Abstract

Dependence on fossil fuels for energy purposes leads to the global energy crises due to the nonrenewable nature and high CO2 production for environmental pollution. Therefore, new ways of nanocatalysis for environmental remediation and sustainable energy resources are being explored. Herein, we report a facile surfactant free, low temperature, and environmentally benign hydrothermal route for development of pure and (5, 10, 15, and 20 mol %) Ta-doped horizontally and vertically interwoven NaNbO3 nanohierarchitecture photocatalysts. To the best of our knowledge, such a type of hierarchical structure of NaNbO3 has never been reported before, and changes in the microstructure of these nanoarchitectures on Ta-doping has also been examined for the first time. As-synthesized nanostructures were characterized by different techniques including X-ray diffraction analysis, electron microscopic studies, X-ray photoelectron spectroscopic studies, etc. Ta-doping considerably affects the microstructure of the nanohierarchitectures of NaNbO3, which was analyzed by FESEM analysis. The UV–visible diffused reflectance spectroscopy study shows considerable change in the band gap of as-synthesized nanostructures and was found to be ranging from 2.8 to 3.5 eV in pure and different mole % Ta-doped NaNbO3. With an increase in dopant concentration, the surface area increases and was equal to 5.8, 6.8, 7.0, 9.2, and 9.7 m2/g for pure and 5, 10, 15, and 20 mol % Ta-doped NaNbO3, respectively. Photocatalytic activity toward the degradation of methylene blue dye and H2 evolution reaction shows the highest activity (89% dye removal and 21.4 mmol g–1 catalyst H2 evolution) for the 10 mol % NaNbO3 nanostructure which was attributed to a change in the conduction band maximum of the material. At 100 °C and 500 kHz, the dielectric constants of pure and 5, 10, 15, and 20 mol % Ta-doped NaNbO3 were found to be 111, 510, 491, 488, and 187, respectively. The current study provides the rational insight into the design of nanohierarchitectures and how microstructure affects different properties of the material upon doping.
1. Introduction
The demand for environmentally benign materials for water remediation, energy generation, and storage applications has increased vastly due to the population explosion in last few decades.1,2 Therefore, to meet the challenges due to exponential growing population, different new methods of environment remediation, green energy generation, and storage are being explored.3−5 Among different techniques, photocatalytic remediation of wastewater and photocatalytic production of hydrogen from water splitting has gained prime importance due to being environment friendly and the renewable nature of water.6,7 An important application of photocatalysis is the removal of hazardous organic pollutants from the water. To carry out the photocatalytic water splitting and degradation of organic pollutants from wastewater, different inorganic nanophotocatalysts like metal oxides (NaNbO3, NaTaO3, TiO2, Cu2O, Ag, KNbO3, La1–xSrxCoO3, Pr2Sn2O7, holmium oxide), metal tungstites, and molybdates have been used.8−20 The use of diverse inorganic photocatalysts starts from the pioneering work carried out by Fujishima using TiO2 as a photocatalyst for water splitting.10 Among different explored photocatalysts, perovskite photocatalysts like NaNbO3, NaTaO3, KNbO3, etc. have gained a lot of interest as an alternative to TiO2 due to their environment friendly nature and comparable band gap to TiO2.8,9,11,12,21−23
With NaNbO3 being a typical perovskite structure having a large pool of important properties like cost-effective, abundant, high crystallinity, environmentally friendly, and high chemical stability.24,25 These properties make NaNbO3 a fascinating material having great scientific, research, and technological interest. However, the large band gap associated with NaNbO3 limits the prospect of utilizing it as an efficient photocatalyst. To have an excellent photocatalytic application of NaNbO3, strenuous efforts are being made to improve the light absorption range and to limit the recombination rate of photogenerated charge carriers. In this context, different strategies like development of one-dimensional nanostructures (which show continuous electron transport thus retards the charge recombination), development of heterostructures between different photocatalysts26 (which reduces the band gap and thus improves the absorption range of photocatalysts) and doping of the “Na” site or “Nb” site with different elements is being developed to improve the activity of NaNbO3.27,28 To control the band structure of the target photocatalysts, doping with foreign element is recognized as one of the potential strategies to improve the photocatalytic activity. The ionic charge balanced doping of Na site in NaNbO3 perovskite has been mostly employed in improving the activity of the photocatalyst. Similarly, Jana et al., have used doping of rare earth metal to improve the photocatalytic activity of NaTaO3. However, such a type of doping has little impact on the band structure.29 In NaNbO3, the conduction band is mainly constituted of the Nb5+ orbital and thus to change the electronic properties significantly doping of Nb site is more favorable. Jana et al. have studied the effect of doping of isovalent Nb5+ at the Ta5+ site on photocatalytic properties of NaTaO3.29 Also, Toresspardo et al. have studied the effect of substitution of Nb by isovalent Ta on electric and dielectric properties of NaNb1–xTaxO3.30 To the best of our knowledge, NaNb1–xTaxO3 has not been investigated for its photocatalytic activity. However, the investigation of effect of doping of Nb by isovalent Ta on dielectric properties of NaNb1–xTaxO3 has been done previously, and the method used for synthesis of NaNb1–xTaxO3 include high temperature solid state reactions.31 Also, to improve the properties the development of nanodimensional materials using different synthesis approaches like the solvothermal, polymeric precursor, hydrothermal, and reverse micellar methods and the use of different structure, morphology, and shape controlling agents have been employed.32−38
In addition to the development of new ways to generate energy, the demand for efficient energy storage materials is also increasing. More research and technological efforts are being made to develop high energy and power density materials which could further help in integration of miniaturized electronic devices. For this purpose, ceramic dielectric material has shown fascinating application due to their high chemical, mechanical, and thermal stabilities and high permittivity with high working temperature.
In this paper, low-temperature hydrothermal synthesis and structural characterization of pure NaNbO3 and NaNb1–xTaxO3 were carried out. The photocatalytic applications of synthesized hierarchical interwoven nano NaNb1–xTaxO3 was screened by carrying out the degradation of organic pollutant and by carrying out H2 evolution from the water splitting reaction. With varying the concentration of Ta doping, the interwoven nanohierarchitecture of NaNb1–xTaxO3 showed improved photocatalytic activity compared to pure NaNbO3. In addition to photocatalytic application, the effect of doping of isovalent Ta at the Nb site on dielectric properties of NaNbO3 was also investigated. From the investigation, it was observed that doping of Nb by isovalent Ta improves both photocatalytic activity and dielectric properties of NaNbO3 nanoparticles. The changes in both photocatalytic activity and dielectric properties of Ta doped NaNbO3 were correlated with the possible changes in the band structure and microstructure taken place due to doping of Ta.
2. Experimental Section
2.1. Materials
All the chemicals used in the synthesis process were of analytical grade. To synthesize pure NaNbO3 and NaNb1–xTaxO3, NaOH (Merck, 97%) was used as a source of Na. Nb2O5 (Alfa Aesar, 99%) was used as a precursor for Nb, and Ta2O5 (Alfa Aesar) was used as the Ta source. To check the photocatalytic removal of organic pollutants, methylene blue dye (MB) (Merck) was used as a model pollutant. Other chemicals used include silver nitrate (Merck, 99.5%), benzoquinone (Merck, 97%), ammonium oxalate (Merck, 30%), and isopropanol (RANKEM, 99%). All the chemicals were used as received without further purification.
2.2. Synthesis of Sodium Niobate Nanohierarchitecture
Sodium niobate nanoparticles with hierarchitectured morphology were first time synthesized by using a simple low-temperature hydrothermal route. The hydrothermal route employed for the synthesis of pure NaNbO3 nanoparticles is reported somewhere else.25 In a typical synthesis process, 0.025 mol of Nb2O5 was added in 10 M NaOH solution. The solution was stirred at room temperature for 4 h. After that, the solution was transferred to a 75 mL Teflon lined autoclave and was heated at 180 °C for 4 h. The solution could cool naturally and was centrifuged and then washed several times, first with distilled water and then with ethanol.
