Abstract
Introduction:
In the early stages of the global COVID-19 pandemic hospital admissions for acute ischemic stroke (AIS) decreased substantially. As health systems have become more experienced in dealing with the pandemic, and as the proportion of the population vaccinated rises, it is of interest to determine whether the prevalence of AIS hospitalization and outcomes from hospitalization have returned to normal.
Patients and methods:
In this observational, retrospective cohort study, we compared the prevalence and outcomes of AIS during the first four waves of the pandemic to corresponding pre-pandemic periods in 2019 using administrative data collected from a nationwide network of 76 hospitals that manages 7% of all in-hospital cases in Germany.
Results:
We included 25,821 AIS cases in the study period (2020/2021) and used 26,295 AIS cases as controls (2019). Compared to pre-pandemic numbers, mean daily AIS admissions decreased only during wave 1 (from 39.6 to 34.1; p < 0.01) and wave 2 (from 39.9 to 38.3; p = 0.03) and returned to normal levels during waves 3 and 4. AIS case fatality increased in wave 1 only (from 6.0% to 7.6%; p = 0.03). We observed a consistent decrease in the prevalences of arterial hypertension, diabetes, and obesity among AIS cases throughout the pandemic and no changes in rates of systemic thrombolysis, mechanical thrombectomy, or decompressive craniectomy. The rate of transfer to stroke units increased only during waves 2 (by 4.6%; p < 0.01) and 3 (by 3.0%; p < 0.01). The proportion of patients with coinciding SARS-CoV-2 and AIS was low, peaking at 3.4% in wave 2 and subsequently decreasing to 0.4% in wave 4.
Conclusion:
In Germany, the COVID-19 pandemic seems to have had a larger effect on nationwide in-hospital AIS care during the early pandemic stages, in which AIS case numbers decreased and case fatality rose. This may reflect a nationwide “learning curve” within health care systems in providing AIS care in times of a pandemic.
Keywords: Acute ischemic stroke, COVID-19, SARS-CoV-2, case fatality
Introduction
During the early stages of the global COVID-19 pandemic, the prevalence of hospitalization for acute ischemic stroke (AIS) decreased and, in some countries, AIS case fatality rose.1–9 These findings are unusual given that the overwhelming majority of cases were SARS-CoV-2-negative patients and hospitals in most parts of the world were accessible for emergency stroke care without significant interruption of service.1,10–12 The most commonly suggested explanation is that fear of hospital-acquired COVID-19 caused AIS patients to delay or defer hospital presentation.13–19 As health systems and countries have become more skilled at dealing with the pandemic, and as the proportion of the population vaccinated rises, it is of interest to determine whether the prevalence of AIS hospitalization and outcomes from hospitalization have returned to normal.
We compared the prevalence and case fatality rate of AIS during early and more recent phases of the pandemic to corresponding pre-pandemic periods in 2019 using data collected from a nationwide network of hospitals in Germany.
Patients and methods
Patient population and data extraction
In this observational, retrospective cohort study, we included administrative data of all patients hospitalized for AIS at 76 hospitals within the Helios network in Germany between January 1, 2020 and October 26, 2021. We present a population study including all patients within the Helios network, which is the largest private healthcare provider in the country. It comprises hospitals in rural and urban areas in 13 of the 16 federal states of Germany and accounts for about 7% of patient hospitalizations nationwide. The Helios hospital network admits patients of all health insurance funds available in Germany (public and private). This improves the generalizability of our findings on health care processes and outcomes to the entire population of Germany, as there is no selection for insurance fund associated subpopulations with specific profiles of comorbidities and risk factors. According to the pandemic waves and the relatively long non-wave period between waves 1 and 2, the study period was subdivided as follows:
‒ leading up to wave 1 (January 1 to March 12, 2020),
‒ wave 1 (March 13 to May 25, 2020),
‒ between waves 1 and 2 (May 26 to September 19, 2020),
‒ wave 2 (September 20, 2020 to February 24, 2021),
‒ wave 3 (February 25, 2021 to June 20, 2021),
‒ wave 4 (June 21, 2021 to October 26, 2021).
Data from each phase of the study period were compared to corresponding periods in 2019 (control period). Primary diagnosis of AIS was made according to International Statistical Classification of Diseases and Related Health Problems ((ICD-10-GM (German Modification)) codes using the main codes I63.0–I63.9. Procedures and treatment paths for neurosurgical interventions (decompressive craniectomies: 5-012.0, 5-010.00-.03, 5.010.10-.13) as well as for thrombolysis (8-020.8, 8-020.d, 8-836.70, 8-836.71), thrombectomy (8-836.80, 8-836.81), and mechanical ventilation (OPS 8-70x, 8-71x, or duration of ventilation > 0) were identified via the Operations and Procedures codes (OPS (German adaptation of the International Classification of the Procedures in Medicine of the World Health Organization, version 2017)). We also examined the prevalence of transfer to stroke units among patients without mechanical ventilation using code 8-981x.
