Abstract
Objectives
This study examined the effect of using patient-reported outcome measures (PROMs) routinely to assess and address depressive symptoms and diabetes distress among adults with type 2 diabetes.
Design
A systematic review of published peer-reviewed studies.
Data sources
Medline, Embase, CINAHL Complete, PsycINFO, The Cochrane Library and Cochrane Central Register of Controlled Trials were searched.
Eligibility criteria
Studies including adults with type 2 diabetes, published in English, from the inception of the databases to 24 February 2022 inclusive; and where the intervention included completion of a PROM of depressive symptoms and/or diabetes distress, with feedback of the responses to a healthcare professional.
Data extraction and synthesis
Using Covidence software, screening and risk of bias assessment were conducted by two reviewers independently with any disagreements resolved by a third reviewer.
Results
The search identified 4512 citations, of which 163 full-text citations were assessed for eligibility, and nine studies met the inclusion criteria. Five studies involved assessment of depressive symptoms only, two studies assessed diabetes distress only, and two studies assessed both. All studies had an associated cointervention. When depressive symptoms were assessed (n=7), a statistically significant between-group difference in depressive symptoms was observed in five studies; with a clinically significant (>0.5%) between-group difference in HbA1c in two studies. When diabetes distress was assessed (n=4), one study demonstrated statistically significant difference in depressive symptoms and diabetes distress; with a clinically significant between-group difference in HbA1c observed in two studies.
Conclusion
Studies are sparse in which PROMs are used to assess and address depressive symptoms or diabetes distress during routine clinical care of adults with type 2 diabetes. Further research is warranted to understand how to integrate PROMs into clinical care efficiently and determine appropriate interventions to manage identified problem areas.
PROSPERO registration number
CRD42020200246.
Keywords: %20Type%202">Diabetes Mellitus, Type 2, Depression, Patient Reported Outcome Measures
Strengths and limitations of this study.
The review focuses on depressive symptoms and diabetes distress in people with type 2 diabetes, an important aspect of diabetes management.
Systematic searching of six databases with independent review of abstracts and studies by two reviewers.
Meta-analysis was not possible due to heterogeneity in method and frequency of patient-reported outcome measure (PROM) completion, communication of PROM responses to healthcare professionals and differing associated cointerventions.
Introduction
Type 2 diabetes is a global health priority, with an estimated 463 million people with diabetes in 2017, set to rise to 700 million people in 2045.1 Up to four in ten adults with type 2 diabetes experience emotional health problems, such as depression, anxiety and diabetes distress.2 3 While depression is a general negative affect; diabetes distress is the negative emotional or affective response specific to the day-to-day living with diabetes.3–5 The relationship between diabetes distress and depressive symptoms is bidirectional: elevated diabetes distress is a predictor of future depression, and depression predicts future diabetes distress.6 7 While early studies have linked depressive symptoms to sub-optimal glycaemia8; more recent research has demonstrated that diabetes distress affects glycaemia more than depressive symptoms.5 9 Elevated depressive symptoms and diabetes distress are associated with reduced diabetes self-care and increased risk of diabetes-related complications, impaired quality of life, mortality and an estimated 50% increase in healthcare costs.6 10–15 Recent systematic reviews have focused on interventions for the management of diabetes distress; however, the first step is to identify people with depressive symptoms or diabetes distress requiring interventions in clinical practice.16–18
Guidelines have acknowledged the importance of assessing psychological well-being as part of diabetes care for over 25 years.19 Given the growing evidence that diabetes-tailored psychological interventions reduce elevated distress and glycaemia, international diabetes guidelines have issued recommendations for routine assessment of depressive symptoms and diabetes distress.16 20–25 Guidelines vary in terms of the specific patient-reported outcome measures (PROMs) recommended to assess depressive symptoms or diabetes distress. PROMs are standardised, validated questionnaires to assess latent constructs such as emotional well-being, treatment satisfaction, perceived health or functional status or health-related quality of life.26 Recent consensus from the International Consortium of Health Outcomes Measurement (ICHOM) recommends standardising the assessment of diabetes distress, depressive symptoms and general emotional well-being—with use of the Problem Areas In Diabetes (PAID) scale, Patient Health Questionnaire-9 (PHQ-9) and WHO-Five Well-Being Index (WHO-5), respectively—within clinical diabetes care.27
Despite these recommendations for using PROMs, 60% of healthcare professionals only discuss emotional issues if initiated by the person with diabetes.28 Healthcare professionals need efficient systems to both assess and address depressive symptoms and diabetes distress as part of routine diabetes care.3 For healthcare professionals to use PROMs, they need to understand the utility of PROMs in supporting people with type 2 diabetes clinically, not just for audit or research purposes,29 30 and they need guidance in how to use and interpret PROM responses in clinical consultations.31 32
Thus, the aim of this systematic review is to examine the effect of using PROMs routinely to assess and address depressive symptoms and/or diabetes distress among adults with type 2 diabetes on: (1) glycaemia as measured by HbA1c; (2) self-reported depressive symptoms or diabetes distress; (3) self-reported general emotional well-being or health-related quality of life; (4) self-reported diabetes self-management; (5) referrals for psychiatric or psychological therapy; (6) self-reported quality of patient-professional communication and (7) self-reported satisfaction with the consultation.
Methods
The protocol for this systematic review has been published,33 and the methods are summarised below. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.34
Eligibility criteria
Inclusion criteria
Studies were eligible if: the design was a randomised controlled trial (RCT), interrupted time-series study, (prospective or retrospective) cohort study, case–control study or analytical cross-sectional study; participants were adults (18 years or older) with type 2 diabetes from any country; interventions involved (1) participants completing a PROM for depressive symptoms and/or diabetes distress and (2) use of PROM responses by the healthcare professional in consultation with the person with type 2 diabetes.
