Abstract
Background:
Epilepsy is characterized by two or more unprovoked recurrent seizures, which often respond to available antiseizure medications. However, seizure control among epileptic patients in the developing world is low. Factors determining seizure control among epileptic patients were not evidently explored in the study setting.
Objectives:
This study aimed to determine the magnitude of uncontrolled seizures and associated factors among epileptic patients at the University of Gondar hospital.
Methods:
This cross-sectional study was conducted at the University of Gondar hospital, Northwest Ethiopia. A convenience sampling method was used to recruit study subjects. Controlled seizure was defined as seizure freedom for the past 1 year. Logistic regression analysis was used to identify factors associated with seizure control. A p-value < 0.05 was used to declare a significant association.
Results:
A total of 320 study subjects were included in the study. The mean (±SD) age of patients was 27.5 ± 7.6 years. More than half (182/320, 57%) of epileptic patients had uncontrolled seizures. Five or more pretreatment seizure episodes (adjusted odds ratio = 3.98, 95% confidence interval: 1.81–8.75, p = 0.001), less than 2 years on anti-seizure medications (adjusted odds ratio = 8.64, 95% confidence interval: 3.27–22.85, p < 0.001), taking 2 or more ASMs (adjusted odds ratio = 2.48, 95% confidence interval: 1.23–5.02, p = 0.011), poor adherence to ASMs (adjusted odds ratio = 9.37, 95% confidence interval: 4.04–21.75, p < 0.001), and living at a single trip distance from hospital equaled 1 h or more (adjusted odds ratio = 4.20, 95% confidence interval: 2.11–8.41, p < 0.001) were significantly associated with uncontrolled seizures.
Conclusion:
The dose of a preferred anti-seizure medication should be optimized before combinations of anti-seizure medications are used. Adherence to anti-seizure medications should be reinforced for better seizure control. Epilepsy care should be integrated into primary health care services in the catchment region.
Keywords: Epilepsy, seizure control, antiseizure medications, Northwest Ethiopia
Introduction
Epilepsy is a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures.1,2 Epilepsy affects people of all ages, gender, races, social classes, and geographic locations.3–6 It has affected more than 69 million people worldwide.1–6 Nearly 80% of people with epilepsy are from developing countries, majority from sub-Saharan Africa (SSA).5–8 In SSA countries, the annual incidence and age-adjusted prevalence of epilepsy is 70–80 per 100,000 and 13.2 per 1000, respectively, two to three times higher than reports from the developed world.9–11 Likewise, Ethiopia is affected by epilepsy, with a reported annual incidence of 64 per 100,000 and prevalence of 5.2 per 1000. 12 These epidemiological findings could be partly attributed to the higher occurrence of perinatal or birth injuries, CNS infections and infestations, and traumatic brain injuries in SSA countries.2,3,6–8 With available antiseizure medications (ASMs), up to 70% of patients living with epilepsy (PWE) attain prolonged seizure remission.1–6 In SSA countries, more than 80% of PWE are not receiving their medication appropriately or are not on treatment.2,4,7,8,13–15 The plausible explanation for the high epilepsy “treatment gap” was the lack of skilled health personnel to diagnose and treat epilepsy, the unavailability and cost of ASMs, poor health system infrastructure, and negative cultural beliefs.6–16 The goal of ASMs is to maintain complete seizure freedom with no or minimal drug side effects. The selection of ASM is based on efficacy against seizure types and adverse effect profile. Older ASMs have a comparable seizure control rate, while newer ASMs are chosen for their better side effect profile. Older ASMs are frequently used in SSA countries for their long-term experience, lower cost, and known efficacy.3,4,8,13–18 Sociodemographic, clinical, behavioral, and treatment-related factors play a major role in epilepsy treatment outcome. Age of seizure onset, prolonged duration of epilepsy, focal or multiple seizure types, frequent pretreatment seizure episodes, symptomatic epilepsy, abnormal electroencephalography (EEG) findings, MRI lesions, poor adherence to ASMs, family history of epilepsy, coexisting psychiatric problems, and alcohol consumption were condemned as risk factors for poor seizure control.11,17–36 Poorly controlled seizures lead to excessive bodily injury, psychological distress, lower educational achievements, lower employment opportunities, reduced marriage prospects, social stigma and discrimination, impairment of quality of life and shortened lifespan.3–11,15,17 The study aimed to find strategies for optimal seizure control in the study setting and similar institutions.
Methods
Study settings
A hospital-based cross-sectional study was conducted between 1 January 2021 and 31 October 2021 at the Neurology Clinic, University of Gondar hospital, Northwest Ethiopia. The Neurology Clinic provides health care services for outpatients with neurological disorders who have follow up at the clinic. The clinic was run by neurologists, internists, medical residents, medical practitioners, and unit nurses. Patients were appointed at the clinic every 1–3 months based on the severity of their illness.
Study population and study subjects
Study population
Adult patients, who had follow up at the Neurology Clinic, University of Gondar hospital.
Study subjects
Adult patients, who had follow up at the Neurology Clinic, University of Gondar hospital, and were on ASMs for at least 1 year before the study period.
