Key Points
Question
How do the characteristics and outcomes of patients randomized to receive antibiotics for appendicitis compare with those of patients who declined randomization and self-selected their treatment?
Findings
In this secondary analysis of the CODA randomized clinical trial (RCT), including 1094 randomized patients and a contemporaneously recruited self-selection cohort consisting of 510 patients who declined randomization, clinical characteristics and most outcomes were similar between RCT participants and those who selected treatment. Several sociodemographic characteristics differed between the RCT and self-selection cohorts.
Meaning
Substantially similar outcomes across the randomized and self-selection cohorts of CODA suggest that the RCT results are generalizable to the community at large.
This secondary analysis of a randomized clinical trial compares the characteristics and outcomes of patients with appendicitis who were randomized to receive antibiotics compared with those who declined randomization and selected their own treatment.
Abstract
Importance
For adults with appendicitis, several randomized clinical trials have demonstrated that antibiotics are an effective alternative to appendectomy. However, it remains unknown how the characteristics of patients in such trials compare with those of patients who select their treatment and whether outcomes differ.
Objective
To compare participants in the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) randomized clinical trial (RCT) with a parallel cohort study of participants who declined randomization and self-selected treatment.
Design, Setting, and Participants
The CODA trial was conducted in 25 US medical centers. Participants were enrolled between May 3, 2016, and February 5, 2020; all participants were eligible for at least 1 year of follow-up, with all follow-up ending in 2021. The randomized cohort included 1094 adults with appendicitis; the self-selection cohort included patients who declined participation in the randomized group, of whom 253 selected appendectomy and 257 selected antibiotics. In this secondary analysis, characteristics and outcomes in both self-selection and randomized cohorts are described with an exploratory analysis of cohort status and receipt of appendectomy.
Interventions
Appendectomy vs antibiotics.
Main Outcomes and Measures
Characteristics among participants randomized to either appendectomy or antibiotics were compared with those of participants who selected their own treatment.
Results
Clinical characteristics were similar across the self-selection cohort (510 patients; mean age, 35.8 years [95% CI, 34.5-37.1]; 218 female [43%; 95% CI, 39%-47%]) and the randomized group (1094 patients; mean age, 38.2 years [95% CI, 37.4-39.0]; 386 female [35%; 95% CI, 33%-38%]). Compared with the randomized group, those in the self-selection cohort were less often Spanish speaking (n = 99 [19%; 95% CI, 16%-23%] vs n = 336 [31%; 95% CI, 28%-34%]), reported more formal education (some college or more, n = 355 [72%; 95% CI, 68%-76%] vs n = 674 [63%; 95% CI, 60%-65%]), and more often had commercial insurance (n = 259 [53%; 95% CI, 48%-57%] vs n = 486 [45%; 95% CI, 42%-48%]). Most outcomes were similar between the self-selection and randomized cohorts. The number of patients undergoing appendectomy by 30 days was 38 (15.3%; 95% CI, 10.7%-19.7%) among those selecting antibiotics and 155 (19.2%; 95% CI, 15.9%-22.5%) in those who were randomized to antibiotics (difference, 3.9%; 95% CI, −1.7% to 9.5%). Differences in the rate of appendectomy were primarily observed in the non-appendicolith subgroup.
Conclusions and Relevance
This secondary analysis of the CODA RCT found substantially similar outcomes across the randomized and self-selection cohorts, suggesting that the randomized trial results are generalizable to the community at large.
Trial Registration
ClinicalTrials.gov Identifier: NCT02800785
Introduction
Randomized clinical trials (RCTs) are the criterion standard for comparing interventions, but narrow inclusion and exclusion criteria and self-selection frequently fail to reflect the at-risk population. Selection bias leading to differences between study and target populations can limit the generalizability of trial findings and may explain differential outcomes between RCTs and self-selection cohort studies. For treating appendicitis in adults, 9 RCTs1,2,3,4,5,6,7,8,9 have shown that appendectomy can usually be avoided with antibiotics. In the only large-scale US trial to date, 71% of participants treated with antibiotics did not undergo appendectomy by 90 days.10 The largest European trial conducted in a lower-risk group (excluding those with appendicolith) found 61% avoided surgery by 5 years.11 In contrast, a large observational cohort study based on commercial claims data found avoidance of appendectomy after antibiotics in nearly 94% of patients over 3 years.12 Another small observational study using clinical data13 found that among 159 patients treated with antibiotics, by 2 years, appendectomy was avoided in 86%.
Although selection bias may explain some of these differences, the lower rate of appendectomy identified in observational studies of antibiotics may also relate to patient belief that the treatment they select will work for them.14 Given that patients usually select their own treatment, observational studies may provide context for the results of RCTs. Including participants in research who decline randomization but agree to participate in a parallel observational cohort study can help identify the magnitude of any selection bias and potentially characterize the influence of treatment choice on outcomes. A parallel observational (self-selection) cohort may be especially informative when treatment arm crossover is possible or when a patient’s subjective response is the outcome of interest.
The Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial—a pragmatic, nonblinded, noninferiority RCT—randomized 1552 patients with the type of appendicitis commonly treated with appendectomy to either appendectomy or antibiotics.10,15 In CODA, antibiotics were found to be noninferior to appendectomy when considering a 5-domain generic global health-status measure (EQ-5D)16 at 30 days, with a similar rate of serious adverse events across study groups (2%).10 CODA included a parallel self-selection cohort of 510 patients who declined randomization but agreed to join a self-selection study of their experience.15 Here, we aim to describe (1) differences in characteristics of participants enrolled in the self-selection cohort compared with a contemporaneously recruited subset of the RCT, (2) outcomes in these 2 cohorts, and (3) reasons for appendectomy in those randomized to antibiotics vs those who selected antibiotics. Our investigation is primarily a descriptive study that includes an exploratory, post hoc analysis (without specific hypothesis testing) to consider the association between self-selection cohort status and the rate of appendectomy after initiating antibiotics.
Methods
The Patient-Centered Outcomes Research Institute (PCORI)–funded CODA RCT and its self-selection cohort have been previously described.15 The University of Washington Institutional Review Board approved the study with reciprocal or parallel approvals from the institutional review boards of the CODA sites; the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline was followed. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was followed for the self-selection cohort study. Only 23 of 25 CODA sites participated in both the RCT and self-selection cohort study (eAppendix 2 in Supplement 1). Recruitment of sites was staggered, with patients enrolled in the CODA trial from May 3, 2016, to February 5, 2020. Recruitment for the self-selection cohort ended on March 5, 2019. Participant follow-up was ended in 2021. All participants were eligible for at least 1 year of follow-up.
