Abstract
Liver fibrosis is a common pathological process of chronic liver diseases, including viral hepatitis and alcoholic liver disease, and ultimately progresses to irreversible cirrhosis and cancer. Hepatic stellate cells (HSCs) are activated to produce amounts of collagens in response to liver injury, thus triggering the initiation and progression of fibrogenesis. Natural killer (NK) cells serve as the essential component of hepatic innate immunity and are considered to alleviate fibrosis by killing activated HSCs. Current antifibrotic interventions have improved fibrosis, but fail to halt its progression in the advanced stage. Clarifying the interaction between NK cells and HSCs will provide clues to the pathogenesis and potential therapies for advanced liver fibrosis.
Keywords: natural killer cells, fibrosis, hepatic stellate cells, crosstalk, TGF-β
1. Introduction
Liver fibrosis presents as a common pathological process characterized by the overproduction of extracellular matrix and impaired matrix degradation in the progression of different liver diseases, such as viral hepatitis and alcoholic liver disease (1). Hepatic stellate cells (HSCs) are the predominant non-parenchymal cells which are activated to repair tissue in response to liver injury; however, hyperactivation of HSCs results in extracellular matrix deposition and thus hepatic fibrosis (2). As an essential component of innate immunity in the liver, natural killer (NK) cells play a critical role in the removal of pathogens, including bacteria, virus, and cancer cells (3). Manipulation of NK cell activation has become a potential cancer immunotherapy, such as adoptive transfer of allogeneic NK cells, genetic engineered NK cells, NK cell-targeted chemotherapy and others (4). Several previous reviews have revealed that NK cells exert antifibrotic functions through their cytotoxicity against activated HSCs and production of various cytokines that further intensify this effect (5). Thus, the activation of NK cells is regarded as a novel therapeutic strategy for hepatic fibrosis (6). However, dysfunction of NK cells in the advanced stage of diseases, including liver fibrosis and tumors, limits its efficacy and clinical application, and the mechanisms of NK cell dysfunction are still elusive. Further clarifying the molecular mechanism of NK cells affecting HSCs during different stages of fibrosis is still required in this research field. Currently, many treatments have improved fibrosis at the early stage of this disease, but fail to halt fibrosis progression in the advanced stage (7). One reason for this is that the cytotoxic function of NK cells is compromised and HSCs become resistant to NK cell killing (8). Therefore, understanding the crosstalk between NK cells and HSCs is vital for developing novel therapeutic strategies. This review concisely summarizes the role of HSCs in fibrogenesis, and the phenotypic and functional characteristics of NK cells in the liver. It emphasizes the research progression on the crosstalk between NK cells and HSCs, and the molecular mechanism of NK cell dysfunction in advanced fibrosis, as well as discusses the therapeutic potential of the combination of activating NK cell therapies with TGF-β inhibitors for advanced liver fibrosis.
2. Review criteria
In order to summarize the interactions between NK cells and HSCs in liver fibrosis, a PubMed search was performed in March 2022. Articles containing the following key words were considered for inclusion: ‘natural killer’ (or ‘NK’) AND ‘hepatic stellate cells’ (or ‘HSCs’) AND ‘liver fibrosis’ (or ‘hepatic fibrosis’). Relevant articles were also identified from a manual search of reference lists within those included. The abstracts of identified articles were screened and classified for inclusion in the review. To be included, the article must have described original data concerning the crosstalk between NK cells and HSCs in liver fibrosis, and have been published in a peer-reviewed journal and written in English.
3. Role of HSCs in liver fibrosis
The activation of HSCs is regarded as the initial event in liver fibrosis. Various pathological agents, such as viruses, ethanol, and lipids, induce persistent hepatic damage, which is primarily responsible for HSC activation and transformation (9). Although signals that trigger the activation of HSCs are intricate and remain incompletely elucidated, it has been demonstrated that several compounds released from dead hepatocytes, including apoptotic bodies, DNA and inflammasomes can promote HSC activation (10). Moreover, inflammatory cells are recruited to lesions and produce numerous cytokines and growth factors, thereby accelerating HSC proliferation and differentiation (11). For instance, the platelet-derived growth factor (PDGF) is the key factor driving HSC proliferation, while transforming growth factor (TGF)-β promotes HSC transformation into myofibroblast-like cells that produce a large amount of collagens, whose deposition beyond degradation by matrix metalloproteases (MMPs) leads to extracellular matrix accumulation and thus fibrosis (1).
In healthy livers, HSCs are normally quiescent, while resident HSCs are activated during liver injury. Activated HSCs become resistant to Fas ligand (FasL) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, thereby aggravating fibrogenesis (12). Persistent inflammatory and immune responses in the liver commonly establish an inflammatory microenvironment that results in the expansion of fibrosis, which makes it susceptible to progression into cirrhosis and cancer (13). Therefore, removal of activated HSCs is essential for a reduction in collagen deposition and suppression of hepatic fibrosis.
