Abstract
Introduction:
Formaldehyde is still the method of choice for fumigation of rooms and HEPA filters at high- and maximum-containment facilities because of its proven track record and low cost. However, formaldehyde has been shown to be carcinogenic and should ideally be replaced by other, less hazardous methods. This change has in part been hampered by the relatively high cost of alternative methods.
Methods:
Here, we provide examples of room fumigations using aerosolized hydrogen peroxide showing not only that it can be used economically but also that it is a versatile method and may be used under circumstances not normally suited for fumigation.
Results and Discussion:
Four examples of fumigation setups are presented that illustrate the versatility, ease of use, and adaptability of aerosolized hydrogen peroxide as a fumigant. In addition, we demonstrate that aerosolized hydrogen peroxide passes through HEPA filters in biological safety cabinets and individually ventilated cage racks.
Conclusions:
Considering that the fumigation method presented here is simple and highly effective, we expect it to serve as a relatively cost-effective alternative to formaldehyde fumigation for disinfecting potentially contaminated rooms and surfaces.
Keywords: hydrogen peroxide fumigation, formaldehyde fumigation, versatility, economical, leaky rooms
Formaldehyde (FA) has been the method of choice for room and equipment fumigation for decades.1 Besides its ease of use, a major contributor to the success of FA as a fumigant has been its low cost. A number of facilities worldwide simply use electrical frying pans to boil off FA and to release it inside the fumigation zone (G. Smith, PhD, Australian Animal Health Laboratory, personal communication, February 2010), keeping equipment costs well below US $100. Even commercial generators will not cost more than a few thousand dollars. This is in stark contrast to equipment costs for hydrogen peroxide vapor or chlorine dioxide, which are sometimes well in excess of US $50,000.
Regardless of cost, FA is known to be a human sensitizer and carcinogen and can leave undesirable residues if its vapor is poorly delivered or not evacuated from the treated area within a defined period of time.2,3 In addition, decisions at the European Union level, under the Biocidal Products Directive (Directive 98/8/EC, 1998), have led to restrictions on its use. Thus, alternative methods have been developed and tested recently, including hydrogen peroxide as either vapor or aerosol.4 -10 Contrary to FA, hydrogen peroxide produces nontoxic by-products (water and oxygen) and thus is ecologically safer, is less hazardous to personnel, and requires no postprocess neutralization or cleaning. As stated previously, the equipment used for hydrogen peroxide fumigation can be costly compared with FA.4,8,10 In addition, fumigation cycles may have to be set up individually in sometimes lengthy processes for every room and every situation. Furthermore, technical design specifications play an important role in the success of the fumigation.8
Hydrogen peroxide, either in its vapor or aerosolized form, has been shown to be just as effective as FA for decontaminating Foot-and-Mouth Disease Virus.10 In addition, several manufacturers now offer equipment in the same price range as FA generators, thus making hydrogen peroxide fumigation more economic. Ultimately, the versatility of hydrogen peroxide as a fumigant may well be its trump card. Here, we would like to demonstrate this versatility by presenting successful fumigations of different room setups, some of which could not be fumigated using traditional methods such as FA. It is this versatility, we believe, that will make aerosolized hydrogen peroxide the fumigant of choice for a number of institutions in the future.
Materials and Methods
Fumigation Setup for a New Rodent Facility
A newly refurbished rodent facility was fumigated prior to operation. The facility was divided into 5 fumigation zones (Figure 1). None of the rooms could be isolated from the heating, ventilation, and air conditioning (HVAC) system via air-tight dampers, and none of the doors were air tight. Thus, supply and exhaust air vents as well as doors were sealed using duct tape. During renovation, walls and ceilings were painted using dispersion paint, which does not confirm with international standards for laboratory wall coatings.11 Dispersion paint does not provide a smooth and easy-to-clean surface, is not impermeable to liquids, and is said to be nonresistant to H2O2. The rooms contained various pieces of equipment including individually ventilated cage (IVC) racks (without cages) and their respective ventilator units including the HEPA filters on the air inlets (Blue Line, Tecniplast, Buguggiate, Italy).
