This cohort study assesses the risk of hematologic cancer and the diagnostic utility of lactate dehydrogenase level in patients with undifferentiated pruritus.
Key Points
Question
What is the risk of hematologic cancer and the diagnostic utility of serum lactate dehydrogenase testing among patients with undifferentiated pruritus?
Findings
In this cohort study of 327 502 patients and 327 502 matched controls, patients with undifferentiated pruritus had an increased risk of being diagnosed with a hematologic cancer in the first year, and serum lactate dehydrogenase level was not associated with increased risk of hematologic cancer.
Meaning
Clinicians should consider a thorough review of symptoms and assessment of cancer risk factors when deciding on workup for patients presenting with undifferentiated pruritus.
Abstract
Importance
Although pruritus is common in patients with hematologic cancers, it is unknown whether patients with undifferentiated pruritus have higher risk of developing hematologic cancer. Furthermore, it is unclear whether serum lactate dehydrogenase (LDH) level, commonly ordered for cancer workup, has diagnostic utility in patients with pruritus.
Objective
To assess the risk of hematologic cancer and the diagnostic utility of LDH level in patients with undifferentiated pruritus.
Design, Setting, and Participants
This retrospective population-level cohort analysis was conducted using the TriNetX Research Network, a global health records database encompassing more than 69 million patients, from 2002 to 2020. The study included 327 502 eligible patients diagnosed with unspecified pruritus, excluding those with existing chronic pruritic dermatoses or systemic diseases known to cause pruritus, along with 327 502 matched controls.
Exposures
Development of hematologic cancer within 1 year, 5 years, and 10 years following unspecified pruritus diagnosis.
Main Outcomes and Measures
Primary study outcomes were 1-year, 5-year, and 10-year relative risks (RRs) for development of 9 hematologic cancers in patients with pruritus compared with control patients. Secondary outcomes were 1-year, 5-year, and 10-year RRs for any hematologic cancer at different LDH cutoffs (250 U/L and 500 U/L).
Results
After matching, the pruritus and control cohorts each had 327 502 patients (68.1% female patients; 0.4% American Indian or Alaska Native patients; 3.5% Asian patients; 22.2% Black patients; 0.1% Native Hawaiian or Pacific Islander patients; 59.3% White patients; mean [SD] age, 42.2 [22] years). Patients with pruritus had increased 1-year risk of Hodgkin lymphoma (RR, 4.42; 95% CI, 2.83-6.88), myeloid leukemia (RR, 2.56; 95% CI, 1.79-3.67), multiple myeloma (RR, 2.38; 95% CI, 1.66-3.41), non-Hodgkin lymphoma (RR, 2.35; 95% CI, 1.96-2.82), monoclonal gammopathy (RR, 1.90; 95% CI, 1.55-2.32), myelodysplastic syndrome (RR, 1.74; 95% CI, 1.14-2.64), and lymphocytic leukemia (RR, 1.47; 95% CI, 1.07-2.02). After 12 months, the cancer risk was comparable with that of controls. Patients with pruritus had increased LDH levels, which were not associated with increased hematologic cancer risk.
Conclusions and Relevance
In this cohort study, the RR of hematologic cancer in patients with undifferentiated pruritus was highest in the first 12 months, and LDH level had limited diagnostic utility in these patients. Clinicians should consider a thorough review of symptoms and assessment of cancer risk factors when deciding on workup for patients presenting with undifferentiated pruritus.
Introduction
Pruritus is a common complaint in dermatology and is associated with psychiatric, neurologic, and systemic conditions.1,2 Pruritus can be an early symptom in patients with hematologic cancers, in whom the prevalence of pruritus approaches 30%.3 However, the risk of future cancer among patients with pruritus is not yet established; studies assessing this association have been limited by cross-sectional designs, small sample sizes, and limited follow-up periods.4,5,6,7 Furthermore, the evaluation of patients with pruritus for cancer involves measuring serum lactate dehydrogenase (LDH), a nonspecific marker of cell turnover, as LDH level is elevated in multiple cancers.8 However, the diagnostic utility of LDH level in patients with pruritus remains unclear. This study assesses the risk of developing hematologic cancer in patients presenting with undifferentiated pruritus, and the utility of serum LDH level as a prognostic marker for cancer in patients with pruritus.
