Abstract
Cholangiocarcinoma (CCA) is an intractable malignant tumour with a high degree of malignancy that is asymptomatic in the early stages. Exosomes have been shown in numerous studies in recent years to be effective delivery vehicles for chemotherapy drugs to suppress tumour proliferation and growth in vivo and in vitro. In order to explore the inhibition of 5-fluorouracil (5-Fu)-loaded exosomes on CCA growth, the present study used human bone marrow mesenchymal stem cell-derived exosomes, as well as incubation and sonication methods for 5-Fu loading into exosomes, to treat CCA in vitro. The findings demonstrated that exosomes isolated from mesenchymal stem cells have typical exosome characteristics. Both the incubation and sonication methods successfully loaded 5-Fu into the exosomes (5-Fu-Exos), with the sonication method having a higher loading efficiency than the incubation method. When compared to the free 5-Fu group, the 5-Fu-Exos group significantly inhibited the viability of CCA cells (P<0.01), indicating that 5-Fu-Exos can be an effective chemotherapy drug for CCA treatment.
Keywords: cholangiocarcinoma, exosome, drug delivery, 5-fluorouracil
Introduction
Cholangiocarcinoma (CCA) is rare cancer, but its incidence is rising worldwide. Because CCA is frequently asymptomatic in the early stages, it is commonly diagnosed at an advanced stage, which has a significant impact on the therapeutic selection (1,2). Despite significant advances in CCA diagnosis and treatment over the last decade, there has been no significant improvement in patient prognosis with a 5-year survival of 7–20% (3–10). Surgery is one of the most primary therapeutic options for CCA. However, the vast majority of patients (~70%) are not indicated for resection when diagnosed (11). Moreover, even after surgery, the tumour recurrence rate is quite high (12).
As a result, chemotherapy remains the treatment of choice for the vast majority of CCA patients. 5-Fluorouracil (5-Fu) is one of the drugs that can be used to treat CCA. Because of its low cost, it is a type of chemotherapy drug that is widely used in the treatment of digestive system neoplasms. Nonetheless, 5-Fu has some drawbacks, including a short biological half-life (10–20 min), volatility in absorption, distribution, metabolism and pharmacokinetics. Approximately 90% of the injected dose will eventually be converted into inactive materials due to the metabolism (13), limiting its efficacy. As a result, maintaining the therapeutic serum concentrations necessitates long-term administration of a high-dose of the drug, which results in severe toxic and side effects (14). Approximately 10–20% of patients treated with standard 5-Fu exhibit a serious toxic reaction, such as myelosuppression, gastrointestinal side effects, neurologic and cutaneous adverse reaction, and others (15). Therefore, other techniques to improve the effectiveness of 5-Fu treatment are urgently needed to extend its effective time, improve its drug application efficiency, and reduce its relevant side effects.
Exosomes, a type of extracellular vesicles (EV), are now regarded as drug carriers with great potential and have been extensively studied, owing to their ability to transport therapeutic molecules such as proteins, nucleic acids, and drugs. Exosomes can be found in a variety of body fluids and tissues, including blood, saliva, urine, breast milk, cerebrospinal fluid, adipose tissue (16–18), and even plants and milk (19). Exosomes play a role in physiological and pathological processes, as well as serving as a critical link in cell-cell communication and material interchange. Exosomes also have distinct advantages, such as natural sources, excellent biocompatibility, organ propensity, and low immunogenicity. They can deliver bioactive substances and therapeutic molecules in a variety of ways and sites, as well as participate in cell modulation with target cells, which includes tissue repair, tumour diagnosis and treatment, and immunomodulation (20–24). Taking into account the properties of exosomes, this study used exosomes as a chemotherapy drug carrier, and 5-Fu was loaded into the exosomes (5-Fu-Exos) via sonication and incubation methods. The experimental results showed that 5-Fu-Exos are more effective than free 5-Fu in inhibiting CCA cell viability, implying that exosomes could be used as a new targeted chemotherapy drug carrier for CCA treatment.
Materials and methods
Cell culture
Human bone marrow mesenchymal stem cells (HBMSCs) and the human CCA cell line QBC939 were obtained from Shanghai Cell Bank, Chinese Academy of Sciences. Both cell lines were grown in DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, and 100 U/ml streptomycin. HBMSCs were found to be uniformly distributed at the bottom of the culture flask and were incubated in a constant temperature incubator at 37°C with 5% CO2. When the cells reached 70–80% confluency, they were digested with trypsin digestive juice (Gibco; Thermo Fisher Scientific, Inc.) and passaged at a 1:4 ratio under an inverted microscope. For this experiment, cells from generations 3–5 were chosen.
