Abstract
Thermophilic anaerobic biodegradation of tetrachloroethene (PCE) was investigated with various inocula from geothermal and nongeothermal areas. Only polluted harbor sediment resulted in a stable enrichment culture that converted PCE via trichloroethene to cis-1,2-dichloroethene at the optimum temperature of 60 to 65°C. After several transfers, methanogens were eliminated from the culture. Dechlorination was supported by lactate, pyruvate, fructose, fumarate, and malate as electron donor but not by H2, formate, or acetate. Fumarate and l-malate led to the highest dechlorination rate. In the absence of PCE, fumarate was fermented to acetate, H2, CO2, and succinate. With PCE, less H2 was formed, suggesting that PCE competed for the reducing equivalents leading to H2. PCE dechlorination, apparently, was not outcompeted by fumarate as electron acceptor. At the optimum dissolved PCE concentration of ∼60 μM, a high dechlorination rate of 1.1 μmol h−1 mg−1 (dry weight) was found, which indicates that the dechlorination is not a cometabolic activity. Microscopic analysis of the fumarate-grown culture showed the dominance of a long thin rod. Molecular analysis, however, indicated the presence of two dominant species, both belonging to the low-G+C gram positives. The highest similarity was found with the genus Dehalobacter (90%), represented by the halorespiring organism Dehalobacter restrictus, and with the genus Desulfotomaculum (86%).
Tetrachloroethene (PCE) is an organic solvent that is widely used for dry cleaning of textiles and degreasing of machines and metal parts. This highly toxic compound has been released into the environment for decades, and therefore, it has become one of the most common contaminants of soils and groundwater. In the past, several anaerobic mixed cultures that are able to reductively dechlorinate PCE to lower-chlorinated compounds, like trichloroethene (TCE), dichloroethene (DCE), vinyl chloride (VC), and even ethene, have been described, indicating that complete anaerobic detoxification of PCE is possible (2, 3, 7, 11, 28). Dechlorination of PCE to TCE can be performed at a low rate via cometabolic processes, as was shown previously for some pure methanogenic and acetogenic cultures (5, 8). On the other hand, several isolates that are able to use PCE as terminal electron acceptor, and possibly to use this type of respiration for energy conservation and growth, have recently been obtained (14, 20). Dehalobacter restrictus, initially described as a highly purified enrichment culture, was the first microorganism that was reported to perform this so-called halorespiration of PCE. It is a strict anaerobic bacterium that exclusively uses H2 as electron donor and PCE or TCE as electron acceptor (13, 15). In contrast, Dehalospirillum multivorans can utilize a range of electron donors, such as organic acids and alcohols, as well as H2. Moreover, it can use fumarate or nitrate as electron acceptor instead of PCE (25). Such versatility was also observed for Desulfitobacterium strain PCE1, which may use chlorophenols or sulfite as electron acceptors as well as PCE (12). Recently, a facultative aerobic Enterobacter-like bacterium that rapidly converts high concentrations of PCE was described also (26). A variety of carbohydrates, fatty acids, and amino acids but not H2 could act as electron donor, while O2 or nitrate could be used as electron acceptor in addition to PCE. Furthermore, an anaerobic PCE dechlorinator which uses acetate as electron donor, and which can use ferric nitriloacetate or fumarate as electron acceptor, has been described (18). All these isolates dechlorinate PCE only to TCE or cis-1,2-DCE. Complete anaerobic dechlorination to ethene was recently described for a single organism, tentatively named Dehalococcoides ethenogenes 195 (19). Evidently, these examples show that a variety of microorganisms that are able to reductively dechlorinate PCE exist. However, the strains obtained so far are all mesophiles, with temperature optima ranging from 25 to 37°C.
For the present paper, we investigated the possibility of reductive dechlorination under thermophilic conditions. Thermophilic microbial dechlorination may have several advantages over mesophilic treatment, since mass transfer processes proceed at higher rates due to higher diffusion coefficients and lower viscosity. In addition, the growth rate of thermophiles is generally higher than that of their mesophilic counterparts (27). Finally, the high optimal growth temperature may allow for the use of such organisms in the treatment of waste streams directly at the source, where often higher temperatures exist. Here we describe the enrichment, physiology, and phylogenetic analysis of an anaerobic culture that is able to convert PCE via TCE to cis-1,2-DCE at an optimum temperature of dechlorination of 60 to 65°C.