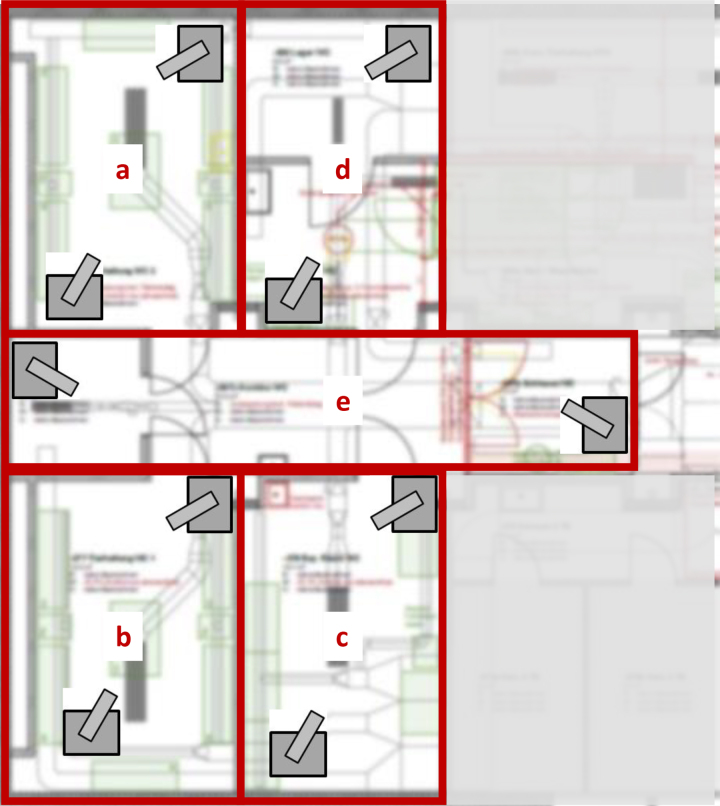
Figure 1.
Setup of fumigation zones for the new rodent facility. The facility was divided into 5 fumigation zones (a-e) ranging from 50 to 70 m3 in volume. Zone (a) contained 3 individually ventilated cage (IVC) racks with their ventilator units and a table; zone (b) was equipped with 2 IVC racks and their ventilator units as well as a table; zone (c) contained cupboards, tables, and chairs; zone (d) consisted of 2 rooms connected via a door and was equipped with a table, cage racks, and a autoclave loading trolley; while zone (e) consisted of a small storage room, the corridor, and the airlock. Aerosolized H2O2 was generated inside the fumigation envelopes. The generators were placed on pedestals facing each other. Biological indicator (BI) pouches were placed in all 4 corners on floor and ceiling levels as well as in and around equipment or furniture present in each zone. Thirteen BIs were placed in zone (a) and (b), 17 in zone (c), 20 in zone (d), and 28 in zone (e).
H2O2 aerosol fumigation was performed by placing 2 generators (Q-Jet Superior, Sanosil AG, Hombrechtikon, Switzerland) inside the fumigation zone on pedestals approximately 800 mm above floor level at opposite ends (Figure 1). Uniform distribution of the aerosol was achieved by either letting the IVC ventilator (where available) run during fumigation and/or by directing the spray of the 2 generators toward each other.
Fumigation Setup for 2 Climate Chambers
Two climate chambers for the cultivation of plants had to be fumigated because of prior fungal contamination. None of the rooms could be isolated from the HVAC system via air-tight dampers, nor were any of the doors air tight. One climate chamber aerated only via an overflow grid at the door into the adjacent corridor. Thus, rooms were sealed using duct tape as described above.
The rooms contained several wall racks as well as smaller pieces of equipment (Figure 2). In addition, each room was equipped with a circulating air-conditioning unit that had to be decontaminated as well.
Figure 2.

Setup of fumigation zones for the climate chambers. The facility was divided into 2 fumigation zones (a-b), each approximately 50 m3 in volume. Both zones contained several wall racks as well as a circulating air-conditioning unit. Zone (a) could be only passively aerated as the room was not connected to the exhaust ventilation system but instead aerated into the adjacent corridor via an overflow grid in the door. Aerosolized H2O2 was generated inside the fumigation envelopes. The generators were placed on pedestals facing each other. Biological indicator (BI) pouches were placed in all 4 corners on floor and ceiling levels as well as along shelves of wall racks at different levels. Fifteen BIs were placed in zone (a) and 13 in zone (b).
The H2O2 aerosol fumigation was performed by placing 2 generators (Q-Jet Superior, Sanosil AG) inside the fumigation zone on pedestals approximately 800 mm above floor level at opposite ends (Figure 2). Uniform distribution of the aerosol was achieved by letting the air-conditioning unit run during fumigation and by directing the spray of the 2 generators toward each other.
