Abstract
Prevalence of skin sores and scabies in remote Australian Aboriginal communities remains unacceptably high, with Group A Streptococcus (GAS) the dominant pathogen. We aim to better understand the drivers of GAS transmission using mathematical models. To estimate the force of infection, we quantified the age of first skin sores and scabies infection by pooling historical data from three studies conducted across five remote Aboriginal communities for children born between 2001 and 2005. We estimated the age of the first infection using the Kaplan–Meier estimator; parametric exponential mixture model; and Cox proportional hazards. For skin sores, the mean age of the first infection was approximately 10 months and the median was 7 months, with some heterogeneity in median observed by the community. For scabies, the mean age of the first infection was approximately 9 months and the median was 8 months, with significant heterogeneity by the community and an enhanced risk for children born between October and December. The young age of the first infection with skin sores and scabies reflects the high disease burden in these communities.
Key words: Skin Sores, scabies, northern territory, aboriginal health, age of first infection
Introduction
Infections with scabies and Group A Streptococcus (GAS) remain highly endemic among Australian Aboriginal and Torres Strait Islander peoples (hereafter referred to as Aboriginal Australians), particularly those living in remote communities. This high disease burden remains despite improvements in other indicators of the health of Aboriginal Australian populations over recent years and demonstrated short-term effectiveness of treatment interventions in trials and focused programmes [1–6].
Observational studies in remote Northern Territory communities have reported skin sore prevalence as high as 45% [7, 8], one of the highest recorded globally. Age-specific prevalence of skin sores among children of up to 14 years of age is high, but remarkably consistent across the age groups studied [2]. Skin sores in these settings are primarily caused by GAS, with Staphylococcus aureus as a common secondary pathogen and are frequently associated with scabies [9, 10]. GAS infections have the potential to become invasive and can lead to serious post-infectious sequelae of glomerulonephritis and rheumatic heart disease, that contribute substantially to the observed health disparity between Aboriginal and non-Aboriginal Australians [11].
Although individual studies have reported that presentation to Aboriginal community health centres with skin sores and scabies is common in the first year of life [9, 10, 12], the age of the first infection has not been quantified across multiple studies. Estimation of the mean age of infection, in particular, allows direct calculation of the force of infection, the primary driver of endemic and epidemic dynamics in mathematical models of infectious disease [13].
This study aims to calculate the mean and median age of the first infection for both skin sores and scabies, using combined data from overlapping observational studies conducted in remote Northern Territory communities [9, 10, 12]. The potential influences of the community of residence, gender, seasonal and temporal factors are explored.
Methods
Study populations
Data were originally collected as part of the East Arnhem Healthy Skin Project (EAHSP), which took place between 2004 and 2007, by the Menzies School of Health Research, in partnership with five remote communities. Active surveillance of skin infections was conducted over the 3-year period, with an aim to reduce the prevalence of skin infections including scabies and skin sores. This active surveillance included an annual ‘healthy skin day’, which provided mass community treatment for scabies and referral to the public health clinic for further treatment if required. For infants and young children whose carers provided consent, all primary health care visits from birth up to 4.75 years of age for children born January 2001–December 2005 were retrospectively audited [2].
Analysis of three separate but overlapping cohorts has been published previously (Table 1) [9, 10, 12]. The three studies were merged into a single dataset, duplicate entries were removed and children actively recruited between 2004 and 2005 included. This synthesis resulted in a total of 365 unique children. For each child, the earliest recorded date of infection for skin sores and scabies was identified. For children not experiencing infection, the date of the last primary health care presentation in the pooled dataset was used as the censoring date.
Table 1.
