Abstract
Background:
Despite policy efforts to prevent overdose, accidental overdoses among individuals prescribed opioids continue to occur. Guided by Rhodes’ Risk Environment Framework, we examined the unintended consequences of restrictive policies by identifying macro policy and micro-level contextual factors that patients prescribed opioids for pain identified as contributing to overdose events.
Methods:
Semi-structured interviews were conducted with 31 patients prescribed opioids who experienced an accidental opioid overdose between April 2017 and June 2019 in two health systems.
Results:
We identified three interrelated factors that emerged within an evolving risk environment and may have increased patients’ vulnerability for an accidental opioid overdose: desperation from persistent pain and comorbidities; limited knowledge about opioid medication safety and effectiveness; and restrictive opioid prescribing policies that exacerbated stigma, fear and mistrust and prevented open patient-clinician communication. When experiencing persistent pain, patients took matters into their own hands by taking more medications or in different intervals than prescribed, mixing them with other substances, or using illicitly obtained opioids.
Conclusion:
For some patients, macro-level policies and guidelines designed to reduce opioid overdoses by restricting opioid supply may have paradoxically created a micro-level risk environment that contributed to overdose events in a subset of patients.
Keywords: Opioid Overdose, Policy, Risk Environment, Qualitative, Opioid Tapering
Background
The opioid overdose epidemic has been traced back to policies and medical practices of the early 1990s (Jones et al., 2018). In the 1990s, policy-makers and clinicians emphasized pain treatment in response to the American Pain Society campaign to raise awareness of ‘pain as the fifth vital sign’ (Tompkins et al., 2017), and influential regulatory bodies mandated that hospitals assess and treat pain to receive federal dollars (Tompkins et al., 2017). At the same time, concerns about safety were minimized after the publication of two influential retrospective studies that showed low rates of addiction among patients prescribed opioids for pain (Foley, 1985; Portenoy & Foley, 1986). In addition, a decline in operational multidisciplinary pain treatment clinics throughout the 1980s and 1990s and aggressive marketing of new sustained-release oxycodone products led health care facilities and clinicians to turn increasingly to opioids to control chronic, non-cancer pain (Tompkins et al., 2017; Van Zee, 2009). These events resulted in a policy environment in which patients experiencing pain increasingly received opioids, which contributed to the escalation of prescription opioid overdose rates in the early phase of the opioid epidemic (Ciccarone, 2019; Dasgupta et al., 2018).
Faced with growing concerns over increases in opioid overdose deaths, government agencies, health systems and clinicians developed and implemented policies and clinical practice innovations aimed at changing opioid prescribing patterns and reducing the risk of overdose (Dowell et al., 2016; “Substance use-disorder prevention that promotes opioid recovery and treatment (support) for patients and communities act (public law 115-271),” 2018). For example, the Drug Enforcement Administration reclassified hydrocodone-containing combination products from schedule III to the more restrictive schedule II (Drug Enforcement Administration, 2014; Raji et al., 2018). In 2016, the Centers for Disease Control and Prevention (CDC) also published a guideline for clinicians prescribing opioids for chronic non-cancer pain to improve prescribing practices (Dowell et al., 2016). Clinicians were advised to carefully weigh the benefits and harms of prescribing opioids, to avoid increasing dosages above certain thresholds, and to consider tapering patients who were no longer making clinical improvements. In addition, state Medicaid programs introduced prior authorization opioid prescribing policies to limit high dosage prescribing (Hartung et al., 2017).
Despite the introduction of these guidelines and policies, high opioid overdose rates persist. Many researchers and public health authorities have expressed concerns that policies themselves may have unintended adverse consequences that increase overdose risk (Health Professionals for Patients in Pain, March 6, 2019; Kertesz, 2016; Rich, 2020). It is therefore important to explore the policy and social contexts in which overdoses occur to better understand such high rates. Specifically, more research is needed to understand how well-intended policies translate to local clinical settings and interact with social environments to influence patient-provider interactions and increase vulnerability for opioid overdose. (Rhodes, 2009; Rhodes et al., 2005)
The Risk Environment Framework offers a theoretical lens to investigate the spaces (both social and physical), domains (economic, legal and policy) and levels (micro, meso, macro) at which factors exogenous to individuals interact to increase individuals’ susceptibility and vulnerability to drug-related harms (Rhodes, 2009; Rhodes et al., 2005). While overdoses occur within complex risk environments (Green et al., 2009; Koester et al., 2017; McLean, 2016; Rhodes, 2002), many contextual factors that contribute to overdose risk may be undetectable by traditional quantitative epidemiological studies that guidelines and policies rely upon when formulating recommendations. Qualitative research methods are well-suited to explore complex patterns of drug use, unintended effects of policies and interventions and the social and cultural risk environments in which they occur (Rhodes et al., 2010).
