Abstract
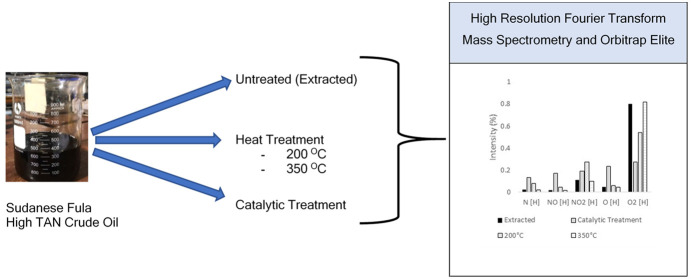
Sudanese Fula crude oil, from the western region, is considered highly viscous and acidic and contains high amounts of heteroatoms (N and O) but a low sulfur content. This work presents an original and comprehensive analysis of its molecular composition in addition to an investigation of the effect of temperature and catalyst on the treatment of the acid fraction. The analysis was performed using a high-resolution Fourier transform mass spectrometer and Orbitrap-Elite with different ionization methods. The results reveal that the Fula crude oil contains a high abundance of nitrogen composition homologue classes N[H], NO2[H], and NO[H]. Their hydrocarbon composition includes low to high aromatic hydrocarbons. The number of oxygen classes varies from acids containing monocarboxylic acids of O2 to acids of multiple carboxylic and phenolic group (CxHyO3 to CxHyO15) classes, which indicate a high content of acidic moiety of 0.765%. In addition to oxygen classes, the acidic fraction that is present as a NOx series indicates the presence of carboxylic carbazole acidic fraction. Low-temperature crude oil treatment at 200 °C decreases the intensity of acids. No significant reduction to low masses was observed; however, there was a clear reduction to high masses. At a high temperature of 350 °C, the carboxylic acid intensity increases (O2 classes), and thus, heating crude oil to 350 °C is unfavorable as it increases the amount of monocarboxylic acids, which are primarily responsible for corrosion in refinery units. Predicted TAN values of residual samples show a reduction in TAN of 62% using thermal treatment at 200 °C, whereas there is an increase in TAN of 5% at 350 °C. A great reduction in acidity results from catalytic treatment with a transition metal catalyst of cobalt and iridium complex. A reduction in all acidic oils is observed; however, the greater reduction is found in mono- and dicarboxylic acids. Catalytic treatment is shown to result in an 85% reduction in predicted TAN values.
1. Introduction
The depletion of highly valuable crude oil, with low hydrocarbon molecular weight, low viscosity, and low amounts of heteroatoms (e.g., nitrogen, oxygen, and sulfur) and metals, is driving the global consideration of heavy oil with lower quality.1 Sudanese crude oil is considered as one of the heaviest crude oils in the world, with a high acid content of 3.68 mg KOH.2 This value is considerably high as a total acid number (TAN) of greater than 0.5 mg KOH/g is considered as highly acidic and thus more corrosive.3,4 High-TAN crude oil results in an increased rate of corrosion of refinery units where highly acidic oil is treated at high temperatures to produce valuable hydrocarbons. High acidity constitutes one of the main challenges faced by the oil and gas industry.5
Sudanese Fula crude oil samples, from the western region, were used. The source of the crude oil is originally from Muglad basin. It is formed from inland fluvial deposition. Reservoirs in Muglad basin are considered as conventional reservoirs. They are thin sandstone layer intersects with shale in depths varying from 700 to 3000 m. The crude oil produced from Fula oilfields is paraffinic oil in nature. The acids are present in different structures, varying from mono- and dicarboxylic acids to cyclic and aromatic acids. It is clear that revealing the structure of those acids helps in their treatment.6,7 The reported acidic distribution of western Sudanese crude oil8 describes the distribution of O2 class only. As it is a highly acidic oil, one may expect to see more acidic fractions in this type of oil. Li et al.9 characterized acids in Muglad basin, Fula sub-basin, Sudan, using FT-IR, FT-ICR-MS, and GC-MS. They revealed a correlation of TAN and bulk and molecular composition with reservoir depth. Acids from O-O4 were determined with a high abundance of O2 class.
