Abstract
Cancer-associated cachexia (CC) is a pathological condition characterized by sarcopenia, adipose tissue depletion, and progressive weight loss. CC is driven by multiple factors such as anorexia, excessive catabolism, elevated energy expenditure by growing tumor mass, and inflammatory mediators released by cancer cells and surrounding tissues. In addition, endocrine system, systemic metabolism, and central nervous system (CNS) perturbations in combination with cachexia mediators elicit exponential elevation in catabolism and reduced anabolism in skeletal muscle, adipose tissue, and cardiac muscle. At the molecular level, mechanisms of CC include inflammation, reduced protein synthesis, and lipogenesis, elevated proteolysis and lipolysis along with aggravated toxicity and complications of chemotherapy. Furthermore, CC is remarkably associated with intolerance to anti-neoplastic therapy, poor prognosis, and increased mortality with no established standard therapy. In this context, we discuss the spatio-temporal changes occurring in the various stages of CC and highlight the imbalance of host metabolism. We provide how multiple factors such as proteasomal pathways, inflammatory mediators, lipid and protein catabolism, glucocorticoids, and in-depth mechanisms of interplay between inflammatory molecules and CNS can trigger and amplify the cachectic processes. Finally, we highlight current diagnostic approaches and promising therapeutic interventions for CC.
Keywords: Glycolysis, gluconeogenesis, cachexia, anorexia, anabolism, catabolism
Impact statement
Cancer cachexia is a sequela of catabolism leading to poor muscle strength, reduced locomotion, weight loss, and intolerance to therapy. Greater than 80% of advanced cancer patients display cachexia, and it is a major cause of cancer-related deaths. Cancer cells harbor host tissue metabolism by secretory molecules and cargo packaged extracellular vesicles (EVs), and skew the entire host metabolism toward catabolism while themselves being anabolic. Currently, there is no standard therapy available that can effectively reverse cancer-related cachexia. Therefore, robust preclinical research in the context of etiology, initiation, progression, and therapy followed by robust clinical validation are warranted. In this regard, our present review discusses the imbalance of host metabolism, early changes during precachexia, and highlights the molecular pathways. In addition, we shed a light upon current diagnostic and therapeutic challenges associated with cancer-associated cachexia.
Introduction
Reprogramming of metabolism is one of the crucial hallmarks of cancer. 1 Elevated glycolysis, hepatic gluconeogenesis, lipolysis, and proteolysis of skeletal muscles result in reduced body fat and progressive weight loss in cancer patients. 1 This systemic tissue wasting sequela termed CC diminishes locomotion leading to poor quality of life, poor prognosis, and increased mortality. More than 80% of advanced-stage cancer patients display cachexia, and it accounts for about 25% of cancer-related deaths.2,3 Nearly 30% of the CC patients have associated cardiac disorders with the risk of developing cardiac cachexia. 4 Cachexia might be present in the early-stage development of cancer even before the onset of signs and symptoms of malignancy. 2 Initially, cachexia was thought to be a muscle protein and fat depletion metabolic disorder.5,6 However, recent studies have established that cachexia is a multifactorial pathological condition associated with hypoxia, tissue pH, anorexia, altered metabolism, and chronic inflammation.6,7 Hypoxia-inducible factor 1-α (HIF-1α) and hypoxia-inducible factor 2-α (HIF-2α) have been revealed to stimulate CC through diverse mechanisms comprising loss of appetite, activation of phosphoenolpyruvate carboxykinase (PEPCK), and the Cori cycle.8,9 Indeed, pro-inflammatory cytokines such as tumor necrosis factor–alpha (TNF-α) and interleukin-6 (IL-6) were shown to be elevated in various CC in vitro and in vivo models and are proposed to be diagnostic markers of CC.8,10–12 Studies conducted by Oliff et al. 13 and Baltgalvis et al. 14 demonstrated that supraphysiological IL-6 and TNF-α levels led to early CC and deaths in animal models. So far, monotherapies targeting numerous inflammatory cytokines have failed to efficaciously treat CC, owing to its multifactorial landscape.15–17 Two independent studies by Stovroff et al. 18 and Vaisman and Hahn 19 demonstrated that TNF-α induces corticotrophin-releasing hormone, and it increases the firing of glucose-sensitive neurons leading to anorexia followed by cachexia. Furthermore, the cytokine surge is known to induce depression-induced anorexia, lipolysis via Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3), and Nuclear factor kappa- light chain enhancer of activated B cells (NF-κB) pathways and β-adrenergic activation connect CC with the central nervous system (CNS).20,21 Nevertheless, loss of appetite is not a primary cause of cachexia during cancer progression, as the accompanying engrossment of massive skeletal muscle wasting does not occur during anorexia, and nutritional supplementation fails to impede loss of lean body mass.22–24 Currently, the precise mechanisms that promote and govern CC are yet to be deduced, thereby limiting the therapeutic targets for the treatment of CC. Therefore, the current review provides in-depth information about spatio-temporal changes in tissue microenvironment and systemic metabolism during the initial stages of CC development. We also focus on the energetics of the cancer cell and surrounding stromal microenvironment, rate-limiting steps, and the mechanism of absconding checkpoints. Furthermore, we provide a comprehensive overview of molecular insights into tumor cell anabolism and normal tissue catabolism status during progressive CC. Finally, we highlight the current difficulties and complications in the early diagnosis and treatment of CC.
Spatio-temporal changes during cachexia progression in cancer patients
CC is divided into three successive clinical stages such as precachexia, cachexia, and refractory cachexia (RC), although not all the patients progress through this continuum.21,22 The degree of clinical manifestations and the condition of patients, including locomotory index and probable survival period, are taken into account while staging the disease. 3 The staging of CC has crucial implications for management and treatment which go beyond merely an understanding of alleviating symptoms.
Cancer precachectic state
Precachexia is associated with less than 5% of unintentional total body weight loss in 6 months. Cancer precachectic state (CPC) is accompanied by underlying chronic disease, inflammation, loss of appetite, and/or metabolic alterations. 3 Notably, CPC patients display heterogeneity, with some progressing rapidly, while others remain weight stable. In all preclinical and clinical settings, CPC displays systemic involvement of inflammation with elevated levels of TNF-α and IL-6, insulin resistance, in addition to sarcopenia and weight loss. 25 An important distinction between cachexia and anorexia is that weight loss during cachexia is not solely confined to reduced calorie intake. Indeed, it is noteworthy that not all the malnourished patients are cachectic, while all cachectic patients are malnourished.3,26 Malnutrition during cancer progression can even be due to the direct impact of tumors on the gastrointestinal tract, therapy-induced nausea, satiety, and impaired appetite regulation. The current understanding of metabolic balance and body weight is largely derived from the integrative physiological research into obesity and its associated etiopathologies. 27 The interplay of chronic inflammation and immune system modulation with dysregulated metabolism in obesity extensively involves inflammatory mediators that overlap with potential molecular drivers of CPC, suggesting the involvement of similar flawed signaling pathways, albeit with dramatically contradicting end-stage clinical manifestations.28,29 Higher glucose demands are met in the initial stages of CC by glycogenolysis and reduced glycogen storage in muscle, and preceding massive gluconeogenesis.30,31 During CPC, muscle protein synthesis and degradation have been found to be normal, with normal atrogens, while myogenesis and muscle contractility have been found to be reduced.30,31 Oxidative stress, inflammatory mediators, and reactive oxygen species (ROS) emission have been found to be elevated with unaltered autophagy and mitophagy.32,33 Similarly, elevated mitochondrial ROS generation and oxidative stress have been shown to precede terminal cachexia development in tumor-bearing mice. 34
Cachexia
Cachexia is diagnosed by >5% unintentional total body weight loss over the previous 6 months despite the normal nutritional food intake, or the combination of >2% ongoing weight loss with body mass index (BMI) < 20 or sarcopenia. 3
Protein homeostasis in skeletal muscle is imbalanced toward decreased protein synthesis and elevated breakdown in response to cancer.30,31 The hyperactivated ubiquitin-proteasome and autophagy pathways are primarily responsible for this unevenness.30,31 Transcriptional upregulation of numerous E3 ligases, including muscle-specific RING finger protein 1 (MURF1), muscle atrophy F-box protein (MAFBX), FBXO30, and FBXO31, and increased turnover of myofibrillar proteins result in muscle atrophy during CC.35–37 These amino acids are directed mainly toward gluconeogenesis to meet the requirements of growing tumor mass. Furthermore, patients with CC often display insulin resistance. Under normal physiology, insulin regulates carbohydrate metabolism and muscle protein balance to maintain blood glucose levels.38,39 Insulin resistance mobilizes proteins from muscle to liver through the suppression of the PI3K-Akt pathway and by upregulation of the proteasomal pathway.40,41 These amino acids released from muscle protein turnover are directed to the liver for gluconeogenesis as an alternate source of energy production during the period of glucose scarcity. 42 These dysregulated energy-cumbersome metabolic activities in the cancer cells can henceforth withstand energy depletion, necessitating more muscle breakdown as cancer progresses.
Furthermore, increased lipolysis leading to elevated levels of fatty acids or glycerol has been reported to fuel liver gluconeogenesis and insulin resistance in CC patients. Insulin resistance during CC dysregulates the PI3K/Akt/mTOR pathway in adipose tissues resulting in increased adipose tissue wasting. 43 Altogether, the complete metabolism of cancer cells concentrates on anabolism while the surrounding host tissues and systemic metabolism of the host under continuous catabolism lead to profound muscle and weight loss in CC patients.
RC
RC is notable for its poor World Health Organization (WHO) physical performance score and survival expectancy of <3 months, and these patients are suited only for psychosocial support and palliative care. 3 The severity of cachexia increases as the disease progresses to metastasize. Bodyweight and circulating albumin (ALB) levels of patients with RC were significantly lower compared to patients with CPC while C-reactive protein (CRP) was found to be profoundly increased in these patients.44,45 Recently, Suno et al. 46 demonstrated that increased plasma fentanyl levels could be the biomarker for RC. In this study, the authors analyzed the fentanyl levels and hence the exclusion criteria of the patients selected in the study was use of drugs, as supportive therapy, that might affect the metabolism of CYP3A4, and antipsychotic drugs. Suno and co-workers have shown the remarkable association of dose-adjusted fentanyl concentrations with refractory cancer cachexia. RC is significantly associated with cardiac atrophy, arrhythmias, and electrolyte imbalance that increase the risk of thromboembolic events, cardiac arrest, breathing difficulties, aspiration pneumonia, swallowing difficulties, reduced joint movements, gastrointestinal muscle atrophy, poor wound healing, and sepsis. Not surprisingly, clinical data showed high mortality within a few weeks of the development of RC. 47
The detailed differences between CPC, CC, and RC are summarized in Table 1.14,30,31,35,37,44,46,48–73
Table 1.
Characterization of different phases of cachexia.
| Condition/molecule | Precachexia | Cachexia | Refractory Cachexia | Reference |
|---|---|---|---|---|
| Fat loss | ↔ | ↓ | ↓↓ | 44,48 |
| Muscle strength, contractility | ↓ | ↓ | ↓↓ | 48,49 |
| Food intake and appetite | ↔ | ↓ | ↓↓ | 44 |
| Protein synthesis | ↔ | ↓ | ↓↓ | 51 |
| Protein degradation | ↔ | ↑ | ↑↑ | 51 |
| Regeneration | ↔ | ↓ | ↓↓ | 52,53 |
| Atrogenes (Atrogin-1 and MuRF-1) | ↔ | ↑ | ↑ | 31 |
| Ubiquitin protein ligase | ↔ | ↑ | ↑ | 35,37 |
| Cell cycle and myogenesis | ↓ | ↔ | ? | 30,55 |
| Apoptosis | ? | ↑ | ↑ | 14,54,55 |
| Autophagy | ↔ | ↑ | ↑ | 30,56,73 |
| Mitophagy | ↔ | ↑ | ↑ | 57,58 |
| ATP production | ↔ | ↑ | ↑ | 59 |
| Inflammation | ↑ | ↑↑ | ↑ | 60,61 |
| TNF-α, IL-6, IL-1β, neuropeptide Y | ↑ | ↑↑ | ↑ | 54,62,63 |
| CRP | ? | ↑ | ↑↑ | 46 |
| MAPK | ? | ↑ | ↑ | 63,71 |
| NFκB | ? | ↑ | ↑ | 64,65 |
| STAT3 signaling | ↑ | ↑↑ | ↑ | 66 |
| ROS | ↑ | ↑ | ↑ | 67,68 |
| Calcium homeostasis | ? | ↑ | ↑ | 69,70 |
| Oxidative stress | ↑ | ↑ | ↑ | 71 |
| Mitochondrial fission | ↔ | ↑ | ↑ | 72,73 |
| Mitochondrial fusion | ↓ | ↓ | ↓ | 73 |
ATP: adenosine triphosphate; TNF-α: tumor necrosis factor–alpha; IL-6: interleukin-6; CRP: C-reactive protein; MAPK: mitogen activated protein kinase; STAT3: signal transducer and activator of transcription 3; ROS: reactive oxygen species.
↔: unaltered; ↑: increased; ↓: decreased; ?: not reported.
Energetics of cancer cells and host tissues
Increased catabolism is observed in cancer patients leading to unsustainable muscle and fat loss, thereby causing high morbidity and mortality.7,74 The factors such as old age, tumor progression, comorbid conditions, nutritional deficiency, drugs, and medical interventions were found to be the major determinants of the resting energy expenditure (REE). 75 Tumors demand a continuous supply of glucose and energy for their uninterrupted growth and proliferation and alter the energy balance of host tissue by eliciting inflammatory pathways which augment both systemic and locally mediated catabolic events. 76 Tumors also consume the increased amount of macronutrients directly. When tumors reach the size >0.75 kg, the energy consumption by the tumor is quantitatively important. 74 The average daily energy expenditure of cancer patients is between 1600 and 1800 kCal. 77 Lieffers et al. 78 demonstrated that in metastatic colorectal cancer patients, there is a cumulative increment in REE which accounts for about ~17,700 kCal over 3 months, and this high demand of REE itself can contribute to a substantial reduction in body weight. In this study, the authors have tried to link the REE to energetically demanding tissues such as liver, spleen, skeletal muscles, adipose tissues, and tumor mass with advanced cancer and cancer cachexia–associated weight loss. Another mechanism that amplifies catabolic signals while desensitizing muscles to anabolic signals is physical inactivity; for instance, Kortebein et al. 79 reported that bed rest for 10 days causes a 6% loss in lower limb muscle, a 30% drop in muscle protein synthesis, and a 16% loss in isokinetic muscle strength in otherwise healthy adults over 65 years of age. Bed rest elevates catabolic responsiveness of the muscles to cortisol by threefold. 80 Muscle anabolism was induced by physical exercise in these patients and though fatigue was not reduced, quality of life was improved. 81 Although bed rest in healthy elderly subjects leads to signs and symptoms of disuse atrophy, more studies are needed to understand the molecular mechanisms governing disuse atrophy and CC, and exploring whether they are similar or not.
Imbalance of anabolism and catabolism: insight into the molecular level
Our present understanding of the basic mechanisms that promote CC is based on three lines of experimental evidence. First, Ni et al. 82 demonstrated that surgical removal of cachexia-related tumors entirely (when practicable) could reverse CC in pancreatic xenograft models. Tumors are thus required not only for the induction of cachexia but also for its maintenance. Second, Norton et al. 83 showed pro-cachectic factors could be transferred between tumor-bearing rats to non-tumor-bearing rats when both are connected surgically via circulation, indicating the humoral nature of these circulatory factors. Succeeding investigations showed that humoral factors are secreted either directly by cancer cells or by stromal cells in the tumor microenvironment, or by distant organs. Primary tumors, as well as metastatic cachexia models, have revealed that cachexia-induced circulating components include hormones, growth factors, metal ions, and both pro- and anti-inflammatory cytokines.84,85 Third, these circulating factors cause cachexia by two separate pathways, either directly by interacting with myocytes and activating muscle catabolic pathways and by restricting muscle protein synthesis, or indirectly through metabolic changes in secondary organs, which leads to muscle atrophy.84–86 Table 2 summarizes the mechanism of cachexia.13,14,34,87–124 The broad picture of CC is summarized in Figure 1.
Table 2.
