Abstract
We report a novel anhydride derivative, 3-acetoxy-2-methylbenzoic anhydride (AMA), obtained from the interaction of 3-acetoxy-2-methylbenzoyl chloride with 3-acetoxy-2-methylbenzoic acid. The synthesized compound was characterized by elemental analysis, IR, 1H NMR, and 13C NMR spectroscopic studies and single-crystal X-ray crystallography which revealed the crystallization of AMA as monoclinic with space group P21/c. A Hirshfeld surface analysis was performed to record various intermolecular interactions, indicating the stabilization of the AMA structure by the intermolecular weak C–H···O hydrogen bonds and π···π interactions. The title compound was screened for antibacterial and antifungal activities using a serial dilution technique under aseptic conditions. The results indicate that the title compound has significant antibacterial properties but showed no antifungal behavior.
Introduction
The carboxylic anhydride series is a very diverse class of organic compounds that serve both as a precursor for the synthesis of esters, amides, drugs, and peptides and as a versatile reagent for numerous reactions including free carboxylic acids with alcohols, silyl esters, chemoselective esterification, lactonization, and conversion of silyl esters to lactones.1−10 A functional group in an organic acid anhydride is highly reactive, making it rare in nature. Cantharidin, a terpene found in many species of blister beetles, is a naturally occurring anhydride of carboxylic acids.11 Shellfish naturally contains maleic anhydride, which is produced by the bacteria Streptomyces spiroverticillatus in the form of the natural compound tautomycin.12,13
Anhydrides are compounds with two carbonyl groups attached to the same oxygen. There are many methods for synthesizing various symmetrical and unsymmetrical anhydrides in the literature.14−16 The most common anhydrides in organic chemistry are those derived from carboxylic acids and the dehydration of carboxylic acids in the presence of dehydrating reagents like carbodiimides,17 thionyl chlorides,18 and isocyanates.19 In addition, the synthesis of anhydrides has been reported by the acylation of acyl halides and anhydrides with carboxylates.20
Carboxylic anhydrides have been used as plasticizers for PVC and other plastics, particularly where temperature stability is required, such as in wire and cable coatings. In addition, these compounds possess a broad spectrum of antimicrobial activity.21 Benzoic anhydride, which is added to low-density polyethylene (LDPE) during film manufacture, can control mold growth on the surface of foods such as cheese and thus have antifungal activity.22,23 The naphthalene tetracarboxylic dianhydrides produced as precursors for the manufacture of naphthalenediimides (NDIs) have been reported to have important applications in materials science and artificial photosynthesis.24 In the present study, we used a known and common method to synthesize the title anhydride compound. The structure of the compound was characterized using elemental analyses, IR, 1H and 13C NMR spectroscopy, single-crystal X-ray diffraction, and Hirshfeld surface analysis. The in vitro antimicrobial activity of the title anhydride was determined against six bacterial and two fungal species.
Experimental Section
Materials and Methods
The chemicals used in the experiment were procured from commercial sources and were not further purified. The solvents were of analytical grade. A Stuart SMP 30 Advanced Digital Melting Point apparatus was used to determine the melting point of the title compound. A Bruker Vertex 80 V spectrometer was used to record FT-IR spectra. Bruker/Ultraschilt spectrometers were used to record 1H and 13C NMR spectra. The 1H spectrum was recorded at 300 MHz and the 13C spectrum at 75 MHz. Elemental analyses were carried out at ODUMARAL at Ordu University using an Elementar Vario Micro Cube (Germany) elemental analyzer.
Synthesis of 3-Acetoxy-2-methylbenzoic Anhydride (AMA)
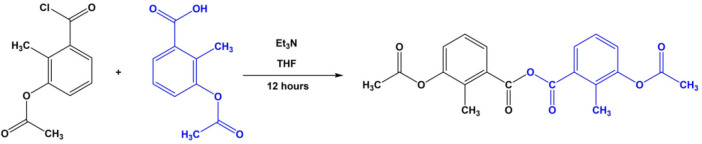
Triethylamine (2.1 mL, 15 mmol) was gradually added to a solution of 3-acetoxy-2-methylbenzoic acid dissolved (15 mmol) in THF (7.5 mL), followed by the addition of 3-acetoxy-2 methylbenzoyl chloride (2.55 g, 12 mmol) dissolved in the same solvent (7.5 mL). The resultant reaction mixture was stirred at room temperature for 12 h, resulting in a white precipitate which was removed by filtration. An amount of 150 mL of deionized water was added to the resulting solution, yielding a product that was washed with deionized water to eliminate triethylamine hydrochloride. The product gives beautiful crystals when crystallized in ethanol. Scheme 1 shows a pictorial representation of the synthesis of the title compound. They were prepared with minor modifications according to a reported procedure.25 Yield: 3.33 g, 75%; mp 118–120 °C. Anal. calcd for C20H18O7: C, 64.86; H, 4.90. Found C, 64.90; H, 4.84.
