Abstract
Background:
Despite substantial attention underscoring the importance of exogenous dietary and endogenous serum cholesterol to human health, thorough evaluation of the associations is lacking. Our study objective was to examine overall and cause-specific mortality in relation to dietary and serum cholesterol, as well as egg consumption, and conduct an updated meta-regression analysis of cohort studies.
Methods:
We conducted a prospective analysis of 27,078 men in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study. Multivariable-controlled cause-specific Cox proportional hazards regression models calculated hazard ratios (HR) and 31-year absolute mortality risk differences (ARD). A systematic review and meta-analysis of cohort studies was also performed (PROSPERO: CRD42021272756).
Results:
Based on 482,316 person-years of follow-up, we identified 22,035 deaths, including 9,110 deaths from cardiovascular disease (CVD). Greater dietary cholesterol and egg consumption were associated with increased risk of overall and CVD mortality. HRs for each additional 300 mg cholesterol intake per day were 1.10 and 1.13 for overall and CVD mortality (respectively), and for each additional 50 g egg consumed daily HRs were 1.06 and 1.09, respectively, for overall and CVD mortality (all P-values<0.0001). After multivariable adjustment, higher serum total cholesterol concentrations were associated with increased risk of CVD mortality (HRs per 1-SD increment: 1.14; P-value<0.0001). The observed associations were generally similar across cohort subgroups. The updated meta-analysis of cohort studies based on 49 risk estimates, 3,601,401 participants, and 255,479 events) showed consumption of one additional 50 g egg daily was associated with significantly increased CVD risk: pooled RR=1.04 (95% CI: 1.00, 1.08); I2=80.1%). In the subgroup analysis of geographical regions (P-value-for-interaction=0.02), an increase of 50 g egg consumed daily was associated with a higher risk of CVD among US cohorts (pooled-RR=1.08, 95% CI: 1.02, 1.14), appeared related to a higher CVD risk in European cohorts with a borderline significance (pooled-RR=1.05), but was not associated with CVD risk in Asian cohorts.
Conclusions:
In this prospective cohort study and updated meta-analysis, greater dietary cholesterol and egg consumption were associated with increased risk of overall and CVD mortality. Our findings support restricted consumption of dietary cholesterol as a means to improve long-term health and longevity.
Keywords: egg consumption, dietary cholesterol intake, serum cholesterol, mortality, systematic review and meta-analysis
Introduction
Despite substantial attention underscoring the importance of exogenous dietary and endogenous serum cholesterol to human health, a thorough, comprehensive examination of their associations with long-term health outcomes is not available. Dietary cholesterol is consumed in foods including eggs, beef, fish, and pork, whereas endogenous serum cholesterol is synthesized in the liver and extrahepatic tissues and circulates in the bloodstream.1 Cholesterol plays an important role in cellular membrane structure and signal transduction, and engages in essential regulatory functions including nutrient absorption, glucose metabolism, reproductive biology, and stress-related responses.1, 2 Furthermore, laboratory evidence demonstrates that cholesterol can have cytotoxic activity; i.e., that cellular cholesterol accumulation can induce membrane disruption, apoptosis, inflammation, and other stress-related responses.2, 3 Earlier experimental and observational studies highlighting the importance of cholesterol homeostasis for proper cellular and physiological functions supported the hypothesis that impairment of cholesterol metabolism can be involved in the development of chronic diseases including cardiovascular disease (CVD) and cancer.4–7
As a result, longstanding dietary guidelines recommended a daily limit of 300 mg for dietary cholesterol to improve cardiovascular health.8 However, the Scientific Report of the 2015 Dietary Guidelines Advisory Committee states that (1) “cholesterol intake need not be limited because there is only a weak relationship between cholesterol intake and serum cholesterol concentrations”, (2) “maintain a minimum dietary cholesterol while consuming a healthy eating pattern”, and (3) “egg consumption should be considered part of a healthy diet”.9, 10 As a very common and affordable food, eggs are one of the main sources of dietary cholesterol, with 186 mg of cholesterol in a large-size boiled egg, while at the same time eggs also contain a wide range of high quality nutrients, including protein, fatty acids, vitamins and minerals.11 The updated Scientific Report of the 2020 Dietary Guidelines Advisory Committee mentioned that 1) “it seems prudent to recommend lower intake of foods high in dietary cholesterol”, and 2) “the lack of studies evaluating a number of outcomes highlights the need for additional research (on dietary cholesterol)”.
For over two decades, epidemiological studies have evaluated the associations between higher dietary cholesterol and egg consumption and disease risk, with conflicting findings. Some studies demonstrate increased risk of CVD 6, 7, 12 and mortality,6, 13–15 while other show null associations for CVD 15–17 and mortality,17–19 or inverse associations.20, 21 The reported risk estimates and directionality vary considerably, however, in part according to study design, number of events, source population, consumption levels, control for confounding, and length of follow-up, leaving the associations unclear.
In an effort to provide evidence from a more comprehensive assessment relevant to dietary guidelines and healthy dietary patterns, we examined the associations between dietary cholesterol, serum cholesterol, and egg consumption and risk of overall and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study of 27,000 participants followed for more than three decades. Based on the findings, we performed an updated meta-analysis of the association between egg consumption and risk of CVD and CVD mortality, that included the current study.
Methods
Data Sharing
Because of previously enacted EU Data Protection Regulation (GDPR) privacy rules and an existing data use agreement between Finland and the U.S. National Cancer Institute, the ATBC Study data and materials described in the manuscript may not be made indiscriminately publicly available for the purposes of reproducing the findings. Please contact the ATBC Study Principal Investigators for appropriate specific data requests (https://atbcstudy.cancer.gov/). Also, in order to minimize the possibility of unintentionally sharing information that can be used to re-identify private information, a subset of the data generated for this study would be made available first upon reasonable request.
Study Population
The ATBC Study was a controlled, 2×2 factorial primary prevention trial originally conducted to evaluate whether supplementation with alpha-tocopherol (50 mg/d), beta-carotene (20 mg/d), or both, could decrease cancer incidence. The ATBC Study recruited 29,133 Finnish male smokers between 1985 and 1988, aged 50 to 69 years, from 14 study centers in southwest Finland. Intervention continued for 5 to 8 years, until the end of intervention (April 30, 1993). Overnight fasting blood samples were collected and stored at −70 °C, and blood pressure, height and weight were measured by skilled research nurses. The study was approved by institutional review boards at the US National Cancer Institute and the Finnish National Public Health Institute. All cohort participants provided written informed consent.
Exposure Assessment
Serum total cholesterol and HDL (high-density lipoprotein) concentrations were determined enzymatically (CHODPAP method, Boehringer Mannheim). Serum concentrations of alpha-tocopherol, beta-carotene, and retinol were assayed by high-performance liquid chromatography.22 Cohort members were invited to complete a food frequency questionnaire, which included dietary information on portion size and frequency of 276 food and beverage items in the past 12 months. In the food frequency questionnaire, participants were asked: (1) How often did you consume eggs, including boiled eggs, fried eggs or omelettes in the past 12 months: 1= More than once a day; 2= Once a day; 3= Nearly every day; 4= Several times a week; 5= Once a week; 6= Once or several times a month; 7= Rarely or Never. (2) How many eggs did you eat each time. A color picture booklet was distributed to all participants for assistance in portion size estimation. Daily nutrient intakes were calculated using the food composition database of the Finnish National Public Health Institute.23 The validity and reproducibility of the food use questionnaire has been previously examined and reported, with intraclass correlation coefficients ranging from 0.6–0.7 for most dietary variables, including 0.66 and 0.58 for dietary cholesterol intake and egg consumption, respectively.24 In the ATBC Study, 27,111 participants (93%) completed the FFQ thoroughly for subsequent analysis. In the present study, 27,078 participants were retained in the final analytical cohort after excluding individuals with missing values for serum total cholesterol (n=36) or dietary cholesterol or egg consumption (n=2,022). The primary dietary sources for cholesterol intake (mean percentage of total daily dietary cholesterol) included eggs (43.6%), butter (13.2%), milk (8.2%), sausages (7.4%), fish (5.9%), pork (5.5%), cheese (3.3%), beef (2.9%) and other food items combined (10.1%).
Cohort Follow-up and Mortality Assessment
Participants were followed from their study entry date in 1985 to 1988 until death or the end of follow-up (December 31, 2015), whichever came first. Vital status of participants was determined through linkage to the Causes of Death Registry, Statistics Finland (for details, see Methods in Supplemental Data).
Statistical Analysis
We used age-stratified, cause-specific Cox proportional hazards regression models (cause-specific hazard models to control for the competing risks) with attained person-time as the underlying time metric to determine hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for the associations between dietary cholesterol (per 300 mg/day), serum total cholesterol (per 1-SD), or egg consumption (per 50 g/day) and risk of death, including overall and cause-specific mortality, respectively. For the latter analysis, mortality other than the outcome of interest was censored by the date of death. No violations were found for the tests of the proportional hazards assumption that modelled the interaction between intervals of follow-up observation time (categorial variable) and dietary cholesterol, serum total cholesterol, or egg consumption (modelled linearly). Regarding the functional forms, a squared term of the given covariate (test for all continuous covariates) was included in the multivariable-adjusted model, to check if model fit was significantly improved, or risk estimate was significantly changed. In these cases, we found that model fit and risk estimates did not change. The age-adjusted model included baseline age and total energy intake. Multivariable models were further adjusted for body mass index (BMI), cigarettes smoked per day, years of smoking, serum HDL cholesterol, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, history of diabetes, education, physical activity, serum concentrations of alpha-tocopherol, beta-carotene and retinol, and alcohol, and percentage of energy from protein, carbohydrates, saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids. Mutual adjustment was performed for dietary cholesterol and serum total cholesterol. For egg consumption, regression models with or without dietary cholesterol were conducted separately. For each obtained HR from the primary models, adjusted absolute risk differences (ARDs) were computed for the tested exposure variables at the end of follow-up of 31 years. The corresponding 95% CIs for each ARD were estimated using 300 bootstrap samples.
We conducted stratified analyses of overall and CVD mortality based on other exposure variables (for details, see Methods in Supplemental Data).We further performed eight sensitivity analyses: (1) To control residual confounding, we adjusted for propensity scores that reflected the associations between the primary exposure variables and the above-mentioned potential confounding covariates.25 (2) To decrease reverse causality bias, we excluded the first 2 and 5 years of follow-up. (3) To minimize bias from potential influence from preexisting illness on exposure variables, we excluded individuals with self-reported history of diabetes at baseline. (4) To test more parsimonious models, we adjusted for age, cigarettes smoked per day, years of smoking, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, education, physical activity, levels of serum alpha-tocopherol, beta-carotene and retinol, and daily dietary total energy and alcohol. (5) To winsorize the distributions of dietary cholesterol, serum total cholesterol, and egg consumption at the 0.5 and 99.5 percentiles before modeling. (6) To obtain risk estimates using different increment units: each additional 50 mg dietary cholesterol per day up to 600 mg per day; each additional 0.5 mmol/L (19.34 mg/dL) up to 3 mmol/L (116 mg/dL) serum total cholesterol; and, each additional 25 g of eggs consumed per day up to 200 g per day. (7) To estimate mortality risk for dietary cholesterol, serum total cholesterol and egg consumption using quintile categories. (8) To apply diet/nutrient density models, dietary cholesterol and egg consumption were regressed on total energy intake (grams per 1,000 kcal) along with energy intake in the models. (9) To control for potential confounding from specific foods, we further adjusted for daily intakes of vegetables, fruit, legumes, whole grains, red and processed meat, fish and potatoes (as quintile categories) in the models.
