Abstract
Chronic and unhealed wound is a serious public problem, which brings severe economic burdens and psychological pressure to patients. Various botanical drugs in traditional Chinese medicine have been used for the treatment of wounds since ancient time. Nowadays, multiple wound healing therapeutics derived from botanical drugs are commercially available worldwide. An increasing number of investigations have been conducted to elucidate the wound healing activities and the potential mechanisms of botanical drugs in recent years. The aim of this review is to summarize the botanical drugs in traditional Chinese medicine with wound healing properties and the underlying mechanisms of them, which can contribute to the research of wound healing and drug development. Taken together, five botanical drugs that have been developed into commercially available products, and 24 botanical drugs with excellent wound healing activities and several multiherbal preparations are reviewed in this article.
Keywords: wound healing, botanical drugs, multiherbal preparations, anti-inflammatory, proangiogenic, antibacterial
Introduction
Recent years, chronic wounds such as venous leg ulcer, diabetic foot ulcer, and pressure ulcer have become tough issues since they are unhealed, and the course of diseases are repeated (Bowers and Franco, 2020). Nearly six million people suffer from chronic wounds worldwide (Shukla et al., 2005). Nowadays, the main treatments for chronic wounds can be divided into two main parts, surgical debridement and growth factor medicines (Powers et al., 2016). The question of surgical debridement is that it cannot cure the chronic radically. The shortcomings of growth factor medicines are the high price and side effects, which will cause more problems to patients (Knight et al., 1998; Papanas and Maltezos, 2008). Thus, development of novel wound healing therapeutics is still a research hotspot in order to provide more selections for patients with wounds.
The usage of traditional Chinese medicines for the treatment of wounds can be traced back to ancient times. According to ancient Chinese medical textbooks, various botanical drugs in traditional Chinese medicine are recommended for the treatment of wounds. As time goes on, great progress was made in demonstrating the wound healing activities of these botanical drugs and clarifying the potential mechanisms of them. Hence, in this article, we are going to make a summarization of the botanical drugs in traditional Chinese medicine with wound healing properties.
Methods
Literatures published in English from 2000 to the present were searched in the PubMed, Web of Science, and Google Scholar databases. The search terms employed for this review included “wound healing, “ulcer,” “diabetic foot,” and “skin injure,” combined with “Chinese medicine,” “herbs,” and “medicinal plants”. Articles that included botanical drugs which were not widely distributed in China were excluded. Articles focusing on the wound healing activities of Chinese medicines which were derived from medical animals were also excluded. Meanwhile, ancient Chinese medical textbooks, including Shen Nong’s Herbal Classic (written by unknown authors from the Han dynasty in about 225 BC), Compendium of Material Medica (compiled by Li Shizhen in the Ming dynasty in 1578), and Tang Materia Medica (compiled by Su Jing in the Tang dynasty in 659 AD) were reviewed and botanical drugs recorded for the treatment of wounds were summarized. Among all the botanical drugs recorded, only those ones whose wound healing activities were evaluated by modern pharmacological studies were selected. Finally, five botanical drugs that have been developed into commercially available products, and 24 botanical drugs and several multiherbal preparations were reviewed in this article.
The General Process of Wound Healing
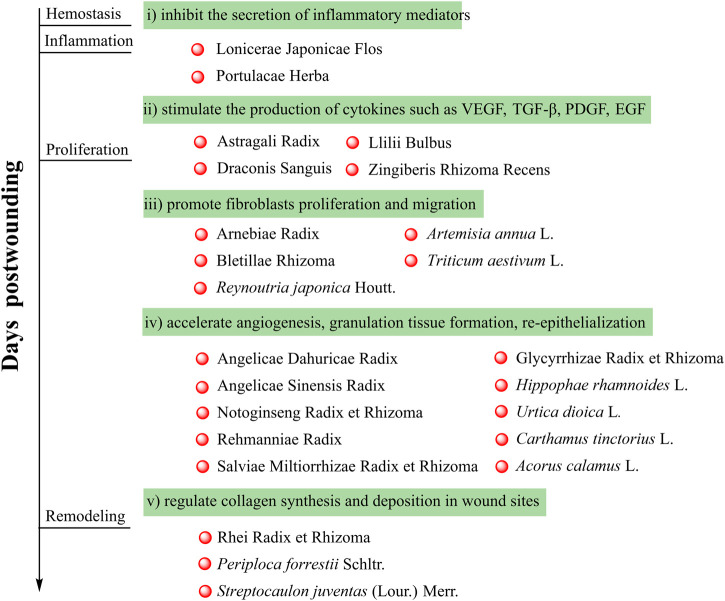
When the skin is injured, it will trigger a well-orchestrated and complex cascade of cellular and biochemical events that aimed at repairing the damaged tissues (Wilkinson and Hardman, 2020). The wound healing process can be divided into four distinct but overlapping phases, hemostasis, inflammation, proliferation, and remodeling. After injury, hemostasis occurs immediately and blood clot will be formed serving as scaffolding for cell migration. In the inflammation phase, vascular permeability will increase, cytokines will be released, and neutrophils and macrophages will migrate to the wound site. Then, the proliferation phase will occur and this phase is characterized by the release of growth factors and cytokines, which will promote the proliferation of fibroblasts and endothelial cells. Meanwhile, collagen fibers and granulation tissue are also produced in the proliferation phase. In the remodeling phase, collagen deposition starts and the scar tissue forms. Any failure or prolongation in any phase may result in delay of healing (Janis and Harrison, 2016). In other words, botanical drugs able to accelerate any phase are potential to be wound healing agents.
Commercially Available Products for Wound Healing Derived From Botanical Drugs
Among all the botanical drugs in traditional Chinese medicine reported to possess wound healing activities, several drugs had been extensively investigated and clinically proven to be effective, such as Centellae Herba, birch bark, calendula, aloe vera, and Curcumae longae rhizoma. In this section, those botanical drugs which have been developed into commercially available products or been clinically proven effective are summarized.
Centellae Herba
Centellae Herba, the dry whole plant of Centella asiatica (L.) Urb. [Apiaceae], is a traditional Chinese medicine which grows widely in China. According to Tang Materia Medica, Centellae Herba was recommended to be used in the treatment of ulcers and other wounds. Nowadays, the wound healing activity of Centellae Herba has been proven in various wound models through both topical and systemic routes. Products derived from Centellae Herba, such as Madecassol® and Emdecassol®, were already on the market for the treatment of skin problems. Several reviews were published focusing on the wound healing activity of Centellae Herba and its potential mechanisms (Brinkhaus et al., 2000; Bylka et al., 2014). Centellae Herba had been revealed to be able to promote the proliferation of fibroblast, increase the collagen synthesis, increase the intracellular fibronectin content, and inhibit the inflammation and all these attributed to its wound healing activity. Thus, readers could refer to the aforementioned reviews about the wound healing activity of Centellae Herba in animal models. In this section, we place the emphasis on the clinical trials about products derived from Centellae Herba.
In Iran, an ointment prepared with fresh leaves of Centella asiatica (L.) Urb. named Centiderm was tested for the efficacy against partial thickness burning in patients (Saeidinia et al., 2017). After the patients were treated with Centiderm, the burn wounds showed better healing in terms of pliability, vascularity, pigmentation etc, and the wounds required less days for re-epithelialization and complete healing (Saeidinia et al., 2017). Additionally, compared with silver sulfadiazine, Centiderm showed much better efficacy, indicating that Centiderm might be more suitable for the treatment of burn (Saeidinia et al., 2017). In Thailand, the efficacy of a standardized extract of Centellae Herba, named ECa 233 gel (contained madecassoside 51% and asiaticoside 38% as determined) on postlaser resurfacing wound healing was investigated in patients with atrophic acne scars (Damkerngsuntorn et al., 2020). After treated with ECa 233 gel, the wounds exhibited improved skin erythema, crusting, and wound appearance, according to the physicians’ assessment. The mechanism study showed that ECa 233 promoted the migration of keratinocyte via the activation of the FAK and Akt pathways, resulting in the increased expression of Rac1 and RhoA proteins, which were crucial for the formation of filopodia (Singkhorn et al., 2018). Additionally, ECa 233 could also induce cell migration via the activation of the ERK1/2 and p38 MAPK signaling pathways (Singkhorn et al., 2018). Due to the excellent wound healing activity of Centellae Herba, several researchers put their efforts on developing the Centellae Herba contained wound dressing. Several Centellae Herba-loaded dressings have been developed, including the hydrocolloid, electrospun double-layered nanocomposites membrane and electrospun gelatin nanofibre (Jin et al., 2015; Yao et al., 2017; Mouro et al., 2020). All these Centellae Herba-loaded dressings showed excellent wound healing activities, which indicated that Centellae Herba-loaded dressings were potential therapeutics for wounds.
Birch Bark
Birch Bark is the cortex of various birch species, such as Betula pendula Roth [Betulaceae], Betula pendula subsp. mandshurica (Regel) Ashburner & McAll [Betulaceae], and Betula pubescens Ehrh. [Betulaceae]. According to Compendium of Material Medica, birch bark was used to treat the acute mastitis traditionally. Nowadays, birch bark is clinically proven to be effective for wound healing. Oleogel-S10 (Episalvan®) is a drug containing triterpene-rich extract from birch bark which had been approved for treatment of partial thickness wounds in adults in the European Union (Scheffler, 2019). Mechanism studies conducted in primary human keratinocytes revealed that birch bark triterpene extract could upregulate various mediators involved in the inflammatory phase of wound healing, resulting in a temporary inflammation (Ebeling et al., 2014). As we all know, the excessive and prolonged inflammatory phase leads to chronic wounds, whereas a temporary inflammation is necessary for wound healing (Landen et al., 2016). Meanwhile, birch bark triterpene extract could also stimulate the migration of keratinocytes and the epidermal differentiation (Ebeling et al., 2014).
Several clinical results provided the evidence that Oleogel-S10 accelerated wound healing. In the Metelmann’s study, a phase II clinical trial was conducted in patients requiring split-thickness skin graft transplantation to assess the wound healing activity of Oleogel-S10 (Metelmann et al., 2015). No adverse events were reported in the involved 24 patients, and Oleogel-S10 markedly accelerated re-epithelialization at the donor sites. In another phase III trial conducted by Frew et al., the wound healing efficacy of Oleogel-S10 for the treatment of superficial partial thickness burn wounds was evaluated and compared with Octenilin® intra-patient (Frew et al., 2019). Results suggested that Oleogel-S10 significantly facilitated the healing of superficial partial thickness burn wounds and it was superior to Octenilin® in terms of efficacy and tolerability. Oleogel-S10 was also clinically proven to be effective for epidermolysis bullosa by phase II and III trials, and the mechanism was to promote the re-epithelialization of wounds (Schwieger-Briel et al., 2017; Kern et al., 2019; Schwieger-Briel et al., 2019).
Calendula
Calendula [Asteraceae; Calendula officinalis L.] or marigold is a medical plant mainly distributed in China, Europe, India, and the United States. Various research studies proved that calendula possesses excellent wound healing activities (Givol et al., 2019). In vitro studies demonstrated that calendula significantly stimulated the proliferation and migration of fibroblasts, upregulated the expression of growth factors (CTGF, TGFβ1, and bFGF), and α-smooth muscle actin (α-SMA) (Fronza et al., 2009; Dinda et al., 2015; Dinda et al., 2016; Hormozi et al., 2019). In vivo studies found that it can accelerate re-epithelization, enhance angiogenesis, and promote collagen deposition (Preethi and Kuttan, 2009; Parente et al., 2011).
Since the mid-20th century, the Cicaderma ointment, prepared with Calendula officinalis L [Asteraceae], Hypericum perforatum L [Hypericaceae], and Achillea millefolium L [Asteraceae] extracts, has been marketed in Europe for the treatment of wounds, insect bites, and so on (Morin et al., 2012). Boiron Calendula cream/gel is another commercialized product in France for adjuvant treatment of irritant dermatitis and superficial burns (Pommier et al., 2004). Meanwhile, calendula was also proven to be effective for the treatment of venous leg ulcer (Buzzi et al., 2016), diabetic foot ulcer (Carvalho et al., 2016), transected tendon (Aro et al., 2015), and acute dermatitis during irradiation for breast cancer (Pommier et al., 2004).
Aloe vera
Aloe vera [Asphodelaceae; Aloe vera (L.) Burm. f.] is a traditionally used medicinal plant for various skin lesions. According to Compendium of Materia Medica, aloe vera was used to treat exudative dermatitis in ancient China. Nowadays, it is widely used for skin problems and various types of aloe vera gels are commercially available.
Numerous research studies have proven that aloe vera possesses excellent wound healing activities with negligible toxicity via modulating the inflammation, increasing wound contraction, and epithelialization (Burusapat et al., 2018; Sanchez et al., 2020). Boudreau’s research found out glucomannan was a core composition of aloe vera, which stimulated the proliferation of fibroblasts and in turn improved collagen production and secretion (Boudreau and Beland, 2006).
Curcumae Longae Rhizoma
Curcumae longae rhizoma, the dried rhizome of Curcuma longa L [Zingiberaceae], is a traditionally used botanical drug as a wound healer in ancient China and India. Curcumin is the main active component and has been proven to be able to arouse earlier re-epithelialization, improve neovascularization, and increase migration of various cells including dermal myofibroblasts, fibroblasts, and macrophages into the wound bed (Aggarwal et al., 2007; Akbik et al., 2014). Even though curcumin possesses powerful wound healing activities, curcumin is limited by its low bioavailability, poor solubility, and rapid metabolism (Stanic, 2017). Thus, novel formulations were explored and wound healing products derived from curcumin including CumarGOLD Gel® and Psoria-Gold® Curcumin Gel had been marketed in various countries.
Botanical Drugs in Traditional Chinese Medicine With Wound Healing Activities
In addition to the aforementioned ones, numerous botanical drugs in traditional Chinese medicine have been traditionally used for the treatment of wounds, and their potential molecular mechanisms were elucidated by various studies. In this section, 24 botanical drugs (Table 1) and their wound healing activities are reviewed.
TABLE 1.
Chinese botanical drugs and their wound healing activities.
