Abstract
Endothelial cell (EC) dysfunction is one of the initiating factors of atherosclerosis. EC dysfunction is primarily caused by oxidative damage and inflammation. As a classic non-specific antioxidant and anti-inflammatory drug, curcumin has been widely used in studies of lipid metabolism disorders. However, whether curcumin is able to alleviate H2O2-induced EC damage and its related mechanisms has remained to be elucidated. The present study confirmed the protective effects of curcumin on human umbilical vein endothelial cells (HUVECs). A HUVEC injury model was established using H2O2 and the optimal concentrations and time of curcumin to achieve therapeutic effects were explored. Curcumin was observed to inhibit H2O2-induced pyroptosis by inhibiting the activation of NOD-, LRR- and pyrin domain-containing protein 3. In addition, curcumin improved HUVEC function by restoring αvβ3 and reducing endothelin-1 expression. In conclusion, the results of the present study revealed the mechanism through which curcumin inhibits pyroptosis and indicated that curcumin may have a potential utility in treating diseases of EC dysfunction.
Keywords: curcumin, hydrogen peroxide, pyroptosis, atherosclerosis, endothelial cell
Introduction
Atherosclerosis (AS) is a chronic progressive inflammatory disease (1). Inflammation and cell death are two key pathological mechanisms of AS (2). In general, cell death may occur via apoptosis, autophagy and necrosis; however, other forms of cell death have been identified, including pyroptosis (3). Pyroptosis is a form of inflammasome-mediated cell death that is dependent on the activation of caspase-1. The maturation of pro-IL-1β and pro-IL-18 is induced by caspase-1 cleavage (4). Pyroptosis has been indicated to be associated with the death of human macrophages due to oxidative damage, suggesting that pyroptosis has an important role in AS development (5).
Pyroptosis is a type of programmed cell death that accompanies an inflammatory response (3). Previous studies have reported that inflammation, and even pyroptosis, have an important role in the progression of cardiovascular diseases, including atherosclerosis, diabetic cardiomyopathy, ischemia-reperfusion injury, heart failure and myocardial infarction (6,7). Pyroptosis differs from apoptosis and necrosis in that it mainly manifests as inflammasome formation, caspase and gasdermin activation and the formation of multiple protein holes in the cell membrane, leading to the rapid loss of cell membrane integrity and the release of a large number of pro-inflammatory factors (8). Consequently, pyroptosis may have a prominent role in AS-related inflammation. However, it has remained elusive whether it is possible to target and regulate pyroptotic cells in atherosclerotic lesions to treat AS.
Endothelial cell (EC) dysfunction and even death are a key and initial stage in the development of AS (9). Vascular endothelial dysfunction is the first step in a complex and multifaceted process that ultimately leads to the formation of a plaque, as well as the formation of atherosclerotic lesions and their complications (10). Studies have indicated that caspase-1 activation in ECs is able to promote endothelial activation and monocyte recruitment, leading to AS (11). Curcumin (Fig. 1A), a polyphenolic compound, is the principal curcuminoid derived from the rhizomes of turmeric (12); it is able inhibit lipopolysaccharide-induced NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome, thereby inhibiting the activation of IL-1β and caspase-1 (13,14). In addition, curcumin also exhibits antioxidant and anti-tumor properties (15–17).
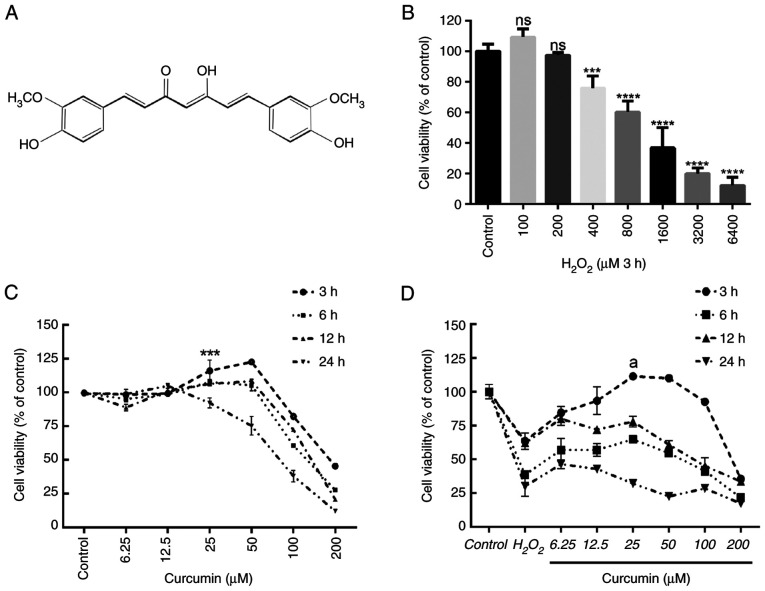
Figure 1.
Curcumin suppresses H2O2-induced viability impairment of HUVECs. The effects of increasing concentrations of H2O2 and curcumin on HUVEC viability were determined. (A) Curcumin contains a variety of functional groups, including the β-diketo group, carbon-carbon double bonds and phenyl rings containing varying amounts of hydroxyl and methoxy substituents. Viability of HUVECs treated with increasing doses of (B) H2O2 (0–6,400 µM) for 3 h and (C) curcumin (0–200 µM) for 3, 6, 12 or 24 h, and (D) different concentrations of curcumin (0–200 µM) for 3, 6, 12 or 24 h after 3 h of pretreatment with H2O2 (800 µM). Cell viability was detected using an MTT assay. Values are expressed as the mean ± standard deviation of three independent experiments. ***P<0.001, ****P<0.0001 vs. the control group; aP<0.001 vs. the H2O2 group; ns, no significance. HUVECs, human umbilical vein endothelial cells.
