Key Points
Question
Are there biomarkers that may predict pathologic response after neoadjuvant therapy (NAT) for pancreatic ductal adenocarcinoma (PDAC)?
Findings
In this prognostic study, RNA sequencing of 23 pretreatment fine-needle aspiration (FNA) biopsy specimens from NAT-naive patients with PDAC found that higher matrix metalloproteinase 7 (MMP-7) expression was associated with unfavorable pathologic response to NAT. Analysis of FNA biopsy specimens from a validation cohort of 80 patients also found that increased MMP-7 expression had a positive predictive value of 88.2% for a negative response to NAT.
Meaning
This study suggests that assessment of MMP-7 expression on FNA biopsy specimens at the time of diagnosis may help identify patients with PDAC who would benefit the most from NAT.
Abstract
Importance
The use of neoadjuvant therapy (NAT) in resectable pancreatic ductal adenocarcinoma (PDAC) remains controversial. A favorable pathologic response (complete or marked tumor regression) to NAT is associated with better outcomes in patients with resected PDAC. The role of NAT for early systemic control compared with immediate surgical resection for PDAC is under investigation. In the era of precision medicine, biomarkers for patient selection and prediction of therapy response are crucial.
Objective
To evaluate the use of assessment for protein expression on fine-needle aspiration (FNA) biopsy specimens in predicting pathologic response to NAT in treatment-naive patients.
Design, Setting, and Participants
This was a single-institution prognostic study from a high-volume center for pancreatic cancer. All specimens were obtained between January 1, 2009, and December 31, 2018, with a median (SE) follow-up of 20.2 (1.4) months. Analysis of the data was performed from October 1, 2019, to April 30, 2021. Targeted RNA sequencing of frozen FNA biopsy specimens from a discovery cohort of 23 patients was performed to identify genes with aberrant expression that was associated with patients’ pathologic response to NAT. Immunohistochemical staining was performed on an additional 80 FNA biopsy specimens to assess expression of matrix metalloproteinase 7 (MMP-7) and its association with pathologic response. Receiver operating characteristic curves for prediction of favorable pathologic response were determined.
Results
In the discovery cohort (12 [52.1%] male; 3 [13.0%] Black and 20 [86.9%] White), RNA sequencing showed that lower MMP-7 expression was associated with favorable pathologic response (College of American Pathologists system scores of 0 [complete response] and 1 [marked response]). In the validation cohort (40 [50.0%] female; 9 [11.3%] Black and 71 [88.7%] White), patients with negative MMP-7 expression were significantly more likely to have a favorable pathologic response (odds ratio, 21.25; 95% CI, 6.19-72.95; P = .001). Receiver operating characteristic curves for prediction of favorable pathologic response from multivariable Cox proportional hazards regression modeling showed that MMP-7 expression increased the area under the curve from 0.726 to 0.906 (P < .001) even after stratifying by resectability status. The positive predictive value and negative predictive value of MMP-7 protein expression on FNA biopsy specimens in predicting unfavorable pathologic response (scores of 2 [partial response] or 3 [poor or no response]) were 88.2% and 73.9%, respectively.
Conclusions and Relevance
Assessment of MMP-7 expression on FNA biopsy specimens at the time of diagnosis may help identify patients who would benefit the most from NAT.
This single-center prognostic study of biopsy specimens from treatment-naive patients with pancreatic ductal adenocarcinoma assesses the association of aberrant gene expression as well as expression of matrix metalloproteinase 7 with pathologic response to preresection neoadjuvant therapy.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is associated with a dismal 5-year survival rate of approximately 10%.1 Multimodal treatment strategies have been used to provide a better chance of cure and enhance survival.2,3 The neoadjuvant paradigm has increased the widespread implementation of chemotherapy, radiotherapy, or a combination of both to be administered prior to surgery.4 It is hypothesized that incorporating multiagent chemotherapy such as FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) not only treats occult metastases and may downstage disease to improve surgical resectability but also permits early systemic control of subclinical micrometastatic deposits.5,6
Neoadjuvant therapy (NAT) is currently recommended by the National Comprehensive Cancer Network for borderline-resectable PDAC and localized disease with vessel involvement.7 In this era of precision medicine with its goal of targeted therapy, biomarkers for patient selection as well as predictors of response to multiagent therapy are still lacking. A trial by Versteijne et al investigated the role of preoperative therapy in resectable or borderline-resectable disease to determine who may benefit from early systemic control or immediate surgical resection.8 Although results did not show significant benefit of NAT in improving overall survival, an increase in the rate of margin-free resection was evident.8 Therefore, methods to determine which patients are best suited for receiving NAT are of high value.
A number of biomarkers have been described to be prognostic and predictive for PDAC.9 These biomarkers have been discovered on cytological specimens, such as fine-needle aspiration (FNA) biopsy specimens, or liquid biopsies.10,11,12,13,14 In fact, recent studies15,16 have found that, in addition to their diagnostic value in PDAC, FNA biopsy specimens obtained endoscopically under ultrasound guidance may help in determining the course of treatment and predicting response to therapy. Thus, there is an urgent need to identify biomarkers in these specimens for favorable or unfavorable pathologic response to neoadjuvant chemotherapy, which is a robust surrogate for outcomes in patients with PDAC.17 The central aim of this study was to apply RNA sequencing to FNA biopsy specimens from treatment-naive patients at the time of diagnosis and investigate its role in predicting pathologic response to neoadjuvant chemotherapy, which could potentially guide therapeutic clinical decision-making in the treatment of patients with PDAC.
Methods
This study was approved by the Johns Hopkins University Institutional Review Board, and written informed consent was obtained from all patients. This study follows the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline. The available specimens of the patients in this study were included based on approval by the investigators. The current analysis was conducted from October 1, 2019, to April 30, 2021. Further details are available in eMethods in the Supplement.