| Botanical drug and Source | Family | Extract/component/product | Experimental model | Dose and route of administration | Wound healing activity and potential mechanism | Reference |
|---|---|---|---|---|---|---|
| Angelicae dahuricae radix [Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav. or Angelica dahurica var. formosana (H.Boissieu) Yen] | Apiaceae | 70% ethanol extract | Diabetic rats with full-thickness wound | 1.2 g/kg once daily by oral gavage | Promoted diabetic wound closure via inducing angiogenesis and granulation tissue formation | Zhang et al. (2017b) |
| db/db mice with full-thickness wound | 1.8 g/kg once daily by oral gavage | Reduced wound area, increased in neovascularization, increased PDGF-β expression, and capillary formation | Guo et al. (2020) | |||
| Human umbilical vein endothelial cells (HUVECs) | 10–400 μg/ml | Induced cell proliferation, migration, and tube formation via the activation of ERK1/2, Akt, eNOS. Increased NO production and the expression of VEGF. | Zhang et al. (2017b) | |||
| HUVECs | 50–300 μg/ml | Promoted angiogenesis of HUVECs via activation of the HIF-1α/PDGF-β pathway | Guo et al. (2020) | |||
| Angelicae sinensis radix [Angelica sinensis (Oliv.) Diels] | Apiaceae | Extract containing 60% polysaccharide | HUVECs | 11.1–100 μg/ml | Stimulated the proliferation and migration of HUVECs via the activation of the JNK 1/2 and p38 signal pathways | Lam et al. (2008) |
| — | Zebrafish embryos | 50–400 μg/ml | Increased in angiogenic phenotype in subintestinal vessels | Lam et al. (2008) | ||
| SBD.4 | db/db mice with full-thickness wound and human skin grafted on SCID mice with full-thickness wound | Topically applied SBD.4 (2 mg per wound) in 2% carboxymethyl cellulose | In db/db mice, SBD.4 stimulated wound healing with complete remodeling of the wounds. In SCID mice, SBD.4 increased granulation tissue formation and assisted in the remodeling of wounds | Zhao et al. (2006) | ||
| SBD.4 (1%)-nanosilver hydrocolloid dressing | Chronic ulcer patients | External application | All patients’ wounds healed at the end of the treatment (day 30) | Zhao et al. (2012) | ||
| Astragali radix [Astragalus mongholicus Bunge] | Fabaceae | Formononetin | Mice with full-thickness wound | Formononetin (50 μM) was injected into dermis nearby wound | Accelerated wound-closure rate | Huh et al. (2011) |
| — | HUVECs | 0.1–50 μM | Promoted endothelial repair via the regulation of Egr-1 through the ERK and p38 MAPK pathways | — | ||
| Astragaloside IV (AS-IV) | Diabetic mice with full thickness wound | 10 μL AS-IV (100 mM) once daily by local delivery | Narrower wounds gaping and augmented re-epithelialization | Luo et al. (2016) | ||
| APS2-1 (polysaccharide) | Human skin fibroblasts | 1, 5, and 25 μg/ml | Promoted cell proliferation and migration | Zhao et al. (2017) | ||
| — | Scalded mice model | Topically applied 0.5 g ointment (containing 1.55% APS2-1) | Accelerated wound healing via reducing inflammatory response and promoting the expression of TGF-β1, bFGF, and EGF. | Zhao et al. (2017) | ||
| Draconis sanguis [Calamus draco Willd.] | Arecaceae | Dracorhodin perchlorate (DP) | Rats with full-thickness wound | Topical applied DP ointment (200 μg of DP DMSO solution mixed with 16 g of Vaseline) | Reduced inflammation via inhibiting IL-1α and TNF-α secretion. Increased VEGF and TGF expression, and collagen deposition | Jiang et al. (2017) |
| HaCaT keratinocytes | 1 μg/ml and 2 μg/ml | Promoted the wound healing of HaCaT keratinocytes via β-catenin, ERK, p38 MAPK, and AKT pathways | Lu et al. (2021) | |||
| HUVECs under high-glucose stimulation | 7.5 μM | Promoted the angiogenesis via the activation of the Ras/MAPK pathway | Li et al. (2016a) | |||
| NIH/3T3 fibroblasts | 0.5–4 μg/ml | Promoted fibroblast proliferation through the activation of the ERK-CREB and PI3K/Akt/mTOR pathways | Liu et al. (2019) | |||
| Notoginseng Radix et Rhizoma [Panax notoginseng (Burkill) F.H.Chen] | Araliaceae | Notoginsenoside R1 | HUVECs | 10 μg/ml | Stimulated the proliferation of HUVECs and enhanced its ability of tube formation | Yang et al. (2016) |
| Ginsenoside Rg1 | Excision diabetic foot ulcer | 150 mg/kg (i.p.) | Accelerated wound healing in diabetic ulcer through NO pathway via miR-23a | Cai et al. (2019) | ||
| Notoginsenoside Ft1 | HUVECs | 0.25–10 μM | Stimulated angiogenesis via HIF-1α-mediated VEGF expression, with PI3K/AKT and Raf/MEK/ERK concurrently participating | Shen et al. (2012) | ||
| — | Excisional wound in db/db mice | Topically applied 15 μL solution (6.7 mg/ml) once daily | Promoted the neovascularization accompanied with increased VEGF, PDGF, and FGF at either mRNA or protein levels. Reduced inflammation via downregulating TNF-α and IL-6 expressions | Zhang et al. (2016a) | ||
| Ginsenoside Rb1 | A rat model of second-degree burn injury | Topical application of ointment at the dose of 1.25, 2.5, or 5 g/kg | Accelerated burn wound healing via upregulating FGF-2/PDGF-BB/PDGFR-β gene and protein expressions | Zhang et al. (2021) | ||
| 20(S)-Protopanaxadiol | Excisional wound splinting model in db/db mice | Topically applied of 15 μL solution (0.6, 6 and 60 mg/ml) once daily | Enhanced angiogenesis via HIF-1α-mediated VEGF expression by the activation of p70S6K via PI3K/Akt/mTOR and Raf/MEK/ERK signaling pathways | Zhang et al. (2017a) | ||
| Arnebiae Radix [Arnebia euchroma (Royle ex Benth.) I.M.Johnst., Arnebia guttata Bunge or Lithospermum erythrorhizon Siebold and Zucc.] | Boraginaceae | A/S-based ointment | Dogs with full-thickness skin defect on forelimb | Topically applied a thin layer of the ointment | Promoted wound angiogenesis, collagen production, and epithelialization | Karayannopoulou et al. (2011) |
| Shikonin | Human gingival fibroblasts | 1 and 10 μM | Promoted fibroblast proliferation and migration via ERK 1/2 signaling pathway | Imai et al. (2019) | ||
| Human keratinocytes and HDFs | 1 μM | Promoted cell proliferation and showed anti-inflammatory activity via inhibiting the NF-κB signaling pathway | Yan et al. (2015) | |||
| Bletillae Rhizoma [Bletilla striata (Thunb.) Rchb.f.] | Orchidaceae | Polysaccharides | Mice with full-thickness wound | 12.5%-crosslinked polysaccharides hydrogel topically applied | Reduced inflammatory cells, decreased TNF-α, and increased EGF secretion in polysaccharides-treated wounds | Luo et al. (2010) |
| Diabetic mice with full-thickness wound | 5% polysaccharides solution (50 μL) treatment once daily | Accelerated wound healing, suppressed macrophage infiltration, promoted angiogenesis via inhibiting the high glucose-activated NLRP3 inflammasome | Zhao et al. (2021) | |||
| Rhei Radix et Rhizoma [Rheum palmatum L., Rheum tanguticum (Maxim. ex Regel) Balf. or Rheum officinale Baill.] | Polygonaceae | Emodin | Rats with full-thickness wound | Topically applied at the dose of 100–400 μg/ml | Enhanced cutaneous wound healing via regulating the Smads-mediated TGF-β1 signaling pathway | Tang et al. (2007) |
| Rehmanniae Radix [Rehmannia glutinosa (Gaertn.) DC.] | Orobanchaceae | Aqueous extract | Diabetic foot ulcer rat model | Topically applied at the dose of 1.85 g/kg | Better developed scars and epithelialization, and improved formation of capillaries with enhanced VEGF expression | Lau et al. (2009b) |
| Norviburtinal | Zebrafish embryo model | 50 μg/ml | An increase in capillary sprouts formation in SIV. | Liu et al. (2011) | ||
| Acteoside | HDFs | 6.3–100 µM | The activation of proMMP-2 along with an increase in MT1-MMP expression through a PI3K signal pathway | Nan et al. (2018) | ||
| Salviae Miltiorrhizae Radix et Rhizoma [Salvia miltiorrhiza Bunge] | Lamiaceae | Nonalcoholic solution produced with a 1:3 dry herb/menstruum ratio | Rats with burn wound | Orally administered at the dose of 1 g/kg/day for 14 days | Decreased the amount of necrosis in burn wounds | Irmak et al. (2018) |
| Cryptotanshinone | db/db mice with excisional wound | 300 mg/kg/d by gavage for 16 days | Accelerated wound closure and increased re-epithelialization and granulation tissue formation | Song et al. (2020) | ||
| Danshensu and salvianolic acid B | Detroit 551 human normal fibroblasts | 25–200 μM and 0.1 mM | Increased cell proliferation (25–200 µM) and promoted collagen synthesis (0.1 mM) | Chen et al. (2014) | ||
| Llilii Bulbus [Lilium lancifolium Thunb., Lilium brownii var. viridulum Baker or Lilium pumilum Redouté] | Liliaceae | Steroidal glycoside 1 and 2 | 3T3 murine fibroblasts | 5 µM | Induced production of NO and increased mRNA level of TGF-β Type I receptor, which played important roles in early wound healing | Esposito et al. (2013) |
| Steroidal glycoside 1 | Primary human dermal fibroblasts | 5 µM | Downregulated gene expression of inflammatory, chemokine, and tissue remodeling, upregulate ECM and cell adhesion related genes to regulate basic functions of cells in wound healing | Di et al. (2020) | ||
| Glycyrrhizae Radix et Rhizoma [Glycyrrhiza uralensis Fisch. ex DC., Glycyrrhiza inflata Batalin or Glycyrrhiza glabra L.] | Fabaceae | Collagen sponge loaded with 72 µg soluble polysaccharide in microcapsule | Rat trauma model | Topically applied | Increased the content of hydroxyproline, promoted the proliferation of capillaries and fibroblasts, and increased number of microvessels in wound site through activating the expression of p-STAT3 and VEGF and upregulating the tanscription levels of VEGF mRNA and miRNA-21 genes | Hao et al. (2020) |
| Isoliquiritin | Zebrafish skin wound model | 100 or 200 μg/ml | Promoted inflammation response and facilitated angiogenesis | Liu et al. (2020) | ||
| Zingiberis Rhizoma Recens [Zingiber officinale Roscoe] | Zingiberaceae | 10-shogaol | Human normal epidermal keratinocytes and dermal fibroblasts | 2 and 10 µM | Enhanced the production of TGF-β, PDGF-αβ, and VEGF. | Chen et al. (2012a) |
| 6-dehydrogingerdione | Fibroblasts | 2 and 10 µM | Upregrulated the production of growth factor, and accelerated cellular proliferation, and migration through blocking the MAPK pathway by supressing c-Jun protein levels and ERK phosphorylation | Chen et al. (2013a) | ||
| Lonicerae Japonicae Flos [Lonicera japonica Thunb.] | Caprifoliaceae | 95% ethanol extract | Rat excision wound model | 10% (w/w) extract ointment topically applied | Promoted wound healing, elevated the production of IL-10, and suppressed the production of TNF-α and IL-6 | Chen et al. (2012b) |
| Portulacae Herba [Portulaca oleracea L.] | Portulacaceae | Fresh plant homogenate | Mouse excision wound model | A single dose of 50 mg or a twice dose of 25 mg topically applied | Stimulated wound contraction and increased the strength of wound | Rashed et al. (2003) |
| Hippophae rhamnoides L | Elaeagnaceae | Leaves extract | Rats with full-thickness wound | 1% aqueous extract prepared in propylene glycol topically applied twice daily | Reduced wound area and increased the hydroxyproline and protein contents in wounds | Gupta et al. (2005) |
| Rats with burn wound | 5% extract prepared in petroleum jelly topically applied twice daily for 7 days | Faster reduction in the wound area, increased collagen synthesis, promoted angiogenesis, and increased levels of antioxidants in wounds | Upadhyay et al. (2011) | |||
| Seed oil | Rats with burn wound | Co-administered by two routes at the dose of 2.5 ml/kg (p.o.) and 200 µL (topical) for 7 days | Increased expression of MMP-2 and 9, collagen III and VEGF in granulation tissue | Upadhyay et al. (2009) | ||
| Sheep with 3rd degree flame burns | 20 ml seed oil topically applied | Shorter complete epithelization time | Ito et al. (2014) | |||
| Urtica dioica L | Urticaceae | Crude saponins extract | Rats with full-thickness wound | 20% saponins extract was prepared in vaseline and topically applied once daily | Promoted the wound healing with shorten heal time | Razika et al. (2017) |
| Dried leaves extract | Rats with full-thickness wound | 10% extract dissolved in glycerol topically applied on the wound at the dose of 50 µL/mm2 | Accelerated wound healing with fast wound closure and improved hydroxyproline content | Zouari Bouassida et al. (2017) | ||
| Periploca forrestii Schltr | Apocynaceae | 65% ethanol eluted fraction (EPFE65) derived from macroporous resin column | Rats with full-thickness wound and mouse fibroblasts | 50 μg/ml for in vitro studies and 0.1% EPFE65 hydrogel for animal studies | Promoted wound healing via enhancing the re-epithelialization, promoting fibroblast proliferation, migration, and stimulating the collagen synthesis | Li et al. (2019a) |
| Periplocin | Rats with full-thickness wound and mouse fibroblasts | 5–20 μg/ml | Promoted wound healing via the activation of Src/Erk and PI3K/Akt signaling pathways | Chen et al. (2019) | ||
| Streptocaulon juventas (Lour.) Merr | Apocynaceae | Ethanol extract | Mice with full-thickness wound | Topically applied at the dose of 100 mg/kg/day | Promoted wound healing via inducing the fibroblast proliferation and angiogenesis, and inhibiting the inflammation | Nguyen et al. (2017) |
| Carthamus tinctorius L | Asteraceae | Hydroxysafflor yellow A | HUVECs and human epithelial keratinocytes | 0.4, 0.8 and 1.6 mM | Enhance keratinocytes migration in a dose-dependent manner (0.4–1.6 mM). Increased the tube formation of HUVECs at 0.4 mM | Gao et al. (2018) |
| Splinted excisional wound model in diabetic rats | 0.2 mg locally applied once daily | Promoted wound closure and elevated the levels of VEGF and TGF-β1 in treated wounds | Gao et al. (2018) | |||
| Reynoutria japonica Houtt | Polygonaceae | 20% or 40% ethanol extract or 60% acetone extract | Human gingival fibroblasts (HGFs) | 50 μg/ml | Stimulated cell proliferation, migration, and collagen III synthesis | Nawrot-Hadzik et al. (2021) |
| Artemisia annua L | Asteraceae | Artemisia annua L.-containing nanofibers | Mouse fibroblasts | Fibroblasts were seeded onto the dressing | Encouraged the attachment, spreading, and proliferation of the fibroblast cells | Mirbehbahani et al. (2020) |
| Acorus calamus L | Acoraceae | 80% ethanol extract of dried leaves | Rats with excision or incision wound | 20% or 40% ointment topically applied once daily | Accelerated wound closure, less inflammatory cells, and higher collagen in treated wounds | Jain et al. (2010) |
| 70% ethanol extract of rhizome | Rats with excision wound | 40 mg/kg topically applied once daily | Increased the levels of collagen, hexosamical, and uronic acid in treated wounds | Ponrasu et al. (2014) | ||
| Triticum aestivum L | Poaceae | Exosomes | HDFs, HUVECs, human keratinocytes | 30–200 μg/ml | Promoted cell proliferation and migration at 30–200 μg/ml. Increased tube formation in HUVECs and promoted collagen synthesis in HDFs at 200 μg/ml | Sahin et al. (2019) |
| A small peptide (YDWPGGRN) | Rats with full thickness wound | Topically applied 20 μL peptide (250 μM) | Stimulated angiogenesis and collagen production in wounds | Sui et al. (2020) |
Angelicae Dahuricae Radix
Angelicae dahuricae radix, the dried root of Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav [Apiaceae] or Angelica dahurica var. formosana (H.Boissieu) Yen [Apiaceae], is a widely used traditional Chinese medicine, which had been reported to possess wound healing activities. In the study conducted by Zhang et al., Angelicae dahuricae radix 70% ethanolic extract (1.2 g/kg once daily by oral gavage) was proven to be able to accelerate diabetic wound healing via inducing angiogenesis (Zhang XN. et al., 2017). Another study conducted in db/db mice with full-thickness cutaneous wound found that Angelicae dahuricae radix 70% ethanolic extract treatment (1.8 g/kg once daily by oral gavage) resulted in the reduced wound area, increased neovascularization, elevated PDGF-β expression, and increased capillary formation (Guo et al., 2020). Its potential mechanisms were studied using human umbilical vein endothelial cells (HUVECs) and results showed that Angelicae dahuricae radix extract stimulated the angiogenesis via the activation of ERK1/2, PI3K/Akt, and eNOS/NO signaling pathways (Zhang XN. et al., 2017) as well as the HIF-1α/PDGF-β signaling pathway (Guo et al., 2020).
Angelicae Sinensis Radix
Angelicae sinensis radix, the dried root of Angelica sinensis (Oliv.) Diels [Apiaceae], is a famous botanical drug, which has been used for a long time in East Asia. Nowadays, Angelicae sinensis radix is also used as a functional food in Europe and America. Promoting angiogenesis is one of the biological activities of Angelicae sinensis radix (Majewska and Gendaszewska-Darmach, 2011). Thus, increasing attention is drawn on the wound healing activities of Angelicae sinensis radix. In the research conducted by Lam et al. the angiogenic activities of Angelicae sinensis radix extract (containing 60% polysaccharide) were studied in both HUVECs and the zebrafish model (Lam et al., 2008). Angelicae sinensis radix extract at the dose of 11.1–100 μg/ml significantly stimulated the proliferation, migration, and tube formation of HUVECs via the activation of the JNK 1/2 and p38 signal pathways. In the zebrafish model, Angelicae sinensis radix extract (50–400 μg/ml) treatment stimulated angiogenesis in the subintestinal vessels. In Hsiao’s research, the proteomic approach was applied to elucidate the wound healing mechanism of Angelicae sinensis radix (Hsiao et al., 2012a). Fifty-one differentially expressed protein spots were observed after human embryonic skin fibroblasts were treated with Angelicae sinensis radix extract (300 μg/ml). Among all the differentially expressed proteins, peroxiredoxins (PRDX-2, PRDX-4, and PRDX-6), Parkinson’s disease protein 7 (PARK7), and glutathione S-transferase Pi (GSTP1) were the ones involved in antioxidant activity. Proteomic results showed that Angelicae sinensis radix exhibited antioxidative activity via regulating the expression of PRDX-2, PRDX-4, PRDX-6, PARK7, and GSTP1 (Hsiao et al., 2012a). Additionally, Angelicae sinensis radix upregulated HSPB1 to inhibit cell apoptosis and regulate cell mobility; upregulated annexin A2 and VAT-1 to regulate calcium ion; downregulated NDKB and MARE1 to enhance the migration of fibroblasts; upregulated CAPNS1 to promote cell growth; and upregulated glycolysis to provide extra energy for wound healing processes (Hsiao et al., 2012a).
SBD.4 was a low-molecular weight fraction prepared from Angelicae sinensis radix (Zhao et al., 2006). In both the genetically diabetic mouse wound model and the human skin/severe-combined immunodeficiency (SCID) mouse wound model, the topical application of SBD.4 (2 mg per wound) in 2% carboxymethyl cellulose showed excellent wound healing activities (compared with becaplermin). In another study conducted by Zhao’s team, wound healing activity of SBD.4 on tissue healing was evaluated under the form of a SBD.4-nanosilver hydrocolloid wound dressing (Zhao et al., 2012). Results showed that SBD.4 dressing (containing 1% SBD.4) can increase type I collagen production in human dermal fibroblasts (HDFs), induce angiogenesis in zebrafish, and promote the healing of patients with chronic lower extremity ulcers. To sum up, SBD.4 formulated in wound dressings may benefit the patients with chronic ulcers.
Astragali Radix
Astragali radix, the dried root of Astragalus mongholicus Bunge [Fabaceae], is a traditional Chinese medicine used to enhance immune system function and promote wound healing (Han et al., 2009). Several components had been isolated from Astragali radix and studied for the wound healing activities including formononetin (Huh et al., 2011), astragaloside IV (Luo et al., 2016), and a polysaccharide named APS2-1 (Zhao et al., 2017).
Formononetin is a phytoestrogen isolated from Astragali radix. According to Huh’s research, formononetin (50 μM) was injected into dermis that is nearby wound, and it significantly promoted the wound healing in mouse full-thickness excisional wound (Huh et al., 2011). Mechanism studies revealed that growth factors including TGF-β1, VEGF, PDGF, and bFGF as well as Egr1 were significantly increased in HUVECs after formononetin treatment (0.1–50 μM) (Huh et al., 2011). Furthermore, western blot assays proved that the activation of Egr-1 by formononetin could be suppressed by ERK inhibitor and p38 inhibitor, suggesting that formononetin promoted wound healing by the regulation of Egr-1 via the ERK and p38 MAPK pathways (Huh et al., 2011).