Fumigation Setup for a BSL-3 Laboratory and Its Ante-room
A Biosafety Level 3 (BSL-3) laboratory and its ante-room at the Institute of Veterinary Bacteriology at the Vetsuisse Faculty of the University of Bern, Switzerland, were fumigated prior to a maintenance shut down. The rooms could not be isolated from the HVAC system using air-tight dampers. Instead, duct tape was used to seal supply and exhaust air vents. Furthermore, the rooms were not fitted with air-tight doors. Thus, the door was sealed using duct tape as well. The facility’s walls and ceiling were coated with dispersion paint. The laboratory contained 1 IVC rack (including cages) connected to a ventilator unit equipped with a HEPA filter for the inlet air (Blue Line, Tecniplast, Buguggiate, Italy), a biological safety cabinet (BSC; Skan, Allschwil, Switzerland), and several larger pieces of equipment, such as freezers, incubators, and an autoclave (Figure 3). The ante-room contained cupboards and a sink. The door between the laboratory and the ante-room was left open during fumigation.
Figure 3.
Setup of fumigation zone for the BSL-3 laboratory and its ante-room. The 2 rooms have a combined volume of approximately 60 m3, were equipped with 1 individually ventilated cage (IVC) rack and its ventilator unit, a biological safety cabinet (BSC), and several larger pieces of equipment such as freezers, incubators, and an autoclave as well as cupboards in the ante-room. Aerosolized H2O2 was generated inside the fumigation envelope. The generators were placed on pedestals in the laboratory as well as the ante-room. Biological indicator (BI) pouches were placed in positions 1 to 9 (floor) and 10 to 18 (ceiling). In addition, 2 BIs were placed in the cupboards in the ante-room (19-20), 4 in and around the BSC (21-24), 4 inside IVC cages (25-28), 4 in and around the ventilator unit including the HEPA filter (29-32), 3 around the laboratory (33-35), and 1 each on the windows of the laboratory and the ante-room, respectively (36-37).
The H2O2 aerosol fumigation was performed by placing 1 generator (Q-Jet Superior, Sanosil AG) inside the laboratory and 1 inside the ante-room on pedestals approximately 800 mm above floor level (Figure 3). Uniform distribution of the aerosol was achieved by placing 2 ventilators (Modell Steba Standventi VT5) facing the generators inside the laboratory. In addition, the BSC as well as the IVC ventilator unit were left running during fumigation.
Fumigation Setup for 2 Connected BSL-3 Units
Two connected BSL-3 units at the Institute of Virology and Immunology in Mittelhäusern, Switzerland, had to be fumigated before starting yearly maintenance work. Both units had epoxy-coated walls, floors, and ceilings and stainless-steel doors with silicon gaskets. They could be isolated individually from the HVAC system via air-tight dampers, and all doors were air tight. The 2 BSL-3 laboratories, fully equipped with BSCs, CO2 incubators, –70°C freezers, and additional equipment, were connected via a shared inner change room (Figure 4).
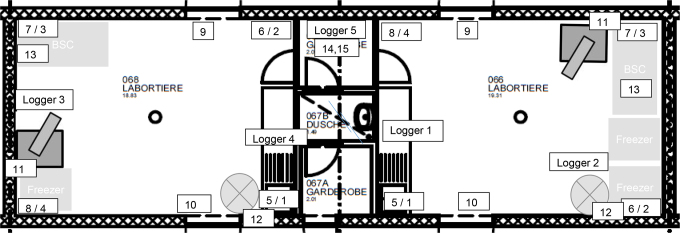
Figure 4.
Setup of fumigation zone for the BSL-3 laboratory. The 2 connected rooms are approximately 19 m3 in volume each, equipped with 1 biosafety cabinet each and 3 freezers in total. Aerosolized H2O2 was generated inside the fumigation envelope. In each room, the generator was placed in a corner. Ventilators were used to aid in the distribution of the aerosolized H2O2. Biological indicator (BI) pouches were placed in positions 1 to 4 (ceiling, 106) and 5 to 8 (floor, 104, 105, and 106 spores) in both rooms. In addition, 2 BIs were placed on the door windows to the corridors (9-10, 106 spores), 1 behind the Q-Jet (11, 106 spores), 1 behind the ventilator (12, 106 spores), 1 underneath the work surface of the BSC (13, 104, 105, and 106 spores), and 2 in the shared inner wardrobe (14 and 15, 106 spores). H2O2 chemical indicators were placed with every BI. The temperature/humidity loggers were placed on the floor in both rooms and 1 in the shared inner wardrobe.