Study Population
Research coordinators evaluated the electronic medical records of all English- or Spanish-speaking adults (aged ≥18 years) with imaging-confirmed appendicitis in emergency departments (EDs). Exclusion criteria included contraindications to surgery, septic shock, diffuse peritonitis, radiographic evidence of severe phlegmon (if the surgeon determined that a more extensive resection was likely), walled-off abscess, significant amounts of free air or ascites, suspicion of neoplasm, or recurrent appendicitis. Sites were regularly audited to confirm that all patients with appendicitis were screened. Sociodemographic, clinical, and radiographic characteristics were assessed through patient self-report and electronic medical records and have been previously described.10,15 To more completely describe the patient population, self-reported race and ethnicity were included in Table 1.
Table 1. Baseline Participant Characteristics of the Self-selection and Randomized Clinical Trial Cohortsa.
| Characteristic | RCT subset (n = 1094)b | All self-selection (n = 510) | Self-selection-antibiotics (n = 257) | Self-selection-appendectomy (n = 253) |
|---|---|---|---|---|
| Age, mean (95% CI), y | 38.2 (37.4-39.0) | 35.8 (34.5-37.1) | 37.1 (35.2-39.0) | 34.4 (32.8-36.1) |
| Sex, % (95% CI) | ||||
| Male | 65 (62-67) | 57 (53-61) | 60 (54-66) | 54 (48-60) |
| Female | 35 (33-38) | 43 (39-47) | 40 (34-46) | 46 (40-52) |
| Race, % (95% CI)c | ||||
| American Indian or Alaska Native | 2 (1-3) | 1 (0-2) | 0 (0-2) | 1 (0-3) |
| Asian | 6 (5-8) | 10 (8-13) | 14 (10-19) | 7 (4-11) |
| Black | 8 (6-10) | 6 (5-9) | 8 (5-12) | 5 (3-8) |
| Native Hawaiian or Pacific Islander | 0.5 (0-1) | 0 | 0 | 0 |
| White | 59 (56-62) | 61 (56-65) | 57 (51-63) | 65 (59-71) |
| Other | 21 (19-24) | 17 (14-21) | 16 (12-21) | 19 (14-24) |
| Multiple | 4 (3-5) | 5 (3-7) | 5 (3-9) | 4 (2-7) |
| Hispanic ethnicity, % (95% CI) | ||||
| No | 57 (54-60) | 70 (66-74) | 74 (69-79) | 66 (60-72) |
| Yes | 43 (40-46) | 30 (26-34) | 26 (21-31) | 34 (28-40) |
| Primary language, % (95% CI) | ||||
| English | 64 (61-67) | 72 (68-76) | 72 (66-77) | 72 (66-77) |
| Spanish | 31 (28-34) | 19 (16-23) | 15 (11-20) | 24 (19-29) |
| Other | 5 (4-6) | 9 (6-11) | 13 (9-17) | 4 (2-8) |
| Employment, % (95% CI) | ||||
| Employed | 73 (70-76) | 70 (65-73) | 66 (60-72) | 73 (67-78) |
| Student | 6 (5-7) | 10 (8-13) | 13 (9-17) | 8 (5-11) |
| Retired or other | 21 (19-24) | 20 (17-24) | 21 (17-27) | 19 (15-25) |
| Education, % (95% CI) | ||||
| Some college or more | 63 (60-65) | 72 (68-76) | 71 (66-77) | 72 (66-77) |
| No college | 37 (35-40) | 28 (24-32) | 29 (23-34) | 28 (23-34) |
| Insurance, % (95% CI) | ||||
| Commercial | 45 (42-48) | 53 (48-57) | 52 (46-58) | 53 (47-59) |
| Medicare or Tricare | 13 (11-15) | 12 (10-16) | 11 (8-16) | 14 (10-19) |
| Medicaid or state | 17 (15-19) | 14 (11-17) | 13 (10-18) | 14 (11-19) |
| Other or none | 25 (22-27) | 21 (18-25) | 24 (19-29) | 19 (15-24) |
| Below the federal poverty level or a Medicaid beneficiary, % (95% CI) | ||||
| No | 57 (53-60) | 64 (59-68) | 64 (57-70) | 63 (56-70) |
| Yes | 43 (40-47) | 36 (32-41) | 36 (30-43) | 37 (30-44) |
| Worried about out-of-pocket bills, % (95% CI) | ||||
| No | 31 (28-34) | 41 (36-45) | 40 (34-46) | 41 (35-47) |
| Yes | 69 (66-72) | 59 (55-64) | 60 (54-66) | 59 (53-65) |
| No. of dependents, % (95% CI) | ||||
| 0 | 37 (34-40) | 43 (39-47) | 42 (36-48) | 44 (38-50) |
| ≥1 | 63 (60-66) | 57 (53-61) | 58 (52-64) | 56 (50-62) |
| Body mass index, mean (95% CI)d | 28.9 (28.5-29.3) | 27.6 (27.0-28.3) | 27.3 (26.5-28.2) | 27.9 (26.9-28.9) |
| Average pain in the previous 7 d, mean (95% CI)e | 5.3 (5.1-5.5) | 5.2 (4.9-5.4) | 4.9 (4.5-5.2) | 5.5 (5.1-5.9) |
| Maximum pain recorded in the emergency department, mean (95% CI) | 7.2 (7.1-7.4) | 7.0 (6.8-7.3) | 6.6 (6.3-6.9) | 7.5 (7.3-7.8) |
| Appendicolith present on radiological imaging, % (95% CI) | ||||
| No | 74 (72-77) | 70 (66-74) | 75 (69-80) | 65 (59-70) |
| Yes | 26 (23-28) | 30 (26-34) | 25 (20-31) | 35 (30-41) |
| Appendiceal width on radiological imaging, mean (95% CI) | 11.4 (11.2-11.6) | 11.3 (11.0-11.5) | 11.2 (10.8-11.6) | 11.4 (11.0-11.7) |
| EQ-5D, mean (95% CI) | 0.69 (0.68-0.71) | 0.66 (0.64-0.69) | 0.71 (0.69-0.74) | 0.61 (0.58-0.65) |
| Alvarado score, mean (95% CI)f | 6.6 (6.5-6.7) | 6.6 (6.4-6.7) | 6.3 (6.1-6.5) | 6.8 (6.6-7.0) |
Abbreviations: EQ-5D, EuroQoL Five-Dimension; RCT, randomized clinical trial.
