This classification study measures the agreement and correlation between 3 systems to classify topical corticosteroid potency.
Key Points
Question
How can investigators meaningfully and transparently classify topical corticosteroid (TCS) potency for pharmacoepidemiologic research?
Findings
In this classification study, a comprehensive list of 232 TCS formulations was classified into 3 systems. There was low-to-moderate agreement but strong correlation between the potency classification systems.
Meaning
The method used to classify TCS potency in pharmacoepidemiologic research could alter the results and interpretation of studies; investigators need to transparently report their TCS potency classifications and consider alternative classifications in sensitivity analyses.
Abstract
Importance
Topical corticosteroids (TCSs) are available in multiple potencies that alter their effectiveness and safety. Pharmacoepidemiologic studies on TCSs are hampered by the absence of a universal potency classification system, limiting comparisons across studies, robust exposure classification, and clinical interpretation.
Objective
To classify TCSs into 3 commonly used potency classification systems and evaluate the agreement and correlation between the 3 systems.
Design, Setting, and Participants
In this classification study, a comprehensive list of TCS formulations was compiled using sources identified in the literature, the Ontario Drug Benefit Formulary, a recent Cochrane review on the use of TCSs in people with eczema, and the Anatomical Therapeutic Classification (ATC) of the World Health Organization from August 11, 2021, to January 6, 2022. Topical corticosteroid potency classifications were assigned and compared using the 7-category US classification system, a 4-category classification from a recent Cochrane review largely based on the UK formulary, and the 4-category ATC classification. To facilitate comparisons across systems, the 7-category US system was consolidated into 4 categories.
Main Outcomes and Measures
Cohen weighted κ (κw) and Spearman rank correlation coefficients (r) were computed to examine agreement and correlation between the classification systems.
Results
A total of 232 unique TCS formulations (ATC, n = 231; US classification, n = 232; Cochrane review, n = 89) were included. Overall, there was low-to-moderate agreement but strong correlation between the classification systems. The US classification had weak agreement with the ATC system (κw, 0.53; 95% CI, 0.45-0.60) and moderate agreement with the Cochrane review classification (κw, 0.60; 95% CI, 0.48-0.73); there was weak agreement between the ATC and Cochrane review classifications (κw, 0.58; 95% CI, 0.46-0.71). The US classification strongly correlated with the ATC system (r, 0.77; 95% CI, 0.71-0.82) and Cochrane review classification (r, 0.74; 95% CI, 0.62-0.82). There was also a strong correlation between the Cochrane review and ATC classifications (r, 0.71; 95% CI, 0.58-0.80).
Conclusions and Relevance
This classification study used multiple resources to classify 232 TCS formulations into 3 potency classifications. Because these systems are often incongruent, they may yield different results in pharmacoepidemiologic studies; investigators need to be transparent in their classification approach and consider alternative potency definitions in sensitivity analyses.
Introduction
Topical corticosteroids (TCSs) are a commonly prescribed, first-line treatment for many inflammatory skin diseases. Topical corticosteroids have been prescribed since the 1950s, with excellent effectiveness and a favorable safety record. They have been associated with predominantly local adverse effects, including skin atrophy, changes in pigmentation, steroid-induced acne, and rosacea.1 Increasing TCS potency, application frequency, and duration of use may increase the risk of these adverse events. High-potency TCSs have also been associated with extracutaneous adverse events, including adrenal suppression,2 diabetes,3 osteoporosis, and major osteoporotic fractures.4 Given how commonly TCSs are prescribed and the concerns of clinicians, patients, and caregivers,5 incorporating TCS potency into pharmacoepidemiologic studies is crucial.
Topical corticosteroids are available in many formulations, including different corticosteroid molecules, concentrations, and vehicles. These factors alter potency, leading to differences in effectiveness and safety. Classifying TCS potency for research is hindered by the lack of a universal classification system. If TCS potency classification systems vary and are discrepant, an investigator’s choice of classification system could alter the study’s results and interpretation. To our knowledge, no studies have systematically examined differences between TCS potency classification systems. To better characterize these potential discrepancies, the aim of this study was to classify TCS formulations using 3 commonly used classification systems and assess their agreement and correlation.
