Abstract
A number of stimuli-responsive-based hydrogels has been widely explored in biomedical applications in the last few decades because of their excellent biodegradability and biocompatibility. The development of synthetic chemistry and materials science leads to the emergence of in situ stimuli-responsive hydrogels. In this regard, several synthetic and natural polymers have been synthesized and utilized to prepare temperature-sensitive in situ forming hydrogels. This could be best used via injections as temperature stimulus could trigger in situ hydrogels gelation and swelling behaviors. There are many smart polymers available for the formulation of the in situ based thermoresponsive injectable hydrogel. Among these, poly (ε-caprolactone) (PCL) polymer has been recognized and approved by the FDA for numerous biomedical applications. More specifically, the PCL is coupled with polyethylene glycol (PEG) to obtain amphiphilic thermosensitive “smart” copolymers (PCL-PEG), to form rapid and reversible physical gelation behavior. However, the chemical structure of the copolymer is a critical aspect in determining water solubility, thermo-gelation behavior, drug release rate, degradation rate, and the possibility to deliver a diverse range of drugs. In this review, we have highlighted the typical PCL-PEG-based thermosensitive injectable hydrogels progress in the last decade for tissue engineering and localized drug delivery applications to treat various diseases. Additionally, the impact of molecular weight of PCL-PEG upon gelling behavior has also been critically highlighted for optimum hydrogels properties for potential pharmaceutical and biomedical applications.
Keywords: Injectable hydrogel, Thermoresponsive, PCL/PEG copolymer, Tissue engineering, Scaffold
1. Introduction
“Smart” materials, i.e., materials that can respond to environmental stimuli have recently emerged showing immense potentialities in the biomedical fields. Among them, smart polymeric materials have gained considerable attention in the field of biomedical engineering as a result of their ease of use when compared with smart metals or ceramics [1]. Polymeric smart materials can respond to different external stimuli based on their chemical structure and composition, such as ionic strength, temperature, pH, light, electrical, magnetic, chemical and biological stimulations [2,3]. Therefore, these polymeric materials can be exploited in the development of sensors, drug carrier systems and as well as scaffolds in the field of tissue engineering [4–6]. As a result of the temperature difference outside and within the human body (from ambient to physiological), polymer hydrogels that are triggered by temperature changes have been widely studied [7–9].
In detail, hydrogels are a form of polymeric materials with the ability to imbibe and swell in aqueous solutions. They form three-dimensional polymeric networks with the capability of retaining a large volume of water in their swollen state. The extremely hydrated nature of hydrogels resembling the extracellular matrix characteristics makes them biomimetic materials for tissue engineering [10]. Thermosensitive hydrogels are particularly interesting because a simple temperature change can easily trigger gelation and swelling behaviors. These systems can also be exploited for in situ hydrogel formation, for example as drug delivery injectable carriers for local delivery in a minimally invasive manner [11–13]. The drug delivery with PCL-PEG injectable thermoresponsive hydrogel system has many advantages such as high miscibility of the drug in aqueous form due to its low viscosity below body temperature, localized delivery via injection enables targeted and time-sensitive delivery of the drug, PCL-PEG makes easier to weigh and disperse due to its powdery crystalline form as compared to the paste-like polyphosphazene and PEG-PLGA [14]. This powder crystalline form results in improved technical convenience.
The drug-loaded PCL-PEG hydrogels also have their limitations as it needs to be formulated above the LCST. Also, homogeneity and poor drug loading into the PEG-PCL hydrogel remain a challenge for scientists. This process is also not suitable for thermolabile drugs. Along with this PCL possesses high crystallinity and hydrophobicity which could hinder the drug release from the hydrogel matrix [15].
To overcome such limitations many researchers have explored the different dimensions associated with the formulations of stable thermoresponsive hydrogel network systems. In general, based on the polymeric source applied for the development of a stable hydrogel network, injectable hydrogels are classified into natural, hybrid or synthetic. To better adjust the properties of hydrogels, they can be cross-linked chemically (i.e., covalently) or physically (i.e., by non-covalent interactions) [16–18]. Various natural and synthetic thermosensitive hydrogels have been extensively investigated, such as chitosan-based [19–21] and polyester copolymer-based hydrogels [22,23], respectively. Polyester-based thermosensitive hydrogels, based on PGA,1 PLA,2 PVL,3 PLGA,4 and PCL5 typically arranged by joining polyester chain terminals with the ends of polyethylene glycol (PEG) molecules [24].
Among them, PCL6 is the most preferred one which is also approved by the FDA because of its excellent biodegradability and biocompatibility making it more suitable for application in health care [25]. Generally, it is used alone or in combination with other biocompatible polymers to prepare scaffolds for tissue engineering [26], such as guided bone regeneration membranes [27], carriers for drug delivery [28], and sutures [29] When PCL, coupled with the hydrophilic PEG, it is possible to obtain amphiphilic thermosensitive “smart” copolymers (PCL-PEG), able to undergo rapid and reversible physical gelation by changing the temperature. By proper selection of block molecular weight and relative amount, PCL-PEG hydrogels may undergo sol-to-gel transition with the rise in temperature ranging from ambient temperature to 37 °C (Fig. 1) [30,31]. The chemical structure of the copolymer is a critical aspect in determining, water solubility, thermo-gelation behavior, drug release rate, degradation rate, and the possibility to deliver a diverse range of drugs, including proteins and bioactive molecules [32,33]. (See Table 1.)
Fig. 1.
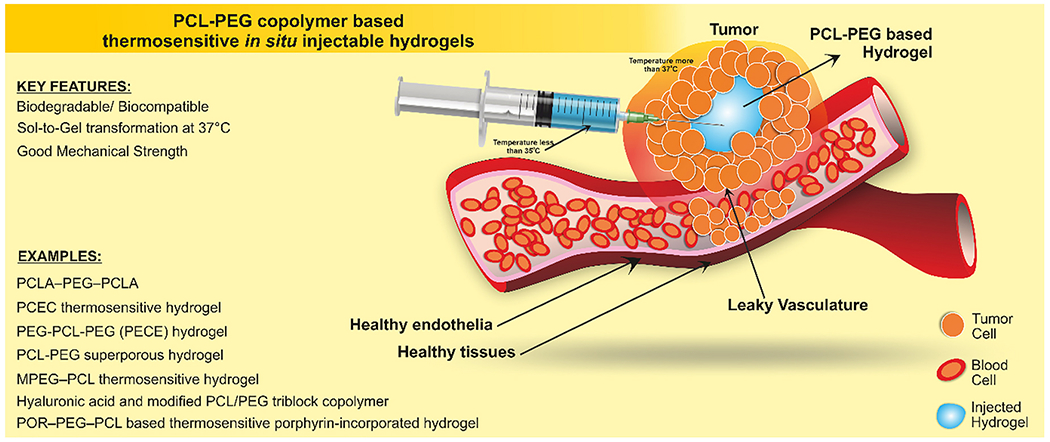
PCL-PEG copolymer based thermosensitive injectable hydrogel. The elevated temperature at the site of the tumor gelled the hydrogel instantly to minimize the initial burst of the drug at the site of delivery.
Table 1.
Versatile design strategies to develop PEG-PCL copolymer based thermoresponsive injectable hydrogels as novel drug delivery system.
| Type of polymer | Stimulus | Molecular weight | Drug | Sol-gel-sol transition temperature | Drug release behavior | Application | Reference |
|---|---|---|---|---|---|---|---|
| Triblock copolymer | Temperature | (PCLA1730-PEG1500-PCLA1730) | – | 37 °C | – | Prevention of post-operative adhesion | [245] |
| Triblock copolymer | Temperature | PEG960-PCL2448-PEG-960 | 5-Flurouracil | 37 °C | Sustained release | Colorectal peritoneal carcinomatosis | [246] |
| Triblock copolymer | Temperature | PEG2000-PCL2000-PEG2000 | Paclitaxel | 37 °C | Controlled release | Preventing local breast cancer recurrence and improving incision wound healing in a mouse model | [247] |
| Tri-block polyols | Temperature | PEG500-PCL2000-PEG500 | Polyurethane | – | – | Human tissue engineering applications | [248] |
| Triblock copolymer | Temperature | PECT | Doxorubicin hydrochloride and Paclitaxel | 37 °C | Doxorubicin hydrochloride – initial burst release and Paclitaxel – sustain release | Anti-tumor efficiency | [249] |
| Triblock copolymer | Temperature | PEG550-PCL2400-PEG550 | Honokiol | 37 °C | sustain release | Cancer chemotherapy | [70] |
| Triblock copolymer | Temperature | PCL1900–PEG2000–PCL1900 for micelles and PCL2100–PEG1000–PCL2100 for hydrogel | Paclitaxel and 5-Flurouraxcil | 37 °C | Sustained release of PTX and 5-FU, the hydrophilic 5-FU was completely release over a period of 1 week, and the hydrophobic PTX was released slowly with lower cumulative release rate | Colorectal peritoneal carcinomatosis | [250] |
| Triblock copolymer | Temperature | PCL1000-PEG1000-PCL1000 | (TGF)-β1 | 37 °C | Local sustainable drug delivery system for TGF-β1 | In vivo cartilage repair | [251] |
| Pentablock copolymer | Temperature and pH | PCL1845–PEG2050–PCL1845 | Insulin loaded chitosan particle | 37 °C | The cumulative release of insulin was around 80% after 30 days, ideal for the treatment of diabetes | Insulin dependent diabetes | [252] |
| Pentablock copolymer | Temperature | PNIPAAm-PCL-PEG-PCL-PNIPAAm | Cellular culture scaffolds for wound healing | 37 °C | Real Time-PCR results shows that 10 and 20% of hydrogel can significantly increase collagen I α1 and collagen III mRNA expression on exposure to hydrogel in fibroblast cells. | Cell scaffold in skin tissue engineering and wound healing | [253] |
| Diblock copolymer | Temperature | POR-PEG1000-PCL | Porphyrin | 37 °C | The fluorescence imaging on the treated animal shows irregular fluorescence imaging of hydrogel along with the transition from the liquid state to the solid gel. After the permeation and distribution for 1 day, the hydrogel shrinks and forma a regular gel. | Real time imaging ability conferred to the hydrogels. | [235] |
| Diblock copolymer | Hydrolytic degradation | PBT-PEG4000 | – | 37 °C | – | Potential application in biomedical field | [254] |
| Diblock copolymers | Temperature | MPEG-PCL-RGD [Mn = 750 to 2400] | Bone marrow-derived mesenchymal stem cells (BMSCs) | 37 °C | Significant increase in the expression levels of collagen 1 and osteocalcin in BMSCs in the the hydrogel. | Bone tissue engineering | [255] |
| Diblock copolymer | Temperature | PEG-PCL [14:6] | Cultivated articular chondrocytes | 37 °C | 14:6 weight ratio of PEG:PCL scaffolds were optimal for cartilage tissue formation in terms of collagen type II expression. | Cell growth and tissue regeneration | [14] |
| Triblock copolymer | Temperature | PEG-PCL-PEG [Mn = 3150] | Intrinsic osteoinductive acellular bone matrix (ABM) | 37 °C | Thermosensitive ABM/PECE composites were highly effective towards the repairing of cranial defects with exceptional osteoinductive activity | Bone regeneration | [101] |
| Triblock copolymer | Temperature | PEG-PCL-PEG | Bevacizumab | 37 °C | Sustained release of bevacizumab intracameral injection | Management of postoperative scarring after glaucoma filtration surgery | [256] |
| Triblock copolymer | Temperature | PEG-PCL-PEG | Collagen and nano hydroxyapatite | 37 °C | The hydrogel composite showed excellent capability of guided bone regeneration as compared to the self process, due to the support of collagen scaffold for new bone tissue in-growth and osteoconduction of HA. | Bone regeneration | [112] |
| Triblock copolymer | Temperature | MPEG-PCL-MPEG | Bone marrow-derived stem cells (BMSCs) | 37 °C | The intramyocardial injection of BMSCs loaded hydrogel increased the cell retention and vessel density around the infract and also prevented the expansion of scar in comparison to the BMSCs injection alone. | Cellular transplantation therapy for myocardial infraction | [257] |
It showed Effect of molecular wight of PCL-PEG block copolymers on sol-gel transition and its application.
Like PCL, PEGs are also well recognized and approved by FDA to be outstanding biodegradable and biocompatible materials for numerous biomedical applications [34–36]. Recently, thermoresponsive PCL-PEG hydrogels have attracted extensive attention as a promising alternative to the commonly investigated hydrogels based on PLGA-PEG co-polymers. However, PLGA-PEG block copolymers suffer from some drawbacks, such as the formation of acidic degradation products upon their rapid degradation, sticky appearance at room temperature making handling difficult, longer dissolution time in the water, and weak hydrogel mechanical properties [37–39]. Due to their crystallinity, PCL-based copolymers have a stable morphology with a significant improvement in handling and transfer [40,41]. Compare to PLGA-PEG hydrogels, PCL-PEG hydrogels have superior rheological properties, slower degradation rate and more extended in vivo stability, hence reducing inflammatory reaction and improving the stability of incorporated bioactive molecules [40,42,43]. In situ hydrogels are prepared from physical and chemical crosslinking for effective localized drug delivery. Physically crosslinked hydrogels are formed from hydrogen bonding between water-soluble polymers in response to environmental stimuli [44]. On the other hand, chemically crosslinked hydrogels are prepared from Michael’s addition reaction, photopolymerization, and enzymatic reactions [45–48]. These hydrogels are prepared from FDA-approved polymers such as PCL/PEG for biomedical applications [49]. The PCL-PEG copolymer and drugs are dissolved above the melting point of PCL and allowed to form a gel. Hence as mentioned above the PCL-PEG hydrogels are not suitable for thermosensitive drugs for localized drug delivery applications [15]. Moreover, such hydrogels may allow a diffusion-controlled drug release. Thus, thermosensitive hydrogels based on PCL-PEG block copolymers have exciting applications in the biomedical field like scaffolds meant for soft and hard tissue engineering and delivery system for drugs and proteins too. In this review article, we have emphasized the various developments in the area of PCL/PEG copolymer-based thermoresponsive injectable hydrogels.
2. Historical perspectives in the synthesis routes
The slow degradation of PCL polymer, due to its higher degree of hydrophobicity and crystallinity, restricts its clinical application. PCL copolymerization can accelerate the material degradation rate, preserving biodegradability, biocompatibility, and excellent mechanical properties [50–53]. The hydrogels based on amphiphilic PCL-PEG copolymers have numerous biomedical applications, as explained in the following paragraph. The first scientists who prepared the PCL/PEG block copolymers series were Perret and Skoulios [54]. They practically proved the anionic polymerization in the presence of naphthalene-sodium complex as a catalyst for the synthesis. Later, Cerrai et al. introduced a new polymerization method for the synthesize of PCL/PEG copolymer in the absence of a catalyst [55]. In the PCL-PEG copolymers, PCL is the hydrophobic block (A-block) while PEG is the hydrophilic block (B-block). Based on their structure, PCL-PEG copolymers, designated as AB di-block, ABA or BAB tri-block, and star-shaped block structures. For the synthesis of PCL-PEG di-block copolymer, the most recommended method is ring-opening copolymerization from monomethoxy-PEG (MPEG) and ε-CL in the presence of a catalyst [56–60]. Also, PCL-PEG triblock copolymers can be categorized into two forms: the ABA type which comprises PCEC7 copolymers and the BAB type comprising PECE8 copolymers. By the application of ring-opening polymerization, PCEC based copolymers are synthesized from ε-CL monomers using a dihydroxy PEG as an initiator [61–63]. On the other hand, using coupling reaction, PECE copolymers are synthesized from MPEG–PCL di-block copolymers with a coupling agent, which is a diisocyanate, for example, IPDI9 and HMDI10 [35]. These multiblock PCL-PEG copolymers can be synthesized by any of the following methods, e.g., by a two-step reaction, involving the initial synthesis of an isocyanate (−NCO) end-capped prepolymer, by reaction of PCL-diol with a coupling agent (e.g., l-lysine methyl ester diisocyanate), followed by reaction with PEG diol [64]. Also, the PCL-PEG star-shaped polymers, synthesized by various methods, e.g., synthetic procedure, convergent, and divergent methods, proposed by Kim et al. [62,65,66]. For the synthesis of PCL-PEG copolymers, different reactants and catalysts can be used to produce various types of di-, tri- or multi-block copolymers of PCL-PEG [31,67].
3. Critical gelation concentration
In contrast to the gelation mechanism of hydrogel via a chemical method, the mild condition, kept for the sol-to-gel transition of temperature. Under these mild conditions (nontoxic), toxic reagents like organic solvents, residual unreacted monomers, catalysts, crosslinkers, etc., are not used for the formulation [68,69]. The chemical composition of the copolymers and the manner of treatment of the hydrous copolymers can bring about a significant change in the sol-gel transition behavior. Gong et al. investigated the sol-gel transition and the factors that govern the alteration of the transition diagram. It has been seen that with the increase in the hydrophobic PCL block length, the gelation occurred at lower concentrations and lower temperatures. This may be due to the improved hydrophobicity of the copolymer backbone. Hence it can be attributed that the hydrophobic interactions bring about the sol-gel transition in an aqueous PECE system [70]. As the molecular weight increases, the sol-gel-sol curve shifts towards the elevated temperature, despite the same molar ratio of PCL to PEG [71]. Hence PEG block length is a critical parameter that influences the sol-gel-sol transition curve of the PECE triblock copolymer. The thermoresponsive polymers exhibit their sol-to-gel transition above UCGT11 or LCGT12 depending upon the molecular weight of the polymer segment, ratio of hydrophobic and hydrophilic blocks [69]. The orientation of the micelles occurs at a particular temperature and concentration above CGT.13 For instance, the triblock copolymers consisting of PEG and PCL comprising of BAB or PECE and ABA or PCEC tends to form a hydrophobic PCL micelle core and a hydrophilic PEG shell. The ABA-type PCEC copolymers form a micelle core with inter-micellar bridges, which helps in the gelation of such copolymers at lower temperatures compared to the BAB-type copolymers, which form a hydrophilic shell with a regular hydrophobic core [72]. Apart from this, inverse thermoresponsive hydrogels are comprised of LCGT, a more preferred choice over the UGCT for the application in biomedical. In mild conditions, only hydrogels with LCGT behavior allow the loading of cells and drugs at ambient temperature in an aqueous disperse solution [69].
4. Synthesis
This particular section will cover the synthesis approach responsible triblock and multiblock copolymers. These syntheses depend upon the arrangement of the polymer segments of PCL (A-block) and PEG (B-block) into the form of diblock (AB), PCEC (ABA), or PECE (BAB) triblock, star-shaped, multiblock and graft polymers. In the case of the diblocks, most frequently the ring-opening polymerization is applied in the presence of the catalyst SnOct214 from MPEG15 and ε-CL16 [73]. At high temperatures (185 °C), the diblock of PCL and PEG can be synthesized even in the absence of a catalyst and any solvent [74]. However, by the use of a suitable coupling agent (aliphatic and aromatic diisocyanates), PECE copolymers can be synthesized [75]. The lysine diisocyanate, 1,4-butane diisocyanate, and hexamethylene diisocyanate are some of the most common aliphatic diisocyanates [76]. Another method is to use the equimolar quantity of PCL diols and PEG diacids for the condensation reaction at room temperature [77,78]. Similarly, in the presence of a catalyst and a coupling agent (diisocyanate), PEG/PCL multiblock can be prepared by one-step copolymerization of PCL and PEG. Also, a two-step process was developed for the synthesis of PEG/PCL. This method requires the reaction of PCL diol with a coupling agent (diisocyanate) to initially synthesis an isocyanate (−NCO) end-capped prepolymer under nitrogen followed by the addition of PEG, and the reaction is allowed to continue for 24 h [64]. Another method for the two-step process is coupling the hydroxyl groups of PCEC molecules using terephtaloyl chloride [31,79]. However, these generally used organometallic catalysts for the synthesis of copolymers which are not suitable for biomedical applications. Hence the residual metal present as a contaminant form should be removed completely from the final resulting copolymer [80]. Even though stannous octoate SnOct2 is an accepted food additive by the American Food and Drug Administration (USFDA), its cytotoxicity is of great concern for the biosafety of the materials synthesized using this catalyst. Discovering novel tin-free catalysts of high biosafety is another challenge for this field [81]. In line with this, the application of metal-free strategies to carry out the ring-opening polymerization reactions has been developed to synthesize polymers with high biosafety. Additionally, the cost of copolymer production can also be lowered by eliminating the process used for the removal of metal impurities [80]. Enzymatic polymerization is another approach reported by Figueiredo et al., where they employed thermophilic carboxylesterase and Lipase B from Candida antarctica to synthesize amphiphilic polyesters for biomedical application [82]. Recently, Qindeel et al. reported a new economical and organic solvent free approach for the synthesis of copolymer using reflux assembly without nitrogen environment and used ice cold double distilled water for extraction and purification of the end product [83]. However, scale up of such ecofriendly approaches for synthesis of PCL/PEG amphiphilic block copolymers remains a major challenge.
5. Biomedical applications
Smart, intelligent injectable temperature triggered in situ hydrogels, investigated in the form of physical and chemical gels for various biomedical applications. Drug delivery and tissue engineering are the more widely explored areas related to PCL-based injectable hydrogels [84]. This section illustrates the recent developments in the field of PCL-PEG-based injectable thermosensitive hydrogels.
