Abstract
Background and purpose
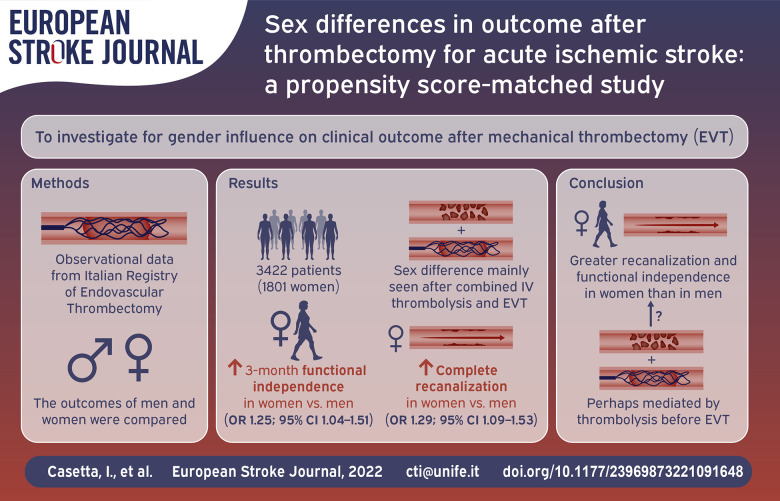
We sought to investigate whether there are gender differences in clinical outcome after stroke due to large vessel occlusion (LVO) after mechanical thrombectomy (EVT) in a large population of real-world patients.
Methods
From the Italian Registry of Endovascular Thrombectomy, we extracted clinical and outcome data of patients treated for stroke due to large vessel occlusion. We compared clinical and safety outcomes in men and women who underwent EVT alone or in combination with intravenous thrombolysis (IVT) in the total population and in a Propensity Score matched set.
Results
Among 3422 patients included in the study, 1801 (52.6%) were women. Despite older age at onset (mean 72.4 vs 68.7; p < 0.001), and higher rate of atrial fibrillation (41.7% vs 28.6%; p < 0.001), women had higher probability of 3-month functional independence (adjusted odds ratio-adjOR 1.19; 95% CI 1.02–1.38), of complete recanalization (adjOR 1.25; 95% CI 1.09–1.44) and lower probability of death (adjOR 0.75; 95% CI 0.62–0.90). After propensity-score matching, a well-balanced cohort comprising 1150 men and 1150 women was analyzed, confirming the same results regarding functional outcome (3-month functional independence: OR 1.25; 95% CI 1.04–1.51), and complete recanalization (OR 1.29; 95% CI 1.09–1.53).
Conclusions
Subject to the limitations of a non-randomized comparison, women with stroke due to LVO treated with mechanical thrombectomy had a better chance to achieve complete recanalization, and 3-month functional independence than men. The results could be driven by women who underwent combined treatment.
Keywords: Ischemic stroke, thrombectomy, sex
Graphical abstract.
Introduction
Several studies have reported remarkable sex differences in epidemiology, pathophysiology, treatments, and outcomes in stroke.1–7 While some analyses, based on pooled data from randomized clinical trials, showed no influence of sex on r-tPA effect, 7 other papers have suggested sex differences in recanalization and outcome after intravenous thrombolysis (IVT) with a greater treatment benefit in women 8 that was hypothesized to be due to a faster and more complete recanalization of arterial occlusive lesions vessels after IV. 9 No differences in short-term clinical and angiographic outcomes between men and women who received IA thrombolysis for acute ischemic stroke were observed in a relatively small single-center study, 10 while a sex by prourokinase treatment interaction was observed in the PROACT-2 trial, 11 with women showing a larger treatment effect compared with men. There are conflicting results also with regard to sex-specific outcome after thrombectomy.12–19 The aim of this study was to investigate whether there are sex differences in clinical outcome after acute ischemic stroke (AIS) due to large vessel occlusion (LVO) treated with mechanical thrombectomy in a large population of real-world patients.