Methods
In this classification study, we compiled a list of unique TCS formulations using the Ontario Drug Benefit Formulary,6 the Anatomical Therapeutic Classification (ATC) of the World Health Organization,7 a recent Cochrane review,8 and other published sources from August 11, 2021, to January 6, 2022. Unique formulations were ascertained by the combination of corticosteroid molecule, concentration, and vehicle. In accordance with Article 2.1 of the Tri-Council Policy Statement, this study was exempt from review by the Women’s College Hospital Research Ethics board because it was not human participant research. This study followed the Guidelines for Reporting Reliability and Agreement Studies (GRRAS).
The US classification system is a 7-category system (1, super potent; 2, potent; 3, upper midstrength; 4, midstrength; 5, lower midstrength; 6, mild; 7, least potent) that ranks vasoconstrictive properties9 and clinical effectiveness.10 This system incorporates the formulation’s corticosteroid molecule, concentration, and vehicle. We developed a comprehensive classification list by combining data from multiple 7-category potency lists (eFigure 1 in the Supplement). A dermatologist (A.M.D.) and an epidemiologist (A.C.B.) assigned previously unclassified TCSs and those with incoherent classifications to appropriate potency categories (eTable 1 in the Supplement).
The ATC classifies dermatologic corticosteroids (ATC group D07)7 using a 4-category hierarchical classification system (1, mild; 2, moderate; 3, potent; 4, very potent) and has been used in pharmacoepidemiologic studies.3,4 This system is primarily useful for drug use studies (rather than clinical use) and classifies TCSs by their primary ingredients (eg, betamethasone) without consideration of the salt (eg, valerate vs dipropionate), vehicle, or concentration.
A different 4-category classification system used in the UK (1, mild; 2, moderate; 3, potent; 4, very potent) classifies potency by vasoconstrictive properties and concentration.11 The earliest publication of this classification described TCSs in the British National Formulary,12 which continues to publish guidance using this system today. This system, supplemented by a hierarchy of additional sources, formed the basis of a 4-category potency classification in a recent Cochrane review on TCSs for eczema.8
Statistical Analysis
We calculated agreement to evaluate the similarity of classification systems and calculated correlation to assess their directional relationship (eMethods in the Supplement). Although agreement and correlation are similar concepts, they often diverge, and their relative importance differs depending on how the classification systems are being used in a given study.13
We reverse-coded the US classifications and consolidated them into 4 categories to analyze agreement (eTable 2 in the Supplement). We assessed agreement between the consolidated US, ATC, and Cochrane review classifications using the Cohen κ statistic (κw) with linear weighting and correlation between the reverse-coded 7-category US classification and the 4-category ATC and Cochrane review classifications using Spearman rank correlation coefficients (r). All statistical analyses were conducted using SAS software, version 9.04 (SAS OnDemand for Academics).
Results
A total of 232 unique TCS formulations were categorized using the 3 classification systems (ATC, 231 formulations; US classification, 232 formulations; Cochrane review, 89 formulations) (eFigure 2 and eTable 3 in the Supplement). The consolidated US classification had weak agreement with the ATC (κw, 0.53; 95% CI, 0.45-0.60; Table); 54.5% (126 of 231) of the classifications were concordant, and 37.7% (87 of 231) US potency classifications were lower than those assigned by the ATC (Figure, A). There was moderate agreement between the consolidated US and Cochrane review classifications (κw, 0.60; 95% CI, 0.48-0.73); 64.0% (57 of 89) of the formulation potency classification units were concordant, whereas 33.7% (30 of 89) of the consolidated US potency classifications were lower in potency (Figure, B). There was weak agreement between the Cochrane review and ATC (κw, 0.58; 95% CI, 0.46-0.71); 64.0% (57 of 89) of the classifications were concordant, and 19.1% (17 of 89) of the ATC categories were higher in potency than those in the Cochrane review (Figure, C).