One H2O2 generator (Q-Jet Superior, Sanosil AG) was placed in every laboratory in a way that the nozzle of the generator was directed toward the connecting change room (Figure 4). In addition, a ventilator (Modell Steba Standventi VT5) was placed in every room in a way that the ventilator was directed across the H2O2 stream. The BSCs were left running throughout the fumigation to aid with the distribution of the aerosol as well as to allow for fumigation of the BSCs.
Program Cycles
A 2-step cycle was used for the fumigation of the BSL-3 laboratories in Mittelhäusern: injection of 7.5% H2O2 (Sanosil S015, Sanosil AG) to achieve an end concentration of 12 mL/m3, holding time of 60 minutes, followed by another injection of 7.5% H2O2 and a 2-hour holding time. Following the final holding time, supply and exhaust dampers were opened to purge the rooms using the building’s HVAC system for approximately 18 hours.
The cycle was based on previous experiments validating H2O2 fumigation for Foot-and-Mouth Disease Virus.10
A 3-step cycle was used for the fumigation of the rodent facility, the BSL-3 laboratory at the Vetsuisse Faculty, and the climate chambers: injection of 7.5% H2O2 (Sanosil S015, Sanosil AG) to achieve an end concentration of between 10 mL/m3 and 14 mL/m3, holding time of 45 minutes, repeat of the initial injection and holding time, followed by another injection of 7.5% H2O2 and a 1-hour holding time. Following the final holding time, the fumigation zone was accessed in full personal protective equipment (PPE) consisting of a DuPont Tychem C suit, full facial mask (3M gas and particle filter series 6000, 3M, Rüschlikon, Switzerland), and nitrile gloves, to remove the duct tape from the air vents and to allow the room to aerate for approximately 18 hours. In the case of the climate chamber, which could not be actively aerated, the room was left as is overnight to let the H2O2 degrade over time.
Process Controls and Monitoring
Temperature and relative humidity in the BSL-3 laboratories in Mittelhäusern were monitored and logged (Ebro Electronic GmbH & Co KG, Ingolstadt, Germany). A total of 5 loggers were placed at different positions throughout the BSL-3 laboratories (Figure 4).
The H2O2 chemical indicators (PCC051, Steris, Mentor, OH) were placed at different locations (varying numbers depending on room size and equipment present) in all fumigation zones to visualize the extent of H2O2 distribution during aerosol fumigation (Figures 1 –4).
Sterility Validation with Bacterial Spores
To determine the extent and efficacy of the aerosol fumigation process, triplicate biological indicator (BI) pouches containing >104, 105, or 106, respectively, spores of Geobacillus stearothermophilus 12980 dried on stainless-steel metal discs sealed in Tyvek (LOG-456, MesaLabs, Bozeman, MT) were placed, together with the chemical indicators, at different locations within the fumigation zones for the animal rooms, the climate chambers, and the BSL-3 laboratory with its ante-room (varying numbers depending on room size and equipment present; Figures 1 –3). For the fumigation of the BSL-3 units in Mittelhäusern, BIs with a single pouch containing 106 spores were also used (Figure 4). Several batches of BIs were used; their lot numbers and D-values (in 2 mg/L gaseous H2O2) were P0318 and 0.3, 0.3, and 0.8 minutes, respectively, for the 3 concentrations; AP-002 and 0.9, 0.8, and 1.6 minutes; and H2057 and 0.8 for the indicators containing 106 spores. Upon completion of the fumigation cycle, indicators were retrieved and opened, and the discs were transferred into Purple Releasat Culture Medium (MesaLabs) and incubated at 56°C for up to 7 days.
Results and Discussion
Fumigation of a New Rodent Facility
Fumigations were set up as outlined in the Materials and Methods section (Figure 1). No ventilators were used to aid with the distribution of the aerosolized H2O2. Furthermore, after discussions with the users, it was decided to aim for a log5 reduction, which was thought to be achievable without prior cycle development or validation based on previous experiences.