Methods
This retrospective cohort study used the TriNetX Research Network, a global health research network comprising deidentified electronic medical records from more than 69 million patients, particularly in the US and Europe. Participants from 2002 to 2020 were identified using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code for unspecified pruritus (L29.9). Diagnoses prior to the introduction of ICD-10 were classified using General Equivalence Mappings.9 Patients with existing chronic pruritic dermatoses (atopic dermatitis, psoriasis, prurigo nodularis, lichen planus) or systemic diseases associated with pruritus (kidney disease, hepatobiliary disease, malignant neoplasms) were excluded. Similar exclusion criteria were applied to controls, who lacked a diagnosis of unspecified pruritus. Patients with pruritus and controls were 1:1 propensity score matched by age, sex, race and ethnicity, smoking status, alcohol use, and body mass index (calculated as weight in kilograms divided by height in meters squared) using a greedy nearest neighbor matching algorithm with a caliper of 0.1 pooled SDs. All demographic and clinical classification data were obtained from the TriNetX database. The study design is depicted in the eFigure in the Supplement. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
For the primary analysis, we assessed the risks of developing Hodgkin lymphoma, non-Hodgkin lymphoma, lymphocytic leukemia, myeloid leukemia, myelodysplastic syndrome, multiple myeloma, Waldenström macroglobulinemia, monoclonal gammopathy, and polycythemia vera. To allow for full 1-year, 5-year, and 10-year observation periods, only patients with index dates prior to 2020, 2016, and 2011, respectively, were included. Patients with missing covariate data or who were lost to follow-up were excluded. Absolute risks and cumulative relative risks (RRs) with 95% CIs for each observation period were conducted through analysis of all patients in the data set. Serum LDH levels were assessed in patients with available values within 3 months of the index date. Cancer risk in patients with elevated LDH level was assessed using 2 cutoffs: 250 U/L and 500 U/L (to convert to µkat/L, multiply by 0.0167). Continuous and categorical variables were compared using the t test and a Z-test for proportions, respectively. The Benjamini-Hochberg method was used to adjust P values for multiple hypothesis testing, with an a priori α of .05. Statistical analyses were performed using the TriNetX platform. Because TriNetX contains only deidentified data, this study was exempt from institutional review board approval and patient informed consent.
