This cohort study compares the overall survival between patients who currently or formerly smoked with patients who never smoked and initiated pembrolizumab monotherapy as first-line treatment for advanced non–small lung cancer.
Key Points
Question
Is smoking status associated with overall survival among patients with advanced non–small cell lung cancer (NSCLC) treated with first-line (1L) pembrolizumab monotherapy in a clinical setting?
Findings
This cohort study of 1166 patients with advanced NSCLC who received 1L pembrolizumab monotherapy in routine clinical care in the US found that, after adjusting for patient characteristics, patients who currently or formerly smoked were associated with having a significantly prolonged overall survival compared with those without any history of smoking.
Meaning
These findings suggest that patients with advanced NSCLC without a history of smoking may not derive the same overall survival benefit from treatment with 1L pembrolizumab as individuals who currently or formerly smoked.
Abstract
Importance
There is a need to tailor treatments to patients who are most likely to derive the greatest benefit from them to improve patient outcomes and enhance cost-effectiveness of cancer therapies.
Objective
To compare overall survival (OS) between patients with a current or former history of smoking with patients who never smoked and initiated pembrolizumab monotherapy as first-line (1L) treatment for advanced non–small lung cancer (NSCLC).
Design, Setting, and Participants
This retrospective cohort study compared patients diagnosed with advanced NSCLC aged 18 or higher selected from a nationwide real-world database originating from more than 280 US cancer clinics. The study inclusion period was from January 1, 2011, to October 1, 2019.
Exposures
Smoking status at the time of NSCLC diagnosis.
Main Outcomes and Measures
OS measured from initiation of 1L pembrolizumab monotherapy.
Results
In this retrospective cohort study, a total of 1166 patients (median [IQR] age, 72.9 [15.3] years; 581 [49.8%] men and 585 [50.2%] women) were assessed in the primary analysis, including 91 patients [7.8%] with no history of smoking (ie, never-smokers) and 1075 patients [92.2%] who currently or formerly smoked (ie, ever-smokers). Compared with ever-smokers, never-smokers were older (median age [IQR] of 78.2 [12.0] vs 72.7 [15.5] years), more likely to be female (61 [67.0%] vs 524 [48.7%]) and to have been diagnosed with nonsquamous tumor histology (70 [76.9%] vs 738 [68.7%]). After adjustment for baseline covariates, ever-smokers who initiated 1L pembrolizumab had significantly prolonged OS compared to never-smokers (median OS: 12.8 [10.9-14.6] vs 6.5 [3.3-13.8] months; hazard ratio (HR): 0.69 [95% CI, 0.50-0.95]). This trend was observed across all sensitivity analyses for the 1L pembrolizumab cohort, but not for initiators of 1L platinum chemotherapy, for which ever-smokers showed significantly shorter OS compared with never-smokers (HR, 1.2 [95% CI, 1.07-1.33]).
Conclusions and Relevance
In patients with advanced NSCLC who received 1L pembrolizumab monotherapy in routine clinical practices in the US, patients who reported a current or former history of smoking at the time of diagnosis had consistently longer OS than never-smokers. This finding suggests that in never-smoking advanced NSCLC, 1L pembrolizumab monotherapy may not be the optimal therapy selection, and genomic testing for potential genomically matched therapies should be prioritized over pembrolizumab in never-smokers.
Introduction
Tobacco smoking is the number 1 risk factor for lung cancer and is associated with over 80% to 90% of lung cancers. People who smoke are more than 15 to 30 times more likely to die of lung cancer than people who do not smoke.1 Smoking is known to affect patient health and quality of life and may influence the effectiveness of some lung cancer treatments.2 The mutations induced by carcinogens in cigarettes can lead to higher numbers of neoantigens, which may make smoking-induced lung cancer more sensitive to immunotherapy.3 In contrast, the efficacy of immunotherapy is comparatively lower in patients who have never smoked, despite variability in programmed death-ligand 1 (PD-L1) expression.4,5 Two recent meta-analyses of randomized clinical trials reported significantly higher efficacy of programmed death-1 (PD-1) inhibitors pembrolizumab and nivolumab relative to chemotherapy regimens among people who currently or formerly smoked compared with those without a history of smoking,6,7 motivating additional investigation. The effect of smoking status in a clinical setting was studied by estimating the association between smoking status and overall survival (OS) in patients with advanced non–small cell lung cancer (NSCLC) managed with first-line (1L) pembrolizumab monotherapy in routine US clinical practice.
