Abstract
Somato-dendritic secretion was first demonstrated over 30 years ago. However, although its existence has become widely accepted, the function of somato-dendritic secretion is still not completely understood. Hypothalamic magnocellular neurosecretory cells were among the first neuronal phenotypes in which somato-dendritic secretion was demonstrated and are among the neurones for which the functions of somato-dendritic secretion are best characterised. These neurones secrete the neuropeptides, vasopressin and oxytocin, in an orthograde manner from their axons in the posterior pituitary gland into the blood circulation to regulate body fluid balance and reproductive physiology. Retrograde somato-dendritic secretion of vasopressin and oxytocin modulates the activity of the neurones from which they are secreted, as well as the activity of neighbouring populations of neurones, to provide intra-and inter-population signals that coordinate the endocrine and autonomic responses for the control of peripheral physiology. Somato-dendritic vasopressin and oxytocin have also been proposed to act as hormone-like signals in the brain. There is some evidence that somato-dendritic secretion from magnocellular neurosecretory cells modulates the activity of neurones beyond their local environment where there are no vasopressin- or oxytocin-containing axons but, to date, there is no conclusive evidence for, or against, hormone-like signalling throughout the brain, although it is difficult to imagine that the levels of vasopressin found throughout the brain could be underpinned by release from relatively sparse axon terminal fields. The generation of data to resolve this issue remains a priority for the field.
Keywords: oxytocin, paraventricular nucleus, somato-dendritic secretion, supraoptic nucleus, vasopressin
1 |. INFORMATION TRANSFER IN THE CENTRAL NERVOUS SYSTEM
The classical understanding of communication in the nervous system is of synaptic transmission in a unidirectional manner within networks from presynaptic neurones to postsynaptic neurones. However, it has become clear that information transfer in the central nervous system is more complex than simple point-to-point, unidirectional transmission between neurones at synapses. Among the additional mechanisms that contribute to information transfer in the nervous system is somato-dendritic secretion. Unlike classical synaptic transmission by neurotransmitters such as glutamate and GABA, which signal between pre- and postsynaptic neurones with spatial precision and high temporal resolution, somato-dendritic secretion causes longer-term changes that alter the overall excitability of neurones by modulating the strength of synaptic inputs and/or by modulating the baseline membrane potential. These effects can be autocrine or paracrine, on the neurone from which somato-dendritic secretion occurs or on nearby neurones, and might spread over relatively long distances to modulate the activity of neurones in brain areas distant from the site of secretion.
Somato-dendritic secretion occurs in many types of neurone and can involve many types of transmitter molecule.1 Magnocellular neurosecretory cells (MNCs) of the hypothalamic supraoptic nucleus (SON) and paraventricular nucleus (PVN) are among those for which the mechanisms and consequences of somato-dendritic secretion are best characterised. This review focusses on studies from the authors’ laboratories, some of which were presented at the 22nd International Symposium on Regulatory Peptides, which have contributed to our understanding of how somato-dendritic secretion from MNCs contributes to endocrine and autonomic regulation of peripheral physiology in health and disease.
2 |. THE MAGNOCELLULAR NEUROSECRETORY SYSTEM
The magnocellular neurosecretory system comprises MNCs that predominantly secrete either vasopressin (antidiuretic hormone, ADH) or oxytocin into the general circulation from the posterior pituitary gland (neurohypophysis). The principal function of vasopressin is to maintain body fluid balance and blood pressure by activation of renal V2-receptors to increase water reabsorption from the urine and, when blood pressure/volume is decreased, by activation of vascular V1a-receptors (V1aRs) to cause vasoconstriction.2 The best-characterised physiological functions of oxytocin are to trigger uterine contractions during birth and milk ejection during lactation.2 However, oxytocin also contributes to body fluid balance by promoting natriuresis in the kidney3 and by stimulating atrial natriuretic peptide secretion.4
The human hypothalamus contains over 100 000 MNCs,5 with approximately 10 000 in the rat, that are principally located in the SON and PVN, as well as in several accessory nuclei.6 MNCs each project a single axon to the posterior pituitary gland (Figure 1) and each axon branches extensively to form several thousand neurosecretory axon swellings and terminals7 that are each tightly packed with dense-core vesicles containing approximately 85 000 molecules of vasopressin or oxytocin in rats.8 Hormone secretion is triggered by action potential invasion of the neurosecretory swellings and terminals. It has been estimated that each MNC contains approximately 10 million dense-core vesicles and secretes between 100 and 10 000 dense-core vesicles from the posterior pituitary gland every minute to maintain basal hormone concentrations in the circulation.9 Hence, the sustained output of the hormones, and the consequent regulation of peripheral physiology, depends on the average action potential discharge from the population.2,10
FIGURE 1.
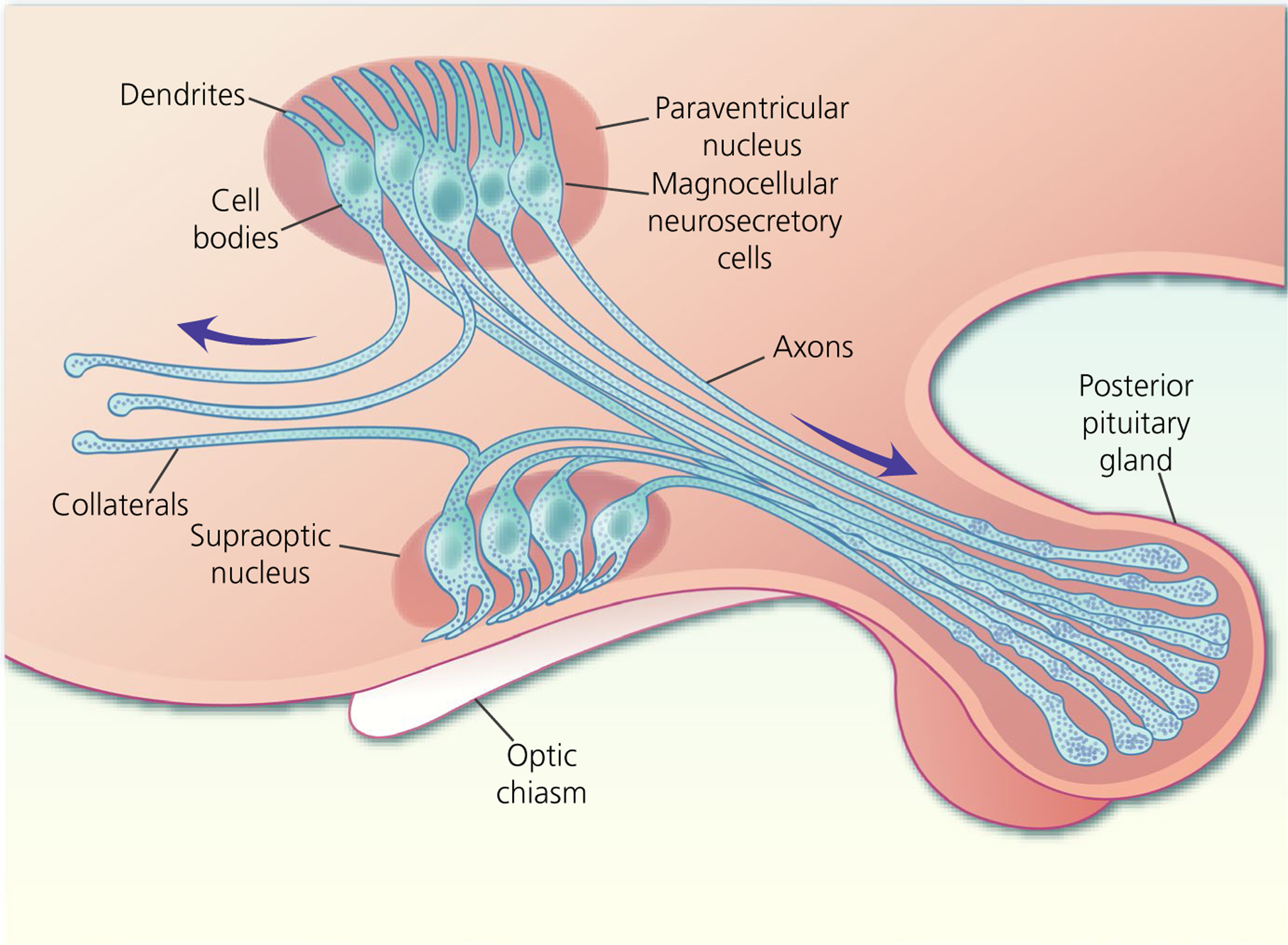
Magnocellular neurosecretory cells (MNCs) of the hypothalamic supraoptic nucleus and paraventricular nucleus each possess one to three dendrites and project a single axon to the posterior pituitary gland where they secrete either oxytocin or vasopressin into the circulation. Some MNC axons project axon collaterals to other brains areas
MNCs also synthesise lesser amounts of other neurotransmitters and neuromodulators that can be contained in the same dense-core vesicles as vasopressin or oxytocin,11 as well as glutamate-containing microvesicles.12 To date, the only evidence of effects of these other neurotransmitters and neuromodulators on peripheral physiology is for secretin, which increases renal antidiuresis.13 Rather, their principal function appears to be modulation of hormone secretion at the level of the posterior pituitary gland, which has been comprehensively reviewed elsewhere,14 and at the level of the somata and dendrites, as we describe in the present review.
Some MNCs project axon collaterals to other brain areas. Originally, these were assumed to remain proximal to the SON15 and PVN,16 projecting to local interneurones as part of a proposed local feedback loop. More recently, it was shown that some MNC axon collaterals project more broadly throughout the brain, with oxytocin MNCs projecting to the medial amygdala (MeA), central amygdala (CeA), nucleus accumbens17 and the lateral septum,18 and vasopressin MNCs to the medial and lateral preoptic area, suprachiasmatic nucleus, lateral habenula, CeA, MeA,19,20 locus coeruleus21 and arcuate nucleus (ARC).22 These axon collaterals have been implicated in the modulation of different behaviours, although it remains to be established how secretion from axon collaterals to modulate behaviour relates to secretion from the posterior pituitary gland to modulate peripheral physiology.
MNCs possess one to three thick, varicose, aspiny dendrites of a few hundred micrometres in length. MNCs of the SON extend their dendrites to the ventral surface of the nucleus, where the dendrites bundle together within the ventral glial lamina (a layer of astrocytes on the ventral surface of the brain within the SON)23 and MNCs of the PVN extend their dendrites towards the subependymal region of the third ventricle.24 In addition to being the site of afferent synaptic input, MNC dendrites are active players in shaping MNC activity through exocytosis of vasopressin and oxytocin (as well as other neurotransmitters/neuromodulators) into the extracellular space of the SON and PVN.
3 |. SOMATO-DENDRITIC SECRETION FROM MAGNOCELLUL AR NEUROSECRETORY CELLS
The somata and dendrites of MNCs are tightly packed with dense-core vesicles containing either vasopressin or oxytocin (Figure 2), which undergo exocytosis to secrete their major neuropeptides25,26 along with lesser amounts of other co-packaged neurotransmitters and neuromodulators.11 Tannic acid capture of somato-dendritic secretion reveals that the entire vesicle content is released from MNCs.25
FIGURE 2.
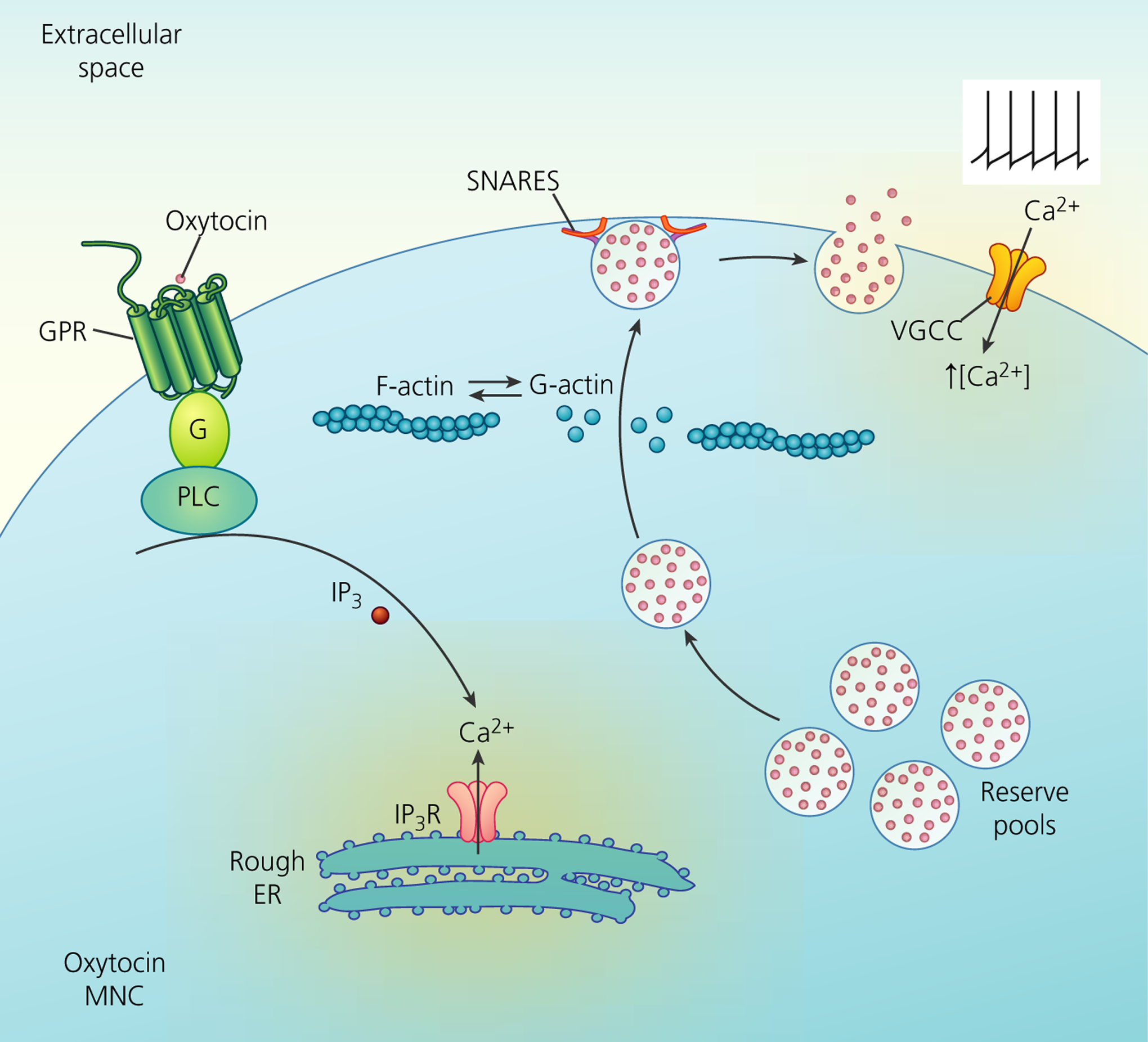
Mechanisms of somato-dendritic release of oxytocin from magnocellular neurosecretory cells (MNCs). Neuropeptides are synthesised and packaged in the soma and stored in dendrites in a reserve pool containing large numbers of large dense-core vesicles (LDCVs). Depolarisation-induced calcium entry through voltage-gated calcium channels (VGCCs) stimulates peptide release by exocytosis of LDCVs. This requires the depolymerisation of F-actin to G-actin. Furthermore, the stimulation of G-protein coupled receptors (GPR), such as the oxytocin receptor, stimulates the mobilisation of Ca2+ from inositol trisphosphate (IP3)-dependent intracellular stores of the rough endoplasmic reticulum (ER) and an increase in the number of LDCVs at the plasma membrane, thus priming the exocytosis machinery for subsequent activity-dependent release. Although some members of the soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) family are detectable by immunocytochemistry, there appears to be a lack of vesicle-associated membrane protein-2, synaptosomal-associated protein 25 and synaptotagmin-1 in the somata and dendrites, with their function presumably being replaced by other SNARE proteins. IP3R, inositol trisphosphate receptor; PLC, phopholipase C
Unlike synaptic transmission by classical neurotransmitters, dense-core vesicle exocytosis from the somata and dendrites of MNCs requires a sustained increase in intracellular calcium27,28 and calcium buffering limits increases in cytoplasmic calcium to restrain the activation of somato-dendritic secretion from MNCs.29 MNCs express different arrays of voltage-gated calcium channels in their somata and axon terminals.30 Relative to other voltage-gated calcium channels, N-type calcium channels (Cav2.2) carry a comparatively small current in MNC somata, although they nevertheless contribute most significantly to somato-dendritic oxytocin secretion.31 Although the primary trigger for somato-dendritic secretion is the influx of extracellular calcium, intracellular calcium release also contributes to somato-dendritic secretion from MNCs.27,28
Action potential invasion triggers exocytosis from axon terminals and MNC dendrites and appears to support depolarisation-induced calcium spikes.32 Capacitance measurements from isolated MNCs suggest that single action potentials trigger somato-dendritic secretion.33 However, functional studies suggest that sustained intracellular calcium release is required to trigger somato-dendritic secretion.27,28 Furthermore, if every action potential fired by each MNC triggered somato-dendritic secretion of a single dense-core vesicle, the brain would be awash with vasopressin and oxytocin.34 Exocytosis of approximately 6000 dense-core vesicles per second has been calculated to be sufficient to maintain the concentrations of vasopressin and oxytocin measured in the rat hypothalamus.34 There are approximately 10 000 MNCs in the rat hypothalamus.6 Although approximately 25% of MNCs are silent under basal conditions, active MNCs display a mean firing rate of approximately 5 Hz under basal conditions.35 Hence, up to 37 500 action potentials are fired by MNCs every second, which is almost 10-fold more than the number of dense-core vesicles secreted. Hence, trains of action potentials that cause a more sustained depolarisation and calcium influx are probably required to trigger somato-dendritic secretion from MNCs. Indeed, under basal conditions, some stimuli reduce the oxytocin MNC action potential firing rate but increase somato-dendritic oxytocin secretion,36,37 and it was shown recently that action potential firing alone at physiological firing rates is insufficient to trigger measurable somato-dendritic secretion from individual vasopresin MNCs.38
In addition to permeation through voltage-gated calcium channels, calcium influx also occurs through NMDA receptors (NMDARs), and synaptic NMDA receptors would be expected to further increase cytoplasmic calcium concentrations during action potential firing. Furthermore, MNCs express extrasynaptic NMDARs.39–41 Although these extrasynaptic NMDARs are activated by basal glutamate levels in vitro,39 they are not activated under basal conditions in vivo but they are activated under stimulated conditions35 and trigger somato-dendritic peptide secretion.38
In addition to triggering somato-dendritic secretion, increased cytoplasmic calcium also promotes movement of dense-core vesicles from the reserve pool toward the cell surface,42 where they are ready for secretion in response to subsequent stimuli that raise cytoplasmic calcium. In parallel, increased intracellular calcium also promotes the recruitment of N-type calcium channels31 to make the system more sensitive to subsequent cytoplasmic calcium increases. Hence, this ‘priming’ increases somato-dendritic secretion triggered by subsequent signals that increase cytoplasmic calcium.