MATERIALS AND METHODS
Medium composition and cultivation.
A standard phosphate-bicarbonate-buffered anaerobic medium was used with a low chloride content, as described before (13), except that the amount of vitamins was increased 10-fold. In addition, the medium contained 0.1 μM sodium tungstate, 0.1 μM sodium selenate, and either yeast extract or fermented yeast extract (1 g/liter). Fermented yeast extract was prepared as described before (13). Routine culturing was performed in 117-ml serum vials closed with viton stoppers (Maag; Technic AG, Dübendorf, Switzerland), which contained 20 or 40 ml of medium. N2-CO2 (4:1; 1.7 × 105 Pa) was used as gas phase. Occasionally, 1,200-ml serum bottles were used, containing 80 ml of medium. These large bottles enabled the addition of higher amounts of PCE, while the concentration in the liquid phase remained below inhibitory levels. Incubations were routinely performed at 62°C. PCE was added separately by syringe from a stock solution of PCE-saturated anoxic water (approximately 0.84 mM [24]) or as pure PCE (large bottles). Lactate or fumarate was routinely added at a concentration of 5 mM. Other electron donors were tested at a concentration of 10 mM.
Analyses.
The dechlorination was monitored by headspace analysis. Gas samples were analyzed by gas chromatography with a Chrompack CP9000 apparatus equipped with a capillary column (25 m by 0.32 mm; Sil 5CB; 1.22 μm) connected to a flame ionization detector. H2 was analyzed on a molecular sieve column, connected to a thermal conductivity detector. Amounts of chlorinated and gaseous compounds were expressed as the absolute amount present per bottle. For the calculation of the actual concentration of dissolved PCE, a nondimensional Henry’s coefficient of 2.76 was calculated at 62°C, according to the method described by Peng and Wan (22). Dissolved compounds (sugars and organic acids) were analyzed by high-pressure liquid chromatography according to standard techniques as described previously (16).
Isolation of nucleic acids.
Total DNA was extracted from a fumarate-grown PCE-dechlorinating enrichment by a guanidium thiocyanate method adapted from the work of Pitcher et al. (23). A 40-ml culture sample was centrifuged for 20 min at 17,250 × g. The pellet was resuspended in 500 μl of GES reagent and transferred to a 1.5-ml microcentrifuge tube, containing 0.4 g of glass beads (0.11 mm). GES reagent consisted of 5 M guanidium thiocyanate, 100 mM EDTA, and 0.5% (wt/vol) Sarkosyl. Cells were disrupted by bead beating for 1 min in an MSK cell homogenizer (Braun, Melsungen, Germany). The cell lysates were cooled on ice, and 0.25 ml of cold 7.5 M ammonium acetate was added. After 10 min on ice, 0.5 ml of chloroform was added, mixed, and centrifuged for 15 min (16,000 × g). The supernatant (500 μl) was transferred to a microcentrifuge tube, and nucleic acids were precipitated overnight with 50 μl of 3 M sodium acetate–1 ml of ice-cold ethanol (96%). After centrifugation, the DNA pellet was washed with 70% ethanol, dried, and resuspended in 100 μl of Tris-EDTA buffer. The DNA obtained was used for PCR, cloning, and temperature gradient gel electrophoresis (TGGE).
Amplification, cloning, and sequencing of 16S ribosomal DNA (rDNA).
Amplification of 16S rDNA sequences was performed with a GeneAmp PCR system 2400 thermocycler (Perkin-Elmer Cetus, Norwalk, Conn.), with 35 cycles of 94°C for 10 s, 54°C for 20 s, and 68°C for 2 min. The PCR mixtures (100 μl) contained 10 mM Tris-HCl (pH 8.3); 50 mM KCl; 3 mM MgCl2; 0.05% detergent W-1; 150 μM (each) dATP, dCTP, dGTP, and dTTP; 100 pmol of primers 8f and 1512r (9); 2.5 U of Taq DNA polymerase; and 1 μl of template DNA. The amount and size of the amplification products were analyzed by 1.2% agarose gel electrophoresis. Subsequently, the amplified DNA was separated from primers and deoxynucleoside triphosphates on a low-melting-point agarose gel, recovered, and cloned in pGEM-T linear plasmid vector and Escherichia coli JM109 competent cells according to the manufacturer’s instructions. Positive clones (white colonies) were taken up with a sterile toothpick and transferred to a 1.5-ml microcentrifuge tube containing 50 μl of Tris-EDTA buffer. The tube was heated for 15 min at 95°C and then chilled on ice. Cloned 16S rRNA inserts were identified by a PCR check and then sequenced as described before (10). The resulting sequences were compared with the 16S rRNA sequences available in the EMBL database by using the FASTA program of the GCG package (4).