Cancer cachexia models and mechanisms.
| Cancer type | In vitroIn vivo | Model | Mechanism of action or outcome | References |
|---|---|---|---|---|
| Breast cancer | Ex vivo | Tissue samples | ↑FATP1, CD36, UCP1, p-p38, ↓PPARγ, p-PPARγ |
87 |
| In vitro | C2C12 cells treated with exosomes from MCF-7 and MDA-MB-231 cells | ↑UCP3, p-p38, exosomal miR-155, ↓PPARγ, p-PPARγ, p-ERK1/2 |
87 | |
| Ex vivo | Tissue samples | ↑gelsolin, calponin2, tropomyosin 2, vimentin, P4HAI,
PKM2 annexin A1, annexin A2, ARHGDIB, PGK1, LDHA, ALDOA, GPD2, ENO1, TPI1, PGAM1, EEF1D, PRDX1, CAT, tenascin C, SPARC, COL1A1, COL1A2, alpha fetoprotein, metastasis tumor recurrence, ↓Cav-1, survival |
88 | |
| In vitro | Cav-1 deficient fibroblasts | ↑PKM2, LDHA, reactive oxygen species | 88 | |
| In vitro | MCF-7 | ↑leptin, mitochondrial respiration, PYC, G6PD, cell proliferation | 89 | |
| Colorectal cancer | In vitro | H9c2 cells | ↑Oxidative stress ↓Basal, maximum and spare respiration, ATP, mitochondrial membrane potential and volume |
90 |
| In vivo | Mouse cachexia model | ↑4-HNE ↓cardiac weight, myocardial area, ATP, SDS-MYL1 |
90 | |
| Ex vivo | Plasma | ↑exosomes | 91 | |
| In vitro | Exosomes from CT26 | ↑IL-6, atrogin-1, MURF1, myotube atrophy, ↓myotube diameters | 91 | |
| In vitro | C2C12 myoblasts | ↑IL-6, atrogin-1 | 92 | |
| In vivo | ApcMin/+ mouse | ↑IL-6, atrogin-1, gastrocnemius muscle wasting | 14 | |
| In vivo | C26 mouse model | ↑LIF, STAT3, p-STAT3, atrophy of myotubes | 14 | |
| In vivo | C26, ApcMin mouse model | ↑IL-6, STAT3, p-STAT3, atrophy of muscle fiber | 92 | |
| In vivo | ApcMin mouse model | ↑IL-6, p-AMPK, ↓ mTOR | 93 | |
| In vivo | C26 mouse model | ↑LC3bII/LC3BI, p62, muscle atrophy | 94 | |
| In vivo | C26 mouse model | ↑Pax7, p65, delayed muscle regeneration | 95 | |
| In vivo | C26 mouse model | ↑UCP1, PBE, CPT1α, BAT temperature | 96 | |
| In vitro | 3T3-L1 | ↑ZAG, p62, muscle fiber atrophy | 97 | |
| In vivo | MAC16 mouse model | ↑ZAG, weight loss, lipolysis | 97 | |
| In vivo | MAC16 and MAC13 mouse model | ↑FDG uptake, succinate level, phosphocholine, weight
loss, MURF1, Serum LDL |
98 | |
| In vitro | C26 conditioned C2C12
myoblasts |
↑Ddit4/REDD1, Akt1, mTOR, LC3 II, p62 ↓p-Akt1, p-mTOR, p-4E-BP1, p-p70S6K |
99 | |
| In vivo | C26 xenograft model | ↑Ddit4, Akt1, Akt2, PDPK1 ↓p-Akt1, p-mTOR, p-4E-BP1, p-p70S6K |
99 | |
| In vitro | C26 conditioned AML2 | ↓mitochondrial membrane potential, H2O2
production, MFN2, DRP1, FIS1, PINK |
34 | |
| In vivo | HCT-116 mouse model | ↑exosome density, miR-146b-5p, UCP1, PRDM6 ↓leptin, ADIPSIN |
100 | |
| In vivo | C26 mouse model | ↑exosomes, miR-195a-5p, miR-125b-1-3p, muscle
atrophy ↓Bcl-2 |
101 | |
| Kidney cancer | In vitro | RXF393, SKRC39, A498, 786-O, SN12C | ↓MyoD1, pan-MHC, mTOR, TGF-β | 102 |
| In vivo | RXF393, SKRC39, A498, 786-O, SN12C mouse model | ↓MyoD1, glycolytic signatures, body weight ↑lipolysis, p38 MAPK |
102 | |
| Lung cancer | Ex vivo | serum | ↑TNF-α, ROS, GSH, vitamin E, IL-6 | 91 |
| In vitro | Exosomes from LLC | ↑IL-6, atrogin-1, MURF1, myotube atrophy, ↓myotube diameters | 91 | |
| In vivo | LLC cells | ↑gp130, STAT3, p-STAT3, atrogin-1, p38 | 103 | |
| In vitro | PC-3, H1299 | ↓cavin-3, p-ERK, EGFR1, glucose uptake, lactate
production ↑p-Akt, HIF1α, pS6K, survivin |
104 | |
| In vivo | Mouse fetus | ↓cavin-3, p-ERK, PTEN, body weight ↑p-Akt, HIF1α, glycolysis, lipodystrophy |
104 | |
| In vitro | LLC conditioned C2C12 myoblasts | ↑REDD1, ↓ myotube diameter, muscle loss, p-Akt1, p-mTORC1 |
105 | |
| In vitro | Extracellular vesicles from LLC | ↑lipolysis, p-HSL, UCP1, PTHrP | 106 | |
| In vivo | C57BL/6 | ↑lipolysis, PTHR, PTHrP | 106 | |
| In vivo | LLC | ↑IL-6, STAT3, atrophy ↓muscle weight, fat weight, BM-MNCs, NFATc1 |
107 | |
| In vivo | LLC | ↑RAGE, S100B, HMGB1, IL-6, IL-3, IL-9, IL-12p40,
IL-17A, IFNγ, TNF-α, splenomegaly, muscle wasting ↓body weight, survival, IL-1α, MyoD, MyHC |
108 | |
| Ex vivo | Patient serum, muscle biopsies | ↑miR-424-5p, miR-424-3p, miR-450a-5p ↓miR-451a, miR-144-5p, muscle strength |
109 | |
| In vivo | LLC | ↑SAA, IL-6 ↓PON1 |
110 | |
| Pancreatic cancer | In vitro | MiaPaCa2, AsPC1, BxPC3, HPAF-2, Panc-1, C2C12 |
↑TNF-α, IL-1β, IFN-γ | 111 |
| In vivo | MiaPaCa2 and AsPC1 xenograft | ↑PCB, G-6-Pase, skeletal muscle proteolysis, ↓myosin, body weight, hepatic glycogen, ATGL, atrogin-1, MURF1 |
111 | |
| In vitro | MiaPaCa2, AsPC1 | ↑Cav-1, IGF1R, IR, glycolysis | 112 | |
| In vivo | MiaPaCa2 cells | ↑ Cav-1, IGF1R, IR, skeletal muscle proteolysis,
↑lipolysis, ↓body weight |
112 | |
| Ex vivo | Serum | ↑GLP-1, apoC-II, apoC-III | 113 | |
| Ex vivo | Serum | ↑ IL-6, glucocorticoids, ↓ ketone levels, food intake |
114 | |
| In vivo | KPC | ↑IL-6, glucocorticoids ↓ food intake, ketones, PPAR-α, ACADM, HMGCS2 |
114 | |
| In vivo | C57BL/6J | ↑LCN2, E3 ubiquitin ligase, MAFBX, MURF1, FOXO1 ↓ BNIP3, CTSL, GABARAPL, PPAR-γ, food intake |
115 | |
| Ex vivo | Human tissue | ↑ IL-6, cachexia | 116 | |
| In vitro | KPC 32908, C2C12, 3T3 | ↑ IL-6, p-STAT3, E3 ubiquitin ligase, atrogin-1, MAFBX, TRIM63/MURF1 ↓ myotube diameter |
116 | |
| In vivo | C57BL/6 | ↑IL-6, muscle wasting ↓ survival |
116 | |
| In vitro | C2C12 | ↑atrogin-1, MURF1 ↓MyHC |
117 | |
| In vivo | C57BL/6 | ↑macrophage mediated STAT3 activation ↓body weight, body strength, IL-6, TNF-α, IL-1α, IL-1β |
117 | |
| In vitro | C2C12 | ↑Sirt1, TRIM63, FBXO32, atrogin-1, NOX4 ↓myotube width, total protein content, MyHC |
118 | |
| In vivo | KPC mouse model S2-013 orthotopic model |
↑Sirt1, MURF1, atrogin-1, ZAG, UCP2, ↓body weight, forelimb grip strength, fat content, MyHC |
118 | |
| In vivo | Pan02, FC1242 bearing mice | ↑ZIP4, Zinc | 119 | |
| Other | In vivo | Walker 256 cells | ↓response to glucagon, isoproterenol, cAMP,
phenylephrine, ↓liver ATP |
120 |
| In vivo | Walker 256 cells | ↑leucine rich diet, ↓tumor FDG uptake, metastasis | 121 | |
| In vitro | C2C12 myoblasts | ↑ atrogin-1, MURF1, E214k, FOXO1, USP2, UBC ↓ myoD, Pax3, p-Akt, MYH2, TNNC1, TPM3, TCAP, |
122 | |
| In vivo | Myostatin cachexia model |
↓myoD, Pax3, ↑ atrogin-1, MURF1, E214k | 122 | |
| In vivo | CHO cells | ↑TNF-20, weight loss, progressive death | 13 | |
| Ex vivo | Serum from cancer patients | ↑ IL-15 | 123 | |
| In vivo | MC38, LLC, CT26 bearing mice |
↑PLA2G7 | 124 |
FATP1: fatty acid transport protein 1; CD36: cluster determinant 36; UCP1: uncoupling protein 1; p-p38: phospho p38; PPARγ: peroxisome proliferator-activated receptor gamma; p-PPARγ: phospho peroxisome proliferator-activated receptor gamma; UCP3: uncoupling protein 3; ERK: extracellular signal-regulated kinase; p-ERK: phospho extracellular signal-regulated kinase; P4HAI: prolyl 4-hydroxylase subunit alpha 1; PKM2: pyruvate kinase M2; ARHGDIB: rho GDP dissociation inhibitor beta; PGK1: phosphoglycerate kinase 1; LDHA: lactate dehydrogenase A; ALDA: aldolase, fructose bisphosphate A; GPD2: glycerol-3-phosphate dehydrogenase 2; ENO1: enolase 1; TPI1: triosephosphate isomerase 1; PGAM1: phosphoglycerol mutase 1; EEF1D: eukaryotic translation elongation factor 1 delta; PRDX1: peroxiredoxin 1; CAT: catalase; SPARC: secreted protein acidic and cysteine rich; COL1A1: collagen type 1 alpha 1 chain; COL1A2: collagen type 1 alpha 2 chain; Cav-1: caveolin 1; PYC: pyruvate carboxylase; G6PD: glucose-6-phosphate dehydrogenase; 4-HNE: 4-hydroxynonenal; ATP: adenosine triphosphate; SDS-MYL1: SDS-soluble myosin light chain 1; IL-6: interleukin-6; MURF1/TRIM63: muscle-specific RING finger protein 1/tripartite motif containing 63; LIF: leukemia inhibitory factor; STAT3: signal transducer and activator of transcription 3; p-STAT3: phospho signal transducer and activator of transcription; p-AMPK: phospho AMP-activated catalytic subunit; mTOR: mechanistic target of rapamycin kinase; p-mTOR: phospho mechanistic target of rapamycin kinase; Pax7: paired box 7; LC3bII/LC3BI: light chain 3 B 1/light chain 3 B 2; PBE: peroxisomal bifunctional enzyme; CPT1α: carnitine palmitoyltransferase 1 alpha; BAT: brown fat tissue; ZAG: zinc-alpha-2 glycoprotein; FDG: fluorodeoxyglucose; LDL: low-density lipoprotein; Ddit4/REDD1: DNA damage inducible transcript 4/regulated in development and DNA damage responses 1; Akt1: Akt serine/threonine kinase 1; p-Akt1: phospho Akt serine/threonine kinase 1; p-4E-BP1: phospho eukaryotic translation initiator factor 4E binding protein 1; p-p70S6K: P70 ribosomal S6 kinase; PDPK1: 3-phophoinositide dependent protein kinase 1; H2O2: hydrogen peroxide; MFN2: mitofusin 2; DRP1: dynamin-related protein 1; FIS1: fission mitochondrial 1; PINK: PTEN induced kinase; PRDM6: PR/SET domain 6; Bcl-2: B-cell CLL/lymphoma 2; TGF-β: transforming growth factor–beta; MHC: myocin heavy chain; MyoD1: myogenic differentiation 1; MAPK: mitogen activated protein kinase; TNF-α: tumor necrosis factor–alpha; ROS: reactive oxygen species; GSH: glutathione; gp130: glycoprotein 130; cavin-3: caveolae associated protein 3; EGFR1: epidermal growth factor receptor 1; HIF1-α: hypoxia inducible factor subunit alpha; pS6K: phospho S6 kinase; PTEN: phosphatase d tensin homolog; HSL: hormone sensitive lipase; p-HSL: phospho hormone sensitive lipase; PTHR: parathyroid hormone receptor; PTHrP: parathyroid hormone receptor–related protein; BM-MNCs: bone marrow–derived mononuclear cells; NFATc1: nuclear factor of activated T cells 1; RAGE: receptor for advanced glycation end product; S100B: S100 calcium binding protein B; HMGB1: high mobility group box 1; IL-3: interleukin-3; IL-9: interleukin-9; IL-12p40: interleukin-12 subunit p40; IL-17A: interleukin-17A; IFNγ: interferon gamma; IL-1α: interleukin-1 alpha; IL-1β: interleukin-1 beta; SAA: serum amyloid A1; PON1: paraoxonase 1; PCB: pyruvate carboxylase; G6pase: glucose 6 phosphatase; ATGL: adipose triglyceride lipase; IGF1R: insulin like growth factor 1 receptor; IR: insulin receptor; GLP1: glucagon like peptide 1; Apo CII: apolipoprotein C2; Apo CIII: apolipoprotein C3; PPAR-α: peroxisome proliferator-activated receptor alpha; ACADM: acyl CoA dehydrogenase medium chain; HMGCS2: 3-hydroxy-3-methylglutaryl-CoA synthase 2; LCN2: lipocalin 2; MAFBX/FBXO32: muscle atrophy F-box protein/F-box protein 32; FOXO1: Forkhead box O1; BNIP3: Bcl-2 interacting protein 3; CTSL: cathepsin L; GABARAPL: GABA type A receptor associated protein like; Sirt1: sirtuin 1, NOX4: NADPH oxidase 4; ZIP4: Zrt/Irt-like protein 4; cAMP: cyclic adenosine mono phosphate; E214K: ubiquitin conjugating enzyme E2 14 kDa; USP2: ubiquitin specific peptidase 2; UBC: ubiquitin C; Pax3: paired box 3; MYH2: myosin heavy chain 2; TNNC1: troponin C1; TPM3: tropomyosin 3; TCAP: titin-cap; TNF-20: tumor necrosis factor–20; IL-15: interleukin-15; PLA2G7: phospholipase A2 group 7.
Figure 1.
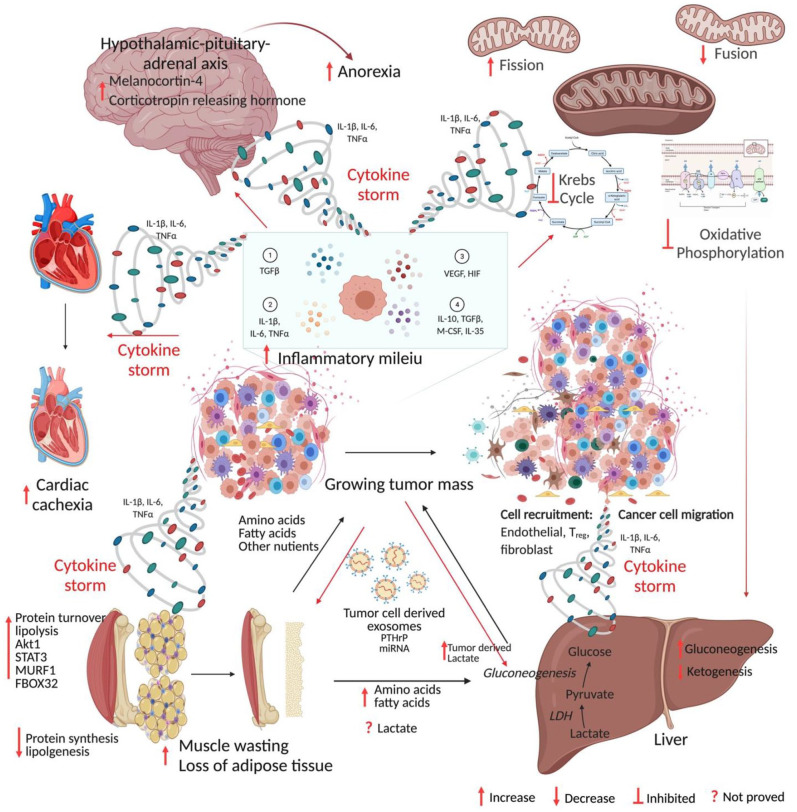
Mechanism of cancer cachexia. Anabolic tumor tissue continuously demands glucose, amino acids, fatty acids, and other nutrients. The cytokine storm generated from the growing mass of tumors acts on host tissue leading to catabolism and cachexia. The figure was created in BioRender.com. (A color version of this figure is available in the online journal.)