Inclusion and exclusion criteria
Inclusion criteria
Epileptic patients 18 years old or above, and were on antiepileptic drugs for at least 1 year before the study period.
Exclusion criteria
Epileptic women who were pregnant; patients with acute symptomatic seizures; patients with incomplete medical records; and patients who did not give consent were excluded from the study.
Study variables
Dependent variable: seizure control among epileptic patients
Independent variables: (1) Socio-demographic variables: Age, sex, residence, distance from hospital, religion, marital status, occupation, educational status, and economic status; (2) Behavioral factors: Alcohol intake, cigarette smoking, and khat chewing; (3) Clinical factors: Age at epilepsy onset, type of epilepsy, pretreatment duration of epilepsy, frequency of pretreatment seizure episodes, presence of comorbidities, and family history of epilepsy; (4) Treatment-related factors: Type of ASMs, number of ASMs taken, access to ASMs, ASM-related side effects, and adherence to ASMs.
Sample size and sampling procedure
The sample size was calculated using a single population proportion formula with the assumption of 95% confidence level, 5% margin of error, and taking 30% for seizure control in recent study in Ethiopia. 20 A convenience sampling method was used to recruit 320 study subjects.
Data collection instrument and procedures
Data were collected through an investigator administered predesigned, semistructured questionnaire. The questionnaire was prepared in English and translated into the local language (Amharic) for data collection, and then retranslated back to English with maintaining its consistency. The questionnaire had been pretested on 32 patients in a similar setup before the actual data collection was commenced to check for consistency and reliability of the questionnaire. Patients were interviewed to obtain sociodemographic data. Relevant medical history and laboratory parameters were obtained from patients’ records. One supervisor (medical resident) and two data collectors (medical practitioners) participated in the data collection process. The quality of the data was ensured through the training and supervising of data collectors.
Statistical analysis
Data were entered into EPI Info version 4.4.1 and transported to SPSS version 20 for analysis.
Patient characteristics were reported as counts (percentages) for categorical variables, and mean with standard deviation for continuous variables. The results were summarized using frequency, tables, and graphs. Bivariate and multivariate logistic regression models were constructed to identify factors associated with seizure control among epileptic patients. The goodness of fit of the model was judged from the Hosmer–Lemeshow test. The fit of the model was considered acceptable (p-value = 0.67). The crude odds ratio (COR) and adjusted odds ratio (AOR) were reported. p-value < 0.05 was used to declare a significant association.
Ethical considerations
The research protocol complied with the Declaration of Helsinki, and ethical clearance was obtained from the Institutional Review Board (IRB) of the College of Medicine and Health Sciences, University of Gondar (date: 19 May 2021, ref no. 615/05/2021). Study subjects were recruited only after written informed consent was obtained. All data obtained were treated confidentially.
Definition of terms
Epilepsy is a disease of the brain defined by the following conditions: (1) At least two unprovoked (or reflex) seizures occurring > 24 h apart; (2) One unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years; (3) Diagnosis of an epilepsy syndrome.1,2
Epilepsy treatment gap: the difference between the number of people with active epilepsy and the number whose seizures are treated appropriately in a given population at a given time, expressed as percentage. It reflects the proportion seeking treatment for epilepsy and who adhere to the prescribed treatment.3,4
Controlled seizure: The patient had no seizure over the past 1 year before the study period. 37
Uncontrolled seizure: The patient experienced at least one episode of seizure over the past 1 year before the study period. 37
Drug adherence: “continuous, single interval measure of medication gaps” was used to assess medication refill for ASMs. It was calculated as the sum of the days a patient was late for ASMs pick-up appointments in each month of the year, divided by the total number of days between pickup periods in the year of study. Nonadherence was defined as more than one-third of days late for ASMs pick-up appointments. 38
Results
Sociodemographic characteristics of study participants
A total of 320 study participants were included in the study. The mean (±SD) age of patients was 27.5 ± 7.6 years.
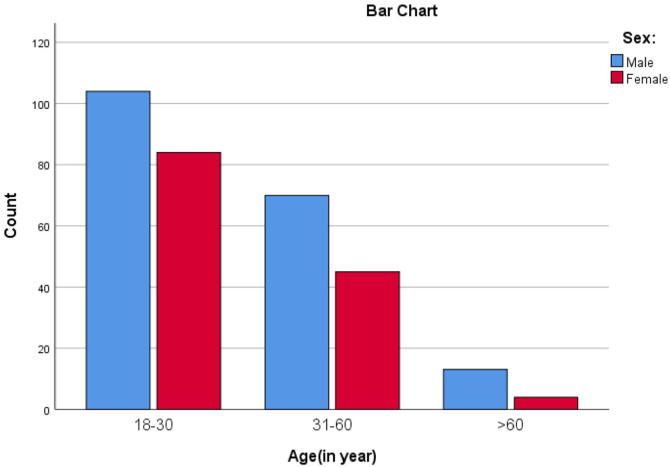
More than half of the study subjects were in the age range of 18–30 years (188/320, 59%), and were males (187/320, 58%) (Figure 1). Majority of study participants were urban dwellers (176/320, 55%), and had formal education (209/320, 65%). Half of the study subjects (161/320, 50%) were married, and most of them (274/320, 86%) were Orthodox Christian followers. A few of study subjects (24/320, 8%) had history of alcohol consumption (Table 1).