Twelve participants in the RCT subset and self-selection participants in the self-selection cohort (3 antibiotics, 2 surgery) were missing data on race; 1 in the self-selection cohort (antibiotics arm) was missing data on Hispanic ethnicity; 5 in the RCT subset and 3 in the self-selection cohort (2 antibiotics, 1 surgery) were missing data on employment; 16 in the RCT subset and 15 in the self-selection cohort (5 antibiotics, 10 surgery) were missing data on education; 24 in the RCT subset and 17 in the self-selection cohort (6 antibiotics, 11 surgery) were missing data on insurance; 238 in the RCT subset and 107 in the self-selection cohort (53 antibiotics, 54 surgery) were missing data on being below the federal poverty level or a Medicaid beneficiary; 26 in the RCT subset and 14 in the self-selection cohort (5 antibiotics, 9 surgery) were missing data on whether they were worried about out-of-pocket bills; 38 in the RCT subset and 22 in the self-selection cohort (9 antibiotics, 13 surgery) were missing data on number of dependents; 170 in the RCT subset and 86 in the self-selection cohort (63 antibiotics, 23 surgery) were missing data on body mass index; 35 in the RCT subset and 15 in the self-selection cohort (8 antibiotics, 7 surgery) were missing data on average pain in the previous 7 days; 142 in the RCT subset and 62 in the self-selection cohort (26 antibiotics, 36 surgery) were missing data on maximum pain recorded in the emergency department; 140 in the RCT subset and 76 in the self-selection cohort (41 antibiotics, 35 surgery) were missing data on appendiceal width; and 65 in the RCT subset and 25 in the self-selection cohort (9 antibiotics, 16 surgery) were missing data on the EQ-5D.
Contemporaneously recruited participants in the RCT.
Participants who reported more than 1 race are listed as multiracial; those who reported just 1 race are listed as having that race. Two hundred ninety-eight (93%) of those reporting “other” race were Hispanic.
Calculated as weight in kilograms divided by height in meters squared.
Ranges from 0 to 10, where 0 is “no pain” and 10 is “worst pain imaginable.”
Ranges from 0 to 10, with higher scores indicating a higher probability of appendicitis.
Consent and Randomization Process
As part of the consent process, eligible patients viewed a standardized informational video (or pamphlet) in English17 or Spanish.18 Patients provided written consent. Those consenting to the RCT were assigned to treatment based on a randomization independently provided by the data coordinating center. Those who declined randomization but met the same eligibility criteria were offered participation in a companion self-selection cohort study (eFigure in Supplement 1).15 By design, the self-selection cohort study included approximately 250 patients in each treatment group, which we limited to a fixed number enrolled each quarter and in each treatment group to ensure contemporaneous recruitment with the RCT. Two sites began enrollment in the RCT after self-selection cohort study enrollment was completed; these RCT participants are not included in this analysis.
Treatment
Antibiotics
A minimum of 24 hours receiving an intravenous antibiotic formulation was required (given as a q4-q24–hour regimen) followed by oral antibiotics, for a 10-day course. Clinical teams selected antibiotics from the Surgical Infection Society and the Infectious Disease Society of America guidelines for intra-abdominal infections.19,20 Standard discharge criteria were applied to all patients: tolerance of liquids, adequate pain control, and improving clinical condition. Some patients met the criteria for discharge without hospital admittance, leaving the ED once discharge criteria were met. Appendectomy was recommended if diffuse peritonitis or septic shock developed at any time, if worsening signs and symptoms developed after 48 hours of antibiotics, or if the patient declined to continue in the randomized group. Absent these conditions, participants in both the randomized and self-selection studies were encouraged to continue taking antibiotics; the decision to perform appendectomy was ultimately made by the treating surgeon.
Appendectomy
Minimally invasive and open approaches were allowed. Preoperative antibiotics and usual postoperative care and discharge criteria were used, including the option of discharge from recovery room to observational unit or home.
Outcomes and Measures
Participants were contacted by telephone, mail, or email at 24 hours after discharge and were surveyed at 1, 2, and 4 weeks, then quarterly for 1 year, at 18 months, and then yearly thereafter. The EuroQoL Five-Dimension (EQ-5D),16 the primary analytic outcome, was assessed at 4 weeks. Other outcomes included severe adverse events and complications of appendectomy defined using National Surgical Quality Improvement Program criteria.21 Additionally, antibiotic-related events were defined as involving an in-person health care encounter, appendectomy, prescriptions for antibiotics beyond index treatment, ED and urgent care visits for related signs and symptoms, hospitalizations, and days of missed work or school (for patient and/or caregiver); all events were assessed. All severe adverse events were adjudicated by an independent safety monitor to determine if they were related to appendicitis and/or treatment.
Statistical Analysis
Demographic details, clinical characteristics, and participant perceptions of treatment success and safety were collected at baseline and were described using proportions and corresponding 95% CIs for categorical variables. Means and CIs were calculated for continuous measures. As this was intended to be a descriptive analysis of characteristics and outcomes for those in the contemporaneously recruited RCT and self-selection cohorts and between those who selected antibiotic treatment and appendectomy, the sample size of the self-selection cohort was not determined to power comparisons between these groups.
Outcomes are presented using an intention-to-treat framework. Confidence intervals were calculated using the t test, Wilson method for proportions, and Poisson exact counts for continuous, binary, and count data, respectively. All comparisons were unadjusted, except where noted, and those who were lost to follow-up or did not report data were generally excluded from analyses of those outcomes. A Kaplan-Meier cumulative incidence curve was used to estimate the incidence of appendectomy in the antibiotics arms of both cohorts, overall and by appendicolith status (prespecified analysis). Appendectomy rates through 2 years are shown. A post hoc analysis assessed the association between sociodemographic variables and the odds of participating in the randomized cohort (vs self-selection) with and without direct covariate adjustment for site. Another post hoc analysis assessed the association between self-selection cohort status (compared with RCT participation) and the odds of appendectomy at 30 days and 1 year, adjusting for site and appendicolith on imaging. Multiple imputation using chained equations algorithms in R software (eAppendix 1 in Supplement 1) was used to account for missing data. Data are current as of March 28, 2021. All analyses were performed using R statistical software, version 4.0.3 (R Foundation for Statistical Computing).