Results
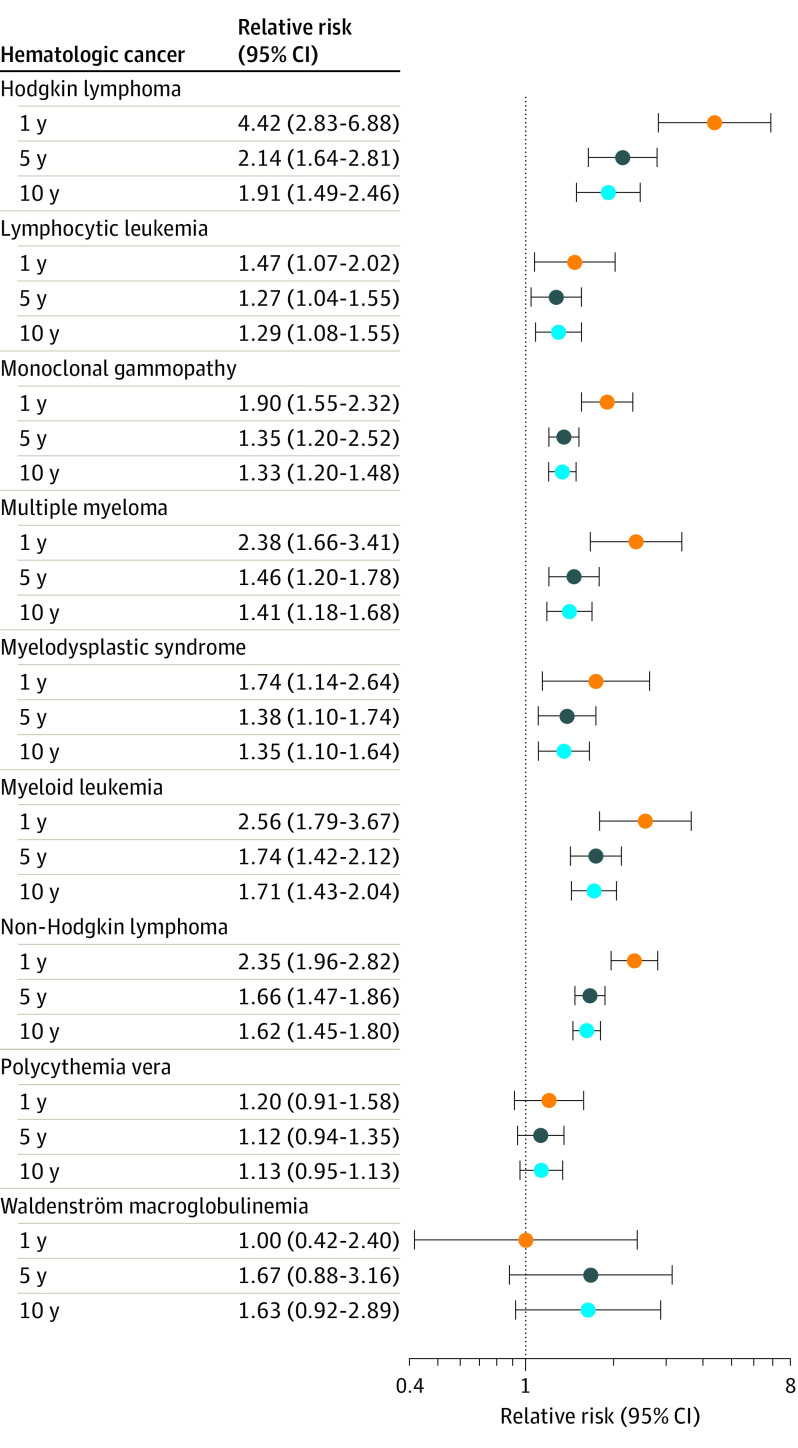
We identified 327 502 patients with undifferentiated pruritus, who were matched to 327 502 controls. Baseline characteristics for the study cohorts are depicted in Table 1. Sensitivity analysis to account for differences in health care utilization identified comparable mean (IQR) numbers of ambulatory visits (3.0 [2.0-6.0]) between patients with pruritus and controls (3.0 [1.0-5.0]), and the same frequency of emergency visits (1.0 [1.0-2.0]). The cumulative RRs of diagnosis for each hematologic cancer are shown in the Figure and Table 2. At 1 year, undifferentiated pruritus was associated with an increased risk of Hodgkin lymphoma (RR, 4.42; 95% CI, 2.83-6.88), myeloid leukemia (RR, 2.56; 95% CI, 1.79-3.67), multiple myeloma (RR, 2.38; 95% CI, 1.66-3.41), non-Hodgkin lymphoma (RR, 2.35; 95% CI, 1.96-2.82), monoclonal gammopathy (RR, 1.90; 95% CI, 1.55-2.32), myelodysplastic syndrome (RR, 1.74; 95% CI, 1.14-2.64), and lymphocytic leukemia (RR, 1.47; 95% CI, 1.07-2.02). At 5 years, undifferentiated pruritus was associated with an increased risk of Hodgkin lymphoma (RR, 2.14; 95% CI, 1.64-2.81), myeloid leukemia (RR, 1.74; 95% CI, 1.42-2.12), non-Hodgkin lymphoma (RR, 1.66; 95% CI, 1.47-1.86), multiple myeloma (RR, 1.46; 95% CI, 1.20-1.78), myelodysplastic syndrome (RR, 1.38; 95% CI, 1.10-1.74), monoclonal gammopathy (RR, 1.35; 95% CI, 1.20-2.52), and lymphocytic leukemia (RR, 1.27; 95% CI, 1.04-1.55). At 10 years, undifferentiated pruritus was associated with an increased risk of Hodgkin lymphoma (RR, 1.91; 95% CI, 1.49-2.46), myeloid leukemia (RR, 1.71; 95% CI, 1.43-2.04), non-Hodgkin lymphoma (RR, 1.62; 95% CI, 1.45-1.80), multiple myeloma (RR, 1.41; 95% CI, 1.18-1.68), myelodysplastic syndrome (RR, 1.35; 95% CI, 1.10-1.64), monoclonal gammopathy (RR, 1.33; 95% CI, 1.20-1.48), and lymphocytic leukemia (RR, 1.29; 95% CI, 1.08-1.55). Patients with pruritus had the highest absolute risk of diagnosis with non-Hodgkin lymphoma in 1 year (0.117% vs 0.050%; P < .001) and 5 years (0.222% vs 0.134%; P < .001), as well as monoclonal gammopathy in 5 years (0.196% vs 0.145%; P < .001) and 10 years (0.235% vs 0.176%; P < .001) (Table 2).
