Abstract
Many older adults report difficulty performing one or more activities of daily living. These difficulties may be attributed to cognitive decline and as a result, measuring cognitive status among aging adults may help provide an understanding of current functional status. The purpose of the present investigation was to determine the association between cognitive status and measures of physical functioning. Seventy-six older adults participated in this study; 41 were categorized as normal memory function (NM) and 35 were poor memory function (PM). NM participants had significantly higher physical function as measured by Short Physical Performance Battery (SPPB; 9.4 ± 2.2 vs. 8.4 ± 2.0; p = .03) and peak velocity (0.67 ± 0.16 vs. 0.56 ± 0.19; p = .04) during a quick sit-to-stand task. Dual-task walking velocities were 22% and 126% slower between cognitive groups for the fast and habitual trials, respectively when compared to the single-task walking condition. Significant correlations existed between measures of memory and physical function. The largest correlations with memory were for peak (r = 0.42) and average (r = 0.38) velocity. The results suggest a positive relationship between physical function and cognitive status. However, further research is needed to determine the mechanism of the underlying relationships between physical and cognitive function.
Keywords: Aging; Physical function; Cognition, movement velocity, dual-task, memory
1. Introduction
In the United States, the number of older adults (>65 years) has increased by 34% since 2008 and is expected to nearly double by 2060 (Administration on Aging, 2018). Among adults over 70 years of age, approximately 30% report difficulty performing at least one activity of daily living (ADL) (Fried et al., 2004; Manini, 2011). The reduced ability to perform ADLs significantly impairs independence and quality of life (Bowling et al., 2007), while increasing admittance into long-term care facilities (Clouston et al., 2013). Older adults requiring assistance with ADLs contribute, individually, more than $36,000 to annual healthcare costs (Manini, 2011). Additionally, dementia-related diseases are among the costliest age-related illnesses (Prince et al., 2016) adding an estimated $305 billion in healthcare expenses in 2020 (Alzheimer’s Association, 2019). Without changes to the current trajectory, projected healthcare expenditures will reach $1.1 trillion by 2050 (Alzheimer’s Association, 2019).
Physical function is the amalgamation of both physical and cognitive performance (Petrella et al., 2004) and directly relates to the ability to carry out ADLs (Forte and Macaluso, 2008; Gray and Paulson, 2014b; McGough et al., 2011; Sprague et al., 2019). Physical function declines with age, presenting difficulties in activities such as rising from a chair, walking, and ascending stairs, leading to reduced physical independence and quality of life (Forte and Macaluso, 2008; Gray and Paulson, 2014b; Lindle et al., 1997). Recently, reports have made connections between physical decline and adverse cognitive outcomes (Atkinson et al., 2009; Sobol et al., 2016; Sprague et al., 2019; Sunderaraman et al., 2019). It is unclear if the factors contributing to physical dysfunction are most closely related to peripheral physiological or cognitive decline, although there is a clear association between mobility disability and poor cognitive function (Clouston et al., 2013; Demnitz et al., 2016). It is also unclear which change precipitates the other, although it is hypothesized that changes in cognitive function occur years before physical function changes are realized (Atkinson et al., 2009). Atkinson et al. (2009) found that significant declines occurred in cognitive function over a 6-year period among community-dwelling older adults (age = 70.3 ± 3.7 years), yet negligible changes occurred in physical function over that same time period. Thus, further investigation is warranted to elucidate the relationships between these two variables.
Age-related cognitive decline occurs across many domains including: reaction time, processing speed, working/episodic memory, and attention (Petrella et al., 2004). Mobility, which is also associated with age-related declines, requires complex and coordinated movements to produce the desired result without adverse effects (Buchman et al., 2011). An additional area of interest for age-related decline is executive function, which is the ability to plan, initiate, and execute specific behaviors (Vazzana et al., 2010) such as physical activities known to improve or maintain physical mobility. Impairments in executive function can result in reduced physical mobility (e.g., difficulty walking on uneven surfaces, poor balance, increased falls) over time due to reduced capacity to perform complex and challenging, real-life activities of daily living. While each component of executive function may contribute to physical mobility (Donoghue et al., 2012), specifically memory has been shown to positively relate with physical mobility among community-dwelling older adults (Donoghue et al., 2012; Watson et al., 2010); yet this relationship is not widely accepted. Thus, it is suggested that cognitive performance across the various domains be assessed at the onset of physical mobility decline (Vazzana et al., 2010).
Many mobility tasks require both cognitive and physical tasks to be completed simultaneously (e.g., cooking, driving). These are known as dual-tasks and are completed frequently throughout the day, requiring an individual to divide their attention between motor and cognitive tasks (Theill et al., 2011). These tasks rely on executive function and share similar neural pathways causing an individual to subconsciously favor either the cognitive or the physical task (Sakurai et al., 2018). With age, the ability to adequately divide attention while performing simultaneous motor and cognitive tasks deteriorates, resulting in performance declines on either the cognitive task, the motor task, or both (Priest et al., 2008; Theill et al., 2011). Not surprisingly, reductions in cognitive ability result in diminished dual-task performance (Casas-Herrero et al., 2013; Sakurai et al., 2018; Sobol et al., 2016). This is an important concept when understanding the role of cognition in the ability to plan and execute physical activity and ADLs. This paradigm suggests even small changes in cognitive decline may lead to larger accumulated negative effects on physical function (Buchman et al., 2011; McGough et al., 2011). Adults with cognitive decline are more likely to have slower gait speed (Casas-Herrero et al., 2013; Demnitz et al., 2016) and increased mobility disability (Taylor et al., 2019; Vazzana et al., 2010) leading to reduced quality of life.
Another area affected by age-related decline and associated with physical disability is muscular power. Muscular power is a combination of the movement velocity and strength of a particular muscle group. Specifically, lower-extremity muscular power is positively associated with both physical function (Glenn et al., 2017; Glenn et al., 2016b; Gray and Paulson, 2014a; Gray and Paulson, 2014b) and cognition (Cherup et al., 2018; Petrella et al., 2004). Among older adults, muscular power is more strongly related to both physical function and cognition when compared to muscular strength (Casas-Herrero et al., 2013; Petrella et al., 2004), suggesting that the velocity of the movement is the more important aspect of the muscular power equation among this population. Additionally, previous research suggests reductions in neural conduction velocity may contribute to these changes in muscular power by reducing the ability to perform rapid movements (Palve and Palve, 2018). Thus, further identifying the specific movement type most closely associated with physical function and cognition is imperative for developing future intervention strategies that mitigate declines.
It is apparent that physical function is positively associated with cognition, but there are multiple approaches for determining both physical and cognitive function. The present investigation aimed to determine which measures of physical function are most highly correlated with memory function among community-dwelling older adults. We hypothesize NM participants will have greater physical function and there will be a positive association between memory and physical function performance.