Study Population and Tissue Specimens
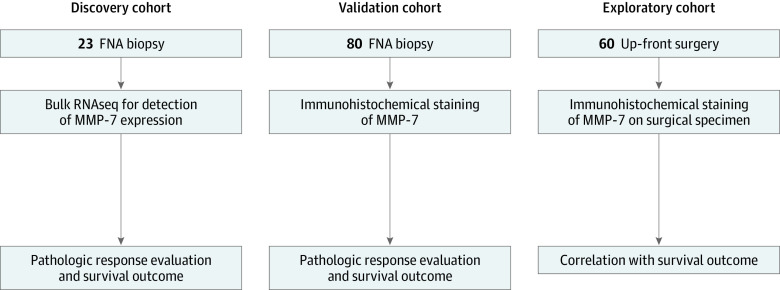
This study was conducted using a discovery cohort (n = 23) (eTable 1 in the Supplement), a validation cohort (n = 80), and an exploratory cohort (n = 60) comprising patients from whom tissue specimens were available in the biobank at The Johns Hopkins Hospital between January 1, 2009, and December 31, 2018 (Figure 1). The FNA biopsy specimens of histologically confirmed PDAC from the 23 patients in the discovery cohort were collected at the time of diagnosis (prior to NAT) and stored at the biobank for research purposes. All 23 patients successfully completed NAT as planned and proceeded to surgical resection. Pathologic response using the current tumor regression grading system was assessed by an independent pathologist through examination of resected primary tumor surgical specimens according to the College of American Pathologists (CAP) system score; the pathologic response was listed as either favorable (score of 0 [complete response] or 1 [marked response]) or unfavorable (score of 2 [partial response] or 3 [poor or no response]).18 Next-generation DNA sequencing was performed on all 23 FNA biopsy specimens for confirmation of the presence of tumor variants associated with PDAC, including KRAS, TP53, SMAD4, and CDKN2A. Targeted RNA sequencing was performed on the same specimens (the complete list of the 472 targeted genes is in eTable 2 in the Supplement).
Figure 1. Study Design.
FNA indicates fine-needle aspiration; MMP-7, matrix metalloproteinase 7.
When RNA sequencing revealed that matrix metalloproteinase 7 (MMP-7) protein expression may be associated with treatment response (see Results), we performed immunohistochemical (IHC) staining on specimens from a validation cohort of 80 patients who underwent NAT to assess the association of MMP-7 protein expression with pathologic response to neoadjuvant treatment. Fine-needle aspiration biopsy specimens archived as formalin-fixed, paraffin-embedded blocks at the time of diagnosis (prior to NAT) were obtained for tissue analysis. Baseline demographic, clinical, and pathologic characteristics were recorded, including age, sex, race and ethnicity, type of NAT, use of chemoradiotherapy or chemotherapy alone, duration of NAT, level of cancer antigen (CA) 19-9, tumor size, pathologic T stage, grade of differentiation, presence of lymphovascular invasion, presence of perineural invasion, margin class, use of adjuvant chemotherapy, and pathologic response score (0, 1, 2, or 3).
Pathologic Assessment and Longitudinal Follow-up
All pathologic diagnoses at The Johns Hopkins Hospital were staged using the American Joint Committee on Cancer 8th edition staging system.19 Resection margin was defined as R1 whenever cancer cells were microscopically visible within 1 mm of the resection margin. Our primary end point was favorable vs unfavorable pathologic response. Overall survival (OS) was defined as survival from time of diagnosis to date of death or last follow-up. Patient’s last date of known follow-up was used as the censoring event for OS analysis. Follow-up data for OS were collected using the National Death Index records of December 31, 2018, through the hospital’s electronic medical record, or through a search of online obituaries, with a median (SE) follow-up of 20.2 (1.4) months. Recurrence-free survival (RFS) was defined as length of survival without disease recurrence from the time of diagnosis to date of recurrence (evaluated on surveillance imaging) or cancer-related death, whichever came first. For RFS analysis, the last date of known radiological follow-up was used as the censoring event. For survival analyses, patients who died within 90 days of diagnosis were excluded; 2 patients died as a result of immediate technical complications of surgery, and their outcomes were not related to biological characteristics of their tumors.
Statistical Analysis
For univariable analysis, a χ2 or Fisher exact test was used for categorical variables, and the Mann-Whitney U test was used for continuous variables. Univariable logistic regression was carried out to evaluate the role of preoperative variables in predicting pathologic response, which determined the variables chosen for subsequent multivariable regression (there is currently little evidence to guide the choice of such variables). The clinical and pathologic characteristics included in multivariable logistic regression modeling were neoadjuvant chemoradiotherapy, a baseline CA 19-9 level of 200 U/mL or greater (normal, 0 to 37 U/mL), tumor size of 3.5 cm or larger, poorly differentiated tumor, and MMP-7 status. The Hosmer-Lemeshow test was used to confirm goodness of fit for each logistic regression model. Receiver operating characteristic (ROC) curves were generated based on risk-adjusted multivariable logistic regression modeling to evaluate the role of MMP-7 as a prognostic test for favorable pathologic response. The comparison of the area under the curve (AUC) for the 2 ROC curve models (with and without MMP-7) was carried out using a paired design to test for statistical significance. Risk-adjusted hazard ratios (HR) with 95% CIs were calculated using a Cox proportional hazards regression model. A backward conditional selection method was applied for the multivariable Cox proportional hazards regression analysis including only those variables showing statistical significance (P < .05) from univariable Cox proportional hazards regression analysis. After the multivariable Cox proportional hazards regression analysis (backward method) was carried out, only those variables that remained significant at the end were presented in each table. All time-to-event analyses (OS and RFS) were expressed as Kaplan-Meier curves, which were compared using the log-rank test. Kaplan-Meier curves were truncated when fewer than 5% of the patients remained at risk. A 2-tailed P < .05 was used to determine statistical significance. All statistical analyses were performed using SPSS software, version 25 (IBM Inc).