Isolation of the exosomes
Ultracentrifugation was used to separate exosomes from the conditioned medium. The HBMSC medium was replaced with high-glucose DMEM containing 10% exosome-depleted FBS and cultured for 48 h. The cultured fluid was collected and centrifuged at 800 × g for 5 min. The supernatant was collected and filtered using a 0.22-µm filter. The supernatant was centrifuged at 300 × g for 10 min to collect the supernatant, 2,000 × g for 10 min to precipitate dead cells, and 10,000 × g for 30 min to remove cell debris at 4°C. Following that, the exosomes were pelleted by ultracentrifugation at 100,000 × g for 70 min and washed with PBS to remove the contaminated cells. The collected solution was then centrifuged at 100,000 × g for 70 min before being resuspended in PBS, with the supernatant removed, to obtain final exosomes, which were then stored in sub-packages at −80°C for the next experimental stage.
Exosome characterization
The morphology of the exosomes was studied using a transmission electron microscope (TEM). An amount of 10 µl exosomes was collected by ultracentrifugation, dropped onto a copper grid, and dried with filter paper after 1 min. The grid was then stained with 2% phosphotungstic acid and dried with filter paper after 1 min. Afterward, it was dried with a filament lamp before being observed and photographed under a TEM. Western blot analysis was used to identify exosome-specific proteins. To determine the concentration of proteins derived from the HBMSCs, the BCA Protein Assay Kit (Beyotime Institute of Biotechnology) was used. After dissociation by sodium dodecyl sulfate-poly-acrylamide gel electrophoresis (SDS-PAGE), proteins in the gel were transferred to a PVDF membrane, sealed with dry skim milk for 1 h at room temperature, and incubated overnight at 4°C with tumor susceptibility 101 (TSG101) (dilution 1:500, cat. no. ab125011; Abcam), and CD9 (dilution 1:500, cat. no. ab236630; Abcam) monoclonal antibodies. After washing the membrane (10 min × 3 times) with Tris-buffered saline with Tween-20 (TBST), goat anti-rabbit secondary antibody (dilution 1:5,000, cat. no. ab150077; Abcam) was applied for 2 h before rewashing with TBST (10 min × 3 times). Finally, the protein was coloured using a chemiluminescent ECL kit (Beyotime Institute of Biotechnology). Nanoparticle tracking analysis (NTA) of exosome size distribution curves was performed using Zetaview (PMX 110, Particle Metrix) in accordance with the manufacturer's instructions. PBS was used to properly dilute exosomes (1 mg/ml) (1:1,000). Zetaview Software 8.02.31 was used to record and analyse particle sizes and numbers from all 11 positions.
Drug entrapment
Exosomes were mixed with 5-Fu in PBS at a protein concentration of 1 mg/ml. The exosome solution (5 ml/1 mg/ml) and the 5-Fu solution (5 ml/1 mg/ml) were mixed uniformly. Two loading methods were used: direct incubation and sonication. The mixture was incubated in an incubation shaker at 37°C for 1 h for the direct incubation method. The ultrasonic probe (Ultrasonic Cell Breaker 705, Thermo Fisher Scientific, Inc.) was immersed in the mixture for the sonication method. The entire procedure was carried out in an ice bath in six cycles (frequency 20 kHz, amplitude 20%, 30 sec on and 30 sec off for each cycle, 2-min interval between each cycle). The solution was sonicated and then incubated at 37°C for 30 min to recover the exosomal membrane. The two types of solutions were centrifuged and washed with PBS to collect exosomes and 5-Fu-loaded exosomes (5-Fu-Exos).
