Abstract
Breast cancer is one of the most common malignant tumors in women. Cell division cycle-associated 5 (CDCA5) is closely associated with the behavior of various cancer types. The aim of the present study was to explore the effect of CDCA5 on breast cancer. Western blot analysis and reverse transcription-quantitative PCR were used to detect the expression level of CDCA5 in human normal mammary cells and human breast cancer cell lines. To determine its function in MDA-MB-231 cells, CDCA5 was silenced in MDA-MB-231 cells by transient short hairpin RNA transfection. Cell Counting Kit-8 and clonogenicity assays were used to evaluate cell proliferation. Wound healing and Transwell assays were used to detect cell invasion and migration. Western blot analysis was used to detect the protein expressions of Ki67 and PCNA associated with proliferation, MMP2 and MMP9 associated with migration. CDCA5 was found to be markedly increased in breast cancer cell lines. CDCA5 knockdown was able to suppress cell proliferation, invasion and migration. CDCA5 inhibition downregulated PDS5 cohesin-associated factor A (PDS5A) expression in breast cancer cells. PDS5A overexpression was found to reverse the effect of CDCA5 inhibition on breast cancer cell proliferation and migration. CDCA5 knockdown was shown to suppress the malignant progression of breast cancer cells by regulating PDS5A. The present findings may provide new potential targets for breast cancer therapy.
Keywords: breast cancer, cell division cycle associated 5, PDS5 cohesin-associated factor A, cell proliferation, cell invasion, cell migration
Introduction
Breast cancer is the most common type of cancer among women worldwide. It is estimated that ~1.4 million women are diagnosed with breast cancer every year, and 458,000 women succumb to the disease, making it the second most frequently diagnosed type of cancer worldwide (1). The incidence of early-onset breast cancer in America is 10.3% of all new female breast cancer cases in 2012–2016 and has markedly increased among young women aged 18–45 years (2). Breast cancer involves a multi-step process associated with the abnormal expression of several oncogenes and tumor suppressor genes (3). Therefore, the study of biomarkers and active molecules in the initial stages of breast cancer can provide a theoretical basis for the prevention and early detection of breast cancer (4–7).
Cell division cycle-associated 5 (CDCA5), also known as sororin, is the encoding gene of CDCA5, which is located on chromosome 11. CDCA5 is a transcriptional protein with a molecular weight of ~27 kDa (8,9) that is widely expressed in bone marrow, testes, lymph nodes, bladder, lung, breast, stomach, and other tissues and organs (10,11). CDCA5 has been reported to promote cancer (10,12,13). The following genes have been shown to be significantly upregulated in breast cancer tissues, which lead to a decrease in the survival rates of patients: Kinetochore scaffold 1 (CASC5), cytoskeleton-associated protein 2 like (CKAP2L), family with sequence similarity 83 member D (FAM83D), kinesin family member 18B (KIF18B), kinesin family member 23 (KIF23), spindle and kinetochore-associated complex subunit 1 (SKA1), GINS complex subunit 1 (GINS1), CDCA5 and minichromosome maintenance complex component 6 (MCM6) (14). However, to the best of our knowledge, the role of CDCA5 in breast cancer has not been studied to date.
PDS5A, a sister chromatid cohesion protein PDS homolog A, is responsible for unloading auxin from chromatin, while CDCA5 (sororin) is involved in establishing sister chromatid cohesion (SCC) (15). Alterations in PDS5A expression levels have been observed in breast, kidney, esophagus, stomach, liver and colon cancer (15,16). PDS5A has been found to be significantly upregulated at both the mRNA and protein levels, and this upregulation is positively correlated with World Health Organization glioma grade (17). PDS5A is upregulated in nasopharyngeal carcinoma tumor tissue, and PDS5A overexpression in 293T cells promotes proliferation and clone formation (18).
In the present study, the effect of CDCA5 and PDS5A on breast cancer cell proliferation, migration and invasion was determined. The present findings may provide a new target and strategy for the clinical treatment of breast cancer.
Materials and methods
Cell culture and cell transfection
Human normal mammary MCF-10A and human breast cancer MCF-7, MDA-MB-231, SUM190PT and SK-BR-3 cell lines were purchased from BeNa Culture Collection and cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) under constant conditions at 37°C in a humidified atmosphere with 5% CO2.
Short hairpin RNAs (shRNAs) against CDCA5 (shRNA-CDCA5-1, 5′-CCGGCCAAAGTACCATAGCCAGTTTCTCGAGAAACTGGCTATGGTACTTTGGTTTTTG-3′; and shRNA-CDCA5-2, 5′-CCGGGAGCAGTTTGATCTCCTGGTTCTCGAGAACCAGGAGATCAAACTGCTCTTTTTG-3′), scrambled shRNA [transfected with empty vector; shRNA-negative control (NC), 5′-TTCGGGTCATCCGATGGGCC-3′], adenovirus overexpressing PDS5A (pcDNA3.1-PDS5A) and control adenovirus (pcDNA3.1-NC) were constructed by Hanbio Biotechnology Co., Ltd. A total of 0.4 µg shRNA/adenovirus was transfected into 1×105 cells using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) and incubated for 10 min at room temperature, according to the manufacturer's instructions. At 48 h post-transfection, the transfected cells were used for subsequent experiments.
