Abstract
Introduction:
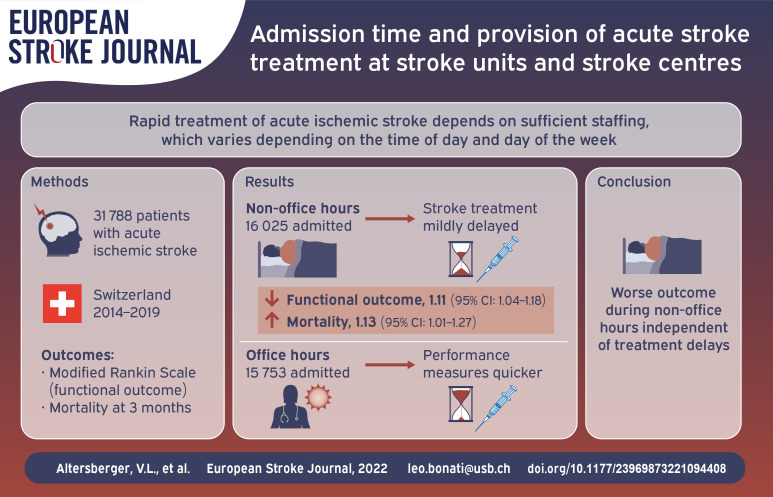
Rapid treatment of acute ischemic stroke (AIS) depends on sufficient staffing which differs between Stroke Centers and Stroke Units in Switzerland. We studied the effect of admission time on performance measures of AIS treatment and related temporal trends over time.
Patients and methods:
We compared treatment rates, door-to-image-time, door-to-needle-time, and door-to-groin-puncture-time in stroke patients admitted during office hours (Monday–Friday 8:00–17:59) and non-office hours at all certified Stroke Centers and Stroke Units in Switzerland, as well as secular trends thereof between 2014 and 2019, using data from the Swiss Stroke Registry. Secondary outcomes were modified Rankin Scale and mortality at 3 months.
Results:
Data were eligible for analysis in 31,788 (90.2%) of 35,261 patients. Treatment rates for IVT/EVT were higher during non-office hours compared with office hours in Stroke Centers (40.8 vs 36.5%) and Stroke Units (21.8 vs 18.5%). Door-to-image-time and door-to-needle-time increased significantly during non-office hours. Median (IQR) door-to-groin-puncture-time at Stroke Centers was longer during non-office hours compared to office hours (84 (59–116) vs 95 (66–130) minutes). Admission during non-office hours was independently associated with worse functional outcome (1.11 [95%CI: 1.04–1.18]) and increased mortality (1.13 [95%CI: 1.01–1.27]). From 2014 to 2019, median door-to-groin-puncture-time improved and the treatment rate for wake-up strokes increased.
Discussion and Conclusion:
Despite differences in staffing, patient admission during non-office hours delayed IVT to a similar, modest degree at Stroke Centers and Stroke Units. A larger delay of EVT was observed during non-office hours, but Stroke Centers sped up delivery of EVT over time. Patients admitted during non-office hours had worse functional outcomes, which was not explained by treatment delays.
Keywords: Stroke, admission time, service provision, outcome, quality of care
Graphical abstract.
Introduction
Intravenous thrombolysis (IVT) and endovascular recanalization therapy (EVT) reduce disability in patients with acute ischemic stroke (AIS).1,2 Rapid delivery of treatment is crucial and depends – among other factors – on the presence of experienced staff and access to infrastructure. In addition, acute stroke care has become more and more complex due to recent extension of time windows and imaging eligibility criteria in both IVT- and EVT-treated patients.3,4 Furthermore, according to most stroke guidelines patients presenting with so called wake-up strokes can also benefit from acute reperfusion therapies depending on certain imaging features.5–7 These aspects represent a considerable challenge for staff involved in the acute treatment of AIS.
However, staff levels and availability of infrastructure may vary depending on time of the day and day of the week, and differ between Stroke Centers and Stroke Units. In Switzerland, certification guidelines require a 24/7 attendance of a stroke neurologist at Stroke Centers whereas an on-call service is permitted at night and on weekends at Stroke Units following the guidelines of the European Stroke Organization. 8 While both, Stroke Centers and Stroke Units, deliver IVT and provide continuous physiological monitoring, EVT is exclusively performed in Stroke Centers in Switzerland. Understanding the effect of day and time of admission on delivery and functional outcome of acute stroke care is relevant for service providers and health policy makers.
Previous research on the effect of admission during “office-hours” versus “non-office hours” on the speed of delivery and outcomes of IVT was done in heterogeneous settings and yielded controversial results.9–13 Importantly, most of the previous research investigated patient cohorts when neither EVT per se, nor reperfusion treatment for AIS in the extended time window were widely implemented in everyday practice. The increasing proportion of stroke patients receiving EVT poses greater demands on staff and infrastructure.
In order to consolidate and extend the evidence on diurnal and weekday variations of acute stroke treatment and to examine possible changes following recent modifications in therapeutic concepts, we conducted the present study using prospectively collected data from the Swiss Stroke Registry (SSR) between 01/2014 and 12/2019.
Methods
All data and materials can be accessed by request from the corresponding author (leo.bonati@usb.ch).