Exclusion criteria
Studies were excluded if they involved: people under 18 years of age, type 1 diabetes or gestational diabetes; or the collection of PROM data but no use of the data in the clinical consultation.
Data sources and searches
A systematic search strategy was used to identify studies. The initial search was on 3 August 2020 and repeated on24 February 2022 using the same search terms (online supplemental file 1). The search was limited to papers published in English and before 24 February 2022. The search strategy was developed in consultation with a librarian from a biomedical library (complete search strategy: online supplemental document 1). Databases searched included MEDLINE (Ovid), EMBASE (Ovid), CINAHL Complete (EBSCO), APA PsycINFO (Ovid), The Cochrane Library (Ovid) and Cochrane Central Register of Controlled Trials (Ovid).
bmjopen-2021-054650supp001.pdf (117.2KB, pdf)
Study selection and data extraction
Following the initial search on 3 August 2020, two reviewers (RM and a second member of the review team (J-AM-N, BH, LC, DK or FCSH)) screened studies independently based on the inclusion criteria using Covidence software. Both reviewers screened the title and abstract of all eligible studies, followed by full-text screening of the shortlisted studies. Any disagreements about selection, assessment and data extraction in the included studies were discussed between the two reviewers, and if required, a third reviewer was involved in the discussion. Following the updated search on 24 February 2022, RM screened additional identified title and abstract independently, with full-text screening of the shortlisted studies by RM. Reference lists were not checked for studies. Data extraction was undertaken by RM with 20% checked by LC or DK. The extracted data were: study settings, participants, description of the interventions, comparators, study duration, length of follow-up and outcome measures. The authors of the selected studies were contacted for additional data (when published details were insufficient), with 1 month allowed for response.
Quality assessment
Eligible studies were assessed for risk of bias by two reviewers (RM and a second member of the review team (J-AM-N, BH or DK)) independently using the Cochrane Risk of Bias 2 tool or ROBINS-I.35 36 Any disagreements were discussed between the two reviewers, and if required, a third reviewer was involved in the discussion.
Data synthesis
Due to heterogeneity regarding method and frequency of PROM completion, communication of PROM responses to healthcare professionals and differing associated cointerventions (actions based on PROM responses) it was not possible to conduct a meta-analysis. Therefore, the results are summarised narratively.
Patient and public involvement
Patients or public were not involved in the conduct of this systematic review.
Results
The systematic search identified 4512 citations, of which 163 full-text citations were assessed for eligibility, and 9 studies met the inclusion criteria (figure 1).
Figure 1.
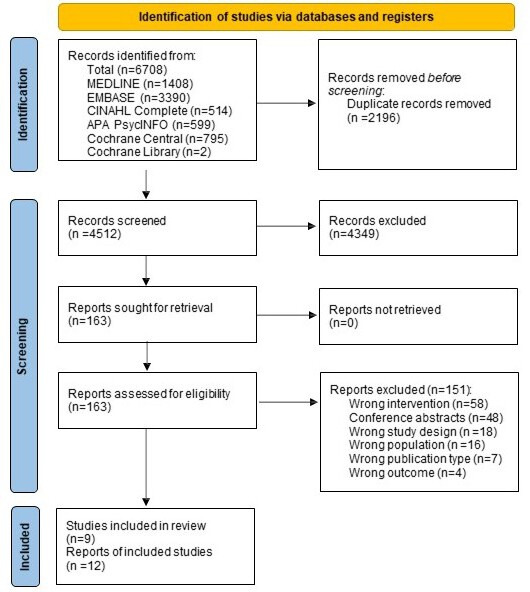
PRISMA flow diagram.34 PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of included studies
The nine included studies were published between 2009 and 2020 (table 1). The overall number of participants across all nine studies was N=3325, ranging from N=40 to N=1306 per study. Six of the nine studies were conducted in the USA,37–42 with the remainder conducted in Australia,43 Germany44 and Iceland.45 Most study designs were RCTs (n=6),37 38 one of which was a pilot study (n=1).43 The remaining three studies included case control study (n=2)41 42 and an observational study (n=1).39 Clinical settings varied across studies, including: general practice (n=4)38 40–42; both primary care and hospital clinics (n=2)37 39; specialist outpatient clinic (n=2)43 45 and a specialist rehabilitation service (n=1).44
Table 1.