Table 1. Baseline Demographic Characteristics of Patients With Undifferentiated Pruritus and Control Patients After Propensity Score Matching.
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| Pruritus (n = 327 502) | Control (n = 327 502) | ||
| Age at index, mean (SD), y | 42.2 (22.1) | 42.2 (22.1) | .98 |
| Sex | |||
| Female | 223 109 (68.1) | 223 109 (68.1) | .96 |
| Male | 104 327 (31.9) | 104 327 (31.9) | .98 |
| Race and ethnicitya | |||
| American Indian or Alaska Native | 1243 (0.4) | 1236 (0.4) | .89 |
| Asian | 11 310 (3.5) | 11 283 (3.5) | .86 |
| Black | 72 674 (22.2) | 72 706 (22.2) | .92 |
| Native Hawaiian or Pacific Islander | 470 (0.1) | 453 (0.1) | .58 |
| White | 194 011 (59.2) | 194 032 (59.3) | .96 |
| Risk factors | |||
| Smoking | 28 091 (8.6) | 28 094 (8.6) | .99 |
| Alcohol use disorder | 6915 (2.1) | 6900 (2.1) | .90 |
| BMI, mean (SD) | 28.4 (7.7) | 27.9 (7.7) | .98 |
Abbreviation: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared.
The remainder 14.6% signifies unknown category. The TriNetX database does not distinguish between unknown races and ethnicities and missing information.
Figure. Cumulative Relative Risks of Hematologic Cancers After 1 Year, 5 Years, and 10 Years.
Cumulative relative risks of hematologic cancers measured in patients following the initial visit for undifferentiated pruritus, compared with matched control patients.
Table 2. Absolute and Cumulative Relative Risks of Hematologic Cancers After 1 Year, 5 Years, and 10 Years.
| Hematologic cancer | Absolute risk, No. (%) | Relative risk (95% CI) | P value | |
|---|---|---|---|---|
| Pruritus | Controls | |||
| Hodgkin lymphoma, y | ||||
| 1 | 106 (0.032) | 24 (0.007) | 4.42 (2.83-6.88) | <.001 |
| 5 | 165 (0.050) | 77 (0.024) | 2.14 (1.64-2.81) | <.001 |
| 10 | 178 (0.054) | 93 (0.028) | 1.91 (1.49-2.46) | <.001 |
| Lymphocytic leukemia, y | ||||
| 1 | 94 (0.029) | 64 (0.020 | 1.47 (1.07-2.02) | .02 |
| 5 | 221 (0.067) | 174 (0.053) | 1.27 (1.04-1.55) | .02 |
| 10 | 269 (0.082) | 208 (0.064) | 1.29 (1.08-1.55) | .005 |
| Monoclonal gammopathy, y | ||||
| 1 | 273 (0.083) | 144 (0.044) | 1.90 (1.55-2.32) | <.001 |
| 5 | 642 (0.196) | 476 (0.145) | 1.35 (1.20-2.52) | <.001 |
| 10 | 769 (0.235) | 577 (0.176) | 1.33 (1.20-1.48) | <.001 |
| Multiple myeloma, y | ||||
| 1 | 100 (0.031) | 42 (0.013) | 2.38 (1.66-3.41) | <.001 |
| 5 | 241 (0.074) | 165 (0.050) | 1.46 (1.20-1.78) | <.001 |
| 10 | 293 (0.089) | 208 (0.064) | 1.41 (1.18-1.68) | <.001 |
| Myelodysplastic syndrome, y | ||||
| 1 | 59 (0.018) | 34 (0.010) | 1.74 (1.14-2.64) | .009 |
| 5 | 177 (0.054) | 128 (0.039) | 1.38 (1.10-1.74) | .005 |
| 10 | 224 (0.068) | 166 (0.051) | 1.35 (1.10-1.64) | .003 |
| Myeloid leukemia, y | ||||
| 1 | 105 (0.032) | 41 (0.013) | 2.56 (1.79-3.67) | <.001 |
| 5 | 262 (0.080) | 151 (0.046) | 1.74 (1.42-2.12) | <.001 |
| 10 | 330 (0.101) | 193 (0.059) | 1.71 (1.43-2.04) | <.001 |
| Non-Hodgkin lymphoma, y | ||||
| 1 | 383 (0.117) | 163 (0.050) | 2.35 (1.96-2.82) | <.001 |