Action potential-mediated depolarisation is not the only trigger for somato-dendritic secretion from MNCs. Vasopressin and oxytocin MNCs express their respective receptors43,44 and activation of these receptors increases cytoplasmic calcium concentrations45 to trigger somato-dendritic secretion.27,28 Although vasopressin and oxytocin trigger somato-dendritic secretion from vasopressin and oxytocin MNCs without a prior stimulus to prime the system, once the MNCs are primed, the peptides can trigger a much greater somato-dendritic secretion.27,28
There is an elaborate network of actin and microtubules in MNC somata and dendrites.46,47 Cortical F-actin regulates somato-dendritic exocytosis; F-actin polymerisation inhibits and F-actin depolymerisation increases somato-dendritic secretion from MNCs.48 Presumably, F-actin depolymerisation increases access to the plasma membrane and this process might account for the requirement for a sustained increase in intracellular calcium to trigger somato-dendritic secretion because calcium causes F-actin depolymerisation. Unlike synaptic transmission, there does not appear to be any specific structure on the soma or dendrites that is specialised for somato-dendritic secretion,49 although it remains to be determined whether there are regions of the cortical F-actin network that are more readily depolymerised to allow dense-core vesicles preferential access to the plasma membrane at specific sites for secretion.
Exocytosis can occur once the dense-core vesicles reach the plasma membrane, which requires exocytotic machinery. The involvement of the soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) complex in exocytosis from axon terminals is well established.50 Although less well characterised, it appears that somato-dendritic secretion is also mediated by SNARE proteins. MNCs express SNARE proteins51; however, although vesicle-associated membrane protein-2 (VAMP-2) and synaptosomal-associated protein 25 (SNAP-25) are both expressed in the axon terminals,52,53 they are not expressed in the somata or dendrites of MNCs.54 Hence, the suite of SNARE complex proteins for somato-dendritic exocytosis probably differs from that for axon terminal secretion from MNCs.
4 |. STIMULATION OF SOMATO-DENDRITIC SECRETION BY NEUROTRANSMITTERS, PEPTIDES AND HORMONES
Noradrenergic afferents from the ventrolateral medulla (VLM) A1 cell group and the nucleus of the tractus solitarius (NTS) A2 cell group make prominent projections to MNCs.2 NTS noradrenergic afferents are activated during birth and lactation55 as part of the Ferguson reflex,56 and noradrenaline facilitates somato-dendritic oxytocin secretion in late pregnancy and lactation.57–59 Oxytocin also increases noradrenaline secretion within the SON,60 which presumably establishes a local positive-feedback loop that reinforces oxytocin MNC excitation and promotes oxytocin secretion into the circulation to trigger uterine contractions during birth and milk ejection during lactation (Figure 3).
FIGURE 3.
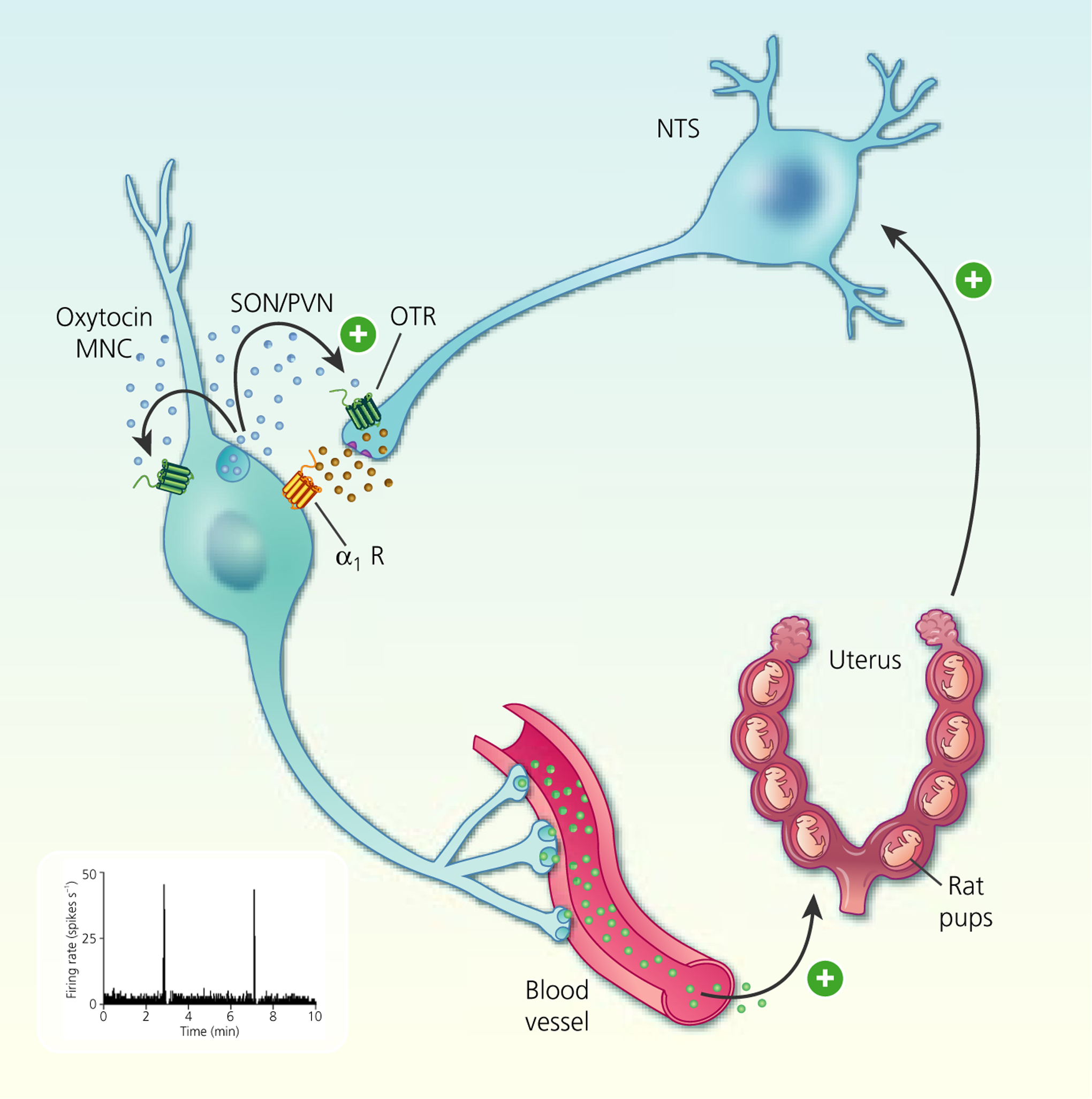
Autocrine modulation of burst firing in oxytocin magnocellular neurosecretory cells (MNCs). Cervical stretch during birth activates stretch receptors to activate A2 noradrenergic neurones in the nucleus tractus solitarius (NTS) that, in turn, activate somato-dendritic oxytocin secretion from oxytocin MNCs. Oxytocin feeds back on oxytocin MNCs to increase excitability. Oxytocin also increases noradrenaline secretion within the supraoptic nucleus (SON) to establishe a local positive feedback loop that reinforces oxytocin MNC excitation and promotes oxytocin secretion into the circulation to trigger uterine contractions during birth. OTR, oxytocin receptor; PVN, paraventricular nucleus
Pro-opiomelanocortin (POMC) afferents from the ARC project to the SON and PVN, particularly to regions of the SON and PVN that are enriched in oxytocin MNCs.61 POMC neurones secrete α-melanocyte stimulating hormone (α-MSH), which acts on melanocortin-4 (MC4-R) receptors in the SON and PVN.62 Although α-MSH inhibits oxytocin secretion into the circulation, it increases somato-dendritic oxytocin secretion.37,63 MC4-R activation increases intracellular calcium in oxytocin MNCs to trigger somato-dendritic oxytocin secretion, as well as endocannabinoid secretion, which inhibits the activity of the MNCs to reduce axonal oxytocin secretion into the blood.37,63 Remarkably, α-MSH inhibition of oxytocin MNC activity is lost in mid-pregnancy,62 although it has yet to be determined whether this represents a switch from pre-pregnancy inhibition to stimulation during lactation, as is seen for the effects of prolactin on oxytocin MNCs.64 Furthermore, it is not known whether α-MSH effects on somato-dendritic oxytocin secretion change in pregnancy and lactation.
In addition to neurotransmitters, other hormones can also trigger somato-dendritic secretion from MNCs. The orexigenic hormone ghrelin is synthesised by oxyntic cells in the gastric mucosa, although not in the brain.65 Central ghrelin administration increases vasopressin secretion into the circulation via activation of neuropeptide Y neurones.66 In addition, ghrelin stimulates somato-dendritic vasopressin secretion, which increases ATP release from astrocytes to increase presynaptic GABA release onto the vasopressin MNCs67 (Figure 4).
FIGURE 4.
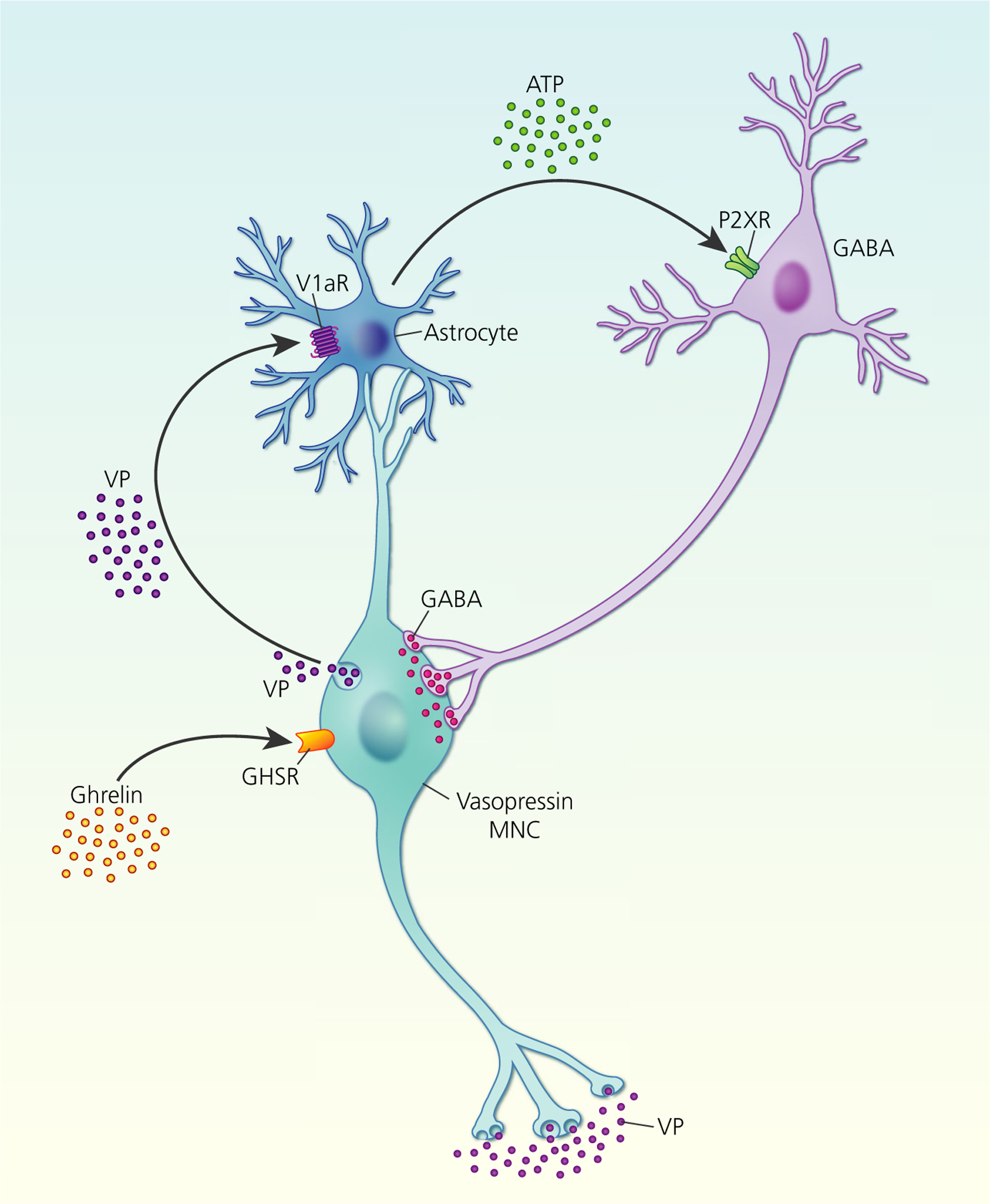
Ghrelin stimulation of somato-dendritic vasopressin (VP) secretion. Ghrelin activation of growth hormone secretagogue receptors (GHSR) on vasopressin magnocellular neurosecretory cells (MNCs) induces somato-dendritic vasopressin secretion, which activates V1a receptors (V1aRs) on neighbouring astrocytes to increase intracellular calcium. Increased astrocytic calcium triggers release ofthe gliotransmitter, ATP, which activates ionotropic P2X receptors (P2XR) on GABA interneurones that project back to vasopressin MNCs
5 |. AUTOCRINE/PARACRINE MODULATION OF VASOPRESSIN MAGNOCELLULAR NEUROSECRETORY CELL ACTIVITY
The effects of vasopressin, oxytocin and other neurotransmitters and neuromodulators secreted from the somata and dendrites of MNCs can be broadly categorised as autocrine, regulating the activity of the MNC from which secretion occurs, and paracrine, regulating the activity of neighbouring neurones, including other neuronal populations.
V1aR and V1b receptors (V1bR) are expressed in the membranes of vasopressin-containing dense-core vesicles68 and are presumably inserted into the plasma membrane during somato-dendritic vasopressin secretion. Hence, vasopressin receptors newly trafficked to the plasma membrane will be exposed to high concentrations of vasopressin to underpin activity-dependent autocrine feedback regulation of vasopressin MNC activity.