Study design
For this cohort study, we used prospectively collected data from the Swiss Stroke Registry (SSR). The SSR is a national web-based registry designed to facilitate multi-centric research in acute stroke and assure the provision and quality of acute stroke care in Switzerland, which started in January 2014. 14 The registry collects a standardized dataset of all patients with acute stroke, TIA and other acute cerebrovascular events including a follow-up assessment after 3 months. The registry is compulsory for all hospitals certified as Stroke Units or Stroke Centers in Switzerland, in line with the European Stroke Organization criteria. 15 The database is managed by the Clinical Trial Unit (CTU) of the University of Basel. Data collection is done locally in each Stroke Center/each Stroke Unit. All patients with ischemic stroke admitted between 01.01.2014 and 31.12.2019 were included.
Parameters of interest for the present study were age, sex, National Institutes of Health Stroke Scale (NIHSS) score, 16 date and time of stroke onset (or last seen well), of hospital admission, of first image and of treatment initiation (IVT and/or EVT), presence of wake up stroke, blood pressure prior to IVT treatment, glucose levels in blood serum on admission, vascular risk factors according to predefined criteria 17 and prior treatment with anticoagulation as well as pre-stroke functional status measured by the modified Rankin Scale (mRS). 18 Wake-up stroke is defined as a stroke with symptoms that were present when the patient awoke but not prior to falling asleep. Clinical data, neurologic and functional outcomes during hospitalization and at 3 months after stroke were also collected. Clinical evaluations, as well as NIHSS and mRS assessments, were performed by certified stroke neurologists as part of their clinical activity. If an in-person visit was not possible at 3 months, mRS score was assessed by a phone interview with mRS-trained examiners.
Outcomes
Primary outcomes were the rate of patients with acute reperfusion therapy (i.e., the proportion of patients with AIS receiving IVT and/or EVT) and in-hospital performance measures in patients receiving IVT and/or EVT), defined as the following time intervals: (i) from hospital admission to brain imaging in IVT/EVT (“door-to-image-time” (DIT)) (ii) from hospital admission to start of IVT (“door-to-needle time” (DNT)) (iii) from hospital admission to start of EVT (“door-to-groin-puncture time” (DPT)). As secondary outcomes, we investigated functional status defined by the mRS, as well as mortality at 3 months.
Statistical analyzes
Statistical analyzes were performed with R version 3.6.3 (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/). The database is implemented in the commercial software secuTrial (interActive Systems GmbH, Germany) and is managed by the Clinical Trial Unit (CTU) of the University of Basel aided by the secuTrialR package. 19
We investigated differences in primary and secondary outcomes between patients admitted during “office hours” (OH) (Monday–Friday 8:00–17:59) and patients admitted during “non-office hours” (NH) (Monday–Friday 18:00–07:59, Saturday, Sunday, national holidays), at Stroke Centers and Stroke Units separately. Continuous data were summarized as median and interquartile range (IQR). We compared the rates of acute reperfusion therapy using Chi 2 -test and the performance measures using Wilcoxon test. Performance measures during OH and NH were additionally compared using a linear mixed model where the respective center was included as random effect. Performance measures were log-transformed to better meet the normality assumption.
In a subgroup, the association between admission time and functional outcome as well as mortality was estimated by calculating odds ratios (OR) with 95% confidence intervals (95% CI), using ordered logistic regression models and binary logistic regression, respectively. Analyses were done both unadjusted and adjusted for baseline NIHSS, age, pre-stroke mRS, and stroke-onset-to-treatment time (for patients receiving acute reperfusion therapy). Patients with missing data on the mRS at 3 months were excluded from this subanalysis. Furthermore, we evaluated the change over time from 2014 until 2019 regarding in-hospital performance measures (DIT, DNT, DPT) to investigate any learning curve effects in Stroke Units and Stroke Centers separately in descriptive analyzes. Performance measures displayed as median and IQR were analyzed each year beginning in 01/2014. Patients referred to hospital with symptoms of wake-up stroke as well as patients with in-hospital strokes were excluded from these analyses.
As an exploratory analysis, we also investigated the rate of patients treated with IVT or EVT for wake-up strokes for each year.
Role of the funding source/ethics
No sponsor was involved in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
The study complied with the Declaration of Helsinki. The study was classified as a Quality Assurance Study by the responsible ethics committee and the necessity for formal review was waived. In accordance with national law, patients were informed about the use of their routinely collected data for research purposes. Patients who denied use of their data were excluded from the analysis. Anonymized data will be shared on request from any qualified investigator. The analysis code is available on GitHub: https://github.com/PatrickRWright/Publications_code
Results
Data were eligible for analysis in 31,788 (90.2%) of 35,261 AIS patients. Reasons for excluding patients were missing data on DIT, DNT or DPT (n = 3473; 9.8%).
Baseline characteristics
In Stroke Centers, 11,844 patients (48.7%) were admitted during office-hours (OH) and 12,471 (51.3%) during non-office hours (NH). In Stroke Units, 3919 (52.4%) arrived during OH and 3554 (47.6%) during NH. Overall, there was no substantial difference in characteristics of AIS patients arriving during OH and those arriving during NH: age, sex, stroke severity, pre-stroke disability as well as the prevalence of cardiovascular risk factors were similar between groups. Stroke-onset-to-admission time was higher during OH in Stroke Centers and Stroke Units (Table 1). Among patients receiving acute reperfusion therapy, baseline characteristics were evenly distributed (Supplemental Table I).