Therefore, it is conceivable that EC dysfunction and atherogenesis may be associated with inflammation-related death pathways. However, whether the effect of curcumin on EC function is related to pyroptosis remains to be elucidated. In the present study, the effects of curcumin on H2O2-induced pyroptosis of human umbilical vein ECs (HUVECs) were explored and a caspase-1 inhibitor (VX-765) and NLRP3 inhibitor (MCC950) were used to corroborate the results. The role of curcumin in EC dysfunction was also explored.
Materials and methods
Cell culture and treatment agents
The HUVEC line (immortal cells; no. C-003-5C) was purchased from The Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences and cultured in RPMI-1640 medium (cat. no. C11875500BT, Gibco; Thermo Fisher Scientific, Inc.), 10% FBS (cat. no. FSP500; Excell Biotechnology Co., Ltd.) and 1% penicillin/streptomycin at 37°C in a humidified atmosphere with 5% CO2. Curcumin and H2O2 were purchased from MilliporeSigma. VX-765 and MCC950 were purchased from APExBIO Technology LLC. For experiments involving pharmacological reagents, according to the different experimental groups, HUVECs were treated with PBS (control), H2O2 (800 µM) for 3 h, curcumin (25 µM) for 3 h, VX-765 (10 µM) for 1 h or MCC950 (10 µM) for 2 h.
Cell viability assay
An MTT assay (cat. no. C0009S; Beyotime Institute of Biotechnology) was performed to evaluate the viability of HUVECs according to the manufacturer's protocol. A total of 2×103 HUVECs per well were seeded in 96-well plates in complete medium and treated with different concentrations of curcumin (0–200 µM) for 3–24 h or H2O2 (0–6,400 µM) for 3–24 h. Next, 10 µl MTT reagent solution was added to each well and the cells were incubated for a further 4 h at 37°C. The absorbance was measured at 490 nm using a Gen5 microplate reader (BioTek Instruments).
Hematoxylin and eosin (H&E) staining
The coverslip was placed in a six-well plate and HUVECs (2×105 cells/well) were added to it and cultured for 12 h. After HUVECs were attached to the coverslip, the cells were fixed with 4% paraformaldehyde at room temperature (25°C) for 30 min. The cells were stained with hematoxylin solution included in a kit (cat. no. C0105S; Beyotime Institute of Biotechnology) at room temperature for 3 min and then placed under running tap water at room temperature for 5 min. The cells were next stained in working eosin Y solution from the kit at room temperature for 2 min. A drop of neutral balsam mounting medium was placed over the cells on each slide and the coverslip was added. The slides were then observed using a microscope (Axiolab 5; Carl Zeiss Suzhou Co., Ltd.).
DAPI/propidium iodide (PI) fluorescent staining
In order to assess pyroptosis, cells were double-stained with DAPI and PI (cat. no. G1012; Wuhan Servicebio Technology Co., Ltd.). HUVECs (2×105 cells/well) were cultured in a 6-well plate. HUVECs were treated with H2O2 for 3 h and then either treated with 25 µM curcumin for 3 h or left untreated (control), whereas for the VX-765 group, HUVECs were pretreated with caspase-1 inhibitor VX-765 (10 µM) for 1 h and then incubated with H2O2 (800 µM) for 3 h. Following treatment, the cells in each group were washed with PBS three times and stained with 50 µl PI (100 µg/ml) for 30 min and 50 µl DAPI for 5 min at 4°C in the dark. The stained cells were examined under a fluorescent microscope (DS-Ri2; Nikon Corporation) at a magnification of ×400.
Immunofluorescence
Immunofluorescence was performed to detect the expression of caspase-1 in HUVECs. In brief, the cells were fixed with 4% paraformaldehyde at room temperature for 30 min, permeabilized with 0.6% Triton X-100 at room temperature for 0.5 h and then blocked with goat serum (cat. no. C0265; Beyotime Institute of Biotechnology) at room temperature for 0.5 h. Subsequently, the cells were incubated with an anti-caspase-1 antibody (cat. no. ARG30330; dilution, 1:2,000; Arigo Biolaboratories) or an anti-αvβ3 antibody (cat. no. ab190147; dilution, 1:2,000; Abcam) at 4°C overnight, followed by incubation with an FITC-conjugated secondary antibody (cat. no. GB22303; dilution, 1:2,000; Wuhan Servicebio Technology Co., Ltd.) or a CY3-conjugated secondary antibody (cat. no. GB21301; dilution, 1:2,000; Wuhan Servicebio Technology Co., Ltd.) in the dark at room temperature for 1 h. The nuclei were stained with DAPI at room temperature for 20 min. The cells were then imaged under a fluorescence microscope (FV300; Olympus Corporation).
Analysis of DNA fragmentation
TUNEL staining was performed to detect DNA fragmentation of HUVECs. In brief, HUVECs (2×105 cells/well) were cultured on coverslips in a 6-well plate. Following the designated treatments, cells were fixed with 4% paraformaldehyde and permeabilized at room temperature for 30 min with 0.1% Triton X-100, followed by incubation with TUNEL reaction mixture (cat. no. G1502; Wuhan Servicebio Technology Co., Ltd.) at 37°C in the dark for 1 h. Cells were then stained at room temperature for 5 min with DAPI and examined under a fluorescent microscope (DS-Ri2; Nikon Corporation).
ELISA
The relative concentrations of IL-1β and IL-18 in the culture medium following the treatment of HUVECs with H2O2, curcumin or VX-765 were determined using specific ELISA kits (cat nos. EHC127.96 and EHC002b.96; Neobioscience Technology Co., Ltd.), according to the manufacturer's protocols.