Although missing values for all covariables were less than 5% of the study population, a missing value indicator variable was generated for each before modeling. All analyses were conducted using SAS software, version 9.4 (SAS Institute Inc.). All reported P values are two-sided at a type I error rate of 0.05. To control for multiple comparisons, the Bonferroni correction threshold was used to define statistical significance: 0.05/15=0.0033 for primary and secondary tests [5 tests for three examined exposure variables], and 0.05/14=0.0036 for the interaction tests in stratified subgroups.
Systematic review and meta-analysis of associations between egg consumption and risk of CVD and CVD mortality
We performed a systematic search and updated meta-analysis including the present data, as well as previous prospective cohort analyses that examined the association of egg consumption with risk of CVD and CVD mortality in the general population. The meta-analysis was conducted based on the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA),26 and the systematic review protocol was registered at the international prospective register (PROSPERO: CRD42021272756). The systematic search was completed through 5 August 2021 of online databases, including Web of Science, PubMed, and Embase.
Two reviewers (LG and JH) performed article searches independently based on predefined criteria. Data were obtained based on the eligible articles, including first author name, publication year, study population and cohort name, country where the cohort was conducted, sample size, follow-up duration, baseline age ranges of participants, approaches for dietary assessment, methods for outcome ascertainment, egg consumption categories, and adjustment for potential confounders. We further extracted data for risk estimates and their 95% confidence intervals from the fully adjusted models (for details, see Methods in Supplemental Data). In terms of egg consumption, we used the median value or the midpoint of upper and lower bound of the intake category when data were available. If the upper bound was not reported for the highest category, the upper bound was estimated by multiplying lower bound by 1.75. In the meta-analysis, we used lowest category of egg consumption as the reference group.27 When the included studies did not present person-years for each consumption category, we imputed it based on available data.6, 15, 17, 19, 28–33 When the dose-response estimates were missing for a study, we computed the relevant estimates of relative risk (RR) based on the trend of log-relative risk.34
We calculated the RR of CVD (including CVD mortality) associated with egg consumed per day (per 50 gram/day) for each study, and used the random-effects models of meta-analysis to compute the pooled relative risk estimate. Heterogeneity was examined using the Cochran Q-test and the I2 statistic. Each individual study was excluded from the overall meta-analysis individually and the RRs were recalculated to determine which if any studies drove the heterogeneity. Univariate meta-regression was conducted using study-level data to evaluate potential causes of heterogeneity. We further conducted subgroup meta-analysis stratifying by sex, number of participants, duration of follow-up, number of events, geographical location, risk of bias, dietary assessment, as well as CVD outcome (incident CVD and CVD mortality).
Egger’s tests and funnel plots were computed to examine potential publication bias. We applied the Newcastle-Ottawa scale to evaluate possible biases for the eligible studies. Stata version 16.0 was used to conduct statistical analyses for the meta-analysis.
Results
Median daily dietary cholesterol intake and egg consumption in ATBC were 538 mg (mean value: 582 mg) and 44.6 g (mean value: 53.3 g), respectively, and median serum total cholesterol was 240 mg/dL (6.2 mmol/L). Participants with greater cholesterol intake were more likely to have lower serum vitamin E concentrations, less education, and lower prevalence of diabetes and CVD (Table 1, Table I in the Supplemental Data; for details, see Results in Supplemental Data). Spearman correlation coefficients for dietary cholesterol, serum total cholesterol and egg consumption and a spectrum of dietary factors are presented in Table II in the Supplemental Data.
Table 1.
Baseline Characteristics of Cohort Participants According to Quintile Categories of Dietary Cholesterol, Serum Total Cholesterol, and Egg Consumption in the ATBC Study *
| Dietary cholesterol (quintile) | Serum total cholesterol (quintile) | Egg consumption (quintile) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 1 | 3 | 5 | 1 | 3 | 5 | |
| Age, y | 57.7 (5.2) | 57.2 (5.1) | 56.5 (4.8) | 57.5 (5.2) | 57.0 (5.0) | 57.0 (4.9) | 57.7 (5.2) | 57.2 (5.0) | 56.5 (4.8) |
| Cigarettes/day | 19.5 (8.7) | 20.4 (8.7) | 21.8 (9.1) | 20.6 (9.0) | 20.2 (8.8) | 20.4 (8.7) | 20.1 (8.9) | 20.2 (8.6) | 21.4 (9.0) |
| Years of smoking, y | 36.1 (8.8) | 36.0 (8.3) | 35.6 (8.3) | 36.2 (8.7) | 35.7 (8.4) | 35.9 (8.3) | 36.4 (8.7) | 36.0 (8.3) | 35.5 (8.3) |
| Systolic blood pressure, mm Hg | 143 (26) | 142 (20) | 141 (19) | 141 (23) | 142 (23) | 143 (19) | 143 (26) | 142 (19) | 142 (19) |
| Diastolic blood pressure, mm Hg | 88 (21) | 87 (11) | 88 (11) | 87 (16) | 88 (16) | 88 (11) | 88 (24) | 87 (11) | 88 (11) |
| Serum total cholesterol, mg/dl | 239.3 (46.0) | 241.2 (65.7) | 242.0 (44.1) | 181.7 (17.8) | 239.7 (6.2) | 305.4 (30.2) | 243.6 (46.4) | 239.7 (46.4) | 239.7 (42.5) |
| Serum HDL cholesterol, mg/dl | 47.6 (89.7) | 46.8 (53.0) | 47.2 (12.4) | 46.4 (53.0) | 46.0 (12.0) | 47.6 (73.5) | 45.6 (12.4) | 46.0 (12.4) | 46.8 (12.4) |
| Serum alpha-tocopherol, mg/L | 12.4 (4.2) | 11.9 (3.4) | 11.6 (3.3) | 9.3 (2.3) | 11.8 (2.5) | 15.0 (4.6) | 12.1 (4.1) | 11.9 (3.3) | 11.9 (3.5) |
| Serum beta-carotene, μg/L | 211 (226) | 212 (161) | 210 (171) | 167 (148) | 212 (173) | 255 (194) | 219 (222) | 211 (168) | 201 (166) |
| Serum retinol, μg/L | 588 (137) | 586 (125) | 591 (131) | 546 (127) | 591 (124) | 627 (132) | 586 (134) | 587 (126) | 593 (133) |
| BMI, kg/m2 | 26.2 (4.2) | 26.4 (4.4) | 26.6 (4.4) | 26.1 (4.6) | 26.3 (3.8) | 26.5 (3.9) | 26.1 (4.6) | 26.3 (3.8) | 26.5 (3.9) |
| Education, % >elementary school | 24.7 | 21.2 | 18.8 | 23.6 | 21.4 | 19.9 | 22.1 | 22.4 | 21.8 |
| Physically active, % | 19.4 | 22.7 | 20.9 | 19.6 | 21.1 | 21.5 | 19.8 | 21.6 | 20.6 |
| History of CVD, % | 47.6 | 39.7 | 38.0 | 42.2 | 41.3 | 41.3 | 46.9 | 40.4 | 39.3 |
| History of diabetes mellitus, % | 5.6 | 4.0 | 3.8 | 6.0 | 3.5 | 3.6 | 5.2 | 3.7 | 4.1 |
| Daily dietary intake | |||||||||
| Energy, kcal | 2,005 (447) | 2,632 (488) | 3,485 (793) | 2,685 (764) | 2,694 (750) | 2,680 (738) | 2,267 (594) | 2,663 (646) | 3,173 (835) |
| Energy from saturated fatty acids, % | 14.8 (4.1) | 17.7 (4.1) | 19.1 (4.1) | 16.8 (4.4) | 17.4 (4.3) | 17.9 (4.4) | 17.0 (4.9) | 17.5 (4.2) | 17.5 (4.1) |
| Alcohol, g | 17.2 (22.5) | 17.4 (20.6) | 20.3 (22.7) | 18.5 (22.9) | 18.1 (21.6) | 17.3 (21.1) | 17.0 (22.0) | 17.4 (20.5) | 20.7 (22.8) |
| Fruit, g | 106 (91) | 126 (94) | 155 (117) | 131 (102) | 130 (102) | 126 (100) | 109 (94) | 127 (97) | 149 (114) |
| Vegetables, g | 100 (68) | 113 (67) | 131 (78) | 115 (73) | 114 (71) | 112 (69) | 100 (69) | 113 (67) | 127 (78) |
| Red meat, g | 54.1 (23.9) | 71.0 (30.6) | 89.2 (41.6) | 69.6 (33.6) | 71.3 (33.8) | 73 (34.6) | 61.7 (30.9) | 70.9 (32.0) | 80.8 (38.1) |
| Alternate Mediterranean Diet Score | 25.9 (5.1) | 24.9 (5.0) | 24.1 (5.0) | 24.9 (5.2) | 25.0 (5.0) | 25.0 (5.1) | 24.9 (5.3) | 24.9 (5.0) | 24.8 (5.0) |
Values are means (SD) or percentages as indicated.
Based on 31 years of cohort follow-up, an average of 18.2 years and 482,316 person-years, there were 22,035 deaths, including 9,110 from CVD (7,450 heart disease and 1,621 strokes), and 7,213 deaths from cancer. Each additional 300 mg dietary cholesterol daily intake increment was significantly associated with increased risk of age-adjusted overall, CVD, heart disease and cancer mortality, representing 8%–10% higher risks. Adjustment for several other risk factors strengthened the cholesterol-mortality risk estimates somewhat, with HRs of 1.10, 1.13 and 1.13 for overall, CVD and heart disease, respectively (all P values <0.0001). Corresponding adjusted ARDs (95% CIs) per each additional 300 mg cholesterol daily intake at 31 years of follow-up were of 1.80% (1.23%, 2.39%), 1.83% (1.14%, 2.48%) and 1.76% (1.19%, 2.45%) for mortality from overall, CVD and heart disease, respectively. By contrast, the multivariable-adjusted positive association between cholesterol intake and cancer and stroke mortality did not reach the Bonferroni correction P-value threshold of 0.0033 (Table 2).
Table 2.
