Abstract
Hyperandrogenism and hyperinsulinemia have resulted from dysfunction of the theca cell of the ovary and adipose tissue and each one potentiates the other in patients with androgen excess disorders e.g., polycystic ovary disease and idiopathic hirsutism. Possible external and/or internal triggers can produce such cellular dysfunction. There is evidence that sodium valproate acts as a trigger of cellular dysfunction and produces both hyperinsulinemia and hyperandrogenism. Therefore, the elimination of these triggers can help the patients to recover from hyperinsulinemia, insulin resistance and hyperandrogenism.
Keywords: Hyperandrogenism, Hyperinsulinism, Central triggers, Polycystic ovary disease
Core Tip: There is a close relationship between hyperinsulinemia and androgen excess in patients with androgen excess disorders. These disorders result from the dysfunction of gonad and adipose cells under the influence of a specific trigger. Sodium valproate is an example of an external trigger that produces concomitant hyperinsulinemia and androgenism leading to polycystic ovary syndrome. Therefore, elimination of the triggers can lead to recovery from antiepileptic drugs while using insulin sensitizers and/or anti-androgens can help to solve this pathological problem.
TO THE EDITOR
I read with great interest an elegant review by Unluhizarci et al[1] who presented the role of insulin in the androgen excess disorders (AEDs) taking polycystic ovary syndrome (PCOS) and idiopathic hirsutism as examples of AEDs. The authors filled the gap about the relationship between hyperandrogenism and hyperinsulinism and they highlighted the following important points: (1) The severity of insulin resistance is related to the phenotype of PCOS; (2) Hyperinsulinemia promotes the ovarian androgen synthesis in a mechanism not related to the gonadotropins; and (3) Using sodium valproate can cause androgen excess and hirsutism. Therefore, according to these important points, it is possible to consider that PCOS is a functional disease of concomitant dysregulation of androgen excess and dysfunction of the adipose tissue which is triggered by exogenous and/or endogenous insult at the hypothalamus-pituitary-target organs (gonads and adrenals)[2,3]. Some authors believe dysregulation of the androgen secretion in the theca cell of the ovary and adrenal gland can produce functional ovarian and adrenal hyperandrogenism, which not necessarily leads to hyperinsulinism and insulin resistance, while dysfunction of the adipose tissue can cause hyperinsulinism and insulin resistance[4]. Therefore, a question has arisen about which factor, trigger substance or event that causes the dysfunction of the theca cells and adipose tissue is still unknown.
So, any therapeutic intervention at the ovarian cell or adipose tissue will ultimately affect the other factor, because each factor potentiates the effect of another factor as Unluhizarci et al[1] mentioned in their review (Figure 1). Therefore, the use of insulin sensitizers and/or anti-androgens are of value in ameliorating the biochemical and clinical features of PCOS[5,6], but these medicines, when used as monotherapy, cannot correct hyperandrogenism and hyperinsulinemia at the same time.
Figure 1.
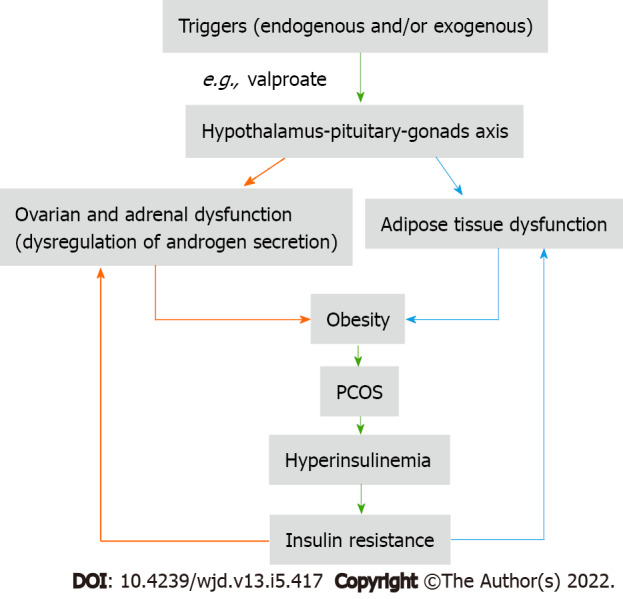
Interaction between hyperinsulinemia and hyperandrogenism as a result of an insult at the hypothalamic-pituitary-gonad axis. PCOS: Polycystic ovary syndrome.