2. Methodology
2.1. Participants and procedures
The current investigation implemented a cross-sectional design where participants were tested on a single occasion. A total of 85 community-dwelling older adults were recruited through flyers, website announcements, and word of mouth. Of the 85 recruited participants, 76 completed all assessments and were included in analyses. Adults over 60 years of age who were able to read and understand English were eligible for participation. Individuals were excluded from the present study if they met any of the following criteria: diagnosed with Attention Deficit Disorder or Attention Deficit Hyperactivity Disorder, known learning disability, disabling vision loss, inability to complete calibration process for the digital cognitive assessment, and/or diagnosed neurological (stroke, tremor) or psychiatric illness. This study was approved by the Institutional Review Board at a land-grant institution in the Midwest. Assessments were completed only after signing an approved informed consent document.
2.2. Biometric assessments
Biometric assessments included height, weight, and body mass index. Height was measured with a standing stadiometer (Detecto; Webb City, MO) to the nearest 0.1 cm. Weight was measured with a balance-beam scale (Detecto; Webb City, MO) to the nearest 0.1 kg. Body mass index was calculated as a ratio between weight and height (kg/m2).
2.3. Cognition
2.3.1. Visual Paired Comparison
Declarative memory was assessed using the Visual Paired Comparison (VPC) assessment (Neurotrack Technologies, Inc.). VPC collects eye tracking data and recognition accuracy to determine declarative memory of participants. VPC has demonstrated convergent validity with standard neuropsychological assessments including Digit Symbol Coding (processing speed and memory) (Bott et al., 2018), Pattern Comparison Processing Speed tests from the NIH Toolbox Cognitive Battery (Bott et al., 2018; Gills et al., 2019), pattern copying assessments (Crutcher et al., 2009), Word List Memory (Crutcher et al., 2009), and declarative memory (Manns et al., 2000; McKee and Squire, 1993). Additionally, previous VPC studies predict declines in the Mini-mental State Examination (Chau et al., 2017) and conversion to MCI or AD within a 3-year period (Zola et al., 2013).
Details of the VPC have been described elsewhere (Gills et al., 2019), briefly, the VPC assessment began with a calibration phase that required participants to follow, with only their eyes, a blue circle at various locations on the computer screen for 30 s. The calibration phase was followed by the first familiarization/learning phase where participants were introduced to 20 pairs of identical images displayed on the right and left sides of the computer screen. Each image pair was presented for 5 s with a 2-second delay between each stimulus presentation. Immediately after the familiarization/learning phase, the first testing phase began. During this phase, participants were presented a series of disparate image pairs on the screen (right and left sides), each consisting of one familiar image from the familiarization phase and one novel image. Participants were instructed to focus their gaze on the novel image (the one they had not seen before). The proportion of time a participant spent gazing at the novel image relative to the total viewing time produced a novelty preference score, with higher scores representing better declarative memory. Next, participants completed a second familiarization/learning phase. This phase of the assessment included presenting the same series of disparate images from the first testing phase and participants were instructed to remember which images were paired together. Immediately after familiarization/learning phase two, the second testing phase began. This final testing phase presented a series of disparate image pairs, some of which were from the second familiarization/learning phase and some were not. Participants were instructed to discriminate between image pairs that exactly matched previously viewed pairs and those that did not by selecting (touching the corresponding box) ‘Yes’ for disparate images previously presented and ‘No’ for images not previously presented together. Fifty trials were completed including a mix of previously viewed image pairs (20 targets), altered image pairs from the previous familiarization phase (20 foils), and entirely new image pairs (10 shams). Outcome variables included accuracy of target, foil, and sham image pairs reported as a percentage of correct selections.
Intraclass correlation (ICC) for VPC is 0.91 (Gills et al., 2019). All norms are gender- and age-specific, with age grouped by decile (60–69, 70–79, etc.). The cutoffs were determined based on standard neuropsychological practice. Any score that is average, above average, or <1 SD below average is considered to be within the normal range and was categorized as normal memory. Any score that is ≥1 SD below mean normative performance was considered to be low and is categorized as poor memory.
2.4. Physical assessments
2.4.1. Short Physical Performance Battery (SPPB)
SPPB is a battery of three subtests designed to evaluate physical function in older adults (Gomez et al., 2013; Guralnik et al., 1994; Pavasini et al., 2016). Poor SPPB performance is associated with negative health outcomes including loss of ADL performance, physical disability, mobility limitations, and nursing home admittance (Guralnik et al., 1994; Pavasini et al., 2016). Detailed methods and scoring details for SPPB are described elsewhere (Guralnik et al., 1994); briefly, the three subtests include gait speed, standing balance, and lower-extremity muscular strength. Gait speed was assessed over a 4-meter distance. The walking path was free from obstacles and performed in a well-lit area. Standing balance included side-by-side, semi-tandem, and tandem stands. Lower-extremity muscular strength was assessed using a 5-time sit-to-stand test. Elapsed time for each assessment was recorded and used to create a composite score. SPPB was scored by tallying the subtests to calculate an overall score (Guralnik et al., 1994). Scores ranged from 0 to 12 with higher values representing better physical function.
2.4.2. Dual-task
Dual-task walking is a measure of attention and executive function (Brustio et al., 2017; Yogev-Seligmann et al., 2008). Participants were instructed to walk 10 m at their usual and fast speeds. There was a 3-meter distance before and after the 10-meter distance to account for acceleration and deceleration (Glenn et al., 2014). For the usual speed assessment, participants were instructed to walk the total 16-meter distance at a pace they would use to walk across their house for no particular reason. For the fast speed, participants were instructed to walk as quickly and as safely as possible without running. During dual-task conditions, participants were instructed to perform the same walking conditions while simultaneously performing serial subtractions (Hausdorff et al., 2001). A random 3-digit number was selected (100–999) and participants were instructed to subtract three from each number while performing each walking condition (usual and fast). Additionally, serial subtractions were performed as a single task. To control for time, all trials were conducted for the same amount of time taken to complete the single motor task (10-m walking) and the dual-task trials. Since, time to complete the dual-task trials differed significantly (p < .001) between conditions (habitual and fast), values were normalized by calculating number of correct answers per second. All conditions were completed twice, averaged separately, and used for analysis. Dual-task is a valid and highly reliable method for assessing working memory in young and older adults (McCulloch et al., 2009; Montero-Odasso et al., 2012).
2.4.3. Lower-extremity muscular power
Muscular power was measured during a sit-to-stand test (Glenn et al., 2017; Glenn et al., 2016a; Glenn et al., 2016b; Gray et al., 2016; Gray and Paulson, 2014a; Gray and Paulson, 2014b; Vincenzo et al., 2018). During this assessment participants had a Kevlar string attached to a Tendo Weightlifting Analyzer (TENDO Sports Machines, London, UK) on their left side. Participants sat on a standard chair (0.43 m seat height) with their arms crossed over their chest and were instructed on the command “Go” to stand up as quickly as possible. The Tendo produces four variables for each stand: peak power and velocity and average power and velocity. Peak variables represent the highest velocity or power measure during the stand; average variables are calculated as the mean movement velocity or power generated throughout the entire stand. Five repetitions were completed, and the averages of all four variables were used for analyses. The Tendo sit-to-stand assessment is a valid and reliable assessment of lower-body muscular power and velocity of the sit-to-stand movement among older adults (Gray and Paulson, 2014a).