Of 91 BIs used during the fumigation over all 5 fumigation zones, 31 showed a log6 reduction, 57 a log5 reduction, 1 a log4 reduction, and 2 a reduction of less than log4. The BI showing only a log4 reduction was placed at ceiling level in the ante-room (Figure 1, fumigation zone e) above the generator and behind a cupboard and HVAC ducts. It is possible that a higher reduction could have been achieved with a better distribution of the H2O2 using ventilators. The 2 BIs that showed less than log4 reduction were placed above an HVAC duct and after a HEPA filter of an IVC rack ventilator facing away from the airstream. In addition, electrical power was lost toward the end of the second injection phase because of circumstances beyond our influence. Despite this, log5 reduction was achieved throughout, except for the 2 mentioned BIs. After discussions with the users, it was decided that a repetition of the fumigation was not necessary.
The results show the versatility of the fumigation system. However, they also show the limits and the need for better distribution in rooms containing a lot of equipment and rooms where the 2 generators are placed far apart, as in fumigation zone e. Further tests and adjustments to the fumigation cycle and setup may lead to an overall reduction of log6 in all fumigation zones.
Fumigation of 2 Climate Chambers
Fumigations were set up as outlined in the Materials and Methods section (Figure 2). No ventilators were used to aid with the distribution of the aerosolized H2O2. Furthermore, following discussions with the users, it was decided to aim for a log5 reduction, which was thought to be achievable without prior cycle development or validation.
Of a total of 28 BIs used during the fumigations, 1 BI showed a reduction of only log4, 26 of log5, and 1 of log6. One BI, therefore, did not fulfill the requirements set forth together with the users. That particular BI had been placed in a bottom corner, underneath the shelf of the wall rack, and behind the aerosol generator. Potentially, log5 and maybe even log6 reduction could have been achieved with better distribution using ventilators additionally placed inside the fumigation zones. Also, even though 1 climate chamber could not be actively aerated following fumigation, the following morning H2O2 concentrations were below the 1 ppm threshold set forth by Swiss authorities. Several aspects may explain this: (1) the system uses 7.5% H2O2, which is a lower concentration than most other manufacturers use or recommend, and (2) having left the air-conditioning unit running may contribute to the reduction in H2O2. H2O2 breaks down spontaneously when coming into contact with surfaces. This effect would certainly be increased when it passes through an air-conditioning unit, where it comes into contact with large surfaces at the heat exchangers. Furthermore, as H2O2 is actively dispersed inside the fumigation room, surface contact will also be increased.
Fumigation of a BSL-3 Laboratory and Its Ante-room
The fumigations were set up as outlined in the Materials and Methods section (Figure 3). As with the previous fumigations, it was decided, together with the users, to aim for a log5 reduction, which was thought to be achievable without prior cycle development or validation.
Of a total of 37 BIs placed inside the fumigation zone, 16 showed a log6 reduction, 20 a log5 reduction, and only 1 a log4 reduction. The 1 BI not achieving the set reduction limit had been placed inside a cupboard with PPE in front of it and, by mistake, the door almost closed. If not due to a handling error, all BIs would likely have confirmed at least a log5 reduction as agreed upon with the users.
The results show that the system can be used to decontaminate BSCs with their HEPA filters as all BIs placed inside the BSC showed a log5 reduction. The 2 BIs placed inside the BSC on the side windows would have been exposed to the aerosol only by H2O2 passing through the workspace HEPA filter. In addition, all BIs placed inside the IVCs achieved the same results.
Fumigation of 2 Connected BSL-3 Units
The fumigation was set up as outlined in the Materials and Methods section (Figure 4). Five triplicate BIs (104, 105, or 106 spores) and 7 single BIs (106 spores) were distributed per BSL-3 unit, together with a chemical indicator at every position. In the connecting inner wardrobe, 2 BIs containing 106 spores were placed.
Temperature and relative humidity in the BSL-3 laboratories were monitored and logged. Two loggers were placed on the floor of each BSL-3 unit and 1 in the inner wardrobe.
Following incubation at 56°C, all BIs showed a complete inactivation by the H2O2, and no growth of spores occurred. All chemical indicators turned from pink to yellow, meaning that the concentration of H2O2 had been high enough in every part of the room.
By means of the data of the relative humidity, the injection phase was clearly detectable and showed that the process proceeded according to the programming of the Q-Jet Superior. A maximum relative humidity of 93% was measured, and condensation was not detected.
The results show that the fumigation with aerosolized H2O2 is an option for these BSL-3 units. An additional advantage compared with the fumigation with FA performed previously is the reduced aeration time and thus the immediate start of maintenance work the following day. Fumigation with FA required a 3-day aeration before maintenance work could commence.