One of the methods of treatment is decarboxylation with the use of a catalyst. This method has shown wide success, however with some restrictions. The use of a metal oxide catalyst is proven for low acidic crude oil.10 The use of a Cu/Ce/Al2O3 catalyst calcined at 1000 °C11 is effective in decreasing the high TAN of crude oil to less than 1. However, addition of many dopants, required as metal oxides, and high calcination temperatures are not recommended in large-scale treatment. A proton reduction catalyst has been studied earlier12,13 in stoichiometric addition of oxidants such as lead tetraacetate14 to convert a carboxylic compound to olefins. Sun et al.15 used a dehydrogenative decarboxylative catalyst in nonstoichiometric addition to produce alkenes from carboxylic acids. The main benefit of this method is its applicability in a large scale. Here, we apply this method to our large classes of acids to reduce their presence in the oil. In this study, Sudanese crude oil from Fula basin is studied in detail. The use of ultrahigh-resolution mass spectrometric methods provides a clear advantage over other methods in terms of higher resolving power, mass accuracy, and sensitivity. The Orbitrap-Elite technology enables the discovery of new acid classes, which will facilitate the future treatment of high-TAN crude oil.
2. Materials and Methods
2.1. Identification of Nitrogen-Containing Compounds Using Electrospray Ionization (+) Methods
The Fula crude oil sample was stored under argon. A concentration of 250 μg/mL was prepared for all samples by taking 25 mg of the sample, which was dissolved in toluene (25 mL, HPLC grade, Overlack, Germany, 99%) to obtain a concentration of 1000 ppm. A 250 mL solution was diluted with 750 mL of methanol (HPLC grade, Baker, Germany, 99.8%) + toluene to reach a final concentration of 250 μg/mL. Mass spectrometric analysis was performed on a 7 T linear trap quadrupole (LTQ) FT-ICR mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with an ESI source with a resolving power of R = 400,000 (FWHM at m/z 400, 1.5 s transient). Electrospray (ESI) in positive ion mode was used as an ionization method. The ionization was performed with a stainless steel needle capillary with a voltage of 4 kV for the crude oil with a molecular weight of 200–1200. Nebulization was assisted by a sheath gas flow of 7 a.u. (arbitrary units), while the auxiliary and sweep gas flow rates were set to 5.0 a.u. for Fula crude oil and the temperature was set at 275 °C.
2.2. Identification of Hydrocarbon Compounds Using Atmospheric Pressure Photoionization (APPI) Methods
APPI (+) was analyzed using an Orbitrap-Elite (Thermo Fisher, Bremen, Germany) with a resolving power of R = 480,000 (FWHM at m/z 400, 1.5 s transient). The sample was infused with the flow rate of 20 μL/min for APPI (+) measurements and evaporated at 275 °C with a continuous sheath gas flow of 25 a.u. (arbitrary units) and auxiliary gas flow of 10 a.u. In the APPI (+) measurement, an APPI lamp was connected to the Orbitrap with a continuous sheath gas flow of 40 a.u. (arbitrary units) and an auxiliary gas flow of 20 a.u. with a molecular weight of 200–1200. The shift to the Orbitrap-Elite method was mainly due to the high ppm error of the resulted composition. Consequently, a much higher accuracy was achieved.
2.3. Identification of Oxygen-Containing Compounds Using Electrospray Ionization (+) Methods
2.3.1. Extraction Methods
Liquid–liquid extraction was conducted to extract water-soluble acid compounds. One milliliter of crude oil was mixed with NH4OH at a ratio of (1:9 v/v) to pH = 12 for 1 h and mixed at 10,000 rpm using a magnetic stirrer. Subsequently, the mixture was left standing for 24 h to allow the phases to separate. Re-extraction was performed five times via liquid–liquid extraction with dichloromethane (DCM). The solvent was then evaporated, and the residual oil was collected and redissolved in 500 μL of MeCN/DCM (2:1 v/v) supplement with 1 vol % formic acid for negative mode measurement.
The crude oil sample was heated to 200 °C and then extracted prior to injection to the mass spectrometer. To treat acids at 350 °C, the acids were condensed under argon for 6 h. The acids were extracted with dichloromethane and then injected to the mass spectrometer after evaporating the solvents with a rotatory evaporator.