4. NK cells in the liver
NK cells are cytotoxic large granular lymphocytes that secrete various cytokines and kill target cells infected by different pathogens, playing a vital role in the innate immune system. Human liver NK cells were first described as ‘pit cells’ by electron microscopy and were found to reside in liver sinusoids (14,15). Liver-resident NK cells nearly account for 50% of intrahepatic lymphocytes in the human, and the percentage of NK cells in the liver is higher than that observed in the spleen and the peripheral blood (5). Compared with circulating NK cells, liver NK cells are less mature under the resting condition, but become more activated in response to pathogenic stimulus and present higher cytotoxicity (16). These results suggest that the enrichment of NK cells in the liver might occur in preparation for exerting particular functions to resist against liver damage. NK cells are classified into CD56bright and the CD56dim subsets based on the expression levels of the activation marker CD69, and CD56bright NK cells display more responsiveness to cytokines while CD56dim NK cells harbor more cytotoxicity against pathogens (17). In the liver, the percentage of these two types of NK cells is equal (18). However, it remains unknown what regulates the frequency and function of these two types of NK cells in the liver.
Once hepatocyte damage is triggered by pathogens such as alcohol and viruses, NK cells are recruited to the hepatic lesions. Within the hepatic microenvironment, the recruitment and retention of NK cells are regulated by chemokine receptors expressed on the surface of NK cells, including CXCR6 and CCR5, which are engaged in the binding with their cognate ligands expressed on hepatocytes, thus mediating their migration to the liver and exerting their immune and cytotoxic functions (19). The main functions of NK cells are to kill target cells through receptor-ligand interactions and to secrete a variety of cytokines for assisting the cytotoxicity. In response to virus infection or tumorigenesis, numerous genomes that are responsible for encoding activating and inhibitory receptors are induced to regulate the opposing receptor-ligand signals to kill target cells (20). When the activating receptors and inhibitory receptors expressed on NK cells bind to the corresponding ligands expressed on target cells, NK cells are either activated or suppressed, respectively (21). Therefore, the activation of NK cells depends on the balance between the signals from the stimulatory and inhibitory receptors. Currently, increasing studies have identified various receptor-ligand signals between NK cells and their target cells (21), and several crucial signals will be discussed below. The activated NK cells kill the target cells through several mechanisms: granule exocytosis, death receptor-mediated apoptosis (22,23), and cytokine secretion (24). Thus, activation of NK cells is expected to becoming a promising strategy to halt the progression of liver diseases.
5. Interaction between HSCs and NK cells
The interaction between NK cells and HSCs is manipulated by various stimulatory and inhibitory receptors on NK cells and the corresponding ligands on HSCs. Among activating NK cell receptors, NK group 2D (NKG2D) and NKp46 have been extensively studied in liver fibrosis. It has been reported that expression levels of NKG2D ligands, including major histocompatibility complex (MHC) class I polypeptide-related sequence A (MICA) and UL16 binding proteins (ULBPs), are increased in senescent HSCs that are more susceptible to NK cell killing by granule exocytosis (25). These ligands bind to NKG2D on NK cells and subsequently induce the activation of NK cells, which leads to the production of a variety of cytotoxic mediators and ultimately to HSC death. With the progression of fibrosis, the expression level of NKp46 is upregulated to kill activated HSCs by binding to its ligand NCR1 (26). However, the expression level of NKp46 and cytolytic activity of NK cells are reduced with the development of fibrosis into the advanced stage (27,28). This result suggests that interactions between activating NK cell receptors and ligands are compromised in the advanced stage of liver fibrosis. In addition, emerging activating receptors are found to be involved in the NK cell-mediated killing of HSCs, such as NKp44, killer cell lectin-like receptor G1 (KLRG1), and Toll-like receptor 9 (TLR-9) (29–31). Under physiological conditions, decreased major histocompatibility complex (MHC) I expression activates HSCs, which are further induced to undergo apoptosis by NK cells (32). Experimentally, the genetic deletion of specific inhibitory receptors such as killer cell immunoglobulin-like receptors (KIRs) and Ly49 increases the NK toxicity to HSCs and attenuates hepatic fibrosis (33). As a consequence, disruption of the interaction between inhibitory NK cell receptors and ligands on HSCs could increase the killing of HSCs and alleviate fibrosis.
6. Cytotoxicity of NK cells to HSCs
Quiescent HSCs
HSCs are the predominant non-parenchymal cells in the liver and regulate the blood flow in hepatic sinusoids. They are also potent liver-resident antigen presenting cells that express MHC I molecules to enhance antigen presentation (34,35). According to the missing-self hypothesis, NK cells target abnormal cells that lack or express low levels of MHC class I, since MHC I recognizes NK cell inhibitory receptors and thus dampens their effector function (36). It is generally accepted that the amount of MHC I expressed on target cells is proportional to the degree of inhibition. Consequently, NK cells fail to kill quiescent HSCs under normal condition. In response to liver injury, HSCs are activated to downregulate the expression of MHC I, which reduces NK cell suppression and increases the cytotoxicity of NK cells against activated HSCs (32,33). Compared with activated HSCs, quiescent HSCs generate less retinoic acid and have decreased expression of retinoic acid early transcript-1 (RAET1E), a ligand for activating NK cell receptors, thereby preventing them from NK cell-mediated killing (37).