Determination of 5-Fu concentration
A high-performance liquid chromatography (HPLC) system (High-Performance Liquid Chromatography Analyser, Agilent Technologies, Inc.) was used to determine the amount of 5-Fu loaded into exosomes. Bond Elut LRC C-18 solid-phase disposable extraction column (Varian) was loaded with 5-Fu solution and exosome dispersion containing 100 µg protein. The extract was evaporated after extraction, and the residue was mixed with 200 µl methanol. HPLC was used to determine the 5-Fu content under the following chromatographic conditions: chromatographic column (Zorbax RX-C18; Agilent Technologies, Inc.); sample volume, 20 µl; move phase, 40% water-60% methanol; detecting wavelength, 265 nm; column temperature, 30°C. PBS standard solutions with 5-Fu concentrations of 0.5, 1.0, 2.5, 5.0, 10.0, and 20.0 µg/ml were prepared, and the 5-Fu standard solutions were determined by HPLC to obtain varying concentrations and their corresponding peak areas, resulting in a standard curve. The peak area in the standard curve was used to calculate the concentration of 5-Fu in the sample.
The uptake of exosomes by CCA QBC939 cells
PKH26 (cat. no. PKH26GL-1KT red, Sigma-Adrich; Merck KGaA) was used to label exosomes that were collected. After co-culturing the stained exosomes and the QBC929 cell line for 24 h, the cell nucleus was stained with DAPI dye (cat. no C1002, Beyotime Institute of Biotechnology) for 10 min. In the subsequent observation, a laser scanning confocal microscope was used.
Cytotoxicity of 5-Fu
The CCK-8 assay was used to determine the cytotoxicity of 5-Fu-Exos and free 5-Fu on the CCA QBC939 cell line. After being resuspended in PBS and medium, the QBC939 cells were counted and were seeded at a density of 5×104/ml in 96-well plates with 100 µl (5×103 cells) per well, three compound wells in each group. For 24 h, the cells were grown in complete DMEM containing 10% (v/v) FBS at 37°C. After three washes with PBS, the DMEM with 10% (v/v) exosome-depleted FBS was added. 5-Fu-Exos were filtered through a 0.22-µm aseptic filter and diluted into nutrient solutions with varying 5-Fu concentrations (0, 0.1, 0.5, 1.0, 2.0, 5.0 and 10.0 µg/ml) using DMEM. One hundred microliters of the above-mentioned 5-Fu-Exos at various concentrations were added to the seeded QBC939 cells. The drug control group received DMEM containing varying concentrations of 5-Fu (0, 0.1, 0.5, 1.0, 2.0, 5.0 and 10.0 µg/ml). The culture plate was incubated at 37°C for 24 h. Incubation was carried out for 1 h after adding 20 µl CCK-8 solvent. Following colour change, absorbance at 450 nm was detected to measure the cell viability using an enzyme labelling instrument (BIO-TekELx800 automatic enzyme labelling instrument, Bio-Rad Laboratories, Inc.). Exosome toxicity against QBC939 cells was determined using the same method as described above. QBC939 cells were co-incubated with pure DMEM, pure HBMSC-Exos, and 5-Fu-Exos.
Statistical analysis
All data were statistically analysed using GraphPad Prism 7.0 (GraphPad Software, Inc.). The data are presented as a mean value ± standard deviation (SD). For inter-group and multiple-group comparisons, the t-test and one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test was used with a significance level of P<0.05.
Results
Identification of the exosomes
Exosomes were isolated from the HBMSCs using ultracentrifugation, and the morphology and size of the exosomes were examined using a transmission electron microscope. Round and small vesicles with a double-layer structure were detected, with a size of approximately 100 nm (Fig. 1A), which was consistent with previous reports (25). Then, exosome-specific proteins were identified using western blot analysis. The findings revealed that CD9 and TSG101 were highly expressed (Fig. 1B). The aforementioned findings indicated that exosomes were successfully purified and separated. Exosomes from the HBMSCs had a narrow size distribution, with an average diameter of 127.8±2.3 nm, according to NTA measurements (Fig. 1C).
Figure 1.