Period, size and duration of the three separate studies which were pooled to form the combined dataset. The three studies conducted retrospective clinic audits across five remote communities (here coded as A, B, C, D and E to protect community privacy), extracting information for the period of January 2001–December 2007
| Study | Period | Size | Duration | Communities |
|---|---|---|---|---|
| Kearns et al. | January 2001–May 2007 | 320 | 0–12 months of age | A, B, C, D, E |
| McMeniman et al. | February 2002–December 2007 | 99 | 0–24 months of age | C |
| Clucas et al. | January 2002–September 2005 | 174 | 0–60 months of age | A, E |
Ethics approval for reuse of existing data was obtained from The Human Research Ethics Committee of the Northern Territory Department of Health and Community Services and Menzies School of Health Research (Ethics approval number 2015-2516). Permission was also obtained from the custodians of each dataset. This project has been conducted in association with an Indigenous Reference Group, as well as an ongoing stakeholder group which contains Aboriginal Australian community members.
Analysis methods
We utilised three separate models to explore aspects of the population-level age of the first infection: Kaplan–Meier estimator, a parametric exponential mixture model and a Cox proportional hazards model. The Kaplan–Meier estimator provides a baseline estimate of the burden of disease by reporting the overall proportion of the population infected by the end of the follow-up period. The parametric exponential mixture model,
provides an estimate of mean age of the first infection (1/λ) which cannot be calculated using the Kaplan–Meier estimator. This form of survival function allows a proportion of individuals (1-α) to experience a constant hazard (λ) and the remainder (α) to experience zero risk (i.e. remain uninfected) over their lifetime [14]. The median age of the first infection, Am, can be extracted from the parametric model using the formula,
Note that if α = 0, this expression is equivalent to the median of a standard exponential distribution.
The Cox proportional hazards model was fit, using the Kaplan–Meier estimator as the baseline hazard, to determine the impact of any covariates, such as differences between gender, community, birth season, birth year or birth quarter on the median age of the first infection. Healthy Skin Days were held in these communities in September 2005 and 2006, with the Healthy Skin Day covariate referring to whether an individual's date of birth was prior to their implementation. The Cox model produces a hazard ratio (HR) for each covariate. A HR greater than 1 implies the covariate is associated with increased infection pressure, resulting in a shorter time to the first infection.
Both the Kaplan–Meier estimator and the Cox proportional hazards model were computed using R v3.3.3 and the package ‘survival’ v2.40.1. Parameter estimates for the parametric model were obtained using maximum likelihood estimation, within the ‘expsurv’ package which was developed alongside this work and released at https://github.com/MikeLydeamore/exponentialsurvival. For each model, the median age of the first infection was calculated, as well as the confidence interval. For the parametric model, these confidence intervals were obtained using bootstrapping with 200 replications.
Results
Study population
There were 365 children included in the dataset with slightly more males than females (Table 2). Individual followup time differed based on the study protocol under which they were recruited (Table 1). The median number of primary health care presentations for a child was 19 (interquartile range (IQR) 13-28) times in the first year of life and 15 (IQR 8-22) in the second year of life.
Table 2.