Vasopressin MNCs express a range of activity patterns under basal conditions; some are silent throughout recordings, some display irregular activity, some are continuously active (typically at approximately 6 spikes s−1) and some display rhythmic ‘phasic’ firing.69 Phasic firing is characterised by bursts of activity that last more than 15 seconds, after which bursts stop randomly.70 Each burst is followed by inactivity for at least 10 seconds, after which the next burst starts randomly.70 At burst onset, vasopressin MNCs can reach firing rates of approximately 15–25 spikes s−1 for the first 5–10 seconds, before spike frequency adaptation occurs to a steady-state firing rate of approximately 6 spikes s−1 for the remainder of the burst.71,72
Of the different activity patterns recorded in vasopressin MNCs, phasic bursting is the most efficient pattern for vasopressin secretion into the circulation because vasopressin secretion is maximal at approximately 13 spikes s−1,73,74 which is typically only achieved by phasically firing MNCs and only during the first 5–10 seconds of each phasic burst. Vasopressin secretion from the posterior pituitary gland rapidly fatigues during continuous stimulation, although this fatigue is reversed when stimulation is stopped for a few tens of seconds.75 Hence, vasopressin MNCs firing continuously at high frequency do not secrete as much vasopressin into the circulation as do phasic MNCs firing at the same frequency because the silent periods between bursts in phasic MNCs reset the system for efficient vasopressin secretion at the onset of the next burst, when the typical firing frequency is again in the range that is most efficient for vasopressin secretion. The importance of phasic activity for efficient vasopressin secretion into the circulation is highlighted by the changes in activity patterning that occur under chronically stimulated conditions, such as prolonged osmotic stimulation. Although burst duration does increase during shorter periods of stimulation, prolonged osmotic stimulation leads to an increase in firing rate within bursts, whereas the burst duration and inter-burst interval remain similar to those seen under basal conditions.76–79
V1aR antagonists consistently increase the activity of phasic MNCs when administered into the SON,80 suggesting that somato-dendritic vasopressin mediates feedback inhibition of vasopressin MNCs via V1aR activation (Figure 5). This feedback inhibition probably involves direct autocrine actions on the MNC that secretes vasopressin because V1aR activation reduces excitatory postsynaptic potential amplitude in vasopressin MNCs.81 However, autocrine activation of V1aRs does not mediate autoregulation of vasopressin MNC activity alone because vasopressin also increases inhibitory postsynaptic potential frequency82 via stimulation of astrocytic ATP release, which acts as a gliotransmitter at P2X receptors on presynaptic GABA neurones to increase GABA release.67 Hence, somato-dendritic vasopressin secretion appears to contribute to the generation of phasic activity in vasopressin MNCs via a combination of autocrine actions on the MNC from which secretion occurs and paracrine actions on nearby cells that modulate the activity of the MNC from which secretion occurs.
FIGURE 5.
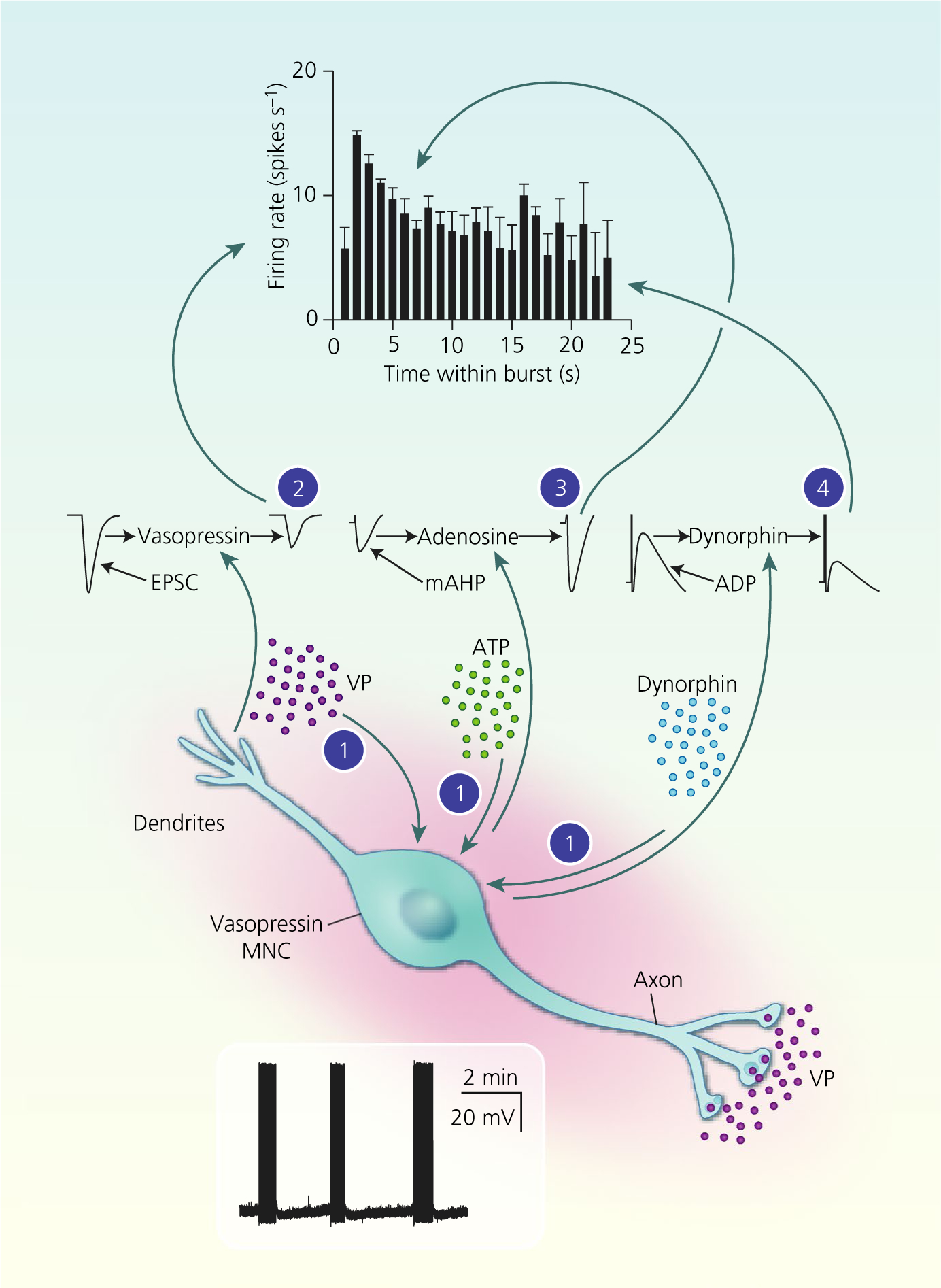
Autocrine modulation of vasopressin magnocellular neurosecretory cell (MNC) activity. Vasopressin (VP) MNCs secrete vasopressin, ATP and dynorphin (and other transmitters) from their somata and dendrites. Endogenous arginine vasopressin (AVP) (2) inhibits spike discharge throughout bursts via inhibition of the excitory post-synaptic current (EPSC) amplitude. Endogenous ATP is rapidly converted to adenosine (3), which enhances the medium afterhyperpolarisation (mAHP) amplitude over the first few seconds of bursts to contribute to spike frequency adaptation. Endogenous dynorphin (4) inhibition of the afterdepolarisation (ADP) increases progressively over the course of bursts, eventually resulting in burst termination. Combined, these autocrine feedback effects of somato-dendritic vasopressin and co-secreted transmitters shape phasic activity for efficient secretion vasopressin into the circulation from the posterior pituitary gland
Although V1aR activation mediates autocrine and paracrine inhibition of phasic activity, local application of exogenous vasopressin was first reported to inhibit highly active phasic MNCs and stimulate weakly active phasic MNCs.83 Vasopressin MNCs also express V1bR68 and, although it has yet to be determined whether V1bR activation also contributes to autocrine regulation of vasopressin MNCs, it might underpin the excitatory effects of vasopressin evident in weakly active vasopressin MNCs. Regardless of the vasopressin excitation of weakly active phasic MNCs (perhaps via V1bR), such an action of endogenous vasopressin would presumably increase peripheral vasopressin secretion to cause robust vasoconstriction that is not present under basal conditions.84 Hence, it appears that any contribution of somato-dendritic vasopressin secretion to peripheral vasopressin secretion by feedback excitation is probably overridden by the V1aR-mediated feedback inhibition.
Although somato-dendritic vasopressin secretion functions as a negative-feedback regulator of vasopressin MNC activity at the single cell level, the important output of the system is overall hormone secretion, which depends on the integrated activity of the MNCs at a population level.10 Some of the earliest work on somato-dendritic secretion showed that osmotic stimulation of vasopressin MNCs increases vasopressin levels in the circulation before levels increase in the SON,85 which is consistent with somato-dendritic vasopressin secretion acting as a negative-feedback regulator of vasopressin MNC activity at a population level to modulate overall vasopressin secretion into the circulation.
6 |. AUTOCRINE/PARACRINE MODULATION OF VASOPRESSIN MAGNOCELLULAR NEUROSECRETORY CELL ACTIVITY BY CO-SECRETED TRANSMITTERS
Vasopressin MNCs also synthesise and secrete a number of other neurotransmitters and neuromodulators, including apelin,86 ATP,87 carbon monoxide (CO),88 dynorphin,89 endocannabinoids,90–92 galanin,93 neuroendocrine regulatory peptides (NERPs),94,95 nitric oxide (NO),96 pituitary adenylate cyclase-activating polypeptide (PACAP)97 and secretin.13
Most neuropeptides synthesised by MNCs are packaged within the same dense-core vesicles as either vasopressin or oxytocin. However, apelin and galanin are differentially packaged in vasopressin MNCs. Apelin is packaged in dense-core vesicles that do not contain vasopressin.98 Although galanin is packaged in some dense-core vesicles that contain vasopressin, it is also packaged in others that do not contain vasopressin and some dense-core vesicles contain vasopressin but no galanin. Presumably, differential packaging in dense-core vesicles might allow for secretion of separate pools that contain vasopressin or their co-expressed neuropeptides. Indeed, dense-core vesicles containing galanin alone are trafficked to the dendrites, whereas those that contain only vasopressin are trafficked to the axon terminals in the posterior pituitary gland.93
Vasopressin MNCs express apelin receptors (APJ receptors)99 and centrally administered apelin inhibits vasopressin MNCs86 to decrease basal vasopressin secretion.86,100 However, systemic apelin administration increases vasopressin secretion101 and chronic infusion of apelin into the PVN also increases vasopressin secretion.102 Furthermore, administration of apelin directly into the SON increases the activity of phasic MNCs (and presumably vasopressin secretion into the circulation) via non-specific cation channel activation, although it reduces somato-dendritic vasopressin secretion,99 which presumably weakens vasopressin-mediated autoregulation to disinhibit and thus further excite vasopressin MNCs.
Vasopressin MNCs express galanin receptor-1 on their somata and dendrites.103 Although centrally administered galanin increases vasopressin secretion into the circulation in vivo,104,105 it inhibits vasopressin secretion from isolated neurohypophyses or hypothalamic-neurohypophysial explants in vitro,106 suggesting that the direct effects of galanin are inhibitory, despite the reduced somato-dendritic vasopressin secretion. Indeed, galanin directly inhibits vasopressin MNCs in vitro by inducing hyperpolarisation and reducing the slow afterdepolarisation (sADP),107 which is a prominent excitatory post-spike potential in vasopressin MNCs.108 Galanin also reduces excitory post-synaptic current (EPSC) frequency,109 suggesting that it might also have paracrine effects after somato-dendritic secretion by retrograde inhibition of excitatory synaptic transmission.
Similarly to vasopressin receptors, κ-opioid receptors (KORs) are expressed in the membranes of vasopressin-containing dense-core vesicles110 and, unlike apelin and galanin, the endogenous opioid peptide (EOP) ligand for KORs, dynorphin, is packaged with vasopressin in the same dense-core vesicles.111 Hence, KORs newly trafficked to the vasopressin MNC plasma membrane will be exposed to high concentrations of dynorphin upon somato-dendritic secretion of dense-core vesicles.
KOR agonists inhibit vasopressin MNCs in vivo69,112 and in vitro.113,114 More importantly, antagonism of SON KORs increases burst duration in phasic MNCs under basal conditions in vivo69,70,115 and in vitro,70,116 showing that an endogenous KOR agonist inhibits phasic bursts. Phasic bursts are underpinned by the summation of sADPs to form a plateau potential that maintains a depolarised membrane potential to sustain further firing during bursts, and KOR activation causes activity-dependent sADP inhibition116 to progressively decrease the plateau potential amplitude, which eventually leads to burst termination.70 Furthermore, KOR desensitisation prevents phasic activity in vasopressin MNCs, even when intensely stimulated.69 Hence, an endogenous KOR agonist inhibits phasic MNCs by autocrine inhibition of the sADP in the MNC from which dynorphin is secreted and this inhibition appears to be necessary for the expression of phasic activity by vasopressin MNCs.
In addition to sADP inhibition, KOR agonists reduce excitory postsynaptic potential (EPSP) and inhibitory postsynaptic potential (IPSP) amplitude114,117 and the delayed rectifier potassium current,118 at the same time as increasing the transient A-type potassium current118 in vasopressin MNCs, although it has yet to be established whether these effects also contribute to the generation of phasic activity.
Although KOR activation inhibits continuously active vasopressin MNCs, KOR antagonism does not affect continuously active vasopressin MNCs, even when they are strongly excited.76 Hence, it appears that continuously active vasopressin MNCs express KORs but do not release sufficient dynorphin to affect activity. Some vasopressin MNCs display irregular activity and these MNCs appear to be even more strongly excited by KOR antagonism than phasic MNCs.76 Taken together, this pattern-dependent sensitivity to KOR inhibition suggests that somato-dendritic dynorphin secretion might determine the firing pattern of vasopressin MNCs and that transitions between firing patterns in individual vasopressin MNCs might result from changes in somato-dendritic dynorphin secretion.119
MNCs also express receptors for PACAP, which they also synthesise and secrete.97 PACAP increases somato-dendritic vasopressin secretion120 by a direct depolarisation through activation of non-specific cation channels.121
NERP-1–3 are packaged with vasopressin in the SON and PVN.94,95 NERP-1 has paracrine effects on vasopressin MNC activity by retrograde inhibition of excitatory synaptic transmission, whereas paracrine inhibition of vasopressin MNCs by NERP-2 is mediated by activation of upstream GABAergic interneurones that inhibit glutamatergic neurones that project to vasopressin MNCs.122 By contrast to NERP-1 and −2, NERP-3 stimulates vasopressin secretion from the isolated posterior pituitary gland.95
Vasopressin MNCs express secretin receptors and central secretin administration increases plasma vasopressin concentrations,13 suggesting that somato-dendritic secretin might stimulate systemic vasopressin secretion. However, secretin is also released from afferent inputs to the SON123 and systemic secretin administration excites vasopressin (and oxytocin) MNCs via noradrenergic afferent inputs,124 suggesting that its actions might be mediated by afferent inputs rather than somato-dendritic secretion. Although its role as a neurohypophysial hormone has not yet been definitively established, secretin is expressed in the posterior pituitary gland13 and increases insertion of aquaporin-2 into the luminal membrane of the kidney to increase water reabsorption.125 Hence, secretin synthesised by vasopressin MNCs might act as a neurohypophysial hormone after secretion from the posterior pituitary gland rather than as an autoregulatory factor secreted from the somata and dendrites.
Vasopressin MNCs express P2X and P2Y receptors126,127 and injection of ATP into the SON induces antidiuresis.128 ATP is packaged in vasopressin dense-core vesicles.87 ATP depolarises MNCs129 and increases vasopressin secretion from hypothalamic-neurohypophysial explants.130 ATP also increases glutamate and GABA release at synapses on MNCs.131 Hence, somato-dendritic ATP secretion might excite vasopressin MNCs by autocrine actions on the MNC from which it is secreted and paracrine actions on afferent inputs to the MNC from which it is secreted. However, MNCs are also excited by ATP released by astrocytes as a gliotransmitter,67,132 as well as by ATP released from noradrenergic afferent inputs.133
Although somato-dendritic ATP secretion might modulate vasopressin MNC activity, ATP is rapidly catabolised to adenosine in the extracellular space.134 Vasopressin MNCs express adenosine A1 and A2A receptors135 and A1 receptor antagonism excites phasic MNCs in vivo, although it does not affect the firing rate of continuously active vasopressin MNCs.136 A1 receptor antagonism reduces activity-dependent inhibition of EPSCs and inhibitory post-synaptic currents (IPSCs),137 as well as activity-dependent enhancement of the medium afterhyperpolarisation (mAHP) in vasopressin MNCs.138 mAHP activation induces spike frequency adaptation at the onset of phasic bursts139 and so endogenous adenosine enhancement of the mAHP increases spike frequency adaptation, thereby shortening bursts in phasic MNCs.136 Although A2 receptor activation depolarises MNCs to increase firing rate,135 vasopressin MNCs are inhibited when adenosine uptake is blocked. Hence, the overall effect of endogenous adenosine appears to be vasopressin MNC inhibition.140
In addition to somato-dendritic exocytosis, vasopressin MNCs also release the gaseous transmitters, NO and CO, by diffusion after synthesis by NO synthase and haem-oxygenase I, respectively. NO inhibits vasopressin MNCs141,142 by increasing IPSC amplitude and frequency,142,143 whereas CO excites vasopressin MNCs.88
Somato-dendritic modulation of vasopressin MNC activity appears to impact hormone secretion into the circulation through a complex interplay of excitatory- (apelin, PACAP, ATP, NO and perhaps secretin) and inhibitory- (galanin, dynorphin, NERPs, adenosine and CO) feedback that might fine tune the activity of individual MNCs to prevent any one MNC bearing too much of the secretory load for too long under basal conditions. It appears to be counter-intuitive that the autoregulatory effects of (at least some) co-secreted transmitters appear greater than that of vasopressin itself, which is secreted in vastly greater quantities. Perhaps the autoregulatory effects of co-secreted transmitters are magnified by activation of both paracrine and autocrine mechanisms. In addition, it is possible that the major role of vasopressin is paracrine inhibition of the population as a whole to prevent over-secretion of vasopressin into the circulation in response to perturbations of body fluid balance and/or blood pressure/volume.