5.1. Injectable hydrogels for hard tissue therapy
Reconstruction of damaged/ diseases bones by the use of biomaterials has been widely investigated using bone substitutes [85–88]. In a few studies, ABM17 (bone substitute) is widely used exhibiting low immunogenicity [89–94]. Various animal models have demonstrated the success of ABM application to support osteoinduction [95]. However, the brittle ABM granules may be released from the defective cavity causing severe inflammation, with consequent pain to the patients [96–98]. In the series of linear thermosensitive hydrogels, PECE triblock copolymers were applied for bone regeneration [95,99,100]. In particular, Ni et al. have combined osteoinductive ABM, and PECE (PEG-PCL-PEG, Mn = 3150), forming injectable thermosensitive ABM/PECE hydrogel for the regeneration of cranial bone defects (Fig. 2(G–H).
Fig. 2.
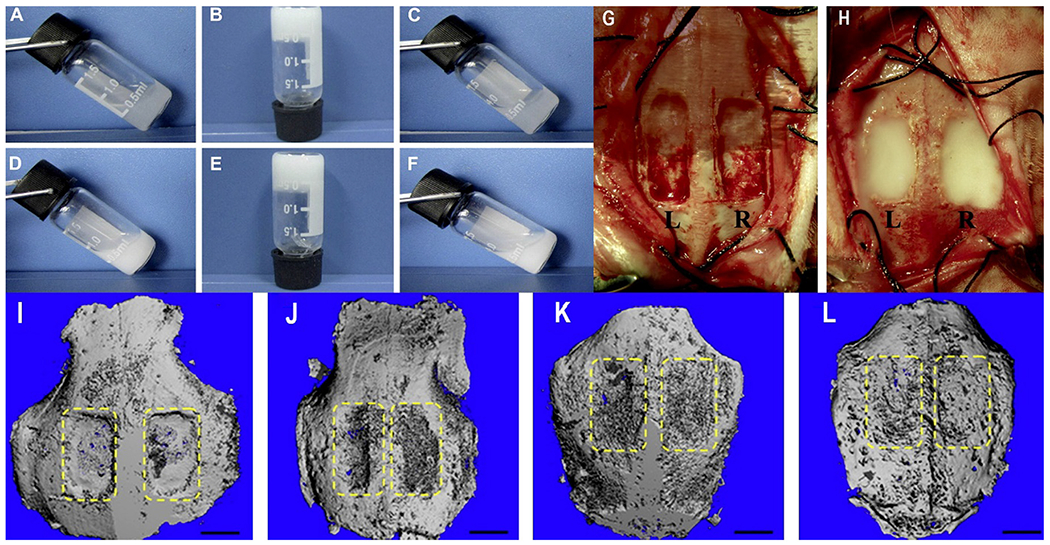
(A-F): Thermosensitivity of ABM/PECE hydrogels at various temperatures. The composites can be seen in sol state at 10 °C (A&D), opaque at physiological temperature 37 °C (B&E) and return to free-flowing sol at 10 °C (C&F). A-C: Pure PECE; D-F: ABM, 30 wt%. G&H: Surgical procedure. G: Filling of the ¼ rectangular cranial defects (12 mm × 8 mm) in New Zealand White rabbits labeled as L left, pure PECE; R ¼ right, ABM/PECE composite. Both the pure PECE (G) and the ABM/PECE composite (H) were gelled in a few minutes at the body temperature. I-L: 3D Micro-computed tomography of cranial defect shows the healing of the cranial defect at various stages of treatment, (I) Surgery moment, (J) 4 weeks, (K) 12 weeks, (L) 20 weeks. [Adopted and modified with permission from Ni et al. [101]].
The ABM/PECE composite hydrogel was in the sol state at 10 °C, formed an opaque gel at 37 ° C and returned a freely flowing solution at 10 °C (Fig. 2(A–F). The X-radiological examination revealed that the ABM/PECE-based injectable hydrogels can successfully heal the cranial defect in treated rabbits. The degree of grey-scale displayed by the x-ray imaging and the amount of new bone regeneration was more prominent as compared to its left control. Additionally, the micro-computed tomography validated the findings of the X-radiological examination. The treated right side showed excellent recovery at the 20th week as inferences from the micro-CT images (Fig. 2(I–L). Also, the prepared ABM/PECE composite was biocompatible and biodegradable having minimal host response as examined after the 4th week of the treatment. Along with this, ABM/PECE-based hydrogels offer various other advantages such as greater ease of handling, broader gelation time (body temperature), noncytotoxic with ROS osteoblasts (in vitro) and surrounding (in vivo) [102].
Additionally, bone tissue engineering has gained tremendous interest in the field of bone regeneration for various orthopedic surgeries [103–110]. In this context, Fu et al. have prepared a thermoresponsive hydrogel utilizing the triblock PECE copolymer. The PECE solution was added with nanohydroxyapatite (n-HA) to develop a thermosensitive PECE/n-HA hydrogel composite. At the same time, the PECE/Collagen/n-HA tricomponent injectable system has been made to improve cellular attachment [111].
The PECE/Collagen/n-HA composite freely flowed at room temperature and was gradually converted into a gel by increasing the temperature. In detail, PECE/Collagen/n-HA (Fig. 3A) composite system behaved like a low viscous solution (with the low elastic modulus (G′) and viscous modulus (G″)) at room temperature. As the temperature tends to increase G′ and G″ increased up to a maximum value at 37 °C (Fig. 3B), exhibiting gel formation. Further, raising the temperature caused a rapid decrease in G′ and G″ (Fig. 3C). Additionally, in vivo experiments have been performed to study the degradation of the hydrogel composite post-surgery effect in a muscle pouch for 7 to 14 days. The composite was degraded entirely after 12 weeks post-implantation. Thus, the injectable PECE/Collagen/n-HA hydrogel represents a potential smart composite injectable hydrogel for reconstructive and orthopedic surgery.
Fig. 3.
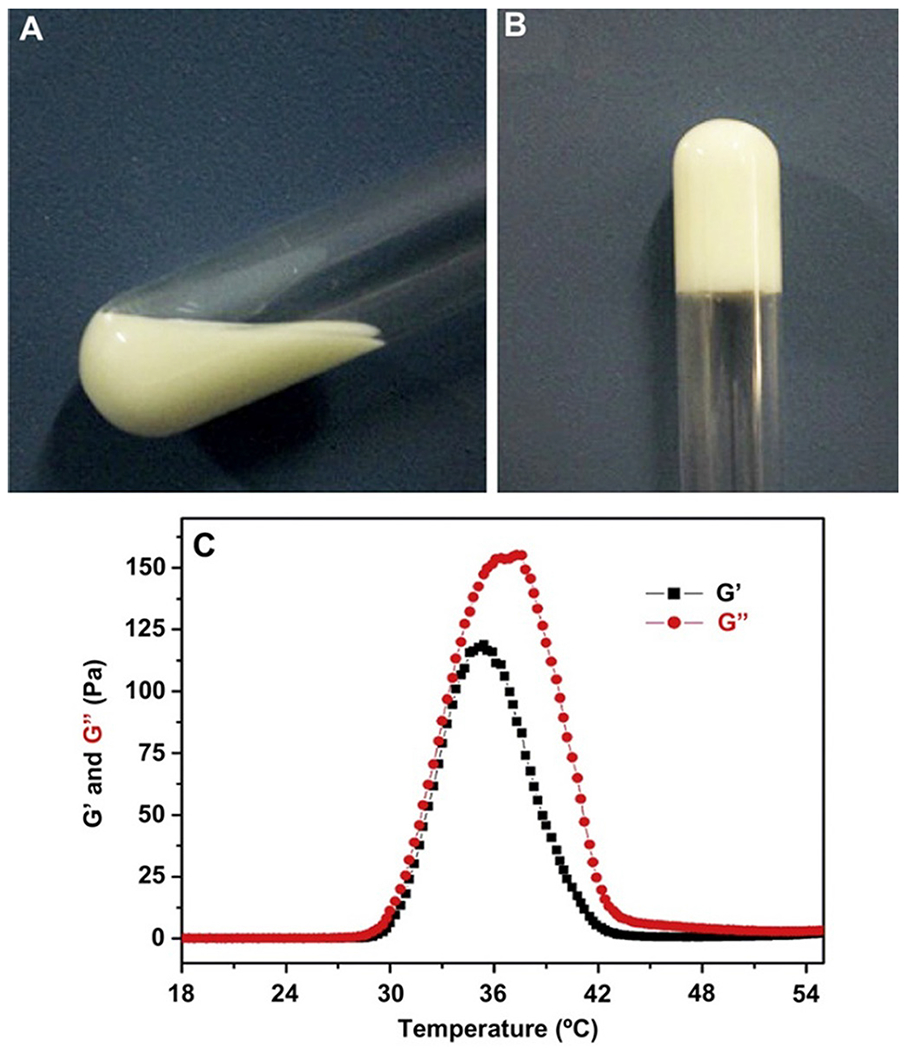
Injectable hydrogel composite is showing thermal sensitivity. Fig. 3 (A) and (B) show gel formation at 20 °C and 37 °C respectively. Fig. 3(C) demonstrates the rheological behavior of G’ and G” for the hydrogel composite upon increasing the temperature. [Adopted with permission from Fu et al.[112]].
Similarly, guided bone regeneration was achieved by fabricating a bilayer composite material with nano calcium phosphate (CaP) incorporated into PCEC block copolymer and covered with an additional PCL layer. This structural difference compared to the pristine PCEC nanocomposite provided an occlusive barrier for the connective tissue, owing to the presence of the PCL layer. This directs the bone tissue regeneration by hydrophilic CaP incorporated in PCEC layer. The addition of the CaP can reinforce the nanocomposite’s mechanical strength, which is crucial for load-bearing bone regeneration. Additionally, the prepared nanocomposites, due to the presence of the CaP provided the nucleation sites for the biological minerals and triggered the mineralization. This, in turn, improved the osteoinductive and osteoconductive properties of the membranes. Hence, it can be said that in addition to the PECE nanocomposites, the PCEC nanocomposites, modified with CoP and extra PCL layer, are potential biomaterials for guided bone regeneration [113].
Apart from these, synthetic thermo-responsible biodegradable polymeric systems based on polyethylene glycol-polypropylene glycol block copolymers (PEO-PPO-PEO) and poly (N-isopropylacrylamide) (PNIPA or PNIPAAM) are being used for delivering the pre differentiated bone marrow stem cells. However, these materials lack osteoinductive and osteoconductive properties; hence, PCEC/PECE-based copolymers are the preferred candidates for hard tissue therapy application[114].
5.2. Injectable hydrogels for soft tissue therapy
Hydrogels are used for various biomedical uses directly just after their preparation (with or without cell) or used after the scaffold preparation. This is because of their unique ability to adopt the characteristic property of the natural tissue. A hydrogel scaffold is meant to support the mechanical structure of a repaired tissue by adhering to the 3D hydrogel framework. Among so many advantages of hydrogels scaffolds, there are associated limitations too. These include irregular seeding owing to the deficient spatial, temporal control; the reduced mechanical strength of the hydrogels limits their use for soft and non-load bearing tissues, and the issue of microvasculature leading to a loss of functional and viability of the seeded cell owing to the insufficient transportation of signaling molecule and nutrients. Apart from these limitations, the hydrogel scaffold remains an interesting topic in tissue engineering. Notably, in the case of repairing articular cartilage lesions by the aid of a combined biodegradable scaffold with chondrocytes [115–118]. In such type of work, small clusters of articular chondrocytes were extracted from the individual patient followed by large-scale cultivation and finally seeded to biodegradable porous polymeric scaffolds [119]. These cell-embedded scaffolds were applied to the defective site for regeneration of articular cartilage. These scaffolds facilitate the mass transfer of nutrients and oxygen via their porous structure for sufficient cell seedlings. The addition of ammonium bicarbonate salt particles into the scaffolds produces a highly interconnected pore structure for high cell seeding density [120–122]. For example, Park et al. developed a biodegradable flexible hydrogel scaffold by modulating the block composition and ratio to get properties like swelling ratio, biodegradability, flexibility, elasticity and hydrophilicity [123]. In order to encapsulate the chondrocytes in media, a 3-D culture was prepared to comprise a porous hydrogel scaffold to facilitate penetration of the inner state of the scaffold [124–126]. This precludes the adaptation of the chondrocytes once implanted inside the body [127,128]. The author preferred to use a radical cross-linking reaction of PEG-DA18 and PCL-DA19 with sodium chloride salt particulates. This salt leaching produces a highly porous structure of the hydrogel. In addition, both in vitro and in vivo results (cell culture with rabbit chondrocytes) demonstrate that the cells and tissues exhibited different swelling ratios in the scaffold to facilitate tissue regeneration by efficient cell growth. Hence, in their work, they prove that PEG/PCL-based hydrogel scaffolds could be used for soft tissue regeneration.
In another piece of work, Oroojalian et al. reported the use of thermosensitive PNIPAAm based hydrogel for wound healing. PNIPAAm is a thermosensitive polymer with a transition temperature of 37 °C. However, the hydrogel prepared using only PNIPAAm was fragile and did not provide sufficient mechanical strength [129]. Thus, the author synthesized penta-block copolymer (PNIPAAm-PCL-PEG-PCL-PNIPAAm) for the preparation of hydrogel to achieve sufficient mechanical strength. On the other hand, the investigators synthesized a star-shaped copolymer [poly(CL—Co—LA)-b-PEG] and compared the wound healing potential of both kinds of polymer-based hydrogels. The cell proliferation and fibroblast cultivation were higher in 20%w/w Penta-block copolymer hydrogel than 10%w/w hydrogel. The prepared Penta-block copolymer showed stable depot on Sprague-Dawley rats and degraded over a month. Besides, the star-shaped copolymer was not recommended for skin tissue engineering as it has low molecular weight and poor mechanical strength, whereas Penta-block copolymer at 20%w/w remained a potential scaffold for wound healing and tissue engineering [130].
In another tissue engineering application of the hydrogel system, PCL-PEG diblock has been merged with another PCL to get a PCL-PEG-PCL triblock copolymer for better cellular compatibility. Noormohammadi et al. have developed polyurethane (PU) (0.1 and 0.25%) based nanocomposites with PCL500-PEGX-PCL500 for tissue engineering applications. The molecular weight of PEG used in this study was 1000 and 2000 g/mol to prepare triblock with PCL. In addition, cellulose nanowhisker (CNW) was utilized as a crosslinker, and the prepared modified PU (0.25%) with PCL-PEG-PCL showed better cell compatibility in cellular studies than PU (0.1%) and can be used for tissue engineering [131].
Similarly, Wang and their team investigated on BMSCs20 to survive and to produce good cell retention to suppress infarction expansion with inhibition of left ventricular (LV) remodeling [132]. These BMSCs were encapsulated in an injectable MPEG–PCL–MPEG21 hydrogel. They refer to this strategy more because the available implantation techniques are not suitable to offer cell custody in culture medium via coronary injection or intramyocardial injection. In addition, these old technique applications are limited because of low cell retention and its survival mainly due to the lack of ECM22 in the infarcted myocardium [133,134].
In contrast, MPEG–PCL–MPEG hydrogel quickly solidifies to support the internal structure to cure impaired cardiac function and at the same time to reduce the cardiac remodeling too, when injected intramyocardially [135]. Significant cell retention and survival (100%) was observed when performed on rabbit for in vivo studies and for in vitro MTT assay was used to validate cytotoxicity of the hydrogels concerning BMSCs proliferation. As reported earlier in their studies, α-CD/MPEG-PCL-MPEG hydrogel composite is not accountable for angiogenesis. However, a hydrogel holding BMSCs upsurges the vessel density in the region of the infarct as compared to BMSCs implanted without hydrogel. Thus, it is clear that intramyocardial injection of BMSCs with hydrogel composite could upsurge the cell holding and vessel density nearby the area of infarction, and prevent LV remodeling thereof.
Interestingly, transforming growth factor- β1 (TGF β1) has been reported to have a vital role in the process of chondrogenesis. It is associated with chondrocyte growth, along with the processes involved in the differentiation and augmentation of the biomechanical properties of neocartilage [136–138]. In line with this Tengfei et al. (2017) prepared TGF-β1 loaded PCEC based hydrogel to be exploited in cartilage repair for the first time (Fig. 4A). The sol-gel transition temperature was found to be in the range of 20–50 °C (Fig. 4B–C) with excellent syringeability and in vitro injectability (Fig. 4D). The storage and loss modulus study reveal the viscoelastic properties of the hydrogel as it represents the elasticity part (G′) and viscosity of the hydrogel, further it confirms the TGF-β1 loaded hydrogel stayed at gel phase at 37 °C (Fig. 4E). The study claimed 20% degradation within 3 weeks when the hydrogel was given subcutaneously in rat knee. Also, a sustained release of TGF β1 was seen throughout 120 h without any initial burst release, while 30% of its part remained inside the hydrogel matrix. Additionally, the macroscopic evaluation study proved that the treatment with TGF β1 loaded hydrogel resulted in the superior deposition of similar peri-native cartilage tissue, indicating its potential in cartilage defect repair as compared to control over an 8 weeks treatment period (Fig. 4F–K) [139]. However, Saghebasl et al., in their comparative evaluation, found the dominancy of PNIPAAm-PCEC-PNIPAAm over PCEC-based thermosensitive hydrogel towards the cartilage repair. They demonstrated the increased survival rate in cells coated and seeded on scaffolds based on PNIPAAm-PCEC-PNIPAAm hydrogel compared to the PCEC gel alone. Additionally, the PNIPAAM-PCEC hydrogel enhanced the expression of Sox-6, collage-II, and COMP genes after 10 days of in vitro study[140].
Fig. 4.
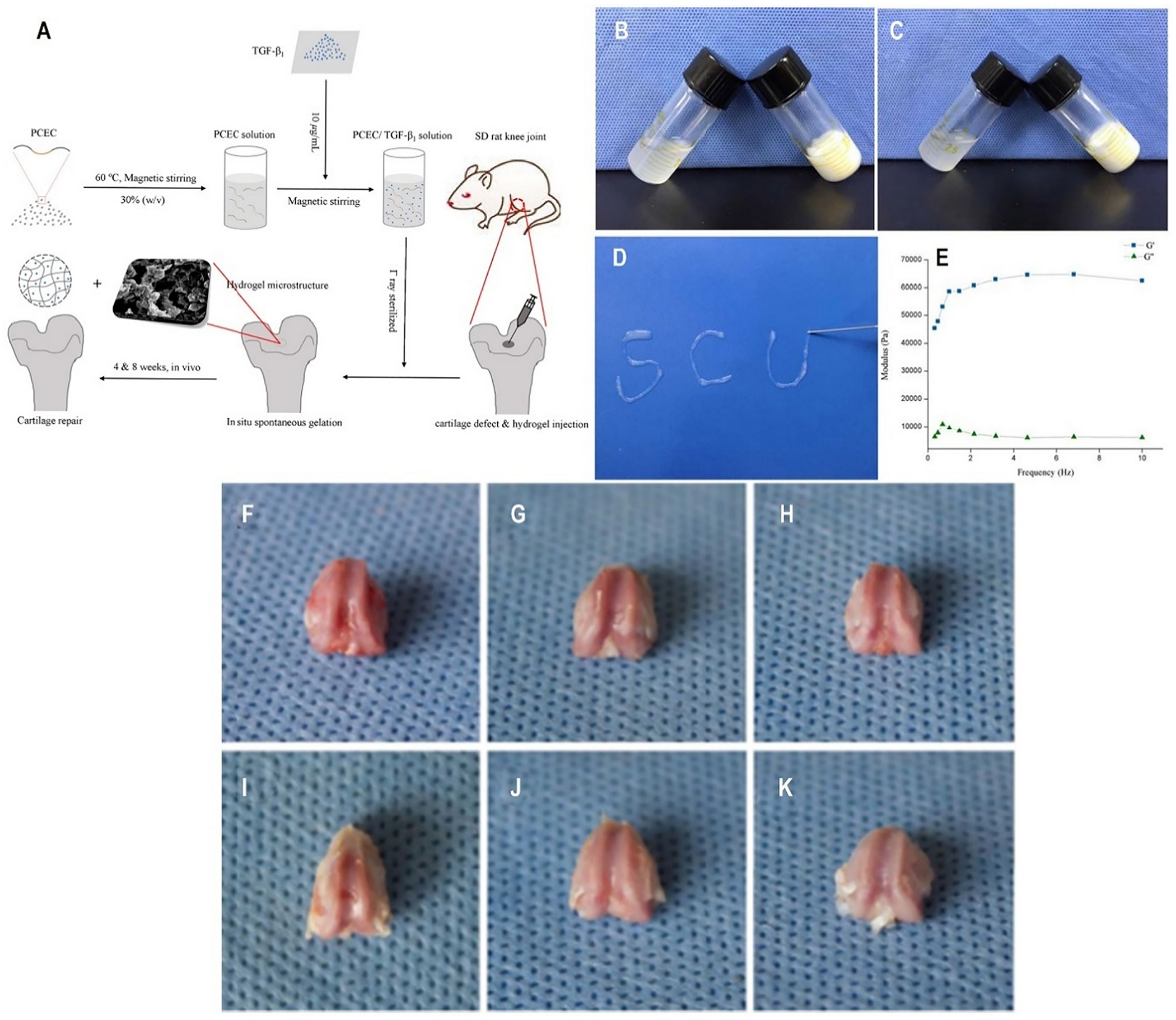
A-K: (A) Schematic illustration of preparation process of TGF-β1/PCEC hydrogel system fabricated with simple procedures; (B) Sol-gel transition of bare PCEC hydrogel. (C) The sol-gel transition of TGF-β1 loaded PCEC hydrogel. (D) In vitro injectability test of TGF-β1 loaded PCEC hydrogel; (E) Rheology test of TGF-β1 loaded PCEC hydrogel (G’: storage modulus, G”: loss modulus); (F-H) Frontal views of rat knee joint defect samples at 4 weeks, with control, bare PCEC hydrogel and TGF-β1 loaded hydrogel groups arranged from left to right. Defects still caved in, with rough nascent tissue grown in. (I-K) Frontal views of rat knee joint defect samples at 8 weeks, with control, bare PCEC hydrogel and TGF-β1 loaded hydrogel groups arranged from left to right. Cartilage-like tissue filled in defects with gradually obscured boundaries, except for the control group. [Adopted and modified with permission from Zhou et al. [141]]
5.3. Injectable hydrogels for cancer therapy
Cancer represents the prominent reasons for mortality and morbidity throughout the world. As per the report of Global Cancer Observatory by WHO, 2020, cancer is the most prevalent disease with the highest mortality rate and considerably increasing cases year by year. In the year 2020, there are 19,292,789 new cases and 9,958,133 mortalities reported throughout the world. These statistics included the number of all kinds of cancer reported in both sexes all around the globe [142,143]. Numerous treatment options are available like radiation, surgery, hormonal therapy, chemotherapy, immunotherapy, and gene therapy [144,145]. Among these, chemotherapy is the most preferred one to exploit using a cytotoxic drug against cancer cells for the treatment of cancer and inhibition of tumor recurrence [146–148]. The localized drug delivery technique is the most effective one among traditional chemotherapies as it reduces the normal tissue toxicity by avoiding the presence of chemotherapeutics in the systemic circulation. Also, at the same time, it also provides localized action with the sustained release of chemotherapeutics [149,150]. To achieve a localized therapeutic response, a hydrogel carrier is the best approach for efficient drug delivery. Intratumor drug delivery through injectable biodegradable hydrogels remains the most attractive approach which provides a sustained and controlled drug delivery directly into the tumor site and at the same time reduces the systemic toxicity. It also minimizes the associated problem of the chemotherapeutic agents, like poor solubility, and reduces the amount of drug to be loaded into the hydrogel [21,84,151–153]. The requirement of elevated temperature for the unstable anticancer drugs is unsuitable for preparing hydrogel formulation [154]. To translate the thermosensitive injectable hydrogel for anticancer drug from bench to bedside a lot of strategies need to be deployed to overcome the formulation factors like sterilization, scale-up, shelf-life, and improve patient compliance [155]. Additionally, many approved and undertrial anticancer drugs are hydrophobic in nature and most of the injectable hydrogels are hydrophilic in nature. This in turn leads to poor drug loading and entrapment in the hydrogel exhibiting insufficient drug release profile thereof [156]. Also, in order to improve the bioavailability many times high dose of such hydrophobic agents are required to achieve desired effect at the injection site [157,158]. Hence to address the above in this section, we have highlighted the recent findings towards the PCL-PEG based in situ injectable hydrogels for the treatment of cancer.