2.4.4. Timed-Up-and-Go (TUG)
TUG is an assessment used to predict falls (Shumway-Cook et al., 2000) and physical mobility of older adults (Podsiadlo and Richardson, 1991). In the present investigation, TUG was completed on a 3-m track. Participants were instructed on the command “Go”, to stand up from a seated position, walk around a cone placed 3 m from the edge of the chair as their usual pace, and return to their seated position. TUG was completed twice, and the average was used in analyses.
2.5. Statistical analysis
Univariate analyses of covariance (ANCOVA) were utilized to determine differences in functional measures between cognitive groups (NM vs. PM). Cognitive groups were determined by VPC scores; individuals scoring 1SD or more below the mean were categorized as PM and individuals more than 1SD below the mean were categorized as NM. Dependent variables included in the analyses were: SPPB, 10-m walking speed (habitual and fast), dual-task (habitual and fast) performance, single-task cognitive performance (raw scores and normalized scores; habitual and fast), lower-extremity power and velocity (average and peak), and TUG. All analyses were performed while controlling for sex, education, and age. In order to determine which measures of physical function are most highly correlated with cognitive function, associations between the VPC and dependent variables were conducted using Pearson-product moment correlations. Correlation between VPC and SPPB (ordinal data) was determined with a Spearman Rho correlation. Statistical significance was set at α = 0.05; to account for multiple univariate test the Bonferroni procedure was implemented for the following variables: walking speed, dual-task performance, lower extremity power, and lower extremity velocity (α = 0.025). Demographic information is reported as mean ± SD.
3. Results
A total of 76 (80.7 ± 5.5 years) community-dwelling older adults participated in the current investigation. Of the participants, 74% were women and 81% had at least a college degree. Classification into cognitive groups was determined by VPC performance. Table 1 displays demographic information for each cognitive group.
Table 1.
Characteristics of the study cohort.
| High cognitive function (n = 41) | Low cognitive function (n = 35) | p-Values | |
|---|---|---|---|
| Age (years) | 79.9 years (5.0) | 82.0 years (5.7) | p = .08 |
| Sex | |||
| Female (%) | 79.5 | 68.3 | p = .24 |
| Education | |||
| College graduates or higher (%) | 83.3 | 77.5 | p = .94 |
| Race | |||
| European-American (%) | 97.6 | 95.1 | p = .49 |
| Biometric | |||
| Height (cm) | 161.35 (7.06) | 162.79 (10.71) | p = .28 |
| Weight (kg) | 71.84 (12.51) | 75.52 (17.64) | p = .48 |
| BMI (kg/m2) | 27.42 (4.48) | 28.35 (5.23) | p = .39 |
Note. Values are presented as means (SD). p-Values represented independent samples t-test differences between groups. BMI = body mass index.
SPPB scores were significantly different between groups (p = .03; Fig. 1). NM participants had average SPPB scores of 9.4 ± 2.2 compared to 8.4 ± 2.0 (Table 2) in the PM group (Cohen’s d = 0.48). Among participants in the PM group, 65% had SPPB values less than 10, indicating mobility disability, compared to only 39% of participants in the NM group.
Fig. 1.
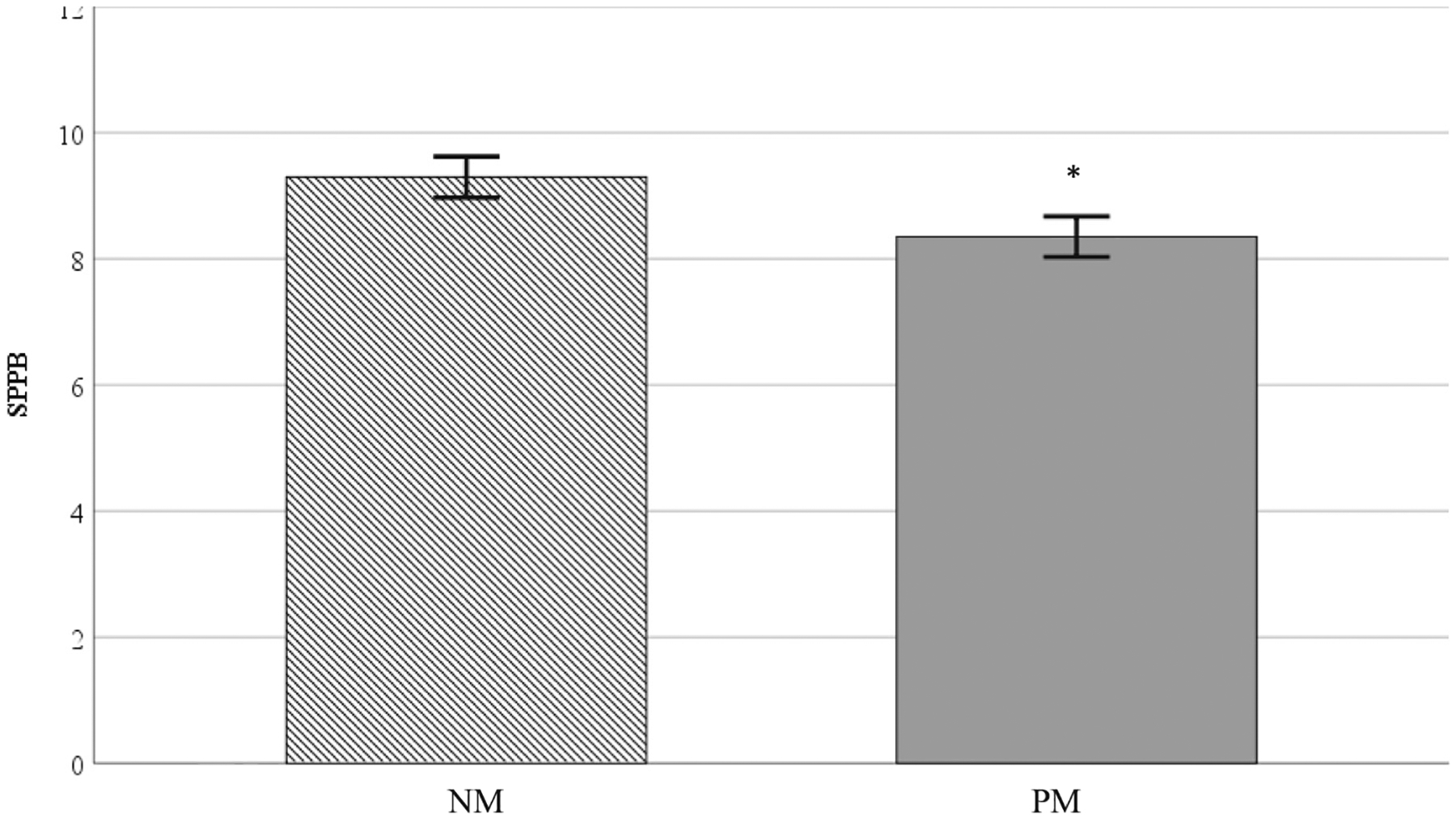
Note. SPPB = Short Physical Performance Battery; NM = Normal memory function; PM Poor memory function. * represents significant difference from HCF group. Values are reported as means ± SD.