Results
Discovery Cohort and RNA Sequencing of FNA Biopsy Specimens
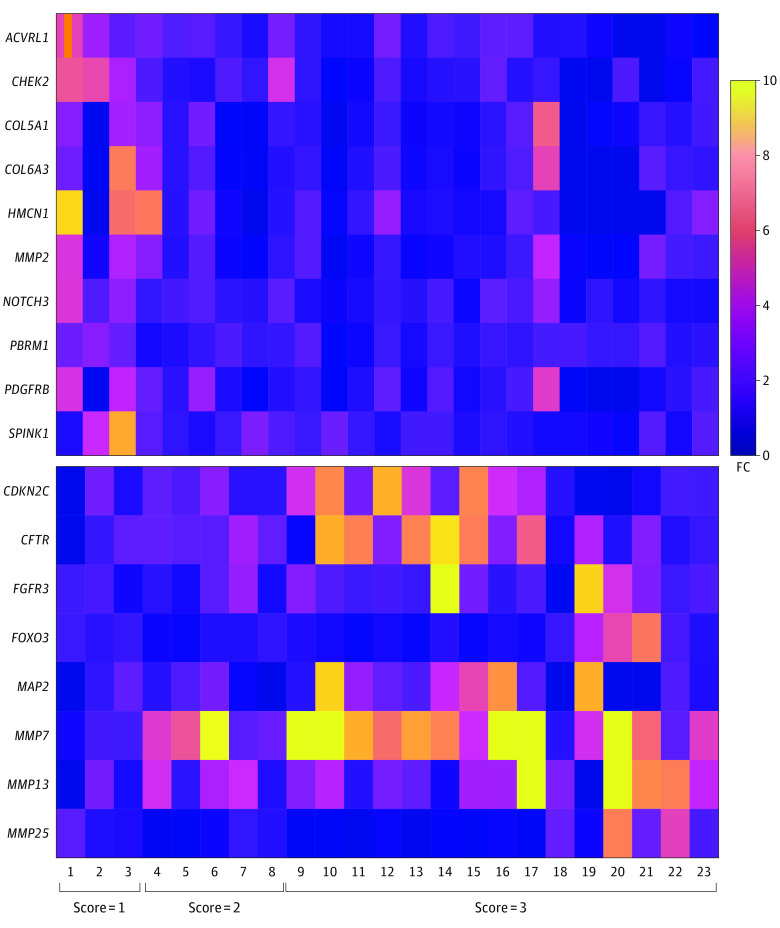
There were 23 patients in the discovery cohort (12 male [52.1%] and 11 female [47.9%]; 3 Black [13.0%] and 20 White [86.9%]); 3 patients (13.0%) were found to have nearly complete pathologic response (score of 1 [none had a score of 0]), 5 (21.7%) had a partial response (score of 2), and 15 (65.2%) had a poor response or no response (score of 3). The clinicopathologic characteristics of this initial (discovery) cohort are summarized in eTable 1 in the Supplement. Targeted RNA sequencing found 18 candidates with aberrant expression, including 10 that overexpressed (ACVRL1 [OMIM 601284], CHEK2 [OMIM 604373], COL5A1 [OMIM 120215], COL6A3 [OMIM 120250], HMCN1 [OMIM 608548], MMP2 [OMIM 120360], NOTCH3 [OMIM 600276], PBRM1 [OMIM 606083], PDGFRB [OMIM 173410], and SPINK1 [OMIM 167790]) and 8 that underexpressed (CDKN2C [OMIM 603369], CFTR [OMIM 602421], FGFR3 [OMIM 134934], FOXO3 [OMIM 602681], MAP2 [OMIM 157130], MMP7 [OMIM 178990], MMP13 [OMIM 600108], and MMP25 [OMIM 608482]), in patients with a pathologic response score of 1 compared with those with a pathologic response score of 2 or 3, as shown in the heat map (Figure 2). MMP7 was further analyzed, and 80.0% of both CAP score 2 samples (4 of 5 samples) and CAP score 3 samples (12 of 15 samples) overexpressed MMP-7 with a fold change greater than 1.5 compared with CAP score 1 samples (3 of 3 samples).
Figure 2. Heat Map of the Dysregulated Genes From Fine-Needle Aspiration Biopsy Specimens by RNA Sequencing Heat Map of Genes With Protein Overexpression or Underexpression on RNA Sequencing.
The heat map represents the 10 most highly expressed (top) and the 8 least expressed (bottom) signals on RNA sequencing of frozen fine-needle aspiration biopsy specimens from the 23 patients in the discovery cohort. Scores indicate association with pathologic response: 1 = marked response, 2 = partial response, and 3 = poor or no response. FC indicates fold change.
Validation Cohort and IHC Staining of FNA Biopsy Specimens
The validation cohort comprised 80 patients (40 [50.0%] and 40 female [50.0%]; 9 Black [11.3%] and 71 White [88.7%]) who underwent NAT followed by surgical resection. (The clinicopathologic characteristics of the validation cohort are summarized in eTable 1 in the Supplement.) Fine-needle aspiration biopsy specimens were obtained from all 80 patients at the time of diagnosis, prior to NAT, and used for MMP-7 IHC staining (eFigure 1 in the Supplement). Specimens from 46 patients (57.5%) were positive for MMP-7 protein expression. There were no significant differences in demographic and clinical characteristics between the discovery and validation cohorts (eTable 1 in the Supplement). Univariable comparisons of basic demographic and clinical characteristics are summarized in Table 1. Pathologic T and N stages were significantly different between the 2 groups, with more advanced stages present in the group with positive MMP-7 expression. For example, in the group with positive MMP-7 expression, there were more patients with pT2 (17 [37.0%]), pT3 (11 [23.9%]), and pT4 (7 [15.2%]) tumors than in the group with negative MMP-7 expression, which had 7 patients with pT2 (20.6%), 5 with pT3 (14.7%), and 0 with pT4 tumors. Perineural invasion was also more prevalent among the MMP-7–positive group than among the MMP-7–negative group (33 patients [71.7%] vs 10 patients [29.4%]; P = .001). All 8 patients with a pathologic response score of 0 were negative for MMP-7 on IHC staining.
Table 1. Patient Demographic and Clinical Characteristics and Tumor Characteristics of FNA Biopsy Specimens From Patients in the Validation Cohort Prior to Neoadjuvant Therapy (n = 80).
