Abstract
Thrombocytopenia is common in patients with invasive bacterial infections. Bacteria can activate platelets, but it is unclear if this affects platelet count. The aim of this study was to examine whether bacteraemia with Staphylococcus aureus, which readily activate human platelets, was more likely to be complicated by thrombocytopenia than bacteraemia with Escherichia coli or Streptococcus pneumoniae with different abilities to activate platelets.
We compared information from 600 adult patients with community-acquired bacteraemia with S. aureus (n = 140), E. coli (n = 420) and S. pneumoniae (n = 40) in Southern Sweden, 2012, linking information on positive blood cultures from microbiological databases and medical charts. The proportion of patients with thrombocytopenia (platelet count <150 × 109/ml) was calculated. Logistic regression was used to estimate the odds ratios (OR) for thrombocytopenia according to bacterial species adjusted for confounders.
The proportion of thrombocytopenia was 29% in S. aureus, 28% in E. coli and 20% in S. pneumonia bacteraemia (P = 0.50), corresponding to an OR of 1.2 (95% confidence interval 0.7–1.9) for thrombocytopenia for S. aureus as compared with E. coli or S. pneumoniae, adjusted for confounders.
This study indicates that platelet activation by bacteria is not a major causative mechanism in sepsis-associated thrombocytopenia.
Key words: Bacteraemia, Escherichia coli, platelet aggregation, sepsis, Staphylococcus aureus, Streptococcus pneumoniae, thrombocytopenia
Introduction
Invasive bacterial infections can cause sepsis, a clinical syndrome with high mortality, reflecting an inflammatory response to an infectious agent [1]. Among the most prevalent causative organisms in community-acquired sepsis are Staphylococcus aureus, Escherichia coli and Streptococcus pneumoniae [2–4].
Thrombocytopenia (platelet count <150 000/μl) is common in patients with sepsis [5]. It is a marker of organ dysfunction and has been associated with poor prognosis [6, 7]. The cause of sepsis-associated thrombocytopenia is not fully understood, although many mechanisms have been proposed [5]. Research has mostly focused on the interplay of platelets with the immune system. Platelets can interact directly with microbial pathogens leading to activation with the release of cytokines and antimicrobial peptides [8, 9]. The capability of S. aureus to activate and aggregate platelets is particularly well characterised [10, 11]. For other major pathogens, less is known about bacterial–platelet interactions. Streptococcus pneumoniae has been shown to aggregate platelets in some studies [12], but failed to activate platelets from patients with bacteraemia [13]. Less is known about interactions between Gram-negative bacteria and platelets; however, some strains of E. coli were recently shown to be able to activate platelets in vitro [14, 15]. There is no direct evidence that platelet activation by bacteria occurs to such an extent that platelet count may be affected significantly. To our knowledge, no study has systematically investigated the incidence of thrombocytopenia in invasive infections caused by different microbes. Our objective was to examine whether bacteraemic infections with S. aureus, which readily activate human platelets, are more likely to be complicated by thrombocytopenia than bacteraemia with E. coli and S. pneumoniae that have different abilities to activate platelets.
Methods
Study design
We conducted a retrospective cohort study, assessing the risk of thrombocytopenia in adults aged ⩾18 years with community-acquired S. aureus bacteraemia compared with patients with bacteraemia caused by E. coli and S. pneumoniae in the Region of Skåne (1.3 million inhabitants) in Southern Sweden, from May 2012 to December 2012, linking information on positive blood cultures from microbiological databases and medical charts.
Information on blood cultures was retrieved from databases from the microbiological laboratories serving the 10 hospitals in Skåne. Linkage of information from the different databases was possible due to the personal identification number that is assigned to all Swedish citizens for life [16]. The study was approved by the local Ethics Committee (Dnr: 2013:31).
Data collection
Charts were systematically reviewed and data were collected according to a pre-specified protocol. Data collected included date of birth, sex, underlying diseases, physiological parameters at admission, laboratory values at admission and the most extreme laboratory values, site of infection, admission to an intensive care unit (ICU) and the type of ICU care that was given, length of ICU and hospital stay, in-hospital mortality and the registered diagnosis as well as the reported cause of death.