Table 2.
Mean physical function differences between memory groups.
| Normal memory function (n = 41) | Poor memory function (n = 35) | p-Values | |
|---|---|---|---|
| SPPB | 9.40 (2.16) | 8.35 (2.03) | p = .03* |
| TUG (sec) | 9.40 (3.23) | 9.95 (2.33) | p = .52 |
| 10-m habitual (sec) | 9.27 (2.93) | 9.98 (2.15) | p = .26 |
| 10-m fast (sec) | 6.79 (2.00) | 7.60 (1.78) | p = .07 |
| DT habitual (sec) | 9.77 (3.22) | 11.08 (3.02) | p = .08 |
| DT fast (sec) | 8.58 (2.83) | 9.86 (2.56) | p = .06 |
| Average power (W) | 294.37 (94.04) | 252.77 (112.98) | p = .19 |
| Peak power (W) | 519.75 (181.70) | 462.65 (295.24) | p = .53 |
| Average velocity (m/s) | 0.42 (0.11) | 0.35 (0.12) | p = .055 |
| Peak velocity (m/s) | 0.67 (0.16) | 0.56 (0.19) | p = .04* |
Note. Values are reported as means (standard deviation). SPPB = Short Physical Performance Battery; TUG = Timed Up-and-Go; 10-m = 10-meter walking speed; DT = Dual-task. Univariate analyses were used to determine differences between groups; control variable used were age, sex, and education level.
represents significant correlations between variables
Peak movement velocity during the sit-to-stand task was significantly faster (p = .04; Fig. 2) among NM older adults compared to PM participants (0.67 ± 0.16 vs. 0.56 ± 0.19, respectively) with a moderate effect size (Cohen’s d = 0.63). NM participants moved 16% faster when compared to participants in the PM group during the power sit-to-stand task. Average velocity was 17% faster among participants with NM compared to PM trending toward statistical significance (0.42 ± 0.11 vs. 0.35 ± 0.12; p = .055). There was a moderate effect for average velocity (d = 0.61). Lower-extremity muscular power (peak and average) was similar between groups (p > .05; Table 2).
Fig. 2.
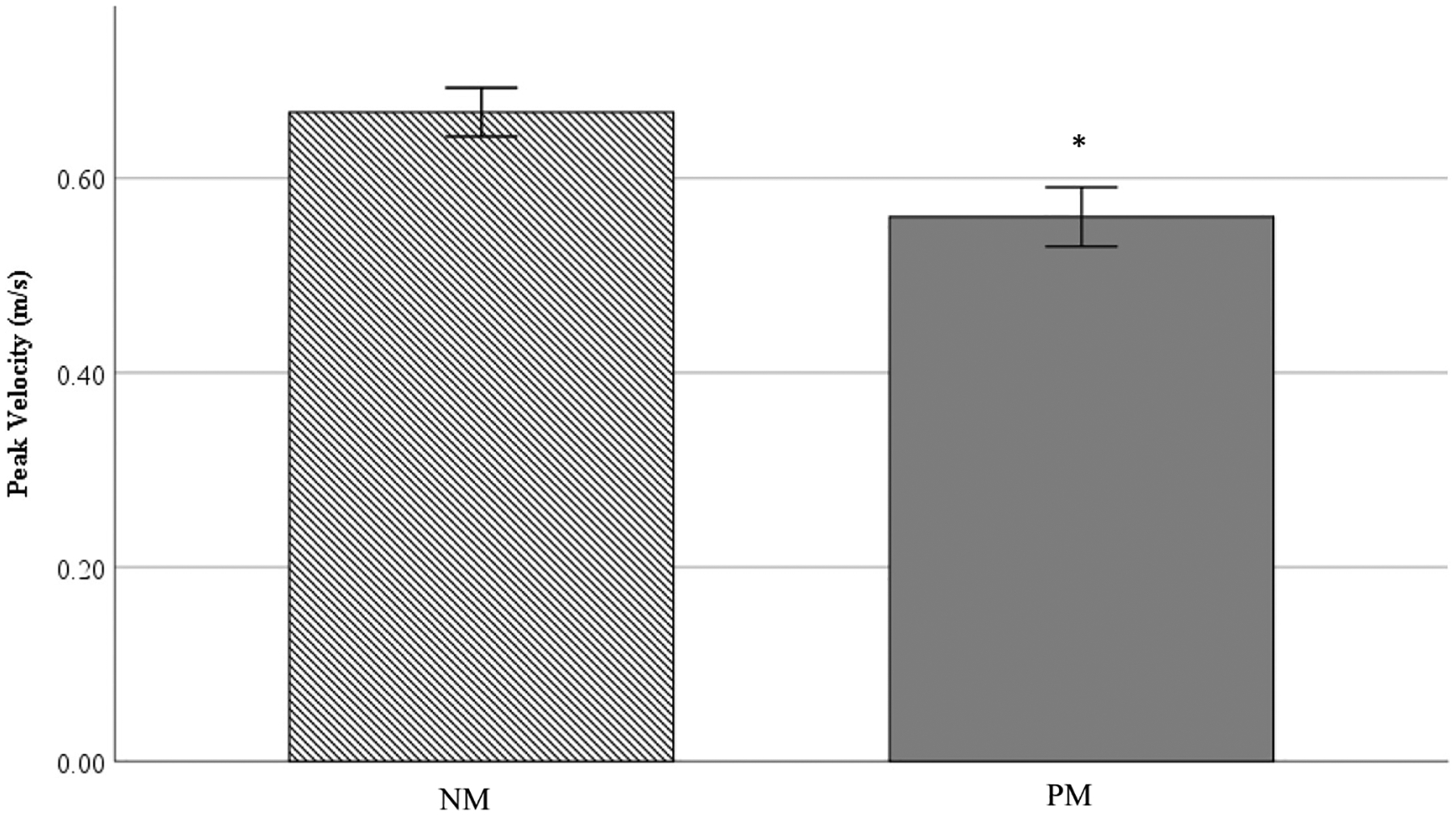
Note. SPPB = Short Physical Performance Battery; NM = Normal memory function; PM = Poor memory function. * represents significant difference from HCF group. Values are reported as means ± SD.