Cytotoxicity assay
HUVECs were treated with curcumin, VX-765, MCC950 and H2O2. Cytotoxicity was determined by measuring the lactate dehydrogenase (LDH) released from cells using an LDH assay kit (cat. no. C0016; Beyotime Institute of Biotechnology) according to the manufacturer's protocol. The absorbance was determined at a wavelength of 450 nm using a spectrophotometric microplate reader.
Western blot analysis
Cells were collected and proteins were extracted using RIPA lysis buffer (cat. no. CW2333S; CoWin Biosciences), and protease and phosphatase inhibitors were added. Protein concentrations were determined using a BCA Protein Assay kit (Bio-Rad Laboratories, Inc.). After loading 30 µg protein per lane, proteins were separated using SDS-PAGE (10% separation gel and 5% stacking gel) and transferred onto PVDF membranes (cat. no. ISEQ00010; MilliporeSigma) followed by blocking with 5% skimmed milk (cat. no. P0216; Beyotime Institute of Biotechnology.) at room temperature for 2 h. Subsequently, the membranes were incubated with primary antibodies against NLRP3 (cat. no. 19771-1-AP; dilution, 1:1,000; ProteinTech Group, Inc.); apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC; cat. no. 10500-1-AP; dilution, 1:1,000; ProteinTech Group, Inc.); caspase-1, gasdermin D (GSDMD) and IL-1β (cat. no. ARG30330; dilution, 1:1,000; Arigo Biolaboratories); and ET-1 (cat. no. 12191-1-AP; dilution, 1:1,000; ProteinTech Group, Inc.) at 4°C overnight. Following washing with Tris-buffered saline with 0.1% Tween-20 detergent (TBST) three times, the membranes were incubated with an HRP-conjugated anti-rabbit or anti-mouse IgG secondary antibody (cat. no. GB23301 and GB23303; dilution, 1:5,000; Wuhan Servicebio Technology Co., Ltd.) for 1 h. Following washing with TBST three times, the immunoreactive bands were detected using chemiluminescence reagent (cat. no. CW0049S; CoWin Biosciences) and visualized using a chemiluminescence gel imager (5200MuIti; Tanon Science and Technology Co., Ltd.). The relative intensity was then measured and analyzed using ImageJ 1.43u software (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0 software (GraphPad Software, Inc.) and values are expressed as the mean ± standard deviation. Differences between groups were determined using one-way ANOVA and Dunnett's multiple-comparisons post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Curcumin alleviates H2O2-induced EC injury
First, the effects of different concentrations of H2O2 (0, 200, 400, 800, 1,600, 3,200 and 6,400 µM) on HUVEC viability were examined after 3 h. According to the results of the MTT analysis, 800 µM H2O2 treatment for 3 h was selected as the optimal concentration and time and used in the following experiments (Fig. 1B). Cell viability was measured after incubation with various concentrations of curcumin (0–200 µM) for different durations (3–24 h). Concentrations of >50 µM had an obvious toxic effect, with longer treatments leading to higher toxicity (Fig. 1C). Similarly, 25 µM curcumin was used to incubate HUVECs for different durations (3–24 h) to determine cell viability following pretreatment with H2O2 for 3 h. Curcumin treatment restored H2O2-induced cell damage after 3 h. However, longer treatments did not increase cell viability (Fig. 1D). Therefore, treatment with 25 µM curcumin for 3 h was selected as the optimal concentration and time and was used in subsequent experiments.
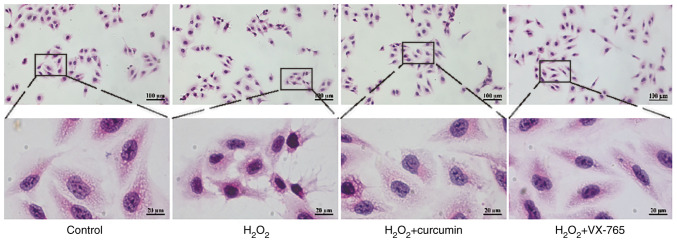
To determine the role of curcumin during H2O2-induced HUVEC injury, H&E staining was performed on HUVECs. Cells were divided into the control, H2O2, curcumin and VX-765 pre-treatment groups. The results indicated that H2O2 treatment led to rupture of the cell membrane of HUVECs and karyopyknosis (Fig. 2). In comparison, curcumin reduced H2O2-induced HUVEC damage.
Figure 2.
Curcumin repairs H2O2-induced HUVEC damage, as indicated by H&E staining. HUVECs were treated with H2O2 for 3 h and then either treated with 25 µM curcumin for 3 h or left untreated (control), whereas in the VX-765 group, HUVECs were pretreated with caspase-1 inhibitor VX-765 (10 µM) for 1 h and then incubated with H2O2 (800 µM) for 3 h (magnification, ×200; scale bar, 100 µm). The areas indicated by the boxes are enlarged regions for each experimental condition (magnification, ×1,000; scale bar, 20 µm). HUVECs, human umbilical vein endothelial cells.