Methods
Approval for this cohort study was granted by the WIRB-Copernicus Group institutional review board. Informed consent was waived because the data were deidentified, in accordance with 45 CFR §46. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
At the initiation date of 1L pembrolizumab (ie, index date), eligible patients were aged 18 years or older, had a documented smoking status, and had been diagnosed with locally advanced or metastatic NSCLC between January 1, 2011, and October 30, 2019. Patients who currently or formerly smoked were considered ever-smokers, and patients without reported smoking history were considered never-smokers. Patients were excluded if they had (1) previously tested positive for driver alterations in ALK or EGFR; (2) reported specific comorbidities (eAppendix in the Supplement); (3) a gap of more than 90 days between advanced NSCLC diagnosis and first documented visit in the database8; (4) or less than 6 months of potential follow-up at the study cut-off date of March 1, 2020, to account for the COVID-19 pandemic.
Identical eligibility criteria were used to select patients who received 1L platinum chemotherapy for comparison. Patients were selected from the Flatiron Health database, a nationwide longitudinal electronic health record-derived database comprising deidentified patient-level structured and unstructured data curated via technology-enabled abstraction from approximately 280 cancer clinics and 800 sites of care in the US.9,10 The endpoint of interest was OS, defined as time (in months) from 1L initiation to all-cause death. Patients missing a date of death were censored at their last recorded visit in the database or March 1, 2020, the data cut and study cut-off date, whichever was earlier.
To adjust for differences in patient characteristics between never-smoker and ever-smoker groups, inverse probability treatment weighting (IPTW) was used.11 Potential confounders included in the logistic regression model used to estimate propensity scores were—age, sex, ECOG PS, cancer stage, histology, and PD-L1 positivity. Patients were weighted by the stabilized inverse probability of being an ever-smoker. Unadjusted and IPTW-adjusted hazard ratios (HR) for all-cause mortality were estimated using Cox proportional-hazards models. Unadjusted and IPTW-adjusted Kaplan-Meier curves were used to estimate median OS time. Statistical significance was set at a 5% level; hence 95% CIs were derived using a robust variance estimator when IPTW was used. Covariates with residual imbalance after IPTW (standardized mean difference [SMD] > 0.1)11 were controlled by including them as covariates in the Cox model, apart from metastases which are known to be underrecorded in this data set. Multiple imputation using chained equations and delta-based tipping point analysis were used for sensitivity analyses of missing data assumptions as described previously.12 Statistical analyses were performed using the R programming environment (version 3.6.3). Details are in the eMethods in the Supplement.
Results
A total of 1166 patients (median [IQR] age, 72.9 [15.3] years; 581 [49.8%] men; and 585 [50.2%] women) were assessed in the primary analysis, including 91 never-smokers and 1075 ever-smokers. Compared with ever-smokers (1075 [92.2%]), never-smokers (91 [7.8%]) were older (median age [IQR] of 78.2 [12.0] vs 72.7 [15.5] years), more likely to be female (61 [67.0%] vs 524 [48.7%]) and tended to have nonsquamous tumor histology (70 [76.9%] vs 738 [68.7%]) (Table). Prior to adjustment for baseline covariates, OS measured from 1L pembrolizumab initiation was similar between ever- vs never-smokers (median OS: 12.1 [95% CI, 6.6-19.2] months vs 12.5 [95% CI, 10.7-14.5] months; HR, 0.97 [95% CI, 0.73-1.28]) (Figure). Following adjustment, ever-smokers had significantly longer OS compared with never-smokers (median OS: 12.8 [95% CI, 10.9-14.6] vs 6.5 [3.3-13.8] months; HR, 0.69 [0.50-0.95]) (Figure). No evidence of violation of the proportional-hazards assumption was identified (eFigure 1 in the Supplement). The distribution of IPTW weights is shown in eFigure 2 in the Supplement. As sensitivity analyses, adjusting for central nervous system metastases yielded an adjusted HR of 0.71 (95% CI, 0.52-0.98), and when retaining patients with comorbidities, the adjusted HR 0.79 (95% CI, 0.58-1.07).