Results
The self-selection cohort included 510 patients (mean age, 35.8 years [95% CI, 34.5-37.1 years]; 218 female [43%; 95% CI, 39%-47%] and 292 male [57%; 95% CI, 53%-61%]; 3 American Indian or Alaska Native [1%; 95% CI, 0-2%], 52 Asian [10%; 95% CI, 8%-13%], 32 Black [6%; 95% CI, 5%-9%], 307 White [61%; 95% CI, 56%-65%], 88 of other race [17%; 95% CI, 14%-21%], and 23 of multiple races [5%; 95% CI, 3%-7%]) recruited at 23 study sites who selected appendectomy (n = 253) or antibiotics (n = 257). Using only RCT data from sites that were contemporaneously recruiting patients for the RCT and self-selection cohort, 5679 patients were screened, and 31.9% of 3429 eligible participants agreed to randomization to appendectomy (544) or antibiotics (550). These 1094 RCT participants represent 70.5% of the previously described RCT cohort of 1552 participants and had substantially similar clinical and demographic characteristics compared with the full RCT cohort (mean age, 38.2 years [95% CI, 37.4-39.0 years]; 386 female [35%; 95% CI, 33%-38%] and 708 male [65%; 95% CI, 62%-67%]; 17 American Indian or Alaska Native [2%; 95% CI, 1%-3%], 66 Asian [6%; 95% CI, 5%-8%], 86 Black [8%; 95% CI, 6%-10%], 5 Native Hawaiian or other Pacific Islander [0.5%; 95% CI, 0%-1%], 638 White [59%; 95% CI, 56%-62%], 231 of other race [21%; 95% CI, 19%-24%], and 39 of multiple races [4%; 95% CI, 3%-5%]).10
Compared with those who were randomized, participants who declined randomization and opted for the self-selection cohort (Table 1) were younger (mean age, 35.8 [95% CI, 34.5-37.1] vs 38.2 [95% CI, 37.4-39.0] years), more often female (218 [43%; 95% CI, 39%-47%] vs 386 [35%; 95% CI, 33%-38%]), less often Hispanic (152 [30%; 95% CI, 26%-34%] vs 471 [43%; 95% CI, 40%-46%]), less often Spanish speakers (99 [19%; 95% CI, 16%-23%] vs 336 [31%; 28%-34%]), and more often students (51 [10%; 95% CI, 8%-13%] vs 64 [6%; 95% CI, 5%-7%]); reported more formal schooling (some college or more, 355 [72%; 95% CI, 68%-76%] vs 674 [63%; 95% CI, 60%-65%]); more often had commercial insurance (259 [53%; 95% CI, 48%-57%] vs 486 [45%; 95% CI, 42%-48%]); and had lower body mass index (27.6 [95% CI, 27.0-28.3] vs 28.9 [28.5-29.3], calculated as weight in kilograms divided by height in meters squared), fewer out-of-pocket health care concerns (295 [59%; 95% CI, 55%-64%] vs 738 [69%; 66%-72%]), and fewer dependents (667 [57%; 53%-61%] vs 279 [63%; 60%-66%]).
Conversely, clinical characteristics, including signs and symptoms and laboratory values that are presumed markers of appendicitis severity (eg, white blood cell count [eTable 1 in Supplement 1]), were similar across the 2 cohorts. A post hoc analysis of sociodemographic characteristics found that a high school degree or less (unadjusted OR, 0.66; 95% CI, 0.52-0.83; OR adjusted for site, 0.85; 95% CI, 0.64-1.13) and commercial insurance (unadjusted OR, 1.33; 95% CI, 1.07-1.65; OR adjusted for site, 0.96; 95% CI, 0.72-1.28) were associated with cohort (self-selection vs randomized), but those associations were attenuated after adjusting for site. Spanish as primary language, however, was less common in the self-selection cohort than in the randomized cohort, even after adjustment for site (unadjusted OR, 0.50; 95% CI, 0.37-0.65; OR adjusted for site, 0.66; 95% CI, 0.47-0.94).
In the self-selection cohort, the antibiotics and appendectomy groups (Table 1) had similar demographic characteristics (eTable 1 in Supplement 1). Those in the self-selection appendectomy group reported greater maximum pain in the ED (7.5 [95% CI, 7.3-7.8] vs 6.6 [95% CI,6.3-6.9] points; scores range from 0 to 10, where 0 is “no pain” and 10 is “worst pain imaginable”), had a higher Alvarado score (6.8 [95% CI, 6.6-7.0] vs 6.3 [95% CI, 6.1-6.5] points; scores range from 0 to 10, with higher scores indicating a higher probability of appendicitis), more rebound pain (65 patients [26%; 95% CI, 21%-31%] vs 37 patients [14%; 95% CI, 11%-19%]), and a higher white blood cell count (13.6 [95% CI, 13.1-14.1] vs 13.0 [95% CI, 12.5-13.5]) than those in the self-selection antibiotics group; the clinical importance of some of these differences is uncertain. Appendicolith was more common in the self-selection appendectomy group than in the self-selection antibiotics group (65 [35%; 95% CI, 30%-41%] vs 89 [25%; 95% CI, 20%-31%]), and the baseline EQ-5D score was also worse in the self-selection appendectomy group (0.61 [95% CI, 0.58-0.65] vs 0.71 [95% CI, 0.69-0.74]). See eTable 2 in Supplement 1 for characteristics of the contemporaneously recruited RCT cohort by group.
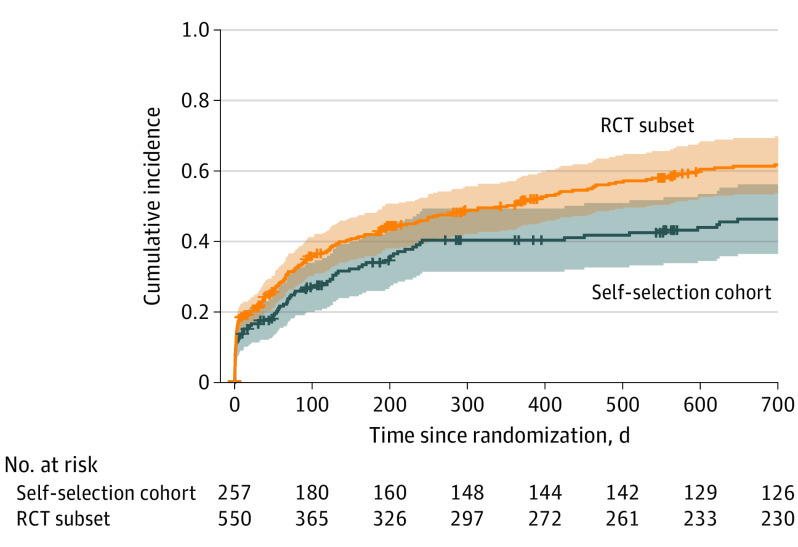
Most outcomes at 30 days were similar between the self-selection cohort and the RCT cohort (Table 2). The 30-day EQ-5D was nearly identical in both the self-selection antibiotics and RCT antibiotics groups, as well as in the self-selection appendectomy and RCT appendectomy groups. Time until resolution of symptoms was similar between treatment groups, whether patients selected or were randomized to treatment. There appeared to be differences between participants in the self-selection and RCT cohorts regarding length of index hospitalization, but ED visits and the number of ED or urgent care visits were small with overlapping CIs. Compared with those participants randomized to antibiotics, those who chose antibiotics appeared to have fewer hospitalizations after initial treatment. Among those who selected antibiotics, 38 had undergone appendectomy by 30 days (15.3%; 95% CI, 10.7%-19.7%), compared with 155 patients among those randomized to antibiotics (19.2%; 95% CI, 15.9%-22.5%), for an absolute difference of 3.9% (95% CI, −1.7% to 9.5%). At 1 year, the absolute difference in the rate of appendectomy was 6.7% (95% CI, −0.8% to 14.2%). Observed differences (Figure) across study groups appeared to be associated with the nonappendicolith subgroup rather than the appendicolith subgroup (Table 3). To better understand this observed difference, we used an exploratory post hoc conditional logistic regression analysis combining the self-selection and contemporaneous RCT cohorts to evaluate the association of self-selection cohort status and appendectomy by 30 days and 1 year. After adjusting for site and appendicolith status, the OR for appendectomy among those receiving antibiotics in the self-selection cohort vs those receiving antibiotics in the RCT at 30 days was 0.88 (95% CI, 0.56-1.37) and at 1 year was 0.72 (95% CI, 0.51-1.03).