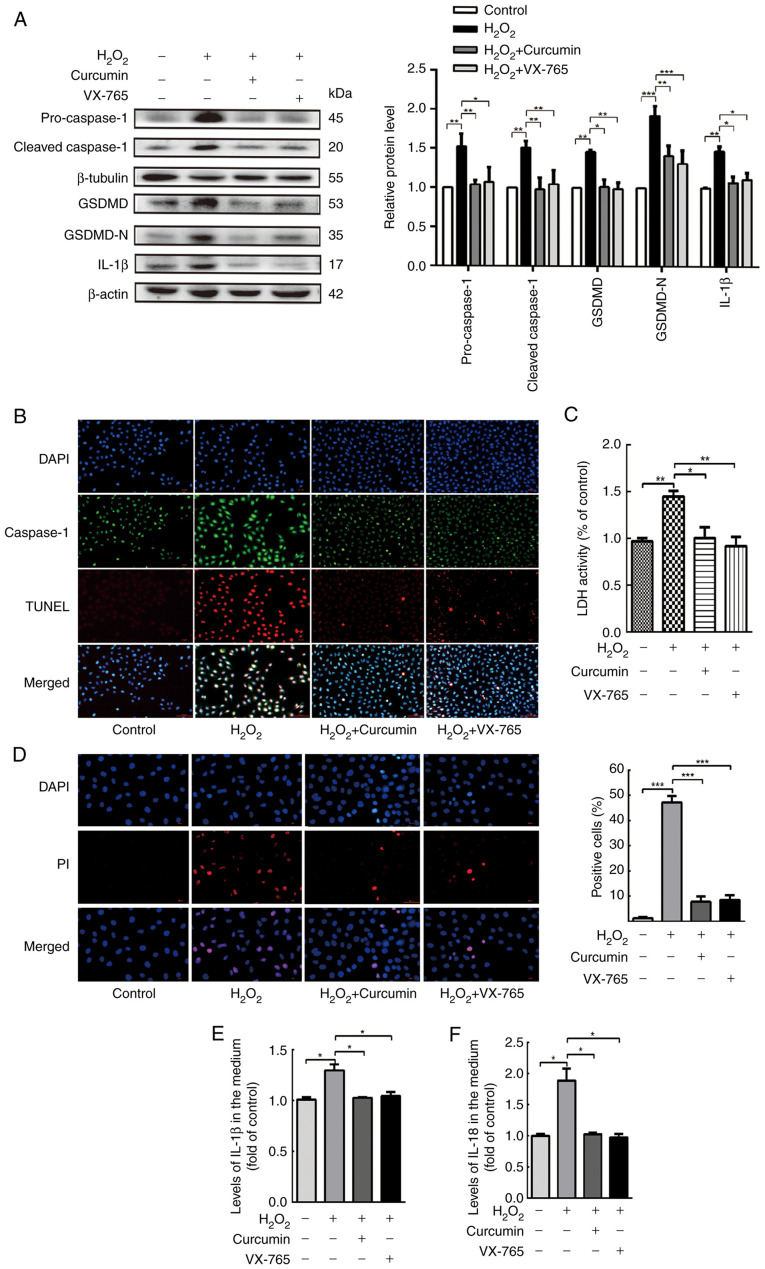
Curcumin suppresses H2O2-induced HUVEC pyroptosis
Pyroptosis is uniquely dependent on the activation of caspase-1, which is able to process cytokines IL-1β and IL-18 into their active forms and then induce pyroptotic cell death. To elucidate the association between curcumin and H2O2-induced pyroptosis, a variety of experiments were performed. Cells were divided into a control, H2O2, curcumin and VX-765 pre-treatment groups. Western blot analysis revealed that caspase-1, GSDMD and IL-1β were all activated and enhanced in H2O2-treated HUVECs (Fig. 3A). Caspase-1/TUNEL double staining was performed in HUVECs (Fig. 3B), indicating that the number of caspase-1 and TUNEL-positive cells were both markedly increased in the presence of H2O2 in HUVECs, and the number of caspase-1 and TUNEL-positive cells were both markedly decreased in the presence of curcumin in HUVECs. Treatment of HUVECs with H2O2 significantly increased the release of LDH (Fig. 3C) and the proportion of cells with PI-positive staining was markedly improved (Fig. 3D). However, this phenomenon was counteracted by curcumin treatment. Similar changes in IL-1β and IL-18 expression were observed in the H2O2-, curcumin- and VX-765-treated and untreated HUVECs (Fig. 3E and F). The results indicated that the selective inhibitor of caspase-1 (VX-765) reduced the level of activated caspase-1 and inhibited the maturation of GSDMD and IL-1β. This indicated that the H2O2-induced pyroptosis of HUVECs was dependent on caspase-1.
Figure 3.
Curcumin suppresses H2O2-induced pyroptosis of HUVECs. HUVECs were treated with H2O2 for 3 h and then either treated with 25 µM curcumin for 3 h or left untreated (control), whereas for the VX-765 group, HUVECs were pretreated with caspase-1 inhibitor VX-765 (10 µM) for 1 h and then incubated with H2O2 (800 µM) for 3 h. (A) Western blot analysis revealed that the protein levels of pro-caspase-1, caspase-1, pro-GSDMD, GSDMD and IL-1β were increased in HUVECs following treatment with H2O2 for 3 h. VX-765 and curcumin inhibited the protein expression of pro-caspase-1, caspase-1, pro-GSDMD, GSDMD and IL-1β. β-actin or β-tubulin was used as an internal control. (B) Compared with the H2O2 group, caspase-1 (green) and TUNEL (red) double-positive cells were decreased in the presence of curcumin or VX-765. The nuclei were stained blue with DAPI (magnification, ×400; scale bar, 50 µm). (C) The relative release of LDH in H2O2-treated HUVECs was increased, while that in curcumin- and VX-765-treated HUVECs was decreased (n=3). (D) The percentage of PI-positive HUVECs (red) was increased following H2O2 treatment, while it decreased following curcumin and VX-765 treatment (left, representative images; right, quantification of PI-positive cells; magnification, ×400; scale bar, 50 µm). Relative concentration of (E) IL-1β and (F) IL-18 in the culture medium following the treatment of HUVECs with H2O2, curcumin or VX-765, as determined by ELISA. Values are expressed as the mean ± standard deviation from three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 as indicated. HUVECs, human umbilical vein endothelial cells; GSDMD, gasdermin D; LDH, lactate dehydrogenase; PI, propidium iodide.