Systemic metabolic dysfunction
Cancer cells remodel their metabolic processes to meet the increased demand of proliferative and bioenergetic needs while disrupting host systemic metabolism.1,85 As mentioned above, protein homeostasis is skewed toward the elevated breakdown of muscle proteins and reduced synthesis. 35 Constitutively active ubiquitin-proteasome and insulin signaling pathways are primarily responsible for this. Under anabolic conditions, the PI3K–Akt pathway protects the muscle from undergoing atrophy by inhibiting Forkhead box O (FOXO)-mediated activation of the genes encoding MURF1 and MAFBX or FBXO32. Akt also stimulates the serine/threonine-protein kinase mTOR complex 1 (mTORC1), which activates the S6 kinase-1 (S6K1), leading to anabolic effects on muscle tissues.35–37 Reduced Akt activity has been reported in CC patients to cause the FOXO proteins to be dephosphorylated, allowing transcription of Tripartite motif containing 63 (TRIM63) and FBXO32, which in turn is involved in the myosin heavy chain degradation.36,37 During the progression of CC, hyperactivation of genes encoding MURF1, MAFBX, FBXO30, FBXO31, and increased turnover of myofibrillar proteins were reported, which results in muscle wasting.125,126 Interestingly, it was shown that CC patients often present insulin resistance.38,39 Besides controlling the carbohydrate metabolism, insulin modulates muscle protein breakdown and synthesis to maintain stable blood glucose levels. Physiologically, insulin resistance speeds up muscle proteolysis by suppressing the PI3K–Akt pathway and counter activation of ubiquitin-proteasome pathway.40,41
This mechanism is important in CC since insulin resistance and/or disrupted insulin signaling has been shown in CC murine and Drosophila models in vivo.127–130 Furthermore, in the murine colon 26 (C26) CC model, treatment with the insulin sensitizer rosiglitazone reduced weight loss and anorexia, and in the hepatoma rat cachexia model, this drug hampered weight loss and prolonged survival.131,132 In the Walker 256 cancer, a commonly used in vitro cachexia model, insulin resistance and muscular breakdown were also exacerbated by impaired insulin synthesis from the pancreas. 130 Recent studies involving the fruit fly Drosophila melanogaster conducted by Figueroa-Clarevega and Bilder 129 revealed that tumors release ImpL2, an insulin-like binding protein and a powerful antagonist of insulin signaling, causing systemic metabolic disruption and muscle atrophy. Besides ubiquitin and insulin signaling, a growing body of evidence suggests that the upregulation of autophagy pathways is also crucial for muscle wasting.56,71 A study conducted on lung cancer cohorts presented elevated autophagy mediators such as Bcl2-interacting protein 3 (BNIP3), LC3B (light chain 3 beta), and transcription factors that promote autophagy such as FOXO1. 133 Tardif et al. 134 reported autophagy pathway deregulation in cachectic esophageal cancer patients versus non-cancerous subjects. In addition, Johns et al. 135 documented that increased expression of autophagy-related genes such as microtubule-associated proteins 1A/1B light chain 3B (MAP1LC3B), autophagy protein 5, and beclin 1 in muscles was associated with the CC. Finally, Stephens et al. 136 showed elevated expression of GABA type A receptor associated protein like 1 (GABARAPL1), an autophagy inducer, in cachectic gastrointestinal cancer patients (n = 92) compared to healthy controls. Taken together, these studies indicate that derailed insulin signaling in cancer can have a deleterious influence on muscle hypertrophy and function.
Inflammatory mediators
As cancer advances, cancer cells, infiltrated immune cells, and other stromal cells in the tumor microenvironment secrete enormous amounts of cytokines into the circulation. 137 A vast body of research has established that cytokines including TNF-α, transforming growth factor–β (TGF-β), and IL-6 stimulate muscle fiber disintegration. TNF-α and TNF-related weak inducer of apoptosis (TWEAK) have been shown to directly activate the NF-κB pathway in differentiated muscle cells resulting in the activation of E3 ligases and proteasome-mediated muscle catabolism.138,139 Guttridge et al. 65 showed that NF-κB-induced suppression of muscle cell terminal differentiation is through loss of MyoD. In addition, Fukawa et al. 102 demonstrated that exposure of muscle cells to cancer cell-conditioned medium containing inflammatory molecules, including TNF-α, IL-1β, IL-6, IL-8, LIF and angiogenic factor VEGF, induced fatty acid oxidation resulting in oxidative stress via activating the p38 stress response pathway and impeding the growth of myotube. Tumor-derived IL-6 has been shown to reduce ketogenesis by suppressing peroxisome proliferator-activated receptor–alpha (PPAR-α) in vivo resulting in the marked elevation of glucocorticoids, systemic metabolic stress, reduced food intake, and poor response to anti-cancer immunotherapy. 114 Furthermore, chronic IL-6 is sufficient to induce cachexia in mice, lipolysis in cultured adipocytes, and atrophy in myocytes.12,14,140 Rupert et al. 116 demonstrated that depletion of IL-6 from the malignant pancreatic cells resulted in a dramatic reduction in adipose tissue wasting, myosteatosis, dysregulated metabolism, and eliminated muscle atrophy. Pharmacological inhibition of STAT3 activation has been shown to suppress caspase 3 and ubiquitin-proteasome system resulting in prevention of muscle protein breakdown and cachexia. 66 Recently, Niu et al. 141 demonstrated that HSP90-mediated STAT3 activation induces muscle wasting, weight loss, and catabolism in both in vitro and in vivo models of colon adenocarcinoma, and pharmacological inhibition of HSP90 reversed this effect. Similarly, cancer cachexia has been linked to multiple members of the TGF family. Myostatin (MSTN), for example, interacts with the activin type II receptors ACVR2 and ACVR2B, and stimulates the SMAD2/3 signaling pathway in normal mice, causing significant fat and muscle loss. In several mouse tumor models, administration of ACVR2B prevented cancer cachexia.142–144 Metastatic lesions have been shown to induce the secretion of TGF-β from the bone matrix into circulation, which then stimulates SMAD2/3 in skeletal muscles, in turn inducing the transcription of NADPH oxidase 4 (Nox4). 145 Nox4 induces oxidation and stabilization of ryanodine receptor 1 (Ryr1) leading to aberrant calcium leakage from muscle endoplasmic reticulum, subsequently leading to muscle weakness. 145 Two more TGF superfamily members, namely, growth differentiation factor 11 (GDF11) and GDF15, have subsequently been established as mediators of appetite and cachexia indirectly by controlling the food intake through their action on the hypothalamus.146–148 Elevated serum level of GDF15 was shown to be correlated with increased incidence of CC and poor patient outcome in pancreatic cancer. 63 In addition, transcription factors such as NF-κB, STAT3, and CAAT/enhancer-binding protein-β regulate expression of E3 ubiquitin ligases and autophagy genes.55,65,66,139,149 These studies suggest that inflammatory mediators possibly cause skeletal muscle weakness and atrophy to accelerate CC-associated muscle dysfunction.
Extracellular vesicle mediated regulation
Extracellular vesicles (EVs) are secreted vesicles that aid in intercellular communication by carrying DNA, RNA, proteins, lipids, and metabolites. 150 Several experiments have shown that conditioned medium from cancer cell lines, including LLC, H1299, C26, and AGS secreted EVs carrying heat shock proteins such as HSP70 and HSP90, resulting in induction of cachexia symptoms both in vitro and in vivo.151–154 Mechanistic studies conducted by Zhang et al. 155 showed that EV-derived HSP70 and HSP90 activate Toll-like receptor 4 (TLR4) and p38–MAPK pathways resulting in CC, and that this is prevented by either neutralizing or silencing HSP70 and HSP90 in LLC cells. Recently, Hu et al. 106 demonstrated that LLC cell-derived EVs induce lipolysis both in vitro and in vivo by delivering the parathyroid hormone–related protein (PTHrP) which interacts with its receptor parathyroid hormone receptor (PTHR) to exert downstream effects. Similar observations were made by Yang et al. 156 in 2019 in pancreatic tumor xenograft models. This study further showed that a zinc finger transporter ZIP4 stimulated EVs secretion by cancer cells via Ras-related protein 27B (RAB27B) GTPase, and tumor xenograft models bearing ZIP4 knockdown pancreatic cancer cell lines displayed better body weight and survival than mice with functional ZIP4 bearing tumors. 156 Using human pancreatic cachexia inducing cancer cell lines and patient sera, it has been demonstrated that TLR7/8/9 antagonist IMO-8503 reverses tumor-derived EVs-induced myoblast apoptosis and CC. 157 Waning et al. 145 demonstrated that the TGF-β secretion was induced by fusing PDAC-derived exosomes with liver Kupffer cells, which induce cachexia in metastatic bone disease. Tumor-secreted EVs carrying miR-21 stimulated apoptosis in myoblast cells via TLR7 signaling, leading to muscle atrophy. 158 In addition, Miao et al. 101 demonstrated that exosome-derived miR-195a-5p and miR-125b-1-3p downregulated Bcl-2, and thereby induced muscle fiber breakdown and cachexia in a mouse colorectal cancer model. Recently, Gao et al. 159 found that esophageal squamous cell carcinoma–derived EVs carrying prolyl 4-hydroxylase beta (P4HB) induced skeletal muscle cell apoptosis in a mouse xenograft model. Mechanistic studies by the authors have revealed that P4HB induced apoptosis via activating the ubiquitin-proteasomal pathway and regulating the stability of Bcl-2 and phosphoglycerate dehydrogenase. The authors further proved that the inclusion of CCF642, a P4HB inhibitor, suppressed apoptosis of muscle cells in vitro and prevented muscle atrophy and weight loss in an esophageal squamous cell carcinoma–induced cachexia mouse model. 159
These studies have delineated how cancer cells harbor host tissue metabolism by secretory molecules and cargo packaged EVs and skew the entire host metabolism toward catabolism while themselves being anabolic.
Loss of white adipose tissue
Turnover of adipose tissue, especially white adipose tissue (WAT), has been commonly observed in cancer patients. Early research found that knocking down the gene encoding adipose triglyceride lipase (ATGL), an enzyme that catalyzes triacylglycerol hydrolysis, prevented tumor-bearing mice from losing their WAT and reduced skeletal muscle atrophy. 160 During precachexia, elevated lipolysis, energy expenditure, and markers of adipose tissue thermogenesis have been reported. In a variety of in vivo tumor models, including colon, lung, liver, and pancreatic cancer models, browning of WAT was discovered to be the primary response during CC development that induces lipid mobilization.21,161 Mechanistic studies have shown that pro-inflammatory cytokine IL-6 induces uncoupling protein 1 (UCP1) in WAT, promoting thermogenesis by uncoupling mitochondrial respiration from adenosine triphosphate (ATP) production. 162 Blockade of inflammation either by blocking IL-6 signaling or by blocking β-adrenergic neurons or by using anti-inflammatory treatments resulted in a remarkable reduction of WAT browning. 21 In the in vivo LLC model, tumor cell-secreted PTHrP binds to receptor PTHR on WAT and promotes WAT browning, while PTHrP neutralization preserved skeletal muscles, preventing CC. 162
Furthermore, these circulatory factors alter liver functions to meet their energy demand. 84 Bioluminescence-based metabolic imaging and subsequent experimental evidence have shown that elevated lactate levels in primary tumor tissues of human cervical cancer were significantly correlated with increased risk of metastasis and poor outcome. 163 Through gluconeogenesis, the liver converts lactate obtained from the blood into glucose, which is subsequently reintroduced into the circulation and used for energy sources by other tissues. This is referred to as the Cori cycle and is an energy-inefficient process.42,164 Despite the lack of experimental evidence, it is believed that the high metabolic demands of the tumor, together with an intensified Cori cycle, cause a 40% increase in energy consumption in individuals with advanced CC. 164 The amino acids released through muscle breakdown have been postulated to be converted to glucose via gluconeogenesis in the liver during glucose scarcity.165–167 Furthermore, cachectic muscle metabolic profiling from mice with lung cancer revealed low amounts of ketone bodies in serum despite the fact that prolonged starvation usually stimulates ketogenesis. Reduced ketogenesis combined with restricted food intake significantly raised glucocorticoid levels, a trend which was seen in various CC models.114,168 In a genetically engineered lung CC mouse model, augmenting ketogenesis with the PPAR agonist fenofibrate prevented muscle atrophy by lowering systemic glucocorticoids. 168 In addition, tumor-derived IL-6 reduced peroxisome proliferator-activated receptor-α (PPAR-α)-controlled ketogenesis in the colon, liver, and pancreatic cancer models, and the consequent increase in glucocorticoid levels suppressed intratumoral immunity during caloric restriction.114,168,169 During CC, malfunction of these general energy-inefficient metabolic activities in the liver might prolong energy shortage which necessitates additional muscle breakdown.
Thus, cancer cachexia is likely to be governed by an imbalance in the homeostasis of anabolic and catabolic processes.