Distribution of the individual-level covariates for individuals born between January 2001 and December 2005 who were included in this study. As well as gender and community of residence, we considered the influences of enrolment before or after a regional ‘Healthy Skin Day’ intervention. The effects of year, quarter and season of birth were explored
| Gender | N | (%) |
|---|---|---|
| Male | 204 | 56 |
| Female | 161 | 44 |
| Community | ||
| A | 53 | 14 |
| B | 91 | 25 |
| C | 99 | 27 |
| D | 40 | 11 |
| E | 82 | 23 |
| Healthy Skin Day | ||
| Pre | 287 | 78 |
| Post | 78 | 22 |
| Birth Year | ||
| 2001 | 50 | 14 |
| 2002 | 78 | 21 |
| 2003 | 103 | 28 |
| 2004 | 84 | 23 |
| 2005 | 50 | 14 |
| Birth Quarter | ||
| January–March | 103 | 28 |
| April–June | 91 | 25 |
| July–September | 85 | 23 |
| October–December | 86 | 24 |
| Birth Season | ||
| Wet | 179 | 51 |
| Dry | 186 | 49 |
Primary health care presentations
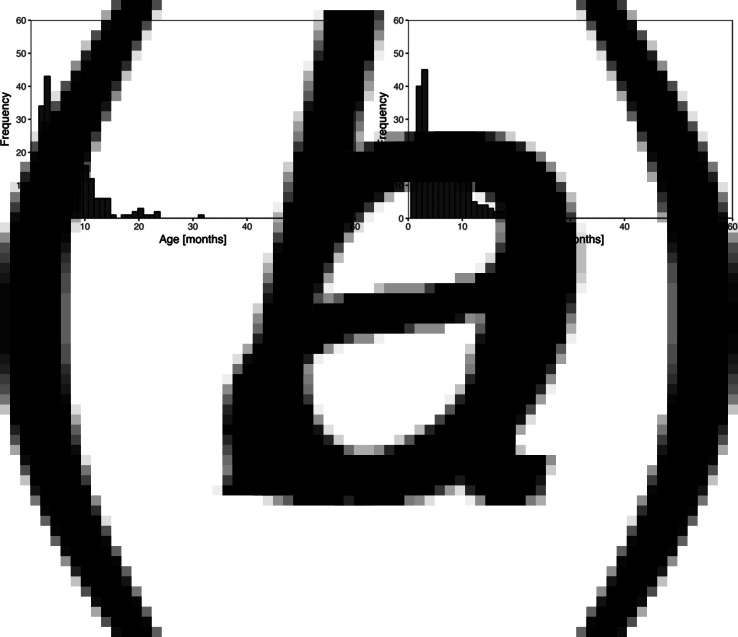
Skin sores were recorded at least once for 307 (84%) of the 365 children and scabies for 260 (71%) children. Of these, 251 (69%) child health records reported at least one episode of scabies and at least one episode of skin sores. The most frequent age of the first infection for skin sores and scabies was similar at 3–4 months with no new first infections reported after the age of 32 months (Fig. 1).
Fig. 1.
Histogram of recorded ages of the first infection for skin sores (a) and scabies (b), using public health network presentation data, for children born between January 2001 and December 2005 across five remote communities.
The curves showing best model fit from the Kaplan–Meier estimates of the survival curve, the parametric model and the Cox model by the community, reveal similarities in the age of acquisition of skin sores across all communities, but demonstrated differences in the age of acquisition of scabies (Figs 2 and 3).
Fig. 2.
Comparison of the distribution of age of the first infection for skin sores for children born between January 2001 and December 2005 across five remote communities (here coded as A, B, C, D and E to protect community privacy). The distributions are estimated using the Kaplan–Meier estimator, the parametric model and the Cox model fitted by the community.
Fig. 3.
Comparison of the distribution of age of the first infection for scabies for children born between January 2001 and December 2005 across five remote communities (here coded as A, B, C, D and E to protect community privacy). The distributions are estimated from the Kaplan–Meier estimator, the parametric model and the Cox model fitted by the community.
Skin sores
The parametric model identified the mean age of the first infection with skin sores as 10.2 months (95%CI 9.4–11.8), with a median seven months (95%CI 6.9–9.5). These values were remarkably consistent across all communities with widely overlapping confidence intervals and almost no children remained uninfected in the first 60 months of life (Tables 3 and Fig. 4).
Table 3.
Presentations with skin sores, by a community of residence (here coded as A, B, C, D and E to protect community privacy). The proportion of children remaining uninfected by the end of the study period is reported, together with the age of first skin sore clinic presentation. Ages are reported as means and medians in months, with 95% confidence intervals calculated using the parametric model.
| Community | Uninfected proportion | Mean age of first infection (months) | Median age of first infection (months) | |||
|---|---|---|---|---|---|---|
| A | <0.001 | (0, 0.11) | 11.0 | (8.8, 14.5) | 7.6 | (6.7, 10.7) |
| B | <0.001 | (0, 0.08) | 10.1 | (8.2, 12.8) | 7.0 | (6.1, 9.1) |
| C | <0.001 | (0, 0.08) | 9.8 | (8.2, 12.5) | 6.8 | (6.1, 9.1) |
| D | <0.001 | (0, 0.10) | 9.2 | (6.8, 12.2) | 6.5 | (5.2, 8.9) |
| E | <0.001 | (0, 0.10) | 10.1 | (8.2, 12.6) | 7.0 | (6.0, 9.1) |
Fig. 4.