2.4. Catalytic Treatment of Acidic Crude Oil
To a 20 mL headspace crimp-top vial equipped with a stir bar were added Ir[dF(CF3)ppy]2(dtbpy)PF6 (2) (3.4 mg, 3.0 μmol, 1.0 mol %), Co(dmgH)2(4-OMe-py)Cl (1) (6.5 mg, 15 μmol, 5.0 mol %), Cs2CO3 (19.5 mg, 60.0 μmol, 20.0 mol %) (12), and carboxylic acid of Fula crude oil (0.30 mmol, 1.0 equiv). The vial was evacuated and filled with argon. This procedure was repeated twice. Dimethyl ether (DME) (3 mL, 0.1 M) was added, followed by H2O (162 mg, 162 μL, 9.00 mmol, 30.0 equiv). The vial was placed 2 cm away from two 34 W blue LEDs. The temperature was kept at approximately 35 °C by cooling with a fan. After being stirred for 15 h (12), the sample was diluted to 250 ppm and analyzed via an ESI (−) Orbitrap-Elite mass spectrometer.
2.5. Prediction of TAN Values
The TAN values of crude oil residues, following thermal and catalytic treatment, were predicted based on the relation proposed by Terra et al.,16 which relates TAN values to the % composition of O2 class in the sample. As the analysis in this study is much more extensive, TAN value estimation is based on the % of O2 class distribution presented by the authors, which includes N[H], NO[H], NO2[H], O[H], O2[H], N2[H], and others. N2[H] was excluded as it was not detected by ESI (−). As for the other compounds, they were omitted as the specific classes were not stated by the authors. Moreover, they were insignificant and accounted for only around 3% of the total intensity.
3. Results and Discussion
3.1. Identification of Nitrogen-Containing Compounds Using Electrospray (+) Ionization FT-ICR-MS
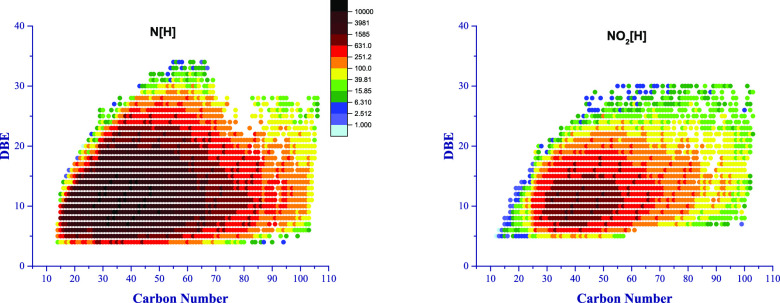
Figure 1 shows N[H] as the dominant class with the highest intensity. The distribution of double-bond equivalents of N[H] and NO2[H] versus their carbon number is shown in a heat map in Figure 2. The double-bond equivalents (DBEs), ranging from 4 to 34 and with a carbon number of up to 100, indicate that the presence of [N]H composition is highly aromatic with a high carbon number. Minor traces of NO, NS, and NO2 components were detected. Figure 3 illustrates all nitrogen-containing compositions, showing the difference in intensity for more clearance.
Figure 1.
Intensity-based distribution in Fula crude oil showing the most abundant group determined by ESI (+) FT-ICR-MS.
Figure 2.
Double-bond equivalents (DBEs) vs m/z distribution of nitrogen-containing compounds of Fula crude oil.
Figure 3.
Heat map comparison of intensity-based distribution of Fula crude oil showing N[H] as the most abundant group.
The data was collected in a full mass range. Figure 4 shows the spectrum of molecular composition assigned by ESI (+). The spectrum is expanded to show the highest intensity peak of nitrogen compositions C27H38N and C52H88N.
Figure 4.
Expanded mass scale of ESI (+) full range: low mass range (top) and high mass range (bottom).