Materials and methods
The source of data is the Italian Registry of Endovascular Treatments in Acute Stroke (IRETAS), a multicenter, prospective, observational internet-based registry which includes patients treated with endovascular thrombectomy (EVT) since 2011.20–22 The purposes, organization, and structure of the Registry were described in more details elsewhere. 20 We analyzed data of patients with LVO of the anterior circulation (intracranial carotid artery – ICA-M1 segment of the middle cerebral artery – MCA) documented on CT angiography (CTA), baseline CT Alberta Stroke Program Early CT Score (ASPECTS) score ⩾6, and pre-stroke mRS⩽2, treated with endovascular therapy. For each patient, demographics, stroke risk factors, pre-stroke mRS, stroke severity (NIHSS at admission), time metrics, baseline neuroimaging and data of endovascular treatment were collected. Clinical follow-up was assessed by mRS at 3 months. The primary outcome measure was the score on mRS at 90 days, as assessed by a local trained neurologist through in-person visit, or through a phone standardized interview when a face to face assessment was not possible. We examined the following dichotomizations of the mRS: 0–1 versus 2–6, 0–2 versus 3–6, and 0–3 versus 4–6. For efficacy measures, arterial recanalization was rated according to the thrombolysis in cerebral infarction (TICI) score. 23 Successful recanalization was defined as TICI score 2b or 3, while TICI 3 was classified as complete recanalization. Symptomatic intracranial hemorrhage (sICH) was defined as any intracranial hemorrhage associated with 4 points increase at 24 h NIHSS, according to ECASS II definition, 24 and death rate were considered as safety measures.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Statistical analysis
Data were presented as absolute numbers, percentages, mean± Standard Deviation (SD) if normally distributed or median and interquartile ranges (IQR) as appropriate on the basis of data distribution. Comparison between the two genders was performed using a two-tailed, independent samples student t-test or Mann Whitney U test as appropriate according to the data distribution for continuous variables. Dichotomous variables were compared using the chi squared test. A multivariable logistic regression analysis, to adjust for age, history of hypertension, diabetes, dyslipidemia, atrial fibrillation (known before or detected after stroke, during the hospital stay/3-month follow-up), smoke (current or former), NIHSS and ASPECTS score at entry, stroke etiology according to the TOAST definition, 25 site of occlusion, type of treatment, onset to groin puncture and onset to final recanalization time, was also run to compare outcome and safety measures. The functional outcome was further evaluated with ordinal logistic regression, taking the whole range of mRS into account as dependent variable, adjusted for the above variables. The adjusted common odds ratio and corresponding 95% confidence interval (95% CI) for a shift on the modified Rankin scale, was therefore calculated. To reduce the unbalance and heterogeneity potentially arising from the differences in clinico-demographic characteristics between males and females, estimated propensity scores were used to match patients according to sex, and a logistic regression model was built adjusting for all baseline potential confounders (see above). Propensity scores underwent 1:1 nearest neighbor matching of the logit of the propensity score with a caliper width of 0.01. Matching was performed without replacement and unpaired subjects not meeting matching criteria were excluded. Since previous studies found a greater benefit in women treated with IVT, 8 we planned an exploratory subgroup analysis to assess a potential heterogeneity of sex-related outcome according to treatment (combined treatment – IVT and EVT – vs primary EVT). Hence, the odds to achieve complete revascularization after treatment (TICI 3), 3-month functional independence (mRS 0–2), and the probability of death, were evaluated in the subgroups of patients who underwent either IVT before EVT or EVT alone. p-values for sex/treatment interaction were also provided. Statistical significance was defined as a two-sided p-value < 0.05 for all analyses (carried out using SPSS Statistics Software Version 18 and Stata version 13.1).
Results
A total of 3422 patients were included in this study (1801 women and 1621 men). Table 1 shows the demographic and baseline clinical characteristics of the total patient population, and of the PS-matched pairs.
Table 1.