2.3. Synthesis of Ta Doped Sodium Niobate Nanohierarchitecture
NaNbO3 doped with different concentrations of Ta was synthesized by a similar hydrothermal route except the addition of Ta2O5 as a precursor for Ta. In a typical synthesis process, 0.025 mol of Nb2O5 was added to 10 M solution of NaOH followed by addition of 5, 10, 15, and 20 mol % of Ta2O5 in the reaction mixture. The whole reaction mixture was stirred for 4 h and transferred to a 75 mL Teflon lined autoclave. The reaction mixture was heated at 180 °C for 4 h and then cooled to room temperature. To get the final product, the reaction mixture was centrifuged at 7000 rpm and was washed with distilled water and ethanol several times. The final product was dried in a vacuum oven at 60 °C and was used for further analysis. All the synthesized samples were coded as (NN) for pure NaNbO3 and NN1, NN2, NN3, and NN4 for 5%, 10%, 15% and 20% Ta doped NaNbO3, respectively.
2.4. Characterization
Powder X-ray diffraction analysis (XRD) was carried out by employing a Rigaku diffractometer using Cu Kα radiation having a wavelength equal to 1.5406 Å. All the samples were scanned with a scan rate and step size equal to 5°/min and 0.02°, respectively. X-ray diffraction analysis was carried out with 2θ ranging from 10 to 70°. Fourier transformation infrared spectroscopic (FTIR) analysis was carried out by using pellets of as synthesized samples in the presence of KBr. FTIR analysis was carried out using a PerkinElmer FT-IR spectrophotometer model IR affinity1. Transmission electron microscope (TEM) analysis was carried out by using the samples dispersed in ethanol and mounted on a carbon coated copper grid. The grids for TEM analysis were prepared by carrying out the drop casting of the sample on the copper grid. TEM analysis was done using a TELOS instrument with an accelerating voltage of 200 kV. For field emission scanning electron microscopy (FESEM), samples were mounted in the form of powder on the grid with gold coating. FESEM analysis was carried out using FEI NOVA NanoSEM 450 having an accelerating voltage of 20 kV. In addition to FESEM, EDAX and elemental mapping of the samples was carried out using FEI NOVA NanoSEM 450. The binding energy and oxidation state analysis of the samples was carried out by using X-ray photoelectron spectroscopy (XPS). XPS analysis was carried out in survey mode by carrying out surface charge neutralization. For XPS analysis operating a flood gun having 30 eV pass energy, a 0.01 eV step size was used. All the peaks in the XPS spectra were calibrated with respect to C 1s having a peak position at 284.8 eV. XPS measurements were carried out using Thermoscientific XPS having Al Kα radiation, with hυ = 1486.6 eV. The band gap was determined by using diffused reflectance UV–vis spectroscopy (DRS). Reflectance analysis of the samples was carried out using a PerkinElmer Lambda365 UV–vis spectrophotometer with BaSO4 as a reference sample in the range of 200–800 nm. By employing reflectance data, a band gap was calculated using the Kubelka–Munk equation. Surface area analysis was carried out by using the Brunauer–Emmett–Teller (BET) surface area analysis technique. For surface area analysis, a Nova 2000e (Quantachrome Instruments Limited, USA) was used. All the measurements were carried out in the presence of liquid nitrogen having a bath temperature of 77 K. Before carrying out the analysis, all the samples were degassed under vacuum conditions at 150 °C for 12 h to remove the unwanted adsorbed gases on the samples. The weight of the samples was measured before and after degassing to calculate the actual amount of sample having no adsorbed gases on their surfaces. Photoluminescence (PL) analysis of the synthesized samples was carried out by using an F-7000 Hitachi fluorescence spectrophotometer with an excitation wavelength ranging from 400 to 500 nm.
2.5. Photocatalytic Studies
Degradation of methylene blue was carried out in the presence of sunlight to elucidate the photocatalytic activity of as-prepared nanoparticles. To carry out the analysis, a stock solution of MB dye having a concentration equal to 1 × 10–5 M was prepared in aqueous medium. A total of 20 mg of nanoparticles was dispersed in 50 mL of the dye solution and was kept in the dark for 1 h to ensure adsorption–desorption equilibrium between the dye and the nanocatalysts. The dye-catalyst suspension was then exposed to sunlight irradiation to initiate the photocatalytic degradation reaction. After every 10 min, adequate aliquots were taken and centrifuged to remove the suspended catalyst particulates for spectral analysis. Similar experiments were carried out either in the dark or without catalysts to confirm the degradation process is solely photocatalytic driven. The efficiency of the catalyst for photodegradation process was monitored by a change in intensity of the characteristic absorption peak of MB at ∼664 nm using a PerkinElmer Lambda365 UV–vis spectrophotometer. The percentage removal of the dye was computed by using eq 1.
| 1 |
where Ci is the initial concentration of MB dye after adsorption–desorption equilibrium before irradiation and Cf is the concentration of dye after time interval t (in minutes). In addition to UV–visible spectra, liquid chromatography-mass spectrometry (LC-MS) of the dye solution was carried out to attest whether the dye has been degraded or not. Mass spectral studies were carried out by using an API2000 Applied Biosystems LC/MS/MS/MS instrument.
2.6. Photocatalytic Water Splitting Measurements
The photocatalytic activities for hydrogen generation were also studied for all synthesized samples in an external irradiation cell with a light source of a 200 W Hg (Xe) lamp (New Port, Mercury–Xenon, 200 W model 66906-200HXF-R15 ozone-free) at an intensity of 190 W without a cutoff filter under full arc irradiation. For the photocatalytic H2 evolution experiments, a 100 mL cylindrical quartz cell with its flat surfaces being exposed to the light source was charged by 20 mg of the photocatalyst and 0.128 moles of Na2S, 0.079 moles of Na2SO3 as the sacrificial agents were dispersed uniformly in 50 mL of double distilled water. The cell containing the solution was closed with an airtight rubber septum at the top and kept in an inert atmosphere by purging N2 gas into the cell for half an hour prior to carrying out the measurements and to remove any impurities of the gases. After degassing, the solution mixture was placed under light on constant stirring and kept at a constant temperature of around (25 °C). The quantification of the amount of evolved H2 gas was done by using gas chromatography (PerkinElmer, Clarus 590 GC) equipped with a TCD detector using N2 as the carrier gas. The gaseous components were monitored by per hour sampling, and evolved H2 was estimated with the reference hydrogen gas by comparing the GC plot.
2.7. Dielectric Measurements
Dielectric properties of the as-prepared samples were measured using 6505 P, Wayne Kerr Electronics, U.K. Dielectric properties of the samples were analyzed as a function of frequency and temperature ranging from 20 Hz to 1 MHz and 50–500 °C respectively. To study the dielectric properties of the synthesized samples, a parallel plate capacitor was developed by using sample disks having a 8 mm diameter and 0.8 mm thickness and coated with a silver conducting surface which acts as an electrode. For the preparation of pellets, poly(vinyl alcohol) (PVA) was used as a binder. All the synthesized samples were mixed with 5% PVA in a mortar and pestle, and pellets were formed by applying pressure equal to 5 tons using a technosearch KBr press model M-5. All the pellets were annealed at 800 °C before dielectric measurements were carried out.
