Abstract
The anatomical structure of the pancreaticoduodenal region is complex and closely related to the surrounding vessels. A variant of the hepatic artery, which is not a rare finding during pancreatic surgery, is prone to intraoperative injury. Inadvertent injury to the hepatic artery may affect liver perfusion, resulting in necrosis, liver abscess, and even liver failure. The preoperative identification of hepatic artery variations, detailed planning of the surgical approach, careful intraoperative dissection, and proper management of the damaged artery are important for preventing hepatic hypoperfusion. Nevertheless, despite the potential risks, planned artery resection has become acceptable in carefully selected patients. Arterial reconstruction is sometimes essential to prevent postoperative ischemic complications and can be performed using various methods. The complexity of procedures such as pancreatectomy with en bloc celiac axis resection may be mitigated by the presence of an aberrant right hepatic artery or a common hepatic artery originating from the superior mesenteric artery. Here, we comprehensively reviewed the anatomical basis of hepatic artery variation, its incidence, and its effect on the surgical and oncological outcomes after pancreatic resection. In addition, we provide recommendations for the prevention and management of hepatic artery injury and liver hypoperfusion. Overall, the hepatic artery variant may not worsen surgical and oncological outcomes if it is accurately identified pre-operatively and appropriately managed intraoperatively.
Keywords: Hepatic artery, Pancreatectomy, Pancreaticoduodenectomy, Arterial reconstruction, Celiac axis resection, Outcome, Prognosis
Core Tip: Variations in hepatic artery anatomy are not rare during pancreatic surgery and have a significant impact on planning and performance of the procedure. Inadvertent intraoperative injury to hepatic artery may affect liver perfusion and result in ischemic complications. Detailed knowledge and awareness of its anatomical variants are critical, and thorough pre- and intraoperative planning is important to prevent hepatic hypoperfusion. This article comprehensively reviews the hepatic artery anatomy and variations, highlights its impact on surgical and oncological outcomes after pancreatic resection, and discusses prevention and management of hepatic artery injury and liver hypoperfusion.
INTRODUCTION
The mortality rate of pancreatectomy has decreased in recent years; however, several complex pancreatic resections such as the pancreaticoduodenectomy (PD), total pancreatectomy (TP), and modified Appleby procedure have high complication rates[1,2]. The anatomy of the pancreaticoduodenal region is complex and closely related to the surrounding blood vessels. Therefore, this region is vulnerable to injury during surgery. Moreover, the presence of vascular anatomical variants makes PD more challenging. Aberrant hepatic artery (AHA) is not rare in patients undergoing PD, with an incidence ranging from 15.1% to 24.8%[3-6]. This variant increases the risk of iatrogenic vascular injury and the incidence of postoperative hepatobiliary ischemic events, inducing complications such as bilioenteric anastomotic leakage and liver abscess[5,7,8]. Therefore, a thorough understanding of the anatomy of the hepatic artery is essential for safer pancreatic resection[9]. Herein, we provide a comprehensive review of the clinical significance of hepatic artery variants in pancreatic surgery.
LITERATURE SEARCH
A thorough search was performed in the PubMed database for relevant studies on hepatic artery variants and pancreatic resection. The search terms used were “aberrant” or “variant” or “anomaly” AND “hepatic artery” AND “pancreaticoduodenectomy” or “pancreatectomy”. All available studies published in English or Chinese between January 2000 and November 2021 (131 articles) were screened.
EMBRYOLOGY
It is important to understand the embryology of the hepatic artery to determine the origin of these variants. During embryogenesis, four visceral roots, known as ventral segmental arteries, originate from the dorsal aorta[10]. These ventral vessels are interconnected by longitudinal anastomoses[11,12]. The second and third roots and their connections, usually disappear during embryonic development. Thereafter, the celiac artery (CA) develops from the junction of the first three vessels and forms the left gastric artery (LGA), splenic artery, and common hepatic artery (CHA). The fourth trunk becomes the superior mesenteric artery (SMA). As the embryo grows, the channel between the roots of the CA and SMA fades away, resulting in anatomic division of the CA from the SMA[13]. This process can lead to several abnormalities in the CA and SMA. The associated changes are thought to depend on the degree of degeneration and longitudinal connectivity of primitive splanchnic vessels[10,11,13,14].
In addition, the hepatobiliary-pancreaticoduodenal region has the largest number of organs and the densest vascular branches. It is located at the junction of the foregut and midgut and is bound by the duodenal papilla. The vessels in this region frequently intersect between the CA and SMA, resulting in vascular variations. The three branches of the CA, from anterior to posterior are the LGA, splenic artery, and CHA. The CHA and its branches, which primarily maintain the blood supply between the foregut and midgut, are the most prone to variation.
ANATOMY
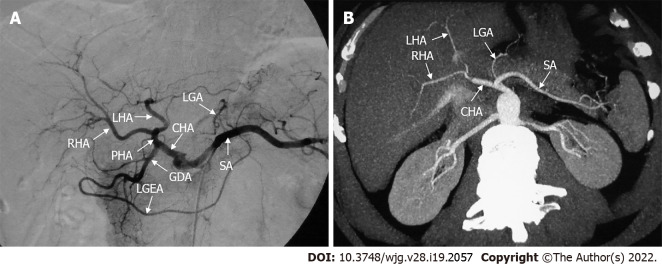
The normal anatomy of the hepatic artery refers to the CHA originating from the CA and crossing from the retroperitoneum toward the liver (Figure 1). This gives rise to a branch at the superior margin of the pancreatic head called the gastroduodenal artery (GDA). In this process, the CHA passes in front of the portal vein (PV), which is also known as the preportal route. Subsequently, it gives rise to the right gastric artery (RGA) and becomes the proper hepatic artery (PHA). Thereafter, it bifurcates into the left and right hepatic arteries (LHA/RHA). However, this so-called normal anatomy of the hepatic vessels is observed in only 55%-75.5% of individuals[15].
Figure 1.
Classic hepatic arterial anatomy. A: Digital subtraction angiography showed normal anatomy of the hepatic arteries; B: Axial maximum intensity projection image showed normal anatomy of the hepatic arteries. CHA: Common hepatic artery; GDA: Gastroduodenal artery; LGA: Left gastric artery; LGEA: Left gastroepiploic artery; LHA: Left hepatic artery; PHA: Proper hepatic artery; RHA: Right hepatic artery; SA: Splenic artery.
A series of terms are used to describe hepatic artery anomalies: “Aberrant”, “anomalous”, “accessory”, and “replaced”[16], and the definitions of these terms vary between studies. An aberrant or anomalous hepatic artery originates outside the typical route. It includes “accessory” and “replaced” hepatic arteries[17]. The term "replaced" RHA (rRHA) or LHA (rLHA) is used when the normal artery is absent, and a replaced artery arises from another artery and supplies blood to the corresponding hepatic lobe. The term "accessory" RHA (aRHA) or LHA (aLHA) is used when an additional artery originating from another source and a standard artery is present[16-18].
TYPES AND INCIDENCE
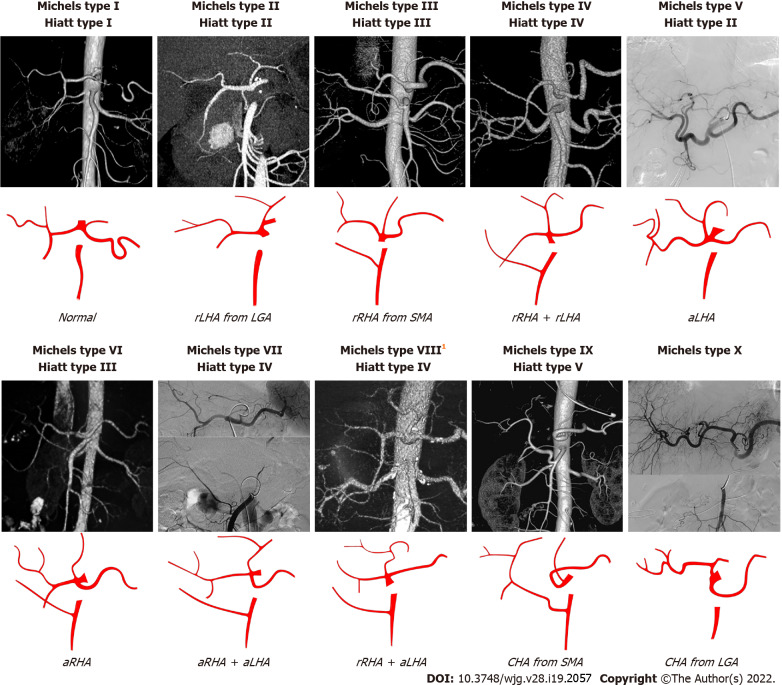
In terms of the anatomical classification of hepatic arteries, the Michels and Hiatt systems are well-established (Figure 2)[19,20]. The Michels system[20] is more extensive, classifying hepatic arteries into 10 types based on their origins. Hiatt et al[19] simplified the Michels system into six types. In addition, Garg et al[21] advocated a new classification that highlighted the distal branches of the hepatic arteries. However, despite these classifications of the hepatic arteries, several infrequent and unclassified hepatic artery variations have been detected in clinical practice. For example, an aberrant RHA may arise directly from the CA (Figure 3), which is an important anomaly to take note of during hepato-pancreato-biliary surgery because of the high risk of inadvertent injury[22]. More rarely, the origins of the CHA, splenic artery, and SMA mix seamlessly to form a hepato-spleno-mesenteric trunk (Figure 4), with a prevalence ranging from 0.67% to 0.90%[23,24]. The presence of a hepato-spleno-mesenteric trunk implies that only one trunk is responsible for the majority of the blood supply to abdominal organs, complicating operations around it[12].
Figure 2.
The Michels and Hiatt classifications of hepatic artery. 1Michels type VIII includes replaced right hepatic artery (rRHA) + accessory left hepatic artery (aLHA) or replaced left hepatic artery (rLHA) + accessory right hepatic artery (aRHA). The common hepatic artery (CHA) from aorta is classified as Hiatt type VI. LGA: Left gastric artery; SMA: Superior mesenteric artery; rRHA: Replaced right hepatic artery; aLHA: Accessory left hepatic artery; rLHA: Replaced left hepatic artery; CHA: Common hepatic artery; aRHA: Accessory right hepatic artery.
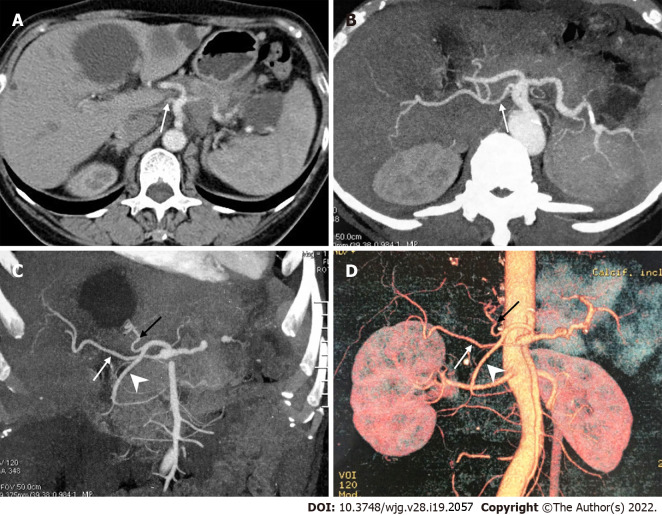
Figure 3.