Activated HSCs
Generally, the activation of HSCs induced by hepatocyte damage is attributed to increased NK cell stimulation and decreased NK cell inhibition (3). Activated NK cells produce a large amount of perforin and granzyme and induce activated HSC apoptosis (32). At the early stage of fibrosis, NK cells are recruited to the liver and harbor potent degranulation activity that are responsible for the removal of activated HSCs, thus delaying the progression of fibrogenesis (38). With the development of this disease, the degranulation activity of NK cells is impaired in the advanced fibrosis stage (39). These findings suggest that activated HSCs are sensitive to NK killing at the initial stage but become blunt in the advanced stage of fibrosis. In addition, activation of HSCs upregulates the expression of TRAIL receptor and Fas, which interact with NK cells by TRAIL/TRAIL receptor and Fas/FasL signaling, leading to enhanced cytotoxicity of NK cells against activated HSCs. For example, activated HSCs highly express RAE-1, and are killed by activated NK cells through TRAIL-mediated apoptosis (40). Increased TRAIL expression on NK cells promotes the killing attack against activated HSCs (41,42). A clinical investigation also confirmed that NK cells from HCV-infected patients induced apoptosis of activated HSCs in the TRAIL and FasL-dependent manners (43). These results are consistent with the fact that administration of TRAIL induces apoptosis of activated HSCs and thus alleviates liver fibrosis (44). Furthermore, activated NK cells secrete various antifibrotic cytokines to induce activated HSC death. Several studies have revealed that IFN-γ released from NK cells mediates cell cycle arrest and proliferation inhibition of activated HSCs (41,45,46).
Senescent HSCs
Senescence is a cellular process that irreversibly blocks cell proliferation. Senescent HSCs have reduced viability, decreased production of extracellular matrix component, enhanced secretion of extracellular matrix-degrading enzymes, and increased immune surveillance, resulting in limited fibrosis (47). The senescence of activated HSCs renders them susceptible to killing by NK cells, thereby facilitating the resolution of fibrosis (48). In liver injury, intrahepatic NK cells are activated to produce high levels of interleukin (IL)-10 and IL-22, which are able to trigger the innate immune response, protection from damage, and regeneration (49,50). Several studies have shown that both IL-10 and IL-22 promote the senescence of activated HSCs via the STAT3/p53/p21 pathway, attenuating liver fibrosis (51–53). A recent study demonstrated that activation of this pathway accelerates HSC senescence and makes them more vulnerable to being eliminated by NK cells (54). These findings indicate that NK cells produce cytokines to facilitate the senescence of activated HSCs and further to promote cell death. In addition, senescent HSCs induced by the natural product curcumin through the PPARγ/P53 signaling, can be easily removed by NK cells (25,55), suggesting that curcumin serves as a senescence inducer to play a protective role in fibrosis. The mechanism by which NK cells kill senescent HSCs is still unclear, but a study revealed that the lack of granule exocytosis in NK cells resulted in excessive accumulation of senescent HSCs and aggravated liver fibrosis, indicating that NK cells kill senescent HSCs by granule exocytosis; moreover, senescent HSCs with high Dcr2 expression are killed by NK cells through granule exocytosis (56). Therefore, further clarifying the mechanisms of the killing of senescent HSCs by NK cells may provide an effective therapeutic strategy for liver fibrosis.
7. Resistance of HSCs to NK cells
The initial activation of NK cells in the early stage of fibrosis is believed to eliminate activated HSCs and restrict fibrogenesis; however, these effects are weakened and HSCs become resistant to the killing of NK cells in the advanced stage. Previous research has revealed that NK cells from patients with cirrhosis directly contact cultured HSCs and are subsequently engulfed by HSCs via the Rac1 and Cdc42 pathways (57). The phagocytosis of NK cells by HSCs is referred as emperipolesis that is mediated by TGF-β, an immunosuppressive cytokine derived from HSCs. This process promotes NK cell apoptosis and contributes to NK cell depletion, impairing the anti-fibrosis capacity of NK cells (58). Phagocytosis of apoptotic NK cells further makes HSCs resistant to FasL and TRAIL-induced apoptosis via the JAK/STAT and Akt/NF-kB-dependent pathways (12). These findings suggest that TGF-β-mediated emperipolesis of NK cells exacerbates liver fibrosis. Indeed, TGF-β is also known to suppress NK cell-induced cytotoxicity and cytokine production (59). Data from a pre-clinical setting demonstrated that overproduction of TGF-β by HSCs renders them resistant to NK cell killing and inhibits the anti-fibrotic effect of NK cells in advanced liver fibrosis where NK cells have reduced expression of NKG2D, TRAIL and interferon (IFN)-γ (8,60). The mechanisms by which TGF-β impairs the cytotoxicity of NK cells against HSCs remain to be investigated. A recent study has shown that the defective capacity to produce IFN-γ in NK cells is reversed by TGF-β blockade in patients with chronic hepatitis B virus infection (61). It can be assumed that the increased TGF-β levels inhibit the production of IFN-γ in NK cells and makes HSCs resistant to IFN-γ-induced cell cycle arrest and apoptosis in the liver with advanced fibrosis. Moreover, a series of studies have uncovered that elevated TGF-β suppresses the antifibrotic function of NK cells via downregulation of NKG2D and 2B4 surface expression (62–64), thereby reducing the activation of NK cells. Therefore, TGF-β derived from HSCs mediates the resistance to NK cell cytotoxicity in advanced liver fibrosis. Blockade of TGF-β might provide a promising therapeutic strategy for this disease.