Table 1.
Baseline characteristics of all ischemic stroke patients.
| Stroke center | Stroke unit | |||
|---|---|---|---|---|
| Office hours | Non-office hours | Office hours | Non-office hours | |
| Patients, n (%) | 11,844 (48.7) | 12,471 (51.3) | 3919 (52.4) | 3554 (47.6) |
| Demographics | ||||
| Age, years, median [IQR] | 75 [65−83] | 75 [63−83] | 76 [66−84] | 75 [63−83] |
| Male sex, n (%) | 6739 (57.0) | 7145 (57.4) | 2170 (55.5) | 2036 (57.3) |
| Independent prior to stroke (pre-mRS 0–2), median[IQR] | 9091 (88.1) | 9745 (88.9) | 2909 (89.3) | 2657 (90.0) |
| Stroke characteristics | ||||
| NIHSS, median [IQR] | 4 [1−9] | 4 [2−11] | 3 [1−6] | 3 [1−7] |
| NIHSS, patients with acute reperfusion therapy, median [IQR] | 9 [4−16] | 9 [5−16] | 6 [3−11] | 6 [4−12] |
| Onset-to-admission time, min, median [IQR] | 276 [86−982] | 208 [81−645] | 510 [108−1330] | 238 [85−766] |
| Medical history | ||||
| Hypertension, n (%) | 8676 (74.7) | 8996 (73.5) | 2863 (76.9) | 2560 (75.9) |
| Diabetes mellitus, n (%) | 2347 (20.2) | 2495 (20.4) | 802 (21.5) | 752 (22.3) |
| Coronary artery disease, n (%) | 2040 (17.7) | 2217 (18.2) | 598 (16.8) | 610 (18.8) |
| Atrial fibrillation, n (%) | 3031 (24.8) | 2791 (24.0) | 816 (22.0) | 779 (23.2) |
| Prior stroke, n (%) | 2168 (18.7) | 2274 (18.6) | 657 (17.7) | 617 (18.3) |
| Systolic blood pressure, mmHg, median [IQR] | 153 [137−172] | 155 [138−175] | 160 [140−180] | 160 [141−180] |
| Glucose, mmol/l, median [IQR] | 6.3 [5.5−7.6] | 6.5 [5.7−7.9] | 6.3 [5.5−7.6] | 6.5 [5.7−8.0] |
| Medication | ||||
| Prior anticoagulation, n (%) | 1755 (21.7) | 1872 (22.0) | 486 (17.8) | 477 (19.5) |
Patients in Stroke Centers had higher baseline stroke severity, were more likely to suffer from atrial fibrillation and more frequently under anticoagulation at the time of their stroke than patients treated in Stroke Units (Table 1).
The baseline characteristics of the excluded AIS patients are presented in Supplemental Table II. 378 (10.9%) of the excluded patients received acute reperfusion therapy.
Primary outcomes: Rate of acute reperfusion therapy and in-hospital performance measures
Patients with AIS arriving during NH at Stroke Centers received acute reperfusion therapy with IVT or EVT more often than during OH (40.8% vs 36.5%, p < 0.001). Likewise, patients being admitted during NH to Stroke Units were more likely to be treated with IVT than during OH (NH 21.8% vs OH 18.5%, p < 0.001) (Table 2).
Table 2.
Thrombolysis rate and performance measures.
| Office hours | Non-office hours | Office vs non-office hours, p-value | ||
|---|---|---|---|---|
| Acute reperfusion therapy, n (%) † | Stroke Center | 4322 (36.5) | 5090 (40.8) | <0.001 |
| Stroke Unit | 724 (18.5) | 773 (21.8) | <0.001 | |
| Door-to-image time, min, median (IQR) ‡ | Stroke Center | 22 (16−30) | 23 (17−31) | <0.001 |
| Stroke Unit | 17 (11−25) | 19 (13−27) | <0.01 | |
| Door-to-IVT time, min, median (IQR) | Stroke Center | 37 (27−54) | 43 (30−61) | <0.001 |
| Stroke Unit | 39 (29−53) | 45 (32−65) | <0.001 | |
| Door-to-EVT time, min, median (IQR) | Stroke Center | 84 (59−116) | 95 (66−130) | <0.001 |
Including wake-up strokes.
In patients treated with acute reperfusion therapy.
Median DIT in patients treated with acute reperfusion therapy was faster during OH: Stroke Centers, DIT 23 vs 22 minutes, p < 0.001; Stroke Units, DIT 19 vs 17 minutes; p < 0.01. Fittingly, median DNT was significantly increased in patients arriving during NH compared to arriving during OH, both at Stroke Centres (43 vs 37 minutes, p < 0.001) and Stroke Units (45 vs 39 minutes, p < 0.001). Median DPT at Stroke Centres was longer in patients arriving during NH compared to OH (95 vs 84 minutes, p < 0.001) (Table 2).
The time differences between OH and NH for each primary outcome remained significant after calculating a linear mixed model with Stroke Center or Stroke Unit included as random effect (Supplemental Table III).