Scheme 1. Synthesis of the Title Compound.
Crystal Structure Determination
An appropriate crystal of size 0.10 × 0.06 × 0.05 mm3 was selected to collect X-ray intensity data at 296 K (λ = 0.71073 Å) with a Bruker diffractometer equipped with Mo Kα radiation. Bruker SAINT software was used to collect rotational mode (f and ω scanning mode) reflectance measurements to compute cell parameters.26 The integration method was used to correct for absorption (μ = 0.08 mm–1). The crystal structure of the compound was solved directly using SHELXT.27 The non-hydrogen atom positional and anisotropic temperature parameters were refined with SHELXL, using a full-matrix least-squares method, yielding 317 crystallographic parameters.28 Anisotropic refinement of the non-hydrogen atom parameters was conducted, and a difference Fourier map was used to determine hydrogen atom positions. Various freely refined coordinates and Uiso(H) values were applied. Using a threshold of I > 2σ(I) to refine the structure, Rint = 0.0647 with 2041 reflections was observed. WinGX (version 2018.3)29 and Mercury30 software was used to plot numbers and obtain data in tables. Table 1 lists all the refinement details for the title compound.
Table 1. Structure Refinement Details for AMA.
| CCDC | 2058057 |
| chemical formula | C20H18O7 |
| temperature (K) | 296 |
| space group | P21/c |
| crystal system | monoclinic |
| Mr | 370.34 |
| a, b, c (Å) | 6.1033 (10), 40.920 (7), 7.8767 (14) |
| α, β, γ (deg) | 90, 111.350 (5), 90 |
| volume, V (Å3) | 1832.2 (6) |
| crystal size (mm) | 0.10 × 0.06 × 0.05 |
| calculated density (Mg/m3) | 1.343 |
| F000 | 776 |
| μ (mm–1) | 0.10 |
| Z | 4 |
| diffractometer | Bruker APEX3 CCD |
| θ range (deg) | 2.8 ≤ θ ≤ 25.8 |
| wavelength (Å) | 0.71073 |
| measurement method | ω scan |
| absorption correction | multiscan |
| hmin, hmax | –8, 8 |
| kmin, kmax | –54, 54 |
| lmin, lmax | –10, 10 |
| Rint | 0.046 |
| reflections collected | 51787 |
| independent reflections | 4571 |
| observed reflections [I > 2σ(I)] | 2041 |
| refinement method | SHELXL18/3 |
| parameters | 317 |
| R[F2 > 2σ(F2)] | 0.065 |
| wR(F2) | 0.234 |
| GooF = S | 1.07 |
| Δρmin, Δρmax (e/Å3) | –0.31, 0.31 |
Antimicrobial Activity
The antimicrobial activity of the title compound was evaluated in in 96-well microplates using the broth microdilution technique. The minimum inhibitory concentration (MIC) method was employed to measure the antimicrobial activity.31−33 The pathogenic microorganisms tested are the Gram-positive strain (Bacillus subtilis ATCC 6633; Staphylococcus aureus ATCC 25923; Enterococcus faecalis ATCC 29212) and Gram-negative strain (Escherichia coli ATCC 25922; Klebsiella pneumoniae ATCC 70060; Pseudomonas aeruginosa ATCC 27853; Aspergillus niger ATCC 16404; Candida albicans ATCC 1023). Dimethyl sulfoxide was used to dilute and dissolve the compound. The stock concentration was prepared as 2000 g/mL. All bacterial strains were cultured in broth following a 24 h incubation period at 37 °C. The fungi were incubated for 24 h at 28 °C and then kept in nutrient solution. Homogenization of bacterial and fungi cells was carried out in nutritional solution. A turbidity of approximately 106 cells/mL was achieved for bacterial and fungal suspensions. The only controls used were inoculated broths. The wells were filled with 100 μL each of the microorganism suspensions and 100 μL of the compound suspension to be tested. The MIC, expressed in μg/mL, was determined by recording the growth rate of microorganisms on the microtiter plate. Amoxicillin and tetracycline were used as antibacterial reference standards, with ketoconazole serving as an antifungal reference standard.