A peculiar replaced right hepatic artery arising directly from the celiac artery. A: Axial contrast enhanced computed tomography scan; B-D: Axial maximum intensity projection (MIP, B), coronal MIP (C) and 3D-volume rendering (D) images showed that the replaced right hepatic artery (white arrow) originated directly from the celiac artery, and the common hepatic artery divided into the left hepatic artery (black arrow) and gastroduodenal artery (arrowhead).
Figure 4.
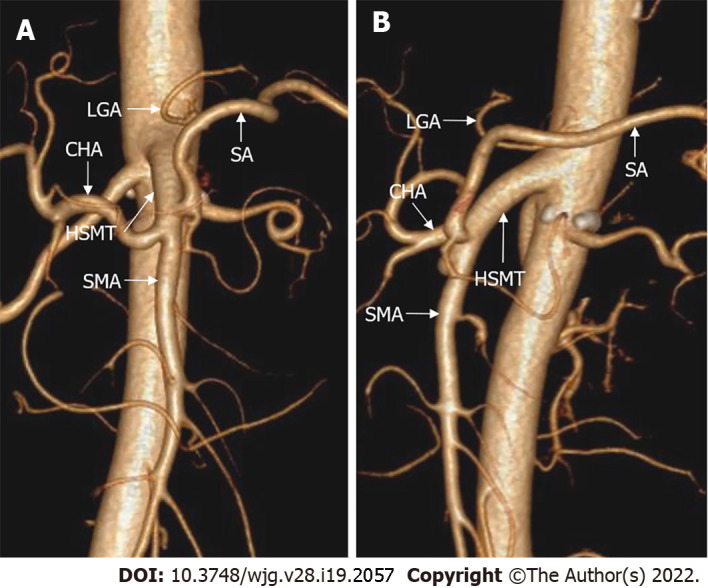
The hepato-spleno-mesenteric trunk. 3D-volume rendering computed tomography images showed that the hepato-spleno-mesenteric trunk was formed by the common hepatic artery, splenic artery and superior mesenteric artery, and the left gastric artery (LGA) originated from the aorta. A: Coronal view; B: Sagittal view. HSMT: Hepato-spleno-mesenteric trunk; CHA: Common hepatic artery; SA: Splenic artery; SMA: Superior mesenteric artery; LGA: Left gastric artery.
AHAs can be categorized into three types based on their origin: Type I, an AHA originating from the SMA; type II, an AHA originating from the LGA; and type III, an AHA originating from other arteries such as the GDA, abdominal aorta, and right renal artery. Numerous studies[3,5,7,25] have reported that the most frequently encountered AHA type during PD is type I, with an aberrant RHA from the SMA accounting for most of the cases. This anomalous hepatic artery is of great concern because it can be close to or cross the pancreatic head, and lie posterior to the common bile duct[26]. Most frequently, the aberrant RHA runs from behind the pancreatic head to the posterior and lateral sides of the main PV, before reaching the liver. However, despite the extremely low frequency of intrapancreatic routes, PD becomes complicated when an aberrant RHA or CHA is found traversing the pancreatic parenchyma[27]. Therefore, variants involving the RHA or CHA should be assessed carefully when performing PD[3-5,7,9,17,25,26,28,29], although the incidence may not be high (Table 1)[30-34]. Unfortunately, the actual incidence of AHA may be underestimated because of the limited accuracy of the current preoperative diagnostic methods. Although a preoperative diagnosis of AHA may be negative, its presence should be suspected intraoperatively.
Table 1.
Incidences of aberrant right and common hepatic artery during pancreaticoduodenectomy in the English literature (> 50 cases)
|
Ref.
|
Year
|
Total, n
|
Male, n
|
PDAC, n
|
a/rRHA, n (%)
|
aRHA1, n (%)
|
rRHA
1
, n (%)
|
rCHA, n (%)
|
Detecting methods
|
| Wang et al[7] | 2021 | 576 | 317 | 149 | 72 (12.5) | 27 (4.7) | 28 (4.9) | 20 (3.5) | CTA |
| Mansour et al[5] | 2021 | 202 | NA | NA | 37 (18.3) | 3 (1.5) | 29 (14.4) | 0 | Surgical finding |
| Giani et al[25] | 2021 | 270 | NA | NA | 66 (24.4) | 3 (1.1) | 39 (14.4) | 10 (3.7) | CT |
| Zhang et al[3] | 2020 | 218 | 122 | 61 | 36 (16.5) | 13 (6.0) | 11 (5.1) | 7 (3.2) | CTA |
| Crocetti et al[34] | 2019 | 297 | 178 | 297 | 44 (14.8) | NA | NA | NA | CTA, surgical finding |
| Alexakis et al[4] | 2019 | 232 | NA | NA | 32 (13.8) | 19 (8.2) | 13 (5.6) | 1 (0.4) | Surgical finding |
| Balzan et al[9] | 2019 | 200 | 93 | NA | 26 (13.0) | 2 (1.0) | 17 (8.5) | 7 (3.5) | CT |
| Kim et al[33] | 2016 | 73 | 35 | 24 | 15 (20.5) | 4 (5.5) | 11 (15.1) | NA | CT, surgical finding |
| Nguyen et al[31] | 2015 | 142 | 76 | 57 | 16 (11.3) | 0 | 14 (9.9) | 9 (6.3) | CT, surgical finding |
| Yang et al[32] | 2015 | 458 | 242 | 291 | 54 (11.8) | 15 (3.3) | 29 (6.3) | 10 (2.2) | CT, DSA, surgical finding |
| Rammohan et al[17] | 2014 | 225 | 167 | 31 | 42 (18.7) | 10 (4.4) | 31 (13.8) | 1 (0.4) | CT, surgical finding |
| Kim et al[26] | 2014 | 289 | 158 | 289 | 38 (13.1) | 3 (1.0) | 31 (10.7) | 2 (0.7) | Surgical finding |
| Sulpice et al[30] | 2013 | 213 | NA | NA | 29 (13.6) | NA | NA | NA | CT, surgical finding |
| Turrini et al[29] | 2010 | 471 | NA | 471 | 47 (10.0) | 2 (0.4) | 44 (9.3) | NA | CT, surgical finding |
| Lee et al[28] | 2009 | 103 | 73 | 0 | 15 (14.6) | 0 | 12 (11.7) | 0 | MRA, surgical finding |
| Total | 3969 | 1461 | 1670 | 540 (14.4) | 101 (2.9) | 309 (8.9) | 67 (2.3) |
These columns contain only variants of the right hepatic artery originating from the superior mesenteric artery.
a/rRHA: Accessory/replaced right hepatic artery; aRHA: Accessory right hepatic artery; CT: Computed tomography; CTA: Computed tomography angiography; CHA: Common hepatic artery; DSA: Digital subtraction angiography; NA: Not available; MRA: Magnetic resonance angiography; PDAC: Pancreatic ductal adenocarcinoma; rCHA: Replaced common hepatic artery; RHA: Right hepatic artery; rRHA: Replaced right hepatic artery.
COLLATERAL PATHWAYS
In general, the blood flow in the hepatic artery is predominantly from the CA. However, when the hepatic artery is involved in the tumor, retrograde flow from the SMA may develop (Figure 5). Similarly, celiac artery stenosis (CAS), which is not rare in patients undergoing PD (2%-11%)[35-37], may alter the hemodynamic profile of the hepatic artery. Retrograde blood flow in the CHA is a strong predictor of CAS[38,39]. In patients with CAS, the arterial blood supply to the upper abdominal organs is generally maintained by a steadily growing system of collaterals. Compensatory circulation between the CA and SMA is important in patients with hepatic artery stenosis or CAS because PD may disrupt these collaterals. Four possible collaterals have been reported: The pancreaticoduodenal arcade (key collateral supplying the liver and stomach), the dorsal pancreatic artery, the arc of Bühler, and the connection of the RHA and SMA[35,40-44], as shown in Figure 6. It is noteworthy that the pancreaticoduodenal and right gastroepiploic arcades are vital to the gastric and hepatic blood supply following pancreatectomy with en bloc celiac axis resection[45,46].
Figure 5.
Retrograde flow from the superior mesenteric artery due to tumor involvement of the hepatic artery. A: Computed tomography scan showed a low-density mass in the pancreatic head with common hepatic artery involvement (arrow); B: Selective celiacography showed involvement of the hepatic artery from the origin of the celiac artery to the bifurcation of the left and right hepatic arteries, as well as involvement of the gastroduodenal artery. Additionally, an accessory right hepatic artery was seen originating from the gastroduodenal artery; C: Superior mesenteric angiography showed retrograde flow to the liver from the superior mesenteric artery via the pancreaticoduodenal arcade.
Figure 6.
Collateral pathways due to celiac axis stenosis. A: Computed tomography scan showed a mass in the pancreatic head with dilated biliary-pancreatic duct, and celiac axis stenosis (CAS, arrow); B: Sagittal maximum intensity projection image demonstrated CAS (arrow); C: Selective celiac angiographic examination revealed the left gastric artery and splenic artery, with no hepatic arteries depicted, which is compatible with occlusion of the common hepatic artery; D: Superior mesenteric arteriogram demonstrated retrograde filling of the celiac branches via the pancreaticoduodenal arcades (arrow) by way of the gastroduodenal artery and anomalous blood vessels traversing the pancreas (arrowhead).
However, few studies have focused on collateral circulation in patients diagnosed with both CAS and hepatic artery variants. In patients with an aberrant RHA arising from the SMA, intrahepatic collaterals may develop between RHA and LHA. In cases of PHA or CHA originating from the SMA, the peribiliary vascular plexus in the hepatoduodenal ligament, which connects the central hepatic artery and GDA, is subsequently enlarged. These patients have a compensatory circulation that interconnects the LHA and LGA, and the RGA and LGA[40,43]. During PD, most of these collaterals are sacrificed, inducing an ischemic threat to the midgut viscera, especially to the liver and common bile duct. Thus, the presence of CAS is an important finding during PD, regardless of external compression, which may require preoperative endovascular stenting or intraoperative revascularization[35,36].
PREOPERATIVE DIAGNOSIS
The importance of preoperative identification of AHA cannot be overemphasized. Although digital subtraction angiography (DSA) is considered the gold standard for the assessment of visceral anatomy and variation, its use is limited because of its invasive nature, minor but non-negligible risk of complications, and high cost[47]. Multidetector computed tomography angiography (MDCTA) is one of the most widely used modalities for diagnosis and identification of AHA and has been shown to reduce the incidence of iatrogenic injuries and postoperative complications[48-51]. Furthermore, magnetic resonance angiography (MRA) provides diagnostic image quality in 72% of the hepatic and visceral arteries and enables the delineation of hepatic arterial anatomical features with high accuracy[47]. Significant advances in computed tomography angiography (CTA) and MRA have led to the marginalization of DSA[52]. However, considering the relatively high human and financial resources required to visualize the vasculature, it is impossible to subject every patient to CTA or MRA. Yang et al[32] showed that multidetector computed tomography (MDCT) without arterial reconstruction aided in the evaluation of aberrant RHA for preoperative planning of pancreatic surgery. Therefore, conventional MDCT combined with meticulous analysis may be helpful for preoperative detection of AHA.