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Studies
The phase composition, phase purity, crystal structure, and crystallinity of pure NaNbO3 and NaNb1–xTaxO3 having different compositions of Ta (5–20 mol %) was determined by XRD analysis as shown in Figure 1a. From the XRD patterns, it was observed that pure monophasic NaNbO3 having an orthorhombic crystal structure was synthesized using the hydrothermal route. All the reflection peaks observed in the XRD were matched with the JCPDS card no. 89-5173. The resultant Ta doped NaNbO3 samples have quite identical isostructural orthorhombic crystal structures. Peaks at 2θ values of 22.89°, 32.77°, 46.85°, 52.61°, 58.06°, and 68.19° correspond to (100), (110), (200), (210), (211), and (220) crystal planes, respectively. With an increase in concentration of Ta in NaNbO3, the peaks are little shifted toward higher 2θ values as shown in Figure 1b, and this apparent shifting in peaks was attributed to the small ionic radii of Ta (0.64 nm) as compared to Nb (0.72 nm) thus causing the lattice contraction in Ta-doped NaNbO3 samples.39 The XRD results obtained were employed to determine the crystallite size of the as-synthesized nanoparticles. To determine the crystallite size, Scherrer’s equation was used, and the calculated crystallite sizes were equal to 23.1, 27.9, 24.9, 24.6, and 20.3 nm for NN, NN1, NN2, NN3, and NN4 samples, respectively. Using XRD results, the crystal parameters and crystal structure of as-synthesized nanoparticles were deduced by using Quantum espreso software. Using the XRD results along with the crystallography open database (COD) file for NaNbO3, the crystal structure of the as synthesized NaNbO3 was deduced. The obtained crystal structures are shown in Figure 2a,b for pure NaNbO3 and Ta-doped NaNbO3 nanostructures, respectively.
Figure 1.
(a) XRD patterns of the as-synthesized NN, NN1, NN2, NN3, and NN4 nanostructures and (b) zoomed in XRD pattern showing shifting of peaks with Ta doping.
Figure 2.
Crystal structure of (a) pure NaNbO3 and (b) Ta-doped NaNbO3 obtained by using Quantum espresso software and (c) FTIR spectra of as synthesized samples (NN, NN1–NN4).
3.2. Fourier Transformation Infrared Spectroscopic (FTIR) Studies
FTIR studies were carried out to determine the formation of M–O bond using KBr as a carrier for as synthesized samples. Figure 2c represents the IR spectra (500–4000 cm–1) of as synthesized samples. From the obtained results, the peak present between 500 and 650 cm–1 confirms the formation of a metal oxide (M–O) bond. In all the synthesized samples, the IR peak is present at 625 cm–1 and thus confirms the successful formation of Ta–O or Nb–O bonds, and no other prominent peaks were observed in any of the synthesized samples.
3.3. Transmission Electron Microscopic (TEM) Studies
Figure 3 represents the TEM micrographs of the as-prepared samples. From TEM analysis, it was observed that typical single layered pure NaNbO3 nanoflakes were synthesized successfully as evident in Figure 3a. With the addition of Ta as a dopant, the formation of the layered structure is restricted as observed in Figure 3b–e. From TEM analysis, it was observed that above 10 mol % Ta doping, the small flake shaped particles start to appear as shown in Figure 3d,e. From the TEM micrographs of the as-synthesized samples, the average particle size distribution was calculated and the obtained histogram is represented in the inset of Figure 3a–e. The average particle sizes for the samples NN, NN1, NN2, NN3, and NN4 were found to be 100, 90, 60, 70, and 40 nm, respectively. To the best of our knowledge, no such flake-like layered nanoparticles of pure NaNbO3 and Ta-doped NaNbO3 have been reported previously.
Figure 3.
TEM micrographs and particle size distribution histograms (inset) of (a) NN, (b) NN1, (c) NN2, (d) NN3, and (e) NN4 nanostructures.
3.4. Field Emission Scanning Electron Microscopic (FESEM) Studies
The FESEM technique was used to investigate the microstructure and micromorphology of as-prepared pure NaNbO3 and Ta-doped NaNbO3 samples. FESEM micrographs of all the samples are shown in Figure 4. It is observed that as-prepared pure NaNbO3 shows large building blocks with hierarchical arrangement of intercrossed horizontal and vertical nanoflakes (Figure 4a). Similarly, in 5% Ta doped NaNbO3, the interwoven nanoflakes are present but the hierarchy is slightly disturbed. With a further increase in concentration of Ta to 10 mol %, intercrossed nanoflakes decrease and little particle formation starts to appear. A further increase in the concentration to 15 and 20 mol %, the needle shaped particles with flowerlike structures are formed. The magnified FESEM micrographs of as-synthesized nanostructures are shown in Figure S1a–e. The detailed observation of the single building blocks showed that these blocks are composed of several small flakes. These small flakes are interconnected horizontally and vertically, thus forming a hierarchical structure. It was also observed that the surface of small flakes was not smooth, which was attributed to Ostwald ripening/coarsening.40Figure S1f–j further demonstrate that the nanoflakes were composed of small nanoparticles.
Figure 4.
FESEM micrographs of (a) NN, (b) NN1, (c) NN2, (d) NN3, and (e) NN4 nanostructures.
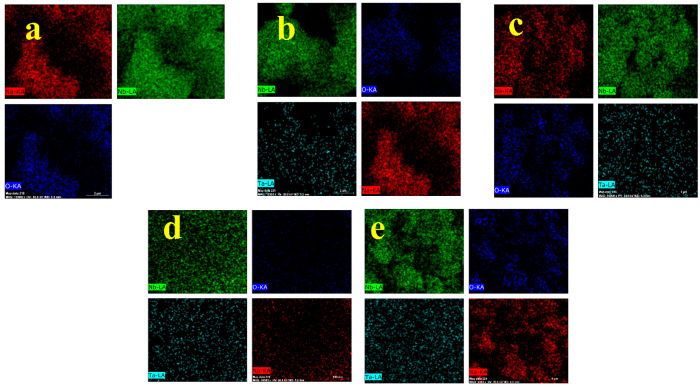
To determine the elemental composition of the as-synthesized pure and Ta-doped NaNbO3 nanoflakes, EDAX analysis was carried out. From the EDAX study, it was observed that peaks in the spectra correspond to Na, Nb, and O with no impure peak present in the NaNbO3 sample as shown in Figure S2a. EDAX of the NaNbO3 samples doped with Ta show the presence of Ta in the spectra (Figure S2b–e). Also, from the EDAX of the samples, it was observed that with increasing the concentration of Ta, the atomic weight percent of Nb decreases which is due to the fact that Ta is doped at Nb sites of the samples. The atomic weight percentage of different constituent elements of the as synthesized nanostructures is given in Table S1. In addition to EDAX analysis, elemental mapping of the as-prepared samples was carried out. Figure 5a–e shows the elemental mapping of pristine NaNbO3 (NN) and Ta-doped NaNbO3 (NN1–NN4) samples. From element mapping of all the samples, it was seen that Na, Nb, and O are distributed uniformly in pristine NaNbO3 sample, whereas, in the samples doped with Ta, coexistence of Ta along with other elements was observed. It was also observed that the density of Ta distribution in all Ta-doped samples increases with an increase in concentration of Ta.
Figure 5.
Elemental mapping of (a) NN, (b) NN1, (c) NN2, (d) NN3, and (e) NN4 nanostructures.