Table 2. Intention-to-Treat Comparison of Patient-Reported Outcomes, Clinical Outcomes, and Time Spent in Health Care Between Those in the Self-selection Cohort and the Contemporaneously Recruited Randomized Clinical Trial Subseta.
| Antibiotics | Appendectomy | |||
|---|---|---|---|---|
| Self-selection | RCT subsetb | Self-selection | RCT subsetb | |
| Quality of life at 30 d | ||||
| EQ-5D, mean (95% CI) | 0.92 (0.90-0.94) | 0.93 (0.91-0.94) | 0.92 (0.90-0.94) | 0.92 (0.91-0.93) |
| Resolution of symptoms, % (95% CI) | ||||
| By 7 d | 53 (47-59) | 49 (45-53) | 44 (37-50) | 51 (47-55) |
| By 14 d | 72 (66-78) | 67 (63-71) | 62 (55-68) | 67 (63-71) |
| By 30 d | 72 (66-78) | 71 (67-75) | 66 (59-72) | 72 (67-75) |
| Time in health care | ||||
| Days from emergency department arrival to discharge for index treatment, mean (95% CI) | 1.51 (1.31-1.71) | 1.47 (1.33-1.61) | 1.76 (1.49-2.03) | 1.61 (1.50-1.72) |
| Any hospitalization after index treatment through 90 d, % (95% CI) | 11 (8-16) | 26 (22-30) | 6 (4-11) | 5 (3-8) |
| Days in hospital after index treatment through 90 d (95% CI) | 0.23 (0.17-0.31) | 0.72 (0.65-0.81) | 0.08 (0.04-0.13) | 0.11 (0.08-0.14) |
| Any visit to an emergency department or urgent care clinic after index treatment within 90 d, % (95% CI) | 7 (4-12) | 9 (7-13) | 6 (4-11) | 5 (3-8) |
| Visits to emergency department or urgent care clinic after index treatment within 90 d, No. (95% CI) | 0.07 (0.04-0.12) | 0.11 (0.08-0.14) | 0.07 (0.03-0.11) | 0.04 (0.03-0.07) |
| Days missed work within 90 d (95% CI) | ||||
| Participant | 3.85 (3.54-4.18) | 5.68 (5.42-5.95) | 8.12 (7.59-8.68) | 7.86 (7.54-8.18) |
| Caregiver | 0.96 (0.81-1.13) | 1.46 (1.33-1.59) | 2.2 (1.93-2.49) | 2.03 (1.87-2.19) |
| Serious adverse events within 90 d | ||||
| No. of events per 100 participants (95% CI) | 3.65 (1.58-7.2) | 3.93 (2.37-6.14) | 3.32 (1.33-6.84) | 3.49 (1.99-5.66) |
| Any, % (95% CI) | 3 (1-6) | 3 (2-5) | 3 (2-7) | 3 (2-5) |
| No. of NISQIP events per 100 participants within 90 d (95% CI) | 2.74 (1.01-5.96) | 9.11 (6.62-12.23) | 4.27 (1.95-8.10) | 3.92 (2.32-6.20) |
Abbreviations: EQ-5D, EuroQoL Five-Dimension; NISQIP, National Surgical Quality Improvement Program; RCT, randomized clinical trial.
Two hundred twenty-three participants in the self-selection antibiotics, 493 in the RCT antibiotics subset, 211 in the self-selection surgery, and 467 in the RCT surgery subset groups contributed data to the analysis of EQ-5D at 30 days; 230 in the self-selection antibiotics, 515 in the RCT antibiotics subset, 216 in the self-selection surgery, and 498 in the RCT surgery subset groups contributed data to the analysis of resolution of symptoms by 7 days; 218 in the self-selection antibiotics, 497 in the RCT antibiotics subset, 213 in the self-selection surgery, and 498 in the RCT surgery subset groups contributed data to the analysis of resolution of symptoms by 14 days; 221 in the self-selection antibiotics, 484 in the RCT antibiotics subset, 209 in the self-selection surgery, and 468 in the RCT surgery subset groups contributed data to the analysis of resolution of symptoms by 30 days; 257 in the self-selection antibiotics, 549 in the RCT antibiotics subset, 252 in the self-selection surgery, and 541 in the RCT surgery subset groups contributed data to the analysis of index stay days; 205 in the self-selection antibiotics, 448 in the RCT antibiotics subset, 193 in the self-selection surgery, and 421 in the RCT surgery subset groups contributed data to the analysis of any hospitalization after index through 90 days; 202 in the self-selection antibiotics, 438 in the RCT antibiotics subset, 190 in the self-selection surgery, and 417 in the RCT surgery subset groups contributed data to the analysis of days in hospital after index treatment through 90 days; 193 in the self-selection antibiotics, 434 in the RCT antibiotics subset, 186 in the self-selection surgery, and 415 in the RCT surgery subset groups contributed to the analysis of any visit to an emergency department or urgent care clinic after index treatment within 90 days; 192 in the self-selection antibiotics, 432 in the RCT antibiotics subset, 184 in the self-selection surgery, and 410 in the RCT surgery subset groups contributed to the analysis of number of visits to an emergency department or urgent care clinic after index treatment within 90 days; 145 in the self-selection antibiotics, 314 in the RCT antibiotics subset, 106 in the self-selection surgery, and 298 in the RCT surgery subset groups contributed data to the analysis of days the participant missed work; 144 in the self-selection antibiotics, 335 in the RCT antibiotics subset, 112 in the self-selection surgery, and 318 in the RCT surgery subset groups contributed data to the analysis of days the participant’s caregiver missed work; and 219 in the self-selection antibiotics, 483 in the RCT antibiotics subset, 211 in the self-selection surgery, and 459 in the RCT surgery subset groups contributed data to the analysis of number of serious adverse events within 90 days.
Contemporaneously recruited participants in the RCT.
Figure. Cumulative Incidence of Appendectomy in Self-selection Antibiotics Cohort and Antibiotics–Randomized Clinical Trial (RCT) Subset Cohort.
Includes all appendectomies, even those performed for nonclinical reasons. The RCT subset consists of those participants in the RCT who were recruited contemporaneously with participants in the self-selection cohort from the sites that participated in both studies. Shaded areas represent 95% CIs.