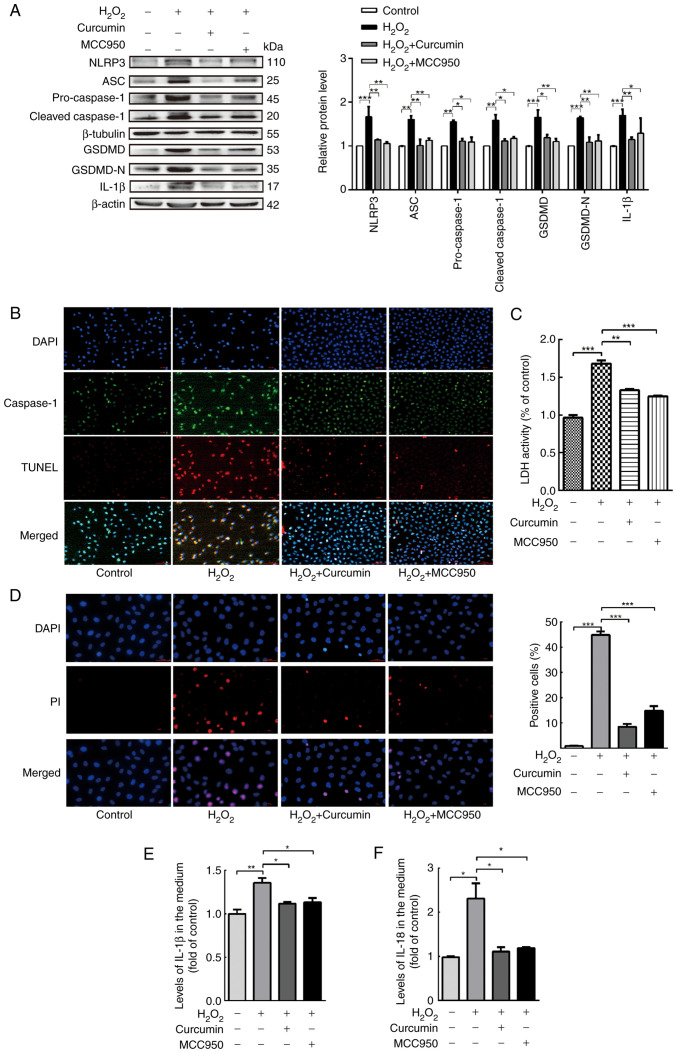
Curcumin alleviates NLRP3 inflammasome activation, which is involved in pyroptosis
Caspase-1 activation requires a protein complex known as the inflammasome. The NLRP3 inflammasome is the most extensively studied type of inflammasome. An increase in the protein levels of cleaved caspase-1 and mature IL-1β/18 is a hallmark of NLRP3 inflammasome activation. NLRP3 recruits caspase-1 through ASC, allowing activated caspase-1 to cleave pro-IL-1β and pro-GSDMD, thus leading to the maturation of IL-1β and GSDMD. Therefore, the expression levels of NLRP3, ASC, caspase-1, GSDMD and IL-1β in HUVECs were measured following treatment with curcumin. In addition, the NLRP3 inhibitor MCC950 was used (4).
As presented in Fig. 4A, the inflammasome-associated protein levels following treatment with H2O2 were measured using western blot analysis. NLRP3 and ASC, as well as the core component of pyroptosis, cleaved caspase-1, were markedly upregulated by H2O2. Conversely, curcumin treatment significantly blocked NLRP3 inflammasome activation. Furthermore, curcumin and MCC950 weakened the ability of H2O2 to induce HUVEC pyroptosis, as evidenced by the decrease in the number of TUNEL and caspase-1 double-positive cells (Fig. 4B). Curcumin and MCC950 also abrogated the release of LDH and the increase in PI-positive cells, indicating inhibition of cell lysis and pyroptotic cell death by curcumin (Fig. 4C and D). Similarly, curcumin and MCC950 also significantly suppressed the expression of caspase-1 and the production of IL-1β and IL-18 (Fig. 4E and F), along with the inhibition of caspase-1-dependent cell death.
Figure 4.
Curcumin alleviates NLRP3 inflammasome activation, which is involved in pyroptosis. HUVECs were treated with H2O2 for 3 h and then either treated with 25 µM curcumin for 3 h or left untreated (control), whereas for the MCC95 group, HUVECs were pretreated with NLRP3 inhibitor (MCC950; 10 µM) for 2 h and then incubated with H2O2 (800 µM) for 3 h. (A) Western blot analysis indicated that the protein levels of NLRP3, ASC, pro-caspase-1, caspase-1, pro-GSDMD, GSDMD and IL-1β were upregulated in HUVECs following treatment with H2O2 for 3 h and that MCC950 and curcumin inhibited the protein expression of NLRP3, ASC, pro-caspase-1, caspase-1, pro-GSDMD, GSDMD and IL-1β. β-Actin or β-tubulin was used as an internal control. (B) As compared with the H2O2 group, caspase-1 (green) and TUNEL (red) double-positive cells were decreased in the presence of curcumin or MCC950. The nuclei were stained blue with DAPI (magnification, ×400; scale bar, 50 µm). (C) The relative release of LDH by H2O2-treated HUVECs was increased, while that of curcumin- and MCC950-treated HUVECs was decreased (n=3). (D) The percentage of PI (red)-positive H2O2-treated HUVECs was increased, while that among curcumin- and MCC950-treated cells was decreased (left, representative images; right, quantification of PI-positive cells) (magnification, ×400; scale bar, 50 µm). Relative concentration of (E) IL-1β and (F) IL-18 in the culture medium following H2O2, curcumin or MCC950 treatment of HUVECs, as determined by ELISA. Values are expressed as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01, ***P<0.001. HUVECs, human umbilical vein endothelial cells; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; GSDMD, gasdermin D; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; LDH, lactate dehydrogenase; PI, propidium iodide.