Relevant stroke comorbidities (such as congestive heart failure, cardiac arrhythmias, peripheral vascular disorder, hypertension, diabetes mellitus, and obesity) were identified from encoded secondary diagnoses at hospital discharge and used to calculate the Elixhauser comorbidity Index (ECI). To assess the amount of resources allocated to AIS treatment as a rough proxy for case severity, we present the case mix index (CMI). In Germany, the CMI is relevant to the reimbursement of health care providers for cases treated by assessing the complexity and severity of each case. For this, based on the sum of ICD-10 codes, each case is assigned a Diagnosis-Related Group (DRG). Each DRG has a specific “relative weight,” which then determines the amount of reimbursement. The CMI is calculated by dividing the sum of all relative weights in a group of cases (in our study: AIS cases) by the number of cases per time period studied. All data were stored in pseudonymized form, and data use was in accordance with national data protection standards. Informed consent was waived due to the retrospective nature of this study. The study was approved by the ethics committee of the University of Leipzig on January 28, 2021 (490/20-ek).
Statistical analysis
Administrative data were extracted using QlikView (QlikTech, Radnor, Pennsylvania, USA). Inferential statistics were based on generalized linear mixed models (GLMM) specifying hospitals as random factor.20,21 We employed Poisson GLMMs with log link function for count data. Effects were estimated with the lme4 package (version 1.1-21) 22 in the R environment for statistical computing (version 4.0.2, 64-bit build). 23 In all models, we specified varying intercepts for the random factor. Incidence-rate ratios (IRR) for the different periods (waves) were based on different models comparing the study and the control period. IRRs were calculated by exponentiation of the regression coefficients; we also provide 95% confidence intervals and p-values. For all tests a two-tailed 5% error criterion for significance was applied.
For the description of the patient characteristics of the cohorts, we employed χ2-tests for binary variables and analysis of variance for numeric variables. Here, we report proportions, means, standard deviations, and p-values. For the comparison of proportions of selected treatments and outcomes in the different cohorts, we used logistic GLMMs with logit link function. Here, we report proportions, odds ratios together with confidence intervals and p-values. The analysis of the outcome variable “duration of stay” was performed via linear mixed models. For this purpose, duration of stay was log-transformed due to its skewed distribution. We report means, standard deviations, and p-values. The ECI and its items was calculated based on the AHRQ algorithm. 24
Results
AIS hospitalizations
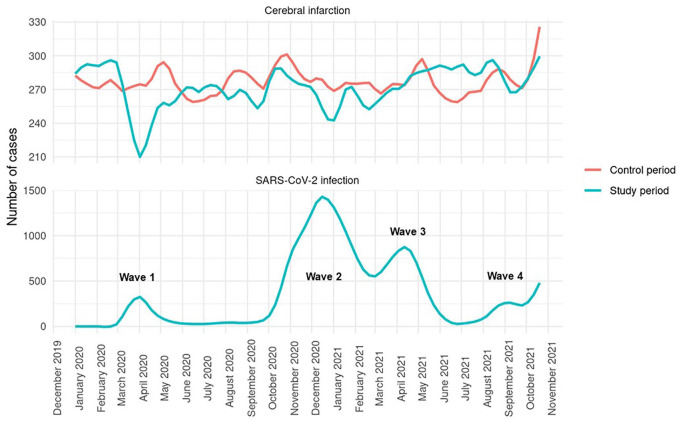
From 76 hospitals nationwide, we included 25,821 AIS cases in the study period (2020/2021) and used 26,295 AIS cases as controls (2019). Compared to corresponding pre-pandemic control periods, mean daily admissions were significantly decreased during waves 1 and 2, but not during waves 3 and 4 or during any of the examined non-wave phases (Table 1). Figure 1 depicts weekly admission rates for AIS during study and control periods in relation to the total number of COVID-19 hospitalizations within the Helios network. Even though wave 1 was less pronounced than all of the later waves, corresponding drop-offs in AIS admission rates were most substantial in wave 1, compared to corresponding control periods.
Table 1.
Daily admissions for AIS.
| Mean daily admissions | IRR (95% CI) | p-Value | ||
|---|---|---|---|---|
| Control period | Study period | |||
| Leading up to wave 1 | 39.7 | 41.7 | 1.06 (0.92–1.23) | 0.39 |
| Wave 1 | 39.6 | 34.1 | 0.74 (0.63–0.88) | < 0.01 |
| Between waves 1 and 2 | 39.0 | 38.2 | 0.94 (0.79–1.11) | 0.45 |
| Wave 2 | 39.9 | 38.3 | 0.79 (0.64–0.97) | 0.03 |
| Wave 3 | 39.6 | 39.4 | 0.81 (0.64–1.04 | 0.10 |
| Wave 4 | 39.5 | 40.9 | 0.94 (0.74–1.20) | 0.62 |
IRR: incidence rate ratio; CI: confidence interval.
Figure 1.
