We are in the midst of a pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus. 1 At the time of writing, there were 2.17 million global coronavirus disease 2019 (COVID-19) cases with 146,000 deaths. 2 Unfortunately, some of these deaths represent health care workers and first responders. One major challenge in preventing the occupational spread of SARS-CoV-2 is the lack of personal protective equipment (PPE), 3 particularly filtering facepiece respirators (FFRs). (Our use of the phrase “surgical mask design or type” below is to denote the appearance of the mask. It is the textile that we believe makes this design of mask an FFR, as it creates a negative pressure environment that filters Bitrex particles out of the air that the user is breathing. 4 )
We acknowledge up front that we do not have enough data to submit a research article yet; we are pursuing standard FFR (quantitative) testing. However, in this time of pandemic crisis, we recognize the severe lack of PPE on the front lines of the pandemic and thus offer an option that might meet responder needs. We hope that this information is helpful for both research and practice while we further study these materials.
Herein, we report efforts to provide a “crisis” alternative mask that provides improved fit compared to a surgical mask (SM), being more like an FFR. We adapted and evaluated the use of a medical-grade textile for mask development. Initially proposed by University of Florida researchers, the 2-ply spun-polypropylene sterilization wrap, Halyard H600 (Owens & Minor), was suggested to serve as a viable material for mask creation. 5 We created 26 sewn masks that resembled typical SM designs (Figure 1, A, B, C, and D models) and 2 designs that resemble N95s (Figure 1, E and F models) from Halyard textiles (H200, H300, H600, or H650). Variations of these designs were sewn from at least two 7 × 8 in. (“larger” size) or two 7 × 6.5 in. (“smaller” size) for SM-style designs. In addition, up to 4 layers of textiles were tested for fit, comfort, and resistance to breathing. The H600 textile used as a surgical wrap is 2-ply. We started with this thickness and increased the thickness sequentially.
Figure 1.
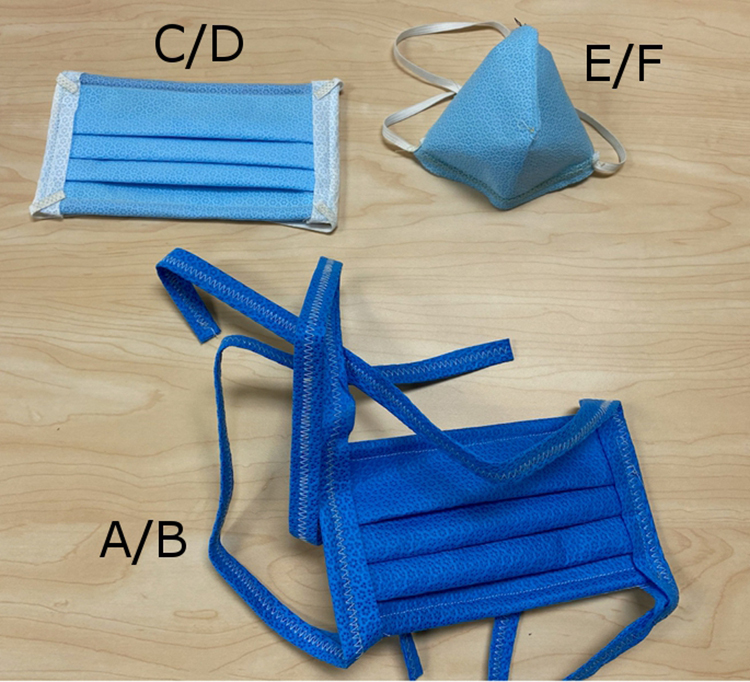
Surgical or SM masks (A = larger size with straps; B = smaller size with straps; C = larger size with elastic; D = smaller size with elastic) and N95-style (E = larger size with straps; F = smaller size with elastic) models of face masks composed of varying Halyard textiles.
Masks were secured to the face by elastic headbands or tied with textile straps. Masks had pliable metal stays inserted to assist sealing at the nose bridge and chin. We used qualitative fit testing to determine which masks “sealed” to the face as determined by Bitrex exclusion (ie, the test is passed if the subject fails to taste the Bitrex). 6 Qualitative testing of the masks also included evaluating their comfort and wearability (1 = best; 3 = worst) and resistance to breathing or breathability (1 = easiest; 3 = hardest). One author with a “larger face” and 2 authors with “smaller” faces evaluated the mask designs by qualitative fit testing, supervised by a certified nurse. We present the results of those masks that passed the fit test in Table 1.
Table 1.
Results of the Masks That Passed the Fit Test.
| No. | Model | Material/layers | Wearability/comfort | Breathability | |||
|---|---|---|---|---|---|---|---|
| 200 | 300 | 600 | 650 | ||||
| 2 | SM-C | 2 | 1 | 1 | |||
| 4 | SM-C | 2 | 1 | 1 | |||
| 6 | SM-C | 2 | 1 | 2 | |||
| 8 | SM-C | 2 | 1 | 3 | |||
| 10 | SM-C | 3 | 1 | 2 | |||
| 12 | SM-C | 3 | 1 | 2 | |||
| 14 | SM-C | 4 | 2 | 3 | |||
| 16 | SM-C | 1 | 1 | 1 | 2 | ||
| 18 | SM-C | 2 | 1 | 2 | 2 | ||
| 20 | SM-C | 1 | 1 | 2 | 3 | ||
| 22 | SM-C | 1 | 1 | 2 | 3 | ||
| 24 | SM-C | 1 | 2 | 2 | 3 | ||
| 27 | 95-E | 2 | 2 | 3 | |||
| 28 | 95-F | 2 | 2 | 1 | |||
Abbreviation: SM, surgical mask.