Astragaloside IV (AS-IV) is a saponin derived from Astragali radix. Results of Luo’s study indicated that local delivery of AS-IV (100 mM) once daily promoted the wound closure in the diabetic mice skin wounds (Luo et al., 2016). On day 10 after wounds, wound closures in AS-IV-treated mice and vehicle treated mice were 72 ± 4.1% and 48.2 ± 3.1%, respectively. In-depth study found that AS-IV promoted wound healing in four parts: re-epithelialization, extracellular matrix deposition, neovascularization, and alternatively activated macrophages development. In the part of re-epithelialization, the AS-IV-treated group showed higher re-epithelialization rate (about 75%) than the untreated group (about 50%) (Luo et al., 2016). In the part of extracellular matrix deposition, the AS-IV-treated group increased the production of collagen compared to the untreated control group on Day 3 (Luo et al., 2016). In the part of neovascularization, more CD31 (a marker of angiogenesis) was detected in AS-IV-treated wounds (Luo et al., 2016). In the part of alternatively activated macrophages development, AS-IV-treated group increased the number of F4/80+CD206+ cells (an alternatively activated macrophages) and the expression of arginase-1 and Ym1 (Luo et al., 2016).
APS2-1 is a polysaccharide isolated from Astragali radix, which can promote wound healing via reducing the inflammatory response, promoting cell cycle progression, and the secretion of cytokines (Zhao et al., 2017). In the part of inflammatory, IκBα is a proinflammatory cytokine and APS2-1 (25 μg/ml) treatment significantly decreased the level of phosphorylation of IκBα in human skin fibroblast cells (Zhao et al., 2017). In the part of cell cycle progression, APS2-1 (1, 5 and 25 μg/ml) decreased the percentage of human skin fibroblast cells in the G0/G1 phase and increased the percentage of cells in the S and G2/M phases, leading to the promoted cell cycle progression significantly (Zhao et al., 2017). In the part of secretion of cytokines, the topical application of 0.5 g ointment (containing 1.55% APS2-1) increased the secretion of EGF, bFGF, and TGF-β1 in mice wounds (Zhao et al., 2017).
Draconis Sanguis
According to Chinese Pharmacopoeia, Draconis sanguis is a red resin obtained from Calamus draco Willd. [Arecaceae]. In other cultures, red resins obtained from Dracaena cochinchinensis (Lour.) S.C.Chen [Asparagaceae], Croton salutaris Casar [Euphorbiaceae], Pterocarpus officinalis Jacq [Fabaceae] etc. were also considered as Draconis sanguis, and they have been widely used in traditional medicine since ancient times (Pona et al., 2019). According to Compendium of Materia Medica, Draconis sanguis showed wound healing activity via promoting tissue regeneration. In vitro and in vivo wound healing activities of Draconis sanguis had been proven by several studies, and Draconis sanguis exhibited wound healing activities via promoting angiogenesis, collagen deposition, epithelization, and wound contraction (Liu et al., 2013b; Apaza Ticona et al., 2020). Additionally, in a case report, a patient who suffered from chronic pressure ulcer with tunneling received an external application of Draconis sanguis powder (Ji et al., 2015). In addition to Draconis sanguis powder treatment, conventional treatments such as local oxygen therapy and anti-infection therapy were also used in this patient. The patient’s integrative treatment program led to complete amelioration of the pressure ulceration (Ji et al., 2015).
By bio-guided isolation, bexarotene, taspine, and 2-hydroxy-1-naphthaldehyde isonicotinoyl hydrazone were isolated from Draconis sanguis and considered to be the active constituents (Apaza Ticona et al., 2020). NF-κB inhibitory activities of these three constituents in THP-1 (human peripheral blood monocyte), HaCaT (human skin keratinocyte), and NIH-3T3 (mouse embryo fibroblast) cells were studied. Results showed that bexarotene, taspine, and 2-hydroxy-1-naphthaldehyde isonicotinoyl hydrazone exhibited NF-κB inhibitory activity in all these 3 cell lines with IC50 values of 0.10–4.78 μM (Celastrol as positive control with an IC50 of 7.96 μM). The agar well diffusion method was used to study the antimicrobial activities of these three constituents. Results showed that bexarotene, taspine, and 2-hydroxy-1-naphthaldehyde isonicotinoyl hydrazone showed higher antimicrobial activities than ofloxacin (positive drug) against S. aureus, E. coli, and C. albicans strains (Apaza Ticona et al., 2020). MIC values of bexarotene, taspine, 2-hydroxy-1-naphthaldehyde isonicotinoyl hydrazone, and ofloxacin were 0.12–0.16, 0.31–0.39, 3.96–3.99, and 27.56 μM, respectively. Meanwhile, bexarotene, taspine, and 2-hydroxy-1-naphthaldehyde isonicotinoyl hydrazone could also stimulate the proliferation of fibroblasts (NIH3T3 cells) and keratinocytes (HaCaT cells) (Apaza Ticona et al., 2020).
Dracorhodin perchlorate (DP), a synthetic analog of dracorhodin, which is isolated from Draconis sanguis, showed various pharmacological activities. Animal studies showed that DP was able to promote wound healing in rats via inhibiting the section of IL-1α and TNF-α, stimulating the expression of VEGF and TGF, and regulating fibroblast proliferation (Jiang et al., 2017; Jiang et al., 2018). In vitro studies demonstrated that DP (1 and 2 μg/ml) enhanced HaCaT keratinocytes wound healing via β-catenin, ERK/p38, and AKT signaling pathways (Lu et al., 2021). On human umbilical vein endothelial cells cultured under high glucose (25 mM) stimulation, DP (7.5 μM) exhibited angiogenic activity via the Ras/MAPK pathway (Li F. et al., 2016). Meanwhile, DP (0.5–4 μg/ml) could also stimulate fibroblast proliferation via the activation of EGFR and its downstream ERK/CREB and PI3K/AKT/mTOR pathways (Liu et al., 2019). Taken together, DP may be developed into a potential lead compound for the treatment of skin wounds.
Notoginseng Radix et Rhizoma
Notoginseng Radix et Rhizoma [Araliaceae; Panax notoginseng (Burkill) F.H.Chen] has been used for hundreds of years in traumatic injuries and various kinds of bleeding (Ren et al., 2020). Notoginseng Radix et Rhizoma is an important component of Yunnan Baiyao, and Yunnan Baiyao is a famous commercial medicinal product in China, which is widely used for wounds (Yao et al., 2021). Saponins isolated from Notoginseng Radix et Rhizoma are the active components. Saponins could enhance angiogenesis on HUVECs in vitro and zebrafish in vivo via the activation of the VEGF-KDR/Flk-1 and PI3K-Akt-eNOS signaling pathways (Hong et al., 2009). Another mechanism of promoting angiogenesis activity is related to AMPK and eNOS-dependent pathways (Wang et al., 2017). Yu’s research found out that saponins can promote the proliferation of anterior cruciate ligament fibroblasts and increase the expression of collagen and fibronectin via enhancing the phosphorylation of PI3K, AKT, and ERK (Yu et al., 2015). Si-ye’s investigation showed saponins can inhibit scar formation through suppressing the proliferation of fibroblast in vitro and reducing the expression of α-SMA in a murine model of cutaneous wound (Men et al., 2020). Meanwhile, saponins can inhibit the formation of hypertrophic scar through inhibiting extracellular matrix deposition and stimulating cell apoptosis by modulating the PIK3/AKT signaling pathway (Zhi et al., 2021).
Arnebiae Radix
Arnebiae radix is the dried root of Arnebia euchroma (Royle ex Benth.) I.M.Johnst [Boraginaceae], Arnebia guttata Bunge [Boraginaceae] or Lithospermum erythrorhizon Siebold & Zucc [Boraginaceae]. Arnebia euchroma (Royle ex Benth.) I.M.Johnst [Boraginaceae] mainly grows in Xinjiang of China. Arnebia guttata Bunge [Boraginaceae] mainly grows in inner Mongolia of China, and Lithospermum erythrorhizon Siebold & Zucc [Boraginaceae] grows widely in China, Korea, and Japan. Present research studies proved that Arnebiae radix and shikonin, an important component of it, possessed the activity of wound healing (Andujar et al., 2013).
In Hsiao’s research, a proteomic platform was applied to explore the wound healing activity of Lithospermum erythrorhizon Siebold & Zucc. (Hsiao et al., 2012b). Results showed that 95% ethanol extract of Lithospermum erythrorhizon Siebold & Zucc. (2.5–20 μg/ml) can promote cell viability, increase antioxidant capacity, and reduce cell mobility of fibroblasts. The molecular mechanism study revealed that Lithospermum erythrorhizon Siebold & Zucc., 95% ethanol extract can downregulate 10-MARE1 and 11-CLIC1 to inhibit cell mobility, upregulate 13-NME1 and p-p38 to promote cell proliferation, upregulate 20-PRDX4 to produce antioxidant activity, and upregulate 21PGK1 and downregulate 18-PSME1 to coordinate metabolism (Hsiao et al., 2012b).
In a study conducted by Karayannopoulou et al., the wound healing activity of an alkannins/shikonins (A/S)-based ointment was evaluated in dogs with surgically created full-thickness skin defects. A/S-based ointment was a pharmaceutical formulation, containing isohexenylnaphthazarins approved by Hellenic Health Authorities. In dog full-thickness cutaneous wounds, the topical application of A/S-based ointment significantly promoted angiogenesis, collagen production, and epithelialization in wounds, but the wound healing time was not shortened (Karayannopoulou et al., 2011). One of the shikonin’s mechanisms in wound healing was anti-inflammatory. Yan’s research showed that shikonin (1 μM) can inhibit the translocation of NF-κB from cytoplasm to nucleus induced by TNF-α in fibroblasts (Yan et al., 2015). Imai’s team focused on the pharmacologicial activities of shikonin in human gingival fibroblasts (Imai et al., 2019). Results showed shikonin (1 and 10 μM) can promote the growth and migration of human gingival fibroblasts. Meanwhile, shikonin (1 μM) increased the production of Type I collagen and the gene expression of VEGF and FN (a cell adhesion factor) in human gingival fibroblasts (Imai et al., 2019). By using the ERK1/2 inhibitor PD98059, the aforementioned activities of shikonin were reduced significantly, indicating that the mechanisms of the biological activities were associated with the activation of the ERK1/2 signaling pathway (Imai et al., 2019). Epithelial–mesenchymal transition (EMT) is another factor that contributes to wound healing (Lamouille et al., 2014). According to Yin’s research, shikonin upregulated the specific EMT regulatory molecules and downregulated the expression of microRNA-205 and other microRNAs in mice wounds, which indicated that shikonin can stimulate EMT and suppress the expression of the associated microRNAs in skin wound healing (Yin et al., 2013).
Bletillae Rhizoma
Bletillae Rhizoma is the tuber of Bletilla striata (Thunb.) Rchb. f [Orchidaceae], which has been widely used in China for the treatment of hemoptysis, traumatic bleeding, ulcers, and chapped skin (He et al., 2017). Phytochemical studies revealed that polysaccharides were the major chemical constituents and Bletillae Rhizoma polysaccharides (BRP) not only promoted wound healing but also showed excellent performance as a promising natural biomaterial (He et al., 2017).
In Zhang’s research, the wound healing activity of BRP was investigated both in vitro and in vivo (Zhang C. et al., 2019). In in vitro, BRP (5 and 10 μg/ml) enhanced the proliferation and migration of mouse fibroblast cells. In in vivo, BRP hydrogel topically applied once daily at the dose of 0.4 g/kg significantly promoted the wound healing process in mouse full-thickness excision wound. In Luo’s study, BRP hydrogel was prepared by the oxidation and crosslinking method and 12.5%-crosslinked BRP hydrogel was applied on the mouse cutaneous wound bed (Luo et al., 2010). In the BRP hydrogel-treated wound tissues, the number of inflammatory cells and the level of TNF-α were significantly decreased and the re-epithelization was improved. In vitro mechanism studies showed that BRP at 80 μg/ml promoted the vascular endothelial cell proliferation and VEGF expression (Wang et al., 2006). In RAW264.7 cells, BRP (5–200 μg/ml) stimulated the expression of iNOS, TNF-α, and IL-1β in concentration-dependent manner (Diao et al., 2008). A recent study conducted in the diabetic mouse model found that 5% BRP solution (50 μl) treatment once daily could accelerate the diabetic wound healing via inhibiting the high glucose-activated NLRP3 inflammasome (Zhao et al., 2021). Additionally, BRP could also be used for the treatment of oral ulcer (Liao et al., 2019). Would dressings based on BRP such as chitosan-Ag nanoparticles and chitosan-BSP spongy bilayer dressing, BRP/carboxymethyl chitosan/Carbomer 940 hydrogel, and probiotic-bound oxidized BRP-chitosan composite hydrogel were all promising wound dressings which could be applied for wound healing (Ding et al., 2017; Huang Y. et al., 2019; Zhang Q. et al., 2019; Yang L. et al., 2020).
Rhei Radix et Rhizoma
Rhei Radix et Rhizoma, the dried root and rhizome of Rheum palmatum L [Polygonaceae], Rheum tanguticum (Maxim. ex Regel) Balf [Polygonaceae], or Rheum officinale Baill [Polygonaceae], is a traditional botanical drug recorded in Chinese Pharmacopoeia, which is widely distributed in China and famous for its remarkable pharmacological activities, such as anti-inflammatory, antimicrobial, and hemostatic activities (Wang et al., 2012). Emodin is an anthraquinone derivative isolated from Rhei Radix et Rhizoma, which has been proven to possess wound healing activity. Wounds treated with topical emodin (100–400 μg/ml) showed higher content of hydroxyproline and more tensile strength (Tang et al., 2007). Further molecular mechanism study indicated that emodin could stimulate the tissue regeneration via regulating the Smads-mediated TGF-β1 signaling pathway (Tang et al., 2007).
In the studies conducted by Yang et al., Rhei Radix et Rhizoma and Angelicae dahuricae radix 70% ethanol extract were mixed together at the ratio of 1:1 as a mixture. The wound healing activity of the mixture was evaluated in rat excisional wound non-infected or infected with Staphylococcus aureus (Yang et al., 2017; Yang WT. et al., 2020). In non-infected wound, the topical application of the mixture significantly promoted the wound healing, and more collagen, myofibroblasts, and inflammatory cell infiltration were observed in the wound site tissues treated with the mixture. Increased plasma IL-6 level and decreased TGF-β1 level were also observed in the group treated with the mixture (Yang et al., 2017). In vitro disc diffusion test results showed that 11.02 μg/disc of mixture solid had an average 8.13 ± 0.05 mm inhibition zone against Staphylococcus aureus ATCC 29213 (Yang WT. et al., 2020). In Staphylococcus aureus-infected wound, the mixture also exhibited excellent wound healing activity (Yang WT. et al., 2020). Taken together, the mixture of Rhei Radix et Rhizoma extract and Angelicae dahuricae radix extract could accelerate the bacterial-infected wound healing.
Rehmanniae Radix
Rehmanniae radix is the root tuber of Rehmannia glutinosa (Gaertn.) DC [Orobanchaceae], which is widely distributed in China. According to Compendium of Materia Medica, Rehmanniae radix is recommended to treat acute mastitis. Nowadays, Rehmanniae radix has been widely applied in traditional Chinese medicine prescriptions for the treatment of wound healing. In Lau’s research, the wound healing activity of Rehmanniae radix was evaluated with a diabetic foot ulcer rat model (Lau et al., 2009b). By comparing H&E staining photographs of wound tissues from rats treated with water or Rehmanniae radix extract (1.85 g/kg), the granulation tissue treated with Rehmanniae radix extract was more solid than that treated with water, which implied that Rehmanniae radix extract can facilitate tissue regeneration. Meanwhile, Rehmanniae radix extract increased the production of VEGF, indicating RR can enhance angiogenesis (Lau et al., 2009b).
In order to further investigate the angiogenesis activity of Rehmanniae radix, a zebrafish model was applied (Liu et al., 2011; Liu et al., 2014). Several Rehmanniae radix fractions were obtained and sub-fraction C2 (6.25–12.5 μg/ml) showed the most potent angiogenesis activity (Liu et al., 2011). Then, sub-fraction C2 was proceeded for further isolation and the major compound was identified as norviburtinal. In the zebrafish model, norviburtinal showed significant angiogenesis activity at the concentration of 50 μg/ml (Liu et al., 2011). Acteoside is another active component isolated from Rehmanniae radix, which exhibits wound healing activity (Nan et al., 2018). After normal human dermal fibroblasts were treated with acteoside (6.3–100 µM), proMMP-2 was activated along with an increase in MT1-MMP expression via the PI3K signal pathway.
Salviae Miltiorrhizae Radix et Rhizoma
Salviae Miltiorrhizae Radix et Rhizoma, a traditional Chinese botanical drug, is the root and rhizome of Salvia miltiorrhiza Bunge [Lamiaceae], which has been widely applied for the treatment of various diseases, including traumatic injuries, cardiovascular, and cerebrovascular diseases. In Irmak’s research, the rat burn model was applied to investigate the wound healing activity of Salviae Miltiorrhizae Radix et Rhizoma (Irmak et al., 2018). Nonalcoholic Salviae Miltiorrhizae Radix et Rhizoma solution was orally administered at the dose of 1 g/kg/day for 14 days, and the solution was commercially produced with a 1:3 dry herb/menstruum ratio. Results showed that the average of the neovascularization score of the Salviae Miltiorrhizae Radix et Rhizoma solution treated group (2 ± 0.66) was significantly increased when compared with the control group (1.4 ± 0.51). Meanwhile, an increase in the tissue perfusion was observed in the Salviae Miltiorrhizae Radix et Rhizoma solution treated group. Numerous reports have demonstrated that inadequate tissue perfusion exacerbated the tendency toward burn wound deepening (Singh et al., 2007). The aforementioned results suggested that Salviae Miltiorrhizae Radix et Rhizoma can decrease the amount of necrosis in burn wounds and promote wound healing.
