsequently detoxified by catalases to water and oxygen. Table 3 presented the SODs and CATs activity in worms pretreated by EPOs. In general, most vegetable edible oils showed a significant inducing effect on SODs activity, in agreement with stress tolerance and intracellular ROS elimination results. Oil-tea seed oil exerted the highest effect on promoting the SODs activity of 145.72 U/mg protein, followed by sesame seed oil, safflower seed oil, and olive oil, ranging from 85.25 to 91.34 U/mg protein. However, in the measurement of the CATs activity, only 12 EPOs exhibited statistically significant induction effect. Olive oil, grape seed oil, and oil-tea seed oil demonstrated a relatively stronger promoting effect on the CATs activity of 20.67, 19.96, and 19.39 U/mg protein, respectively. Followed by pumpkin seed oil, pretreated of which elevated the CATs activity to 16.27 U/mg protein. The closed cluster distance in the correlation heatmap Fig. 1 revealed a similar composition pattern contributing to the activity of SODs and CATs. As shown in Fig. 1, oleic acid (r = 0.47, p < 0.05) and MUFA (r = 0.52, p < 0.05) were positively correlated to the SODs activity, while polyphenol (r = 0.48, p < 0.05) were the major affector for the CATs activity.
MDA, a metabolite of prostaglandin endoperoxides, is widely used as an indicator of lipid peroxidation. Since EPOs exerted beneficial effects on resistance tolerance and in vivo antioxidant property, their inhibition ability on lipid peroxidation products, MDA, was assessed in worms. The results were expressed as the mean inhibition percentage normalized by control groups in Table 3. PNSO, HSO, WO, GSO, PLSO, and FO significantly suppressed the MDA production to 9.25%, 9.97%, 11.36%, 12.49%, 13.94%, and 14.69%, respectively, compared to the untreated control group. Correlations analysis revealed that PUFA (r = 0.61, p < 0.01) and linolenic acid (r = 0.46, p < 0.05) were positively correlated to the MDA inhibition. This result may be because polyunsaturated fatty acids exhibit relatively lower BDE than other lipid components, making them particularly susceptible to lipid radicals to cease lipid peroxidation.
3.6. Chemometric analysis
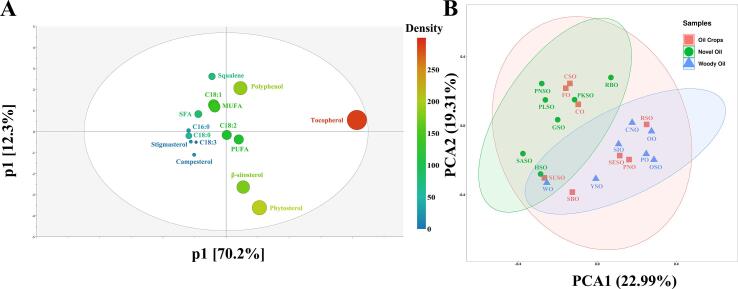
To explore the interrelationships among chemical composition and biological activities in different EPOs, principal component analysis (PCA) and hierarchical cluster analysis (HCA) were employed to reveal the distinctiveness and similarity based on chemical profiles of 22 tested oils, including fatty acid compositions, polyphenol, tocopherol, squalene, and phytosterols. As shown in Fig. 2A, the contents of tocopherol, phytosterol, polyphenol, and β-sitosterol predominantly contributed to the first two principal components. Principle component 1 (PC1) and principal component 2 (PC2) accounted for 82.5% of the total variance. In Fig. 2B, the score plot of 22 oils was generated from PC1 and PC2, which explained the 42.30% of the variability. The novel oils extracted from unconventional oilseed crops, like rice bran oil, pumpkin seed oil, hemp seed oil, perilla seed oil, peony seed oil, safflower seed oil, and grape seed oil, were located in the left, representing the similarity of chemical profiles. The woody oil, including walnut oil, palm oil, coconut oil, olive oil, oil-tea seed oil, yellowhorn seed oil, and sacha inchi oil, shared similar chemical composition features, grouping together in the bottom right. While, the conventional edible oils, such as peanut oil, soybean oil, corn oil, flaxseed oil, rapeseed oil, sunflower seed oil, sesame seed oil, and cotton seed oil, possessed a wide variety of oil ingredients which was separated through the score plot.