Table. Agreement and Correlation Between US, ATC, and Cochrane Review Classification Systems.
| Classification system | κ agreement (95% CI)a | Spearman rank correlation coefficient (95% CI) | ||
|---|---|---|---|---|
| US classificationb | ATC | US classificationc | ATC | |
| ATC | 0.53 (0.45-0.60) | NA | 0.77 (0.71-0.82) | NA |
| Cochrane review | 0.60 (0.48-0.73) | 0.58 (0.46-0.71) | 0.74 (0.62-0.82) | 0.71 (0.58-0.80) |
Abbreviations: ATC, Anatomical Therapeutic Classification; NA, not applicable.
Linear weighting was used.
Consolidated (4-category scale).
Reverse-coded (7-category scale).
Figure. Mean Difference in Potency for Topical Corticosteroids With the Same Primary Ingredient.
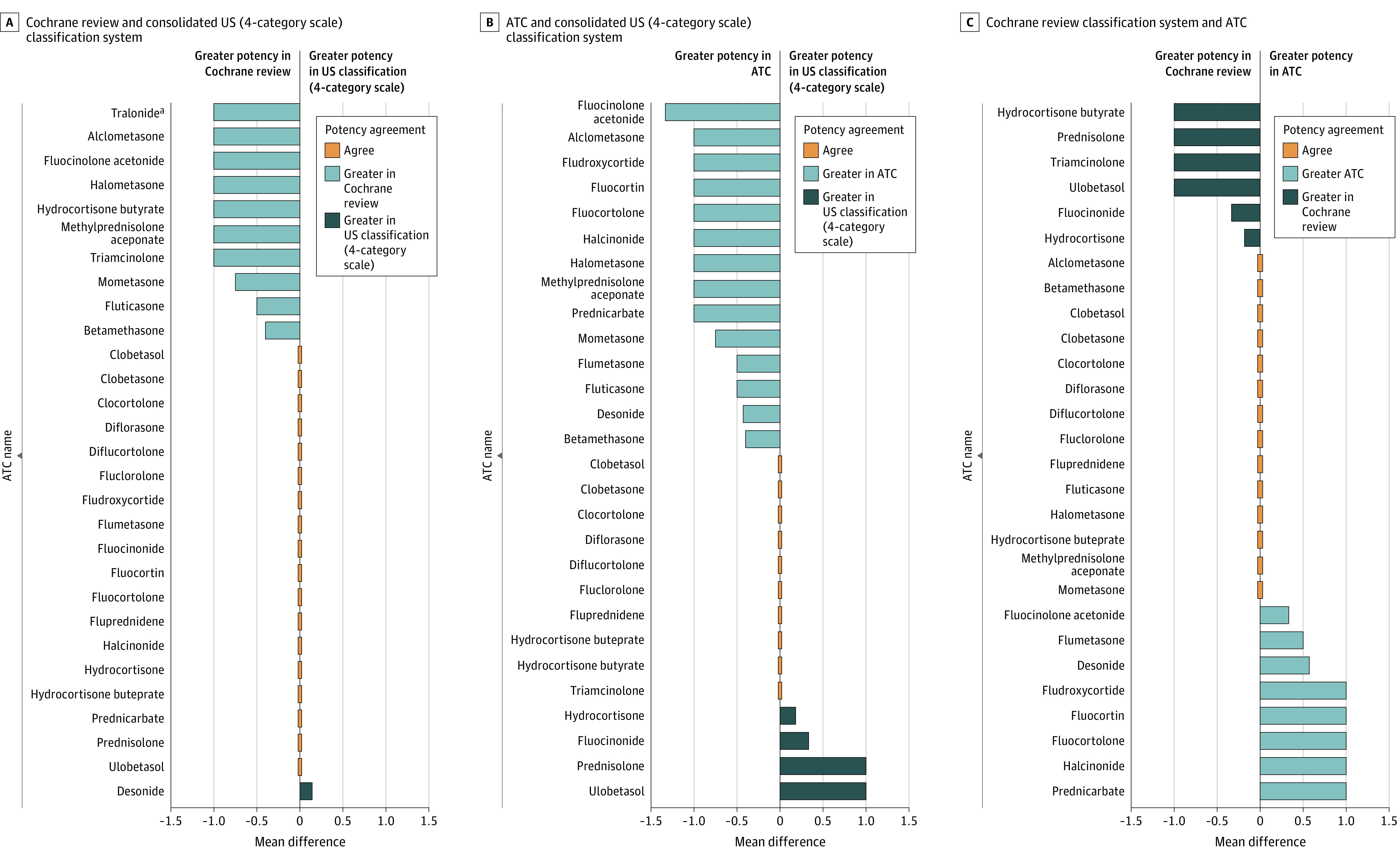
The unit of measure for average difference is potency classification units. ATC indicates Anatomical Therapeutic Classification.