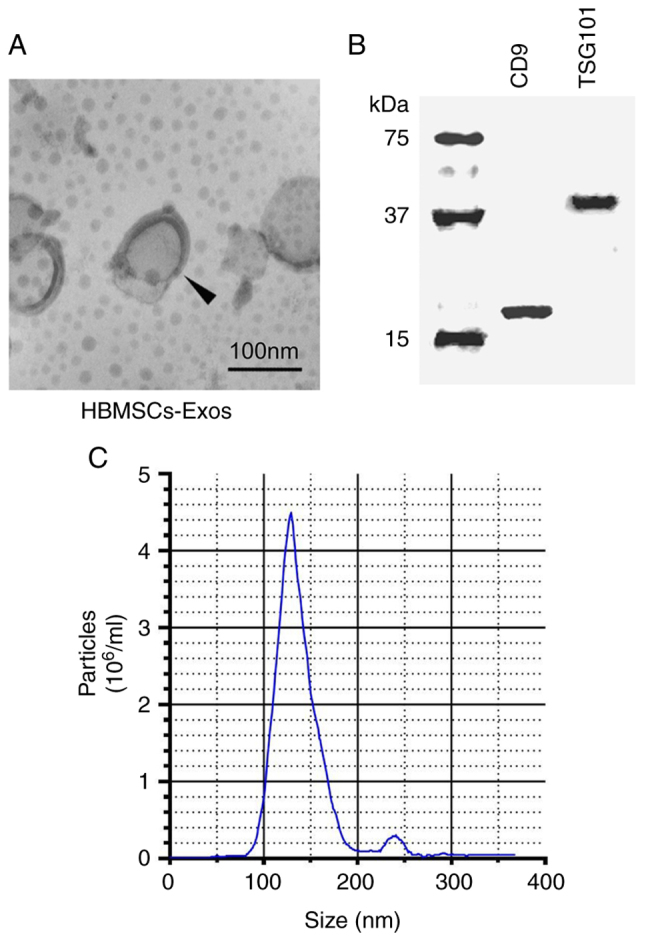
Identification of Exosomes. (A) The TEM image of blank HBMSC-Exos (magnification, ×20,000). (B) Expression of HBMSC-Exos-specific proteins (CD9, TSG101) analyzed using western blot analysis. (C) Size analysis of exosomes from HBMSCs was performed using nanoparticle tracking analysis. TEM, transmission electron microscope; HBMSCs, human bone marrow mesenchymal stem cells; Exos, exoxomes; TSG101, tumor susceptibility 101.
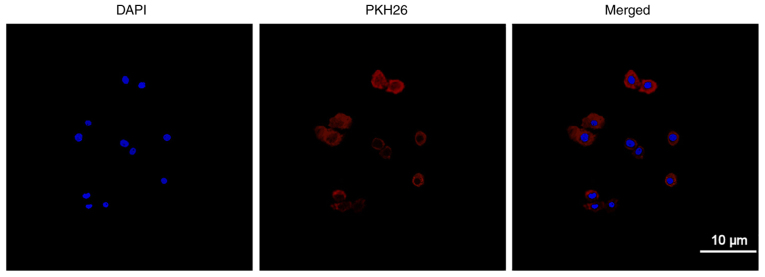
Uptake of exosomes
Exosomes were labelled with PKH26 and incubated with QBC939 cells before being observed using laser confocal scanning microscopy to determine the efficiency of the QBC939 cellular uptake of exosomes. Fig. 2 shows the cell nucleus stained with DAPI (blue) and exosomes labelled with PKH26 (red). Red fluorescence can be seen in the cytoplasm after confluence, revealing the QBC939 cellular uptake of the exosomes.
Figure 2.
Uptake of Exos. Uptake of Exos in CCA QBC939 cells is observed using laser confocal microscope. Exosomes were isolated from HBMSCs and labeled with PKH26 (red). The stained exosomes were co-cultured with QBC929 cells for 24 h, and then stained with DAPI (blue) (magnification, ×400). CCA, cholangiocarcinoma; HBMSCs, human bone marrow mesenchymal stem cells; Exos, exoxomes.
Efficiency of 5-Fu loaded in the exosomes
The protein content in exosomes was determined using a BCA kit, and the amount of 5-Fu encapsulated in the exosome was determined using HPLC to determine the loading efficiency of different loading methods (sonication and incubation). The following equation was used to calculate the drug loading rate (DL%): DL (%)=(Weight of drug-loaded/Weight of exosomes) ×100%. Fig. 3 depicts the result. In comparison to the incubation DL of 4.10%, the DL of sonication reached 6.79%.
Figure 3.