Figure 5 shows possible chemical structures for nitrogen-containing compounds. Compound (1) (C15H25N) is a pyridine derivative with DBE = 4 and Mwt = 119.20 commonly found in crude oil.17 Compound (2) (C14H17N) with DBE = 7 and Mwt = 199.14 is a quinoline derivative. Compound (3) (C16H17N) is a carbazole derivative with DBE = 12 and Mwt = 208.127. Due to their polarities, pKa = 6.8 for carbazole18 and pKa = 4.9 for quinolone19 are clearly detected by ESI (+). Compound (4) is C14H17NO.
Figure 5.
Some possible chemical structures for nitrogen-containing compounds: 1, pyridine derivative; 2, quinoline derivative; 3, carbazole derivative; 4, piperidine derivative.
3.2. Identification of Hydrocarbon-Containing Compounds Using APPI (+) Methods Employing the Orbitrap-Elite
Figure 6 shows [HC] as the dominant class with the highest intensity. Figure 7 shows the distribution of double-bond equivalents of the most dominant nonpolar hydrocarbons versus their carbon number.
Figure 6.

Intensity-based distribution in Fula crude oil showing HC[H] as [M + H]+, a cation form, as the most abundant group determined by the Orbitrap-Elite.
Figure 7.
Double-bond equivalents (DBEs) vs m/z distribution of hydrocarbon-containing compounds of Fula crude oil.
Using photoionization techniques such as APPI (1-photon VUV ionization), compounds of nonpolar hydrocarbons, naphthenes, and polyaromatic heterocycles (PAHS and PASHS) such as benzo- and dibenzothiophenes or dibenzofurans with low to medium polarity are effectively ionized by producing ions in radical M+• or protonated [M + H]+ form. Figure 8 shows the spectrum of molecular composition assigned by APPI (+), which was expanded to show the highest intensity peak of hydrocarbon compositions C22H12 and C49H84.
Figure 8.
Expanded mass scale of full mass range (Fula basin) from low mass range (top) and high mass range (bottom) compounds. The most abundant hydrocarbon compounds (PAHS) are shown.
The [O] class is considered as a benzketone group, which can be represented as a radical cation [M+•] to be determined by APPI (+). This benzketone is a derivative of ketone compound detected by APPI (+) due to the low acidity and basicity of the delocalized π-bond attached to the ketone group, thus decreasing the basic properties.
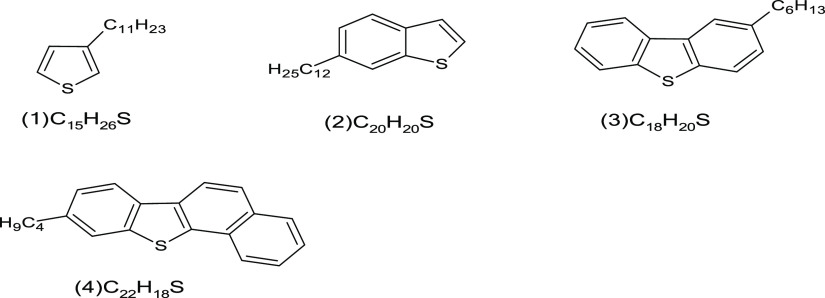
The [HC] group is a hydrocarbon that appears in the highest intensities. It ranges from aliphatic hydrocarbon with DBE = 1 to highly aromatic compounds with DBE = 50. Figure 9 shows the core structures for polycyclic aromatic sulfur heterocycles (PASH): 1, thiophene; 2, benzo[b]thiophene; 3, dibenzo[b,d]thiophene; 4, benzo[b]-naphtho[2,1-d]thiophene. The core structures for polycyclic aromatic hydrocarbons (PAHS) are as follows (Figure 10): naphthalene (DBE = 7) compound (5), anthracene (DBE = 10) compound (6), phenenthrene (DBE = 10) compound (7), pyrene (DBE = 12) compound (8), and benz[a]pyrene (DBE = 15) compound (9).
Figure 9.
Core structures for polycyclic aromatic sulfur heterocycles (PASH): 1, thiophene; 2, benzo[b]thiophene; 3, dibenzo[b,d]thiophene; 4, benzo[b]-naphtho[2,1-d]thiophene.
Figure 10.