CNS in cachexia
Our understanding of the CNS role in CC pathogenesis largely depends on observations from animal CC models. An increasing body of evidence suggests that the paracrine inflammatory milieu generated from peripheral inflammation during CPC is amplified and modified within the medio-basal hypothalamus, leading to an alteration in the activity of neurons involved in the regulation of appetite and metabolic processes.60,170,171 Inflammatory stimuli including TNF-α and IL-1β have been shown to initiate a feed-forward loop by acting upon receptors on hypothalamic neurons—pro-opiomelanocortin, agouti-related protein neurons—resulting in acute illness response, loss of appetite, weight reduction, and muscle protein degradation. 61 CNS/IL-1β induced hypothalamic-pituitary-adrenal axis activation evokes rapid muscle atrophy and is blocked by adrenalectomy or by the muscle-specific knockout of glucocorticoid receptors. 61 IL-1β has been shown to stimulate melanocortin-4, corticotropin-releasing hormone, adrenocorticotropic hormone, and cortisol, thereby reducing appetite and promoting the catabolic effects.172,173 To date, the clinical studies on CNS-regulated cachexia are limited to investigations on circulatory levels or administration of inflammatory molecules or neuromodulatory peptides such as ghrelin. 174
Cardiac muscle atrophy
Currently, a countable number of studies are available focusing on the effects of CC on vital organs. Cardiac muscle performs a vital role and was assumed to be spared since it cannot be exploited as amino acid or fat repository like skeletal muscles during normal physiology. Although the cardiac muscle atrophy in CC remains to be evaluated, research on animal cachexia models has shown substantial loss of cardiac muscle fibers and functional impairment of cardiac muscles by echocardiography. 4 In vivo studies have proved that mechanisms that contribute to cardiac muscle atrophy are similar to those in skeletal muscles including elevated protein degradation, reduced protein synthesis, inflammation, and autophagy.175–177 Although adequate knowledge of mechanisms is lacking, it has been shown that RC is significantly associated with cardiac arrhythmias and arrest in cancer patients. 175
Others
Recently, “omic” studies have been extended to find the molecular mechanisms, pathways, and promising biomarkers for CC. Integrative transcriptomic analysis of cachectic muscle and extensive bioinformatics approaches by Niu et al. 99 revealed that 371 genes were up-regulated and 422 were downregulated with the enrichment of genes involved in extracellular matrix reorganization, muscle system processes, muscle differentiation, muscle tissue development, JAK-STAT signaling, cytokine-cytokine receptor signaling, HIF-1 signaling, and so on. Using in vitro knockdown models, authors further showed that p38 induced expression of Ddit4 which in turn inhibited phosphorylation of Akt1, mTOR, 4E-BP1, and p70SK6. 99 Deletion of REDD1 in LLC-conditioned C2C12 myoblasts showed that elevated phosphorylation of FOXO3A, Akt1, mTORC1 prevented cachexia. 105 Furthermore, knockout of lipocalin 2 (LCN2) in pancreatic cachexia mice models showed the suppression of food intake by directly acting on CNS upon crossing the blood-brain barrier. 115 Recently, transcriptome profiling of rectus abdominis muscle from pancreatic cancer patients showed 340 differentially expressed genes, including FOXO1, FOXO3, phosphoinositide-3-kinase regulatory subunit 1 (PIK3RI), glutamate-ammonia ligase (GLUL), interleukin-6 receptor (IL-6R), ZIP14, protein phosphatase 1 regulatory subunit 8 (PPP1R8), apoptosis enhancing nuclease (AEN), coiled-coil domain containing 68 (CCDC68), Wnt family member 9A (WNT9A), protein O-mannosyl transferase 2 (POMT2), sestrin 1 (SESN1), ring finger protein 207 (RNF207), and dystonin (DST) were found to be correlated with an increasing grade of weight loss in cancer patients. 178
Diagnosis and treatment
Diagnosis of CC is challenging due to the display of multiple symptoms and accordingly, each patient must be critically evaluated for BMI, nutritional status, and locomotory index prior to the optimization of precision medicine.3,25 Cachexia might be under-recognized in certain cases including epidemic obesity. Even at the initial time of cancer diagnosis, obese patients can have substantial ongoing muscle depletion known as sarcopenic obesity. In addition to obesity, underlying cachexia can be masked by weight gain due to ascitic fluid accumulation or peripheral edema.74,78,179 Hence, the effective early diagnosis of cachexia includes careful monitoring of body composition, fat mass, and muscle mass. The Patient-Generated Subjective Global Assessment (PG-SGA) is an approved and validated method for malnutrition in patients with CC. 180 PG-SGA contains a comprehensive questionnaire that assesses calorie intake, body weight, muscle mass, body fat mass, temperature, functional status of locomotory organs, and whether systemic or local edema is present. 180 The Mini-Nutritional Assessment (MNA) is a rapid nutritional screening tool that evaluates dietary history, nutritional risk factors, body weight, and mid-arm circumference. 181 However, these screening tools do not measure muscle mass or composition. 182 Regularly used parameters to evaluate body composition in cancer patients include anthropometric methods, bioelectrical impedance analysis (BIA), computed tomography (CT), and dual-energy X-ray absorptiometry (DXA). The anthropometric method evaluates body composition by measuring height, weight, skinfolds, and body circumference. It is one of the oldest and simplest methods, exhibiting poor accuracy, and it cannot distinguish between fat content and lean body mass. 183 BIA, on the contrary, can be used to assess the percentage of total body fat, fat-free mass, and to calculate body fluid based on the electrical properties. 184 Nonetheless, BIA is not as accurate as DXA, which predominantly determines appendicular muscle mass. Although DXA is cost-effective and requires a very low level of radiation, it does not distinguish subsets of adipose tissues into visceral, subcutaneous, and intramuscular. 185 Meanwhile, recent advancements in CT scans allow assessment of axial muscle mass. This approach offers a high level of sensitivity and specificity and is a gold standard for evaluating body composition. 186 Magnetic resonance imaging (MRI) has a higher specificity and accuracy, equivalent to that of CT, in measuring the body composition, and does not expose the patients to ionizing radiation, although it is more expensive than CT.187,188 Upper arm grip assessment, psychosocial and physical activity are the few parameters to measure muscle strength and functionality of patients with CC.3,189 Screening for the novel biomarkers of CC is the research hotspot. A variety of inflammatory markers and cytokines are proposed to be biomarkers of CC, including hemoglobin content, ALB content, CRP, leptin (LEP), adiponectin, ghrelin, insulin-like growth factor-1 (IGF-1), IL-1, IL-6, and TNF-α.190,191 Moreover, a few cancer patients might be less susceptible to cachexia development. For instance, cachexia is less likely in patients with a loss of function mutation in protein P selectin (SELP). 192 Recently, using an aptamer-based discovery platform, Narasimhan et al. 193 demonstrated the expression of 71 proteins that correlated with pancreatic cancer cachexia, including known cachexia markers such as LEP, MSTN, and ALB as well as novel cachexia markers such as lymphatic vessel endothelial hyaluronan captor 1 (LYVE1), complement C7 (C7), and coagulation factor 2 (F2). However, these biomarkers are affected by several factors such as age, sex, inflammation, and other underlying diseases. 194 In order to measure the CC systematically, systematic scoring methods have been evolved including the CC scoring system (CASCO), the modified Glasgow prognostic score (mGPS), and the cachexia staging score (CSS). The score corresponds with either non-malignant CPC or CC and RC and effectively predicts the survival of CC patients.194–197 More recently, Anderson et al. demonstrated that CC patients display reduced total lean body mass, stair climb power (SCP), upper body strength, and bioavailable testosterone, and increased REE, cytokines levels, and fatigue compared to the cancer patients without cachexia and weight stable patients without cancer. This study also showed that SCP is a better marker of CC with 78% sensitivity and 77% specificity at a cut-off of 336 watts. 198
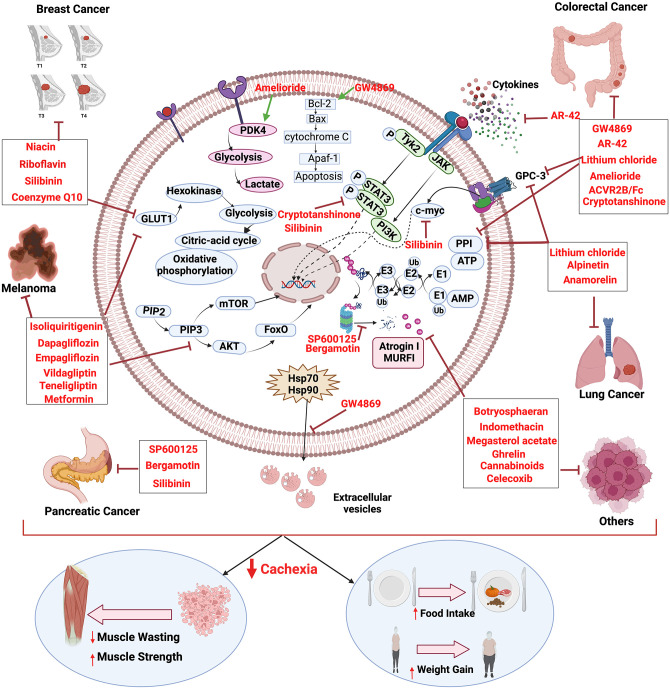
Over the years, studies have proven that CC can be differentiated from the underlying other muscle wasting disorders by the mechanical characteristics, and the targeted therapies that prevented CC prolonged the survival and improved the quality of life, while tumor cells continue to propagate.143,199 Multidimensionality of CC demands personalized and systematized treatment strategies. Nevertheless, RC is rebellious to treat, and palliative treatments are often preferred over the other therapy options. The first line of choice to prevent further deterioration during CC is to treat the patients with catabolism inhibitory drugs alongside nutrition supplementation, exercise, and psychological counseling.2,200,201 Table 3 and Figure 2 summarize drugs under various clinical trials and their mechanisms.91,92,100–102,174,199,202–219
Table 3.
Preclinical and clinical trials to treat cachexia.
| Cancer type | Drug or treatment | In vitro/in vivo/clinical | Model | Mechanism of outcome | References |
|---|---|---|---|---|---|
| Breast | Vitamins—riboflavin, Niacin, coenzyme Q10 | In vivo | Sprague-Dawely rats | ↑ Krebs cycle, oxidative phosphorylation | 202 |
| Silibinin | In vitro | MDA-MB-231, MDA-MB-468, BT549, BT474, SKBR3 cells |
↓anaerobic glycolysis, GLUT-1, HK-II, p-EGFR, c-MYC,
stemness ↑cellular biomass |
203 | |
| Colorectal | AR-42 | In vivo | C26 model | ↑restoration glycolytic intermediates, KY, GDAP1, KCNH2, ACTC1,
FOXO1 ↑PDE4A, TNMD, CASR, SYPL2, IGFBP5 ↓catabolic muscle phenotype, weight loss, glycogen intermediates, ↓IL-6, LIF, EDEM1, FBXO6, XIRP1, CDKN1A, DAXX, MYLK2, NUB1 ↓NUB1 |
199 |
| Amelioride | In vivo | CT26 model | ↑PDK4 ↓exosome release from tumors, muscle atrophy, catabolism, glycolysis, OXCT1, BDH2 |
91 | |
| Lithium chloride | In vitro | C2C12 cells | ↑ myocyte differentiation, MyHC, GSK3β ↓Pax-7, atrogin-1, IL-1β, IL-6, iNOS |
204 | |
| In vivo | CT26 model | ↑body weight ↓ atrogin-1, MURF1, iNOS, muscle wasting, tumor growth |
204 | ||
| GW4869 | In vitro | HCT-116 cells | ↑Leptin, ADIPSIN ↓exosome secretion, UCP1, PRDM16, WAT browning |
100 | |
| GW4869 | In vivo | C26 model | ↑Bcl-2 ↓exosomes, miR-195a-5p, miR-125b-1-3p, muscle atrophy |
101 | |
| ACVR2B/Fc | In vivo | HCT-116 model | ↑muscle strength and function, cardiac strength ↓ fat loss, bone loss, |
205 | |
| Cryptotanshinone | In vitro | C2C12 cells | ↓ atrogin-1, MURF1, STAT3, atrophy | 206 | |
| In vivo | CT26 model | ↓STAT3, atrogin-1, MURF1, weight loss, muscle wasting | 206 | ||
| Lung | Alpinetin | In vitro | LLC cells | ↓E3 ubiquitin ligase, atrogin-1, MURF1, myoatrophy | 207 |
| In vivo | C57BL/6 | ↑body weight, PPAR-γ ↓spleen weight, IL-1β, NF-κB |
207 | ||
| Anamorelin |
Phase II trial | Advanced NSCLC patients | ↑ lean body mass, body weight | 208 | |
| Melanoma | Isoliquiritigenin | In vitro | A375 cell line | ↓glucose uptake, lactate levels, GLUT1, HK-II, ATP, mTOR,
p-mTOR, RICTOR, p-Akt, p-GSK3β ↑oxygen consumption |
209 |
| Metformin, teneligliptin, vildagliptin, empagliflozin, dapagliflozin | In vivo | B16F1 mouse melanoma cells | ↑ body weight, feed intake, water intake, serum GLP-1, glycogen
storage skeletal muscle weight, locomotor count, perirenal fat, visceral fat, subcutaneous fat, serum triglyceride, serum HDL, serum VLDL ↓ ghrelin, TNF- α, IL-6, CRP, serum glucose, serum insulin, serum LDH, liver weight/body weight ratio, kidney weight/body weight ratio, spleen weight/ body weight ratio, muscle fibrosis |
210 | |
| Pancreatic | Silibinin | In vitro | S2-013, T3M4, PANC-1, BxPC-3, AsPC-1, MiaPaCa-2 |
↓c-MYC, STAT3 | 211 |
| In vivo | Orthotopic nude mice | ↑body weight ↓c-MYC, p-STAT3, GLUT-1, MURF1, atrogin-1, myofiber degradation, TNF-α, IL-6 |
211 | ||
| Bergamotiin | In vitro | Pancreatic cancer cell conditioned C2C12, 3T3L1 |
↑MyHC, aP2, adiponectin, resistin ↓MURF1, atrogin-1, cell viability, LC3-II, p-STAT3, p-FOXO4, p-Akt, ZAG, HSL |
212 | |
| In vivo | MiaPaCa-2 cells | ↑body weight, C/EBP- α, PPAR-γ ↓atrophy, MURF1, atrogin-1 |
212 | ||
| SP600125 | In vitro | Pancreatic cancer cell conditioned C2C12 | ↓TRIM63, FBXO32, myotube atrophy, myosin heavy chain turnover | 213 | |
| In vivo | Orthotopic cachexia Mouse model |
↑forelimb grip strength, body weight, carcass weight,
gastrocnemius weight ↓TRIM63, FBXO32 |
213 | ||
| Other | Botryosphaeran Monoclonal Antibody Against GFRAL (3P10) |
In vivo | Walker-256 model | Corrected insulin resistance, normalized glucose and cholesterol
levels ↑leukocytes, lymphocytes ↓tumor growth, cachexia |
214 |
| In vivo | Patient-derived xenograft model | ↑forelimb grip strength, body weight ↓FOXO32, BNIP3, GADD45A, lipid mobilization and oxidation |
215 | ||
| Indomethacin | In vivo | C57BL/6J | ↑food intake, weight ↓ IL-1α, IL-1β, IL-2, TNF-α, |
216 | |
| Ghrelin | In vivo | F344/NTafBR | ↑weight gain, lean body mass, neuropeptide Y, agouti
gene-related peptide ↓ IL-1β, |
174 | |
| Megestrol acetate | Clinical trial | Cachectic cancer patients (n = 133) | ↑weight gain, appetite, food intake | 217 | |
| Cannabinoids | Clinical trial | Advanced stage cancer patients (n = 24) | ↑food intake, appetite, quality of sleep, improved chemosensory perception | 218 | |
| Celecoxib | Clinical trial | Cachectic cancer Patients (n = 11) |
↑BMI, body weight, quality of life, physical performance | 219 |
GLUT-1: glucose transporter 1; HK-II: hexokinase 2; p-EGFR: phospho epidermal growth factor receptor 1; c-MYC: c-MYC proto oncogene; KY: kyphoscoliosis peptidase; GDAP1: ganglioside induced differentiation associated protein 1; KCNH2: potassium voltage gated channel subfamily H member 2; ACTC1: actin alpha cardiac muscle 1; PDE4A: phosphodiesterase 4A; TNMD: tenomodulin; CASR: calcium sensing receptor; SYPL2: synaptophysin 2; IGFBP5: insulin like growth factor binding protein 2; IL-6: interleukin-6; LIF: leukemia inhibitory factor; EDEM1: ER degradation enhancing alpha mannosidase-like protein 1; FBXO6: F-box protein 36; XIRP1: xin actin binding protein repeat containing 1; CDKN1A: cyclin-dependent kinase inhibitor 1A; DAXX: death domain associated protein; MYLK2: myosin light chain kinase 2; NUB1: negative regulator of ubiquitin-like proteins 1; MyHC: myocin heavy chain; GSK3β: glycogen synthase kinase 3 beta; p-GSK3β: phosphoglycogen synthase kinase 3 beta; Paired box 7; IL-1β: interleukin-1 beta; iNOS: inducible nitric oxide synthase; MURF1/TRIM63: muscle-specific RING finger protein 1/tripartite motif containing 63; UCP1: uncoupling protein 1; PRDM16: PR/SET domain 16; WAT: white adipose tissue; Bcl-2: B-cell CLL/lymphoma 2; STAT3: signal transducer and activator of transcription 3; p-STAT3: phospho signal transducer and activator of transcription 3; NF-κB: nuclear factor kappa—light chain enhancer of activated B cells; ATP: adenosine triphosphate; mTOR: mechanistic target of rapamycin kinase; p-mTOR: phospho mechanistic target of rapamycin kinase; TNF-α: tumor necrosis factor–alpha; CRP: C-reactive protein; GLP1: glucagon like peptide 1; HDL: high-density lipoprotein; VLDL: very low density lipoprotein; RICTOR: RPTOR independent companion of mTOR complex 2; Akt1: Akt serine/threonine kinase 1; p-Akt1: phospho Akt serine/threonine kinase 1; LC3bII/LC3BI: light chain 3 B 1/light chain 3 B 2; p-FOXO4: phospho Forkhead box O4; ZAG: zinc-alpha-2 glycoprotein; HSL: hormone sensitive lipase; C/EBP- α: CAAT enhancer binding protein alpha; PPARγ: phospho peroxisome proliferator-activated receptor gamma; FBXO32: F-box protein 32; BNIP3: Bcl-2 interacting protein 3; GADD45A: growth arrest and DNA damage inducible alpha.
Figure 2.
Molecular insight to cachexia and mechanism of cancer cachexia inhibition by various drugs. The figure was created in BioRender.com. (A color version of this figure is available in the online journal.)