Table. Baseline Patient Characteristics Before and After IPTW Adjustment of the Primary Analysisa.
| Characteristic | Unadjusted, No. (%) | SMD | Post-IPTW adjustment, No. (%) | SMD | ||
|---|---|---|---|---|---|---|
| Never-smokers | Ever-smokers | Never-smokers | Ever-smokers | |||
| Sample size (unadjusted) | 91 | 1075 | NA | |||
| ESS (post-IPTW adjustment) | 60 | 1072 | NA | |||
| Age, <65 | 12 (13.2) | 301 (28.0) | 0.37 | 23.4 (26.1) | 288.6 (26.8) | 0.02 |
| Sex | 0.38 | 0.04 | ||||
| Female | 61 (67.0) | 524 (48.7) | 43.4 (48.3) | 539.3 (50.2) | ||
| Male | 30 (33.0) | 551 (51.3) | 46.5 (51.7) | 535.7 (49.8) | ||
| Race | 0.15 | 0.21c | ||||
| White | 61 (67.0) | 790 (73.5) | 57.6 (64.1) | 791.2 (73.6) | ||
| Other racesb | 21 (23.1) | 192 (17.9) | 21.7 (24.2) | 190.4 (17.7) | ||
| Unknown | 9 (9.9) | 93 (8.7) | 10.5 (11.7) | 93.5 (8.7) | ||
| Histology | 0.21 | 0.01 | ||||
| Nonsquamous | 70 (76.9) | 738 (68.7) | 61.9 (68.9) | 744.9 (69.3) | ||
| Squamous | 17 (18.7) | 294 (27.3) | 24.3 (27.1) | 286.8 (26.7) | ||
| Not otherwise specified | 4 (4.4) | 43 (4.0) | 3.6 (4.0) | 43.4 (4.0) | ||
| Cancer stage at diagnosis | 0.16 | 0.05 | ||||
| Stage I-III | 25 (27.5) | 372 (34.6) | 28.6 (31.8) | 365.7 (34.0) | ||
| Stage IV | 66 (72.5) | 703 (65.4) | 61.3 (68.2) | 709.4 (66.0) | ||
| ECOG | 0.29 | 0.12c | ||||
| 0 | 30 (33.0) | 251 (23.3) | 21.3 (23.7) | 258.9 (24.1) | ||
| 1 | 42 (46.2) | 477 (44.4) | 44.9 (50.0) | 478.9 (44.6) | ||
| ≥2 | 19 (20.9) | 347 (32.3) | 23.7 (26.3) | 337.2 (31.4) | ||
| Time since diagnosis, median (range), mo | 0.89 (0.28-4.32) | 0.95 (0-5.01) | 0.14 | 0.92 (0.28-4.32) | 0.95 (0-5.01) | 0.07 |
| No. of sites of metastases, median (range) | 0 (0-2) | 0 (0-3) | 0.01 | 0 (0-2) | 0 (0-3) | 0.05 |
| CNS metastases, none | 2 (18.2) | 48 (29.3) | 0.26 | 1.9 (17.8) | 48.1 (29.5) | 0.28c |
| Liver metastases, none | 88 (96.7) | 1026 (95.4) | 0.07 | 87.2 (97) | 1026.3 (95.5) | 0.08 |
| Lung metastases, none | 88 (96.7) | 1045 (97.2) | 0.03 | 87.3 (97.1) | 1045.0 (97.2) | 0.01 |
| Other metastases, none | 73 (80.2) | 841 (78.2) | 0.05 | 73.0 (81.2) | 840.9 (78.2) | 0.07 |
| Recorded PD-L1 positivity, absent | 24 (26.4) | 229 (21.3) | 0.12 | 20.2 (22.5) | 233.3 (21.7) | 0.02 |
| Insurance status, insured | 66 (72.5) | 787 (73.2) | 0.02 | 66.8 (74.3) | 786.3 (73.1) | 0.03 |
| Practice type | 0.01 | 0.01 | ||||
| Academic | 9 (9.9) | 109 (10.1) | 9.5 (10.6) | 109.7 (10.2) | ||
| Community | 82 (90.1) | 966 (89.9) | 80.3 (89.4) | 965.3 (89.8) | ||
Abbreviations: CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group Performance Score; ESS, effective sample size; IPTW, inverse probability treatment weighting; PD-L1, programmed death-ligand 1; SMD, standardized mean difference.