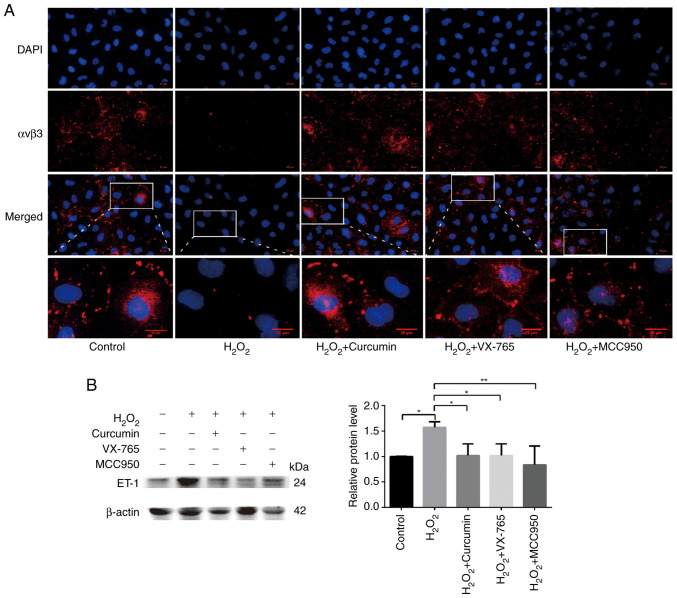
Curcumin improves H2O2-induced functional damage of HUVECs
Endothelial cell adhesion molecules, including integrin αvβ3 and E-selectin, are involved in angiogenesis (18). A previous study reported that decreased expression of αvβ3 in atherosclerotic mice led to decreased recruitment of endothelial progenitor cells (EPCs) (19); αvβ3 has an important role in the EPC-led restoration of the biological function of ECs. Endothelin (ET)-1 is a potent vasoconstrictor peptide produced and released primarily by ECs. In addition to its vasoregulatory properties, ET-1 overexpression is associated with the development and progression of atherosclerosis and is generally considered to be an atherogenic peptide (20,21). Therefore, immunofluorescence was used to detect the expression levels of αvβ3 and western blot analysis to quantify ET-1-related protein levels. Cells were divided into the control, H2O2, curcumin, VX-765 pre-treatment and MCC950 pre-treatment groups. H2O2 significantly inhibited the expression of αvβ3 (green), which was restored following the addition of curcumin (Fig. 5A). At the same time, high ET-1 expression was observed in the H2O2 treatment group, while low ET-1 expression was present in the curcumin, inhibitor and control groups (Fig. 5B). Curcumin was thus further confirmed to improve H2O2-induced cell damage.
Figure 5.
Curcumin improves H2O2-induced functional damage in HUVECs. HUVECs were pretreated with caspase-1 inhibitor VX-765 (10 µM) for 1 h or NLRP3 inhibitor (MCC950; 10 µM) for 2 h and then incubated with H2O2 (800 µM) for 3 h. HUVECs were treated with H2O2 for 3 h and then either treated with 25 µM curcumin for 3 h or left untreated (control). (A) αvβ3-positive cells (red) were increased following pretreatment with curcumin and inhibitor. The nuclei were stained blue with DAPI. The regions indicated by boxes are enlarged regions for each experimental condition (scale bars, 10 µm). (B) The protein expression levels of ET-1 were upregulated in HUVECs following treatment with H2O2 for 3 h. VX-765, MCC950 and curcumin inhibited the protein expression of ET-1, as indicated by the western blot analysis. β-actin was used as an internal control. Values are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 and **P<0.01. HUVECs, human umbilical vein endothelial cells; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; αvβ3, alpha v beta 3; ET-1, endothelin 1.
Discussion
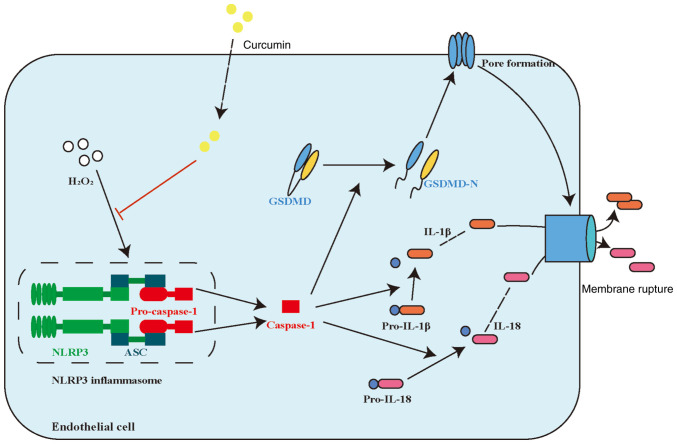
The results of the present study suggested that curcumin has an important role in restoring H2O2-induced pyroptosis in HUVECs. Curcumin inhibited both the H2O2-induced and NLRP3 inflammasome activation pathways in HUVECs. Curcumin ameliorated pyroptosis of HUVECs by inhibiting NLRP3 inflammasome activation. These results suggested that curcumin may be a promising therapeutic agent for AS. A schematic summary of the proposed mechanism through which curcumin modulates the function of HUVECs challenged with H2O2 is provided in Fig. 6.