Thrombocytopenia was defined as a platelet count below 150 × 109/l. Hypotension was defined as systolic blood pressure <90 mmHg or treatment with vasopressors. Hypoperfusion was defined as maximum P-lactate level of >3.2 mmol/l or minimum base excess value <−5 in patients without chronic kidney failure. Organ dysfunction was defined as follows: respiratory dysfunction – oxygen saturation of ⩽86% or ⩽78% if pneumonia or mechanical ventilation; renal dysfunction – S-creatinine increase >45 µmol/l in patients without chronic renal disease, or treatment with continuous renal replacement therapy (CRRT) or haemodialysis; liver dysfunction – S-bilirubin >45 µmol/l in patients without liver disease, acute cholecystitis or cholangitis; neurological dysfunction – altered mental status; coagulopathy – activated partial thromboplastin time (APTT) >60 s in patients not treated with heparin or international normalised ratio (INR) >1.5 in patients not treated with warfarin (platelet counts were not included).
Hypoperfusion and organ dysfunction were calculated in patients with at least one measured and interpretable value, missing data were regarded as within the normal range.
Statistical methods
The χ2, rank-sum, Kruskal–Wallis tests and analysis of variance were used to assess the distribution of risk factors for thrombocytopenia between different bacterial species and between different exposure groups. The proportion of thrombocytopenia was calculated for the three bacterial species. We used logistic regression to estimate the odds ratios (OR) for thrombocytopenia according to bacterial species. The aim of the study was to explore if bacterial species affected the frequency of thrombocytopenia and not to predict the risk factors for thrombocytopenia per se. We therefore selected potential confounders to this association a priori from factors known or suspected to be associated with thrombocytopenia and invasive bacterial infection from S. aureus, E. coli or S. pneumoniae [6]. The covariates were first examined separately in univariable models and were then entered into different models including age, sex, comorbidities, markers of severity of disease (organ dysfunction, hypoperfusion, hypotension and ICU care) with bacterial species as the main explanatory variable [17]. Models were compared using the Akaike's information criterion; goodness of fit of the final model was assessed using the Hosmer–Lemeshow test; the overall explanatory power of the model was assessed by calculating the area under the receiver operator curve curve.
ORs were also estimated in subgroups of participants classified according to age, sex, severe sepsis and ICU care. Homogeneity of estimates across subgroups was assessed using likelihood ratio tests. All analyses were performed using STATA/SE (version 13.1 for Windows; StataCorp LP, College Station, TX, USA).
Results
Cohort selection
In total, we identified 300 patients with S. aureus, 719 with E. coli and 65 with S. pneumoniae bacteraemia in the microbiological database. Of these 1084 patients, 139 were excluded because their charts were incomplete or inaccessible; 149 episodes were not community-acquired (blood cultures were drawn 48 h or more after admission to a hospital); 25 patients were younger than 18 years; 125 patients had not had their platelet count measured; and 44 patients were excluded since their platelet counts were not evaluable due to haematologic disease or to chemotherapy treatment. In two patients who experienced recurrent bacteraemia, only the first episode was included. Finally, 600 episodes of bacteraemia (140 S. aureus, 420 E. coli, 40 S. pneumoniae) were included in the analyses.
Overall, 48% patients were female and median age was 77 (interquartile range (IQR) 65–84). In-hospital mortality was 10%, and median length of stay was 8 days (IQR 5–13). Eight per cent were admitted to an ICU. Basic demographic and clinical factors and patient data according to the bacterial species are shown in Table 1.
Table 1.