Fig. 2.
(A)The loading scatter plot for the first two principal components of the PCA model. (B) The score plot of the 22 edible oils.
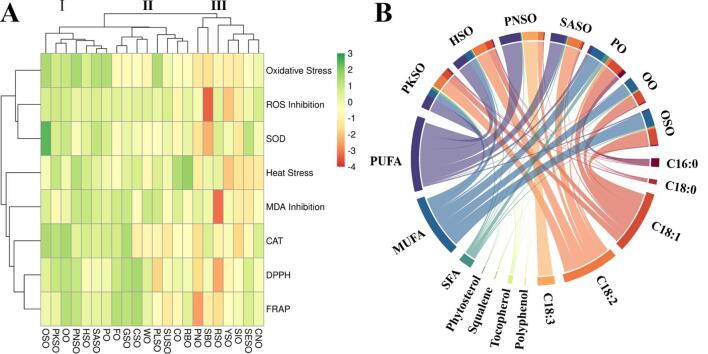
Fig. 3A, 22 edible oils were grouped into 3 clusters according to their in vitro antioxidant activity and in vivo healthspan promoting effects. Oil-tea seed oil, pumpkin seed oil, olive oil, peony seed oil, hemp seed oil, safflower seed oil, and palm oil were grouped into Cluster 1 characterized by the remarkably ameliorative effect against oxidative stress and inductive effect of antioxidant enzymes. Heat stress resistance and free radical scavenging capacity were treated as primary responses for grouping Cluster 2. A circus map (Fig. 3B) was constructed based on the hierarchical cluster analysis to provide an easy-to-read illustration to highlight the shared composition characteristic among oils in cluster 1. It could be easily observed that oleic acid, linoleic acid, MUFA, and PUFA, were the high-abundance ingredients in selected oil. Woody oil, including OSO, OO, and PO, possessed high amounts of oleic acid and MUFA, while PKSO, HSO, PNSO, and SASO were mainly comprised of linoleic acid and PUFA. The chord diagram demonstrated the relationships between chemical composition and oils exerting remarkably health-beneficial effects.
Fig. 3.
(A)The cluster heatmap of different oils based on biological activities. (B) Chord diagram of pumpkin seed oil (PKSO), hemp seed oil (HSO), peony seed oil (PNSO), safflower seed oil (SASO), palm oil (PO), olive oil (OO), and oil-tea seed oil (OSO) demonstrating the chemical composition feature.
4. Conclusion
In the present study, the fatty acid composition and minor compounds (polyphenol, tocopherol, squalene, and phytosterol) in 22 edible vegetable oil were analyzed. The chemical composition of different oil varied dramatically. CNO contained the highest SFA content due to the existence of specific medium-chain SFAs, including octanoic acid (C8:0), decanoic acid (C10:0), lauric acid (C12:0), and myristic acid (C14:0). Camellia Oleifera seed oil, olive oil, and palm oil contained a comparatively high proportion of oleic acid (C18:1) and MUFA. The PUFA, including linoleic acid (C18:2) and linolenic acid (C18:3), was mainly presented in PNSO, PLSO, FO, GSO, and WO. For the minor oil-derived ingredients, GSO possessed the highest total polyphenol contents. Tocopherol and squalene enriched in PKSO and OO, respectively. In addition, RBO was the typical phytosterol abundant oil. Based on the tocopherol and phytosterol contents, except WO, the woody oils (PO, CNO, OO, OSO, YSO, and SIO) could be clearly discriminated from oils extracted from unconventional oilseed crops (RBO, PKSO, HSO, PLSO, PNSO, SASO, and GSO) by PCA and HCA analysis. To further compare the health-beneficial effects of different oils, the free radical scavenging capacity, stress resistance, antioxidant enzyme induction, and MDA inhibition effect of 22 oils were systematically assessed in worms. According to Pearson's correlation analysis, oleic acid and MUFA were positively correlated to SODs activity. Linoleic acid and PUFA significantly contributed to the MDA inhibition effect. The polyphenol content was significantly correlated to in vitro antioxidant activity and CATs activity. The tocopherol content was positively correlated to thermotolerance, DPPH, and FRAP. A significant correlation coefficient was obtained between phytosterol and heat stress. This study comprehensively evaluated the contribution of specific components in edible oils to health-span promoting effects. The findings proved a theoretical basis for further exploiting novel edible oils with health-beneficial effects in the functional food industry.