Some possible chemical structures for hydrocarbon compounds. Core structures for polycyclic aromatic hydrocarbons (PAH): 5, naphthalene; 6, anthracene; 7, phenanthrene; 8, pyrene; 9, benz[a]pyrene.
Sulfur compositions (Figure 6) have a very low intensity in Fula crude oil. Those compositions have a carbon number from 10 to 60, although they have lower molecular weight than the other compounds detected by APPI (+). The DBE values of 3, 6, 9, and 12 are commonly attributed to the thiophene compound (1) (Figure 9), benzothiophene compound (2), dibenzothiophene compound (3), and benzonaphthothiophene compound (4), respectively.
3.3. Identification of Oxygen-Containing Compounds Using the ESI (−) Orbitrap-Elite
Figure 11 shows O4[H] as the dominant class with the highest intensity. Figure 12 shows the distribution of double-bond equivalents of the most dominant oxygen classes versus their carbon number.
Figure 11.
Intensity-based distribution of Fula crude oil showing the most abundant group determined by the ESI (−) Orbitrap-Elite.
Figure 12.
Double-bond equivalents (DBEs) vs m/z distribution of oxygen-containing compounds of Fula crude oil.
Figure 13 shows the spectrum of molecular composition assigned by ESI (−). The spectrum is expanded to show the highest intensity peak of oxygen compositions C18H36O2 and C44H83O4. The mass spectrum is shown in a full spectrum for Fula crude oil, which is then expanded to show all molecular compositions detected by ESI (−). It is expanded at the compositions C15H35O2 and C44H83O4 with the highest intensity (Figure 13). The negative ESI is highly selective for the acidic group, and as a result, the oxygen group is dominant. In Figure 14, compounds (1)–(4) belong to the O2[H] group. Compound (1) is C14H27O2 with DBE = 1, which is an aliphatic carboxylic acid or fatty acid. Compound (2) is C13H24O2, a cyclic carboxylic acid with DBE = 2. Compound (3) is C13H18O2 with DBE = 5, which is a derivative of carboxylic acid. Compound (4) is C13H16O2 with DBE = 6, which belongs to the naphthalene carboxyl group. O4[H] has the highest intensity in Fula crude oil, represented as compound (5) (C10H18O4) with DBE = 2, which is known as glutanic acid. Compound (6) (C11H12O4) with DBE = 6 is known as phthalic acid. Compound (7) is C16H10O4 with DBE = 12, which is known as anthracene dicarboxylic acid. Compounds (8) and (9) represent the aldehyde and ketone group O[H]. The [O] class was detected in low intensities in Fula crude oil by ESI (−), which is considered as a benzketone group with DBE = 10, which is best represented as a radical cation M+• to be detected better by APPI (+), (see Figure 14, compound (9)).
Figure 13.
Expanded mass scale of ESI (−) full range: low mass range (top) and high mass range (bottom).
Figure 14.
Some possible structures for chemical compounds of oxygen-containing compounds. Compounds (1)–(4) represent the O2[H] group of dicarboxylic acid. Compounds (5)–(7) represent O4[H]. Compounds (8) and (9) represent the aldehyde and ketone group O[H].
3.4. Temperature Treatment for Acids
Figure 15 shows O2[H] as the class gaining the highest intensity at 350 °C, while O4[H] gains the highest intensity at room temperature. Figures 16 and 17 compare the distribution of double-bond equivalents of the most dominant oxygen classes at three different temperatures (extracted acid at room temperature, 200 °C, and 350 °C).
Figure 15.

Heteroatom groups of Fula acids and their heated forms at 200 and 350 °C.
Figure 16.
Iso-abundance plots of DBE versus the carbon number of the O2[H] species for Fula crude oil at 25 °C (left), the oil phase at 200 °C (middle), and the oil phase at 350 °C (right).
Figure 17.

Iso-abundance plots of DBE versus the carbon number of the O4[H] species for Fula crude oil at 25 °C (left), the oil phase at 200 °C (middle), and the oil phase at 350 °C (right).