Moving average weekly admissions for AIS during study period (upper blue line) and control period (red line, superimposed from 2019) and for all SARS-CoV2-positive cases admitted to Helios hospitals nationwide (lower blue line).
Baseline characteristics
On admission, there were no differences between study and control periods in patient sex and age distribution, except for in waves 3 and 4, where we observed a significant decrease in AIS hospitalizations among patients aged 70–79 years (Table 2). Across all examined phases of the pandemic, AIS cases generally showed a lower prevalence of comorbidities, compared to pre-pandemic levels. This effect was especially consistent with regard to congestive heart failure, peripheral vascular disorders, arterial hypertension, diabetes, renal failure, and obesity. The ECI showed a significant decrease only in the phase leading up to wave 1 and during wave 4, but not in any other phase of the pandemic. The proportion of patients with coinciding SARS-CoV-2 and AIS remained in low single digit percentages throughout all phases of the study period (Table 3).
Table 2.
Baseline characteristics and comordities of the total cohort as a comparison of the control period and the study period.
| Leading up to wave 1 | Wave 1 | Between waves 1 and 2 | Wave 2 | Wave 3 | Wave 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control period | Study period | p-Value | Control period | Study period | p-Value | Control period | Study period | p-Value | Control period | Study period | p-Value | Control period | Study period | p-Value | Control period | Study period | p-Value | |
| Number of cases | 2857 | 3004 | 2928 | 2523 | 4558 | 4474 | 6306 | 6050 | 4595 | 4567 | 5051 | 5233 | ||||||
| Age | ||||||||||||||||||
| Mean (SD) | 74.2 ± 12.8 | 74.7 ± 12.4 | 0.11 | 73.9 ± 12.6 | 73.8 ± 13.1 | 0.68 | 73.6 ± 12.9 | 73.7 ± 13.1 | 0.70 | 74.3 ± 12.8 | 74.2 ± 12.7 | 0.58 | 73.8 ± 12.8 | 73.8 ± 12.9 | 0.88 | 73.9 ± 12.9 | 73.7 ± 13.2 | 0.54 |
| ⩽59 years | 13.2% (376) | 12.9% (389) | 0.84 | 13.8% (404) | 14.8% (373) | 0.32 | 14.6% (667) | 15.3% (684) | 0.40 | 13.8% (872) | 14.0% (844) | 0.86 | 14.2% (654) | 14.2% (649) | 1.00 | 14.0% (706) | 14.9% (778) | 0.21 |
| 60−69 years | 18.6% (530) | 17.2% (518) | 0.20 | 19.4% (568) | 18.4% (464) | 0.36 | 18.8% (857) | 18.3% (820) | 0.58 | 17.4% (1,098) | 17.7% (1,072) | 0.67 | 18.8% (862) | 19.1% (874) | 0.66 | 18.5% (934) | 18.8% (982) | 0.74 |
| 70−79 years | 27.9% (796) | 27.7% (831) | 0.89 | 27.4% (801) | 26.9% (678) | 0.71 | 28.2% (1,284) | 26.4% (1,183) | 0.07 | 27.4% (1,728) | 26.4% (1,597) | 0.22 | 28.2% (1,295) | 25.8% (1,180) | 0.01 | 27.8% (1,406) | 25.8% (1,349) | 0.02 |
| ⩾80 years | 40.4% (1,155) | 42.1% (1,266) | 0.19 | 39.4% (1,155) | 40.0% (1,008) | 0.72 | 38.4% (1,750) | 39.9% (1,787) | 0.14 | 41.4% (2,608) | 41.9% (2,537) | 0.53 | 38.8% (1,784) | 40.8% (1,864) | 0.05 | 39.7% (2,005) | 40.6% (2,124) | 0.37 |
| Sex | ||||||||||||||||||
| Male | 52.0% (1,487) | 50.5% (1,517) | 53.6% (1,570) | 54.1% (1,366) | 53.9% (2,458) | 54.0% (2,418) | 52.2% (3,290) | 52.1% (3,152) | 52.8% (2,428) | 53.8% (2,455) | 53.3% (2,694) | 52.7% (2,760) | ||||||
| Female | 48.0% (1,370) | 49.5% (1,487) | 0.25 | 46.4% (1,358) | 45.9% (1,157) | 0.72 | 46.1% (2,100) | 46.0% (2,056) | 0.93 | 47.8% (3,016) | 47.9% (2,898) | 0.95 | 47.2% (2,167) | 46.2% (2,112) | 0.39 | 46.7% (2,357) | 47.3% (2,473) | 0.56 |
| Comorbidities | ||||||||||||||||||