Associations Between Dietary Cholesterol, Serum Total Cholesterol and Overall and Cause-Specific Mortality in the ATBC Study
| Cause of death | Dietary cholesterol (per 300 mg) * | Serum total cholesterol (per 1-SD) † | ||||
|---|---|---|---|---|---|---|
| ARD, % (95% CI) | HR (95% CI) | P value | ARD, % (95% CI) | HR (95% CI) | P value | |
| All causes | ||||||
| Age adjusted | 1.83 (1.41, 2.27) | 1.09 (1.07, 1.12) | <0.0001 | −0.48 (−0.79, −0.21) | 0.98 (0.96, 0.99) | 0.0008 |
| Multivariable ‡ | 1.80 (1.23, 2.39) | 1.10 (1.06, 1.13) | <0.0001 | 0.35 (−0.085, 0.91) | 1.02 (1.00, 1.04) | 0.05 |
| CVD | ||||||
| Age adjusted | 1.48 (0.95, 1.99) | 1.10 (1.06, 1.14) | <0.0001 | 1.26 (0.90, 1.59) | 1.08 (1.06, 1.11) | <0.0001 |
| Multivariable ‡ | 1.83 (1.14, 2.48) | 1.13 (1.08, 1.18) | <0.0001 | 1.96 (1.57, 2.40) | 1.14 (1.11, 1.17) | <0.0001 |
| Heart disease | ||||||
| Age adjusted | 1.40 (0.87, 1.92) | 1.10 (1.06, 1.14) | <0.0001 | 1.64 (1.29, 1.98) | 1.12 (1.10, 1.15) | <0.0001 |
| Multivariable ‡ | 1.76 (1.19, 2.45) | 1.13 (1.08, 1.19) | <0.0001 | 2.09 (1.71, 2.51) | 1.16 (1.13, 1.20) | <0.0001 |
| Stroke | ||||||
| Age adjusted | 0.45 (−0.03, 0.88) | 1.08 (0.99, 1.17) | 0.069 | −0.52 (−0.84, −0.22) | 0.92 (0.87, 0.96) | 0.0007 |
| Multivariable ‡ | 0.55 (−0.13, 1.13) | 1.10 (0.99, 1.22) | 0.088 | 0.17 (−0.26, 0.59) | 1.03 (0.96, 1.10) | 0.40 |
| Cancer | ||||||
| Age adjusted | 1.18 (0.62, 1.76) | 1.08 (1.04, 1.13) | <0.0001 | −0.79 (−1.19, −0.41) | 0.95 (0.93, 0.97) | <0.0001 |
| Multivariable ‡ | 0.87 (0.17, 1.63) | 1.06 (1.01, 1.12) | 0.02 | −0.59 (−1.12, −0.018) | 0.96 (0.93, 0.99) | 0.0083 |
Abbreviations: ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention, CVD = cardiovascular disease, HDL = high-density lipoprotein, HR = hazard ratio, SD = standard deviation
Absolute risk differences and hazard ratios (HRs) of overall and cause-specific mortality are for each 300 mg increment of dietary cholesterol consumption per day.
Absolute risk differences and HRs of cardiovascular disease (CVD) mortality are for 1-SD increment of serum total cholesterol.
Models adjusted for baseline age, body mass index, cigarettes smoked per day, years of smoking, serum HDL cholesterol, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, diabetes, education, physical activity, levels of serum alpha-tocopherol, beta-carotene and retinol, and daily dietary total energy, alcohol, and percentage of energy from protein, carbohydrates, saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids. Mutual adjustment was performed for dietary cholesterol and serum total cholesterol. Total event number for all-cause-, CVD-, heart disease-, stroke-, and cancer-death is 22035, 9110, 7450, 1621 and 7213, respectively.
Higher concentrations of serum total cholesterol were significantly inversely associated with age-adjusted mortality from overall, stroke and cancer, but positively associated with CVD and heart disease (Table 2). Following adjustment for several potential confounding factors, however, the associations for overall and stroke mortality were attenuated and that with cancer mortality did not achieve the Bonferroni correction threshold. By contrast, multivariable adjustment strengthened the positive risk estimates for CVD and heart disease mortality, with the HRs (95% CIs) per 1-SD increment of serum total cholesterol being 1.14 (1.11, 1.17) and 1.16 (1.13, 1.20), respectively (all P values <0.0001). At the time of follow-up through 31 years, the corresponding adjusted ARDs (95% CIs) per 1-SD increment were of 1.96% (1.57%, 2.40%) and 2.09% (1.71%, 2.51%) for mortality from CVD and heart disease, respectively (Table 2).
Consumption of one additional 50 g egg per day was associated with significantly increased age-adjusted overall, CVD and heart disease mortality (Table 3). After multivariable adjustment (model 2), the respective risk estimates of 6%, 9% and 9% remained statistically significant (all P values<0.0001), with corresponding adjusted ARDs of 1.19% (95% CIs: 0.75%, 1.65%), 1.25% (95% CIs: 0.72%, 1.74%) and 1.20% (95% CIs: 0.69%, 1.71%). After further adjustment for dietary cholesterol (model 3), the egg consumption associations with overall, CVD and heart disease mortality were no longer significant, however, with HRs of 0.91, 0.92 and 0.91 (all P values >0.0033). Egg consumption was not related to stroke or cancer mortality (all P values >0.0033) (Table 3).
Table 3.
Associations Between Daily Egg Consumption and Overall and Cause-Specific Mortality in the ATBC Study
| Cause of death | Egg consumption (per 50 g/day) * | ||
|---|---|---|---|
| ARD, % (95% CI) | HR (95% CI) | P value | |
| All causes | |||
| Model 1: Age adjusted | 1.18 (0.81, 1.56) | 1.06 (1.04, 1.08) | <0.0001 |
| Model 2: Multivariable † | 1.19 (0.75, 1.65) | 1.06 (1.04, 1.09) | <0.0001 |
| Model 3: Multivariable ‡ | −1.83 (−3.50, −0.14) | 0.91 (0.84, 0.99) | 0.029 |
| CVD | |||
| Model 1: Age adjusted | 1.00 (0.55, 1.44) | 1.07 (1.03, 1.10) | <0.0001 |
| Model 2: Multivariable † | 1.25 (0.72, 1.74) | 1.09 (1.05, 1.12) | <0.0001 |
| Model 3: Multivariable ‡ | −1.33 (−3.51, 0.62) | 0.92 (0.81, 1.04) | 0.18 |
| Heart disease | |||
| Model 1: Age adjusted | 0.94 (0.49, 1.38) | 1.07 (1.03, 1.10) | <0.0001 |
| Model 2: Multivariable † | 1.20 (0.69, 1.71) | 1.09 (1.05, 1.13) | <0.0001 |
| Model 3: Multivariable ‡ | −1.34 (−3.57, 0.70) | 0.91 (0.79, 1.05) | 0.19 |
| Stroke | |||
| Model 1: Age adjusted | 0.32 (−0.11, 0.70) | 1.06 (0.98, 1.13) | 0.13 |
| Model 2: Multivariable † | 0.37 (−0.15, 0.80) | 1.06 (0.98, 1.15) | 0.13 |
| Model 3: Multivariable ‡ | −0.46 (−2.50, 1.41) | 0.93 (0.68, 1.25) | 0.62 |
| Cancer | |||
| Model 1: Age adjusted | 0.58 (0.087, 1.10) | 1.04 (1.01, 1.08) | 0.021 |
| Model 2: Multivariable † | 0.56 (0.022, 1.16) | 1.04 (1.00, 1.08) | 0.047 |
| Model 3: Multivariable ‡ | −1.04 (−3.10, 1.12) | 0.93 (0.81, 1.08) | 0.34 |
Abbreviations: ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention, CVD = cardiovascular disease, HDL = high-density lipoprotein, HR = hazard ratio, SD = standard deviation
Absolute risk differences and HRs of overall and cause-specific mortality are for each 50 g increment of egg consumption per day.
Models were adjusted for age, body mass index, cigarettes smoked per day, years of smoking, serum total and HDL cholesterol, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, diabetes, education, physical activity, levels of serum alpha-tocopherol, beta-carotene and retinol, and daily dietary total energy, alcohol, and percentage of energy from protein, carbohydrates, saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids. Total event number for all-cause-, CVD-, heart disease-, stroke-, and cancer-death is 22035, 9110, 7450, 1621 and 7213, respectively.
Models were further adjusted for dietary cholesterol.
Figures 1 (a, b, c)–2 (a, b) present cohort subgroup mortality findings for dietary cholesterol, serum total cholesterol and egg consumption. Overall, we observed similar risk estimates across strata of age, cigarettes per day, BMI, cardiovascular disease history, trial intervention arms, diet quality, total energy intake, dietary cholesterol, saturated fatty acid intake, serum total cholesterol, serum concentrations of alpha-tocopherol, beta-carotene, and retinol, and years of follow-up (all P values >0.0036, the Bonferroni correction threshold).
Figure 1. Association between Cholesterol and Mortality by Selected Risk Factors in the ATBC Study.