The modified hydrogel system was also explored to improve the efficacy of drugs and to get sustained release. With this objective, a dual drug-loaded thermoresponsive hydrogel was developed by encapsulating one hydrophobic and one hydrophilic drug, paclitaxel (PTX), and doxorubicin (DOX), respectively. For the development of thermoresponsive hydrogel PEG-PCL was modified into poly(e-caprolactone-co-1,4,8-trioxa[4.6]spiro-9-undecanone)-poly(ethylene glycol)-poly(ecaprolaone-co-1,4,8-trioxa[4.6]spiro-9-undecanone) (PECT) by ring-opening copolymerization method. The dual drugs PTX and DOX were loaded into the hydrogel system without affecting the thermo-sensitive property. The increase in viscosity of hydrogel was observed at 31 °C and observed maximum in the range of 35–38 °C. The test tube inverting method showed gel formation at 37 °C. The hydrogel system released DOX by initial burst effect while PTX in a sustained manner. The PTX-DOX hydrogel system offers a fast and intense tumor suppression activity due to burst release of DOX which was prolonged to subsequent sustained release of PTX [159]. Similarly, Yu et al. prepared a thermogelling system based on PLGA-PEG-PLGA block copolymer to sustain DOX delivery. The prepared gel was retained at the target site for more than 20 days upon a subcutaneous injection. However, the viscosity of the hydrogel system was significantly increased after the DOX loading, which is a concerning issue for the injectability of the hydrogel[160]. Also, DOX has been incorporated into the poly(N-isopropylacrylamide) (PNIPAAm) star polymer having an β-CD core and adamantyl-terminated poly(ethylene glycol) based hydrogel. PNIPAAm can strengthen the structural stability of the hydrogels when the temperature is raised from 25 °C to 37 °C. The hydrogel was dissolved very slowly and released the pseudo block copolymer in the form of micelles which further sustained the DOX release encapsulated in the hydrophobic core. This resulted in better cellular uptake even for the multidrug-resistant cancer cells[161]. Wu et al. have worked on the development of thermosensitive injectable hydrogel for the loading of the dual drug, cisplatin (DDP) and paclitaxel (PTX) [162]. These drugs were selected as useful chemotherapeutic agents for the treatment of lung cancer. In general, degradable polymers are preferred for the preparation of the NDDS.23 Also, MPEG–PCL copolymers are extensively used for the development of micelles towards the delivery of an antitumor drug. In such copolymers, PCL is used as a hydrophobic segment and forms a micelle core for hydrophobic drugs. On the other hand, PEG being a hydrophilic segment forms an envelope and remains as an excellent stabilizing interface [163,164]. Thus, MPEG-PCL micelles represent water-based formulations for the encapsulation of hydrophobic drugs. Micelles may improve the therapeutic efficacy by EPR.24 Micelles, included in a thermosensitive PECE hydrogel. The objective of the work was the administration of the drug via intratumoral injection directly to the lung using PECE hydrogels for controlled drug release of drug-loaded micelles. In detail, PECE hydrogel including DDP and MPEG–PCL micelles loaded with PTX (Fig. 5A) prepared. The resulting hydrogel, coded as PDMP.
Fig. 5.
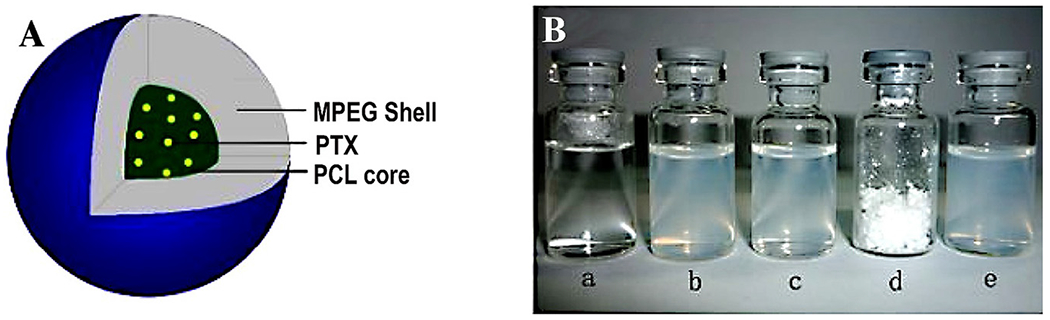
(A) illustration of PTX micelles; (B) (a) normal saline (NS), (b) only micelles (blank), (c) MPEG-PCL/PTX micelles, (d) lyophilized powder of PTX micelles, and (e) re-dissolved freeze-dried PTX micelles. [Adopted and modified with permission from Wu et al.[165]].
To validate micelles, a comparative in vitro release test of PTX from free PTX solution and MPEG-PCL/PTX micelles (Fig. 5B) through a modified dialysis membrane. Nearly 100% of the free PTX got released within 4 days, indicating no effect on the dialysis membrane. On the other hand, less than 65% of the drug, released from the MPEG–PCL/PTX micelles up to 2 weeks. This release pattern confirmed the sustained release as compared to free PTX. This feature further established the stable structure of polymeric micelles. To study the gelling kinetics of PECE alone and PDMP hydrogel composite, storage modulus (G′) and loss modulus (the G″) were measured. At low temperatures, G′ and G″ of PDMP were low, showing low viscosity and fluidity of the composite. As the temperature increases, the solution transformed to an elastic-like gel at the crossover point of G′ and G″. The composite viscosity was highest at a temperature above 40 °C. Thus, the PDMP hydrogel composite exhibited thermosensitivity. During this study, a consistent thermo-sensitive property of PDPM hydrogel composite as compared to blank PECE hydrogel was observed. This gelling behavior of PDPM hydrogel composite suggested its appropriate administration via intratumoral injection. Among various available PCL-PEGs based thermoresponsive hydrogel, PECE composites remain the most preferred one. The PECE exhibits excellent biocompatibility, and at the same time, they are biodegradable too. These composite can very well reduce the initial burst of the drug especially, in the case of an anticancer drug to reduce hemolysis (in the case of an alkylating agent) [21,153]. The PECE composite is therefore very well exploited for the treatment of cancer, also because of its good syringeability and injectability to provide local action. In a similar study, Shim et al. prepared sulfamethazine conjugated PCLA-PEG-PCLA based pH and thermoresponsive block copolymer to achieve PTX sustained release upto 1 month without any burst release. However, the properties of the hydrogel could only be conserved upto 5 mg/ml of PTX loading[166].
Fang et al. developed a delivery system to sustain the release of a hydrophobic anticancerous drug, the honokiol (HK) [167]. In the case of various cancer patients, the common occurrences of malignant pleural effusion are noticed in many cases. This becomes very difficult to treat thereof. The first improved the poor solubility of the HK by converting it into a stable nanoparticle (NP) by using the solvent evaporation method, which is readily dispersed in water [168]. The prepared HK-NP concentration was 25 mg/ml with a size range of about 33 nm exhibiting a good zeta potential of −0.385 mv which is close to neutral. After that, the prepared HK-NP was loaded into the PECE hydrogel synthesized by ring-opening copolymerization technique to produce HK-hydrogel. The PCL/PEG molecular weight was 3408, and the ratio of the triblock copolymer was PEG480-PCL2448-PEG480. An effective administration of the HK hydrogel through intrapleural injection would be responsible for the tumor cell death. This reduced the permeability of pleural vascular and prevented the formation of any more blood vessels in pleural tumors [167]. The release of HK from the HK-hydrogel was only 10% up to 72 h would prevent the exposure of the HK with the healthier tissue and enhance its accumulation at the target site. Thus, the HK-hydrogel efficiently successfully decreased the density of microvessels in the pleural tumors. Similarly, Gong et al. have developed the same thermoresponsive composite of PECE triblock copolymer comprising of different ratios of PCL/PEG to deliver HK [169]. They prepared biodegradable HK-loaded PECE micelles by ultrasonication method and kept the triblock ratio of PCL/PEG as PEG5000-PCL5000-PEG5000 in the absence of any organic solvent. HK micelles were found to be small (~59 nm) and neutral (zeta potential was −0.385 mV) making it more competent to sustain the release of the HK by self-assembly. The cellular uptake of the HK micelles exhibited excellent cytotoxicity towards the cancerous cells, and at the same time, the sustained release was also obtained at a low concentration within the first 48 h. This, in turn, lowers the exposure to the healthy tissue and provides a local effect by increasing the accumulation of the drug at the tumor site. Later on, Wang et al. have also recommended the potential application of the PECE triblock copolymer composite hydrogel as a prominent delivery system for the controlled release of 5-fluorouracil (5-FU) [170]. Wang and their team noticed that peritoneal carcinomatosis (PC) of cancers required an efficient delivery system that would not only deliver the drug at the tumor site but can also reduce the systemic toxicity too [171]. In this context, they selected intraperitoneal chemotherapy as a route to deliver 5-FU to cure colorectal cancer [172]. They synthesized a PECE copolymer composite in the ratio of PEG960-PCL2448-PEG960. The in vitro drug release suggested that a lower dose (0.5 mg) of 5-FU from PECE hydrogel exhibited an initial burst (26%) of the drug within 1 h and higher drug release (~80%) in 1 day. This drug release behavior is essential for intraperitoneal chemotherapy, i.e., delayed-release with prolonging retention and effective drug permeation to control the drug release. In addition, the 5-Fu-hydrogel remains an injectable solution at 32 °C and transforms sol-gel at 37 °C confirming the controlled release of the drug thereof. Thus, to control the release of 5-FU the biodegradable PECE hydrogel remains the most prominent delivery system. In another instance, Chantal et al., developed a Poloxamer 407 (PEO98–PPO67–PEO98, MW 12.6 kDa) and Poloxamer 188 (PEO80–PPO30-PEO80; MW 8.4 kDa) based thermosensitive hydrogel to incorporate the 5-fluorouracil as neoadjuvant or adjuvant therapy in colorectal cancer. The Tsol-gel transition was found at 26 °C and drug release behavior from hydrogel demonstrated an initial burst release of 50% within 1 h which further completely released in 9 h. Also, the in vivo study in the mice after intra tumoral injection exhibited reduced systemic toxicity and increased the concentration of the drug at the site of action. However, the mice survival was increased in the adjuvant therapy (after tumor excision) when treated with 5-Fluorouracil hydrogel compared with the 5- Fluorouracil free hydrogel. This study reveals the efficiency of both Poloxamer 407 and Poloxamer 188 based thermosensitive hydrogels for cancer treatment [173].
Similarly, Lei et al. expanded the therapeutic efficacy of PECE hydrogel for drug targeting to achieve the local effect with excellent anti-tumor activity especially in the case of malignant diseases.[174] They investigated the recurrence of the tumor growth in the locoregional after tumor removal especially, in the case of breast cancer [175–178]. To confirm the study, they selected PTX as a model drug and further to increase its solubility, it is converted to micelle first. Thereafter, to deliver the PTX micelle in the target site and loaded to PECE hydrogel (PTX-PECE hydrogel) with different block ration of PEG2000-PCL2000-PEG2000. To confirm the in vitro anti-tumor activity, the 4 T1 cell line was used for 3 days. Significant cytotoxicity towards the tumor cell was observed in the case of PTX-PECE hydrogel. To investigate the recurrence of breast cancer, they selected the mice model, and only PTX-PECE hydrogel could delay the recurrence time up to an 18th day. The drug concentration was significantly increased (70 folds higher than the free PTX group) at the local site in the case of PTX-PECE hydrogel. This study confirms the efficiency of PTX-PECE hydrogel towards the prevention of the recurrence of breast cancer after the tumor resection in the case of the mouse model.
Additionally, injectable PCL-PEG hydrogels were developed for intraperitoneal chemotherapy which remains a promising strategy over conventional intravenous chemotherapy [179,180]. Intraperitoneal combinational therapy requires a suitable delivery system, loaded with the drug. Thus, an ideal delivery system must be able to transport both hydrophilic and hydrophobic drugs [181]. In this context, the dual drug delivery system (DDDS) like thermosensitive hydrogels composed of block copolymers are suitable to protect the drug from degradation, prolong the drug release, and above all to maintain their bioactivity. Based on that, Gong et al. developed a blend of biodegradable PCEC copolymers to produce thermosensitive hydrogels. The authors used a hydrophilic (Fluorouracil (Fu)) and hydrophobic PTX drug, loaded into the PCEC hydrogel [182]. Firstly, PTX was encapsulated into the PCEC copolymer solution in the absence of any surfactant to produce PCEC micelles (PTX encapsulated). Likewise, the PCEC solution was added with Fu [183,184]. Then, the prepared PTX micelle was finally encapsulated into the thermoresponsive Fu-hydrogel forming a stable homogeneous solution. A temperature triggered a noteworthy alteration in G′ and G″ in the case of PTX-micelles loaded Fu-hydrogel was detected. The hydrophilic/ hydrophobic molecular structure balance and macromolecular weight of PECE copolymers significantly influenced the phase transitions. In vivo studies validated the formation of the hydrogel when injected subcutaneously into KunMing mice. Following subcutaneous injection, the images were taken on different days ranging from day 1 to day 14 and observed a quick formation of a gel with a spherical shape. To underline the effectiveness, the gel was compared with the Pluronic (F127) gel which wholly dissolved within a few hours. Hydrophilic drug almost got released entirely within a week from hydrogel with high initial burst release; in contrast, the hydrophobic drug showed slower release up to 14 days. The driving forces behind this drug release pattern from hydrogel were diffusion-controlled and degradation/ erosion of the hydrogel [185]. Hence, such hydrogels, used for controlled and sustained drug release.
5.4. Injectable hydrogel for intracameral application
To decrease IOP25 and at the same time maintain the vision, GFS26 is the most recommended one [186]. The first line prescribed pharmacologic modulation drugs inhibit fibroblast proliferation. This interferes with the wound healing process and thus diminishes the post-operative wound-healing response. Thus, intracameral injection in the anterior chamber facilitates the diffuse distribution of the drug.
Antiadhesive injectable materials can be useful for ocular applications. For instance, to reduce the IOP for proper vision, glaucoma filtration surgery remains the most effective method [186,187]. Also, postoperative adhesion, avoided by improved surgical techniques. However, postoperative marks with scars are the primary cause of failure; hence modulating agents have to be applied. These agents cause premature wound healing generally by hampering fibroblast proliferation; this decreases postoperative wound-healing development thereof. Therefore repeated/frequent conjunctival injections of the drugs are preferred. Also, modulating agents like 5-FU27 and MMC28 cause cell death and apoptosis ensuing in the increased application of angiogenesis inhibiting compounds, acting in contradiction of VEGF,29 commonly known as anti-VEGF compounds [188–194]. Bevacizumab (humanized monoclonal antibody), applied against VEGF-A [195–197]. Its administration into the anterior chamber remains uncertain while subconjunctival injection produces a secondary therapeutic effect and repetitive injections are thus required [198]. Therefore, to overcome this problem, Han et al. have reported a method for the preparation of an injectable PECE thermosensitive hydrogel [199]. Mainly, intracameral injection of bevacizumab (antifibrotic drug) loaded PECE hydrogel is highly recommended in the postoperative scarring management after GFS in the rabbits and has the potential to avoid postoperative adhesions too [200,201].
In vivo, animal studies were performed before and after GFS. With particular concern to filtration bleb and anterior chamber, eyes were observed for post-operative 28 days. The changes seen after PECE thermoresponsive hydrogel is shown in Fig. 6. PECE thermoresponsive hydrogel was utterly absorbed within 3 weeks with no corneal edema. Thus, bevacizumab-loaded PECE hydrogels represent a potential sustained drug delivery system for intracameral injection to prevent post-operative adhesions. However, Rauck et al. studied the bevacizumab release from the PEG-poly-(serinolhexamethylene urethane) (ESHU) hydrogel for 9 weeks. The bevacizumab concentration in the eyes was found to be 4 times higher than the bolus injection. Moreover, the claimed superiority and biocompatibility of ESHU hydrogel over PECE or PEG-PLA-PEG-based hydrogels was more in terms of lactic acid formation as degradation products[203].
Fig. 6.
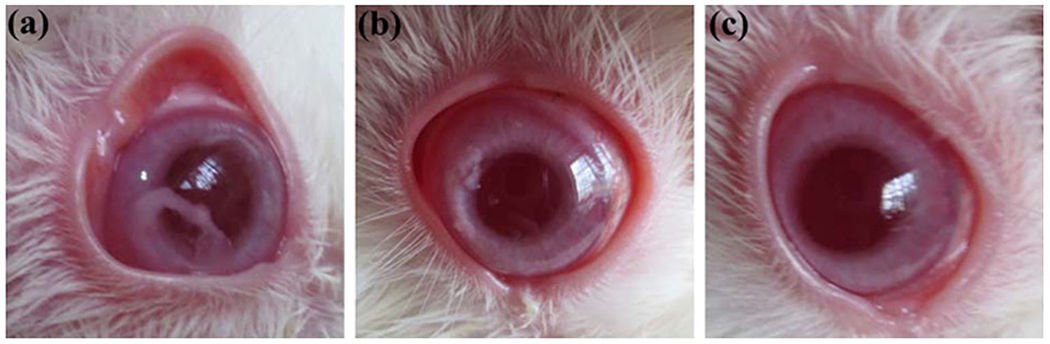
PECE hydrogel degrades in the anterior chamber. (a) PECE, sol-to-gel transition just after administration into the anterior chamber just above the iris; (b) PECE hydrogel, in 7 days got absorbed in the rabbit eyes; (c) PECE hydrogel completely vanished (absorbed) after 21 days. [Adopted with permission from Han et al. [202]].
5.5. Injectable hydrogels for controlled drug delivery
In situ thermoresponsive injectable hydrogels are extremely useful when controlled, and a sustained drug release profile is required. The modulation of rheological and drug release as well as degradation properties is necessary for proper hydrogel design. The molecular weight and composition of the copolymer can be varied to modulate the rheological properties [204]. Amphiphilicity is a crucial factor that controls the sol-to-gel transition. Thus, there is a need for a balanced molecular weight of hydrophilic and hydrophobic blocks in the copolymers to have injectability.
The family of Poloxamer or Pluronic copolymers, chemically known as PEG-PPG-PEG30 triblock copolymers, possesses thermo-responsive behavior and is one of the most explored in the developments of novel carrier systems [12,205]. Nevertheless, the reduced hydrophobicity of PPG block results in a high CMC, which is one of the major problems of such copolymers. Additionally, Pluronic hydrogels are fast eroding and non-biodegradable that may cause accumulation of plasma cholesterol and triglycerol in the body thereby inducing systemic toxicity [206]. Such limitations persuaded the search for substitution of PPG block in Pluronic copolymer backbone. The PCL substituted co-polymer solved the limitations resulting in reduced CMC, inferior molecular weight after degradation and possible elimination of degradation products from the body [34,207]. PCL is an FDA-approved polymer that is biodegradable and biocompatible and possesses excellent permeability. Following this substitution, Gong et al. have prepared a biodegradable, thermosensitive, injectable hydrogel consuming PECE copolymers for controlled drug delivery [208]. The synthesis of PECE triblock copolymer is a multi-step process that initially involves the synthesis of PEG-PCL diblock copolymer using ε-CL as a precursor compound. The ring-opening copolymerization was the method of choice, and the process was initiated by MPEG using stannous octoate as a catalyst. After that, this PEG-PCL di-block was further used to synthesize PECE triblock biodegradable polymer by using HMDI as a coupling reagent [182]. The PECE copolymer hydrogel showed a concentration-dependent CGC, LCGT, and UCGT in an aqueous solution. The sol-to-gel transition of PECE hydrogels is thus triggered by the accumulation and packing of micelles above CGC at LCGT [207,209]. A further rise in the surrounding temperature, increases the molecular movement of the PCL block, stimulating reverse transition or gel-to-sol transition [210]. A broader gelation region of PECE, observed with the increasing length of PCL block. Higher molecular weight PCL block caused a decrease in the LCGT (from 35 to 31 °C) and CGC, whereas only slightly increased the UCGT of PECE hydrogel. A hydrogel solution, subcutaneously injected into KunMing mice, was converted into a gel within a few minutes and the gel was stable for 13 days. In the biological environment, the gel started degrading with time to release the drug entrapped, and at 14 days, almost entirely dissolved.