Comparison of the estimated median age of the first infection for skin sores under the Kaplan–Meier estimator, the Cox model and the parametric model, across five remote communities (here coded as A, B, C, D and E to protect community privacy) using public health network presentation data for children born between January 2001 and December 2005.
Despite overlapping confidence intervals, the Cox model identified community of residence as having a marginally significant influence on the median age of the first skin sore presentation for children in community D (HR 1.59, CI (1.01, 2.48), P = 0.043 giving median age of the first infection of 5.68 months (reduction by 2.3 months)) (Table 4). A clear increase in infection hazard was observed for children born between October and December (HR 1.50, CI (1.10, 2.04), P = 0.010 giving the median age of the first infection of 5.5 months (reduction by 1.9 months)) (Table 4). This association was present both before and after the healthy skin programme (September 2004 and 2005), demonstrating that the observed birth quarter effect was not due to the intervention (data not shown).
Table 4.
Estimated values for the coefficients of a Cox regression model for the age of the first infection with skin sores, using public health network presentation data for children born between January 2001 and December 2005. Baseline covariates were Gender = Male, Community = A, After Healthy Skin Program = false, Birth Year = 2001, Birth Quarter = 1 and Birth Season = Dry. The bold rows indicate covariates which were identified as significant at the 5% level.
| Covariate | Hazard Ratio | 95% CI | P-value | |
|---|---|---|---|---|
| Gender | Male | 1.00 | Reference | |
| Female | 0.92 | (0.74, 1.16) | 0.499 | |
| Community | A | 1.00 | Reference | |
| B | 1.21 | (0.83, 1.78) | 0.299 | |
| C | 1.19 | (0.83, 1.70) | 0.338 | |
| D | 1.59 | (1.01, 2.48) | 0.043 | |
| E | 1.33 | (0.92, 1.92) | 0.135 | |
| After Healthy Skin Program | False | 1.00 | Reference | |
| True | 1.23 | (0.93, 1.63) | 0.146 | |
| Birth Year | 2001 | 1.00 | Reference | |
| 2002 | 0.90 | (0.61, 1.34) | 0.612 | |
| 2003 | 0.85 | (0.59, 1.23) | 0.382 | |
| 2004 | 0.91 | (0.62, 1.33) | 0.625 | |
| 2005 | 1.10 | (0.71, 1.71) | 0.654 | |
| Birth Quarter | January–March | 1.00 | Reference | |
| April–June | 1.07 | (0.79, 1.47) | 0.637 | |
| July–September | 0.87 | (0.63, 1.19) | 0.380 | |
| October–December | 1.50 | (1.10, 2.04) | 0.010 | |
| Birth Season | Dry | 1.00 | Reference | |
| Wet | 0.86 | (0.68, 1.07) | 0.179 |
Scabies
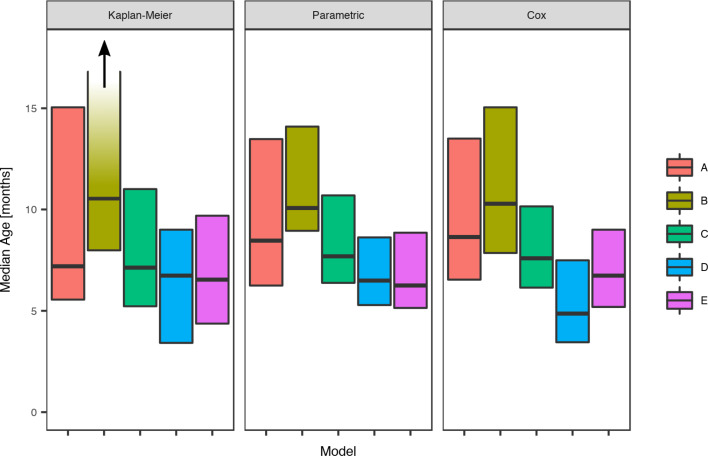
For scabies, the parametric model's estimate of the mean age of first infection was 8.6 months (95% CI 7.8–9.6) and the median 7.6 months (95% CI 7.1–9.0). Differences in the mean and median ages of first scabies infection were observed by a community of residence, with means ranging from 7.0 to 14.5 months and medians between 6.2 and 10.1 months, with non-overlapping confidence intervals (Fig. 5). It was estimated that more than 20% of children remained uninfected in some communities and none in others (Table 5).
