Abstract
Introduction:
Animal carcasses differ in composition from other types of solid waste, and through prior testing it was determined that cycle parameters applied to general, solid biohazardous waste did not ensure proper sterilization of ferret carcasses.
Objectives:
The goals of this study were to develop and validate an autoclave cycle that would ensure the decontamination of infectious animal carcasses before removal from an animal biosafety level 2/3 containment suite for downstream disposal and to test different ways to prepare and package animal carcasses for autoclaving.
Methods:
Intact ferret carcasses were implanted with biological indicators, and the carcasses were placed in biohazard bags, then into metal pans. To test the efficacy of the autoclave cycle on larger biomasses, 1, 2, or 4 ferret carcasses were placed in a biohazard bag. A total of 4 carcasses were placed in each pan. An autoclave cycle was created to begin the study. After initial tests, minor modifications to the initial test cycle parameters were made, and a new cycle was validated for ferret carcasses up to 2 kg each. Parameters for the validated cycle were as follows: sterilization time 240 minutes, temperature 125°C, 5 prevacuum pulses, and chamber pressure 15 psi.
Results:
The results of this study indicate that an extended sterilization time is required to successfully decontaminate animal carcasses compared with regular, solid, and biohazardous waste.
Conclusions:
This study demonstrates that it is possible to sterilize multiple intact ferret carcasses per load under validated autoclave cycle conditions.
Keywords: animal biosafety, biocontainment, sterilization, biosafety level 3, decontamination
The Regional Biocontainment Laboratory (RBL) at Duke University includes approximately 6,525 ft2 of biosafety level 3 (BSL-3) containment space. Construction of the RBL was funded primarily by the National Institute of Allergy and Infectious Disease to support basic and translational research to make drugs, vaccines, and diagnostics to protect society from emerging infections and biothreats. Work is currently conducted with virulent strains of Mycobacterium tuberculosis, Yersinia pestis, Francisella tularensis, West Nile virus, yellow fever virus, influenza virus, and other pathogens.
The RBL incorporates multiple enhancements over standard BSL-3 containment, including a double-door changing room at the BSL-3 facility entrance for gown-in, individual anterooms for each BSL-3 laboratory and animal suite, shower-out capacity for laboratory and animal suites, effluent waste treatment for the whole BSL-3 containment area, HEPA filtration of all exhaust air from the BSL-3 containment area, a ventilation control system to prevent positive pressurization of BSL-3 rooms, autoclaves for each laboratory and animal suite, redundant bulk autoclaves for animal cages and waste, HEPA-filtered negative-pressure cage racks, all BSL-3 room penetrations confirmed to be sealed, and a validated vaporized hydrogen peroxide decontamination sequence for laboratory and animal suites.1,2
Pathogenesis studies and experimental vaccine and therapeutic testing with well-characterized small-animal challenge models are a significant component of the RBL’s research portfolio. Currently, mice, rabbits, and ferrets are used humanely and in full accordance with Duke’s Institutional Animal Care and Use Committee (IACUC) requirements for research purposes. Safe and compliant processing of animal carcasses is a critical component of daily operations at the RBL. Animal experiments involving risk group 3 infectious materials, including select agents, are assigned to animal BSL-3 (ABSL-3) containment. Infectious carcasses are autoclaved within the animal suite where the experimental procedures are conducted and then transported to an adjacent building, which is not a high-containment facility, for alkaline hydrolysis tissue digestion. This practice is consistent and compliant with federal guidelines, which communicate an expectation to decontaminate all potentially infectious materials (including animal tissues and carcasses) by an appropriate method before removal from the area where infectious materials are manipulated.1 In addition, this practice is consistent with the Federal Select Agent Program’s requirements for restricting access to select agents and toxins. Carcasses infected with a select agent may be possessed (eg, ability to carry, use, or manipulate) only by those individuals currently approved under the federal laws governing select agents (42 CFR Part 73 and 9 CFR Part 121).3
Animal carcasses differ in composition from other types of solid waste, and through previous testing at the RBL, it was determined that the standard cycle parameters used for solid biohazardous waste (sterilization time 90 minutes, temperature 121°C, 3 prevacuum pulses, chamber pressure 15 psi) did not ensure proper sterilization of intact ferret carcasses. A literature search yielded few results on suggested autoclave cycles sufficient to sterilize ferret or ferret-sized carcasses. Previous studies using other small and medium-sized carcasses did provide a starting point for selecting possible cycle times and temperatures.4,5 As such, the purpose of the study presented in this report was to determine and validate an autoclave cycle that would ensure the sterilization of infectious ferret carcasses before removal from the biohazard containment area for downstream disposal.