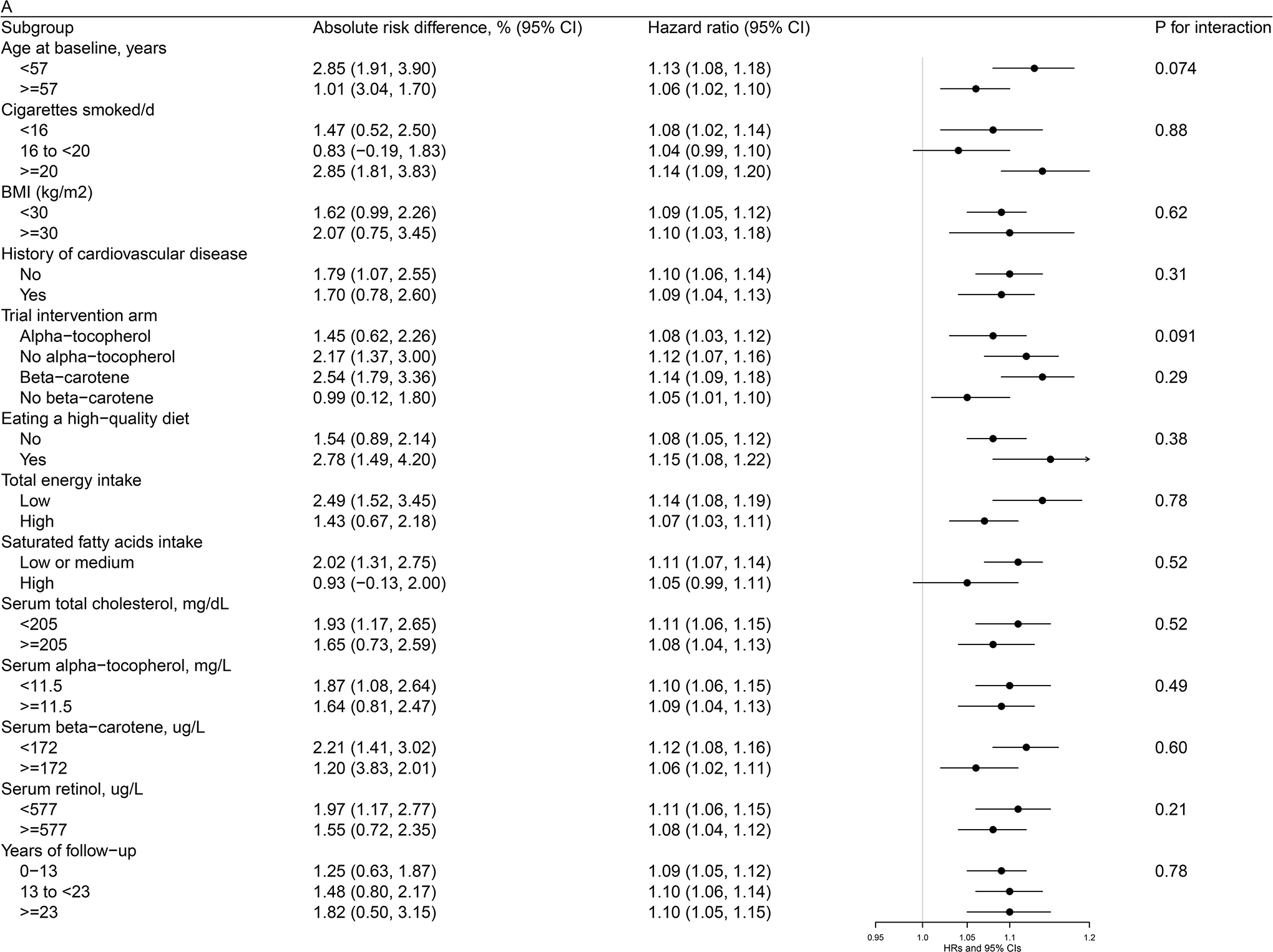
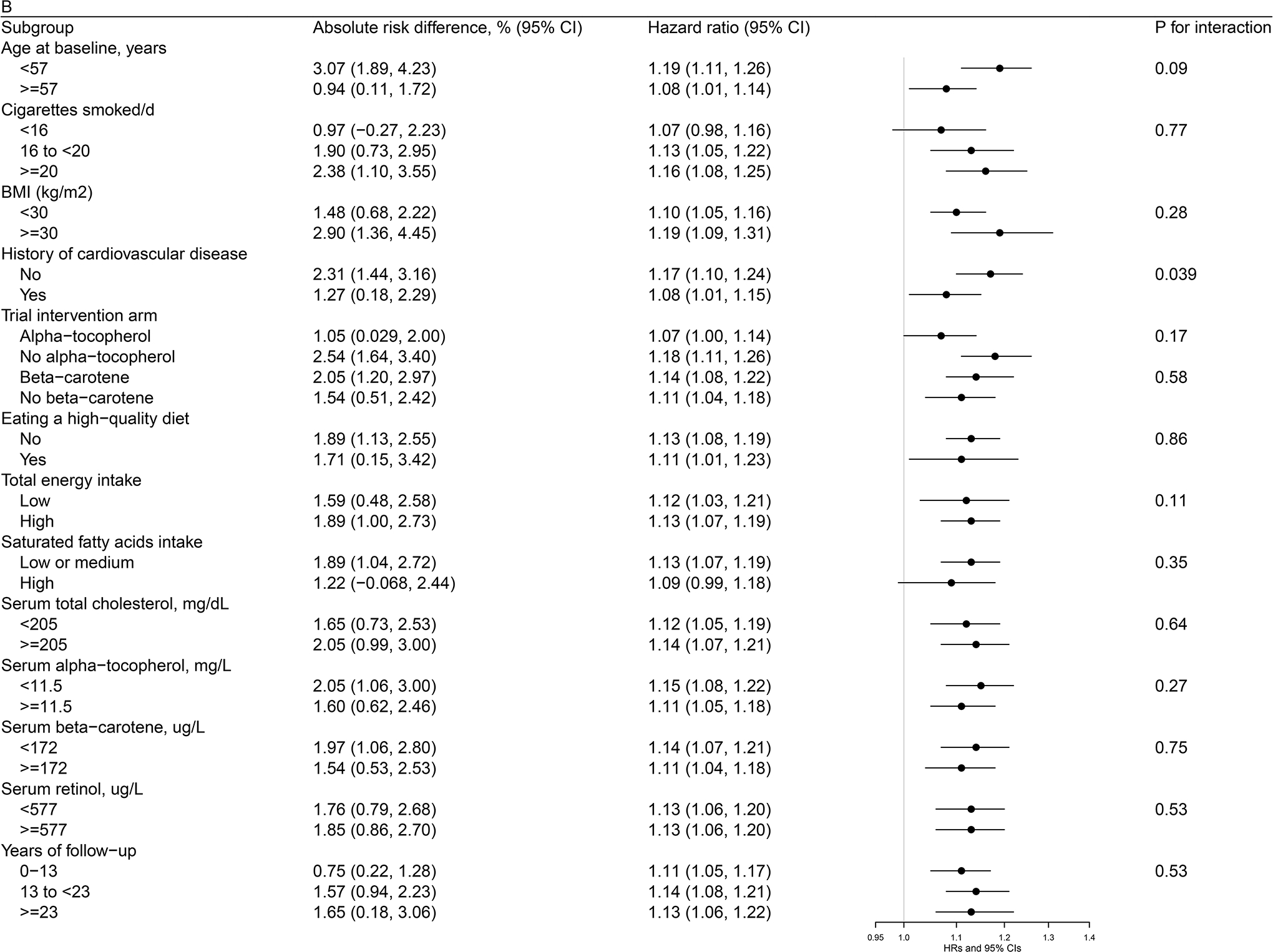
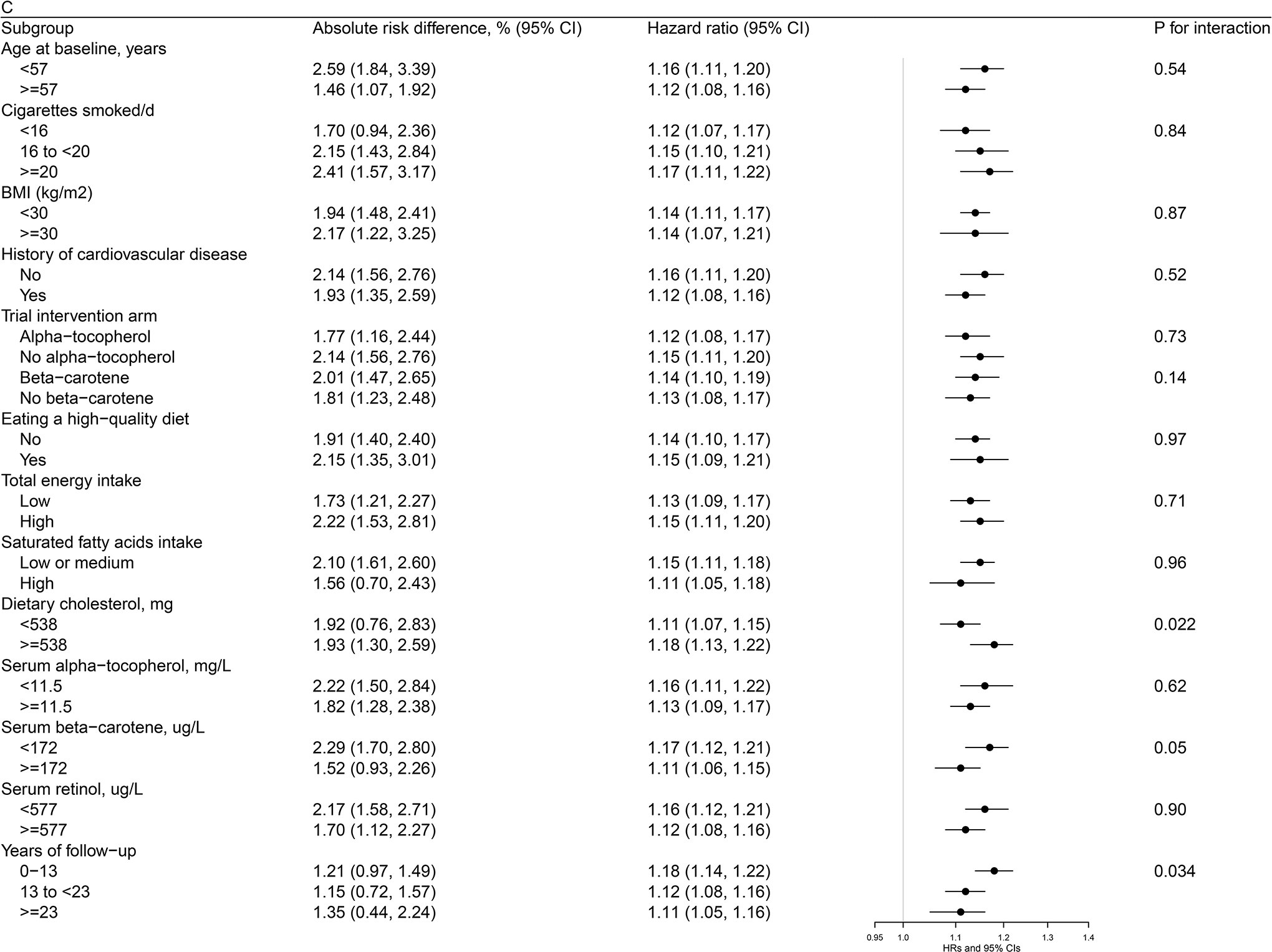
Figure 1a. Association between Dietary Cholesterol Intake (per day) and Overall Mortality by Selected Risk Factors in the ATBC StudyAbbreviations: ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention, HDL = high-density lipoprotein, HR = hazard ratioAbsolute risk differences and HRs of overall mortality are for each 300 mg increment of dietary cholesterol consumption per day. Models were adjusted for age, body mass index, cigarettes smoked per day, years of smoking, serum total and HDL cholesterol, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, diabetes, education, physical activity, levels of serum alpha-tocopherol, beta-carotene and retinol, and dietary intakes of energy, alcohol, and percentage of energy from protein, carbohydrates, saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids. Figure 1b. Association between Dietary Cholesterol Intake (per day) and CVD Mortality by Selected Risk Factors in the ATBC StudyAbbreviations: ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention, CVD = cardiovascular disease, HDL = high-density lipoprotein, HR = hazard ratioAbsolute risk differences and HRs of CVD mortality are for each 300 mg increment of dietary cholesterol consumption per day. Models were adjusted for age, body mass index, cigarettes smoked per day, years of smoking, serum total and HDL cholesterol, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, diabetes, education, physical activity, levels of serum alpha-tocopherol, beta-carotene and retinol, and dietary intakes of energy, alcohol, and percentage of energy from protein, carbohydrates, saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids. Figure 1c. Association between Serum Total Cholesterol (per 1-SD) and Cardiovascular Disease Mortality by Selected Risk Factors in the ATBC StudyAbbreviations: ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention, CVD = cardiovascular disease, HDL = high-density lipoprotein, HR = hazard ratio, SD = standard deviationAbsolute risk differences and HRs of CVD mortality are for 1-SD increment of serum total cholesterol. Models were adjusted for age, body mass index, cigarettes smoked per day, years of smoking, serum HDL cholesterol, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, diabetes, education, physical activity, levels of serum alpha-tocopherol, beta-carotene and retinol, and dietary intakes of energy, alcohol, and percentage of energy from protein, carbohydrates, saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids.