Partial 16S rRNA amplification and TGGE.
The PCR template was DNA directly extracted from the enrichment culture or from the cultured transformants. A TGGE-suitable 16S rDNA amplicon was generated with 35 cycles of 94°C for 10 s, 56°C for 20 s, and 68°C for 40 s. The PCR mixtures (10 to 20 μl) contained 10 mM Tris-HCl (pH 8.3); 50 mM KCl; 3 mM MgCl2; 0.05% detergent W-1; 50 μM (each) dATP, dCTP, dGTP, and dTTP; 100 pmol of primers U968/GC and L1401 (9); 0.5 U of Taq DNA polymerase; and 1 μl of template DNA. The Diagen TGGE system was used for sequence-specific separation of PCR products. Electrophoresis was performed with a 0.8-mm polyacrylamide gel (6% [wt/vol] acrylamide, 0.1% [wt/vol] bisacrylamide, 8 M urea, 20% [vol/vol] formamide, 2% [vol/vol] glycerol) with 1× TA buffer (40 mM Tris-acetate, pH 8.0) at a fixed current of 9 mA (approximately 120 V) for 16 h. A temperature gradient from 38 to 47°C was established in the direction of electrophoresis. After electrophoresis, the gel was silver stained (6).
Materials.
All chemicals were of analytical grade. PCE and TCE were from Merck (Darmstadt, Germany). cis-1,2-DCE was obtained from Aldrich Chemie (Axel, The Netherlands), and trans-1,2-DCE was from Janssen Chimica (Beerse, Belgium). All gases were supplied by Hoek-Loos (Schiedam, The Netherlands). Taq DNA polymerase and detergent W-1 were from Life Technologies (Paisley, United Kingdom). The pGEM-T linear plasmid vector and E. coli JM109 competent cells were from Promega (Madison, Wis.).
Nucleotide sequence accession numbers.
The accession number in the EMBL database for sequence ST10 is AJ131536, and that for sequence ST12 is AJ131537.
RESULTS
Enrichment of thermophilic PCE-dechlorinating bacteria.
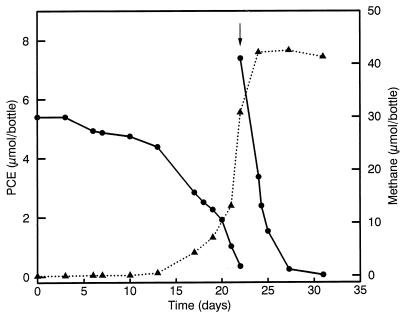
Thermophilic PCE-degrading microorganisms were enriched at 60°C under methanogenic conditions with lactate as electron donor and inoculated with samples from geothermally heated sites in Iceland (Hveragerdi), New Zealand (Whaka Stream), Italy (Naples-Stuferone), and Turkey (Yozgat). However, in none of these incubations was the concentration of PCE found to decrease significantly. In contrast, the use of PCE-polluted sediment from the harbor of Rotterdam, The Netherlands, resulted in a rapid stoichiometric transformation of PCE to TCE and cis-1,2-DCE within 3 weeks (Fig. 1). This culture also showed methane formation, but during subculturing in the presence of the methanogenic inhibitor bromoethanesulfonate (5 mM), this activity was easily lost, without affecting the dechlorinating activity. The culture could be repeatedly transferred, and the lactate-dependent dechlorinating activity was retained. Through serial dilution, fast-growing lactate-fermenting microorganisms were lost from the culture.
FIG. 1.