Results and Discussion
Vibrational Frequencies
The C=O stretching vibrations were identified as distinguishing absorption bands at 1790 and 1759 cm–1 in the IR spectra. These two bands were observed as the symmetric stretching mode at the higher frequency and the asymmetric stretching mode at the lower frequency. For the −CO–O–CO– system, the symmetric stretching band is relatively weaker with the lower asymmetric stretching. The C=O stretching mode of the acetoxy groups appeared at 1721 cm–1. The aromatic and aliphatic CH vibrations were observed at 3060–2974 and 2926–2835 cm–1, respectively. The aromatic strong ν(−C–O) stretch was detected at 1210 cm–1, as shown in Figure 1. The stretch of the aliphatic ν(−C–O) was noticed at 1004 cm–1. These findings are similar to those reported previously for comparable compounds.14,34−37
Figure 1.
IR spectrum of the title compound.
1H NMR Spectrum
The 1H NMR spectrum of the title compound was measured in deuterated chloroform. A singlet of the methyl proton was observed at 2.52 ppm (s, 3H, −OCOCH3) and linked to the ester carbonyl ring due to oxygen and carbonyl groups contained in the ester carbonyl group, whereas the methyl proton at the 2-position on the phenyl ring was observed at 2.39 ppm (s, 3H, Ar–CH3). The protons (H1–H3) of the phenyl rings were found at 7.95–7.31 ppm in the title compound, as illustrated in Figure 2. The H1 and H2 protons, which were coupled to the H3 proton and each other, resonated as quintet peaks at 7.38–7.31 ppm (q, J = 8.0 Hz, 2H). In addition, the H1 proton is identical to the H1′ proton, and H2 and H3 protons are identical to H2′ and H3′ protons. These values appear to be consistent with values previously reported for similar compounds.14,34,35
Figure 2.
1H spectrum of the compound, AMA.
13C NMR Spectrum
The 13C NMR spectra of the title compound, measured in CDCl3, showed 10 distinct resonances that are consistent with the target compound. The carbonyl (C=O) peak of the anhydride was detected at 169.06 ppm, while the measured carbon signal due to the ester group appeared at 162.14 ppm. The C3 aromatic carbon signal occurred at 150.19 ppm. The existence of the carbonyl group on the phenyl ring causes the C1 carbon signal to move downfield and appear at 134.52 ppm. The other aromatic ring carbons ranged from 129.67 to 126.53 ppm. The C2, C4, C5, and C6 carbons were observed at 129.67, 127.69, 129.07, and 126.53 ppm, respectively. The signal at 20.77 ppm is attributed to the methyl carbon linked to the ester carbonyl group (−OCOCH3), whereas the signal at 13.58 ppm is assigned to the methyl group bound phenyl ring, as illustrated in Figure 3. These values agree with previously reported values for similar compounds.14,34,35
Figure 3.
13C NMR spectrum of the compound.
Crystal Structure of AMA, C20H18O7
The molecular structure of AMA with atom numbering is shown in Figure 4. A hydrogen atom is shown as a small sphere of arbitrary radius, and the other atoms are ellipsoids with displacement probabilities of 30%. AMA is crystallized in a monoclinic space group P21/c with Z = 4 (Table 1). There is an independent molecule in the asymmetric unit. The title compound has nearly planar molecular geometry with a dihedral angle of 5.26° between the planes of the C1/C6 and C7/C12 six-membered aromatic rings. Noncovalent interactions such as hydrogen bonding, van der Waals interactions, and π···π influence the molecular conformation. Two intermolecular C–H···O bonds and two π···π contacts stabilize the crystalline packing of the compound in a three-dimensional network. Table 2 summarizes various contact lengths, angles, and noncovalent interactions. As illustrated in Figure 5, the intermolecular C4–H4···O2i hydrogen bonds in crystal packing form a one-dimensional structure along the [101] direction, whereas the C14–H14···O4ii hydrogen bond forms a chain along the [001] direction. In addition, the π···π interactions shown in Figure 6 also generate a chain motif along [100]. These weak π···π interactions are Cg1···Cg2 (x + 1, y, z) = 3.8453 (7) Å and Cg2···Cg1 (x–1, y, z) = 3.8453 (7) Å; Cg1 and Cg2 are the centroids of the C1–C6 and C7–C12 rings, respectively. The arrangements of O3 = C10–O7 and O4 = C11–O7 groups are not planar with respect to their carrier benzene rings (C1/C6 and C7/C12), with dihedral angles of 15.8(4)° and 19.3(30)°, respectively. The central anhydride moieties C1–C10–O3–O7 and C12–C11–O4–O7 are twisted with a dihedral angle of 34.8(2)°. The torsion angles of O3–C10–O7–C11, O4–C11–O7–C10, C2–C1–C10–O3, and C17–C12–C11–O4 are 19.7(5)°, 23.1(5)°, −17.2(5)°, and −20.1(5)°, respectively. It is clearly seen that the central anhydride group is not planar with these angle values. C–O double bond lengths are in the range of 1.184–1.199 Å. Some selected bong lengths and angles are given in Table 3. When compared with the recently reported phthalic anhydride containing studies, C–O double bond lengths appear to be in agreement with the values in this study.38,39
Figure 4.