Figure 1.
Age and sex distribution of study participants.
Table 1.
Sociodemographic characteristics of study participants on seizure control and its associated factors among epileptic patients at Neurology Clinic, University of Gondar hospital, Northwest Ethiopia, 1 January 2021 to 31 October 2021.
| Characteristics | Frequency | Percentage |
|---|---|---|
| Age | ||
| 18–30 | 188 | 58.8 |
| 31–60 | 115 | 35.9 |
| >60 | 17 | 5.3 |
| Sex | ||
| Male | 187 | 58.4 |
| Female | 133 | 41.6 |
| Residence | ||
| Rural | 144 | 45 |
| Urban | 176 | 55 |
| Distance from hospital (single trip in hrs) | ||
| <1 h | 110 | 34.4 |
| ⩾1 h | 210 | 65.6 |
| Religion | ||
| Orthodox | 274 | 85.6 |
| Muslim | 40 | 12.5 |
| Protestant | 6 | 1.9 |
| Marital status | ||
| Married | 161 | 50.3 |
| Single | 146 | 45.6 |
| Widowed | 8 | 2.5 |
| Divorced | 5 | 1.6 |
| Occupation | ||
| Farmer | 72 | 22.5 |
| Housewife | 37 | 11.6 |
| Unemployed | 38 | 11.9 |
| Student | 66 | 20.6 |
| Merchant | 32 | 10.0 |
| Daily laborer | 33 | 10.3 |
| Employed | 42 | 13.1 |
| Educational status | ||
| Not educated | 64 | 20.0 |
| Can read and write | 47 | 14.7 |
| Elementary school | 87 | 27.2 |
| Secondary school | 76 | 23.7 |
| College and above | 46 | 14.4 |
| Monthly income (Eth birr) | ||
| <1500 birr | 180 | 56.2 |
| ⩾1500 birr | 140 | 43.8 |
Eth: Ethiopia.
Clinical characteristics of study participants
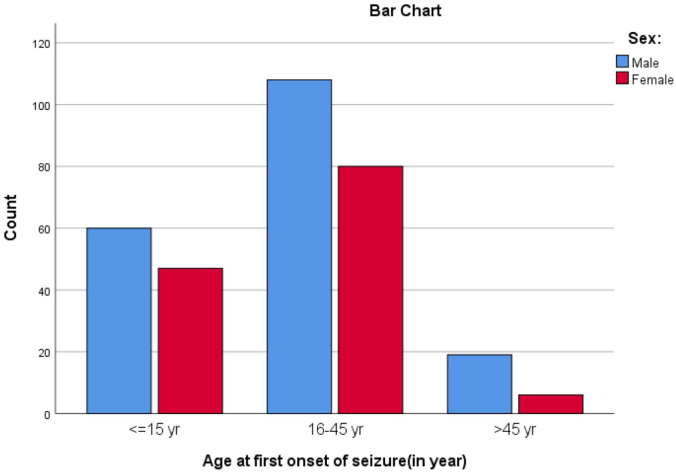
Among 320 study subjects, most (305/320, 95%) of study participants had generalized tonic clonic seizures. One-third (107/320, 33%) had their first seizure when they were 15 years old or younger, and two-thirds (188/320, 59%) had their first seizure when they were 16–45 years old (Figure 2). More than two-thirds (219/320, 68%) had duration of epilepsy more than 5 years. More than half (182/320, 57%) of epileptic patients had uncontrolled seizures. Among patients who had uncontrolled seizure, about 132 (41%) patients had 1–5 episodes of seizure, 40(13%) patients had 6–10 episodes of seizure, and 10 (3%) patients had >10 episodes of seizure over 1 year period before recruitment. One-tenth of patients (32/320, 10%) had concomitant comorbid illnesses. Among the comorbidities, 11/32 (34%) had psychiatric disorders (8 patients had major depression and 3 patients had schizophrenia). EEG abnormalities were found in two-thirds (39/65, 60%) of patients who had EEG determination. Among patients who had a brain imaging (computed tomography (CT) scan), 15% (10/66) of them had focal lesions. One-tenth (36/320, 11%) of patients had family history of epilepsy (Table 2).
Figure 2.
Age at onset of epileptic seizure of study participants.
Table 2.
Clinical characteristics of study participants on seizure control and its associated factors among epileptic patients at Neurology Clinic, University of Gondar hospital, Northwest Ethiopia, 1 January 2021 to 31 October 2021.