Habitual and fast 10-m walking speeds were similar between cognitive groups (Table 2), although there was a trend for the NM participants to walking quicker during the fast condition (p = .07) with a moderate effect size (Cohen’s d = 0.43). Results for the dual-task conditions followed a similar pattern. Dual-task (habitual and fast conditions) trended toward significance (p = .08 and 0.06, respectively). Both dual-task conditions had moderate effects (Cohen’s d = 0.42 & 0.47 for habitual and fast conditions, respectively) with NM participants out-performing the PM participants. There were no differences between memory groups for the serial subtraction single task during either condition for raw scores (Table 3). Significant differences existed between memory groups only during the fast condition (p = .03). When comparing scores after normalizing for walk time (#correct/s), significant differences existed for both habitual (p = .02) and fast (p = .001) conditions for serial subtraction (Table 3). When comparing dual-task accuracy scores normalized to walking time, differences between groups were 28% and 40% for cognitive accuracy scores when completing the dual-task habitual and fast conditions, respectively. No differences existed for TUG scores between groups (p = .52; Table 2).
Table 3.
Serial subtraction differences between memory groups.
| Normal memory function (n = 41) | Poor memory function (n = 35) | p-Values | |
|---|---|---|---|
| Serial subtraction ST habitual (# correct) | 3.1 (1.7) | 3.4 (1.6) | p = .48 |
| Serial subtraction ST fast (# correct) | 3.2 (1.5) | 2.8 (1.8) | p = .32 |
| Serial subtraction DT habitual (# correct) | 4.5 (2.6) | 3.8 (2.1) | p = .21 |
| Serial subtraction DT fast (# correct) | 4.4 (2.0) | 3.2 (2.3) | p = .03 |
| Serial subtraction DT habitual (#correct/sec) | 0.50 (0.29) | 0.36 (0.21) | p = .02 |
| Serial subtraction DT fast (#correct/sec) | 0.57 (0.25) | 0.34 (0.22) | p < .001 |
Note. ST = single task; serial subtraction only; DT = dual task; serial subtraction accuracy during motor task. Values reported as means (SD).
Significant correlations existed between VPC scores and measures of physical function among the entire sample (Table 4). The greatest correlations existed between VPC and peak (r = 0.42, p < .001) and average (r = 0.38, p < .001) velocity during the power sit-to-stand assessment. These results suggest speeded movements are more strongly associated with cognition than slower or more habitual movements, such as the 10-m walk (r = 0.27, p = .02).
Table 4.
Correlations between VPC physical function.
| VPC | |
|---|---|
| SPPB (units) | 0.32** |
| TUG (s) | −0.19 |
| 10-m habitual (s) | −0.27* |
| 10-m fast (s) | −0.32** |
| DT habitual (s) | −0.28* |
| DT fast (s) | −0.33** |
| Average power (W) | 0.33** |
| Peak power (W) | 0.24* |
| Average velocity (m/s) | 0.38** |
| Peak velocity (m/s) | 0.42** |
Note. VPC = Visual Paired Comparison: percentile; SPPB = Short Physical Performance Battery; TUG = Timed Up-and-Go; 10-m = 10-m walking speed; DT = Dual-task.
p < .05.
p < .01.
4. Discussion
The present investigation aimed to determine which measures of physical function are most strongly correlated with cognition among community-dwelling older adults. Results demonstrate differences in physical function (as measured by SPPB and peak sit-to-stand velocity) between the NM and PM groups. This is the first investigation known to evaluate differences in physical function between PM and NM participants using VPC as the cognitive measure.
4.1. SPPB
Currently evidence exists supporting a positive relationship between cognitive function and physical performance (Demnitz et al., 2016; Pedersen et al., 2014) using traditional paper-pencil assessments. Among community-dwelling older adults, SPPB performance predicts mobility disability (Guralnik et al., 2000), hospital admittance, mortality, and cognitive dysfunction (Corsonello et al., 2012). SPPB is comprised of three assessments including standing balance, lower extremity strength, and gait speed resulting in a composite score ranging from 0 to 12 (Guralnik et al., 2000). Pedersen et al. (2014) found a 1.6-unit (11%) difference in SPPB scores between community-dwelling older adults diagnosed with mild cognitive impairment versus participants with normal cognitive function after neuropsychological screening. In the present investigation, the absolute difference in SPPB performance between cognitive groups was considered substantial at 1.0 units (11%). This is important to note, because clinically meaningful differences for SPPB are 0.3–0.5 points (minimal differences) and 1.0–1.9 points (substantial differences) (Perera et al., 2006).
4.2. Lower-extremity muscular power
Muscular power is positively associated with cognitive function among older adults (van Dam et al., 2018). In the present investigation, peak and average muscular power was similar between groups. This may be explained, in part, by the fact that standing from a seated position is a task that is relatively automated requiring little higher order processing from the brain (Hausdorff et al., 2005). Additionally, while VPC has shown to have convergent validity with executive function (Montreal Cognitive Assessment and NIH Toolbox Cognitive Battery), it is an assessment of declarative memory. Memory is a component of executive function but has yet to be studied relative to lower-extremity muscular power until now.
Upon further investigation, a moderate correlation existed between average lower-extremity muscular power and VPC scores (r = 0.33). These results are similar to those reported by Cherup et al. (2018) who found moderate correlations between global cognition and lower-extremity muscular power. Similarly, Petrella et al. (2004) found a significant positive correlation (r = 0.41) between processing speed and lower-extremity muscular power (Petrella et al., 2004). The relationship between muscular power and cognitive function is important when considering that lower-extremity muscular power is strongly associated with functional outcomes and physical independence of older adults (Glenn et al., 2017; Gray and Paulson, 2014b; Reid and Fielding, 2012). Notably, reductions in both muscular power and cognition are inter-twined with no clear direction whether one parameter is driving change in the other. Fortunately, interventions designed to increase muscular power have been shown to increase executive function (Yoon et al., 2017).
Additionally, peak lower-extremity velocity was significantly correlated with VPC (r = 0.42) and was 16% faster among NM participants when compared to individuals in the PM group (p = .04). To our knowledge, this is the first study to associate lower-extremity movement velocity with cognition. These results are promising, and further investigation into the effects of specific velocity training programs on cognitive function is warranted.
4.3. Dual-task
Compared to young adults, older individuals have greater activation of the prefrontal cortex during dual-task activities when compare to young adults (Ohsugi et al., 2013). Therefore, the addition of a subsequent motor task is likely to require a greater proportion of the brain beyond what is typically engaged during a single task (Li et al., 2018). Due to this change in cognitive function, dual-task performance may be particularly impacted among older adults. Impaired dual-task performance among older adults is associated with a significant increase in falls, fall risk (Li et al., 2018; Muir-Hunter and Wittwer, 2016), and physical disability (Guedes et al., 2014). Additionally, dual-task performance is associated with cognitive decline and cognitive impairment among older adults (Theill et al., 2011).