SURGICAL AND ONCOLOGICAL OUTCOMES
Studies examining the effect of AHA on the perioperative outcomes of either open PD (OPD) or minimally invasive PD (MIPD) have reported negative outcomes (Table 2)[3-5,7,17,25,26,30,31,33,34,53]. Although duration of surgery can be prolonged[3,17,26], the AHA does not jeopardize surgical outcomes of PD[5,7,31,33]. Nevertheless, the presence of AHA may predispose patients to intraoperative injury, increasing the risk of hepatic hypoperfusion and ultimately leading to necrosis, liver abscess, and even liver failure. Nearly 10% of patients undergoing PD have a vascular injury, with the incidence of hepatic artery injury ranging from 0.5% to 1.7%[54-56]. Although the PV supply, extrahepatic collaterals, and interlobular communicating arteries may compensate to some extent for hepatic hypoperfusion, the incidence of catastrophic complications in the postoperative period is approximately 20%[34,57,58]. Liver ischemia after pancreatic resection is classified into four types: Hypoperfusion without major hepatic vessel occlusion, arterial occlusion, PV occlusion, and PV plus arterial occlusion[59]. This classification helps surgeons identify the degree of liver ischemia and escalate treatment appropriately. In addition to the liver, the biliary tree may be involved in an AHA injury. The extrahepatic biliary system receives its blood supply from the retroduodenal artery, RHA, and GDA[26,60]. After ligation of the GDA in PD, the remaining bile duct relies mainly on the RHA for its blood supply. Therefore, bile leakage may occur because of possible ischemia of the bilioenteric anastomosis[6].
Table 2.
Surgical outcomes of pancreaticoduodenectomy in patients with and without aberrant hepatic artery (> 50 cases)
|
Ref.
|
Total, n
|
AHA, n
|
Morbidity, n (%)
|
POPF, n (%)
|
PPH, n (%)
|
DGE, n (%)
|
Reoperation, n (%)
|
Mortality, n (%)
|
||||||
|
AHA
|
n-AHA
|
AHA
|
n-AHA
|
AHA
|
n-AHA
|
AHA
|
n-AHA
|
AHA
|
n-AHA
|
AHA
|
n-AHA
|
|||
| OPD | ||||||||||||||
| Rammohan et al[17] | 225 | 43 | 26 (60.5) | 111 (61.0) | 2 (4.7) | 9 (4.9) | 1 (2.3) | 4 (2.1) | 23 (53.4) | 98 (53.8) | NA | NA | 1 (2.3) | 3 (1.7) |
| Kim et al[26] | 249 | 371 | 92 (24.3) | 882 (41.5) | 1 (2.7) | 12 (5.6) | 1 (2.7) | 16 (7.6) | 4 (10.8) | 16 (7.6) | NA | NA | 0 | 1 (0.5) |
| Sulpice3 et al[30] | 84 | 291 | 14 (48.3) | 32 (58.2) | 3 (10.3) | 9 (16.4) | 4 (13.8) | 9 (16.4) | 10 (34.5) | 24 (43.6) | NA | NA | 3 (10.3) | 3 (5.5) |
| Eshuis et al[53] | 758 | 143 | 80 (55.9) | 303 (49.3) | 18 (12.6) | 87 (14.1) | 11 (7.7) | 44 (7.2) | 48 (33.6) | 193 (31.4) | 10 (7.0) | 68 (11.0) | 2 (1.4) | 13 (2.1) |
| Mansour3 et al[5] | 82 | 41 | 22 (53.7) | 27 (65.9) | 8 (19.5) | 12 (29.3) | 4 (9.8) | 3 (7.3) | 6 (14.6) | 7 (17.1) | 0 | 0 | 1 (2.4) | 0 |
| Crocetti et al[34] | 297 | 441 | 32 (72.7) | 185 (73.1) | 5 (11.3) | 27 (10.7) | 2 (4.5) | 12 (4.7) | 5 (11.4) | 37 (14.6) | 3 (6.8) | 28 (11.7) | 7 (15.9) | 33 (13.0) |
| Alexakis3 et al[4] | 105 | 35 | 4 (11.4) | 19 (27.1) | 2 (5.7) | 9 (12.9) | 0 | 2 (2.9) | 1 (2.9) | 1 (1.4) | 1 (2.9) | 5 (7.1) | 2 (5.7) | 4 (5.7) |
| LPD | ||||||||||||||
| Wang et al[7] | 576 | 127 | NA | NA | 21 (16.5) | 68 (15.1) | 11 (9.0) | 37 (8.2) | 3 (2.5) | 9 (2.0) | 4 (3.1) | 24 (5.3) | 4 (3.1) | 12 (2.7) |
| Giani et al[25] | 72 | 141 | 5 (35.7) | 34 (58.6) | 2 (14.3) | 10 (17.2) | 2 (14.3) | 10 (17.2) | 2 (14.3) | 14 (24.1) | NA | NA | 0 | 4 (6.9) |
| Zhang et al[3] | 218 | 54 | 22 (40.7) | 70 (42.7) | 5 (2.3) | 21 (9.6) | 5 (2.3) | 15 (6.9) | NA | NA | NA | NA | 3 (3.7) | 7 (4.3) |
| RPD | ||||||||||||||
| Nguyen et al[31] | 142 | 30 | 19 (63.3) | 74 (66.0) | 4 (13.3) | 9 (8.1) | NA | NA | 3 (10.0) | 26 (23.2) | NA | NA | 2 (6.7) | 3 (2.7) |
| Kim et al[33] | 73 | 151 | 5 (33.3) | 29 (50.0) | 2 (13.3) | 5 (8.6) | 1 (6.7) | 5 (8.6) | NA | NA | 2 (13.3) | 4 (6.9) | 0 | 2 (3.4) |
AHA in these studies included only right hepatic artery variants.
The P value for this comparison was 0.04, while all others were > 0.05.
These studies provided matched analysis.
AHA: Aberrant hepatic artery; DGE: Delayed gastric emptying; LPD: Laparoscopic pancreaticoduodenectomy; n-AHA: Non-aberrant hepatic artery; NA: Not available; OPD: Open pancreaticoduodenectomy; POPF: Postoperative pancreatic fistula; PPH: Post-pancreatectomy hemorrhage; RPD: Robotic pancreaticoduodenectomy.
Ischemic complications of PD can occur in patients with hepatic artery injury or CAS. Even in the absence of arterial stenosis or intraoperative arterial trauma, calcified plaques or atherosclerosis in the CHA can cause fatal complications[37]. Compression by a CHA pseudoaneurysm due to postoperative pancreatic fistula may cause ischemia of the abdominal organs, sepsis, and multiorgan dysfunction syndrome, which can be life threatening[61].
Most studies reported no significant differences in R0 resection rates between patients with and without AHA. Furthermore, the median overall survival and number of lymph nodes harvested did not differ significantly between the two groups. Based on these results, the presence of AHA does not seem to affect the radical outcome of pancreatic cancer surgery. The effects of AHA on oncological outcomes in pancreatic cancer, such as R0 resection, median overall survival, and number of lymph nodes harvested, are listed in Table 3[3,4,7,17,25,26,28-31,34,62].
Table 3.
Oncological outcomes of pancreaticoduodenectomy in patients with and without aberrant hepatic artery (> 50 cases)
| Author | Total, n | AHA, n |
R0 resection, n (%)
|
Median OS (mo)
|
Median HLN, n
|
|||
|
AHA
|
n-AHA
|
AHA
|
n-AHA
|
AHA
|
n-AHA
|
|||
| OPD | ||||||||
| Crocetti et al[34] | 297 | 441 | 42 (95.5) | 220 (87.0) | 30 | 25 | NA | NA |
| Alexakis2 et al[4] | 105 | 35 | 29 (82.9) | 56 (80.0) | NA | NA | NA | NA |
| Rubio-Manzanares-Dorado et al[62] | 151 | 11 | 11 (100.0) | 116 (82.9) | NA | NA | 10 | 12 |
| Rammohan et al[17] | 225 | 43 | 40 (93.1) | 169 (92.9) | NA | NA | 12 | 12 |
| Kim et al[26] | 249 | 371 | 33 (89.2) | 178 (84.0) | 17 | 23 | 17 | 17 |
| Sulpice2 et al[30] | 84 | 291 | 25 (86.2) | 49 (89.1) | 21.8 | 23.6 | 16 | 26 |
| Turrini et al[29] | 62 | 31 | 25 (80.7) | 26 (83.9) | 23 | 17 | 11 | 13 |
| Lee et al[28] | 103 | 151 | 12 (80.0) | 66 (75.0) | 29.8 | 28.7 | NA | NA |
| LPD | ||||||||
| Wang et al[7] | 576 | 127 | NA | NA | 28 | 32 | 15 | 14 |
| Giani et al[25] | 72 | 141 | 11 (84.6) | 50 (86.2) | NA | NA | 223 | 173 |
| Zhang et al[3] | 218 | 54 | 54 (100.0) | 163 (99.4) | NA | NA | 18.59 | 18.26 |
| RPD | ||||||||
| Nguyen et al[31] | 115 | 27 | 25 (92.6) | 77 (87.5) | NA | NA | 22.3 | 17.5 |
AHA in these studies included only right hepatic artery variants.
These studies provided matched analysis.
The P value for this comparison was 0.032, while all others were > 0.05.
AHA: Aberrant hepatic artery; HLN: Number of harvested lymph nodes; LPD: Laparoscopic pancreaticoduodenectomy; n-AHA: Non-aberrant hepatic artery; NA: Not available; OPD: Open pancreaticoduodenectomy; OS: Overall survival; RPD: Robotic pancreaticoduodenectomy.