Study characteristics
| Author (year) Country | Clinical setting | Study design and n per arm | Intervention PROM | Method and frequency of PROM completion | Summary of actions based on PROM responses | Control arm |
| Cummings et al40 (2019) USA |
Adults with symptoms of distress and/or depression attending general practice | 12-month RCT: Intervention n=67/usual care n=72 | PHQ-9* DDS-17† | In-person completion with trained study team member twice, 6 months apart | Stratified treatment to 16 sessions of cognitive behavioural therapy or lifestyle coaching based on PROM responses | Educational materials and usual care with GP. |
| Dobler et al44 (2018) Germany |
Adults attending specialist outpatient clinic, recruitment during inpatient rehabilitation stay | 12-month RCT: Intervention n=98/Control n=101 | PAID†, WHO-5, PHQ-9* | Telephone completion with trained study team member, monthly | Behaviour motivation plan developed. Monthly follow-up telephone calls using PHQ-2 (with progression to PHQ-9 if PHQ score >3) to identify and address emotional problems. Severity of symptoms guided counselling techniques, increase in call frequency or referral | Written information on diet, physical activity by mail at 3 and 9 months. |
| Fortmann et al42 (2020) USA |
Adults attending two primary care clinics | 12-month case control study: Intervention n=236/n=239 |
PHQ-2*, PHQ-9* | In-person completion with the registered nurse or certified diabetes educator, once | Positive screening on PROM resulted in referral to depression care manager with group-based cognitive behavioural therapy. Depression screening was part of a collaborative care model focused on cardiometabolic targets | Standard diabetes care without depression screening. |
| Ell et al38 (2011) USA |
Adults with PHQ9 response ≥10, attending primary care safety net clinics |
24-month RCT: Intervention n=193/Enhanced usual care n=194 | PHQ-9* | Telephone completion with trained study team member once | Collaborative care model using structured stepped-care algorithm, with patient preferences for problem-solving therapy or antidepressants guiding treatment | Standard care, depression educational pamphlets and social resource list. GPs informed of depression diagnosis. |
| Johnson et al41 (2014) USA |
Adults with PHQ >10, attending general practice | 12-month case control: Intervention n=95/Active control n=62/usual care n=71 | PHQ-9* | Telephone completion with trained study team member at least monthly until PHQ-9 <10 | Case-managers delivered individualised care, in collaboration with psychiatrist and endocrinologist, with treatment recommendations to GP based on a treatment algorithm and PROM responses | GP notified by letter of elevated PHQ-9 responses. |
| Naik et al37 (2019) USA |
Adults attending hospital and outpatient community Veterans Affairs clinics | 12-month RCT: Intervention n=136/Enhanced usual care (EUC) n=89 | PHQ-9* | Telephone completion with trained study team member once | Nine telephone coaching sessions with trained study members using workbooks guiding the discussion and tracking progress to set and assess goals related to wellness, diet, exercise medication management. | Participants informed of PHQ-9 responses with educational materials. |
| Rees et al43 (2017) Australia |
Adults with diabetes related retinopathy and moderate diabetes distress attending specialist outpatient clinic | 6-month pilot RCT: Intervention n=21/control n=19 | DDS† | In-person completion with trained study member once | PROM responses guided eight 45–60 min problem solving therapy sessions | Pamphlets on diabetes-specific topics |
| Sigurdardottir et al 45 (2009) Iceland |
Adults attending specialist outpatient clinic | 6-month RCT: Intervention n=28/Control n=25 | PAID† DKT, DES, Summary of diabetes self-care measure |
In-person completion at clinic with diabetes educator once | Diabetes educators delivered individual educational sessions based on empowerment theory. PROM responses identified barriers to goals with a weekly follow-up call for 5 weeks | Information booklet about T2D and attended usual diabetes clinics. |
| Wu et al39 (2018) USA |
Adults attending primary care or hospital-based safety net clinics | 6-month observational: Technology-facilitated care n=432/supported care n=461/usual care n=416 | PHQ-2, PHQ-9* | Initially completed via telephone with trained study member. Then monthly—quarterly completion via automated calls | PROM responses linked to clinical decision support that generated action reminders for healthcare professionals depending on PROM responses | Standard primary care. GPs offered optional training. |
*Depression.
†Diabetes distress.
DDS, Diabetes Distress Scale; DES, Diabetes Empowerment Scale; DKT, Diabetes Knowledge Test; GP, general practitioner; PAID, Problem Area In Diabetes scale; PHQ, Patient Health Questionnaire (2 items or 9 items); PROM, patient-reported outcome measure; RCT, randomised controlled trial; T2D, type 2 diabetes; WHO-5, WHO five-item Well-Being Index.
Risk of bias of included studies
Four of the nine studies were rated as having a low risk of bias (online supplemental file 2).38 40 41 43 45 Three studies were non-randomised studies of interventions, and at moderate risk of bias due to risk of baseline confounding.39 41 42 Methodological concerns were observed in three studies.37 39 44 Döbler et al reported outcomes for 98 of the 123 participants randomised to the intervention group and did not state how missing outcomes were dealt with; intention to treat was not reported.44 Naik et al reported 12-month outcome data for only 90 of the 136 intervention participants; intention to treat was not reported.37 In most studies, due to the study design, participants and clinical study team members delivering the intervention could not be blinded to participants’ group allocation. Two studies were pilot studies with small sample sizes.43 45 Despite being a pilot study, the Rees et al had sufficient power to detect differences in glycaemia, but lower power for depressive symptoms or diabetes distress.43 Sigurdardottir et al did not include power calculations.45
bmjopen-2021-054650supp002.pdf (55.9KB, pdf)
Intervention
Interventions to assess depressive symptoms and/or diabetes distress
Five of the nine studies assessed depressive symptoms alone,37–39 41 42 two assessed depressive symptoms and diabetes distress,40 44 and two assessed diabetes distress alone.43 45 All seven studies assessing depressive symptoms used the PHQ.37–42 44 One study used the PHQ-2 for brief screening with responses of more than three proceeding to the PHQ-9.39 Diabetes distress was assessed in two studies using the Diabetes Distress Scale (DDS)40 43 and in two studies using the PAID scale.44 45
PROMs were completed either in-person (n=5),40–44 or via telephone (n=4).37–39 45 In six studies, PROM responses were collected by study team members not involved in ongoing clinical care,37 38 40 41 43 44 either via telephone,37 38 41 44 or at the clinic with a study team member.40 43 One study collected PROM responses using automated calls.39 In two study, PROM completion was at the clinic with the diabetes educator.42 45
Feedback of PROM responses provided to treating healthcare professionals varied. Three studies trained case managers in making treatment recommendations to primary care health professionals based on case collaboration and treatment algorithms.38 41 42 In studies where trained study members collected PROM responses, the mechanism by which PROM data were provided to the treating healthcare professionals was not reported.43 44 In the Naik et al study, the general practitioner received a secure message notifying the HbA1c results and PHQ-9 response.37 Wu et al used PHQ-9 responses to generate action reminders integrated with the disease management registry for healthcare professionals to review.39
Cointervention associated with PROM responses
Each of the nine studies had a cointervention associated with the PROM completion (see table 1), which included telephone-assisted psychological therapy or coaching interventions,37 40 43–45 or healthcare professional interventions of collaborative team care with case management and stepped care treatment algorithms.38 41 42 Wu et al linked PROM responses to a clinical decision support tool that generated action reminders for healthcare professionals based on PROM responses within a disease management register.39
Outcomes
Reported outcomes across studies are detailed in table 2. Referrals to psychology or psychiatry services were not reported. In three studies, in the control arm, healthcare professionals were informed of the elevated depressive symptoms.37 38 41 In no study were healthcare professionals informed about elevated diabetes distress of participants in the control group.