Table 3. Cumulative Incidence (95% CI) of Appendectomy in Self-selection and Randomized Clinical Trial (RCT) Subset Cohorts.
| Appendectomy incidence | Cumulative incidence, % (95% CI) | ||
|---|---|---|---|
| Self-selection | RCT subseta | Difference | |
| Overall | |||
| 48 h | 10.5 (6.6 to 14.2) | 11.1 (8.5 to 13.7) | 0.6 (−4.0 to 5.2) |
| 30 d | 15.3 (10.7 to 19.7) | 19.2 (15.9 to 22.5) | 3.9 (−1.7 to 9.5) |
| 1 y | 33.2 (27 to 38.9) | 39.9 (35.6 to 43.9) | 6.7 (−0.8 to 14.2) |
| 2 y | 37.1 (30.6 to 43) | 46.1 (41.6 to 50.3) | 9 (0.9 to 17.1) |
| Among those without appendicolith on radiological imaging | |||
| 48 h | 7.0 (3.2 to 10.5) | 7.9 (5.2 to 10.5) | 0.9 (−3.6 to 5.4) |
| 30 d | 11.2 (6.6 to 15.7) | 15.4 (11.8 to 18.9) | 4.2 (−1.6 to 10) |
| 1 y | 26.5 (19.7 to 32.6) | 35.3 (30.3 to 39.8) | 8.8 (0.6 to 17) |
| 2 y | 30.2 (23.1 to 36.7) | 42.8 (37.5 to 47.6) | 12.6 (3.5 to 21.7) |
| Among those with appendicolith on radiological imaging | |||
| 48 h | 21.3 (10.3 to 30.9) | 20.3 (13.4 to 26.6) | −1.0 (−13.1 to 11.1) |
| 30 d | 27.9 (15.7 to 38.3) | 30.1 (22.1 to 37.2) | 2.2 (−11.3 to 15.7) |
| 1 y | 54.1 (39.3 to 65.3) | 53.0 (43.8 to 60.6) | −1.1 (−16.7 to 14.5) |
| 2 y | 58.4 (43.2 to 69.6) | 55.5 (46.3 to 63.1) | −2.9 (−19.1 to 13.3) |
Contemporaneously recruited participants in the RCT.
The distribution of reasons for appendectomy (confirmed by the participant) were similar between the RCT and self-selection cohorts, with 10 (29%; 95% CI, 16%-45%) of all appendectomies in the self-selection cohort vs 24 (23%; 95% CI, 16%-32%) in the RCT cohort reported for nonclinical reasons. Pathology reports confirmed the diagnosis of appendicitis in 32 (97%; 95% CI, 92%-99%) of the antibiotics-assigned participants and 99 (91%; 95% CI, 78%-97%) of those who selected antibiotics. A repeated course of antibiotics was administered in 18 patients receiving antibiotics (11%) in the RCT and 54 (8%) in the self-selection cohorts at 4 weeks.
Discussion
The CODA RCT and parallel cohort composed of those who declined randomization but agreed to join the self-selection study represent a diverse group of participants. Those declining randomization more often had characteristics associated with better access to health care services (eg, commercial insurance, higher income, English speaking). For most outcomes (including EQ-5D and resolution of symptoms), results were similar between the RCT and the self-selection cohort, supporting the generalizability of the RCT results to similar populations. Some clear differences in outcomes were observed (eg, health care utilization), and other outcomes with smaller differences may be variably interpreted (appendectomy rate after initiating antibiotics). Some outcomes, such as complications, occurred too infrequently to compare rates between cohorts. The reasons behind observed differences and the extent to which such differences will affect patient and clinical decision-making remain to be determined.
Parallel observational cohort studies have been deployed in tandem with RCTs for multiple interventions (tonsillitis,22 pregnancy,23,24 Achilles’ tendon rupture,25 ankle fracture,26 diverticulitis,27 and various spine operations).28,29,30 They can provide context and external validity for RCTs31 and address population differences as well as the association of treatment selection with outcomes. Parallel observational cohorts are especially relevant for small RCTs when selection factors may be more extreme. In a prior larger-scale pragmatic RCT (SPORT trial of spine surgery), the parallel observational cohort was important in understanding outcomes because of high rates of crossover.28,29 A recent meta-analysis found that observational cohorts conducted in parallel with RCT studies had higher participation rates and populations more reflective of the at-risk group than the RCT.32 Delevry et al33 also examined the influence of treatment selections, finding improved outcomes among patients who received their chosen therapy compared with those who were randomized. To our knowledge, our study is the first randomized trial of appendicitis treatment with a parallel observational (self-selection) cohort.
A first goal in recruiting a parallel self-selection cohort was to give context to the population willing to randomize, recognizing that demographic and clinical features of those who randomize may be different from the general at-risk population. Although this self-selection cohort may provide context, it, too, was not composed of the general, at-risk population. Instead, this group included those who were eligible for the CODA trial, observed a consent video15 presenting risks and benefits of both treatments, declined participation in the RCT, and then agreed to join an observational research study. The extent to which these aspects of cohort recruitment affected their characteristics or outcome is unknown, but we observed that practice site was important. Site effects may have included varied recruitment practices and their influence on enrollment in the RCT. Furthermore, cultural norms and perceptions of power and privilege may have resulted in some people seeking out clinical trials while others may have been uncomfortable refusing involvement. Explaining this observation is made more complex by underinsurance and systemic barriers to health care. Some who are underinsured or historically and currently marginalized may anticipate benefit from trial participation (eg, perception of better follow-up and access to innovation). The findings of overrepresentation of some minoritized groups, including people experiencing poverty, in the randomized group is in distinction to the observation that Black individuals, Indigenous individuals, those of other minoritized racial and ethnic groups, individuals who speak Spanish, and those living in poverty34 are often underrepresented in RCTs. It remains to be determined whether specific aspects of the CODA recruitment strategy (eg, requiring refusal to participate in the RCT before offering the self-selection cohort), differential interest in obtaining the antibiotic option, or other aspects of care affected higher proportional recruitment to the RCT in these subgroups. Health care access and patient experience is influenced by systematic barriers (including racism and xenophobia) within our health systems. Ensuring that people from diverse backgrounds have the opportunity to participate in clinical trials is important to understanding disparate experiences and clinical outcomes, and it is a critical piece in advancing health equity.35 In addition, it is essential that all potential participants are empowered to refuse participation.