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| MMP-7 negative (n = 34) | MMP-7 positive (n = 46) | ||
| Age, median (IQR), y | 62.8 (54.4-72.4) | 68.3 (61.3-73.2) | .26 |
| Sex | |||
| Female | 19 (55.9) | 21 (45.7) | .49 |
| Male | 15 (44.1) | 25 (54.3) | |
| Race and ethnicity | |||
| Black | 3 (8.8) | 6 (13.0) | .72 |
| White | 31 (91.2) | 40 (87.0) | |
| Type of neoadjuvant | |||
| 5-Fluorouracil based | 23 (67.6) | 33 (71.7) | .71 |
| Gemcitabine based | 9 (26.5) | 9 (19.6) | |
| Other | 2 (5.9) | 4 (8.7) | |
| Chemoradiotherapy | 28 (82.4) | 32 (69.6) | .29 |
| Chemotherapy alone | 7 (20.6) | 14 (30.4) | |
| Duration of neoadjuvant therapy, median (IQR), mo | 3.6 (3.2-4.5) | 3.9 (2.2-5.1) | .26 |
| CA 19-9, median (IQR), U/mLa | 111 (19.4-362.2) | 256 (29.7-884.5) | .36 |
| Tumor size (largest measurement), median (IQR), cm | 3.7 (2.7-4.7) | 3.6 (2.5-4.4) | .97 |
| Vascular involvement | 32 (94.1) | 41 (89.1) | .71 |
| Tumor located in pancreas head/neck region | 26 (76.5) | 25 (54.3) | .07 |
| Pathologic T stage | |||
| pT0 | 7 (20.6) | 0 | .001 |
| pT1 | 15 (44.1) | 11 (23.9) | |
| pT2 | 7 (20.6) | 17 (37.0) | |
| pT3 | 5 (14.7) | 11 (23.9) | |
| pT4 | 0 | 7 (15.2) | |
| Pathologic N stage | |||
| pN0 | 26 (76.5) | 23 (50.0) | .04 |
| pN1 | 7 (20.6) | 17 (37.0) | |
| pN2 | 1 (2.9) | 6 (13.0) | |
| Grade | |||
| Well differentiated | 4 (11.8) | 2 (4.3) | .37 |
| Moderately differentiated | 19 (55.9) | 31 (67.4) | |
| Poorly differentiated | 11 (32.4) | 13 (28.3) | |
| Lymphovascular invasion | 5 (14.7) | 15 (32.6) | .07 |
| Perineural invasion | 10 (29.4) | 33 (71.7) | .001 |
| Margin class of R0b | 31 (91.2) | 40 (87.0) | .72 |
| Adjuvant chemotherapy | 20 (58.8) | 33 (71.7) | .24 |
| Pathologic response (CAP score)c | |||
| 0 | 8 (23.5) | 0 | .001 |
| 1 | 22 (64.7) | 12 (26.1) | |
| 2 | 1 (2.9) | 12 (26.1) | |
| 3 | 3 (8.8) | 22 (47.8) | |
Abbreviations: CA 19-9, cancer antigen 19-9; CAP, College of American Pathologists; FNA, fine-needle aspiration; MMP-7, matrix metalloproteinase 7.
Normal, 0 to 37 U/mL.
R0 corresponds to a goal of resection for cure or complete remission.
CAP score of 0 indicates complete response; 1, marked response; 2, partial response; and 3, poor or no response.
Association of MMP-7 Expression With Favorable Pathologic Response (Validation Cohort)
Univariable and multivariable logistic regression modeling was performed to analyze the association of demographic and clinical factors at the time of diagnosis with CAP scores as the primary end point of our study (eTable 3 in the Supplement). On univariable analysis, patients undergoing preoperative radiotherapy in addition to chemotherapy were more likely to have a favorable pathologic response than those undergoing chemotherapy alone (odds ratio [OR], 4.82; 95% CI, 1.54-15.06; P = .007). In addition, patients with negative MMP-7 expression were significantly more likely to have a favorable pathologic response (OR, 21.25; 95% CI, 6.19-72.95; P = .001).
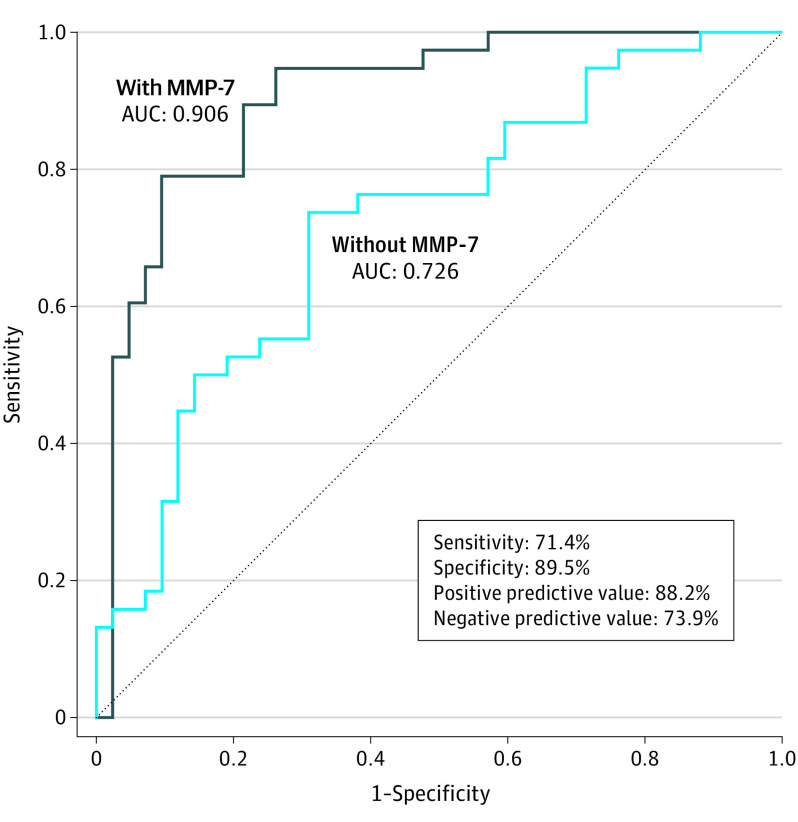
To further investigate whether MMP-7 expression may be used a biomarker to inform the chances of a favorable pathologic response, we used risk-adjusted multivariable logistic regression modeling for favorable pathologic response. The first model excluded MMP-7 expression and comprised variables assessed at the time of diagnosis: chemoradiotherapy, baseline CA 19-9, baseline tumor size on imaging, and histologic grade of differentiation (Hosmer-Lemeshow goodness-of-fit test: χ2 = 11.01; P = .20; df = 8). The second model included the same parameters as well as MMP-7 status on IHC staining of FNA biopsy specimens (Hosmer-Lemeshow goodness -of-fit test: χ2 = 6.58; P = .58; df = 7). Chemoradiotherapy compared with chemotherapy alone (OR, 7.42; 95% CI, 1.55-35.38; P = .01) and negative MMP-7 expression (OR, 32.85; 95% CI, 7.22-149.41; P = .001) emerged as independent predictors of a favorable pathologic response. Furthermore, the 2 models were compared using their ROCs (Figure 3). The addition of MMP-7 expression significantly increased the AUC from 0.726 to 0.906 (P < .001). To further assess the role of MMP-7 in predicting pathologic response in different clinical presentations, we applied the same multivariable modeling with and without MMP-7 expression after stratification by resectability status (resectable, borderline resectable, or locally advanced). There was a statistically significant increase in the AUC on addition of MMP-7 status in the predictive model, regardless of resectability status (eTable 5; eFigure 2 in the Supplement).