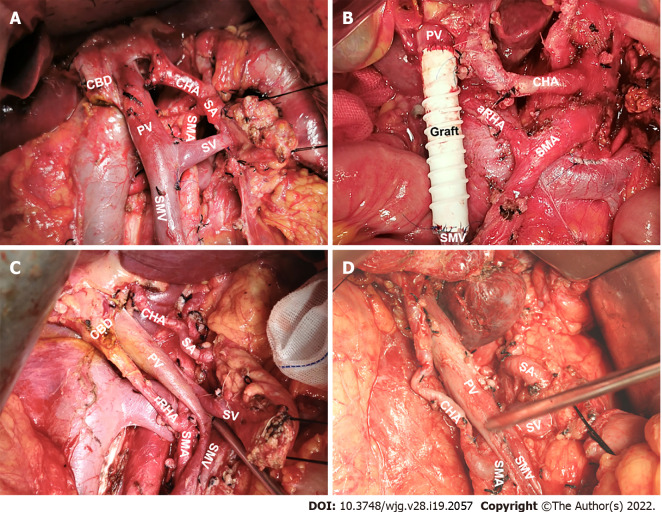
PREVENTION AND MANAGEMENT OF INTRAOPERATIVE AHA INJURY
Among the types of AHA, aberrant RHA or CHA from the SMA may increase the difficulty of PD; therefore, these should be of greater concern during surgery (Figure 7). Even when using radiological techniques with high sensitivity, an AHA may still be missed preoperatively. Hence, the anatomy of the hepatic artery should be determined by performing extended kocherization before dissection. There are various approaches to manage anomalous vessels. Of these, traction away from the ongoing surgical site and revascularization are of paramount importance[17]. Artery-first approaches, such as the posterior and inferior infracolic “SMA first” approach, can be used to identify AHAs[63]. The direct tactile feedback of arterial pulsation is a unique advantage of OPD. For example, normal PHA pulsation can be felt intraoperatively at the left margin of the hepatoduodenal ligament. If an anomaly in this normal condition is detected, surgeons should assess whether an AHA is present. Arterial pulsation behind the pancreatic head or PV may be an aberrant RHA or CHA[64]. The AHA originating from the LGA is vulnerable to injury during partial gastrectomy and may be palpated at the gastrohepatic ligament[18]. Therefore, during distal gastrectomy in PD, the LGA branch should be ligated away from the origin of the aberrant artery or close to the gastric wall[65].
Figure 7.
Significant hepatic artery variants during open pancreaticoduodenectomy. A: Normal anatomy of hepatic artery; B: Accessory right hepatic artery from the superior mesenteric artery (SMA); C: Replaced right hepatic artery from the SMA; D: Common hepatic artery from the SMA. CBD: Common bile duct; PV: Portal vein; SA: Splenic artery; SV: Splenic vein; SMV: Superior mesenteric vein.
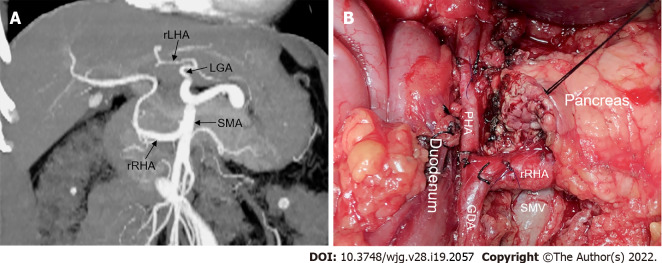
In addition, some specific AHAs may have embarrassing pitfalls. One such AHA is the aberrant RHA arising from the GDA, which has been reported in 3.5% of patients undergoing PD[66]. This variant sets an obstacle to achieving R0 resection because its origin is close to the pancreatic head. It can be accidentally ligated if the GDA is divided close to the CHA. In this case, a clamping test should be performed before transection of the GDA, followed by palpation of the hepatic artery[18]. Another pitfall is the aberrant retropancreatic RHA originating from the SMA. To avoid intraoperative injury to this artery, dissection of the hepatoduodenal ligament above the pancreas is essential after detecting the aberrant RHA by palpation or intraoperative ultrasonography. Skeletonization of the common bile duct may prevent it from being mistakenly ligated[67]. Rarely, an rRHA from the SMA can give rise to GDA after coursing to the right in front of the superior mesenteric vein (Figure 8). This type of aberrant RHA is of real concern because it is vulnerable to pancreatic neck dissection. It can be protected during OPD by accurate preoperative identification, intraoperative tactile feedback of arterial pulsation, and Doppler ultrasonography.
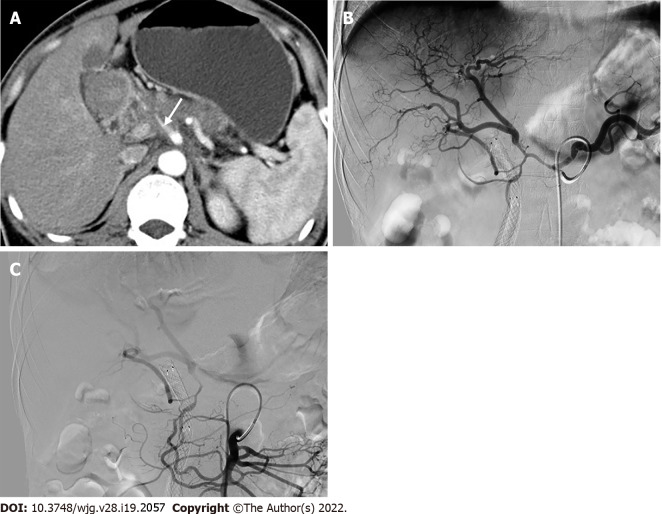
Figure 8.
A rare replaced right hepatic artery from the superior mesenteric artery susceptible to injury during pancreaticoduodenectomy. A: Coronal maximum intensity projection image showed Michels type IV hepatic artery variant; B. Intraoperative image showed that replaced right hepatic artery (rRHA) originated from the superior mesenteric artery and traveled to the right behind the inferior margin of the pancreatic neck via the superior mesenteric vein anteriorly and gave rise to the gastroduodenal artery and proper hepatic artery. LGA: Left gastric artery; rLHA: Replaced left hepatic artery; SMA: Superior mesenteric artery; GDA: Gastroduodenal artery; SMV: Superior mesenteric vein; PHA: Proper hepatic artery; rRHA: Replaced right hepatic artery.
In recent years, with the rapid development of surgical techniques, MIPD has been shown to be as safe as OPD[68]. However, the absence of tactile feedback from arterial pulsation during MIPD complicates the entire procedure, particularly in response to AHA[31]. Can et al[69] provided a series of tips to avoid AHA injury during laparoscopic PD (LPD), including the use of the hepatoduodenal ligament as a reliable marker to detect AHA during the dissection of the uncinate process, moderate retraction, and skillful differentiation of the AHA from arterial-like lymphatics and elongated lymph nodes. In terms of the surgical approach, the posterior approach is most often used for LPD[3,65]. This approach can be applied to all patients, regardless of the presence of AHA, because it adheres to the principle of "artery first"[3,65].
Although the feasibility and safety of robotic PD (RPD) have been confirmed[70], a major limitation is the lack of tactile feedback. It has been suggested that aberrant RHA should be suspected when the tissue thickness on the right side of the PV increases or the CHA becomes thin[33]. In such cases, careful dissection is required to prevent vascular injury. During RPD, artery injury can be avoided by strict adherence to the no-touch technique[31]. An additional robotic arm provides sufficient tension to the surrounding tissue to ensure safe dissection. Combining a low-energy robotic hook with a bipolar grabber promises effective handling of tiny branches of the uncinate process with minimal heat dissipation to anomalous vessels[31].
Although many studies have analyzed the clinical outcomes of AHA in pancreatic resection, few studies have reported the management of unexpected intraoperative AHA injuries. The early detection of accidental artery injuries is crucial. Remedial reconstruction of a damaged hepatic artery is considered a reliable option[54]. No significant difference in surgical outcomes has been reported between intentional and unintentional revascularization[55]. Ligation of inadvertently injured hepatic arteries may be attempted in view of the presence of collaterals, such as the subcostal arteries and the right inferior phrenic artery. This is like a planned resection of tumor-involved AHA without revascularization[55]. In brief, timely identification and proper management of an inadvertently damaged AHA are important to prevent hepatic hypoperfusion.
MANAGEMENT OF TUMOR INVOLVEMENT OF THE AHA
Intraoperative preservation of the AHA is an ideal surgical option, provided that radical surgical treatment is guaranteed[18]. Vascular invasion is common in patients with pancreatic cancer. In cases where tumor involvement of the AHA is suspected preoperatively, neoadjuvant therapy is recommended to allow translational surgery[71-73]. Unfortunately, even with neoadjuvant chemoradiation, it is sometimes still difficult to preserve the AHA involved in R0 resection[74,75]. However, despite the potential risks, planned artery resection has become acceptable in carefully selected patients[76,77]. If the cancer is located less than 1 cm from the origin of the AHA or if the AHA penetrates through the tumor, it should be resected to achieve R0 resection[7]. After clamping the AHA, intraoperative Doppler ultrasound can be used to assess the hepatic blood supply and determine the requirement for arterial reconstruction[7,78]. Revascularization may not be mandatory in some cases; for example, when an accessory hepatic artery is present, the diameter of the involved branch is thin, and adequate hepatic blood supply is confirmed after dissection[26,55,77].
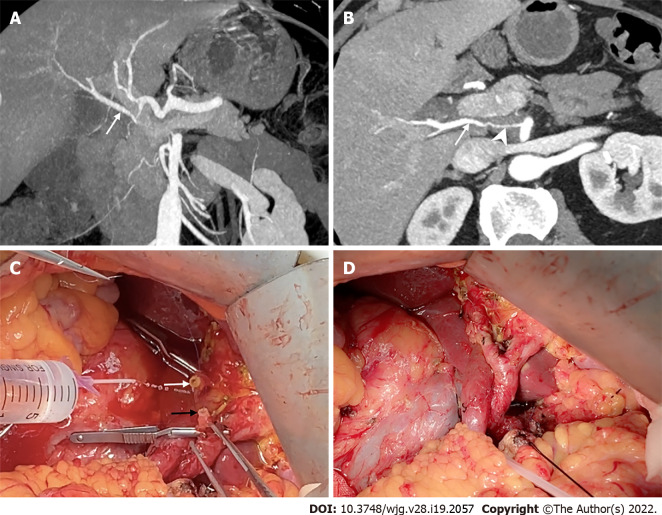
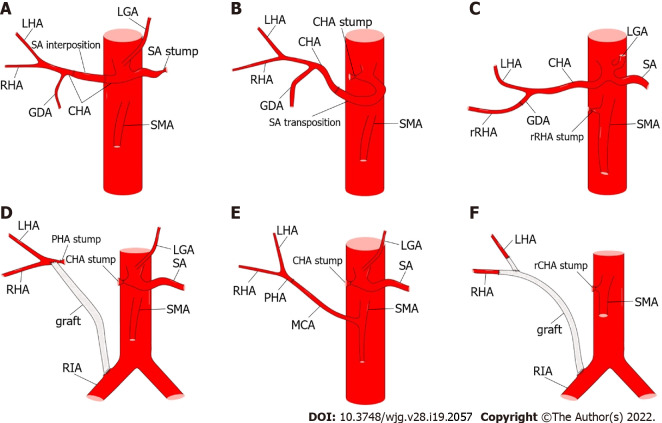
However, hepatic artery reconstruction is sometimes necessary to prevent postoperative liver ischemia[79]. If the two stumps of the artery are less than 1-2 cm apart, most of them are amenable to primary end-to-end anastomosis[18,72,80]. Tension-free anastomosis is necessary because of the potential risk of pseudoaneurysms[18]. If the distance between the stumps is too long to achieve primary anastomosis, transposition or graft interposition may be an option[18,81]. When compared, transposition may be more effective (Figure 9). In addition, the middle colonic, splenic, and dorsal pancreatic arteries are alternative options for anastomosis[81-83]. However, graft interposition is sometimes unavoidable and can be achieved using a saphenous vein or prosthetic graft[80]. In summary, various methods of hepatic artery reconstruction have been reliably performed, as illustrated by Figure 10[71,72,80-83].
Figure 9.