8. Therapy implications
With liver disease progression to fibrosis, the effector function of NK cells is activated to eliminate HSCs at the early stage but exhausted at the advanced stage. Therefore, the initial intensification of the antifibrotic function of NK cells can halt the development of liver fibrosis. Experimentally, several studies have unveiled that upregulation of the expression level of NKG2D in NK cells by natural products, such as Mycelium cordyceps sinensis and Salvia miltiorrhiza, alleviates liver fibrosis (65,66). Using siRNA to downregulate the expression of KIR also limits fibrosis (33). These findings imply that modulation of the expression of activating and inhibitory NK cell receptors may be a promising strategy to activate NK cells. In addition, an enhancement of NK cell-derived antifibrotic cytokines has been confirmed to be effective in killing HSCs. For instance, the herbal agent Yu Gan Long was found to increase the expression level of IFN-γ and facilitate extracellular matrix degradation (67). Exogenous IFN-γ supplementation delivered to HSCs directly elevates the antifibrotic potency of NK cells (68). Thus, activation of NK cells is recognized as a novel therapeutic strategy for treatment of liver fibrosis (5). However, in the advanced fibrosis stage, the expression of activating NK cell receptors, the production of IFN-γ, the degranulation activity, and the cytotoxic function of NK cells are diminished after interaction with activated HSCs (39,69,70), suggesting a functional exhaustion of NK cells. Unfortunately, as concluded above, HSCs become resistant to these exhausted NK cells in the advanced stage of disease. Only the activation of NK cells is insufficient to recover their cytotoxic function in advance fibrosis. A recent study found that fasudil facilitates the apoptosis and inhibits the proliferation of HSCs by decreasing TGF-β (71). Thus, NK cell activation combined with TGF-β blockade may be effective to restrict fibrogenesis in the advanced stage of the disease.
9. Future directions
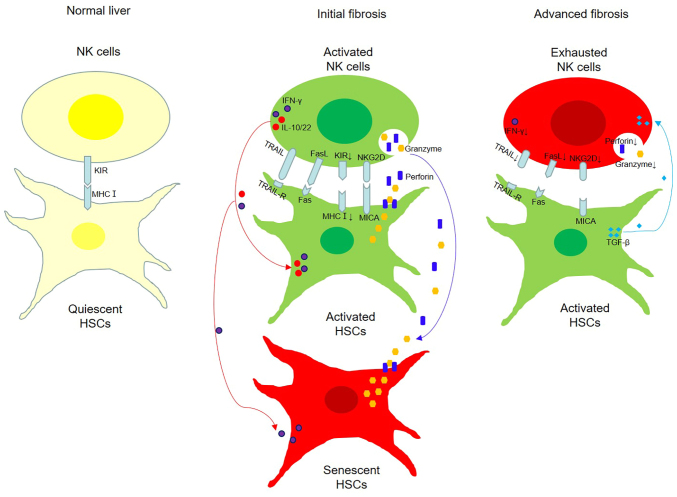
As the initial event in fibrogenesis, HSCs are activated to tissue repair in liver injury, but hyperactivation of HSCs produces excessive collagens and contributes to fibrosis. Hepatic NK cells, an essential component of the innate immunity, are activated to eliminate HSCs through receptor-ligand interactions and to produce various antifibrotic cytokines to assist their cytotoxicity. These effects are modulated by both activating and inhibitory NK cell receptors. Under physiological condition, quiescent HSCs fail to be targeted by NK cells, but activated and senescent HSCs can be killed by activated NK cells in the early stage of fibrosis through granule exocytosis, death receptor-mediated pathways, and production of antifibrotic cytokines; however, in the advanced stage, the cytotoxicity of NK cells against HSCs is defective (Fig. 1). A summary of the crosstalk between HSCs and NK cells in fibrosis of various liver diseases is described in Table I (25,32,33,37,40,43,46,54,56–58,60).
Figure 1.
Crosstalk between NK cells and HSCs in different stages of fibrosis. Under normal condition, NK cells fail to kill quiescent HSCs that express MHC I, which binds to inhibitory NK cell receptors (KIRs). In the initial stage of fibrosis, activated NK cells express activating receptors (NKG2D) that bind the corresponding ligands (MICA) expressed in activated HSCs thus leading to their death through TRAIL/FasL-mediated apoptosis, granule exocytosis and IFN-γ secretion. Moreover, interleukin (IL)-10 and IL-22 released from activated NK cells promote the senescence of HSCs, which are more susceptible to the killing mediated by granule exocytosis and IFN-γ. In the advanced stage of fibrosis, NK cells present functional exhaustion and TGF-β produced by HSCs further diminishes the expression of activating receptors and the cytotoxic effect of NK cells. NK, natural killing; HSCs, hepatic stellate cells; NKG2D, NK group 2D; MICA, major histocompatibility complex (MHC) class I polypeptide-related sequence A; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; IFN-γ, interferon-γ.
Table I.