Table 2.
Follow-up study outcomes between intervention and control groups
| Author (year) country |
Intervention PROM | Length of follow-up | HbA1c | Depressive symptoms | Diabetes distress | Other PROM outcomes | Self-management |
| Cummings et al40 (2019) USA |
PHQ-9* DDS-17† | 12 months | 8.9% (2.1) vs 9% (2.2) p=0.06 |
PHQ-9: 6.3 (5.9) vs 7.9 (7) p=0.01 |
DDS (RDD): 2.1 (1.2) vs 2.6 (1.3) p=0.0001 |
Not assessed | SDSCA: 4.3 (1.4) vs 3.98 (1.3) p=0.03 |
| Dobler et al44 (2018) Germany |
PAID†, PHQ-9* | 12 months | mean change −0.7% (1.4) vs 0.1% (1.7) p=0.006 |
PHQ-9: mean change −1.35 (4.3) vs −0.23 (4.9) p=0.057 |
PAID: mean change −4.77 (14.4) vs −1.4 (17) p=0.069 |
WHO-5: 1.23 (5.7) vs 0.1 (5.8) p=0.044 |
Not assessed |
| Ell et al38 (2011) USA |
PHQ-9* | 24 months | 9.1% (0.29) vs 8.9% (0.29) p=0.42 |
PHQ-9 (reported as >50% reduction): adjusted OR=1.87, 95% CI (1.05 to 3.32) p=0.03 |
Not assessed | SF-12 mental: 44.76 (1.150) vs 42.48 (1.17) p=0.001 |
SDSCA: 3.6 (0.15) vs 3.41 (0.2) p=0.26 |
| Fortmann et al 42 (2020) USA |
PHQ-2, PHQ-9* | 12 months | Mean change: −0.5% vs 0.0% p=0.011 | Only assessed in intervention arm | Only assessed in intervention arm | Not assessed | Only assessed in intervention arm |
| Johnson et al41 (2014) USA |
PHQ-9* | 12 months | Mean change: −0.2% (1.3) vs −0.2% (1.1) p=0.47 |
PHQ-9: 7.1 (5.4) vs 9.4 (5.9) p≤0.001 |
PAID-5: mean change −0.6 (0.8) vs 0.2 (0.9) p=0.03 |
EQ-5D: mean change 0.03 (0.1) vs 0.04 (0.12) p=0.23 |
Not assessed |
| Naik et al37 (2019) USA |
PHQ-9* | 12 months | 8.7% (1.6) vs 8.9% (2) p=0.83 |
PHQ-9: 10.1 (6.9) vs 12.6 (6.5) p=0.03 |
Not assessed | Not assessed | Not assessed |
| Rees et al 43 (2017) Australia |
DDS† | 6 months | 7.1% (1.1) vs 8.4% (2.5) p=0.093 |
PHQ-9: 6.7 (5.9) vs 9.9 (6.5) p=0.144 |
DDS: 2.2 (1.1) vs 2.5 (0.8) p=0.427 |
Not assessed | SDSCA diet: 6.1 (1.1) vs 5 (1.5) p=0.026 |
| Sigurdardottir et al45 (2009) Iceland |
PAID† | 6 months | 8.0% (1.16) vs 7.8% (.081) p=0.399 |
Not assessed | PAID: 19.1 (12.9) vs 13.8 (12.6) p=0.239 |
WBQ-12: 28.4 (6.1) vs 27.4 (5.6) p=0.544 |
SDSCA diet: 3.6 (0.4) vs 3.4 (0.5) p=0.122 |
| Wu et al39 (2018) USA |
PHQ-2, PHQ-9* | 6 months | 8.1% (0.16) vs 8.0% (0.17) p=0.57 |
PHQ-9: 5.16 (0.48) vs 6.35 (0.49) p=0.02 |
Not assessed | SF-12 mental: 49.87 (1.02) vs 48.38 (1.04) p=0.17 Satisfaction with diabetes care 4.20 (0.09) vs 4.01 (0.09) p=0.05 |
SDSCA: 4.78 (0.12) vs 4.66 (0.13) p=0.38 |
Outcome data are always presented as intervention versus control. Note, Johnson et al41 was a case–control study involving three groups, with data related to intervention and active control represented here. Wu et al39 was an observational study involving three groups, with data related to intervention versus usual care represented here.
Other PROM outcomes included general emotional well-being, mental health and health status, as well as satisfaction with diabetes care.
*indicates PROM related to depressive symtpoms.
†indicates PROM related to diabetes distress.
DDS, Diabetes Distress Scale; HbA1c, Glycated hemoglobin; 5-level EQ-5D, EuroQoL Five Dimensions; PAID, Problem Area in Diabetes scale; PHQ, Patient Health Questionnaire; PROM, patient-reported outcome measure; RDD, Regimen-related Diabetes Distress (a subscale of the DDS); SDSCA, Summary of Diabetes Self-Care Activities; SF-12, 12-Item Short-Form Survey; WBQ, Well-being Questionnaire; WHO-5, WHO Five-item Well-Being Index.