On the contrary, the gel prepared from Pluronic (F127) got dissolved in a few seconds only. Such prolonged effect of PECE hydrogel was attributed to reduced degradation time and increased molecular weight with respect to Pluronic F127. The decreased degradation rate of PECE was due to the behavior of polyester or polyester copolymers [211–214]. Accordingly, the PECE-based injectable thermosensitive hydrogel remained used as carriers for sustained and controlled drug delivery.
There are numerous works reported in the field of thermoresponsive injectable hydrogels in recent past years. Some of the investigators have pointed out the difficulty in injectability of the thermoresponsive hydrogel due to the increased temperature of the needle during insertion into the body [206,215,216]. To overcome this problem, Huynh et al. explored the potential capabilities of pH/temperature-sensitive hydrogels towards successful drug delivery. However, there are a few shortcomings too in using these hydrogels like the drug release pattern through hydrogel follows diffusion-controlled behavior failing to prevent an initial burst release of the drug [217,218]. To prepare this dual-functional system of injectable pH/temperature-sensitive hydrogel, poly (b-amino ester) (PAE), used as a pH-sensitive block and after that incorporated with an amphiphilic PEG-PCL-PEG triblock thermosensitive copolymer. The prepared injectable hydrogel (pH/temperature-sensitive) is a result of the formation of Penta-block copolymer PAE1258-PCL1584-PEG1650-PCL1584-PAE1258. At a low pH (6.5) the solubility of the PAE block increases due to ionization, and at the same time, PEG/PCL exhibited hydrophilicity at low temperature. As the pH and temperature increase (7.4 and 37 °C, respectively) PAE and PCL block tend to increase their hydrophobicity due to the rise in pH and temperature, respectively. This results in the formation of a micelle structure in the presence of the aqueous environment with PAE-PCLs in the core region surrounded by the hydrophilic PEG groups to form a stable gel phase. To study the drug release behavior, insulin added, and notice that the loading of insulin to the hydrogel, the lower critical gel temperature (CGT) the sol-gel transition decreases with increased insulin concentration. In contrast, the upper CGT and critical gel pH (CGpH) remain unchanged. Further, the in vivo studies were performed on male Sprague-Dawley (SD) rats, demonstrating a diffusion-controlled release of insulin from the hydrogel for more than 15 days followed by subcutaneous injection.
In another study, Kim et al. have evaluated RGD peptides conjugated thermosensitive mPEG-PCL hydrogel to study adhesion-dependent cellular behavior. This hydrogel-based RGD peptide was prepared using their own patented technology, and the conjugation of RGD to mPEG (750 g/mol)-PCL was achieved using a nucleophilic reaction. The hydrogel formulation retains as a suspension at ambient temperature. Sol-gel phase transition occurred when the temperature increased at 37 °C with a 2.4 mM concentration of RGD peptide-containing hydrogel system. The RGD conjugated peptide significantly enhanced the focal adhesion and thus facilitated the cytoskeleton reorganization through an integrin-mediated cell signaling mechanism. The mPEG-PCL injectable hydrogel provided sustained release [219].
Later on, similarly, Huynh et al. extended this investigation to examine the efficacy of the delivery system on streptozotocin (STZ)-induced diabetic rats [220]. Briefly, STZ was administered to SD rats by intraperitoneal injection to damage insulin secretory cells in the pancreatic islets. The results showed that only 30 wt% of copolymer solution (Penta-block) after subcutaneous injection of a small dose of insulin (10 mg/mL) into STZ-induced diabetic rats successfully maintained the glucose level in blood in a healthy range up to 7 days, with no loss in the body weight.
Utilizing the PCL/PEG blocks ahead for the drug delivery, Khodaverdi et al. synthesized a PCL-PEG-PCL triblock copolymer by both conventional and a rapid microwave-assisted method to produce an injectable solution to control the release of drug from gelling matrix [221]. To validate the delivery system, AMPB31 and VANH32 were used as model drugs. For synthesis, the molecular weight of triblock copolymer solution was kept as PCL1500-PEG1500-PCL1500 and PCL1000-PEG2000-PCL1000 both by a conventional method and microwave-assisted method. Among these two PCL1500-PEG1500-PCL1500 exhibited higher sol-gel transition found to be good and selected for further study due to shorter PCL segments and low PCL/PEG ratio. The chosen copolymer solution releases the drugs by bulk erosion more in spite of the diffusion control from the copolymer. The PCL1500-PEG1500-PCL1500 hydrogel successfully released the active ingredients for more than 15 days. The nature of the drug however affected the drug release like, VANH being a hydrophilic drug easily bound to the PEG hydrophilic segments which in turn reduced the number of PEG segments and increases the formation of the hydrophobic bond at a lower temperature and vice versa is for AMPB. Thus, the PCL1500-PEG1500-PCL1500 injectable thermogelling system is more capable of controlling the release of the drug. Extending the remarkable efficiency of PECE thermoresponsive injectable hydrogels, Gong et al. synthesized PECE triblock copolymer in the ratio of PEG550-PCL2000-PEG550 by ring-opening copolymerization of ε-CL [99]. Briefly, the synthesize of PEG-PCL di-block was undergone in the presence of MPEG using catalyst stannous octoate, and this PEG-PCL di-block was used for further synthesis using HMDI as a coupling agent to produce PECE triblock. This PECE hydrogel exhibited a sol-gel transition at 37 °C. To understand the drug release behavior Vitamin B12 (VB12) was used as a model drug. This hydrophilic drug molecule exhibited complete release almost in 7 days with a higher release rate (90% in 24 h) with high initial burst release (20% in 1 h) from PECE hydrogel. This drug release mechanism from the PECE hydrogel is mainly due to the erosion and diffusion effect of the hydrogel [185]. In this series, Gong et al. also worked on successfully validating the gel formation and its degradation by injecting the PECE solution subcutaneously into KunMing mice [208].
Buwalda et al. have explored another dimension not only to increase the mechanical strength but also to improve the stability of the hydrogel systems. The work relates to the extension of the polymeric chain/block to strengthen the entire hydrogel system. Interestingly, an 8-armed star block copolymer hydrogel system is deployed with the initial replacement of the PLA with the PCL group. The stannous octoate catalyzed ring-opening polymerization of the e-caprolactone method was used to synthesis 8-armed PEG-PCL star block copolymers linked with amide group between PEG and PCL in toluene at 110 °C. The gel stiffness strongly depends on the hydrophobic block length. Also, the increase in G′ is due to an increase in caprolactone block which remains more densely crosslinked. This is mostly due to the formation of intermolecular H-bonds between amide groups. Normally, Tsol-gel is increased is due to higher concentration and temperature, between the cross-linked micelles. Physical crosslinking of micelle plays a vital role in gel-sol transformation, as at higher concentrations gel to sol transition is observed with an increase in temperature. In vitro stability of PEG-PCL star block copolymer hydrogels was significantly higher than PEG-PLA star copolymers hydrogels due to a lower rate of hydrolytic degradation of PCL than PLA.
Steinman et al. have studied the effect of increasing molecular weight of the polymer (PEG) on gelling properties, rheology, and stability of the hydrogels. For this study, PCL-PEG-PCL triblock copolymers were synthesized by ring-opening polymerization of caprolactone with a different molecular weight of PEG blocks like PEG 2000, 4000, and 8000 Da. It was observed that increasing the molecular weight of PEG blocks results in the formation of stiff gels upon increasing temperature. However, solubility will be compromised upon increasing the molecular weight of the copolymer which will enhance its hydrophobicity. Based on rheological studies the higher molecular weight PEG (i.e 8000 Da) shows stronger G′ (storage modulus) than the low molecular weight PEG (i.e 4000 Da) and indicates the Pseudo plastic type of flow behavior. However, the investigator found that with low molecular weight PEG (i.e., 2000 Da) the hydrogel was not formed and had no gelling effect. The Tsol-gel transition was found after heating at 25 °C with high molecular weight PEG (i.e 8000 Da). In all, replacing the hydrophobic PLGA blocks with PCL gives advantages such as increased mechanical strength, biodegradability, and a better safety profile.
Utilizing this strategy, Nguyen and the group developed in situ injectable hydrogels for the controlled delivery of insulin. The in situ gelations occurred upon injection into the body in response to the endogenous stimuli, pH and temperature, by altering the Penta-block copolymers of PCL-PEG. The synthesis of Penta-block copolymer takes place by conjugation between the hydroxyl group of thermoresponsive triblock copolymers (poly(ε-caprolactone)-b-poly(ethylene glycol)-b-poly(ε-caprolactone)) (PCL1845–PEG2050–PCL1845) and carboxylic group of oligo serine. Owing to the stimuli-responsive properties, the penta-block copolymers form a gel at physiological pH and temperature (7.4 and 37 °C) while remained soluble at higher pH. It was observed that a higher concentration (30% w/w) of the Penta-block copolymer resulted in stable gel which showed sustained degradation. On another side, insulin-loaded chitosan nanoparticles were prepared using the electrospraying method and mixed with the Penta-block copolymers for controlled delivery. The insulin-loaded chitosan nanoparticles with Penta-block copolymers showed release over one month in a controlled manner with inhibition of initial burst release of insulin. The investigators believe that this formulation might also control the release of other proteins with the reduction of early burst release [222]. The PCEC-based hydrogel is more superior in delivering insulin than the PLGA1000-PEG1500-PLGA1000 based hydrogel (ReGel®), where its drug release was controlled only for up to 15 days[223].
PCL-PEG hydrogels have been developed in the form of supermolecular hydrogels for drug delivery. The responsible phenomena for supramolecular hydrogel systems are well-organized noncovalent intermolecular binding interactions between two or more linear polymers and CDs.33 These supramolecular hydrogels are explored as CDDS.34 Here an example of supermolecular hydrogels for lysozyme delivery is described. Lysozyme has mostly been applied in medicine, food and pharmaceutical industries because of its antibacterial ability against Gram-positive bacteria [224]. It is also responsible for the modulation of the TNF,35 stimulation of some interleukins and initiating the activity of phagocytizing cells [225]. However, it is also responsible for antiviral activity against several viral strains. The only limitation associated with its use is the complex physical and chemical instabilities, which remains the biggest challenge for its formulation and delivery. Thus, a suitable technique is required which involves no chemical reaction, no organic solvent, and ease in the formulation. In the same context, there is a need to fabricate supramolecular hydrogels [226]. Working on this concept, Ma et al. successfully encapsulated lysozymes to sustain its release from a supramolecular hydrogel [227]. To prepare this supramolecular hydrogel, α-cyclodextrin (α-CD) and PCL-b-PEG diblock copolymer were prepared in the presence of chicken egg, lysozyme. This hydrogel did not require any crosslinking agent and was formed at room temperature as supramolecular hydrogel (Fig. 7).
Fig. 7.
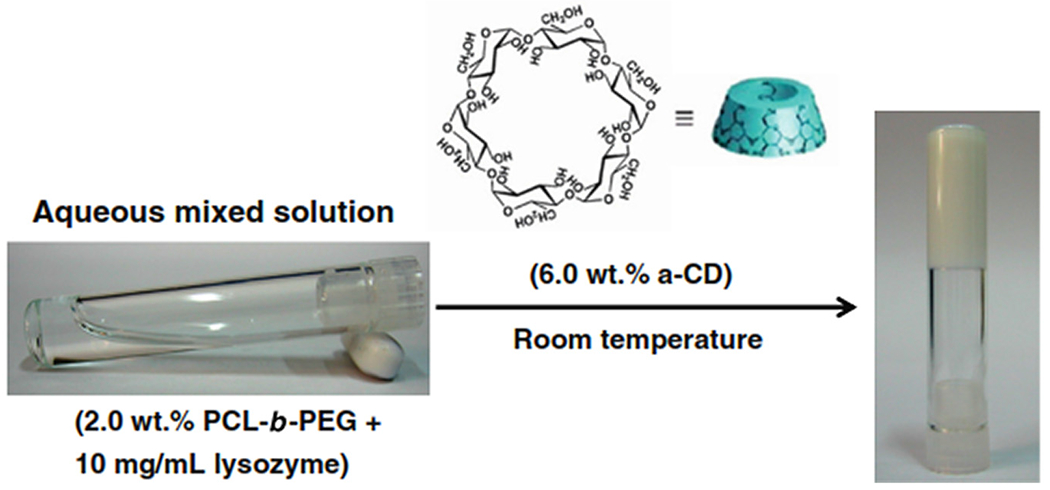
Sol-to gel transition of PCL-b-PEG/lysozyme after addition of α-CD. [Adopted with permission from Ma et al.[228]].
A crystalline inclusion complex was formed by α-CD and low molecular weight of PEG or PEO36 in an aqueous solution. The hydrogel’s physical formation was due to the aggregation into microcrystals of PEG blocks in PCL-b-PEG copolymer and also to the aggregate of α-CD inclusion complexes [229–231]. A low amount of PCL-b-PEG and α-CD led to the formation of the low viscous solution, and the solution transforms into a gel after 12 h. On the contrary, in the case of optimized higher concentration of PCL-b-PEG and α-CD, gelation time was rapid (2 min). The formation of the polymeric network in the supramolecular hydrogel controlled the release of the encapsulated lysozyme and produced a stable hydrogel with excellent biocompatibility and excellent mechanical properties. Medical imaging has an essential role in implant research. The implant can be monitored in vivo by the use of US,37 CT,38 and MRI.39 Additionally, fluorescence imaging can be applied because of its high sensitivity, low cost, low level of radiation and non-invasiveness.
Lv et al. synthesized a PEG–PCL copolymer-based thermosensitive porphyrin hydrogel [232]. In this system, a fluorescence tag for in vivo implant imaging, i.e., porphyrin (POR) was included, and the material was tested in a mice model. POR can be used for the formulation of hydrogels by coupling POR with PEG (Fig. 8). A similar strategy was also explored by Lovell et al. to develop a hydrogel by forming Pd and Cu complexes using PEG diamine and mesotetra(4-carboxyphenyl) porphyrin [233,234]. POR-conjugated PEG started the ring-opening copolymerization of ε-CL40 using (Sn (Oct)2)41 catalyst to synthesize POR–PEG1000–PCL copolymer (Fig. 8).
Fig. 8.
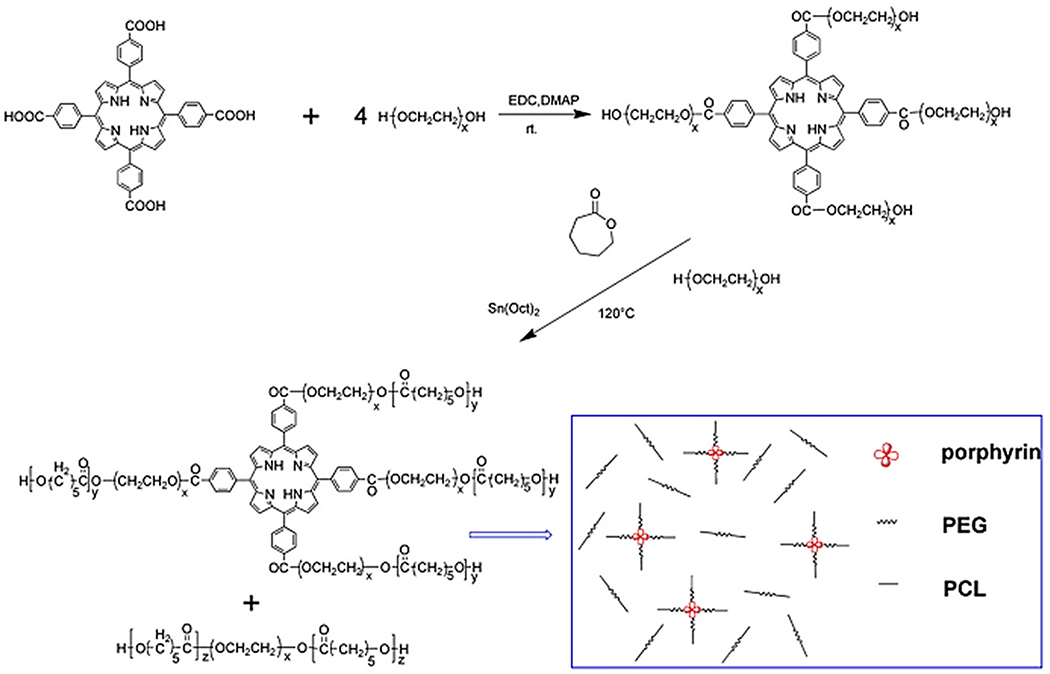
A synthetic route for the synthesis of a porphyrin-incorporated copolymer. [Adopted with permission from Lv et al.[235]].
The four functional groups present in the porphyrin easily substitute the PEG after copolymerization by PCL. Due to this conjugation of the porphyrin, POR-PEG-PCL hydrogel displayed sol-to-gel transition behavior (at 30 °C) and PCL-PEG-PCL hydrogel present the same (32 °C). This approach could be exploited to regulate final molecular weight and to improve fluorescence intensity. The tube inverting test method was used for the estimation of sol-gel-sol phenomena of POR-PEG-PCL copolymers. An intermediate concentration of copolymer resulted in the quick gel formation of triblock copolymer solution of POR-PEG-PCL within a minute. This was because of the star structure (with high molecular weight) and macrocycle structure of porphyrin. In conclusion, the porphyrin-incorporated thermosensitive hydrogel could form fluorescent injectable materials. Such hydrogels are extremely useful for preclinical testing allowing monitoring of hydrogel degradation and hydrogel molecule migrations to different organs, after injection.
Injectable copolymers were also formed by coupling Pluronic polymers and PCL, obtaining PCL-Pluronic-PCL copolymers [236,237]. In detail, Liu et al. successfully synthesized a series of the PCL-Pluronic-PCL biodegradable block copolymers. These triblock copolymers are synthesized by ring-opening polymerization of ε-CL initiated by a macromer PEG-PPG-PEG (Pluronic) (Fig. 9) [238]. The formulated copolymers showed a temperature-dependent sol-to-gel transition in water.
Fig. 9.
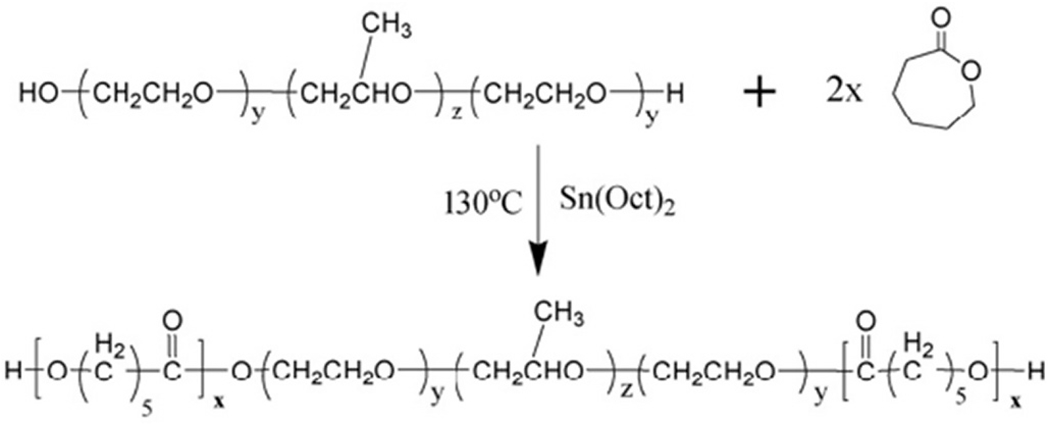
Schematic representation of the synthesis of PCL-Pluronic (L-35)-PCL copolymers. [Adopted with permission from Liu et al.[238]].
By increasing the molecular weight of PCL blocks, the LCTST42 increased because of an increase in the melting point of associated copolymers. However, the UCTST43 decreases due to an increase in hydrophobic interaction among the copolymers. The sol-to-gel transformation was based on the thermogelation mechanism like partial crystallization, micelle packing, coil-to-helix transition, hydrophobic association [239,240]. The author proposed a double layer shell-core structure of copolymer micelle responsible for the sol-to-gel transition of PCL-Pluronic-PCL copolymer (Fig. 10). Accordingly, strong hydrophobic PCL blocks due to hydrophobic interaction constituted the core, on the other hand, hydrated PEG block and weak hydrophobic PPG block formed the inner shell and the outer shell, respectively. The entrapped water is present in the entangled PEG block, and little water is present in the PEG block [241] and the untwisted PPG blocks [242,243]. Thus, in this work, the authors have developed a method to produce a double layer shell-core structure of PCL-Pluronic (L35)-PCL micelles, explaining the possible mechanism responsible for sol-to-gel transition, and proposed its use as an aqueous injectable system designed for a controlled drug carrier. Additionally, Jun Li et al. prepared the supramolecular hydrogel system, suitable for the sustained release of drugs. The hydrogel was self-assembled between a triblock copolymer of PEO-poly[(R)-3-hydroxybutyrate]-PEO. (PEO-PHB-PEO) and α-cyclodextrin. Two triblock copolymers were prepared with different molecular weights such as PEO-PHB-PEO (5000–3140-5000) and PEO-PHP-PEO (5000–17,500-5000) and resulted in gel formation at the body temperature. Dextran was used as the model drug. The controlled release dextran FITC was found with the high concentration of α–CD (9.7 wt%) of α–CD-PEO-PHB-PEO (5000–3140-5000) hydrogel for 1 month. The prepared supramolecular hydrogel has the potential to get the sustained/controlled release of high molecular weight drugs with injectable thermosensitive hydrogels [244].