Potential confounders included in the logistic regression model used to estimate propensity scores were—age, sex, ECOG PS, cancer stage, histology, and PD-L1 positivity. Race and ECOG PS were included in the Cox model for further adjustment, whereas CNS metastases is underrecorded and so was used for IPTW adjustment in a sensitivity analysis.
Other races encompasses Asian, Black or African American, Hispanic or Latino, or other (not specified).
Residual imbalances after IPTW (SMD).
Figure. Kaplan-Meier Curves for Never-Smokers vs Ever-Smokers Who Initiated First-line Pembrolizumab Monotherapy Showing Unadjusted and Inverse Probability Treatment Weighting–Adjusted Comparisons.
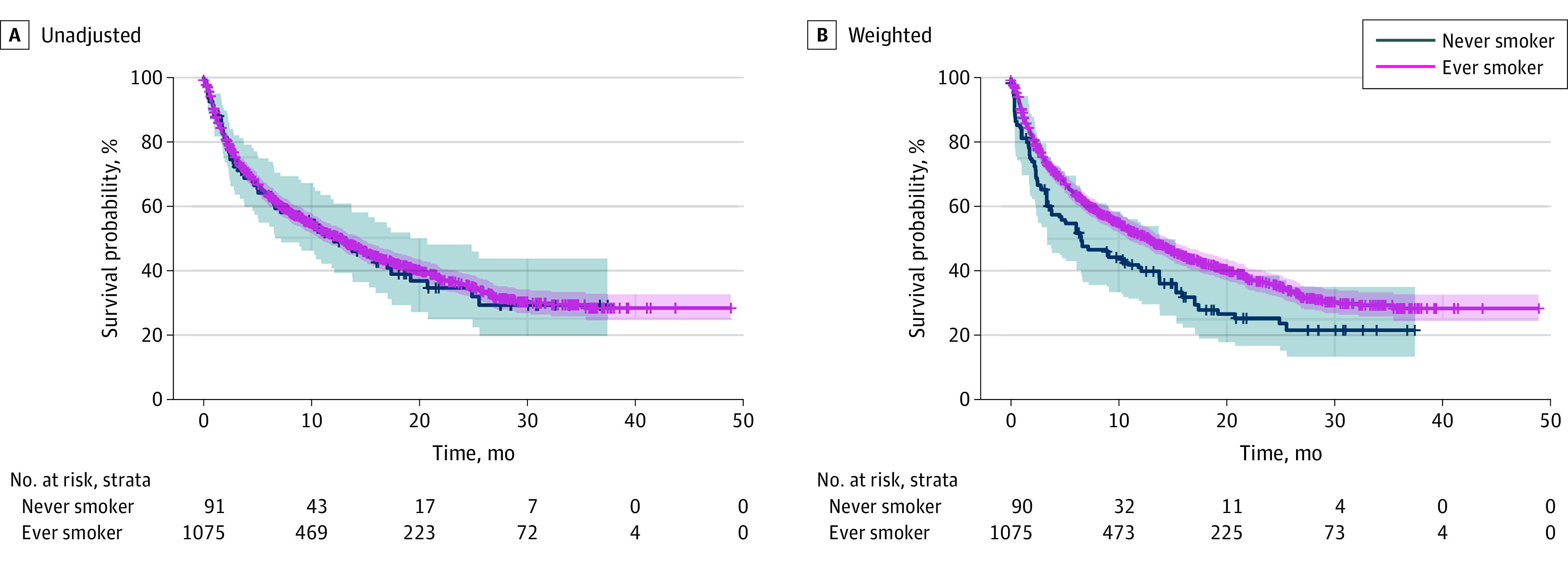
Individuals in the ever-smokers group had a current or former history of smoking, and individuals in the never-smokers group had no history of smoking. The gray shading indicates the 95% CI for never-smokers and red shading indicates the 95% CI for ever-smokers.