Secondary outcomes: Functional outcome and mortality
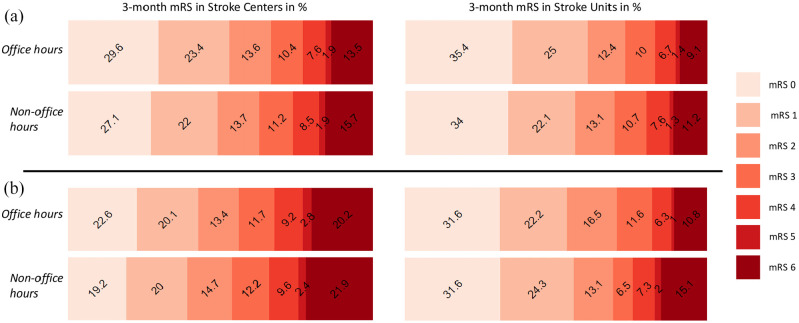
The mRS at 3 months was missing in 7489 AIS patients (23.6%, 5332 Stroke Center patients and 2157 Stroke Unit patients). After adjustment for age, baseline NIHSS, pre-stroke mRS and acute reperfusion treatment, AIS patients arriving during NH in Stroke Centers had 1.11 (95% CI 1.04–1.18) times the odds of having a worse functional outcome at 3 months and OR 1.13 (95% CI 1.01–1.27) times the odds for mortality at 3 months compared with arriving during OH. Admission during NH in Stroke Units also increased the odds for worse functional outcome (1.12 (95% CI 0.99–1.27)) and mortality (1.17 (95% CI 0.90–1.52)) at 3 months without reaching statistical significance.
Among patients receiving acute reperfusion therapy, admission during NH was again associated with worse outcome (1.18 (95% CI 1.07–1.31)) and mortality (1.18 (95% CI 1.01–1.38)) at 3 months in Stroke Centers, after adjustment for patient characteristics and onset-to-treatment time. At Stroke Units, arrival during NH increased the point estimate similarly for worse outcome (1.12 (95% CI 0.87–1.46)) and mortality (1.48 (95% CI 0.89–2.47)) without reaching statistical significance (Table 3, Figure 1).
Table 3.
Multivariable analysis of outcomes. Odds ratio (95% confidence interval), p-value.
| Worse outcome | Mortality | |||
|---|---|---|---|---|
| All ischemic stroke patients † | Non-office hours vs office hours | Stroke Center | 1.11 (1.04−1.18) † p = 0.002 | 1.13 (1.01−1.27) † p = 0.037 |
| Stroke Unit | 1.12 (0.99−1.27) † p = 0.061 | 1.17 (0.90−1.52) † p = 0.243 | ||
| Ischemic stroke patients with acute reperfusion therapy ‡ | Non-office hours vs office hours | Stroke Center | 1.18 (1.07−1.31) ‡ p < 0.001 | 1.18 (1.01−1.38) ‡ p = 0.034 |
| Stroke Unit | 1.12 (0.87−1.46) ‡ p = 0.374 | 1.48 (0.89−2.47) ‡ p = 0.131 |
Age, baseline NIHSS, pre-mRS, acute reperfusion treatment.
Age, baseline NIHSS, pre-mRS, onset-to-treatment time.
Figure 1.
Modified Rankin Scale (mRS) at 3 months. (a) mRS at 3 months in all ischemic stroke patients. (b) mRS at 3 months in ischemic stroke patients treated with acute reperfusion therapy.
Temporal trends in performance measures and treatment of wake-up strokes
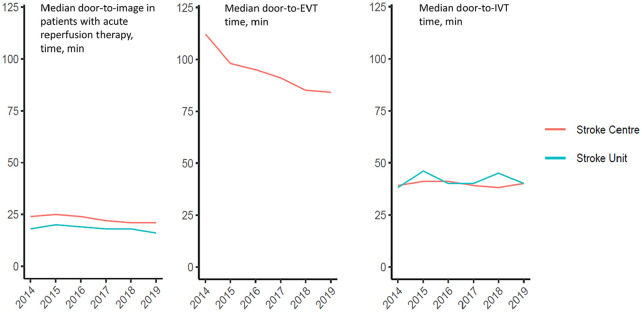
The median DIT for patients with acute reperfusion therapy remained relatively constant from 2014 to 2019 at Stroke Centers and Stroke Units. Similarly, the median DNT remained relatively stable (Stroke Center: 2014 39 vs 2019 40 minutes; Stroke Unit: 2014 38 vs 2019 40 minutes). However, a considerable decrease of DPT at Stroke Centers (2014 112 vs 2019 84 minutes) over time became apparent (Figure 2).
Figure 2.
Performance measures over time (years).
The probability for acute reperfusion treatment of wake-up stroke with IVT and/ or EVT increased over time from 13.2% in 2014 to 25.0% in 2016, and to 31.7% in 2019 (Figure 3).
Figure 3.