7 |. AUTOCRINE/PARACRINE MODULATION OF OXYTOCIN MAGNOCELLULAR NEUROSECRETORY CELL ACTIVITY
Similar to somato-dendritic vasopressin secretion, somato-dendritic oxytocin secretion also has autocrine and paracrine actions that modulate oxytocin MNC activity. By contrast to vasopressin, the autocrine and paracrine effects of oxytocin are arranged in series rather than in parallel; somato-dendritic oxytocin secretion activates oxytocin receptors (OTRs) on oxytocin MNCs to increase intracellular calcium, which has various actions, including the release of endocannabinoids that cause retrograde inhibition of excitatory synaptic transmission under basal conditions.90 Although the oxytocin-stimulated retrograde endocannabinoid suppression of excitatory synaptic input is expected to inhibit oxytocin MNCs (Figure 6), the best characterised effect of somato-dendritic oxytocin secretion is excitation, although only under specific (patho)physiological conditions. Hence, it has been proposed that endocannabinoid inhibition might occur over a longer timescale than autocrine effects of oxytocin to shape activity patterning in oxytocin MNCs during birth and lactation,144 although it has yet to be established whether this occurs in vivo. Alternatively, the excitatory effects of oxytocin might involve a switch from endocannabinoid inhibition to excitation during pregnancy, perhaps by enhanced expression/activation of excitatory transient receptor potential vanilloid-1 channels145 for which the endocannabinoid, anandamide, is an endogenous ligand.146 Additionally, spillover of the endocannabinoid 2-arachi-donoylglycerol from glutamate onto GABA synapses and a resulting suppression of inhibitory synaptic input has been observed in MNCs following glial retraction induced by salt loading.91 This endocannabinoid spillover could also occur with glial retraction during parturition and lactation to reduce inhibitory synaptic transmission, although this remains to be determined. Finally, it is also possible that oxytocin-stimulated endocannabinoid retrograde modulation of excitatory and inhibitory synapses might be overridden by changes in the postsynaptic properties of oxytocin MNCs,147,148 increased excitatory afferent inputs,149–153 or a switch in GABA signalling from inhibitory to excitatory, or less inhibitory, during pregnancy.64,154
FIGURE 6.
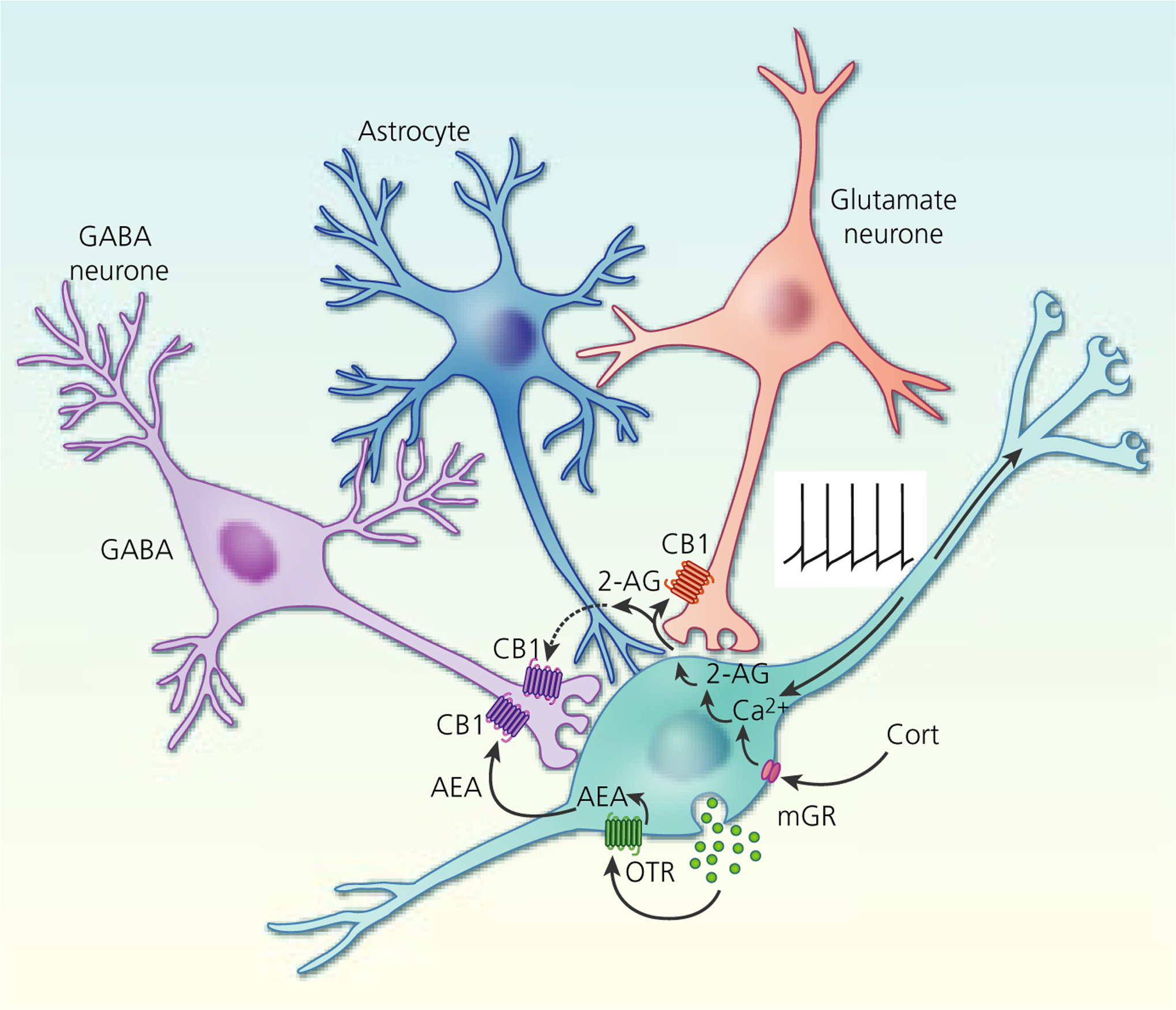
Endocannabinoid modulation of excitatory and inhibitory synapses on magnocellular neurosecretory cells (MNCs). Oxytocin activation of autocrine oxytocin receptors (OTR) on oxytocin neurones leads to a tonic basal release of the endocannabinoid anandamide (AEA) at GABA synapses, which tonically suppresses synaptic inhibitory input to oxytocin neurones by activating presynaptic CB1 receptors. Depolarisation (eg, via action potential generation) or corticosteroid (Cort) activation of membrane glucocorticoid receptors (mGR) (eg, during stress) leads to a calcium-dependent release of the other main endocannabinoid, 2-arachidonoylglycerol (2-AG), at glutamate synapses, which suppresses synaptic excitation of both oxytocin and vasopressin MNCs by activating presynaptic CB1 receptors. Glial retraction induced by salt loading allows the 2-AG released at glutamate synapses to spill over onto GABA synapses and suppress synaptic inhibition via CB1 receptor activation. Tonic AEA occupation of CB1 receptors at GABA synapses is non-saturating, allowing additional suppression of GABA release following phasic 2-AG release and synaptic spillover
Oxytocin MNCs typically exhibit continuous activity at approximately 1–5 spikes s−1 under basal conditions to maintain circulating oxytocin concentrations of approximately 1–3 pg mL−1, with higher concentrations during sleep.2 However, oxytocin is best known for its stimulation of rhythmic uterine contraction during birth and of episodic milk ejection during suckling. Uterine contractions and mammary duct contraction each occur at intervals of several minutes and each contraction is triggered by a coordinated, high frequency burst of activity across the population of oxytocin MNCs155 to secrete a discrete pulse of oxytocin into the circulation, which transiently increases intrauterine pressure during birth156 and intra-mammary pressure during suckling.157
Somato-dendritic oxytocin secretion increases immediately preceding each burst of activity in lactating rats158 and bursts are blocked by OTR antagonist administration,159 as is the rise in somato-dendritic oxytocin secretion,160 suggesting that somato-dendritic oxytocin secretion is required for bursts to occur. However, the mechanisms by which somato-dendritic oxytocin secretion promotes burst firing in oxytocin MNCs are not fully understood and it is probably not the only contributor; synchronised volleys of EPSPs,161 rebound depolarisation after bursts of IPSPs162 and enhancement of the sADP147,163 have all been proposed to trigger or sustain firing during bursts in oxytocin MNCs.
In brain slices from male rats that do not normally fire bursts, α1-adrenoceptor activation in low calcium can induce bursts in oxytocin MNCs reminiscent of those seen during birth and milk ejection.164 Hence, noradrenergic inputs might trigger bursts. Consistent with this hypothesis, noradrenergic innervation of oxytocin MNCs is increased in late pregnancy152 and these afferent inputs are activated during birth55 to increase noradrenaline release into the SON.165
Noradrenergic stimulation of bursts in oxytocin MNCs might be mediated by somato-dendritic oxytocin release because noradrenergic receptor stimulation is required for suckling-induced somato-dendritic oxytocin release,58 which might be part of a positive-feedback loop that builds towards bursts during continuous suckling. Indeed, burst-like activity can also be induced in virgin rats in vivo by coordinated activation of neighbouring oxytocin MNCs, which induces priming of somato-dendritic dense-core vesicles for subsequent secretion.28 Once primed, high-frequency electrical stimulation induces bursting in oxytocin MNCs of virgin rats.28 Hence, continuous suckling might trigger tonic noradrenaline release onto oxytocin MNCs that triggers increasing somato-dendritic oxytocin secretion, which could prime further somato-dendritic oxytocin secretion until a tipping-point is reached to induce each burst.
In addition to facilitating burst firing in individual oxytocin MNCs, somato-dendritic oxytocin secretion might also help coordinate the timing of bursts across the population of oxytocin MNCs. Oxytocin injection into one SON increases the frequency of milk ejection bursts in the contralateral SON.166 Oxytocin MNCs have one to three dendrites148 that are normally separated from neighbouring dendrites by astrocytic processes. However, in late pregnancy and lactation, astrocytes withdraw their processes from between oxytocin MNC dendrites, which then form bundles of approimately 10 closely apposed dendrites.167,168 A mathematical model in which oxytocin MNCs send each dendrite to different dendritic bundles to form a sparse network of interactions emulates burst firing in which each burst is initiated randomly at any of the dendritic bundles and spreads rapidly through the oxytocin MNC population.144
However, this model does not account for coordination of bursts across the bilateral SONs and PVNs, which might be mediated by noradrenergic inputs that project bilaterally to the SON.169 Indeed, sectioning the optic chiasm or mammillary body disrupts coordination of bursts between oxytocin MNCs in the left and right SON, suggesting that burst coordination across the magnocellular nuclei involves projections through these areas.169,170 Furthermore, the perinuclear zone that lies immediately dorsal to the SON sends prominent projections to the SON171 and PVN172,173 that might also contribute to coordination of bursts across the four main magnocellular nuclei.
8 |. AUTOCRINE/PARACRINE MODULATION OF OXYTOCIN MAGNOCELLULAR NEUROSECRETORY CELL ACTIVITY BY CO-SECRETED TRANSMITTERS
Oxytocin MNCs also synthesise other transmitters that are probably secreted from their somata and dendrites, although the effects of these co-transmitters are not as well characterised as for those released from vasopressin MNCs.
Oxytocin MNCs express μ-opioid receptors (MORs) and KORs,174,175 and MOR or KOR activation inhibits oxytocin MNCs.69,176 Although oxytocin MNCs synthesise μ- and κ-EOPs,177,178 neither MOR, nor KOR antagonists affect the activity of oxytocin MNCs in vivo.69,179 Hence, it appears that, if EOPs undergo somato-dendritic secretion with oxytocin, they do not modulate oxytocin MNC activity to any appreciable extent under basal conditions. MOR-mediated EOP inhibition of somato-dendritic oxytocin secretion and oxytocin MNC activity is increased in late pregnancy,180 although this modulation is probably mediated by afferent inputs.181
By contrast to the central actions of MOR activation, KOR activation appears to restrain secretion into the bloodstream at the posterior pituitary gland182 and this effect also increases in late pregnancy.183 KOR restraint of oxytocin secretion might build up stores of oxytocin for birth and lactation and might potentiate secretion during bursts because KORs are desensitised on the day of birth.184,185
Oxytocin MNC dense-core vesicles also contain ATP, which is presumably secreted along with oxytocin from the somata and dendrites. Co-secreted ATP does not modulate oxytocin MNC activity via adenosine receptor activation,136 but it might excite oxytocin MNC via P2X receptor activation.130,131
Oxytocin MNCs also express NO synthase96 and NO appears to restrain the activity of oxytocin MNCs, particularly under stimulated conditions,186,187 suggesting that NO is an inhibitory autocrine/paracrine modulator of oxytocin MNC activity.
Remarkably, chronic MOR activation by the opioid alkaloid agonist, morphine (but not EOPs188), induces tolerance and dependence in oxytocin MNCs.189 Tolerance is revealed as loss of inhibition to acute administration of morphine176 and dependence is revealed by a sustained hyperexcitation upon withdrawal of chronic morphine administration.112 Somato-dendritic oxytocin secretion is increased during morphine withdrawal and OTR antagonism reduces morphine withdrawal-induced excitation of oxytocin MNCs.190 Hence, somato-dendritic oxytocin secretion appears to contribute to morphine withdrawal-induced excitation of oxytocin MNCs, although the mechanism by which it does so has yet to be identified.
9 |. PARACRINE MODULATION OF PARVOCELLULAR PARAVENTRICULAR NUCLEUS NEURONE ACTIVITY BY SOMATO-DENDRITIC SECRETION FROM MAGNOCELLULAR NEUROSECRETORY CELLS
Although the SON essentially contains only MNCs (as well as glia and cells of the vasculature), the PVN also contains parvocellular neurones. Parvocellular neurones are subdivided by their projections and functions: neurosecretory parvocellular neurones project to the hypothalamic median eminence, where they secrete hormones into the hypophysial portal blood vessels to control hormone secretion from the anterior pituitary gland; preautonomic parvocellular neurones project to the brainstem and spinal cord to modulate parasympathetic and sympathetic nervous system activity191,192; the remaining parvocellular neurones project to various brain areas to modulate behaviour.
It has long been hypothesised that somato-dendritic secretion from MNCs modulates the activity of parvocellular neurones but only recently has definitive evidence to support this hypothesis been generated for somato-dendritic vasopressin.38,193,194 For paracrine effects on other neuronal phenotypes to occur, vasopressin (or oxytocin) must first diffuse through the parenchyma to reach other neurones. The effective diffusion distance for somato-dendritic vasopressin was determined under basal conditions using Chinese hamster ovary cells transfected with human V1aRs and a calcium indicator to generate biosensor ‘sniffer’ cells with a threshold detection level of 0.5 nmol L−1 and an EC50 of 7.2 nmol L−1 for vasopressin.38 Using sniffer cells that were dispersed over the PVN in hypothalamic slices, it was shown that activation of an individual MNC induces sufficient somato-dendritic vasopressin secretion to induce intracellular calcium increases for tens of seconds in sniffer cells over 100 μm from the soma of the activated MNC.38 Similar results were seen using HEK-293 sniffer cells in the SON.194 Hence, somato-dendritic vasopressin release from an individual MNC diffuses through the PVN at sufficient concentration to activate V1aRs expressed on parvocellular neurone somata or dendrites under basal conditions.38,193 Remarkably, astrocytes withdraw their processes from between MNCs when chronically stimulated,167,168,195,196 which reduces the tortuosity of the extracellular space and probably increases the effective diffusion distance for somato-dendritic vasopressin and oxytocin through the parenchyma.197
Some preautonomic parvocellular neurones project to the rostral ventrolateral medulla (RVLM) in the brainstem, which projects to sympathetic ganglia to regulate sympathetic nerve activity.198 RVLM-projecting parvocellular neurones express V1aRs and their dendrites are intermingled with vasopressin MNC somata and dendrites in the PVN.193 Hence, the architecture is in place to provide for somato-dendritic vasopressin modulation of autonomic function by paracrine modulation of preautonomic neurones within the PVN (Figure 7). Activation of individual vasopressin MNCs by uncaging NMDA increases action potential firing in RVLM-projecting parvocellular neurones beyond 100 μm from the activated MNC193 and this activation is much more potent than that elicited by action potential firing alone,38 suggesting that dendritic NMDARs might be a main driver of somato-denritic secretion. The responses of RVLM-projecting parvocellular neurones to vasopressin MNC activation occur after a delay of several seconds, are blocked by superfusion of a V1aR antagonist and are enhanced by a peptidase inhibitor.193 Most importantly, the depolarisation of RVLM-projecting parvocellular neurones in response to vasopressin MNC activation is not affected by blockade of action potential firing with tetrodotoxin. Taken together, the data demonstrate that the excitation of RVLM-projecting parvocellular neurones is mediated by diffusion of somato-dendritic vasopressin through the extracellular space of the PVN.