Aside from ECOG PS (Eastern Cooperative Oncology Group Performance Score), none of the patients were missing data for all other potential confounders of interest. Baseline ECOG PS was missing for a nonnegligible proportion of patients (397 [25.4%]). To assess the sensitivity of results to missing data assumptions, adjusted HRs were estimated under missing at random (MAR) and missing not at random (MNAR). After multiple imputation of missing ECOG PS under MAR, the pooled and adjusted HR was 0.85 (95% CI, 0.67-1.07). Tipping point-based bias analysis assuming nonrandom missingness for ECOG PS in the ever-smokers group found that, although not statistically significant, HR point estimates remained less than 1 (ie, unfavorable for never-smokers) under all scenarios tested (eFigure 3 in the Supplement). Assessing generalizability of our findings to other treatment settings, we repeated the primary analysis for 8375 patients who received 1L platinum chemotherapy (7803 ever-smokers [93.2%] and 572 of never-smokers [6.8%]). Within this patient group, the adjusted HR was 1.2 (95% CI, 1.06-1.33), representing a significant relative OS benefit for never-smokers instead.
Discussion
In this retrospective study of patients with EGFR and ALK wild-type advanced NSCLC who received 1L pembrolizumab monotherapy, OS was compared between never-smokers and ever-smokers after direct adjustment for differences in prognostic patient characteristics. To our knowledge, this is the first direct comparison suggesting that smoking status is predictive of OS after receiving 1L pembrolizumab monotherapy in a large, nationally representative real-world US cohort of patients with advanced NSCLC. The distribution of baseline patient covariates before IPTW-adjustment was similar between ever-smokers and never-smokers (SMD < 0.4). Never-smokers were older than ever-smokers, more likely to be women and be diagnosed with nonsquamous tumor histology, which is consistent with publications of clinical features of nonsmoking patients with NSCLC.13 Although ever-smokers and never-smokers had similar OS before adjustment for patient characteristics, adjusted comparisons showed that ever-smokers had significantly better OS with 1L pembrolizumab than never-smokers. Although statistical significance was not achieved for all sensitivity analyses, they all recapitulated the trend of a relative benefit for ever-smokers receiving 1L pembrolizumab, but not 1L platinum chemotherapy, relative to never-smokers. This study’s results were consistent with subgroup analyses of smoking status in matched case-controls comparing 1L pembrolizumab vs 1L platinum chemotherapy from clinical practices in Italy14 and previous meta-analyses of randomized trials.6,7
Limitations
This study had limitations. As with any observational study, unmeasured differences between never-smoker and ever-smoker groups may exist. Statistical significance was not achieved for all sensitivity analyses, possibly because of smaller sample sizes for never-smokers. A larger number of never-smokers would also reduce the width of estimated CI. Another limitation is that metastases and comorbidities are underrecorded in this dataset, which makes appropriate adjustment of these variables difficult. However, because our overall results agree with previously published reports,6,7,14 we expect that external validity of our conclusions holds.