| Variables | Frequency | Percentage | |
|---|---|---|---|
| Types of seizure | |||
| GTC | 305 | 95.3 | |
| Focal | 12 | 3.8 | |
| Absence | 3 | 0.9 | |
| Age at onset of epilepsy(years) | |||
| ⩽15 | 107 | 33.4 | |
| 16–45 | 188 | 58.8 | |
| >45 | 25 | 7.8 | |
| Duration of epilepsy (years) | |||
| 1–5 | 102 | 31.9 | |
| >5 | 218 | 68.1 | |
| Seizure attack in the past 1 year | |||
| Yes | 182 | 56.9 | |
| No | 138 | 43.1 | |
| Number of seizure attack in past 1 year | |||
| 1–5 | 132 | 41.3 | |
| 6–10 | 40 | 12.5 | |
| >10 | 10 | 3.1 | |
| History of comorbidities | |||
| Yes | DM | 2 | 0.6 |
| HTN | 6 | 1.9 | |
| HIV | 6 | 1.9 | |
| Stroke | 5 | 1.6 | |
| Psychiatric disorders | 11 | 3.4 | |
| Others | 4 | 1.3 | |
| Total | 32 | 10 | |
| No | 288 | 90 | |
| EEG done | |||
| Yes, then any EEG abnormality | Yes | 39 | 12.2 |
| No | 26 | 8.1 | |
| Total | 65 | 20.3 | |
| No done | 255 | 79.7 | |
| Brain imaging(CT/MRI) done | |||
| Yes, then any imaging abnormality | Yes | 10 | 3.1 |
| No | 56 | 17.5 | |
| Total | 66 | 20.6 | |
| Not done | 254 | 79.4 | |
| Family history of epilepsy | |||
| Yes | 36 | 11.3 | |
| No | 284 | 88.7 | |
EEG: electroencephalography; DM: diabetes mellitus; GTC: generalized tonic clonic epilepsy; HTN: hypertension.
Treatment-related characteristics of study participants
Among study participants, most (266/320, 83%) were taking ASMs for 2 or more years. More than two-thirds (222/320, 69%) had >5 pretreatment seizure episodes. More than half (172/320, 54%) of patients had started ASMs within 1 year of seizure onset. The majority (228/320, 71%) of patients were on a single ASM. Two-thirds (209/320, 66%) were on phenobarbital, as a preferred ASM or combined with other ASMs. Less than 10% (25/320, 8%) of patients had history of drug-related side effects over the past 1 year. One-third (88/320, 28%) of patients had poor adherence to ASMs. More than half (178/320, 56%) patients accessed medication via health insurance. The remaining (142/320, 44%), accessed their medication via payment (Table 3).
Table 3.
Treatment-related characteristics of study participants on seizure control and its associated factors among epileptic patients at Neurology Clinic, University of Gondar hospital, Northwest Ethiopia, 1 January 2021 to 31 October 2021.
| Variables | Frequency | Percentage | |
|---|---|---|---|
| Time since ASM started (years) | |||
| <2 | 54 | 16.9 | |
| ⩾2 | 266 | 83.1 | |
| Number of seizure attack before ASM started | |||
| 1–5 | 98 | 30.6 | |
| >5 | 222 | 69.4 | |
| Duration of epilepsy before ASM started (years) | |||
| <1 | 172 | 53.8 | |
| ⩾1 | 148 | 46.2 | |
| Numbers of ASM taking | |||
| One | 228 | 71.3 | |
| Two or more | 92 | 28.7 | |
| Types of ASM taken | |||
| Phenytoin | 144 | 45.0 | |
| Phenobarbital | 209 | 65.3 | |
| Valporic acid | 31 | 9.7 | |
| Carbamazepine | 34 | 10.6 | |
| Lamotrigine | 5 | 1.6 | |
| ASM dose tapering in past 1 year | |||
| Yes | 29 | 9.1 | |
| No | 291 | 90.9 | |
| Having regular follow-up visit | |||
| Yes | 311 | 97.2 | |
| No | 9 | 2.8 | |
| Access to ASMs | |||
| Health insurance | 178 | 55.6 | |
| With payment | can afford | 130 | 40.6 |
| cannot afford | 12 | 3.8 | |
| Total | 142 | 44.4 | |
| History of drug-related side effects | |||
| Yes | Epigastric pain | 7 | 2.2 |
| Gingival swelling | 3 | 0.9 | |
| Fatigue | 5 | 1.6 | |
| Blurring of vision | 1 | 0.3 | |
| Headache | 5 | 1.6 | |
| Nightmare | 3 | 0.9 | |
| Skin rash | 1 | 0.3 | |
| irritability | 1 | 0.3 | |
| Confusion | 5 | 1.6 | |
| Total | 25 | 7.8 | |
| No | 295 | 92.2 | |
| ASM adherence status | |||
| Good | 232 | 72.5 | |
| Poor (nonadherent) | 88 | 27.5 | |
ASM: antiseizure medications.
Factors associated with uncontrolled seizure among epileptic patients
A binary logistic regression analysis was done to determine the association between each explanatory variable and seizure control among epileptic patients. In bivariate analysis, sociodemographic variables such as age of patient, distance from hospital, occupation, educational status, and economic status were associated with seizure control. Among clinical and treatment-related factors, age at epilepsy onset, frequency of pretreatment seizure episodes, pretreatment duration of epilepsy, family history of epilepsy, number of ASMs taken, and adherence to ASMs were associated with seizure control. When variables with p-value < 0.25 in bivariate analysis were regressed into multivariate analysis, >5 pretreatment seizure episodes (AOR = 3.98, 95% CI: 1.81–8.75, p = 0.001), <2 years on ASMs (AOR = 8.64, 95% CI: 3.27–22.85, p < 0.001), taking 2 or more ASMs (AOR = 2.48, 95% CI: 1.23–5.02, p = 0.011), poor adherence to ASMs (AOR = 9.37, 95% CI: 4.04–21.75, p < 0.001), and living at single trip distance from hospital equaled 1 h or more (AOR = 4.20, 95% CI: 2.11–8.41, p < 0.001) were significantly associated with uncontrolled seizure among epileptic patients (Table 4).