FIGURE 7.
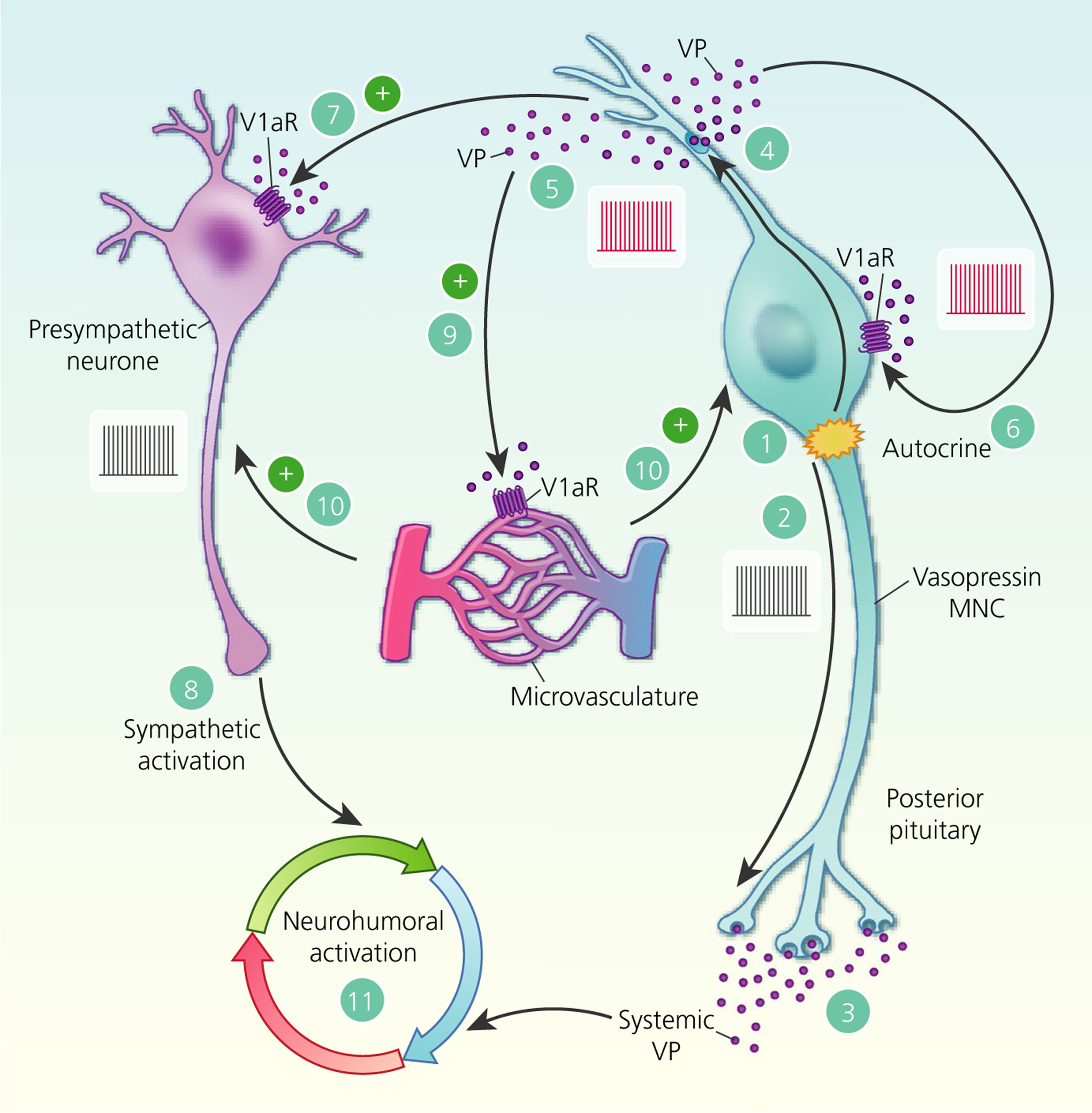
Paracrine actions of somato-dendritic vasopressin (VP) secretion. Activation of neurosecretory vasopressin magnocellular neurosecretory cells (MNCs) (1) triggers action potential firing (2) to release vasopressin into the circulation from the posterior pituitary gland (3). In parallel, action potentials back-propagate into the dendrites (4) to trigger somato-dendritic vasopressin secretion (5). In addition to autocrine feedback inhibition of vasopressin MNC activity via V1a receptors (V1aRs) (6), somato-dendritic vasopressin diffuses through the extracellular space to bind to V1aRs on presympathetic paraventricular nucleus neurones (7) to increase action potential firing (8) and therefore increase sympathetic outflow to peripheral organs. Somato-dendritic vasopressin also activates V1aRs on local blood vessels (9) to cause vasoconstriction, which is predicted to inhibit vasopressin MNCs at a population level (10) by restricting the availability of oxygen and nutrients. Hence, somato-dendritic vasopressin secretion coordinates neurohumoral responses to (patho)physiological activation (11)
Given that activation of an individual vasopressin MNC can excite preautonomic neurones within a radius of at least 100 μm, somato-dendritic secretion from the population of MNCs could function as a population-to-population signal to recruit preautonomic neurones as a whole. Indeed, administration of a V1aR antagonist alone reduces preautonomic neurone activity and the more active the MNC, the more effective is the antagonist at reducing preautonomic neurone activity,193 suggesting that preautonomic neurones are under tonic modulation by vasopressin MNCs to coordinate the humoral (circulating vasopressin) and neuronal (sympathetic nerve activity) responses to changes in body fluid balance and to pathophysiological conditions, such as hypertension, myocardial infarction and chronic heart failure.
Hyperosmolality increases vasopressin secretion into the circulation by MNCs and increases renal sympathetic nerve activity, both of which increase water retention to protect body fluid balance. The mechanisms that underpin these responses to hyperosmolality have been reviewed in detail elsewhere.199,200 Although these mechanisms occur in parallel, somato-dendritic vasopressin secretion probably coordinates the responses because intracarotid infusion of hyperosmotic saline causes a dose-dependent increase in renal sympathetic nerve activity that is accompanied by increased somato-dendritic vasopressin secretion, and bilateral injection of a V1aR antagonist into the PVN abolishes the renal sympathetic nerve activation.193 Hence, it appears that somato-dendritic vasopressin secretion coordinates the humoral and neuronal responses to increased osmolality.
PVN-driven sympathoexcitation is a key pathophysiological mechanism in hypertension,201,202 acute myocardial infarction192,203 and heart failure204,205 that contributes to morbidity and mortality.206 Vasopressin MNCs are activated in hypertension,207,208 acute myocardial infarction209 and heart failure.210,211 Although the increased circulating vasopressin levels contribute directly to detrimental myocardial effects,212,213 increased vasopressin MNC activity probably also increases somato-dendritic vasopressin secretion to contribute to the pathophysiological sympathoexcitation via activation of PVN preautonomic neurones.
Although less well established than for vasopressin effects on preautonomic neurones, it appears that somato-dendritic oxytocin secretion might also modulate the activity of a neighbouring population of neurones within the PVN, corticotrophin-releasing hormone (CRH) neurones. Stress-induced CRH secretion stimulates adrenocorticotrophic hormone secretion from the anterior pituitary gland,214 which, in turn, increases adrenal corticosteroid secretion to mediate the response to the stressor. CRH neurones express mRNA for OTRs215 and oxytocin inhibits EPSC frequency (but not amplitude) in CRH neurones.216 Oxytocin MNC and CRH neurone dendrites are intermingled within the PVN,216 allowing for den-dro-dendritic interactions between the two populations. Hence, somato-dendritic oxytocin might suppress CRH neurone excitability by presynaptic inhibition of excitatory synaptic inputs to reduce activation of the stress axis. However, OTR antagonism has no effect on CRH neurones in brain slices,216 suggesting that, unlike vasopressin modulation of preautonomic neurone activity, there is no OTR tone on CRH neurones, at least under in vitro basal conditions.
10 |. PARACRINE MODULATION OF ARTERIOLAR VASOCONSTRICTION IN THE SUPRAOPTIC NUCLEUS BY SOMATO-DENDRITIC SECRETION FROM MAGNOCELLULAR NEUROSECRETORY CELLS
Classically, neuronal activity is considered to dilate arterioles and thereby increase local cerebral blood flow to meet the metabolic demands of active brain areas; this ‘neurovascular coupling’ is generally accepted to result from glutamatergic synaptic transmission that evokes the release of vasoactive substances from neurones and astrocytes to relax vascular smooth muscle cells.217 However, vascular smooth muscle cells express V1aRs, providing a target for somato-dendritic vasopressin, at least within the SON and PVN. Vasoconstriction can be elicited in SON arterioles by stimulation of an individual vasopressin MNC, an effect that is blocked by V1aR antagonism218 (Figure 7). Consistent with its effects on preautonomic neurones, somato-dendritic vasopressin can induce responses in arterioles beyond 100 μm from the activated MNC under basal conditions.218 Importantly, this V1aR-mediated vasoconstriction is overridden in hyperosmotic conditions by parallel release of NO, which causes vasodilation of local arterioles.218 Presumably, the NO-induced vasodilation increases blood flow through the SON when increased vasopressin MNC activity is required to protect from further fluid loss and maintain blood pressure in the general circulation through vasopressin secretion from the posterior pituitary gland.
11 |. PARACRINE MODULATION OF NEURONAL ACTIVITY BEYOND THE PARAVENTRICULAR NUCLEUS BY SOMATO-DENDRITIC SECRETION FROM MAGNOCELLULAR NEUROSECRETORY CELLS
Although paracrine modulation of parvocellular neurones within the PVN by somato-dendritic secretion from MNCs is now well characterised, it has yet to be definitively established whether neuropeptides secreted from MNC somata and dendrites can affect the activity of neurones outside the PVN. Nevertheless, there is some evidence that somato-dendritic oxytocin might act on neurones in brain areas relatively close to the SON and/or PVN, particularly for brain areas that receive little or no axonal projections from MNCs or from vasopressin or oxytocin parvocellular neurones.
The central effects of the primary anorexigenic hormone, leptin, are mediated by oxytocin, at least in part.219 Leptin is sensed by ARC POMC neurones that, as described above, project to the SON and PVN,61 where they secrete α-MSH to activate MC4-Rs62 and thereby increase somato-dendritic oxytocin secretion.63,220 Oxytocin inhibition of food intake is mediated, in part, by the ventromedial hypothalamus (VMH) because oxytocin injection into the VMH decreases food intake that is driven by energy balance rather than palatability.221 Although OTRs are highly expressed in the VMH,222 there are essentially no oxytocin MNC axons in the VMH.219 Given that vasopressin released from a single MNC can activate cells over 100 μm from the MNC soma from which it is secreted38,193 and the VMH is situated roughly between the SON and PVN, it is possible that somato-dendritic oxytocin release from MNCs could diffuse through the parenchyma in sufficient quantities to activate OTRs in the VMH, which have nanomolar affinity for oxytocin.223 It is also possible that OTR-expressing astrocytes in the SON and PVN could expand the spatial domain of the dendritically released oxytocin signalling by relaying the signals through astrocytic networks, as has been reported for vasopressin release from vasopressin MNC dendrites67 and CRH neurone dendrites.224
Although oxytocin MNCs inhibit fear responses via axon collaterals to the CeA,17 SON somato-dendritic oxytocin secretion probably enhances social recognition via actions in the MeA. The CeA contains oxytocin MNC axon collaterals, although there are no oxytocin (MNC or parvocellular) neurone axons in the MeA.17 OTRs are highly expressed in the MeA222 and OTR antagonist injection into the MeA reduces social recognition induced by SON activation.225 However, MeA OTRs are not directly activated by oxytocin secreted into the CeA from MNC axon collaterals.17 The MeA lies immediately lateral to the SON and, even if somato-dendritic oxytocin secreted within the SON does not reach the MeA, some oxytocin MNC dendrites project to the MeA,225 which might deliver sufficient oxytocin to the MeA to promote social recognition. Alternatively, OTRs might be activated by vasopressin MNC axon collaterals in the MeA19 because vasopressin has appreciable activity at OTRs.223
12 |. HORMONE-LIKE MODULATION OF NEURONAL ACTIVITY IN DISTANT BRAIN AREAS BY SOMATO-DENDRITIC SECRETION FROM MAGNOCELLULAR NEUROSECRETORY CELLS
It has been hypothesised that somato-dendritic vasopressin and oxytocin from MNCs comprise a hormone-like signal in the brain with widespread effects on distant populations of neurones.226 However, accumulating evidence of the functional impact of MNC axon collaterals on behaviour via direct projections to distant brain areas17–21 have led to this hypothesis being challenged.227
The half-lives of vasopressin and oxytocin are approximately 20 minutes in the cerebrospinal fluid (CSF),228 giving time for diffusion through the ventricular system, particularly downstream. However, vasopressin (at least) has a half-life of less than 1 minute in the parenchyma.229 Given that the paracrine effects of somato-dendritic vasopressin on preautonomic neurones that are only approximately 100 μm away is delayed by 2–5 seconds,193 it is improbable that the neuropeptides could diffuse long distances through the brain to act as a hormone-like signal. However, dense-core vesicle exocytosis is a slow process compared to microvesicle fusion at the synapse, with latencies of several seconds in hippocampal neurones,230 which could account for much of the latency of the preautonomic neurone response to somato-dendritic vasopressin secreted by MNCs in the PVN. Furthermore, vasopressin and oxytocin are secreted in sufficient quantities to be measured in dialysates collected from the SON and PVN,231 as well as in other brain areas232–234 and in the CSF,235 suggesting that they diffuse through the parenchyma sufficiently to reach the CSF. Indeed, the microdialysis probes used to measure vasopressin and oxytocin in many experiments have a recovery rate of <10% for vasopressin in the SON and PVN.236 Hence, the actual concentrations of vasopressin and oxytocin present in the parenchyma and CSF are probably appreciably higher than those measured in dialysates. A further factor to be considered is retraction of astrocytic processes from around MNCs in dehydration and pregnancy,167,168,195,196 which decreases tortuosity in the extracellular space, presumably allowing more ready escape of somato-dendritic vasopressin and oxytocin from the SON and PVN. It is difficult to imagine that the relatively sparse terminal fields of MNC axon collaterals and parvocellular vasopressin and oxytocin MNCs could release sufficient vasopressin and oxytocin to maintain the ambient levels of the neuropeptides found in the brain and CSF.
Although there are clear examples of axon collaterals from sub-populations of MNCs affecting neuronal activity beyond the SON and PVN,237 this does not preclude the possibility that there is also long-distance inter-population signalling mediated by somato-dendritic volume transmission of vasopressin and oxytocin over a longer timescale, particularly in brain regions in which there are neuropeptide receptors but no neuropeptide axons, such as in the olfactory bulb. Nevertheless, there is still no compelling evidence for (or against) distal hormone-like signalling by somato-dendritic vasopressin and oxytocin transmission, although the levels of oxytocin and vasopressin present in the cerebrospinal fluid and in brain areas devoid of oxytocin and vasopressin axon terminals appear to be much higher than could be achieved from axon terminal release from MNC and parvocellular axon collaterals. The resolution of this debate remains an ongoing challenge for the field.
13 |. CONCLUDING REMARKS
Autocrine/paracrine modulation of MNC activity by somato-dendritic vasopressin and oxytocin release has been extensively studied and is broadly accepted as a major function of somato-dendritic secretion from MNCs.238 Although it is clear that co-secreted transmitters also modulate MNC activity, there is no evidence of paracrine actions on other neurones, even other MNCs. Indeed, somato-dendritic dynorphin terminates bursts in the MNC from which it is secreted,70 although there is no correlation between burst termination in phasic MNCs in paired recordings using a single electrode in which the recorded MNCs would be at most tens of micrometres apart,71 which is well within the range of somato-dendritic vasopressin but evidently beyond the range of somato-dendritic dynorphin. Hence, it is possible that co-secreted transmitters act as autocrine/paracrine modulators of the individual MNC from which they are secreted, whereas the much higher levels of vasopressin or oxytocin secreted modulate the activity of the population as a whole to regulate peripheral physiology.