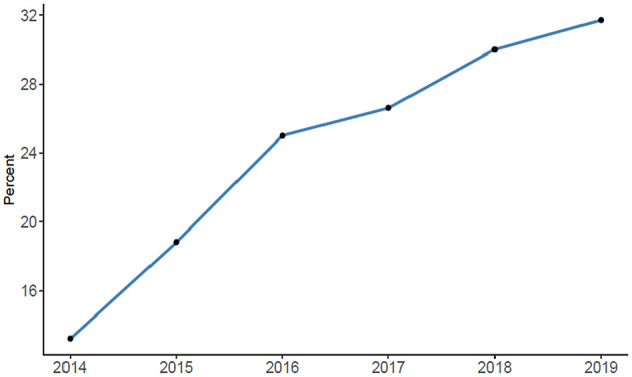
Rate of wake-up strokes with acute reperfusion treatment.
Discussion
In our study population about 50% of all ischemic stroke patients were admitted during NH, where the rate of acute reperfusion therapy (IVT or EVT) turned out to be higher than during OH, both at Stroke Centers (40.8% vs 36.5%) and Stroke Units (21.8% vs 18.5%). We observed that AIS patients were admitted considerably faster during NH than during OH, which may have contributed to the higher reperfusion therapy rate.
We found a statistically significant delay in delivery of IVT during NH, both at Stroke Centers and Stroke Units. Previous studies on diurnal variations of service provision in acute stroke treatment have yielded inconsistent results: A Swedish study found that DNT within 30 minutes was less likely during NH. 12 In line, Reuter et al. found the longest DNT time and the lowest IVT rate between 03:01 and 06:00 am. 9 Furthermore, Kristiansen et al. found in-hospital performance measures to be worse in patients admitted during NH. 20 Other studies, however, showed no association of DNT with time of hospital arrival 11 and no deterioration of acute reperfusion therapy rates in candidates for thrombolytic therapy. 21
Differences in staffing of emergency and radiology departments (independent of acute stroke services) might contribute to the higher DNT during NH by delaying image acquisition and decision-making. 12 However, the delays in DIT in the present study during NH were very minor. Some studies have also reported that patients admitted during NH may have more severe strokes and more comorbidities, potentially rendering treatment decisions more difficult.12,22 Yet in our study, baseline characteristics regarding comorbidities and stroke severity were well balanced between patients arriving during OH and NH. As there is little difference in DIT between NH and OH, the procedural step most sensitive to delay DNT during NH appears to be the decision whether to administer IVT. In Stroke Units the treating physician is obliged to make contact with the on-call neurologist during NH which takes additional time. In Stroke Centers, certification criteria in Switzerland require a 24/7 presence of a stroke neurologist. Despite these differences in staff requirements, the delay in DNT during NH compared with OH was similar at Stroke Centers and Stroke, and only moderate in extent. It has to be noted that patients admitted to Stroke Centers for IVT were more likely to take oral anticoagulation than patients treated at Stroke Units, which may cause additional delays in treatment during NH, depending on the availability of coagulation tests.
Regarding the speed of EVT at Stroke Centers, we found a substantial, 11 minute in-house treatment delay during NH compared with admission during OH. One previous study analyzing data from 2013 to 2014 found longer door-to-reperfusion times for patients admitted during night-time and weekends. 23 Another study reported significantly longer image-to-treatment times during NH. 24 Recently, it was suggested that EVT in the morning is associated with good and EVT at the end of the workday with poor functional outcome. 25 In the same study DPT was also increased during night-time. Optimizing EVT performance during NH is crucial because it has already been suggested that the majority of EVTs occur during NH, when transfer to hospital was reported to be delayed.24,26 EVT is a staff-intensive procedure that requires presence of nurses, medical technical assistants, anesthesiologists and neurointerventionalists. Furthermore, during NH the neurointerventionalist and other on-call staff have to travel to the hospital from home, in most settings. 27 Overall, we observed a clear reduction in DPT over the years, indicating that Stroke Centers have continuously optimized their in-house procedures to deliver EVT (Figure 2). The same secular trend could not be observed for DNT, possibly indicating a certain ceiling effect in the way that IVT pathways were already optimized at the beginning of the capture period. In Stroke Units DNT showed fluctuations over time with increases in 2015 and 2018. Thus, counteracting trends prolonging DNT must also be considered, such as the increasing proportion of patients on oral anticoagulation receiving IVT after emergency coagulation checks (which take time). 28 Over time, DIT remained stable with no considerable fluctuations suggesting that DIT was close to optimal already or that some gains are still possible. Furthermore, Stroke Centers and Stroke Units appear to have quickly adopted the recent evidence and treatment recommendations for wake-up stroke, indicated by a more than doubling in the proportion of patients with wake-up stroke receiving acute recanalization therapy from 2014 to 2019 (Figure 3).
Admission during NH resulted in higher odds for worse outcome and mortality in ischemic stroke patients similar to other studies investigating outcomes of patients suffering from AIS as well as other diseases during NH.12,13,29 Regarding EVT one study found no association between patients receiving EVT during the weekend and in-hospital death or functional status at discharge while in another study patients who were treated with EVT in nonteaching hospitals during the weekend had worse functional status at discharge.30,31 However, these studies only distinguished between weekend and weekdays and outcome measures were in-hospital based without a three month follow-up. The worse outcome during NH in our study was not accounted for by differences in measured patient characteristics, rates of or delays in recanalization therapy.