Baseline characteristics of patients according to bacteraemia pathogen
| Staphylococcus aureus | Escherichia coli | Streptococcus pneumoniae | P | Missing values | |
|---|---|---|---|---|---|
| No of individuals | 140 | 420 | 40 | % | |
| Age, median (IQR) | 76 (64–85) | 77 (66–84) | 68 (60–79) | 0.03 | 0 |
| Male sex (%) | 66.4 | 46.9 | 55.0 | <0.01 | 0 |
| Co-morbidity | |||||
| Pulmonary disease, % | 10.0 | 15.2 | 25.0 | 0.05 | 0 |
| Cardiovascular disease, % | 42.9 | 41.2 | 30.0 | 0.33 | 0 |
| Diabetes, % | 27.1 | 26.0 | 17.5 | 0.46 | 0 |
| Chronic kidney disease, % | 24.3 | 7.9 | 2.5 | <0.01 | 0 |
| Rheumatic disease, % | 3.6 | 8.6 | 10.0 | 0.12 | 0 |
| Immunosuppression, % | 5.7 | 4.3 | 2.5 | 0.59 | 0 |
| Malignancy, % | 14.3 | 13.1 | 7.5 | 0.53 | 0 |
| Neurologic disease, % | 19.3 | 11.2 | 2.5 | <0.01 | 0 |
| Urologic disease, % | 11.4 | 12.1 | 5.0 | 0.40 | 0 |
| Haemo- or peritoneal dialysis, % | 7.9 | 0.2 | 0 | <0.01 | 0 |
| Prosthetic joint, % | 27.9 | 11.9 | 7.5 | <0.01 | 0 |
| Clinical signs | |||||
| Respiratory rate, median (IQR) | 22 (18–28) | 22 (19–28) | 22 (20–29) | 0.30 | 14.8 |
| Heart rate, median (IQR) | 96 (82–114) | 100 (87–113) | 102 (90–110) | 0.30 | 4.0 |
| Systolic blood pressure, median (IQR) | 150 (110–145) | 128 (110–146) | 126 (110–150) | 0.72 | 3.5 |
| Temperature, median °C (IQR) | 38.0 (37–38.7) | 38.4 (37.8–39.2) | 37.8 (37.3–38.6) | <0.01 | 3.7 |
| Oxygen saturation, median (IQR) | 96 (93–98) | 95 (92–97) | 93 (87–96) | <0.01 | 7.3 |
| RLS, median (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.70 | 18.7 |
| Laboratory testsa | |||||
| Platelet count <100 × 109/l, % | 9.3 | 10.5 | 7.5 | 0.79 | 0 |
| Platelet count <150 × 109/l, % | 29.3 | 28.3 | 20.0 | 0.50 | 0 |
| Platelet count, ×109/l, median (IQR) | 200 (137–294) | 192 (140–249) | 219 (159–239) | 0.28 | 0 |
| Leucocyte count, ×109/l, median (IQR) | 14.2 (9.6–17.5) | 13.0 (9.1–17.6) | 17.8 (10.7–23) | <0.01 | 1.7 |
| Lactate level, median (IQR) | 2.0 (1.6–3) | 2.1 (1.4–3.4) | 2.4 (1.7–3.7) | 0.29 | 24.5 |
| Base excess, median (IQR) | 0 (−2 to 2) | 0 (−2.3 to 2) | 0.8 (−2.1 to 3) | 0.76 | 26.5 |
| Bilirubin, median (IQR) | 10 (8–16) | 15 (9–29) | 15.5 (9–26) | <0.01 | 52.3 |
| Creatinine, median (IQR) | 128 (83–218) | 104 (83–160) | 96 (72–160) | 0.02 | 0 |
| Organ dysfunctionb | |||||
| Liver, % | 0 | 8.1 | 8.3 | 0.05 | |
| Kidney, % | 8.7 | 6.8 | 4 | 0.63 | |
| Altered mental status, % | 8.4 | 5.8 | 10.3 | 0.58 | |
| Hypoxemia, % | 5.4 | 6.5 | 28.2 | <0.01 | |
| Coagulopathy, % | 6.5 | 5.3 | 6.5 | 0.88 | |
| Hypotension, % | 5.1 | 12.9 | 18.0 | 0.02 | |
| Hypoperfusion, % | 24.5 | 27.9 | 27.8 | 0.79 | |
| Treatment | |||||
| Treatment in ICU, % | 3.6 | 8.1 | 27.5 | <0.01 | |
| Mechanical ventilation, % | 1.4 | 1.7 | 22.5 | <0.01 | |
| Vasopressor treatment, % | 2.1 | 6.0 | 15 | <0.01 | |
| Dialysis/CRRT, %c | 0.7 | 0.2 | 7.5 | <0.01 | |
| Outcome | |||||
| Mortality, % | 21.4 | 6.0 | 10.0 | 0.01 | |
| Days to death, median (IQR) | 10 (5–15) | 4 (3–14) | 12 (1–50) | 0.19 | |
| Length of stay among survivors, median (IQR) | 13 (10–25) | 7 (5–10) | 7 (5–12) | <0.01 |
Minima and maxima values.