Table 4.
Bivariate and multivariate regression analysis of factors associated with seizure control among epileptic patients at Neurology Clinic, University of Gondar hospital, Northwest Ethiopia, 1 January 2021 to 31 October 2021.
| Variables | Seizure control | COR (95%) | AOR (95%) | p-value | |
|---|---|---|---|---|---|
| Uncont rolled | controlled | ||||
| Age (years) | |||||
| 18–30 | 115 | 73 | 1 | 1 | |
| 31–60 | 60 | 55 | 0.69 (0.43–1.11) | 0.98 (0.43–2.22) | 0.966 |
| >60 | 7 | 10 | 0.44 (0.16–1.22) | 1.25 (0.13–12.46) | 0.847 |
| Distance from hospital (single strip in hour) | |||||
| <1 | 39 | 71 | 1 | ||
| ⩾1 | 143 | 67 | 3.88 (2.39–6.32) | 4.20 (2.11–8.41) | <0.001 |
| Occupation | |||||
| Employed | 13 | 29 | 1 | 1 | |
| Daily laborer | 23 | 10 | 5.13 (1.91–13.80) | 1.52 (0.36–6.41) | 0.570 |
| Merchant | 13 | 19 | 1.53 (0.58–3.99) | 1.51 (0.38–5.98) | 0.561 |
| Student | 41 | 25 | 3.66 (1.61–8.32) | 1.07 (0.31–3.64) | 0.918 |
| Unemployed | 26 | 12 | 4.83 (1.87–12.45) | 1.48 (0.39–5.62) | 0.563 |
| Housewife | 20 | 17 | 2.62 (1.05–6.58) | 1.36 (0.32–5.66) | 0.676 |
| Farmer | 46 | 26 | 3.95 (1.75–8.89) | 0.72 (0.17–2.96) | 0.649 |
| Educational status | |||||
| College and above | 17 | 29 | 1 | 1 | |
| Secondary school | 41 | 35 | 1.99 (0.94–4.23) | 0.90 (0.31–2.62) | 0.848 |
| Primary school | 52 | 35 | 2.53 (1.21–5.29) | 0.74 (0.24–2.27) | 0.597 |
| Can read and write | 24 | 23 | 1.78 (0.78–4.07) | 1.05 (0.28–3.86) | 0.941 |
| Not educated | 48 | 16 | 5.12 (2.25–11.66) | 1.44 (0.32–6.48) | 0.633 |
| Monthly income (Eth birr) | |||||
| ⩾1500 | 49 | 91 | 1 | 1 | |
| <1500 | 133 | 47 | 5.25 (3.25–8.50) | 2.11 (0.98–4.55) | 0.057 |
| Age at first onset of seizure (years) | |||||
| ⩽15 | 72 | 35 | 1 | 1 | |
| 16–45 | 101 | 87 | 0.56 (0.34–0.93) | 0.52 (0.23–1.13) | 0.097 |
| >45 | 9 | 16 | 0.27 (0.11–0.68) | 0.39 (0.06–2.63) | 0.332 |
| Family history | |||||
| No | 157 | 127 | 1 | 1 | |
| Yes | 25 | 11 | 1.84 (0.87–3.88) | 2.31 (0.81–6.56) | 0.116 |
| Time since ASM started (years) | |||||
| ⩾2 | 138 | 128 | 1 | 1 | |
| <2 | 44 | 10 | 4.08 (1.97–8.45) | 8.64 (3.27–22.85) | <0.001 |
| Pretreatment number of seizure | |||||
| 1–5 | 31 | 67 | 1 | 1 | |
| >5 | 151 | 71 | 4.59 (2.76–7.66) | 3.98 (1.81–8.75) | 0.001 |
| Pretreatment duration of epilepsy | |||||
| <12 months | 82 | 90 | 1 | 1 | |
| ⩾12 months | 100 | 48 | 2.29 (1.45–3.61) | 1.05 (0.52–2.13) | 0.894 |
| Numbers of ASM taken | |||||
| One | 112 | 116 | 1 | 1 | |
| Two or more | 70 | 22 | 3.29 (1.91–5.68) | 2.48 (1.23–5.02) | 0.011 |
| ASM adherence status | |||||
| Good | 105 | 127 | 1 | 1 | |
| Poor | 77 | 11 | 8.47 (4.28–16.76) | 9.37 (4.04–21.75) | < 0.001 |
COR: crude odds ratio; AOR: adjusted odds ratio; ASM: antiseizure medications.
All variables having p < 0.2 in bivariate analysis, then was taken for multivariate analysis.