| 5 | 727 (0.222) | 439 (0.134) | 1.66 (1.47-1.86) | <.001 |
| 10 | 846 (0.258) | 524 (0.160) | 1.62 (1.45-1.80) | <.001 |
| Polycythemia vera, y | ||||
| 1 | 114 (0.035) | 95 (0.029) | 1.20 (0.91-1.58) | .19 |
| 5 | 245 (0.075) | 218 (0.067) | 1.12 (0.94-1.35) | .21 |
| 10 | 289 (0.088) | 256 (0.078) | 1.13 (0.95-1.13) | .16 |
| Waldenström macroglobulinemia, y | ||||
| 1 | 10 (0.003) | 10 (0.003) | 1.00 (0.42-2.40) | >.99 |
| 5 | 25 (0.008) | 15 (0.005) | 1.67 (0.88-3.16) | .11 |
| 10 | 31 (0.009) | 19 (0.006) | 1.63 (0.92-2.89) | .09 |
Patients with pruritus were at the highest risk of hematologic cancer within 12 months of initial diagnosis. Following the first year, the risks were still significantly higher, but more comparable with that of control patients. Patients with pruritus had a higher mean (SD) serum LDH (297.55 [310.99] U/L) compared with controls (255.51 [223.73] U/L) (P < .001). However, patients with serum LDH level greater than the cutoff values of 250 U/L and 500 U/L did not have significantly higher risks of hematologic cancer diagnosis after 1, 5, or 10 years (eTable in the Supplement).
Discussion
This study highlights that clinicians should have a heightened concern for underlying hematologic cancer in patients with undifferentiated pruritus, especially within the first 12 months of presentation. These findings align with prior studies of increased risks of Hodgkin lymphoma, non-Hodgkin lymphoma, and myeloid leukemia in patients with pruritus.10 We additionally found increased risks of multiple myeloma, myelodysplastic syndrome, lymphocytic leukemia, and monoclonal gammopathy. Pruritus in cancer is thought to arise from systemic inflammation; in some hematologic cancers, such as Hodgkin lymphoma, there is increased release of proinflammatory and pruritogenic cytokines, including interleukin-6, interleukin-31, and thymic stromal lymphopoietin.11,12,13
Although patients with undifferentiated pruritus had higher serum LDH levels, higher LDH levels were not associated with increased risk of hematologic cancer. Current guidelines from the British Association of Dermatologists and the National Comprehensive Cancer Network in the US recommend LDH testing to evaluate for certain hematopoietic cancers in patients with pruritus.14,15 Given the findings of the present study, serum LDH levels have limited diagnostic utility in the initial workup for pruritus.
Limitations
Limitations of this study include the use of ICD-10 codes to identify patient diagnoses, which may be liable to misclassification and can affect determination of the timing of cancer development, and the inability to distinguish between generalized and localized pruritus. Furthermore, despite the identified elevated RRs of cancer, absolute risk differences are low, and most patients with undifferentiated pruritus will not have an underlying hematologic cancer. Finally, causal relationships between pruritus and hematologic cancers cannot be determined.