Fig. 10.
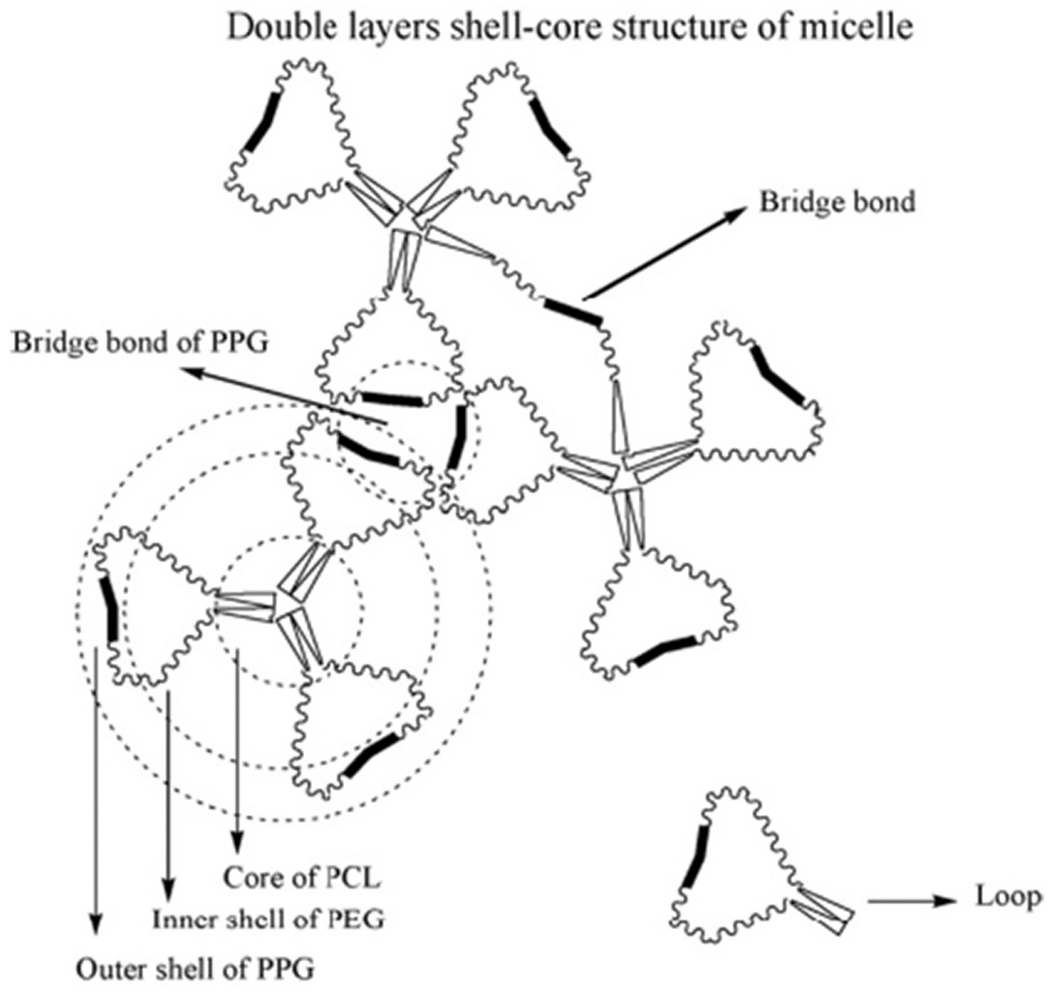
Double layers of shell-core structure of PCL-Pluronic (L35)-PCL micelles. [Adopted with permission from Liu et al.[238]].
5.6. Injectable hydrogels for post-operative treatment
To prevent postoperative intestinal adhesion, the biodegradable and thermoreversible PCLA1730-PEG1500-PCLA1730 triblock copolymer hydrogel was prepared by Zhang et al. (2011) (Fig. 11A). Ring-opening polymerization methods were used for the synthesis of PCLA1730-PEG1500-PCLA1730 triblock copolymer hydrogels. The hydrophobic blocks were formed by adding ℇ-Caprolactone (CL) with D, l-lactide (LA) to reduce the acidic effect of material degradation. The Tsol-gel transition of the concentrated PCLA1730-PEG1500-PCLA1730 aqueous solution takes place with a temperature shift from room to body temperature (Fig. 11B & E).
Fig. 11.
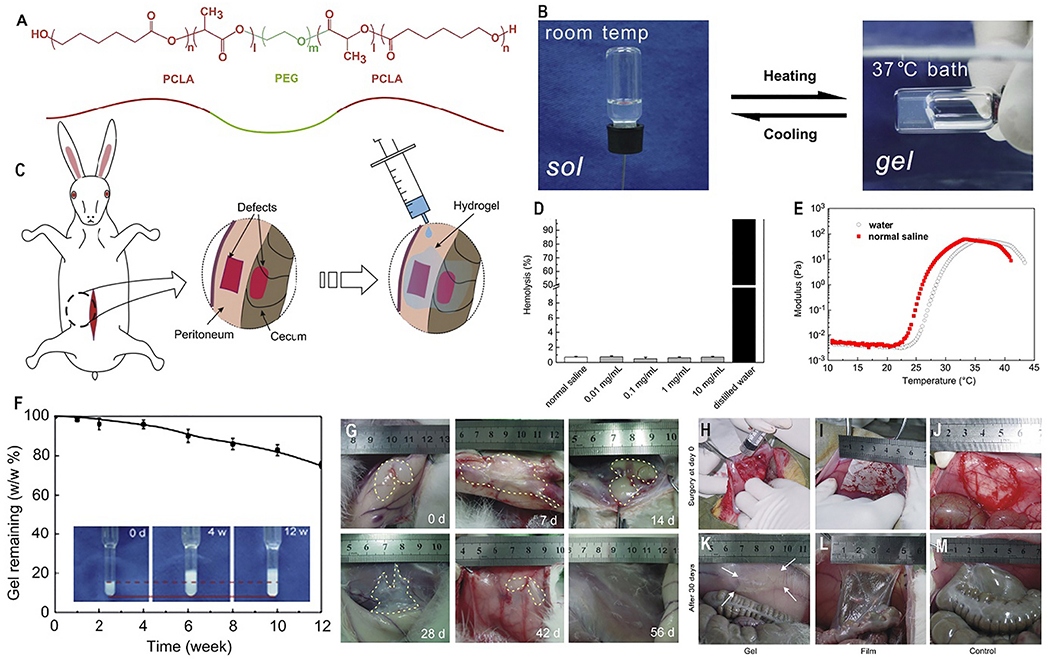
(A) Chemical structure of PCLA-PEG-PCLA hydrogel. (B) Tsol-gel behavior, exhibiting sol at room temperature and gel after heating to the body temperature. (C) PCLA-PEG-PCLA hydrogel onto a peritoneal wall defect of a rabbit. (D) Hemolysis study of hydrogel, E) Storage modulus (G’) of PCLA-PEG-PCLA solution. (F) In vitro degradation of PCLA-PEG-PCLA hydrogels mass of remaining after 12 weeks. (G) In vivo degradation study of hydrogels after subcutaneous injection of 20 wt% hydrogels into the back of the rabbits. The gel remaining indicated by dashed lines. (H) Animal experiments of post-operative adhesions after 30 days, (H & K). Hydrogels applied on the injury sites; (I & L) PLA films applied on the injury sites; (J & M) no barrier materials were used after defects. [Adopted and modified with permission from Zhang et al. [245]].
The hydrogel was formed within 10–20s after injecting the aqueous solution into the rabbit model (Fig. 11C). The in situ hydrogel was found stable for about 12 weeks in vitro (Fig. 11F) and 6 weeks in vivo. In this study, the investigator found that the prepared gel starts degrading after 28 days upon subcutaneous injection into the in vivo rabbit model while taking around 56 days in the in vitro studies (Fig. 11G). Alongside, it demonstrated minimum hemolytic (Fig. 11D) and cytotoxic effects. The effect of prevention of post-operative adhesion was evaluated in a rabbit model of sidewall defect and bowel abrasion and it was confirmed that after surgery for thirty days, the control group and the commercialized anti-adhesion film fails to the prevention of adhesion as compared with the hydrogel (Fig. 11H–M). This behavior indicates the injectability of the developed tri-block polymer PCLA1730-PEG1500-PCLA1730 [204]. Thus, the PCLA1730-PEG1500-PCLA1730 thermosensitive hydrogel remains potential for the prevention of post-operative adhesion as it is convenient in operation and more effective in reducing the formation of intraperitoneal post-operative intestinal adhesion.
6. Future perspectives and challenges
PCL-based in situ injectable thermoresponsive hydrogels are a broad class of biomaterials that share a high degree of biocompatibility and biodegradability and suitability for minimally invasive treatments. Such hydrogels are prepared by a simple method, involving dissolution in water solution followed by gelification through a change in the temperature, with no toxic or side effects during in vivo hydrogel formation. In recent years, the scientific community has widely dealt with the development of in situ injectable thermoresponsive hydrogels for various biomedical applications, including cancer therapy, drug release, and tissue regeneration. Since the methods of preparation of these hydrogels are simple, they could be widely applied in clinics in the future, if available at low cost. Hence, efforts should be made to minimize the cost of copolymer synthesis. Up to now, mainly pre-clinical investigation has been carried out on such hydrogels with ample evidence of their promising properties. These hydrogels are still in a ray of hope towards success in clinical trials. By this review article, we would like to draw the attention of the scientists working in this area to make available these injectable systems in the market at the lowest price, compared to the conventional drug dosage forms like tablets, capsules, etc. These injectable systems for drug delivery could be advantageous for local drug therapies, e.g., for the controlled release of chemotherapeutics after tumor surgery. Furthermore, they can be applied in combination with bioactive molecules for soft and hard tissue regeneration, as injectable scaffolds, or vice versa as non-adhesive injectable systems to inhibit tissue adhesion. All of these approaches are advantageous for their minimal invasiveness. Additionally, thermosensitive polymers, including those based on PCL-PEG copolymers, could be explored for new emerging applications such as the additive manufacturing of scaffolds with controlled architecture for tissue regeneration or tissue modeling [258]. Despite these advantages, hydrogels are associated with several limitations like lack of mechanical strength in aqueous media, which further affects its load-bearing capacity. Also, the hydrophilic chain of polymers and excessive water content are thermodynamically incompatible with the hydrophobic moieties and hence these are not suitable for the delivery of hydrophobic drug cargo [259].
7. Guideline for the future
In the development of an in situ thermoresponsive hydrogel, different polymers have been investigated for various biomedical applications like hard/ soft tissue engineering and drug delivery systems. Among these, PCL copolymer-based thermoresponsive hydrogels remain the most promising due to their excellent biocompatibility and biodegradability. PCL copolymerization with PEG allows tailoring the hydrophilicity and crystallinity degree of the material, enhancing biocompatibility and increasing the degradation rate while preserving good mechanical properties. PCL-PEG-based thermoresponsive injectable hydrogels have been proven as efficient carrier systems for various biomedical applications because of their ease of formulation, amphiphilic nature, better stability in biological milieu, enhanced cargo loading, and excellent biocompatibility. These hydrogels are very well exploited as a carrier for cells in tissue engineering, self-healing material and a promising carrier system for the delivery of biomolecules and drugs. Hydrogel gel strength should be regulated depending on the tissue to be treated, for example in the case of bone regeneration; injectable composite hydrogels can be used. The type of PCL-PEG copolymer (di-, tri- or multi-block copolymer) can affect the resulting mechanical strength, due to differences in molecular weight and crystallization degree of the copolymers. Additionally, different types of copolymers possess different degradation rates making them suitable for various applications. Hydrogel physicochemical properties can be easily altered by the blending or functionalization with bioactive molecules guiding cell behavior, such as proteins or bioactive peptides. Besides the possibility to engineer injectable scaffolds, PCL-PEG hydrogels can be exploited for the preparation of novel scaffolds with rapid prototyping. Such applications will deserve further investigations in the future.
Acknowledgment
This present compilation is possible because of the facilities and infrastructural support provided by the National Institute of Pharmaceutical Education and Research, Guwahati, Assam, 781101. One of the author wants to acknowledge funding from National Institute of Health, USA (R15NS108815 and R01AI147731).
Footnotes
Declaration of Competing Interest
No conflict of interest.
polyglycolide
polylactide
poly(δ-valerolactone)
poly(lactide-co-glycolide)
poly(ε-caprolactone)
poly(ε-caprolactone)
PCL-PEG-PCL
PEG-PCL-PEG
isophorone diisocyanate
hexamethylene diisocyanate
upper critical gelation temperature
lowercritical gelation temperature
critical gelation temperature
Stannous octoate
monomethoxy-PEG
ε-caprolactone
acellular bone matrix
Poly ethylene glycol diacrylate
Polycaprolactone diacrylate
bone marrow-derived stem cells
α-cyclodextrin/poly (ethylene glycol)-b-polycaprolactone-(dodecanedioic acid)-polycaprolactone–poly (ethylene glycol)
extracellular matrix
novel drug delivery systems
enhancing permeability and retention
intraocular pressure
glaucoma filtration surgery
5-fluorouracil
mitomycin-C
vascular endothelial growth factor
poly(ethylene glycol)-poly(propylene glycol)-poly(ethylene glycol)
amphotericin B
vancomycin Hydrochloride
cyclodextrins
controllable drug delivery systems
tumor necrosis factor
poly (ethylene oxide)
Ultrasonography
tomography
magnetic resonance imaging
ε-caprolactone
stannous octoate
lower critical transparent sol temperature
upper critical transparent sol temperature
References
- [1].Galaev IY, Mattiasson B, ‘Smart’ polymers and what they could do in biotechnology and medicine, Trends Biotechnol. 17 (1999) 335–340. [DOI] [PubMed] [Google Scholar]
- [2].Liu F, Urban MW, Recent advances and challenges in designing stimuli-responsive polymers, Prog. Polym. Sci 35 (2010) 3–23. [Google Scholar]
- [3].Alexander A, Ajazuddin, Khan J, Saraf S, Saraf S, Polyethylene glycol (PEG)-poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications, Eur. J. Pharm. Biopharm 88 (2014) 575–585. [DOI] [PubMed] [Google Scholar]
- [4].Gandhi A, Paul A, Sen SO, Sen KK, Studies on thermoresponsive polymers: phase behaviour, drug delivery and biomedical applications, Asian J. Pharm. Sci 10 (2015) 99–107. [Google Scholar]
- [5].Twaites BR, de Las Heras C, Alarcon M, Lavigne A, Saulnier SS, Pennadam D, Cunliffe DC, Alexander Gorecki C, Thermoresponsive polymers as gene delivery vectors: cell viability, DNA transport and transfection studies, J. Control. Release 108 (2005) 472–483. [DOI] [PubMed] [Google Scholar]
- [6].Kim MS, Kim JH, Min BH, Chun HJ, Han DK, Lee HB, Polymeric scaffolds for regenerative medicine, Polym. Rev 51 (2011) 23–52. [Google Scholar]
- [7].Yang J-A, Yeom J, Hwang BW, Hoffman AS, Hahn SK, In situ-forming injectable hydrogels for regenerative medicine, Prog. Polym. Sci 39 (2014) 1973–1986. [Google Scholar]
- [8].Ajazuddin, Alexander A, Khan J, Giri TK, Tripathi DK, Saraf S, Saraf S, Advancement in stimuli triggered in situ gelling delivery for local and systemic route, Expert Opin. Drug Deliv 9 (2012) 1573–1592. [DOI] [PubMed] [Google Scholar]
- [9].Alexander A, Dwivedi S, Ajazuddin TK, Giri S, Saraf S, Tripathi Saraf DK, Approaches for breaking the barriers of drug permeation through transdermal drug delivery, J. Control. Release 164 (2012) 26–40. [DOI] [PubMed] [Google Scholar]
- [10].Giri TK, Thakur A, Alexander A, Ajazuddin H, Tripathi Badwaik DK, Modified chitosan hydrogels as drug delivery and tissue engineering systems: present status and applications, Acta Pharm. Sin. B 2 (2012) 439–449. [Google Scholar]
- [11].Buwalda SJ, Boere KW, Dijkstra PJ, Feijen J, Vermonden T, Hennink WE, Hydrogels in a historical perspective: from simple networks to smart materials, J. Control. Release 190 (2014) 254–273. [DOI] [PubMed] [Google Scholar]
- [12].Alexander A, Saraf S, Saraf S, Understanding the role of poloxamer 407 based thermoreversible in situ gelling hydrogel for delivery of PEGylated Melphalan conjugate, Curr. Drug Deliv 13 (2016) 621–630. [DOI] [PubMed] [Google Scholar]
- [13].Zhou S, Zheng X, Zheng C, Qu F, Cai X, Xu J, A thermosensitive gel formulation of an empirical traditional Chinese prescription for treating cervical erosion, Acta Pharm. Sin. B 2 (2012) 495–501. [Google Scholar]
- [14].Hyun H, Kim YH, Song IB, Lee JW, Kim MS, Khang G, Park K, Lee HB, In vitro and in vivo release of albumin using a biodegradable MPEG-PCL diblock copolymer as an in situ gel-forming carrier, Biomacromolecules 8 (2007) 1093–1100. [DOI] [PubMed] [Google Scholar]
- [15].Deng H, Dong A, Song J, Chen X, Injectable thermosensitive hydrogel systems based on functional PEG/PCL block polymer for local drug delivery, J. Control. Release 297 (2019) 60–70. [DOI] [PubMed] [Google Scholar]
- [16].Kim DY, Kwon DY, Kwon JS, Kim JH, Min BH, Kim MS, Stimuli-responsive injectable in situ-forming hydrogels for regenerative medicines, Polym. Rev 55 (2015) 407–452. [Google Scholar]
- [17].Priya James H, John R, Alex A, Anoop KR, Smart polymers for the controlled delivery of drugs – a concise overview, Acta Pharm. Sin. B 4 (2014) 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun DD, Lee PI, Crosslinked hydrogels—a promising class of insoluble solid molecular dispersion carriers for enhancing the delivery of poorly soluble drugs, Acta Pharm. Sin. B 4 (2014) 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Park KM, Lee SY, Joung YK, Na JS, Lee MC, Park KD, Thermosensitive chitosan – Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration, Acta Biomater. 5 (2009) 1956–1965. [DOI] [PubMed] [Google Scholar]
- [20].Niranjan R, Koushik C, Saravanan S, Moorthi A, Vairamani M, Selvamurugan N, A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering, Int. J. Biol. Macromol 54 (2013) 24–29. [DOI] [PubMed] [Google Scholar]
- [21].Alexander A, Saraf S, Saraf S, A comparative study of chitosan and poloxamer based thermosensitive hydrogel for the delivery of PEGylated melphalan conjugates, Drug Dev. Ind. Pharm 41 (2015) 1954–1961. [DOI] [PubMed] [Google Scholar]
- [22].Zhang Z, Ni J, Chen L, Yu L, Xu JW, Ding JD, Biodegradable and thermoreversible PCLA-PEG-PCLA hydrogel as a barrier for prevention of postoperative adhesion, Biomaterials 32 (2011) 4725–4736. [DOI] [PubMed] [Google Scholar]
- [23].Abandansari HS, Aghaghafari E, Nabid MR, Niknejad H, Preparation of injectable and thermoresponsive hydrogel based on penta-block copolymer with improved sol stability and mechanical properties, Polymer 54 (2013) 1329–1340. [Google Scholar]
- [24].Buwalda SJ, Dijkstra PJ, Calucci L, Forte C, Feijen J, Influence of amide versus ester linkages on theproperties of eight-armed PEGPLA star block copolymer hydrogels, J. Biomacromolecules 11 (2010) 224–232. [DOI] [PubMed] [Google Scholar]
- [25].Dabbaghi A, Ramazani A, Farshchi N, Rezaei A, Bodaghi A, Rezayati S, Synthesis, physical and mechanical properties of amphiphilic hydrogels based on polycaprolactone and polyethylene glycol for bioapplications: a review, J. Ind. Eng. Chem 101 (2021) 307–323. [Google Scholar]
- [26].Chen S-H, Chen C-H, Shalumon KT, Chen J-P, Preparation and characterization of antiadhesion barrier film from hyaluronic acid-grafted electrospunpoly(caprolactone) nanofibrous membranes for prevention of flexor tendon postoperative peritendinous adhesion, Int. J. Nanomedicine 22 (2014) 4079–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fujihara K, Kotaki M, Ramakrishna S, Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers, Biomaterials 26 (2005) 4139–4147. [DOI] [PubMed] [Google Scholar]
- [28].Gou M, Gong C, Zhang J, Wang X, Wang X, Gu Y, Guo G, Chen L, Luo F, Zhao X, Wei Y, Qian Z, Polymeric matrix for drug delivery: honokiol-loaded PCL-PEG-PCL nanoparticles in PEG-PCL-PEG thermosensitive hydrogel, J. Biomed. Mater. Res. A 93 (2010) 219–226. [DOI] [PubMed] [Google Scholar]
- [29].Masket S, Hovanesian JA, Levenson J, Tyson F, Flynn W, Endl M, Majmudar P, Modi S, Chu R, Raizman MB, Lane SS, Kim T, Hydrogel sealant versus sutures to prevent fluid egress after cataract surgery, J Cataract Refract Surg 40 (2014) 2057–2066. [DOI] [PubMed] [Google Scholar]
- [30].Mishra GP, Tamboli V, Mitra AK, Effect of hydrophobic and hydrophilic additives on sol–gel transition and release behavior of timolol maleate from polycaprolactone-based hydrogel, Colloid Polym. Sci 289 (2011) 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Boffito M, Sirianni P, Di Rienzo AM, Chiono V, Thermosensitive block copolymer hydrogels based on poly(varepsilon-caprolactone) and polyethylene glycol for biomedical applications: state of the art and future perspectives, J. Biomed. Mater. Res. A 103 (2015) 1276–1290. [DOI] [PubMed] [Google Scholar]
- [32].Fan RR, Deng XH, Zhou LX, Gao X, Fan M, Wang YL, Guo G, Injectable thermosensitive hydrogel composite with surface-functionalized calcium phosphate as raw materials, Int. J. Nanomedicine 9 (2014) 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Agrawal M, Ajazuddin, Tripathi DK, Saraf S, Saraf S, Antimisiaris SG, Mourtas S, Hammarlund-Udenaes M, Alexander A, Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease, J. Control. Release 260 (2017) 61–77. [DOI] [PubMed] [Google Scholar]
- [34].Kissel T, Li Y, Unger F, ABA-triblock copolymers from biodegradable polyester A-blocks and hydrophilic poly(ethylene oxide) B-blocks as a candidate for in situ forming hydrogel delivery systems for proteins, Adv. Drug Deliv. Rev 54 (2002) 99–134. [DOI] [PubMed] [Google Scholar]
- [35].ChangYang G, ZhiYong Q, CaiBing L, MeiJuan H, YingChun G, YanJun W, Bing K, Ke W, Mei D, XingYi L, MaLing G, MingJing T, YuQuan W, A thermosensitive hydrogel based on biodegradable amphiphilic poly(ethylene glycol)–polycaprolactone–poly(ethylene glycol) block copolymers, Smart Mater. Struct 16 (2007) 927. [Google Scholar]