Calculated among patients with a measured and interpretable value. Kidney dysfunction was defined as an increase >45 µmol/l in S-creatinine in patients without chronic kidney dysfunction or treatment with dialysis or CRRT; liver dysfunction was defined as: S-bilirubin >45 mmol/l in patients without chronic liver disease or diagnosis of acute pancreatitis, cholecystitis or cholangitis; hypoxemia was defined as oxygen saturation <87% or <79% if pneumonia or mechanical ventilation; coagulation dysfunction was defined as: INR>1.5 or APTT>60 s in patients without warfarin or heparin treatment. Platelet count was not included in the definition; hypoperfusion was defined as any lactate >3.2 mmol/l or base excess ⩽−5 in patients without chronic kidney failure; hypotension was defined as systolic blood pressure <90 mmHg and/or treatment with vasopressors.
Continous renal replacement therapy.
Thrombocytopenia
Patients with S. aureus bacteraemia had a median nadir platelet count of 200 × 109/l (IQR 137–294), E. coli 192 × 109/l (IQR 140–249) and S. pneumoniae 219 × 109/l (IQR 159–239) (P = 0.28). There was no significant difference in the proportion of patients with thrombocytopenia among the different bacterial species, 29 in S. aureus, 28% in E.coli and 20% in S. pneumoniae (P = 0.50). Thrombocytopenia was associated with male sex, underlying kidney disease, admission temperature, respiratory rate, leucocyte count, lactate, liver failure, kidney failure, hypotension, hypoperfusion and ICU care. Pulmonary disease was associated with not having thrombocytopenia (see Supplementary Table S1).
We found no evidence of an increased risk of thrombocytopenia in S. aureus bacteraemia as compared with bacteraemia with E. coli or S. pneumoniae when adjusting for potential confounders in a multivariable logistic regression model, OR was 1.2 (95% confidence interval (95% CI) 0.7–1.9), adjusted for age, sex, comorbidities, hypotension, hypoperfusion, organ failure and ICU care, see Table 2.
Table 2.
Risk of thrombocytopenia according to bacterial species
| Univariable analysis | Multivariable analysesa | ||||
|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | Odds ratio | 95% Confidence interval | P value | |
| E. coli and S. pneumoniae bacteraemia | 1.0 | Ref. | 1.0 | Ref. | |
| S. aureus bacteraemia | 1.1 | 0.7–1.7 | 1.2 | 0.7–1.9 | 0.58 |
| Age, per 1 year increase | 1.0 | 1.0–1.0 | 1.0 | 1.0–1.0 | 0.66 |
| Male sex | 1.5 | 1.0–2.1 | 1.9 | 1.2–2.9 | 0.01 |
| Pulmonary disease | 0.6 | 0.3–1.0 | 0.5 | 0.3–1.0 | 0.05 |
| Kidney disease | 1.7 | 1.0–2.9 | 2.0 | 1.0–3.8 | 0.04 |
| Hypotensionb | 3.2 | 1.9–5.6 | 1.8 | 0.9–3.6 | 0.13 |
| Hypoperfusionc | 1.9 | 1.3–2.9 | 1.4 | 0.8–2.2 | 0.23 |
| Organ dysfunctiond | 2.1 | 1.40–3.0 | 1.6 | 1.0–2.5 | 0.08 |
| ICU care | 3.7 | 2.1–6.7 | 2.0 | 0.9–4.4 | 0.11 |
Estimated by logistic regression in 462 subjects with complete information, adjusted for all other variables in the table.
Defined as systolic blood pressure <90 mmHg and/or treatment with vasopressors.
Defined as any lactate >3.2 mmol/l or base excess ⩽−5 (in patients without chronic kidney failure).