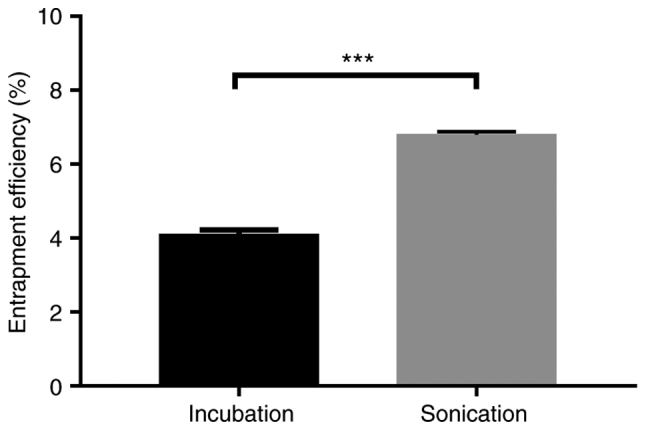
Determination of the loading efficiency of 5-Fu into HBMSC-derived exosomes. Comparison of the entrapment efficiency of 5-Fu into HBMSC-derived exosomes by sonication with incubation. Data were analyzed by Student's t-test; Significance level; ***P<0.001. HBMSCs, human bone marrow mesenchymal stem cells; 5-Fu, 5-fluorouracil.
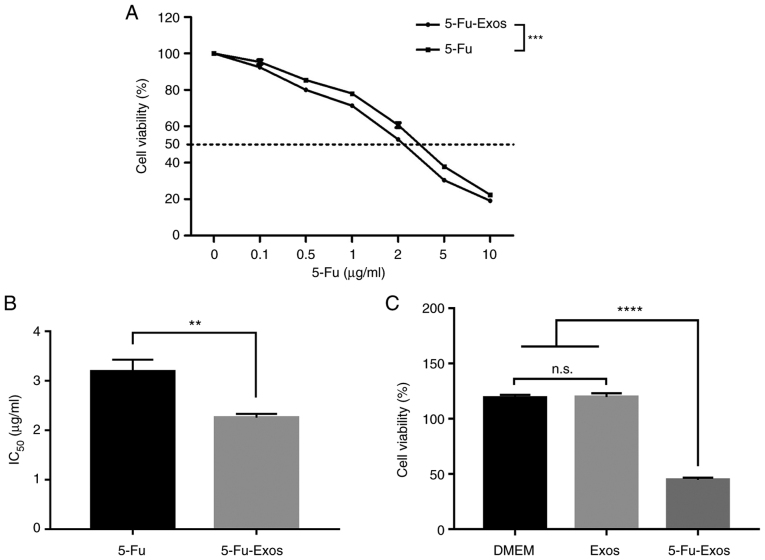
Effect of 5-Fu-Exos on the cell viability of QBC939 cells
The CCK-8 test was used to assess the effect of the drug-loaded exosomes on tumour proliferation. Fig. 4 depicts the cytotoxic properties of 5-Fu and 5-Fu-Exos. After 24 h of incubation with QBC939 cells, the 5-Fu-Exos group significantly inhibited tumour cell viability more than the 5-Fu group (Fig. 4A) and had a lower half-maximal inhibitory concentration (IC50) (Fig. 4B). The cytotoxicity of the exosome control groups (DMEM) revealed that naive exosomes (Exos) had no cytotoxic effect on QBC939 cells (Fig. 4C).
Figure 4.
Effect of HBMSC-derived Exo-5-Fu, free 5-Fu, DMEM and naive exosomes on the viability of CCA QBC939 cells. (A) QBC939 cells were treated with free 5-Fu, and Exo-5-Fu. The cells were further incubated for 24 h, and the viability assay was performed following CCK-8 assay protocol. (B) The half-maximal inhibitory concentration (IC50) of free 5-Fu and Exo-5-Fu, where the value was calculated according to A. (C) The naive exosomes from HBMSCs had no cytotoxic effect against QBC939 cells. Data were analyzed by one-way ANOVA with Bonferroni's post hoc test. Significance level; **P<0.01, ***P<0.001, ****P<0.0001, n.s., not significant. CCA, cholangiocarcinoma; HBMSCs, human bone marrow mesenchymal stem cells; Exos, exoxomes; 5-Fu, 5-fluorouracil.
Discussion
5-Fluorouracil (5-Fu) is currently one of the most commonly used chemotherapy drugs for cholangiocarcinoma (CCA) treatment, but its efficacy and clinical application are limited due to its dose-dependent nature. As a classic chemotherapy drug, 5-Fu promotes cell apoptosis by releasing metabolites after entering cells to disrupt RNA synthesis and the action of thymidylate synthase (26). Therefore, the apoptosis experiment was not repeated in this study. As for whether 5-Fu-Exos have any other different modulation actions, we will investigate them in future research.