Recently, compelling evidence has emerged indicating that somato-dendritic secretion from MNCs modulates the arteriole diameter in the SON,218 as well as the activity of neurones, particularly RVLM-projecting preautonomic neurones in the parvocellular PVN193 (and perhaps also CRH neurones216). This inter-population cross-talk between MNCs and preautonomic neurones probably coordinates the hormonal (vasopressin secretion into the circulation) and neural (sympathetic nerve activation) response to perturbations of body fluid balance and blood pressure/volume.239
To date, there is no definitive evidence that somato-dendritic vasopressin and oxytocin have actions beyond the SON and PVN and it remains to be determined whether these neuropeptides act as hormone-like signals after secretion from MNC somata and dendrites. Nevertheless, although much of the data remains circumstantial, somato-dendritic oxytocin release probably modulates the activity of neurones in some nearby brain areas that express OTRs but do not contain oxytocin (MNC or parvocellular) neurone projections, specifically the VMH219,222 and MeA.222,225 The confirmation (or refutation) of the effects of somato-dendritic oxytocin on the VMH and/or MeA is required to resolve the ongoing debate within this field.
REFERENCES
- 1.Ludwig M Dendritic Neurotransmitter Release. New York: Kluwer Academic Publishers; 2004. [Google Scholar]
- 2.Brown CH. Magnocellular neurons and posterior pituitary function. Compr Physiol. 2016;6:1701–1741. [DOI] [PubMed] [Google Scholar]
- 3.Verbalis JG, Mangione MP, Stricker EM. Oxytocin produces natriuresis in rats at physiological plasma concentrations. Endocrinology. 1991;128:1317–1322. [DOI] [PubMed] [Google Scholar]
- 4.Haanwinckel MA, Elias LK, Favaretto AL, Gutkowska J, McCann SM, Antunes-Rodrigues J. Oxytocin mediates atrial natriuretic peptide release and natriuresis after volume expansion in the rat. Proc Natl Acad Sci USA. 1995;92:7902–7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manaye KF, Lei DL, Tizabi Y, Davila-Garcia MI, Mouton PR, Kelly PH. Selective neuron loss in the paraventricular nucleus of hypothalamus in patients suffering from major depression and bipolar disorder. J Neuropathol Exp Neurol. 2005;64:224–229. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes CH, Morrell JI, Pfaff DW. Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. J Comp Neurol. 1981;198:45–64. [DOI] [PubMed] [Google Scholar]
- 7.Tweedle CD, Smithson KG, Hatton GI. Neurosecretory endings in the rat neurohypophysis are en passant. Exp Neurol. 1989;106:20–26. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann JJ, Morris JF. Method for quantitating the molecular content of a subcellular organelle: hormone and neurophysin content of newly formed and aged neurosecretory granules. Proc Natl Acad Sci USA. 1984;81:180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol. 2008;586:5625–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng G, Brown C, Sabatier N, Scott V. Population dynamics in vasopressin cells. Neuroendocrinology. 2008;88:160–172. [DOI] [PubMed] [Google Scholar]
- 11.Brown CH, Ruan M, Scott V, Tobin VA, Ludwig M. Multi-factorial somato-dendritic regulation of phasic spike discharge in vasopressin neurons. Prog Brain Res. 2008;170:219–228. [DOI] [PubMed] [Google Scholar]
- 12.Meeker RB, Swanson DJ, Greenwood RS, Hayward JN. Ultrastructural distribution of glutamate immunoreactivity within neurosecretory endings and pituicytes of the rat neurohypophysis. Brain Res. 1991;564:181–193. [DOI] [PubMed] [Google Scholar]
- 13.Chu JY, Lee LTO, Lai CH et al. Secretin as a neurohypophysial factor regulating body water homeostasis. Proc Natl Acad Sci USA. 2009;106:15961–15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemos JR, Ortiz-Miranda SI, Cuadra AE et al. Modulation/physiology of calcium channel sub-types in neurosecretory terminals. Cell Calcium. 2012;51:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason WT, Ho YW, Hatton GI. Axon collaterals of supraoptic neurones: anatomical and electrophysiological evidence for their existence in the lateral hypothalamus. Neuroscience. 1984;11:169–182. [DOI] [PubMed] [Google Scholar]
- 16.Hatton GI, Cobbett P, Salm AK. Extranuclear axon collaterals of paraventricular neurons in the rat hypothalamus: intracellular staining, immunocytochemistry and electrophysiology. Brain Res Bull. 1985;14:123–132. [DOI] [PubMed] [Google Scholar]
- 17.Knobloch HS, Charlet A, Hoffmann LC et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. [DOI] [PubMed] [Google Scholar]
- 18.Menon R, Grund T, Zoicas I et al. Oxytocin signaling in the lateral septum prevents social fear during lactation. Curr Biol. 2018;28:1066–78.e6. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez VS, Vazquez-Juarez E, Marquez MM, Jauregui-Huerta F, Barrio RA, Zhang L. Extra-neurohypophyseal axonal projections from individual vasopressin-containing magnocellular neurons in rat hypothalamus. Front Neuroanat. 2015;9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez VS, Hernandez OR, de la Mora M Perez et al. Hypothalamic vasopressinergic projections innervate central amygdala GABAergic neurons: Implications for anxiety and stress coping. Front Neural Circuits. 2016;10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez-Perez OR, Hernandez VS, Nava-Kopp AT et al. A synaptically connected hypothalamic magnocellular vasopressin-locus coeruleus neuronal circuit and its plasticity in response to emotional and physiological stress. Front Neurosci. 2019;13:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeo SH, Kyle V, Blouet C, Jones S, Colledge WH. Mapping neuronal inputs to Kiss1 neurons in the arcuate nucleus of the mouse. PLoS ONE. 2019;14:e0213927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong WE, Scholer J, McNeill TH. Immunocytochemical, golgi and electron microscopic characterization of putative dendrites in the ventral glial lamina of the rat supraoptic nucleus. Neuroscience. 1982;7:679–694. [DOI] [PubMed] [Google Scholar]
- 24.Hatton GI. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog Neurogibol. 1990;34:437–504. [DOI] [PubMed] [Google Scholar]
- 25.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig M, Apps D, Menzies J, Patel JC, Rice ME. Dendritic release of neurotransmitters. Compr Physiol. 2017;7:235–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig M, Bull PM, Tobin VA et al. Regulation of activity-dependent dendritic vasopressin release from rat supraoptic neurones. J Physiol. 2005;564:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. [DOI] [PubMed] [Google Scholar]
- 29.Dayanithi G, Forostyak O, Ueta Y, Verkhratsky A, Toescu EC. Segregation of calcium signalling mechanisms in magnocellular neurones and terminals. Cell Calcium. 2012;51:293–299. [DOI] [PubMed] [Google Scholar]
- 30.Fisher TE, Bourque CW. Calcium-channel subtypes in the somata and axon terminals of magnocellular neurosecretory cells. Trends Neurosci. 1996;19:440–444. [DOI] [PubMed] [Google Scholar]
- 31.Tobin VA, Douglas AJ, Leng G, Ludwig M. The involvement of voltage-operated calcium channels in somato-dendritic oxytocin release. PLoS ONE. 2011;6:e25366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bains JS, Ferguson AV. Activation of N-methyl-D-aspartate receptors evokes calcium spikes in the dendrites of rat hypothalamic paraventricular nucleus neurons. Neuroscience. 1999;90:885–891. [DOI] [PubMed] [Google Scholar]
- 33.de Kock CP, Wierda KD, Bosman LW et al. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown CH, Scott V, Ludwig M, Leng G, Bourque CW. Somatodendritic dynorphin release: orchestrating activity patterns of vasopressin neurons. Biochem Soc Trans. 2007;35:1236–1242. [DOI] [PubMed] [Google Scholar]
- 35.Joe N, Scott V, Brown CH. Glial regulation of extrasynaptic NMDA receptor-mediated excitation of supraoptic nucleus neurons during dehydration. J Neuroendocrinol. 2014;26:35–42. [DOI] [PubMed] [Google Scholar]
- 36.Sabatier N alpha-Melanocyte-stimulating hormone and oxytocin: a peptide signalling cascade in the hypothalamus. J Neuroendocrinol. 2006;18:703–710. [DOI] [PubMed] [Google Scholar]
- 37.Sabatier N, Caquineau C, Dayanithi G et al. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitra S, Zhang M, Cauley E, Stern JE. NMDA receptors potentiate activity-dependent dendritic release of neuropeptides from hypothalamic neurons. J Physiol. 2019;597:1735–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol. 2011;589:3929–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naskar K, Stern JE. A functional coupling between extrasynaptic NMDA receptors and A-type K+ channels under astrocyte control regulates hypothalamic neurosecretory neuronal activity. J Physiol. 2014;592:2813–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M, Biancardi VC, Stern JE. An increased extrasynaptic NMDA tone inhibits A-type K(+) current and increases excitability of hypothalamic neurosecretory neurons in hypertensive rats. J Physiol. 2017;595:4647–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobin VA, Hurst G, Norrie L, Dal Rio FP, Bull PM, Ludwig M. Thapsigargin-induced mobilization of dendritic dense-cored vesicles in rat supraoptic neurons. Eur J Neurosci. 2004;19:2909–2912. [DOI] [PubMed] [Google Scholar]
- 43.Hurbin A, Boissin-Agasse L, Orcel H et al. The V1a and V1b, but not V2, vasopressin receptor genes are expressed in the supraoptic nucleus of the rat hypothalamus, and the transcripts are essentially colocalized in the vasopressinergic magnocellular neurons. Endocrinology. 1998;139:4701–4707. [DOI] [PubMed] [Google Scholar]
- 44.Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–5104. [DOI] [PubMed] [Google Scholar]
- 45.Dayanithi G, Sabatier N, Widmer H. Intracellular calcium signalling in magnocellular neurones of the rat supraoptic nucleus: understanding the autoregulatory mechanisms. ExpPhysiol. 2000;85:75S. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Kindrat AN, Sharif-Naeini R, Bourque CW. Actin filaments mediate mechanical gating during osmosensory transduction in rat supraoptic nucleus neurons. J Neurosci. 2007;27:4008–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prager-Khoutorsky M, Khoutorsky A, Bourque CW. Unique interweaved microtubule scaffold mediates osmosensory transduction via physical interaction with TRPV1. Neuron. 2014;83:866–878. [DOI] [PubMed] [Google Scholar]
- 48.Tobin VA, Ludwig M. The role of the actin cytoskeleton in oxytocin and vasopressin release from rat supraoptic nucleus neurons. J Physiol. 2007;582:1337–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris JF, Pow DV. Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytoses. AnatRec. 1991;231:437. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Q, Zhou P, Wang AL et al. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature. 2017;548:420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jurgutis P, Shuang R, Fletcher A, Stuenkel EL. Characterization and distribution of SNARE proteins at neuroendocrine nerve endings. Neuroendocrinology. 1996;64:379–392. [DOI] [PubMed] [Google Scholar]
- 52.Pupier S, Leveque C, Marqueze B, Kataoka M, Takahashi M, Seagar MJ. Cysteine string proteins associated with secretory granules of the rat neurohypophysis. J Neurosci. 1997;17:2722–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyata S, Takamatsu H, Maekawa S et al. Plasticity of neurohypophysial terminals with increased hormonal release during dehydration: ultrastructural and biochemical analyses. J Comp Neurol. 2001;434:413–427. [DOI] [PubMed] [Google Scholar]
- 54.Tobin V, Schwab Y, Lelos N, Onaka T, Pittman QJ, Ludwig M. Expression of exocytosis proteins in rat supraoptic nucleus neurones. J Neuroendocrinol. 2012;24:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meddle SL, Leng G, Selvarajah JR, Bicknell RJ, Russell JA. Direct pathways to the supraoptic nucleus from the brainstem and the main olfactory bulb are activated at parturition in the rat. Neuroscience. 2000;101:1013–1021. [DOI] [PubMed] [Google Scholar]
- 56.Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9:11–25. [DOI] [PubMed] [Google Scholar]
- 57.Lipschitz DL, Crowley WR, Bealer SL. Differential sensitivity of intranuclear and systemic oxytocin release to central noradrenergic receptor stimulation during mid- and late gestation in rats. Am J Physiol Endocrinol Metab. 2004;287:E523–E528. [DOI] [PubMed] [Google Scholar]
- 58.Bealer SL, Crowley WR. Noradrenergic control of central oxytocin release during lactation in rats. Am J Physiol. 1998;274:E453–E458. [DOI] [PubMed] [Google Scholar]
- 59.Bealer SL, Armstrong WE, Crowley WR. Oxytocin release in magnocellular nuclei: neurochemical mediators and functional significance during gestation. Am J Physiol Regul Integr Comp Physiol. 2010;299:R452–R528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Onaka T, Ikeda K, Yamashita T, Honda K. Facilitative role of endogenous oxytocin in noradrenaline release in the rat supraoptic nucleus. Eur J Neurosci. 2003;18:3018–3026. [DOI] [PubMed] [Google Scholar]
- 61.Bagnol D, Lu XY, Kaelin CB et al. Anatomy of an endogenous antagonist: Relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:Rc26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ladyman SR, Augustine RA, Scherf E, Phillipps HR, Brown CH, Grattan DR. Attenuated hypothalamic responses to alpha-melanocyte stimulating hormone during pregnancy in the rat. J Physiol. 2016;594:1087–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sabatier N, Leng G. Presynaptic actions of endocannabinoids mediate alpha-MSH-induced inhibition of oxytocin cells. Am J Physiol Regul Integr Comp Physiol. 2006;290:R577–R584. [DOI] [PubMed] [Google Scholar]
- 64.Augustine RA, Ladyman SR, Bouwer GT et al. Prolactin regulation of oxytocin neurone activity in pregnancy and lactation. J Physiol. 2017;595:3591–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakata I, Nakano Y, Osborne-Lawrence S et al. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishizaki S, Murase T, Sugimura Y et al. Role of ghrelin in the regulation of vasopressin release in conscious rats. Endocrinology. 2002;143:1589–1593. [DOI] [PubMed] [Google Scholar]
- 67.Haam J, Halmos KC, Di S, Tasker JG. Nutritional state-dependent ghrelin activation of vasopressin neurons via retrograde trans-neuronal-glial stimulation of excitatory GABA circuits. J Neurosci. 2014;34:6201–6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hurbin A, Orcel H, Alonso G, Moos F, Rabie A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143:456–466. [DOI] [PubMed] [Google Scholar]
- 69.Brown CH, Ludwig M, Leng G. Kappa-opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. J Neurosci. 1998;18:9480–9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown CH, Leng G, Ludwig M, Bourque CW. Endogenous activation of supraoptic nucleus kappa-opioid receptors terminates spontaneous phasic bursts in rat magnocellular neurosecretory cells. J Neurophysiol. 2006;95:3235–3244. [DOI] [PubMed] [Google Scholar]
- 71.Brown CH. Rhythmogenesis in vasopressin cells. J Neuroendocrinol. 2004;16:727–739. [DOI] [PubMed] [Google Scholar]
- 72.Brown CH, Bourque CW. Mechanisms of rhythmogenesis: insights from hypothalamic vasopressin neurons. Trends Neurosci. 2006;29:108–115. [DOI] [PubMed] [Google Scholar]
- 73.Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. J Exp Biol. 1988;139:51–65. [DOI] [PubMed] [Google Scholar]
- 74.Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bicknell RJ, Brown D, Chapman C, Hancock PD, Leng G. Reversible fatigue of stimulus-secretion coupling in the rat neurohypophysis. J Physiol. 1984;348:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott V, Bishop VR, Leng G, Brown CH. Dehydration-induced modulation of kappa-opioid inhibition of vasopressin neurone activity. J Physiol. 2009;587:5679–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978;148:425–440. [DOI] [PubMed] [Google Scholar]
- 78.Dyball RE, Pountney PS. Discharge patterns of supraoptic and paraventricular neurones in rats given a 2 per cent NaCl solution instead of drinking water. J Endocrinol. 1973;56:91–98. [DOI] [PubMed] [Google Scholar]