- [36].Liu CB, Gong CY, Huang MJ, Wang JW, Pan YF, Zhang YD, Li GZ, Gou ML, Wang K, Tu MJ, Wei YQ, Qian ZY, Thermoreversible gel–sol behavior of biodegradable PCL-PEG-PCL triblock copolymer in aqueous solutions, J. Biomed. Mater. Res. B Appl. Biomater 84B (2008) 165–175. [DOI] [PubMed] [Google Scholar]
- [37].Tarasevich BJ, Gutowska A, Li XS, Jeong BM, The effect of polymer composition on the gelation behavior of PLGA-g-PEG biodegradable thermoreversible gels, J. Biomed. Mater. Res. A 89 (2009) 248–254. [DOI] [PubMed] [Google Scholar]
- [38].Bonacucina G, Cespi M, Mencarelli G, Giorgioni G, Palmieri GF, Thermosensitive self-assembling block copolymers as drug delivery systems, Polymers 3 (2011) 779–811. [Google Scholar]
- [39].Lin G, Cosimbescu L, Karin NJ, Tarasevich BJ, Injectable and thermosensitive PLGA-g-PEG hydrogels containing hydroxyapatite: preparation, characterization and in vitro release behavior, Biomed. Mater 7 (2012), 024107. [DOI] [PubMed] [Google Scholar]
- [40].Nguyen MK, Lee DS, Injectable biodegradable hydrogels, Macromol. Biosci 10 (2010) 563–579. [DOI] [PubMed] [Google Scholar]
- [41].Woodruff MA, Hutmacher DW, The return of a forgotten polymer—Polycaprolactone in the 21st century, Prog. Polym. Sci 35 (2010) 1217–1256. [Google Scholar]
- [42].Bae SJ, Joo MK, Jeong Y, Kim SW, Lee W-K, Sohn YS, Jeong B, Gelation behavior of poly(ethylene glycol) and polycaprolactone triblock and multiblock copolymer aqueous solutions, Macromolecules 39 (2006) 4873–4879. [Google Scholar]
- [43].Zhu GZ, Mallery SR, Schwendeman SP, Stabilization of proteins encapsulated in injectable poly(lactide-co-glycolide), Biotechnology 18 (2000) 52–57. [DOI] [PubMed] [Google Scholar]
- [44].Hennink WE, van Nostrum CF, Novel crosslinking methods to design hydrogels, Adv. Drug Deliv. Rev 64 (2012) 223–236. [DOI] [PubMed] [Google Scholar]
- [45].Nuttelman CR, Henry SM, Anseth KS, Synthesis and characterization of photocrosslinkable, degradable poly(vinyl alcohol)-based tissue engineering scaffolds, Biomaterials 23 (2002) 3617–3626. [DOI] [PubMed] [Google Scholar]
- [46].Parhi R, Cross-linked hydrogel for pharmaceutical applications: a review, Adv. Pharm. Bull 7 (2017) 515–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Guaresti O, Basasoro S, González K, Eceiza A, Gabilondo N, In situ cross–linked chitosan hydrogels via Michael addition reaction based on water–soluble thiol–maleimide precursors, Eur. Polym. J 119 (2019) 376–384. [Google Scholar]
- [48].Zhang H, Zhang F, Wu J, Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique, React. Funct. Polym 73 (2013) 923–928. [Google Scholar]
- [49].Gong C, Shi S, Dong P, Kan B, Gou M, Wang X, Li X, Luo F, Zhao X, Wei Y, Qian Z, Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel, Int. J. Pharm 365 (2009) 89–99. [DOI] [PubMed] [Google Scholar]
- [50].Koenig MF, Huang SJ, Biodegradable blends and composites of polycaprolactone and starch derivatives, Polymer 36 (1995) 1877–1882. [Google Scholar]
- [51].Chen DR, Bei JZ, Wang SG, Polycaprolactone microparticles and their biodegradation, Polym. Degrad. Stab 67 (2000) 455–459. [Google Scholar]
- [52].Moon HT, Lee YK, Han JK, Byun Y, Improved blood compatibility by sustained release of heparin-deoxycholic acid conjugates in a PCL-PEG multiblock copolymer matrix, J. Biomater. Sci. Polym. Ed 13 (2002) 817–828. [DOI] [PubMed] [Google Scholar]
- [53].Huang MH, Li S, Hutmacher DW, Schantz JT, Vacanti CA, Braud C, Vert M, Degradation and cell culture studies on block copolymers prepared by ring opening polymerization of epsilon-caprolactone in the presence of poly(ethylene glycol), J. Biomed. Mater. Res. A 69 (2004) 417–427. [DOI] [PubMed] [Google Scholar]
- [54].Perret R, Skoulios A, Synthèse et caractérisation de copolymères séquencés polyoxyéthylène/poly-ε-caprolactone, Die Makromolekulare Chemie 156 (1972) 143–156. [Google Scholar]
- [55].Cerrai P, Tricoli M, Andruzzi F, Paci M, Paci M, Polyether-polyester block copolymers by non-eatalysed polymerization of ε-caprolactone with poly (ethylene glycol), Polymer 30 (1989) 338–343. [Google Scholar]
- [56].Jedliński Z, Kurcok P, Walach W, Janeczek H, Radecka I, Polymerization of lactones, 17. Synthesis of ethylene glycol-L-lactide block copolymers, Die Makromolekulare Chemie 194 (1993) 1681–1689. [Google Scholar]
- [57].Youxin L, Kissel T, Synthesis and properties of biodegradable ABA triblock copolymers consisting of poly(l-lactic acid) or poly (1-lactic-co-glycolic acid) A-blocks attached to central poly ( oxyethylene ) B-blocks, J. Control. Release 27 (1993) 247–257. [Google Scholar]
- [58].Cerrai P, Guerra GD, Lelli L, Tricoli M, Sbarbati Del Guerra R, Cascone MG, Giusti P, Poly(ester-ether-ester) block copolymers as biomaterials, J. Mater. Sci. Mater. Med 5 (1994) 33–39. [Google Scholar]
- [59].Gan Z, Zhang J, Jiang B, Poly(ϵ-caprolactone)/poly(ethylene oxide) diblock copolymer II. Nonisothermal crystallization and melting behavior, J. Appl. Polym. Sci 63 (1997) 1793–1804. [Google Scholar]
- [60].Rashkov I, Manolova N, Li SM, Espartero JL, Vert M, Synthesis, characterization, and hydrolytic degradation of PLA/PEO/PLA triblock copolymers with short poly(l-lactic acid) chains, Macromolecules 29 (1996) 50–56. [Google Scholar]
- [61].Cohn D, Stern T, González MF, Epstein J, Biodegradable poly(ethylene oxide)/poly(ϵ-caprolactone) multiblock copolymers, J. Biomed. Mater. Res 59 (2002) 273–281. [DOI] [PubMed] [Google Scholar]
- [62].Kim KH, Cui GH, Lim HJ, Huh J, Ahn C-H, Jo WH, Synthesis and micellization of star-shaped poly(ethylene glycol)-block-poly(ε-caprolactone), Macromol. Chem. Phys 205 (2004) 1684–1692. [Google Scholar]
- [63].Gou ML, Huang MJ, Gou ML, Huang MJ, Qian ZY, Gou ML, Huang MJ, Qian ZY, Yang L, Gou ML, Huang MJ, Qian ZY, Yang L, Dai M, Li XY, Wang K, Wen YJ, Li J, Zhao X, Wei YQ, Preparation of anionic poly (s-caprolactone)-poly( ethylene glycol)-poly(ε-caprolactone) copolymeric nanoparticles as basic protein antigen carrier, Growth Factors 25 (2007) 202–208. [DOI] [PubMed] [Google Scholar]
- [64].Zhang Y, Zhuo RX, Synthesis and in vitro drug release behavior of amphiphilic triblock copolymer nanoparticles based on poly (ethylene glycol) and polycaprolactone, Biomaterials 26 (2005) 6736–6742. [DOI] [PubMed] [Google Scholar]
- [65].Gao X, Wang B, Wei X, Rao W, Ai F, Zhao F, Men K, Yang B, Liu X, Huang M, Gou M, Qian Z, Huang N, Wei Y, Preparation, characterization and application of star-shaped PCL/PEG micelles for the delivery of doxorubicin in the treatment of colon cancer, Int. J. Nanomedicine 8 (2013) 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhao J, Wang Y, Luan L, Star-shaped polycaprolactone-polyethyleneglycol copolymer micelle-like nanoparticles for picropodophyllin delivery, J. Biomed. Nanotechnol 10 (2014) 1627–1634. [DOI] [PubMed] [Google Scholar]
- [67].Wei X, Gong C, Gou M, Fu S, Guo Q, Shi S, Luo F, Guo G, Qiu L, Qian Z, Biodegradable poly(ε-caprolactone)–poly(ethylene glycol) copolymers as drug delivery system, Int. J. Pharm 381 (2009) 1–18. [DOI] [PubMed] [Google Scholar]
- [68].Ruel-Gariépy E, Leroux J-C, In situ-forming hydrogels—review of temperature-sensitive systems, Eur. J. Pharm. Biopharm 58 (2004) 409–426. [DOI] [PubMed] [Google Scholar]
- [69].Nguyen MK, Lee DS, Injectable biodegradable hydrogels, Macromol. Biosci 10 (2010) 563–579. [DOI] [PubMed] [Google Scholar]
- [70].Gong C, Shi S, Dong P, Kan B, Gou M, Wang X, Li X, Luo F, Zhao X, Wei Y, Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel, Int. J. Pharm 365 (2009) 89–99. [DOI] [PubMed] [Google Scholar]
- [71].Feng Z, Zhao J, Li Y, Xu S, Zhou J, Zhang J, Deng L, Dong A, Temperature-responsive in situ nanoparticle hydrogels based on hydrophilic pendant cyclic ether modified PEG-PCL-PEG, Biomater. Sci 4 (2016) 1493–1502. [DOI] [PubMed] [Google Scholar]
- [72].Zamani S, Khoee S, Preparation of core–shell chitosan/PCL-PEG triblock copolymer nanoparticles with ABA and BAB morphologies: effect of intraparticle interactions on physicochemical properties, Polymer 53 (2012) 5723–5736. [Google Scholar]
- [73].Wei X, Gong C, Gou M, Fu S, Guo Q, Shi S, Luo F, Guo G, Qiu L, Qian Z, Biodegradable poly(epsilon-caprolactone)-poly(ethylene glycol) copolymers as drug delivery system, Int. J. Pharm 381 (2009) 1–18. [DOI] [PubMed] [Google Scholar]
- [74].Shin IG, Kim SY, Lee YM, Cho CS, Sung YK, Methoxy poly(ethylene glycol)/epsilon-caprolactone amphiphilic block copolymeric micelle containing indomethacin. I. Preparation and characterization, J. Control. Release 51 (1998) 1–11. [DOI] [PubMed] [Google Scholar]
- [75].Woodhouse K, Lamda NMK SL Cooper, Polyurethanes in Biomedical Applications, CRC Press, Boca Raton, 1998. [Google Scholar]
- [76].Wang C, Li H, Zhao X, Ring opening polymerization of L-lactide initiated by creatinine, Biomaterials 25 (2004) 5797–5801. [DOI] [PubMed] [Google Scholar]
- [77].Manolova N, Petrova T, Rashkov I, Li S, Vert M, Synthesis and characterization of poly(oxyethylene)–poly(caprolactone) multiblock copolymers, Polym. Int 45 (1998) 419–926. [Google Scholar]
- [78].Garreau H, Li S, Vert M, Petrova T, Manolova N, Rashkov I, Hydrolytic degradation of poly(oxyethylene)–poly(E-caprolactone) multiblock copolymers, J. Appl. Polym. Sci 68 (1998) 989–998. [Google Scholar]
- [79].Bae SJ, Joo MK, Jeong Y, Kim SW, Lee W-K, Sohn YS, Jeong B, Gelation behavior of poly(ethylene glycol) and polycaprolactone triblock and multiblock copolymer aqueous solutions, Macromolecules 39 (2006) 4873–4879. [Google Scholar]
- [80].Bhatt S, Pulpytel J, Mirshahi M, Arefi-Khonsari F, Catalyst-free plasma-assisted copolymerization of poly (ε-caprolactone)-poly (ethylene glycol) for biomedical applications, ACS Macro Lett. 1 (2012) 764–767. [DOI] [PubMed] [Google Scholar]
- [81].Boffito M, Sirianni P, Di Rienzo AM, Chiono V, Thermosensitive block copolymer hydrogels based on poly (ε-caprolactone) and polyethylene glycol for biomedical applications: state of the art and future perspectives, J. Biomed. Mater. Res. A 103 (2015) 1276–1290. [DOI] [PubMed] [Google Scholar]
- [82].Figueiredo P, Almeida BC, Carvalho AT, Enzymatic polymerization of PCL-PEG co-polymers for biomedical applications, Front. Mol. Biosci 6 (2019) 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Qindeel M, Ahmed N, Shah KU, Ullah N, New, environment friendly approach for synthesis of amphiphilic PCL–PEG–PCL triblock copolymer: an efficient carrier for fabrication of nanomicelles, J. Polym. Environ 28 (2020) 1237–1251. [Google Scholar]
- [84].Alexander A, Ajazuddin J, Khan S, Saraf S, Saraf, formulation and evaluation of chitosan-based long-acting injectable hydrogel for PEGylated melphalan conjugate, J. Pharm. Pharmacol 66 (2014) 1240–1250. [DOI] [PubMed] [Google Scholar]
- [85].Hajiali F, Tajbakhsh S, Shojaei A, Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review, Polym. Rev 58 (2018) 164–207. [Google Scholar]
- [86].Dong SW, Ying DJ, Duan XJ, Xie Z, Yu ZJ, Zhu CH, Yang B, Sun JS, Bone regeneration using an acellular extracellular matrix and bone marrow mesenchymal stem cells expressing Cbfa1, Biosci. Biotechnol. Biochem 73 (2009) 2226–2233. [DOI] [PubMed] [Google Scholar]
- [87].Kruyt MC, Dhert WJ, Yuan H, Wilson CE, van Blitterswijk CA, Verbout AJ, de Bruijn JD, Bone tissue engineering in a critical size defect compared to ectopic implantations in the goat, J. Orthop. Res 22 (2004) 544–551. [DOI] [PubMed] [Google Scholar]
- [88].Orsini M, Orsini G, Benlloch D, Aranda JJ, Lazaro P, Sanz M, De Luca M, Piattelli A, Comparison of calcium sulfate and autogenous bone graft to bioabsorbable membranes plus autogenous bone graft in the treatment of intrabony periodontal defects: a split-mouth study, J. Periodontol 72 (2001) 296–302. [DOI] [PubMed] [Google Scholar]
- [89].Damsky CH, Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling, Bone 25 (1999) 95–96. [DOI] [PubMed] [Google Scholar]
- [90].Horton JM, Summers AP, The material properties of acellular bone in a teleost fish, J. Exp. Biol 212 (2009) 1413–1420. [DOI] [PubMed] [Google Scholar]
- [91].Sun XJ, Peng W, Yang ZL, Ren ML, Zhang SC, Zhang WG, Zhang LY, Xiao K, Wang ZG, Zhang B, Wang J, Heparin-chitosan-coated acellular bone matrix enhances perfusion of blood and vascularization in bone tissue engineering scaffolds, Tissue Eng. Part A 17 (2011) 2369–2378. [DOI] [PubMed] [Google Scholar]
- [92].Liao W, Yang Z, Deng L, Li X, Sun T, Luo J, Qin T, Xie H, Morphological and biomechanical study on in vivo osteogenesis after repair of cranial defects with plastic engineered bone in rabbits, Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 19 (2005) 460–463. [PubMed] [Google Scholar]
- [93].Qi C, Yang Z, Huang F, Qin T, Li X, Luo J, Cai Y, Experimental study on repair of goat tibia defect with marrow stromal cell and bio-derived bone, Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 19 (2005) 90–94. [PubMed] [Google Scholar]
- [94].Song K, Yang Z, Liu T, Zhi W, Li X, Deng L, Cui Z, Ma X, Fabrication and detection of tissue-engineered bones with bio-derived scaffolds in a rotating bioreactor, Biotechnol. Appl. Biochem 45 (2006) 65–74. [DOI] [PubMed] [Google Scholar]
- [95].Ni PY, Fan M, Qian ZY, Luo JC, Gong CY, Fu SZ, Shi S, Luo F, Yang ZM, Synthesis and characterization of injectable, thermosensitive, and biocompatible acellular bone matrix/poly(ethylene glycol)-poly (epsilon-caprolactone)-poly (ethylene glycol) hydrogel composite, J. Biomed. Mater. Res. A 100 (2012) 171–179. [DOI] [PubMed] [Google Scholar]
- [96].Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA, Cell-based bone tissue engineering, PLoS Med. 4 (2007), e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Nishida J, Shiraishi H, Okada K, Ehara S, Shimamura T, Vascularized iliac bone graft for iliosacral bone defect after tumor excision, Clin. Orthop. Relat. Res 447 (2006) 145–151. [DOI] [PubMed] [Google Scholar]
- [98].Reichert JC, Saifeadeh S, Wullschleger ME, Epari DR, Schutz MA, Duda GN, Schell H, van Griensven M, Redl H, Hutmacher DW, The challenge of establishing preclinical models for segmental bone defect research, Biomaterials 30 (2009) 2149–2163. [DOI] [PubMed] [Google Scholar]
- [99].Gong CY, Dong PW, Shi S, Fu SZ, Yang JL, Guo G, Zhao X, Wei YQ, Qian ZY, Thermosensitive PEG-PCL-PEG hydrogel controlled drug delivery system: sol-gel-sol transition and in vitro drug release study, J. Pharm. Sci 98 (2009) 3707–3717. [DOI] [PubMed] [Google Scholar]
- [100].Gong CY, Wu QJ, Dong PW, Shi S, Fu SZ, Guo G, Hu HZ, Zhao X, Wei YQ, Qian ZY, Acute toxicity evaluation of biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PEG-PCL-PEG hydrogel, J Biomed Mater Res B Appl Biomater 91 (2009) 26–36. [DOI] [PubMed] [Google Scholar]
- [101].Ni P, Ding Q, Fan M, Liao J, Qian Z, Luo J, Li X, Luo F, Yang Z, Wei Y, Injectable thermosensitive PEG–PCL–PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects, Biomaterials 35 (2014) 236–248. [DOI] [PubMed] [Google Scholar]
- [102].Ni P, Ding Q, Fan M, Liao J, Qian Z, Luo J, Li X, Luo F, Yang Z, Wei Y, Injectable thermosensitive PEG-PCL-PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects, Biomaterials 35 (2014) 236–248. [DOI] [PubMed] [Google Scholar]
- [103].Moreau JL, Xu HHK, Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate - chitosan composite scaffold, Biomaterials 30 (2009) 2675–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hench LL, Polak JM, Third-generation biomedical materials, Science 295 (2002) 1014–1017. [DOI] [PubMed] [Google Scholar]
- [105].Laurencin CT, Ambrosio AM, Borden MD, Cooper JA Jr., Tissue engineering: orthopedic applications, Annu. Rev. Biomed. Eng 1 (1999) 19–46. [DOI] [PubMed] [Google Scholar]
- [106].Fu S, Guo G, Wang X, Zhou L, Liu T, Dong P, Luo F, Gu Y, Shi X, Zhao X, Wei Y, Qian Z, Preparation and characterization of n-hydroxyapatite/PCL-pluronic-PCL nanocomposites for tissue engineering, J. Nanosci. Nanotechnol 10 (2010) 711–718. [DOI] [PubMed] [Google Scholar]
- [107].Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N, Hasirci V, Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering, Biomaterials 30 (2009) 3551–3559. [DOI] [PubMed] [Google Scholar]
- [108].Nie H, Soh BW, Fu YC, Wang CH, Three-dimensional fibrous PLGA/HAp composite scaffold for BMP-2 delivery, Biotechnol. Bioeng 99 (2008) 223–234. [DOI] [PubMed] [Google Scholar]
- [109].Lee SH, Shin H, Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering, Adv. Drug Deliv. Rev 59 (2007) 339–359. [DOI] [PubMed] [Google Scholar]
- [110].Schneider OD, Weber F, Brunner TJ, Loher S, Ehrbar M, Schmidlin PR, Stark WJ, In vivo and in vitro evaluation of flexible, cottonwool-like nanocomposites as bone substitute material for complex defects, Acta Biomater. 5 (2009) 1775–1784. [DOI] [PubMed] [Google Scholar]
- [111].Fu S, Ni P, Wang B, Chu B, Zheng L, Luo F, Luo J, Qian Z, Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration, Biomaterials 33 (2012) 4801–4809. [DOI] [PubMed] [Google Scholar]
- [112].Fu S, Ni P, Wang B, Chu B, Zheng L, Luo F, Luo J, Qian Z, Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration, Biomaterials 33 (2012) 4801–4809. [DOI] [PubMed] [Google Scholar]
- [113].Türkkan S, Pazarçeviren AE, Keskin D, Machin NE, Duygulu Ö, Tezcaner A, Nanosized CaP-silk fibroin-PCL-PEG-PCL/PCL based bilayer membranes for guided bone regeneration, Mater. Sci. Eng. C 80 (2017) 484–493. [DOI] [PubMed] [Google Scholar]