Clinico-demographic and baseline characteristic by sex in the total population and in the PS-matched pairs.
| Variable | Total population | PS-matched pairs | ||||
|---|---|---|---|---|---|---|
| Women (1801) | Men (1621) | p | Women (1150) | Men (1150) | p | |
| Age (mean ± SD) | 72.4 ± 13.6 | 68.7 ± 13.3 | <0.001 | 70.2 (12.4) | 70.6 (14.3) | |
| History (n/N; %) | ||||||
| Hypertension | 1170/1772; 66.0 | 929/1586; 58.6 | <0.001 | 71,161.8 | 717, 62.3 | 0.8 |
| Diabetes | 243/1772; 13.7 | 276/1586;17.4 | <0.01 | 18,215.8 | 168, 14.6 | 0.4 |
| Atrial fibrillation | 739/1772; 41.7 | 454/1586; 28.6 | <0.001 | 38,133.1 | 391, 34 | 0.7 |
| Dyslipidemia | 384/1772; 21.7 | 402/1586; 25.3 | <0.05 | 270, 23.5 | 258, 22.4 | 0.6 |
| Smoke (current/former) | 177/1771; 10.0 | 395/1586; 24.9 | <0.001 | 145, 12.6 | 151, 13.1 | 0.7 |
| Pre-stroke mRS 0–1 | 1642/1801; 91.2 | 1485/1621; 91.6 | n.s. | 1049, 91.3 | 1052, 91.5 | |
| Baseline data | ||||||
| Median NIHSS (IQR) | 17 (13–21) | 17 (13–21) | n.s. | 17 (13–21) | 17 (13–21) | 1 |
| ASPECTS 10 (n, %) | 1048, 58.2 | 981, 60.5 | n.s. | 675, 58.7 | 688, 59.8 | 0.58 |
| Internal carotid artery occlusion (n, %) | 559, 31.0 | 641, 39.5 | <0.001 | 403, 35 | 403, 35 | 1 |
| M1 occlusion 2222 (n, %) | 1242, 69.0 | 980, 60.5 | <0.001 | 747, 65 | 747, 65 | 1 |
| Previous IVT (n, %) | 921, 51.5 | 807, 49.8 | n.s. | 588, 51.1 | 591, 51.4 | 0.9 |
| Median (IQR) onset to groin puncture | 235 (180–209.5) | 239 (180–305) | n.s. | 235 (185–305) | 230 (179–290.25) | 0.6 |
| Median (IQR) onset to revascularization time | 312 (243.5–390) | 316 (250–390) | n.s. | 312 (250–383) | 310 (248–375) | 0.3 |
mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; IVT: intravenous thrombolysis.
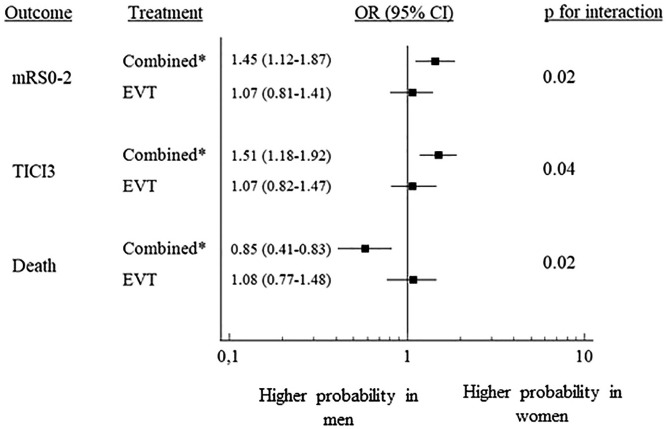
In the original cohort, women were significantly older than men (mean ± SD age 72.4 ± 13.6, vs 68.7 ± 13.3; p < 0.001). Atrial fibrillation was significantly more frequent in women (41.7% vs 28.6%; p < 0.001), as was history of hypertension (66 vs 58.6; p < 0.01), while diabetes, and dyslipidemia, were more common in male sex (p < 0.01, 0.05, and 0.001 respectively). Moreover, men were more likely to be smokers (p < 0.001). Pre-existing disability was comparable between the two genders. Time metrics were similar for men and women, in terms onset to groin puncture time and onset to revascularization/end of the procedure time. Median NIHSS at admission was similar. M1 occlusion was more frequent in women (p < 0.001), while intracranial carotid artery occlusion in men (p < 0.001). The proportion of patients who underwent combined treatment (intravenous thrombolysis followed by thrombectomy) was similar