For dual-task assessments in the present investigation, the greatest differences in walking velocity existed between NM and PM groups for the habitual condition. PM individuals slowed their fast walking velocity by 11% when performing serial subtractions compared to 5% in the NM group. Theill et al. (2011) reported a 28% reduction in walking velocity under a dual-task condition between cognitively normal and PM older adults. While dual-task methodologies vary widely between researchers, one result has been commonly found: the more mentally taxing the cognitive task, the more severely motor performance suffers (Brustio et al., 2017; Hausdorff et al., 2005; Neider et al., 2011; Riby et al., 2010). The present investigation used a serial three subtraction condition where participants were instructed to walk at their usual and fast speeds while counting backward by 3 s (Glenn et al., 2014; Smith et al., 2016). Among cognitively intact adults, dual-task is expected to decline by approximately 10–16% when compared to a single walking task; this is termed dual-task cost (Guedes et al., 2014; Theill et al., 2011). In the present investigation differences in dual-task cost of the walking task between NM and PM participants during the fast condition was 22%; however, this increased to 126% difference during the habitual trial. These results are similar to Sobol et al. (2016) who found a 22% decline when participants with Alzheimer’s disease completed a walking task while counting backward by 1 s. Similarly, previous research has found a 10–40% reduction in usual walking velocity when completing a serial subtraction task among older adults (Brustio et al., 2017; Priest et al., 2008; Smith et al., 2016). Differences may be attributed to the specific methodology used to assess dual-task cost between the studies. We used a serial three subtraction task, a more difficult condition when compared to serial one subtraction assessments. During the motor task, serial subtraction accuracy was similar between memory groups when evaluating raw scores. However, significant differences arose when controlling for 10-m walk time for each dual-task condition (habitual and fast). These results directly conflict with Corp et al. (2018) who suggest older adults will sacrifice walking speed to preserve cognitive outcomes during a dual-task condition. In the present investigation, motor function (walking velocity) was maintained while cognitive accuracy during the dual-task assessment declined. Difference in results may be an effect of the specific instructions given to the participants. The present investigation provided no specific direction on which task should be prioritized to mimic more naturally occurring events (i.e., walking and talking).
4.4. Timed-Up-and-Go
The TUG assessment is predictive of falls (Asai et al., 2018) among community-dwelling adults, as well as hospital readmission rates among previously hospitalized older adults (Aubert et al., 2017). Although TUG is an assessment of functional mobility, it also poses a significant cognitive challenge (Ibrahim et al., 2017; Lee et al., 2018). The TUG requires an individual to transfer, walk, and turn (Ibrahim et al., 2017) while putting the list of instructions into the appropriate sequence of events (e.g., transfer, walking, turning). In the present investigation, although statistically insignificant, TUG values were 0.60s (6%) slower among the PM group when compared to the NM participants. Previous investigations have consistently reported slower TUG times in PM compared to NM individuals (Ibrahim et al., 2017; Rajtar-Zembaty et al., 2019). Specifically, differences ranged from 0.30 s to 1.11 s when comparing the two cognitive groups (Ibrahim et al., 2017; Rajtar-Zembaty et al., 2019). The variation in results between studies could be a result of how individuals are classified into PM and NM groups. Ibrahim et al. (2017) categorized PM older adults if they self-reported worry about memory, while Rajtar-Zembaty et al. (2019) defined PM as a score of less than 23 on the Mini-Mental State Exam. The present investigation used a digital form of a VPC task to assess declarative memory function. This objective assessment is reliable and valid for assessing cognitive function (Gills et al., 2019).
5. Limitations
There are a few limitations associated with the present investigation. First, results were likely impacted by the study population. All participants were volunteers who were fully aware of the study’s purpose before enrolling. This could have swayed enrollment toward adults with higher physical capability and/or cognitive capacity. Additionally, participants that completed assessments with an assistive device were removed from statistical analysis based on current testing recommendations; however, this could falsely inflate physical function scores. Lastly, this was a cross-sectional study; thus, determining the causative nature of either physical or cognitive decline is not possible.
6. Conclusion
Based on the current results, differences exist between cognitive groups on various parameters of physical function among older adults, specifically SPPB and lower-extremity movement velocity. The nature of the relationship remains unclear. However, preclinical declines in cognition may lead to reduced capacity to perform complex planning and execution of physical activity regimens in order to maintain adequate physical function and prevent physical disability. Further research is needed to examine the direction of the potentially causal relationship between cognitive decline and physical function. Longitudinal studies are warranted to determine interventions to impact both cognition and physical function. High-velocity resistance training programs significantly improve physical function among older adults; however, the effect on cognitive change is unknown. Thus, future studies are necessary to ascertain effects of these intervention strategies on cognitive performance potentially leading to advances in exercise programming for this population.
Acknowledgments
This work was supported, in part, by the Translational Research Institute grant KL2 TR003108 through the National Center for Advancing Translational Sciences of the National Institutes of Health.