Bioinformatics
STRING (https://string-db.org/) is a database that can be used to predict interactions among proteins (accession date, September 2020, version. 11.0). The differences in PDS5A expression between breast cancer and normal tissues were predicted using Encyclopaedia of RNA Interactomes website (https://starbase.sysu.edu.cn/) and Gene Expression Profiling Interactive Analysis (GEPIA) database (gepia.cancer-pku.cn/), and Pearson was used as the statistical tests.
Cell proliferation assays
For Cell Counting Kit-8 (CCK-8) assay, MDA-MB-231 cells (1×103 cells/well) were seeded in 96-well plates and cultured for 24 h. At 24, 48 and 72 h after incubation, CCK-8 solution (Merck KGaA) was added to each well. The cells were incubated for an additional 1 h to determine cell viability. Optical density was determined at 450 nm using a microtiter plate reader.
For colony formation assay, transfected MDA-MB-231 cells (1×105 cells/well) were seeded into 6-well plates, and the MDA-MB-231 cells treated with sh-NC or pcDNA3.1 were used as the negative controls. Following an incubation period of 14 days at 37°C, the colonies were fixed with 100% methanol for 15 min at room temperature and stained with 0.1% crystal violet at room temperature in absolute ethanol for 15 min. Visible colonies of >50 cells were counted and analyzed under an inverted light microscope.
Wound healing and Transwell assays
For the wound healing assay, transfected MDA-MB-231 cells (1×105 cells/well) were cultured in 6-well plates until they reached 70–80% confluence, and then wounded using a 200-µl sterile pipette tip. Upon washing with PBS, the cells were cultured in serum-free DMEM. Images were acquired using a light microscope at each 0 and 24 h. The cell migration ratio was calculated as the percentage of remaining cell-free area compared to the area of the initially scratched area.
For the Transwell assay, MDA-MB-231 cells (3×104 cells/well) were cultured in serum-free DMEM. The upper chamber of 24-well Transwell was pre-coated with Matrigel (Merck KGaA) at 37°C for 30 min. The cells were then cultured in the upper chamber with 0.1 ml cell suspension in each well, while the lower chamber was filled with cell culture DMEM containing 20% FBS. The cells were cultured for 24 h at 37°C. Subsequently, the membrane in the lower chamber was collected and cleaned, followed by staining with 0.5% crystal violet dye (Merck KGaA) at room temperature for 10 min. The stained cells were counted using an optical microscope.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from 1×104 MDA-MB-231 cells using TRIzol® reagent (Invitrogen, cat.no.15596018). The total RNA concentration was assessed with NanoDrop 2000™ (NanoDrop Technologies; Thermo Fisher Scientific, Inc.) before being reverse transcribed to cDNA using Titan One Tube RT-PCR reagent (Merck KGaA; cat. No. 11939823001), according to the manufacturer's instructions. Subsequently, RT-qPCR was performed using a QuantiTect SYBR Green PCR kit (Qiagen GmbH), according to the manufacturer's instructions. The following thermocycling conditions were used for qPCR: Initial denaturation at 95°C for 5 min, followed by 30 cycles of 95°C for 10 sec and 60°C for 45 sec. The following primers (GenScript) were used for RT-qPCR: CDCA5 forward, 5′-GGCCAGAGACTTGGAAATGT-3′ and reverse, 5′-GGCCAGAGACTTGGAAATGT-3′; PDS5A forward, 5′-GATCACCACGGACGAGATGA-3′ and reverse, 5′-AAGGCTAGTGGGAGATACTGCTGTT-3′; and GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′. mRNA expression levels were quantified using the 2−ΔΔCq method (19) and normalized to the internal reference gene GAPDH.
Western blotting
Total protein was extracted from 1×106 MDA-MB-231 cells using RIPA lysis buffer (Elabscience, cat. No. E-BC-R327) and quantified using a Pierce™ BCA Protein Assay Kit (Pierce; Thermo Fisher Scientific, Inc.). Following denaturation, electrophoresis of 30 µg protein/lane was performed using 12% SDS-PAGE. Following gel transfer onto PVDF membranes, the membranes were blocked in 5% fat-free milk for 2 h at room temperature. Subsequently, the membranes were incubated overnight at 4°C with the following primary antibodies: Anti-MMP2 (1:1,000; cat. no. ab215986; Abcam), anti-MMP9 (1:1,000; cat. no. ab219372; Abcam), anti-GAPDH (1:1,000; cat. no. ab181602; Abcam), anti-Ki67 (1:1,000; cat. no. ab92742; Abcam), anti-proliferating cell nuclear antigen (PCNA; 1:1,000; cat. no. ab92552; Abcam), anti-CDCA5 (1:1,000; cat. no. ab192237; Abcam) and anti-PDS5A (1:500; cat. no. 203627-T34; Sino Biological Inc.). Next, the membranes were incubated with a goat anti-rabbit HRP-conjugated IgG secondary antibody (1:2,000; cat. no. ab6721; Abcam) at room temperature for 4 h. The protein bands were visualized using an enhanced chemiluminescence reagent (cat. no. abs920, Absin). The protein expression levels were semi-quantified using ImageJ software (v1.46; National Institutes of Health) with GAPDH as the loading control.