Discussion
A total of 320 PWE were included in the study. The rate of controlled seizures among PWE was 43%. A recent hospital-based meta-analysis report on the prevalence of controlled seizure in the country was 46%. 12 A hospital-based study in India, Nigeria, and Ethiopia reported seizure remission rate of 40%–60%.18,20–23,39 The low magnitude of seizure control in developing countries could be explained by economic, social, and cultural issues like availability and cost of ASMs, poor adherence to ASMs, poor health care facilities, inadequate community awareness and attitudes, and social stigma. Generalized tonic clonic seizure accounted for most of seizure types, while two-thirds of epilepsies in adults were known to be focal types.2–6 This discrepancy might be due to witnessing the conspicuous motor picture of convulsive epilepsy, and subtle findings of focal seizure might not be noticed. Because brain imaging and EEG facilities are scarce in SSA countries, classification of epilepsy is largely clinical.3,4 A multicenter study in Africa revealed that more than half of EEG-proven focal types were documented to have generalized convulsive epilepsy.17,40 Two-thirds of patients with controlled seizure achieved seizure control with single ASM use, which was congruent with reports from other sub-Saharan African countries.16–18,21–23 Several Western studies have shown that the response to the first ASM is the best predictor of long-term outcome. Seizures that continued despite adequate doses of an appropriate had a lower chance of subsequent seizure remission.24–26 Two-thirds of the patients used phenobarbital as the therapy of choice for their epilepsy. It is the World Health Organization’s3,4 drug of choice for treating epilepsy in developing countries. Several studies found it to be widely used in SSA countries.12,16,18,20–23 On multivariate logistic regression analysis, the odds of an uncontrolled seizure was fourfold higher in patients with more than five pretreatment seizure episodes as compared with those who had five or less (AOR = 3.98, 95% CI: 1.81–8.75, p = 0.001). Hospital-based studies in Italy, Scotland, India, and Ethiopia showed that frequent seizures before treatment were related to poor seizure control.22–24,26,39 It is known that prolonged and recurrent seizures contribute to further neuronal damage and the development of new epileptic foci, which could increase the risk of uncontrolled seizures. In addition, a high number of pretreatment seizures may correlate with the intrinsic severity of the disease, which is more likely to give a poor response to ASMs. 27 Patients who were on ASMs for less than 2 years had a ninefold higher risk of uncontrolled seizures as compared with patients who were on ASMs for 2 or more years (AOR = 8.64, 95% CI: 3.27–22.85, p < 0.001). This finding was consistent with studies done in Ethiopia, which revealed that less period on ASMs was a predictor of uncontrolled seizures.28,29 A possible explanation might be that the dosage of their ASMs might not be optimized, or patients might have less experience on drugs and might be nonadherent to their medication. The odds of uncontrolled seizures was 2.5-fold higher in epileptic patients on 2 or more ASMs as compared with those on single ASMs (AOR = 2.48, 95% CI: 1.23–5.02, p = 0.011). This finding was supported by studies done in the United Kingdom (UK), Saudi Arabia, and Ethiopia which showed taking two or more ASMs was a predictor of uncontrolled seizure.29,30,41 The plausible explanation could be that patients on dual or polytherapy might have more pill burden, more drug-related side effects, and more financial expenses which predispose to poor ASM adherence. Polypharmacy causes drug–drug interactions and altered drugs metabolism. In addition, PWE on poly-therapy might have inherent intractable epilepsy, and might require other interventions. All these clinical scenarios could lead to uncontrolled seizure in epileptic patients. Patients with poor drug adherence had more than a ninefold higher risk of uncontrolled seizure as compared with those with good adherence (AOR = 9.37, 95% CI: 4.04–21.75, p < 0.001). Studies done in Ethiopia and South Africa showed that poor ASMs adherence was a predictor of uncontrolled seizures.21–23,28,29,32,33,39,40 Patients who do not adhere to their medication achieve suboptimal serum level, and then predisposes to uncontrolled seizure. Drug adherence is a crucial issue for good treatment outcome. Patients who spent 1 h or more in single trip to the hospital had more than fourfold higher risk of uncontrolled seizure (AOR = 4.20, 95% CI: 2.11–8.41, p < 0.001). This finding was supported by studies in Kenya and Ethiopia, in which living far away from the health facility site was related to poor treatment outcome.15,42 The long distance from the hospital was a barrier for adherence to ASMs and missing regular follow up visits, especially when it was accompanied by a scarcity of road transportation. Among 24 epileptic patients who had a history of alcohol intake, 22 (92%) of them had uncontrolled seizures. Even though the sample size was too small to compute regression analysis because of small count of those with alcohol intake and good seizure control, alcohol intake as a predictor for uncontrolled seizure was supported by a meta-analysis report by Samokhvalov et al. 31 and a study from Ethiopia. The sound explanation would be that alcohol intake could lead to sleep deprivation, missing meals, missing medications, and increased drug side effects, which were reported as triggering factors for seizures among epileptic patients.
Limitation of the study
Since it was a cross-sectional study, the determined seizure control among epileptic patients might not be a true reflection of what happens all the time. Generalizability to the study population was limited, since a nonprobability sampling method was used to recruit study subjects.