Conversion of PCE (●) and formation of methane (▴) in the first enrichment culture, inoculated with Rotterdam harbor sludge and incubated at 60°C. The formation of TCE and cis-DCE is not shown. At day 22, PCE was readded. The arrow indicates readdition of PCE.
Effect of electron donors and medium components.
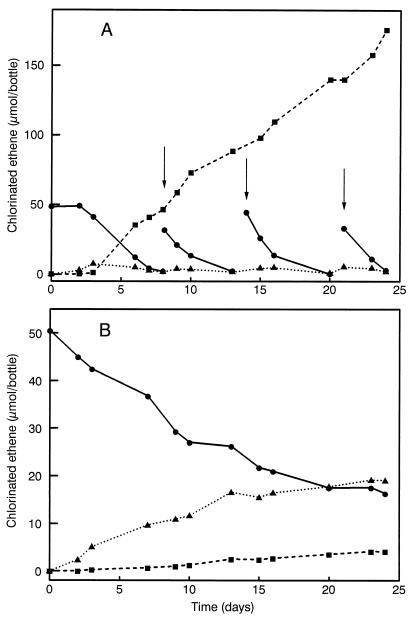
For the enrichment and routine subculturing, lactate was used as electron donor and carbon source. In the absence of lactate, no dechlorination occurred. This indicated that the fermented yeast extract present in the medium does not cause dechlorination. Simple electron donors like H2 or formate, often found to sustain dechlorination in other studies, did not result in PCE conversion. In the presence of acetate, which may be required as carbon source, H2 or formate was also ineffective. Acetate alone and also propionate, butyrate, succinate, oxalate, citrate, isovalerate, isobutyrate, glycerol, glucose, ethanol, or carbon monoxide (7% to gas phase) were unable to bring about dechlorination. However, pyruvate, fructose, fumarate, and l-malate could replace lactate as electron donor for dechlorination. Notably, fumarate was found to be a better electron donor than lactate, since the rate of dechlorination was higher and PCE could be repeatedly added without causing a decrease in the rate of dechlorination (Fig. 2). Moreover, TCE hardly accumulated but was rapidly converted to cis-1,2-DCE under these conditions. For this reason, fumarate was used as substrate for further enrichment instead of lactate. Dechlorination rates amounted to 800 μM day−1 or 1.1 μmol h−1 mg−1 (dry weight). Besides cis-1,2-DCE, small amounts of trans-1,2-DCE (0.5 to 1%) were also formed. Formation of vinyl chloride, ethene, or ethane was never observed. Remarkably, when TCE was added instead of PCE, dechlorination did not occur, suggesting that PCE was required for the conversion of TCE.
FIG. 2.
Dechlorination of PCE and product formation by the thermophilic enrichment culture at 62°C in the presence of fumarate (A) or lactate (B). ●, PCE; ▴, TCE; ■, cis-1,2-DCE. The arrow indicates readdition of PCE. In this case, 1,200-ml bottles were used, which enabled the addition of higher amounts of PCE.
Slightly oxidized cultures, as indicated by the pink color of the redox indicator resazurin, did not convert PCE.
In the absence of fermented or nonfermented yeast extract, dechlorination did not occur with lactate as substrate. However, when fumarate was used, yeast extract could be omitted, although the lag phase was extended and the rate of dechlorination was slightly decreased (data not shown). Apparently, fermented yeast extract provided the medium with some unknown growth factor that could also be formed by the enrichment culture itself. In the absence of vitamins, no dechlorination was found, but the effect of the separate vitamins was not examined.
The standard medium was bicarbonate buffered, and therefore CO2 had to be present in the gas phase. However, when a MOPS (3[N-morpholino]propanesulfonate) buffer was used instead, and CO2 was omitted from the gas phase, dechlorination no longer took place (data not shown). Addition of CO2 to the gas phase of the MOPS-buffered medium resulted again in dechlorination. This indicated that CO2 was necessary for dechlorination.
Effect of the PCE concentration.
The use of different concentrations of PCE in the presence of lactate showed that above ∼10 μM (actual concentration in liquid phase) PCE inhibited the dechlorination process (data not shown). However, when fumarate was used as electron donor, PCE was less inhibitory, and concentrations up to 60 μM could be applied (data not shown). At PCE concentrations of 360 μM and higher, dechlorination was not observed. This concentration is in the same range as that found for other dechlorinating microorganisms, with the exception of a facultative aerobic Enterobacter-like organism, which can convert up to 1 mM PCE (26).