3.5. X-ray Photoelectron Spectroscopy (XPS) Study
Oxidation state and the surface chemical composition of the synthesized photocatalyst samples were elucidated by employing the XPS technique. The binding energies of all the samples were calibrated with respect to C 1s having a binding energy centered at 285.8 eV. Figure 6a represents the survey spectra of pure NaNbO3 and Ta doped (with different mole %) NaNbO3 nanoparticles. From the survey scan of pure NaNbO3, the peaks corresponding to Nb, Na, and O were present. While as in addition to the peaks corresponding to Na, Nb, and O, a peak corresponding to Ta was also present in the samples doped with 10, 15, and 20 mol % Ta. From the XPS analysis, it was observed that no peak corresponding to Ta in the sample with 5 mol % of Ta doping was observed. This was due to a low concentration of Ta present on the surface of the sample. High-resolution XPS of all the samples was carried out as shown in Figure 6. Figure 6b represents the high-resolution XPS spectra of Na with peak centered at 1071 eV, which corresponds to Na 1s.8 The peak present at 1071 eV shows that Na is present in +1 oxidation in all the synthesized samples. Figure 6c represents the high-resolution XPS spectra of O 1s, which is centered at a binding energy equal to 529 eV confirming the −2 oxidation state of O.41 The peak corresponding to O 1s originates due to −Nb–O in pure NaNbO3 or due to −Ta–O present in Ta-doped NaNbO3 samples. Figure 6d shows the high-resolution XPS spectra of Nb element present in the synthesized photocatalysts. The peak corresponding to Nb 3d was deconvoluted into two peaks centered at 206.3 and 209.5 eV, which corresponds to Nb 3d5/2 and Nb 3d3/2, respectively.42 The difference between the two deconvoluted peaks corresponding to Nb was found to be 3 eV, which evidenced that Nb is present in the +5 oxidation state in all the synthesized samples.43Figure 6e corresponds to the high-resolution XPS spectra of Ta. Ta present in the prepared samples shows a doublet of peaks of spin orbit coupling (7/2 and 5/2). The peaks centered at 25.6 and 27.7 eV correspond to the Ta 4f7/2 and Ta 4f5/2, respectively. The binding energy corresponding to 7/2 (25.6 eV) is consistent with previously reported data and confirms the +5 oxidation state of Ta cations.44 From the XPS results, it was confirmed that Ta was successfully incorporated at Nb sites upon doping in NaNbO3 photocatalysts.
Figure 6.
(a) Full range XPS spectra of the as-synthesized photocatalysts (NN–NN4). High-resolution XPS spectra of (b) Na 1s, (c) O 1s, (d) Nb 3d, and (e) Ta 4f present in the NN, NN1, NN2, NN3, and NN4 nanostructures.
3.6. Diffused UV–Visible Reflectance Spectroscopy (DRS) Study
UV–vis DRS spectra of the synthesized samples are shown in Figure S3. Figure S3a shows the reflectance spectra of the as-synthesized samples. The obtained reflectance data was used to calculate the band gap of the materials using the Kubelka–Munk equation. Reflectance was converted to absorbance of the samples using eq 2.
| 2 |
In eq 2, F(R∞) represents the Kubelka–Munk function and is equal to the diffused reflectance of the infinite thick sample, the terms F, α, s, and R represent the Kubelka–Munk function, absorption coefficient, scattering factor, and reflectance of the material, respectively. Figure S3b represents the plot of band gap energy versus (F(R∞)hυ)1/n. From the DRS study, it was observed that pure NaNbO3 shows a band gap equal to 2.89 eV, whereas the photocatalysts doped with 5, 10, 15, and 20 mol % Ta have band gaps equal to 2.92, 3.0, 3.18, and 3.2 eV, respectively. The band gap calculated in this report for pure NaNbO3 is in accordance to the previously reported results.45 From the calculated bandgap, it is observed that the band gap of Ta-doped NaNbO3 photocatalysts is more than pure NaNbO3. The increase in the band gap is due to substitution of Ta5+ at Nb5+ sites in the pristine NaNbO3 photocatalyst. In most of the metal oxides, the O 2p and d orbital of the transition metal constitute the valence and conduction bands, respectively. Thus, the top of the valence band of each prepared sample comprises the O 2p orbital. By substitution of Ta at Nb sites, the conduction potential of Ta-doped NaNbO3 becomes more negative, thus resulting in an increase in the band gap of the as-synthesized Ta-doped nanostructures. This more negative conduction potential is considered as beneficial for driving and separation of photogenerated charge carriers, which could be highly useful for improved photocatalytic activity of the synthesized nanoparticles. However, the large bandgap of a material decreases the absorption range of a photocatalytic material which could reduce the activity of a material as a photocatalyst.
3.7. Brunauer–Emmett–Teller (BET) Surface Area Analysis
Surface area of the sample plays vital role in the catalytic activity of the materials. BET surface area analysis was used to determine the surface area of the synthesized samples. Figure 7a shows the BET N2 adsorption–desorption isotherm of as prepared photocatalysts. From the BET surface area measurements, it was observed that all the samples show type IV adsorption isotherms with a H3 hysteresis loop which confirms the as prepared nanoparticles are having a well-defined mesoporous structure.46 This type of isotherm indicates that multilayer adsorption is taking place followed by condensation. The isotherm possesses a hysteresis loop which is formed because of condensation process taking place on mesoporous solids.47 Using desorption points, the pore size distribution of as-prepared samples was evaluated from the Barrett–Joyner–Halenda (BJH) plot as shown in Figure 7b. From the BJH plot, it was observed that all the samples show monomodal pore distribution centered between 15 and 20 Å. The average pore size of all the samples was deduced from adsorption desorption points of the isotherm by using Dubinin–Astakhov (DA) analysis. Figure 7c represents the DA plot of synthesized NaNbO3 and Ta doped NaNbO3 nanoparticles. The specific surface area, DA average pore size, and BJH pore radius of the samples calculated from BET analysis is given in Table 1. From the BET studies, it was observed that using the hydrothermal route, nanoparticles with enhanced surface area were obtained compared to the samples prepared by the conventional solid state route.44 Also, it was observed that with an increase in the amount of Ta, the surface area increases which was attributed to formation of small particles as observed in TEM and FESEM analysis.
Figure 7.
(a) N2 adsorption desorption isotherms, (b) BJH plots, and (c) DA plots of as-prepared nanostructures.
Table 1. BET Surface Area, DA Pore Size, and BJH Pore Size Distribution of As-Synthesized Nanohierarchitectures.
| sample | BET surface area (m2/g) | DA average pore size (Å) | BJH pore size distribution (Å) |
|---|---|---|---|
| NN | 5.8 | 11.6 | 18.7 |
| NN-1 | 6.8 | 13.3 | 18.8 |
| NN-2 | 7 | 11.3 | 18.7 |
| NN-3 | 9.2 | 11.1 | 18.8 |
| NN-4 | 9.7 | 11.7 | 18.8 |
3.8. Photocatalytic Studies
3.8.1. Photocatalytic Dye Degradation Studies
To check the photocatalytic activity of as-prepared nanoparticles, methylene blue (MB) was used as a model pollutant. A 1 × 10–5 M stock solution of MB was prepared followed by addition of 20 mg of as-prepared photocatalysts. The dye solution along with the photocatalyst nanoparticles were kept under dark condition for 1 h to attain adsorption desorption equilibrium. From the adsorption study, it was observed that the negligible amount of dye has been adsorbed on the catalyst surface. Also, the dye solution without photocatalyst was exposed to sunlight to check the photolysis of MB dye. After adsorption–desorption equilibrium, the dye solution was exposed to solar radiations. After every 10 min, 3 mL of dye solution was extracted to check the change in the absorption maxima of the MB dye in the presence of solar radiation and photocatalyst. The process of photocatalysis was carried out for 80 min. Figure S4a–e represents the UV–visible absorption spectra of MB dye in the presence of as-synthesized photocatalysts and light. From Figure S4a–e, it was observed that with an increase in exposure time, the intensity of absorption maxima of MB dye decreases continuously, confirming the degradation of MB dye with time in the presence of sunlight and as-synthesized photocatalysts. From UV–vis spectra it was also observed that with an increase in Ta doping up to 10 mol %, the photocatalytic activity of NaNbO3 increases, and with a further increase in concentration of dopant the photocatalytic activity decreases. Using UV–visible absorption spectra, percentage removal of MB dye was calculated as represented in Figure 8a. From the obtained results, a maximum decrease in the concentration of MB dye was observed in 10 mol % Ta doped NaNbO3 photocatalyst. From Figure 8a, maximum degradation of 89% was observed in 10 mol % Ta doped NaNbO3. The percentage removal of MB dye using different photocatalysts is tabulated in Table 2. To determine the kinetics of the photocatalytic degradation reaction of MB dye using as-synthesized nanoparticles, different models were used. From the kinetic study, it was observed that all the synthesized photocatalysts obey the Langmuir–Hinshelwood mechanism as shown in Figure 8b. The kinetics of the photocatalysts was determined by using eq 3.