Previous qualitative studies have drawn on the Risk Environment Framework to examine contextual factors contributing to opioid overdose (Rhodes, 2002; Rhodes, 2009). One study explored the role macro-level economic factors play in producing an environment contributing to illicit opioid use and overdose (McLean, 2016), while others have highlighted the influence of historical and structural socio-economic factors (Garcia, 2010; Green et al., 2009; Moore, 2004), and physical properties of heroin types in heroin-related overdoses (Mars et al., 2015). However, many of these studies examined illicit opioid overdoses that occurred prior to 2012, predating many of the public health policies and interventions put in place to reduce overdose risk. Few qualitative studies have used the Risk Environment Framework to examine the risk environment for accidental opioid overdoses in later phases of the opioid overdose epidemic.
To examine factors that contribute to overdose risk and better inform policies designed to reduce overdose risk, we conducted a qualitative study of patients prescribed opioids for pain who experienced an accidental opioid overdose and their caregivers or family members (Rhodes, 2002; Rhodes, 2009). Leveraging in-depth interviews and the Risk Environment Framework, we examine the potential unintended consequences of restrictive opioid prescribing policies and describe patients’ perceptions of overdose events to identify factors that emerge across evolving macro policy and micro social risk environments that exacerbate patients’ vulnerability to an accidental opioid overdose.
Methods
Study Design
We conducted a qualitative study to explore patients’ perspectives on overdose events and contextual factors and conditions that contributed to such events (Gale et al., 2013; Ward et al., 2013). We used the Risk Environment Framework to guide the analyses, frame the results and identify areas for interventions that may create “enabling environments” to reduce harm. This framework allowed for a consideration of how overdose events occurred within the evolving social, structural, and environmental contexts in which patients are embedded (Rhodes, 2009).
Study Setting
Between June 2018 and August 2019, we recruited patients from two Colorado health systems that represent a diverse patient population and have robust programs designed to prevent overdose and overdose fatalities. The rates of opioid overdose within both health systems are congruent with Colorado and national rates (Centers for Disease Control and Prevention & National Center for Health Statistics; National Institute on Drug Abuse).
Prior to patient recruitment, both health systems had implemented clinical care initiatives to reduce adverse events from opioids. These initiatives were strongly influenced by the Policy for Prescribing and Dispensing Opioids issued by the Colorado Department of Regulatory Agencies in 2014 (“State member board briefs,” 2014), among others. This policy recommended that clinicians carefully consider implementing risk mitigation strategies when prescribing opioids at doses > 120 morphine milligram equivalents (MME), long-acting and extended-release opioid formulations, or opioids in combination with benzodiazepines (“State member board briefs,” 2014). In 2017, the Colorado Department of Health Care Policy and Financing implemented a policy that restricted daily maximum dose and the number of pills dispensed, reducing the number of opioid pills prescribed and number of people taking opioids within the Colorado Medicaid program by 30 percent in subsequent years (Colorado Department of Health Care Policy and Financing, September 2018; Williams, October 31, 2018). These local initiatives were also influenced by the CDC prescribing guideline (Dowell et al., 2016), which recommended clinicians limit opioid doses to the lowest effective dose, check the prescription drug monitoring program (PDMP) at regular intervals, conduct urine drug screens for illicit drug use and the presence of prescribed opioids, and taper or discontinue opioids when the risks of opioids outweigh the benefits.
Both health systems also allowed pharmacists to dispense naloxone, an opioid antagonist that can reverse respiratory depression associated with overdose, under a standing order and implemented policies to increase naloxone distribution to at-risk patients. One health system implemented a pain management program that offered both counseling and multidisciplinary pain treatment. Several clinics within the other health system implemented opioid safety clinic visits for patients receiving chronic opioid therapy in which patients meet with a clinician to receive education on opioid safety and a prescription for naloxone.
Participants
We recruited patients 18 and older who experienced an accidental opioid overdose to participate in semi-structured interviews through purposive sampling. Patients were eligible for interviews if they had a prescription opioid overdose or had an illicit opioid overdose with a history of receiving chronic opioid therapy. Eligible patients were first identified in electronic health records using International Classification of Diseases, Tenth Revision (ICD-10) codes for opioid overdoses (Appendix 1) that occurred between April 2017-June 2019. Pharmacy records were used to identify patients who received three or more opioid dispensings in 90 days, consistent with the definition of chronic opioid therapy (Glanz et al., 2018). We then conducted a medical record review to confirm that the overdose involved opioids and to exclude overdoses with suicidal intent. We also recruited patients who reported experiencing an accidental overdose emergency in the prior four months on surveys that were routinely collected as part of two pragmatic trials (NCT03337009 and NCT03337100) designed to prevent fatal opioid overdoses among patients prescribed opioids.
Patients were recruited using mail, email and phone. To help participants recall the details of the overdose event, patients who agreed to participate were asked to invite a family member, friend, caregiver or other bystander who was present at the time of the event to participate in the interview.