Figure 2. Association between Egg Consumption and Mortality by Selected Risk Factors in the ATBC Study.
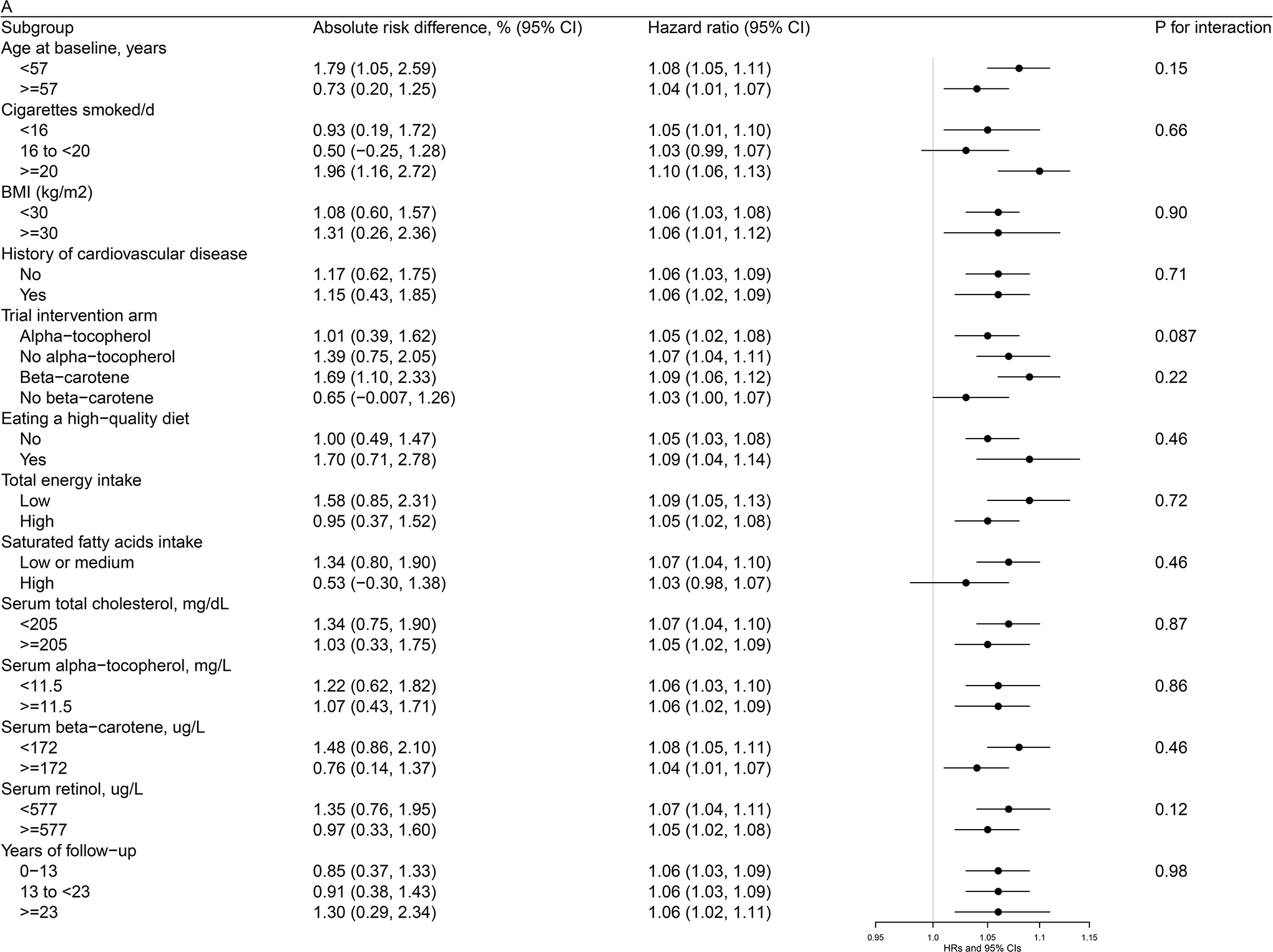
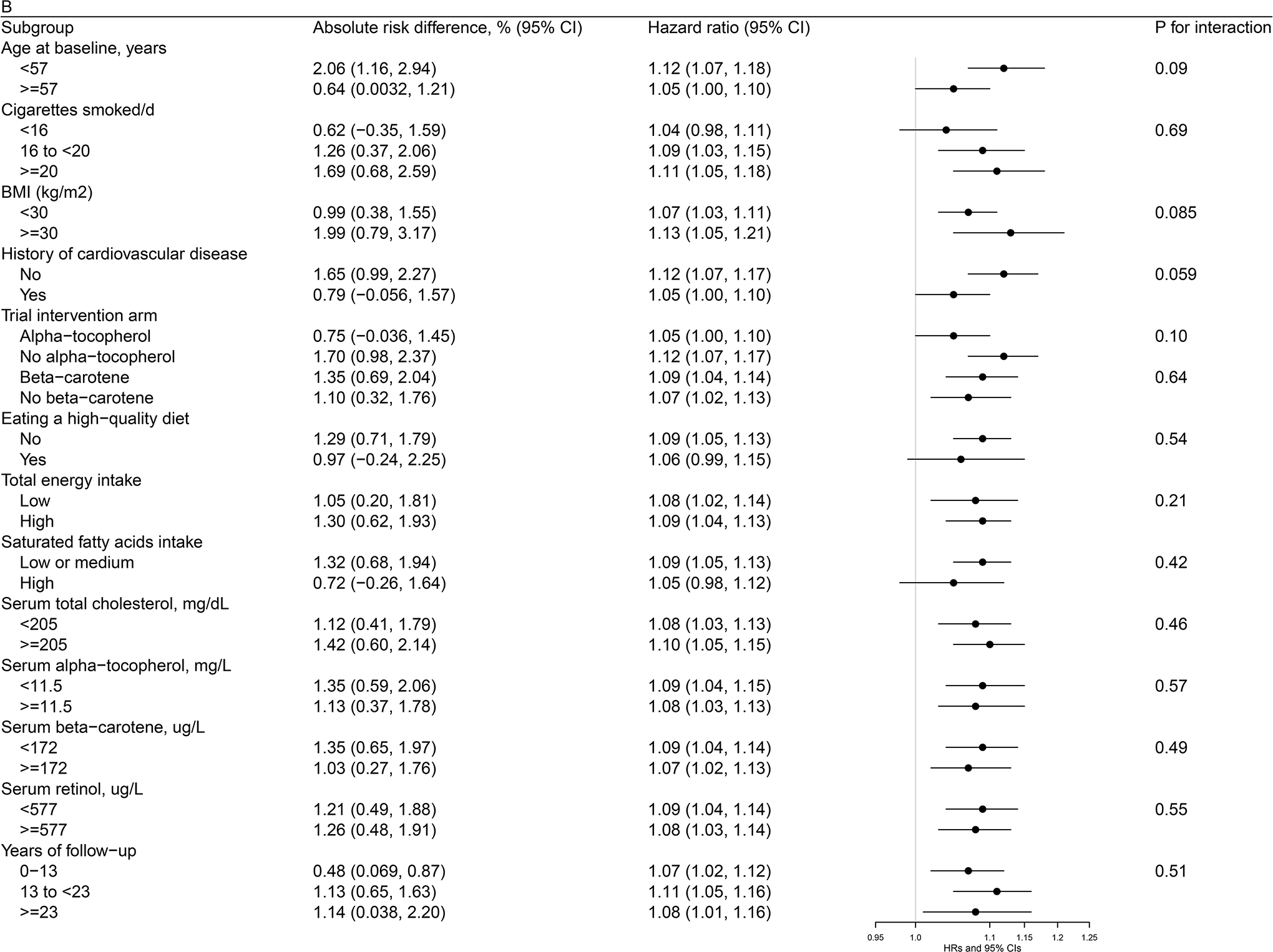
Figure 2a. Association between Egg Consumption (per day) and Overall Mortality by Selected Risk Factors in the ATBC StudyAbbreviations: ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention, HDL = high-density lipoprotein, HR = hazard ratioAbsolute risk differences and HRs of overall mortality are for each 50 g increment of egg consumption per day. Models were adjusted for age, body mass index, cigarettes smoked per day, years of smoking, serum total and HDL cholesterol, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, diabetes, education, physical activity, levels of serum alpha-tocopherol, beta-carotene and retinol, and dietary intakes of energy, alcohol, and percentage of energy from protein, carbohydrates, saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids.Figure 2b. Association between Egg Consumption (per day) with CVD Mortality by Selected Risk Factors in the ATBC StudyAbbreviations: ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention, CVD = cardiovascular disease, HDL = high-density lipoprotein, HR = hazard ratioAbsolute risk differences and HRs of CVD mortality are for each 50 g increment of egg consumption per day. Models were adjusted for age, body mass index, cigarettes smoked per day, years of smoking, serum total and HDL cholesterol, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, diabetes, education, physical activity, levels of serum alpha-tocopherol, beta-carotene and retinol, and dietary intakes of energy, alcohol, and percentage of energy from protein, carbohydrates, saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids.
Propensity score adjustment did not materially change the risk estimates of dietary cholesterol, serum total cholesterol or egg consumption with overall and CVD mortality (all P values<0.0001; Table III in the Supplemental Data). All risk estimates remained essentially the same in the lag analyses (all P values<0.0001; Table IV in the Supplemental Data). Excluding participants with self-reported CVD histories also did not alter the observed associations (all P values<0.0001; Table V in the Supplemental Data). Our findings remained essentially unchanged using parsimonious models (Table VI in the Supplemental Data) or Winsorized distributions of dietary cholesterol, serum total cholesterol and egg at the 0.5 and 99.5 percentiles (Table VII in the Supplemental Data). The multivariable-adjusted HRs and corresponding 95% CIs of overall and CVD mortality according to gradual increment units for dietary cholesterol, serum total cholesterol and egg consumption are presented in the Tables VIII–X in the Supplemental Data. Our findings remained largely unchanged according to the quintiles of exposure variables (P for trend≤0.003; Table XI in the Supplemental Data). Quintile models for dietary cholesterol and egg consumption were further adjusted for energy intake using the nutrient density method, and our findings were not materially altered (P for trend≤0.0002; Table XII in the Supplemental Data). Our findings remained essentially unchanged after further adjustment for specific foods including vegetables, fruit, legumes, whole grains, red and processed meat, fish and potatoes (Supplemental Table XIII) (for details, see Results in Supplemental Data).