Three different experiments at three different temperatures were scanned with negative electrospray in almost the same conditions, and the data was recorded in the full spectrum (Figure 18). The results indicate that the high mass is mostly affected by temperature. The acids were studied under two temperatures (200 and 350 °C). The main objective was to investigate the possibility of acid decarboxylation under moderate to high temperatures. Extracted acids contain the highest amount of acids with 29.670 compositions of the oxygen group from O1-O15, while heated crude had an oxygenated group from O1-O9 with 20.794 assigned compositions at 200 °C and 6625 at 350 °C (Figure 18). The spectrum was zoomed out of the full spectrum to identify the differences in intensities of molecular composition at three different temperatures. The peaks were expanded at low and high masses as shown in Figures 19 and 20.
Figure 18.
Full mass range of extracted acids and thermally treated acids at 200 and 350 °C.
Figure 19.
Expanded mass scale for low masses zoomed at molecular composition C28H53O4 (Mwt = 453.4) at three different temperatures (25 °C, top; 200 °C, middle; 350 °C, bottom).
Figure 20.
Intensity of some oxygen heteroatoms at high mass (highlighted in orange color) at three different temperatures (25 °C, top; 200 °C, middle; 350 °C, bottom).
3.4.1. O2 Classes
The O2 species in petroleum and bitumen are presumably carboxylic (“naphthenic”) acids, which are a common constituent in young and immature crude oils. The O2[H] group is a carboxylic acid in extracted acids. It has a DBE of 1–30 and a carbon count of 15–80. The highest intensity group has an Mwt of 200–350 and a DBE of 1–10. The intensity of the monocarboxylic group decreases when heating the oil to 200 °C where the intensity is shown by a change in color. The high intensity of extracted acid changes to very low intensity when heating the acid to 200 °C; however, heating to 350 °C increases the intensity of monocarboxylic acids to about 60%. At temperatures above 230 °C, corrosion induced by NAs in distillation units is enhanced. On the other hand, the group O7-O15 appears only in extracted acids at 25 °C and disappears when heating the oil to 200 and 350 °C, although it is unfavorable to heat the crude oil to above 200 °C as this leads to the increasing concentration of the monocarboxylic acids. This could be due to evaporation of CO2 resulting from the group O9-O15 as monocarboxylic acids. With the expanded mass spectrum (Figure 20), we realize that many carboxylic acids of high molecular weight are highlighted in pink color, which disappear when heating the acid to 200 and 350 °C.
3.4.2. O4 Classes
O4 species are considered as dicarboxylic acids. In the extracted acid, it has a DBE that expands over 1–30. A DBE of 2 is likely to indicate a dicarboxylic saturated fatty acid as it has the highest intensity over heated carboxylic acids. The species with DBEs of 5 and 6 were the second-most abundant compounds in degraded oil and are likely to be dicarboxylic acids with two and three naphthenic rings, respectively. With the expanded mass spectrum (Figure 19), C28H53O4 with DBE = 3 exists when heating the crude oil to two different temperatures with almost the same intensity, although the effect of temperature is not effective in low molecular weight.
3.5. Catalytic Dehydrogenative Decarboxylation of Acidic Crude Oil by Using Negative Electrospray Orbitrap-Elite Mass Spectrometry
Figure 21 compares the intensities of catalyzed oxygenated groups with the acid group in the extracted acid. In the carboxylic acid classes of extracted acid, the intensity decreases for the treated acidic sample. Figure 22 compares the distribution of double-bond equivalents of the most dominant oxygen classes for catalyzed and uncatalyzed acids. Comparing the molecular composition of DBE versus a carbon number, one realizes that the highest intensity ranges from DBE 1 to 15 and carbon number C12 to C35, while for the same range when treated with the catalyst, the intensity is greatly decreased. For dicarboxylic classes (CxHyO4), the highest intensity classes range from DBE 1 to 25 and carbon number C15 to C40, while for the same range when treated with the catalyst, the intensity decreases due to catalytic transformation of the carboxylic group to alkene. By using a catalyst, for extracted samples of Fula crude oil, a great reduction of mono- and dicarboxylic acids (CxHyO2) is achieved. The reduction in the intensity of the acidic class is also observed in other classes. This is due to the ability of cobaloxime Co(dmgH)2(4-OMe-py)Cl to reduce a proton from a carboxylic group.