| Elixhauser comorbidity index, mean (SD) | 10.3 ± 10.0 | 9.8 ± 10.1 | 0.03 | 10.4 ± 10.0 | 9.9 ± 9.8 | 0.11 | 10.1 ± 10.0 | 9.8 ± 10.0 | 0.12 | 9.8 ± 9.8 | 9.9 ± 10.0 | 0.58 | 10.6 ± 10.2 | 10.3 ± 10.3 | 0.23 | 9.8 ± 9.9 | 9.2 ± 9.7 | <0.01 |
| Congestive heart failure | 20.6% (589) | 17.8% (534) | < 0.01 | 20.7% (606) | 16.8% (424) | <0.01 | 21.2% (967) | 17.3% (773) | <0.01 | 18.7% (1,181) | 16.1% (973) | <0.01 | 21.6% (992) | 16.0% (731) | <0.01 | 20.0% (1008) | 14.8% (775) | <0.01 |
| Cardiac arrhythmias | 35.9% (1,027) | 36.5% (1,095) | 0.71 | 35.8% (1,048) | 34.2% (862) | 0.22 | 33.7% (1,534) | 32.1% (1,437) | 0.13 | 33.9% (2,139) | 35.0% (2,120) | 0.20 | 35.5% (1,629) | 32.6% (1,488) | <0.01 | 33.6% (1,698) | 32.0% (1,674) | 0.08 |
| Peripheral vascular disorders | 10.0% (286) | 7.2% (216) | < 0.01 | 9.2% (268) | 7.3% (184) | 0.01 | 9.9% (452) | 7.6% (338) | <0.01 | 8.9% (559) | 6.7% (400) | <0.01 | 9.4% (433) | 6.9% (313) | <0.01 | 9.4% (474) | 6.7% (352) | <0.01 |
| Hypertension, uncomplicated | 65.8% (1881) | 69.3% (2083) | < 0.01 | 65.5% (1918) | 68.3% (1723) | 0.03 | 61.8% (2815) | 66.0% (2952) | <0.01 | 65.6% (4137) | 67.6% (4090) | 0.02 | 64.1% (2945) | 67.2% (3067) | <0.01 | 62.9% (3,175) | 65.8% (3,443) | <0.01 |
| Hypertension, complicated | 15.4% (441) | 12.6% (379) | < 0.01 | 15.9% (465) | 14.3% (362) | 0.12 | 16.8% (766) | 13.9% (621) | <0.01 | 14.4% (908) | 12.0% (728) | <0.01 | 16.1% (739) | 11.5% (527) | <0.01 | 16.3% (821) | 11.2% (586) | <0.01 |
| Diabetes, uncomplicated | 18.6% (532) | 21.5% (645) | < 0.01 | 19.9% (582) | 20.6% (520) | 0.52 | 19.6% (895) | 21.1% (946) | 0.08 | 20.0% (1,262) | 21.5% (1,301) | 0.04 | 20.1% (924) | 20.6% (941) | 0.57 | 19.7% (993) | 20.8% (1,091) | 0.!4 |
| Diabetes, complicated | 12.6% (361) | 9.9% (296) | < 0.01 | 11.1% (324) | 8.5% (214) | <0.01 | 10.6% (485) | 8.9% (396) | <0.01 | 10.6% (667) | 9.5% (576) | 0.05 | 11.4% (524) | 9.0% (409) | <0.01 | 10.3% (521) | 8.1% (423) | <0.01 |
| Obesity | 12.8% (367) | 10.1% (304) | < 0.01 | 12.9% (378) | 9.1% (229) | <0.01 | 12.5% (569) | 10.3% (463) | <0.01 | 12.1% (766) | 9.6% (581) | <0.01 | 13.1% (602) | 8.9% (406) | <0.01 | 12.4% (628) | 9.5% (499) | <0.01 |
| Renal failure | 31.3% (894) | 25.9% (777) | < 0.01 | 30.1% (881) | 25.4% (642) | <0.01 | 29.1% (1325) | 26.2% (1171) | <0.01 | 27.8% (1755) | 26.9% (1626) | 0.24 | 30.4% (1399) | 29.5%(1348) | 0.34 | 28.4% (1435) | 24.0%(1257) | <0.01 |
SD: standard deviation.
Table 3.
Rate of SARS-CoV-2 infections among AIS cases during study period.
| Rate of SARS-CoV-2 infections | ||
|---|---|---|
| Leading up to wave 1 | 2/3004 | 0.1% |
| Wave 1 | 25/2523 | 1.0% |
| Between waves 1 and 2 | 5/4474 | 0.1% |
| Wave 2 | 208/6050 | 3.4% |
| Wave 3 | 65/4567 | 1.4% |
| Wave 4 | 22/5233 | 0.4% |
Treatment and outcomes
Throughout all phases of the COVID-19 pandemic, the rates of systemic thrombolysis, mechanical thrombectomy, and decompressive craniectomy remained stable in patients with AIS, compared to 2019 (Table 4). The rate of transfer to stroke unit increased only during waves 2 and 3 and remained stable in all other periods. Rates of mechanical ventilation showed a decrease in waves 2 and 4. AIS case fatality rates increased only during wave 1 (by 1.6%) but not in any of the other examined phases. Compared to 2019, the duration of hospital stay was significantly shorter throughout the pandemic. During all pandemic waves, the CMI was significantly decreased, compared to pre-pandemic levels.