Exosomes have been widely studied in targeted delivery systems in recent years due to their high biocompatibility, low cytotoxicity, and low immunogenicity. Exosomes can be loaded with a variety of therapeutic molecules to improve drug efficacy and inhibit tumour cell proliferation. According to Wei et al, loading doxorubicin (DOX) into exosomes derived from mesenchymal stem cells to inhibit osteosarcoma cell viability in vitro exhibited a better antitumor effect than free DOX (27). Exosomes are also important in the treatment of drug-resistant tumours. Liang et al discovered that electroporation and transfection of tumour-derived exosomes loaded with miR-21 inhibitor and 5-Fu could effectively reverse drug resistance and improve therapeutic outcomes in 5-Fu-resistant colon carcinoma cells (28). In the present study, exosomes derived from human bone marrow mesenchymal stem cells (HBMSCs) and loaded with 5-Fu were used to treat CCA. The exosome medication group significantly increased cytotoxicity in CCA cells and decreased drug concentration when compared to free 5-Fu.
Exosomes are gaining popularity as a carrier for drug and gene therapy. However, the problem of mass production of exosome loading systems remains difficult, with drug loading being an important component. Sonication is a popular loading method because it is simple and efficient. Exosomes have been found to be capable of carrying small-molecule drugs, proteins and small nucleic acids (22). Kim et al loaded paclitaxel into macrophage-derived engineered exosomes with a loading efficiency of 33% (29). The sonication and standard incubation methods were used to load 5-Fu in this experiment, and it was discovered that the sonication method had a higher loading efficiency. The sonication method can also be used to load small nucleic acids. According to research, the sonication approach has higher loading efficiency and less siRNA aggregation when loading siRNAs by electroporation and sonication (30). Furthermore, Thakur et al demonstrated that sonication and microfluidic technology are more efficient than traditional incubation and electroporation methods in loading DOX into exosomes. In addition, it was discovered that the sigmoid-type microfluidic structure outperformed the linear microfluidic structure when it came to drug loading (31). As a result, further research into optimising loading methods to improve exosome loading efficiency is warranted.
In conclusion, the present study successfully loaded the anti-cholangiocarcinoma drug 5-Fu into exosomes derived from HBMSCs using sonication and incubation methods. In terms of loading efficiency, sonication outperformed incubation, and 5-Fu-Exos had higher antineoplastic activity than free 5-Fu. Therefore, the goal of this study was to develop an exosome-based drug delivery system for the targeted delivery of 5-Fu for CCA treatment. It is expected that further research on mouse models of CCA will be conducted in the future to confirm the safety and efficacy of exosome-loading drugs in the treatment of CCA.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MC and JZ designed the research, performed the experiments, analyzed the data and wrote the manuscript. YL and NM collected the data. MC and JZ confirm the authenticity of all the raw data. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work (including the provided data) are appropriately investigated and resolved. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by The Ethics Review Committee at Taizhou People's Hospital, Taizhou, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031.e15. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European network for the study of cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 3.Strijker M, Belkouz A, van der Geest LG, van Gulik TM, van Hooft JE, de Meijer VE, Haj Mohammad N, de Reuver PR, Verheij J, de Vos-Geelen J, et al. Treatment and survival of resected and unresected distal cholangiocarcinoma: A nationwide study. Acta Oncol. 2019;58:1048–1055. doi: 10.1080/0284186X.2019.1590634. [DOI] [PubMed] [Google Scholar]