ORTEP diagram of the compound with atomic numbers.
Table 2. Noncovalent Interactions for AMA (Å, deg).
| D–H···A | D–H | H···A | D···A | D–H···A | symmetry code |
|---|---|---|---|---|---|
| C4–H4···O2i | 0.93 | 2.59 (4) | 3.424 (6) | 149 (3) | (i) x, –y + 1/2, z – 1/2 |
| C14–H14···O4ii | 0.93 | 2.58 (4) | 3.253 (6) | 130 (3) | (ii) x – 1, y, z – 1 |
Figure 5.
A view crystal packing of AMA, showing C4–H4···O2i and C14–H14···O4ii hydrogen bonds.
Figure 6.
Π···π interactions in AMA shown along the [100] axis.
Table 3. Selected Geometric Parameters of AMA (Å, deg).
| geometric
parameters |
geometric parameters |
||
|---|---|---|---|
| bond lengths (Å) | X-ray | bond angles (deg) | X-ray |
| C8–O1 | 1.349 (5) | O1–C8–O2 | 121.7 (4) |
| C3–O1 | 1.411 (4) | O7–C10–O3 | 121.6 (3) |
| C8–O2 | 1.184 (5) | O7–C11–O4 | 121.4 (3) |
| C10–O3 | 1.199 (4) | O5–C19–O6 | 122.0 (3) |
| C11–O4 | 1.195 (3) | C3–O1–C8 | 118.3 (3) |
| C16–O5 | 1.409 (3) | C16–O5–C19 | 118.1 (2) |
| C19–O5 | 1.357 (4) | C1–C10–O7 | 111.1 (3) |
| C19–O6 | 1.196 (4) | C12–C11–O7 | 111.2 (3) |
| C10–O7 | 1.381 (4) | torsion angles (deg) | |
| C11–O7 | 1.393 (4) | C1–C2–C3–O1 | 176.4 (3) |
| C2–C7 | 1.509 (5) | C14–C15–C16–O5 | –176.3 (3) |
| C8–C9 | 1.499 (6) | C2–C1–C10–O7 | 165.9 (3) |
| C19–C20 | 1.485 (5) | C17–C12–C11–O7 | 164.3 (3) |
Hirshfeld Surface Analyses
The Hirshfeld surface was analyzed with CrystalExplorer21 to identify various intermolecular contacts in the crystal structure.40−46 The analysis of the 3D Hirshfeld surface (HS) and 2D fingerprint plots (FPs) were performed in order to describe the molecular structure of AMA. The surfaces mapped over dnorm, shape index, and curvedness were shown in Figure 7. Complementary pairs of triangles are seen on both surfaces of the molecule, for the shape index surface, indicating that the respective π-stacking interactions are present in the crystal packing for the title compound. In Figure 7, the π···π interactions for the compound correspond to the relatively large green flat regions on the curvedness surfaces and are evident on both sides of the rotated molecule. Like the title compound, in the 1-acyl-thiourea derivative recently reported, namely, 1-(2-oxo-2H-chromene-3-carbonyl)-3-(2-methoxyphenyl)thiourea, the molecule is linked by the π···π interactions.47 Similarly, the shape index surface has a connecting complementary pair of triangles, and the curvedness surface has large green flat regions. This means that the corresponding π-stacking interactions are present in the crystal packing for the given structure. The 3D dnorm surface is mapped to a fixed color scale from −0.1183 to 1.3829 Å, showing that the largest contribution to crystal packing comes from interactions between H atoms, covering a significant region of the entire HS (45.5%). Figure 8 shows the fingerprint plots with two symmetrical peaks associated with O···H interactions (29.5%), correlating with intermolecular hydrogen bonds between C4–H4···O2i and C14–H14···O4ii. The asymmetric contact wings of C···H with a relative contribution of 12.4% can be seen on both sides of the relevant plots. C···O (6.2%), C···C (4.4%), and O···O (2%) are the less important interactions.