Conclusions
In this cohort study, findings show that undifferentiated pruritus was associated with an elevated risk of diagnosis with hematologic cancers, especially within the first 12 months of presentation. Clinicians should conduct a thorough review of symptoms and assessment of cancer risk factors when evaluating patients presenting with undifferentiated pruritus. Future studies should examine covariates, such as age or patient-specific risk factors, for the development of specific hematologic cancers in patients with pruritus, as well as the optimal diagnostic and prognostic hematologic workup.
eFigure. Study Flow Diagram
eTable. Relative Risks of Hematologic Cancer Development Based on Serum Lactate Dehydrogenase Levels
References
- 1.Roh YS, Choi J, Sutaria N, Kwatra SG. Itch: epidemiology, clinical presentation, and diagnostic workup. J Am Acad Dermatol. 2022;86(1):1-14. doi: 10.1016/j.jaad.2021.07.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whang KA, Khanna R, Williams KA, Mahadevan V, Semenov Y, Kwatra SG. Health-related QOL and economic burden of chronic pruritus. J Invest Dermatol. 2021;141(4):754-760.e1. doi: 10.1016/j.jid.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 3.Larson VA, Tang O, Ständer S, Kang S, Kwatra SG. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80(4):931-937. doi: 10.1016/j.jaad.2018.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belzberg M, Larson VA, Khanna R, et al. Association between itch and cancer in 3836 pediatric pruritus patients at a tertiary care center. Medicines (Basel). 2019;6(4):E99. doi: 10.3390/medicines6040099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fett N, Haynes K, Propert KJ, Margolis DJ. Predictors of malignancy development in patients with chronic pruritus. J Dermatol Sci. 2016;82(2):123-128. doi: 10.1016/j.jdermsci.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 6.Sommer F, Hensen P, Böckenholt B, Metze D, Luger TA, Ständer S. Underlying diseases and co-factors in patients with severe chronic pruritus: a 3-year retrospective study. Acta Derm Venereol. 2007;87(6):510-516. doi: 10.2340/00015555-0320 [DOI] [PubMed] [Google Scholar]
- 7.Zirwas MJ, Seraly MP. Pruritus of unknown origin: a retrospective study. J Am Acad Dermatol. 2001;45(6):892-896. doi: 10.1067/mjd.2001.117732 [DOI] [PubMed] [Google Scholar]
- 8.Livesey A, Garty F, Shipman AR, Shipman KE. Lactate dehydrogenase in dermatology practice. Clin Exp Dermatol. 2020;45(5):539-543. doi: 10.1111/ced.14134 [DOI] [PubMed] [Google Scholar]
- 9.Topaloglu U, Palchuk MB. Using a federated network of real-world data to optimize clinical trials operations. JCO Clin Cancer Inform. 2018;2:1-10. doi: 10.1200/CCI.17.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johannesdottir SA, Farkas DK, Vinding GR, et al. Cancer incidence among patients with a hospital diagnosis of pruritus: a nationwide Danish cohort study. Br J Dermatol. 2014;171(4):839-846. doi: 10.1111/bjd.13157 [DOI] [PubMed] [Google Scholar]
- 11.Biggar RJ, Johansen JS, Smedby KE, et al. Serum YKL-40 and interleukin 6 levels in Hodgkin lymphoma. Clin Cancer Res. 2008;14(21):6974-6978. doi: 10.1158/1078-0432.CCR-08-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferretti E, Hohaus S, Di Napoli A, et al. Interleukin-31 and thymic stromal lymphopoietin expression in plasma and lymph node from Hodgkin lymphoma patients. Oncotarget. 2017;8(49):85263-85275. doi: 10.18632/oncotarget.19665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe B, Yosipovitch G. Malignancy-associated pruritus. Eur J Pain. 2016;20(1):19-23. doi: 10.1002/ejp.760 [DOI] [PubMed] [Google Scholar]
- 14.Millington GWM, Collins A, Lovell CR, et al. British Association of Dermatologists’ guidelines for the investigation and management of generalized pruritus in adults without an underlying dermatosis, 2018. Br J Dermatol. 2018;178(1):34-60. doi: 10.1111/bjd.16117 [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): primary cutaneous lymphomas (version 2.2021). Accessed July 4, 2021. https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Study Flow Diagram
eTable. Relative Risks of Hematologic Cancer Development Based on Serum Lactate Dehydrogenase Levels