Footnotes
CRediT authorship contribution statement
Michelle Gray, Ph.D.: Conceptualization, Methodology, Formal Analysis, Investigation, Data Curation, Writing – Original Draft, Visualization, Supervision, Project Administration
Joshua L. Gills, M.S.: Investigation, Data Curation, Writing – Review & Editing
Jordan M. Glenn, Ph.D.: Conceptualization, Methodology, Investigation, Writing – Review & Editing
Jennifer Vincenzo, P.T., MPH., Ph.D.: Investigation, Writing – Review & Editing, Partial Funding acquisition
Christopher S. Walter, PT, DPT, Ph.D.: Investigation, Writing – Review & Editing
Erica Madero, M.S., MPH: Investigation, Data Curation, Writing – Review & Editing, Project administration
Aidan Hall, B.A.: Investigation, Writing – Review & Editing
Nami Fuseya, MSW: Investigation, Writing – Review & Editing
Nick T. Bott, Psy.D.: Investigation, Data Curation, Writing – Review & Editing, Project administration
References
- Administration on Aging, 2018. In: Services. U.S.D.o.H.a.H (Ed.), Profile of Older Americans. U.S. Department of Health and Human Services. [Google Scholar]
- Alzheimer’s Association, 2019. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 15, 321–387. [Google Scholar]
- Asai T, Oshima K, Fukumoto Y, Yonezawa Y, Matsuo A, Misu S, 2018. Association of fall history with the Timed Up and Go test score and the dual task cost: a cross-sectional study among independent community-dwelling older adults. Geriatr Gerontol Int 18, 1189–1193. [DOI] [PubMed] [Google Scholar]
- Atkinson HH, Rapp SR, Williamson JD, Lovato J, Absher JR, Gass M, et al. , 2009. The relationship between cognitive function and physical performance in older women: results from the Women’s Health Initiative Memory Study. The Journals of Gerontology: Series A 65A, 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert CE, Folly A, Mancinetti M, Hayoz D, Donzé JD, 2017. Performance-based functional impairment and readmission and death: a prospective study. BMJ Open 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott N, Madero EN, Glenn J, Lange A, Anderson J, Newton D, et al. , 2018. Device-embedded cameras for eye tracking–based cognitive assessment: validation with paper-pencil and computerized cognitive composites. J. Med. Internet Res 20, e11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A, Seetai S, Morris R, Ebrahim S, 2007. Quality of life among older people with poor functioning. The influence of perceived control over life. Age Ageing 36, 310–315. [DOI] [PubMed] [Google Scholar]
- Brustio PR, Magistro D, Zecca M, Rabaglietti E, Liubicich ME, 2017. Age-related decrements in dual-task performance: comparison of different mobility and cognitive tasks. A cross sectional study. PLoS One 12, e0181698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Leurgans SE, Barnes LL, Bennett DA, 2011. Cognitive function is associated with the development of mobility impairments in community-dwelling elders. Am. J. Geriatr. Psychiatry 19, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas-Herrero A, Cadore EL, Zambom-Ferraresi F, Idoate F, Millor N, Martinez-Ramirez A, et al. , 2013. Functional capacity, muscle fat infiltration, power output, and cognitive impairment in institutionalized frail oldest old. Rejuvenation Res. 16, 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau SA, Sherman C, Lanctôt KL, Herrmann N, Chung J, Eizenman M, et al. , 2017. Visual selective attention toward novel stimuli predicts cognitive decline in Alzheimer’s disease patients. J. Alzheimers Dis 55, 1339–1349. [DOI] [PubMed] [Google Scholar]
- Cherup N, Roberson K, Potiaumpai M, Widdowson K, Jaghab AM, Chowdhari S, et al. , 2018. Improvements in cognition and associations with measures of aerobic fitness and muscular power following structured exercise. Exp. Gerontol 112, 76–87. [DOI] [PubMed] [Google Scholar]
- Clouston SA, Brewster P, Kuh D, Richards M, Cooper R, Hardy R, et al. , 2013. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol. Rev 35, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corp DT, Youssef GJ, Clark RA, Gomes-Osman J, Yücel MA, Oldham SJ, et al. , 2018. Reduced motor cortex inhibition and a ‘cognitive-first’ prioritisation strategy for older adults during dual-tasking. Exp. Gerontol 113, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsonello A, Lattanzio F, Pedone C, Garasto S, Laino I, Bustacchini S, et al. , 2012. Prognostic significance of the short physical performance battery in older patients discharged from acute care hospitals. Rejuvenation Res. 15, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutcher MD, Calhoun-Haney R, Manzanares CM, Lah JJ, Levey AI, Zola SM, 2009. Eye tracking during a visual paired comparison task as a predictor of early dementia. Am. J. Alzheimers Dis. Other Dement 24, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demnitz N, Esser P, Dawes H, Valkanova V, Johansen-Berg H, Ebmeier KP, et al. , 2016. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait & posture 50, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue OA, Horgan NF, Savva GM, Cronin H, O’Regan C, Kenny RA, 2012. Association between timed up-and-go and memory, executive function, and processing speed. J. Am. Geriatr. Soc 60, 1681–1686. [DOI] [PubMed] [Google Scholar]
- Forte R, Macaluso A, 2008. Relationship between performance-based and laboratory tests for lower-limb muscle strength and power assessment in healthy older women. J. Sports Sci 26, 1431–1436. [DOI] [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G, 2004. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J. Gerontol. Ser. A Biol. Med. Sci 59, M255–M263. [DOI] [PubMed] [Google Scholar]
- Gills JL, Glenn JM, Madero EN, Bott NT, Gray M, 2019. Validation of a digitally delivered visual paired comparison task: reliability and convergent validity with established cognitive tests. Geroscience 41, 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn JM, Vincenzo J, Canella CK, Binns A, Gray M, 2014. Habitual and maximal dual-task gait speeds among sedentary, recreationally active, and masters athlete late-middle aged adults. J. Aging Phys. Act 433–437. [DOI] [PubMed] [Google Scholar]
- Glenn JM, Gray M, Jensen A, Stone MS, Vincenzo JL, 2016a. Acute citrulline-malate supplementation improves maximal strength and anaerobic power in female, masters athletes tennis players. Eur. J. Sport Sci 1–9. [DOI] [PubMed] [Google Scholar]
- Glenn JM, Gray M, Vincenzo JL, Stone MS, 2016b. Functional lower-body power: a comparison study between physically inactive, recreationally active, and masters athlete late-middle-aged adults. J. Aging Phys. Act 24, 501–507. [DOI] [PubMed] [Google Scholar]
- Glenn JM, Gray M, Binns A, 2017. Relationship of sit-to-stand lower-body power with functional fitness measures among older adults with and without sarcopenia. J. Geriatr. Phys. Ther 40, 42–50. [DOI] [PubMed] [Google Scholar]
- Gomez JF, Curcio CL, Alvarado B, Zunzunegui MV, Guralnik J, 2013. Validity and reliability of the Short Physical Performance Battery (SPPB): a pilot study on mobility in the Colombian Andes. Colombia medica (Cali, Colombia) 44, 165–171. [PMC free article] [PubMed] [Google Scholar]
- Gray M, Paulson S, 2014a. Developing a measure of muscular power during a functional task for older adults. BMC Geriatr. 14, 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M, Paulson S, 2014b. The importance of muscular power among community-dwelling older adults. International Journal of Aging and Society 4, 1–11. [Google Scholar]
- Gray M, Glenn JM, Binns A, 2016. Predicting sarcopenia from functional measures among community-dwelling older adults. Age 38, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes RC, Dias RC, Pereira LS, Silva SL, Lustosa LP, Dias JM, 2014. Influence of dual task and frailty on gait parameters of older community-dwelling individuals. Braz J Phys Ther 18, 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. , 1994. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol 49, M85–M94. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. , 2000. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed along compared with short physical short performance battery. Journal of Gerontology: Medical Sciences 55A, M221–M231. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Nelson ME, Kaliton D, Layne JE, Bernstein MJ, Nuernberger A, et al. , 2001. Etiology and modification of gait instability in older adults: a randomized controlled trial of exercise. J. Appl. Physiol 90, 2117–2129. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N, 2005. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp. Brain Res 164, 541–548. [DOI] [PubMed] [Google Scholar]