Figure 21.

Comparison of the relative intensity of the oxygen group of acidic crude oil and the catalyzed product using ESI (−) Orbitrap-Elite MS (R = 480,000, FWHM at m/z 400).
Figure 22.
Iso-abundance plots of DBE versus the carbon number compared to DBE vs m/z plots of acidic crude oil treated with a cobalt catalyst (uncatalyzed (left) and catalyzed (right)).
3.6. TAN Values of Thermally and Catalytically Treated Samples
Figure 23 shows the class distribution of the untreated and treated crude oil samples as categorized by Terra et al.16 The measured and predicted TAN values are shown in Table 1. It is clear that the catalytic treatment results in the most significant reduction in TAN with a value of 85% relative to the predicted TAN value for the untreated sample. Heat treatment of samples at 200 °C results in a 62% reduction in TAN value, whereas heat treatment at 350 °C results in an increase of 5% in TAN value relative to the predicted value for the untreated sample.
Figure 23.
Class distribution from the ESI (−) FT-ICR-MS data of crude oil samples.
Table 1. Measured and Predicted TAN Values.
| TAN (mg KOH g–1) |
|||
|---|---|---|---|
| crude oil sample | measured | predicted | % reduction in TAN values relative to the predicted untreated sample |
| untreated | 3.8 | 3.6 | |
| heat-treated (200 °C) | 1.36 | 62% | |
| heat-treated (350 °C) | 3.76 | –5% | |
| catalytic treatment | 0.54 | 85% | |
4. Conclusions
Sudanese crude oil contains a highly aromatic hydrocarbon composition with the existence of nitrogen composition homologue groups with high intensity. Their oxygenated composition extends from O[H] to O15[H], which indicates the high presence of acidic composition. The low molecular weight of dicarboxylic acid O4[H] is mostly responsible for acid corrosion; however, it is unaffected by temperature. A high temperature affects carboxylic acids at high molecular weights. The largest effect of decarboxylation is realized when acids are treated with dehydrogenative decarboxylative catalysts.
Acknowledgments
We are grateful and indebted to Professor Dr. Wolfgang Schrader for providing the lab facility and equipment to complete this work at the Max-Planck-Institut für Kohlenforschung.
The authors declare no competing financial interest.
References
- Johnson D.; McAteer G.; Zuk H.. The safe processing of high naphthenic acid content crude oils refinery experience and mitigations studies. Proc. Corros.03, NACE International: Houston, TX. 2003, Paper No. 03645. [Google Scholar]
- Baukal C. E., Jr.The John Zink Combustion Handbook (Industrial Combustion); CRC Press: Boca Raton, 2001. [Google Scholar]
- Simanzhenkov V.; Idem R.. Crude oil Chemistry; CRC Press: Boca Raton, 2003. [Google Scholar]
- Hsu C. S.; Dechert G. J.; Robbins W. K.; Fukuda E. K. Naphthenic acids in crude oils characterized by mass spectrometry. Energy Fuels 2000, 14, 217–223. 10.1021/ef9901746. [DOI] [Google Scholar]
- Varadaraj R.; Brons C. Molecular origins of crude oil interfacial activity part 3: Characterization of the complex fluid rag layer formed at crude oil-water interfaces. Energy Fuels 2007, 21, 1617–1621. 10.1021/ef0606299. [DOI] [Google Scholar]
- Schmitter J. M.; Arpino P.; Guiochon G. Investigation of highmolecular-weight carboxylic acids in petroleum by different combinations of chromatography (gas and liquid) and mass spectrometry (electron impact and chemical ionization). J. Chromatogr. A 1978, 167, 149–158. 10.1016/S0021-9673(00)91154-3. [DOI] [Google Scholar]