aNot classified in the ATC.
The US classification was strongly correlated with the ATC (r, 0.77; 95% CI, 0.71-0.82) and Cochrane review classifications (r, 0.74; 95% CI, 0.62-0.82). The Cochrane review classification and ATC were also strongly correlated (r, 0.71; 95% CI, 0.58-0.80).
Discussion
We used multiple sources to classify 232 TCSs using 3 potency classification systems. The classifications showed weak-to-moderate agreement despite strong correlation, suggesting that TCS potency classifications are inconsistent between systems. Consequently, the classification system chosen for a given pharmacoepidemiologic study could substantially alter its results and clinical interpretation.
For studies on commonly prescribed medications available in different formulations, or those that are taken as needed, substituting a defined daily dose method with a more robust approach has been suggested14; in the context of TCSs, this includes potency classifications. Given how commonly TCSs are prescribed, transparent classification is necessary to strengthen dermatologic outcomes and treatment. The 3 potency classification systems have potential strengths and weaknesses. The 7-category US system is more nuanced and may better reflect the continuous nature of TCS potency than 4-category systems, but it may be difficult to interpret because the importance of a 1-level difference in classification is unclear; for example, are differences between upper midstrength and midstrength TCSs clinically meaningful? The ATC classification is straightforward but overly simplistic by combining formulations with potentially different potencies. For example, in the US classification, betamethasone dipropionate, 0.05%, is classified as potent as an ointment and upper midstrength as a cream; betamethasone valerate, 0.05%, is classified as midstrength as an ointment and lower midstrength as a cream; the ATC system classifies these formulations together as potent. Variation across classifications may limit the comparability of findings across studies; for example, fluocinolone acetonide, 0.01%, cream is categorized as mild in the US system, moderate in the Cochrane review classification, and potent in the ATC system.
Limitations
This study has limitations. We identified potency from lists published in the literature, most of which did not describe how classifications were ascertained. Some existing classification systems, including the 5-category Japanese classification system, were not evaluated. Most classification systems were based on the vasoconstriction assay, which does not always correlate with clinical treatment effects.15 Future research on the clinical validity and applicability of TCS potency classification systems is needed.
Conclusions
Variability between classification systems found in this classification study suggests that research using different potency classifications could yield different results. We offer a comprehensive list of TCS potency classifications to support future pharmacoepidemiologic studies and potentially strengthen evidence on TCS effectiveness and safety. Investigators need to transparently report how they categorize TCS potency and consider sensitivity analyses with alternative classifications to examine the robustness of their findings.
eMethods.
eTable 1. Topical Corticosteroids Manually Assigned to a 7-Category Potency Classification.
eTable 2. Reorganized 7-Category Potency Classifications.
eTable 3. Final List of Topical Corticosteroid Potency Classifications.
eFigure 1. Flowchart Outlining the Process of Developing a TCS List and Assigning a 7-Category Potency Classification Based on the US system.