Table 4.
Rates of treatment, case fatality, duration of stay, and case mix index.
| Control period | Study period | Odds ratio (95% CI) | p-Value | |
|---|---|---|---|---|
| Systemic thrombolysis | ||||
| Leading up to wave 1 | 14.3% (408) | 13.8% (414) | 0.97 (0.84–1.13) | 0.695 |
| Wave 1 | 14.3% (419) | 15.2% (383) | 1.06 (0.91–1.23) | 0.487 |
| Between waves 1 and 2 | 15.1% (686) | 15.4% (691) | 1.01 (0.90–1.14) | 0.802 |
| Wave 2 | 14.4% (907) | 15.1% (911) | 1.03 (0.93–1.14) | 0.548 |
| Wave 3 | 15.0% (690) | 14.5% (660) | 0.90 (0.79–1.01) | 0.068 |
| Wave 4 | 14.4% (725) | 14.1% (736) | 0.98 (0.87–1.09) | 0.690 |
| Mechanical thrombectomy | ||||
| Leading up to wave 1 | 5.8% (165) | 6.9% (206) | 1.24 (1.00–1.55) | 0.055 |
| Wave 1 | 6.7% (195) | 6.1% (153) | 0.89 (0.71–1.13) | 0.338 |
| Between waves 1 and 2 | 6.5% (298) | 7.4% (332) | 1.11 (0.94–1.32) | 0.222 |
| Wave 2 | 6.6% (416) | 6.9% (418) | 1.02 (0.87–1.18) | 0.838 |
| Wave 3 | 6.6% (302) | 5.8% (263) | 0.88 (0.73–1.05) | 0.155 |
| Wave 4 | 6.3% (210) | 6.1% (320) | 0.95 (0.80–1.12) | 0.552 |
| Decompressive craniectomy | ||||
| Leading up to wave 1 | 0.4% (11) | 0.4% (12) | 1.05 (0.46–2.39) | 0.906 |
| Wave 1 | 0.4% (11) | 0.4% (11) | 1.15 (0.49–2.66) | 0.751 |
| Between waves 1 and 2 | 0.5% (22) | 0.3% (14) | 0.65 (0.33–1.27) | 0.204 |
| Wave 2 | 0.4% (24) | 0.4% (25) | 1.06 (0.60–1.86) | 0.842 |
| Wave 3 | 0.4% (18) | 0.5% (23) | 1.37 (0.73–2.56) | 0.322 |
| Wave 4 | 0.5% (24) | 0.6% (32) | 1.38 (0.81–2.36) | 0.238 |
| Transfer to stroke unit among patients without mechanical ventilation | ||||
| Leading up to wave 1 | 59.8% (1,636) | 58.5% (1,681) | 0.99 (0.84–1.16) | 0.872 |
| Wave 1 | 60.9% (1,707) | 62.5% (1,507) | 1.17 (0.98–1.39) | 0.076 |
| Between waves 1 and 2 | 61.6% (2,670) | 63.7% (2,716) | 1.12 (0.98–1.28) | 0.103 |
| Wave 2 | 59.3% (3,552) | 63.9% (3,707) | 1.31 (1.31–1.31) | <0.001 |
| Wave 3 | 60.9% (2,670) | 63.9% (2,798) | 1.21 (1.06–1.38) | 0.006 |
| Wave 4 | 61.7% (2,963) | 61.5% (3,099) | 0.97 (0.86–1.09) | 0.578 |
| Mechanical ventilation | ||||
| Leading up to wave 1 | 4.3% (123) | 4.4% (131) | 1.02 (0.79–1.31) | 0.892 |
| Wave 1 | 4.3% (127) | 4.4% (112) | 1.01 (0.78–1.31) | 0.936 |
| Between waves 1 and 2 | 5.0% (227) | 4.7% (210) | 0.93 (0.77–1.14) | 0.498 |
| Wave 2 | 4.9% (312) | 4.2% (252) | 0.82 (0.69–0.98) | 0.027 |
| Wave 3 | 4.5% (208) | 4.2% (190) | 0.96 (0.78–1.18) | 0.701 |
| Wave 4 | 5.0% (252) | 3.7% (195) | 0.74 (0.61–0.90) | 0.003 |
| In-hospital case fatality | ||||
| Leading up to wave 1 | 6.2% (160) | 6.4% (175) | 1.05 (0.84–1.31) | 0.691 |
| Wave 1 | 6.0% (160) | 7.6% (177) | 1.27 (1.02–1.59) | 0.033 |
| Between waves 1 and 2 | 6.4% (264) | 5.4% (220) | 0.83 (0.69–1.00) | 0.045 |
| Wave 2 | 6.7% (383) | 7.1% (390) | 1.06 (0.92–1.23) | 0.418 |
| Wave 3 | 6.2% (258) | 6.0% (245) | 0.97 (0.81–1.16) | 0.710 |
| Wave 4 | 6.5% (294) | 5.4% (253) | 0.82 (0.69–0.98) | 0.025 |
| Duration of hospital stay (mean, days) | ||||
| Leading up to wave 1 | 9.2 ± 9.6 | 9.9 ± 9.7 | – | <0.01 |
| Wave 1 | 9.7 ± 9.6 | 8.3 ± 8.3 | – | <0.01 |
| Between waves 1 and 2 | 9.2 ± 8.9 | 8.7 ± 9.5 | – | <0.01 |
| Wave 2 | 9.7 ± 9.6 | 9.1 ± 10.9 | – | <0.01 |
| Wave 3 | 9.6 ± 9.1 | 8.8 ± 9.6 | – | <0.01 |
| Wave 4 | 9.3 ± 9.1 | 8.9 ± 8.4 | – | <0.01 |
| Case mix index (mean) | ||||
| Leading up to wave 1 | 1.68 ± 1.3 | 1.68 ± 1.2 | 0.66 | |
| Wave 1 | 1.71 ± 1.4 | 1.68 ± 1.6 | <0.01 | |
| Between waves 1 and 2 | 1.70 ± 1.2 | 1.70 ± 1.3 | 0.37 | |
| Wave 2 | 1.69 ± 1.3 | 1.62 ± 1.4 | <0.01 | |
| Wave 3 | 1.71 ± 1.3 | 1.55 ± 1.2 | <0.01 | |
| Wave 4 | 1.71 ± 1.2 | 1.54 ± 1.2 | <0.01 | |
CI: confidence interval.