Methods
Ferret Carcasses
Ferret carcasses were obtained after use and euthanasia for purposes directly related to their respective IACUC protocols, and therefore no IACUC approval was required for this postmortem study.
Biological Indicators
All cycles tested in this study were validated using ProSpore Geobacillus stearothermophilus, 106 spores/vial (Mesa Laboratories, Omaha, NE), biological indicators (BIs). After each autoclave cycle, the BIs were incubated in a 55°C incubator for 48 hours. Positive growth was indicated by turbidity and a color change from purple to yellow. Negative growth, indicating a successful cycle, was shown by no turbidity and a purple color. In all experiments, the BIs not subjected to autoclave cycle showed a turbid, yellow color after incubation at 55°C for 48 hours. The BIs that were negative for growth did show some darker purple or brown coloring, but this discoloration was due to the caramelization of the growth medium from extended exposure to high heat and does not affect the ability of G stearothermophilus to grow.6
Water Tests
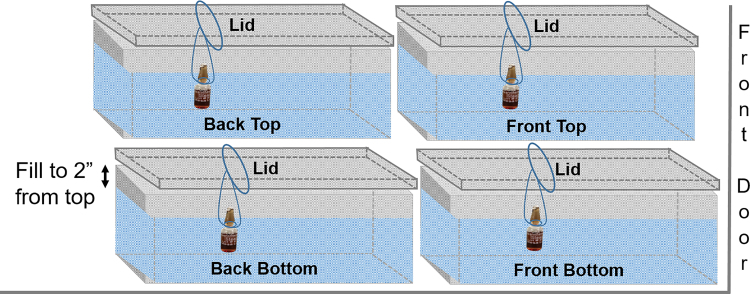
A steam sterilizing autoclave (model PSS5-B-MSDD, Primus Sterilizer, Omaha, NE) was validated in this study. The cycle parameters (cycle A, Table 1) were first tested using 21-qt, 22-gauge stainless steel pans (20 in width × 12 in length × 6 in height; Central Restaurant Products, Indianapolis, IN) filled with cold tap water to within 2 inches of the top of the pan. The pan lids were placed upside down on each pan; this placement allowed a piece of string to be looped around the pan lid. The string was used to suspend a BI halfway into the pan (Figure 1). Four pans were placed in the autoclave, stacked 2 on 2, and the cycle was run.
Table 1.
Autoclave Parameters Tested.
| Cycle Name | Experiment | Sterilizing Temperature, °C | Pulse Pressure, psi | Number of Prevacuum Pulses | Sterilizing Time, min |
|---|---|---|---|---|---|
| Cycle A | Water pan tests and ferret test 1 | 123.1 | 20 | 3 | 240 |
| Cycle B | Ferret tests 2 and 3 | 125 | 15 | 5 | 240 |
Figure 1.
Configuration of water pans in the autoclave. Pans were stacked one on top of the other with the biological indicators suspended halfway into the water.
Often carcasses are frozen for multiple reasons prior to autoclaving. To mimic the conditions of frozen carcasses, the pans were filled with cold tap water and placed in a –80°C chest freezer until frozen. The pans were removed from the freezer, and a drill was used to core a 1-inch diameter hole, approximately 6 inches deep, into the center of the ice. The hole was then filled with cold tap water and the BI suspended inside the hole as described for the water tests. The pans were configured inside the autoclave as indicated in Figure 1, and cycle A was used for the initial water test.
Ferret Carcass Test 1
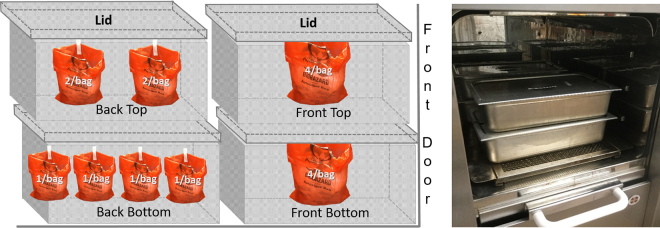
Sixteen ferret carcasses, each weighing approximately 1.5 to 2 kg, were used to validate autoclave cycle A. BIs were labeled and implanted into the carcasses (Figure 2). To test different bag-load capacities, 1, 2, or 4 carcasses were placed inside 2 autoclave bags. The bags were placed inside the steel pans, to a total of 4 bagged carcasses per pan (Figure 3). The 4 pans were placed inside the autoclave (Figure 4), and the cycle was run (cycle A, Table 1).