Cryptotanshinone (CT) is a terpenoid isolated from Salviae Miltiorrhizae Radix et Rhizoma. Min’s team designed a study using the excisional wound splinting model in db/db mice to evaluate the wound healing activity of CT (Song et al., 2020). Results showed that 300 mg/kg/d CT by gavage for 16 days can significantly accelerate the rate of wound closure, which displayed in the increase of re-epithelialization and granulation tissue formation. Mechanism studies showed that in diabetic mice, CT can depress leukocyte infiltration, decrease the expression of chemokine, increase eNOS phosphorylation, increase the protein expression of VEGF, Ang-1, inhibit MMP2 and MMP9 protein expression, and increase fibroblasts translation, leading to improved angiogenesis and collagen deposition (Song et al., 2020). Meanwhile, CT could also prevent scarring via decreasing the excessive deposition of extracellular matrix components (Li Y. et al., 2016). In addition to CT, Danshensu (3,4-dihydroxyphenyllactic acid) and salvianolic acid B were also proven to be active components in Salviae Miltiorrhizae Radix et Rhizoma for wound healing (Chen et al., 2014). In Detroit 551 human normal fibroblast cells, danshensu (25–200 µM) or salvianolic acid B (25–200 µM) treatment significantly promoted the cell proliferation. Danshensu (0.1 mM) or salvianolic acid B (0.1 mM) treatment increased the collagen production in fibroblasts. These results indicated that danshensu and salvianolic acid B could be utilized as wound healing agents.
Lilii Bulbus
Lilii Bulbus, the dried fleshy petal-like layers of Lilium lancifolium Thunb [Liliaceae], Lilium brownii var. viridulum Baker [Liliaceae], or Lilium pumilum Redouté [Liliaceae], is a famous botanical drug widely used in China. Lilii Bulbus possesses anti-inflammatory and antibacterial activities, which are useful in wound repair (Zhou et al., 2021). In Esposito’s research, two steroidal glycosides from the Lilii Bulbus can accelerate wound closure in the 3T3 murine fibroblast cell line. Steroidal glycoside 1 was identified as (22R, 25R)-spirosol-5-en-3β-yl O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranosyl-(1→4)-β-D-glucopyranoside and steroidal glycoside 2 was an acetylated derivative of steroidal glycoside 1, which was identified as (22R, 25R)-spirosol-5-en-3β-yl O-α-L-rhamnopyranosyl-(1→2)-[6-O-acetyl-β-D-glucopyranosyl-(1→4)]-β-D-glucopyranoside. Steroidal glycoside 1 accelerated the scratch wound closure in skin fibroblasts at the concentration of 0.2, 1, and 5 μM, and steroidal glycoside 2 showed wound healing activity at a concentration of 5 μM. The mechanism study showed that these two steroidal glycosides (5 μM) could induce the production of NO and increase the mRNA level of TGF-β Type I receptor, which played important roles in early wound healing (Esposito et al., 2013). The wound healing activity of steroidal glycoside 1 was further studied (Di et al., 2020). After the wounded fibroblast cells were treated with steroidal glycoside 1 at 5 μM, genes related to inflammation, chemokine, and tissue remodeling were downregulated. The decreases in the expression of key genes contributed to an early resolution of the inflammation and shorten the early phase of wound healing (Di et al., 2020). Also, steroidal glycoside 1 upregulated the extracellular matrix and cell adhesion-related genes to regulate basic functions of cells in wound healing (Di et al., 2020).
Glycyrrhizae Radix et Rhizoma
Glycyrrhizae Radix et Rhizoma is the dried root and rhizome of Glycyrrhiza uralensis Fisch. ex DC [Fabaceae], Glycyrrhiza inflata Batalin [Fabaceae] or Glycyrrhiza glabra L [Fabaceae]. It is the most common botanical drug in China, which can be used to treat hepatitis, cough, gastric ulcer, and wound (Kotian et al., 2018). It is also widely used in cosmetics and food ingredients and the major components of it are triterpene saponins and flavonoids (Li et al., 2020). Hao’s research showed that in the rat wound model, the topical application of Glycyrrhizae Radix et Rhizoma soluble polysaccharide collagen sponge (containing 72 μg polysaccharide) could increase the content of hydroxyproline in wounds, promote the proliferation of capillaries and fibroblasts in granulation tissues, and increase the number of microvessels in wound sites through activating the expression of p-STAT3 and VEGF and upregulating the transcription levels of VEGF mRNA and miRNA-21 genes (Hao et al., 2020). Another active component is isoliquiritin, an isoflavonoid isolated from Glycyrrhizae Radix et Rhizoma. According to Liu’s investigation, isoliquiritin at 100 or 200 μg/ml can accelerate the healing of zebrafish skin wound via promoting inflammation response and facilitating angiogenesis (Liu et al., 2020).
Zingiberis Rhizoma Recens
Zingiberis Rhizoma Recens is the fresh rhizoma of Zingiber officinale Roscoe [Zingiberaceae], which is widely cultivated in China. According to Compendium of Materia Medica, Zingiberis Rhizoma Recens can promote the generation of new tissues. Plenty of studies revealed that gingerols, essential oils, and diarylheptanoids were the major components of Zingiberis Rhizoma Recens and Zingiberis Rhizoma Recens showed excellent antioxidant, antibacterial, antitumor, and antiinflammatory activities (Li et al., 2021). In the acetic acid-induced gastric ulcer rat model, orally administration of Zingiberis Rhizoma Recens aqueous extract (1.25, 2.5, and 5 g/kg) reduced the gastric ulcer area in dose-dependent manner via inhibiting the expression of the chemokines and TNF-α (Ko and Leung, 2010). In vitro, 10-shogaol from Zingiberis Rhizoma Recens promoted the proliferation of human normal epidermal keratinocytes and dermal fibroblasts and 2 μM of 10-shogaol showed the highest increase in the cells viability. In both keratinocytes and fibroblasts, 10-shogaol (2 and 10 μM) significantly enhanced the production of TGF-β, PDGF-αβ, and VEGF (Chen CY. et al., 2012). 6-dehydrogingerdione, another component of Zingiberis Rhizoma Recens also can upregulate the growth factor production, and accelerate cellular proliferation and migration at 2 or 10 μM. The mechanism study revealed that 6-dehydrogingerdione could block the MAPK pathway by suppressing c-Jun protein level and ERK phosphorylation in fibroblasts (Chen CY. et al., 2013).
Lonicerae Japonicae Flos
Lonicerae Japonicae Flos is the dried flower bud or opening flower of Lonicera japonica Thunb [Caprifoliaceae]. According to Shen Nong’s Herbal Classic, it was used in clinical applications for clearing heat and detoxification and it could also be used for the treatment of swelling and ulcer on the body surface. Modern research studies showed that Lonicerae Japonicae Flos possesses anti-inflammatory, antivirus and antioxidant activities, and organic acids, iridoid glycosides, flavonoids, and saponins were the main active compounds (Li J. et al., 2019). Wound healing activity of Lonicerae Japonicae Flos in the rat excision wound model was investigated by Chen WC. et al. (2012), and 10% (w/w) Lonicerae Japonicae Flos extract ointment was prepared by incorporating 10 g Lonicerae Japonicae Flos extract (90% ethanol) into 100 g of ointment base. Results showed that topical administration of 10% Lonicerae Japonicae Flos extract ointment significantly reduced the wound size, promoted the tissue regeneration, enhanced angiogenesis, and increased collagen deposition. Meanwhile, the elevated production of anti-inflammatory cytokine (IL-10) and suppressed production of proinflammatory cytokines (TNF-α and IL-6) were observed in rat blood samples after treated with Lonicerae Japonicae Flos extract ointment (Chen WC. et al., 2012). Since chlorogenic acid was the main active component of Lonicerae Japonicae Flos, another study conducted by Chen’s team investigated the wound healing activity of chlorogenic acid. In rats with excision wounds, topical application of 1% (w/w) chlorogenic acid ointment accelerated the wound healing through the upregulation of TNF-α and TGF-β (Chen WC. et al., 2013). Another study revealed that intraperitoneal administration of chlorogenic acid (25–200 mg/kg) could also accelerate wound healing (Bagdas et al., 2014).
Portulacae Herba
Portulacae Herba is the dried aerial part of Portulaca oleracea L [Portulacaceae], which is widely distributed in the tropical and subtropical areas of the world. According to Tang Materia Medica, Portulacae Herba could be used for wounds. Modern pharmacological research studies demonstrated that Portulacae Herba possesses anti-inflammatory, antimicrobial, antioxidant, and antiulcerogenic activities (Zhou J. et al., 2015). Rashed’s team evaluated the preliminary wound healing activity of Portulacae Herba with the mouse excision wound model. Fresh Portulacae Herba homogenate (single dose of 50 mg or a twice dose of 25 mg topically applied) can stimulate wound contraction and increase the breaking strength of the treated wounds (Rashed et al., 2003). Additionally, a clinical study was conducted in lactating women to study the effects of Portulaca oleracea L. cream on the healing of nipple fissure (Niazi et al., 2019). The mean score of breast fissures significantly decreased and no complications were observed after the patients were treated with Portulaca oleracea L. cream (2%). It is worth noting that adverse events such as itching and tingling of whole body, dyspnea and tachycardia were reported after the systemic administration of Portulaca oleracea seed extract (Mirabzadeh et al., 2013).
Hippophae rhamnoides L
Hippophae rhamnoides L [Elaeagnaceae] is a shrub native to various countries, including China, India, Nepal, and Russia. Hippophae rhamnoides L. has been widely used for the treatment of various diseases, such as cough, skin diseases, and inflammation (Pundir et al., 2021). It has been reported that the leaves, fruits, and seeds of Hippophae rhamnoides L. all possess wound healing activities (Gupta et al., 2005; Gupta et al., 2006; Upadhyay et al., 2009; Ito et al., 2014).
In rat full-thickness wound models, Hippophae rhamnoides L. leave extract significantly promoted the wound healing (Gupta et al., 2005; Kim and Lee, 2017). Mechanism studies conducted in rat burn wounds found that it showed positive pharmacological effects on different phases of the wound healing process (Upadhyay et al., 2011). After the burn wounds were treated with Hippophae rhamnoides L. leave extract (5% prepared in petroleum jelly) twice daily for 7 days, no evidence of wound bleeding, exudates, pus, or inflammation were observed at any time (Upadhyay et al., 2011). In the rat burn wound model, Hippophae rhamnoides L. leave extract could promote the angiogenesis via upregulating the expression of VEGF in regenerated tissue, stimulate the collagen synthesis, and increase the levels of antioxidants such as glutathione, superoxide dismutase, catalase, glutathione-S-transferase, and vitamin C in wounds (Upadhyay et al., 2011). Meanwhile, the Hippophae rhamnoides L. fruit pulp flavone and the seed oil also showed excellent wound healing activities (Gupta et al., 2006; Upadhyay et al., 2009; Ito et al., 2014).
Urtica dioica L
Urtica dioica L [Urticaceae] is a botanical drug widely used for the treatment of various diseases, such as diabetes, hypertension, cardiovascular diseases, and prostate cancer (El Haouari and Rosado, 2019). Recently, Urtica dioica L. has been proven to possess wound healing activity. In Razika’s study, crude saponins were extracted from the leaves of Urtica dioica L. and topical application of the extract (20% in Vaseline) significantly promoted the wound healing process of rat excision wounds (Razika et al., 2017). The antioxidant activity of the crude saponins extract was also evaluated by in vitro diphenyl-picryl-hydrazyl test (DPPH) and excellent antioxidant activity (IC50 = 0.159 mg/ml), which was similar to that of ascorbic acid (p > 0.05) was observed (Razika et al., 2017). In another study conducted by Zouari Bouassida et al., hydroethanolic extract of Urtica dioica L. was proven to show hemostatic and wound healing activities (Zouari Bouassida et al., 2017). Lupeol was identified as an active component in the extract, and lupeol had been reported to be able to improve re-epithelization during the wound healing process (Ammar et al., 2015). Thus, it was suggested that Urtica dioica L. showed wound healing activity, and lupeol was the potential therapeutic material basis.
Periploca forrestii Schltr
Periploca forrestii Schltr [Apocynaceae] is a Chinese folk medicine, which has been historically used for the treatment of traumatic injuries (Huang M. et al., 2019). Recently, the molecular mechanism of its wound healing activities and the active components of it were revealed by the Liu’s group (Chen et al., 2018; Li Y. et al., 2019; Chen et al., 2019). Periploca forrestii Schltr. extract was separated by a macroporous resin column, and 65% ethanol eluted fraction (EPFE65) was tested for the wound healing activity. In their in vitro study, EPFE65 (50 μg/ml) significantly promoted the proliferation and migration of mouse fibroblast and stimulated the collagen synthesis in mouse fibroblast. In the in vivo study, topical application of EPFE65 hydrogel (containing 0.1% EPFE65) exhibited wound healing activity via enhancing the re-epithelialization and promoting the formation of complete dermis (Li J. et al., 2019). Further studies demonstrated that the regulation of Src mediated Mek/Erk and PI3K/Akt signaling pathways was the potential mechanism of the wound healing, and cardiac glycosides were the potential active components (Li Y. et al., 2019). Periplocin, the cardiotonic steroide isolated from Periploca forrestii Schltr., exhibited wound healing activity via the activation of Src/Erk and PI3K/Akt pathways both in vitro and in vivo (Chen et al., 2019).
Streptocaulon juventas (Lour.) Merr
Streptocaulon juventas (Lour.) Merr [Apocynaceae] is a widely used folk medicine of the Dai minority in China and it is famous for its anti-inflammatory, anticancer, and wound healing activities (Anaya-Eugenio et al., 2019). In the research conducted by the Nguyen’s group, the wound healing activity of Streptocaulon juventas (Lour.) Merr. root ethanolic extract was evaluated in the mouse excision wound model (Nguyen et al., 2017). Results showed that topical administration of the extract (100 mg/kg/day) remarkably reduced the wound closure time. Meanwhile, the reduced expression of TNF-α and NF-κB1 genes and enhanced angiogenesis were observed in the wound granulation tissues treated with Streptocaulon juventas (Lour.) Merr. extract (Nguyen et al., 2017). Taken together, it was deduced that Streptocaulon juventas (Lour.) Merr. extract exerted wound healing activity via inducing the fibroblast proliferation and angiogenesis, and inhibiting the inflammation.
Carthamus tinctorius L
Carthamus tinctorius L [Asteraceae] is a traditional botanical drug for treating blood stasis and painful menstrual problems (Zhang LL. et al., 2016). The flower and seed are the main medicinal parts of Carthamus tinctorius L. Flavonoids, alkaloids, and organic acids are the components responsible for most of its pharmacological activities and hydroxysafflor yellow A (HSYA) is the most popular component (Zhang LL. et al., 2016). The wound healing activity of HSYA both in vitro and in vivo was evaluated by Gao et al. (2018). In vitro, HSYA (0.4, 0.8 and 1.6 mM) promoted the migration of human epithelial keratinocytes in a dose-dependent manner. Low concentration of HSYA (0.4 mM) increased the tube formation of HUVECs and high concentration of HSYA (1.6 mM) impaired the tube formation. In diabetic rats with splinted excisional wound, HSYA topically applied at the dose of 0.2 mg significantly promoted wound closure, re-epithelialization and angiogenesis. Meanwhile, the higher collagen content and elevated VEGF and TGF-β1 levels were observed in HSYA-treated wounds (Gao et al., 2018).
Reynoutria japonica Houtt
Reynoutria japonica Houtt [Polygonaceae] is a popular botanical drug widely distributed in East China, Central South, and Southwest China. Since ancient time Reynoutria japonica Houtt. has been used for the treatment of jaundice, scald, inflammation, and favus (Peng et al., 2013). Izabela’s team did the research to evaluate the wound healing activity of Reynoutria japonica Houtt. by using human gingival fibroblasts (HGFs). The results showed the various extracts of Reynoutria japonica Houtt. (25% ethanol, 40% ethanol, or 60% acetone) all able to stimulate HGFs proliferation and migration, and increase collagen III synthesis at the dose of 50 μg/ml (Nawrot-Hadzik et al., 2021). Various compounds have been isolated from Reynoutria japonica Houtt, including quinones, stilbenes, flavonoids, coumarins, and other polyphenolic compounds (Peng et al., 2013). Resveratrol is one of the active components of Reynoutria japonica Houtt, which is used widely in skin care products to improve the overall condition of the skin (Igielska-Kalwat et al., 2019). Nowadays, various investigations have been conducted to demonstrate the mechanism for resveratrol’s wound healing activity. It is believed that resveratrol promotes the wound healing via attenuating the oxidative stress levels and increasing cell proliferation and migration (Kamolz et al., 2020).
Artemisia annua L
In line with Compendium of Material Medica, Artemisia annua L [Asteraceae] could be used for wounds. Artemisia annua L. has been proven to possess antibacterial, antifungal, anti-inflammatory, and antioxidant activities by various studies (Ćavar et al., 2012). Owning to these properties, Artemisia annua L. has been used in novel wound dressings. In the study conducted by Mirbehbahani et al., Artemisia annua L.-containing nanofibers were prepared, and the nanofibers promoted wound healing via encouraging the attachment, spreading and proliferation of the fibroblasts and exhibited antibacterial properties against Staphylococcus aureus (Mirbehbahani et al., 2020).
Acorus calamus L
Acorus calamus L [Acoraceae] is a medicinal plant widely distributed in China. As recorded in Compendium of Materia Medica, in ancient times, Acorus calamus L. was a botanical drug used for traumatic injury. In traditional folk medicine of China and Indonesia, it was used for the treatment of inflammation, depression, hemorrhoids, skin diseases etc., and phenylpropanoids, sesquiterpenoids, and monoterpenes were the major constituents (Sharma et al., 2020). In rat excision and incision wounds, 80% ethanol extract of Acorus calamus L. dried leaves (20% or 40% ointment topically applied once daily) promoted wound healing via enhancing wound contraction, decreasing epithelialisation time, and increasing hydroxyproline content (Jain et al., 2010). The rhizoma of Acorus calamus L. also exhibited wound healing activity. Ponrasu’s team proved that after the dermal wounds in rat were treated with 70% ethanolic extract of Acorus calamus L. rhizoma (40 mg/kg topically applied once daily), the tensile strength of wounds was increased and granulation tissue was formed with more collagen, hexosamine, and uronic acid (Ponrasu et al., 2014).