Figure 6.
Proposed model of curcumin alleviating H2O2-induced pyroptosis of human umbilical vein endothelial cells. NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; GSDMD, gasdermin D; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain.
Reactive oxygen species (ROS) are products of a one-electron reduction of a type of oxygen in the body (22). H2O2 is able to undergo a spontaneous conversion to OH through the Fenton reaction (22). Vascular wall cells are directly damaged by oxidative stress and EC damage is a key driver of early AS (22). Excessive accumulation of ROS in mitochondria is able to cause programmed cell death (23). Studies have indicated that ROS, as an important molecular upstream regulator of the NLRP3 inflammasome, is able to activate the NLRP3 inflammasome (6,24). In the present study, H2O2 was used to create a model of oxidative damage in HUVECs and the effect of curcumin on the H2O2-induced pyroptosis of HUVECs was explored. The results suggested that curcumin significantly reduced H2O2-induced pyroptosis, inflammasome activation and release of inflammatory factors (including IL-1β and IL-18). In addition, The AMP-activated protein kinase (AMPK) pathway was reported to prevent and treat AS by promoting cholesterol efflux, inhibiting inflammation and accelerating fatty acid oxidation (25). Studies have also indicated that the activation of the AMPK and sirtuin 1 (SIRT1) pathway not only inhibits processes of AS by inhibiting oxidative stress and apoptosis in ECs, but also has an effective protective role in a variety of inflammation-related diseases (26,27). However, whether curcumin is able to reduce oxidative stress by activating the MAPK/SIRT1 pathway remains elusive and should be further studied.
Integrins not only mediate physical cell adhesion but also initiate signaling events, alone or in combination with growth factor receptor-mediated signals, to promote basic cell functions such as cell migration, proliferation, survival and differentiation. The integrin αvβ3 is able to bind to multiple ligands in an Arg-Gly-Asp-dependent manner (28). It also enhances the biological functions of ECs by mediating the binding of EPCs to ECs and promotes the phosphorylation of VEGF receptor 2 and activation of the ERK1/2/MAPK signaling pathway (29). In addition, tissue ET-1 levels are significantly increased in the early and late stages of coronary artery disease and are associated with its severity (30). A study indicated that ET-1 tissue immunoreactivity predicts AS progression in patients with chronic kidney disease (31). Consistent with the findings of that study, the present results indicated that curcumin restores αvβ3 expression and inhibits ET-1 overexpression. Although ET-1-mediated EC dysfunction may be involved in the development of AS (21), the direct effects of ET-1 on EC function remain elusive.
Previous studies have demonstrated that curcumin has multiple biological activities (15–17). In the present study, it was confirmed that curcumin has significant anti-inflammatory and anti-pyroptosis effects. However, although curcumin has a wide range of potential beneficial pharmacological activities, numerous questions regarding the fate of the compound in mammalian organisms remain unanswered. Further studies are required to improve the current understanding of curcumin and promote its application in human diseases.
In conclusion, the present study proved for the first time that curcumin is able to inhibit H2O2-induced inflammation and pyroptosis in HUVECs. Therefore, curcumin is a potential drug for the treatment of AS. However, further studies are required to verify the mechanisms through which curcumin inhibits NLRP3 to improve EC pyroptosis. In addition, the role of curcumin in angiogenesis mediated by EC adhesion molecule αvβ3, thereby stabilizing atherosclerotic plaques and reducing adverse cardiovascular events, remains to be further experimentally explored.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the Natural Science Foundation of Hunan Province (grant nos. 2018JJ2346 and 2018JJ2348).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YY and SL conceptualized the study. YY, CZ and SL designed the study. YY, LY, QZ and YL carried out the experiments and curated the data. YY, QZ, YH and YL analyzed the data. YY wrote the manuscript. CZ and YH reviewed and edited the manuscript. YY and SL checked and approved the authenticity of the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welsh P, Grassia G, Botha S, Sattar N, Maffia P. Targeting inflammation to reduce cardiovascular disease risk: A realistic clinical prospect? Br J Pharmacol. 2017;174:3898–3913. doi: 10.1111/bph.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin J, Shou X, Mao X, Dong J, Mohabeer N, Kushwaha KK, Wang L, Su Y, Fang H, Li D. Oxidized low density lipoprotein induced caspase-1 mediated pyroptotic cell death in macrophages: implication in lesion instability? PLoS One. 2013;8:e62148. doi: 10.1371/journal.pone.0062148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, Tang Q. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. doi: 10.1016/j.redox.2019.101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang F, Qin Y, Lv J, Wang Y, Che H, Chen X, Jiang Y, Li A, Sun X, Yue E, et al. Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis. 2018;9:1000. doi: 10.1038/s41419-018-1029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, He WT, Hu L, Li J, Fang Y, Wang X, Xu X, Wang Z, Huang K, Han J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007–1020. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mollace V, Gliozzi M, Musolino V, Carresi C, Muscoli S, Mollace R, Tavernese A, Gratteri S, Palma E, Morabito C, et al. Oxidized LDL attenuates protective autophagy and induces apoptotic cell death of endothelial cells: Role of oxidative stress and LOX-1 receptor expression. Int J Cardiol. 2015;184:152–158. doi: 10.1016/j.ijcard.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Jensen HA, Mehta JL. Endothelial cell dysfunction as a novel therapeutic target in atherosclerosis. Expert Rev Cardiovasc Ther. 2016;14:1021–1033. doi: 10.1080/14779072.2016.1207527. [DOI] [PubMed] [Google Scholar]