Temperature effect.
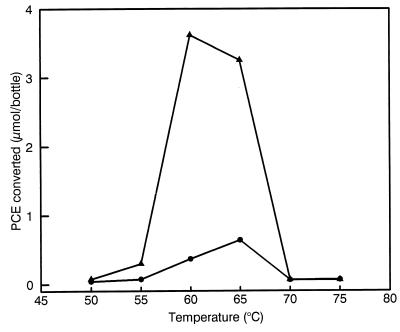
Dechlorination was determined at temperatures ranging from 50 to 75°C. The highest rate of dechlorination was found at approximately 60 to 65°C. Above 65°C, the dechlorinating activity decreased rapidly. Therefore, 62°C was chosen for routine culturing. At 50 and 70°C, no dechlorination and no growth occurred (Fig. 3). Heat treatment of the culture (30 min; 90°C) destroyed the dechlorinating ability of the culture, indicating that the responsible organism is probably not spore forming.
FIG. 3.
Effect of temperature on the dechlorination of PCE by the thermophilic enrichment culture. Lactate was used as electron donor. The amount of PCE converted after 2 (●) and 5 (▴) days of incubation is shown.
The fate of fumarate in the enrichment culture.
The conversion of fumarate was not dependent on the presence of PCE. To determine the influence of PCE on the fumarate conversion, enrichment cultures converting fumarate in the presence and absence of PCE were analyzed repeatedly for fermentation products (both liquid and gas phase). In the absence of PCE, fumarate was fermented to acetate, succinate, and H2. Propionate was never observed. The amount of CO2 could not be determined because of the bicarbonate buffer but was assumed to be twice the amount of acetate formed. The fermentation balances of a typical experiment are given in Table 1. From 1 mol of fumarate, approximately 0.5 mol of succinate, 0.5 mol of acetate, and 0.5 mol of H2 were formed. In the presence of PCE, stoichiometrically less H2 was formed, indicating that reducing equivalents were now shuttled toward PCE.
TABLE 1.
Typical fermentation pattern for the conversion of fumarate in the presence and absence of PCEa
| Presence of PCE | PCE converted (mM) | TCE formed (mM) | DCE formed (mM) | Fumarate converted (mM) | Malate formed (mM) | Succinate formed (mM) | Acetate formed (mM) | H2 formed (mM) | C balance (%) | H balanceb (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| + | 2.2 | 0.08 | 2.1 | 12.2 | 0.09 | 5.31 | 6.19 | 4.19 | 0.95 | 0.97 |
| − | 10.9 | 0.21 | 4.97 | 6.12 | 7.14 | 1.03 | 1.03 |
For comparison with dissolved compounds, the amounts of chlorinated ethenes are expressed as if completely dissolved in the liquid phase.
For calculation of the available hydrogen balance, it was assumed that for the formation of 1 mol of TCE and DCE, 1 and 2 mol of H2, respectively, are required.
Apparently, fumarate fermentation was not obligately coupled to dechlorination. Dechlorination may, however, be dependent on some fermentation product of fumarate, instead of fumarate itself. The addition of hydrogen plus fumarate, or succinate plus fumarate, however, did not stimulate the dechlorination.
Bacterial composition and molecular analysis of the enrichment culture.