between the two genders. In the PS-matched groups (1150 men and 1150 women), the baseline characteristics were well balanced (Table 1) without significant difference in any variable. Table 2 shows the results of sex comparisons on outcome. In the total original cohort, after adjusting for all covariates, functional independence at 3 months was more likely in women than in men (OR 1.19; 95% CI 1.02–1.38), as were rates of successful or complete recanalization (TICI 2b3: OR 1.18; 95% CI 1.03–1.38 and TICI 3: 1.25; 95% CI 1.09–1.44). The probability of sICH was similar by sex. Three-month death was significantly less frequent in women (OR 0.75; 95% CI 0.62–0.90). In the matched pairs, we confirmed a higher probability of favorable outcome (OR 1.25; 95% CI 1.04–1.51), and complete recanalization in women (TICI 3: 1.29; 95% CI 1.09–1.53). On the whole, the magnitude of the association was similar to that observed when considering the original cohort. In the pre-specified subgroup analysis (Figure 1), female sex was also associated with higher probability of favorable outcome (OR 1.45; 95% CI 1.12–1.87), complete recanalization (OR 1.51; 95% CI 1.18–1.92), and lower probability of death (OR 0.58; 95% CI 0.41–0.83) in patients who underwent combined treatment, with a significant sex/treatment interaction (Figure 1)
Table 2.
Sex comparisons on outcome.
| Total population | PS-matched pairs | |
|---|---|---|
| adjOR* (95% CI) | OR (95% CI) | |
| mRS 0–1 | 1.17 (0.99–1.37) | 1.19 (0.99–1.45) p = 0.07 |
| mRS 0–2 | 1.19 (1.02–1.38) § | 1.25 (1.04-1.51) § |
| mRS 0–3 | 1.13 (0.97–1.31) | 1.09 (0.92–1.32) |
| Death | 0.75 (0.62–0.90) §§ | 0.81 (0.63–1.02) p = 0.055 |
| sICH | 0.83 (0.65–1.06) | 0.82 (0.59–1.12) |
| TICI 2b–3 | 1.18 (1.03–1.38) § | 1.19 (0.92–1.36) |
| TICI 3 | 1.25 (1.09–1.44) § | 1.29 ( 1.09-1.53) §§ |
| Shift analysis | 0.94 (0.82–1.06) | 0.92 (0.79–1.06) |
Source: Male sex was the reference group for OR calculation.
mRS, modified Rankin Scale; sICH, symptomatic intracranial hemorrhage; TICI, thrombolysis in cerebral infarction score.
Adjusted for age, history of hypertension, diabetes, dyslipidemia, atrial fibrillation, smoke (current or former), NIHSS and ASPECTS score at entry, stroke etiology, site of occlusion, type of treatment, onset to groin puncture, and onset to final recanalization. § p < 0.05; §§ p < 0.01
Figure 1.
The forest plot shows gender comparisons in outcome of PS-matched patients treated either with EVT alone or combined treatment (IVT and EVT). The squares with horizontal lines are adjusted odds ratios (OR) and corresponding 95% confidence interval (95% CI). The right column shows p values for interaction.