Figure 3. Positive and Negative Predictive Value of Matrix Metalloproteinase 7 (MMP-7) for Favorable Pathologic Response.
Receiver operating characteristic curves based on multivariable logistic regression modeling with and without MMP-7 represent the role of MMP-7 protein expression status in predicting favorable pathologic response. Among 34 MMP-7–negative fine-needle aspiration specimens, 30 (88.2%) were associated with a favorable pathologic result. Among 46 MMP-7–positive specimens, 12 (26.1%) were associated with a favorable pathologic result. AUC indicates area under the curve.
In addition, we analyzed the diagnostic value of MMP-7 expression as a predictive tool for pathologic response. The sensitivity and specificity of MMP-7 protein expression on FNA biopsy specimens in predicting an unfavorable pathologic response (scores of 2 and 3) were 71.4% and 89.5%, respectively; the positive predictive value and negative predictive value were 88.2% and 73.9%, respectively (Figure 3).
Association of MMP-7 Protein With Survival Outcome (Validation Cohort)
Patients with FNA biopsy specimens showing negative MMP-7 expression on IHC staining had a significantly higher median RFS of 37.3 months compared to 13.8 months in the group with positive MMP-7 expression (P = .03) (eFigure 3 in the Supplement). In addition, the median OS of the negative MMP-7 subgroup also demonstrated superiority over the positive MMP-7 subgroup (38.2 vs 27.6 months, P = .049). Multivariable Cox proportional hazards regression analysis was carried out with a backward conditional selection method including only the variables that were statistically significant on univariable Cox proportional hazards regression. This showed that the value of MMP-7 expression as a predictor of OS and RFS is incorporated under a favorable pathologic response, which itself remains an independent predictor of an improved OS and RFS with an HR (95% CI) of 0.48 (0.26-0.87) and 0.44 (0.21-0.88), respectively (Table 2).
Table 2. Backward-Entry Method Multivariable Cox Proportional Hazards Regression Depicting Role of MMP-7 in Predicting RFS and OS in the Validation Using FNA Biopsy Specimens Prior to Neoadjuvant Therapy Cohort (n = 80).
| Characteristic | Recurrence-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable Cox proportional hazards regression | Multivariable Cox proportional hazards regression | Univariable Cox proportional hazards regression | Multivariable Cox proportional hazards regression | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age ≥65 y | 1.01 (0.58-1.73) | .99 | NA | NA | 1.47 (0.79-2.72) | .22 | NA | NA |
| Sex | ||||||||
| Female | 1 [Reference] | .21 | NA | NA | 1 [Reference] | .40 | NA | NA |
| Male | 1.42 (0.81-2.46) | NA | NA | 1.3 (0.70-2.39) | NA | |||
| Race and ethnicity | ||||||||
| Black | 1 [Reference] | .18 | NA | 1 [Reference] | .37 | |||
| White | 0.58 (0.26-1.29) | NA | NA | 0.65 (0.25-1.66) | NA | |||
| Neoadjuvant treatment | .41 | NA | NA | .56 | NA | NA | ||
| Treatment type | ||||||||
| Chemotherapy alone | 1 [Reference] | NA | NA | 1 [Reference] | NA | NA | ||
| Chemoradiotherapy | 0.82 (0.44-1.51) | .53 | NA | NA | 0.51 (0.26-1.00) | .05 | NA | NA |
| Chemotherapy regimen | ||||||||
| 5-Fluorouracil based | 1 [Reference] | NA | NA | 1 [Reference] | NA | NA | ||
| Gemcitabine based | 0.61 (0.29-1.26) | .19 | NA | NA | 0.66 (0.30-1.43) | .30 | NA | NA |
| Other | 0.83 (0.29-2.33) | .73 | NA | NA | 0.76 (0.18-3.19) | .71 | NA | NA |
| Baseline CA 19-9 ≥200a | 1.64 (0.94-2.84) | .08 | NA | NA | 1.29 (0.71-0.23) | .40 | NA | NA |
| Baseline tumor size (largest dimension) ≥3.5 cm | 1.55 (0.87-2.65) | .14 | NA | NA | 1.48 (0.79-2.80) | .22 | NA | NA |
| Tumor grade of poor | 1.14 (0.63-2.07) | .65 | NA | NA | 1.64 (0.86-3.13) | .13 | NA | NA |
| T stage ≥T3 | 1.86 (1.04-3.31) | .04 | NA | NA | 2.61 (1.38-4.92) | .003 | NA | NA |
| N stage ≥N1 | 2.51 (1.44-4.38) | .001 | NA | NA | 3.20 (1.72-5.92) | .001 | 2.58 (1.25-5.41) | .01 |
| Lymphovascular invasion | 2.32 (1.27-4.21) | .006 | 2.33 (1.19-4.57) | .01 | 3.02 (1.50-6.10) | .002 | 2.46 (1.12-5.41) | .02 |
| Perineural invasion | 1.96 (1.11-3.46) | .02 | 2.63 (1.37-5.04) | .003 | NA | NA | ||
| Margin class of R0b | 0.47 (0.22-1.02) | .06 | 0.35 (0.16-0.76) | .008 | 0.96 (0.42-2.18) | .93 | NA | NA |
| MMP-7 negative | 0.51 (0.28-0.93) | .03 | NA | 0.52 (0.27-0.00) | .049 | NA | NA | |
| Favorable pathologic response (CAP score of 0 or 1)c | 0.39 (0.21-0.70) | .002 | 0.48 (0.26-0.87) | .02 | 0.30 (0.15-0.57) | .001 | 0.44 (0.21-0.88) | .02 |
| Adjuvant chemotherapy | 0.75 (0.42-1.34) | .34 | NA | NA | 0.56 (0.35-0.72) | .02 | 0.40 (0.19-0.83) | .01 |
Abbreviations: FNA, fine-needle aspiration; HR, hazard ratio; MMP-7, matrix metalloproteinase 7; NA, not applicable; OS, overall survival; RFS, recurrence-free survival.