Systematic review and meta-analysis
Overall, our initial search included 1,036 articles, and 40 studies (41 including the present study) met the predefined inclusion criteria and were kept in the final meta-analysis (Figure I in the Supplemental Data and Tables XIV–XV in the Supplemental Data). The covariates in the multivariable models of each individual study were presented in Tables XV–XVI in the Supplemental Data. For the Newcastle-Ottawa scale assessment, 17 studies (20 risk estimates) received a score of seven or greater which suggested low risk of bias (Table XVII in the Supplemental Data).
The meta-analysis for the association of daily egg consumption and risk of CVD (including CVD mortality) contained 49 risk estimates, 3,601,401 participants, and 255,479 events (Figure 3). In the pooled RR of the meta-analysis, consumption of one additional 50 g egg per day was associated with significantly increased risk of CVD (pooled RR=1.04, 95% CI: 1.00, 1.08, Figure 3). We did not observe any evidence of publication bias for the association between egg consumption and CVD risk (Figure II in the Supplemental Data). However, evidence of substantial heterogeneity existed between studies (I2=80.1%, Figure 3). On the other hand, no individual study disproportionately influenced the heterogeneity alone (Figure III in the Supplemental Data). There were no statistically significant interactions in the predefined subgroup meta-analyses (Table XVIII and Figure IV in the Supplemental Data), with the exception of geographical region (US, Europe and Asia, Figure V in the Supplemental Data), with a P value for interaction of 0.02. An increase of 50 g egg consumed per day was associated with a higher risk of CVD among cohorts conducted in the US (RR=1.08, 95% CI: 1.02, 1.14), and appeared to be related to a higher CVD risk in European cohorts with a borderline significance (RR=1.05, 95% CI: 0.98, 1.14), but not associated with CVD risk in Asian cohorts (RR=0.96, 95% CI: 0.87, 1.06).
Figure 3: Association of egg consumption with cardiovascular disease risk for one egg per day increase using random effects meta-analysis.
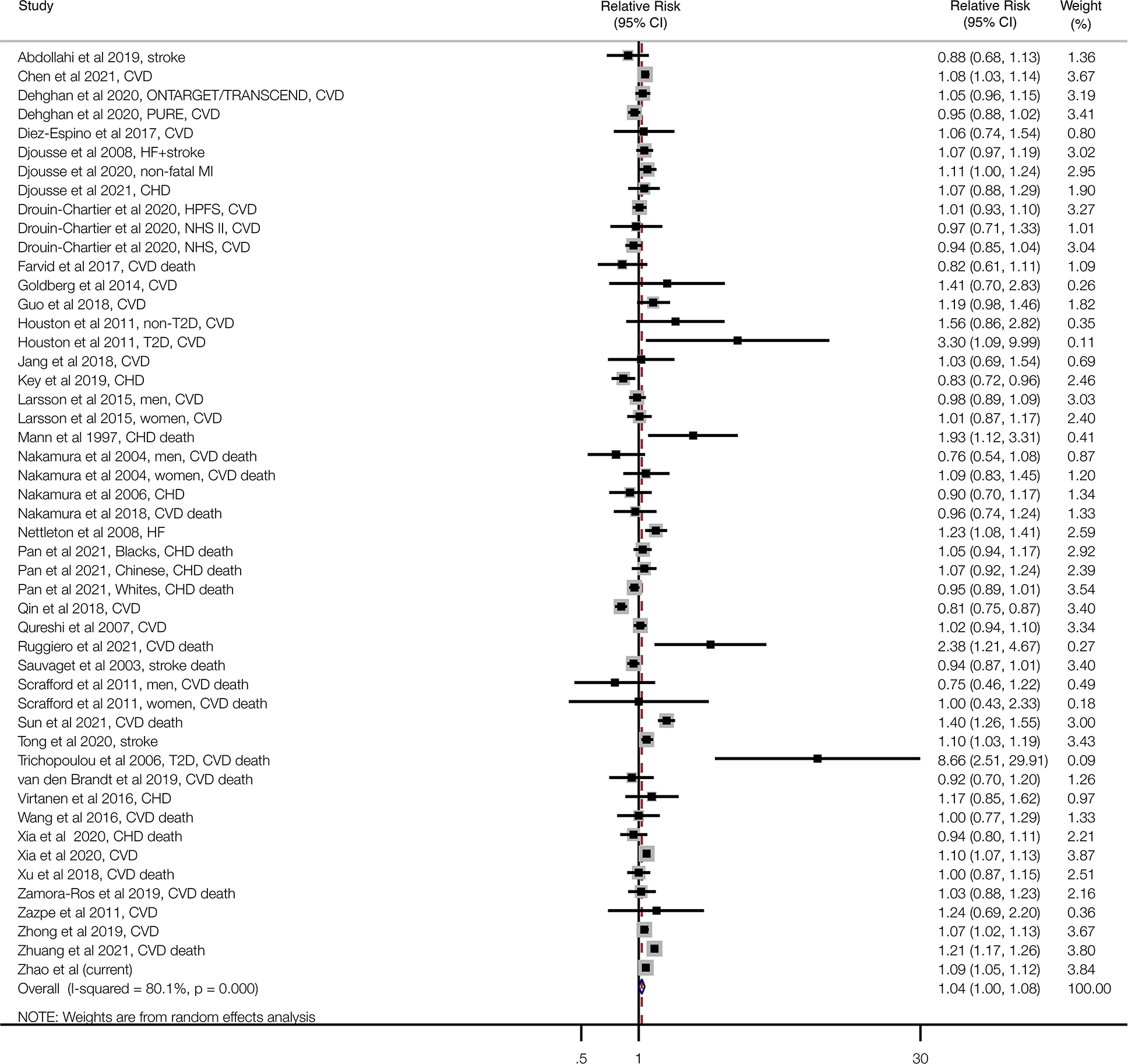
Squares reflect study-specific relative risk. Grey square areas are proportional to the individual study weight for the overall meta-analysis. Horizontal lines denote 95% CIs. I-squared refers to the proportion of heterogeneity among studies. CHD=coronary heart disease; CI=confidence interval; CVD=cardiovascular disease; HF=heart failure; i-stroke=ischemic stroke; MI: myocardial infarction; T2D=type 2 diabetes
Discussion
In this large cohort of 27,078 Finnish men followed for up to 31 years, participants with greater consumption of dietary cholesterol and egg experienced modest but significant increases in risk of overall, CVD and heart disease mortality independent of other risk factors including serum total cholesterol. The increased risk of mortality was similar across cohort subgroups. The observed egg-mortality associations were diminished after adjustment for dietary cholesterol. Findings from the updated meta-regression analysis provided strong support for the overall positive association between egg consumption (e.g., one egg per day) and risk of CVD, including CVD mortality. We did observe some evidence of heterogeneity in the association across the studies (I2=80.1%), however, finding that population geographic location was associated with study differences in the US, Europe and Asia. Whereas there was a significant positive association between egg consumption (50 g per day) and CVD risk in the US cohorts, we found a marginal positive association in the European cohorts, and no association in the Asian cohorts. The number of participants in Asian cohorts (n=921,563) were fewer than those in the U.S. cohorts (n=1,388,758), which may have limited power to examine the associations. Additionally, whether the egg-mortality associations are modified by cooking practices (e.g., boiling versus frying) may need further investigation. The other predefined subgroup meta-analyses revealed stable positive egg-CVD risk associations, including for sex, cohort size, duration of follow-up, number of events, risk of study bias, and method of dietary assessment.
A recent pooled analysis of three U.S. cohorts including the Nurses’ Health Study (NHS), NHS II Study and the Health Professionals’ Follow-Up Study (HPFS) found that moderate egg consumption (<1 egg per day) was not associated with CVD risk, even with adjustment for history of hypercholesterolemia and use of lipid lowering medications.16 Similarly, a large analysis from the Prospective Urban Rural Epidemiology (PURE) study also showed null associations for egg consumption and mortality.17 The China Kadoorie Biobank Study, a large Asian cohort, with nearly 0.5 million adults demonstrated an inverse association between moderate consumption of egg and risk of CVD, with a HR of 0.89 (95% CI: 0.87, 0.92) for daily egg consumers compared with non-consumers.21 The NIPPON DATA (ND) 90 Study of >4,000 Japanese women reported that those who consumed more than 2 eggs per day had increased overall mortality when compared with those consuming less than 1 egg per week, but showed no association for CVD mortality.14 With respect to dietary cholesterol and CVD risk, a recent meta-analysis did not demonstrate a conclusive association based on sparse data and heterogeneity across the available studies.35 By contrast, a recent study of 30,000 participants from six U.S. cohorts followed-up for up to 31 years showed dose-response associations such that each additional 300 mg of cholesterol intake daily was associated with 17% and 18% increased risk for CVD and overall mortality, and that eating half an egg more per day was associated with 6% and 8% increased risk of CVD and overall mortality.6 The sample sizes of the Zhong study and our study were similar, while the former included a more diverse population comprised of 55% women and 31% of black participants. Although the dietary cholesterol-mortality risk estimates were slightly higher than the present findings, the results of the two studies are essentially consistent, showing that the egg-mortality associations were attenuated after adjusting for dietary cholesterol. Also, both studies utilized competing risk models (i.e., cause-specific hazard models). Of note, the dietary cholesterol-mortality risk estimates were higher among women than men in the study of Zhong and colleagues (HRs=1.28 and 1.14 for women and men, respectively; P for interaction=0.02),6 with the risk estimate among men being very similar to that in our study. It should be noted, however, that the average daily cholesterol intake of 582 mg and egg consumption of 53.3 g were relatively high in our cohort as compared with corresponding values of 293 mg and 25.5 g in the general U.S. population based on the latest released data from National Health and Nutrition Examination Survey.36 Thus, our data provide mortality risk estimates for relatively higher consumption levels, which may partially account for some differences from the earlier studies in addition to population characteristics and length of follow-up. For example, average weekly consumption was 2.9 eggs for NHS, 1.3 eggs for NHS II, 2.4 eggs for HPFS, 3.3 eggs for the China Kadoorie Biobank Study, 3.9 eggs for PURE, and 7.5 eggs for our 1980’s cohort. Of note, cholesterol is contained in the yolk of the egg, and it is estimated that a whole egg contains 372 mg cholesterol on average,37 while egg whites contain primarily proteins (i.e., albumin).38 Our findings, as well as those from other groups, show attenuation of the egg consumption-mortality association after adjusting for dietary cholesterol, supporting the hypothesis that it is the increased cholesterol intake from eggs that plays a key role and accounts for the elevated mortality associations. Additional investigations are warranted to explore the likely different biological roles of egg yolks versus egg whites and whole eggs.