| 3 |
where C and C0 represents concentration after time t and initial concentration at t = 0, respectively, and R is the rate constant of the photocatalytic reaction. From the kinetic study, it was observed that degradation process carried out by as-synthesized photocatalysts follow pseudo first order kinetics, which was confirmed by the straight line obtained from the semilogarithmic plot of concentration versus irradiation time. The rate constant of the degradation process was determined by using linear fitting of the curves. Table 2 shows the rate constant and R2 values of the photocatalytic degradation process of the MB dye in the presence of pure and Ta-doped NaNbO3 photocatalysts.
Figure 8.
(a) Percentage removal efficiency and (b) kinetic plots of the photocatalytic degradation reaction using the as-synthesized pure and Ta-doped NaNbO3 photocatalysts.
Table 2. Percentage Removal of MB Dye and Rate Constant of Photocatalytic Reactions Carried out by the Synthesized Nanocatalysts.
| sample | percentage removal | rate constant | R2 |
|---|---|---|---|
| NN | 44.90 | 0.12 | 0.97 |
| NN1 | 44.90 | 0.13 | 0.99 |
| NN2 | 89 | 0.36 | 0.98 |
| NN3 | 74.84 | 0.21 | 0.97 |
| NN4 | 60.96 | 0.16 | 0.96 |
In addition to UV–visible spectral studies, the degradation of MB dye was confirmed by using LC-MS. The samples with least intensity (obtained after 80 min) in the UV–vis spectra were used to carry out the LC-MS analysis. LC-MS spectra of MB dye after carrying out photocatalytic degradation using as-synthesized photocatalysts are shown in Figure S5a–e. The possible chemical formulas and structure of degradation fragments obtained from LC-MS spectra were deduced using the Chemdraw structural tool and are shown in Figure S6a–e. The role of different active species (OH•, O2•–, e–, H+) generated during the photocatalytic process was elucidated by carrying out the quenching process using different scavengers as discussed in the Supporting Information of the manuscript. Figure S7 represents the effect of different scavengers on photocatalytic activity of as-synthesized photocatalysts. Using the results obtained from the quenching experiments, the following possible mechanism for photocatalytic activity was deduced.
where NN corresponds to pure NaNbO3 and NN1–NN4 corresponds to 5–20 mol % Ta-doped NaNbO3, respectively. The electrons in the conduction band after photoexcitation are represented by eCB–, and the holes in the valence band are designated by hVB+.
3.8.2. Photocatalytic Water Splitting Studies
The photocatalytic activity for hydrogen generation using water splitting phenomenon was evaluated for all the samples. The evolution of hydrogen was measured as a function of time for pure NaNbO3 and NN1–NN4 compositions. Herein, we have reported the H2 evolution for pure sample and NN2 sample with the highest activity and for NN4 to show with a further increase in Ta concentration photocatalytic activity decreases. Figure 9a shows the rates of H2 generation of pure NaNbO3, 10 mol % Ta-doped NaNbO3 (NN2), and 20 mol % Ta-doped NaNbO3 (NN4) samples under full arc irradiation. During the investigation, it was observed that the amount of H2 generated was equal to 1.7, 21.4, and 5.5 mmol g–1 in the presence of pure NaNbO3, NN2, and NN4 respectively, in 8 h. From the obtained results the highest H2 production rate was observed in the NN2 sample. With the increasing concentration of Ta, the photocatalytic H2 evolution activity increases until 10 mol % as sample NN2 shows the highest activity. Thus, the improvement in photocatalytic activity could be attributed to the increase in surface area and change in the reduction potential of the conduction band. With addition of Ta at the Nb sites, the position of the conduction band is altered and is shifted to a more negative reduction potential, which favors the easy separation of photogenerated charge carriers, while as with a further increase in Ta concentration, photocatalytic activity decreases, which is attributed to an increase in band gap due to which photoabsorption activity of a material is reduced.
Figure 9.
(a) Photocatalytic hydrogen evolution of pure and Ta-doped NaNbO3 photocatalysts with irradiation time using Na2S and Na2SO3 as sacrificial agents, and (b) PL spectra of pure and Ta-doped NaNbO3 nanoparticles.
To further investigate the reason responsible for enhanced photocatalytic activity of as synthesized Ta-doped NaNbO3 nanostructures, photoluminescence (PL) investigation was carried out. Figure 9b represents the PL spectra of the synthesized pure NaNbO3 and different mole % Ta-doped NaNbO3 samples. From the PL spectra, the excitation peak for all the samples was observed at 472 nm. It was also observed that with an increase in Ta doping, the intensity of the peak present at 472 nm decreases, thus confirming the delay in the recombination of photogenerated charge carriers due to the entrapment of excitons.48
3.9. Dielectric Studies
Variation of dielectric properties including dielectric constant (ε) and dielectric loss (D) of all the synthesized samples was evaluated with frequency ranging from 20 Hz to 1 MHz at 100 °C. Also, the variation of (ε) and (D) of pristine NaNbO3 and Ta-doped NaNbO3 nanoparticles with a temperature range (50–500 °C) was measured at 500 kHz. Figure 10a–e shows the variation of (ε) and (D) of pure NaNbO3 and 5, 10, 15, and 20 mol % Ta doped NaNbO3 nanoparticles with frequency at 100 °C. From Figure 10, it was observed that with an increase in frequency, the dielectric constant of as-prepared nanoparticles decreases rapidly at lower frequencies and remains stable and constant at higher frequencies. At lower frequencies, all these nanoparticles show high dielectric dispersion. Similar behavior is demonstrated by dielectric loss, which shows high value at lower frequency and decreases with an increase in frequency. The whole dielectric behavior can be explained based on Maxwell–Wagner interfacial polarization theory of dielectrics.49,50 At lower frequencies, different structures including space charge, dipoles, and ions contribute to the overall polarization of the materials. At lower frequencies, these structures have enough time to undergo the relaxation process and follow the external applied electric field. With an increase in frequency, the space charge polarization does not get enough time to follow the applied electric field and therefore is relaxed out at higher frequencies. According to Koop’s theory, another important reason for the decrease in the dielectric constant with frequency is contribution from grain boundaries and grains which are relaxed out at higher frequencies.51 The energy dissipation or dielectric loss of all the synthesized samples decreases with increase in frequency because of the contribution of dipoles toward polarization. The variation of the dielectric loss curve follows similar behavior as that of the dielectric constant. The high value to dielectric loss at lower frequency is due to high resistive grain boundaries which are more prominent than grains.
Figure 10.
Variation of dielectric constant and dielectric loss with frequency of (a) NN, (b) NN1, (c) NN2, (d) NN3, and (e) NN4 samples at 100 °C.