Conclusion
The magnitude of seizure control among epileptic patients was low. Frequent pretreatment seizure episodes, less than 2 years on ASMs, being on 2 or more ASMs, poor adherence to ASMs, and living far away from a health facility site were independently associated with uncontrolled seizures among epileptic patients. The dose of a preferred ASM should be optimized before combinations of medications are used. Adherence to medication should be reinforced for better seizure control. Epilepsy care should be integrated into primary health care services in the catchment region.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121221100612 for Seizure control and its associated factors among epileptic patients at Neurology Clinic, University of Gondar hospital, Northwest Ethiopia by Dawit Zena, Abilo Tadesse, Nebiyu Bekele, Samson Yaregal, Nuria Sualih and Edilawit Worku in SAGE Open Medicine
Acknowledgments
The authors are grateful to thank the study participants and their health personnel.
Footnotes
Author contributions: D.Z. contributed to the conception, design, data collection, analysis, writing, and review of the manuscript. A.T. contributed to the conception, design, analysis, writing and review of the manuscript. N.B., S.Y., N.S., and E.W. contributed to conception, design, analysis and review of the manuscript. All authors read and approved the final manuscript and approved its submission for publication.
Availability of data and materials: All data generated and analyzed are included in this research article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for research was obtained from the “Research and Publication Office” of College of Medicine and Health Sciences, University of Gondar. The funding body had no role in the design of the study, data collection, analysis and interpretation of the data.
Ethical approval and consent to participate: Ethical approval was obtained from the Institutional Review Board of College of Medicine and Health Sciences, University of Gondar (date: 19/05/2021, ref no. 615/05/2021). Formal letter of permission was obtained from the University of Gondar hospital administrative body. Study subjects were recruited only after informed written consent was obtained.
Informed consent: Written informed consent was obtained from all subjects before the study.
Consent for publication: Written informed consent for publication was obtained from study subjects.
ORCID iDs: Dawit Zena  https://orcid.org/0000-0003-0711-1320
https://orcid.org/0000-0003-0711-1320
Abilo Tadesse  https://orcid.org/0000-0002-3966-4969
https://orcid.org/0000-0002-3966-4969
Supplemental material: Supplemental material for this article is available online.
References
- 1. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55(4): 475–482. [DOI] [PubMed] [Google Scholar]
- 2. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017; 58(4): 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO). Atlas: epilepsy care in the world. Geneva: WHO, 2005. [Google Scholar]
- 4. World Health Organization (WHO). Epilepsy in the WHO African region (Bridging the gap): the global campaign against epilepsy (out of the shadows). Amsterdam: WHO, 2004. [Google Scholar]
- 5. Beghi E. The epidemiology of epilepsy. Neuroepidemiol 2020; 54: 185–191. [DOI] [PubMed] [Google Scholar]
- 6. De Boer HM, Mula M, Sander JW. The global burden and stigma of epilepsy. Epilepsy Behav 2008; 12(4): 540–546. [DOI] [PubMed] [Google Scholar]
- 7. Espinosa-Jovel C, Toledano R, Aledo-Serrano Á, et al. Epidemiological profile of epilepsy in low income populations. Seizure 2018; 56: 67–72. [DOI] [PubMed] [Google Scholar]
- 8. Ba-Diop A, Marin B, Druet-Cabanac M, et al. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol 2014; 13(10): 1029–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winkler AS, Kerschbaumsteiner K, Stelzhammer B, et al. Prevalence, incidence, and clinical characteristics of epilepsy—a community-based door-to-door study in northern Tanzania. Epilepsia 2009; 50(10): 2310–2313. [DOI] [PubMed] [Google Scholar]
- 10. Houinato D, Yemadje LP, Glitho G, et al. Epidemiology of epilepsy in rural Benin: prevalence, incidence, mortality, and follow-up. Epilepsia 2013; 54(4): 757–763. [DOI] [PubMed] [Google Scholar]
- 11. Wagner RG, Kabudula CW, Forsgren L, et al. Epilepsy care cascade, treatment gap and its determinants in rural South Africa. Seizure 2020; 80: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tekle-Haimanot R, Forsgren L, Ekstedt J. Incidence of epilepsy in rural central Ethiopia. Epilepsia 1997; 38(5):541–546. [DOI] [PubMed] [Google Scholar]
- 13. Meyer AC, Dua T, Ma J, et al. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ 2010; 88(4): 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chin JH. Epilepsy treatment in sub-Saharan Africa: closing the gap. Afr Health Sci 2012; 12(2): 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mbuba CK, Ngugi AK, Fegan G, et al. Risk factors associated with the epilepsy treatment gap in Kilifi, Kenya: a cross-sectional study. Lancet Neurol 2012; 11(8): 688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Osemeke NP, Azuoma AL, Obunike AE, et al. Utilization of antiepileptic drug use and seizure control among people with epilepsy in a sub urban community in Southern Nigeria. Afri J Neurol Sci 2012; 3(2): 36–42. [Google Scholar]