All nine studies reported glycaemia, measured by HbA1c, as an outcome measure. Where PROM assessed depressive symptoms (n=7), a clinically significant between-group difference in HbA1c was observed in two studies.42 44 Where diabetes distress was assessed (n=4), a clinically significant between-group difference in HbA1c was observed in two studies.43 44 Each of these studies had a cointervention involving a series of psychological therapy sessions.43 44 Only one of three studies using PROMs as part of stepped care algorithms with care coordination demonstrated a statistically significant glycaemic reduction.42
All but two studies examined the impact of PROMs use on depressive symptoms.42 45 Across all seven studies, depressive symptoms (measured with the PHQ-9) reduced in both arms. Where the intervention included assessment of depressive symptoms (n=7), statistically significant difference in depressive symptoms between groups was observed in five studies.37–41 Where diabetes distress was assessed during the intervention (n=4),40 43–45 three studies40 43 44 reported depressive symptoms as an outcome measure, with a significant difference in depressive symptoms between groups observed in one study.40 Five studies reported diabetes distress as an outcome measure.40 41 43–45 Diabetes distress reduced in both the intervention and control arms across all five studies.40 41 43–45 The difference between groups, favouring the intervention, was statistically significant in two studies.40 41
In the Cummings et al study, when therapy was stratified based on elevated levels of depressive symptoms or diabetes distress, improved diabetes self-management was reported.40 Similarly, in the Rees et al study, when cointerventions focused on people with type 2 diabetes with elevated distress levels receiving individual psychological therapy, an improvement in diabetes self-management was reported.43 General emotional well-being, mental health and health status were reported using various measures, including the WHO-5, Well-being Questionnaire (W-BQ), 12-Item Short-Form Survey and EQ-5D. No study reported patient–professional communication as an outcome. The Wu et al study was the only one to assess satisfaction with diabetes care, and a statistically significant improvement in the intervention arm was observed.39
Discussion
Main findings
To our knowledge, this is the first systematic review to synthesise the evidence related to PROM use to assess and address depressive symptoms and/or diabetes distress in type 2 diabetes care, despite diabetes guidelines recommending this practice for the past 25 years.20–25 The key finding is that very few studies have examined the use of PROMs to assess and address depressive symptoms and/or diabetes distress during routine type 2 diabetes care. When depressive symptoms were assessed (n=7), a statistically significant between-group difference in HbA1c was observed in two studies.42 44 A statistically significant between-group difference in depressive symptoms was observed in five of six studies where depressive symptoms were assessed during the intervention.37–41 Where diabetes distress was assessed, a clinically significant between-group difference in HbA1c (glycated hemoglobin) was observed in two of four studies,43 44 and a statistically significant difference in both depressive symptoms and diabetes distress was observed in one study.40 Two studies targeting people with elevated diabetes distress or depressive symptoms demonstrated statistically and clinically significant reductions in glycaemia.43 44 This review found little evidence of the best-associated cointervention for people identified by PROMs with elevated depressive symptoms or diabetes distress despite guideline recommendations.20–25
Similar to this review’s findings, a Cochrane review of PROM completion and feedback to healthcare professionals in the treatment of mental health conditions found insufficient evidence of impact on patient outcomes.46 However, the interventions included in the Cochrane review were limited to PROM feedback to the healthcare professional, not linked to interventions.46 While healthcare professionals frequently treat coexisting depression and type 2 diabetes, emotional issues such as diabetes distress are discussed less frequently.28 While over 238 unique PROMs for people with type 2 diabetes have been identified, the most effective intervention to implement and then address PROM-identified elevated depressive symptoms or diabetes distress remains unclear.47 Details about how precisely PROMs were used by healthcare professionals in discussion with people with type 2 diabetes were lacking. Further exploration of how PROMs can be integrated into routine clinical practice with the escalation of care for people with elevated depressive symptoms or distress is needed. Considering the recent recommendations from ICHOM for PROM use during diabetes care,27 healthcare professionals need guidance on the appropriate evidence-based intervention for elevated depressive symptoms or diabetes distress identified using a PROM in clinical practice.29 30
Studies demonstrating improved glycaemia had cointerventions of targeting people with elevated distress levels or depressive symptoms.43 44 Döbler et al increased frequency of follow-up counselling if elevated depressive symptoms were identified using the PHQ-9.44 Sturt’s systematic review regarding the effectiveness of interventions to reduce diabetes distress showed that interventions delivered by a general healthcare professional demonstrate an improvement in glycaemia and reduce diabetes distress.17 However, participants included in Sturt’s review had low levels of diabetes distress, and a further systematic review in 2018 identified that severe diabetes distress reduced with diabetes-specific psychological interventions.16 Evidentially, targeted interventions are needed stratified on the basis of severity of distress.
Studies have reported that completing a measure of diabetes distress before a consultation can improve glycaemia and patient satisfaction among adults with type 1 and type 2 diabetes.48 However, only Wu et al39 explored changes in patient satisfaction with care—which is an important measure considering PROMs are reported as enablers of person-centred care.39 49 No studies in our review explored the impact on patient-professional communication in the consultation, despite evidence suggesting PROM use in other clinical settings (oncology) improves communication, with PROMs initiating discussion of issues not otherwise addressed.50
Studies have also indicated that completion of a diabetes distress measure before a consultation, and discussion of those responses during the consultation, improves glycaemia and reduces diabetes distress among adults with type 1 and type 2 diabetes in specialist diabetes clinics.7 48 Pouwer et al’s study of people with type 1 and type 2 diabetes found monitoring of well-being, using the W-BQ, during diabetes care resulted in improved mood.51 While PROMs in these studies were embedded in routine care, they included people with type 1 and type 2 diabetes (without separate sub-group analyses) and were not conducted in general practice, where most type 2 diabetes care occurs.52 In our review, PROMs were completed most frequently with a trained study team member, not by a healthcare professional involved in the person’s clinical care.37 38 40 41 43 44 While this may replicate the likely real-world administration of PROMs (eg, by a receptionist, on arrival at the clinic), it is suggested that screening for depressive symptoms is best performed as part of collaborative care by the treating doctor or diabetes educator.53 In the future, it would be useful to explore models based on depressive symptoms or diabetes distress identified by the usual healthcare professional with stratification of actions based on responses.