ASCO guidelines recommend dietary assessment and psychological counseling as a crucial part of the treatment besides the drug-based treatments. 220 Artificial feeding with priority given to the enteral route has been the preferred way to treat terminally ill cancer patients. 221 It is becoming increasingly obvious that cancer cachexia is a multiorgan disease involving a variety of causes, and hence needs combined therapeutic approaches such as nutrient supply, physical activity, and drugs for its management. 222 Nutritional supplementation is inevitable in cancer patients, as food intake is compromised secondary to anorexia, oral mucositis, and vomiting. 223 For effective anabolic resistance, an increase in caloric intake of 300–400 kcal/day and 50% extra protein intake is essential. 224 High calorie and proteinaceous diet along with oral supplementation of β-hydroxy-β-methyl butyrate (HMB), omega-3 fatty acids, L-carnitine, and eicosatetraenoic acid have shown to be beneficial in patients with CPC and CC.225–229 A study conducted on lung cancer patients showed that oral administration of omega-3 fatty acids increases lean body mass and improves the cachectic conditions in these patients. 230 In addition, pharmacological agents such as those that target proinflammatory cytokines, non-steroidal anti-inflammatory drugs (NSAIDs), and cannabinoids have been widely used to treat CC. 224 Anti-TNF-α agents such as etanercept and infliximab, thalidomide, and pentoxifylline showed a modest gain in lean body mass with no noteworthy clinical benefits.15,16,231–235 Clazakizumab humanized anti-IL-6 monoclonal antibody increased the hemoglobin and ALB levels and relieved fatigue in advanced-stage cancer patients. 236 Administration of ghrelin daily for 8 weeks in low dose (0.7µg/kg body weight) or high dose (13µg/kg body weight) subcutaneously improved the appetite and reduced fat loss in gastrointestinal cancer patients. 237 In a phase II randomized clinical trial, intravenous administration of ghrelin increased the food intake and appetite and reduced chemotherapy-induced nausea in esophageal cancer patients. 238 In addition, progesterone derivatives including megestrol and medroxyprogesterone have been shown to inhibit proinflammatory cytokines such as TNF-α, IL-6, and IL-1 thereby reducing cancer-related anorexia and cachexia.239–241 Various corticosteroids such as dexamethasone (3–6 mg/day), methylprednisolone (12 mg/day), and prednisone (15 mg/day) have been shown to increase appetite and weight gain in cancer patients.242,243 However, these hormonal derivatives have shown long-term side effects, including insulin resistance, myopathy, fluid retention, adrenal inefficiency, and sleep disorders.243,244 In addition, administration of NSAIDs such as celecoxib showed a significant increase in body weight, BMI, and quality of life in head and neck and gastrointestinal cancer patients. 219 A phase III clinical trial showed that administration of phytocannabinoid, tetrahydrocannabinol (THC), increases appetite, body fat, body weight, and quality of life. 245 Recent studies have shown that Kampo medicine, a traditional Japanese medicine, improves CC condition.247–249 Kampo formulae including Hochuekkito (HET), Juzentaihoto (JTT), Ninjin’yoeito (NYT), Seishoekkito (SET), Shosaikoto (SSK), and Rikkunshito (RKT) improved appetite, anemia, protein synthesis, ghrelin levels, and reduced protein breakdown, inflammation, and adipose tissue loss in various preclinical and clinical settings of CC.248–259
Recently, in December 2020, Japan approved anamorelin (non-peptide ghrelin analog) for cancer cachexia treatment based on phase II trials conducted in Japanese advanced gastrointestinal and lung cancer patients, creating the hope for the development of novel drugs.208,260,261 In addition, physical exercise has shown beneficial effects, including reduced systemic inflammation, reduced catabolism, and preserving muscle mass in CC patients along with psychological stability.262–264 Recent studies have highlighted the considerations for multimodal treatment protocol owing to the complexity of pathogenesis in CC. A phase II clinical trial conducted by Solheim et al. 265 used oral nutritional supplements, resistance training, and celecoxib for CC in patients with incurable lung and pancreatic cancers. Next, a randomized controlled clinical trial by Uster et al. 266 implementing nutritional supplements with 60 min of exercise twice a week showed increased protein intake, and reduced nausea and vomiting in palliative cancer patients. Since no major side effects were observed during these studies, multimodal treatment approaches might be considered safe and feasible.
Lack of adequate early diagnosis, prognosis, and therapeutic algorithms creates great difficulty in preventing cachexia development and effective treatments during cancer progression.
Conclusions
Overall, progressive cachexia has deleterious and lethal consequences for cancer patients. The widespread failure of upliftment of CC patients by the usage of anti-cancer therapies led to the realization that critical mediators and mechanisms that induce cachexia could be distinct from those that drive primary tumor progression and metastasis. As mentioned earlier, our current knowledge about cachexia is widely accepted from the studies on genetically engineered and orthotopic animal cachexia models which do not recapitulate systemic and molecular changes occurring in CC patients. Moreover, the vast majority of murine models used for studying CC are LLC in C57BL/6, colon-26 in Balb/c, tumor cell injection, and transgenic mice C57BL/6 APC+/min models. Different strains and different cancer types need to be employed to reveal the relevant cachectic mechanisms. Nevertheless, these observations prompt future studies to aim for screening of relevant early diagnostic and prognostic biomarkers and clinical validation of promising therapies that can effectively treat cachexia alongside the tumor. In addition, it is important to distinguish precachexia from anorexia and later-stage cachexia events. Present knowledge gained from animal studies and a few clinical studies are insufficient to comment on potential mediators of precachexia alone and is a subject of future investigations. Clinical trials on RC patients are lacking due to increased dropout, missing data, and avoiding issues of confounding death. Currently, there is no standard therapy that can effectively reverse the CC. Given the complexity of CC, rigorous preclinical studies in the context of etiology, initiation, progression, and therapy followed by robust clinical validation are all warranted for the effective management of CC.
Footnotes
Authors’ Contributions: MH contributed to the conceptualization, drafting of the manuscript, visualization, and overall editing; UD, SG, and AK supplied critical overall manuscript revision and editing, and ABK contributed to the conceptualization, funding, and overall supervision, and supported review development and overall editing.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Authors acknowledge DBT-AIST International Center for Translational and Environmental Research (DIACENTER) awarded to Prof Ajaikumar B. Kunnumakkara (grant number BT/BI/14/042/2017). Mangala Hegde acknowledges Science and Engineering Board (SERB)-National Post Doctoral Fellowship (NPDF) (PDF/2021/004053). Uzini Devi Daimary and Aviral Kumar acknowledge the Prime Minister’s Research Fellowship (PMRF) program, Ministry of Education (MoE), Government of India for providing them the fellowship.
ORCID iD: Ajaikumar B. Kunnumakkara  https://orcid.org/0000-0001-9121-6816
https://orcid.org/0000-0001-9121-6816
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74 [DOI] [PubMed] [Google Scholar]
- 2. von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 2010;1:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–95 [DOI] [PubMed] [Google Scholar]
- 4. Kazemi-Bajestani SMR, Becher H, Fassbender K, Chu Q, Baracos VE. Concurrent evolution of cancer cachexia and heart failure: bilateral effects exist. J Cachexia Sarcopenia Muscle 2014;5:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacDonald N. Cancer cachexia and targeting chronic inflammation: a unified approach to cancer treatment and palliative/supportive care. J Support Oncol 2007;5:157–64; discussion 164–6, 183 [PubMed] [Google Scholar]
- 6. Rydén M, Arner P. Fat loss in cachexia—is there a role for adipocyte lipolysis. Clin Nutr 2007;26:1–6. [DOI] [PubMed] [Google Scholar]
- 7. Siddiqui JA, Pothuraju R, Jain M, Batra SK, Nasser MW. Advances in cancer cachexia: intersection between affected organs, mediators, and pharmacological interventions. Biochim Biophys Acta Rev Cancer 2020;1873:188359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vincent EE, Sergushichev A, Griss T, Gingras M-C, Samborska B, Ntimbane T, Coelho PP, Blagih J, Raissi TC, Choinière L, Bridon G, Loginicheva E, Flynn BR, Thomas EC, Tavaré JM, Avizonis D, Pause A, Elder DJE, Artyomov MN, Jones RG. Mitochondrial phosphoenolpyruvate carboxykinase regulates metabolic adaptation and enables glucose-independent tumor growth. Mol Cell 2015;60:195–207 [DOI] [PubMed] [Google Scholar]
- 9. Schwartsburd P. Cancer-induced reprogramming of host glucose metabolism: “Vicious Cycle” supporting cancer progression. Front Oncol 2019;9:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costelli P, Carbó N, Tessitore L, Bagby GJ, Lopez-Soriano FJ, Argilés JM, Baccino FM. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest 1993;92:2783–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tessitore L, Costelli P, Baccino FM. Humoral mediation for cachexia in tumour-bearing rats. Br J Cancer 1993;67:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen JL, Walton KL, Qian H, Colgan TD, Hagg A, Watt MJ, Harrison CA, Gregorevic P. Differential effects of IL6 and activin A in the development of cancer-associated cachexia. Cancer Res 2016;76:5372–82 [DOI] [PubMed] [Google Scholar]
- 13. Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, Wolfe A, Socher SH. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell 1987;50:555–63 [DOI] [PubMed] [Google Scholar]
- 14. Baltgalvis KA, Berger FG, Peña MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc ( Min/+) mouse. Pflugers Arch 2009;457:989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jatoi A, Dakhil SR, Nguyen PL, Sloan JA, Kugler JW, Rowland KM, Soori GS, Wender DB, Fitch TR, Novotny PJ, Loprinzi CL. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central Cancer Treatment Group. Cancer 2007;110:1396–403 [DOI] [PubMed] [Google Scholar]
- 16. Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, Mattar BI, Loprinzi CL. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 2010;68:234–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wiedenmann B, Malfertheiner P, Friess H, Ritch P, Arseneau J, Mantovani G, Caprioni F, Van Cutsem E, Richel D, DeWitte M, Qi M, Robinson D, Jr, Zhong B, De Boer C, Lu JD, Prabhakar U, Corringham R, Von Hoff D. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol 2008;6:18–25 [PubMed] [Google Scholar]
- 18. Stovroff MC, Fraker DL, Swedenborg JA, Norton JA. Cachectin/tumor necrosis factor: a possible mediator of cancer anorexia in the rat. Cancer Res 1988;48:4567–72 [PubMed] [Google Scholar]
- 19. Vaisman N, Hahn T. Tumor necrosis factor—alpha and anorexia—cause or effect? Metabolism 1991;40:720–3 [DOI] [PubMed] [Google Scholar]
- 20. Gilabert M, Calvo E, Airoldi A, Hamidi T, Moutardier V, Turrini O, Iovanna J. Pancreatic cancer-induced cachexia is Jak2-dependent in mice. J Cell Physiol 2014;229:1437–43 [DOI] [PubMed] [Google Scholar]
- 21. Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 2014;20:433–47 [DOI] [PubMed] [Google Scholar]
- 22. Tisdale MJ. Cancer cachexia. Langenbecks Arch Surg 2004;389:299–305 [DOI] [PubMed] [Google Scholar]
- 23. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381–410 [DOI] [PubMed] [Google Scholar]
- 24. Tisdale MJ. Cancer anorexia and cachexia. Nutrition 2001;17:438–42 [DOI] [PubMed] [Google Scholar]
- 25. Martin L. Diagnostic criteria for cancer cachexia: data versus dogma. Curr Opin Clin Nutr Metab Care 2016;19:188–98 [DOI] [PubMed] [Google Scholar]
- 26. Kasvis P, Vigano M, Vigano A. Health-related quality of life across cancer cachexia stages. Ann Palliat Med 2019;8:33–42 [DOI] [PubMed] [Google Scholar]
- 27. Nicolini A, Ferrari P, Masoni MC, Fini M, Pagani S, Giampietro O, Carpi A. Malnutrition, anorexia and cachexia in cancer patients: a mini-review on pathogenesis and treatment. Biomed Pharmacother 2013;67:807–17 [DOI] [PubMed] [Google Scholar]
- 28. Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev 2010;38:168–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loumaye A, Thissen J-P. Biomarkers of cancer cachexia. Clin Biochem 2017;50:1281–8 [DOI] [PubMed] [Google Scholar]
- 30. Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Cancer cachexia–pathophysiology and management. J Gastroenterol 2013;48: 574–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown JL, Lee DE, Rosa-Caldwell ME, Brown LA, Perry RA, Haynie WS, Huseman K, Sataranatarajan K, Van Remmen H, Washington TA, Wiggs MP, Greene NP. Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice. J Cachexia Sarcopenia Muscle 2018;9:987–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guigni BA, Callahan DM, Tourville TW, Miller MS, Fiske B, Voigt T, Korwin-Mihavics B, Anathy V, Dittus K, Toth MJ. Skeletal muscle atrophy and dysfunction in breast cancer patients: role for chemotherapy-derived oxidant stress. Am J Physiol Cell Physiol 2018;315:C744–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brown JL, Rosa-Caldwell ME, Lee DE, Blackwell TA, Brown LA, Perry RA, Haynie WS, Hardee JP, Carson JA, Wiggs MP, Washington TA, Greene NP. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle 2017;8:926–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Visavadiya NP, Pena GS, Khamoui AV. Mitochondrial dynamics and quality control are altered in a hepatic cell culture model of cancer cachexia. Mol Cell Biochem 2021;476:23–34 [DOI] [PubMed] [Google Scholar]
- 35. Baracos VE, DeVivo C, Hoyle DH, Goldberg AL. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol 1995;268:E996–1006 [DOI] [PubMed] [Google Scholar]
- 36. Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest 2004;114:370–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 2007;6:376–85 [DOI] [PubMed] [Google Scholar]
- 38. Honors MA, Kinzig KP. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J Cachexia Sarcopenia Muscle 2012;3:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heber D, Byerly LO, Chlebowski RT. Metabolic abnormalities in the cancer patient. Cancer 1985;55:225–9 [DOI] [PubMed] [Google Scholar]
- 40. Cahill GF, Jr, Aoki TT, Brennan MF, Müller WA. Insulin and muscle amino acid balance. Proc Nutr Soc 1972;31:233–8 [DOI] [PubMed] [Google Scholar]
- 41. Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006;147: 4160–8 [DOI] [PubMed] [Google Scholar]
- 42. Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, Mueller-Klieser W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 2000;60:916–21 [PubMed] [Google Scholar]
- 43. Tsoli M, Schweiger M, Vanniasinghe AS, Painter A, Zechner R, Clarke S, Robertson G. Depletion of white adipose tissue in cancer cachexia syndrome is associated with inflammatory signaling and disrupted circadian regulation. PLoS ONE 2014;9:e92966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou T, Yang K, Thapa S, Liu H, Wang B, Yu S. Differences in symptom burden among cancer patients with different stages of cachexia. J Pain Symptom Manage 2017;53:919–26 [DOI] [PubMed] [Google Scholar]
- 45. Bland KA, Zopf EM, Harrison M, Ely M, Cormie P, Liu E, Dowd A, Martin P. Prognostic markers of overall survival in cancer patients attending a cachexia support service: an evaluation of clinically assessed physical function, malnutrition and inflammatory status. Nutr Cancer 2021;73:1400–10 [DOI] [PubMed] [Google Scholar]
- 46. Suno M, Endo Y, Nishie H, Kajizono M, Sendo T, Matsuoka J. Refractory cachexia is associated with increased plasma concentrations of fentanyl in cancer patients. Ther Clin Risk Manag 2015;11:751–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle 2013;4:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van der Meij BS, Schoonbeek CP, Smit EF, Muscaritoli M, van Leeuwen PAM, Langius JAE. Pre-cachexia and cachexia at diagnosis of stage III non-small-cell lung carcinoma: an exploratory study comparing two consensus-based frameworks. Br J Nutr 2013;109:2231–9 [DOI] [PubMed] [Google Scholar]
- 49. Kilgour RD, Vigano A, Trutschnigg B, Lucar E, Borod M, Morais JA. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer 2013;21:3261–70 [DOI] [PubMed] [Google Scholar]