Defined as either kidney dysfunction (increase >45 µmol/l in S-creatinine in patients without chronic kidney dysfunction or CRRT or dialysis treatment), liver dysfunction (S-bilirubin >45 mmol/l in patients without chronic liver disease or diagnosis of acute pancreatitis, cholecystitis or cholangitis), hypoxemia (oxygen saturation <87% or <79% if pneumonia or mechanical ventilation); altered mental status; coagulation dysfunction (INR>1.5 or APTT>60 s in patients without warfarin or heparin treatment).
There was no difference in the crude bacteria–thrombocytopenia association between the subgroups with any or no missing values, ORs were 1.0 (95% CI 0.7–2.5) vs. 1.3 (95% CI 0.6–1.9), respectively (P = 0.60).
Subgroup analyses
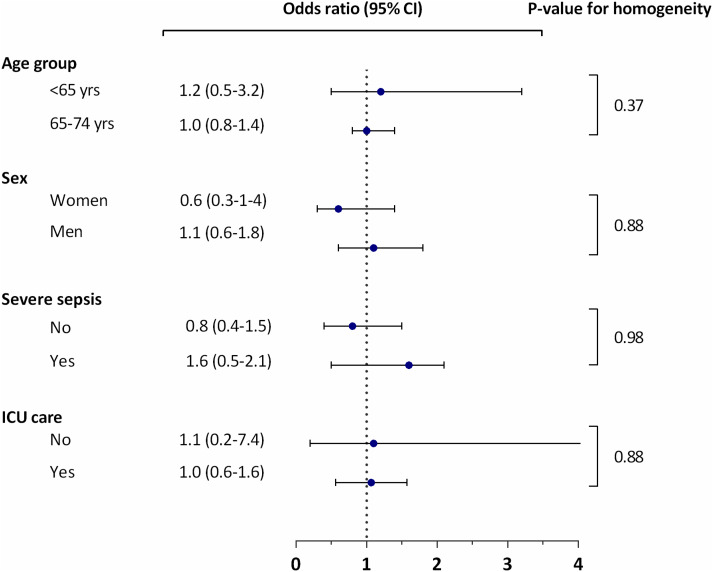
We did not observe any statistically significant differences in the ORs of thrombocytopenia according to the bacterial species in the different subgroups of age (P for test of homogeneity = 0.88), sex (P = 0.37), severe sepsis (P = 0.88) or treated in an ICU (P = 0.98) (see Figure 1).
Fig. 1.
Thrombocytopenia in Staphylococcus aureus bacteraemia vs. Escherichia coli and Streptococcus pneumoniae according to subgroups.
Thrombocytopenia as a risk factor for death
Patients who were alive at discharge had a median nadir platelet count of 199 × 109/l (IQR 144–260), whereas patients who died during hospitalisation had a median platelet count of 166 × 109/l (IQR 115–218) (P = 0.02). However, after adjusting for age, sex, comorbidities, hypotension, hypoperfusion, organ failure and ICU care, no significant difference in in-hospital mortality could be detected, OR for death in thrombocytopenic vs. non-thrombocytopenic was 1.0 (95% CI 0.4–2.1).
Discussion
We investigated the frequency of thrombocytopenia in 600 consecutive adult patients with community-acquired bacteraemia caused by S. aureus as compared with E. coli and S. pneumoniae [2–4]. In our study, up to a third of the patients had a decreased platelet count, but there was no association between bacterial species and the occurrence of thrombocytopenia.
Thrombocytopenia is common in invasive bacterial infections [5], and a drop in platelet count is used as one of several markers of organ dysfunction which in turn are diagnostic criteria for sepsis [18, 19]. However, the causes of sepsis-associated thrombocytopenia are not entirely elucidated [5]. One of the proposed mechanisms is direct interaction between bacteria and platelets [20]. A number of pathogens that cause sepsis have been shown to activate and aggregate platelets [8]. In most of these pathogens, including in S. pneumoniae and E. coli, this ability seems to be confined to some or even few strains [12, 14, 15]. Contrarily, it appears that most clinical isolates of S. aureus from bacteraemic infections readily aggregate platelets [13]. Gafter-Gvili et al. investigated thrombocytopenia in bacteraemia with S. aureus and found a strong correlation between thrombocytopenia and increased mortality, leading to the speculation that thrombocytopenia in part might be attributed to platelet aggregation induced by the bacterium [21].