Co-immunoprecipitation (co-IP) assay
Total protein was isolated from 1×107 MDA-MB-231 cells using RIPA lysis buffer (Beyotime Institute of Biotechnology), followed by centrifugation at 14,000 × g for 15 min at 4°C and quantified using a BCA kit (Beyotime Institute of Biotechnology). For the co-IP assay, 400 µg protein was incubated with 2 µg the appropriate antibodies including anti-PDS5A (1:500, 203627-T34, Sino Biological), anti-CDCA5 (1:500; FineTest, FNab01542), Goat Anti-Rabbit IgG (1:2000, ab6721, Abcam) overnight at 4°C. Subsequently, 30 µl Protein G/A agarose beads (Invitrogen; Thermo Fisher Scientific, Inc.) were added to the cell lysates for a 3-h incubation at 4°C. Following three washes with PBS, the precipitated proteins were resuspended in 5X SDS-PAGE loading buffer (cat. no. ZY81204, Shanghai Zeye Biological Inc.), boiled for 5 min at 99°C and eluted from the beads with 1 ml lysis buffer for 3 times. Finally, western blotting was used to detect the co-IP products in the way as aforementioned.
Statistical analysis
All experiments were repeated at least three independent times, and the results are expressed as the mean ± SD. Statistical analysis was performed using SPSS 19.0 software (IBM Corp.). Unpaired Student's t-test and one-way ANOVA followed by Tukey's post-hoc test were used to determine statistical significance. Correlation between CDCA5 and PDS5A was evaluated using Pearson's correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
CDCA5 is upregulated in human breast cancer tissues and cell lines
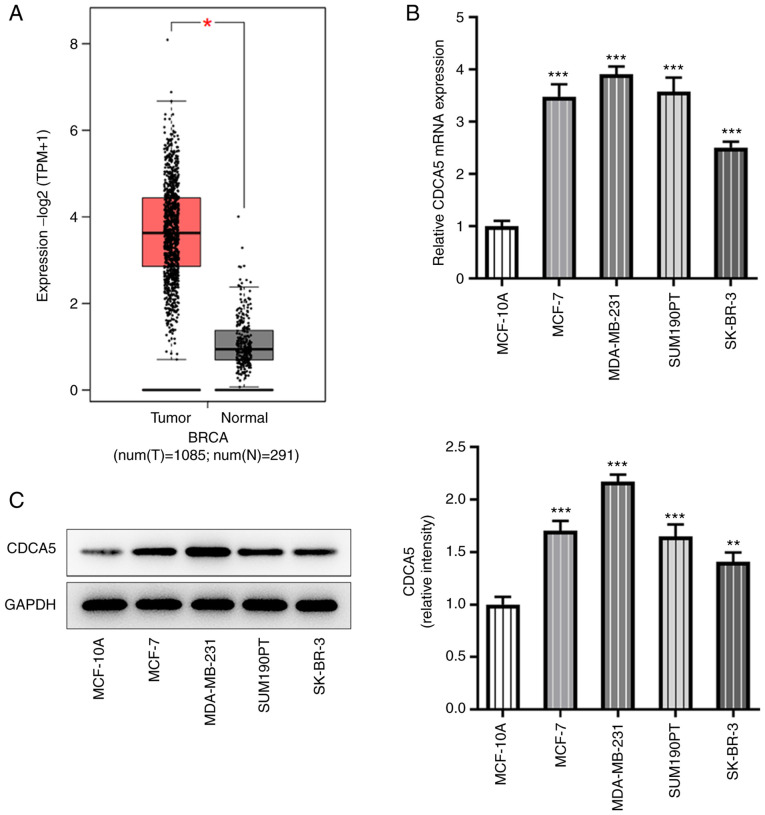
According to GEPIA database (gepia.cancer-pku.cn/) prediction, CDCA5 expression was increased in breast cancer tissues (Fig. 1A). Further RT-qPCR (Fig. 1B) and western blotting (Fig. 1C) were performed to detect the expression levels of CDCA5 in breast cancer cells. The results indicated that CDCA5 was upregulated in breast cancer cells, particularly in MDA-MB-231 cells, compared with the expression levels in the breast cancer lines MCF-7, SUM190T and SK-BR-3. The MDA-MB-231 cell line was therefore selected for subsequent experiments.
Figure 1.
CDCA5 is upregulated in breast cancer tissues and cell lines. (A) Gene Expression Profiling Interactive Analysis database (http://gepia.cancer-pku.cn/) indicated that CDCA5 was upregulated in the tissues of patients with breast cancer compared with normal tissue adjacent to the cancer. (B) Reverse transcription-quantitative PCR and (C) western blotting were performed to detect the expression level of CDCA5 in breast cancer cells. *P<0.05, **P<0.01 and ***P<0.001 vs. MCF-10A or Normal. CDCA5, cell division cycle-associated 5; BRCA, breast cancer.