- 50. Han Y, Smith MT. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front Pharmacol 2013;4:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith KL, Tisdale MJ. Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br J Cancer 1993;67:680–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White JP, Puppa MJ, Narsale A, Carson JA. Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biol Open 2013;2:1346–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Talbert EE, Guttridge DC. Impaired regeneration: a role for the muscle microenvironment in cancer cachexia. Semin Cell Dev Biol 2016;54:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bilir C, Engin H, Can M, Temi YB, Demirtas D. The prognostic role of inflammation and hormones in patients with metastatic cancer with cachexia. Med Oncol 2015;32:56. [DOI] [PubMed] [Google Scholar]
- 55. Marchildon F, Lamarche É, Lala-Tabbert N, St-Louis C, Wiper-Bergeron N. Expression of CCAAT/enhancer binding protein beta in muscle satellite cells inhibits myogenesis in cancer cachexia. PLoS ONE 2015;10:e0145583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Milan G, Romanello V, Pescatore F, Armani A, Paik J-H, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, Blaauw B, DePinho RA, Sandri M. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun 2015;6:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ballarò R, Beltrà M, De Lucia S, Pin F, Ranjbar K, Hulmi JJ, Costelli P, Penna F. Moderate exercise in mice improves cancer plus chemotherapy-induced muscle wasting and mitochondrial alterations. FASEB J 2019;33:5482–94 [DOI] [PubMed] [Google Scholar]
- 58. Ballarò R, Lopalco P, Audrito V, Beltrà M, Pin F, Angelini R, Costelli P, Corcelli A, Bonetto A, Szeto HH, O’Connell TM, Penna F. Targeting mitochondria by SS-31 ameliorates the whole body energy status in cancer- and chemotherapy-induced cachexia. Cancers (Basel) 2021;13:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Beijer S, Hupperets PS, van den Borne BE, Eussen SR, van Henten AM, van den Beuken-van Everdingen M, de Graeff A, Ambergen TA, van den Brandt PA, Dagnelie PC. Effect of adenosine 5′-triphosphate infusions on the nutritional status and survival of preterminal cancer patients. Anticancer Drugs 2009;20:625–33 [DOI] [PubMed] [Google Scholar]
- 60. Burfeind KG, Michaelis KA, Marks DL. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin Cell Dev Biol 2016;54:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Braun TP, Zhu X, Szumowski M, Scott GD, Grossberg AJ, Levasseur PR, Graham K, Khan S, Damaraju S, Colmers WF, Baracos VE, Marks DL. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic-pituitary-adrenal axis. J Exp Med 2011;208:2449–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D, Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer 2003;97:1211–6 [DOI] [PubMed] [Google Scholar]
- 63. Lerner L, Hayes TG, Tao N, Krieger B, Feng B, Wu Z, Nicoletti R, Chiu MI, Gyuris J, Garcia JM. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle 2015;6:317–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. He WA, Berardi E, Cardillo VM, Acharyya S, Aulino P, Thomas-Ahner J, Wang J, Bloomston M, Muscarella P, Nau P, Shah N, Butchbach MER, Ladner K, Adamo S, Rudnicki MA, Keller C, Coletti D, Montanaro F, Guttridge DC. NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest 2013;123:4821–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 2000;289:2363–6 [DOI] [PubMed] [Google Scholar]
- 66. Silva KAS, Dong J, Dong Y, Dong Y, Schor N, Tweardy DJ, Zhang L, Mitch WE. Inhibition of Stat3 activation suppresses caspase-3 and the ubiquitin-proteasome system, leading to preservation of muscle mass in cancer cachexia. J Biol Chem 2015;290:11177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. MacDonald AJ, Johns N, Stephens N, Greig C, Ross JA, Small AC, Husi H, Fearon KCH, Preston T. Habitual myofibrillar protein synthesis is normal in patients with upper GI cancer cachexia. Clin Cancer Res 2015;21:1734–40 [DOI] [PubMed] [Google Scholar]
- 68. Sakellariou GK, Pearson T, Lightfoot AP, Nye GA, Wells N, Giakoumaki II, Vasilaki A, Griffiths RD, Jackson MJ, McArdle A. Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci Rep 2016;6:33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Conte E, Camerino GM, Mele A, De Bellis M, Pierno S, Rana F, Fonzino A, Caloiero R, Rizzi L, Bresciani E, Ben Haj Salah K, Fehrentz JA, Martinez J, Giustino A, Mariggiò MA, Coluccia M, Tricarico D, Lograno MD, De Luca A, Torsello A, Conte D, Liantonio A. Growth hormone secretagogues prevent dysregulation of skeletal muscle calcium homeostasis in a rat model of cisplatin-induced cachexia. J Cachexia Sarcopenia Muscle 2017;8:386–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Isaac ST, Tan TC, Polly P. Endoplasmic reticulum stress, calcium dysregulation and altered protein translation: intersection of processes that contribute to cancer cachexia induced skeletal muscle wasting. Curr Drug Targets 2016;17:1140–6 [DOI] [PubMed] [Google Scholar]
- 71. McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol 2010;298:C542–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lerner L, Tao J, Liu Q, Nicoletti R, Feng B, Krieger B, Mazsa E, Siddiquee Z, Wang R, Huang L, Shen L, Lin J, Vigano A, Chiu MI, Weng Z, Winston W, Weiler S, Gyuris J. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J Cachexia Sarcopenia Muscle 2016;7:467–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Penna F, Ballarò R, Martinez-Cristobal P, Sala D, Sebastian D, Busquets S, Muscaritoli M, Argilés JM, Costelli P, Zorzano A. Autophagy exacerbates muscle wasting in cancer cachexia and impairs mitochondrial function. J Mol Biol 2019;431:2674–86 [DOI] [PubMed] [Google Scholar]
- 74. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–9 [DOI] [PubMed] [Google Scholar]
- 75. Purcell SA, Baracos VE, Chu QSC, Sawyer MB, Severin D, Mourtzakis M, Lieffers JR, Prado CM. Profiling determinants of resting energy expenditure in colorectal cancer. Nutr Cancer 2020;72:431–8 [DOI] [PubMed] [Google Scholar]
- 76. Purcell SA, Elliott SA, Baracos VE, Chu QSC, Prado CM. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur J Clin Nutr 2016;70:1230–8 [DOI] [PubMed] [Google Scholar]
- 77. Moses AWG, Slater C, Preston T, Barber MD, Fearon KCH. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer 2004;90:996–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CMM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr 2009;89:1173–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007;297:1772–4 [DOI] [PubMed] [Google Scholar]
- 80. Ferrando AA, Stuart CA, Sheffield-Moore M, Wolfe RR. Inactivity amplifies the catabolic response of skeletal muscle to cortisol. J Clin Endocrinol Metab 1999;84:3515–21 [DOI] [PubMed] [Google Scholar]
- 81. Deaver JW, Greene NP. Emerging mechanisms for exercise effects on muscle wasting and anabolic resistance. Sports Med Health Sci 2020; 2:175–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ni X, Yang J, Li M. Imaging-guided curative surgical resection of pancreatic cancer in a xenograft mouse model. Cancer Lett 2012;324: 179–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Norton JA, Moley JF, Green MV, Carson RE, Morrison SD. Parabiotic transfer of cancer anorexia/cachexia in male rats. Cancer Res 1985; 45:5547–52 [PubMed] [Google Scholar]
- 84. Argilés JM, Stemmler B, López-Soriano FJ, Busquets S. Nonmuscle tissues contribution to cancer cachexia. Mediators Inflamm 2015;2015:182872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fearon KCH, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–66 [DOI] [PubMed] [Google Scholar]
- 86. Argilés JM, López-Soriano FJ, Busquets S. Mediators of cachexia in cancer patients. Nutrition 2019;66:11–5 [DOI] [PubMed] [Google Scholar]
- 87. Wu Q, Sun S, Li Z, Yang Q, Li B, Zhu S, Wang L, Wu J, Yuan J, Wang C, Li J, Sun S. Breast cancer-released exosomes trigger cancer-associated cachexia to promote tumor progression. Adipocyte 2019;8:31–45 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88. Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009;8:3984–4001 [DOI] [PubMed] [Google Scholar]
- 89. Blanquer-Rosselló MDM, Oliver J, Sastre-Serra J, Valle A, Roca P. Leptin regulates energy metabolism in MCF-7 breast cancer cells. Int J Biochem Cell Biol 2016;72:18–26 [DOI] [PubMed] [Google Scholar]
- 90. Nukaga S, Mori T, Miyagawa Y, Fujiwara-Tani R, Sasaki T, Fujii K, Mori S, Goto K, Kishi S, Nakashima C, Ohmori H, Kawahara I, Luo Y, Kuniyasu H. Combined administration of lauric acid and glucose improved cancer-derived cardiac atrophy in a mouse cachexia model. Cancer Sci 2020;111:4605–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhou L, Zhang T, Shao W, Lu R, Wang L, Liu H, Jiang B, Li S, Zhuo H, Wang S, Li Q, Huang C, Lin D. Amiloride ameliorates muscle wasting in cancer cachexia through inhibiting tumor-derived exosome release. Skelet Muscle 2021;11:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab 2012;303:E410–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. White JP, Puppa MJ, Gao S, Sato S, Welle SL, Carson JA. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab 2013;304:E1042–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pigna E, Berardi E, Aulino P, Rizzuto E, Zampieri S, Carraro U, Kern H, Merigliano S, Gruppo M, Mericskay M, Li Z, Rocchi M, Barone R, Macaluso F, Di Felice V, Adamo S, Coletti D, Moresi V. Aerobic exercise and pharmacological treatments counteract cachexia by modulating autophagy in colon cancer. Sci Rep 2016;6:26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Coletti D, Aulino P, Pigna E, Barteri F, Moresi V, Annibali D, Adamo S, Berardi E. Spontaneous physical activity downregulates Pax7 in cancer cachexia. Stem Cells Int 2016;2016:6729268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tsoli M, Moore M, Burg D, Painter A, Taylor R, Lockie SH, Turner N, Warren A, Cooney G, Oldfield B, Clarke S, Robertson G. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res 2012;72:4372–82 [DOI] [PubMed] [Google Scholar]
- 97. Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, Tisdale MJ, Trayhurn P. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci USA 2004;101:2500–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Penet M-F, Gadiya MM, Krishnamachary B, Nimmagadda S, Pomper MG, Artemov D, Bhujwalla ZM. Metabolic signatures imaged in cancer-induced cachexia. Cancer Res 2011;71:6948–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Niu M, Li L, Su Z, Wei L, Pu W, Zhao C, Ding Y, Wazir J, Cao W, Song S, Gao Q, Wang H. An integrative transcriptome study reveals Ddit4/Redd1 as a key regulator of cancer cachexia in rodent models. Cell Death Dis 2021;12:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Di W, Zhang W, Zhu B, Li X, Tang Q, Zhou Y. Colorectal cancer prompted adipose tissue browning and cancer cachexia through transferring exosomal miR-146b-5p. J Cell Physiol 2021;236:5399–410 [DOI] [PubMed] [Google Scholar]
- 101. Miao C, Zhang W, Feng L, Gu X, Shen Q, Lu S, Fan M, Li Y, Guo X, Ma Y, Liu X, Wang H, Zhang X. Cancer-derived exosome miRNAs induce skeletal muscle wasting by Bcl-2-mediated apoptosis in colon cancer cachexia. Mol Ther Nucleic Acids 2021;24:923–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fukawa T, Yan-Jiang BC, Min-Wen JC, Jun-Hao ET, Huang D, Qian C-N, Ong P, Li Z, Chen S, Mak SY, Lim WJ, Kanayama H-O, Mohan RE, Wang RR, Lai JH, Chua C, Ong HS, Tan K-K, Ho YS, Tan IB, Teh BT, Shyh-Chang N. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat Med 2016;22:666–71 [DOI] [PubMed] [Google Scholar]
- 103. Puppa MJ, Gao S, Narsale AA, Carson JA. Skeletal muscle glycoprotein 130’s role in Lewis lung carcinoma-induced cachexia. FASEB J 2014;28:998–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hernandez VJ, Weng J, Ly P, Pompey S, Dong H, Mishra L, Schwarz M, Anderson RGW, Michaely P. Cavin-3 dictates the balance between ERK and Akt signaling. Elife 2013;2:e00905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hain BA, Xu H, VanCleave AM, Gordon BS, Kimball SR, Waning DL. REDD1 deletion attenuates cancer cachexia in mice. J Appl Physiol (1985) 2021;131:1718–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hu W, Xiong H, Ru Z, Zhao Y, Zhou Y, Xie K, Xiao W, Xiong Z, Wang C, Yuan C, Shi J, Du Q, Zhang X, Yang H. Extracellular vesicles-released parathyroid hormone-related protein from Lewis lung carcinoma induces lipolysis and adipose tissue browning in cancer cachexia. Cell Death Dis 2021;12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yu J, Choi S, Park A, Do J, Nam D, Kim Y, Noh J, Lee KY, Maeng CH, Park K-S. Bone marrow homeostasis is impaired via JAK/STAT and glucocorticoid signaling in cancer cachexia model. Cancers (Basel) 2021;13:1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chiappalupi S, Sorci G, Vukasinovic A, Salvadori L, Sagheddu R, Coletti D, Renga G, Romani L, Donato R, Riuzzi F. Targeting RAGE prevents muscle wasting and prolongs survival in cancer cachexia. J Cachexia Sarcopenia Muscle 2020;11:929–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. van de Worp WRPH, Schols AMWJ, Dingemans A-MC, Op den Kamp CMH, Degens JHRJ, Kelders MCJM, Coort S, Woodruff HC, Kratassiouk G, Harel-Bellan A, Theys J, van Helvoort A, Langen RCJ. Identification of microRNAs in skeletal muscle associated with lung cancer cachexia. J Cachexia Sarcopenia Muscle 2020;11:452–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lim S, Dunlap KR, Rosa-Caldwell ME, Haynie WS, Jansen LT, Washington TA, Greene NP. Comparative plasma proteomics in muscle atrophy during cancer-cachexia and disuse: the search for atrokines. Physiol Rep 2020;8:e14608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang F, Liu H, Hu L, Liu Y, Duan Y, Cui R, Tian W. The Warburg effect in human pancreatic cancer cells triggers cachexia in athymic mice carrying the cancer cells. BMC Cancer 2018;18:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hu L, Xu X, Li Q, Chen X, Yuan X, Qiu S, Yao C, Zhang D, Wang F. Caveolin-1 increases glycolysis in pancreatic cancer cells and triggers cachectic states. FASEB J 2021;35:e21826 [DOI] [PubMed] [Google Scholar]
- 113. Felix K, Fakelman F, Hartmann D, Giese NA, Gaida MM, Schnölzer M, Flad T, Büchler MW, Werner J. Identification of serum proteins involved in pancreatic cancer cachexia. Life Sci 2011;88:218–25 [DOI] [PubMed] [Google Scholar]
- 114. Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, Fearon DT. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab 2016;24:672–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Olson B, Zhu X, Norgard MA, Levasseur PR, Butler JT, Buenafe A, Burfeind KG, Michaelis KA, Pelz KR, Mendez H, Edwards J, Krasnow SM, Grossberg AJ, Marks DL. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat Commun 2021;12:2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rupert JE, Narasimhan A, Jengelley DHA, Jiang Y, Liu J, Au E, Silverman LM, Sandusky G, Bonetto A, Cao S, Lu X, O’Connell TM, Liu Y, Koniaris LG, Zimmers TA. Tumor-derived IL-6 and trans-signaling among tumor, fat, and muscle mediate pancreatic cancer cachexia. J Exp Med 2021;218:e20190450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shukla SK, Markov SD, Attri KS, Vernucci E, King RJ, Dasgupta A, Grandgenett PM, Hollingsworth MA, Singh PK, Yu F, Mehla K. Macrophages potentiate STAT3 signaling in skeletal muscles and regulate pancreatic cancer cachexia. Cancer Lett 2020;484:29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dasgupta A, Shukla SK, Vernucci E, King RJ, Abrego J, Mulder SE, Mullen NJ, Graves G, Buettner K, Thakur R, Murthy D, Attri KS, Wang D, Chaika NV, Pacheco CG, Rai I, Engle DD, Grandgenett PM, Punsoni M, Reames BN, Teoh-Fitzgerald M, Oberley-Deegan R, Yu F, Klute KA, Hollingsworth MA, Zimmerman MC, Mehla K, Sadoshima J, Tuveson DA, Singh PK. SIRT1-NOX4 signaling axis regulates cancer cachexia. J Exp Med 2020;217:e20190745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shakri AR, Zhong TJ, Ma W, Coker C, Kim S, Calluori S, Scholze H, Szabolcs M, Caffrey T, Grandgenett PM, Hollingsworth MA, Tanji K, Kluger MD, Miller G, Biswas AK, Acharyya S. Upregulation of ZIP14 and altered zinc homeostasis in muscles in pancreatic cancer cachexia. Cancers (Basel) 2019;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Biazi GR, Frasson IG, Miksza DR, de Morais H, de Fatima Silva F, Bertolini GL, de Souza HM. Decreased hepatic response to glucagon, adrenergic agonists, and cAMP in glycogenolysis, gluconeogenesis, and glycolysis in tumor-bearing rats. J Cell Biochem 2018;119:7300–9. [DOI] [PubMed] [Google Scholar]