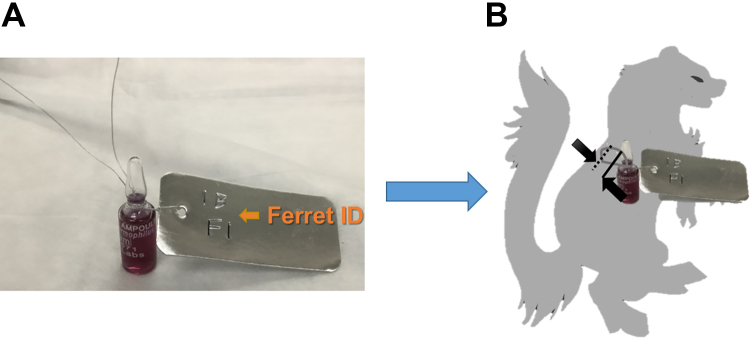
Figure 2.
A 1- to 2-inch incision was made between the ribs on either side of the thoracic vertebrae, approximately 2 to 3 inches below the neck area (opposite the chest/abdominal cavity area). Scalpels were used to cut into the flesh and through to the chest cavity, taking care to minimize the width of the incision hole. To implant the biological indicators (BIs) inside the abdominal cavity of the carcass, a flexible wire write-on metal tag (VWR, Radnor, PA) was wrapped around the BI (A), then the BI was fed through one of the incisions made on the back of the carcass and fixed into place by twisting the wire around the spine through the second incision hole (B).
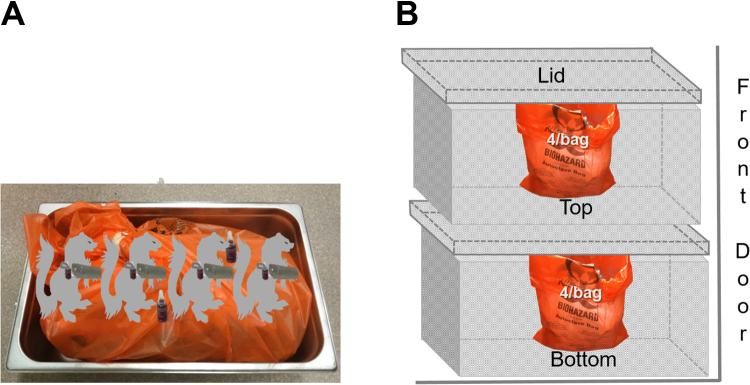
Figure 3.

Placement of ferret carcasses inside pans. Each pan contained 4 carcasses with either (A) a single carcass per bag, (B) 2 carcasses per bag, or (C) 4 carcasses per bag. Each carcass was implanted with a biological indicator (BI). An additional BI was placed between the 2 middle carcasses in the 4-per-bag configuration.
Figure 4.
Configuration of pans within the autoclave. The 4-per-bag carcass pans were placed one on top of the other at the front of the autoclave. The 2-per-bag carcass pan was placed on top of the single-bagged carcass pan at the back of the autoclave. The picture on the right shows how much space the pans use within the autoclave.
Once the cycle was completed, the pans were removed from the autoclave and allowed to cool for 4 hours. The BIs were removed from the carcasses and cleaned with 70% isopropanol, dirty tags were removed and replaced with fresh labeled tape, and BIs were incubated for 48 hours at 55°C.
Ferret Carcass Tests 2 and 3
For test 2, 8 carcasses were prepared as indicated above. Four carcasses were placed inside a double bag and placed inside a steel pan. Two BIs were placed between the carcasses (Figure 5). On the basis of the results of the first carcass test, an augmented cycle (cycle B, Table 1) was established.
Figure 5.
Configuration of carcasses in autoclave for second ferret test. (A) Four carcasses were placed in a double-bag with 2 biological indicators placed between the carcasses. (B) Two pans, each containing 4 carcasses, were stacked and placed inside the autoclave.
For test 3, a 6-inch section of a 25-mL disposable serological pipette (with a diameter slightly wider than the BIs) was placed inside the chest cavity of each carcass to maintain a spot to place the BI after freezing. Eight carcasses were first frozen by placing in a –80°C freezer overnight (a minimum of 8 hours). The carcasses were removed from the freezer, a BI was placed inside the chest cavity through the pipette, and 4 carcasses were placed inside a double bag and into a metal pan. Two BIs were placed between the carcasses, and autoclave cycle B was run as indicated for test 2 (Figure 5).