CDCA5 knockdown inhibits proliferation, invasion and migration in MDA-MB-231 cell
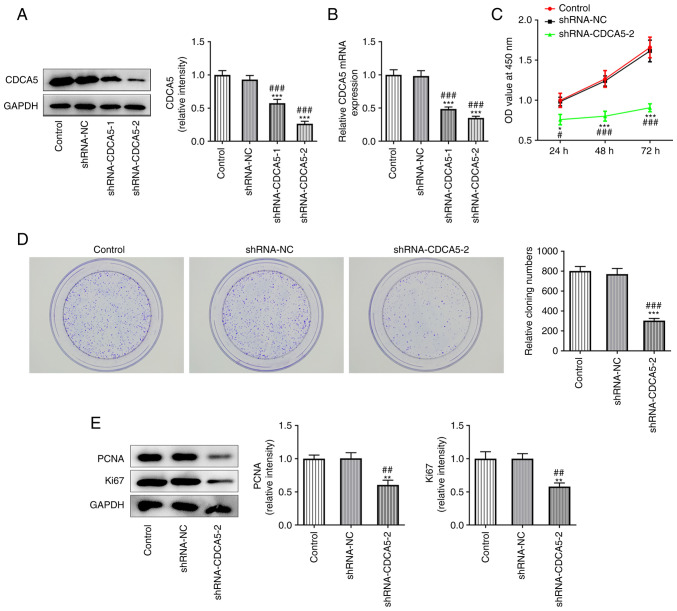
To further explore the role of CDCA5 in breast cancer, CDCA5 knockdown plasmids were constructed. The inhibition level of CDCA5 was subsequently detected by RT-qPCR and western blotting. As presented in Fig. 2A and B, CDCA5 was significantly inhibited in the shRNA-CDCA5-1/2 groups compared with the shRNA-NC group, indicating that the plasmid-mediated inhibition of CDCA5 had been successful. shRNA-CDCA5-2 showed a better knockdown than shRNA-CDCA5-1 and was selected for subsequent experiments.
Figure 2.
CDCA5 knockdown inhibits the proliferation of MDA-MB-231 cells. (A) Western blotting and (B) reverse transcription-quantitative PCR were performed to detect CDCA5 expression. (C) Cell Counting Kit-8 assay was used to detect the level of cell proliferation. (D) Colony formation assay was used to detect the colony formation ability of the cells. (E) The expression levels of the proliferation-related proteins Ki67 and PCNA were reduced upon CDCA5 silencing, as indicated by western blotting. *P<0.05, **P<0.01 and ***P<0.001 vs. control; #P<0.05, ##P<0.01 and ###P<0.001 vs. shRNA-NC. CDCA5, cell division cycle-associated 5; sh, short hairpin; NC, negative control; PCNA, proliferating cell nuclear antigen; OD, optical density.
CCK-8 assay was performed to detect the level of cell proliferation (Fig. 2C) and a colony formation experiment was performed to detect the colony formation potential of MDA-MB-231 cells (Fig. 2D). The results indicated that CDCA5 knockdown inhibited the proliferation of MDA-MB-231 cells. The expression of the proliferation-related proteins Ki67 and PCNA was also decreased, as shown by western blot analysis, which confirmed the aforementioned observations (Fig. 2E).
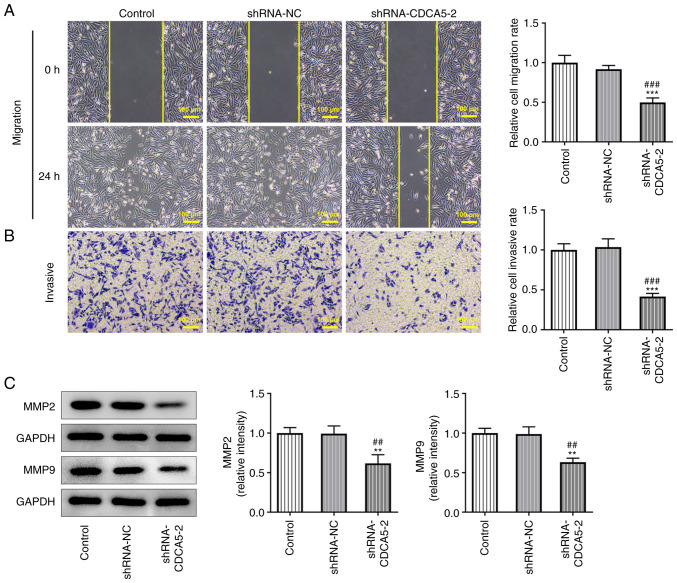
Furthermore, wound healing and Transwell assays detected a marked decrease in the migration (Fig. 3A) and invasion (Fig. 3B) of MDA-MB-231 cells. In addition, the expressions of the migration-related proteins MMP2 and MMP9 were reduced in MDA-MB-231 cells (Fig. 3C). The results indicated that CDCA5 knockdown inhibited the invasion and migration of breast cancer cells.
Figure 3.
CDCA5 knockdown inhibits the invasion and migration of breast cancer cells. (A) Wound healing and (B) Transwell assays were used to detect the level of migration and invasion, respectively, of MDA-MB-231 cells. (C) The expression levels of the migration-related proteins MMP2 and MMP9 were detected by western blotting. **P<0.01 and ***P<0.001 vs. control; ##P<0.01 and ###P<0.001 vs. shRNA-NC. CDCA5, cell division cycle-associated 5; sh, short hairpin; NC, negative control.