Each SM design made from 2- or more layers of Halyard textiles and secured with elastic straps (Figure 1, model SM-C) excluded Bitrex when fit tested on adults of typical (“larger”) face size. The elastic straps appeared to hold masks tighter to the face, thus excluding Bitrex even with up-down and side-to-side head movements (and other exercises) during each 20- to 30-minute test. In addition, each N95 design made from 2 layers of Halyard textiles secured with elastic, or textile, straps excluded Bitrex (with head movement) on “larger” and “smaller” faces (Figure 1, models 95-E and F, respectively).
However, no SM design of 2- or more layer combination of Halyard products secured with textile straps (Figure 1, models A and B), consistently excluded Bitrex as determined by tester grimace. In addition to straps feeling less comfortable and less secure, these masks often initially excluded Bitrex and then failed to exclude it with head movement, as gaps between skin and mask occurred. Also, no single combination of Halyard products made with elastic or textile straps fitted in the larger or smaller SM designs excluded Bitrex on “smaller” faces (Figure 1, models A-D) due to failure to achieve a good seal. Finally, masks created with Halyard materials using either an SM or N95 design were generally more difficult to seal at the nose bridge, were more uncomfortable, and had increased resistance to breathing with thickness (eg, H200 to H600) or density (eg, 2 layers to 4 layers).
We are experiencing a PPE shortage, which is putting health care workers and first responders at occupational risk of exposure to infection from COVID-19 patients. Our results have shown that some combinations of sewn (Halyard) surgical wrap can exclude Bitrex, are comfortable to wear, and have nominal breathing resistance. Moreover, unlike FFRs approved by the National Institute for Occupational Safety and Health (NIOSH), 7 which are in short supply, surgical wrap material can be readily obtained and sewn into an SM in about 10 minutes (at a beginner-level sewing knowledge) and around 20 minutes for an N95-style mask (with intermediate sewing knowledge). This means there is a high likelihood of rapid production and mass dissemination to health care workers and first or emergency responders for use in low-risk situations.
Additional quantitative FFR testing will determine conditions under which these “crisis” SM- and N95-style designs can provide protection from respiratory droplets or aerosols. Groups that follow these patterns and sew their own masks or use any nonapproved materials should conduct a thorough risk assessment of workplace hazards before their use. However, we share these qualitative data prior to the completion of quantitative testing to alert mask users of additional mask construction options during global PPE shortages.
In conclusion, we strongly recommend the use of NIOSH-approved FFRs in SARS-CoV-2 and other high-risk environments. However, in their absence, the aforementioned textiles and designs may offer benefit to frontline responders in low risk settings until quantitative testing proves their utility in higher-risk environments or N95 supply chain pressures are relieved.
Acknowledgments
We thank Georgetta Graham and Sonia D. Mehta, MD, University of Florida, for their original sewing designs that were adapted for this study; Arthur Lee, MD, Cardiac & Vascular Institute in Gainesville, Florida, for study and design feedback; MedWish International for its partnership and for supplying the surgical wrap; the Kent State University DeWeese Health Center for supplying fit testing materials; and J. R. Campbell and the Kent State PPE Production Team for their continued work and support of this research.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Janet Reed  https://orcid.org/0000-0003-3905-4156
https://orcid.org/0000-0003-3905-4156
References
- 1. World Health Organization. Coronavirus disease (COVID-19) pandemic. Published 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 1 April 2020).
- 2. World Health Organization. Coronavirus disease 2019 (COVID-19) situation reports. Published 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed 9 April 2020).
- 3. World Health Organization. Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19). Published 2020. https://apps.who.int/iris/bitstream/handle/10665/331498/WHO-2019-nCoV-IPCPPE_use-2020.2-eng.pdf (accessed 1 April 2020).
- 4. US Department of Labor, Occupational Safety and Health Administration. Respiratory Protection (29 CFR 1910.134). Published 2011. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134 (accessed 1 April 2020).
- 5. Bulletti L. UF Health anesthesiology team devises respirator mask made from existing hospital materials. Published 2020. https://ufhealth.org/news/2020/uf-health-anesthesiology-team-devises-respirator-mask-made-existing-hospital-materials (accessed 1 April 2020).
- 6. Radonovich L. Elastomeric and powered-air purifying respirators in US healthcare. Published 2017. https://www.cdc.gov/niosh/npptl/pdfs/ElastomericPAPR-Healthcare-508.pdf (accessed 1 April 2020).
- 7. US Department of Labor, Occupational Safety and Health Administration. Respiratory protection (29 CFR 1910.134(d)(1)(ii)). Published 2011. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134 (accessed 1 April 2020).