Crosstalk between HSCs and NK cells in the fibrosis of various liver diseases.
| Research finding | Study design | Refs. |
|---|---|---|
| Senescent HSCs highly express MICA and are more susceptible to NK cell killing by granule exocytosis HSCs are activated with decreased MHC I expression, and are induced apoptosis by NK cells | CCl4-induced liver fibrosis, LX2 and NK-92 cell line culture | (25) |
| CCl4-induced liver fibrosis, primary cell culture form human | (32) | |
| HSCs are killed by NK cells with iKIR knockdown | CCl4-induced liver fibrosis, cell culture from HCV-infected patients | (33) |
| Quiescent HSCs have a low expression of RAE-1, and are fail to be killed by NK cells | Primary cell culture from mice | (37) |
| Activated HSCs highly express RAE-1, and are killed by activated NK cells through TRAIL-mediated apoptosis | DDC and CCl4-induced liver fibrosis | (40) |
| NK cells are activated to induce apoptosis of activated | Cell culture from HCV-infected patients | (43) |
| HSCs in TRAIL and FasL-dependent manners | ||
| Activated NK cells kill activated HSCs via producing IFN-γ | S. japonicum-induced liver fibrosis | (46) |
| Senescent HSCs are more vulnerable to NK cell killing | S. japonicum-induced liver fibrosis | (54) |
| Senescent HSCs with high Dcr2 expression are killed by NK cells through granule exocytosis | CCl4-induced liver fibrosis, primary cell culture form human | (56) |
| NK cells in advanced liver fibrosis are engulfed by HSCs via the Rac1 and Cdc42 pathways | Cell culture from HBV- and HCV-infected patients | (57) |
| Activated HSCs induce NK cell apoptosis by TGF-β-mediated emperipolesis | Cell culture from HBV-infected patients | (58) |
| HSCs are resistant to the killing of NK cells that have reduced expression of NKG2D, TRAIL and IFN-γ | Alcohol and CCl4-induced liver fibrosis | (60) |
NK, natural killer; HSCs, hepatic stellate cells; RAE-1, retinoic acid early inducible gene 1; MICA, major histocompatibility complex class I polypeptide-related sequence A; KIRs, killer cell immunoglobulin-like receptors; TGF-β, transforming growth factor-β; NKG2D, NK group 2D; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; IFN-γ, interferon-γ.
Experimentally, a series of studies demonstrated that activating NK cells in the initial stage of disease postpone the development of liver fibrosis, but fail to halt fibrogenesis in the advanced stage (8,38,39,60). The mechanism of dysfunction in NK cells is still elusive, but it is partially attributed to the fact that HSCs become resistant to exhausted NK cells in a TGF-β-dependent manner. This may provide an additional clue to explain why activating NK cells fail in progressive fibrosis. In this content, activating NK cell therapies combined with a TGF-β inhibitor will likely represent a promising therapeutic strategy in the disease, and merits further verification in clinical investigations. In addition, the cytotoxic function of NK cells is regulated by a complex network of activating and inhibitory receptors. Therefore, further clarification of NK cell receptors and their cytotoxic functions will provide new insight in the etiology of fibrogenesis, as potential therapies for liver fibrosis.
Acknowledgements
Not applicable.
Funding Statement
This work was funded by the National Natural Science Foundation of China (grant no. 81973601) and TCM Research Projects of Heilongjiang Province (grant nos. ZHY18-029, ZHY19-027, ZHY19-062 and ZHY2020-041).
Availability of data and materials
All information provided in this review is documented by relevant references.
Authors' contributions
XY and BW designed the review. YZ, YW and WS wrote the review in light of the literature findings. All authors read and approved the final manuscript for publication. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
- 1.Gan J, Mao XR, Zheng SJ, Li JF. Invariant natural killer T cells: Not to be ignored in liver disease. J Dig Dis. 2021;22:136–142. doi: 10.1111/1751-2980.12968. [DOI] [PubMed] [Google Scholar]
- 2.Yan Y, Zeng J, Xing L, Li C. Extra- and intra-cellular mechanisms of hepatic stellate cell activation. Biomedicines. 2021;9:1014. doi: 10.3390/biomedicines9081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Highton AJ, Schuster IS, Degli-Esposti MA, Altfeld M. The role of natural killer cells in liver inflammation. Semin Immunopathol. 2021;43:519–533. doi: 10.1007/s00281-021-00877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St-Pierre F, Bhatia S, Chandra S. Harnessing natural killer cells in cancer immunotherapy: A review of mechanisms and novel therapies. Cancers (Basel) 2021;13:1988. doi: 10.3390/cancers13081988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng M, Sun H, Tian Z. Natural killer cells in liver diseases. Front Med. 2018;12:269–279. doi: 10.1007/s11684-018-0621-4. [DOI] [PubMed] [Google Scholar]
- 6.Gao B, Radaeva S, Jeong WI. Activation of natural killer cells inhibits liver fibrosis: A novel strategy to treat liver fibrosis. Expert Rev Gastroenterol Hepatol. 2007;1:173–180. doi: 10.1586/17474124.1.1.173. [DOI] [PubMed] [Google Scholar]