Strengths and limitations
One strength of the present study is the large sample size (n = 35,261) with low number of missing data on primary outcomes (9.8%). We used key performance measures as well as functional outcome at 3 months as outcomes in a prospectively collected dataset. Furthermore, data collection was performed from 01/2014 up to 12/2019 resulting in up-to-date analyzes reflecting the current situation of real world stroke service provision.
Our study has some limitations: Apart from general limitations of registry based, retrospective studies we were unable to clarify why performance measures and outcomes are worse during NH and can only make assumptions. It is possible that unmeasured differences between OH and NH were missed in patients or settings, leading to residual confounding. We were not able to investigate if the improvement of DPT over time was associated with increased economic costs. As we do not know the exact staff levels of each participating center we were not able to calculate optimal staffing during NH. Also, our results may not be generalizable to countries with different organization of acute stroke care, different distribution of acute stroke patients (in our study most patients were admitted to a Stroke Center) and more limited availability of infrastructure and staff.
Summary/conclusions
The delivery of acute recanalization therapy at Swiss Stroke Centers and Stroke Units is moderately delayed during non-office hours. Patients admitted during NH have a worse functional outcome which is not explained by availability or delay of recanalization therapy. Recent evidence and recommendations for treatment of wake-up stroke have been quickly adopted over the past few years. Overall, our findings show that Stroke Centers and Stroke Units certified in accordance to European guidelines are capable of providing round-the-clock acute stroke care, which may inform the planning of service provision in other health care systems.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873221094408 for Effect of admission time on provision of acute stroke treatment at stroke units and stroke centers—An analysis of the Swiss Stroke Registry by Valerian L Altersberger, Patrick R Wright, Sabine A Schaedelin, Gian Marco De Marchis, Henrik Gensicke, Stefan T Engelter, Marios Psychogios, Timo Kahles, Martina Goeldlin, Thomas R Meinel, Pasquale Mordasini, Johannes Kaesmacher, Alexander von Hessling, Jochen Vehoff, Johannes Weber, Susanne Wegener, Stephan Salmen, Rolf Sturzenegger, Friedrich Medlin, Christian Berger, Ludwig Schelosky, Susanne Renaud, Julien Niederhauser, Christophe Bonvin, Michael Schaerer, Marie-Luise Mono, Biljana Rodic, Guido Schwegler, Nils Peters, Manuel Bolognese, Andreas R Luft, Carlo W Cereda, Georg Kägi, Patrick Michel, Emmanuel Carrera, Marcel Arnold, Urs Fischer, Krassen Nedeltchev and Leo H Bonati in European Stroke Journal
Acknowledgments
None
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VLA, SAS, PRW, MP, TK, AvH, TM, EC, SS, RS, JV, JN, LS, SR, MS, MLM, BR, GS report no conflicting interests. GMDM has received support from the Swiss National Science Foundation; Spezialprogramm Nachwuchsförderung Klinische Forschung, University of Basel; Science Funds of the University Hospital Basel; Swiss Heart Foundation; Bangerter-Rhyner-Stiftung; Swisslife Jubiläumsstiftung for Medical Research; Swiss Neurological Society; Fondazione Dr Ettore Balli; De Quervain research grant; Thermo-Fisher-GmbH; consultant honoraria by Bayer; speaker honoraria by Medtronic and BMS/Pfizer. HG has received research support from the Swiss National Science Foundation, advisory board honoraria from Daiichi-Sankyo and funding for travel from BMS/Pfizer. STE has received funding for travel or speaker honoraria from Bayer Boehringer-Ingelheim, and Daiichi-Sankyo. He has served on scientific advisory boards for Bayer, Boehringer-Ingelheim, BMS/Pfizer, MindMaze, the editorial board of Stroke. He has received an educational grant from Pfizer and research support from the Science Funds of the University Hospital Basel, the University Basel, the Swiss Heart Foundation and the Swiss National Science Foundation. KN received speaker’s fees from Abbott. MA received Speaker honoraria from Bayer, Boehringer-Ingelheim, and Covidien; Scientific advisory board honoraria from Amgen, Bayer, Boehringer-Ingelheim, BMS, Pfizer, Covidien, Daichy Sankyo and Nestlé Health Science. Research grants from the Swiss Heart Foundation and the Swiss National Science Foundation. UF has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation and Medtronic; he is a consultant for Medtronic, Stryker, and CSL-Behring. PM received speaker honoraria from Medtronic, Stryker. Consultant for Medtronic, Cerenovus, Phenox, Microvention, research grants from the Swiss Heart Foundation, Siemens and iSchemview. MG reports grants from Bangerter-Rhyner-Foundation. JK reports grants from the Swiss Stroke Society and the Swiss Academy of Medical Sciences/Bangerter Foundation. PM has received has received through his institution research grants from the Swiss National Science Foundation, the Swiss Heart Foundation and the ERISTA program (Pfizer/BMS); consulting fees from Medtronic. All this support goes to his institution