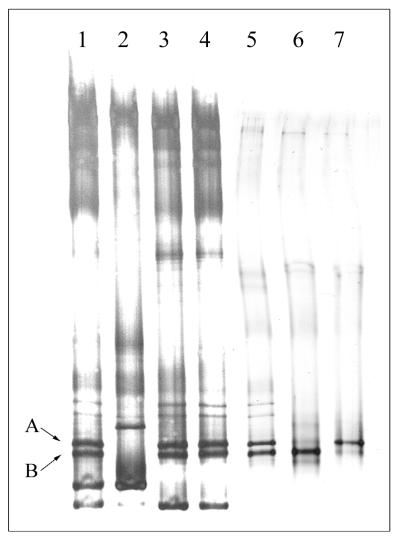
Cell counts of approximately 2 × 108 cells ml−1 have been reached in the fumarate-grown enrichments. Phase-contrast microscopy revealed the dominance of a thin rod, next to low numbers of a small, motile vibrio (<1%). From the microscopic analysis of dechlorinating and nondechlorinating cultures obtained under various conditions, it was concluded that the thin rod is most probably responsible for the dechlorination. Despite the predominance of this morphotype, dilution series did not yet result in a pure culture, and no colonies were obtained in agar roll tubes. Therefore, the dechlorinating culture was characterized by a molecular approach. For this purpose, DNA was isolated from different cultures and used as template for the amplification of the V6 to V8 region of the 16S rRNA. Subsequent separation by TGGE revealed that the compositions of dechlorinating and nondechlorinating cultures differed significantly. Two prominent bands, close to each other (A and B), were present in all dechlorinating cultures (Fig. 4). As expected from this prominence, the 16S rDNA amplicons corresponding to these bands were readily obtained in a clone library (ST10 and ST12) (Fig. 4). Sequence analysis showed only remote similarity (78% nucleotide sequence identity) between the two sequences. Clone ST10 revealed the highest sequence similarity (90%) to the mesophilic Dehalobacter restrictus within the group of low-G+C gram positives. Clone ST12 also fell into the group of low-G+C gram positives, but the highest sequence similarity was found with the thermophilic Desulfotomaculum thermosapovorans (86%).
FIG. 4.
TGGE patterns of amplified of 16S rDNA (V6 to V8 region) derived from various thermophilic enrichment cultures. Lanes 1 to 4 show amplicons derived from earlier transfers: 1, dechlorinating culture grown on fumarate and lactate; 2, culture grown on lactate without PCE; 3, dechlorinating culture grown on fumarate; 4, culture grown on fumarate without PCE. Lane 5 shows the amplicons derived from a recent dechlorinating culture grown on fumarate. Lane 6 and 7 contain the amplicons derived from clones ST12 and ST10, respectively. The dominant bands A and B, corresponding to the bands of the clones, are indicated.
DISCUSSION
Recently, several pure cultures that are able to reductively dechlorinate PCE to lower-chlorinated ethenes have been described. All the isolates obtained are mesophiles with temperature optima ranging from 25 to 37°C. The present paper describes a highly enriched culture that also dechlorinates PCE via TCE to cis-1,2-DCE, at an optimum growth temperature of 60 to 65°C. Besides its temperature optimum, the culture differs from many other previously described bacteria in that it cannot use H2 as electron donor for dechlorination, although it can use fumarate, malate, fructose, lactate, or pyruvate. Through fermentation, these compounds predominantly lead to the formation of acetate, H2, and CO2. Since acetate and H2 (or formate) did not cause dechlorination, we assume that the dechlorinating organism is able to utilize the substrates directly as actual electron donor for dechlorination, and not some fermentation product derived from them.
The use of fumarate as electron donor under anaerobic conditions is rather unusual. In fact, fumarate is a well-known electron acceptor, and fumarate respiration is the most widespread type of respiration among anaerobes (17). In the PCE-dechlorinating Dehalospirillum multivorans, fumarate inhibits the conversion of PCE, possibly by competing for the reducing equivalents (25). In our case, fumarate apparently does not outcompete PCE dechlorination. Moreover, the addition of PCE hardly affects the amount of succinate formed out of fumarate. In contrast, PCE influences the amount of H2 formed, suggesting that PCE competes for reducing equivalents at a lower redox level. Apparently, these reducing equivalents are not easily used for fumarate reduction, which is confirmed by the 1:1:1 ratio of the products succinate, acetate, and H2.
Using fumarate as substrate, dechlorination rates of up to 1.1 μmol h−1 mg−1 (dry weight) were obtained. These high rates indicate that the dechlorination is probably not a cometabolic process, i.e., catalyzed as a side activity of the normal enzymatic equipment of an organism. Such cometabolic dechlorination has been reported for certain Methanosarcina species, having rates below 0.02 nmol h−1 mg−1 (dry weight) (7, 8). The dechlorination rate found here is in the same range as that reported for several of the recently described halorespiring bacteria. For Dehalobacter restrictus, Desulfitobacterium strain PCE1, and Dehalospirillum multivorans, dechlorination rates of 5, 1.5, and 1.5 μmol h−1 mg−1 (dry weight), respectively, were calculated from the available data (12, 13, 25). Whether the responsible dechlorinating organism in the enrichment is able to obtain energy from the dechlorination process by electron transport phosphorylation cannot as yet be determined, because the fermentation of the organic electron donors will in any case enable substrate-level phosphorylation.