Triticum aestivum L
According to Compendium of Material Medica, Triticum aestivum L [Poaceae] (Wheat) had been used for wound healing since ancient time. Sahin’s team took the research in the wheat exosomes, and the wound healing activities of wheat grass juice-derived exosomes were evaluated in vitro. Results revealed that wheat exosomes (30–200 μg/ml) showed astonishing proliferative and migratory activities on HDFs, HUVECs, and human keratinocytes. Wheat exosomes significantly increased the expression level of collagen type I in HDFs and promoted the formation of tube-like structure of in HUVECs at the dose of 200 μg/ml (Sahin et al., 2019). Another interesting fraction of wheat is a small peptide named YDWPGGRN, which can accelerate wound healing in a rat model with a full thickness dermal wound through stimulating angiogenesis and collagen production in wounds (Sui et al., 2020).
Multiherbal Preparations With Wound Healing Properties
It is believed that multiple botanical drug compositions in a particular ratio will reduce toxicity and give a better therapeutic effect. Thus, multiherbal preparations are widely used in Chinese hospitals. Many of the multiherbal preparations have been proven for their wound healing activities, such as Shiunko (Zi Yungao in Chinese) and NF3. Shiunko is a formulation consists of Arnebiae radix and Angelicae sinensis radix, which is developed during the Ming dynasty in China and widely used in China and Japan for the treatment of wounds (Chak et al., 2013). Mechanism studies revealed that Shiunko promoted the epithelization, angiogenesis, and granulation tissue formation of wounds (Huang et al., 2004; Lu et al., 2008; Chak et al., 2013). Recently, clinical trials proved that Shiunko might be also effective for radiation-induced dermatitis in patients with breast cancer and localized cutaneous leishmaniasis (Na-Bangchang et al., 2016; Kim et al., 2021). NF3, a two-botanical drug formulation comprised Astragali radix and Rehmanniae radix, is another one which has been extensively investigated for the treatment of diabetic foot ulcer (Lau et al., 2008; Lau et al., 2009a; Zhang et al., 2011; Lau et al., 2012). In the human skin fibroblast cell Hs27, NF3 (4 mg/ml) enhanced wound healing via activating the TGF-β pathway and promoting ECM deposition (Zhang et al., 2012). In human vascular endothelial cells, NF3 promoted cell migration at 75–300 μg/ml and enhanced tubule formation via MAPK and Akt pathways at 75 and 150 μg/ml (Liu et al., 2013a). A recent proof-of-concept study showed that NF3 treatment (5 g/sachet, two sachets daily) significantly reduced the foot ulcer area in Chinese patients with diabetes (Ko et al., 2014). Other multiherbal preparations with wound healing properties are summarized in Table 2.
TABLE 2.
Multiherbal preparations and their wound healing activities.
| Multiherbal preparation | Composition | Source or preparation procedure | Experimental model | Doses and route | Wound healing activity and potential mechanism | Reference |
|---|---|---|---|---|---|---|
| Jing Wan Hong ointment | 30 drugs including Ampelopsis japonica (Thunb.) Makino [Vitaceae], Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav. [Apiaceae], Lobelia chinensis Lour. [Campanulaceae], Cinnamomum camphora (L.) J.Presl [Lauraceae], Atractylodes lancea (Thunb.) DC. [Asteraceae], Paeonia lactiflora Pall. [Paeoniaceae], and Conioselinum anthriscoides 'Chuanxiong’ [Apiaceae] | Commercially available and obtained from Tianjin Darentang Jingwanhong Pharmaceutical Co., Ltd | Nerve injury diabetic foot ulcer in rats | Topically applied with gauze immersed with the ointment | Promoted the foot ulcer healing through tissue regeneration, angiogenesis, and inflammation control, which was dependent on the increased expression of PDGF mRNA. | Jin et al. (2014) |
| Liu-He-Dan | More than 10 botanical drugs including but not limited to Rheum officinale Baill. [Polygonaceae], Phellodendron amurense Rupr. [Rutaceae], Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav. [Apiaceae], smoked plum, and Bletilla striata (Thunb.) Rchb.f. [Orchidaceae] | Drugs were powdered and mixed with water and honey into a paste | Patients with radiodermatitis associated with breast cancer | 5 g Liu-He-Dan past applied externally once daily | Decreased inflammatory seepage and alleviated pain | Zhou et al. (2015b) |
| Tuo-Li-Xiao-Du-San | Comprised Angelica sinensis (Oliv.) Diels [Apiaceae], Astragalus mongholicus Bunge [Fabaceae], Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav. [Apiaceae], and thorns of Gleditsia sinensis Lam. [Fabaceae] in the ratio of 5:5:4:4 | Crude botanical drugs were powered and then extracted by 70% ethanol for three times | Diabetic rats with full-thickness wound | Orally dosed once daily (1.5, 1.5, 1.2, and 1.2 g/kg for the four drugs) | Promoted diabetes-impaired wound healing via reducing inflammation, increasing angiogenesis and collagen deposition | Zhang et al. (2016c) |
| Ulcer oil | Comprised Phellodendron amurense Rupr. [Rutaceae] and Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav. [Apiaceae] | Obtained from the Beijing University of Chinese Medicine Dongzhimen Hospital | Diabetic rats with full-thickness wound | Topically applied (1 ml/cm2) once daily | Promoted wound healing via downregulating PTPIB and AGEs and upregulating VEGF and PDGF. | Jia et al. (2018) |
| Shengfu Oil | Comprised Phellodendron amurense Rupr. [Rutaceae], Rheum officinale Baill. [Polygonaceae], Astragalus mongholicus Bunge [Fabaceae], Coptis chinensis Franch. [Ranunculaceae], Scutellaria baicalensis Georgi [Lamiaceae], Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav. [Apiaceae], Boswellia sacra Flück. [Burseraceae], Sanguisorba officinalis L. [Rosaceae], Lithospermum erythrorhizon Siebold and Zucc. [Boraginaceae], Commiphora myrrha (T.Nees) Engl. [Burseraceae], Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae], Bletilla striata (Thunb.) Rchb.f. [Orchidaceae], Vincetoxicum atratum (Bunge) C.Morren and Decne. [Apocynaceae], Angelica sinensis (Oliv.) Diels [Apiaceae], and Cinnamomum camphora (L.) J.Presl [Lauraceae] | These botanical drugs were powdered and soaked in five times volume of sesame oil for 7 days at room temperature and then further incubated for another 2 h at 90°C. The filtered supernatant was Shengfu oil | Rabbit with full-thickness scalded wound | Topically applied at the dose of 0.15 ml/cm2 three times per day | Showed anti-inflammatory, analgesic, and antimicrobial activities. Promoted wound healing via regulating the expression of β-catenin, Dlk1, and COX-2 | Chen et al. (2017) |
| Sheng-ji Hua-yu formula | Comprised eight drugs including Astragalus mongholicus Bunge [Fabaceae], Salvia miltiorrhiza Bunge [Lamiaceae], Rheum palmatum L. [Polygonaceae], Calamus draco Willd. [Arecaceae], Arnebia euchroma (Royle ex Benth.) I.M.Johnst. [Boraginaceae], Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav. [Apiaceae], and Nacre and Calamine. The amount of these drugs were 60, 15, 15, 10, 30, 30, 30, and 30 g, respectively | The ethanol extract of the drugs was prepared and then extracted with chloroform for four times. The extract was mixed, concentrated, and then mixed with carbomer | Diabetic mice with full-thickness wound | Topically applied the ointment at 0.5 g/cm2/day | Accelerated re-epithelialization and healing time of diabetic wounds via decreasing the high expression of activin/follistatin | (Kuai et al., 2018; Xiang et al., 2021) |
| Zhangpi Ointment | Comprised six botanical drugs and two minerals including Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae], Angelica sinensis (Oliv.) Diels [Apiaceae], Arnebia euchroma (Royle ex Benth.) I.M.Johnst. [Boraginaceae], Rheum officinale Baill. [Polygonaceae], Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae], and Lycium barbarum L. [Solanaceae], rubber powder and calomel | Obtained from Shanghai Ninth People’s Hospital | Hydroxyurea-induced leg ulcers in patients with myeloproliferative neoplasms | About 1 mm thick ointment was applied to the surface of the ulcer | Compared with the control group, patients treated with Zhangpi Ointment achieved a significant higher rate of wound healing | Pang et al. (2018) |
| ANBP | Comprised Agrimonia eupatoria L. [Rosaceae], Nelumbo nucifera Gaertn. [Nelumbonaceae], Boswellia sacra Flück. [Burseraceae], and the pollen of Typha angustifolia L. [Typhaceae] | Powdered the drugs with ultralow temperature broken method | Rabbit ear hypertrophic scar model | Topically applied the powder | Promoted wound healing and reduced scar formation via the regulation of the TGF-b1/Smad pathway | Hou et al. (2014) |
| Huiyang Shengji decoction | Comprised Astragalus mongholicus Bunge [Fabaceae] 30 g, Atractylodes macrocephala Koidz. [Asteraceae] 10 g, Atractylodes lancea (Thunb.) DC. [Asteraceae] 10 g, Poria 10 g, Cinnamomum verum J.Presl [Lauraceae] 10 g, Aconitum carmichaeli Debeaux [Ranunculaceae] 10 g, Cervi Cornu Degelatinatum 10 g, Chaenomeles lagenaria (Loisel.) Koidz. [Rosaceae] 10 g, Sinapis alba L. [Brassicaceae] 10 g, Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae] 10 g, Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav. [Apiaceae] 10 g, and Glehnia littoralis (A.Gray) F.Schmidt ex Miq. [Apiaceae] 15 g | 155 g drugs were soaked for 1 h and then extracted with water. The extract was filter and concentrated, and the concentration was 1.993 g/ml.Then the solution was freeze-dried into powder | db/db mice with full-thickness wound | Orally dosed of the decoction at 19.93 g/kg | Promoted the proliferation of epidermal cells in wounds via increasing EGFR expression and the activation of PI3K/AKT pathway | Liu et al. (2021) |
Discussion
As described in this review, anti-inflammatory, antibacterial, antioxidant, proangiogenic, promoting fibroblast proliferation, and pro-collagen synthesis activities of botanical drugs contribute to the wound healing process. Botanical drugs in traditional Chinese medicine with these properties could i) inhibit the secretion of inflammatory mediators; ii) stimulate the production of cytokines such as VEGF, TGF-β, PDGF, and EGF; iii) promote fibroblasts proliferation and migration; iv) accelerate angiogenesis, granulation tissue formation, and re-epithelialization; and v) regulate collagen synthesis and deposition in wound sites, and all these lead to a promoted wound healing (Figure 1). Meanwhile, to maximize the therapeutic benefits of botanical drugs, different types of wound dressings include hydrogel, hydrocolloids, scaffolds, nanocomposites membranes, and nanofibers are used. Advanced wound dressings loaded with botanical drugs would be new treatment options.
FIGURE 1.
Botanical drugs in traditional Chinese medicine and their wound healing properties.
Even though numerous investigations proved that botanical drugs are promising therapeutics for wound healing, there are still some limitations needed to be pointed out. First, for most of the botanical drugs, investigations about the wound healing activities remain at preclinical studies. Clinical trials are needed to prove the safety and effectiveness. There are many wound-related diseases in clinical, such as diabetic foot, venous leg ulcer, ulcerative bedsore, and burn ulcer. Well-designed clinical trials could provide scientific basis for further development and application of the botanical drugs for the treatment of wounds. Second, as for some botanical drugs, the molecular mechanisms are unclear. Along with the deepening of research, an increasing number of targets and pathways are demonstrated to be associated with wound healing. To better understand the wound healing activities of botanical drugs, further studies which are able to reveal the mechanism on the molecular biological levels are still needed. Third, extracts are commonly used to evaluate the wound healing activities of botanical drugs; however, components responsible for the activity are rarely identified (Figure 2), and the preparation of the extracts is usually not standardized. Due to the fact that botanical drugs obtained from different origins, habitats, and harvest time could be different in chemical profiles, it is important to identify the therapeutic basis or standardize the preparation of the extracts. Fourth, it is a general belief that botanical drugs display fewer side effects. Thus, most of the published investigations focused on the wound healing activities of botanical drugs, and the safety of using them for wounds was less discussed. Studies able to assess the potential adverse effects of botanical drugs for wounds are still needed. Taken together, botanical drugs are important sources of wound healing therapeutics and more efforts should be paid to bring these therapeutics earlier to patients.
FIGURE 2.
Chemical structures of components isolated from botanical drugs which possess wound healing activities.
Conclusion
To sum up, botanical drugs in traditional Chinese medicine are powerful alternatives for the treatment of wounds. This review summarized the wound healing activities of traditionally used Chinese botanical drugs and multiherbal preparations. It provides valuable information for the development of effective wound healing drugs.
Author Contributions
SN collected the literatures and wrote the original draft. JZ and BZ collected literatures. XF and FQ reviewed and revised the manuscript. All authors approved the published version of this manuscript.