Fig. 5.
Comparison of the estimated median age of the first infection for scabies under the Kaplan–Meier estimator, the Cox model and the parametric model, across five remote communities (here coded as A, B, C, D and E to protect community privacy) using public health network presentation data for children born between January 2001 and December 2005. Note that for Community B, the upper confidence interval for the Kaplan–Meier model is infinite.
Table 5.
Presentations with scabies, by a community of residence (here coded as A, B, C, D and E to protect community privacy). The proportion of children remaining uninfected by the end of the study period is reported, together with the age of first scabies clinic presentation. Ages are reported as means and medians in months, with 95% confidence intervals calculated using the parametric model
| Community | Uninfected proportion | Mean age of first infection (months) | Median age of first infection (months) | |||
|---|---|---|---|---|---|---|
| A | 0.22 | (0.13, 0.36) | 8.3 | (6.0, 11.4) | 8.5 | (6.3, 13.9) |
| B | <0.001 | (0, 0.15) | 14.5 | (11.2, 18) | 10.1 | (8.9, 13.6) |
| C | 0.14 | (0.09, 0.25) | 8.9 | (7.1, 10.6) | 7.7 | (6.4, 10.0) |
| D | <0.001 | (0, 0.11) | 9.4 | (7.1, 12.6) | 6.5 | (5.2, 8.9) |
| E | 0.15 | (0.09, 0.26) | 7.0 | (5.4, 8.8) | 6.2 | (4.9, 8.5) |
Community was the only influential covariate in the Cox model, with scabies infections occurring at a significantly younger age among children living in community D (HR 1.74, 95%CI (1.09, 2.79), P = 0.021 giving median age of the first infection of 4.86 months (reduction by 3.8 months)) (Table 6). The overall incidence of scabies was markedly higher in this community than all others (Fig. 3).
Table 6.
Estimated values for the coefficients for a Cox regression model for the age of the first infection with scabies, using public health network presentation data for children born between January 2001 and December 2005. Baseline covariates were Gender = Male, Community = A, After Healthy Skin Program = false, Birth Year = 2001, Birth Quarter = January–March and Birth Season = Dry
| Covariate | Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|---|
| Gender | Male | 1.00 | Baseline | |
| Female | 0.90 | (0.70, 1.15) | 0.404 | |
| Community | A | 1.00 | Baseline | |
| B | 0.86 | (0.56, 1.31) | 0.477 | |
| C | 1.14 | (0.77, 1.69) | 0.522 | |
| D | 1.74 | (1.09, 2.79) | 0.021 | |
| E | 1.30 | (0.87, 1.96) | 0.200 | |
| After Healthy Skin Program | False | 1.00 | Baseline | |
| True | 1.09 | (0.80, 1.48) | 0.599 | |
| Birth Year | 2001 | 1.00 | Baseline | |
| 2002 | 1.10 | (0.73, 1.67) | 0.649 | |
| 2003 | 0.96 | (0.65, 1.42) | 0.825 | |
| 2004 | 0.86 | (0.57, 1.31) | 0.485 | |
| 2005 | 0.95 | (0.60, 1.53) | 0.846 | |
| Birth Quarter | January–March | 1.00 | Baseline | |
| April–June | 1.03 | (0.74, 1.44) | 0.840 | |
| July–September | 0.87 | (0.62, 1.23) | 0.423 | |
| October–December | 1.01 | (0.72, 1.42) | 0.938 | |
| Birth Season | Dry | 1.00 | Baseline | |
| Wet | 1.02 | (0.80, 1.30) | 0.875 |
Discussion
We have estimated the age of the first infection for both skin sores and scabies among children from five remote communities in the Northern Territory, to inform the development of transmission models of GAS and scabies [15]. Differences in the mean and median age of first scabies infection were observed across communities, revealing heterogeneity in the force of infection. Despite this fact, remarkable consistency for the mean age of skin sore infection was observed, with a lower median observed in the one community with heightened scabies risk relative to the others. Anecdotally, this heightened risk may have been associated with a case of crusted scabies in that community.