Normal, 0 to 37 U/mL.
R0 corresponds to resection for cure or complete remission.
CAP score of 0 indicates complete response; 1, marked response.
Association of MMP-7 Expression on Primary Tumor Surgical Specimens With Survival Outcome (Exploratory Cohort)
A cohort of 60 patients who did not receive NAT and underwent up-front surgical resection was identified. Comparisons of basic demographic and clinical characteristics are summarized in eTable 4 in the Supplement. Formalin-fixed, paraffin-embedded blocks of primary tumor surgical specimens were used for IHC staining of MMP-7 (eFigure 1 in the Supplement). Survival analysis of the chemotherapy-naive cohort showed similar results to the cohort undergoing NAT prior to resection. Patients with negative MMP-7 expression on IHC staining had significantly higher median (SE) RFS compared with the group with positive MMP-7 expression (18.2 [2.4] vs 13.4 [1.5] months; P = .001) (eFigure 3 in the Supplement). Similarly, the median (SE) OS of the negative MMP-7 group was also superior to the positive MMP-7 group (27.6 [2.5] vs 18.7 [2.0] months, P = .03). On multivariable Cox proportional hazards regression analysis, negative MMP-7 expression remained an independent predictor of an improved OS (HR, 0.23; 95% CI, 0.11-0.49) and RFS (HR, 0.44; 95% CI, 0.23-0.85) (eTable 6 in the Supplement).
Discussion
This prognostic study is unique in using RNA sequencing in combination with IHC staining on FNA biopsy specimens to evaluate the role of MMP-7. To our knowledge, it is also the first study to assess the role of MMP-7 expression in predicting pathologic response, which is known to be a strong predictor of oncologic outcomes. Our results are especially timely in light of the neoadjuvant paradigm. Previous studies18,20 demonstrated that favorable pathologic response, defined as the combination of CAP scores of 0 and 1, was associated with superior outcomes compared with scores of 2 and 3, which informed our choice to investigate favorable pathologic response as the primary end point. In addition, MMPs are known to be key players in the pathogenesis and progression of PDAC.21 The role of different MMPs (MMP-1, MMP-2, MMP-7, MMP-9, and MMP-14) have been evaluated in different pancreatic cancer experimental models.21,22 These efforts have translated to an established association of different MMPs with outcome.21 The role of MMP-7 in particular within the tumor microenvironment occurs through upregulated mitogen-activated protein kinase–dependent pathways and has been associated with the increased invasiveness of PDAC by stimulating epidermal growth factor receptor–mediated pathways.22,23,24,25,26,27,28,29,30 Likewise, elevated MMP-7 serum levels have been associated with advanced disease, local invasion, lymph node metastasis, and decreased RFS.27
Our findings are supportive of MMP-7 not only as a prognostic biomarker, but also as a predictive variable for pathologic response to neoadjuvant treatment. The ROC curves that we used using baseline data obtained at the time of diagnosis to determine the association of MMP-7 expression status with response to NAT suggest a potential benefit of checking MMP-7 expression status from FNA biopsy specimens. Although negative node status and lower T stage have been shown to predict better pathologic response in PDAC, preoperative MMP-7 expression may independently predict pathologic response without any other postoperative tumor characteristics being available.31,32,33 The high diagnostic specificity (89.5%) and positive predictive value (88.2%) of MMP-7 expression as a tool for predicting pathologic response at the time of diagnosis suggests its promise as a clinical biomarker to inform precision medicine. Furthermore, given that MMP-7 expression was positive in more than half of the FNA biopsy specimens obtained from patients with verified PDAC, it may be a valuable biomarker to test for at the time of diagnosis. Therefore, MMP-7 expression may have validity in predicting disease response, although further prospective validation is needed.
In the current study, MMP-7 expression was an independent predictor of RFS and OS. In the cohort undergoing NAT, an association was observed through including MMP-7 status and pathologic response in the same models predicting RFS and OS. Although favorable pathologic response remained an independent predictor of improved RFS and OS, MMP-7 expression was no longer significant, which was largely because of the collinearity between MMP-7 and a favorable pathologic response predicting the same outcome. This suggests the possibility of MMP-7 status in treatment-naive patients serving as a robust surrogate for pathologic response. In the treatment-naive cohort, MMP-7 remained a strong independent predictor of both RFS and OS, which is concordant with other reports in the literature.22,28
Limitations
This study has several limitations. First, a relatively small number of patients were included in the study, largely because of the limited availability of FNA biopsy specimens available for research purposes, although the FNA biopsy samples were selected consecutively to prevent case-selection bias. This particularly applies to our discovery cohort, on whose tissue RNA sequencing was carried out; however, the panel of 472 genes included could potentially compensate for this limitation. Second, there could be selection bias because obtaining FNA biopsy specimens with enough cellularity is not always feasible in the clinical setting, in which patients may have poorly identified or inaccessible tumors. This has also limited the number of available FNA biopsy specimens for retrospective research, given that they were usually obtained for clinical purposes. Third, although this study did provide evidence supporting the selection of MMP-7 to validate the hypothesis, potential statistical multiplicity should be addressed regarding multiple gene candidates. Other candidates from the RNA sequencing data require further research and explorations of their mechanisms, which we hope to address in future studies. Fourth, risk-adjusted multivariable modeling carried out for logistic regression as well as Cox proportional hazards regression analysis included variables that have commonly been used in literature or that we thought had potential to be predictive of an outcome. However, other possible explanatory variables that were not included in the modeling process warrant evaluation in future studies. Fifth, there could be further selection bias because the patients in this cohort successfully completed their course of NAT with tumor response allowing curative surgical resection; however, a significant portion of patients receiving NAT experience toxicity or disease progression that precludes surgical resection. Thus, a prospective study with a larger cohort is warranted to further validate our findings.