Healthcare professionals need PROMs that provide responses that provoke action. However, the effective interventions in this study were resource-intensive, which will be difficult to replicate and sustain in routine clinical practice. Only one study used electronic prompts to healthcare professionals based on PHQ responses.39 Several studies have highlighted that clinical systems for PROM response delivery to healthcare professionals need to fit with clinical workflow.54–56 Even with the electronic delivery of PROM responses, the large volume of responses for healthcare professionals to review and the difficulty accessing PROM responses (due to storage on a dashboard separate from the electronic medical record) contribute to low use of PROMs in clinical settings.55–57
Strengths and limitations of the review
Key strengths of this review include adherence to the PRISMA guidelines,34 a comprehensive search strategy of six electronic databases and screening performed independently by two reviewers. The risk of bias was low in most studies, indicating outcomes of this review are based on high-quality studies. Depression and diabetes distress were assessed using well-validated measures, including PHQ, PAID and the DDS. The focus on type 2 diabetes is also a strength, as people with type 2 diabetes receive their care mostly in primary care settings, and their needs and preferences are different from people with type 1 diabetes.58 59
The heterogeneity of included cointerventions, how PROMs were completed, and healthcare professionals received the PROM responses, limits the overall review, making comparisons between studies difficult. It was not possible to conduct a meta-analysis because of the wide range of interventions and cointerventions assessed. Two studies had a small sample size with limited statistical power.43 45 Other limitations include the restriction of our search to published journal articles in the English language. This may explain why all studies included were from high-income or upper-middle-income countries, with no studies from low-middle-income countries identified. The inclusion criteria limited studies to populations with type 2 diabetes only, or where a subgroup analysis of participants with type 2 diabetes was included.
Future directions
Considering the low number of eligible studies, further research is warranted to understand the most efficient cointerventions to associate with PROM responses and how to integrate PROMs to coordinate interventions in general practice where most type 2 diabetes care occurs. The interventions examined as part of this review required significant external staff involvement, while only one study used technology to assist with PROM collection and delivery to healthcare professionals. Future research could focus on similar interventions using technology for self-completing PROMs with actionable outcomes if elevated depressive symptoms or diabetes distress are identified. Further research is needed to explore if PROM assessment of depressive symptoms and diabetes distress in routine type 2 diabetes care impacts communication and patient satisfaction with care.
Conclusions
This systematic review summarised and critiqued studies using PROMs for assessing and addressing depressive symptoms and/or diabetes distress as part of clinical type 2 diabetes care. The findings showed few studies using PROMs, but most are effective in reducing depressive symptoms or diabetes distress, though cointerventions related to PROM use in type 2 diabetes care are heterogeneous. While guidelines recommend the routine assessment of depressive symptoms and diabetes distress using PROMs, a clear mechanism for implementing this in routine diabetes care or the most effective cointervention is yet to be established.
Supplementary Material
Acknowledgments
The authors acknowledge the support of the University of Melbourne librarians Wil Villareal and Patrick Condon with preparing the search strategy
Footnotes
Twitter: @janespeight, @jo_manski
Contributors: RM, J-AM-N, BH, JE, JS and CH conceived the study. RM, J-AM-N, BH, DK, LC and FCSH performed the citation screening and risk of bias assessments. RM extracted the data with 20% also extracted by LC. RM drafted the manuscript and revised it based on the feedback from coauthors. RM is the acting guarantor and accepts full responsibility for the work/manuscript, she has access to data, controlled the decision to publish. All authors approved the manuscript for submission.
Funding: RM receives a PhD scholarship from Australian Rotary Health and the University of Melbourne (Grant number is not applicable). CH and JS are supported by core funding to the Australian Centre for Behavioural Research in Diabetes provided by the collaboration between Diabetes Victoria and Deakin University.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This is a systematic review, ethical approval was not required.
References
- 1.Saeedi P, Petersohn I, Salpea P. Diabetes research and clinical practice. In: Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International diabetes Federation diabetes atlas. 157. 9th edn, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Peyrot M, Rubin RR, Lauritzen T, et al. Psychosocial problems and barriers to improved diabetes management: results of the cross-national diabetes attitudes, wishes and needs (dawn) study. Diabet Med 2005;22:1379–85. 10.1111/j.1464-5491.2005.01644.x [DOI] [PubMed] [Google Scholar]
- 3.Skinner TC, Joensen L, Parkin T. Twenty-Five years of diabetes distress research. Diabet Med 2020;37:393–400. 10.1111/dme.14157 [DOI] [PubMed] [Google Scholar]
- 4.Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol 2015;3:450–60. 10.1016/S2213-8587(15)00135-7 [DOI] [PubMed] [Google Scholar]