Discussion
This analysis is the first to present nationwide data on the impact of COVID-19 on AIS care in all of the four pandemic waves that have occurred in Germany, so far. Our main observation is that AIS outcomes and processes (increased case fatality, reduced hospitalization rate) were initially adversely affected by the pandemic, most prominently in wave 1, but returned to expectations over time during subsequent pandemic waves.
We also found that SARS-CoV-2 infections were uncommon among AIS patients and even decreased during waves 2, 3, and 4. In all phases of the pandemic, AIS patients had fewer comorbidities, compared to pre-pandemic levels, and the prevalence of transfer to a stroke unit was not increased. Rates of interventional or surgical treatment consistently remained at pre-pandemic levels.
Some authors have hypothesized an association between SARS-CoV-2 infections and thromboembolic events, which would predict an increase in AIS incidence. 13 Like other authors, we observed the opposite, namely a decrease in hospitalization rate for AIS during the initial wave, and normal AIS hospitalization rates at later stages of the pandemic.1–9 Certain selective processes during the pandemic are also implied by the fact that comorbidities among AIS patients were consistently less frequent throughout the pandemic and by a decrease in the CMI during all four pandemic waves.
The underlying mechanisms of patient selection at the threshold of hospitals during the COVID-19 pandemic remain uncertain and numerous factors are currently being discussed. Most prominently, it is assumed that fear of acquiring COVID-19 in hospitals may deter AIS patients from presenting, and health care professionals from referring AIS cases.13–19 In our study cohort, AIS patients with known comorbidities may have been more reluctant to present to a hospital, as, even in the early stages of the pandemic, the public had been made aware of a clear association between comorbidities and poor outcome of COVID-19. 25 As social distancing and masking have become normal even outside of actual periods of lock-down, patients with mild forms of AIS may not have come into sufficient contact with friends, family, or neighbors to be made aware of AIS symptoms. 19 Also, masking and social distancing may have prevented viral infections other than SARS-CoV-2 that could have caused AIS.26,27 Other factors, such as a lock-down associated decrease in air pollution, less availability of public transport, a decreased consumption of unhealthy fast food, or improved compliance to medication plans during the pandemic have been suggested.19,26,28,29 Furthermore, the pandemic may also have prevented patients with diseases mimicking AIS from presenting to hospitals. Such mimics may include conversion disorder, migraine, and seizures and have been shown to account for about a fourth of all cases diagnosed as AIS. 30 The fact that mental health presentations declined substantially during the COVID-19 pandemic may further support this hypothesis. 31 Finally, it is worth pointing out that during the pandemic, many elective procedures were canceled. This may have reduced the rate of AIS, as surgical procedures increase the risk of stroke.32–35
In the early stages of the pandemic, utilization of AIS care capacities may have been suboptimal, since the first and smallest wave generated the largest decrease in AIS hospitalizations and a rise in the case fatality rate, even though, in wave 1, the rate of transfer to stroke unit did not differ from pre-pandemic levels. The subsequent change in AIS outcomes and hospitalizations back toward pre-pandemic levels suggests a “learning effect” over time. In spite of the fact that, in waves 2 and 3, which were the most pronounced of all four waves, AIS case fatality did not increase, we observed a simultaneous increase in rates of transfer to stroke units. This may reflect that, during peaks of the pandemic, stroke unit transfer may not be a reliable indicator of stroke severity. In times of larger pressure on in-hospital capacities, as in waves 2 and 3, AIS patients without COVID-19 may have been more frequently transferred from intensive care units (ICU) to stroke units, in order to decrease pressure on ICU capacities allocated to COVID-19 care.