- [114].Girones Molera J, Alberto Mendez J, San Roman J, Bioresorbable and nonresorbable polymers for bone tissue engineering Jordi Girones, Curr. Pharm. Des 18 (2012) 2536–2557. [DOI] [PubMed] [Google Scholar]
- [115].Bonaventure J, Kadhom N, Cohen-Solal L, Ng KH, Bourguignon J, Lasselin C, Freisinger P, Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads, Exp. Cell Res 212 (1994) 97–104. [DOI] [PubMed] [Google Scholar]
- [116].Hauselmann HJ, Fernandes RJ, Mok SS, Schmid TM, Block JA, Aydelotte M, Kuettner KE, Thonar EJ, Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads, J. Cell Sci 107 (Pt 1) (1994) 17–27. [DOI] [PubMed] [Google Scholar]
- [117].Schuman L, Buma P, Versleyen D, de Man B, van der Kraan PM, van den Berg WB, Homminga GN, Chondrocyte behaviour within different types of collagen gel in vitro, Biomaterials 16 (1995) 809–814. [DOI] [PubMed] [Google Scholar]
- [118].Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R, Biodegradable polymer scaffolds for tissue engineering, Biotechnology (N Y) 12 (1994) 689–693. [DOI] [PubMed] [Google Scholar]
- [119].Elvers D, Song CH, Steinbuchel A, Leker J, Technology trends in biodegradable polymers: evidence from patent analysis, Polym. Rev 56 (2016) 584–606. [Google Scholar]
- [120].Baek CH, Ko YJ, Characteristics of tissue-engineered cartilage on macroporous biodegradable PLGA scaffold, Laryngoscope 116 (2006) 1829–1834. [DOI] [PubMed] [Google Scholar]
- [121].Lu L, Peter SJ, Lyman MD, Lai HL, Leite SM, Tamada JA, Uyama S, Vacanti JP, Langer R, Mikos AG, In vitro and in vivo degradation of porous poly (DL-lactic-co-glycolic acid) foams, Biomaterials 21 (2000) 1837–1845. [DOI] [PubMed] [Google Scholar]
- [122].Lu L, Garcia CA, Mikos AG, In vitro degradation of thin poly(DL-lactic-co-glycolic acid) films, J. Biomed. Mater. Res 46 (1999) 236–244. [DOI] [PubMed] [Google Scholar]
- [123].Park JS, Woo DG, Sun BK, Chung HM, Im SJ, Choi YM, Park K, Huh KM, Park KH, In vitro and in vivo test of PEG/PCL-based hydrogel scaffold for cell delivery application, J. Control. Release 124 (2007) 51–59. [DOI] [PubMed] [Google Scholar]
- [124].Im SJ, Choi YM, Subramanyam E, Huh KM, Park K, Synthesis and characterization of biodegradable elastic hydrogels based on poly(ethylene glycol) and poly(ε-caprolactone) blocks, Macromol. Res 15 (2007) 363–369. [Google Scholar]
- [125].Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H, Bone regeneration through controlled release of bone morphogenetic protein-2 from 3-D tissue engineered nano-scaffold, J. Control. Release 117 (2006) 380–386 (232 (2016) 271). [DOI] [PubMed] [Google Scholar]
- [126].Chen FM, Zhao YM, Sun HH, Jin T, Wang QT, Zhou W, Wu ZF, Jin Y, Novel glycidyl methacrylated dextran (Dex-GMA)/gelatin hydrogel scaffolds containing microspheres loaded with bone morphogenetic proteins: formulation and characteristics, J. Control. Release 118 (2007) 65–77. [DOI] [PubMed] [Google Scholar]
- [127].Oudega M, Gautier SE, Chapon P, Fragoso M, Bates ML, Parel JM, Bunge MB, Axonal regeneration into Schwann cell grafts within resorbable poly (alpha-hydroxyacid) guidance channels in the adult rat spinal cord, Biomaterials 22 (2001) 1125–1136. [DOI] [PubMed] [Google Scholar]
- [128].Syftestad GT, Weitzhandler M, Caplan AI, Isolation and characterization of osteogenic cells derived from first bone of the embryonic tibia, Dev. Biol 110 (1985) 275–283. [DOI] [PubMed] [Google Scholar]
- [129].Xu X, Liu Y, Fu W, Yao M, Ding Z, Xuan J, Li D, Wang S, Xia Y, Cao M, Poly (N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications, Polymers (Basel) 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Oroojalian F, Jahanafrooz Z, Chogan F, Rezayan AH, Malekzade E, Rezaei SJT, Nabid MR, Sahebkar A, Synthesis and evaluation of injectable thermosensitive penta-block copolymer hydrogel (PNIPAAm-PCL-PEG-PCL-PNIPAAm) and star-shaped poly(CL horizontal line CO horizontal line LA)-b-PEG for wound healing applications, J. Cell. Biochem 120 (2019) 17194–17207. [DOI] [PubMed] [Google Scholar]
- [131].Noormohammadi F, Gity Mir Mohamad Sadeghi MN, Peng-Yuan Wang HS, The role of cellulose nanowhiskers in controlling phase segregation, crystallization and thermal stimuli responsiveness in PCL-PEGx-PCL block copolymer-based PU for human tissue engineering applications, J. Control. Release 252 (2021) 1–12. [DOI] [PubMed] [Google Scholar]
- [132].Wang T, Jiang XJ, Tang QZ, Li XY, Lin T, Wu DQ, Zhang XZ, Okello E, Bone marrow stem cells implantation with alpha-cyclodextrin/MPEG-PCL-MPEG hydrogel improves cardiac function after myocardial infarction, Acta Biomater. 5 (2009) 2939–2944. [DOI] [PubMed] [Google Scholar]
- [133].Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J, Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution, Circulation 108 (2003) 863–868. [DOI] [PubMed] [Google Scholar]
- [134].Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H, Monitoring of bone marrow cell homing into the infarcted human myocardium, Circulation 111 (2005) 2198–2202. [DOI] [PubMed] [Google Scholar]
- [135].Jiang XJ, Wang T, Li XY, Wu DQ, Zheng ZB, Zhang JF, Chen JL, Peng B, Jiang H, Huang C, Zhang XZ, Injection of a novel synthetic hydrogel preserves left ventricle function after myocardial infarction, J. Biomed. Mater. Res. A 90 (2009) 472–477. [DOI] [PubMed] [Google Scholar]
- [136].Zhong J, Guo B, Xie J, Deng S, Fu N, Lin S, Li G, Lin Y, Cai X, Crosstalk between adipose-derived stem cells and chondrocytes: when growth factors matter, Bone Res. 4 (2016) 15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Shi S, Xie J, Zhong J, Lin S, Zhang T, Sun K, Fu N, Shao X, Lin Y, Effects of low oxygen tension on gene profile of soluble growth factors in co-cultured adipose-derived stromal cells and chondrocytes, Cell Prolif. 49 (2016) 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gong T, Xie J, Liao J, Zhang T, Lin S, Lin Y, Nanomaterials and bone regeneration, Bone Res. 3 (2015) 15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zhou T, Li X, Li G, Tian T, Lin S, Shi S, Liao J, Cai X, Lin Y, Injectable and thermosensitive TGF-β1-loaded PCEC hydrogel system for in vivo cartilage repair, Sci. Rep 7 (2017) (10553–10553). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Saghebasl S, Davaran S, Rahbarghazi R, Montaseri A, Salehi R, Ramazani A, Synthesis and in vitro evaluation of thermosensitive hydrogel scaffolds based on (PNIPAAm-PCL-PEG-PCL-PNIPAAm)/Gelatin and (PCL-PEG-PCL)/Gelatin for use in cartilage tissue engineering, J. Biomater. Sci. Polym. Ed 29 (2018) 1185–1206. [DOI] [PubMed] [Google Scholar]
- [141].Zhou T, Li X, Li G, Tian T, Lin S, Shi S, Liao J, Cai X, Lin Y, Injectable and thermosensitive TGF-β1-loaded PCEC hydrogel system for in vivo cartilage repair, Sci. Rep 7 (2017) 10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].G.C.O, All cancers, in: WHO (Ed.), International Agency for Research on Cancer, 2020. [Google Scholar]
- [143].Kang L, Gao Z, Huang W, Jin M, Wang Q, Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment, Acta Pharm. Sin. B 5 (2015) 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Xue B, Kozlovskaya V, Liu F, Chen J, Williams JF, Campos-Gomez J, Saeed M, Kharlampieva E, Intracellular degradable hydrogel cubes and spheres for anti-cancer drug delivery, ACS Appl. Mater. Interfaces 7 (2015) 13633–13644. [DOI] [PubMed] [Google Scholar]
- [145].Ta HT, Dass CR, Dunstan DE, Injectable chitosan hydrogels for localised cancer therapy, J. Control. Release 126 (2008) 205–216. [DOI] [PubMed] [Google Scholar]
- [146].Xiong L, Luo Q, Wang Y, Li X, Shen Z, Zhu W, An injectable drug-loaded hydrogel based on a supramolecular polymeric prodrug, Chem. Commun 51 (2015) 14644–14647. [DOI] [PubMed] [Google Scholar]
- [147].McGlynn LM, Kirkegaard T, Edwards J, Tovey S, Cameron D, Twelves C, Bartlett JM, Cooke TG, Ras/Raf-1/MAPK pathway mediates response to tamoxifen but not chemotherapy in breast cancer patients, Clin. Cancer Res 15 (2009) 1487–1495. [DOI] [PubMed] [Google Scholar]
- [148].Du YQ, Yang XX, Li WL, Wang J, Huang CZ, A cancer-targeted drug delivery system developed with gold nanoparticle mediated DNA-doxorubicin conjugates, RSC Adv. 4 (2014) 34830–34835. [Google Scholar]
- [149].Tian R, Chen J, Niu R, The development of low-molecular weight hydrogels for applications in cancer therapy, Nanoscale 6 (2014) 3474–3482. [DOI] [PubMed] [Google Scholar]
- [150].Alexander A, Ajazuddin, Patel RJ, Saraf S, Saraf S, Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives, J. Control. Release 241 (2016) 110–124. [DOI] [PubMed] [Google Scholar]
- [151].Wu X, He C, Wu Y, Chen X, Synergistic therapeutic effects of Schiff s base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model, Biomaterials 75 (2016) 148–162. [DOI] [PubMed] [Google Scholar]
- [152].Jeswani G, Alexander A, Saraf S, Saraf S, Qureshi A, Ajazuddin, Recent approaches for reducing hemolytic activity of chemotherapeutic agents, J. Control. Release 211 (2015) 10–21. [DOI] [PubMed] [Google Scholar]
- [153].Alexander A, Ajazuddin, Khan J, Saraf S, Saraf S, Poly(ethylene glycol)-poly (lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications, J. Control. Release 172 (2013) 715–729. [DOI] [PubMed] [Google Scholar]
- [154].Ma G, Miao B, Song C, Thermosensitive PCL-PEG-PCL Hydrogels: Synthesis, Characterization, and Delivery of Proteins 116, 2010, pp. 1985–1993. [Google Scholar]
- [155].Cirillo G, Spizzirri UG, Curcio M, Nicoletta FP, lemma F, Injectable hydrogels for cancer therapy over the last decade, Pharmaceutics 11 (2019) 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].van der Merwe J, Steenekamp J, Steyn D, Hamman J, The Role of Functional Excipients in Solid Oral Dosage Forms to Overcome Poor Drug Dissolution and Bioavailability 12, 2020, p. 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Larrañeta E, Stewart S, Ervine M, Al-Kasasbeh R, Donnelly RF, Hydrogels for hydrophobic drug delivery, Classif. Synth. Appl 9 (2018) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Rafael D, Melendres MMR, Andrade F, Montero S, Martinez-Trucharte F, Vilar-Hernandez M, Durán-Lara EF, Schwartz S Jr., Abasolo I, Thermo-responsive hydrogels for cancer local therapy: challenges and state-of-art, Int. J. Pharm 606 (2021), 120954. [DOI] [PubMed] [Google Scholar]
- [159].Xu S, Wang W, Li X, Liu J, Dong A, Deng L, Sustained release of PTX-incorporated nanoparticles synergized by burst release of DOXHCl from thermosensitive modified PEG/PCL hydrogel to improve anti-tumor efficiency, Eur. J. Pharm. Sci 62 (2014) 267–273. [DOI] [PubMed] [Google Scholar]
- [160].Yu L, Ci T, Zhou S, Zeng W, Ding J, The thermogelling PLGA–PEG–PLGA block copolymer as a sustained release matrix of doxorubicin, Biomater. Sci 1 (2013) 411–420. [DOI] [PubMed] [Google Scholar]
- [161].Song X, Zhang Z, Zhu J, Wen Y, Zhao F, Lei L, Phan-Thien N, Khoo BC, Li J, Thermoresponsive hydrogel induced by dual supramolecular assemblies and its controlled release property for enhanced anticancer drug delivery, Biomacromolecules 21 (2020) 1516–1527. [DOI] [PubMed] [Google Scholar]
- [162].Wu Z, Zou X, Yang L, Lin S, Fan J, Yang B, Sun X, Wan Q, Chen Y, Fu S, Thermosensitive hydrogel used in dual drug delivery system with paclitaxel-loaded micelles for in situ treatment of lung cancer, Colloids Surf. B: Biointerfaces 122 (2014) 90–98. [DOI] [PubMed] [Google Scholar]
- [163].Gong C, Wang Y, Wang X, Wei X, Wu Q, Wang B, Dong P, Chen L, Luo F, Qian Z, Biodegradable self-assembled PEG-PCL-PEG micelles for hydrophobic drug delivery, part 2: in vitro and in vivo toxicity evaluation, J. Nanopart. Res 13 (2011) 721–731. [Google Scholar]
- [164].Nakanishi T, Fukushima S, Okamoto K, Suzuki M, Matsumura Y, Yokoyama M, Okano T, Sakurai Y, Kataoka K, Development of the polymer micelle carrier system for doxorubicin, J. Control. Release 74 (2001) 295–302. [DOI] [PubMed] [Google Scholar]
- [165].Wu Z, Zou X, Yang L, Lin S, Fan J, Yang B, Sun X, Wan Q, Chen Y, Fu S, Thermosensitive hydrogel used in dual drug delivery system with paclitaxel-loaded micelles for in situ treatment of lung cancer, Colloids Surf. B: Biointerfaces 122 (2014) 90–98. [DOI] [PubMed] [Google Scholar]
- [166].Shim WS, Kim J-H, Kim K, Kim Y-S, Park R-W, Kim I-S, Kwon IC, Lee DS, pH- and temperature-sensitive, injectable, biodegradable block copolymer hydrogels as carriers for paclitaxel, Int. J. Pharm 331 (2007) 11–18. [DOI] [PubMed] [Google Scholar]
- [167].Fang F, Gong C, Qian Z, Zhang X, Gou M, You C, Zhou L, Liu J, Zhang Y, Guo G, Gu Y, Luo F, Chen L, Zhao X, Wei Y, Honokiol nanoparticles in thermosensitive hydrogel: therapeutic effects on malignant pleural effusion, ACS Nano 3 (2009) 4080–4088. [DOI] [PubMed] [Google Scholar]
- [168].Gou ML, Dai M, Li XY, Wang XH, Gong CY, Xie Y, Wang K, Zhao X, Qian ZY, Wei YQ, Preparation and characterization of honokiol nanoparticles, J. Mater. Sci. Mater. Med 19 (2008) 2605–2608. [DOI] [PubMed] [Google Scholar]
- [169].Gong C, Shi S, Wang X, Wang Y, Fu S, Dong P, Chen L, Zhao X, Wei Y, Qian Z, Novel composite drug delivery system for honokiol delivery: self-assembled poly(ethylene glycol)-poly(epsilon-caprolactone)-poly(ethylene glycol) micelles in thermosensitive poly(ethylene glycol)-poly(epsilon-caprolactone)-poly(ethylene glycol) hydrogel, J. Phys. Chem. B 113 (2009) 10183–10188. [DOI] [PubMed] [Google Scholar]
- [170].Wang Y, Gong C, Yang L, Wu Q, Shi S, Shi H, Qian Z, Wei Y, 5-FU-hydrogel inhibits colorectal peritoneal carcinomatosis and tumor growth in mice, BMC Cancer 10 (2010) 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Chao GT, Qian ZY, Huang MJ, Kan B, Gu YC, Gong CY, Yang JL, Wang K, Dai M, Li XY, Gou ML, Tu MJ, Wei YQ, Synthesis, characterization, and hydrolytic degradation behavior of a novel biodegradable pH-sensitive hydrogel based on polycaprolactone, methacrylic acid, and poly(ethylene glycol), J. Biomed. Mater. Res. A 85 (2008) 36–46. [DOI] [PubMed] [Google Scholar]
- [172].Yan TD, Black D, Savady R, Sugarbaker PH, Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma, J. Clin. Oncol 24 (2006) 4011–4019. [DOI] [PubMed] [Google Scholar]
- [173].Al Sabbagh C, Seguin J, Agapova E, Kramerich D, Boudy V, Mignet N, Thermosensitive hydrogels for local delivery of 5-fluorouracil as neoadjuvant or adjuvant therapy in colorectal cancer, Eur. J. Pharm. Biopharm 157 (2020) 154–164. [DOI] [PubMed] [Google Scholar]
- [174].Lei N, Gong C, Qian Z, Luo F, Wang C, Wang H, Wei Y, Therapeutic application of injectable thermosensitive hydrogel in preventing local breast cancer recurrence and improving incision wound healing in a mouse model, Nanoscale 4 (2012) 5686–5693. [DOI] [PubMed] [Google Scholar]
- [175].Veronesi U, Saccozzi R, Del Vecchio M, Banfi A, Clemente C, De Lena M, Gallus G, Greco M, Luini A, Marubini E, Muscolino G, Rilke F, Salvadori B, Zecchini A, Zucali R, Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast, N. Engl. J. Med 305 (1981) 6–11. [DOI] [PubMed] [Google Scholar]
- [176].Jacobson JA, Danforth DN, Cowan KH, d’Angelo T, Steinberg SM, Pierce L, Lippman ME, Lichter AS, Glatstein E, Okunieff P, Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer, N. Engl. J. Med 332 (1995) 907–911. [DOI] [PubMed] [Google Scholar]
- [177].Noel G, Mazeron JJ, Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial, Cancer Radiother. 5 (2001) 211–212. [DOI] [PubMed] [Google Scholar]
- [178].Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH, Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer, J. Clin. Oncol 16 (1998) 1689–1696. [DOI] [PubMed] [Google Scholar]
- [179].Petignat P, du Bois A, Bruchim I, Fink D, Provencher DM, Should intraperitoneal chemotherapy be considered as standard first-line treatment in advanced stage ovarian cancer? Crit. Rev. Oncol. Hematol 62 (2007) 137–147. [DOI] [PubMed] [Google Scholar]
- [180].Kehoe SM, Williams NL, Yakubu R, Levine DA, Chi DS, Sabbatini PJ, Aghajanian CA, Barakat RR, Abu-Rustum NR, Incidence of intestinal obstruction following intraperitoneal chemotherapy for ovarian tubal and peritoneal malignancies, Gynecol. Oncol 113 (2009) 228–232. [DOI] [PubMed] [Google Scholar]
- [181].Wei L, Cai C, Lin J, Chen T, Dual-drug delivery system based on hydrogel/micelle composites, Biomaterials 30 (2009) 2606–2613. [DOI] [PubMed] [Google Scholar]
- [182].Gong C, Wang C, Wang Y, Wu Q, Zhang D, Luo F, Qian Z, Efficient inhibition of colorectal peritoneal carcinomatosis by drug loaded micelles in thermosensitive hydrogel composites, Nanoscale 4 (2012) 3095–3104. [DOI] [PubMed] [Google Scholar]
- [183].Gong C, Shi S, Wu L, Gou M, Yin Q, Guo Q, Dong P, Zhang F, Luo F, Zhao X, Wei Y, Qian Z, Biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PCL-PEG-PCL hydrogel. Part 2: sol-gel-sol transition and drug delivery behavior, Acta Biomater. 5 (2009) 3358–3370. [DOI] [PubMed] [Google Scholar]
- [184].de Bree E, Witkamp AJ, Zoetmulder FA, Intraperitoneal chemotherapy for colorectal cancer, J. Surg. Oncol 79 (2002) 46–61. [DOI] [PubMed] [Google Scholar]
- [185].Ron ES, Bromberg LE, Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery, Adv. Drug Deliv. Rev 31 (1998) 197–221. [DOI] [PubMed] [Google Scholar]
- [186].Burr J, Azuara-Blanco A, Avenell A, Tuulonen A, Medical versus surgical interventions for open angle glaucoma, Cochrane Database Syst. Rev (2012) Cd004399. [DOI] [PubMed] [Google Scholar]