- 7.Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68-69:435–451. doi: 10.1016/j.matbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Jeong WI, Park O, Suh YG, Byun JS, Park SY, Choi E, Kim JK, Ko H, Wang H, Miller AM, Gao B. Suppression of innate immunity (natural killer cell/interferon-gamma) in the advanced stages of liver fibrosis in mice. Hepatology. 2011;53:1342–1351. doi: 10.1002/hep.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells. 2020;9:875. doi: 10.3390/cells9040875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wree A, McGeough MD, Inzaugarat ME, Eguchi A, Schuster S, Johnson CD, Peña CA, Geisler LJ, Papouchado BG, Hoffman HM, Feldstein AE. NLRP3 inflammasome driven liver injury and fibrosis: Roles of IL-17 and TNF in mice. Hepatology. 2018;67:736–749. doi: 10.1002/hep.29523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan M, Han J, Ding L, Hu F, Gao P. Novel immune subsets and related cytokines: Emerging players in the progression of liver fibrosis. Front Med. 2021;8:604894. doi: 10.3389/fmed.2021.604894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang JX, Mikami K, Venugopal S, Li Y, Török NJ. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J Hepatol. 2009;51:139–148. doi: 10.1016/j.jhep.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanwar S, Rhodes F, Srivastava A, Trembling PM, Rosenberg WM. Inflammation and fibrosis in chronic liver diseases including non-alcoholic fatty liver disease and hepatitis C. World J Gastroenterol. 2020;26:109–133. doi: 10.3748/wjg.v26.i2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo DZ, Vermijlen D, Ahishali B, Triantis V, Plakoutsi G, Braet F, Vanderkerken K, Wisse E. On the cell biology of pit cells, the liver-specific NK cells. World J Gastroenterol. 2000;6:1–11. doi: 10.3748/wjg.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wisse E, van't Noordende JM, van der Meulen J, Daems WT. The pit cell: Description of a new type of cell occurring in rat liver sinusoids and peripheral blood. Cell Tissue Res. 1976;173:423–435. doi: 10.1007/BF00224305. [DOI] [PubMed] [Google Scholar]
- 16.Tang L, Peng H, Zhou J, Chen Y, Wei H, Sun R, Yokoyama WM, Tian Z. Differential phenotypic and functional properties of liver-resident NK cells and mucosal ILC1s. J Autoimmun. 2016;67:29–35. doi: 10.1016/j.jaut.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Zhang C. The Roles of Liver-resident lymphocytes in liver diseases. Front Immunol. 2019;10:1582. doi: 10.3389/fimmu.2019.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunemann S, Martrus G, Goebels H, Kautz T, Langeneckert A, Salzberger W, Koch M, J Bunders M, Nashan B, van Gisbergen KPJM, Altfeld M. Hobit expression by a subset of human liver-resident CD56bright Natural Killer cells. Sci Rep. 2017;7:6676. doi: 10.1038/s41598-017-06011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudspeth K, Donadon M, Cimino M, Pontarini E, Tentorio P, Preti M, Hong M, Bertoletti A, Bicciato S, Invernizzi P, et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun. 2016;66:40–50. doi: 10.1016/j.jaut.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Hu Y, Xiao W, Tian Z. Chimeric antigen receptor- and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy. Cell Mol Immunol. 2021;18:2083–2100. doi: 10.1038/s41423-021-00732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obajdin J, Davies DM, Maher J. Engineering of chimeric natural killer cell receptors to develop precision adoptive immunotherapies for cancer. Clin Exp Immunol. 2020;202:11–27. doi: 10.1111/cei.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vrazo AC, Hontz AE, Figueira SK, Butler BL, Ferrell JM, Binkowski BF, Li J, Risma KA. Live cell evaluation of granzyme delivery and death receptor signaling in tumor cells targeted by human natural killer cells. Blood. 2015;126:e1–e10. doi: 10.1182/blood-2015-03-632273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saparbay J, Tanaka Y, Tanimine N, Ohira M, Ohdan H. Everolimus enhances TRAIL-mediated anti-tumor activity of liver resident natural killer cells in mice. Transpl Int. 2020;33:229–243. doi: 10.1111/tri.13536. [DOI] [PubMed] [Google Scholar]
- 24.Choi WM, Ryu T, Lee JH, Shim YR, Kim MH, Kim HH, Kim YE, Yang K, Kim K, Choi SE, et al. Metabotropic glutamate receptor 5 in natural killer cells attenuates liver fibrosis by exerting cytotoxicity to activated stellate cells. Hepatology. 2021;74:2170–2185. doi: 10.1002/hep.31875. [DOI] [PubMed] [Google Scholar]
- 25.Jin H, Jia Y, Yao Z, Huang J, Hao M, Yao S, Lian N, Zhang F, Zhang C, Chen X, et al. Hepatic stellate cell interferes with NK cell regulation of fibrogenesis via curcumin induced senescence of hepatic stellate cell. Cell Signal. 2017;33:79–85. doi: 10.1016/j.cellsig.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Gur C, Doron S, Kfir-Erenfeld S, Horwitz E, Abu-Tair L, Safadi R, Mandelboim O. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut. 2012;61:885–893. doi: 10.1136/gutjnl-2011-301400. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Jiang Y, Wang X, Jin J, Qi Y, Chi X, Zhang H, Feng X, Niu J. Natural killer p46 controls hepatitis B virus replication and modulates liver inflammation. PLoS One. 2015;10:e0135874. doi: 10.1371/journal.pone.0135874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nel I, Lucar O, Petitdemange C, Béziat V, Lapalus M, Bédossa P, Debré P, Asselah T, Marcellin P, Vieillard V. Accumulation of intrahepatic TNF-α-producing NKp44+ NK cells correlates with liver fibrosis and viral load in chronic HCV infection. Medicine (Baltimore) 2016;95:e3678. doi: 10.1097/MD.0000000000003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wijaya RS, Read SA, Schibeci S, Eslam M, Azardaryany MK, El-Khobar K, van der Poorten D, Lin R, Yuen L, Lam V, et al. KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B. J Hepatol. 2019;71:252–264. doi: 10.1016/j.jhep.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Tair L, Axelrod JH, Doron S, Ovadya Y, Krizhanovsky V, Galun E, Amer J, Safadi R. Natural killer cell-dependent anti-fibrotic pathway in liver injury via Toll-like receptor-9. PLoS One. 2013;8:e82571. doi: 10.1371/journal.pone.0082571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai X, Wang J, Wang J, Zhou Q, Yang B, He Q, Weng Q. Intercellular crosstalk of hepatic stellate cells in liver fibrosis: New insights into therapy. Pharmacol Res. 2020;155:104720. doi: 10.1016/j.phrs.2020.104720. [DOI] [PubMed] [Google Scholar]
- 33.Muhanna N, Abu Tair L, Doron S, Amer J, Azzeh M, Mahamid M, Friedman S, Safadi R. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011;60:90–98. doi: 10.1136/gut.2010.211136. [DOI] [PubMed] [Google Scholar]
- 34.Schölzel K, Schildberg FA, Welz M, Börner C, Geiger S, Kurts C, Heikenwälder M, Knolle PA, Wohlleber D. Transfer of MHC-class-I molecules among liver sinusoidal cells facilitates hepatic immune surveillance. J Hepatol. 2014;61:600–608. doi: 10.1016/j.jhep.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 35.Arriola Benitez PC, Pesce Viglietti AI, Elizalde MM, Giambartolomei GH, Quarleri JF, Delpino MV. Hepatic stellate cells and hepatocytes as liver Antigen-presenting cells during B. abortus Infection. Pathogens. 2020;9:527. doi: 10.3390/pathogens9070527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Lu D, Churov A, Fu R. Research progress on NK cell receptors and their signaling pathways. Mediators Inflamm. 2020;2020:6437057. doi: 10.1155/2020/6437057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radaeva S, Wang L, Radaev S, Jeong WI, Park O, Gao B. Retinoic acid signaling sensitizes hepatic stellate cells to NK cell killing via upregulation of NK cell activating ligand RAE1. Am J Physiol Gastrointest Liver Physiol. 2007;293:G809–G816. doi: 10.1152/ajpgi.00212.2007. [DOI] [PubMed] [Google Scholar]
- 38.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fugier E, Marche H, Thelu MA, Macek Jílková Z, Van Campenhout N, Dufeu-Duchesne T, Leroy V, Zarski JP, Sturm N, Marche PN, Jouvin-Marche E. Functions of liver natural killer cells are dependent on the severity of liver inflammation and fibrosis in chronic hepatitis C. PLoS One. 2014;9:e95614. doi: 10.1371/journal.pone.0095614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 41.Jeong WI, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44:1441–1451. doi: 10.1002/hep.21419. [DOI] [PubMed] [Google Scholar]
- 42.Ma PF, Gao CC, Yi J, Zhao JL, Liang SQ, Zhao Y, Ye YC, Bai J, Zheng QJ, Dou KF, et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J Hepatol. 2017;67:770–779. doi: 10.1016/j.jhep.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Glassner A, Eisenhardt M, Kramer B, Körner C, Coenen M, Sauerbruch T, Spengler U, Nattermann J. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab Invest. 2012;92:967–977. doi: 10.1038/labinvest.2012.54. [DOI] [PubMed] [Google Scholar]
- 44.Oh Y, Park O, Swierczewska M, Hamilton JP, Park JS, Kim TH, Lim SM, Eom H, Jo DG, Lee CE, et al. Systemic PEGylated TRAIL treatment ameliorates liver cirrhosis in rats by eliminating activated hepatic stellate cells. Hepatology. 2016;64:209–223. doi: 10.1002/hep.28432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rockey DC, Maher JJ, Jarnagin WR, Gabbiani G, Friedman SL. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992;16:776–784. doi: 10.1002/hep.1840160325. [DOI] [PubMed] [Google Scholar]
- 46.Hou X, Yu F, Man S, Huang D, Zhang Y, Liu M, Ren C, Shen J. Negative regulation of Schistosoma japonicum egg-induced liver fibrosis by natural killer cells. PLoS Negl Trop Dis. 2012;6:e1456. doi: 10.1371/journal.pntd.0001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55. doi: 10.1016/j.mam.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Zhang M, Serna-Salas S, Damba T, Borghesan M, Demaria M, Moshage H. Hepatic stellate cell senescence in liver fibrosis: Characteristics, mechanisms and perspectives. Mech Ageing Dev. 2021;199:111572. doi: 10.1016/j.mad.2021.111572. [DOI] [PubMed] [Google Scholar]
- 49.Fan Y, Zhang W, Wei H, Sun R, Tian Z, Chen Y. Hepatic NK cells attenuate fibrosis progression of non-alcoholic steatohepatitis in dependent of CXCL10-mediated recruitment. Liver Int. 2020;40:598–608. doi: 10.1111/liv.14307. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Min J, Ge C, Shu J, Tian D, Yuan Y, Zhou D. Interleukin 22 in liver injury, inflammation and cancer. Int J Biol Sci. 2020;16:2405–2413. doi: 10.7150/ijbs.38925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang YH, Chen MH, Guo QL, Chen YX, Zhang LJ, Chen ZX, Wang XZ. Interleukin-10 promotes primary rat hepatic stellate cell senescence by upregulating the expression levels of p53 and p21. Mol Med Rep. 2018;17:5700–5707. doi: 10.3892/mmr.2018.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang YH, Chen MH, Guo QL, Chen ZX, Chen QD, Wang XZ. Interleukin-10 induces senescence of activated hepatic stellate cells via STAT3-p53 pathway to attenuate liver fibrosis. Cell Signal. 2020;66:109445. doi: 10.1016/j.cellsig.2019.109445. [DOI] [PubMed] [Google Scholar]