eFigure 2. Flowchart of the Process of Assigning the ATC Classifications to the Combined List of TCSs.
eReferences
References
- 1.Mehta AB, Nadkarni NJ, Patil SP, Godse KV, Gautam M, Agarwal S. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82(4):371-378. doi: 10.4103/0378-6323.178903 [DOI] [PubMed] [Google Scholar]
- 2.Tempark T, Phatarakijnirund V, Chatproedprai S, Watcharasindhu S, Supornsilchai V, Wananukul S. Exogenous Cushing’s syndrome due to topical corticosteroid application: case report and review literature. Endocrine. 2010;38(3):328-334. doi: 10.1007/s12020-010-9393-6 [DOI] [PubMed] [Google Scholar]
- 3.van der Linden MW, Penning-van Beest FJ, Nijsten T, Herings RM. Topical corticosteroids and the risk of diabetes mellitus: a nested case-control study in the Netherlands. Drug Saf. 2009;32(6):527-537. doi: 10.2165/00002018-200932060-00008 [DOI] [PubMed] [Google Scholar]
- 4.Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157(3):275-282. doi: 10.1001/jamadermatol.2020.4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatol. 2017;153(10):1036-1042. doi: 10.1001/jamadermatol.2017.2437 [DOI] [PubMed] [Google Scholar]
- 6.Government of Ontario. Ontario drug benefit formulary/comparative drug index. Queen’s Printer for Ontario. Updated December 17, 2021. Accessed September 01, 2021. https://www.formulary.health.gov.on.ca/formulary/
- 7.WHO Collaborating Centre for Drug Statistics Methodology . ATC/DDD index. Updated December 14, 2021. Accessed July 20, 2021. https://www.whocc.no/atc_ddd_index/?code=D07A&showdescription=no
- 8.Lax SJ, Harvey J, Axon E, et al. Strategies for using topical corticosteroids in children and adults with eczema. Cochrane Database Syst Rev. 2022;3(3):CD013356. doi: 10.1002/14651858.CD013356.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenzie AW, Stoughton RB. Method for comparing percutaneous absorption of steroids. Arch Dermatol. 1962;86(5):608-610. doi: 10.1001/archderm.1962.01590110044005 [DOI] [Google Scholar]
- 10.Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121(1):63-67. doi: 10.1001/archderm.1985.01660010067020 [DOI] [PubMed] [Google Scholar]
- 11.Oakley R, Arents BWM, Lawton S, Danby S, Surber C. Topical corticosteroid vehicle composition and implications for clinical practice. Clin Exp Dermatol. 2021;46(2):259-269. doi: 10.1111/ced.14473 [DOI] [PubMed] [Google Scholar]
- 12.Corticosteroids for the skin. Drug Ther Bull. 1977;15(2):5-8. doi: 10.1136/dtb.15.2.5 [DOI] [PubMed] [Google Scholar]
- 13.Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: measures of agreement. Perspect Clin Res. 2017;8(4):187-191. doi: 10.4103/picr.PICR_123_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes KN, Gomes T, Tadrous M. Greater than the sum: applying daily-dose equivalents to antipsychotic prescription claims to study real-world effects. Front Pharmacol. 2021;12:709349. doi: 10.3389/fphar.2021.709349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humbert P, Guichard A. The topical corticosteroid classification called into question: towards a new approach. Exp Dermatol. 2015;24(5):393-395. doi: 10.1111/exd.12677 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Topical Corticosteroids Manually Assigned to a 7-Category Potency Classification.
eTable 2. Reorganized 7-Category Potency Classifications.
eTable 3. Final List of Topical Corticosteroid Potency Classifications.
eFigure 1. Flowchart Outlining the Process of Developing a TCS List and Assigning a 7-Category Potency Classification Based on the US system.
eFigure 2. Flowchart of the Process of Assigning the ATC Classifications to the Combined List of TCSs.
eReferences