- 4.Kamsa-Ard S, Luvira V, Suwanrungruang K, Kamsa-Ard S, Luvira V, Santong C, Srisuk T, Pugkhem A, Bhudhisawasdi V, Pairojkul C. Cholangiocarcinoma trends, incidence, and relative survival in Khon Kaen, Thailand from 1989 through 2013: A population-based cancer registry study. J Epidemiol. 2019;29:197–204. doi: 10.2188/jea.JE20180007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindnér P, Rizell M, Hafström L. The impact of changed strategies for patients with cholangiocarcinoma in this millenium. HPB Surg. 2015;2015:736049. doi: 10.1155/2015/736049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alabraba E, Joshi H, Bird N, Griffin R, Sturgess R, Stern N, Sieberhagen C, Cross T, Camenzuli A, Davis R, et al. Increased multimodality treatment options has improved survival for hepatocellular carcinoma but poor survival for biliary tract cancers remains unchanged. Eur J Surg Oncol. 2019;45:1660–1667. doi: 10.1016/j.ejso.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Groot Koerkamp B, Wiggers JK, Allen PJ, Besselink MG, Blumgart LH, Busch OR, Coelen RJ, D'Angelica MI, DeMatteo RP, Gouma DJ, et al. Recurrence rate and pattern of perihilar cholangiocarcinoma after curative intent resection. J Am Coll Surg. 2015;221:1041–1049. doi: 10.1016/j.jamcollsurg.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komaya K, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nagino M. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: Analysis of a large cohort with a close postoperative follow-up approach. Surgery. 2018;163:732–738. doi: 10.1016/j.surg.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Søreide K, Wigmore SJ, Guest RV. Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma. Ann Surg. 2021;273:240–250. doi: 10.1097/SLA.0000000000003801. [DOI] [PubMed] [Google Scholar]
- 10.Spolverato G, Kim Y, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, Clark Gamblin T, Maithel SK, Pulitano C, Bauer TW, et al. Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection. Ann Surg Oncol. 2016;23:235–243. doi: 10.1245/s10434-015-4642-9. [DOI] [PubMed] [Google Scholar]
- 11.Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39((Suppl 1)):S98–S107. doi: 10.1111/liv.14086. [DOI] [PubMed] [Google Scholar]
- 12.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lollo G, Matha K, Bocchiardo M, Bejaud J, Marigo I, Virgone-Carlotta A, Dehoux T, Rivière C, Rieu JP, Briançon S, et al. Drug delivery to tumours using a novel 5-FU derivative encapsulated into lipid nanocapsules. J Drug Target. 2019;27:634–645. doi: 10.1080/1061186X.2018.1547733. [DOI] [PubMed] [Google Scholar]
- 14.Lee JJ, Beumer JH, Chu E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother Pharmacol. 2016;78:447–464. doi: 10.1007/s00280-016-3054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang CG, Ciccolini J, Blesius A, Dahan L, Bagarry-Liegey D, Brunet C, Varoquaux A, Frances N, Marouani H, Giovanni A, et al. DPD-based adaptive dosing of 5-FU in patients with head and neck cancer: Impact on treatment efficacy and toxicity. Cancer Chemother Pharmacol. 2011;67:49–56. doi: 10.1007/s00280-010-1282-4. [DOI] [PubMed] [Google Scholar]
- 16.Bell BM, Kirk ID, Hiltbrunner S, Gabrielsson S, Bultema JJ. Designer exosomes as next-generation cancer immunotherapy. Nanomedicine. 2016;12:163–169. doi: 10.1016/j.nano.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R, Tewari M, Hladik F. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014;42:7290–7304. doi: 10.1093/nar/gku347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong J, Xia B, Shan S, Zheng A, Zhang S, Chen J, Liang XJ. High-quality milk exosomes as oral drug delivery system. Biomaterials. 2021;277:121126. doi: 10.1016/j.biomaterials.2021.121126. [DOI] [PubMed] [Google Scholar]
- 20.Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, Jiang Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. doi: 10.1038/s41392-020-00261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 2021;329:894–906. doi: 10.1016/j.jconrel.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- 24.Farooqi AA, Desai NN, Qureshi MZ, Librelotto DRN, Gasparri ML, Bishayee A, Nabavi SM, Curti V, Daglia M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv. 2018;36:328–334. doi: 10.1016/j.biotechadv.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, et al. Reassessment of exosome composition. Cell. 2019;177:428–445.e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 27.Wei H, Chen J, Wang S, Fu F, Zhu X, Wu C, Liu Z, Zhong G, Lin J. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int J Nanomedicine. 2019;14:8603–8610. doi: 10.2147/IJN.S218988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, Sun B, Chen B, Xiao Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 2020;18:10. doi: 10.1186/s12951-019-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, Kabanov AV, Batrakova EV. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine. 2018;14:195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol Bioeng. 2016;9:315–324. doi: 10.1007/s12195-016-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thakur A, Sidu RK, Zou H, Alam MK, Yang M, Lee Y. Inhibition of glioma cells' proliferation by doxorubicin-loaded exosomes via microfluidics. Int J Nanomedicine. 2020;15:8331–8343. doi: 10.2147/IJN.S263956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.