In addition, the variation of (ε) and (D) in pure NaNbO3 and Ta-doped NaNbO3 with temperature is shown in Figure 11a–e. The detailed measurements of dielectric properties were carried out in the temperature range of 50–500 °C at a selected frequency of 500 kHz with a temperature interval of 50 °C. From Figure 11, it was observed that at 500 kHz all the samples show an increase in the dielectric constant with an increase in temperature. In the pure NaNbO3 sample, the dielectric constant increases in the temperature range 50–400 °C and shows a maximum value at 400 °C. With a further increase in temperature, the dielectric constant decreases. The temperature having a maximum value of the dielectric constant corresponds to a Curie temperature of the sample.52 From dielectric dependence on temperature, at 500 kHz dielectric constant, maxima were observed in all the samples with different compositions. From the results, it was observed that with an increase in the dopant concentration, the dielectric constant maximum is shifted toward lower temperature. Also, with an increase in the doping concentration, the dielectric constant increases up to 5 mol % doping of Ta in NaNbO3, and with a further increase in dopant concentration, the dielectric constant starts decreasing.
Figure 11.
Variation of dielectric constant and dielectric loss with temperature of (a) NN, (b) NN1, (c) NN2, (d) NN3, and (e) NN4 samples at 500 kHz.
The increase in dielectric constant as compared to pure NaNbO3 up to 5 mol % Ta doping is attributed to the change in the microstructure of the samples. It had been observed that dielectric properties of the material are influenced by grain size, porosity, applied temperature, and substituent/dopant concentration. As investigated by SEM analysis, the concentration of Ta as dopant in NaNbO3 plays an important role in controlling the microstructure. As reported earlier, it is observed that grain size distribution plays an important role in the dielectric properties of the material. The variation of the dielectric constant in Ga doped K1–xNaxNbO3 and BaTiO3 with grain size was reported, and from these results it was explained how domain twining, microstructure, and internal stress at the grain boundaries play an important role in the dielectric constant of the material.53 In this report, we speculate similar behavior responsible for the variation of the dielectric constant with temperature. As observed from dielectric analysis, the sample having 5 mol % Ta doping concentration has the highest value of the dielectric constant and it decreases with a further increase of Ta doping. This behavior is attributed to electronic or ionic defect migration taking place on addition of a dopant in the host material.53
All the synthesized samples with different concentration ranges have comparable dielectric loss all over the measuring temperature range as shown in Figure 11. The increase in dielectric loss with temperature of as-prepared samples is attributed to the change in mobility of ionic defects with an increase in temperature. Table 3 represents the value of the dielectric constant and dielectric loss at 100 °C and 500 kHz frequency of as-prepared samples.
Table 3. Dielectric Constant and Dielectric Loss Values of As-Prepared Samples at 500 kHz and 100 °C.
| sample | dielectric constant | dielectric loss |
|---|---|---|
| NN | 111 | 0.031 |
| NN1 | 510 | 0.06 |
| NN2 | 491 | 0.044 |
| NN3 | 488 | 0.047 |
| NN4 | 188 | 0.05 |
3.10. AC Conductivity Studies
To understand the conduction mechanism and evaluate the parameters controlling the process of conduction for pure and Ta-doped NaNbO3 nanoparticles, AC conductivity measurements were carried in the frequency range 20 Hz to 1 MHz at 50 °C as shown in Figure S8. From conductivity measurements, it was observed that with an increase in frequency, the conductivities of all the samples increase. The presence of hopping channels is responsible for hopping conduction; therefore, with an increase in frequency, the hopping channels are facilitated and becomes more active, which promotes more charge carrier hopping. The Jonscher power law (eq 4) was used to understand the underlying mechanism for the conduction behavior of as-prepared nanoparticles.
| 4 |
where A is a constant, ω represents angular frequency, and exponent n represents the frequency dependent slope. The value of n determines the mechanism responsible for the conduction behavior of the samples. For η < 1 and η > 1 conduction follows the Maxwell–Wagner type mechanism and barrier hopping conduction mechanism, respectively. From fitting of the conductivity plots of different samples, it was observed that all the samples have η < 1 and follow the conduction hopping barrier mechanism, which involves short-range translational hopping along with the small and large polaron hopping mechanisms. From AC conductivity measurements, it was also observed that conductivity first decreases with an increase in Ta %, and with a further increase in Ta above 5 mol %, conductivity increases.
4. Conclusion
Successful synthesis of pristine NaNbO3 and Ta-doped NaNbO3 nanohierarchal building blocks was carried out by a simple hydrothermal route. XRD and electron microscopic investigations confirm the formation of highly crystalline, monophasic, typical single layered nanoflakes of pristine NaNbO3, and with an increase in percentage doping of Ta, thin layered nanosheet formation was restricted. The addition of Ta in NaNbO3 owned a strong influence on its band structure, which obviously improves the photocatalytic activity and dielectric properties of NaNbO3. The highest photocatalytic water splitting activity (21 μmol/g) and 89% removal of MB organic dye was observed in 10 mol % Ta-doped NaNbO3 as compared to pure NaNbO3. Similarly, in 5 mol % Ta-doped NaNbO3, an enhanced dielectric constant of 510 was observed. Improved photocatalytic activity and a dielectric constant of Ta-doped NaNbO3 was attributed to the improvement in the separation of photogenerated charge carriers, absorption ability, and structural changes due to Ta doping.
Acknowledgments
T.A. thanks the SPARC scheme (Grant SPARC/2018-2019/P843/SL) of Ministry of Education, Govt. of India for financial support. U.F. especially thanks UGC, New Delhi for the Research Fellowship. The authors also acknowledge the measurement support provided through the DST PURSE program at CIF, Jamia Millia Islamia, and AIIMS, New Delhi, for electron microscopic studies. Y.M. would like to thank the support by the IIT start-up funds. The authors extend their sincere appreciation to Researchers Supporting Project Number (Grant RSP-2021/29), King Saud University, Riyadh, Saudi Arabia.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c07250.