Another potential factor contributing to changes in in-hospital AIS care, especially during waves 3 and 4, is the growing SARS-CoV-2 vaccination rate. In Germany, full vaccination rates increased from 2.3% at the end of wave 2 to 31.1% in wave 3, and ultimately 66.3% at the end of our study period in wave 4. 36 The observed normalization of AIS hospitalizations in waves 2, 3, and 4 may in part be due to patients being more confident that they will not be infected during a hospital stay.
We acknowledge some limitations of our study. First, our analysis relies on ICD-10 codes to identify AIS, introducing an unknown amount of case misclassification. Nonetheless, diagnostic codes for AIS undergo rigorous screening by in-hospital auditors prior to entry into the hospital system’s database. Second, the database does not capture AIS grade or type, so outcomes other than case fatality are not available. Third, wave 4 of the pandemic is currently still ongoing, so estimating its impact on AIS is not yet complete. Fourth, our data are not able to assess stroke severity and the observed changes in rates of transfer to stroke units may be influenced by changes in in-hospital processes due to the pandemic rather than by stroke severity itself. Fifth, with our data we were not able to further evaluate a potential learning curve in managing AIS during a pandemic by means of time analyses, such as time to first CT, first contact with specialist, or first physiotherapy, mainly since diagnostic and therapeutic processes were coded with a substantial delay. Finally, our study is not able to project long-term effects of the pandemic on AIS care and its results may not be generalizable to health systems outside of Germany.
Conclusion
We found that the COVID-19 pandemic in Germany seems to have had a larger effect on nationwide in-hospital AIS care during the early pandemic stages, in which AIS case numbers decreased and case fatality rose. Many aspects of AIS care normalized during waves 2, 3 and 4, which may reflect a certain learning curve within health care systems in providing AIS care in times of a pandemic. The fact that, among AIS patients, the prevalence of comorbidities and the case mix index decreased during the pandemic suggests selective processes prior to hospitalization that warrant further investigation.
Acknowledgments
We thank the nursing and medical staff and all hygiene specialists in the HELIOS network for their efforts in caring for patients in these difficult times. We also thank Daniel Cher, MD, for external review of the manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JD, KP, FP, SH, VP, MS, BH, AMH, RK, AB, and SR are employees of Helios. RK holds shares in Fresenius AG.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project is funded by the Helios Center for Research and Innovation via a grant (HCRI ID 2020-0458) to JD.
Informed consent: Informed consent was waived due to the retrospective nature of this study.
Ethical approval: The study was approved by the ethics committee of the University of Leipzig on January 28, 2021 (490/20-ek).
Guarantor: JD.
Author contributions: JD and SR conceived the study. JD, BH, and SR wrote the first draft of the manuscript. SH conducted all statistical analyses. JD, KP, FB, AB, RK, and SR were involved in data interpretation. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iD: Julius Dengler  https://orcid.org/0000-0002-6698-8600
https://orcid.org/0000-0002-6698-8600
References
- 1. Uchino K, Kolikonda MK, Brown D, et al. Decline in stroke presentations during COVID-19 surge. Stroke 2020; 51: 2544–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Velilla-Alonso G, García-Pastor A, Rodríguez-López, et al. Acute stroke care during the COVID-19 pandemic: reduction in the number of admissions of elderly patients and increase in prehospital delays. Cerebrovasc Dis 2021; 50: 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Havenon A, Ney JP, Callaghan B, et al. Characteristics and outcomes among US patients hospitalized for ischemic stroke before vs during the COVID-19 pandemic. JAMA Netw Open 2021; 4: e2110314. DOI: 10.1001/jamanetworkopen.2021.10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jaehn P, Holmberg C, Uhlenbrock G, et al. Differential trends of admissions in accident and emergency departments during the COVID-19 pandemic in Germany. BMC Emerg Med 2021; 21: 42. DOI: 10.1186/s12873-021-00436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olié V, Carcaillon-Bentata L, Thiam MM, et al. Emergency department admissions for myocardial infarction and stroke in France during the first wave of the COVID-19 pandemic: national temporal trends and regional disparities. Arch Cardiovasc Dis 2021; 114: 371–380. DOI: 10.1016/j.acvd.