Conclusions
In this retrospective cohort study of patients with advanced NSCLC who received 1L pembrolizumab, never-smokers were associated with a significantly worse OS compared with ever-smokers after adjustment for baseline patient characteristics. This finding adds additional support that 1L pembrolizumab monotherapy may not be the optimal therapy for patients who have never smoked and have advanced NSCLC, a disease enriched for oncogene addiction and with lower tumor mutational burden.15 Efforts to comprehensively profile tumors for genotype-matched treatments should be prioritized.
eAppendix. Variable Definitions and Comments
eMethods.
eFigure 1. Schoenfeld Residuals and Log-Negative-Log (LNL) Survival Curves for Tests of Proportional Hazards Before and After IPTW-Adjustment
eFigure 2. Distribution of IPTW Weights
eFigure 3. Delta-Based Tipping Point Analysis for ECOG PS
References
- 1.Centers for Disease Control and Prevention . What Are the Risk Factors for Lung Cancer? Accessed December 30, 2021. https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm
- 2.Condoluci A, Mazzara C, Zoccoli A, Pezzuto A, Tonini G. Impact of smoking on lung cancer treatment effectiveness: a review. Future Oncol. 2016;12(18):2149-2161. doi: 10.2217/fon-2015-0055 [DOI] [PubMed] [Google Scholar]
- 3.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348(6230):124-128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gainor JF, Rizvi H, Jimenez Aguilar E, et al. Clinical activity of programmed cell death 1 (PD-1) blockade in never, light, and heavy smokers with non–small-cell lung cancer and PD-L1 expression ≥50. Ann Oncol. 2020;31(3):404-411. doi: 10.1016/j.annonc.2019.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharpnack MF, Cho JH, Johnson TS, et al. Clinical and molecular correlates of tumor mutation burden in non–small cell lung cancer. Lung Cancer. 2020;146:36-41. doi: 10.1016/j.lungcan.2020.05.021 [DOI] [PubMed] [Google Scholar]
- 6.Li B, Huang X, Fu L. Impact of smoking on efficacy of PD-1/PD-L1 inhibitors in non–small cell lung cancer patients: a meta-analysis. Onco Targets Ther. 2018;11:3691-3696. doi: 10.2147/OTT.S156421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mo J, Hu X, Gu L, et al. Smokers or non-smokers: who benefits more from immune checkpoint inhibitors in treatment of malignancies: an up-to-date meta-analysis. World J Surg Oncol. 2020;18(1):15. doi: 10.1186/s12957-020-1792-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khozin S, Carson KR, Zhi J, et al. Real-world outcomes of patients with metastatic non–small cell lung cancer treated with programmed cell death protein 1 inhibitors in the year following US regulatory approval. Oncologist. 2019;24(5):648-656. doi: 10.1634/theoncologist.2018-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv. Preprint posted online January 13, 2020. Accessed April 19, 2022. https://arxiv.org/abs/2001.09765
- 10.Ma X, Long L, Moon S, et al. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv medRxiv. Preprint posted online May 30, 2020. Accessed May 6, 2022. doi: 10.1101/2020.03.16.20037143 [DOI]
- 11.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramagopalan S, Gupta A, Arora P, Thorlund K, Ray J, Subbiah V. Comparative effectiveness of atezolizumab, nivolumab, and docetaxel in patients with previously treated non–small cell lung cancer. JAMA Netw Open. 2021;4(11):e2134299. doi: 10.1001/jamanetworkopen.2021.34299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho J, Choi SM, Lee J, et al. Proportion and clinical features of never-smokers with non–small cell lung cancer. Chin J Cancer. 2017;36(1):20. doi: 10.1186/s40880-017-0187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortellini A, De Giglio A, Cannita K, et al. Smoking status during first-line immunotherapy and chemotherapy in NSCLC patients: a case-control matched analysis from a large multicenter study. Thorac Cancer. 2021;12(6):880-889. doi: 10.1111/1759-7714.13852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolle E, Leithner K, Olschewski H. Oncogene addiction and tumor mutational burden in non–small-cell lung cancer: clinical significance and limitations. Thorac Cancer. 2020;11(2):205-215. doi: 10.1111/1759-7714.13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Variable Definitions and Comments
eMethods.
eFigure 1. Schoenfeld Residuals and Log-Negative-Log (LNL) Survival Curves for Tests of Proportional Hazards Before and After IPTW-Adjustment
eFigure 2. Distribution of IPTW Weights
eFigure 3. Delta-Based Tipping Point Analysis for ECOG PS