- 5.Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010;33:23–8. 10.2337/dc09-1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanayakkara N, Pease A, Ranasinha S, et al. Depression and diabetes distress in adults with type 2 diabetes: results from the Australian National diabetes audit (ANDA) 2016. Sci Rep 2018;8:7846–46. 10.1038/s41598-018-26138-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snoek FJ, Kersch NYA, Eldrup E, et al. Monitoring of Individual Needs in Diabetes (MIND)-2: follow-up data from the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) MIND study. Diabetes Care 2012;35:2128–32. 10.2337/dc11-1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lustman PJ, Anderson RJ, Freedland KE, et al. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000;23:934–42. 10.2337/diacare.23.7.934 [DOI] [PubMed] [Google Scholar]
- 9.Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care 2012;35:2472–8. 10.2337/dc12-0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with type 2 diabetes: a longitudinal study. Diabet Med 2009;26:622–7. 10.1111/j.1464-5491.2009.02730.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pintaudi B, Lucisano G, Gentile S, et al. Correlates of diabetes-related distress in type 2 diabetes: findings from the benchmarking network for clinical and humanistic outcomes in diabetes (BENCH-D) study. J Psychosom Res 2015;79:348–54. 10.1016/j.jpsychores.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 12.Goldney RD, Phillips PJ, Fisher LJ, et al. Diabetes, depression, and quality of life: a population study. Diabetes Care 2004;27:1066–70. 10.2337/diacare.27.5.1066 [DOI] [PubMed] [Google Scholar]
- 13.Hutter N, Schnurr A, Baumeister H. Healthcare costs in patients with diabetes mellitus and comorbid mental disorders--a systematic review. Diabetologia 2010;53:2470–9. 10.1007/s00125-010-1873-y [DOI] [PubMed] [Google Scholar]
- 14.Gilmer TP, O'Connor PJ, Rush WA, et al. Predictors of health care costs in adults with diabetes. Diabetes Care 2005;28:59–64. 10.2337/diacare.28.1.59 [DOI] [PubMed] [Google Scholar]
- 15.Park M, Katon WJ, Wolf FM. Depression and risk of mortality in individuals with diabetes: a meta-analysis and systematic review. Gen Hosp Psychiatry 2013;35:217–25. 10.1016/j.genhosppsych.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt CB, van Loon BJP, Vergouwen ACM, et al. Systematic review and meta-analysis of psychological interventions in people with diabetes and elevated diabetes-distress. Diabet Med 2018 10.1111/dme.13709. [Epub ahead of print: 13 Jun 2018]. [DOI] [PubMed] [Google Scholar]
- 17.Sturt J, Dennick K, Hessler D, et al. Effective interventions for reducing diabetes distress: systematic review and meta-analysis. International Diabetes Nursing 2015;12:40–55. 10.1179/2057332415Y.0000000004 [DOI] [Google Scholar]
- 18.Chew BH, Vos RC, Metzendorf M-I, et al. Psychological interventions for diabetes-related distress in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2017;9:CD011469. 10.1002/14651858.CD011469.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speight J, Hendrieckx C, Pouwer F. Back to the future: 25 years of ‘Guidelines for encouraging psychological well-being’ among people affected by diabetes. Diabetic Medicine 2019. 10.1111/dme.14165 [DOI] [PubMed] [Google Scholar]
- 20.Bradley C, Gamsu DS, Psychological Well-being Working Group of the WHO/IDF St Vincent Declaration Action Programme for Diabetes . Guidelines for encouraging psychological well-being. 1994;11:510–6. 10.1111/j.1464-5491.1994.tb00316.x [DOI] [PubMed] [Google Scholar]
- 21.Diabetes Canada Clinical Practice Guidelines Expert Committee . Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2018;42:S1–325.29650079 [Google Scholar]
- 22.Federation ID . Recommendations for managing type 2 diabetes in primary care 2017.
- 23.Garber AJ, Abrahamson MJ, Barzilay JI, et al. consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm - 2019 executive summary. Endocr Pract 2019;25:69–101. 10.4158/CS-2018-0535 [DOI] [PubMed] [Google Scholar]
- 24.Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: a position statement of the American diabetes association. Diabetes Care 2016;39:2126–40. 10.2337/dc16-2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RACGP . General practice management of type 2 diabetes. East Melbourne, Vic, 2016. [Google Scholar]
- 26.Dawson J, Doll H, Fitzpatrick R, et al. The routine use of patient reported outcome measures in healthcare settings. BMJ 2010;340:c186. 10.1136/bmj.c186 [DOI] [PubMed] [Google Scholar]
- 27.Nano J, Carinci F, Okunade O, et al. A standard set of person-centred outcomes for diabetes mellitus: results of an international and unified approach. Diabet Med 2020;37:2009–18. 10.1111/dme.14286 [DOI] [PubMed] [Google Scholar]
- 28.Byrne JL, Davies MJ, Willaing I, et al. Deficiencies in postgraduate training for healthcare professionals who provide diabetes education and support: results from the diabetes attitudes, wishes and needs (DAWN2) study. Diabet Med 2017;34:1074–83. 10.1111/dme.13334 [DOI] [PubMed] [Google Scholar]
- 29.Boyce MB, Browne JP, Greenhalgh J. The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: a systematic review of qualitative research. BMJ Qual Saf 2014;23:508–18. 10.1136/bmjqs-2013-002524 [DOI] [PubMed] [Google Scholar]
- 30.Foster A, Croot L, Brazier J, et al. The facilitators and barriers to implementing patient reported outcome measures in organisations delivering health related services: a systematic review of reviews. J Patient Rep Outcomes 2018;2:46. 10.1186/s41687-018-0072-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendrick T, Stuart B, Leydon GM, et al. Patient-Reported outcome measures for monitoring primary care patients with depression: PROMDEP feasibility randomised trial. BMJ Open 2017;7:e015266. 10.1136/bmjopen-2016-015266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao C-J, Dymek C, Kim B, et al. Advancing the use of patient-reported outcomes in practice: understanding challenges, opportunities, and the potential of health information technology. Qual Life Res 2019;28:1575–83. 10.1007/s11136-019-02112-0 [DOI] [PubMed] [Google Scholar]