While, to our knowledge, no study has compared the incidence of thrombocytopenia in bacteraemia caused by specific species in adults, there are reports on the incidence of thrombocytopenia in Gram-positive and Gram-negative bacteraemia, mainly in neonates. Some of these studies have found thrombocytopenia to be more common in Gram-negative sepsis [22]. In contrast, Manzoni et al. did not find such a correlation [23]. Aydemir et al. reported a longer duration of thrombocytopenia in adults with sepsis (defined as the presence of a systemic inflammatory response syndrome and positive blood cultures) in Gram-negative and fungal infection than in Gram-positive bacteraemia [24]. Since these studies included different proportions of community- and hospital-acquired infections, the distribution of microorganisms varied considerably. This makes it difficult to draw definite conclusions based on previous research on the role of specific organisms in sepsis-associated thrombocytopenia.
In the present study, there was no association between specific bacterial species, with apparently different abilities to induce platelet activation, and thrombocytopenia, indicating that there is no strong causal connection with bacterial platelet activation and a drop in platelet count. It appears likelier that other factors, such as the inflammatory response by the innate immune system, are more important for sepsis-associated thrombocytopenia. This does not, however, exclude a significant role for bacterial activation of platelets in severe bacterial infections. Platelets binding to bacteria increase virulence in animal models of infective endocarditis with S. aureus and Streptococcus gordonii [25–28], and binding to platelets has been shown to promote dissemination of bacteria in a mouse model of sepsis with Streptococcus pyogenes [28].
A strength of our study is that it is population-based, including all consecutive patients in a confined geographical area during the study period. A limitation of the study is the retrospective design, resulting in a large proportion of missing values on lactate and bilirubin. Reassuringly, though, there was no difference in the crude bacteria–thrombocytopenia association across the subgroups of patients with no missing values vs. patients with any missing values. Platelet counts were not available for all patients, but there was no association between missingness and bacterial species.
Bacteraemia with sepsis is common, and only critically ill patients are admitted to an ICU. This likely explains the difference in overall mortality between our cohort and recent studies of severe infections that were carried out in ICUs [6, 7, 29–31]. It should be noted, though, that thrombocytopenia was not associated with mortality in our study, neither among all patients or among those infected with S. aureus.
In conclusion, this study indicates that platelet activation by bacteria is not a main mechanism behind sepsis-associated thrombocytopenia.
Acknowledgement
The authors acknowledge Ellen Beronius and Helena Bernestrå for contributing to data collection. Lena Hyllebusk for help with bacteriology records and Dr Oonagh Shannon for important advice.
Financial support
This work was supported by the Swedish Government Fund for Clinical Research (ALF), the Marianne and Marcus Wallenberg foundation, and the foundations of Österlund and Crafoord. Malin Inghammar is supported by grants from Swedish Government Fund for Clinical Research (ALF). The funders played no role in the design of the study, data collection or analysis, decision to publish or preparation of the manuscript.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268818001206.