The replaced right hepatic artery reconstruction with gastroduodenal artery remnant during pancreaticoduodenectomy. A and B: Coronal and axial maximum intensity projection images showed the replaced right hepatic artery (rRHA, arrow) originated from the superior mesenteric artery and penetrated through the pancreatic head with tumor invasion resulting in significant stenosis (arrowhead); C: Intraoperative image showed a long length of the gastroduodenal artery (GDA) remnant (black arrow) was available for direct end-to-end anastomosis with the rRHA (white arrow); D: The rRHA-GDA anastomosis is complete.
Figure 10.
Methods of hepatic artery reconstruction during pancreaticoduodenectomy. A: Splenic artery (SA) interposition between two ends of the common hepatic artery (CHA); B: SA transposition to be anastomosed with the CHA; C: Direct end-to-end anastomosis between replaced right hepatic artery (rRHA) and gastroduodenal artery (GDA); D: Graft interposition between proper hepatic artery (PHA) and right iliac artery (RIA); E: Direct end-to-end anastomosis between PHA and middle colic artery; F: Graft interposition between RHA and RIA, and end-to-side anastomosis between left hepatic artery and graft. CA: Celiac artery; LGA: Left gastric artery; rCHA: Replaced common hepatic artery; SMA: Superior mesenteric artery; rRHA: Replaced right hepatic artery; CHA: Common hepatic artery; GDA: Gastroduodenal artery; LHA: Left hepatic artery; MCA: Middle colic artery; SA: Splenic artery.
Preoperative arterial embolization is an alternative approach to the prevention of liver ischemia after AHA resection[18,84,85]. Theoretically, preoperative coil embolization may lead to insufficient perfusion of the CHA, causing hepatic artery thrombosis. Migration of embolic material leading to liver ischemia, necrosis, and the risk of bleeding are other possible complications[86]. However, preoperative embolization can improve blood supply to the hepatobiliary system and stomach while providing valuable information to decide whether a partial hepatectomy is required[87]. In short, despite the invasiveness of this approach, it can lead to the prevention of postoperative liver ischemia without causing major complications[18,85].
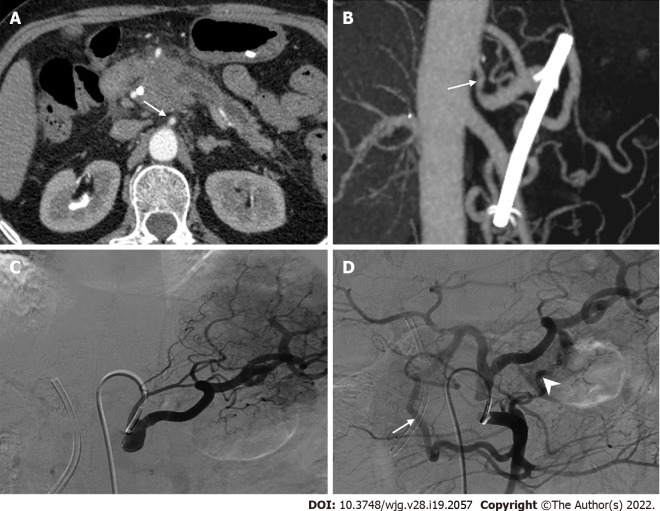
AHA IN PANCREATECTOMY WITH CELIAC AXIS RESECTION
With the increased use of neoadjuvant chemoradiation, pancreatectomy with en bloc celiac axis resection (CAR) has become more commonly used to treat locally advanced pancreatic cancer[46,88]. Distal pancreatectomy with CAR (DP-CAR), also known as the modified Appleby procedure, is widely used due to its acceptable surgical and oncological outcomes[89-91]. Nevertheless, maintenance of visceral blood supply following DP-CAR, especially in the liver and stomach, remains a hot topic. Preoperative embolization has been recommended because it may reduce the incidence of ischemic complications by promoting associated collaterals[87,91,92]. Nevertheless, this may be avoided when the rRHA or CHA originates from the SMA. However, its application has several limitations, including failure to reduce the incidence of postoperative ischemic complications[84,93], coil migration and secondary ischemia[46,90,94], and complicating revascularization[95].
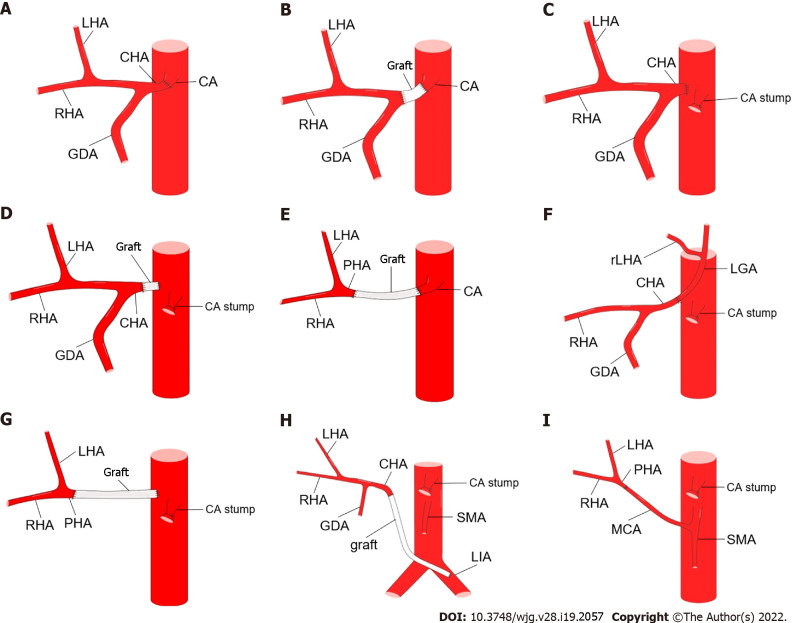
Hepatic artery reconstruction is an option to prevent postoperative ischemic complications and can be performed by anastomosis with the CA stump[71,86,96], splenic artery interposition[88], abdominal aorta[94,97], middle colonic artery[45], and iliac artery[79,98], as shown in Figures 11 and 12. However, the need for hepatic artery reconstruction remains a controversial issue. Liver perfusion can be intraoperatively assessed by palpation, Doppler ultrasonography, and indocyanine green fluorescence angiography[46,84,90,91,94,99]. Mittal et al[86] proposed a novel method to objectively assess liver blood supply by measuring CHA blood pressure. If CHA blood pressure decreases by more than 25% or 18 mmHg, hepatic artery reconstruction should be performed. Although current opinions are not unanimous[79,97], arterial reconstruction may not be required in most cases when liver perfusion is confirmed to be adequate[87,92,94,100].
Figure 11.
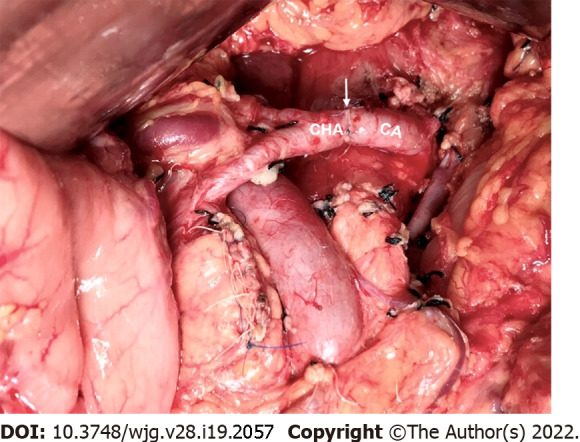
The common hepatic artery-celiac artery anastomosis during the modified Appleby procedure. Intraoperative image showed direct end-to-end anastomosis between common hepatic artery and celiac artery following distal pancreatectomy with celiac axis resection. The white arrow marks the anastomosis. CHA: Common hepatic artery; CA: Celiac artery.
Figure 12.
Methods of hepatic artery reconstruction during pancreatectomy with en bloc celiac axis resection. A: Direct end-to-end anastomosis between common hepatic artery (CHA) and celiac artery (CA); B: Graft interposition between CHA and CA; C: Direct end-to-end anastomosis between CHA and abdominal aorta (AA); D: Graft interposition between CHA and AA; E: Graft interposition between proper hepatic artery (PHA) and CA; F: Direct end-to-end anastomosis between CHA and left gastric artery [LGA, with replaced left hepatic artery (rLHA) from it]; G: Graft interposition between PHA and AA; H: Graft interposition between CHA and left iliac artery; I: Direct end-to-end anastomosis between PHA and middle colic artery. GDA: Gastroduodenal artery; LHA: Left hepatic artery; RHA: Right hepatic artery; SMA: Superior mesenteric artery.
Unfortunately, despite the increasing detection rate of AHAs, few studies have described the effect of AHA on DP-CAR. In contrast to PD, which requires attention to aberrant RHA or CHA from the SMA, DP-CAR requires caution for aberrant LHA originating from the LGA[18]. This variant may result in left hepatic ischemia when the LGA is transected[101]. Therefore, to prevent aberrant LHA damage and preserve the intact hepatic blood supply, surgeons may preserve the tumor-free LGA or perform anastomosis between the LGA and CHA[84,90,96]. The complexity of the procedure may be mitigated by the presence of an aberrant RHA or CHA from the SMA. For example, the presence of Michels type IV may help avoid arterial reconstruction in TP with en bloc CAR (TP-CAR)[102]. Nevertheless, in the absence of this variant (Michels type IV), TP-CAR should be revascularized[88].
CONCLUSION
In conclusion, AHA is not rare in patients undergoing pancreatic resection, and its clinical significance sometimes resembles a double-edged sword. The presence of AHA does not worsen surgical and oncological outcomes, if it is accurately identified preoperatively and appropriately managed intraoperatively. Given the potential risk of hepatic hypoperfusion caused by AHA injury or resection, hepatic artery reconstruction is sometimes necessary.
Footnotes
Conflict-of-interest statement: We declare no conflicts of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 3, 2022
First decision: March 10, 2022
Article in press: April 9, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dilek ON, Turkey; Nah YW, South Korea S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Contributor Information
Ye-Cheng Xu, Department of Pancreatic Surgery, Huashan Hospital, Shanghai 200040, China.
Feng Yang, Department of Pancreatic Surgery, Huashan Hospital, Shanghai 200040, China. yffudan98@126.com.
De-Liang Fu, Department of Pancreatic Surgery, Pancreatic Disease Institute, Huashan Hospital, Shanghai 200040, China.