for stroke education and research. CWC has received modest honoraria for scientific advisory board from Bayer, Boehringer-Ingelheim and iSchemaview; Research grants from the Swiss Heart Foundation. MB has received honoraria for travel from Bayer and for participation in advisory board from AstraZeneca. GK has received modest honoraria for travel and advisory board from Bayer, Medtronic, Alexion, Bial, Boehringer-Ingelheim and Zambon, a research grant from the Swiss Heart Foundation, Swiss Parkinson Foundation, Swiss National Science Foundation. ARL has received modest honoraria for travel and advisory board from Bayer, Moleac and Amgen and research grants from the P&K Pühringer-Foundation. SW received research funds by the Swiss National Science Foundation, the UZH Clinical research priority program (CRPP) stroke, the Swiss Heart foundation, Boehringer-Ingelheim, speakers honorarium from Amgen and a consultancy fee from Bayer. NP has received research funding from the Swiss Heart Foundation and the Swiss National Science Foundation, speaker honoraria from Vifor; served on advisory boards for Bayer, Boehringer-Ingelheim, BMS/Pfizer, Daiichi-Sankyo and AstraZeneca. FM has received research support from the Swiss Heart Foundation and has not received any honoria from industry 2017. CBe received modest honoraria for travel and advisory board from Novartis and Bayer. CBo reports travel and speaker honoraria from Amgen, Bayer, Biogen, Boehringer-Ingelheim, Bristol-Myers-Squibb, Lilly, Merck, Novartis, Pfizer, Roche, Servier, Sanofi, TEVA. LHB has received grants from the Swiss National Science Foundation, the University of Basel, the Swiss Heart Foundation, and the “Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung sowie der medizinischen Bildauswertung.” Unrestricted research grant from AstraZeneca, consultancy or advisory board fees or speaker’s honoraria from Amgen, Bayer, Bristol-Myers-Squibb, Claret Medical, and InnovHeart, and travel grants from AstraZeneca and Bayer.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: In accordance with national law, patients were informed about the use of their routinely collected data for research purposes. Patients who denied use of their data were excluded from the analysis.
Ethical approval: Ethical approval for this study was waived by the EKNZ (Ethikkommission Nordwest- und Zentralschweiz) because the study was classified as a Quality Assurance Study.
Guarantor: LHB
Contributorship: VLA designed/conceptualized the study, interpreted the data, and drafted the manuscript. PRW and SAS performed statistical analyzes, interpreted data, and revised the manuscript. LHB designed/ conceptualized and initiated the study, supervised the study, collected data, interpreted the data, revised the manuscript, and is the Coordinator of the Swiss Stroke Registry. GMDM, HG, STE, MP, UF, TM and JK contributed to the conception and design of the study. KN, TK, MA, UF, MG, TM, PM, JK, EC, PM, CWC, MB, AvH, GK, JV, JW, ARL, SW, NP, SS, RS, FM, CBe, LS, SR, JN, CBo, MS, MLM, BR and GS contributed to drafting the text, preparing the figures, and the acquisition of data.
Trial registration: Not applicable.
ORCID iDs: Valerian L Altersberger  https://orcid.org/0000-0002-0610-9328
https://orcid.org/0000-0002-0610-9328
Gian Marco De Marchis  https://orcid.org/0000-0002-0342-9780
https://orcid.org/0000-0002-0342-9780
Timo Kahles  https://orcid.org/0000-0002-1569-6376
https://orcid.org/0000-0002-1569-6376
Martina Goeldlin  https://orcid.org/0000-0001-5800-116X
https://orcid.org/0000-0001-5800-116X
Thomas R Meinel  https://orcid.org/0000-0002-0647-9273
https://orcid.org/0000-0002-0647-9273
Leo H Bonati  https://orcid.org/0000-0003-1163-8133
https://orcid.org/0000-0003-1163-8133
Supplemental material: Supplemental material for this article is available online.
References
- 1. IST-3 collaborative group, Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 2012; 379: 2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. New Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 3. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. New Engl J Med 2018; 378(1): 11–21. [DOI] [PubMed] [Google Scholar]
- 4. Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. New Engl J Med 2019; 380: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 5. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 6. Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-Guided thrombolysis for stroke with unknown time of onset. New Engl J Med 2018; 379: 611–622. [DOI] [PubMed] [Google Scholar]
- 7. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ringelstein EB, Chamorro A, Kaste M, et al. European stroke organisation recommendations to establish a stroke unit and stroke center.. Stroke 2013; 44: 828–840. [DOI] [PubMed] [Google Scholar]
- 9. Reuter B, Sauer T, Gumbinger C, et al. ; Stroke Working Group of Baden-Wuerttemberg. Diurnal variation of intravenous thrombolysis rates for acute ischemic stroke and associated quality performance parameters. Front Neurol 2017; 8: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zonneveld TP, Curtze S, Zinkstok SM, et al. Non-office-hours admission affects intravenous thrombolysis treatment times and clinical outcome. J Neurol Neurosurg Psychiatry 2018; 89: 1005–1007. [DOI] [PubMed] [Google Scholar]