Discussion
This large, multicenter, prospective, observational real-world study showed that women with AIS from LVO have a better chance of complete recanalization after EVT and better 3-month functional outcome. Whether the gender intrinsically confers different prognosis in stroke patients after thrombectomy remains a matter of debate. A post hoc analysis of the MR CLEAN trial suggested that the treatment effect was greater in men, while women experienced higher 90-day mortality and more serious adverse events after EVT. 13 The pre-specified subgroup analysis by sex of the ESCAPE 26 and SWIFT PRIME 27 trials showed no heterogeneity of effect. The meta-analysis of individual patient data of the HERMES collaboration showed no differences in the adjusted treatment effect of EVT between men and women. 14 A further meta-analysis of the same group, including two additional trials, reported that sex did not influence clinical outcome after EVT in the intervention group and did not modify treatment effect of EVT. 28 More recently, a pooled analysis of the SWIFT, STAR, and SWIFT PRIME trials showed that men and women had comparable functional outcome after EVT, but women had more years of optimal life (DALYs) despite older age an higher rate of atrial fibrillation. 17 These studies relied on data from randomized clinical trials with well-defined selection criteria, which limits the generalizability of the results to the routine clinical practice. Moreover, most of them were underpowered to test for treatment-gender interaction. 7
Few studies on sex differences on AIS patient receiving mechanical thrombectomy were carried out in a real-world setting.15,16,18,19 Carvalho et al. 15 analyzed 145 patients, and reported no gender differences in clinical and safety outcome. On the contrary, Madsen et al. 16 in a cohort of 279 patients reported that females were less likely to achieve functional independence at 90 days, even if both sexes had the same likelihood of being independent at discharge, indicating that gender disparities in outcome were not explained by different response to treatment /recanalization, but rather to other unknown variables that affect recovery after discharge. In prospective multicenter study including 492 patients, Pérez-Sánchez et al. 18 reported a significantly worse outcome in women; however, after adjustment for age, the difference lost statistical significance except for patients admitted to a hospital without a stroke unit. Finally, sex was not an independent predictor of outcome after adjustment for relevant confounders (namely age and pre-stroke functional status) also in the study by Deb-Chatterji et al. 19 on 316 patients included the German Stroke Registry - Endovascular Treatment. More recently, a multicenter study, including only patients treated with EVT in the late windows (beyond 6 h) showed that sex was not associated with functional outcome, but influenced the association between age and safety outcomes, with men experiencing worse outcomes with advancing age. 29
On the whole, available studies reported either no differences in outcome, or a better prognosis in men. The latter however, appears to result from differences in baseline confounding factors,18,19 confirming a crucial role of age, baseline characteristics, and comorbidities in determining the outcome, rather than intrinsic sex-related differences.
We attempted to reduce as much as possible the effects of confounding, and to balance baseline covariates, by applying propensity score matching. The results, substantially consistent with those obtained from the whole cohort, seem to suggest that women with AIS from LVO treated with EVT fare better than men, On the other hand, in some similar analyses on patients who underwent intravenous thrombolysis, women turned out to have a larger margin of benefit than men,8,30 implying that, while untreated women have a poorer outcome compared to men, this negative prognostic sex effect is neutralized by thrombolysis. Along the same line, a large European observational study, analyzing data from the Safe Implementation of Treatments in Stroke-International Stroke Thrombolysis Register, showed comparable functional outcome in the two gender, but a higher mortality in men, although women were older, had a higher prevalence of hypertension and AF, as well as higher median NIHSS at stroke onset. 31
In our prespecified subgroup analysis, we found a significant sex difference in favor of women in patients receiving intravenous rtPA before thrombectomy, but not in patients who underwent primary endovascular treatment, with a significant sex-treatment interaction, suggesting that rtPA pre-treatment rather than EVT per se could exert a greater effect in female gender, in agreement with the above reported studies.8,9,28,29 Several hypotheses have been proposed to explain the observed gender difference in response to tPA, pointing to either a different likelihood of reperfusion or to the response of the brain to ischemia and reperfusion.8,32,33
This study has some limitations, inherent to its observational nature and to the lack a control group of untreated patients; hence, we may only compare functional outcome and differences in risk of mortality and sICH between men and women, but not estimate sex-specific effect of treatment. Moreover, although the propensity score matching allows a selection of comparable patients, there may have been unmeasured confounding variables, and hidden bias due to uncontrolled variables may have remained after matching . In particular, a certain proportion of patients with cryptogenic stroke - about a quarter of our sample- could have been diagnosed as suffering from atrial fibrillation/cardioembolic stroke after the discharge ad 3-month follow-up period thanks to a long-term monitoring. Moreover, although, median age, prestroke functional independence, relevant comorbidities, and stroke characteristics, were well balanced between the two sexes, a more comprehensive multidimensional measure of frailty, recently pointed out as an independent predictor of worse outcome after acute stroke treated with reperfusion therapies, 34 could not be accounted for.