Conclusions
The identification of biomarkers that can predict response to therapy and outcome is a hallmark of precision medicine. The results of this study suggest that FNA biopsy specimens at the time of diagnosis can be used for prediction of pathologic response, which could influence survival outcomes. MMP-7 appears to be an excellent candidate biomarker to identify patients who could benefit from NAT. Further research using FNA biopsy specimens is warranted to identify additional biomarkers that are predictive of treatment outcomes.
eMethods.
eTable 1. Demographics and Tumor Characteristics of FNA Specimens in Initial (Discovery) Cohort Prior to Neoadjuvant Therapy (n = 23)
eTable 2. Targeted RNA NGS List (N = 472)
eTable 3. Univariable and Multivariable Logistic Regression Analyzing the Association of Preoperative Demographic and Clinical Factors With Favorable PR (Score 0 or 1) Showing That MMP-7 Expression Profile on FNA Specimens Prior to Neoadjuvant Therapy Is an Independent Predictor of PR
eTable 4. Demographics and Tumor Characteristics of FFPE Surgical Specimens in Chemotherapy-Naive Cohort (n = 60)
eTable 5. Area Under Curve (AUC) Obtained From ROC Curves (eFigure 2) Based on Multivariable Regression Modeling With and Without MMP-7 After Stratification by Resectability Status at the Time of Diagnosis Showing Role of MMP-7 Protein Expression Profile on FNA Specimens in Predicting Favorable PR
eTable 6. Univariable and Multivariable Cox Regression (Backward-Entry Method) on FFPE Surgical Specimens in Exploratory (Chemotherapy-Naive) Cohort
eFigure 1. IHC Staining of Tissue Biopsy Prior to Neoadjuvant Therapy (Validation Cohort) Representing Negative (A) and Positive (B) MMP-7 Protein Expression; IHC Staining of Primary Tumor Surgical Specimens From Chemotherapy-Naive Cohort (Exploratory Cohort) Showing Negative (C) and Positive (D) MMP-7 Protein Expression
eFigure 2. ROC Curves Based on Multivariable Regression Modeling With and Without MMP-7 After Stratification by Resectability Status at the Time of Diagnosis Showing Role of MMP-7 Protein Expression Profile on FNA Specimens in Predicting Favorable PR
eFigure 3. Kaplan-Meier Overall Survival and Recurrence-Free Survival Curves Comparing MMP-7 Positive and Negative Expression in FNA Specimens From Cohort Undergoing Neoadjuvant Therapy (A, B) and FFPE Surgical Specimens From Chemotherapy-Naive Cohort (C, D)
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846-4861. doi: 10.3748/wjg.v24.i43.4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oba A, Ho F, Bao QR, Al-Musawi MH, Schulick RD, Del Chiaro M. Neoadjuvant treatment in pancreatic cancer. Front Oncol. 2020;10:245. doi: 10.3389/fonc.2020.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16(1):11-26. doi: 10.1038/s41571-018-0112-1 [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Hammel P, Hebbar M, et al. ; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group . FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395-2406. doi: 10.1056/NEJMoa1809775 [DOI] [PubMed] [Google Scholar]
- 6.Motoi F, Unno M. Adjuvant and neoadjuvant treatment for pancreatic adenocarcinoma. Jpn J Clin Oncol. 2020;50(5):483-489. doi: 10.1093/jjco/hyaa018 [DOI] [PubMed] [Google Scholar]
- 7.Raufi AG, Manji GA, Chabot JA, Bates SE. Neoadjuvant treatment for pancreatic cancer. Semin Oncol. 2019;46(1):19-27. doi: 10.1053/j.seminoncol.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 8.Versteijne E, Suker M, Groothuis K, et al. ; Dutch Pancreatic Cancer Group . Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38(16):1763-1773. doi: 10.1200/JCO.19.02274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le N, Sund M, Vinci A; GEMS collaborating group of Pancreas 2000 . Prognostic and predictive markers in pancreatic adenocarcinoma. Dig Liver Dis. 2016;48(3):223-230. doi: 10.1016/j.dld.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 10.Tsai S, Christians KK, George B, et al. A phase II clinical trial of molecular profiled neoadjuvant therapy for localized pancreatic ductal adenocarcinoma. Ann Surg. 2018;268(4):610-619. doi: 10.1097/SLA.0000000000002957 [DOI] [PubMed] [Google Scholar]
- 11.Habib JR, Yin L, Yu J. Pancreatic ductal adenocarcinoma: the role of circulating tumor DNA. J Pancreatol. 2019;2:72-75. doi: 10.1097/JP9.0000000000000021 [DOI] [Google Scholar]
- 12.Poruk KE, Valero V III, Saunders T, et al. Circulating tumor cell phenotype predicts recurrence and survival in pancreatic adenocarcinoma. Ann Surg. 2016;264(6):1073-1081. doi: 10.1097/SLA.0000000000001600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gemenetzis G, Groot VP, Yu J, et al. Circulating tumor cells dynamics in pancreatic adenocarcinoma correlate with disease status: results of the prospective CLUSTER study. Ann Surg. 2018;268(3):408-420. doi: 10.1097/SLA.0000000000002925 [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Sadakari Y, Shindo K, et al. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut. 2017;66(9):1677-1687. doi: 10.1136/gutjnl-2015-311166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semaan A, Bernard V, Lee JJ, et al. Defining the comprehensive genomic landscapes of pancreatic ductal adenocarcinoma using real-world endoscopic aspiration samples. Clin Cancer Res. 2021;27(4):1082-1093. doi: 10.1158/1078-0432.CCR-20-2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Archibugi L, Ruta V, Panzeri V, et al. RNA extraction from endoscopic ultrasound-acquired tissue of pancreatic cancer is feasible and allows investigation of molecular features. Cells. 2020;9(12):1-14. doi: 10.3390/cells9122561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin L, Pu N, Thompson E, Miao Y, Wolfgang C, Yu J. Improved assessment of response status in patients with pancreatic cancer treated with neoadjuvant therapy using somatic mutations and liquid biopsy analysis. Clin Cancer Res. 2021;27(3):740-748. doi: 10.1158/1078-0432.CCR-20-1746 [DOI] [PubMed] [Google Scholar]