Funding
This work was supported by the National Natural Science Foundation of China (31900294).
CRediT authorship contribution statement
Shiling Feng: Methodology. Xiaoyan Xu: Data curation, Visualization. Shengyong Tao: Supervision. Tao Chen: Methodology, Supervision. Lijun Zhou: Conceptualization. Yan Huang: Investigation. Hongyu Yang: Investigation. Ming Yuan: Investigation. Chunbang Ding: Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100341.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- American Oil Chemists' Society Sampling and analysis of commercial fats and oils. AOCS official method Cc. 1997:6–25. [Google Scholar]
- American Oil Chemists' Society Peroxide value acetic acid-isooctane method. AOCS Official Method Cd. 2009:8b–b. [Google Scholar]
- Bondioli P., Mariani C., Lanzani A., Fedeli E., Muller A. Squalene recovery from olive oil deodorizer distillates. Journal of the American Oil Chemists' Society. 1993;70(8):763–766. doi: 10.1007/bf02542597. [DOI] [Google Scholar]
- Calzolari D., Rocchetti G., Lucini L., Amaducci S. The variety, terroir, and harvest types affect the yield and the phenolic and sterolic profiles of hemp seed oil. Food Research International. 2021;142 doi: 10.1016/j.foodres.2021.110212. [DOI] [PubMed] [Google Scholar]
- Chang M., Yang J., Guo X., Zhang T., Liu R., Jin Q., Wang X. Medium/long-chain structured triglycerides are superior to physical mixtures triglycerides in Caenorhabditis elegans lifespan through an AMPK modified pathway. Food Bioscience. 2021;39 doi: 10.1016/j.fbio.2020.100815. [DOI] [Google Scholar]
- Chen A.L., Lum K.M., Lara-Gonzalez P., Ogasawara D., Cognetta A.B., 3rd, To A., Cravatt B.F. Pharmacological convergence reveals a lipid pathway that regulates C. elegans lifespan. Nature Chemical Biology. 2019;15(5):453–462. doi: 10.1038/s41589-019-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Feng S., Jia X., Li Q., Zhou Y., Ding C. Structural characterization and antioxidant activities of polysaccharides extracted from Epimedium acuminatum. Carbohydrate Polymers. 2013;92(1):63–68. doi: 10.1016/j.carbpol.2012.09.051. [DOI] [PubMed] [Google Scholar]
- Deng J., Liu Q., Zhang Q., Zhang C., Liu D., Fan D., Yang H. Comparative study on composition, physicochemical and antioxidant characteristics of different varieties of kiwifruit seed oil in China. Food Chemistry. 2018;264:411–418. doi: 10.1016/j.foodchem.2018.05.063. [DOI] [PubMed] [Google Scholar]
- El-Mallah M., El-Shami S. Effect of chemical refining steps on the minor and major components of cottonseed oil. Agriculture and Biology Journal of North America. 2011;2(2):341–349. doi: 10.5251/abjna.2011.2.2.341.349. [DOI] [Google Scholar]
- Feng S., Cheng H., Xu Z., Yuan M., Huang Y., Liao J., Ding C. Panax notoginseng polysaccharide increases stress resistance and extends lifespan in Caenorhabditis elegans. Journal of Functional Foods. 2018;45:15–23. doi: 10.1016/j.jff.2018.03.034. [DOI] [Google Scholar]
- Gao P., Liu R., Jin Q., Wang X. Comparative study of chemical compositions and antioxidant capacities of oils obtained from two species of walnut: Juglans regia and Juglans sigillata. Food Chemistry. 2019;279:279–287. doi: 10.1016/j.foodchem.2018.12.016. [DOI] [PubMed] [Google Scholar]
- Gu L., Zhang G., Du L., Du J., Qi K., Zhu X., Jiang Z. Comparative study on the extraction of Xanthoceras sorbifolia Bunge (yellow horn) seed oil using subcritical n-butane, supercritical CO2, and the soxhlet method. Lwt-Food Science and Technology. 2019;111:548–554. doi: 10.1016/j.lwt.2019.05.078. [DOI] [Google Scholar]