The strength of this study is the large real-world population included, allowing for highly powerful multivariable analyses and generalizability of the results. We used propensity matching for available demographic, clinical, and procedural data to create groups of women and men with balanced baseline characteristics. The results in direction and magnitude of the association are consistent in the whole cohort and in the propensity-score matched sample.
Even if residual confounding factors cannot be ruled out, the difference in the outcomes we detected could be regarded at least in part as explained by biological sex-related differences. Although subgroup analyses should be considered with caution and regarded only as hypothesis generating, the data from our study seem to indicate that a different sex-related response to thrombolytic agents could account at least in part for sex differences in outcome.
Conclusions
This large, real-practice study, suggests that women with AIS from LVO who underwent EVT had a better chance to achieve complete recanalization and a favorable outcome than men. The differential outcome could be related to the association between thrombolysis and thrombectomy rather than endovascular treatment alone. The latter hypothesis should be considered speculative until confirmed by further focused research.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MB: Consultant for Penumbra Inc., Stryker Italia; AS: Consultant for Stryker; ADV: Consultant for Boehringer Ingelheim, Daichi Sankyo; AZ: speaker fees and consulting fees from Boehringer-Ingelheim, Medtronic, Cerenovus and advisory board from Daiichi Sankyo and Boehringer-Ingelheim and Stryker; MC: speaker fees and consulting fees from Daiichi Sankyo and Bristol Myers Squibb, advisory board from Boehringer-Ingelheim; NPN: Consultant for Penumbra Inc., Acandis GmbH; SS: personal fees and non-financial support from Allergan, Abbott, Eli Lilly, Novartis, TEVA, participation to Advisory Board for Astra Zeneca, and research support from Laborest; AM: Consultant for Boehringer Ingelheim, DR: Proctor for Penumbra. Other Authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project “Registro Nazionale Trattamento Ictus Acuto” (RFPS-2006-1-336562) was funded by grants from the Italian Ministry of Health within the framework of 2006 Finalized Research Programmes (D.Lgs.n.502/1992).
Ethical approval and informed consent: The study (ID 140289) was approved by the ethics Committee of Ferrara, Italy, and informed consent was signed by patients or their next of kin.
Guarantor: Ilaria Casetta
Contributorship: IC, EF, GP, VS, DI, SM, DT conceived the study; IC and EF wrote the first draft of the manuscript; IC and MEF performed the statistical analysis; VS and GP coordinated patient recruitment and data collection; All authors contributed to the data collection and analysis and to the to interpretation of the results. All authors reviewed and edited the manuscript and approved its final version.
ORCID iDs: Ilaria Casetta  https://orcid.org/0000-0003-4099-8875
https://orcid.org/0000-0003-4099-8875
Enrico Fainardi  https://orcid.org/0000-0003-0477-724X
https://orcid.org/0000-0003-0477-724X
Valentina Saia  https://orcid.org/0000-0001-9855-8894
https://orcid.org/0000-0001-9855-8894
Rossana Tassi  https://orcid.org/0000-0002-5906-8718
https://orcid.org/0000-0002-5906-8718
Mauro Bergui  https://orcid.org/0000-0002-5336-695X
https://orcid.org/0000-0002-5336-695X
Alessandro De Vito  https://orcid.org/0000-0002-7572-819X
https://orcid.org/0000-0002-7572-819X
Andrea Zini  https://orcid.org/0000-0003-1486-4507
https://orcid.org/0000-0003-1486-4507
Maria Ruggiero  https://orcid.org/0000-0002-3612-4289
https://orcid.org/0000-0002-3612-4289
Laura Malfatto  https://orcid.org/0000-0003-2552-2385
https://orcid.org/0000-0003-2552-2385