Figure 7.
Hirshfeld surfaces dnorm, shape index, and curvedness for AMA. Molecule is shown in two orientations.
Figure 8.
Two-dimensional fingerprint plots and HS for AMA.
Energy framework analysis was performed by CrystalExplorer21 to obtain the intermolecular interaction energies, allowing analysis and visualization of the three-dimensional crystal packing to analyze and visualize the three-dimensional crystal topology. The interaction energy is calculated using the formula Etot = Eele + Epol + Edis + Erep (where Eele is the electrostatic component, Epol the polarization energy, Edis the dispersion energy, and Erep the exchange repulsion energy). The energy framework is depicted in Figure 8. Clusters of 3.8 Å were formed around each molecule. The tube size used was 100 with a cutoff value of 5 kJ/mol. The thickness of the tube in Figure 9 is proportional to the values of the interaction energies. The total interaction energies are repulsion (Erep = 42.1 kJ/mol), dispersion (Edis = −160.1 kJ/mol), polarization (Epol = −21.6 kJ/mol), electrostatics (Eele = −70 kJ/mol), and total interaction energy (Etot = −209.6 kJ/mol). The dispersion component contributed 63% and the electrostatic component 28% to lattice stabilization. The 9% contribution belongs to the polarization and repulsion energies. For the compound, the sum of dispersion energies (−160 kJ/mol) is greater than that of electrostatic energies (−70 kJ/mol). According to the result obtained from these calculated values, the superiority of the dispersion energy framework over the electrostatic energy framework is clearly seen. In this study, the dispersion energies have a significant contribution in agreement with reported values.48,49
Figure 9.
Energy framework diagram for (a) electrostatic (red), (b) dispersion (green), and (c) total interaction energy (blue) of AMA.
Antimicrobial Activity
The in vitro antimicrobial activity of the anhydride compound was evaluated against Gram-strain positive and negative bacterial strains, as well as two fungal strains. The MIC values were evaluated against Gram-positive and Gram-negative bacteria at doses of 500–1000 μg/mL. The tested compound exhibited excellent antibacterial activity against S. aureus and E. faecalis in comparison to standard amoxicillin, and it was also equally effective against K. pneumoniae and P. aeruginosa. In addition, the title anhydride showed less activity against B. subtilis and E. coli than standards and showed no activity against fungi. The antimicrobial activity of the title anhydride is given in Table 4.
Table 4. Minimum Inhibitory Concentrations (MICs) of the AMA Compounda.
| minimum inhibition concentration (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gram-straining-positive |
Gram-straining-negative |
Fungi |
||||||
| sample | B. subtilis | S. aureus | E. faecalis | E. coli | K. pneumoniae | P. aeruginosa | A. niger | C. albicans |
| 3-acetoxy-2-methylbenzoic anhydride | 1000 | 500 | 500 | 1000 | 1000 | 1000 | - | - |
| amoxicillin | <2 | >1000 | >1000 | 32 | >1000 | >1000 | NT | NT |
| tetracycline | <2 | 8 | 8 | <2 | 8 | 4 | NT | NT |
| ketoconazole | NT | NT | NT | NT | NT | NT | 1 | 2 |
NT: not tested.
Conclusions
A novel 3-acetoxy-2-methylbenzoic anhydride compound was investigated by elemental analysis, FT-IR, 1H and 13C NMR spectroscopy, and single-crystal X-ray crystallography. The X-ray findings show that a molecule has a nearly planar shape with an angle of 5.26° between the planes of the six-membered aromatic rings. There are different types of noncovalent interactions involved in creating the 3D network of the compound. The Hirshfeld surface analysis findings suggest that the H···H (45.5%), O···H (29.5%), and C···H (12.4%) interactions contribute the most to crystal packing. Although the topologies of Edis and Eele are similar in the title compound, the dispersion energy framework makes a larger contribution to the total energy framework. When compared to the amoxicillin standard, AMA demonstrated remarkable antibacterial activity against the Gram-positive strains S. aureus and E. faecalis, as well as being equally efficient against the Gram-negative strains K. pneumoniae and P. aeruginosa. However, it showed less antimicrobial activity than standard antibiotics in the Gram-positive B. subtilis strain and in the Gram-negative E. coli strain but not in fungi.
Acknowledgments
The authors acknowledge the financial support through Researchers Supporting Project number (RSP-2021/147), King Saud University, Riyadh, Saudi Arabia.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00879.