The observation that the dechlorination is dependent on CO2 suggests the involvement of a carboxylation reaction. Alternatively, the dechlorinating organism is a homoacetogenic bacterium, which uses CO2 as electron acceptor. This possibility is supported by the observed substrate spectrum, which is typical for many homoacetogenic bacteria. For instance, Clostridium formicoaceticum also ferments fructose, fumarate, lactate, and pyruvate and also does not utilize H2 (1). According to the product formation, however, homoacetate formation does not occur, and H2 is formed next to acetate.
The molecular analysis by TGGE showed that the culture is highly enriched, with only two very abundant rRNA sequences present. The equal intensity of their TGGE bands, as observed in several TGGE gels, and the fact that the bands were never observed separately, initially suggested that both originated from the same organism, carrying 16S rRNA genes of different sequences. Such sequence heterogeneity of 16S rRNA genes from one single organism has been described before (21). The assumption was also supported by microscopic analysis which revealed only one morphotype (thin rod). However, by subsequent cloning and sequencing, both bands were found to represent quite different species of the low-G+C gram positives. The low sequence similarity of only 78% made the assumption of one source organism rather unlikely. Interestingly, the sequence ST10 was related to Dehalobacter restrictus, a mesophilic halorespiring species. Nevertheless, due to the low sequence similarity of 90%, the thermophilic species would constitute a novel genus. This also holds for the species represented by sequence ST12, which was only 86% identical to Desulfotomaculum thermosapovorans. It remains to be elucidated whether both species are involved in dechlorination and what their exact roles are. In situ hybridization experiments with specific oligonucleotide probes are currently under way to clarify the contribution of both organisms in the enrichment culture.
ACKNOWLEDGMENTS
We thank Wilma Akkermans-van Vliet and Erwin Zoetendal for their help with the molecular analyses.
This work was partly supported by the Environment Programme of the European Union (contract EV5V-CT94-0540).
REFERENCES
- 1.Andreesen J R, Gottschalk G, Schlegel H G. Clostridium formicoaceticum nov. spec. Isolation, description and distinction from C. aceticum and C. thermoaceticum. Arch Mikrobiol. 1970;72:154–174. doi: 10.1007/BF00409521. [DOI] [PubMed] [Google Scholar]
- 2.Belay N, Daniels L. Production of ethane, ethylene, and acetylene from halogenated hydrocarbons by methanogenic bacteria. Appl Environ Microbiol. 1987;53:1604–1610. doi: 10.1128/aem.53.7.1604-1610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Bruin W P, Kotterman M J J, Posthumus M A, Schraa G, Zehnder A J B. Complete biological reductive transformation of tetrachloroethene to ethane. Appl Environ Microbiol. 1992;58:1996–2000. doi: 10.1128/aem.58.6.1996-2000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egli C, Tschan T, Scholtz R, Cook A M, Leisinger T. Transformation of tetrachloromethane to dichloromethane and carbon dioxide by Acetobacterium woodii. Appl Environ Microbiol. 1988;54:2819–2824. doi: 10.1128/aem.54.11.2819-2824.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelen B, Heuer H, Felske A, Nübel U, Smalla K, Backhaus H. Abstracts for the Workshop on Application of DGGE and TGGE in Microbial Ecology 1995. BBA for Agriculture and Forestry. Germany: Braunschweig; 1995. Protocols for the TGGE. [Google Scholar]
- 7.Fathepure B Z, Nengu J P, Boyd S A. Anaerobic bacteria that dechlorinate perchloroethene. Appl Environ Microbiol. 1987;53:2671–2674. doi: 10.1128/aem.53.11.2671-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fathepure B Z, Boyd S A. Dependence of tetrachloroethylene dechlorination on methanogenic substrate consumption by Methanosarcina sp. strain DCM. Appl Environ Microbiol. 1988;54:2976–2980. doi: 10.1128/aem.54.12.2976-2980.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felske A, Rheims H, Wolterink A, Stackebrandt E, Akkermans A D L. Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiology. 1997;143:2983–2989. doi: 10.1099/00221287-143-9-2983. [DOI] [PubMed] [Google Scholar]
