Imagine a city without traffic lights, a postal system without addresses, or yourself at a busy international airport with no gate information. Cells alike, without proper logistics can easily descend into chaos! The regulation of vesicle trafficking is critical for the exchange of information and cellular components between the multiple organelles and compartments of eukaryotic cells.
Vesicle trafficking occurs through the secretory and endocytic pathways that allow matter to move between the cell surface and its interior. Plant endocytic and secretory vesicles are formed from the plasma membrane and endomembranes, respectively, and can carry different types of cargo molecules, including lipids, proteins, and components of the cell wall. The processes of cargo selection, encapsulation in clathrin-coated vesicles (CCVs), and fusion with the membrane of a target compartment are thought to be controlled by accessory proteins. Specific combinations of accessory proteins act as signatures of vesicles allowing for the utmost precision in targeting and regulating the dynamics of vesicle trafficking (Robinson, 2015; Kim and Brandizzi, 2016; Kaksonen and Roux, 2018).
Several systematic studies have provided insight into yeast and mammalian CCV composition (Takamori et al., 2006; Borner et al., 2012). A number of reports shed light on the protein content of specific plant endomembrane compartments, while systematic analyses of CCV composition of plant cells remain lacking. A new study by Dana Dahhan, Gregory Reynolds, and colleagues (Dahhan et al., 2022) bridges this gap by analyzing the proteome of enriched trans-Golgi network/early endosome-derived and endocytic CCVs isolated from suspension-cultured Arabidopsis cells employing an impressive set of techniques. The study yielded a long-awaited comprehensive roadmap of plant clathrin-mediated vesicle trafficking.
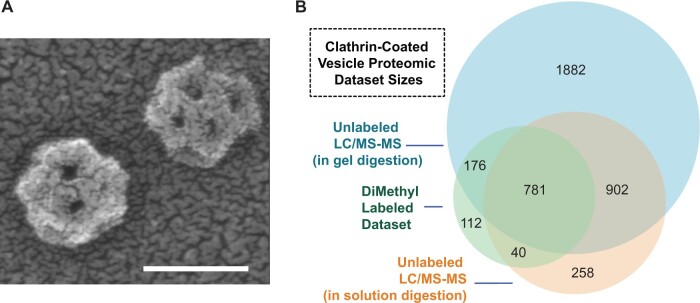
Using pH conditions that inhibit clathrin cage disassembly, CCVs were isolated by differential centrifugation from suspension-cultured Arabidopsis cells (see Figure, panel A). To establish a comprehensive proteome profile of plant CCVs, two independent, parallel workflows were performed on enriched CCV protein samples. First, the authors used in-gel digestion of proteins by trypsin followed by LC/MS-MS analysis. A second method, using trypsin digestion in-solution prior to the separation of recovered peptides by LC/MS-MS, accounted for differences in protein sampling related to 1D SDS-PAGE sample fractionation and the use of detergents. To further refine the CCV proteome and assess the relative enrichment or depletion of proteins identified by mass spectrometry, the authors attempted a third workflow in which they compared the abundance of peptides in pre- and post-gradient samples using a differential labeling strategy with stable isotope dimethyl moieties. The technique resulted in identical peptides from the two types of preparations differing by 4 Daltons and allowed the derivation of quantitative ratios for individual peptides. This approach pointed at general trends of enrichment of CCV-associated proteins and depletion of markers of subcellular compartments not associated with CCV trafficking.
Figure.
A comprehensive plant CCV proteome. A, Negative stain transmission electron micrographs of a CCV preparation used in the study. B, Sizes of and overlaps in proteomic datasets defining proteins associated with CCVs purified from Arabidopsis cells. The sizes of circles and overlaps are proportional to the number of proteins or protein groups contained within. Scale bar, 50 nm. Adapted from Dahhan et al. (2022), Figures 1 and 3.
The study yielded a core set of 781 proteins associated with CCV identified in all three proteomic workflows (see Figure, panel B). The study identified conserved proteins involved in CCV-mediated trafficking between plants and other eukaryotes and key evolutionary divergences, including the association of the adaptor AP-4 specifically with plant CCVs. One should expect a considerable level of divergence that accounts for the involvement of CCVs in plant-specific processes, such as cell wall formation, cytokinesis, and pathogen response. An enormous undertaking, this study yielded a valuable resource providing high-resolution profiling of a plant CCV proteome that could be used by researchers of many fields of plant biology addressing various physiological and developmental processes.
References
- Borner GH, Antrobus R, Hirst J, Bhumbra GS, Kozik P, Jackson LP, Sahlender DA, Robinson MS (2012) Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles. J Cell Biol 197: 141–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahhan DA, Reynolds GD, Cárdenas JJ, Eeckhout D, Johnson A, Yperman K, Kaufmann WA, Vang N, Yan X, Hwang I et al. (2022) Proteomic characterization of isolated Arabidopsis clathrin-coated vesicles reveals evolutionarily conserved and plant-specific components. Plant Cell 34: 2150--2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Roux A (2018) Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 19: 313–326 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Brandizzi F (2016) The plant secretory pathway for the trafficking of cell wall polysaccharides and glycoproteins. Glycobiology 26: 940–949 [DOI] [PubMed] [Google Scholar]
- Robinson MS (2015) Forty years of clathrin‐coated vesicles. Traffic 16: 1210–1238 [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P (2006) Molecular anatomy of a trafficking organelle. Cell 127: 831–846 [DOI] [PubMed] [Google Scholar]