CCDC 2058057 contains all of the crystallographic data and is freely available at www.ccdc.cam.ac.uk/structures (CIF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ogliaruso M. A.; Wolfe J. F.. Synthesis of carboxylic acids, esters, and their derivatives; Wiley: New York, NY, 1991; p 198. [Google Scholar]
- Tarbell D. S. Carboxylic carbonic anhydrides and related compounds. Acc. Chem. Res. 1969, 2, 296–300. 10.1021/ar50022a002. [DOI] [Google Scholar]
- Held H.; Rengstl A.; Mayer D.. Acetic anhydride and mixed fatty acid anhydrides, Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Rebek J.; Feitler D. Mechanism of the carbodiimide reaction. II. Peptide synthesis on the solid phase. J. Am. Chem. Soc. 1974, 96, 1606–1607. 10.1021/ja00812a061. [DOI] [PubMed] [Google Scholar]
- Kocz R.; Roestamadji J.; Mobashery S. A convenient triphosgene-mediated synthesis of symmetric carboxylic acid anhydrides. J. Org. Chem. 1994, 59, 2913–2914. 10.1021/jo00089a046. [DOI] [Google Scholar]
- Kazemi F.; Sharghi H.; Nasseri M. A. A Cheap, Simple and efficient method for the preparation of symmetrical carboxylic acid anhydrides. Synthesis 2004, 205–207. 10.1055/s-2003-44381. [DOI] [Google Scholar]
- Kazemi F.; Kiasat A. R.; Mombaini B. Simple preparation of symmetrical Carboxylic acid anhydrides by means of Na2CO3/SOCl2. Synth. Commun. 2007, 37, 3219–3223. 10.1080/00397910701547904. [DOI] [Google Scholar]
- Kawamura Y.; Sato Y.; Horie T.; Tsukayama M. Dehydration induced by intramolecular redox character of a stable allylidenetributylphosphorane. Tetrahedron Lett. 1997, 38, 7893–7896. 10.1016/S0040-4039(97)10099-5. [DOI] [Google Scholar]
- Keshavamurthy S.; Vankar Y. D.; Dhar D. N. Preparation of acid anhydrides, amides, and esters using chlorosulfonyl isocyanate as a dehydrating agent. Synthesis 1982, 1982, 506–508. 10.1055/s-1982-29859. [DOI] [Google Scholar]
- Clarke P. A.; Kayaleh N. E.; Smith M. A.; Baker J. R.; Bird S. J.; Chan C. A one-step procedure for the monoacylation of symmetrical 1,2-diols. J. Org. Chem. 2002, 67, 5226–5231. 10.1021/jo0257041. [DOI] [PubMed] [Google Scholar]
- Epstein W. L.; Kligman A. M. Treatment of warts with cantharidin. AMA Arch. Derm. 1958, 77, 508–511. 10.1001/archderm.1958.01560050014003. [DOI] [PubMed] [Google Scholar]
- Oikawa M.; Ueno T.; Oikawa H.; Ichihara A. Total synthesis of tautomycin. J. Org. Chem. 1995, 60, 5048–5068. 10.1021/jo00121a026. [DOI] [Google Scholar]
- Cheng X.-C.; Kihara T.; Kusakabe H.; Magae J.; Kobayashi Y.; Fang R.-P.; Ni Z.-F.; Shen Y.-C.; Yamaguchi I.; Isono K. A new antibiotic, tautomycin. J. Antibiot. (Tokyo) 1987, 40, 907–909. 10.7164/antibiotics.40.907. [DOI] [PubMed] [Google Scholar]
- Khatun N.; Santra S. K.; Banerjee A.; Patel B. K. Nano CuO catalyzed cross dehydrogenative coupling (CDC) of aldehydes to anhydrides. Eur. J. Org. Chem. 2015, 2015, 1309–1313. 10.1002/ejoc.201403508. [DOI] [Google Scholar]
- Adib M.; Rajai-Daryasarei S.; Pashazadeh R.; Tajik M.; Mirzaei P. Regioselective transition metal-free acylation of coumarins via cross-dehydrogenative coupling reaction of coumarins and aldehydes. Tetrahedron Lett. 2016, 57, 3701–3705. 10.1016/j.tetlet.2016.06.061. [DOI] [Google Scholar]
- Nuree Y.; Singha R.; Ghosh M.; Roy P.; Ray J. K. Cu (I) catalyzed synthesis of anhydrides from aldehydes via CDC-pathway at ambient temperature. Tetrahedron Lett. 2016, 57, 1479–1482. 10.1016/j.tetlet.2016.02.066. [DOI] [Google Scholar]