Conclusion
In this article, we present 4 different fumigation setups using the same fumigation system based on aerosolized H2O2. Three of the 4 situations represent fumigations of rooms not designed to be fumigated, because of their HVAC system, their leakiness, or their materials, such as paint, used within. The fourth example is more classical in its setup but still demonstrates the robustness and versatility of the system with its complicated floorplan. At no time did we measure any residual H2O2 outside the fumigation zone during fumigations, despite the leakiness of some rooms. Neither did we observe any damage to rooms or equipment, despite some claimed incompatibilities (eg, paint) or hazards (eg, electrical due to equipment running during fumigation). While aerosol is created, one can observe a thin mist forming inside the fumigation zone. If equipment is sprayed directly with aerosol (not recommended), droplets of H2O2 will be observed. However, despite the humidity present, no electrical equipment was damaged during any fumigation, as seen by the BSCs, IVC rack ventilators, stand-alone ventilators, and so forth, during and following fumigation.
It was also demonstrated that aerosolized H2O2 can pass through HEPA filters (BSC and IVC systems) while still being capable of inactivating bacterial spores.
These newer systems coming onto the market10,12 with their simpler setup and controls have a markedly reduced price as compared with older, more established systems. Despite their lower price, they are versatile and may be used in very different situations. Fumigation of rooms not designed for fumigation is easily achieved without too much effort and certainly with only limited risks if any at all.
Cycle development is much easier compared with previous systems. As shown here, the same cycle can be used for largely different room setups (rodent facility, climate chambers, BSL-3 laboratory) without necessitating prior cycle development for each individual fumigation zone.
Of course, this system also has its limits, as has previously been shown.10 Penetration into the duct work or through HEPA filters into the ventilation system is limited and would require additional adjustments such as blowers to help with distribution of the H2O2. We do believe, however, that the lower costs, ease of use, versatility, and robustness largely overcome these limits.
Ethical Statement
Not applicable to this research.
Statement of Human Rights
Not applicable to this research.
Statements of Informed Consent
Not applicable to this research.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Dreyfus W. Review of formaldehyde fumigation. Am J Public Health. 1914;4(11):1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheney JE, Collins CH. Formaldehyde disinfection in laboratories: limitations and hazards. Br J Biomed Sci. 1995;52(3):195–201. [PubMed] [Google Scholar]
- 3. Nelson N, Levine RJ, Albert RE, et al. Contribution of formaldehyde to respiratory cancer. Environ Health Perspect. 1986;70:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beswick AJ, Farrant J, Makison C, et al. Comparison of multiple systems for laboratory whole room fumigation. Appl Biosafety. 2011;16(3):139–157. [Google Scholar]
- 5. Pottage T, Richardson C, Park S, Walker JT, Bennett AM. 2009. Evaluation of hydrogen peroxide gaseous disinfection systems to decontaminate viruses. J Hosp Inf. 2010;74(1):55–61. [DOI] [PubMed] [Google Scholar]
- 6. Heckert RA, Best M, Jordan LT, Dulac GC, Eddington DL, Sterritt WG. Efficacy of vaporized hydrogen peroxide against exotic animal viruses. Appl Environ Microbiol. 1997;63(10):3916–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petit BM, Almeida FC, Uchiyama TR, Lopes FOC, Tino KH, Chewins J. Evaluating the efficacy of hydrogen peroxide vapour against foot-and-mouth disease virus within a BSL4 biosafety facility. Lett Appl Microbiol. 2017;65(4):281–284. [DOI] [PubMed] [Google Scholar]
- 8. Kümin D, Signer J, Portmann J, Beuret C. Of a storm in a teacup and a gutter heater—practical aspects of VHP room fumigation. Appl Biosafety. 2015;20(3):146–154. [Google Scholar]
- 9. Kümin D, Portmann J, Signer J, Beuret C, Strasser M. The IndicatorSafe—a simple tool to confirm successful fumigation of a hepa filter housing. Appl Biosafety. 2015;20(4):179–183. [Google Scholar]
- 10. Kümin D, Gsell-Albert M, Summermatter K. Comparison and validation of three fumigation methods to inactivate foot-and-mouth disease virus . Appl Biosafety. 2018;23(2):70–76. [Google Scholar]
- 11. World Health Organization. Laboratory Biosafety Manual. 3rd ed. Geneva, Switzerland; 2004. [Google Scholar]
- 12. Freyssenet C, Karlen S. Plasma-activated hydrogen peroxide (ahp) in surface inactivation procedures. Appl Biosafety. 2019;24(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]