- 33.McMorrow R, Hunter B, Hendrieckx C, et al. Effect of routinely assessing and addressing depression and diabetes distress using patient-reported outcome measures in improving outcomes among adults with type 2 diabetes: a systematic review protocol. BMJ Open 2021;11:e044888. 10.1136/bmjopen-2020-044888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 36.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naik AD, Hundt NE, Vaughan EM, et al. Effect of Telephone-Delivered collaborative goal setting and behavioral activation vs enhanced usual care for depression among adults with uncontrolled diabetes: a randomized clinical trial. JAMA Netw Open 2019;2:e198634–e34. 10.1001/jamanetworkopen.2019.8634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ell K, Katon W, Xie B, et al. One-Year postcollaborative depression care trial outcomes among predominantly Hispanic diabetes safety net patients. Gen Hosp Psychiatry 2011;33:436–42. 10.1016/j.genhosppsych.2011.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S, Ell K, Jin H, et al. Comparative effectiveness of a Technology-Facilitated depression care management model in safety-net primary care patients with type 2 diabetes: 6-month outcomes of a large clinical trial. J Med Internet Res 2018;20:e147. 10.2196/jmir.7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings DM, Lutes LD, Littlewood K, et al. Randomized trial of a tailored cognitive behavioral intervention in type 2 diabetes with comorbid depressive and/or Regimen-Related distress symptoms: 12-month outcomes from COMRADE. Diabetes Care 2019;42:841–8. 10.2337/dc18-1841 [DOI] [PubMed] [Google Scholar]
- 41.Johnson JA, Al Sayah F, Wozniak L, et al. Collaborative care versus screening and follow-up for patients with diabetes and depressive symptoms: results of a primary care-based comparative effectiveness trial. Diabetes Care 2014;37:3220–6. 10.2337/dc14-1308 [DOI] [PubMed] [Google Scholar]
- 42.Fortmann AL, Walker C, Barger K, et al. Care team integration in primary care improves one-year clinical and financial outcomes in diabetes: a case for value-based care. Popul Health Manag 2020;23:467–75. 10.1089/pop.2019.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rees G, O'Hare F, Saeed M, et al. Problem-Solving therapy for adults with diabetic retinopathy and diabetes-specific distress: a pilot randomized controlled trial. BMJ Open Diabetes Res Care 2017;5:e000307. 10.1136/bmjdrc-2016-000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Döbler A, Herbeck Belnap B, Pollmann H, et al. Telephone-delivered lifestyle support with action planning and motivational interviewing techniques to improve rehabilitation outcomes. Rehabil Psychol 2018;63:170–81. 10.1037/rep0000224 [DOI] [PubMed] [Google Scholar]
- 45.Sigurdardottir AK, Benediktsson R, Jonsdottir H. Instruments to tailor care of people with type 2 diabetes. J Adv Nurs 2009;65:2118–30. 10.1111/j.1365-2648.2009.05040.x [DOI] [PubMed] [Google Scholar]
- 46.Kendrick T, El-Gohary M, Stuart B, et al. Routine use of patient reported outcome measures (PROMs) for improving treatment of common mental health disorders in adults. Cochrane Database Syst Rev 2016;7:CD011119–CD19. 10.1002/14651858.CD011119.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wee PJL, Kwan YH, Loh DHF, et al. Measurement properties of patient-reported outcome measures for diabetes: systematic review. J Med Internet Res 2021;23. 10.2196/25002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chawla A, Saha C, Marrero DG. A novel application of the problem areas in diabetes (paid) instrument to improve glycemic control and patient satisfaction. Diabetes Educ 2010;36:337–44. 10.1177/0145721709354607 [DOI] [PubMed] [Google Scholar]
- 49.Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013;346:167. 10.1136/bmj.f167 [DOI] [PubMed] [Google Scholar]
- 50.Greenhalgh J, Gooding K, Gibbons E, et al. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? A realist synthesis. J Patient Rep Outcomes 2018;2:42. 10.1186/s41687-018-0061-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pouwer F, Snoek FJ, van der Ploeg HM, et al. Monitoring of psychological well-being in outpatients with diabetes: effects on mood, HbA(1c), and the patient's evaluation of the quality of diabetes care: a randomized controlled trial. Diabetes Care 2001;24:1929–35. 10.2337/diacare.24.11.1929 [DOI] [PubMed] [Google Scholar]
- 52.Ventura ADB, Holmes-Truscott JL, Hendrieckx E. Diabetes MILES-2 2016 survey report. Melbourne: Victoria, 2016. [Google Scholar]
- 53.van der Feltz-Cornelis CM. Depression in diabetes mellitus: to screen or not to screen? A patient-centred approach. Br J Diabetes Vasc Dis 2011;11:276–81. 10.1177/1474651411423539 [DOI] [Google Scholar]
- 54.Leydon GM, Dowrick CF, McBride AS, et al. Questionnaire severity measures for depression: a threat to the doctor–patient relationship? British Journal of General Practice 2011;61:117–23. 10.3399/bjgp11X556236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hans PK, Gray CS, Gill A, et al. The provider perspective: investigating the effect of the electronic patient-reported outcome (ePRO) mobile application and portal on primary care provider workflow. Prim Health Care Res Dev 2018;19:151–64. 10.1017/S1463423617000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barr PJ, Berry SA, Gozansky WS, et al. No date for the PROM: the association between patient-reported health events and clinical coding in primary care. J Patient Rep Outcomes 2020;4:17. 10.1186/s41687-020-0183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner GM, Litchfield I, Finnikin S, et al. General practitioners' views on use of patient reported outcome measures in primary care: a cross-sectional survey and qualitative study. BMC Fam Pract 2020;21:14. 10.1186/s12875-019-1077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fenwick EK, Rees G, Holmes-Truscott E, et al. What is the best measure for assessing diabetes distress? A comparison of the problem areas in diabetes and diabetes distress scale: results from diabetes MILES-Australia. J Health Psychol 2018;23:667–80. 10.1177/1359105316642006 [DOI] [PubMed] [Google Scholar]
- 59.Halliday JA, Hendrieckx C, Busija L, et al. Validation of the WHO-5 as a first-step screening instrument for depression in adults with diabetes: Results from Diabetes MILES - Australia. Diabetes Res Clin Pract 2017;132:27–35. 10.1016/j.diabres.2017.07.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-054650supp001.pdf (117.2KB, pdf)
bmjopen-2021-054650supp002.pdf (55.9KB, pdf)
Data Availability Statement
Data are available on reasonable request to the corresponding author.