- 18.Kakar S, Shi C, Adsay NV, et al. ; College of American Pathologists. Protocol for the Examination of Specimens From Patients With Carcinoma of the Pancreas. Version PancreasExocrine 4.0.0.1. 2017. Accessed June 2017. https://documents.cap.org/protocols/cp-pancreas-exocrine-17protocol-4001.pdf
- 19.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 20.Truty MJ, Kendrick ML, Nagorney DM, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2021;273(2):341-349. doi: 10.1097/SLA.0000000000003284 [DOI] [PubMed] [Google Scholar]
- 21.Jakubowska K, Pryczynicz A, Januszewska J, et al. Expressions of matrix metalloproteinases 2, 7, and 9 in carcinogenesis of pancreatic ductal adenocarcinoma. Dis Markers. 2016;2016:9895721. doi: 10.1155/2016/9895721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones LE, Humphreys MJ, Campbell F, Neoptolemos JP, Boyd MT. Comprehensive analysis of matrix metalloproteinase and tissue inhibitor expression in pancreatic cancer: increased expression of matrix metalloproteinase-7 predicts poor survival. Clin Cancer Res. 2004;10(8):2832-2845. doi: 10.1158/1078-0432.CCR-1157-03 [DOI] [PubMed] [Google Scholar]
- 23.Tan X, Egami H, Abe M, Nozawa F, Hirota M, Ogawa M. Involvement of MMP-7 in invasion of pancreatic cancer cells through activation of the EGFR mediated MEK-ERK signal transduction pathway. J Clin Pathol. 2005;58(12):1242-1248. doi: 10.1136/jcp.2004.025338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slapak EJ, Duitman J, Tekin C, Bijlsma MF, Spek CA. Matrix metalloproteases in pancreatic ductal adenocarcinoma: key drivers of disease progression? Biology (Basel). 2020;9(4):80. doi: 10.3390/biology9040080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S-H, Hung W-C, Wang P, Paul C, Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep. 2013;3:1870. doi: 10.1038/srep01870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang SC, Parekh JR, Porembka MR, et al. A pilot study evaluating serum MMP7 as a preoperative prognostic marker for pancreatic ductal adenocarcinoma patients. J Gastrointest Surg. 2016;20(5):899-904. doi: 10.1007/s11605-015-3057-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhlmann KFD, van Till JWO, Boermeester MA, et al. Evaluation of matrix metalloproteinase 7 in plasma and pancreatic juice as a biomarker for pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(5):886-891. doi: 10.1158/1055-9965.EPI-06-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan X, Egami H, Ishikawa S, et al. Involvement of matrix metalloproteinase-7 in invasion-metastasis through induction of cell dissociation in pancreatic cancer. Int J Oncol. 2005;26(5):1283-1289. doi: 10.3892/ijo.26.5.1283 [DOI] [PubMed] [Google Scholar]
- 29.Resovi A, Bani MR, Porcu L, et al. Soluble stroma-related biomarkers of pancreatic cancer. EMBO Mol Med. 2018;10(8):1-14. doi: 10.15252/emmm.201708741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto H, Itoh F, Iku S, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J Clin Oncol. 2001;19(4):1118-1127. doi: 10.1200/JCO.2001.19.4.1118 [DOI] [PubMed] [Google Scholar]
- 31.Fischer LK, Katz MH, Lee SM, et al. The number and ratio of positive lymph nodes affect pancreatic cancer patient survival after neoadjuvant therapy and pancreaticoduodenectomy. Histopathology. 2016;68(2):210-220. doi: 10.1111/his.12732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roland CL, Yang AD, Katz MH, et al. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22(4):1168-1175. doi: 10.1245/s10434-014-4192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perri G, Prakash LR, Katz MHG. Response to preoperative therapy in localized pancreatic cancer. Front Oncol. 2020;10:516. doi: 10.3389/fonc.2020.00516 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Demographics and Tumor Characteristics of FNA Specimens in Initial (Discovery) Cohort Prior to Neoadjuvant Therapy (n = 23)
eTable 2. Targeted RNA NGS List (N = 472)
eTable 3. Univariable and Multivariable Logistic Regression Analyzing the Association of Preoperative Demographic and Clinical Factors With Favorable PR (Score 0 or 1) Showing That MMP-7 Expression Profile on FNA Specimens Prior to Neoadjuvant Therapy Is an Independent Predictor of PR
eTable 4. Demographics and Tumor Characteristics of FFPE Surgical Specimens in Chemotherapy-Naive Cohort (n = 60)
eTable 5. Area Under Curve (AUC) Obtained From ROC Curves (eFigure 2) Based on Multivariable Regression Modeling With and Without MMP-7 After Stratification by Resectability Status at the Time of Diagnosis Showing Role of MMP-7 Protein Expression Profile on FNA Specimens in Predicting Favorable PR
eTable 6. Univariable and Multivariable Cox Regression (Backward-Entry Method) on FFPE Surgical Specimens in Exploratory (Chemotherapy-Naive) Cohort
eFigure 1. IHC Staining of Tissue Biopsy Prior to Neoadjuvant Therapy (Validation Cohort) Representing Negative (A) and Positive (B) MMP-7 Protein Expression; IHC Staining of Primary Tumor Surgical Specimens From Chemotherapy-Naive Cohort (Exploratory Cohort) Showing Negative (C) and Positive (D) MMP-7 Protein Expression
eFigure 2. ROC Curves Based on Multivariable Regression Modeling With and Without MMP-7 After Stratification by Resectability Status at the Time of Diagnosis Showing Role of MMP-7 Protein Expression Profile on FNA Specimens in Predicting Favorable PR
eFigure 3. Kaplan-Meier Overall Survival and Recurrence-Free Survival Curves Comparing MMP-7 Positive and Negative Expression in FNA Specimens From Cohort Undergoing Neoadjuvant Therapy (A, B) and FFPE Surgical Specimens From Chemotherapy-Naive Cohort (C, D)