CDCA5 knockdown downregulates PDS5A expression in breast cancer cells
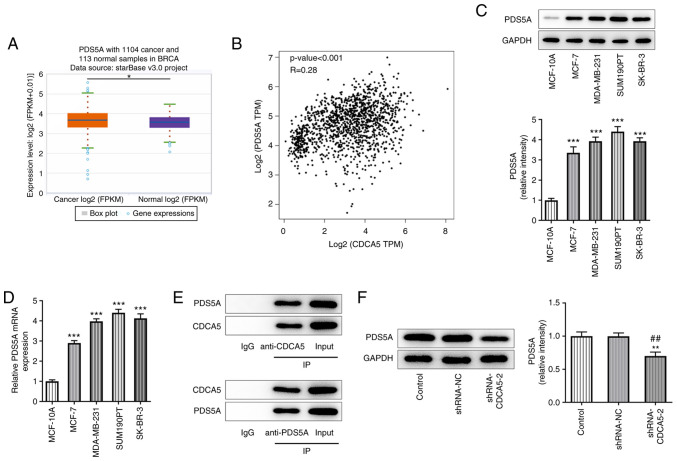
starBase database analysis demonstrated that PDS5A expression was upregulated in breast cancer tissues (Fig. 4A), while the prediction results from the GEPIA website revealed a positive correlation between CDCA5 and PDS5A expression in patients with breast cancer (Fig. 4B). Furthermore, RT-qPCR and western blot analyses were used to detect PDS5A expression in breast cancer cell lines, and PDS5A was found to be upregulated in MDA-MB-231 cells (Fig. 4C and D). Co-IP assay further indicated that CDCA5 could bind to PDS5A (Fig. 4E). In addition, western blot analysis showed that CDCA5 knockdown inhibited PDS5A expression (Fig. 4F).
Figure 4.
CDCA5 knockdown downregulates PDS5A expression in breast cancer cells. (A) starBase website (https://starbase.sysu.edu.cn/) indicated that PDS5A was upregulated in tissues of patients with breast cancer compared with normal tissues adjacent to the cancer. (B) Gene Expression Profiling Interactive Analysis database (http://gepia.cancer-pku.cn/) indicated that the expression of CDCA5 and PDS5A in patients with breast cancer was positively correlated. (C) Western blotting and (D) reverse transcription-quantitative PCR were used to detect the expression level of PDS5A in MDA-MB-231 cells. (E) Co-IP assay further indicated that CDCA5 could bind to PDS5A. (F) Western blotting indicated that knocking down the expression of CDCA5 inhibited the expression of PDS5A. *P<0.05, **P<0.01 and ***P<0.001 vs. control or MCF-10A; ##P<0.01 vs. shRNA-NC. CDCA5, cell division cycle-associated 5; sh, short hairpin; NC, negative control; PDS5A, PDS5 cohesin-associated factor A; IP, immunoprecipitation; log, logarithm; BRCA, breast cancer. TPM, Transcripts Per Million.
PDS5A overexpression reverses the consequences of CDCA5 inhibition on breast cancer cell proliferation and migration
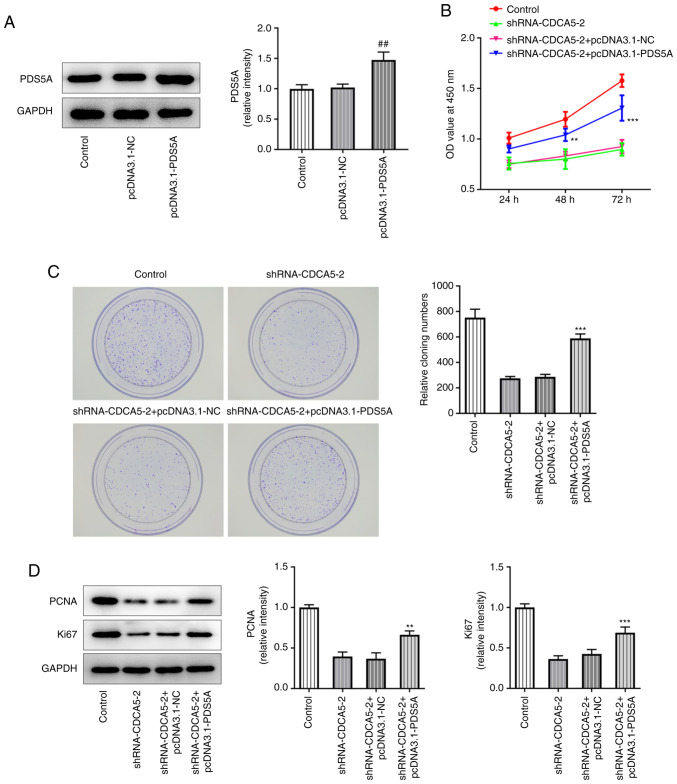
The overexpression of PDS5A in MDA-MB-231 cells by using a pcDNA3.1-PDS5A vector was confirmed by western blotting, which revealed that PDS5A expression was upregulated in the pcDNA3.1-PDS5A group compared with the pcDNA3.1-NC group (Fig. 5A). Furthermore, in the shRNA-CDCA5-2+pcDNA3.1-PDS5A group, CCK-8 assay revealed a marked increase in cell viability (Fig. 5B), increased levels of colony formation (Fig. 5C) and also elevated expression of the proliferation-related proteins Ki67 and PCNA (Fig. 5D), compared to shRNA-CDCA5-2+pcDNA3.1-NCgroup. The results showed that PDS5A overexpression reversed the consequences of CDCA5 inhibition on breast cancer cell proliferation.