Results
Water Tests
All of the test BIs from both the liquid and frozen water tests showed no indication of growth after the autoclave cycle, therefore validating these conditions for testing in actual carcasses.
Ferret Carcass Test 1
Of the 18 test BIs that were exposed to the sterilization cycle, 17 passed validation according to the established criteria. The 1 BI that did show growth was placed between the carcasses from the 4-per-bag configuration in the top front pan (Figure 6).
Figure 6.
Results of ferret carcass test 1. (A) The biological indicator (BI) shown in the red circle failed the sterilization cycle. The control vial is shown at the top right. (B) The location of the failed BI is indicated.
Ferret Carcass Tests 2 and 3
Because of the failed BI from test 1, the validation cycle was altered by increasing the temperature of sterilization from 123.1°C to 125°C, decreasing the pressure of the prevacuum pulses from 20 to 15 psi, and increasing the number of prevacuum pulses to 5 from 3 (see Table 1 for cycle comparison). After completion of the sterilization cycle for both the fresh and frozen carcasses, all test BIs passed and showed no growth. After the sterilization cycle from test 3, the section of serological pipette that was embedded in each carcass was slightly deformed because of the exposure to high temperatures. The BIs that were inside the pipettes remained intact but could not be extracted from the pipettes (Figure 7). The pipette-containing BIs were incubated as is for 48 hours.
Figure 7.
Results of frozen carcass test. (A) On the left, the control biological indicator (BI) exhibited growth (turbid, yellow color) as expected. On the right, the BIs placed between the carcasses exhibited no growth. (B) The BIs placed inside the pipettes exhibited no growth. Shown here is how the pipette deformed and melted around the BI.
Discussion
Decontaminating potentially infectious waste, including carcasses, is critical in a high-containment (ABSL-3) area, and full sterilization of waste is imperative if select agents are involved.3 The parameters should be validated to ensure that laboratories are achieving these goals of their decontamination and sterilization protocols. The validated solid-waste cycle that is typically used in the ABSL-3 containment area is sufficient for most noncarcass solid-waste loads, but previous studies conducted at this facility showed that it was insufficient to inactivate BIs placed inside ferret carcasses (data not shown). The importance of running validation studies to confirm decontamination of new and different types of waste is demonstrated here. It is also important to note that users must be familiar with their specific autoclaves and be familiar with manufacturers’ recommendations for selecting and programming cycles.
Ideally, decontamination and sterilization protocols will be validated prior to initial work with the infectious substances and then confirmed with the agent of interest or a proper analog in the actual conditions for which the protocols are being developed. Identifying a proper model for decontamination and sterilization protocols can prove difficult.4 Studies with mice generally provide abundant carcasses with which to work, but medium-sized to large animal models are quite expensive and as such are performed more rarely with much smaller animal numbers. We would prefer to be able to perform these validation experiments cheaply and without the need for animal carcasses. The water tests described above used physical volumes well in excess that of the ferret carcasses, and testing BIs always proved successful in validating the cycle, even in frozen ice blocks.
However, testing in carcasses was not successful with our first cycle in even room-temperature carcasses. One of the BIs placed between the carcasses failed, which we speculate may have been the result of an insulated air pocket created from overlapping fur between 2 of the carcasses. If the BI was located in this air pocket, the steam may not have fully penetrated this area, resulting in viable G stearothermophilus spores. Another possibility is that the double bags used to contain the carcasses could have created insulating air pockets in the space between the bags or between the bags and the carcasses. Previous studies have shown that air trapped in a load can create insulating pockets that interfere with thermal transfer and result in longer cycle times needed to reach sterilizing temperatures.7,8 Once 2 extra prevacuum pulses were added to the decontamination cycle, all of the BIs passed. Adding extra pulses has been previously shown to reduce the equilibration time needed for thermocouples placed within dry goods loads9 and, in this study, could have better allowed the steam to reach any air pockets between the ferret carcasses. It is also important to note that the physics of thermal conductivity of the tissues and bones compared with that of water may also account for these differences, but this setback highlights the need for better medium-sized to large animal decontamination and sterilization validation models.
The placement of the BIs inside the carcasses was carefully considered. The smallest incisions possible were made to keep the carcasses intact and to mimic an unaltered, whole carcass as much as possible while still adhering to approved euthanasia secondary measure procedures. By keeping the carcasses as intact as possible, the manipulation of infectious carcasses is reduced, therefore mitigating the risk for exposure to personnel.