- 17. Kariuki SM, Matuja W, Akpalu A, et al. Clinical features, proximate causes, and consequences of active convulsive epilepsy in Africa. Epilepsia 2014; 55(1): 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Obiako OR, Sheikh TL, Kehinde JA, et al. Factors affecting epilepsy treatment outcomes in Nigeria. Acta Neurol Scand 2014; 130(6): 360–367. [DOI] [PubMed] [Google Scholar]
- 19. Mohanraj R, Brodie MJ. Early predictors of outcome in newly diagnosed epilepsy. Seizure 2013; 22(5): 333–344. [DOI] [PubMed] [Google Scholar]
- 20. Yazie TS, Kefale B, Molla M. Treatment outcome of epileptic patients receiving antiepileptic drugs in Ethiopia: a systematic review and meta-analysis. Behav Neurol 2021; 2021: 5586041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niriayo YL, Mamo A, Kassa TD, et al. Treatment outcome and associated factors among patients with epilepsy. Sci Rep 2018; 8: 17354–17359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gidey K, Chelkeba L, Gemechu TD, et al. Treatment response and predictors in patients with newly diagnosed epilepsy in Ethiopia: a retrospective cohort study. Sci Rep 2019; 9: 16254–16257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zewudie A, Mamo Y, Feyissa D, et al. Epilepsy treatment outcome and its predictors among ambulatory patients with epilepsy at Mizan-Tepi University Teaching hospital, Southwest Ethiopia. Neurol Res Int 2020; 2020: 8109858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Del Felice A, Beghi E, Boero G, et al. Early versus late remission in a cohort of patients with newly diagnosed epilepsy. Epilepsia 2010; 51(1): 37–42. [DOI] [PubMed] [Google Scholar]
- 25. Schiller Y, Najjar Y. Quantifying the response to antiepileptic drugs: effect of past treatment history. Neurology 2008; 70: 54–65. [DOI] [PubMed] [Google Scholar]
- 26. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000; 342(5): 314–319. [DOI] [PubMed] [Google Scholar]
- 27. Rogawski MA, Johnson MR. Intrinsic severity as a determinant of antiepileptic drug refractoriness. Epilepsy Curr 2008; 8(5): 127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dubale M, Gizaw K, Aklog A, et al. Treatment outcome and associated factors among adult epileptic patients at Hawassa University Specialized Hospital, Southern Ethiopia. J Bioanalysis Biomed 2020; 12(6): 7. [Google Scholar]
- 29. Nasir BB, Yifru YM, Engidawork E, et al. Antiepileptic drug treatment outcomes and seizure-related injuries among adult patients with epilepsy in a Tertiary Care Hospital in Ethiopia. Patient Relat Outcome Meas 2020; 11: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamdy NA, Alamgir MJ, Mohammad EGE, et al. Profile of epilepsy in a regional hospital in Al Qassim, Saudi Arabia. Int J Health Sci 2014; 8(3): 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Samokhvalov AV, Irving H, Mohapatra S, et al. Alcohol consumption, unprovoked seizures, and epilepsy: a systematic review and meta-analysis. Epilepsia 2010; 51(7): 1177–1184. [DOI] [PubMed] [Google Scholar]
- 32. Egenasi C, Steinberg WJ, Raubenheimer JE. Beliefs about medication, medication adherence and seizure control among adult epilepsy patients in Kimberley, South Africa. S Afr Fam Pract 2015; 57(5): 326–332. [Google Scholar]
- 33. Hasiso TY, Desse TA. Adherence to treatment and factors affecting adherence of epileptic patients at Yirgalem General Hospital, Southern Ethiopia: a prospective cross-sectional study. PLoS One 2016; 11(9): e0163040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang HJ, Liao CC, Hu CJ, et al. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PLoS One 2013; 8(4): e59999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. So EL. Predictors of outcome in newly diagnosed epilepsy: clinical, EEG and MRI. Neurol Asia 2011; 16(suppl. 1): 27–29. [Google Scholar]
- 36. Sillanpää M, Schmidt D. Long-term outcome of medically treated epilepsy. Seizure 2017; 44: 211–216. [DOI] [PubMed] [Google Scholar]
- 37. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010; 51(6): 1069–1077. [DOI] [PubMed] [Google Scholar]
- 38. Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006; 40: 1280–1288. [DOI] [PubMed] [Google Scholar]
- 39. Rawat C, Guin D, Talwar P, et al. Clinical predictors of treatment outcome in North Indian patients on antiepileptic drug therapy: a prospective observational study. Neurol India 2018; 66(4): 1052–1059. [DOI] [PubMed] [Google Scholar]
- 40. Kariuki SM, White S, Chengo E, et al. Electroencephalographic features of convulsive epilepsy in Africa: a multicentre study of prevalence, pattern and associated factors. Clin Neurophysiol 2016; 127: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moran NF, Poole K, Bell G, et al. Epilepsy in the United Kingdom: seizure frequency and severity, anti-epileptic drug utilization and impact on life in 1652 people with epilepsy. Seizure 2004; 13(6): 425–433. [DOI] [PubMed] [Google Scholar]
- 42. Berhanu S, Alemu S, Prevett M, et al. Primary care treatment of epilepsy in rural Ethiopia: causes of default from follow-up. Seizure 2009; 18(2): 100–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121221100612 for Seizure control and its associated factors among epileptic patients at Neurology Clinic, University of Gondar hospital, Northwest Ethiopia by Dawit Zena, Abilo Tadesse, Nebiyu Bekele, Samson Yaregal, Nuria Sualih and Edilawit Worku in SAGE Open Medicine