- Guo Z., Jia X., Zheng Z., Lu X., Zheng Y., Zheng B., Xiao J. Chemical composition and nutritional function of olive (Olea europaea L.): A review. Phytochemistry Reviews. 2017;17(5):1091–1110. doi: 10.1007/s11101-017-9526-0. [DOI] [Google Scholar]
- Han S., Schroeder E., Silva-Garcia C.G., Hebestreit K., Mair W.B., Brunet A. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature. 2017;544(7649):185–190. doi: 10.1038/nature21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janu C., Kumar D.R.S., Reshma M.V., Jayamurthy P., Sundaresan A., Nisha P. Comparative study on the total phenolic content and radical scavenging activity of common edible vegetable oils. Journal of Food Biochemistry. 2014;38(1):38–49. doi: 10.1111/jfbc.12023. [DOI] [Google Scholar]
- Jiao J., Li Z., Gai Q., Li X., Wei F., Fu Y., Ma W. Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. Food Chemistry. 2014;147:17–24. doi: 10.1016/j.foodchem.2013.09.079. [DOI] [PubMed] [Google Scholar]
- Lapierre L.R., Hansen M. Lessons from C. elegans: Signaling pathways for longevity. Trends in Endocrinology & Metabolism. 2012;23(12):637–644. doi: 10.1016/j.tem.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Hwang W., Artan M., Jeong D.E., Lee S.J. Effects of nutritional components on aging. Aging Cell. 2015;14(1):8–16. doi: 10.1111/acel.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Feng S., Chen T., Zhou L., Yuan M., Liao J., Ding C. Quality Assessment of Camellia oleifera oil cultivated in southwest China. Separations. 2021;8(9):144. doi: 10.3390/separations8090144. [DOI] [Google Scholar]
- Liu R., Xu Y., Chang M., Tang L., Lu M., Liu R., Wang X. Antioxidant interaction of α-tocopherol, γ-oryzanol and phytosterol in rice bran oil. Food Chemistry. 2021;343 doi: 10.1016/j.foodchem.2020.128431. [DOI] [PubMed] [Google Scholar]
- López-García G., Cilla A., Barberá R., Genovés S., Martorell P., Alegría A. Effect of plant sterol and galactooligosaccharides enriched beverages on oxidative stress and longevity in Caenorhabditis elegans. Journal of Functional Foods. 2020;65 doi: 10.1016/j.jff.2019.103747. [DOI] [Google Scholar]
- Mensink R.P. World Health Organization; 2016. Effects of saturated fatty acids on serum lipids and lipoproteins: A systematic review and regression analysis. [Google Scholar]
- Möller S., Saul N., Cohen A., Köhling R., Sender S., Murua Escobar H., Fuellen G. Healthspan pathway maps in C. elegans and humans highlight transcription, proliferation/biosynthesis and lipids. Aging. 2020;12(13):12534–12581. doi: 10.18632/aging.103514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcillo F., Vaissayre V., Serret J., Avallone S., Domonhédo H., Jacob F., Dussert S. Natural diversity in the carotene, tocochromanol and fatty acid composition of crude palm oil. Food Chemistry. 2021;365 doi: 10.1016/j.foodchem.2021.130638. [DOI] [PubMed] [Google Scholar]
- Narayanankutty A., Illam S.P., Raghavamenon A.C. Health impacts of different edible oils prepared from coconut (Cocos nucifera): A comprehensive review. Trends in Food Science & Technology. 2018;80:1–7. doi: 10.1016/j.tifs.2018.07.025. [DOI] [Google Scholar]
- Przykaza, K .,Nikolaichuk, H., Kozub, K., Tomaszewska, G., Perˇsurí,K., Kraljevi, S., & Fornal, E., (2021). Newly marketed seed oils. What we can learn from the current status of authentication of edible oils. Food Control, 2021,108349. 10.1016/j.foodcont.2021.108349.