- [187].Jampel HD, Initial treatment for open-angle glaucoma- medical, laser, or surgical? Laser trabeculoplasty is the treatment of choice for chronic open-angle glaucoma, Arch. Ophthalmol 116 (1998) 240–241. [DOI] [PubMed] [Google Scholar]
- [188].Chen CW, Huang HT, Bair JS, Lee CC, Trabeculectomy with simultaneous topical application of mitomycin-C in refractory glaucoma, J. Ocul. Pharmacol 6 (1990) 175–182. [DOI] [PubMed] [Google Scholar]
- [189].Greenfield DS, Liebmann JM, Jee J, Ritch R, Late-onset bleb leaks after glaucoma filtering surgery, Arch. Ophthalmol 116 (1998) 443–447. [DOI] [PubMed] [Google Scholar]
- [190].Green E, Wilkins M, Bunce C, Wormald R, 5-fluorouracil for glaucoma surgery, Cochrane Database Syst. Rev (2014) Cd001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Paula JS, Ribeiro VR, Chahud F, Cannellini R, Monteiro TC, Gomes EC, Reinach PS, Rodrigues Mde L, Silva-Cunha A, Bevacizumab-loaded polyurethane subconjunctival implants: effects on experimental glaucoma filtration surgery, J.Ocul. Pharmacol. Ther 29 (2013) 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Rodriguez-Agirretxe I, Vega SC, Rezola R, Vecino E, Mendicute J, Suarez-Cortes T, Acera A, The PLGA implant as an antimitotic delivery system after experimental trabeculectomy, Invest. Ophthalmol. Vis. Sci 54 (2013) 5227–5235. [DOI] [PubMed] [Google Scholar]
- [193].Park HY, Kim JH, Park CK, VEGF induces TGF-betal expression and myofibroblast transformation after glaucoma surgery, Am. J. Pathol 182 (2013) 2147–2154. [DOI] [PubMed] [Google Scholar]
- [194].Zhou M, Chen S, Wang W, Huang W, Cheng B, Ding X, Zhang X, Levels of erythropoietin and vascular endothelial growth factor in surgery-required advanced neovascular glaucoma eyes before and after intravitreal injection of bevacizumab, Invest. Ophthalmol. Vis. Sci 54 (2013) 3874–3879. [DOI] [PubMed] [Google Scholar]
- [195].Grewal DS, Jain R, Kumar H, Grewal SP, Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy a pilot study, Ophthalmology 115 (2008) 2141–2145.e2142. [DOI] [PubMed] [Google Scholar]
- [196].Li Z, Van Bergen T, Van de Veire S, Van de Vel I, Moreau H, Dewerchin M, Maudgal PA, Zeyen T, Spileers W, Moons L, Stalmans I, Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery, Invest. Ophthalmol. Vis. Sci 50 (2009) 5217–5225. [DOI] [PubMed] [Google Scholar]
- [197].Vandewalle E, Abegao Pinto L, Van Bergen T, Spielberg L, Fieuws S, Moons L, Spileers W, Zeyen T, Stalmans I, Intracameral bevacizumab as an adjunct to trabeculectomy: a 1-year prospective, randomised study, Br. J. Ophthalmol 98 (2014) 73–78. [DOI] [PubMed] [Google Scholar]
- [198].Nomoto H, Shiraga F, Kuno N, Kimura E, Fujii S, Shinomiya K, Nugent AK, Hirooka K, Baba T, Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits, Invest. Ophthalmol. Vis. Sci 50 (2009) 4807–4813. [DOI] [PubMed] [Google Scholar]
- [199].Han Q, Wang Y, Li X, Peng R, Li A, Qian Z, Yu L, Effects of bevacizumab loaded PEG-PCL-PEG hydrogel intracameral application on intraocular pressure after glaucoma filtration surgery, J. Mater. Sci. Mater. Med 26 (2015) 225. [DOI] [PubMed] [Google Scholar]
- [200].Yang B, Gong C, Zhao X, Zhou S, Li Z, Qi X, Zhong Q, Luo F, Qian Z, Preventing postoperative abdominal adhesions in a rat model with PEG-PCL-PEG hydrogel, Int. J. Nanomedicine 7 (2012) 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Luo Z, Jin L, Xu L, Zhang ZL, Yu J, Shi S, Li X, Chen H, Thermosensitive PEG-PCL-PEG (PECE) hydrogel as an in situ gelling system for ocular drug delivery of diclofenac sodium, Drug Deliv. 23 (2016) 63–68. [DOI] [PubMed] [Google Scholar]
- [202].Han Q, Wang Y, Li X, Peng R, Li A, Qian Z, Yu L, Effects of bevacizumab loaded PEG-PCL-PEG hydrogel intracameral application on intraocular pressure after glaucoma filtration surgery, J. Mater. Sci. Mater. Med 26 (2015) 225. [DOI] [PubMed] [Google Scholar]
- [203].Rauck BM, Friberg TR, Mendez CAM, Park D, Shah V, Bilonick RA, Wang Y, Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo, Invest. Ophthalmol. Vis. Sci 55 (2014) 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Zhang Z, Ni J, Chen L, Yu L, Xu J, Ding J, Biodegradable and thermoreversible PCLA-PEG-PCLA hydrogel as a barrier for prevention of post-operative adhesion, Biomaterials 32 (2011) 4725–4736. [DOI] [PubMed] [Google Scholar]
- [205].Xiong XY, Tam KC, Gan LH, Synthesis and aggregation behavior of Pluronic F127/poly(lactic acid) block copolymers in aqueous solutions, Macromolecules 36 (2003) 9979–9985. [Google Scholar]
- [206].Jeong B, Lee DS, Shon J-I, Bae YH, Kim SW, Thermoreversible gelation of poly(ethylene oxide) biodegradable polyester block copolymers, J. Polym. Sci. A Polym. Chem 37 (1999) 751–760. [Google Scholar]
- [207].Hwang MJ, Suh JM, Bae YH, Kim SW, Jeong B, Caprolactonic poloxamer analog: PEG-PCL-PEG, Biomacromolecules 6 (2005) 885–890. [DOI] [PubMed] [Google Scholar]
- [208].Gong C, Shi S, Dong P, Kan B, Gou M, Wang X, Li X, Luo F, Zhao X, Wei Y, Qian Z, Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel, Int. J. Pharm 365 (2009) 89–99. [DOI] [PubMed] [Google Scholar]
- [209].Bae SJ, Suh JM, Sohn YS, Bae YH, Kim SW, Jeong B, Thermogelling poly (caprolactone-b-ethylene glycol-b-caprolactone) aqueous solutions, Macromolecules 38 (2005) 5260–5265. [Google Scholar]
- [210].Choi SW, Choi SY, Jeong B, Kim SW, Lee DS, Thermoreversible gelation of poly(ethylene oxide) biodegradable polyester block copolymers. II, J. Polym. Sci. Part A 37 (1999) 2207–2218. [Google Scholar]
- [211].Liu C, Qian Z, Gu Y, Fan L, Li J, Chao G, Jia W, Tu M, Synthesis, characterization, and thermal properties of biodegradable aliphatic copolyester based on ε-caprolactone, adipic acid, and 1,6-hexanediol, Mater. Lett 60 (2006) 31–38. [Google Scholar]
- [212].Qian Z, Li S, He Y, Liu X, Synthesis and in vitro degradation study of poly (ethylene terephthalate)/poly(ethylene glycol) (PET/PEG) multiblock copolymer, Polym. Degrad. Stab 83 (2004) 93–100. [Google Scholar]
- [213].Qian Z, Li S, He Y, Zhang H, Liu X, Hydrolytic degradation study of biodegradable polyesteramide copolymers based on ε-caprolactone and 11-aminoundecanoic acid, Biomaterials 25 (2004) 1975–1981. [DOI] [PubMed] [Google Scholar]
- [214].Chao G, Fan L, Jia W, Qian Z, Gu Y, Liu C, Ni X, Li J, Deng H, Gong C, Gou M, Lei K, Huang A, Huang C, Yang J, Kan B, Tu M, Synthesis, characterization and hydrolytic degradation of degradable poly(butylene terephthalate)/poly(ethylene glycol) (PBT/PEG) copolymers, J. Mater. Sci. Mater. Med 18 (2007) 449–455. [DOI] [PubMed] [Google Scholar]
- [215].Qiu Y, Park K, Environment-sensitive hydrogels for drug delivery, Adv. Drug Deliv. Rev 53 (2001) 321–339. [DOI] [PubMed] [Google Scholar]
- [216].Jeong B, Bae YH, Lee DS, Kim SW, Biodegradable block copolymers as injectable drug-delivery systems, Nature 388 (1997) 860–862. [DOI] [PubMed] [Google Scholar]
- [217].Huynh DP, Shim WS, Kim JH, Lee DS, pH/temperature sensitive poly (ethylene glycol)-based biodegradable polyester block copolymer hydrogels, Polymer 47 (2006) 7918–7926. [Google Scholar]
- [218].Shim WS, Yoo JS, Bae YH, Lee DS, Novel injectable pH and temperature sensitive block copolymer hydrogel, Biomacromolecules 6 (2005) 2930–2934. [DOI] [PubMed] [Google Scholar]
- [219].Kim HJ, You SJ, Yang DH, Chun HJ, Kim MS, Preparation of novel RGD-conjugated thermosensitive mPEG-PCL composite hydrogels and in vitro investigation of their impacts on adhesion-dependent cellular behavior, J. Ind. Eng. Chem 84 (2020) 226–235. [Google Scholar]
- [220].Huynh DP, Im GJ, Chae SY, Lee KC, Lee DS, Controlled release of insulin from pH/temperature-sensitive injectable pentablock copolymer hydrogel, J. Control. Release 137 (2009) 20–24. [DOI] [PubMed] [Google Scholar]
- [221].Khodaverdi E, Akbari A, Tekie FS, Mohajeri SA, Zohuri G, Hadizadeh F, Sustained delivery of amphotericin B and vancomycin hydrochloride by an injectable thermogelling tri-block copolymer, PDA J. Pharm. Sci. Technol 67 (2013) 135–145. [DOI] [PubMed] [Google Scholar]
- [222].Nguyen DT, Phan VHG, Lee DS, Thambi T, Huynh DP, Bioresorbable pH- and temperature-responsive injectable hydrogels-incorporating electrosprayed particles for the sustained release of insulin, Polym. Degrad. Stab 162 (2019) 36–46. [Google Scholar]
- [223].Kim YJ, Choi S, Koh JJ, Lee M, Controlled release of insulin from injectable biodegradable triblock copolymer, Pharm. Res 18 (2001) 548. [DOI] [PubMed] [Google Scholar]
- [224].Proctor VA, Cunningham FE, The chemistry of lysozyme and its use as a food preservative and a pharmaceutical, Crit. Rev. Food Sci. Nutr 26 (1988) 359–395. [DOI] [PubMed] [Google Scholar]
- [225].Malinowski E, Lysozyme dimer in therapy and prophylaxis of animal diseases, in: Nika Health Products Vaduz, Lichtenstein, 2001. [Google Scholar]
- [226].Li J, Loh XJ, Cyclodextrin-based supramolecular architectures: syntheses, structures, and applications for drug and gene delivery, Adv. Drug Deliv. Rev 60 (2008) 1000–1017. [DOI] [PubMed] [Google Scholar]
- [227].Ma D, Zhang LM, Xie X, Liu T, Xie MQ, Tunable supramolecular hydrogel for in situ encapsulation and sustained release of bioactive lysozyme, J. Colloid Interface Sci 359 (2011) 399–406. [DOI] [PubMed] [Google Scholar]
- [228].Ma D, Zhang L-M, Xie X, Liu T, Xie M-Q, Tunable supramolecular hydrogel for in situ encapsulation and sustained release of bioactive lysozyme, J. Colloid Interface Sci 359 (2011) 399–406. [DOI] [PubMed] [Google Scholar]
- [229].Harada A, Li J, Kamachi M, Preparation and properties of inclusion complexes of polyethylene glycol with .alpha.-cyclodextrin, Macromolecules 26 (1993) 5698–5703. [Google Scholar]
- [230].Huh KM, Ooya T, Lee WK, Sasaki S, Kwon IC, Jeong SY, Yui N, Supramolecular-structured hydrogels showing a reversible phase transition by inclusion complexation between poly(ethylene glycol) grafted dextran and α-cyclodextrin, Macromolecules 34 (2001) 8657–8662. [Google Scholar]
- [231].Vyas A, Saraf S, Saraf S, Encapsulation of cyclodextrin complexed simvastatin in chitosan nanocarriers: a novel technique for oral delivery, J. Incl Phenom. Macrocycl. Chem 66 (2010) 251–259. [Google Scholar]
- [232].Lv F, Mao L, Liu T, Thermosensitive porphyrin-incorporated hydrogel with fourarm PEG-PCL copolymer: preparation, characterization and fluorescence imaging in vivo, Mater. Sci. Eng. C Mater. Biol. Appl 43 (2014) 221–230. [DOI] [PubMed] [Google Scholar]
- [233].Lovell JF, Roxin A, Ng KK, Qi Q, McMullen JD, DaCosta RS, Zheng G, Porphyrin-cross-linked hydrogel for fluorescence-guided monitoring and surgical resection, Biomacromolecules 12 (2011) 3115–3118. [DOI] [PubMed] [Google Scholar]
- [234].Huang H, Song W, Chen G, Reynard JM, Ohulchanskyy TY, Prasad PN, Bright FV, Lovell JF, Pd-porphyrin-cross-linked implantable hydrogels with oxygen-responsive phosphorescence, Adv. Healthc. Mater 3 (2014) 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Lv F, Mao L, Liu T, Thermosensitive porphyrin-incorporated hydrogel with fourarm PEG–PCL copolymer: preparation, characterization and fluorescence imaging in vivo, Mater. Sci. Eng. C 43 (2014) 221–230. [DOI] [PubMed] [Google Scholar]
- [236].Xiong XY, Tam KC, Gan LH, Hydrolytic degradation of pluronic F127/poly (lactic acid) block copolymer nanoparticles, Macromolecules 37 (2004) 3425–3430. [Google Scholar]
- [237].Kim SY, Ha JC, Lee YM, Poly(ethylene oxide)-poly(propylene oxide)-poly (ethylene oxide)/poly(epsilon-caprolactone) (PCL) amphiphilic block copolymeric nanospheres. II. Thermo-responsive drug release behaviors, J. Control. Release 65 (2000) 345–358. [DOI] [PubMed] [Google Scholar]
- [238].Liu C, Gong C, Pan Y, Zhang Y, Wang J, Huang M, Wang Y, Wang K, Gou M, Tu M, Wei Y, Qian Z, Synthesis and characterization of a thermosensitive hydrogel based on biodegradable amphiphilic PCL-Pluronic (L35)-PCL block copolymers, Colloids Surf. A Physicochem. Eng. Asp 302 (2007) 430–438. [Google Scholar]
- [239].Lee JW, Hua F, Lee DS, Thermoreversible gelation of biodegradable poly (epsilon-caprolactone) and poly(ethylene glycol) multiblock copolymers in aqueous solutions, J. Control. Release 73 (2001) 315–327. [DOI] [PubMed] [Google Scholar]
- [240].Jeong B, Kim SW, Bae YH, Thermosensitive sol-gel reversible hydrogels, Adv. Drug Deliv. Rev 54 (2002) 37–51. [DOI] [PubMed] [Google Scholar]
- [241].El Eini DID, Barry BW, Rhodes CT, Micellar size, shape, and hydration of long-chain polyoxyethylene nonionic surfactants, J. Colloid Interface Sci 54 (1976) 348–351. [Google Scholar]
- [242].Nivaggioli T, Alexandridis P, Hatton TA, Yekta A, Winnik MA, Fluorescence probe studies of pluronic copolymer solutions as a function of temperature, Langmuir 11 (1995) 730–737. [Google Scholar]
- [243].Alexandridis P, Nivaggioli T, Hatton TA, Temperature effects on structural properties of pluronic P104 and F108 PEO-PPO-PEO block copolymer solutions, Langmuir 11 (1995) 1468–1476. [Google Scholar]
- [244].Li J, Li X, Ni X, Wang X, Li H, Leong KW, Self-assembled supramolecular hydrogels formed by biodegradable PEO–PHB–PEO triblock copolymers and α-cyclodextrin for controlled drug delivery, Biomaterials 27 (2006) 4132–4140. [DOI] [PubMed] [Google Scholar]
- [245].Zhang Z, Ni J, Chen L, Yu L, Xu J, Ding J, Biodegradable and thermoreversible PCLA–PEG–PCLA hydrogel as a barrier for prevention of post-operative adhesion, Biomaterials 32 (2011) 4725–4736. [DOI] [PubMed] [Google Scholar]
- [246].Wang Y, Gong C, Yang L, Wu Q, Shi S, Shi H, Qian Z, Wei Y, 5-FU-hydrogel inhibits colorectal peritoneal carcinomatosis and tumor growth in mice, BMC Cancer 10 (2010) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Lei N, Gong C, Qian Z, Luo F, Wang C, Wang H, Wei Y, Therapeutic application of injectable thermosensitive hydrogel in preventing local breast cancer recurrence and improving incision wound healing in a mouse model, Nanoscale 4 (2012) 5686–5693. [DOI] [PubMed] [Google Scholar]
- [248].Noormohammadi F, Nourany M, Sadeghi GMM, Wang P-Y, Shahsavarani H, The role of cellulose nanowhiskers in controlling phase segregation, crystallization and thermal stimuli responsiveness in PCL-PEGx-PCL block copolymer-based PU for human tissue engineering applications, Carbohydr. Polym 252 (2021), 117219. [DOI] [PubMed] [Google Scholar]
- [249].Xu S, Wang W, Li X, Liu J, Dong A, Deng L, Sustained release of PTX-incorporated nanoparticles synergized by burst release of DOX· HCl from thermosensitive modified PEG/PCL hydrogel to improve anti-tumor efficiency, Eur. J. Pharm. Sci 62 (2014) 267–273. [DOI] [PubMed] [Google Scholar]
- [250].Gong C, Wang C, Wang Y, Wu Q, Zhang D, Luo F, Qian Z, Efficient inhibition of colorectal peritoneal carcinomatosis by drug loaded micelles in thermosensitive hydrogel composites, Nanoscale 4 (2012) 3095–3104. [DOI] [PubMed] [Google Scholar]
- [251].Zhou T, Li X, Li G, Tian T, Lin S, Shi S, Liao J, Cai X, Lin Y, Injectable and thermosensitive TGF-β1-loaded PCEC hydrogel system for in vivo cartilage repair, Sci. Rep 7 (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Nguyen DT, Phan VG, Lee DS, Thambi T, Bioresorbable pH-and temperature-responsive injectable hydrogels-incorporating electrosprayed particles for the sustained release of insulin, Polym. Degrad. Stab 162 (2019) 36–46. [Google Scholar]
- [253].Oroojalian F, Jahanafrooz Z, Chogan F, Rezayan AH, Malekzade E, Rezaei SJT, Nabid MR, Sahebkar A, Synthesis and evaluation of injectable thermosensitive penta-block copolymer hydrogel (PNIPAAm-PCL-PEG-PCL-PNIPAAm) and star-shaped poly (CL— CO— LA)-b-PEG for wound healing applications, J. Cell. Biochem 120 (2019) 17194–17207. [DOI] [PubMed] [Google Scholar]
- [254].Chao G, Fan L, Jia W, Qian Z, Gu Y, Liu C, Ni X, Li J, Deng H, Gong C, Synthesis, characterization and hydrolytic degradation of degradable poly (butylene terephthalate)/poly (ethylene glycol)(PBT/PEG) copolymers, J. Mater. Sci. Mater. Med 18 (2007) 449–455. [DOI] [PubMed] [Google Scholar]
- [255].Kim HJ, You SJ, Yang DH, Eun J, Park HK, Kim MS, Chun HJ, Injectable hydrogels based on MPEG–PCL–RGD and BMSCs for bone tissue engineering, biomaterials, Science 8 (2020) 4334–4345. [DOI] [PubMed] [Google Scholar]
- [256].Han Q, Wang Y, Li X, Peng R, Li A, Qian Z, Yu L, Effects of bevacizumab loaded PEG-PCL-PEG hydrogel intracameral application on intraocular pressure after glaucoma filtration surgery, J. Mater. Sci. Mater. Med 26 (2015) 1–9. [DOI] [PubMed] [Google Scholar]
- [257].Wang T, Jiang X-J, Tang Q-Z, Li X-Y, Lin T, Wu D-Q, Zhang X-Z, Okello E, Bone marrow stem cells implantation with α-cyclodextrin/MPEG–PCL–MPEG hydrogel improves cardiac function after myocardial infarction, Acta Biomater. 5 (2009) 2939–2944. [DOI] [PubMed] [Google Scholar]
- [258].Gioffredi E, Boffito M, Calzone S, Giannitelli SM, Rainer A, Trombetta M, Mozetic P, Chiono V, Pluronic F127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications, Procedia CIRP 49 (2016) 125–132. [Google Scholar]
- [259].Dabbaghi A, Ramazani A, Farshchi N, Rezaei A, Bodaghi A, Rezayati S, Synthesis, physical and mechanical properties of amphiphilic hydrogels based on polycaprolactone and polyethylene glycol for bioapplications: a review, J. Ind. Eng. Chem 101 (2021) 307–323. [Google Scholar]