Alessio Comai  https://orcid.org/0000-0002-0566-395X
https://orcid.org/0000-0002-0566-395X
Emilio Lopuzone  https://orcid.org/0000-0003-3992-9071
https://orcid.org/0000-0003-3992-9071
Edoardo Puglielli  https://orcid.org/0000-0002-3082-249X
https://orcid.org/0000-0002-3082-249X
Giuseppe Ricciardi  https://orcid.org/0000-0001-8536-5794
https://orcid.org/0000-0001-8536-5794
Manuel Cappellari  https://orcid.org/0000-0002-3534-3201
https://orcid.org/0000-0002-3534-3201
Luigi Chiumarulo  https://orcid.org/0000-0001-5790-2353
https://orcid.org/0000-0001-5790-2353
Anna Cavallini  https://orcid.org/0000-0002-5227-1502
https://orcid.org/0000-0002-5227-1502
Nicola Cavasin  https://orcid.org/0000-0001-6246-3543
https://orcid.org/0000-0001-6246-3543
Andrea Giorgianni  https://orcid.org/0000-0002-4035-3172
https://orcid.org/0000-0002-4035-3172
Maurizio Versino  https://orcid.org/0000-0003-1813-9492
https://orcid.org/0000-0003-1813-9492
Francesco Biraschi  https://orcid.org/0000-0001-7660-2401
https://orcid.org/0000-0001-7660-2401
Ettore Nicolini  https://orcid.org/0000-0002-8481-6327
https://orcid.org/0000-0002-8481-6327
Nunzio Paolo Nuzzi  https://orcid.org/0000-0003-2615-8554
https://orcid.org/0000-0003-2615-8554
Simona Sacco  https://orcid.org/0000-0003-0651-1939
https://orcid.org/0000-0003-0651-1939
Marina Mannino  https://orcid.org/0000-0001-7683-7235
https://orcid.org/0000-0001-7683-7235
Salvatore Mangiafico  https://orcid.org/0000-0002-5844-3117
https://orcid.org/0000-0002-5844-3117
References
- 1. Bousser MG. Stroke in women: the 1997 Paul Dudley White International Lecture. Circulation 1999; 99: 463–467. [DOI] [PubMed] [Google Scholar]
- 2. Di Carlo A, Lamassa M, Baldereschi M, et al. ; European BIOMED Study of Stroke Care Group. Sex differences in the clinical presentation, resource use, and 3-Month outcome of acute stroke in Europe. Stroke 2003; 34: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 3. Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008; 7: 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke 2009; 40: 1082–1090. [DOI] [PubMed] [Google Scholar]
- 5. Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr Cardiol Rep 2010; 12: 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haast RA, Gustafson DR, Kiliaan AJ. Sex differences in stroke. J Cereb Blood Flow Metab 2012; 32: 2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bushnell C, Howard VJ, Lisabeth L, et al. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol 2018; 17: 641–650. [DOI] [PubMed] [Google Scholar]
- 8. Kent DM, Price LL, Ringleb P, et al. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: a pooled analysis of randomized clinical trials. Stroke 2005; 36: 62–65. [DOI] [PubMed] [Google Scholar]
- 9. Savitz SI, Schlaug G, Caplan L, et al. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke 2005; 36: 1447–1451. [DOI] [PubMed] [Google Scholar]
- 10. Shah SH, Liebeskind DS, Saver JL, et al. Influence of gender on outcomes after intra-arterial thrombolysis for acute ischemic stroke. Neurology 2006; 66: 1745–1746. [DOI] [PubMed] [Google Scholar]
- 11. Hill MD, Kent DM, Hinchey J, et al. Sex-based differences in the effect of intra-arterial treatment of stroke: analysis of the PROACT-2 study. Stroke 2006; 37: 2322–2325. [DOI] [PubMed] [Google Scholar]
- 12. Arnold M, Kappeler L, Nedeltchev K, et al. Recanalization and outcome after intra-arterial thrombolysis in middle cerebral artery and internal carotid artery occlusion: does sex matter? Stroke 2007; 38: 1281–1285. [DOI] [PubMed] [Google Scholar]