References
- 1.Yang F, Jin C, Warshaw AL, You L, Mao Y, Fu D. Total pancreatectomy for pancreatic malignancy with preservation of the spleen. J Surg Oncol. 2019;119:784–793. doi: 10.1002/jso.25377. [DOI] [PubMed] [Google Scholar]
- 2.Yang F, Jin C, Li J, Di Y, Zhang J, Fu D. Clinical significance of drain fluid culture after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2018;25:508–517. doi: 10.1002/jhbp.589. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Wang K, Liu S, Wang Y, Liu K, Meng L, Chen Q, Jia B, Liu Y. A single-center clinical study of hepatic artery variations in laparoscopic pancreaticoduodenectomy: A retrospective analysis of data from 218 cases. Medicine (Baltimore) 2020;99:e20403. doi: 10.1097/MD.0000000000020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexakis N, Bramis K, Toutouzas K, Zografos G, Konstadoulakis M. Variant hepatic arterial anatomy encountered during pancreatoduodenectomy does not influence postoperative outcomes or resection margin status: A matched pair analysis of 105 patients. J Surg Oncol. 2019;119:1122–1127. doi: 10.1002/jso.25461. [DOI] [PubMed] [Google Scholar]
- 5.Mansour S, Damouny M, Obeid M, Farah A, Halloun K, Marjiyeh R, Ghalia J, Kluger Y, Khuri S. Impact of Vascular Anomalies on Pancreatoduodenectomy Procedure. J Clin Med Res. 2021;13:158–163. doi: 10.14740/jocmr4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan MR, Begum S, Khan DB, Inam Pal KM. Surgical and oncological implications of aberrant arterial anatomy in patients undergoing pancreatoduodenectomy. J Pak Med Assoc. 2020;70:930–934. doi: 10.5455/JPMA.4875. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Chen Q, Liu S, Zhang W, Ji B, Liu Y. The Impact of Aberrant Hepatic Artery on Resection Margin and Outcomes of Laparoscopic Pancreatoduodenectomy: A Single-Center Report. World J Surg. 2021;45:3183–3190. doi: 10.1007/s00268-021-06231-z. [DOI] [PubMed] [Google Scholar]
- 8.Yang F, Long J, Fu DL, Jin C, Yu XJ, Xu J, Ni QX. Aberrant hepatic artery in patients undergoing pancreaticoduodenectomy. Pancreatology. 2008;8:50–54. doi: 10.1159/000114867. [DOI] [PubMed] [Google Scholar]
- 9.Balzan SMP, Gava VG, Pedrotti S, Magalhães MA, Schwengber A, Dotto ML, Krebs CR. Prevalence of hepatic arterial variations with implications in pancreatoduodenectomy. Arq Bras Cir Dig. 2019;32:e1455. doi: 10.1590/0102-672020190001e1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuya K, Einama T, Miyata Y, Iwasaki T, Yamagishi Y, Takihata Y, Morimura F, Edo H, Otsuka Y, Mori S, Tsunenari T, Fujinuma I, Hirose Y, Tsujimoto H, Ueno H, Kishi Y. Destruction of a wandering accessory right hepatic artery in a patient with pancreatic body cancer: a case report. Clin J Gastroenterol. 2021;14:560–565. doi: 10.1007/s12328-020-01304-3. [DOI] [PubMed] [Google Scholar]
- 11.Mrowiec S, Król R, Jabłońska B. Absence of the celiac trunk and anomalous very low origin of the common hepatic artery arising independently from the abdominal aorta just above aortic bifurcation in patient undergoing radical pancreaticoduodenectomy. Surg Radiol Anat. 2021;43:585–588. doi: 10.1007/s00276-020-02666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Losanoff JE, Millis JM, Harland RC, Testa G. Hepato-spleno-mesenteric trunk. J Am Coll Surg. 2007;204:511. doi: 10.1016/j.jamcollsurg.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 13.Namba Y, Oishi K, Okimoto S, Moriuchi T, Bekki T, Mukai S, Saito Y, Fujisaki S, Takahashi M, Fukuda T, Ohdan H. Imaging diagnosis of aberrant proper hepatic and gastroduodenal arteries prior to pancreaticoduodenectomy: A case report. Radiol Case Rep. 2021;16:1650–1654. doi: 10.1016/j.radcr.2021.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Cheng C, Wang L, Li R, Chen JH, Gong SG. Anatomical variations in the origins of the celiac axis and the superior mesenteric artery: MDCT angiographic findings and their probable embryological mechanisms. Eur Radiol. 2014;24:1777–1784. doi: 10.1007/s00330-014-3215-9. [DOI] [PubMed] [Google Scholar]
- 15.Marín-Gómez LM, Gómez-Bravo MA, Bernal-Bellido C, Alamo-Martínez JM, Suárez-Artacho G, Serrano-Díez-Canedo J. Variability of the extrahepatic arterial anatomy in 500 hepatic grafts. Transplant Proc. 2010;42:3159–3161. doi: 10.1016/j.transproceed.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 16.Nakata K, Higuchi R, Ikenaga N, Sakuma L, Ban D, Nagakawa Y, Ohtsuka T, Asbun HJ, Boggi U, Tang CN, Wolfgang CL, Nishino H, Endo I, Tsuchida A, Nakamura M Study Group of Precision Anatomy for Minimally Invasive Hepato-Biliary-Pancreatic surgery (PAM-HBP Surgery. Precision anatomy for safe approach to pancreatoduodenectomy for both open and minimally invasive procedure: A systematic review. J Hepatobiliary Pancreat Sci. 2022;29:99–113. doi: 10.1002/jhbp.901. [DOI] [PubMed] [Google Scholar]
- 17.Rammohan A, Palaniappan R, Pitchaimuthu A, Rajendran K, Perumal SK, Balaraman K, Ramasamy R, Sathyanesan J, Govindan M. Implications of the presence of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy. World J Gastrointest Surg. 2014;6:9–13. doi: 10.4240/wjgs.v6.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swami A, Yadav T, Varshney VK, Sreesanth KS, Dixit SG. Hepatic Arterial Variations and Its Implication During Pancreatic Cancer Surgeries. J Gastrointest Cancer. 2021;52:462–470. doi: 10.1007/s12029-021-00598-x. [DOI] [PubMed] [Google Scholar]
- 19.Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994;220:50–52. doi: 10.1097/00000658-199407000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337–347. doi: 10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- 21.Garg S, Sahni D, Kumar H, Yadav TD, Aggarwal A, Gupta T. The segmental branching of the hepatic arteries in the liver: a cadaveric study. Anat Sci Int. 2019;94:216–223. doi: 10.1007/s12565-018-00475-x. [DOI] [PubMed] [Google Scholar]
- 22.Katagiri H, Sakamoto T, Okumura K, Lefor AK, Kubota T. Aberrant right hepatic artery arising from the celiac trunk: A potential pitfall during laparoscopic cholecystectomy. Asian J Endosc Surg. 2016;9:72–74. doi: 10.1111/ases.12247. [DOI] [PubMed] [Google Scholar]
- 23.Bolintineanu LA, Costea AN, Iacob N, Pusztai AM, Pleş H, Matusz P. Hepato-spleno-mesenteric trunk, in association with an accessory left hepatic artery, and common trunk of right and left inferior phrenic arteries, independently arising from left gastric artery: case report using MDCT angiography. Rom J Morphol Embryol. 2019;60:1323–1331. [PubMed] [Google Scholar]
- 24.Matusz P, Loukas M, Iacob N, Ples H. Common stem origin of left gastric, right and left inferior phrenic arteries, in association with a hepatosplenomesenteric trunk, independently arising from the abdominal aorta: case report using MDCT angiography. Clin Anat. 2013;26:980–983. doi: 10.1002/ca.22204. [DOI] [PubMed] [Google Scholar]
- 25.Giani A, Mazzola M, Morini L, Zironda A, Bertoglio CL, De Martini P, Magistro C, Ferrari G. Hepatic vascular anomalies during totally laparoscopic pancreaticoduodenectomy: challenging the challenge. Updates Surg . 2022;74:583–590. doi: 10.1007/s13304-021-01152-x. [DOI] [PubMed] [Google Scholar]
- 26.Kim PT, Temple S, Atenafu EG, Cleary SP, Moulton CA, McGilvray ID, Gallinger S, Greig PD, Wei AC. Aberrant right hepatic artery in pancreaticoduodenectomy for adenocarcinoma: impact on resectability and postoperative outcomes. HPB (Oxford) 2014;16:204–211. doi: 10.1111/hpb.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishigami K, Nishie A, Asayama Y, Ushijima Y, Takayama Y, Okamoto D, Fujita N, Yoshizumi T, Harimoto N, Ohtsuka T, Nakata K, Honda H. The prevalence of transpancreatic common hepatic artery and coexisting variant anatomy. Clin Anat. 2018;31:598–604. doi: 10.1002/ca.22957. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Lee YJ, Kim CW, Moon KM, Kim MW. Clinical implications of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy. World J Surg. 2009;33:1727–1732. doi: 10.1007/s00268-009-0063-x. [DOI] [PubMed] [Google Scholar]
- 29.Turrini O, Wiebke EA, Delpero JR, Viret F, Lillemoe KD, Schmidt CM. Preservation of replaced or accessory right hepatic artery during pancreaticoduodenectomy for adenocarcinoma: impact on margin status and survival. J Gastrointest Surg. 2010;14:1813–1819. doi: 10.1007/s11605-010-1272-1. [DOI] [PubMed] [Google Scholar]
- 30.Sulpice L, Rayar M, Paquet C, Bergeat D, Merdrignac A, Cunin D, Meunier B, Boudjema K. Does an aberrant right hepatic artery really influence the short- and long-term results of a pancreaticoduodenectomy for malignant disease? J Surg Res. 2013;185:620–625. doi: 10.1016/j.jss.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen TK, Zenati MS, Boone BA, Steve J, Hogg ME, Bartlett DL, Zeh HJ 3rd, Zureikat AH. Robotic pancreaticoduodenectomy in the presence of aberrant or anomalous hepatic arterial anatomy: safety and oncologic outcomes. HPB (Oxford) 2015;17:594–599. doi: 10.1111/hpb.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang F, Di Y, Li J, Wang XY, Yao L, Hao SJ, Jiang YJ, Jin C, Fu DL. Accuracy of routine multidetector computed tomography to identify arterial variants in patients scheduled for pancreaticoduodenectomy. World J Gastroenterol. 2015;21:969–976. doi: 10.3748/wjg.v21.i3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JH, Gonzalez-Heredia R, Daskalaki D, Rashdan M, Masrur M, Giulianotti PC. Totally replaced right hepatic artery in pancreaticoduodenectomy: is this anatomical condition a contraindication to minimally invasive surgery? HPB (Oxford) 2016;18:580–585. doi: 10.1016/j.hpb.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crocetti D, Sapienza P, Ossola P, Tarallo M, Cavallaro G, Serra R, Grande R, Mingoli A, Fiori E, DE Toma G. Does Aberrant Right Hepatic Artery Influence the Surgical Short- and Long-term Outcome of Pancreatoduodenectomy? In Vivo. 2019;33:1285–1292. doi: 10.21873/invivo.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCracken E, Turley R, Cox M, Suhocki P, Blazer DG 3rd. Leveraging Aberrant Vasculature in Celiac Artery Stenosis: The Arc of Buhler in Pancreaticoduodenectomy. J Pancreat Cancer. 2018;4:4–6. doi: 10.1089/pancan.2017.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pallisera A, Morales R, Ramia JM. Tricks and tips in pancreatoduodenectomy. World J Gastrointest Oncol. 2014;6:344–350. doi: 10.4251/wjgo.v6.i9.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaujoux S, Sauvanet A, Vullierme MP, Cortes A, Dokmak S, Sibert A, Vilgrain V, Belghiti J. Ischemic complications after pancreaticoduodenectomy: incidence, prevention, and management. Ann Surg. 2009;249:111–117. doi: 10.1097/SLA.0b013e3181930249. [DOI] [PubMed] [Google Scholar]