- 121. Viana LR, Tobar N, Busanello ENB, Marques AC, de Oliveira AG, Lima TI, Machado G, Castelucci BG, Ramos CD, Brunetto SQ, Silveira LR, Vercesi AE, Consonni SR, Gomes-Marcondes MCC. Leucine-rich diet induces a shift in tumour metabolism from glycolytic towards oxidative phosphorylation, reducing glucose consumption and metastasis in Walker-256 tumour-bearing rats. Sci Rep 2019;9:15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 2006;209: 501–14 [DOI] [PubMed] [Google Scholar]
- 123. Martínez-Hernández PL, Hernanz-Macías Á, Gómez-Candela C, Grande-Aragón C, Feliu-Batlle J, Castro-Carpeño J, Martínez-Muñoz I, Zurita-Rosa L, Villarino-Sanz M, Prados-Sánchez C, Sánchez García-Girón J. Serum interleukin-15 levels in cancer patients with cachexia. Oncol Rep 2012;28:1443–52. [DOI] [PubMed] [Google Scholar]
- 124. Morigny P, Kaltenecker D, Zuber J, Machado J, Mehr L, Tsokanos F-F, Kuzi H, Hermann CD, Voelkl M, Monogarov G, Springfeld C, Laurent V, Engelmann B, Friess H, Zörnig I, Krüger A, Krijgsveld J, Prokopchuk O, Fisker Schmidt S, Rohm M, Herzig S, Berriel Diaz M. Association of circulating PLA2G7 levels with cancer cachexia and assessment of darapladib as a therapy. J Cachexia Sarcopenia Muscle 2021;12:1333–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004;117:399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 2004;14:395–403 [DOI] [PubMed] [Google Scholar]
- 127. Asp ML, Tian M, Wendel AA, Belury MA. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int J Cancer 2010;126:756–63 [DOI] [PubMed] [Google Scholar]
- 128. Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, Perrimon N. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev Cell 2015;33:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Figueroa-Clarevega A, Bilder D. Malignant drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev Cell 2015;33:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Fernandes LC, Machado UF, Nogueira CR, Carpinelli AR, Curi R. Insulin secretion in Walker 256 tumor cachexia. Am J Physiol 1990; 258:E1033–6 [DOI] [PubMed] [Google Scholar]
- 131. Trobec K, Palus S, Tschirner A, von Haehling S, Doehner W, Lainscak M, Anker SD, Springer J. Rosiglitazone reduces body wasting and improves survival in a rat model of cancer cachexia. Nutrition 2014;30:1069–75 [DOI] [PubMed] [Google Scholar]
- 132. Asp ML, Tian M, Kliewer KL, Belury MA. Rosiglitazone delayed weight loss and anorexia while attenuating adipose depletion in mice with cancer cachexia. Cancer Biol Ther 2011;12:957–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Op den Kamp CM, Langen RC, Snepvangers FJ, de Theije CC, Schellekens JM, Laugs F, Dingemans A-MC, Schols AM. Nuclear transcription factor κ B activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am J Clin Nutr 2013;98:738–48 [DOI] [PubMed] [Google Scholar]
- 134. Tardif N, Klaude M, Lundell L, Thorell A, Rooyackers O. Autophagic-lysosomal pathway is the main proteolytic system modified in the skeletal muscle of esophageal cancer patients. Am J Clin Nutr 2013;98:1485–92 [DOI] [PubMed] [Google Scholar]
- 135. Johns N, Hatakeyama S, Stephens NA, Degen M, Degen S, Frieauff W, Lambert C, Ross JA, Roubenoff R, Glass DJ, Jacobi C, Fearon KCH. Clinical classification of cancer cachexia: phenotypic correlates in human skeletal muscle. PLoS ONE 2014;9:e83618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Stephens NA, Skipworth RJE, Gallagher IJ, Greig CA, Guttridge DC, Ross JA, Fearon KCH. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle 2015;6:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309–22 [DOI] [PubMed] [Google Scholar]
- 138. Bhatnagar S, Mittal A, Gupta SK, Kumar A. TWEAK causes myotube atrophy through coordinated activation of ubiquitin-proteasome system, autophagy, and caspases. J Cell Physiol 2012;227:1042–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cai D, Frantz JD, Tawa NE, Melendez PA, Oh B-C, Lidov HGW, Hasselgren P-O, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 2004;119:285–98 [DOI] [PubMed] [Google Scholar]
- 140. Trujillo ME, Sullivan S, Harten I, Schneider SH, Greenberg AS, Fried SK. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J Clin Endocrinol Metab 2004;89:5577–82 [DOI] [PubMed] [Google Scholar]
- 141. Niu M, Song S, Su Z, Wei L, Li L, Pu W, Zhao C, Ding Y, Wang J, Cao W, Gao Q, Wang H. Inhibition of heat shock protein (HSP) 90 reverses signal transducer and activator of transcription (STAT) 3-mediated muscle wasting in cancer cachexia mice. Br J Pharmacol 2021;178:4485–500 [DOI] [PubMed] [Google Scholar]
- 142. Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee S-J. Induction of cachexia in mice by systemically administered myostatin. Science 2002;296:1486–8 [DOI] [PubMed] [Google Scholar]
- 143. Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010;142:531–43 [DOI] [PubMed] [Google Scholar]
- 144. Benny Klimek ME, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun 2010;391:1548–54. [DOI] [PubMed] [Google Scholar]
- 145. Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, Chiechi A, Wright LE, Umanskaya A, Niewolna M, Trivedi T, Charkhzarrin S, Khatiwada P, Wronska A, Haynes A, Benassi MS, Witzmann FA, Zhen G, Wang X, Cao X, Roodman GD, Marks AR, Guise TA. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat Med 2015;21:1262–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK, Foltz LA, Muppidi A, Alsina-Fernandez J, Barnard GC, Tang JX, Liu X, Mao X, Siegel R, Sloan JH, Mitchell PJ, Zhang BB, Gimeno RE, Shan B, Wu X. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 2017;23:1215–9 [DOI] [PubMed] [Google Scholar]
- 147. Yang L, Chang C-C, Sun Z, Madsen D, Zhu H, Padkjær SB, Wu X, Huang T, Hultman K, Paulsen SJ, Wang J, Bugge A, Frantzen JB, Nørgaard P, Jeppesen JF, Yang Z, Secher A, Chen H, Li X, John LM, Shan B, He Z, Gao X, Su J, Hansen KT, Yang W, Jørgensen SB. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med 2017;23:1158–66 [DOI] [PubMed] [Google Scholar]
- 148. Tsai VW-W, Macia L, Johnen H, Kuffner T, Manadhar R, Jørgensen SB, Lee-Ng KKM, Zhang HP, Wu L, Marquis CP, Jiang L, Husaini Y, Lin S, Herzog H, Brown DA, Sainsbury A, Breit SN. TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS ONE 2013;8:e55174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang G, Lin R-K, Kwon YT, Li Y-P. Signaling mechanism of tumor cell-induced up-regulation of E3 ubiquitin ligase UBR2. FASEB J 2013;27:2893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014;3:26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Li X, Wang S, Zhu R, Li H, Han Q, Zhao RC. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFκB-TLR signaling pathway. J Hematol Oncol 2016;9:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer 2010;10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rébé C, Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 2010;120:457–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lv L-H, Wan Y-L, Lin Y, Zhang W, Yang M, Li G-L, Lin H-M, Shang C-Z, Chen Y-J, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 2012;287:15874–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhang G, Liu Z, Ding H, Zhou Y, Doan HA, Sin KWT, Zhu ZJ, Flores R, Wen Y, Gong X, Liu Q, Li Y-P. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat Commun 2017;8:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yang J, Zhang Z, Zhang Y, Ni X, Zhang G, Cui X, Liu M, Xu C, Zhang Q, Zhu H, Yan J, Zhu VF, Luo Y, Hagan JP, Li Z, Fang J, Jatoi A, Fernandez-Zapico ME, Zheng L, Edil BH, Bronze MS, Houchen CW, Li Y-P, Li M. ZIP4 promotes muscle wasting and cachexia in mice with orthotopic pancreatic tumors by stimulating RAB27B-regulated release of extracellular vesicles from cancer cells. Gastroenterology 2019;156:722–34.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Calore F, Londhe P, Fadda P, Nigita G, Casadei L, Marceca GP, Fassan M, Lovat F, Gasparini P, Rizzotto L, Zanesi N, Jackson D, Mehta S, Nana-Sinkam P, Sampath D, Pollock RE, Guttridge DC, Croce CM. The TLR7/8/9 antagonist IMO-8503 inhibits cancer-induced cachexia. Cancer Res 2018;78:6680–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. He WA, Calore F, Londhe P, Canella A, Guttridge DC, Croce CM. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc Natl Acad Sci USA 2014;111:4525–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gao X, Wang Y, Lu F, Chen X, Yang D, Cao Y, Zhang W, Chen J, Zheng L, Wang G, Fu M, Ma L, Song Y, Zhan Q. Extracellular vesicles derived from oesophageal cancer containing P4HB promote muscle wasting via regulating PHGDH/Bcl-2/caspase-3 pathway. J Extracell Vesicles 2021;10:e12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011;333:233–8 [DOI] [PubMed] [Google Scholar]
- 161. Kliewer KL, Ke J-Y, Tian M, Cole RM, Andridge RR, Belury MA. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. Cancer Biol Ther 2015;16:886–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014;513:100–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Schwickert G, Walenta S, Sundfør K, Rofstad EK, Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res 1995;55:4757–9 [PubMed] [Google Scholar]
- 164. Friesen DE, Baracos VE, Tuszynski JA. Modeling the energetic cost of cancer as a result of altered energy metabolism: implications for cachexia. Theor Biol Med Model 2015;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Argilés JM, Fontes-Oliveira CC, Toledo M, López-Soriano FJ, Busquets S. Cachexia: a problem of energetic inefficiency. J Cachexia Sarcopenia Muscle 2014;5:279–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Felig P, Pozefsky T, Marliss E, Cahill GF. Alanine: key role in gluconeogenesis. Science 1970;167:1003–4 [DOI] [PubMed] [Google Scholar]
- 167. Ishikawa E. The regulation of uptake and output of amino acids by rat tissues. Adv Enzyme Regul 1976;14:117–36 [DOI] [PubMed] [Google Scholar]
- 168. Goncalves MD, Hwang S-K, Pauli C, Murphy CJ, Cheng Z, Hopkins BD, Wu D, Loughran RM, Emerling BM, Zhang G, Fearon DT, Cantley LC. Fenofibrate prevents skeletal muscle loss in mice with lung cancer. Proc Natl Acad Sci USA 2018;115:E743–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol 2008;197:1–10 [DOI] [PubMed] [Google Scholar]
- 170. Grossberg AJ, Scarlett JM, Zhu X, Bowe DD, Batra AK, Braun TP, Marks DL. Arcuate nucleus proopiomelanocortin neurons mediate the acute anorectic actions of leukemia inhibitory factor via gp130. Endocrinology 2010;151:606–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Bodnar RJ, Pasternak GW, Mann PE, Paul D, Warren R, Donner DB. Mediation of anorexia by human recombinant tumor necrosis factor through a peripheral action in the rat. Cancer Res 1989;49:6280–4 [PubMed] [Google Scholar]
- 172. Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, Grant WF, Cowley MA, Marks DL. Regulation of central melanocortin signaling by interleukin-1 beta. Endocrinology 2007;148:4217–25 [DOI] [PubMed] [Google Scholar]
- 173. Ericsson A, Kovács KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci 1994;14:897–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, Taylor JE, Halem HA, Dong JZ, Datta R, Culler MD, Marks DL. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology 2007;148:3004–12 [DOI] [PubMed] [Google Scholar]
- 175. Murphy KT. The pathogenesis and treatment of cardiac atrophy in cancer cachexia. Am J Physiol Heart Circ Physiol 2016;310:466–77 [DOI] [PubMed] [Google Scholar]
- 176. Marin-Corral J, Fontes CC, Pascual-Guardia S, Sanchez F, Olivan M, Argilés JM, Busquets S, López-Soriano FJ, Barreiro E. Redox balance and carbonylated proteins in limb and heart muscles of cachectic rats. Antioxid Redox Signal 2010;12:365–80 [DOI] [PubMed] [Google Scholar]
- 177. Belloum Y, Rannou-Bekono F, Favier FB. Cancer-induced cardiac cachexia: pathogenesis and impact of physical activity (Review). Oncol Rep 2017;37:2543–52 [DOI] [PubMed] [Google Scholar]
- 178. Narasimhan A, Zhong X, Au EP, Ceppa EP, Nakeeb A, House MG, Zyromski NJ, Schmidt CM, Schloss KNH, Schloss DEI, Liu Y, Jiang G, Hancock BA, Radovich M, Kays JK, Shahda S, Couch ME, Koniaris LG, Zimmers TA. Profiling of adipose and skeletal muscle in human pancreatic cancer cachexia reveals distinct gene profiles with convergent pathways. Cancers (Basel) 2021;13:1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629–35 [DOI] [PubMed] [Google Scholar]
- 180. Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996;12:S15–9 [DOI] [PubMed] [Google Scholar]
- 181. Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, Albarede JL. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999;15:116–22 [DOI] [PubMed] [Google Scholar]
- 182. Thoresen L, Frykholm G, Lydersen S, Ulveland H, Baracos V, Prado CMM, Birdsell L, Falkmer U. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. Different assessment criteria for nutritional status provide unequal results. Clin Nutr 2013;32:65–72 [DOI] [PubMed] [Google Scholar]
- 183. Harvie MN, Campbell IT, Thatcher N, Baildam A. Changes in body composition in men and women with advanced nonsmall cell lung cancer (NSCLC) undergoing chemotherapy. J Hum Nutr Diet 2003;16:323–6 [DOI] [PubMed] [Google Scholar]
- 184. Cardoso ICR, Aredes MA, Chaves GV. Applicability of the direct parameters of bioelectrical impedance in assessing nutritional status and surgical complications of women with gynecological cancer. Eur J Clin Nutr 2017;71:1278–84 [DOI] [PubMed] [Google Scholar]
- 185. Trutschnigg B, Kilgour RD, Reinglas J, Rosenthall L, Hornby L, Morais JA, Vigano A. Precision and reliability of strength (Jamar vs. Biodex handgrip) and body composition (dual-energy X-ray absorptiometry vs. bioimpedance analysis) measurements in advanced cancer patients. Appl Physiol Nutr Metab 2008;33:1232–9. [DOI] [PubMed] [Google Scholar]
- 186. Prado CMM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care 2009;3:269–75 [DOI] [PubMed] [Google Scholar]
- 187. Bieliuniene E, Brøndum Frøkjær J, Pockevicius A, Kemesiene J, Lukosevičius S, Basevicius A, Atstupenaite V, Barauskas G, Ignatavicius P, Gulbinas A, Dambrauskas Z. CT- and MRI-based assessment of body composition and pancreatic fibrosis reveals high incidence of clinically significant metabolic changes that affect the quality of life and treatment outcomes of patients with chronic pancreatitis and pancreatic cancer. Medicina (Kaunas) 2019;55:E649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lindenberg KS, Weydt P, Müller HP, Bornstedt A, Ludolph AC, Landwehrmeyer GB, Rottbauer W, Kassubek J, Rasche V. Two-point magnitude MRI for rapid mapping of brown adipose tissue and its application to the R6/2 mouse model of Huntington disease. PLoS ONE 2014;9:e105556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009;15:2920–6 [DOI] [PubMed] [Google Scholar]
- 190. Takayoshi K, Uchino K, Nakano M, Ikejiri K, Baba E. Weight loss during initial chemotherapy predicts survival in patients with advanced gastric cancer. Nutr Cancer 2017;69:408–15 [DOI] [PubMed] [Google Scholar]
- 191. Ni J, Zhang L. Cancer cachexia: definition, staging, and emerging treatments. Cancer Manag Res 2020;12:5597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Tan BHL, Fladvad T, Braun TP, Vigano A, Strasser F, Deans DAC, Skipworth RJE, Solheim TS, Damaraju S, Ross JA, Kaasa S, Marks DL, Baracos VE, Skorpen F, Fearon KCH, European Palliative Care Research Collaborative. P-selectin genotype is associated with the development of cancer cachexia. EMBO Mol Med 2012;4:462–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Narasimhan A, Shahda S, Kays JK, Perkins SM, Cheng L, Schloss KNH, Schloss DEI, Koniaris LG, Zimmers TA. Identification of potential serum protein biomarkers and pathways for pancreatic cancer cachexia using an aptamer-based discovery platform. Cancers (Basel) 2020;12:3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Bye A, Wesseltoft-Rao N, Iversen PO, Skjegstad G, Holven KB, Ulven S, Hjermstad MJ. Alterations in inflammatory biomarkers and energy intake in cancer cachexia: a prospective study in patients with inoperable pancreatic cancer. Med Oncol 2016;33:54. [DOI] [PubMed] [Google Scholar]