- 79.Walters JK, Hatton GI. Supraoptic neuronal activity in rats during five days of water deprivation. Physiol Behav. 1974;13:661–667. [DOI] [PubMed] [Google Scholar]
- 80.Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. [DOI] [PubMed] [Google Scholar]
- 81.Kombian SB, Mouginot D, Hirasawa M, Pittman QJ. Vasopressin preferentially depresses excitatory over inhibitory synaptic transmission in the rat supraoptic nucleus in vitro. J Neuroendocrinol. 2000;12:361–367. [DOI] [PubMed] [Google Scholar]
- 82.Hermes ML, Ruijter JM, Klop A, Buijs RM, Renaud LP. Vasopressin increases GABAergic inhibition of rat hypothalamic paraventricular nucleus neurons in vitro. J Neurophysiol. 2000;83:705–711. [DOI] [PubMed] [Google Scholar]
- 83.Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular vasopressin neurons. J Neurosci. 1998;18:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yosten GL, Samson WK. Pressor doses of vasopressin result in only transient elevations in plasma peptide levels. Peptides. 2012;33:342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ludwig M, Callahan MF, Neumann I, Landgraf R, Morris M. Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. J Neuroendocrinol. 1994;6:369–373. [DOI] [PubMed] [Google Scholar]
- 86.De Mota N, Reaux-Le Goazigo A, El Messari S et al. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci USA. 2004;101:10464–10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poisner AM, Douglas WW. Adenosine triphosphate and adenosine triphosphatase in hormone-containing granules of posterior pituitary gland. Science. 1968;160:203–204. [DOI] [PubMed] [Google Scholar]
- 88.Reis WL, Biancardi VC, Son S, Antunes-Rodrigues J, Stern JE. Enhanced expression of heme oxygenase-1 and carbon monoxide excitatory effects in oxytocin and vasopressin neurones during water deprivation. J Neuroendocrinol. 2012;24:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watson SJ, Akil H, Fischli W et al. Dynorphin and vasopressin: common localization in magnocellular neurons. Science. 1982;216:85–87. [DOI] [PubMed] [Google Scholar]
- 90.Hirasawa M, Schwab Y, Natah S et al. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol. 2004;559:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di S, Popescu IR, Tasker JG. Glial control of endocannabinoid heterosynaptic modulation in hypothalamic magnocellular neuroendocrine cells. J Neurosci. 2013;33:18331–18342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iremonger KJ, Kuzmiski JB, Baimoukhametova DV, Bains JS. Dual regulation of anterograde and retrograde transmission by endocannabinoids. J Neurosci. 2011;31:12011–12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Landry M, Vila-Porcile E, Hokfelt T, Calas A. Differential routing of coexisting neuropeptides in vasopressin neurons. Eur J Neurosci. 2003;17:579–589. [PubMed] [Google Scholar]
- 94.Yamaguchi H, Sasaki K, Satomi Y et al. Peptidomic identification and biological validation of neuroendocrine regulatory peptide-1 and −2. J Biol Chem. 2007;282:26354–26360. [DOI] [PubMed] [Google Scholar]
- 95.Fujihara H, Sasaki K, Mishiro-Sato E et al. Molecular characterization and biological function of neuroendocrine regulatory peptide-3 in the rat. Endocrinology. 2012;153:1377–1386. [DOI] [PubMed] [Google Scholar]
- 96.Kadowaki K, Kishimoto J, Leng G, Emson PC. Up-regulation of nitric oxide synthase (NOS) gene expression together with NOS activity in the rat hypothalamo-hypophysial system after chronic salt loading: evidence of a neuromodulatory role of nitric oxide in arginine vasopressin and oxytocin secretion. Endocrinology. 1994;134:1011–1017. [DOI] [PubMed] [Google Scholar]
- 97.Gillard ER, Leon-Olea M, Mucio-Ramirez S et al. A novel role for endogenous pituitary adenylate cyclase activating polypeptide in the magnocellular neuroendocrine system. Endocrinology. 2006;147:791–803. [DOI] [PubMed] [Google Scholar]
- 98.Reaux-Le Goazigo A, Morinville A, Burlet A, Llorens-Cortes C, Beaudet A. Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology. 2004;145:4392–4400. [DOI] [PubMed] [Google Scholar]
- 99.Tobin VA, Bull PM, Arunachalam S, O’Carroll AM, Ueta Y, Ludwig M. The effects of apelin on the electrical activity of hypothalamic magnocellular vasopressin and oxytocin neurons and somatodendritic peptide release. Endocrinology. 2008;149:6136–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reaux A, De Mota N, Skultetyova I et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77:1085–1096. [DOI] [PubMed] [Google Scholar]
- 101.Hus-Citharel A, Bodineau L, Frugiere A, Joubert F, Bouby N, Llorens-Cortes C. Apelin counteracts vasopressin-induced water reabsorption via cross talk between apelin and vasopressin receptor signaling pathways in the rat collecting duct. Endocrinology. 2014;155:4483–4493. [DOI] [PubMed] [Google Scholar]
- 102.Zhang F, Sun HJ, Xiong XQ et al. Apelin-13 and APJ in paraventricular nucleus contribute to hypertension via sympathetic activation and vasopressin release in spontaneously hypertensive rats. Acta Physiol (Oxf). 2014;212:17–27. [DOI] [PubMed] [Google Scholar]
- 103.Burazin TC, Larm JA, Gundlach AL. Regulation by osmotic stimuli of galanin-R1 receptor expression in magnocellular neurones of the paraventricular and supraoptic nuclei of the rat. J Neuroendocrinol. 2001;13:358–370. [DOI] [PubMed] [Google Scholar]
- 104.Ciosek J, Cisowska A. Centrally administered galanin modifies vasopressin and oxytocin release from the hypothalamo-neurohypophysial system of euhydrated and dehydrated rats. J Physiol Pharmacol. 2003;54:625–641. [PubMed] [Google Scholar]
- 105.Cisowska-Maciejewska A, Ciosek J. Galanin influences vasopressin and oxytocin release from the hypothalamo-neurohypophysial system of salt loaded rats. J Physiol Pharmacol. 2005;56:673–688. [PubMed] [Google Scholar]
- 106.Wodowska J, Ciosek J. Galanin and galanin-like peptide modulate vasopressin and oxytocin release in vitro: the role of galanin receptors. Neuropeptides. 2014;48:387–397. [DOI] [PubMed] [Google Scholar]
- 107.Papas S, Bourque CW. Galanin inhibits continuous and phasic firing in rat hypothalamic magnocellular neurosecretory cells. J Neurosci. 1997;17:6048–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. [DOI] [PubMed] [Google Scholar]
- 109.Kozoriz MG, Kuzmiski JB, Hirasawa M, Pittman QJ. Galanin modulates neuronal and synaptic properties in the rat supraoptic nucleus in a use and state dependent manner. J Neurophysiol. 2006;96:154–164. [DOI] [PubMed] [Google Scholar]
- 110.Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. The kappa opioid receptor and dynorphin co-localize in vasopressin magnocellular neurosecretory neurons in guinea-pig hypothalamus. Neuroscience. 2000;96:373–383. [DOI] [PubMed] [Google Scholar]
- 111.Whitnall MH, Gainer H, Cox BM, Molineaux CJ. Dynorphin-A-(1–8) is contained within vasopressin neurosecretory vesicles in rat pituitary. Science. 1983;222:1137–1139. [DOI] [PubMed] [Google Scholar]
- 112.Ludwig M, Brown CH, Russell JA, Leng G. Local opioid inhibition and morphine dependence of supraoptic nucleus oxytocin neurones in the rat in vivo. J Physiol. 1997;505:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Inenaga K, Imura H, Yanaihara N, Yamashita H. Kappa-selective opioid receptor agonists leumorphin and dynorphin inhibit supraoptic neurons in rat hypothalamic slice preparations. J Neuroendocrinol. 1990;2:389–395. [DOI] [PubMed] [Google Scholar]
- 114.Inenaga K, Nagatomo T, Nakao K, Yanaihara N, Yamashita H. Kappa-selective agonists decrease postsynaptic potentials and calcium components of action potentials in the supraoptic nucleus of rat hypothalamus in vitro. Neuroscience. 1994;58:331–340. [DOI] [PubMed] [Google Scholar]
- 115.Brown CH, Ludwig M, Leng G. Temporal dissociation of the feedback effects of dendritically co-released peptides on rhythmogenesis in vasopressin cells. Neuroscience. 2004;124:105–111. [DOI] [PubMed] [Google Scholar]
- 116.Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29:7349–7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muller W, Hallermann S, Swandulla D. Opioidergic modulation of voltage-activated K + currents in magnocellular neurons of the supraoptic nucleus in rat. J Neurophysiol. 1999;81:1617. [DOI] [PubMed] [Google Scholar]
- 119.Scott V, Brown CH. State-dependent plasticity in vasopressin neurones: dehydration-induced changes in activity patterning. J Neuroendocrinol. 2010;22:343–354. [DOI] [PubMed] [Google Scholar]
- 120.Shibuya I, Noguchi J, Tanaka K et al. PACAP increases the cytosolic Ca2+ concentration and stimulates somatodendritic vasopressin release in rat supraoptic neurons. J Neuroendocrinol. 1998;10:31–42. [DOI] [PubMed] [Google Scholar]
- 121.Shibuya I, Kabashima N, Tanaka K et al. Patch-clamp analysis of the mechanism of PACAP-induced excitation in rat supraoptic neurones. J Neuroendocrinol. 1998;10:759–768. [DOI] [PubMed] [Google Scholar]
- 122.Toshinai K, Saito T, Yamaguchi H et al. Neuroendocrine regulatory peptide-1 and −2 (NERPs) inhibit the excitability of magnocellular neurosecretory cells in the hypothalamus. Brain Res. 2014;1563:52–60. [DOI] [PubMed] [Google Scholar]
- 123.Lee VH, Lee LT, Chu JY et al. An indispensable role of secretin in mediating the osmoregulatory functions of angiotensin II. FASEB J. 2010;24:5024–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Velmurugan S, Brunton PJ, Leng G, Russell JA. Circulating secretin activates supraoptic nucleus oxytocin and vasopressin neurons via noradrenergic pathways in the rat. Endocrinology. 2010;151:2681–2688. [DOI] [PubMed] [Google Scholar]
- 125.Chu JY, Chung SC, Lam AK, Tam S, Chung SK, Chow BK. Phenotypes developed in secretin receptor-null mice indicated a role for secretin in regulating renal water reabsorption. Mol Cell Biol. 2007;27:2499–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiang Z, Bo X, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamus. Brain Res. 1998;813:390–397. [DOI] [PubMed] [Google Scholar]
- 127.Song Z, Vijayaraghavan S, Sladek CD. ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;292:R423–R431. [DOI] [PubMed] [Google Scholar]
- 128.Mori M, Tsushima H, Matsuda T. Antidiuretic effects of ATP induced by microinjection into the hypothalamic supraoptic nucleus in water-loaded and ethanol-anesthetized rats. Jpn J Pharmacol. 1994;66:445–450. [DOI] [PubMed] [Google Scholar]
- 129.Hiruma H, Bourque CW. P2 purinoceptor-mediated depolarization of rat supraoptic neurosecretory cells in vitro. J Physiol. 1995;489:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Song Z, Gomes DA, Stevens W, Sladek CD. Multiple alpha1-adrenergic receptor subtypes support synergistic stimulation of vasopressin and oxytocin release by ATP and phenylephrine. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1529–R1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vavra V, Bhattacharya A, Zemkova H. Facilitation of glutamate and GABA release by P2X receptor activation in supraoptic neurons from freshly isolated rat brain slices. Neuroscience. 2011;188:1–12. [DOI] [PubMed] [Google Scholar]
- 132.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. [DOI] [PubMed] [Google Scholar]
- 133.Day TA, Sibbald JR, Khanna S. ATP mediates an excitatory noradrenergic neuron input to supraoptic vasopressin cells. Brain Res. 1993;607:341–344. [DOI] [PubMed] [Google Scholar]
- 134.Song Z, Sladek CD. Does conversion of ATP to adenosine terminate ATP-stimulated vasopressin release from hypothalamo-neurohypophyseal explants? Brain Res. 2005;1047:105–111. [DOI] [PubMed] [Google Scholar]
- 135.Ponzio TA, Wang YF, Hatton GI. Activation of adenosine A2A receptors alters postsynaptic currents and depolarizes neurons of the supraoptic nucleus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R359–R366. [DOI] [PubMed] [Google Scholar]
- 136.Bull PM, Brown CH, Russell JA, Ludwig M. Activity-dependent feedback modulation of spike patterning of supraoptic nucleus neurons by endogenous adenosine. Am J Physiol Regul Integr Comp Physiol. 2006;291:R83–R90. [DOI] [PubMed] [Google Scholar]
- 137.Oliet SH, Poulain DA. Adenosine-induced presynaptic inhibition of IPSCs and EPSCs in rat hypothalamic supraoptic nucleus neurones. J Physiol. 1999;520:815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ruan M, Brown CH. Feedback inhibition of action potential discharge by endogenous adenosine enhancement of the medium afterhyperpolarization. J Physiol. 2009;587:1043–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kirkpatrick K, Bourque CW. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. J Physiol. 1996;494:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ponzio TA, Hatton GI. Adenosine postsynaptically modulates supraoptic neuronal excitability. J Neurophysiol. 2005;93:535–547. [DOI] [PubMed] [Google Scholar]
- 141.Liu QS, Jia YS, Ju G. Nitric oxide inhibits neuronal activity in the supraoptic nucleus of the rat hypothalamic slices. Brain Res Bull. 1997;43:121–125. [DOI] [PubMed] [Google Scholar]
- 142.Stern JE, Ludwig M. NO inhibits supraoptic oxytocin and vasopressin neurons via activation of GABAergic synaptic inputs. Am J Physiol. 2001;280:R1815–R1822. [DOI] [PubMed] [Google Scholar]
- 143.Ozaki M, Shibuya I, Kabashima N et al. Preferential potentiation by nitric oxide of spontaneous inhibitory postsynaptic currents in rat supraoptic neurones. J Neuroendocrinol. 2000;12:273–281. [DOI] [PubMed] [Google Scholar]
- 144.Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput Biol. 2008;4:e1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharif Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–98. [DOI] [PubMed] [Google Scholar]
- 146.Zygmunt PM, Petersson J, Andersson DA et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. [DOI] [PubMed] [Google Scholar]
- 147.Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. J Neurosci. 1996;16:4861–4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stern JE, Armstrong WE. Reorganization of the dendritic trees of oxytocin and vasopressin neurons of the rat supraoptic nucleus during lactation. J Neurosci. 1998;18:841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.El Majdoubi M, Poulain DA, Theodosis DT. The glutamatergic innervation of oxytocin- and vasopressin-secreting neurons in the rat supraoptic nucleus and its contribution to lactation-induced synaptic plasticity. EurJNeurosci. 1996;8:1377–1389. [DOI] [PubMed] [Google Scholar]
- 150.Gies U, Theodosis DT. Synaptic plasticity in the rat supraoptic nucleus during lactation involves GABA innervation and oxytocin neurons: a quantitative immunocytochemical analysis. J Neurosci. 1994;14:2861–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stern JE, Hestrin S, Armstrong WE. Enhanced neurotransmitter release at glutamatergic synapses on oxytocin neurones during lactation in the rat. J Physiol. 2000;526:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Michaloudi HC, El Majdoubi M, Poulain DA, Papadopoulos GC, Theodosis DT. The noradrenergic innervation of identified hypothalamic magnocellular somata and its contribution to lactation-induced synaptic plasticity. J Neuroendocrinol. 1997;9:17–23. [DOI] [PubMed] [Google Scholar]
- 153.Seymour AJ, Scott V, Augustine RA, Bouwer GT, Campbell RE, Brown CH. Development of an excitatory kisspeptin projection to the oxytocin system in late pregnancy. J Physiol. 2017;595:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee SW, Kim YB, Kim JS et al. GABAergic inhibition is weakened or converted into excitation in the oxytocin and vasopressin neurons of the lactating rat. Mol Brain. 2015;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Belin V, Moos F, Richard P. Synchronization of oxytocin cells in the hypothalamic paraventricular and supraoptic nuclei in suckled rats: direct proof with paired extracellular recordings. Exp Brain Res. 1984;57:201–203. [DOI] [PubMed] [Google Scholar]
- 156.Douglas A, Scullion S, Antonijevic I, Brown D, Russell J, Leng G. Uterine contractile activity stimulates supraoptic neurons in term pregnant rats via a noradrenergic pathway. Endocrinology. 2001;142:633–644. [DOI] [PubMed] [Google Scholar]