A second rationale for conducting a parallel observational cohort study with the RCT was to provide context for outcomes identified in the RCT—especially subjective outcomes associated with antibiotics—and to explore differences that might be associated with selecting, rather than being randomized to, treatment.36 A direct comparison of outcomes between the self-selection and RCT cohorts for either treatment is limited by selection bias and confounding. Nonetheless, a description of outcomes in both cohorts and treatment groups found that for most findings—such as overall health status, time to resolution of symptoms, and safety events—results were similar in both cohorts. These similarities speak to the generalizability of the trial results and may be reassuring to those considering antibiotics outside of the RCT framework. There appeared to be a lower rate of subsequent hospitalization in the group that selected antibiotics compared with those randomized to antibiotics. This outcome may be related to a slightly higher number of negative appendectomy findings (no appendicitis identified in pathologic evaluation) and appendectomy for nonclinical reasons in the self-selection cohort, but the infrequency of these events limits interpretation. The 3.9% observed difference in the proportion undergoing appendectomy after initiating antibiotics between those in the self-selection and RCT cohorts prompted a post hoc analysis adjusting for site effects. That analysis did not suggest lower odds of appendectomy among those selecting antibiotic treatment. Interpreting these observed differences is challenging, given the relatively infrequent outcomes, small subgroups, and unbalanced covariates between groups. Although not measured in CODA, potential site effects that may be relevant to understanding the observed difference include ease of access to follow-up care, nausea and pain management strategies, encouragement during the early recovery period, and variation in surgeon threshold for offering surgery.
A challenge of patient-centered outcomes research is the degree to which clinicians and patients interpret and prioritize results differently. When planning CODA, patient advisors encouraged us to include subgroups—even if treatment success might be less than 50% in those groups. The advisors believed patients would want the information to make an informed decision and that many would choose a treatment if there was any option to safely avoid surgery. Supporting that perspective is a recent survey study of 1200 at-risk participants in an online, crowdsourcing community (mTurk). Participants were randomized to review vignettes describing antibiotic treatment that varied the “success rate” in avoiding appendectomy from 90% to as low as 40%. The vast majority of patients (approximately 80%) were interested in trying antibiotics for appendicitis37 irrespective of the chance of success. This finding may provide context for how some clinicians and patients may variably consider differences observed for the rates of appendectomy in the self-selection and randomized cohorts.
Limitations
This study has limitations. To qualify for the self-selection cohort, participants first refused to join the randomized cohort. This requirement may have influenced the observed differences in participant characteristics and outcomes. Second, not all sites contributed to both the RCT and self-selection cohort studies. Because describing differences between the self-selection and randomized cohort was a goal, we created an RCT subgroup drawn only from sites recruiting contemporaneously. This decision may have introduced selection bias with unclear implications. Third, although we theorized that people refusing randomization and selecting their treatment might be different at baseline and/or have different outcomes than those randomized, we did not design the study to explicitly test this hypothesis. We did not predetermine how much of a difference in any baseline characteristic or outcome (eg, rate of appendectomy after starting antibiotics) would be considered meaningful or recruit a specific number in the self-selection cohort to test for such differences. As a result, type I and II errors are a consideration in interpreting these results. Similarly, the regression analysis that explored the association between self-selection vs the RCT cohort status and appendectomy after starting antibiotics—adjusting for site and appendicolith—was post hoc and exploratory. Also, we asked participants only why they did not agree to randomize; we can only speculate on reasons behind observed differences in baseline characteristics between those who did and did not randomize.
Conclusions
Observational cohort studies conducted in parallel to RCTs aim to help future patients and clinicians determine whether trial results are relevant to them. In both its randomized and observational studies, CODA recruited a diverse group of participants across the United States, which should allow future patients to “find themselves” in the data. Rates of appendectomy, patient-reported outcomes, and safety events in the RCT and self-selection cohort suggest that CODA findings should be generalizable to a broad patient population.
eFigure. Study Recruitment Diagram
eTable 1. Additional Baseline Participant Characteristics—Observational vs Randomized Clinical Trial Cohort
eTable 2. Baseline Randomized Clinical Trial Participant Characteristics by Arm
eAppendix 1. Analysis Comparing Odds of Appendectomy Between the Observational Cohort and Contemporaneous RCT Cohort
eAppendix 2. The CODA Trial Sites and Site Leads
Nonauthor Collaborators
References
- 1.Eriksson S, Granström L. Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis. Br J Surg. 1995;82(2):166-169. doi: 10.1002/bjs.1800820207 [DOI] [PubMed] [Google Scholar]
- 2.Styrud J, Eriksson S, Nilsson I, et al. Appendectomy versus antibiotic treatment in acute appendicitis: a prospective multicenter randomized controlled trial. World J Surg. 2006;30(6):1033-1037. doi: 10.1007/s00268-005-0304-6 [DOI] [PubMed] [Google Scholar]
- 3.Turhan AN, Kapan S, Kütükçü E, Yiğitbaş H, Hatipoğlu S, Aygün E. Comparison of operative and non operative management of acute appendicitis. Ulus Travma Acil Cerrahi Derg. 2009;15(5):459-462. [PubMed] [Google Scholar]
- 4.Hansson J, Körner U, Khorram-Manesh A, Solberg A, Lundholm K. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg. 2009;96(5):473-481. doi: 10.1002/bjs.6482 [DOI] [PubMed] [Google Scholar]
- 5.Vons C, Barry C, Maitre S, et al. Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377(9777):1573-1579. doi: 10.1016/S0140-6736(11)60410-8 [DOI] [PubMed] [Google Scholar]
- 6.Salminen P, Paajanen H, Rautio T, et al. Antibiotic therapy vs appendectomy for treatment of uncomplicated acute appendicitis: the APPAC Randomized Clinical Trial. JAMA. 2015;313(23):2340-2348. doi: 10.1001/jama.2015.6154 [DOI] [PubMed] [Google Scholar]
- 7.Talan DA, Saltzman DJ, Mower WR, et al. ; Olive View–UCLA Appendicitis Study Group . Antibiotics-first versus surgery for appendicitis: a US pilot randomized controlled trial allowing outpatient antibiotic management. Ann Emerg Med. 2017;70(1):1-11.e9. doi: 10.1016/j.annemergmed.2016.08.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moris D. Comment on “a randomised clinical trial evaluating the efficacy and quality of life of antibiotic only treatment of acute uncomplicated appendicitis: results of the COMMA trial”. Ann Surg. Published online June 18, 2021. doi: 10.1097/SLA.0000000000005018 [DOI] [PubMed] [Google Scholar]