- Lande S. S.; Kochi J. K. Formation and oxidation of alkyl radicals by cobalt (III) complexes. J. Am. Chem. Soc. 1968, 90, 5196–5207. 10.1021/ja01021a023. [DOI] [Google Scholar]
- Zhen-Hua W. A. N. G. Distribution of Carboxylic Acids in Sudanese Dar Crude Oil: Characterized by Negative-Ion Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. China Pet. Process. Petrochem. Technol. 2011, 13, 15–22. [Google Scholar]
- Li M.; Cheng D.; Pan X.; Dou L.; Hou D.; Shi Q.; Wen Z.; Tang Y.; Achal S.; Milovic M.; Tremblay L. Characterization of petroleum acids using combined FT-IR, FT-ICR-MS and GC-MS: Implications for the origin of high acidity oils in the Muglad Basin, Sudan. Org. Geochem. 2010, 41, 959–965. 10.1016/j.orggeochem.2010.03.006. [DOI] [Google Scholar]
- Shohaimi N. A. M.; Bakar W. A. W. A.; Jaafar J.; Shukri N. M. Treatment of acidic petroleum crude oil utilizing catalytic neutralization technique magnesium oxide catalyst. J. Ind. Eng. Chem. 2014, 20, 2086–2094. 10.1016/j.jiec.2013.09.037. [DOI] [Google Scholar]
- Ding L.; Rahimi P.; Hawkins R.; Bhatt S.; Shi Y. General Naphthenic Acid Removal from Heavy Oils on Alkaline Earth-Metal Oxides and ZnO Catalysts. Appl. Catal., A 2009, 371, 121–130. 10.1016/j.apcata.2009.09.040. [DOI] [Google Scholar]
- Anderson J. M.; Kochi J. K. Silver(i)-catalyzed oxidative decarboxylation of acids by peroxydisulfate. The role of silver (ii). J. Am. Chem. Soc. 1970, 92, 1651–1659. 10.1021/ja00709a039. [DOI] [Google Scholar]
- Dennig A.; Kuhn M.; Sebastian T.; Thiessenhysen A.; Gilch S.; Bülter T.; Haas T.; Hall M.; Faber K. Oxidative decarboxylation of short-chain fatty acids to1-alkenes. Angew. Chem., Int. Ed. 2015, 54, 8819–8822. 10.1002/anie.201502925. [DOI] [PubMed] [Google Scholar]
- Bacha J. D.; Kochi J. K. Alkenes from acids by oxidative decarboxylation. Tetrahedron 1968, 24, 2215–2226. 10.1016/0040-4020(68)88124-4. [DOI] [Google Scholar]
- Sun X.; Chen J.; Ritter T. Catalytic dehydrogenative decarboxyolefination of carboxylic acids. Nat. Chem. 2018, 10, 1229–1233. 10.1038/s41557-018-0142-4. [DOI] [PubMed] [Google Scholar]
- Terra L. A.; Filgueiras P. R.; Tose L. V.; Romão W.; de Souza D. D.; de Castro E. V. R.; de Oliveira M. S. L.; Dias J. C. M.; Poppi R. J. Petroleomics by electrospray ionization FT-ICR mass spectrometry coupled to partial least squares with variable selection methods: prediction of the total acid number of crude oils. Analyst 2014, 139, 4908–4916. 10.1039/c4an00538d. [DOI] [PubMed] [Google Scholar]
- Dzidic I.; Carroll D. I.; Stillwell R. N.; Horning E. C. Comparison of Positive Ions Formed in Nickel-63 and Corona Discharge Ion Sources Using Nitrogen, Argon, Isobutane, Ammonia and Nitric Oxide as Reagents in Atmospheric Pressure Ionization Mass Spectrometry. Anal. Chem. 1976, 48, 1763–1768. 10.1021/ac50006a035. [DOI] [Google Scholar]
- Carroll D. I.; Dzidic I.; Stillwell R. N.; Haegele K. D.; Horning E. C. Atmospheric Pressure Ionization Mass Spectrometry. Corona Discharge Ion Source for Use in a Liquid Chromatograph-Mass Spectrometer-Computer Analytical System. Anal. Chem. 1975, 47, 2369–2373. 10.1021/ac60364a031. [DOI] [Google Scholar]
- Hayen H.; Karst U. Strategies for the Liquid Chromatographic-Mass Spectrometric Analysis of Non-Polar Compounds. J. Chromatogr. 2003, 1000, 549–565. 10.1016/s0021-9673(03)00505-3. [DOI] [PubMed] [Google Scholar]