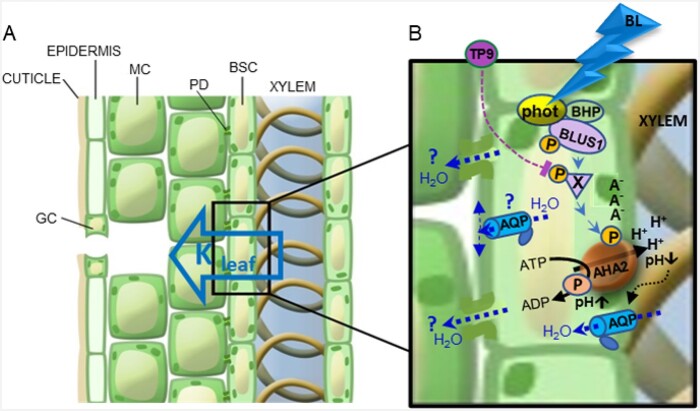
Blue light (BL, 390–550 nm) has been shown to cause stomatal opening and increase leaf hydraulic conductance (Kleaf) in many species. However, the molecular mechanisms of Kleaf regulation remain elusive. Bundle-sheath cells (BSCs), a parenchymal layer surrounding leaf vasculature (see Figure), act as a selective xylem–mesophyll barrier to water and ions. In this issue of The Plant Cell, Yael Grunwald and colleagues (Grunwald et al., 2022) show that BL induces an autonomous signaling pathway in BSCs (like that of guard cells [GCs]), which regulates Kleaf via activation of the BSCs plasma membrane autoinhibited H+-ATPase 2 (AHA2) (see Figure).
Figure.
Proposed model for the BSC-autonomous BL signal transduction pathway regulating Kleaf. A, Radial water movement from xylem to mesophyll via the BSCs. MC, mesophyll cell; PD, plasmodesma. B, BL signaling pathway in a BSC. Reprinted from Grunwald et al. (2022), Figure 9.
In GCs, BL is perceived by the photoreceptor protein kinases phototropin 1 (PHOT1) and PHOT2 (Kinoshita et al., 2001), followed by phosphorylation of downstream signaling kinases BLUS1 and BHP and other yet unknown kinases, which leads to the activation of an H+-ATPase as the endpoint of a phosphorylation cascade, which in turn hyperpolarizes the GCs and acidifies the surrounding apoplast. These pH and membrane potential changes cause ion and water fluxes across the GC membranes that lead to stomatal opening (Hayashi et al., 2017).
Using genetics and a series of pharmacological and physiological assays, Grunwald et al. (2022) investigated BL-induced activation of phototropins in BSCs using artificial microRNA to selectively silence PHOT1 or PHOT2 in BSCs. When illuminated with red light and BL (RL + BL), Kleaf and water potential (Ψleaf) showed a significant reduction in phot mutants relative to wild-type although there was no change in stomatal conductance (gs) or leaf transpiration (E), suggesting that the BSC PHOTs are indispensable for Kleaf regulation.
The kinase inhibitor tyrphostin 9 has been shown to prevent phototropin-mediated phosphorylation of H+-ATPase in GCs (Hayashi et al., 2017), thus disrupting the BL-activated GC-opening signal. To examine whether BSCs possess similar phosphorylation events and whether Kleaf is similarly reduced, Grunwald et al. (2022) applied tyrphostin 9 to wild-type leaves in two ways, xylem fed and leaf spray, followed by RL + BL illumination. When tyrphostin 9 was xylem fed, the Kleaf of illuminated leaves was about 50% lower than that in controls without the inhibitor. In contrast, the leaf spray of inhibitor did not alter the BL-mediated increase in BSC Kleaf, but it did reduce the BL-induced increase in gs, emphasizing the similarity and independence of BL-induced signaling pathways in GCs and BSCs.
Previously, Grunwald and colleagues (Grunwald et al., 2021) demonstrated that the positive relation between xylem acidification by AHA2 and Kleaf is due to an acidification-induced increase in the osmotic water permeability of BSC membranes. To further investigate the BL-induced AHA2-mediated Kleaf increase at a single-cell level, Grunwald et al. (2022) measured membrane potential and cytosolic pH in BSC protoplasts. They found that RL + BL illumination hyperpolarizes BSC protoplasts and increases their cytosolic pH relative to RL alone, supporting the hypothesis that BL-induced activation of AHA2 leads to xylem acidification that subsequently increases water permeability of BSC membranes and thus increases water influx into the leaf.
Together, Grunwald et al. (2022) provided mechanistic insights into the role of an autonomous BL-induced signaling pathway in BSCs which regulates Kleaf with the implication of improving water-use efficiency in plants. The work demonstrates that BSCs, independently of GCs, perceive BL via PHOTs that activate a series of kinases resulting in phosphorylation of AHA2. This autonomous signaling pathway leads to BSCs hyperpolarization and xylem acidification, thus increasing the water permeability of BSC membranes and ultimately increasing radial water influx from the xylem into BSCs and mesophyll cells. It will be intriguing to investigate other yet unknown signaling components such as those involved in direct phosphorylation of AHA2, and the interaction with aquaporins mediating radial water movement to the BSCs and from the BSCs to the mesophyll.
References
- Grunwald Y, Gosa SC, Srivastava TT, Moran N, Moshelion M (2022) Out of the blue: Phototropins of the leaf vascular bundle sheath mediate the regulation of leaf hydraulic conductance by blue light. Plant Cell 34: 2328--2342 [DOI] [PMC free article] [PubMed]
- Grunwald Y, Wigoda N, Sade N, Yaaran A, Torne T, Gosa SC, Moran N, Moshelion M (2021) Arabidopsis leaf hydraulic conductance is regulated by xylem sap pH, controlled, in turn, by a P‐type H+-ATPase of vascular bundle sheath cells. Plant J 106: 301–313 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Inoue S-I, Ueno Y, Kinoshita T (2017) A Raf-like protein kinase BHP mediates blue light-dependent stomatal opening. Sci Rep 7: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]