Comparison of the median age of the first infection in this study with earlier estimates obtained from single cohorts demonstrates the importance of including all available population data, including uninfected individuals and the value of pooling estimates from related studies conducted over different timeframes. Previous analyses of a subset of the data contained here – in which individuals were followed for 12 months – reported the median age of the first infection as 5 months for skin sores and 4 months for scabies [9]. Our analyses provide consistent values utilising a similar subset of data; however, by including longer duration of follow up and censoring, we have estimated a notably later age of the first infection. Using a series of statistical approaches, we have also been able to explore community-level differences in age of the first infection. Reuse of these high-value datasets represents a highly desirable research efficiency.
Although the data on which these findings are based were collected more than a decade ago, the clinical picture of GAS and scabies remains relatively unchanged, despite the short-term demonstration of intervention effectiveness. While recent reports do indicate some improvements in the general health of Aboriginal populations living remotely [1, 16], contemporary prevalence estimates of skin sores and scabies demonstrate an unacceptably high ongoing disease burden [7, 8, 16].
No significant difference by season of birth (wet, dry) in the age of first infection with either skin sores or scabies was observed in this study. This aligns with the analysis of previous prevalence-based studies in our study populations [2] and suggests that the force of infection in the wet and dry season is relatively similar [13]. It is worth noting that historical studies in more temperate climates such as the USA have reported a higher prevalence of GAS in the summer season [17–20], which would indicate an increased force of infection, or conversely a lower force of infection in cooler months when skin is covered [20, 21]. Previous observations of an impact of low humidity on the risk of some skin conditions [22] may explain our finding of increased skin sore (but not scabies) hazard for children born between October and December, as the average age of the first infection for children born in this period is between March and June, which is generally a period of lower humidity. This finding warrants additional investigation to see whether it is reproduced in similar clinic-based cohort studies. Similar to scabies, tinea and fungal infections also create a break in the skin, which may facilitate skin sore infection. There were insufficient data in this study to explore such an association.
The ability to aggregate multiple datasets with different durations of follow up was a key strength of our study. The assembled cohorts represented approximately 20% of all births in the study communities over the period [9]. This analysis also accounted for the right-censored nature of the data, allowing individuals who did not become infected during the observation interval to still inform the age of the first infection in the estimation procedure, a critical step in accurately estimating the age of the first infection [23]. This approach enabled maximal information gains from valuable historical data resources, using a series of complementary analytic techniques.
We acknowledge the challenges involved with the retrospective review of medical records data, particularly where information on clinical presentations was missing and by inference assumed to represent the absence of disease. It is noteworthy, however, that this study drew on information around a period of a mass community intervention that is likely to have improved both presenteeism and reporting for skin disease. In addition, the general consistency of skin sore findings across communities reported here and in a companion manuscript (Cuningham et al., manuscript under review) provided reassurance that ascertainment was not biased by the clinic of attendance. As the involved PHC services were community-based and governed, access to culturally appropriate care was assumed.