click here to view supplementary material
References
- 1.Martin GS (2012) Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Reviews of Anti Infective Therapy 10, 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corona A et al. (2010) Antibiotic use and impact on outcome from bacteraemic critical illness: the BActeraemia Study in Intensive Care (BASIC). Journal of Antimicrobial Chemotherapy 65, 1276–1285. [DOI] [PubMed] [Google Scholar]
- 3.Heffner AC et al. (2010) Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clinical Infectious Disease 50, 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valles J et al. (2011) Health-care-associated bloodstream infections at admission to the ICU. Chest 139, 810–815. [DOI] [PubMed] [Google Scholar]
- 5.Larkin CM et al. (2016) Sepsis-associated thrombocytopenia. Thrombosis Research 141, 11–16. [DOI] [PubMed] [Google Scholar]
- 6.Venkata C et al. (2013) Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. Journal of Intensive Care 1, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsirigotis P et al. (2016) Thrombocytopenia in critically ill patients with severe sepsis/septic shock: prognostic value and association with a distinct serum cytokine profile. Journal of Critical Care 32, 9–15. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald JR, Foster TJ and Cox D (2006) The interaction of bacterial pathogens with platelets. Nature Reviews Microbiology 4, 445–457. [DOI] [PubMed] [Google Scholar]
- 9.Yeaman MR (2014) Platelets: at the nexus of antimicrobial defence. Nature Reviews Microbiology 12, 426–437. [DOI] [PubMed] [Google Scholar]
- 10.Bayer AS et al. (1995) Staphylococcus aureus induces platelet aggregation via a fibrinogen-dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infection and Immunity 63, 3634–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien L et al. (2002) Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Molecular Microbiology 44, 1033–1044. [DOI] [PubMed] [Google Scholar]
- 12.Keane C et al. (2010) Invasive Streptococcus pneumoniae trigger platelet activation via Toll-like receptor 2. Journal of Thrombosis and Haemostis 8, 2757–2765. [DOI] [PubMed] [Google Scholar]
- 13.Johansson D, Shannon O and Rasmussen M (2011) Platelet and neutrophil responses to Gram positive pathogens in patients with bacteremic infection. PLoS ONE 6, e26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriarty RD et al. (2016) Escherichia coli induces platelet aggregation in an FcgammaRIIa-dependent manner. Journal of Thrombosis and Haemostis 14, 797–806. [DOI] [PubMed] [Google Scholar]
- 15.Watson CN et al. (2016) Human platelet activation by Escherichia coli: roles for FcgammaRIIA and integrin alphaIIbbeta3. Platelets 27, 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludvigsson JF et al. (2009) The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. European Journal of Epidemiology 24, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David W and Hosmer SL (2005) Model building strategies. In Walter A and Shewhart SSW (eds), Applied Logistic Regression, 2nd Edn. John Wiley & Sons Inc, pp. 118–128. [Google Scholar]
- 18.Levy MM et al. (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Medicine 29, 530–538. [DOI] [PubMed] [Google Scholar]
- 19.Singer M et al. (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guida JD et al. (2003) Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response? Pediatrics 111, 1411–1415. [DOI] [PubMed] [Google Scholar]
- 21.Gafter-Gvili A et al. (2011) Thrombocytopenia in Staphylococcus aureus bacteremia: risk factors and prognostic importance. Mayo Clinical Proceedings 86, 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhat MA et al. (2009) Organism-specific platelet response and factors affecting survival in thrombocytopenic very low birth weight babies with sepsis. Journal of Perinatology 29, 702–708. [DOI] [PubMed] [Google Scholar]
- 23.Manzoni P et al. (2009) Is thrombocytopenia suggestive of organism-specific response in neonatal sepsis? Pediatric International 51, 206–210. [DOI] [PubMed] [Google Scholar]
- 24.Aydemir H et al. (2015) Platelet and mean platelet volume kinetics in adult patients with sepsis. Platelets 26, 331–335. [DOI] [PubMed] [Google Scholar]
- 25.Moreillon P et al. (1995) Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infection and Immunity 63, 4738–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullam PM et al. (1996) Diminished platelet binding in vitro by Staphylococcus aureus is associated with reduced virulence in a rabbit model of infective endocarditis. Infection and Immunity 64, 4915–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong YQ et al. (2008) Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microbial Pathogenesis 45, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn F, Hurley S and Shannon O (2013) Platelets promote bacterial dissemination in a mouse model of streptococcal sepsis. Microbes and Infection 15, 669–676. [DOI] [PubMed] [Google Scholar]
- 29.Sharma B et al. (2007) Thrombocytopenia in septic shock patients – a prospective observational study of incidence, risk factors and correlation with clinical outcome. Anaesthesia and Intensive Care Journal 35, 874–880. [DOI] [PubMed] [Google Scholar]
- 30.Vandijck DM et al. (2010) Thrombocytopenia and outcome in critically ill patients with bloodstream infection. Heart and Lung 39, 21–26. [DOI] [PubMed] [Google Scholar]
- 31.Thiery-Antier N et al. (2016) Is thrombocytopenia an early prognostic marker in septic shock? Critical Care Medicine 44, 764–772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268818001206.
click here to view supplementary material