- 157.Augustine RA, Seymour AJ, Campbell RE, Grattan DR, Brown CH. Integrative neuro-humoral regulation of oxytocin neuron activity in pregnancy and lactation. J Neuroendocrinol. 2018;30:e12569. [DOI] [PubMed] [Google Scholar]
- 158.Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76:593–602. [DOI] [PubMed] [Google Scholar]
- 159.Freund-Mercier MJ, Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984;352:447–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Neumann I, Douglas AJ, Pittman QJ, Russell JA, Landgraf R. Oxytocin released within the supraoptic nucleus of the rat brain by positive feedback action is involved in parturition-related events. J Neuroendocrinol. 1996;8:227–233. [DOI] [PubMed] [Google Scholar]
- 161.Israel JM, Le Masson G, Theodosis DT, Poulain DA. Glutamatergic input governs periodicity and synchronization of bursting activity in oxytocin neurons in hypothalamic organotypic cultures. Eur J Neurosci. 2003;17:2619–2629. [DOI] [PubMed] [Google Scholar]
- 162.Popescu IR, Buraei Z, Haam J, Weng FJ, Tasker JG. Lactation induces increased IPSC bursting in oxytocinergic neurons. Physiol Rep. 2019;7:e14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Teruyama R, Armstrong WE. Changes in the active membrane properties of rat supraoptic neurones during pregnancy and lactation. J Neuroendocrinol. 2002;14:933–944. [DOI] [PubMed] [Google Scholar]
- 164.Wang YF, Hatton GI. Burst firing of oxytocin neurons in male rat hypothalamic slices. Brain Res. 2005;1032:36–43. [DOI] [PubMed] [Google Scholar]
- 165.Herbison AE, Voisin DL, Douglas AJ, Chapman C. Profile of monoamine and excitatory amino acid release in rat supraoptic nucleus over parturition. Endocrinology. 1997;138:33–40. [DOI] [PubMed] [Google Scholar]
- 166.Moos F, Richard P. Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related. J Physiol. 1989;408:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hatton GI. Astroglial modulation of neurotransmitter/peptide release from the neurohypophysis: present status. J Chem Neuroanat. 1999;16:203–221. [DOI] [PubMed] [Google Scholar]
- 168.Perlmutter LS, Tweedle CD, Hatton GI. Neuronal/glial plasticity in the supraoptic dendritic zone: dendritic bundling and double synapse formation at parturition. Neuroscience. 1984;13:769–779. [DOI] [PubMed] [Google Scholar]
- 169.Moos F, Marganiec A, Fontanaud P, Guillou-Duvoid A, Alonso G. Synchronization of oxytocin neurons in suckled rats: possible role of bilateral innervation of hypothalamic supraoptic nuclei by single medullary neurons. Eur J Neurosci. 2004;20:66–78. [DOI] [PubMed] [Google Scholar]
- 170.Wang YF, Negoro H, Higuchi T. Lesions of hypothalamic mammillary body desynchronise milk-ejection bursts of rat bilateral supraoptic oxytocin neurones. J Neuroendocrinol. 2012;25:67–75. [DOI] [PubMed] [Google Scholar]
- 171.Boudaba C, Di S, Tasker JG. Presynaptic noradrenergic regulation of glutamate inputs to hypothalamic magnocellular neurones. J Neuroendocrinol. 2003;15:803–810. [DOI] [PubMed] [Google Scholar]
- 172.Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332:123–143. [DOI] [PubMed] [Google Scholar]
- 173.Boudaba C, Tasker JG. Internuclear coupling of hypothalamic magnocellular nuclei by glutamate synaptic circuits. Am J Physiol Regul Integr Comp Physiol. 2006;291:R102–R111. [DOI] [PubMed] [Google Scholar]
- 174.Sumner BE, Coombes JE, Pumford KM, Russell JA. Opioid receptor subtypes in the supraoptic nucleus and posterior pituitary gland of morphine-tolerant rats. Neuroscience. 1990;37:635–645. [DOI] [PubMed] [Google Scholar]
- 175.Smith MJ, Wise PM. Localization of kappa opioid receptors in oxytocin magnocellular neurons in the paraventricular and supraoptic nuclei. Brain Res. 2001;898:162–165. [DOI] [PubMed] [Google Scholar]
- 176.Kim JS, Brown CH, Anderson GM. Anti-opioid effects of RFRP-3 on magnocellular neuron activity in morphine-naive and morphine-treated female rats. Endocrinology. 2016;157: 4003–4011. [DOI] [PubMed] [Google Scholar]
- 177.Martin R, Moll U, Voigt KH. An attempt to characterize by immunocytochemical methods the enkephalin-like material in oxytocin endings of the rat neurohypophysis. Life Sci. 1983;33(Suppl. 1):69–72. [DOI] [PubMed] [Google Scholar]
- 178.Eriksson M, Ceccatelli S, Uvnas-Moberg K, Iadarola M, Hokfelt T. Expression of Fos-related antigens, oxytocin, dynorphin and galanin in the paraventricular and supraoptic nuclei of lactating rats. Neuroendocrinology. 1996;63:356–367. [DOI] [PubMed] [Google Scholar]
- 179.Brown CH, Murphy NP, Munro G et al. Interruption of central noradrenergic pathways and morphine withdrawal excitation of oxytocin neurones in the rat. J Physiol. 1998;507:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Douglas AJ, Neumann I, Meeren HK et al. Central endogenous opioid inhibition of supraoptic oxytocin neurons in pregnant rats. J Neurosci. 1995;15:5049–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Douglas AJ, Bicknell RJ, Leng G, Russell JA, Meddle SL. Beta-endorphin cells in the arcuate nucleus: Projections to the supraoptic nucleus and changes in expression during pregnancy and parturition. J Neuroendocrinol. 2002;14:768–777. [DOI] [PubMed] [Google Scholar]
- 182.Zhao BG, Chapman C, Bicknell RJ. Functional kappa-opioid receptors on oxytocin and vasopressin nerve terminals isolated from the rat neurohypophysis. Brain Res. 1988;462:62–66. [DOI] [PubMed] [Google Scholar]
- 183.Leng G, Dye S, Bicknell RJ. Kappa-opioid restraint of oxytocin secretion: plasticity through pregnancy. Neuroendocrinology. 1997;66:378–383. [DOI] [PubMed] [Google Scholar]
- 184.Douglas AJ, Bicknell RJ. Oxytocin nerve terminal desensitization to kappa-opioids at the end of pregnancy. Ann NY Acad Sci. 1993;689:589–592. [DOI] [PubMed] [Google Scholar]
- 185.Douglas AJ, Dye S, Leng G, Russell JA, Bicknell RJ. Endogenous opioid regulation of oxytocin secretion through pregnancy in the rat. J Neuroendocrinol. 1993;5:307–314. [DOI] [PubMed] [Google Scholar]
- 186.Srisawat R, Ludwig M, Bull PM, Douglas AJ, Russell JA, Leng G. Nitric oxide and the oxytocin system in pregnancy. J Neurosci. 2000;20:6721–6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bull PM, Ludwig M, Blackburn-Munro GJ, Delgado-Cohen H, Brown CH, Russell JA. The role of nitric oxide in morphine dependence and withdrawal excitation of rat oxytocin neurons. Eur J Neurosci. 2003;18:2545–2551. [DOI] [PubMed] [Google Scholar]
- 188.Doi N, Brown CH, Cohen HD, Leng G, Russell JA. Effects of the endogenous opioid peptide, endomorphin 1, on supraoptic nucleus oxytocin and vasopressin neurones in vivo and in vitro. Br J Pharmacol. 2001;132:1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Brown CH, Russell JA. Cellular mechanisms underlying neuronal excitability during morphine withdrawal in physical dependence: lessons from the magnocellular oxytocin system. Stress. 2004;7:97–107. [DOI] [PubMed] [Google Scholar]
- 190.Brown CH, Munro G, Johnstone LE, Robson AC, Landgraf R, Russell JA. Oxytocin neurone autoexcitation during morphine withdrawal in anaesthetized rats. NeuroReport. 1997;8:951–955. [DOI] [PubMed] [Google Scholar]
- 191.Pinol RA, Jameson H, Popratiloff A, Lee NH, Mendelowitz D. Visualization of oxytocin release that mediates paired pulse facilitation in hypothalamic pathways to brainstem autonomic neurons. PLoS ONE. 2014;9:e112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Roy RK, Augustine RA, Brown CH, Schwenke DO. Activation of oxytocin neurons in the paraventricular nucleus drives cardiac sympathetic nerve activation following myocardial infarction in rats. Commun Biol. 2018;1:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Son SJ, Filosa JA, Potapenko ES et al. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron. 2013;78:1036–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zaelzer C, Gizowski C, Salmon CK, Murai KK, Bourque CW. Detection of activity-dependent vasopressin release from neuronal dendrites and axon terminals using sniffer cells. J Neurophysiol. 2018;120:1386–1396. [DOI] [PubMed] [Google Scholar]
- 195.Theodosis DT, Poulain DA. Evidence that oxytocin-secreting neurones are involved in the ultrastructural reorganisation of the rat supraoptic nucleus apparent at lactation. Cell Tissue Res. 1984;235:217–219. [DOI] [PubMed] [Google Scholar]
- 196.Tweedle CD, Hatton GI. Ultrastructural changes in rat hypothalamic neurosecretory cells and their associated glia during minimal dehydration and rehydration. Cell Tissue Res. 1977;181:59–72. [DOI] [PubMed] [Google Scholar]
- 197.Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci USA. 2004;101:2151–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989;491:156–162. [DOI] [PubMed] [Google Scholar]
- 199.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. [DOI] [PubMed] [Google Scholar]
- 200.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177:43–55. [DOI] [PubMed] [Google Scholar]
- 201.Gabor A, Leenen FH. Central neuromodulatory pathways regulating sympathetic activity in hypertension. J Appl Physiol. 2012;113:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Han SY, Gray E, Hughes G, Brown CH, Schwenke DO. Increased sympathetic drive during the onset of hypertension in conscious Cyp1a1-Ren2 rats. Pflugers Arch Eur J Physiol. 2014;466:459–466. [DOI] [PubMed] [Google Scholar]
- 203.Jardine DL, Charles CJ, Ashton RK et al. Increased cardiac sympathetic nerve activity following acute myocardial infarction in a sheep model. J Physiol. 2005;565:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Leenen FH. Brain mechanisms contributing to sympathetic hyperactivity and heart failure. Circ Res. 2007;101:221–223. [DOI] [PubMed] [Google Scholar]
- 205.Biancardi VC, Son SJ, Sonner PM, Zheng H, Patel KP, Stern JE. Contribution of central nervous system endothelial nitric oxide synthase to neurohumoral activation in heart failure rats. Hypertension. 2011;58:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cohn JN, Levine TB, Olivari MT et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. [DOI] [PubMed] [Google Scholar]
- 207.Han SY, Bouwer GT, Seymour AJ, Korpal AK, Schwenke DO, Brown CH. Induction of hypertension blunts baroreflex inhibition of vasopressin neurons in the rat. Eur J Neurosci. 2015;42:2690–2698. [DOI] [PubMed] [Google Scholar]
- 208.Korpal AK, Han SY, Schwenke DO, Brown CH. A switch from GABA inhibition to excitation of vasopressin neurons exacerbates the development angiotensin II-dependent hypertension. J Neuroendocrinol. 2018;30:e12564. [DOI] [PubMed] [Google Scholar]
- 209.Roy RK, Augustine RA, Brown CH, Schwenke DO. Acute myocardial infarction activates magnocellular vasopressin and oxytocin neurons. J Neuroendocrinol. 2019;31:e12808. [DOI] [PubMed] [Google Scholar]
- 210.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol. 2012;303:R291–R300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Stern JE, Potapenko ES. Enhanced NMDA receptor-mediated intracellular calcium signaling in magnocellular neurosecretory neurons in heart failure rats. Am Physiol Regul Integr Comp Physiol. 2013;305:R414–R422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Goldsmith SR, Francis GS, Cowley AW Jr, Levine TB, Cohn JN. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol. 1983;1:1385–1390. [DOI] [PubMed] [Google Scholar]
- 213.Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol. 2005;46:1785–1791. [DOI] [PubMed] [Google Scholar]
- 214.Murakami K, Akana S, Dallman MF, Ganong WF. Correlation between the stress-induced transient increase in corticotropin-releasing hormone content of the median eminence of the hypothalamus and adrenocorticotropic hormone secretion. Neuroendocrinology. 1989;49:233–241. [DOI] [PubMed] [Google Scholar]
- 215.Dabrowska J, Hazra R, Ahern TH et al. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jamieson BB, Nair BB, Iremonger KJ. Regulation of hypothalamic corticotropin-releasing hormone neurone excitability by oxytocin. J Neuroendocrinol. 2017;29:e12532. [DOI] [PubMed] [Google Scholar]
- 217.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Du W, Stern JE, Filosa JA. Neuronal-derived nitric oxide and somatodendritically released vasopressin regulate neurovascular coupling in the rat hypothalamic supraoptic nucleus. J Neurosci. 2015;35:5330–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Leng G, Sabatier N. Oxytocin - the sweet hormone? Trends Endocrinol Metab. 2017;28:365–376. [DOI] [PubMed] [Google Scholar]
- 220.Sabatier N, Caquineau C, Douglas AJ, Leng G. Oxytocin released from magnocellular dendrites: a potential modulator of alpha-melanocyte-stimulating hormone behavioral actions? Ann N Y Acad Sci. 2003;994:218–224. [DOI] [PubMed] [Google Scholar]
- 221.Klockars OA, Waas JR, Klockars A, Levine AS, Olszewski PK. Neural basis of ventromedial hypothalamic oxytocin-driven decrease in appetite. Neuroscience. 2017;366:54–61. [DOI] [PubMed] [Google Scholar]
- 222.Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. J Neuroendocrinol. 2003;15:787–793. [DOI] [PubMed] [Google Scholar]
- 223.Manning M, Misicka A, Olma A et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24:609–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chen C, Jiang Z, Fu X, Yu D, Huang H, Tasker JG. Astrocytes amplify neuronal dendritic volume transmission stimulated by norepinephrine. Cell Rep. 2019;29:4349–4361.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Takayanagi Y, Yoshida M, Takashima A et al. Activation of supraoptic oxytocin neurons by secretin facilitates social recognition. Biol Psychiatry. 2017;81:243–251. [DOI] [PubMed] [Google Scholar]
- 226.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. [DOI] [PubMed] [Google Scholar]
- 227.Chini B, Verhage M, Grinevich V. The action radius of oxytocin release in the mammalian CNS: from single vesicles to behavior. Trends Pharmacol Sci. 2017;38:982–991. [DOI] [PubMed] [Google Scholar]
- 228.Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. [DOI] [PubMed] [Google Scholar]
- 229.Stark H, Burbach JP, Van der Kleij AA, De Wied D. In vivo conversion of vasopressin after microinjection into limbic brain areas of rats. Peptides. 1989;10:717–720. [DOI] [PubMed] [Google Scholar]
- 230.Xia X, Lessmann V, Martin TF. Imaging of evoked dense-core-vesicle exocytosis in hippocampal neurons reveals long latencies and kiss-and-run fusion events. J Cell Sci. 2009;122:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Engelmann M, Ebner K, Landgraf R, Wotjak CT. Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm Behav. 2006;50:496–501. [DOI] [PubMed] [Google Scholar]
- 232.Ebner K, Wotjak CT, Landgraf R, Engelmann M. Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. Eur J Neurosci. 2002;15:384–388. [DOI] [PubMed] [Google Scholar]
- 233.Ebner K, Wotjak CT, Landgraf R, Engelmann M. A single social defeat experience selectively stimulates the release of oxytocin, but not vasopressin, within the septal brain area of male rats. Brain Res. 2000;872:87–92. [DOI] [PubMed] [Google Scholar]
- 234.Engelmann M, Ebner K, Landgraf R, Wotjak CT. Swim stress triggers the release of vasopressin within the suprachiasmatic nucleus of male rats. Brain Res. 1998;792:343–347. [DOI] [PubMed] [Google Scholar]
- 235.Ebner K, Wotjak CT, Holsboer F, Landgraf R, Engelmann M. Vasopressin released within the septal brain area during swim stress modulates the behavioural stress response in rats. EurJNeurosci. 1999;11:997–1002. [DOI] [PubMed] [Google Scholar]
- 236.Landgraf R, Ludwig M. Vasopressin release within the supraoptic and paraventricular nuclei of the rat brain: osmotic stimulation via microdialysis. Brain Res. 1991;558:191–196. [DOI] [PubMed] [Google Scholar]
- 237.Althammer F, Grinevich V. Diversity of oxytocin neurons: beyond magno- and parvocellular cell types? J Neuroendocrinol. 2017; 30:e12549. [DOI] [PubMed] [Google Scholar]
- 238.Ludwig M, Sabatier N, Dayanithi G, Russell JA, Leng G. The active role of dendrites in the regulation of magnocellular neurosecretory cell behavior. Prog Brain Res. 2002;139:247. [DOI] [PubMed] [Google Scholar]
- 239.Stern JE. Neuroendocrine-autonomic integration in the paraventricular nucleus: novel roles for dendritically released neuropeptides. J Neuroendocrinol. 2015;27:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]