A recent meta-analysis of 28 studies showed that there was no significant association between moderate egg consumption (i.e., one egg per day) and risk of CVD.16 The consistent results between that meta-analysis and our updated analysis include the considerable heterogeneity across European, US and Asian cohorts. Indeed, the previous meta-analysis suggested that there was a possible moderately increased risk of cardiovascular disease of up to 19% among European cohorts (pooled RR=1.05, 95% CI: 0.92, 1.19),16 and we observed a borderline significant positive association among European cohorts (pooled RR=1.05, 95% CI: 0.98, 1.14), along with an 8% increased risk of CVD with moderate egg consumption (one egg per day) in the US cohorts and no association in Asian cohorts (pooled RR=0.96). Notably, the significant overall positive association between moderate egg consumption and CVD risk in our updated meta-analysis remained stable among subgroup analyses, including when restricted to studies of low potential bias based on the Newcastle-Ottawa Score of seven or higher (pooled RR=1.04, 95% CI: 1.01, 1.08).
The association between dietary cholesterol and CVD mortality has been debated for decades but remains biologically plausible. Although dietary cholesterol and serum cholesterol are only weakly associated, laboratory studies provide evidence that dietary cholesterol may be related to postprandial inflammation, oxidative stress-associated responses and impairment of endothelial function.39, 40 In vivo studies have found that high dietary cholesterol can lead to an increased serum biomarker of chronic systemic inflammation, serum amyloid A (SAA),41 which has been shown to be strongly positively associated with risk of cardiovascular disease,42–45 possibly as a result of SAA binding and inhibiting HDL bioactivity,46, 47 promoting monocyte chemotaxis and adhesion, and fostering pro-inflammatory cytokine production,48, 49 all actions that would facilitate progression of atherosclerosis. Other experimental data demonstrate that higher cholesterol intake results in adipose tissue macrophage accumulation which subsequently contributed to chronic inflammation,42 and that dietary cholesterol withdrawal leads to a reduced inflammatory response and monocyte infiltration of coronary artery plaque with subsequent favorable stabilization.50
Extensive investigation has showed that serum cholesterol does not directly reflect dietary cholesterol in healthy adults, based in part on metabolic cholesterol homeostasis. Dietary cholesterol is absorbed in the small bowel where it enters the portal circulation as chylomicrons which are taken up by the liver where cholesterol is metabolized and used for steroid biosynthesis and other biochemical requirements. The liver controls both endogenously synthesized and exogenous cholesterol and determines the amount of cholesterol released into the bloodstream in lipoproteins.5, 38, 51, 52 In line with substantial previous research,53–56 our findings show that individuals with higher concentrations of serum total cholesterol experience significantly increased risk of CVD mortality. Of note, we found that the serum cholesterol-CVD association is largely independent of several other risk factors, including cholesterol intake. As a leading cause of death worldwide, atherosclerotic disease can be initiated by the aggregation of lipids (including circulating cholesterol) in the arterial wall, which subsequently leads to local chronic inflammation and promotes atherosclerotic plaque progression.57 In additional to the initial plaque build-up, cholesterol can increase the production of oxysterol and aggregation, which activates arterial macrophages and an inflammatory response.58 Circulating cholesterol can also be engulfed by arterial macrophages, promoting inflammasome activation and leading to further pro-inflammatory cytokine production and amplification.3, 58–60 By contrast, our results showed that men with higher serum total cholesterol experienced lower risk of cancer mortality, and these findings were essentially in good agreement with previous studies.61, 62
Important strengths of our study include its prospective design, large sample size and completeness of follow-up for ascertainment of cause-specific mortality via linkage with national registries over a 31-year period. The sample size afforded considerable statistical power for the examined associations across a wide range of cohort subgroups. Our analyses included both exogenous dietary cholesterol and endogenous circulating cholesterol to offer an objective and thorough examination of the associations between cholesterol exposures and long-term health. Several study limitations should also be noted. First, we used a food frequency questionnaire to evaluate cholesterol intake and egg consumption during the previous 12 months, and a single baseline measurement of serum total cholesterol, with the risk of subsequent changes in diet and biochemical status. However, the nondifferential misclassification from inherent measurement errors would serve to underestimate the associations that influence the observed risk estimates and bias them toward the null. On the other hand, an earlier validation study using multiple-day diet records reported correlations of 0.66 for dietary cholesterol (0.67–0.75 after corrected for attenuation) and 0.58 for egg consumption, supporting the validity and reproducibility of our instrument.24 In addition, the correlation of serum total cholesterol between baseline and 3 years was 0.74 (Spearman correlation coefficient, P value<10−10), reflecting its stability over time. Our study was a relatively homogenous male smoker population of European ancestry with relatively high cholesterol and egg intake, which decreases generalizability of the findings to other populations. However, we included the updated meta-analysis that provided comprehensive findings from other populations. Finally, we cannot rule out the potential influence of residual confounding bias of our observed associations. However, our findings remained largely unchanged following careful adjustment for a wide range of potential confounding factors, construction and use of a propensity score to control for variation of these factors among comparison groups, estimating associations based on gradual increment units for exposure variables, and conducting multiple stratified analyses.
In conclusion, findings from the ATBC cohort with over three decades of observation demonstrate that greater consumption of dietary cholesterol and eggs is associated with increased risk of overall, CVD and heart disease mortality. Increased serum total cholesterol was also associated with increased CVD and heart disease mortality. The observed cholesterol-mortality associations were modest and independent of several other CVD risk factors. The results from this updated meta-analysis provide compelling evidence for the association between increased egg consumption and elevated risk of CVD, especially in the US and possibly Europe, but not in Asia. Our data regarding cholesterol intake provide additional evidence relevant to dietary guidelines.
Supplementary Material
Clinical Perspective.
What is new?
Whether dietary cholesterol and egg consumption influence risk of CVD and overall mortality remains unclear.
The present analysis represents both an original cohort study of 27,078 Finnish men and an updated meta-analysis of 41 prospective cohort studies, and it demonstrates that participants with greater consumption of dietary cholesterol and eggs experienced increased risk of overall and CVD mortality.
In the updated meta-analysis, we found a significant positive association for egg consumption and CVD risk in US cohorts, a marginal positive association in European cohorts, and no association in Asian cohorts.
What are the clinical implications?
These findings support restricted consumption of dietary cholesterol as a means to improve long-term cardiovascular health and longevity, and provide compelling evidence relevant to dietary guidelines.
Acknowledgements:
We appreciate all participants in the ATBC cohort for their contribution to the study.
Funding:
The ATBC Study is supported by the Intramural Research Program of the U.S. National Cancer Institute, National Institutes of Health, Department of Health and Human Services. The funders had no role in the design and conduct this present study, including the systematic review and meta-analysis.
Non-standard Abbreviations and Acronyms
- ARD
absolute risk differences
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention
- BMI
body mass index
- CIs
confidence intervals
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- HPFS
NHS II Study and the Health Professionals’ Follow-Up Study
- HR
hazard ratios
- NHS
Nurses’ Health Study
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analysis
- PURE
Prospective Urban Rural Epidemiology
- RR
relative risk
- SAA
serum amyloid A
Footnotes
Competing interests: none.
Supplemental materials:
Supplemental Materials: Expanded Methods, Expanded Results
Tables I – XVIII in the Supplemental Data
Figures I – V in the Supplemental Data
References
- 1.Soliman GA. Dietary Cholesterol and the Lack of Evidence in Cardiovascular Disease. Nutrients. 2018;10:e780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soffientini U and Graham A. Intracellular cholesterol transport proteins: roles in health and disease. Clin Sci (Lond). 2016;130:1843–59. [DOI] [PubMed] [Google Scholar]
- 3.Cortes VA, Busso D, Maiz A, Arteaga A, Nervi F and Rigotti A. Physiological and pathological implications of cholesterol. Front Biosci (Landmark Ed). 2014;19:416–28. [DOI] [PubMed] [Google Scholar]
- 4.Brown MS, Radhakrishnan A and Goldstein JL. Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu Rev Biochem. 2018;87:783–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo J, Yang H and Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21:225–245. [DOI] [PubMed] [Google Scholar]
- 6.Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao L, Norwood AF, Lloyd-Jones DM and Allen NB. Associations of Dietary Cholesterol or Egg Consumption With Incident Cardiovascular Disease and Mortality. JAMA. 2019;321:1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zhou C, Zhou X and Li L. Egg consumption and risk of cardiovascular diseases and diabetes: a meta-analysis. Atherosclerosis. 2013;229:524–30. [DOI] [PubMed] [Google Scholar]
- 8.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW Jr., Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL and Bazzarre TL. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–99. [DOI] [PubMed] [Google Scholar]
- 9.Kanter MM, Kris-Etherton PM, Fernandez ML, Vickers KC and Katz DL. Exploring the factors that affect blood cholesterol and heart disease risk: is dietary cholesterol as bad for you as history leads us to believe? Adv Nutr 2012;3:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.USDA. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. https://ods.od.nih.gov/pubs/2015_dgac_scientific_report.pdf. 2015. [Google Scholar]