Figure S1, detailed view of hierarchitectures of NN, NN1, NN2, NN3, and NN4 samples and magnified micrographs of NN, NN1, NN2, NN3, and NN4 nanohierarchitectures; Figure S2, EDAX spectral analysis of as prepared samples; Table S1, atomic percentage and stoichiometry of as-synthesized samples obtained by using EDAX analysis; Figure S3 and S4, UV–visible DRS spectra, Kubelka–Munk plot of synthesized samples, and UV–visible spectra of dye solution in the presence of light and synthesized photocatalysts; Figure S5, LC-MS spectra of MB dye after photocatalysis; Figure S6, degradation fragments formed from MB dye after the photocatalytic degradation process; section S1, mechanism of the photocatalytic dye degradation process deduced by using different scavengers; Figure S7, variation of photocatalytic activity in the presence of different scavengers; and Figure S8, variation of conduction with frequency for as-synthesized samples at 100 °C (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Gust D.; Moore T. A.; Moore A. L. Mimicking photosynthetic solar energy transduction. Acc. Chem. Res. 2001, 34, 40–48. 10.1021/ar9801301. [DOI] [PubMed] [Google Scholar]
- Li P.; Ouyang S.; Xi G.; Kako T.; Ye J. The effects of crystal structure and electronic structure on photocatalytic H2 evolution and CO2 reduction over two phases of perovskite-structured NaNbO3. J. Phys. Chem. C 2012, 116, 7621–7628. 10.1021/jp210106b. [DOI] [Google Scholar]
- Alshehri S. M.; Ahmed J.; Ahamad T.; Alhokbany N.; Arunachalam P.; Al-Mayouf A. M.; Ahmad T. Synthesis, characterization, multifunctional electrochemical (OGR/ORR/SCs) and photodegradable activities of ZnWO4 nanobricks. J. Sol-Gel Sci. Technol. 2018, 87, 137–146. 10.1007/s10971-018-4698-7. [DOI] [Google Scholar]
- Shi H.; Chen G.; Zhang C.; Zou Z. Polymeric g-C3N4 coupled with NaNbO3 nanowires toward enhanced photocatalytic reduction of CO2 into renewable fuel. ACS Catal. 2014, 4, 3637–3643. 10.1021/cs500848f. [DOI] [Google Scholar]
- Ahmad T.; Farooq U.; Phul R. Fabrication and photocatalytic applications of perovskite materials with special emphasis on alkali-metal-based niobates and tantalates. Ind. Eng. Chem. Res. 2018, 57, 18–41. 10.1021/acs.iecr.7b04641. [DOI] [Google Scholar]
- Gu Q.; Zhu K.; Zhang N.; Sun Q.; Liu P.; Liu J.; Wang J.; Li Z. Modified solvothermal strategy for straightforward synthesis of cubic NaNbO3 nanowires with enhanced photocatalytic H2 evolution. J. Phys. Chem. C 2015, 119, 25956–25964. 10.1021/acs.jpcc.5b08018. [DOI] [Google Scholar]
- Tong H.; Ouyang S.; Bi Y.; Umezawa N.; Oshikiri M.; Ye J. Nano-photocatalytic materials: possibilities and challenges. Adv. Mater. 2012, 24, 229–251. 10.1002/adma.201102752. [DOI] [PubMed] [Google Scholar]
- Farooq U.; Phul R.; Alshehri S. M.; Ahmed J.; Ahmad T. Electrocatalytic and enhanced photocatalytic applications of sodium niobate nanoparticles developed by citrate precursor route. Sci. Rep. 2019, 9, 4488. 10.1038/s41598-019-40745-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehtab A.; Ahmed J.; Alshehri S. M.; Mao Y.; Ahmad T. Rare earth doped metal oxide nanoparticles for photocatalysis: A perspective. Nanotechnology 2022, 33 (1–31), 142001. 10.1088/1361-6528/ac43e7. [DOI] [PubMed] [Google Scholar]
- Fujishima A.; Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Farooq U.; Ahmed J.; Alshehri S. M.; Ahmad T. High-surface-area sodium tantalate nanoparticles with enhanced photocatalytic and electrical properties prepared through polymeric citrate precursor route. ACS Omega 2019, 4, 19408–19419. 10.1021/acsomega.9b02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq U.; Chaudhary P.; Ingole P. P.; Kalam A.; Ahmad T. Development of cuboidal KNbO3@α-Fe2O3 hybrid nanostructures for improved photocatalytic and photoelectrocatalytic applications. ACS Omega 2020, 5, 20491–20505. 10.1021/acsomega.0c02646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad T.; Phul R.; Khan H. Iron oxide nanoparticles: An efficient nano-catalyst. Curr. Org. Chem. 2019, 23 (9), 994–1004. 10.2174/1385272823666190314153208. [DOI] [Google Scholar]
- Mohan S.; Mao Y. Molten salt synthesized submicron perovskite La1-xSrxCoO3 particles as efficient electrocatalyst for water electrolysis. Front. Mater. 2020, 7, 117–126. 10.3389/fmats.2020.00259. [DOI] [Google Scholar]
- Abraham A.; Gupta S. K.; Mohan S.; Abdou H.; Mao Y. Defect induced optical and electrochemical properties of Pr2Sn2O7 nanoparticles enhanced by Bi3+ doping. J. Mater. Res. 2020, 35 (9), 1214–1224. 10.1557/jmr.2020.48. [DOI] [Google Scholar]
- Mortazavi-Derazkola S.; Zinatloo-Ajabshir S.; Salavati-Niasari M. Novel simple solvent-less preparation, characterization and degradation of the cationic dye over holmium oxide ceramic nanostructures. Ceram. Int. 2015, 41 (8), 9593–9601. 10.1016/j.ceramint.2015.04.021. [DOI] [Google Scholar]
- Ahmed J.; Ahamad T.; Alhokbany V.; Almaswari B. M.; Ahmad T.; Hussain A.; Al-Farraj E. S. S.; Alshehri S. M. Molten salts derived copper tungstate nanoparticles as bifunctional electro-catalysts for electrolysis of water and supercapacitor applications. ChemElectroChem. 2018, 5, 3938–3945. 10.1002/celc.201801196. [DOI] [Google Scholar]
- Naaz F.; Farooq U.; Khan M. A. M.; Ahmad T. Multifunctional efficacy of environmentally benign silver nanospheres for organic transformation, photocatalysis and water remediation. ACS Omega 2020, 5 (40), 26063–26076. 10.1021/acsomega.0c03584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phul R.; Khan M. A. M.; Sardar M.; Ahmed J.; Ahmad T. Multifunctional electrochemical properties of synthesized non-precious iron oxide nanostructures. Crystals 2020, 10 (1–14), 751. 10.3390/cryst10090751. [DOI] [Google Scholar]
- Jain S. K.; Fazil M.; Naaz F.; Pandit N. A.; Ahmed J.; Alshehri S. M.; Mao Y.; Ahmad T. Silver doped SnO2 nanostructures for photocatalytic water splitting and catalytic nitrophenol reduction. New J. Chem. 2022, 46, 2846–2857. 10.1039/D1NJ05432E. [DOI] [Google Scholar]
- Ahmed J.; Alam M.; Majeed Khan M.A.; Alshehri S. M. Bifunctional electro-catalytic performances of NiMoO4-NRs@RGO nanocomposites for oxygen evolution and oxygen reduction reactions. J. King Saud Univ. Sci. 2021, 33, 101317. 10.1016/j.jksus.2020.101317. [DOI] [Google Scholar]
- Kato H.; Asakura K.; Kudo A. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089. 10.1021/ja027751g. [DOI] [PubMed] [Google Scholar]
- Bajorowicz B.; Reszczyńska J.; Lisowski W.; Klimczuk T.; Winiarski M.; Słoma M.; Zaleska-Medynska A. Perovskite-type KTaO3–reduced graphene oxide hybrid with improved visible light photocatalytic activity. RSC Adv. 2015, 5, 91315–91325. 10.1039/C5RA18124K. [DOI] [Google Scholar]
- Wang X.; Chen G.; Zhou C.; Yu Y.; Wang G. N-doped Nb2O5 sensitized by carbon nitride polymer–synthesis and high photocatalytic activity under visible light. Eur. J. Inorg. Chem. 2012, 2012 (11), 1742–1749. 10.1002/ejic.201101285. [DOI] [Google Scholar]
- Kumar S.; Khanchandani S.; Thirumal M.; Ganguli A. K. Achieving enhanced visible-light-driven photocatalysis using type-II NaNbO3/CdS core/shell heterostructures. ACS Appl. Mater. Interfaces 2014, 6, 13221–13233. 10.1021/am503055n. [DOI] [PubMed] [Google Scholar]