- 38.Zwolak RM. Can duplex ultrasound replace arteriography in screening for mesenteric ischemia? Semin Vasc Surg. 1999;12:252–260. [PubMed] [Google Scholar]
- 39.Zwolak RM, Fillinger MF, Walsh DB, LaBombard FE, Musson A, Darling CE, Cronenwett JL. Mesenteric and celiac duplex scanning: a validation study. J Vasc Surg. 1998;27:1078–87; discussion 1088. doi: 10.1016/s0741-5214(98)60010-0. [DOI] [PubMed] [Google Scholar]
- 40.Sakorafas GH, Sarr MG, Peros G. Celiac artery stenosis: an underappreciated and unpleasant surprise in patients undergoing pancreaticoduodenectomy. J Am Coll Surg. 2008;206:349–356. doi: 10.1016/j.jamcollsurg.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Kurosaki I, Hatakeyama K, Nihei KE, Oyamatsu M. Celiac axis stenosis in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2004;11:119–124. doi: 10.1007/s00534-003-0871-6. [DOI] [PubMed] [Google Scholar]
- 42.Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G. Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat. 2004;26:239–244. doi: 10.1007/s00276-004-0229-z. [DOI] [PubMed] [Google Scholar]
- 43.Song SY, Chung JW, Kwon JW, Joh JH, Shin SJ, Kim HB, Park JH. Collateral pathways in patients with celiac axis stenosis: angiographic-spiral CT correlation. Radiographics. 2002;22:881–893. doi: 10.1148/radiographics.22.4.g02jl13881. [DOI] [PubMed] [Google Scholar]
- 44.Yang F, Jin C, Fu D. Celiac axis compression syndrome and pancreatic head cancer. Pancreatology. 2014;14:310–311. doi: 10.1016/j.pan.2014.05.795. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki H, Hosouchi Y, Sasaki S, Araki K, Kubo N, Watanabe A, Kuwano H. Reconstruction of the hepatic artery with the middle colic artery is feasible in distal pancreatectomy with celiac axis resection: A case report. World J Gastrointest Surg. 2013;5:224–228. doi: 10.4240/wjgs.v5.i7.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Truty MJ, Colglazier JJ, Mendes BC, Nagorney DM, Bower TC, Smoot RL, DeMartino RR, Cleary SP, Oderich GS, Kendrick ML. En Bloc Celiac Axis Resection for Pancreatic Cancer: Classification of Anatomical Variants Based on Tumor Extent. J Am Coll Surg. 2020;231:8–29. doi: 10.1016/j.jamcollsurg.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Puippe GD, Alkadhi H, Hunziker R, Nanz D, Pfammatter T, Baumueller S. Performance of unenhanced respiratory-gated 3D SSFP MRA to depict hepatic and visceral artery anatomy and variants. Eur J Radiol. 2012;81:e823–e829. doi: 10.1016/j.ejrad.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Costea AN, Iacob N, Pusztai AM, Pleş H, Matusz P. Replaced right hepatic artery arising from inferior pancreaticoduodenal artery, in association with left multiple renal arteries: a case report using MDCT angiography. Rom J Morphol Embryol. 2019;60:971–977. [PubMed] [Google Scholar]
- 49.Gündoğdu E, Kebapçı M. Two novel hepatic arterial variations in a living liver donor detected by multidetector computed tomography angiography. Surg Radiol Anat. 2021;43:1385–1389. doi: 10.1007/s00276-021-02730-9. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi S, Murakami T, Takamura M, Kim T, Hori M, Narumi Y, Nakamura H, Kudo M. Multi-detector row helical CT angiography of hepatic vessels: depiction with dual-arterial phase acquisition during single breath hold. Radiology. 2002;222:81–88. doi: 10.1148/radiol.2221010326. [DOI] [PubMed] [Google Scholar]
- 51.Subbiah Nagaraj S, Kaman L, Dahiya D, Ramavath K, Kalra N, Behera A. Correlation of Multi-Detector Computed Tomography and Intraoperative Variations of the Celiac Trunk and Hepatic Artery in Resectable Hepatobiliary Pancreatic Cancers. Cureus. 2020;12:e12106. doi: 10.7759/cureus.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matoba M, Tonami H, Kuginuki M, Yokota H, Takashima S, Yamamoto I. Comparison of high-resolution contrast-enhanced 3D MRA with digital subtraction angiography in the evaluation of hepatic arterial anatomy. Clin Radiol. 2003;58:463–468. doi: 10.1016/s0009-9260(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 53.Eshuis WJ, Olde Loohuis KM, Busch OR, van Gulik TM, Gouma DJ. Influence of aberrant right hepatic artery on perioperative course and longterm survival after pancreatoduodenectomy. HPB (Oxford) 2011;13:161–167. doi: 10.1111/j.1477-2574.2010.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim AW, McCarthy WJ 3rd, Maxhimer JB, Quiros RM, Hollinger EF, Doolas A, Millikan KW, Deziel DJ, Godellas CV, Prinz RA. Vascular complications associated with pancreaticoduodenectomy adversely affect clinical outcome. Surgery. 2002;132:738–44; discussion 744. doi: 10.1067/msy.2002.127688. [DOI] [PubMed] [Google Scholar]
- 55.Asano T, Nakamura T, Noji T, Okamura K, Tsuchikawa T, Nakanishi Y, Tanaka K, Murakami S, Ebihara Y, Kurashima Y, Shichinohe T, Hirano S. Outcome of concomitant resection of the replaced right hepatic artery in pancreaticoduodenectomy without reconstruction. Langenbecks Arch Surg. 2018;403:195–202. doi: 10.1007/s00423-018-1650-9. [DOI] [PubMed] [Google Scholar]
- 56.Bacalbasa N, Balescu I, Vilcu M, Croitoru A, Dima S, Brasoveanu V, Brezean I, Popescu I. Pancreatoduodenectomy After Neoadjuvant Chemotherapy for Locally Advanced Pancreatic Cancer in the Presence of an Aberrant Right Hepatic Artery. In Vivo. 2020;34:401–406. doi: 10.21873/invivo.11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majno PE, Prêtre R, Mentha G, Morel P. Operative injury to the hepatic artery. Consequences of a biliary-enteric anastomosis and principles for rational management. Arch Surg. 1996;131:211–215. doi: 10.1001/archsurg.1996.01430140101025. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto M, Zaima M, Yamamoto H, Harada H, Kawamura J, Yamada M, Yazawa T, Kawasoe J. Liver necrosis shortly after pancreaticoduodenectomy with resection of the replaced left hepatic artery. World J Surg Oncol. 2017;15:77. doi: 10.1186/s12957-017-1151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hackert T, Stampfl U, Schulz H, Strobel O, Büchler MW, Werner J. Clinical significance of liver ischaemia after pancreatic resection. Br J Surg. 2011;98:1760–1765. doi: 10.1002/bjs.7675. [DOI] [PubMed] [Google Scholar]
- 60.Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379–384. doi: 10.1002/bjs.1800660603. [DOI] [PubMed] [Google Scholar]
- 61.Asai K, Watanabe M, Kusachi S, Matsukiyo H, Saito T, Kodama H, Enomoto T, Nakamura Y, Okamoto Y, Saida Y, Iijima R, Nagao J. Successful treatment of a common hepatic artery pseudoaneurysm using a coronary covered stent following pancreatoduodenectomy: report of a case. Surg Today. 2014;44:160–165. doi: 10.1007/s00595-012-0314-6. [DOI] [PubMed] [Google Scholar]
- 62.Rubio-Manzanares-Dorado M, Marín-Gómez LM, Aparicio-Sánchez D, Suárez-Artacho G, Bellido C, Álamo JM, Serrano-Díaz-Canedo J, Padillo-Ruiz FJ, Gómez-Bravo MÁ. Implication of the presence of a variant hepatic artery during the Whipple procedure. Rev Esp Enferm Dig. 2015;107:417–422. doi: 10.17235/reed.2015.3701/2015. [DOI] [PubMed] [Google Scholar]
- 63.Sanjay P, Takaori K, Govil S, Shrikhande SV, Windsor JA. 'Artery-first' approaches to pancreatoduodenectomy. Br J Surg. 2012;99:1027–1035. doi: 10.1002/bjs.8763. [DOI] [PubMed] [Google Scholar]
- 64.Shukla PJ, Barreto SG, Kulkarni A, Nagarajan G, Fingerhut A. Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol. 2010;17:186–193. doi: 10.1245/s10434-009-0757-1. [DOI] [PubMed] [Google Scholar]
- 65.Ogiso S, Conrad C, Araki K, Basso V, Gayet B. Posterior approach for laparoscopic pancreaticoduodenectomy to prevent replaced hepatic artery injury. Ann Surg Oncol. 2013;20:3120. doi: 10.1245/s10434-013-3058-7. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi T, Hasegawa K, Sauvain MO, Passoni S, Kazami Y, Kokudo T, Cristaudi A, Melloul E, Uldry E, Kobayashi K, Akamatsu N, Kaneko J, Arita J, Sakamoto Y, Demartines N, Kokudo N, Halkic N. An aberrant right hepatic artery arising from the gastroduodenal artery: a pitfall encountered during pancreaticoduodenectomy. Surg Today. 2021;51:1577–1582. doi: 10.1007/s00595-021-02242-4. [DOI] [PubMed] [Google Scholar]
- 67.Jah A, Jamieson N, Huguet E, Praseedom R. The implications of the presence of an aberrant right hepatic artery in patients undergoing a pancreaticoduodenectomy. Surg Today. 2009;39:669–674. doi: 10.1007/s00595-009-3947-3. [DOI] [PubMed] [Google Scholar]
- 68.Wang M, Li D, Chen R, Huang X, Li J, Liu Y, Liu J, Cheng W, Chen X, Zhao W, Tan Z, Huang H, Zhu F, Qin T, Ma J, Yu G, Zhou B, Zheng S, Tang Y, Han W, Meng L, Ke J, Feng F, Chen B, Yin X, Chen W, Ma H, Xu J, Lin R, Dong Y, Yu Y, Zhang H, Qin R Minimally Invasive Treatment Group in the Pancreatic Disease Branch of China's International Exchange and Promotion Association for Medicine and Healthcare (MITG-P-CPAM) Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:438–447. doi: 10.1016/S2468-1253(21)00054-6. [DOI] [PubMed] [Google Scholar]