Figure 5.
PDS5A overexpression reverses the consequences of CDCA5 inhibition in breast cancer cell proliferation. (A) Overexpression of PDS5A was detected by western blotting. (B) Cell Counting Kit-8 assay detected increased level of cell proliferation in MDA-MB-231 cells. (C) Colony formation assay was used to detect the colony formation ability of MDA-MB-231 cells. (D) Expression levels of the proliferation-related proteins Ki67 and PCNA were increased after being initially reduced, as indicated by western blotting. **P<0.01 and ***P<0.001 vs. shRNA-CDCA5-2 + pcDNA3.1-NC; ##P<0.01 vs. pcDNA3.1-NC. CDCA5, cell division cycle-associated 5; sh, short hairpin; NC, negative control; PCNA, proliferating cell nuclear antigen; OD, optical density; PDS5A, PDS5 cohesin-associated factor A.
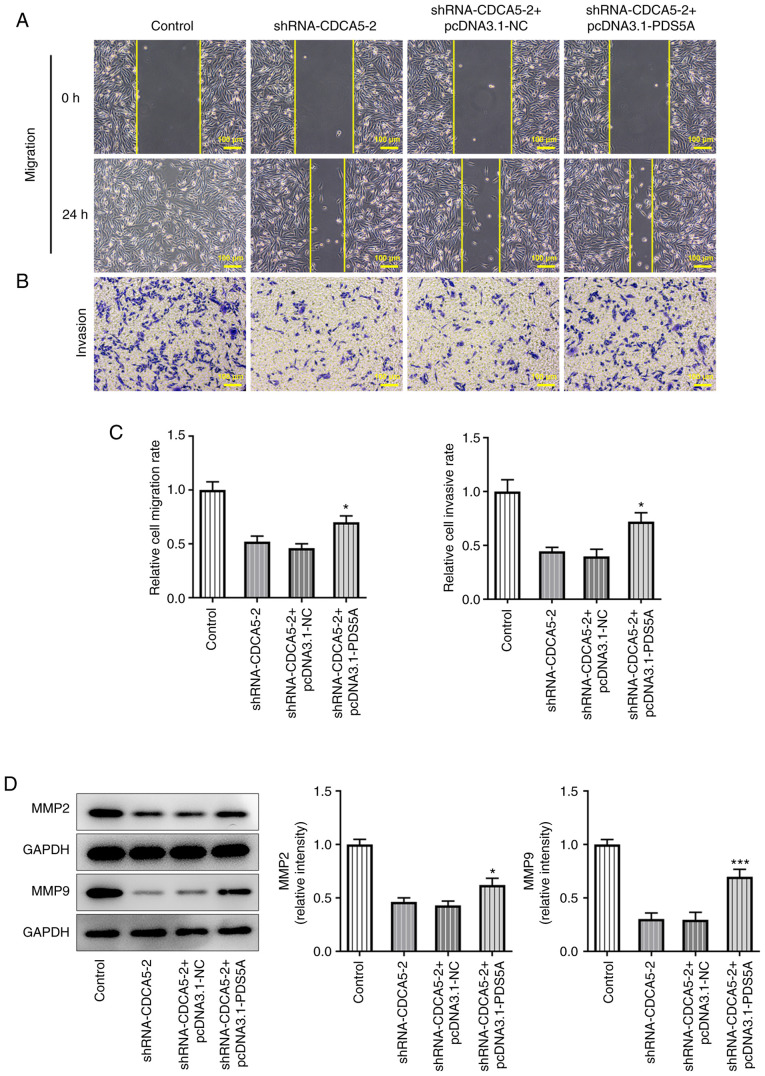
Subsequently, wound healing (Fig. 6A) and Transwell (Fig. 6B) assays were performed. Quantitative analysis of the experimental results (Fig. 6C) indicated that CDCA5 knockdown inhibited the invasion and migration of breast cancer cells, and PDS5A overexpression could reverse this trend. Consistently, migration-associated proteins also showed an upregulation (Fig. 6D). Overall, the present findings demonstrated that PDS5A overexpression reversed the inhibitory effect of CDCA5 interference on breast cancer cell proliferation and migration.
Figure 6.
Overexpression of PDS5A reverses the inhibitory effect of shRNA-CDCA5 on the migration and invasion of breast cancer cells. (A) Wound healing and (B) Transwell assays were used to detect the level of migration and invasion of MDA-MB-231 cells, respectively. (C) Cell migration and invasive rate was increased in MDA-MB-231. (D) Expression levels of the migration-related proteins MMP2 and MMP9 were detected by western blotting. *P<0.05 and ***P<0.001 vs. shRNA-CDCA5-2 + pcDNA3.1-NC. CDCA5, cell division cycle-associated 5; sh, short hairpin; NC, negative control; PDS5A, PDS5 cohesin-associated factor A.