- Presti G., Guarrasi V., Gulotta E., Provenzano F., Provenzano A., Giuliano S., Giacomazza D. Bioactive compounds from extra virgin olive oils: Correlation between phenolic content and oxidative stress cell protection. Biophysical Chemistry. 2017;230:109–116. doi: 10.1016/j.bpc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Rodríguez G., Villanueva E., Cortez D., Sanchez E., Aguirre E., Hidalgo A. Oxidative stability of Chia (Salvia hispanica L.) and sesame (Sesamum indicum L.) oil blends. Journal of the American Oil Chemists' Society. 2020;97(7):729–735. doi: 10.1002/aocs.12357. [DOI] [Google Scholar]
- Rodríguez G., Squeo G., Estivi L., Quezada Berru S., Buleje D., Caponio F.…Hidalgo A. Changes in stability, tocopherols, fatty acids and antioxidant capacity of sacha inchi (Plukenetia volubilis) oil during French fries deep-frying. Food Chemistry. 2021;340 doi: 10.1016/j.foodchem.2020.127942. [DOI] [PubMed] [Google Scholar]
- Shi L., Zheng L., Zhang Y., Liu R., Chang M., Huang J., Wang X. Evaluation and comparison of lipid composition, oxidation stability, and antioxidant capacity of sesame oil: An industrial-scale study based on oil extraction method. European Journal of Lipid Science and Technology. 2018;120(10):1800158. doi: 10.1002/ejlt.201800158. [DOI] [Google Scholar]
- Suealek N., Tharavanij T., Hackman R.M., Keen C.L., Holt R.R., Burawat B.…Rojpibulstit P. Thai Tea seed oil and virgin olive oil similarly reduce plasma lipids: A pilot study within a healthy adult male population. European Journal of Lipid Science and Technology. 2021;123(2):2000126. doi: 10.1002/ejlt.202000126. [DOI] [Google Scholar]
- Sun X., Wang Y., Li H., Zhou J., Han J., Wei C. Changes in the volatile profile, fatty acid composition and oxidative stability of flaxseed oil during heating at different temperatures. Lwt. 2021;151 doi: 10.1016/j.lwt.2021.112137. [DOI] [Google Scholar]
- Tura M., Mandrioli M., Valli E., Rubino R.C., Parentela D., Gallina Toschi T. Changes in the composition of a cold-pressed hemp seed oil during three months of storage. Journal of Food Composition and Analysis. 2022;106 doi: 10.1016/j.jfca.2021.104270. [DOI] [Google Scholar]
- Wang L., Ahmad S., Wang X., Li H., Luo Y. Comparison of antioxidant and antibacterial activities of camellia oil from Hainan with camellia oil from Guangxi, olive oil, and peanut oil. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.667744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang Z., Li H., Hou T., Zhao Y., Li H. Effects of ethanol, activated carbon, and activated kaolin on perilla seed oil: Volatile organic compounds, physicochemical characteristics, and fatty acid composition. Journal of Food Science. 2021;86(10):4393–4404. doi: 10.1111/1750-3841.15907. [DOI] [PubMed] [Google Scholar]