- 9.Ceresoli M, Pisano M, Allievi N, et al. Never put equipoise in appendix! Final results of ASAA (antibiotics vs. surgery for uncomplicated acute appendicitis in adults) randomized controlled trial. Updates Surg. 2019;71(2):381-387. doi: 10.1007/s13304-018-00614-z [DOI] [PubMed] [Google Scholar]
- 10.Flum DR, Davidson GH, Monsell SE, et al. ; CODA Collaborative . A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383(20):1907-1919. doi: 10.1056/NEJMoa2014320 [DOI] [PubMed] [Google Scholar]
- 11.Salminen P, Tuominen R, Paajanen H, et al. Five-year follow-up of antibiotic therapy for uncomplicated acute appendicitis in the APPAC Randomized Clinical Trial. JAMA. 2018;320(12):1259-1265. doi: 10.1001/jama.2018.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sceats LA, Trickey AW, Morris AM, Kin C, Staudenmayer KL. Nonoperative management of uncomplicated appendicitis among privately insured patients. JAMA Surg. 2019;154(2):141-149. doi: 10.1001/jamasurg.2018.4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Saverio S, Sibilio A, Giorgini E, et al. The NOTA Study (Non Operative Treatment for Acute Appendicitis): prospective study on the efficacy and safety of antibiotics (amoxicillin and clavulanic acid) for treating patients with right lower quadrant abdominal pain and long-term follow-up of conservatively treated suspected appendicitis. Ann Surg. 2014;260(1):109-117. doi: 10.1097/SLA.0000000000000560 [DOI] [PubMed] [Google Scholar]
- 14.Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12(3):191-204. doi: 10.1038/nrd3923 [DOI] [PubMed] [Google Scholar]
- 15.Davidson GH, Flum DR, Talan DA, et al. Comparison of Outcomes of antibiotic Drugs and Appendectomy (CODA) trial: a protocol for the pragmatic randomised study of appendicitis treatment. BMJ Open. 2017;7(11):e016117. doi: 10.1136/bmjopen-2017-016117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 17.Comparing Outcomes of Drugs & Appendectomy (CODA) Patient Education Video (English, Version 2). August 7, 2017. Accessed April 19, 2022. https://www.youtube.com/watch?v=e5Vasw7ngH4
- 18.Comparing Outcomes of Drugs & Appendectomy (CODA) Patient Education Video (Spanish, Version 2). August 7, 2017. Accessed April 19, 2022. https://www.youtube.com/watch?v=Gjylvx24qD0
- 19.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt). 2010;11(1):79-109. doi: 10.1089/sur.2009.9930 [DOI] [PubMed] [Google Scholar]
- 20.Mazuski JE, Tessier JM, May AK, et al. The Surgical Infection Society revised guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt). 2017;18(1):1-76. doi: 10.1089/sur.2016.261 [DOI] [PubMed] [Google Scholar]
- 21.American College of Surgeons . National Surgical Quality Improvement Program. User guide for the 2014 ACS NISQIP participant use data file (PUF) October 2015; https://www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2014.ashx.
- 22.Lock C, Wilson J, Steen N, et al. North of England and Scotland Study of Tonsillectomy and Adeno-tonsillectomy in Children(NESSTAC): a pragmatic randomised controlled trial with a parallel non-randomised preference study. Health Technol Assess. 2010;14(13):1-164, iii-iv. iii-iv. [DOI] [PubMed] [Google Scholar]
- 23.Ashok PW, Hamoda H, Flett GM, Kidd A, Fitzmaurice A, Templeton A. Patient preference in a randomized study comparing medical and surgical abortion at 10-13 weeks gestation. Contraception. 2005;71(2):143-148. doi: 10.1016/j.contraception.2004.08.013 [DOI] [PubMed] [Google Scholar]
- 24.Crowther CA, Dodd JM, Hiller JE, Haslam RR, Robinson JS; Birth After Caesarean Study Group . Planned vaginal birth or elective repeat caesarean: patient preference restricted cohort with nested randomised trial. PLoS Med. 2012;9(3):e1001192. doi: 10.1371/journal.pmed.1001192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kearney RS, Achten J, Parsons NR, Costa ML. The comprehensive cohort model in a pilot trial in orthopaedic trauma. BMC Med Res Methodol. 2011;11:39. doi: 10.1186/1471-2288-11-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittal R, Harris IA, Adie S, Naylor JM; CROSSBAT Study Group . Surgery for type B ankle fracture treatment: a combined randomised and observational study (CROSSBAT). BMJ Open. 2017;7(3):e013298. doi: 10.1136/bmjopen-2016-013298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raue W, Langelotz C, Paolucci V, et al. Problems of randomization to open or laparoscopic sigmoidectomy for diverticular disease. Int J Colorectal Dis. 2011;26(3):369-375. doi: 10.1007/s00384-010-1074-7 [DOI] [PubMed] [Google Scholar]
- 28.Weinstein JN, Tosteson TD, Lurie JD, et al. ; SPORT Investigators . Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794-810. doi: 10.1056/NEJMoa0707136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257-2270. doi: 10.1056/NEJMoa070302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Heest AE, Bagley A, Molitor F, James MA. Tendon transfer surgery in upper-extremity cerebral palsy is more effective than botulinum toxin injections or regular, ongoing therapy. J Bone Joint Surg Am. 2015;97(7):529-536. doi: 10.2106/JBJS.M.01577 [DOI] [PubMed] [Google Scholar]
- 31.Olschewski M. Rapid response: comprehensive cohort study: adding evidence from non-randomised patients to a randomised trial. BMJ. Published online April 9, 1999. doi: 10.1136/bmj.318.7187.874a [DOI] [Google Scholar]
- 32.Wasmann KA, Wijsman P, van Dieren S, Bemelman W, Buskens C. Partially randomised patient preference trials as an alternative design to randomised controlled trials: systematic review and meta-analyses. BMJ Open. 2019;9(10):e031151. doi: 10.1136/bmjopen-2019-031151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delevry D, Le QA. Effect of treatment preference in randomized controlled trials: systematic review of the literature and meta-analysis. Patient. 2019;12(6):593-609. doi: 10.1007/s40271-019-00379-6 [DOI] [PubMed] [Google Scholar]
- 34.Washington HA. Medical apartheid: the dark history of medical experimentation on Black Americans from colonial times to the present. Doubleday; 2006. [Google Scholar]
- 35.U.S. Food & Drug Administration. Clinical trial diversity. 2021. Accessed July 27, 2021. https://www.fda.gov/consumers/minority-health-and-health-equity/clinical-trial-diversity
- 36.Menezes P. Trial effect: the road from efficacy to effectiveness. Clin Invest. 2012;2(5):443-445. doi: 10.4155/cli.12.34 [DOI] [Google Scholar]
- 37.Rosen JE, Agrawal N, Flum DR, Liao JM. Willingness to undergo antibiotic treatment of acute appendicitis based on risk of treatment failure. Br J Surg. 2021;108(11):e361-e363. doi: 10.1093/bjs/znab280 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Study Recruitment Diagram
eTable 1. Additional Baseline Participant Characteristics—Observational vs Randomized Clinical Trial Cohort
eTable 2. Baseline Randomized Clinical Trial Participant Characteristics by Arm
eAppendix 1. Analysis Comparing Odds of Appendectomy Between the Observational Cohort and Contemporaneous RCT Cohort
eAppendix 2. The CODA Trial Sites and Site Leads
Nonauthor Collaborators