- 10.Felske A, Wolterink A, van Lis R, Akkermans A D L. Phylogeny of the main bacterial 16S rRNA sequences in Drentse A grassland soils (The Netherlands) Appl Environ Microbiol. 1998;64:871–879. doi: 10.1128/aem.64.3.871-879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman D L, Gossett J M. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol. 1989;55:2144–2151. doi: 10.1128/aem.55.9.2144-2151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerritse J, Renard V, Pedro Gomes T M, Lawson P A, Collins M D, Gottschal J C. Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethene or ortho-chlorinated phenols. Arch Microbiol. 1996;165:132–140. doi: 10.1007/s002030050308. [DOI] [PubMed] [Google Scholar]
- 13.Holliger C, Schraa G, Stams A J M, Zehnder A J B. A highly purified enrichment culture couples the reductive dechlorination of tetrachloroethene to growth. Appl Environ Microbiol. 1993;59:2991–2997. doi: 10.1128/aem.59.9.2991-2997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holliger C, Schumacher W. Reductive dehalogenation as a respiratory process. Antonie Leeuwenhoek. 1994;66:239–246. doi: 10.1007/BF00871642. [DOI] [PubMed] [Google Scholar]
- 15.Holliger C, Hahn D, Harmsen H, Ludwig W, Schumacher W, Tindall B, Vazquez F, Weiss N, Zehnder A J B. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetrachloroethene in an anaerobic respiration. Arch Microbiol. 1998;169:313–321. doi: 10.1007/s002030050577. [DOI] [PubMed] [Google Scholar]
- 16.Kengen S W M, Stams A J M. Formation of L-alanine as a reduced end product in carbohydrate fermentation by the hyperthermophilic archaeon Pyrococcus furiosus. Arch Microbiol. 1994;161:168–175. [Google Scholar]
- 17.Kröger A, Geisler V, Lemma E, Theis F, Lenger R. Bacterial fumarate respiration. Arch Microbiol. 1992;158:311–314. [Google Scholar]
- 18.Krumholz L. Desulfuromonas chloroethenica sp. nov. uses tetrachloroethylene and trichloroethylene as electron acceptors. Int J Syst Bacteriol. 1997;47:1262–1263. [Google Scholar]
- 19.Maymo-Gatell X, Chien Y, Gossett J M, Zinder S H. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science. 1997;276:1568–1571. doi: 10.1126/science.276.5318.1568. [DOI] [PubMed] [Google Scholar]
- 20.McCarty P L. Breathing with chlorinated solvents. Science. 1997;276:1521–1522. doi: 10.1126/science.276.5318.1521. [DOI] [PubMed] [Google Scholar]
- 21.Nübel U, Engelen B, Felske A, Snaidr J, Wieshuber A, Amann R I, Ludwig W, Backhaus H. Sequence heterogeneity of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol. 1996;178:5636–5643. doi: 10.1128/jb.178.19.5636-5643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng J, Wan A. Measurement of Henry’s constants of high-volatility organic compounds using a headspace autosampler. Environ Sci Technol. 1997;31:2998–3003. [Google Scholar]
- 23.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 24.Rippen G. Handbuch Umweltchemikalien. Landsberg, Germany: Ecomed; 1992. [Google Scholar]
- 25.Scholz-Muramatsu H, Neumann A, Mesmer M, Moore E, Diekert G. Isolation and characterization of Dehalospirillum multivorans gen. nov., spec. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol. 1995;163:48–56. [Google Scholar]
- 26.Sharma P K, McCarty P L. Isolation and characterization of a facultative aerobic bacterium that reductively dehalogenates tetrachloroethene to cis-1,2-dichloroethene. Appl Environ Microbiol. 1996;62:761–765. doi: 10.1128/aem.62.3.761-765.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Lier J B. Ph.D. thesis. Wageningen, The Netherlands: Wageningen Agricultural University; 1995. Thermophilic anaerobic wastewater treatment; temperature aspects and process stability; pp. 10–12. [Google Scholar]
- 28.Vogel T M, McCarty P L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985;49:1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]