- Hata T.; Tajima K.; Mukaiyama T. A convenient method for the preparation of acid anhydrides from metallic carboxylates. Bull. Chem. Soc. Jpn. 1968, 41 (11), 2746–2747. 10.1246/bcsj.41.2746. [DOI] [Google Scholar]
- Kazemi F.; Kiasat A. R. Dabco/SOCl2, mild, and convenient reagent for the preparation of symmetrical carboxylic acid anhydrides. Phosphorus Sulfur Silicon Relat. Elem. 2003, 178 (10), 2287–2291. 10.1080/713744563. [DOI] [Google Scholar]
- Olah G. A.; Vankar Y. D.; Arvanaghi M.; Sommer J. Formic anhydride. Angew. Chem., Int. Ed. Engl. 1979, 18 (8), 614–614. 10.1002/anie.197906141. [DOI] [Google Scholar]
- Rambacher P.; Mäke S. Simplified process for preparation of anhydrides of aromatic acids. Angew. Chem., Int. Ed. Engl. 1968, 7 (6), 465–465. 10.1002/anie.196804651. [DOI] [Google Scholar]
- Ogliaruso M. A.; Wolfe J. F. In Acid Derivatives (1979) Supplement B: Part 1; Patai S., Ed.; Wiley: New York, 1979; Vol. 1, p 267. [Google Scholar]
- Chen X.; Zheng Y.; Shen Y. Natural products with maleic anhydride structure: Nonadrides, tautomycin, chaetomellic anhydride, and other compounds. Chem. Rev. 2007, 107, 1777–1830. 10.1021/cr050029r. [DOI] [PubMed] [Google Scholar]
- Weng Y. M.; Hotchkiss J. H. Anhydrides as antimycotic agents added to polyethylene films for food packaging. Packag. Technol. Sci. 1993, 6, 123–128. 10.1002/pts.2770060304. [DOI] [Google Scholar]
- Bhosale S. V.; Jani C. H.; Langford S. J. Chemistry of naphthalene diimides. Chem. Soc. Rev. 2008, 37, 331–342. 10.1039/B615857A. [DOI] [PubMed] [Google Scholar]
- Kawanami Y.; Dainobu Y.; Inanaga J.; Katsuki T.; Yamaguchi M. Synthesis of thiol esters by carboxylic trichlorobenzoic anhydrides. Bull. Chem. Soc. Jpn. 1981, 54 (3), 943–944. 10.1246/bcsj.54.943. [DOI] [Google Scholar]
- APEX2; Bruker AXS Inc.: Madison WI, 2013. [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C: Struct. Chem. 2015, 71, 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. A: Found. Adv. 2015, 71, 3–8. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854. 10.1107/S0021889812029111. [DOI] [Google Scholar]
- Macrae C. F.; Bruno I. J.; Chisholm J. A.; Edgington P. R.; McCabe P.; Pidcock E.; Rodriguez-Monge L.; Taylor R.; van de Streek J.; Wood P. A. Mercury CSD 2.0-new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. 10.1107/S0021889807067908. [DOI] [Google Scholar]
- Sarker S. D.; Nahar L.; Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne P. National committee for clinical laboratory standards. Performance standards for antimicrobial disc susceptibility testing 2002, 12, 01–53. [Google Scholar]
- Wiegand I.; Hilpert K.; Hancock R. E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Li Y.; Xue D.; Wang C.; Liu Z.-T.; Xiao J. Carboxylic acid anhydrides via Pd-catalyzed carbonylation of aryl halides at atmospheric CO pressure. Chem. Commun. 2012, 48, 1320–1322. 10.1039/C2CC16611A. [DOI] [PubMed] [Google Scholar]
- Saberi D.; Shojaeyan F.; Niknam K. Oxidative self-coupling of aldehydes in the presence of CuCl2/TBHP system: direct access to symmetrical anhydrides. Tetrahedron Lett. 2016, 57, 566–569. 10.1016/j.tetlet.2015.12.095. [DOI] [Google Scholar]
- Nakamoto K.Infrared Spectra of Inorganic and Coordination Compounds; Wiley Interscience: New York, 1970; p 317. [Google Scholar]
- Nyquist R. A.Interpreting infrared, Raman, and nuclear magnetic resonance spectra. Chapter 11 - Anhydrides; Academic Press, 2001; Vol. 1, pp 205–212. [Google Scholar]
- Fatima A.; Khanum G.; Sharma A.; Garima K.; Savita S.; Verma I.; Siddiqui N.; Javed S. Computational, spectroscopic, Hirshfeld surface, electronic state and molecular docking studies on phthalic anhydride. J. Mol. Struct. 2022, 1249, 131571. 10.1016/j.molstruc.2021.131571. [DOI] [Google Scholar]