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Douiri A, Muruet W, Bhalla A, et al. Stroke care in the United Kingdom during the COVID-19 pandemic. Stroke 2021; 52: 2125–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Y, Chen F, Sun Z, et al. Impact of the pandemic of COVID-19 on emergency attendance for stroke and acute myocardial infarction in Beijing, China. J Thromb Thrombolysis 2021; 52: 1047–1055. DOI: 10.1007/s11239-021-02385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koge J, Shiozawa M, Toyoda K. Acute stroke care in the with-COVID-19 era: experience at a comprehensive stroke center in Japan. Front Neurol 2021; 11: 611504. DOI: 10.3389/fneur.2020.611504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richter D, Eyding J, Weber R, et al. Analysis of nationwide stroke patient care in times of COVID-19 pandemic in Germany. Stroke 2021; 52: 716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aguiar de Sousa D, van der Worp HB, Caso V, et al. Maintaining stroke care in Europe during the COVID-19 pandemic: results from an international survey of stroke professionals and practice recommendations from the European Stroke Organisation. Eur Stroke J 2020; 5: 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dula AN, Gealogo Brown G, Aggarwal A, et al. Decrease in stroke diagnoses during the COVID-19 pandemic: where did all our stroke patients go? JMIR Aging 2020; 3: e21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoyer C, Ebert A, Huttner HB, et al. Acute stroke in times of the COVID-19 pandemic: a multicenter study. Stroke 2020; 51: 2224–2227. [DOI] [PubMed] [Google Scholar]
- 13. Lyden PD. Stroke, research and science in the time of COVID. Stroke 2020; 51: 2613–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nogueira RG, Qureshi MM, Abdalkader M, et al. Global Impact of COVID-19 on stroke care and IV thrombolysis. Neurology 2021; 96: e2824–e2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke 2020; 51: 2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kansagra AP, Goyal MS, Hamilton S, et al. Collateral effect of COVID-19 on stroke evaluation in the United States. New Engl J Med 2020; 383: 400–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teo KC, Leung WCY, Wong YK, et al. Delays in stroke onset to hospital arrival time during COVID-19. Stroke 2020; 51: 2228–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montaner J, Barragán-Prieto A, Pérez-Sánchez S, et al. Break in the stroke chain of survival due to COVID-19. Stroke 2020; 51: 2307–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diegoli H, Magalhães PSC, Martins SCO, et al. Decrease in hospital admissions for transient ischemic attack, mild, and moderate stroke during the COVID-19 era. Stroke 2020; 51: 2315–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang 2008; 59: 390–412. [Google Scholar]
- 21. Kliegl R, Masson MEJ, Richter EM. A linear mixed model analysis of masked repetition priming. Vis Cogn 2010; 18: 655–681. [Google Scholar]
- 22. Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models usinglme4. J Stat Softw 2015; 67: 1–48. [Google Scholar]
- 23. R Core Team. A language and environment for statistical computing. Vienna: R Foundation for statistical computing, 2020. [Google Scholar]
- 24. Moore BJ, White S, Washington R, et al. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser comorbitiy index. Med Care 2017; 55: 698–705. [DOI] [PubMed] [Google Scholar]
- 25. Nachtigall I, Lenga P, Jóźwiak K, et al. Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID-19 in Germany: an observational study. Clin Microbiol Infect 2020; 26: 1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Demaerschalk BM. Where in the world have all the strokes gone? Neurology 2021; 96: 1069–1070. [DOI] [PubMed] [Google Scholar]
- 27. Grau AJ, Urbanek C, Palm F. Common infections and the risk of stroke. Nat Rev Neurol 2010; 6: 681–694. [DOI] [PubMed] [Google Scholar]
- 28. Schlachetzki F, Wilfling S, Hubert ND, et al. Decline and recurrence of stroke consultations during the COVID-19 pandemic lockdown parallels population activity levels. Cerebrovasc Dis 2021; 50: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Heart Association’s Mission: Lifeline and Get With The Guidelines Coronary Artery Disease Advisory Work Group and the Council on Clinical Cardiology’s Committees on Acute Cardiac Care and General Cardiology and Interventional Cardiovascular Care. Temporary emergency guidance to STEMI systems of care during the COVID-19 pandemic: AHA’s mission: lifeline. Circulation 2020; 142: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garg R, Rech MA, Schneck M. Stroke mimics: an important source of bias in acute ischemic stroke research. J Stroke Cerebrovasc Dis 2019; 28: 2475–2480. [DOI] [PubMed] [Google Scholar]
- 31. Dragovic M, Pascu V, Hall T, et al. Emergency department mental health presentations before and during the COVID-19 outbreak in Western Australia. Australas Psychiatry 2020; 28: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Selim M. Perioperative stroke. New Engl J Med 2007; 356: 706–713. [DOI] [PubMed] [Google Scholar]
- 33. Urbanek C, Palm F, Buggle F, et al. Recent surgery or invasive procedures and the risk of stroke. Cerebrovasc Dis 2014; 38: 370–376. [DOI] [PubMed] [Google Scholar]
- 34. Amukotuwa SA, Bammer R, Maingard J. Where have our patients gone? The impact of COVID-19 on stroke imaging and intervention at an Australian stroke center. J Med Imaging Radiat Oncol 2020; 64: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Macellari F, Paciaroni M, Agnelli G, et al. Perioperative stroke risk in nonvascular surgery. Cerebrovasc Dis 2012; 34: 175–181. [DOI] [PubMed] [Google Scholar]
- 36. RKI. Digitales impfquotenmonitoring zur COVID-19-impfung. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Impfquoten-Tab.htm (2021, accessed 14 November 2021).