- Wang Y., He Y., Rayman M.P., Zhang J. Prospective selective mechanism of emerging senolytic agents derived from flavonoids. Journal of Agricultural and Food Chemistry. 2021;69(42):12418–12423. doi: 10.1021/acs.jafc.1c04379. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zheng C., Huang F., Liu C., Huang Y., Wang W. Effects of radio frequency pretreatment on quality of tree peony seed oils: Process optimization and comparison with microwave and roasting. Foods. 2021;10(12) doi: 10.3390/foods10123062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J.L., Ristow M. Lipid and carbohydrate metabolism in Caenorhabditis elegans. Genetics. 2017;207(2):413–446. doi: 10.1534/genetics.117.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Zhu M., Hu R., Zhao J., Chen Z., Li J., Ni Y. Characterisation of seed oils from different grape cultivars grown in China. Journal of Food Science and Technology. 2016;53(7):3129–3136. doi: 10.1007/s13197-016-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varas Condori M.A., Pascual Chagman G.J., Barriga-Sanchez M., Villegas Vilchez L.F., Ursetta S., Guevara Perez A., Hidalgo A. Effect of tomato (Solanum lycopersicum L.) lycopene-rich extract on the kinetics of rancidity and shelf-life of linseed (Linum usitatissimum L.) oil. Food Chemistry. 2020;302 doi: 10.1016/j.foodchem.2019.125327. [DOI] [PubMed] [Google Scholar]
- Xu D., Hao J., Wang Z., Liang D., Wang J., Ma Y., Zhang M. Physicochemical properties, fatty acid compositions, bioactive compounds, antioxidant activity and thermal behavior of rice bran oil obtained with aqueous enzymatic extraction. Lwt-Food Science and Technology. 2021;149 doi: 10.1016/j.lwt.2021.111817. [DOI] [Google Scholar]
- Yang R., Zhang L., Li P., Yu L., Mao J., Wang X., Zhang Q. A review of chemical composition and nutritional properties of minor vegetable oils in China. Trends in Food Science & Technology. 2018;74:26–32. doi: 10.1016/j.tifs.2018.01.013. [DOI] [Google Scholar]
- Zhang D., Duan X., Wang Y., Shang B., Liu H., Sun H., Wang Y. A comparative investigation on physicochemical properties, chemical composition, and in vitro antioxidant activities of rice bran oils from different japonica rice (Oryza sativa L.) varieties. Journal of Food Measurement and Characterization. 2021;15(2):2064–2077. doi: 10.1007/s11694-020-00806-5. [DOI] [Google Scholar]
- Zhu L., Yang A., Mu Y., Zhang N., Sun L., Rajput S.A., Qi D. Effects of dietary cottonseed oil and cottonseed meal supplementation on the structure, nutritional composition of egg yolk and gossypol residue in eggs. Poultry Science. 2019;98(1):381–392. doi: 10.3382/ps/pey359. [DOI] [PubMed] [Google Scholar]
- Zou X., Huang Y., Li H., Xu T., Fan Y., Li J., Deng Z. Effects of Chinese dietary pattern of fat content, n-6/n-3 polyunsaturated fatty acid ratio, and cholesterol content on lipid profile in rats. BioMed Research International. 2018;2018:1–13. doi: 10.1155/2018/4398086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- Shi L., Mao J., Zheng L., Zhao C., Jin Q., Wang X. Chemical characterization and free radical scavenging capacity of oils obtained from Torreya grandis Fort. ex. Lindl. and Torreya grandis Fort. var. Merrillii: A comparative study using chemometrics. Industrial Crops and Products. 2018;115:250–260. doi: 10.1016/j.indcrop.2018.02.037. [DOI] [Google Scholar]
- Shi L., Zheng L., Mao J., Zhao C., Huang J., Liu R., Wang X. Effects of the variety and oil extraction method on the quality, fatty acid composition and antioxidant capacity of Torreya grandis kernel oils. Lwt-Food Science and Technology. 2018;91:398–405. doi: 10.1016/j.lwt.2018.01.080. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.