Funding
This work was supported by the grant from the National Natural Science Foundation of China (No’s 81973565 and 82030116).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aggarwal B. B., Sundaram C., Malani N., Ichikawa H. (2007). Curcumin: the Indian Solid Gold. Adv. Exp. Med. Biol. 595, 1–75. 10.1007/978-0-387-46401-5_1 [DOI] [PubMed] [Google Scholar]
- Akbik D., Ghadiri M., Chrzanowski W., Rohanizadeh R. (2014). Curcumin as a Wound Healing Agent. Life Sci. 116, 1–7. 10.1016/j.lfs.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Ammar I., Bardaa S., Mzid M., Sahnoun Z., Rebaii T., Attia H., et al. (2015). Antioxidant, Antibacterial and In Vivo Dermal Wound Healing Effects of Opuntia Flower Extracts. Int. J. Biol. Macromol 81, 483–490. 10.1016/j.ijbiomac.2015.08.039 [DOI] [PubMed] [Google Scholar]
- Anaya-Eugenio G. D., Addo E. M., Ezzone N., Henkin J. M., Ninh T. N., Ren Y., et al. (2019). Caspase-Dependent Apoptosis in Prostate Cancer Cells and Zebrafish by Corchorusoside C from Streptocaulon Juventas. J. Nat. Prod. 82, 1645–1655. 10.1021/acs.jnatprod.9b00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andújar I., Ríos J. L., Giner R. M., Recio M. C. (2013). Pharmacological Properties of Shikonin - a Review of Literature since 2002. Planta Med. 79, 1685–1697. 10.1055/s-0033-1350934 [DOI] [PubMed] [Google Scholar]
- Apaza Ticona L., Rumbero Sánchez Á., Sánchez Sánchez-Corral J., Iglesias Moreno P., Ortega Domenech M. (2021). Anti-inflammatory, Pro-proliferative and Antimicrobial Potential of the Compounds Isolated from Daemonorops Draco (Willd.) Blume. J. Ethnopharmacol 268, 113668. 10.1016/j.jep.2020.113668 [DOI] [PubMed] [Google Scholar]
- Aro A. A., Perez M. O., Vieira C. P., Esquisatto M. A., Rodrigues R. A., Gomes L., et al. (2015). Effect of Calendula officinalis Cream on Achilles Tendon Healing. Anat. Rec. (Hoboken) 298, 428–435. 10.1002/ar.23057 [DOI] [PubMed] [Google Scholar]
- Bagdas D., Gul N. Y., Topal A., Tas S., Ozyigit M. O., Cinkilic N., et al. (2014). Pharmacologic Overview of Systemic Chlorogenic Acid Therapy on Experimental Wound Healing. Naunyn Schmiedebergs Arch. Pharmacol. 387, 1101–1116. 10.1007/s00210-014-1034-9 [DOI] [PubMed] [Google Scholar]
- Boudreau M. D., Beland F. A. (2006). An Evaluation of the Biological and Toxicological Properties of Aloe Barbadensis (miller), Aloe Vera. J. Environ. Sci. Health C Environ. Carcinog Ecotoxicol Rev. 24, 103–154. 10.1080/10590500600614303 [DOI] [PubMed] [Google Scholar]
- Bowers S., Franco E. (2020). Chronic Wounds: Evaluation and Management. Am. Fam. Physician 101, 159–166. [PubMed] [Google Scholar]
- Brinkhaus B., Lindner M., Schuppan D., Hahn E. G. (2000). Chemical, Pharmacological and Clinical Profile of the East Asian Medical Plant Centella asiatica . Phytomedicine 7, 427–448. 10.1016/s0944-7113(00)80065-3 [DOI] [PubMed] [Google Scholar]
- Burusapat C., Supawan M., Pruksapong C., Pitiseree A., Suwantemee C. (2018). Topical Aloe Vera Gel for Accelerated Wound Healing of Split-Thickness Skin Graft Donor Sites: A Double-Blind, Randomized, Controlled Trial and Systematic Review. Plast. Reconstr. Surg. 142, 217–226. 10.1097/PRS.0000000000004515 [DOI] [PubMed] [Google Scholar]
- Buzzi M., de Freitas F., de Barros Winter M. (2016). Therapeutic Effectiveness of a Calendula officinalis Extract in Venous Leg Ulcer Healing. J. Wound Care 25, 732–739. 10.12968/jowc.2016.25.12.732 [DOI] [PubMed] [Google Scholar]
- Bylka W., Znajdek-Awiżeń P., Studzińska-Sroka E., Dańczak-Pazdrowska A., Brzezińska M. (2014). Centella asiatica in Dermatology: an Overview. Phytother Res. 28, 1117–1124. 10.1002/ptr.5110 [DOI] [PubMed] [Google Scholar]
- Cai H. A., Huang L., Zheng L. J., Fu K., Wang J., Hu F. D., et al. (2019). Ginsenoside (Rg-1) Promoted the Wound Closure of Diabetic Foot Ulcer through iNOS Elevation via miR-23a/IRF-1 axis. Life Sci. 233, 116525. 10.1016/j.lfs.2019.05.081 [DOI] [PubMed] [Google Scholar]
- Carvalho A. F., Feitosa M. C., Coelho N. P., Rebêlo V. C., Castro J. G., Sousa P. R., et al. (2016). Low-level Laser Therapy and Calendula officinalis in Repairing Diabetic Foot Ulcers. Rev. Esc Enferm USP 50, 628–634. 10.1590/S0080-623420160000500013 [DOI] [PubMed] [Google Scholar]
- Ćavar S., Maksimović M., Vidic D., Parić A. (2012). Chemical Composition and Antioxidant and Antimicrobial Activity of Essential Oil of Artemisia Annua L. From Bosnia. Ind. Crops Prod. 37, 479–485. [Google Scholar]
- Chak K. F., Hsiao C. Y., Chen T. Y. (2013). A Study of the Effect of Shiunko, a Traditional Chinese Herbal Medicine, on Fibroblasts and its Implication on Wound Healing Processes. Adv. Wound Care (New Rochelle) 2, 448–455. 10.1089/wound.2012.0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Cheng K. C., Chang A. Y., Lin Y. T., Hseu Y. C., Wang H. M. (2012a). 10-Shogaol, an Antioxidant from Zingiber Officinale for Skin Cell Proliferation and Migration Enhancer. Int. J. Mol. Sci. 13, 1762–1777. 10.3390/ijms13021762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Chiu C. C., Wu C. P., Chou Y. T., Wang H. M. (2013a). Enhancements of Skin Cell Proliferations and Migrations via 6-dehydrogingerdione. J. Agric. Food Chem. 61, 1349–1356. 10.1021/jf304340q [DOI] [PubMed] [Google Scholar]
- Chen L., Jiang P., Li J., Xie Z., Xu Y., Qu W., et al. (2019). Periplocin Promotes Wound Healing through the Activation of Src/ERK and PI3K/Akt Pathways Mediated by Na/K-ATPase. Phytomedicine 57, 72–83. 10.1016/j.phymed.2018.12.015 [DOI] [PubMed] [Google Scholar]
- Chen L., Li J., Ke X., Sun C., Huang X., Jiang P., et al. (2018). Chemical Profiling and the Potential Active Constituents Responsible for Wound Healing in Periploca Forrestii Schltr. J. Ethnopharmacol 224, 230–241. 10.1016/j.jep.2018.04.023 [DOI] [PubMed] [Google Scholar]
- Chen M. T., Yang Y. J., Li Y. S., Li X. J., Zhang W. K., Wang J. P., et al. (2017). Shengfu Oil Enhances the Healing of Full-Thickness Scalded Skin Accompanying the Differential Regulation of β-Catenin, Dlk1, and COX-2. Front. Pharmacol. 8, 801. 10.3389/fphar.2017.00801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. C., Liou S. S., Tzeng T. F., Lee S. L., Liu I. M. (2013b). Effect of Topical Application of Chlorogenic Acid on Excision Wound Healing in Rats. Planta Med. 79, 616–621. 10.1055/s-0032-1328364 [DOI] [PubMed] [Google Scholar]
- Chen W. C., Liou S. S., Tzeng T. F., Lee S. L., Liu I. M. (2012b). Wound Repair and Anti-inflammatory Potential of Lonicera japonica in Excision Wound-Induced Rats. BMC Complement. Altern. Med. 12, 226. 10.1186/1472-6882-12-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. S., Lee S. M., Lin Y. J., Chiang S. H., Lin C. C. (2014). Effects of Danshensu and Salvianolic Acid B from Salvia Miltiorrhiza Bunge (Lamiaceae) on Cell Proliferation and Collagen and Melanin Production. Molecules 19, 2029–2041. 10.3390/molecules19022029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkerngsuntorn W., Rerknimitr P., Panchaprateep R., Tangkijngamvong N., Kumtornrut C., Kerr S. J., et al. (2020). The Effects of a Standardized Extract of Centella asiatica on Postlaser Resurfacing Wound Healing on the Face: A Split-Face, Double-Blind, Randomized, Placebo-Controlled Trial. J. Altern. Complement. Med. 26, 529–536. 10.1089/acm.2019.0325 [DOI] [PubMed] [Google Scholar]
- Di R., Murray A. F., Xiong J., Esposito D., Komarnytsky S., Gianfagna T. J., et al. (2020). Lily Steroidal Glycoalkaloid Promotes Early Inflammatory Resolution in Wounded Human Fibroblasts. J. Ethnopharmacol 258, 112766. 10.1016/j.jep.2020.112766 [DOI] [PubMed] [Google Scholar]
- Diao H., Li X., Chen J., Luo Y., Chen X., Dong L., et al. (2008). Bletilla Striata Polysaccharide Stimulates Inducible Nitric Oxide Synthase and Proinflammatory Cytokine Expression in Macrophages. J. Biosci. Bioeng. 105, 85–89. 10.1263/jbb.105.85 [DOI] [PubMed] [Google Scholar]
- Dinda M., Dasgupta U., Singh N., Bhattacharyya D., Karmakar P. (2015). PI3K-mediated Proliferation of Fibroblasts by Calendula officinalis Tincture: Implication in Wound Healing. Phytother Res. 29, 607–616. 10.1002/ptr.5293 [DOI] [PubMed] [Google Scholar]
- Dinda M., Mazumdar S., Das S., Ganguly D., Dasgupta U. B., Dutta A., et al. (2016). The Water Fraction of Calendula officinalis Hydroethanol Extract Stimulates In Vitro and In Vivo Proliferation of Dermal Fibroblasts in Wound Healing. Phytother Res. 30, 1696–1707. 10.1002/ptr.5678 [DOI] [PubMed] [Google Scholar]
- Ding L., Shan X., Zhao X., Zha H., Chen X., Wang J., et al. (2017). Spongy Bilayer Dressing Composed of Chitosan-Ag Nanoparticles and Chitosan-Bletilla Striata Polysaccharide for Wound Healing Applications. Carbohydr. Polym. 157, 1538–1547. 10.1016/j.carbpol.2016.11.040 [DOI] [PubMed] [Google Scholar]
- Ebeling S., Naumann K., Pollok S., Wardecki T., Vidal-Y-Sy S., Nascimento J. M., et al. (2014). From a Traditional Medicinal Plant to a Rational Drug: Understanding the Clinically Proven Wound Healing Efficacy of Birch Bark Extract. PLoS One 9, e86147. 10.1371/journal.pone.0086147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Haouari M., Rosado J. A. (2019). Phytochemical, Anti-diabetic and Cardiovascular Properties of Urtica Dioica L. (Urticaceae): A Review. Mini Rev. Med. Chem. 19, 63–71. 10.2174/1389557518666180924121528 [DOI] [PubMed] [Google Scholar]
- Esposito D., Munafo J. P., Jr., Lucibello T., Baldeon M., Komarnytsky S., Gianfagna T. J. (2013). Steroidal Glycosides from the Bulbs of Easter Lily (Lilium Longiflorum Thunb.) Promote Dermal Fibroblast Migration In Vitro . J. Ethnopharmacol 148, 433–440. 10.1016/j.jep.2013.04.032 [DOI] [PubMed] [Google Scholar]
- Frew Q., Rennekampff H. O., Dziewulski P., Moiemen N., Group B. B. W. S., Zahn T., et al. (2019). Betulin Wound Gel Accelerated Healing of Superficial Partial Thickness burns: Results of a Randomized, Intra-individually Controlled, Phase III Trial with 12-months Follow-Up. Burns 45, 876–890. 10.1016/j.burns.2018.10.019 [DOI] [PubMed] [Google Scholar]
- Fronza M., Heinzmann B., Hamburger M., Laufer S., Merfort I. (2009). Determination of the Wound Healing Effect of Calendula Extracts Using the Scratch Assay with 3T3 Fibroblasts. J. Ethnopharmacol 126, 463–467. 10.1016/j.jep.2009.09.014 [DOI] [PubMed] [Google Scholar]
- Gao S. Q., Chang C., Niu X. Q., Li L. J., Zhang Y., Gao J. Q. (2018). Topical Application of Hydroxysafflor Yellow A Accelerates the Wound Healing in Streptozotocin Induced T1DM Rats. Eur. J. Pharmacol. 823, 72–78. 10.1016/j.ejphar.2018.01.018 [DOI] [PubMed] [Google Scholar]
- Givol O., Kornhaber R., Visentin D., Cleary M., Haik J., Harats M. (2019). A Systematic Review of Calendula officinalis Extract for Wound Healing. Wound Repair Regen. 27, 548–561. 10.1111/wrr.12737 [DOI] [PubMed] [Google Scholar]
- Guo J., Hu Z., Yan F., Lei S., Li T., Li X., et al. (2020). Angelica Dahurica Promoted Angiogenesis and Accelerated Wound Healing in Db/db Mice via the HIF-1α/pdgf-β Signaling Pathway. Free Radic. Biol. Med. 160, 447–457. 10.1016/j.freeradbiomed.2020.08.015 [DOI] [PubMed] [Google Scholar]
- Gupta A., Kumar R., Pal K., Banerjee P. K., Sawhney R. C. (2005). A Preclinical Study of the Effects of Seabuckthorn (Hippophae Rhamnoides L.) Leaf Extract on Cutaneous Wound Healing in Albino Rats. Int. J. Low Extrem Wounds 4, 88–92. 10.1177/1534734605277401 [DOI] [PubMed] [Google Scholar]
- Gupta A., Kumar R., Pal K., Singh V., Banerjee P. K., Sawhney R. C. (2006). Influence of Sea Buckthorn (Hippophae Rhamnoides L.) Flavone on Dermal Wound Healing in Rats. Mol. Cel Biochem 290, 193–198. 10.1007/s11010-006-9187-6 [DOI] [PubMed] [Google Scholar]
- Han D. O., Lee H. J., Hahm D. H. (2009). Wound-healing Activity of Astragali Radix in Rats. Methods Find Exp. Clin. Pharmacol. 31, 95–100. 10.1358/mf.2009.31.2.1353846 [DOI] [PubMed] [Google Scholar]
- Hao B., Wang X., Ma X., Jin Y., Fan W., Laba C., et al. (2020). Preparation of Complex Microcapsules of Soluble Polysaccharide from Glycyrrhiza Uralensis and its Application in Wound Repair and Scar Inhibition. Int. J. Biol. Macromol 156, 906–917. 10.1016/j.ijbiomac.2020.03.121 [DOI] [PubMed] [Google Scholar]
- He X., Wang X., Fang J., Zhao Z., Huang L., Guo H., et al. (2017). Bletilla Striata: Medicinal Uses, Phytochemistry and Pharmacological Activities. J. Ethnopharmacol 195, 20–38. 10.1016/j.jep.2016.11.026 [DOI] [PubMed] [Google Scholar]
- Hong S. J., Wan J. B., Zhang Y., Hu G., Lin H. C., Seto S. W., et al. (2009). Angiogenic Effect of Saponin Extract from Panax Notoginseng on HUVECs In Vitro and Zebrafish In Vivo . Phytother Res. 23, 677–686. 10.1002/ptr.2705 [DOI] [PubMed] [Google Scholar]
- Hormozi M., Gholami M., Babaniazi A., Gharravi A. M. (2019). Calendula officinalis Stimulate Proliferation of Mouse Embryonic Fibroblasts via Expression of Growth Factors TGFβ1 and bFGF. Inflamm. Regen. 39, 7. 10.1186/s41232-019-0097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q., He W. J., Hao H. J., Han Q. W., Chen L., Dong L., et al. (2014). The Four-Herb Chinese Medicine ANBP Enhances Wound Healing and Inhibits Scar Formation via Bidirectional Regulation of Transformation Growth Factor Pathway. PLoS One 9, e112274. 10.1371/journal.pone.0112274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C. Y., Hung C. Y., Tsai T. H., Chak K. F. (2012a2012). A Study of the Wound Healing Mechanism of a Traditional Chinese Medicine, Angelica Sinensis, Using a Proteomic Approach. Evid. Based Complement. Alternat Med. 2012, 467531. 10.1155/2012/467531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C. Y., Tsai T. H., Chak K. F. (2012b). The Molecular Basis of Wound Healing Processes Induced by Lithospermi Radix: a Proteomics and Biochemical Analysis. Evid. Based Complement. Alternat Med. 2012, 508972. 10.1155/2012/508972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. F., Hsu Y. C., Lin C. N., Tzeng J. I., Chen Y. W., Wang J. J. (2004). Shiunko Promotes Epithelization of Wounded Skin. Am. J. Chin. Med. 32, 389–396. 10.1142/S0192415X04002041 [DOI] [PubMed] [Google Scholar]
- Huang M., Shen S., Luo C., Ren Y. (2019a). Genus Periploca (Apocynaceae): A Review of its Classification, Phytochemistry, Biological Activities and Toxicology. Molecules 24. 10.3390/molecules24152749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Shi F., Wang L., Yang Y., Khan B. M., Cheong K. L., et al. (2019b). Preparation and Evaluation of Bletilla Striata Polysaccharide/carboxymethyl chitosan/Carbomer 940 Hydrogel for Wound Healing. Int. J. Biol. Macromol 132, 729–737. 10.1016/j.ijbiomac.2019.03.157 [DOI] [PubMed] [Google Scholar]
- Huh J. E., Nam D. W., Baek Y. H., Kang J. W., Park D. S., Choi D. Y., et al. (2011). Formononetin Accelerates Wound Repair by the Regulation of Early Growth Response Factor-1 Transcription Factor through the Phosphorylation of the ERK and P38 MAPK Pathways. Int. Immunopharmacol 11, 46–54. 10.1016/j.intimp.2010.10.003 [DOI] [PubMed] [Google Scholar]
- Igielska-Kalwat J., Firlej M., Lewandowska A., Biedziak B. (2019). In Vivo studies of Resveratrol Contained in Cosmetic Emulsions. Acta Biochim. Pol. 66, 371–374. 10.18388/abp.2019_2838 [DOI] [PubMed] [Google Scholar]
- Imai K., Kato H., Taguchi Y., Umeda M. (2019). Biological Effects of Shikonin in Human Gingival Fibroblasts via ERK 1/2 Signaling Pathway. Molecules 24. 10.3390/molecules24193542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmak F., Kurt Yazar S., Şirvan S. S., Serin M., Özağarı A., Karasoy Yeşilada A. (2018). Beneficial Effects of Salvia Miltiorrhiza in the Healing of Burn Wounds: an Experimental Study in Rats. J. Plast. Surg. Hand Surg. 52, 229–233. 10.1080/2000656X.2018.1461631 [DOI] [PubMed] [Google Scholar]
- Ito H., Asmussen S., Traber D. L., Cox R. A., Hawkins H. K., Connelly R., et al. (2014). Healing Efficacy of Sea Buckthorn (Hippophae Rhamnoides L.) Seed Oil in an Ovine Burn Wound Model. Burns 40, 511–519. 10.1016/j.burns.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Jain N., Jain R., Jain A., Jain D. K., Chandel H. S. (2010). Evaluation of Wound-Healing Activity of Acorus calamus Linn. Nat. Prod. Res. 24, 534–541. 10.1080/14786410802531782 [DOI] [PubMed] [Google Scholar]