- 11.Kuang H, Yang F, Zhang Y, Wang T and Chen G. The Impact of Egg Nutrient Composition and Its Consumption on Cholesterol Homeostasis. Cholesterol. 2018;2018:6303810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khawaja O, Singh H, Luni F, Kabour A, Ali SS, Taleb M, Ahmed H, Gaziano JM and Djousse L. Egg Consumption and Incidence of Heart Failure: A Meta-Analysis of Prospective Cohort Studies. Front Nutr. 2017;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L, Lam TH, Jiang CQ, Zhang WS, Zhu F, Jin YL, Woo J, Cheng KK and Thomas GN. Egg consumption and the risk of cardiovascular disease and all-cause mortality: Guangzhou Biobank Cohort Study and meta-analyses. Eur J Nutr. 2019;58:785–796. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Okamura T, Kita Y, Okuda N, Kadota A, Miura K, Okayama A, Ueshima H and Group NDR. Re-evaluation of the associations of egg intake with serum total cholesterol and cause-specific and total mortality in Japanese women. Eur J Clin Nutr. 2018;72:841–847. [DOI] [PubMed] [Google Scholar]
- 15.Djousse L and Gaziano JM. Egg consumption in relation to cardiovascular disease and mortality: the Physicians’ Health Study. Am J Clin Nutr. 2008;87:964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drouin-Chartier JP, Chen S, Li Y, Schwab AL, Stampfer MJ, Sacks FM, Rosner B, Willett WC, Hu FB and Bhupathiraju SN. Egg consumption and risk of cardiovascular disease: three large prospective US cohort studies, systematic review, and updated meta-analysis. BMJ. 2020;368:m513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehghan M, Mente A, Rangarajan S, Mohan V, Lear S, Swaminathan S, Wielgosz A, Seron P, Avezum A, Lopez-Jaramillo P, Turbide G, Chifamba J, AlHabib KF, Mohammadifard N, Szuba A, Khatib R, Altuntas Y, Liu X, Iqbal R, Rosengren A, Yusuf R, Smuts M, Yusufali A, Li N, Diaz R, Yusoff K, Kaur M, Soman B, Ismail N, Gupta R, Dans A, Sheridan P, Teo K, Anand SS and Yusuf S. Association of egg intake with blood lipids, cardiovascular disease, and mortality in 177,000 people in 50 countries. Am J Clin Nutr. 2020;111:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia PF, Pan XF, Chen C, Wang Y, Ye Y and Pan A. Dietary Intakes of Eggs and Cholesterol in Relation to All-Cause and Heart Disease Mortality: A Prospective Cohort Study. J Am Heart Assoc. 2020;9:e015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo J, Hobbs DA, Cockcroft JR, Elwood PC, Pickering JE, Lovegrove JA and Givens DI. Association between egg consumption and cardiovascular disease events, diabetes and all-cause mortality. Eur J Nutr. 2018;57:2943–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Key TJ, Appleby PN, Bradbury KE, Sweeting M, Wood A, Johansson I, Kuhn T, Steur M, Weiderpass E, Wennberg M, Lund Wurtz AM, Agudo A, Andersson J, Arriola L, Boeing H, Boer JMA, Bonnet F, Boutron-Ruault MC, Cross AJ, Ericson U, Fagherazzi G, Ferrari P, Gunter M, Huerta JM, Katzke V, Khaw KT, Krogh V, La Vecchia C, Matullo G, Moreno-Iribas C, Naska A, Nilsson LM, Olsen A, Overvad K, Palli D, Panico S, Molina-Portillo E, Quiros JR, Skeie G, Sluijs I, Sonestedt E, Stepien M, Tjonneland A, Trichopoulou A, Tumino R, Tzoulaki I, van der Schouw YT, Verschuren WMM, di Angelantonio E, Langenberg C, Forouhi N, Wareham N, Butterworth A, Riboli E and Danesh J. Consumption of Meat, Fish, Dairy Products, and Eggs and Risk of Ischemic Heart Disease. Circulation. 2019;139:2835–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin C, Lv J, Guo Y, Bian Z, Si J, Yang L, Chen Y, Zhou Y, Zhang H, Liu J, Chen J, Chen Z, Yu C, Li L and China Kadoorie Biobank Collaborative G. Associations of egg consumption with cardiovascular disease in a cohort study of 0.5 million Chinese adults. Heart. 2018;104:1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4:1–10. [DOI] [PubMed] [Google Scholar]
- 23.Reinivuo H, Hirvonen T, Ovaskainen ML, Korhonen T and Valsta LM. Dietary survey methodology of FINDIET 2007 with a risk assessment perspective. Public Health Nutr. 2010;13:915–9. [DOI] [PubMed] [Google Scholar]
- 24.Pietinen P, Hartman AM, Haapa E, Rasanen L, Haapakoski J, Palmgren J, Albanes D, Virtamo J and Huttunen JK. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128:655–66. [DOI] [PubMed] [Google Scholar]
- 25.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG and Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamling J, Lee P, Weitkunat R and Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008;27:954–70. [DOI] [PubMed] [Google Scholar]
- 28.Zazpe I, Beunza JJ, Bes-Rastrollo M, Warnberg J, de la Fuente-Arrillaga C, Benito S, Vazquez Z, Martinez-Gonzalez MA and Investigators SUNP. Egg consumption and risk of cardiovascular disease in the SUN Project. Eur J Clin Nutr. 2011;65:676–82. [DOI] [PubMed] [Google Scholar]
- 29.Houston DK, Ding J, Lee JS, Garcia M, Kanaya AM, Tylavsky FA, Newman AB, Visser M, Kritchevsky SB and Health ABCS. Dietary fat and cholesterol and risk of cardiovascular disease in older adults: the Health ABC Study. Nutr Metab Cardiovasc Dis. 2011;21:430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson SC, Akesson A and Wolk A. Egg consumption and risk of heart failure, myocardial infarction, and stroke: results from 2 prospective cohorts. Am J Clin Nutr. 2015;102:1007–13. [DOI] [PubMed] [Google Scholar]
- 31.Virtanen JK, Mursu J, Virtanen HE, Fogelholm M, Salonen JT, Koskinen TT, Voutilainen S and Tuomainen TP. Associations of egg and cholesterol intakes with carotid intima-media thickness and risk of incident coronary artery disease according to apolipoprotein E phenotype in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2016;103:895–901. [DOI] [PubMed] [Google Scholar]
- 32.Zamora-Ros R, Cayssials V, Cleries R, Redondo ML, Sanchez MJ, Rodriguez-Barranco M, Sanchez-Cruz JJ, Mokoroa O, Gil L, Amiano P, Navarro C, Chirlaque MD, Huerta JM, Barricarte A, Ardanaz E, Moreno-Iribas C and Agudo A. Moderate egg consumption and all-cause and specific-cause mortality in the Spanish European Prospective into Cancer and Nutrition (EPIC-Spain) study. Eur J Nutr. 2019;58:2003–2010. [DOI] [PubMed] [Google Scholar]
- 33.Chen GC, Chen LH, Mossavar-Rahmani Y, Kamensky V, Shadyab AH, Haring B, Wild RA, Silver B, Kuller LH, Sun Y, Saquib N, Howard B, Snetselaar LG, Neuhouser ML, Allison MA, Van Horn L, Manson JE, Wassertheil-Smoller S and Qi Q. Dietary cholesterol and egg intake in relation to incident cardiovascular disease and all-cause and cause-specific mortality in postmenopausal women. Am J Clin Nutr. 2021;113:948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenland S and Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 35.Berger S, Raman G, Vishwanathan R, Jacques PF and Johnson EJ. Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2015;102:276–94. [DOI] [PubMed] [Google Scholar]
- 36.Xu Z, McClure ST and Appel LJ. Dietary Cholesterol Intake and Sources among U.S Adults: Results from National Health and Nutrition Examination Surveys (NHANES), 2001–2014. Nutrients. 2018;10:e771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puertas G and Vazquez M. Advances in techniques for reducing cholesterol in egg yolk: A review. Crit Rev Food Sci Nutr. 2019;59:2276–2286. [DOI] [PubMed] [Google Scholar]
- 38.Abeyrathne E, Huang X and Ahn DU. Antioxidant, angiotensin-converting enzyme inhibitory activity and other functional properties of egg white proteins and their derived peptides - A review. Poult Sci. 2018;97:1462–1468. [DOI] [PubMed] [Google Scholar]
- 39.Spence JD, Jenkins DJ and Davignon J. Dietary cholesterol and egg yolks: not for patients at risk of vascular disease. Can J Cardiol. 2010;26:e336–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Njike V, Faridi Z, Dutta S, Gonzalez-Simon AL and Katz DL. Daily egg consumption in hyperlipidemic adults--effects on endothelial function and cardiovascular risk. Nutr J 2010;9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis KE, Kirk EA, McDonald TO, Wang S, Wight TN, O’Brien KD and Chait A. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110:540–5. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A 3rd, Kirk EA, O’Brien KD and Chait A Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jousilahti P, Salomaa V, Rasi V, Vahtera E and Palosuo T. The association of c-reactive protein, serum amyloid a and fibrinogen with prevalent coronary heart disease--baseline findings of the PAIS project. Atherosclerosis. 2001;156:451–6. [DOI] [PubMed] [Google Scholar]
- 44.Ridker PM, Hennekens CH, Buring JE and Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 45.Zhou J, Lu Y, Wang S and Chen K. Association between serum amyloid A levels and coronary heart disease: a systematic review and meta-analysis of 26 studies. Inflamm Res. 2020;69:331–345. [DOI] [PubMed] [Google Scholar]
- 46.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM and Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zewinger S, Drechsler C, Kleber ME, Dressel A, Riffel J, Triem S, Lehmann M, Kopecky C, Saemann MD, Lepper PM, Silbernagel G, Scharnagl H, Ritsch A, Thorand B, de las Heras Gala T, Wagenpfeil S, Koenig W, Peters A, Laufs U, Wanner C, Fliser D, Speer T and Marz W. Serum amyloid A: high-density lipoproteins interaction and cardiovascular risk. Eur Heart J. 2015;36:3007–16. [DOI] [PubMed] [Google Scholar]
- 48.Badolato R, Wang JM, Stornello SL, Ponzi AN, Duse M and Musso T. Serum amyloid A is an activator of PMN antimicrobial functions: induction of degranulation, phagocytosis, and enhancement of anti-Candida activity. J Leukoc Biol. 2000;67:381–6. [DOI] [PubMed] [Google Scholar]
- 49.Song C, Shen Y, Yamen E, Hsu K, Yan W, Witting PK, Geczy CL and Freedman SB. Serum amyloid A may potentiate prothrombotic and proinflammatory events in acute coronary syndromes. Atherosclerosis. 2009;202:596–604. [DOI] [PubMed] [Google Scholar]
- 50.Verhamme P, Quarck R, Hao H, Knaapen M, Dymarkowski S, Bernar H, Van Cleemput J, Janssens S, Vermylen J, Gabbiani G, Kockx M and Holvoet P. Dietary cholesterol withdrawal reduces vascular inflammation and induces coronary plaque stabilization in miniature pigs. Cardiovasc Res. 2002;56:135–44. [DOI] [PubMed] [Google Scholar]
- 51.Lecerf JM and de Lorgeril M. Dietary cholesterol: from physiology to cardiovascular risk. Br J Nutr. 2011;106:6–14. [DOI] [PubMed] [Google Scholar]
- 52.Repa JJ and Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–81. [DOI] [PubMed] [Google Scholar]
- 53.Shin JY, Xun P, Nakamura Y and He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:146–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asia Pacific Cohort Studies C. Joint effects of systolic blood pressure and serum cholesterol on cardiovascular disease in the Asia Pacific region. Circulation. 2005;112:3384–90. [DOI] [PubMed] [Google Scholar]
- 55.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P and Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284:311–8. [DOI] [PubMed] [Google Scholar]
- 56.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [DOI] [PubMed] [Google Scholar]
- 57.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT and Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–21. [DOI] [PubMed] [Google Scholar]
- 58.Tall AR and Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V and Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA and Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH and Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guan XM, Wu SL, Yang XL, Han X, Yang YH, Li XT, Bin Waleed K, Yue D, Zhan SY, Liu Y, Li HH and Xia YL. Association of total cholesterol, low-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol with atherosclerotic cardiovascular disease and cancer in a Chinese male population. Int J Cancer. 2018;142:1209–1217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