- 53.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150–1159. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Xu T, Zhu D, Wang J, Huang C, Lyu L, Hu B, Sun W, Duan Y. Egg antigen p40 of Schistosoma japonicum promotes senescence in activated hepatic stellate cells by activation of the STAT3/p53/p21 pathway. Cell Death Dis. 2016;7:e2315. doi: 10.1038/cddis.2016.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin H, Lian N, Zhang F, Chen L, Chen Q, Lu C, Bian M, Shao J, Wu L, Zheng S. Activation of PPARγ/P53 signaling is required for curcumin to induce hepatic stellate cell senescence. Cell Death Dis. 2016;7:e2189. doi: 10.1038/cddis.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene. 2013;32:1971–1977. doi: 10.1038/onc.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muhanna N, Doron S, Wald O, Horani A, Eid A, Pappo O, Friedman SL, Safadi R. Activation of hepatic stellate cells after phagocytosis of lymphocytes: A novel pathway of fibrogenesis. Hepatology. 2008;48:963–977. doi: 10.1002/hep.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi J, Zhao J, Zhang X, Cheng Y, Hu J, Li Y, Zhao X, Shang Q, Sun Y, Tu B, et al. Activated hepatic stellate cells impair NK cell anti-fibrosis capacity through a TGF-β-dependent emperipolesis in HBV cirrhotic patients. Sci Rep. 2017;7:44544. doi: 10.1038/srep44544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, Jaung MS, Blaser BW, Sun J, Benson DM, Jr, et al. Pro- and antiinflammatory cytokine signaling: Reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity. 2006;24:575–590. doi: 10.1016/j.immuni.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C, Pallant C, Ellis G, Khanna P, Dusheiko G, et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 2010;6:e1001227. doi: 10.1371/journal.ppat.1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dasgupta S, Bhattacharya-Chatterjee M, O'Malley BW, Jr, Chatterjee SK. Inhibition of NK cell activity through TGF-beta 1 by down-regulation of NKG2D in a murine model of head and neck cancer. J Immunol. 2005;175:5541–5550. doi: 10.4049/jimmunol.175.8.5541. [DOI] [PubMed] [Google Scholar]
- 63.Sene D, Levasseur F, Abel M, Lambert M, Camous X, Hernandez C, Pène V, Rosenberg AR, Jouvin-Marche E, Marche PN, et al. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog. 2010;6:e1001184. doi: 10.1371/journal.ppat.1001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, Wei H. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012;8:e1002594. doi: 10.1371/journal.ppat.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng Y, Huang K, Shen L, Tao YY, Liu CH. Cultured mycelium cordyceps sinensis alleviates CCl4-induced liver inflammation and fibrosis in mice by activating hepatic natural killer cells. Acta Pharmacol Sin. 2016;37:204–216. doi: 10.1038/aps.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng Y, Yang T, Huang K, Shen L, Tao Y, Liu C. Salvia miltiorrhiza ameliorates liver fibrosis by activating hepatic natural killer cells in vivo and in vitro. Front Pharmacol. 2018;9:762. doi: 10.3389/fphar.2018.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia Y, Yu B, Ma C, Tu Y, Zhai L, Yang Y, Liu D, Liu Y, Wu H, Dan H, You P. Yu Gan Long reduces rat liver fibrosis by blocking TGF-β 1/Smad pathway and modulating the immunity. Biomed Pharmacother. 2018;106:1332–1338. doi: 10.1016/j.biopha.2018.07.081. [DOI] [PubMed] [Google Scholar]
- 68.van Dijk F, Olinga P, Poelstra K, Beljaars L. Targeted therapies in liver fibrosis: Combining the best parts of Platelet-Derived growth factor BB and interferon gamma. Front Med (Lausanne) 2015;2:72. doi: 10.3389/fmed.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polo ML, Ghiglione YA, Salido JP, Urioste A, Poblete G, Sisto AE, Martinez A, Rolón MJ, Ojeda DS, Cahn PE, et al. Liver cirrhosis in HIV/HCV-coinfected individuals is related to NK cell dysfunction and exhaustion, but not to an impaired NK cell modulation by CD4+ T-cells. J Int AIDS Soc. 2019;22:e25375. doi: 10.1002/jia2.25375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, Wang JJ, Gao S, Liu Q, Bai J, Zhao XQ, Hao YH, Ding HH, Zhu F, Yang DL, Zhao XP. Decreased peripheral natural killer cells activity in the immune activated stage of chronic hepatitis B. PLoS One. 2014;9:e86927. doi: 10.1371/journal.pone.0086927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han QJ, Mu YL, Zhao HJ, Zhao RR, Guo QJ, Su YH, Zhang J. Fasudil prevents liver fibrosis via activating natural killer cells and suppressing hepatic stellate cells. World J Gastroenterol. 2021;27:3581–3594. doi: 10.3748/wjg.v27.i24.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All information provided in this review is documented by relevant references.