- 69.Can MF, Kerem M, Dikmen K. Prevention of inadvertent injury to aberrant hepatic artery arising from superior mesenteric artery during laparoscopic pancreaticoduodenectomy. Turk J Surg. 2018:1–2. doi: 10.5152/turkjsurg.2018.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rice MK, Hodges JC, Bellon J, Borrebach J, Al Abbas AI, Hamad A, Knab LM, Moser AJ, Zureikat AH, Zeh HJ, Hogg ME. Association of Mentorship and a Formal Robotic Proficiency Skills Curriculum With Subsequent Generations' Learning Curve and Safety for Robotic Pancreaticoduodenectomy. JAMA Surg. 2020;155:607–615. doi: 10.1001/jamasurg.2020.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Christians KK, Pilgrim CH, Tsai S, Ritch P, George B, Erickson B, Tolat P, Evans DB. Arterial resection at the time of pancreatectomy for cancer. Surgery. 2014;155:919–926. doi: 10.1016/j.surg.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Q, Wu J, Tian Y, Duan J, Shao Y, Yan S, Wang W. Arterial resection and reconstruction in pancreatectomy: surgical technique and outcomes. BMC Surg. 2019;19:141. doi: 10.1186/s12893-019-0560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuribara T, Ichikawa T, Osa K, Inoue T, Ono S, Asanuma K, Kaneko S, Sano T, Shigeyoshi I, Matsubara K, Irie N, Iai A, Shinobi T, Ishizu H, Miura K. Combined resection of the hepatic artery without reconstruction in pancreaticoduodenectomy: a case report of pancreatic cancer with an aberrant hepatic artery. Surg Case Rep. 2020;6:228. doi: 10.1186/s40792-020-00997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noussios G, Dimitriou I, Chatzis I, Katsourakis A. The Main Anatomic Variations of the Hepatic Artery and Their Importance in Surgical Practice: Review of the Literature. J Clin Med Res. 2017;9:248–252. doi: 10.14740/jocmr2902w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Landen S, Ursaru D, Delugeau V, Landen C. How to deal with hepatic artery injury during pancreaticoduodenectomy. A systematic review. J Visc Surg. 2017;154:261–268. doi: 10.1016/j.jviscsurg.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 76.Bachellier P, Addeo P, Faitot F, Nappo G, Dufour P. Pancreatectomy With Arterial Resection for Pancreatic Adenocarcinoma: How Can It Be Done Safely and With Which Outcomes? Ann Surg. 2020;271:932–940. doi: 10.1097/SLA.0000000000003010. [DOI] [PubMed] [Google Scholar]
- 77.Yang F, Wang X, Jin C, He H, Fu D. Pancreatectomy with Hepatic Artery Resection for Pancreatic Head Cancer. World J Surg. 2019;43:2909–2919. doi: 10.1007/s00268-019-05106-8. [DOI] [PubMed] [Google Scholar]
- 78.Ramanadham S, Toomay SM, Yopp AC, Balch GC, Sharma R, Schwarz RE, Mansour JC. Rare hepatic arterial anatomic variants in patients requiring pancreatoduodenectomy and review of the literature. Case Rep Surg. 2012;2012:953195. doi: 10.1155/2012/953195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Özsoy M, Şahin E, Yavuz M, Özsoy Z, Okur N, Şahin S, Yılmaz S, Arıkan Y. Alternative hepatic arterial reconstruction technique in a case of total pancreaticoduodenectomy after celiac artery resection in pancreas cancer: Iliac-hepatic bypass. Turk J Surg. 2019;35:146–150. doi: 10.5578/turkjsurg.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimura Y, Imamura M, Kuroda Y, Nagayama M, Itoh T, Oota S, Murakami T, Yamaguchi H, Nobuoka T, Kawaharada N, Takemasa I. Clinical usefulness of saphenous vein graft in major arterial reconstruction during extended pancreatectomy. Langenbecks Arch Surg. 2020;405:1051–1059. doi: 10.1007/s00423-020-01947-3. [DOI] [PubMed] [Google Scholar]
- 81.Hackert T, Weitz J, Büchler MW. Splenic artery use for arterial reconstruction in pancreatic surgery. Langenbecks Arch Surg. 2014;399:667–671. doi: 10.1007/s00423-014-1200-z. [DOI] [PubMed] [Google Scholar]
- 82.Tinelli G, Montanari F, Sica S, Razionale F, Ardito F, Minelli F, De Nigris F, Tshomba Y, Giuliante F. Splenic artery transposition for hepatic arterial reconstruction in a locally advanced pancreatic cancer: a case report and literature review. Eur Rev Med Pharmacol Sci. 2021;25:3679–3683. doi: 10.26355/eurrev_202105_25934. [DOI] [PubMed] [Google Scholar]
- 83.Yoshida R, Yagi T, Yasui K, Umeda Y, Yoshida K, Fuji T, Takagi K, Kumano K, Yoshimoto M, Fujiwara T. Usefulness of Middle Colic Artery Transposition Technique for Hepatic Arterial Reconstruction in Conversion Surgery for an Initially Unresectable, Locally Advanced Pancreatic Cancer. Acta Med Okayama. 2021;75:543–548. doi: 10.18926/AMO/62410. [DOI] [PubMed] [Google Scholar]
- 84.Addeo P, Guerra M, Bachellier P. Distal pancreatectomy with en bloc celiac axis resection (DP-CAR) and arterial reconstruction: Techniques and outcomes. J Surg Oncol. 2021;123:1592–1598. doi: 10.1002/jso.26424. [DOI] [PubMed] [Google Scholar]
- 85.El Amrani M, Pruvot FR, Truant S. Management of the right hepatic artery in pancreaticoduodenectomy: a systematic review. J Gastrointest Oncol. 2016;7:298–305. doi: 10.3978/j.issn.2078-6891.2015.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mittal A, de Reuver PR, Shanbhag S, Staerkle RF, Neale M, Thoo C, Hugh TJ, Gill AJ, Samra JS. Distal pancreatectomy, splenectomy, and celiac axis resection (DPS-CAR): common hepatic arterial stump pressure should determine the need for arterial reconstruction. Surgery. 2015;157:811–817. doi: 10.1016/j.surg.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 87.Cesaretti M, Abdel-Rehim M, Barbier L, Dokmak S, Hammel P, Sauvanet A. Modified Appleby procedure for borderline resectable/locally advanced distal pancreatic adenocarcinoma: A major procedure for selected patients. J Visc Surg. 2016;153:173–181. doi: 10.1016/j.jviscsurg.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 88.Aosasa S, Nishikawa M, Noro T, Yamamoto J. Total Pancreatectomy with Celiac Axis Resection and Hepatic Artery Restoration Using Splenic Artery Autograft Interposition. J Gastrointest Surg. 2016;20:644–647. doi: 10.1007/s11605-015-2991-0. [DOI] [PubMed] [Google Scholar]
- 89.Nigri G, Petrucciani N, Belloni E, Lucarini A, Aurello P, D'Angelo F, di Saverio S, Fancellu A, Ramacciato G. Distal Pancreatectomy with Celiac Axis Resection: Systematic Review and Meta-Analysis. Cancers (Basel) 2021;13 doi: 10.3390/cancers13081967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Inoue Y, Oba A, Ono Y, Sato T, Ito H, Takahashi Y. Radical Resection for Locally Advanced Pancreatic Cancers in the Era of New Neoadjuvant Therapy-Arterial Resection, Arterial Divestment and Total Pancreatectomy. Cancers (Basel) 2021;13 doi: 10.3390/cancers13081818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colombo PE, Quenet F, Alric P, Mourregot A, Neron M, Portales F, Rouanet P, Carrier G. Distal Pancreatectomy with Celiac Axis Resection (Modified Appleby Procedure) and Arterial Reconstruction for Locally Advanced Pancreatic Adenocarcinoma After FOLFIRINOX Chemotherapy and Chemoradiation Therapy. Ann Surg Oncol. 2021;28:1106–1108. doi: 10.1245/s10434-020-08740-y. [DOI] [PubMed] [Google Scholar]
- 92.Toguchi M, Tsurusaki M, Numoto I, Hidaka S, Yamakawa M, Asato N, Im S, Yagyu Y, Matsuki M, Takeyama Y, Murakami T. Utility of Amplatzer Vascular Plug with Preoperative Common Hepatic Artery Embolization for Distal Pancreatectomy with En Bloc Celiac Axis Resection. Cardiovasc Intervent Radiol. 2017;40:445–449. doi: 10.1007/s00270-016-1509-9. [DOI] [PubMed] [Google Scholar]
- 93.Gong H, Ma R, Gong J, Cai C, Song Z, Xu B. Distal Pancreatectomy With En Bloc Celiac Axis Resection for Locally Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2016;95:e3061. doi: 10.1097/MD.0000000000003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baumgartner JM, Krasinskas A, Daouadi M, Zureikat A, Marsh W, Lee K, Bartlett D, Moser AJ, Zeh HJ 3rd. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic adenocarcinoma following neoadjuvant therapy. J Gastrointest Surg. 2012;16:1152–1159. doi: 10.1007/s11605-012-1839-0. [DOI] [PubMed] [Google Scholar]
- 95.Ueda A, Sakai N, Yoshitomi H, Furukawa K, Takayashiki T, Kuboki S, Takano S, Suzuki D, Kagawa S, Mishima T, Nakadai E, Miyazaki M, Ohtsuka M. Is hepatic artery coil embolization useful in distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic cancer? World J Surg Oncol. 2019;17:124. doi: 10.1186/s12957-019-1667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deal S, Nathan D, Rocha FG. Modified Appleby procedure for locally advanced pancreatic cancer. Am J Surg. 2018;215:853–855. doi: 10.1016/j.amjsurg.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Ielpo B, Ferri V, Caruso R, Duran H, Diaz E, Fabra I, Oliva C, Olivares S, Quijano Y, Vicente E. Alternative arterial reconstruction after extended pancreatectomy. Case report and some considerations of locally advanced pancreatic cancer. JOP. 2013;14:432–437. doi: 10.6092/1590-8577/1468. [DOI] [PubMed] [Google Scholar]
- 98.Machado MA, Surjan RC, Nishinari K, Makdissi FF, Machado MC. Iliac-hepatic arterial bypass for compromised collateral flow during modified Appleby operation for advanced pancreatic cancer. Eur J Surg Oncol. 2009;35:1124–1127. doi: 10.1016/j.ejso.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 99.Delpero JR, Sauvanet A. Vascular Resection for Pancreatic Cancer: 2019 French Recommendations Based on a Literature Review From 2008 to 6-2019. Front Oncol. 2020;10:40. doi: 10.3389/fonc.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morris M, Price T, Callahan Z, Yeo CJ. Celiac Axis Resection with Distal Pancreatectomy (Modified Appleby Procedure) Allows for R0 Resection of Pancreatic Body and Tail Mass Following Neoadjuvant Therapy: Case Report and Literature Review. Case Rep Pancreat Cancer. 2016;2:53–57. doi: 10.1089/crpc.2016.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cannella R, Borhani AA, Zureikat AH, Tublin ME. Appleby Procedure (Distal Pancreatectomy With Celiac Artery Resection) for Locally Advanced Pancreatic Carcinoma: Indications, Outcomes, and Imaging. AJR Am J Roentgenol. 2019;213:35–44. doi: 10.2214/AJR.18.20887. [DOI] [PubMed] [Google Scholar]
- 102.Nara S, Oguro S, Hata S, Kishi Y, Esaki M, Shimada K, Kosuge T. Total pancreatectomy with en bloc celiac axis resection for a pancreatic adenocarcinoma involving both the gastroduodenal artery and the celiac artery. Hepatogastroenterology. 2012;59:1635–1637. doi: 10.5754/hge11687. [DOI] [PubMed] [Google Scholar]