- Dardeer H. M.; Mahgoub M. Y.; da Silva J. F.; Elboray E. E. Synthesis, crystal structure and supramolecular analysis of chlorendic acid derivatives. J. Mol. Struct. 2021, 1228, 129458. 10.1016/j.molstruc.2020.129458. [DOI] [Google Scholar]
- Spackman P. R.; et al. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. 10.1107/S1600576721002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman M. A.; Byrom P. G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. 10.1016/S0009-2614(97)00100-0. [DOI] [Google Scholar]
- Ashfaq M.; Tahir M. N.; Muhammad S.; Munawar K. S.; Ali A.; Bogdanov G.; Alarfaji S. S. Single-crystal investigation, Hirshfeld surface analysis, and DFT study of third-order NLO properties of unsymmetrical acyl thiourea derivatives. ACS Omega 2021, 6, 31211–31225. 10.1021/acsomega.1c04884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demircioglu Z.; Kastas C. A.; Kastas G.; Ersanlı C. C. Synthesis, crystal structure, computational chemistry studies and Hirshfeld surface analysis of two Schiff bases, (E)-2-[(4-bromo-2-methylphenylimino)methyl]-4-methylphenol and (E)-2-[(4-bromo-2-methylphenylimino)methyl]-6-methylphenol. Molecular Crystals and Liquid Crystals 2021, 723 (1), 45–61. 10.1080/15421406.2020.1871178. [DOI] [Google Scholar]
- Al-Thamili D. M.; Almansour A. I.; Arumugam N.; Kansız S.; Dege N.; Soliman S. M.; Azam M.; Kumar R. S. Highly functionalized N-1-(2-pyridinylmethyl)-3,5-bis[(E)-arylmethylidene]tetrahydro-4(1H)-pyridinones: Synthesis, characterization, crystal structure and DFT studies. J. Mol. Struct. 2020, 1222, 128940. 10.1016/j.molstruc.2020.128940. [DOI] [Google Scholar]
- Boshaala A.; Abrahem A. F.; Almughery A. A.; Al-Zaqri N.; Zorrouk A.; Lgaz H.; Warad I. Spectroscopic insight into tetrahedrally distorted square planar copper(II) complex: XRD/HSA, physicochemical, DFT, and thermal investigations. Crystals 2021, 11, 1179. 10.3390/cryst11101179. [DOI] [Google Scholar]
- Çakmak Ş.; Koşar Kırca B.; Veyisoglu A.; Yakan H.; Ersanlı C. C.; Kütük H. Experimental and theoretical investigations on a furan-2-carboxamide-bearing thiazole: synthesis, molecular characterization by IR/NMR/XRD, electronic characterization by DFT, Hirshfeld surface analysis and biological activity. Acta Crystallogr. Section C: Struct. Chem. 2022, 78 (3), 201–211. 10.1107/S2053229622002066. [DOI] [PubMed] [Google Scholar]
- Saeed A.; Ashraf S.; Florke U.; Delgado Espinoza Z. Y.; Erben M. F.; Perez H. Supramolecular self-assembly of a coumarine-based acylthiourea synthon directed by π-stacking interactions: Crystal structure and Hirshfeld surface analysis. J. Mol. Struct. 2016, 1111, 76–83. 10.1016/j.molstruc.2016.01.074. [DOI] [Google Scholar]
- Saeed A.; Floerke U.; Fantoni A.; Khurshid A.; Pérez H.; Erben M. F. F. A close insight into the nature of intermolecular interactions in dihydropyrimidine-2(1H)-thione derivatives. CrystEngComm 2017, 19, 1495–1508. 10.1039/C6CE02619B. [DOI] [Google Scholar]
- Saeed A.; Khurshid A.; Floerke U.; Echeverria G. A.; Piro O. E.; Gil D. M.; Rocha M.; Frontera A.; Mumtaz A.; El-Seedi H.; Erben M. F. F. Intermolecular interactions in antipyrine-like derivatives 2-halo-N-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1-H-pyrazol-4-yl)benzamides: X-ray structure, Hirshfeld surface analysis and DFT calculations. New J. Chem. 2020, 44, 19541–19554. 10.1039/D0NJ03958F. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.