- Janis J. E., Harrison B. (2016). Wound Healing: Part I. Basic Science. Plast. Reconstr. Surg. 138, 9S–17S. 10.1097/PRS.0000000000002773 [DOI] [PubMed] [Google Scholar]
- Ji S., Zhang G., Hua Y., Jin X. (2015). Sanguis Draconis (Daemonorops Draco): a Case Report of Treating a Chronic Pressure Ulcer with Tunneling. Holist. Nurs. Pract. 29, 48–52. 10.1097/HNP.0000000000000063 [DOI] [PubMed] [Google Scholar]
- Jia H., Yang B., Li Y., Liang C., Lu H., Lin D., et al. (2018). Chinese Medicine Ulcer Oil Promotes the Healing of Diabetic Foot Ulcers. J. Int. Med. Res. 46, 2679–2686. 10.1177/0300060518769529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Liu L., Qiao L., Zhang B., Wang X., Han Y., et al. (2018). Dracorhodin Perchlorate Regulates Fibroblast Proliferation to Promote Rat's Wound Healing. J. Pharmacol. Sci. 136, 66–72. 10.1016/j.jphs.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Jiang X. W., Qiao L., Liu L., Zhang B. Q., Wang X. W., Han Y. W., et al. (2017). Dracorhodin Perchlorate Accelerates Cutaneous Wound Healing in Wistar Rats. Evid. Based Complement. Alternat Med. 2017, 8950516. 10.1155/2017/8950516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Zhang M., Gao Y., Zhang X., Cui G., Zhang Y. (2014). The Efficacy of Jing Wan Hong Ointment for Nerve Injury Diabetic Foot Ulcer and its Mechanisms. J. Diabetes Res. 2014, 259412. 10.1155/2014/259412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. G., Kim K. S., Yousaf A. M., Kim D. W., Jang S. W., Son M. W., et al. (2015). Mechanical Properties and In Vivo Healing Evaluation of a Novel Centella Asiatica-Loaded Hydrocolloid Wound Dressing. Int. J. Pharm. 490, 240–247. 10.1016/j.ijpharm.2015.05.058 [DOI] [PubMed] [Google Scholar]
- Kamolz L. P., Luze H., Nischwitz S. P., Kotzbeck P. (2020). Resveratrol Promotes Wound Healing: A Very Short Overview. Burns 47, 972–973. 10.1016/j.burns.2020.11.020 [DOI] [PubMed] [Google Scholar]
- Karayannopoulou M., Tsioli V., Loukopoulos P., Anagnostou T. L., Giannakas N., Savvas I., et al. (2011). Evaluation of the Effectiveness of an Ointment Based on Alkannins/Shikonins on Second Intention Wound Healing in the Dog. Can. J. Vet. Res. 75, 42–48. [PMC free article] [PubMed] [Google Scholar]
- Kern J. S., Schwieger-Briel A., Löwe S., Sumeray M., Davis C., Martinez A. E. (2019). Oleogel-S10 Phase 3 Study "EASE" for Epidermolysis Bullosa: Study Design and Rationale. Trials 20, 350. 10.1186/s13063-019-3362-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. H., Yoon J. H., Park S. B., Lee J. Y., Chung W. K., Yoon S. W. (2021). Comparative Efficacy of Jaungo, A Traditional Herbal Ointment, and the Water-In-Oil Type Non-steroidal Moisturizer for Radiation-Induced Dermatitis in Patients with Breast Cancer: A Study Protocol for a Prospective, Randomized, Single-Blinded, Pilot Study. Front. Pharmacol. 12, 751812. 10.3389/fphar.2021.751812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lee C. M. (2017). Wound Healing Potential of a Polyvinyl Alcohol-Blended Pectin Hydrogel Containing Hippophae Rahmnoides L. Extract in a Rat Model. Int. J. Biol. Macromol 99, 586–593. 10.1016/j.ijbiomac.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Knight E. V., Oldham J. W., Mohler M. A., Liu S., Dooley J. (1998). A Review of Nonclinical Toxicology Studies of Becaplermin (rhPDGF-BB). Am. J. Surg. 176, 55S–60S. 10.1016/s0002-9610(98)00176-7 [DOI] [PubMed] [Google Scholar]
- Ko C. H., Yi S., Ozaki R., Cochrane H., Chung H., Lau W., et al. (2014). Healing Effect of a Two-Herb Recipe (NF3) on Foot Ulcers in Chinese Patients with Diabetes: a Randomized Double-Blind Placebo-Controlled Study. J. Diabetes 6, 323–334. 10.1111/1753-0407.12117 [DOI] [PubMed] [Google Scholar]
- Ko J. K., Leung C. C. (2010). Ginger Extract and Polaprezinc Exert Gastroprotective Actions by Anti-oxidant and Growth Factor Modulating Effects in Rats. J. Gastroenterol. Hepatol. 25, 1861–1868. 10.1111/j.1440-1746.2010.06347.x [DOI] [PubMed] [Google Scholar]
- Kotian S., Bhat K., Pai S., Nayak J., Souza A., Gourisheti K., et al. (2018). The Role of Natural Medicines on Wound Healing: A Biomechanical, Histological, Biochemical and Molecular Study. Ethiop J. Health Sci. 28, 759–770. 10.4314/ejhs.v28i6.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai L., Zhang J. T., Deng Y., Xu S., Xu X. Z., Wu M. F., et al. (2018). Sheng-ji Hua-Yu Formula Promotes Diabetic Wound Healing of Re-epithelization via Activin/Follistatin Regulation. BMC Complement. Altern. Med. 18, 32. 10.1186/s12906-017-2074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H. W., Lin H. C., Lao S. C., Gao J. L., Hong S. J., Leong C. W., et al. (2008). The Angiogenic Effects of Angelica Sinensis Extract on HUVEC In Vitro and Zebrafish In Vivo . J. Cel Biochem 103, 195–211. 10.1002/jcb.21403 [DOI] [PubMed] [Google Scholar]
- Lamouille S., Xu J., Derynck R. (2014). Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cel Biol 15, 178–196. 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landén N. X., Li D., Ståhle M. (2016). Transition from Inflammation to Proliferation: a Critical Step during Wound Healing. Cell Mol Life Sci 73, 3861–3885. 10.1007/s00018-016-2268-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K. M., Lai K. K., Liu C. L., Tam J. C., To M. H., Kwok H. F., et al. (2012). Synergistic Interaction between Astragali Radix and Rehmanniae Radix in a Chinese Herbal Formula to Promote Diabetic Wound Healing. J. Ethnopharmacol 141, 250–256. 10.1016/j.jep.2012.02.025 [DOI] [PubMed] [Google Scholar]
- Lau T. W., Chan Y. W., Lau C. P., Lau K. M., Lau C. B., Fung K. P., et al. (2009a). Radix Astragali and Radix Rehmanniae, the Principal Components of Two Antidiabetic Foot Ulcer Herbal Formulae, Elicit Viability-Promoting Effects on Primary Fibroblasts Cultured from Diabetic Foot Ulcer Tissues. Phytother Res. 23, 809–815. 10.1002/ptr.2649 [DOI] [PubMed] [Google Scholar]
- Lau T. W., Lam F. F., Lau K. M., Chan Y. W., Lee K. M., Sahota D. S., et al. (2009b). Pharmacological Investigation on the Wound Healing Effects of Radix Rehmanniae in an Animal Model of Diabetic Foot Ulcer. J. Ethnopharmacol 123, 155–162. 10.1016/j.jep.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Lau T. W., Sahota D. S., Lau C. H., Chan C. M., Lam F. C., Ho Y. Y., et al. (2008). An In Vivo Investigation on the Wound-Healing Effect of Two Medicinal Herbs Using an Animal Model with Foot Ulcer. Eur. Surg. Res. 41, 15–23. 10.1159/000122834 [DOI] [PubMed] [Google Scholar]
- Li F., Jiang T., Liu W., Hu Q., Yin H. (2016a). The Angiogenic Effect of Dracorhodin Perchlorate on Human Umbilical Vein Endothelial Cells and its Potential Mechanism of Action. Mol. Med. Rep. 14, 1667–1672. 10.3892/mmr.2016.5442 [DOI] [PubMed] [Google Scholar]
- Li F., Liu B., Li T., Wu Q., Xu Z., Gu Y., et al. (2020). Review of Constituents and Biological Activities of Triterpene Saponins from Glycyrrhizae Radix et Rhizoma and Its Solubilization Characteristics. Molecules 25. 10.3390/molecules25173904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chen L., Xu J., Xie Z., Xu Y., Jiang P., et al. (2019a). Effects of Periploca Forrestii Schltr on Wound Healing by Src Meditated Mek/Erk and PI3K/Akt Signals. J. Ethnopharmacol 237, 116–127. 10.1016/j.jep.2019.03.046 [DOI] [PubMed] [Google Scholar]
- Li X., Ao M., Zhang C., Fan S., Chen Z., Yu L. (2021). Zingiberis Rhizoma Recens: A Review of its Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid. Based Complement. Alternat Med. 2021, 6668990. 10.1155/2021/6668990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li W., Fu C., Song Y., Fu Q. (2019b). Lonicerae Japonicae Flos and Lonicerae Flos: a Systematic Review of Ethnopharmacology, Phytochemistry and Pharmacology. Phytochem. Rev., 1–61. 10.1007/s11101-019-09655-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Shi S., Gao J., Han S., Wu X., Jia Y., et al. (2016b). Cryptotanshinone Downregulates the Profibrotic Activities of Hypertrophic Scar Fibroblasts and Accelerates Wound Healing: A Potential Therapy for the Reduction of Skin Scarring. Biomed. Pharmacother. 80, 80–86. 10.1016/j.biopha.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Liao Z., Zeng R., Hu L., Maffucci K. G., Qu Y. (2019). Polysaccharides from Tubers of Bletilla Striata: Physicochemical Characterization, Formulation of Buccoadhesive Wafers and Preliminary Study on Treating Oral Ulcer. Int. J. Biol. Macromol 122, 1035–1045. 10.1016/j.ijbiomac.2018.09.050 [DOI] [PubMed] [Google Scholar]
- Liu C. L., Cheng L., Kwok H. F., Ko C. H., Lau T. W., Koon C. M., et al. (2011). Bioassay-guided Isolation of Norviburtinal from the Root of Rehmannia Glutinosa, Exhibited Angiogenesis Effect in Zebrafish Embryo Model. J. Ethnopharmacol 137, 1323–1327. 10.1016/j.jep.2011.07.060 [DOI] [PubMed] [Google Scholar]
- Liu C. L., Kwok H. F., Cheng L., Ko C. H., Wong C. W., Ho T. W., et al. (2014). Molecular Mechanisms of Angiogenesis Effect of Active Sub-fraction from Root of Rehmannia Glutinosa by Zebrafish Sprout Angiogenesis-Guided Fractionation. J. Ethnopharmacol 151, 565–575. 10.1016/j.jep.2013.11.019 [DOI] [PubMed] [Google Scholar]
- Liu C. L., Tam J. C., Sanders A. J., Ko C. H., Fung K. P., Leung P. C., et al. (2013a). Molecular Angiogenic Events of a Two-Herb Wound Healing Formula Involving MAPK and Akt Signaling Pathways in Human Vascular Endothelial Cells. Wound Repair Regen. 21, 579–587. 10.1111/wrr.12055 [DOI] [PubMed] [Google Scholar]
- Liu H., Lin S., Xiao D., Zheng X., Gu Y., Guo S. (2013b2013). Evaluation of the Wound Healing Potential of Resina Draconis (Dracaena Cochinchinensis) in Animal Models. Evid. Based Complement. Alternat Med. 2013, 709865. 10.1155/2013/709865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Jiang X., Yu W. (2019). Dracohodin Perochlorate Stimulates Fibroblast Proliferation via EGFR Activation and Downstream ERK/CREB and PI3K/Akt/mTOR Pathways In Vitro . Evid. Based Complement. Alternat Med. 2019, 6027186. 10.1155/2019/6027186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhang J., Han X., Chen J., Zhai Y., Lin Y., et al. (2021). Huiyang Shengji Decoction Promotes Wound Healing in Diabetic Mice by Activating the EGFR/PI3K/ATK Pathway. Chin. Med. 16, 111. 10.1186/s13020-021-00497-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Y., Wu J. Q., Fan R. Y., He Z. H., Li C. Y., He M. F. (2020). Isoliquiritin Promote Angiogenesis by Recruiting Macrophages to Improve the Healing of Zebrafish Wounds. Fish. Shellfish Immunol. 100, 238–245. 10.1016/j.fsi.2020.02.071 [DOI] [PubMed] [Google Scholar]
- Lu C. C., Yang J. S., Chiu Y. J., Tsai F. J., Hsu Y. M., Yin M. C., et al. (2021). Dracorhodin Perchlorate Enhances Wound Healing via β-catenin, ERK/p38, and AKT Signaling in Human HaCaT Keratinocytes. Exp. Ther. Med. 22, 822. 10.3892/etm.2021.10254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P. J., Yang C., Lin C. N., Li C. F., Chu C. C., Wang J. J., et al. (2008). Shiunko and Acetylshikonin Promote Reepithelialization, Angiogenesis, and Granulation Tissue Formation in Wounded Skin. Am. J. Chin. Med. 36, 115–123. 10.1142/S0192415X08005631 [DOI] [PubMed] [Google Scholar]
- Luo X., Huang P., Yuan B., Liu T., Lan F., Lu X., et al. (2016). Astragaloside IV Enhances Diabetic Wound Healing Involving Upregulation of Alternatively Activated Macrophages. Int. Immunopharmacol 35, 22–28. 10.1016/j.intimp.2016.03.020 [DOI] [PubMed] [Google Scholar]
- Luo Y., Diao H., Xia S., Dong L., Chen J., Zhang J. (2010). A Physiologically Active Polysaccharide Hydrogel Promotes Wound Healing. J. Biomed. Mater. Res. A. 94, 193–204. 10.1002/jbm.a.32711 [DOI] [PubMed] [Google Scholar]
- Majewska I., Gendaszewska-Darmach E. (2011). Proangiogenic Activity of Plant Extracts in Accelerating Wound Healing - a New Face of Old Phytomedicines. Acta Biochim. Pol. 58, 449–460. 10.18388/abp.2011_2210 [DOI] [PubMed] [Google Scholar]
- Men S. Y., Huo Q. L., Shi L., Yan Y., Yang C. C., Yu W., et al. (2020). Panax Notoginseng Saponins Promotes Cutaneous Wound Healing and Suppresses Scar Formation in Mice. J. Cosmet. Dermatol. 19, 529–534. 10.1111/jocd.13042 [DOI] [PubMed] [Google Scholar]
- Metelmann H. R., Brandner J. M., Schumann H., Bross F., Fimmers R., Böttger K., et al. (2015). Accelerated Reepithelialization by Triterpenes: Proof of Concept in the Healing of Surgical Skin Lesions. Skin Pharmacol. Physiol. 28, 1–11. 10.1159/000357501 [DOI] [PubMed] [Google Scholar]
- Mirabzadeh M., Rahimi R., Sahraee Z. (2013). Evaluation of Adverse Events Reported in Traditional Iranian Medicine Following Administration of Aqueous Extract of Herba Portulacae Oleraceae Seed. J. Tradit Chin. Med. 33, 535–537. 10.1016/s0254-6272(13)60161-2 [DOI] [PubMed] [Google Scholar]
- Mirbehbahani F. S., Hejazi F., Najmoddin N., Asefnejad A. (2020). Artemisia Annua L. As a Promising Medicinal Plant for Powerful Wound Healing Applications. Prog. Biomater. 9, 139–151. 10.1007/s40204-020-00138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C., Roumegous A., Carpentier G., Barbier-Chassefière V., Garrigue-Antar L., Caredda S., et al. (2012). Modulation of Inflammation by Cicaderma Ointment Accelerates Skin Wound Healing. J. Pharmacol. Exp. Ther. 343, 115–124. 10.1124/jpet.111.188599 [DOI] [PubMed] [Google Scholar]
- Mouro C., Fangueiro R., Gouveia I. C. (2020). Preparation and Characterization of Electrospun Double-Layered Nanocomposites Membranes as a Carrier for Centella asiatica (L.). Polymers (Basel) 12. 10.3390/polym12112653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na-Bangchang K., Ahmed O., Hussein J., Hirayama K., Kongjam P., Aseffa A., et al. (2016). Exploratory, Phase II Controlled Trial of Shiunko Ointment Local Application Twice a Day for 4 Weeks in Ethiopian Patients with Localized Cutaneous Leishmaniasis. Evid. Based Complement. Alternat Med. 2016, 5984709. 10.1155/2016/5984709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan Si. H. K., Okuyama Katsuki., Imada Keisuke., Wang Hongjie., Yang Jian., Zhao Haiyu., et al. (2018). Involvement of Catechols in Acteoside in the Activation of Promatrix Metalloproteinase-2 and Membrane Type-1-Matrix Metalloproteinase Expression via a Phosphatidylinositol-3-Kinase Pathway in Human Dermal Fibroblasts. Biol. Pharm. Bull. 41, 1530–1536. 10.1248/bpb.b18-00107 [DOI] [PubMed] [Google Scholar]
- Nawrot-Hadzik I., Matkowski A., Pitulaj A., Sterczala B., Olchowy C., Szewczyk A., et al. (2021). Vitro Gingival Wound Healing Activity of Extracts from Reynoutria Japonica Houtt Rhizomes. Pharmaceutics 13. 10.3390/pharmaceutics13111764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. C., Le D. T., Kamei K., Dang T. P. T. (2017). Wound Healing Activity of Streptocaulon Juventas Root Ethanolic Extract. Wound Repair Regen. 25, 956–963. 10.1111/wrr.12599 [DOI] [PubMed] [Google Scholar]
- Niazi A., Yousefzadeh S., Rakhshandeh H., Esmaily H., Askari V. R. (2019). Promising Effects of Purslane Cream on the Breast Fissure in Lactating Women: A Clinical Trial. Complement. Ther. Med. 43, 300–305. 10.1016/j.ctim.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Pang Y. Y., Li Y., Kui G., Tang Y., Liao M. J., Wang Y. L., et al. (2018). Efficacy of a Chinese Herbal Medicine Compound Zhangpi Ointment against Hydroxyurea-Induced Leg Ulcers: A Prospective, Randomized, Open-Label, Controlled Clinical Trial. Evid. Based Complement. Alternat Med. 2018, 9329465. 10.1155/2018/9329465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanas N., Maltezos E. (2008). Becaplermin Gel in the Treatment of Diabetic Neuropathic Foot Ulcers. Clin. Interv. Aging 3, 233–240. 10.2147/cia.s1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente L. M., Andrade M. A., Brito L. A., Moura V. M., Miguel M. P., Lino-Júnior Rde. S., et al. (2011). Angiogenic Activity of Calendula officinalis Flowers L. In Rats. Acta Cir Bras 26, 19–24. 10.1590/s0102-86502011000100005 [DOI] [PubMed] [Google Scholar]