- 13. de Ridder IR, Fransen PS, Beumer D, et al. Is intra-arterial treatment for acute ischemic stroke less effective in women than in men? Interv Neurol 2016; 5: 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 15. Carvalho A, Cunha A, Gregório T, et al. Is the efficacy of endovascular treatment for acute ischemic stroke sex-related. Interv Neurol 2018; 7: 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madsen TE, DeCroce-Movson E, Hemendinger M, et al. Sex differences in 90-day outcomes after mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg 2019; 11: 221–225. [DOI] [PubMed] [Google Scholar]
- 17. Sheth SA, Lee S, Warach SJ, et al. Sex differences in outcome after endovascular stroke therapy for acute ischemic stroke. Stroke 2019; 50: 2420–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pérez-Sánchez S, Barragán-Prieto A, Ortega-Quintanilla J, et al. Sex differences by hospital-level in performance and outcomes of endovascular treatment for acute ischemic stroke. J Stroke 2020; 22: 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deb-Chatterji M, Schlemm E, Flottmann F, et al. Sex differences in outcome after thrombectomy for acute ischemic stroke are explained by confounding factors. Clin Neuroradiol 2021; 31: 1101–1109. DOI: 10.1007/s00062-020-00983-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mangiafico S, Pracucci G, Saia V, et al. The Italian registry of endovascular treatment in acute stroke: rationale, design and baseline features of patients. Neurol Sci 2015; 36: 985–993. [DOI] [PubMed] [Google Scholar]
- 21. Casetta I, Pracucci G, Saletti A, et al. ; The Italian Registry of Endovascular Treatment in Acute Stroke. Combined intravenous and endovascular treatment versus primary mechanical thrombectomy. The Italian registry of endovascular treatment in acute stroke. Int J Stroke 2019; 14: 898–907. [DOI] [PubMed] [Google Scholar]
- 22. Casetta I, Fainardi E, Saia V, et al. Italian Registry of Endovascular Treatment in Acute Stroke. Endovascular thrombectomy for acute ischemic stroke beyond 6 hours from onset: a real-world experience. Stroke 2020; 51: 2051-2057. [DOI] [PubMed] [Google Scholar]
- 23. Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 24. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 25. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 26. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 27. Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 28. Chalos V, de Ridder IR, Lingsma HF, et al. HERMES collaborators. Does sex modify the effect of endovascular treatment for ischemic stroke? Stroke 2019; 50: 2413–2419. DOI: 10.1161/STROKEAHA.118.023743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bala F, Casetta I, Nannoni S, et al. Sex-related differences in outcomes after endovascular treatment of patients with late-window stroke. Stroke 2022; 53: 311–318. DOI: 10.1161/STROKEAHA.121.037127 [DOI] [PubMed] [Google Scholar]
- 30. Shobha N, Sylaja PN, Kapral MK, et al. ; Investigators of the Registry of the Canadian Stroke Network. Differences in stroke outcome based on sex. Neurology 2010; 74: 767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lorenzano S, Ahmed N, Falcou A, et al. SITS Investigators. Does sex influence the response to intravenous thrombolysis in ischemic stroke?: answers from safe implementation of treatments in Stroke-International Stroke Thrombolysis Register. Stroke 2013; 44: 3401–3406. [DOI] [PubMed] [Google Scholar]
- 32. Kain K, Carter AM, Bamford JM, et al. Gender differences in coagulation and fibrinolysis in white subjects with acute ischemic stroke. J Thromb Haemost 2003; 1: 390–392. [DOI] [PubMed] [Google Scholar]
- 33. Dula AN, Mlynash M, Zuck ND, et al. ; DEFUSE 3 Investigators. Neuroimaging in ischemic stroke is different between men and women in the DEFUSE 3 Cohort. Stroke 2020; 51: 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pilotto A, Brass C, Fassbender K, et al. Premorbid frailty predicts short- and long-term outcomes of reperfusion treatment in acute stroke. J Neurol 2022. DOI: 10.1007/s00415-022-10966-7 [DOI] [PubMed] [Google Scholar]