The use of primary health care presentation data can underestimate the age of the first infection and infection burden. The high endemic prevalence of scabies and skin sores has resulted in ‘normalisation’ of skin infections [2, 24], leading potentially to a delay in the child presenting to the primary health care service with either skin sores or scabies. If true, this phenomenon would make the true age of the first infection earlier than our estimate, leading us to underestimate the force of infection.
The estimated early mean age of the first infection adds to existing knowledge of the high burden and intensity of transmission of skin pathogens in remote Aboriginal communities in Northern Australia [25]. Further, the influence of community on the force of infection is important to explore in greater depth when planning interventions, which may need to be tailored to the particular drivers in each community. Of particular concern in the Northern Territory are crusted scabies infections that have been identified previously as core transmitters in communities with high scabies prevalence [16, 26]. With new treatments for scabies such as ivermectin and potentially moxidectin becoming the standard [2, 16, 27], recent efforts in controlling disease in these communities are beginning to show some success [1, 11], although strategies for sustained control have remained elusive, likely due to extreme (and heterogeneous) infection pressure [26–28].
Our estimate of the age of the first infection will directly inform quantification of the force of infection in a series of transmission models being developed as part of a larger project to identify intervention strategies for sustained impact against skin disease. These models will incorporate emerging information on the social determinants of transmission intensity. They will provide a useful conceptual framework for the participatory development of innovative disease control measures with affected communities, to reduce the burden of skin disease and health inequalities associated with its sequelae.
Acknowledgements
We would like to thank the Menzies School for Health Research and related project staff, for providing the data from the EAHSP, staff at the primary health care centres and the members of the five remote indigenous communities for their participation. We acknowledge our partners in this work: Northern Territory Remote Health, Aboriginal Medical Services Alliance Northern Territory, Northern Territory Centre for Disease Control, One Disease and Miwatj Health and the NHMRC funded HOT NORTH initiative. We acknowledge the Lowitja Institute and the Cooperative Research Centre for Aboriginal Health who originally funded and lent significant support to the EAHSP. We would like to thank B. J. Currie for his contributions. M. J. Lydeamore is funded by an Australian Postgraduate Research Training Program Scholarship; This work is supported by an NHMRC Project Grant titled ‘Optimising intervention strategies to reduce the burden of Group A Streptococcus in Aboriginal Communities’ (GNT1098319). We thank the NHMRC Centre for Research Excellence in Infectious Diseases Modelling to Inform Public Health Policy (GNT1078068). J. McVernon is supported by an NHMRC Principal Research Fellowship (GNT1117140). S. Tong is supported by an NHMRC Career Development Fellowship (GNT1145033).
Conflict of interest
None.
References
- 1.Australian Health Ministers’ Advisory Council (2014) Aboriginal and Torres Strait Islander Health Performance Framework 2014 Report.
- 2.Andrews RM et al. (2009) A regional initiative to reduce skin infections amongst aboriginal children living in remote communities of the Northern Territory, Australia. PLoS Neglected Tropical Diseases 3, e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carapetis JR et al. (1997) Success of a scabies control program in an Australian Aboriginal community. The Pediatric Infectious Disease Journal 6, 494–499. [DOI] [PubMed] [Google Scholar]
- 4.La Vincente S et al. (2009) Community management of endemic scabies in remote Aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PL0S Neglected Tropical Diseases 3, e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald MI et al. (2008) The dynamic nature of group A streptococcal epidemiology in tropical communities with high rates of rheumatic heart disease. Epidemiology and Infection 136, 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong LC et al. (2001) Outcome of an interventional program for scabies in an Indigenous community. The Medical Journal of Australia 175, 367–370. [DOI] [PubMed] [Google Scholar]
- 7.Romani L et al. (2015) Prevalence of scabies and impetigo worldwide: a systematic review. The Lancet Infectious Diseases 15, 960–967. [DOI] [PubMed] [Google Scholar]
- 8.Bowen AC et al. (2015) The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS ONE 10, e0136789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kearns T et al. (2013) Clinic Attendances during the first 12 months of life for Aboriginal children in five remote communities of northern Australia. PLoS ONE 8, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clucas DB et al. (2008) Disease burden and health-care clinic attendances for young children in remote Aboriginal communities of northern Australia. Bulletin of the World Health Organization 86, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Australia's health (2016) Australia's health series no. 15., Australian Institute of Health and Welfare 2016. Cat. no. AUS 199. Canberra: AIHW.