The decision to test frozen carcasses was made to allow future experiments in which same-day autoclaving of waste is not possible and infectious carcasses must be stored in the containment area for a period of time. Unfortunately, these BIs could not be frozen with the carcasses, because the type of indictor used is no longer efficacious once frozen and could have resulted in a false negative.10 One shortcoming of our method to use the 25-mL serological pipette is that during autoclaving, the BI is not completely encapsulated in the frozen carcass with the open tube allowing access of steam to the BI. Depending on when the pipette tube deformed, this could have sealed off the BI and possibly created a better simulation of the BIs that were sealed inside the nonfrozen carcasses. One follow-up for this study would be to identify a method of either including a freezer-stable BI with the carcass before freezing or another form of postfreeze measurement (ie, a real-time temperature probe to ensure that temperature is achieved at the proper length of time). Another possibility could be to seal off the 25-mL serological pipette with the BI inside before beginning the autoclave cycle.
Future directions include repeating the ferret test studies to ensure the results can be repeated. Finding a better method of implanting the BIs into frozen carcasses is also being explored. Additional studies are planned to look at increasing the number of carcasses and pans per load. Testing the cycles described here with other types of carcasses (eg, rabbits and other medium-sized to large animals) is also planned.
Conclusions
The autoclave cycles described here were validated for a total load of 16 ferret carcasses (of approximately 1.5-2 kg each) with up to 4 carcasses per stainless steel pan and 4 pans per load. The cycle was also validated with previously frozen ferret carcasses, demonstrating that the chosen cycle is sufficient to sterilize even fully frozen carcasses.
Acknowledgments
We would like to thank Elizabeth Fallon for her administrative assistance and Doug Elliott for his technical assistance.
Authors’ Note
All authors contributed equally to this work.
Ethical Approval Statement
Not applicable
Statement of Human and Animal Rights
No live animals or human subjects were involved in this study.
Statement of Informed Consent
Not applicable
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosure
Animal studies were done in the Duke RBL, which received partial support for construction from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (UC6-AI058607).
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Prior Presentation
This work was presented at the 62nd annual biosafety and biosecurity conference (ABSA International), held in Charleston, South Carolina, on October 17, 2018.
References
- 1. Centers for Disease Control and Prevention, Office of Safety, Health and Environment. Biosafety in Microbiological and Biomedical Laboratories (BMBL). 5th ed. 2009. Available at: https://www.cdc.gov/biosafety/publications/bmbl5/ . Accessed January 19, 2019.
- 2. World Health Organization. Laboratory Biosafety Manual. 3rd ed. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 3. Federal Select Agent Program. 2003. Select agents regulations. 42 CFR 73, 9 CFR 121. https://www.selectagents.gov/regulations.html. Accessed February 2019.
- 4. Santacroce JC, Swearengen J, Weaver P. Novel approach for validating autoclave cycles for biomass in BSL-3/-4. Appl Biosaf. 2015;20(3):141–145. [Google Scholar]
- 5. Vijayan V, Ng B. Validating waste management equipment in an animal biosafety level 3 facility. Appl Biosaf. 2016;21(4):185–192. [Google Scholar]
- 6. Mesa Laboratories. ProSpore Media Integrity Study. 2009. Available at: https://biologicalindicators.mesalabs.com/wp-content/uploads/sites/31/2014/02/ProSpore-Media-Integrity.16SEP13.pdf. Accessed January 19, 2019.
- 7. Ozanne G, Huot R, Montpetit C. Influence of packaging and processing conditions on the decontamination of laboratory biomedical waste by steam sterilization. Appl Environ Microbiol. 1993;59(12):4335–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bearss JJ, Honnold SP, Picado ES, Davis NM, Lackemeyer JR. Validation and verification of steam sterilization procedures for the decontamination of biological waste in a biocontainment laboratory. Appl Biosaf. 2017;22(1):33–37. [Google Scholar]
- 9. Shah S, Alayli S, Peng Y. Factors affecting measurement of equilibration time of dry goods loads in autoclaves. PDA J Pharm Sci Technol. 2019. doi:10.5731/pdajpst.2018.009225. [DOI] [PubMed] [Google Scholar]
- 10. Bojanski A. Taking the “mystery” out of biological indicator storage. Spore News. 2014;11(1):1–2. [Google Scholar]