Sodium valproate is a modifiable risk factor for the development of PCOS in epileptic and bipolar disorder women by increasing body weight and androgen production[7,8]. In addition, sodium valproate induces hyperinsulinism by having a direct effect on the beta-cell of the pancreas and an indirect effect by suppressing peripheral insulin-glucose uptake[9]. According to the valproate example, PCOS is the result of the vicious cycle (hyperinsulinism-hyperandrogenism) triggered by external or internal modifiable factors which are producing ovarian cell dysfunction. According to the literature, the triggers that cause dysfunction of the ovaries and adrenal glands act on the hypothalamic-pituitary-gonadal axis, and this explains why valproate can produce manifestations of PCOS in epileptic and bipolar depressed women. This effect seems to be gender-based because the relationship between insulin resistance and circulating androgens in obese young men is significantly inversed, while in PCOS women is significantly positive, indicating that there is a trigger factor that causes specific dysfunction of ovarian cells[10].
ACKNOWLEDGEMENTS
The author expressed his appreciation to the author of the article, Dr. Kursad Unluhizarci and his team, Department of Endocrinology, Erciyes University Medical School, Kayseri, Turkey, for doing this type of research.
Footnotes
Conflict-of-interest statement: The author declares no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: May 10, 2021
First decision: September 5, 2021
Article in press: April 25, 2022
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Iraq
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Balbaa ME, Egypt; Ng HY, China; Xiong YP, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
References
- 1.Unluhizarci K, Karaca Z, Kelestimur F. Role of insulin and insulin resistance in androgen excess disorders. World J Diabetes. 2021;12:616–629. doi: 10.4239/wjd.v12.i5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim JJ, Lima PDA, Salehi R, Lee DR, Tsang BK. Regulation of androgen receptor signaling by ubiquitination during folliculogenesis and its possible dysregulation in polycystic ovarian syndrome. Sci Rep. 2017;7:10272. doi: 10.1038/s41598-017-09880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macut D, Bjekić-Macut J, Rahelić D, Doknić M. Insulin and the polycystic ovary syndrome. Diabetes Res Clin Pract. 2017;130:163–170. doi: 10.1016/j.diabres.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev. 2016;37:467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambineri A, Pelusi C, Genghini S, Morselli-Labate AM, Cacciari M, Pagotto U, Pasquali R. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60:241–249. doi: 10.1111/j.1365-2265.2004.01973.x. [DOI] [PubMed] [Google Scholar]
- 6.Condorelli RA, Calogero AE, Di Mauro M, Mongioi' LM, Cannarella R, Rosta G, La Vignera S. Androgen excess and metabolic disorders in women with PCOS: beyond the body mass index. J Endocrinol Invest. 2018;41:383–388. doi: 10.1007/s40618-017-0762-3. [DOI] [PubMed] [Google Scholar]
- 7.Morrell MJ, Isojärvi J, Taylor AE, Dam M, Ayala R, Gomez G, O'Neill F, Tennis P, Messenheimer J. Higher androgens and weight gain with valproate compared with lamotrigine for epilepsy. Epilepsy Res. 2003;54:189–199. doi: 10.1016/s0920-1211(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 8.Prabhakar S, Sahota P, Kharbanda PS, Siali R, Jain V, Lal V, Khurana D. Sodium valproate, hyperandrogenism and altered ovarian function in Indian women with epilepsy: a prospective study. Epilepsia. 2007;48:1371–1377. doi: 10.1111/j.1528-1167.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 9.Verrotti A, la Torre R, Trotta D, Mohn A, Chiarelli F. Valproate-induced insulin resistance and obesity in children. Horm Res. 2009;71:125–131. doi: 10.1159/000197868. [DOI] [PubMed] [Google Scholar]
- 10.Kurniawan LB, Adnan E Windarwati , Mulyono B. Insulin resistance and testosterone level in Indonesian young adult males. Rom J Intern Med. 2020;58:93–98. doi: 10.2478/rjim-2020-0004. [DOI] [PubMed] [Google Scholar]