Discussion
It has been reported that the CASC5, CKAP2L, FAM83D, KIF18B, KIF23, SKA1, GINS1, CDCA5 and MCM6 genes are significantly upregulated in breast cancer tissues, and that this upregulation leads to a decrease in patient survival rate (14). CDCA5 expression was found to be higher in bladder cancer tissues compared with that in adjacent healthy tissues, and high CDCA5 expression was found to be associated with poor survival in patients with breast cancer (13,20). A previous study indicated that CDCA5 knockout in T24 and 5637 cells reduced cell proliferation and induced cell apoptosis (13). Compared with that in adjacent tissues, CDCA5 expression was upregulated in hepatocellular carcinoma (HCC) tissue and was negatively correlated with HCC patient survival (21). CDCA5 knockdown can inhibit cell proliferation and tumorigenesis, and induce cell apoptosis, suggesting that CDCA5 plays a carcinogenic role in liver cancer (22). The present study also indicated that CDCA5 was upregulated in breast cancer tissue, as queried through the GEPIA website. The present findings also demonstrated that CDCA5 was upregulated in breast cancer cells.
In recent years, the role of CDCA5 in cancer has been gradually revealed (11,13). In a subcutaneous tumorigenesis experiment on nude mice, CDCA5 knockdown could effectively inhibit tumor formation and growth in tumor-bearing mice (10). In a mechanistic study, CDCA5 knockdown was able to block the cell cycle of triple-negative breast cancer cells (14). CDCA5 enhanced the proliferation and inhibited the apoptosis of bladder cancer cells (10,13). CDCA5 promoted bladder cancer cell proliferation by activating the Akt signaling pathway in bladder tumors (9). In the present study, CDCA5 knockdown inhibited breast cancer cell proliferation, and the expression level of proliferation-related proteins was downregulated following CDCA5 knockdown.
A previous study has indicated that PDS5A overexpression in breast cancer cells could increase the number of cells in G1 phase and enhance the activation of caspase 3, leading to tumor cell apoptosis (23). In addition, a decrease in PDS5A mRNA expression was observed in breast and kidney tumor samples compared with that in the corresponding normal tissues (24). Another study reported an increase in PDS5A mRNA expression in esophageal, stomach, liver and transverse colon tumors (25). In conclusion, these data suggested that PDS5A may be highly tissue-specific in the process of tumorigenesis and development, and that, depending on the tissue or cell type, it could inhibit the proliferation or induce the over-proliferation of tumor cells 18,226). In the present study, CDCA5 knockdown downregulated PDS5A expression in breast cancer cells, and PDS5A overexpression reversed the inhibitory effects of CDCA5 knockdown on breast cancer cell proliferation and migration.
In conclusion, CDCA5 could be a valuable oncogene, and it should be further investigated in breast cancer cell progression. However, the present study has certain limitations. Firstly, the drug sensitivity of shRNA-CDCA5-transfected cells was not analyzed. Secondly, the mRNA and protein expression levels of CDCA5 and PDS5A in breast cancer and normal tissue biopsy specimens were not analyzed. In addition, further analysis on whether CDCA5 knockdown causes the inhibition of the Akt signaling pathway in breast cancer cell lines would be helpful for confirming the accuracy of the present experimental results. Therefore, future research should focus on the aforementioned issues.
To conclude, CDCA5 knockdown was found to suppress the malignant progression of breast cancer by regulating PDS5A in the current study. The present findings may provide new potential targets for breast cancer treatment, since CDCA5 was found to be a potential molecular target for breast cancer therapy and diagnosis.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YW and JY designed the study and performed the experiments. YW, XJZ and YLZ drafted and revised the manuscript. XJZ, JL, YJ, FS and YLZ analyzed the data. JL and FS performed the literature search. JL and YW confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Smaili F, Boudjella A, Dib A, Braikia S, Zidane H, Reggad R, Bendib A, Abdelouahab A, Bereksi-Reguig F, Yekrou D, et al. Epidemiology of breast cancer in women based on diagnosis data from oncologists and senologists in Algeria. Cancer Treat Res Commun. 2020;25:100220. doi: 10.1016/j.ctarc.2020.100220. [DOI] [PubMed] [Google Scholar]
- 2.Chelmow D, Pearlman MD, Young A, Bozzuto L, Dayaratna S, Jeudy M, Kremer ME, Scott DM, O'Hara JS. Executive summary of the early-onset breast cancer evidence review conference. Obstet Gynecol. 2020;135:1457–1478. doi: 10.1097/AOG.0000000000003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontomanolis EN, Koutras A, Syllaios A, Schizas D, Mastoraki A, Garmpis N, Diakosavvas M, Angelou K, Tsatsaris G, Pagkalos A, et al. Role of oncogenes and tumor-suppressor genes in carcinogenesis: A Review. Anticancer Res. 2020;40:6009–6015. doi: 10.21873/anticanres.14622. [DOI] [PubMed] [Google Scholar]