- 11. Helsinki Stroke Thrombolysis Registry Group. Does time of day or physician experience affect outcome of acute ischemic stroke patients treated with thrombolysis? A study from Finland. Int J Stroke 2012; 7: 511–516. [DOI] [PubMed] [Google Scholar]
- 12. Darehed D, Blom M, Glader EL, et al. Diurnal variations in the quality of stroke care in Sweden. Acta Neurol Scand 2019; 140: 123–130. [DOI] [PubMed] [Google Scholar]
- 13. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care.. Lancet 2016; 388: 170–177. [DOI] [PubMed] [Google Scholar]
- 14. Manno C, Disanto G, Bianco G, et al. Outcome of endovascular therapy in stroke with large vessel occlusion and mild symptoms. Neurology 2019; 93: e1618–e1626. [DOI] [PubMed] [Google Scholar]
- 15. Waje-Andreassen U, Nabavi DG, Engelter ST, et al. European Stroke Organisation certification of stroke units and stroke centres. Eur Stroke J 2018; 3: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH stroke scale using video training. NINDS TPA Stroke Study Group. Stroke 1994; 25: 2220–2226. [DOI] [PubMed] [Google Scholar]
- 17. Fluri F, Hatz F, Voss B, et al. Restenosis after carotid endarterectomy: significance of newly acquired risk factors. Eur J Neurol 2010; 17: 493–498. [DOI] [PubMed] [Google Scholar]
- 18. Wilson JT, Hareendran A, Hendry A, et al. Reliability of the modified Rankin Scale across multiple raters: benefits of a structured interview. Stroke 2005; 36: 777–781. [DOI] [PubMed] [Google Scholar]
- 19. Wright P, Haynes A, Markovic M. secuTrialR: seamless interaction with clinical trial databases in R. J Open Source Softw 2020; 5: 2816. [Google Scholar]
- 20. Kristiansen NS, Mainz J, Nørgård BM, et al. Off-hours admission and acute stroke care quality: a nationwide study of performance measures and Case-Fatality. Stroke 2014; 45: 3663–3669. [DOI] [PubMed] [Google Scholar]
- 21. Jauss M, Schütz HJ, Tanislav C, et al. Effect of daytime, weekday and year of admission on outcome in acute ischaemic stroke patients treated with thrombolytic therapy. Eur J Neurol 2010; 17: 555–561. [DOI] [PubMed] [Google Scholar]
- 22. Campbell JTP, Bray BD, Hoffman AM, et al. The effect of out of hours presentation with acute stroke on processes of care and outcomes: analysis of data from the Stroke Improvement National Audit Programme (SINAP). PLoS One 2014; 9: e87946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mpotsaris A, Kowoll A, Weber W, et al. Endovascular stroke therapy at nighttime and on weekends-as fast and effective as during normal business hours? J Vasc Interv Neurol 2015; 8: 39–45. [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson TA, Leslie-Mazwi T, Hirsch JA, et al. A multicenter study evaluating the frequency and time requirement of mechanical thrombectomy. J Neurointerv Surg 2018; 10: 235–239. [DOI] [PubMed] [Google Scholar]
- 25. Hajdu SD, Kaesmacher J, Michel PP, et al. Association of time of day when endovascular therapy for stroke starts and functional outcome. Neurology 2021; 96: e1124–e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Regenhardt RW, Mecca AP, Flavin SA, et al. Delays in the air or ground transfer of patients for endovascular thrombectomy. Stroke 2018; 49: 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams MM, Wilson TA, Leslie-Mazwi T, et al. The burden of neurothrombectomy call: a multicenter prospective study. J Neurointerv Surg 2018; 10: 1143–1148. [DOI] [PubMed] [Google Scholar]
- 28. Meinel TR, Branca M, De Marchis GM, et al. Prior anticoagulation in patients with ischemic stroke and atrial fibrillation. Ann Neurol 2021; 89: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou Y, Li W, Herath C, et al. Off-Hour admission and mortality risk for 28 specific diseases: a systematic review and meta-analysis of 251 cohorts. J Am Heart Assoc 2016; 5: e003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grandhi R, Ravindra VM, Ney JP, et al. Investigating the “Weekend Effect” on outcomes of patients undergoing endovascular mechanical thrombectomy for ischemic stroke. J Stroke Cerebrovasc Dis 2021; 30: 106013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saad A, Adil MM, Patel V, et al. Clinical outcomes after thrombectomy for acute ischemic stroke on weekends versus weekdays. J Stroke Cerebrovasc Dis 2014; 23: 2708–2713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873221094408 for Effect of admission time on provision of acute stroke treatment at stroke units and stroke centers—An analysis of the Swiss Stroke Registry by Valerian L Altersberger, Patrick R Wright, Sabine A Schaedelin, Gian Marco De Marchis, Henrik Gensicke, Stefan T Engelter, Marios Psychogios, Timo Kahles, Martina Goeldlin, Thomas R Meinel, Pasquale Mordasini, Johannes Kaesmacher, Alexander von Hessling, Jochen Vehoff, Johannes Weber, Susanne Wegener, Stephan Salmen, Rolf Sturzenegger, Friedrich Medlin, Christian Berger, Ludwig Schelosky, Susanne Renaud, Julien Niederhauser, Christophe Bonvin, Michael Schaerer, Marie-Luise Mono, Biljana Rodic, Guido Schwegler, Nils Peters, Manuel Bolognese, Andreas R Luft, Carlo W Cereda, Georg Kägi, Patrick Michel, Emmanuel Carrera, Marcel Arnold, Urs Fischer, Krassen Nedeltchev and Leo H Bonati in European Stroke Journal