- 12.McMeniman E et al. (2011) Skin disease in the first two years of life in Aboriginal children in East Arnhem Land. Australasian Journal of Dermatology 52, 270–273. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RM et al. (1992) Infectious Diseases of Humans: Dynamics and Control. Oxford University Press. [Google Scholar]
- 14.De Angelis R et al. (1999) Mixture models for cancer survival analysis: application to population-based data with covariates. Statistics in Medicine 18, 441–454. [DOI] [PubMed] [Google Scholar]
- 15.Kermack WO and McKendrick AG (1927) A contribution to the mathematical theory of epidemics. Proceedings of the Royal Society of London A: Mathematical, Physical and Engineering Sciences 115, 700–721. [Google Scholar]
- 16.Kearns TM et al. (2015) Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Neglected Tropical Diseases 9, e0004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddox JS et al. (1985) The natural history of streptococcal skin infection: prevention with topical antibiotics. Journal of the American Academy of Dermatology 13, 207–212. [DOI] [PubMed] [Google Scholar]
- 18.Poon-King T et al. (1967) Recurrent epidemic nephritis in South Trinidad. New England Journal of Medicine 277, 728–733. [DOI] [PubMed] [Google Scholar]
- 19.Dillon HC (1968) Impetigo contagiosa: suppurative and Non-suppurative complications: I. clinical, bacteriologic, and epidemiologic characteristics of impetigo. American Journal of Diseases of Children 115, 530–541. [DOI] [PubMed] [Google Scholar]
- 20.Dajani AS et al. (1972) Natural history of impetigo: II. Etiologic agents and bacterial interactions. Journal of Clinical Investigation 51, 2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anthony BF et al. (1976) The dynamics of streptococcal infections in a defined population of children: serotypes associated with skin and respiratory infections. American Journal of Epidemiology 104, 652–666. [DOI] [PubMed] [Google Scholar]
- 22.Rycroft RJG and Smith WDL (1980) Low humidity occupational dermatoses. Contact Dermatitis 6, 488–492. [DOI] [PubMed] [Google Scholar]
- 23.Cox DR (1992) Regression models and life-tables. In Samuel Kotz and Johnson Norman L. (eds). Breakthroughs in Statistics. New York, NY: Springer, pp. 527–541. [Google Scholar]
- 24.Yeoh DK et al. (2017) Are scabies and impetigo ‘normalised’? A cross-sectional comparative study of hospitalised children in northern Australia assessing clinical recognition and treatment of skin infections. PLOS Neglected Tropical Diseases 11, e0005726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gracey M and King M (2009) Indigenous health part 1: determinants and disease patterns. The Lancet 374, 65–75. [DOI] [PubMed] [Google Scholar]
- 26.Huffam SE and Currie BJ (1998) Ivermectin for Sarcoptes scabiei hyperinfestation. International Journal of Infectious Diseases 2, 152–154. [DOI] [PubMed] [Google Scholar]
- 27.Mounsey KE et al. (2016) Prospects for moxidectin as a new oral treatment for human scabies. PLoS Neglected Tropical Diseases 10, e0004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Currie BJ et al. (2004) First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clinical Infectious Diseases 39, e8–e12. [DOI] [PubMed] [Google Scholar]