- Farooq U.; Naz F.; Phul R.; Pandit N. A.; Jain S. K.; Ahmad T. Development of heterostructured ferroelectric SrZrO3/CdS photocatalysts with enhanced surface area and photocatalytic activity. J. Nanosci. Nanotechnol. 2020, 20, 3770–3779. 10.1166/jnn.2020.17516. [DOI] [PubMed] [Google Scholar]
- Modak B.; Modak P.; Ghosh S. K. Improving visible light photocatalytic activity of NaNbO3: a DFT based investigation. RSC Adv. 2016, 6, 90188–90196. 10.1039/C6RA15024A. [DOI] [Google Scholar]
- Jana P.; Mata Montero C.; Pizarro P.; Coronado J.M.; Serrano D.P.; de la Pena O'Shea V.A. Photocatalytic hydrogen production in the water/methanol system using Pt/RE:NaTaO3 (RE = Y, La, Ce, Yb) catalysts. Int. J. Hydrog. Energy 2014, 39, 5283–5290. 10.1016/j.ijhydene.2013.12.182. [DOI] [Google Scholar]
- Torres-Pardo A.; Jiménez R.; García-González E.; González-Calbet J. M. Phase coexistence in NaNb(1-x)TaxO3 materials with enhanced dielectric properties. J. Mater. Chem. 2012, 22, 14938–14943. 10.1039/c2jm32078a. [DOI] [Google Scholar]
- Yadav A.; Fahad M.; Satapathy S.; Sarun P. M. Effect of tantalum on the temperature dependent electrical characteristics of NaNb1-xTaxO3 (0.0 < x < 0.3) ceramics between 400–560 °C. J. Alloys Compd. 2019, 797, 902–911. 10.1016/j.jallcom.2019.04.142. [DOI] [Google Scholar]
- Fan M.; Hu B.; Yan X.; Song C.; Chen T.; Feng Y.; Shi W. Excellent visible-light-driven photocatalytic performance of Cu2O sensitized NaNbO3 heterostructures. New J. Chem. 2015, 39, 6171–6177. 10.1039/C5NJ00751H. [DOI] [Google Scholar]
- Khatoon S.; Ahmad T. Synthesis, optical and magnetic properties of Ni-doped ZnO nanoparticles. J. Mater. Sci. Eng. B 2012, 2 (6), 325–333. [Google Scholar]
- Ahmad T.; Ganguly A.; Ahmed J.; Ganguli A. K.; Al-Hartomy O. A. Nanorods of transition metal oxalates: A versatile route to the oxide nanoparticles. Arab. J. Chem. 2011, 4, 125–134. 10.1016/j.arabjc.2010.06.041. [DOI] [Google Scholar]
- Ahmad T.; Ganguli A. K. Synthesis of nanometer-sized particles of barium orthotitanate prepared through a modified reverse micellar route: structural characterization, phase stability and dielectric properties. J. Mater. Res. 2004, 19 (10), 2905–2912. 10.1557/JMR.2004.0406. [DOI] [Google Scholar]
- Salavati-Niasari M.; Ghanbari D.; Davar F. Synthesis of different morphologies of bismuth sulfide nanostructures via hydrothermal process in the presence of thioglycolic acid. J. Alloys Compd. 2009, 488, 442–447. 10.1016/j.jallcom.2009.08.152. [DOI] [Google Scholar]
- Ghanbari D.; Salavati-Niasari M.; Sabet M. Preparation of flower-like magnesium hydroxide nanostructure and its influence on the thermal stability of poly vinyl acetate and poly vinyl alcohol. Compos. B. Eng. 2013, 45, 550–555. 10.1016/j.compositesb.2012.09.007. [DOI] [Google Scholar]
- Salavati-Niasari M.; Davar F.; Loghman-Estarki M. R. Controllable synthesis of thioglycolic acid capped ZnS (Pn) 0.5 nanotubes via simple aqueous solution route at low temperatures and conversion to wurtzite ZnS nanorods via thermal decompose of precursor. J. Alloys Compd. 2010, 494, 199–204. 10.1016/j.jallcom.2009.10.265. [DOI] [Google Scholar]
- Zinatloo-Ajabshir S.; Morassaei M. S.; Amiri O.; Salavati-Niasari M. Green synthesis of dysprosium stannate nanoparticles using Ficus carica extract as photocatalyst for the degradation of organic pollutants under visible irradiation. Ceram. Int. 2020, 46, 6095–6107. 10.1016/j.ceramint.2019.11.072. [DOI] [Google Scholar]
- Zhu L. P.; Bing N. C.; Wang L. L.; Jin H. Y.; Liao G. H.; Wang L. J. Self-assembled 3D porous flowerlike α-Fe2O3 hierarchical nanostructures: synthesis, growth mechanism, and their application in photocatalysis. Dalton Trans. 2012, 41, 2959–2965. 10.1039/c2dt11822j. [DOI] [PubMed] [Google Scholar]
- Kruczek M.; Talik E.; Kania A. Electronic structure of AgNbO3 and NaNbO3 studied by X-ray photoelectron spectroscopy. Solid State Commun. 2006, 137, 469–473. 10.1016/j.ssc.2006.01.001. [DOI] [Google Scholar]
- Deng Q.; Li M.; Wang J.; Zhang P.; Jiang K.; Zhang J.; Hu Z.; Chu J. Exploring optoelectronic properties and mechanisms of layered ferroelectric K4Nb6O17 nanocrystalline films and nanolaminas. Sci. Rep. 2017, 7, 1883. 10.1038/s41598-017-01838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumata K.; Cordonier C. E. J.; Shichi T.; Fujishima A. Photocatalytic activity of NaNbO3 thin films. J. Am. Chem. Soc. 2009, 131, 3856–3857. 10.1021/ja900394x. [DOI] [PubMed] [Google Scholar]
- An L.; Onishi H. Electron–hole recombination controlled by metal doping sites in NaTaO3 photocatalysts. ACS Catal. 2015, 5, 3196–3206. 10.1021/acscatal.5b00484. [DOI] [Google Scholar]
- Liu Q.; Zhang Q.; Zhang L.; Dai W.-L. Highly efficient single-crystalline NaNb1-XTaXO3 (x = 0.125) wires: The synergistic effect of tantalum-doping and morphology onphotocatalytic hydrogen evolution. J. Mater. Sci. Technol. 2020, 54, 20–30. 10.1016/j.jmst.2020.05.006. [DOI] [Google Scholar]
- Thommes M.; Kaneko K.; Neimark A. V.; Olivier J. P.; Rodriguez-Reinoso F.; Rouquerol J.; Sing K. S. W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- Kumar S.; Yadav N.; Kumar P.; Ganguli A. K. Design and comparative studies of Z-Scheme and type II based heterostructures of NaNbO3/CuInS2/In2S3 for efficient photoelectrochemical applications. Inorg. Chem. 2018, 57, 15112–15122. 10.1021/acs.inorgchem.8b02264. [DOI] [PubMed] [Google Scholar]
- Phul R.; Shrivastava V.; Farooq U.; Sardar M.; Kalam A.; Al-Sehemi A. G.; Ahmad T. One pot synthesis and surface modification of mesoporous iron oxide nanoparticles. Nano-Struct. Nano-Objects 2019, 19 (1–6), 100343. 10.1016/j.nanoso.2019.100343. [DOI] [Google Scholar]
- Singh Vig A.; Rani N.; Gupta A.; Pandey O.P. Influence of Ca-doped NaNbO3 and its heterojunction with g-C3N4 on the photoredox performance. Sol. Energy 2019, 185, 469–479. 10.1016/j.solener.2019.04.088. [DOI] [Google Scholar]
- Xu J.; Feng B.; Wang Y.; Qi Y.; Niu J.; Chen M. BiOCl decorated NaNbO3 nanocubes: A novel p-n heterojunction photocatalyst with improved activity for ofloxacin degradation. Front. Chem. 2018, 6, 393–402. 10.3389/fchem.2018.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad T.; Ubaidullah M.; Lone I. H.; Kumar D.; Al-Hartomy O. A. Microemulsion synthesis, structural characterization and dielectric properties of Ba1-xPbxZrO3 (0.05 ≤ x ≤ 0.20) nanoparticles. Mater. Res. Bull. 2017, 89, 185–192. 10.1016/j.materresbull.2017.01.044. [DOI] [Google Scholar]
- Koops C. G. On the dispersion of resistivity and dielectric constant of some semiconductors at audiofrequencies. Phys. Rev. 1951, 83, 121–124. 10.1103/PhysRev.83.121. [DOI] [Google Scholar]
- Kwon D.; Goh Y.; Son D.; Kim B.; Bae H.; Perini S.; Lanagan M. Temperature-and frequency-dependent dielectric properties of sol–gel-derived BaTiO3-NaNbO3 solid solutions. J. Electron. Mater. 2016, 45, 631–638. 10.1007/s11664-015-4162-1. [DOI] [Google Scholar]
- Atamanik E.; Thangadurai V. Dielectric properties of Ga-doped Na0.5K0.5NbO3. J. Phys. Chem. C 2009, 113, 4648–4653. 10.1021/jp809905u. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.