- Peng W., Qin R., Li X., Zhou H. (2013). Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: a review. J. Ethnopharmacol 148, 729–745. 10.1016/j.jep.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Pommier P., Gomez F., Sunyach M. P., D'Hombres A., Carrie C., Montbarbon X. (2004). Phase III Randomized Trial of Calendula officinalis Compared with Trolamine for the Prevention of Acute Dermatitis during Irradiation for Breast Cancer. J. Clin. Oncol. 22, 1447–1453. 10.1200/JCO.2004.07.063 [DOI] [PubMed] [Google Scholar]
- Pona A., Cline A., Kolli S. S., Taylor S. L., Feldman S. R. (2019). Review of Future Insights of Dragon's Blood in Dermatology. Dermatol. Ther. 32, e12786. 10.1111/dth.12786 [DOI] [PubMed] [Google Scholar]
- Ponrasu T., Madhukumar K. N., Ganeshkumar M., Iyappan K., Sangeethapriya V., Gayathri V. S., et al. (2014). Efficacy of Acorus calamus on Collagen Maturation on Full Thickness Cutaneous Wounds in Rats. Pharmacogn Mag. 10, S299–S305. 10.4103/0973-1296.133283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J. G., Higham C., Broussard K., Phillips T. J. (2016). Wound Healing and Treating Wounds: Chronic Wound Care and Management. J. Am. Acad. Dermatol. 74, 607–625. quiz 625-606. 10.1016/j.jaad.2015.08.070 [DOI] [PubMed] [Google Scholar]
- Preethi K. C., Kuttan R. (2009). Wound Healing Activity of Flower Extract of Calendula officinalis. J. Basic Clin. Physiol. Pharmacol. 20, 73–79. 10.1515/jbcpp.2009.20.1.73 [DOI] [PubMed] [Google Scholar]
- Pundir S., Garg P., Dviwedi A., Ali A., Kapoor V. K., Kapoor D., et al. (2021). Ethnomedicinal Uses, Phytochemistry and Dermatological Effects of Hippophae Rhamnoides L.: A Review. J. Ethnopharmacol 266, 113434. 10.1016/j.jep.2020.113434 [DOI] [PubMed] [Google Scholar]
- Rashed A. N., Afifi F. U., Disi A. M. (2003). Simple Evaluation of the Wound Healing Activity of a Crude Extract of Portulaca Oleracea L. (Growing in Jordan) in Mus musculus JVI-1. J. Ethnopharmacol 88, 131–136. 10.1016/s0378-8741(03)00194-6 [DOI] [PubMed] [Google Scholar]
- Razika L., Thanina A. C., Nadjiba C. M., Narimen B., Mahdi D. M., Karim A. (2017). Antioxidant and Wound Healing Potential of Saponins Extracted from the Leaves of Algerian Urtica Dioica L. Pak J. Pharm. Sci. 30, 1023–1029. [PubMed] [Google Scholar]
- Ren X., Zhu Y., Xie L., Zhang M., Gao L., He H. (2020). Yunnan Baiyao Diminishes Lipopolysaccharide-Induced Inflammation in Osteoclasts. J. Food Biochem. 44, e13182. 10.1111/jfbc.13182 [DOI] [PubMed] [Google Scholar]
- Saeidinia A., Keihanian F., Lashkari A. P., Lahiji H. G., Mobayyen M., Heidarzade A., et al. (2017). Partial-thickness Burn Wounds Healing by Topical Treatment: A Randomized Controlled Comparison between Silver Sulfadiazine and Centiderm. Medicine (Baltimore) 96, e6168. 10.1097/MD.0000000000006168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin F., Kocak P., Gunes M. Y., Ozkan I., Yildirim E., Kala E. Y. (2019). In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 188, 381–394. 10.1007/s12010-019-02979-2 [DOI] [PubMed] [Google Scholar]
- Sánchez M., González-Burgos E., Iglesias I., Gómez-Serranillos M. P. (2020). Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules 25. 10.3390/molecules25061324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler A. (2019). The Wound Healing Properties of Betulin from Birch Bark from Bench to Bedside. Planta Med. 85, 524–527. 10.1055/a-0850-0224 [DOI] [PubMed] [Google Scholar]
- Schwieger-Briel A., Kiritsi D., Schempp C., Has C., Schumann H. (2017). Betulin-Based Oleogel to Improve Wound Healing in Dystrophic Epidermolysis Bullosa: A Prospective Controlled Proof-Of-Concept Study. Dermatol. Res. Pract. 2017, 5068969. 10.1155/2017/5068969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieger-Briel A., Ott H., Kiritsi D., Laszczyk-Lauer M., Bodemer C. (2019). Mechanism of Oleogel-S10: A Triterpene Preparation for the Treatment of Epidermolysis Bullosa. Dermatol. Ther. 32, e12983. 10.1111/dth.12983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Sharma R., Gautam D. S., Kuca K., Nepovimova E., Martins N. (2020). Role of Vacha (Acorus calamus Linn.) in Neurological and Metabolic Disorders: Evidence from Ethnopharmacology, Phytochemistry, Pharmacology and Clinical Study. J. Clin. Med. 9. 10.3390/jcm9041176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K., Ji L., Gong C., Ma Y., Yang L., Fan Y., et al. (2012). Notoginsenoside Ft1 Promotes Angiogenesis via HIF-1α Mediated VEGF Secretion and the Regulation of PI3K/AKT and Raf/MEK/ERK Signaling Pathways. Biochem. Pharmacol. 84, 784–792. 10.1016/j.bcp.2012.05.024 [DOI] [PubMed] [Google Scholar]
- Shukla V. K., Ansari M. A., Gupta S. K. (2005). Wound Healing Research: a Perspective from India. Int. J. Low Extrem Wounds 4, 7–8. 10.1177/1534734604273660 [DOI] [PubMed] [Google Scholar]
- Singh V., Devgan L., Bhat S., Milner S. M. (2007). The Pathogenesis of Burn Wound Conversion. Ann. Plast. Surg. 59, 109–115. 10.1097/01.sap.0000252065.90759.e6 [DOI] [PubMed] [Google Scholar]
- Singkhorn S., Tantisira M. H., Tanasawet S., Hutamekalin P., Wongtawatchai T., Sukketsiri W. (2018). Induction of Keratinocyte Migration by ECa 233 Is Mediated through FAK/Akt, ERK, and P38 MAPK Signaling. Phytother Res. 32, 1397–1403. 10.1002/ptr.6075 [DOI] [PubMed] [Google Scholar]
- Song M., Chen L., Zhang L., Li C., Coffie J. W., Fang Z., et al. (2020). Cryptotanshinone Enhances Wound Healing in Type 2 Diabetes with Modulatory Effects on Inflammation, Angiogenesis and Extracellular Matrix Remodelling. Pharm. Biol. 58, 845–853. 10.1080/13880209.2020.1803369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic Z. (2017). Curcumin, a Compound from Natural Sources, a True Scientific Challenge - A Review. Plant Foods Hum. Nutr. 72, 1–12. [DOI] [PubMed] [Google Scholar]
- Sui H., Wang F., Weng Z., Song H., Fang Y., Tang X., et al. (2020). A Wheat Germ-Derived Peptide YDWPGGRN Facilitates Skin Wound-Healing Processes. Biochem. Biophys. Res. Commun. 524, 943–950. 10.1016/j.bbrc.2020.01.162 [DOI] [PubMed] [Google Scholar]
- Tang T., Yin L., Yang J., Shan G. (2007). Emodin, an Anthraquinone Derivative from Rheum Officinale Baill, Enhances Cutaneous Wound Healing in Rats. Eur. J. Pharmacol. 567, 177–185. 10.1016/j.ejphar.2007.02.033 [DOI] [PubMed] [Google Scholar]
- Upadhyay N. K., Kumar R., Mandotra S. K., Meena R. N., Siddiqui M. S., Sawhney R. C., et al. (2009). Safety and Healing Efficacy of Sea Buckthorn (Hippophae Rhamnoides L.) Seed Oil on Burn Wounds in Rats. Food Chem. Toxicol. 47, 1146–1153. 10.1016/j.fct.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Upadhyay N. K., Kumar R., Siddiqui M. S., Gupta A. (20112011). Mechanism of Wound-Healing Activity of Hippophae Rhamnoides L. Leaf Extract in Experimental Burns. Evid. Based Complement. Alternat Med. 2011, 659705. 10.1093/ecam/nep189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Sun J., Luo Y., Xue W., Diao H., Dong L., et al. (2006). A Polysaccharide Isolated from the Medicinal Herb Bletilla Striata Induces Endothelial Cells Proliferation and Vascular Endothelial Growth Factor Expression In Vitro . Biotechnol. Lett. 28, 539–543. 10.1007/s10529-006-0011-x [DOI] [PubMed] [Google Scholar]
- Wang D., Jie Q., Liu B., Li Y., Dai L., Luo J., et al. (2017). Saponin Extract from Panax notoginseng Promotesangiogenesis through AMPK- and eNOS-dependent P-athways in HUVECs. Mol. Med. Rep. 16, 5211–5218. 10.3892/mmr.2017.7280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Song H., Yue J., Li J., Hou Y. B., Deng J. L. (2012). Rheum Officinale (A Traditional Chinese Medicine) for Chronic Kidney Disease. Cochrane Database Syst. Rev. CD008000. 10.1002/14651858.cd008000.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H. N., Hardman M. J. (2020). Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 10, 200223. 10.1098/rsob.200223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Kuai L., Ru Y., Jiang J., Li X., Li F., et al. (2021). Transcriptional Profiling and circRNA-miRNA-mRNA Network Analysis Identify the Biomarkers in Sheng-Ji Hua-Yu Formula Treated Diabetic Wound Healing. J. Ethnopharmacol 268, 113643. 10.1016/j.jep.2020.113643 [DOI] [PubMed] [Google Scholar]
- Yan Y., Furumura M., Gouya T., Iwanaga A., Teye K., Numata S., et al. (2015). Shikonin Promotes Skin Cell Proliferation and Inhibits Nuclear Factor-Κb Translocation via Proteasome Inhibition In Vitro . Chin. Med. J. (Engl) 128, 2228–2233. 10.4103/0366-6999.162512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B. R., Hong S. J., Lee S. M., Cong W. H., Wan J. B., Zhang Z. R., et al. (2016). Pro-angiogenic Activity of Notoginsenoside R1 in Human Umbilical Vein Endothelial Cells In Vitro and in a Chemical-Induced Blood Vessel Loss Model of Zebrafish In Vivo . Chin. J. Integr. Med. 22, 420–429. 10.1007/s11655-014-1954-8 [DOI] [PubMed] [Google Scholar]
- Yang L., Han Z., Chen C., Li Z., Yu S., Qu Y., et al. (2020a). Novel Probiotic-Bound Oxidized Bletilla Striata Polysaccharide-Chitosan Composite Hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 117, 111265. 10.1016/j.msec.2020.111265 [DOI] [PubMed] [Google Scholar]
- Yang W. T., Ke C. Y., Wu W. T., Harn H. J., Tseng Y. H., Lee R. P. (2017). Effects of Angelica Dahurica and Rheum Officinale Extracts on Excisional Wound Healing in Rats. Evid. Based Complement. Alternat Med. 2017, 1583031. 10.1155/2017/1583031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. T., Ke C. Y., Wu W. T., Tseng Y. H., Lee R. P. (2020b). Antimicrobial and Anti-inflammatory Potential of Angelica Dahurica and Rheum Officinale Extract Accelerates Wound Healing in Staphylococcus Aureus-Infected Wounds. Sci. Rep. 10, 5596. 10.1038/s41598-020-62581-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C. H., Yeh J. Y., Chen Y. S., Li M. H., Huang C. H. (2017). Wound-healing Effect of Electrospun Gelatin Nanofibres Containing Centella asiatica Extract in a Rat Model. J. Tissue Eng. Regen. Med. 11, 905–915. 10.1002/term.1992 [DOI] [PubMed] [Google Scholar]
- Yao Q., Chang B. T., Chen R., Wei Y. J., Gong Q. J., Yu D., et al. (2021). Research Advances in Pharmacology, Safety, and Clinical Applications of Yunnan Baiyao, a Traditional Chinese Medicine Formula. Front. Pharmacol. 12, 773185. 10.3389/fphar.2021.773185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S. Y., Peng A. P., Huang L. T., Wang Y. T., Lan C. W., Yang N. S. (2013). The Phytochemical Shikonin Stimulates Epithelial-Mesenchymal Transition (EMT) in Skin Wound Healing. Evid. Based Complement. Alternat Med. 2013, 262796. 10.1155/2013/262796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Xie J., Xin N., Wang Z. (2015). Panax Notoginseng Saponins Promote Wound Repair of Anterior Cruciate Ligament through Phosphorylation of PI3K, AKT and ERK. Int. J. Clin. Exp. Pathol. 8, 441–449. [PMC free article] [PubMed] [Google Scholar]
- Zhang C., He Y., Chen Z., Shi J., Qu Y., Zhang J. (2019a). Effect of Polysaccharides from Bletilla Striata on the Healing of Dermal Wounds in Mice. Evid. Based Complement. Alternat Med. 2019, 9212314. 10.1155/2019/9212314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E., Gao B., Yang L., Wu X., Wang Z. (2016a). Notoginsenoside Ft1 Promotes Fibroblast Proliferation via PI3K/Akt/mTOR Signaling Pathway and Benefits Wound Healing in Genetically Diabetic Mice. J. Pharmacol. Exp. Ther. 356, 324–332. 10.1124/jpet.115.229369 [DOI] [PubMed] [Google Scholar]
- Zhang E. Y., Gao B., Shi H. L., Huang L. F., Yang L., Wu X. J., et al. (2017a). 20(S)-Protopanaxadiol Enhances Angiogenesis via HIF-1α-Mediated VEGF Secretion by Activating p70S6 Kinase and Benefits Wound Healing in Genetically Diabetic Mice. Exp. Mol. Med. 49, e387. 10.1038/emm.2017.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Hu Q., Jin H., Yang Y., Yang Y., Yang R., et al. (2021). Effects of Ginsenoside Rb1 on Second-Degree Burn Wound Healing and FGF-2/PDGF-BB/PDGFR-β Pathway Modulation. Chin. Med. 16, 45. 10.1186/s13020-021-00455-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. L., Tian K., Tang Z. H., Chen X. J., Bian Z. X., Wang Y. T., et al. (2016b). Phytochemistry and Pharmacology of Carthamus tinctorius L. Am. J. Chin. Med. 44, 197–226. 10.1142/S0192415X16500130 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Fong C. C., Yu W. K., Chen Y., Wei F., Koon C. M., et al. (2012). Herbal Formula Astragali Radix and Rehmanniae Radix Exerted Wound Healing Effect on Human Skin Fibroblast Cell Line Hs27 via the Activation of Transformation Growth Factor (TGF-β) Pathway and Promoting Extracellular Matrix (ECM) Deposition. Phytomedicine 20, 9–16. 10.1016/j.phymed.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Qi C., Wang H., Xiao X., Zhuang Y., Gu S., et al. (2019b). Biocompatible and Degradable Bletilla Striata Polysaccharide Hemostasis Sponges Constructed from Natural Medicinal Herb Bletilla Striata. Carbohydr. Polym. 226, 115304. 10.1016/j.carbpol.2019.115304 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wei F., Fong C. C., Yu W. K., Chen Y., Koon C. M., et al. (2011). Transcriptional Profiling of Human Skin Fibroblast Cell Line Hs27 Induced by Herbal Formula Astragali Radix and Rehmanniae Radix. J. Ethnopharmacol 138, 668–675. 10.1016/j.jep.2011.08.080 [DOI] [PubMed] [Google Scholar]
- Zhang X. N., Ma Z. J., Wang Y., Li Y. Z., Sun B., Guo X., et al. (2016c2016). The Four-Herb Chinese Medicine Formula Tuo-Li-Xiao-Du-San Accelerates Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats through Reducing Inflammation and Increasing Angiogenesis. J. Diabetes Res. 2016, 5639129. 10.1155/2016/5639129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. N., Ma Z. J., Wang Y., Sun B., Guo X., Pan C. Q., et al. (2017b). Angelica Dahurica Ethanolic Extract Improves Impaired Wound Healing by Activating Angiogenesis in Diabetes. PLoS One 12, e0177862. 10.1371/journal.pone.0177862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Zhang X., Han W., Cheng J., Qin Y. (2017). Wound Healing Effect of an Astragalus Membranaceus Polysaccharide and its Mechanism. Mol. Med. Rep. 15, 4077–4083. 10.3892/mmr.2017.6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Deneau J., Che G. O., Li S., Vagnini F., Azadi P., et al. (2012). Angelica Sinensis Isolate SBD.4: Composition, Gene Expression Profiling, Mechanism of Action and Effect on Wounds, in Rats and Humans. Eur. J. Dermatol. 22, 58–67. 10.1684/ejd.2011.1599 [DOI] [PubMed] [Google Scholar]
- Zhao H., Mortezaei R., Wang Y., Sheng X., Aria F., Bojanowski K. (2006). SBD.4 Stimulates Regenerative Processes In Vitro, and Wound Healing in Genetically Diabetic Mice and in Human Skin/severe-Combined Immunodeficiency Mouse Chimera. Wound Repair Regen. 14, 593–601. 10.1111/j.1743-6109.2006.00166.x [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang Q., Yan S., Zhou J., Huang L., Zhu H., et al. (2021). Bletilla Striata Polysaccharide Promotes Diabetic Wound Healing through Inhibition of the NLRP3 Inflammasome. Front. Pharmacol. 12, 659215. 10.3389/fphar.2021.659215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi Y., Wang H., Huang B., Yan G., Yan L. Z., Zhang W., et al. (2021). Panax Notoginseng Saponins Suppresses TRPM7 via the PI3K/AKT Pathway to Inhibit Hypertrophic Scar Formation In Vitro . Burns 47, 894–905. 10.1016/j.burns.2020.10.003 [DOI] [PubMed] [Google Scholar]
- Zhou J., An R., Huang X. (2021). Genus Lilium: A Review on Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol 270, 113852. 10.1016/j.jep.2021.113852 [DOI] [PubMed] [Google Scholar]
- Zhou J., Fang L., Xie H., Yao W. X., Zhou X., Xiong Z. J. (2015a). A Pilot Study Using the Chinese Herbal Paste Liu-He-Dan to Manage Radiodermatitis Associated with Breast Cancer Radiotherapy. Curr. Oncol. 22, e453–6. 10.3747/co.22.2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. X., Xin H. L., Rahman K., Wang S. J., Peng C., Zhang H. (2015b). Portulaca Oleracea L.: a Review of Phytochemistry and Pharmacological Effects. Biomed. Res. Int. 2015, 925631. 10.1155/2015/925631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouari Bouassida K., Bardaa S., Khimiri M., Rebaii T., Tounsi S., Jlaiel L., et al. (2017). Exploring the Urtica Dioica Leaves Hemostatic and Wound-Healing Potential. Biomed. Res. Int. 2017, 1047523. 10.1155/2017/1047523 [DOI] [PMC free article] [PubMed] [Google Scholar]