- Ibrahim A, Singh DKA, Shahar S, 2017. ‘Timed Up and Go’ test: age, gender and cognitive impairment stratified normative values of older adults. PLoS One 12, e0185641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Shin DW, Jeong SM, Son KY, Cho B, Yoon JL, et al. , 2018. Association between timed up and go test and future dementia onset. J. Gerontol. A Biol. Sci. Med. Sci 73, 1238–1243. [DOI] [PubMed] [Google Scholar]
- Li KZH, Bherer L, Mirelman A, Maidan I, Hausdorff JM, 2018. Cognitive involvement in balance, gait and dual-tasking in aging: a focused review from a neuroscience of aging perspective. Front. Neurol 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, et al. , 1997. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. Jouranl of Applied Physiology 83, 1581–1587. [DOI] [PubMed] [Google Scholar]
- Manini T, 2011. Development of physical disability in older adults. Current aging science 4, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Stark CEL, Squire LR, 2000. The visual paired-comparison task as a measure of declarative memory. Proc. Natl. Acad. Sci 97, 12375–12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch KL, Mercer V, Giuliani C, Marshall S, 2009. Development of a clinical measure of dual-task performance in walking: reliability and preliminary validity of the Walking and Remembering Test. J. Geriatr. Phys. Ther 32, 2–9. [DOI] [PubMed] [Google Scholar]
- McGough EL, Kelly VE, Logsdon RG, McCurry SM, Cochrane BB, Engel JM, et al. , 2011. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed “Up & Go” test. Phys. Ther 91, 1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee RD, Squire LR, 1993. On the development of declarative memory. J. Exp. Psychol. Learn. Mem. Cogn 19, 397–404. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM, 2012. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc 60, 2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir-Hunter SW, Wittwer JE, 2016. Dual-task testing to predict falls in community-dwelling older adults: a systematic review. Physiotherapy 102, 29–40. [DOI] [PubMed] [Google Scholar]
- Neider MB, Gaspar JG, McCarley JS, Crowell JA, Kaczmarski H, Kramer AF, 2011. Walking and talking: dual-task effects on street crossing behavior in older adults. Psychol. Aging 26, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsugi H, Ohgi S, Shigemori K, Schneider EB, 2013. Differences in dual-task performance and prefrontal cortex activation between younger and older adults. BMC Neurosci. 14, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palve SS, Palve SB, 2018. Impact of aging on nerve conduction velocities and late responses in healthy individuals. J Neurosci Rural Pract 9, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, et al. , 2016. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 14, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen MM, Holt NE, Grande L, Kurlinski LA, Beauchamp MK, Kiely DK, et al. , 2014. Mild cognitive impairment status and mobility performance: an analysis from the Boston RISE study. J. Gerontol. A Biol. Sci. Med. Sci 69, 1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera S, Mody SH, Woodman RC, Studenski SA, 2006. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc 54, 743–749. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Miller LS, Cress ME, 2004. Leg extensor power, cognition, and functional performance in independent and marginally dependent older adults. Age & Ageing. 33, 342–348. [DOI] [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S, 1991. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc 39, 142–148. [DOI] [PubMed] [Google Scholar]
- Priest AW, Salamon KB, Hollman JH, 2008. Age-related differences in dual task walking: a cross sectional study. J Neuroeng Rehabil 5, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M, 2016. World Alzheimer report. In: Improving Healthcare for People Living With Dementia: Coverage, Quality, and Costs Now and in the Future. Alzheimer’s Disease International, London, UK. [Google Scholar]
- Rajtar-Zembaty A, Rajtar-Zembaty J, Salkowski A, Starowicz-Filip A, Skalska A, 2019. Global cognitive functioning and physical mobility in older adults with and without mild cognitive impairment: evidence and implications. Folia Med. Cracov 59, 75–88. [PubMed] [Google Scholar]
- Reid KF, Fielding RA, 2012. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc. Sport Sci. Rev 40, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riby L, Perfect T, Stollery B, 2010. The effects of age and task domain on dual task performance: a meta-analysis. Eur. J. Cogn. Psychol 16, 863–891. [Google Scholar]
- Sakurai R, Bartha R, Montero-Odasso M, 2018. Entorhinal cortex volume is associated with dual-task gait cost among older adults with MCI: results from the Gait and Brain Study. The Journals of Gerontology: Series A 74, 698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway-Cook A, Brauer S, Woollacott M, 2000. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys. Ther 80, 896–903. [PubMed] [Google Scholar]
- Smith E, Cusack T, Blake C, 2016. The effect of a dual task on gait speed in community dwelling older adults: a systematic review and meta-analysis. Gait & posture 44, 250–258. [DOI] [PubMed] [Google Scholar]
- Sobol NA, Hoffmann K, Vogel A, Lolk A, Gottrup H, Høgh P, et al. , 2016. Associations between physical function, dual-task performance and cognition in patients with mild Alzheimer’s disease. Aging Ment. Health 20, 1139–1146. [DOI] [PubMed] [Google Scholar]
- Sprague BN, Phillips CB, Ross LA, 2019. Age-varying relationships between physical function and cognition in older adulthood. Journals of Gerontology Series B: Psychological Sciences & Social Sciences 74, 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderaraman P, Maidan I, Kozlovski T, Apa Z, Mirelman A, Hausdorff JM, et al. , 2019. Differential associations between distinct components of cognitive function and mobility: implications for understanding aging, turning and dual-task walking. Front. Aging Neurosci 11, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ME, Boripuntakul S, Toson B, Close JCT, Lord SR, Kochan NA, et al. , 2019. The role of cognitive function and physical activity in physical decline in older adults across the cognitive spectrum. Aging Ment. Health 23, 863–871. [DOI] [PubMed] [Google Scholar]
- Theill N, Martin M, Schumacher V, Bridenbaugh SA, Kressig RW, 2011. Simultaneously measuring gait and cognitive performance in cognitively healthy and cognitively impaired older adults: the Basel motor–cognition dual-task paradigm. J. Am. Geriatr. Soc 59, 1012–1018. [DOI] [PubMed] [Google Scholar]
- van Dam R, Van Ancum JM, Verlaan S, Scheerman K, Meskers CGM, Maier AB, 2018. Lower cognitive function in older patients with lower muscle strength and muscle mass. Dement. Geriatr. Cogn. Disord 45, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazzana R, Bandinelli S, Lauretani F, Volpato S, Di Iorio A, Abate M, et al. , 2010. Trail Making Test predicts physical impairment and mortality in older persons. J. Am. Geriatr. Soc 58, 719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenzo JL, Gray M, Glenn JM, 2018. Validity of a novel, clinically relevant measure to differentiate functional power and movement velocity and discriminate fall history among older adults: a pilot investigation. Innov. Aging 2, igy028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson NL, Rosano C, Boudreau RM, Simonsick EM, Ferrucci L, Sutton-Tyrrell K, et al. , 2010. Executive function, memory, and gait speed decline in well-functioning older adults. The Journals of Gerontology: Series A 65A, 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N, 2008. The role of executive function and attention in gait. Mov. Disord 23, 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon DH, Kang D, Kim HJ, Kim JS, Song HS, Song W, 2017. Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatr Gerontol Int 17, 765–772. [DOI] [PubMed] [Google Scholar]
- Zola SM, Manzanares CM, Clopton P, Lah JJ, Levey AI, 2013. A behavioral task predicts conversion to mild cognitive impairment and Alzheimer’s disease. Am. J. Alzheimers Dis. Other Dement 28, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]