- 4.Falzone L, Grimaldi M, Celentano E, Augustin LSA, Libra M. Identification of modulated micrornas associated with breast cancer, diet, and physical activity. Cancers (Basel) 2020;12:2555. doi: 10.3390/cancers12092555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocco S, Piezzo M, Calabrese A, Cianniello D, Caputo R, Lauro VD, Fusco G, Gioia GD, Licenziato M, De Laurentiis M. Biomarkers in Triple-negative breast cancer: State-of-the-Art and future perspectives. Int J Mol Sci. 2020;21:4579. doi: 10.3390/ijms21134579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y, Wang J, Xu B. Novel biomarkers and prediction model for the pathological complete response to neoadjuvant treatment of triple-negative breast cancer. J Cancer. 2021;12:936–945. doi: 10.7150/jca.52439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sporikova Z, Koudelakova V, Trojanec R, Hajduch M. Genetic markers in triple-negative breast cancer. Clin Breast Cancer. 2018;18:e841–e850. doi: 10.1016/j.clbc.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Zhang N, Pati D. Sororin is a master regulator of sister chromatid cohesion and separation. Cell Cycle. 2012;11:2073–2083. doi: 10.4161/cc.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan PW, Eyster C, Chen J, Pezza RJ, Rankin S. Sororin is enriched at the central region of synapsed meiotic chromosomes. Chromosome Res. 2017;25:115–128. doi: 10.1007/s10577-016-9542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang IW, Lin VC, He HL, Hsu CT, Li CC, Wu WJ, Huang CN, Wu TF, Li CF. CDCA5 overexpression is an indicator of poor prognosis in patients with urothelial carcinomas of the upper urinary tract and urinary bladder. Am J Transl Res. 2015;7:710–722. [PMC free article] [PubMed] [Google Scholar]
- 11.Xu T, Ma M, Dai J, Yu S, Wu X, Tang H, Yu J, Yan J, Yu H, Chi Z, et al. Gene expression screening identifies CDCA5 as a potential therapeutic target in acral melanoma. Hum Pathol. 2018;75:137–145. doi: 10.1016/j.humpath.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Shen M, Zhou G. Upregulation of CDCA5 promotes gastric cancer malignant progression via influencing cyclin E1. Biochem Biophys Res Commun. 2018;496:482–489. doi: 10.1016/j.bbrc.2018.04.160. [DOI] [PubMed] [Google Scholar]
- 13.Fu G, Xu Z, Chen X, Pan H, Wang Y, Jin B. CDCA5 functions as a tumor promoter in bladder cancer by dysregulating mitochondria-mediated apoptosis, cell cycle regulation and PI3k/AKT/mTOR pathway activation. J Cancer. 2020;11:2408–2420. doi: 10.7150/jca.35372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Zhou QZ, Zhang XL, Wang ZZ, Wang P. Identification of hub genes using Co-Expression network analysis in breast cancer as a tool to predict different stages. Med Sci Monit. 2019;25:8873–8890. doi: 10.12659/MSM.919046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang N, Coutinho LE, Pati D. PDS5A and PDS5B in cohesin function and human disease. Int J Mol Sci. 2021;22:5868. doi: 10.3390/ijms22115868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales C, Ruiz-Torres M, Rodríguez-Acebes S, Lafarga V, Rodríguez-Corsino M, Megías D, Cisneros DA, Peters JM, Méndez J, Losada A. PDS5 proteins are required for proper cohesin dynamics and participate in replication fork protection. J Biol Chem. 2020;295:146–157. doi: 10.1074/jbc.RA119.011099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagemann C, Weigelin B, Schommer S, Schulze M, Al-Jomah N, Anacker J, Gerngras S, Kühnel S, Kessler AF, Polat B, et al. The cohesin-interacting protein, precocious dissociation of Sisters 5A/sister chromatid cohesion protein 112, is up-regulated in human astrocytic tumors. Int J Mol Med. 2011;27:39–51. doi: 10.3892/ijmm.2010.551. [DOI] [PubMed] [Google Scholar]
- 18.Zheng MZ, Zheng LM, Zeng YX. SCC-112 gene is involved in tumor progression and promotes the cell proliferation in G2/M phase. J Cancer Res Clin Oncol. 2008;134:453–462. doi: 10.1007/s00432-007-0306-x. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data suing real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Phan NN, Wang CY, Li KL, Chen CF, Chiao CC, Yu HG, Huang PL, Lin YC. Distinct expression of CDCA3, CDCA5, and CDCA8 leads to shorter relapse free survival in breast cancer patient. Oncotarget. 2018;9:6977–6992. doi: 10.18632/oncotarget.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian Y, Wu J, Chagas C, Du Y, Lyu H, He Y, Qi S, Peng Y, Hu J. CDCA5 overexpression is an Indicator of poor prognosis in patients with hepatocellular carcinoma (HCC) BMC Cancer. 2018;18:1187. doi: 10.1186/s12885-018-5072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Chen J, Zhao L, Song W, Xuan Z, Chen J, Li Z, Song G, Hong L, Song P, Zheng S. CDCA5, Transcribed by E2F1, promotes oncogenesis by enhancing cell proliferation and inhibiting apoptosis via the AKT pathway in hepatocellular carcinoma. J Cancer. 2019;10:1846–1854. doi: 10.7150/jca.28809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar D, Sakabe I, Patel S, Zhang Y, Ahmad I, Gehan EA, Whiteside TL, Kasid U. SCC-112, a novel cell cycle-regulated molecule, exhibits reduced expression in human renal carcinomas. Gene. 2004;328:187–196. doi: 10.1016/j.gene.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Hill VK, Kim JS, Waldman T. Cohesin mutations in human cancer. Biochim Biophys Acta. 2016;1866:1–11. doi: 10.1016/j.bbcan.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Put N, Van Roosbroeck K, Vande Broek I, Michaux L, Vandenberghe P. PDS5A, a novel translocation partner of MLL in acute myeloid leukemia. Leuk Res. 2012;36:e87–e89. doi: 10.1016/j.leukres.2011.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.