- 195. Zhou T, Wang B, Liu H, Yang K, Thapa S, Zhang H, Li L, Yu S. Development and validation of a clinically applicable score to classify cachexia stages in advanced cancer patients. J Cachexia Sarcopenia Muscle 2018;9:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Argilés JM, López-Soriano FJ, Toledo M, Betancourt A, Serpe R, Busquets S. The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J Cachexia Sarcopenia Muscle 2011;2:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Argilés JM, Betancourt A, Guàrdia-Olmos J, Peró-Cebollero M, López-Soriano FJ, Madeddu C, Serpe R, Busquets S. Validation of the CAchexia SCOre (CASCO). Staging cancer patients: the use of miniCASCO as a Simplified Tool. Front Physiol 2017;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Anderson LJ, Lee J, Mallen MC, Migula D, Liu H, Wu PC, Dash A, Garcia JM. Evaluation of physical function and its association with body composition, quality of life and biomarkers in cancer cachexia patients. Clin Nutr 2021;40:978–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Tseng Y-C, Kulp SK, Lai I-L, Hsu E-C, He WA, Frankhouser DE, Yan PS, Mo X, Bloomston M, Lesinski GB, Marcucci G, Guttridge DC, Bekaii-Saab T, Chen C-S. Preclinical investigation of the novel histone deacetylase inhibitor AR-42 in the treatment of cancer-induced cachexia. J Natl Cancer Inst 2015;107:djv274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Fearon KCH. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer 2008;44:1124–32. [DOI] [PubMed] [Google Scholar]
- 201. Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, Glare P, Nabal M, Viganò A, Larkin P, De Conno F, Hanks G, Kaasa S, Steering Committee of the European Association for Palliative Care. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol 2005;23:6240–8 [DOI] [PubMed] [Google Scholar]
- 202. Perumal SS, Shanthi P, Sachdanandam P. Energy-modulating vitamins—a new combinatorial therapy prevents cancer cachexia in rat mammary carcinoma. Br J Nutr 2005;93:901–9 [DOI] [PubMed] [Google Scholar]
- 203. Iqbal MA, Chattopadhyay S, Siddiqui FA, Ur Rehman A, Siddiqui S, Prakasam G, Khan A, Sultana S, Bamezai RN. Silibinin induces metabolic crisis in triple-negative breast cancer cells by modulating EGFR-MYC-TXNIP axis: potential therapeutic implications. FEBS J 2021;288:471–85 [DOI] [PubMed] [Google Scholar]
- 204. Lee J-H, Kim S-W, Kim J-H, Kim H-J, Um J, Jung D-W, Williams DR. Lithium chloride protects against sepsis-induced skeletal muscle atrophy and cancer cachexia. Cells 2021;10:1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Huot JR, Pin F, Narasimhan A, Novinger LJ, Keith AS, Zimmers TA, Willis MS, Bonetto A. ACVR2B antagonism as a countermeasure to multi-organ perturbations in metastatic colorectal cancer cachexia. J Cachexia Sarcopenia Muscle 2020;11:1779–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Chen L, Yang Q, Zhang H, Wan L, Xin B, Cao Y, Zhang J, Guo C. Cryptotanshinone prevents muscle wasting in CT26-induced cancer cachexia through inhibiting STAT3 signaling pathway. J Ethnopharmacol 2020;260:113066. [DOI] [PubMed] [Google Scholar]
- 207. Zhang Y, Zhang Y, Li Y, Zhang L, Yu S. Preclinical investigation of alpinetin in the treatment of cancer-induced cachexia via activating PPARγ. Front Pharmacol 2021;12:687491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, Yamada K, Kitajima H, Yoshimori K, Sato K, Saito H, Aoe K, Tsuji T, Takiguchi Y, Takayama K, Komura N, Takiguchi T, Eguchi K. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 2018;124:606–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Chen X-Y, Li D-F, Han J-C, Wang B, Dong Z-P, Yu L-N, Pan Z-H, Qu C-J, Chen Y, Sun S-G, Zheng Q-S. Reprogramming induced by isoliquiritigenin diminishes melanoma cachexia through mTORC2-AKT-GSK3β signaling. Oncotarget 2017;8:34565–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Bora V, Patel BM. Investigation into the role of anti-diabetic agents in cachexia associated with metastatic cancer. Life Sci 2021;274:119329. [DOI] [PubMed] [Google Scholar]
- 211. Shukla SK, Dasgupta A, Mehla K, Gunda V, Vernucci E, Souchek J, Goode G, King R, Mishra A, Rai I, Nagarajan S, Chaika NV, Yu F, Singh PK. Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth. Oncotarget 2015;6:41146–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Jung YY, Ko J-H, Um J-Y, Sethi G, Ahn KS. A novel role of bergamottin in attenuating cancer associated cachexia by diverse molecular mechanisms. Cancers (Basel) 2021;13:1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Mulder SE, Dasgupta A, King RJ, Abrego J, Attri KS, Murthy D, Shukla SK, Singh PK. JNK signaling contributes to skeletal muscle wasting and protein turnover in pancreatic cancer cachexia. Cancer Lett 2020;491:70–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Comiran PK, Ribeiro MC, Silva JHG, Martins KO, Santos IA, Chiaradia AEF, Silva AZ, Dekker RFH, Barbosa-Dekker AM, Alegranci P, Queiroz EAIF. Botryosphaeran attenuates tumor development and the cancer cachexia syndrome in Walker-256 tumor-bearing obese rats and improves the metabolic and hematological profiles of these rats. Nutr Cancer 2021;73:1175–92 [DOI] [PubMed] [Google Scholar]
- 215. Suriben R, Chen M, Higbee J, Oeffinger J, Ventura R, Li B, Mondal K, Gao Z, Ayupova D, Taskar P, Li D, Starck SR, Chen H-IH, McEntee M, Katewa SD, Phung V, Wang M, Kekatpure A, Lakshminarasimhan D, White A, Olland A, Haldankar R, Solloway MJ, Hsu J-Y, Wang Y, Tang J, Lindhout DA, Allan BB. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat Med 2020;26:1264–70 [DOI] [PubMed] [Google Scholar]
- 216. Gelin J, Andersson C, Lundholm K. Effects of indomethacin, cytokines, and cyclosporin A on tumor growth and the subsequent development of cancer cachexia. Cancer Res 1991;51:880–5 [PubMed] [Google Scholar]
- 217. Loprinzi CL, Ellison NM, Schaid DJ, Krook JE, Athmann LM, Dose AM, Mailliard JA, Johnson PS, Ebbert LP, Geeraerts LH. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst 1990;82:1127–32 [DOI] [PubMed] [Google Scholar]
- 218. Brisbois TD, de Kock IH, Watanabe SM, Mirhosseini M, Lamoureux DC, Chasen M, MacDonald N, Baracos VE, Wismer WV. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double-blind, placebo-controlled pilot trial. Ann Oncol 2011;22:2086–93 [DOI] [PubMed] [Google Scholar]
- 219. Lai V, George J, Richey L, Kim HJ, Cannon T, Shores C, Couch M. Results of a pilot study of the effects of celecoxib on cancer cachexia in patients with cancer of the head, neck, and gastrointestinal tract. Head Neck 2008;30:67–74 [DOI] [PubMed] [Google Scholar]
- 220. Roeland EJ, Bohlke K, Baracos VE, Bruera E, Del Fabbro E, Dixon S, Fallon M, Herrstedt J, Lau H, Platek M, Rugo HS, Schnipper HH, Smith TJ, Tan W, Loprinzi CL. Management of cancer cachexia: ASCO guideline. J Clin Oncol 2020;38:2438–53 [DOI] [PubMed] [Google Scholar]
- 221. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48 [DOI] [PubMed] [Google Scholar]
- 222. Aversa Z, Costelli P, Muscaritoli M. Cancer-induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol 2017;9:369–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sadeghi M, Keshavarz-Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: diagnosis, assessment, and treatment. Crit Rev Oncol Hematol 2018;127:91–104 [DOI] [PubMed] [Google Scholar]
- 224. Baba MR, Buch SA. Revisiting cancer cachexia: pathogenesis, diagnosis, and current treatment approaches. Asia Pac J Oncol Nurs 2021;8: 508–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Sánchez-Lara K, Turcott JG, Juárez-Hernández E, Nuñez-Valencia C, Villanueva G, Guevara P, De la Torre-Vallejo M, Mohar A, Arrieta O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr 2014;33:1017–23 [DOI] [PubMed] [Google Scholar]
- 226. Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2012;104:371–85 [DOI] [PubMed] [Google Scholar]
- 227. Balstad TR, Solheim TS, Strasser F, Kaasa S, Bye A. Dietary treatment of weight loss in patients with advanced cancer and cachexia: a systematic literature review. Crit Rev Oncol Hematol 2014;91:210–21 [DOI] [PubMed] [Google Scholar]
- 228. Ries A, Trottenberg P, Elsner F, Stiel S, Haugen D, Kaasa S, Radbruch L. A systematic review on the role of fish oil for the treatment of cachexia in advanced cancer: an EPCRC cachexia guidelines project. Palliat Med 2012;26:294–304 [DOI] [PubMed] [Google Scholar]
- 229. Kraft M, Kraft K, Gärtner S, Mayerle J, Simon P, Weber E, Schütte K, Stieler J, Koula-Jenik H, Holzhauer P, Gröber U, Engel G, Müller C, Feng Y-S, Aghdassi A, Nitsche C, Malfertheiner P, Patrzyk M, Kohlmann T, Lerch MM. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)—a randomized multicentre trial. Nutr J 2012;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. van der Meij BS, Langius JA, Smit EF, Spreeuwenberg MD, von Blomberg BM, Heijboer AC, Paul MA, van Leeuwen PA. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J Nutr 2010;140:1774–80 [DOI] [PubMed] [Google Scholar]
- 231. Gordon JN, Trebble TM, Ellis RD, Duncan HD, Johns T, Goggin PM. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut 2005;54:540–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Mantovani G. Randomised phase III clinical trial of 5 different arms of treatment on 332 patients with cancer cachexia. Eur Rev Med Pharmacol Sci 2010;14:292–301 [PubMed] [Google Scholar]
- 233. Goldberg RM, Loprinzi CL, Mailliard JA, O’Fallon JR, Krook JE, Ghosh C, Hestorff RD, Chong SF, Reuter NF, Shanahan TG. Pentoxifylline for treatment of cancer anorexia and cachexia? A randomized, double-blind, placebo-controlled trial. J Clin Oncol 1995;13:2856–9 [DOI] [PubMed] [Google Scholar]
- 234. Mehrzad V, Afshar R, Akbari M. Pentoxifylline treatment in patients with cancer cachexia: a double-blind, randomized, placebo-controlled clinical trial. Adv Biomed Res 2016;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Yennurajalingam S, Willey JS, Palmer JL, Allo J, Del Fabbro E, Cohen EN, Tin S, Reuben JM, Bruera E. The role of thalidomide and placebo for the treatment of cancer-related anorexia-cachexia symptoms: results of a double-blind placebo-controlled randomized study. J Palliat Med 2012;15:1059–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Clarke SJ, Smith JT, Gebbie C, Sweeney C, Olszewski N. A phase I, pharmacokinetic (PK), and preliminary efficacy assessment of ALD518, a humanized anti-IL-6 antibody, in patients with advanced cancer. JCO 2009;27:3025 [Google Scholar]
- 237. Lundholm K, Gunnebo L, Körner U, Iresjö B-M, Engström C, Hyltander A, Smedh U, Bosaeus I. Effects by daily long term provision of ghrelin to unselected weight-losing cancer patients: a randomized double-blind study. Cancer 2010;116:2044–52 [DOI] [PubMed] [Google Scholar]
- 238. Hiura Y, Takiguchi S, Yamamoto K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Miyata H, Fujiwara Y, Mori M, Kangawa K, Doki Y. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo-controlled phase 2 study. Cancer 2012;118:4785–94 [DOI] [PubMed] [Google Scholar]
- 239. Madeddu C, Macciò A, Panzone F, Tanca FM, Mantovani G. Medroxyprogesterone acetate in the management of cancer cachexia. Expert Opin Pharmacother 2009;10:1359–66 [DOI] [PubMed] [Google Scholar]
- 240. Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort-Marti S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev 2013;2013:CD004310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Mantovani G, Macciò A, Lai P, Massa E, Ghiani M, Santona MC. Cytokine involvement in cancer anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate on cytokine downregulation and improvement of clinical symptoms. Crit Rev Oncog 1998;9:99–106 [DOI] [PubMed] [Google Scholar]
- 242. Tuca A, Jimenez-Fonseca P, Gascón P. Clinical evaluation and optimal management of cancer cachexia. Crit Rev Oncol Hematol 2013;88:625–36 [DOI] [PubMed] [Google Scholar]
- 243. Mantovani G, Madeddu C. Cancer cachexia: medical management. Support Care Cancer 2010;18:1–9 [DOI] [PubMed] [Google Scholar]
- 244. Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, Krook JE, Wilwerding MB, Rowland KM, Jr, Camoriano JK, Novotny PJ, Christensen BJ. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol 1999;17:3299–306 [DOI] [PubMed] [Google Scholar]
- 245. Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko Y-D, Schnelle M, Reif M, Cerny T, Cerny T. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol 2006;24:3394–400 [DOI] [PubMed] [Google Scholar]
- 246. Miyano K, Ohshima K, Suzuki N, Furuya S, Yoshida Y, Nonaka M, Higami Y, Yoshizawa K, Fujii H, Uezono Y. Japanese herbal medicine Ninjinyoeito mediates its orexigenic properties partially by activating Orexin 1 receptors. Front Nutr 2020;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Terawaki K, Kashiwase Y, Sawada Y, Hashimoto H, Yoshimura M, Ohbuchi K, Sudo Y, Suzuki M, Miyano K, Shiraishi S, Higami Y, Yanagihara K, Hattori T, Kase Y, Ueta Y, Uezono Y. Development of ghrelin resistance in a cancer cachexia rat model using human gastric cancer-derived 85As2 cells and the palliative effects of the Kampo medicine rikkunshito on the model. PLoS ONE 2017;12:e0173113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Goswami C, Dezaki K, Wang L, Inui A, Seino Y, Yada T. Ninjin-yoeito activates ghrelin-responsive and unresponsive NPY neurons in the arcuate nucleus and counteracts cisplatin-induced anorexia. Neuropeptides 2019;75:58–64 [DOI] [PubMed] [Google Scholar]
- 249. Ohsawa M, Makino T, Takimoto Y, Inui A. Application of Kampo medicines for the palliation of cancer cachexia. Neuropeptides 2021; 90:102188. [DOI] [PubMed] [Google Scholar]
- 250. Jackson KM, Cole CL, Dunne RF. From bench to bedside: updates in basic science, translational and clinical research on muscle fatigue in cancer cachexia. Curr Opin Clin Nutr Metab Care 2021;24:216–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Ohsawa M, Maruoka J, Inami C, Iwaki A, Murakami T, Ishikura K-I. Effect of Ninjin’yoeito on the loss of skeletal muscle function in cancer-bearing mice. Front Pharmacol 2018;9:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Takayama S, Akaishi T, Nozaki H, Suzuki S, Arita R, Saito N, Tanaka J, Numata T, Kikuchi A, Ohsawa M, Abe M, Ishii T. Characteristics and course of patients treated with Kampo Medicine in the Department of General Medicine. J Gen Fam Med 2020;21:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Choi YK, Jung KY, Woo S-M, Yun YJ, Jun C-Y, Park JH, Shin YC, Cho S-G, Ko S-G. Effect of Sipjeondaebo-tang on cancer-induced anorexia and cachexia in CT-26 tumor-bearing mice. Mediators Inflamm 2014;2014:736563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Cheon C, Yoo J-E, Yoo H-S, Cho C-K, Kang S, Kim M, Jang B-H, Shin Y-C, Ko S-G. Efficacy and safety of Sipjeondaebo-Tang for anorexia in patients with cancer: a pilot, randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med 2017;2017:8780325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Keum J-H, Kang O-H, Kim S-B, Mun S-H, Seo Y-S, Kim M-R, Rho J-R, Lee Y-S, Park C-B, Kim Y-G, Kim Y-I, Han S-H, Kwon D-Y. The anti-inflammatory effect of Cheongseoikki-tang ethanol extract on allergic reactions mediated by bone marrow-derived mast cells. Chin J Integr Med 2013;19:380–6 [DOI] [PubMed] [Google Scholar]
- 256. Wang H, Chan Y-L, Li T-L, Wu C-J. Improving cachectic symptoms and immune strength of tumour-bearing mice in chemotherapy by a combination of Scutellaria baicalensis and Qing-Shu-Yi-Qi-Tang. Eur J Cancer 2012;48:1074–84 [DOI] [PubMed] [Google Scholar]
- 257. Tsubouchi H, Yanagi S, Miura A, Mogami S, Yamada C, Iizuka S, Hattori T, Nakazato M. Rikkunshito ameliorates cachexia associated with bleomycin-induced lung fibrosis in mice by stimulating ghrelin secretion. Nutr Res 2014;34:876–85 [DOI] [PubMed] [Google Scholar]
- 258. Fujitsuka N, Asakawa A, Uezono Y, Minami K, Yamaguchi T, Niijima A, Yada T, Maejima Y, Sedbazar U, Sakai T, Hattori T, Kase Y, Inui A. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry 2011;1:e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Kim A, Im M, Ma JY. Sosiho–tang ameliorates cachexia-related symptoms in mice bearing colon 26 adenocarcinoma by reducing systemic inflammation and muscle loss. Oncol Rep 2016;35:1841–50 [DOI] [PubMed] [Google Scholar]
- 260. Hamauchi S, Furuse J, Takano T, Munemoto Y, Furuya K, Baba H, Takeuchi M, Choda Y, Higashiguchi T, Naito T, Muro K, Takayama K, Oyama S, Takiguchi T, Komura N, Tamura K. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 2019;125:4294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle 2021;12:14–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005;98:1154–62. [DOI] [PubMed] [Google Scholar]
- 263. Lira FS, Neto JCR, Seelaender M. Exercise training as treatment in cancer cachexia. Appl Physiol Nutr Metab 2014;39:679–86 [DOI] [PubMed] [Google Scholar]
- 264. Lira FS, Yamashita AS, Rosa JC, Koyama CH, Caperuto EC, Batista ML, Jr, Seelaender MC. Exercise training decreases adipose tissue inflammation in cachectic rats. Horm Metab Res 2012;44:91–8. [DOI] [PubMed] [Google Scholar]
- 265. Solheim TS, Laird BJA, Balstad TR, Stene GB, Bye A, Johns N, Pettersen CH, Fallon M, Fayers P, Fearon K, Kaasa S. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle 2017;8:778–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Uster A, Ruehlin M, Mey S, Gisi D, Knols R, Imoberdorf R, Pless M, Ballmer PE. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr 2018;37:1202–9 [DOI] [PubMed] [Google Scholar]