- 11.Yin Y, Li X, Sha X, Xi H, Li YF, Shao Y, Mai J, Virtue A, Lopez-Pastrana J, Meng S, et al. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol. 2015;35:804–816. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Yin H, Guo Q, Li X, Tang T, Li C, Wang H, Sun Y, Feng Q, Ma C, Gao C, et al. Curcumin suppresses IL-1β secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. J Immunol. 2018;200:2835–2846. doi: 10.4049/jimmunol.1701495. [DOI] [PubMed] [Google Scholar]
- 14.Saeedi-Boroujeni A, Mahmoudian-Sani MR, Bahadoram M, Alghasi A. COVID-19: A case for inhibiting NLRP3 inflammasome, suppression of inflammation with curcumin? Basic Clin Pharmacol Toxicol. 2021;128:37–45. doi: 10.1111/bcpt.13503. [DOI] [PubMed] [Google Scholar]
- 15.Liang WF, Gong YX, Li HF, Sun FL, Li WL, Chen DQ, Xie DP, Ren CX, Guo XY, Wang ZY, et al. Curcumin activates ROS signaling to promote pyroptosis in hepatocellular carcinoma HepG2 cells. In Vivo. 2021;35:249–257. doi: 10.21873/invivo.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocaadam B, Sanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017;57:2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- 17.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 18.Seguin J, Nicolazzi C, Mignet N, Scherman D, Chabot GG. Vascular density and endothelial cell expression of integrin alpha v beta 3 and E-selectin in murine tumours. Tumour Biol. 2012;33:1709–1717. doi: 10.1007/s13277-012-0428-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippi A, Constantin A, Alexandru N, Voicu G, Constantinescu CA, Rebleanu D, Fenyo M, Simionescu D, Simionescu A, Manduteanu I, Georgescu A. Integrins α4β1 and αVβ3 are reduced in endothelial progenitor cells from diabetic dyslipidemic mice and may represent new targets for therapy in aortic valve disease. Cell Transplant. 2020;29:963689720946277. doi: 10.1177/0963689720946277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brewster LM, Garcia VP, Levy MV, Stockelman KA, Goulding A, DeSouza NM, Greiner JJ, Hijmans JG, DeSouza CA. Endothelin-1-induced endothelial microvesicles impair endothelial cell function. J Appl Physiol (1985) 2020;128:1497–1505. doi: 10.1152/japplphysiol.00816.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew V, Hasdai D, Lerman A. The role of endothelin in coronary atherosclerosis. Mayo Clin Proc. 1996;71:769–777. doi: 10.1016/S0025-6196(11)64842-8. [DOI] [PubMed] [Google Scholar]
- 22.Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 23.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Wang Z, Feng D, Zhao H, Lin M, Hu Y, Zhang N, Lv L, Gao Z, Zhai X, et al. p66Shc contributes to liver fibrosis through the regulation of mitochondrial reactive oxygen species. Theranostics. 2019;9:1510–1522. doi: 10.7150/thno.29620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Q, Xu J, Ma Q, Liu Z, Sudhahar V, Cao Y, Wang L, Zeng X, Zhou Y, Zhang M, et al. PRKAA1/AMPKα1-driven glycolysis in endothelial cells exposed to disturbed flow protects against atherosclerosis. Nat Commun. 2018;9:4667. doi: 10.1038/s41467-018-07132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan SH, Hung CH, Shih JY, Chu PM, Cheng YH, Lin HC, Hsieh PL, Tsai KL. Exercise intervention attenuates hyperhomocysteinemia-induced aortic endothelial oxidative injury by regulating SIRT1 through mitigating NADPH oxidase/LOX-1 signaling. Redox Biol. 2018;14:116–125. doi: 10.1016/j.redox.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton CC, Al-Rashed F, Calay D, Birdsey GM, Bauer A, Mylroie H, Morley BJ, Randi AM, Haskard DO, Boyle JJ, Mason JC. Methotrexate-mediated activation of an AMPK-CREB-dependent pathway: A novel mechanism for vascular protection in chronic systemic inflammation. Ann Rheum Dis. 2016;75:439–448. doi: 10.1136/annrheumdis-2014-206305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felding-Habermann B, Silletti S, Mei F, Siu CH, Yip PM, Brooks PC, Cheresh DA, O'Toole TE, Ginsberg MH, Montgomery AM. A single immunoglobulin-like domain of the human neural cell adhesion molecule L1 supports adhesion by multiple vascular and platelet integrins. J Cell Biol. 1997;139:1567–1581. doi: 10.1083/jcb.139.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao D, Xiao W, Liu R, Kumar P, Li Y, Zhou P, Guo F, Farmer DL, Lam KS, Wang F, Wang A. Discovery and characterization of a potent and specific peptide ligand targeting endothelial progenitor cells and endothelial cells for tissue regeneration. ACS Chem Biol. 2017;12:1075–1086. doi: 10.1021/acschembio.7b00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerman A, Holmes DR, Jr, Bell MR, Garratt KN, Nishimura RA, Burnett JC., Jr Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92:2426–2431. doi: 10.1161/01.CIR.92.9.2426. [DOI] [PubMed] [Google Scholar]
- 31.Noshad H, Argani H, Nezami N, Ghojazadeh M, Zomorrodi A, Bohlouli A, Bonyadi MR, Fakhrjou A, Ghorbanihaghjo A, Gharedaghi A, et al. Arterial atherosclerosis in patients with chronic kidney disease and its relationship with serum and tissue endothelin-1. (corrected) Iran J Kidney Dis. 2009;3:203–209. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.