Data Collection
Semi-structured interviews were conducted at the clinic where participants received primary care or over the phone. Participants were provided a copy of the consent form and verbal consent was obtained before interviews began. The interviewers (SRM, MS, SK, APN), who were all trained and experienced in qualitative methods, emphasized that individual responses would not be shared with their clinicians or other members of the health system outside of the study team. The entire multidisciplinary study team, which included researchers with a background in anthropology, sociology, medicine, epidemiology, and health and behavioral sciences, developed a semi-structured interview guide that included open-ended questions and clarifying probes to explore patients’ history of pain, perceived risks and benefits of opioids, interactions with clinicians, and circumstances that led to the overdose. Interviews lasted between 27 and 93 minutes. At the conclusion of interviews, participants completed a structured survey on demographic and clinical characteristics. Patients and caregivers were each compensated with a $30 gift card to an online retailer or local grocery store for participating. Interviews were audio recorded and transcribed verbatim and a second study team member was present for most interviews to record notes. At the completion of each interview, the interviewer and note-taker recorded primary impressions and identified relationships between emerging codes and themes in a memo (Razaghi et al., 2015). Interviews continued until data saturation was reached (Saunders et al., 2018).
Data Analysis
Interviews were analyzed using the Framework Analysis Method (Gale et al., 2013), an approach that is part of a broad family of methods often referred to as thematic analysis.
To begin, preliminary codes were generated and grouped into broad categories. The interviewers created both inductive codes, reflecting concepts that emerged from the data, and deductive codes, reflecting codes derived from topics of the interview guide (Elo & Kyngas, 2008). The interviewers openly coded three transcripts using ATLAS.ti 8 to augment the preliminary codes (Friese, 2019). Data was also coded for manifest meaning (surface content), and underlying meaning (latent content) (Cho & Lee, 2014).
The interviewers met regularly to discuss codes until there was agreement on the meaning of codes and how they should be applied to the data. Codes were then arranged into a group of categories that comprised the initial analytic framework. These codes and categories were discussed at a meeting with the larger multidisciplinary study team, who provided feedback based on their reading of several transcripts to reach consensus on all codes and categories.
After consensus was reached on codes and categories, the interviewers coded the remaining transcripts using the analytic framework of codes. New codes were created when new attitudes, experiences or behaviors that did not belong to an existing code emerged from the data. The interviewers regularly discussed and agreed upon new codes and categories and applied them to the transcripts. This process was repeated throughout the analysis until no new codes were identified (thematic saturation) (Saunders et al., 2018).
The interviewers then reviewed the coded data for coherency and consistency. They summarized and transferred the data into a matrix in which the columns represented each category of codes and the rows represented each interview. They added illustrative quotations and succinct summaries of each code category to the matrix. The matrix provided a visually straightforward method for identifying connections across codes, categories and participants. Finally, the larger multidisciplinary team identified factors that increased patients’ vulnerability for an accidental overdose using the matrix and refined them in group discussions. Using the Risk Environment Framework, the larger research team situated these factors within the types of environment (physical, social, economic, policy) and levels of influence (micro, meso and macro) (Rhodes, 2009).
Ethical Considerations
Ethical approval was granted by the Kaiser Permanente Colorado Institutional Review Board (#1224275) and Colorado Multiple Institutional Review Board (#17-0108). We received a Federal Certificate of Confidentiality.
Results
Among 130 eligible patients across both healthcare sites who experienced an accidental opioid-related overdose, 31 participated in interviews. Six additional participants included family members or caregivers in interviews. Table 1 shows that patients interviewed had a mean age of 53.2 years, were mostly female, and reported being prescribed opioids for an average of 9.08 years over their lifetime.
Table 1.
Patient Characteristics (N=31)
| Age in years, mean (range) | 53.2 (22-81) |
|
| |
| Female, no. (%) | 18 (58.1) |
|
| |
| Race/ethnicity, no. (%) | |
| White, non-Hispanic | 20 (64.5) |
| Hispanic | 7 (22.5) |
| African American, Asian or American Indian | 4 (12.9) |
|
| |
| Education, no. (%)* | |
| Less than high school graduate | 4 (12.9) |
| High school graduate or GED certificate | 9 (29.0) |
| Some college | 6 (19.3) |
| College graduate | 11 (35.4) |
|
| |
| Lifetime length of time prescribed opioids in years, mean (range) | 9.08 (1 week – 40 years) |
Numbers do not add up to 31 because of missing data
Overall, patients identified three contextual factors that contributed to their accidental opioid overdose events:
Desperation from persistent pain and comorbidities;
Limited knowledge about opioid medication safety and effectiveness
Restrictive opioid prescribing policies that contributed to feelings of stigma, fear and mistrust and prevented open patient-clinician communication
As a result of these factors, patients described taking matters into their own hands by adjusting the way they took medications to achieve greater pain relief, including taking more medications or in different intervals than prescribed, mixing medications with alcohol, or using illicitly obtained opioids, leading to the accidental overdoses identified in the medical records.
Desperation from persistent pain and comorbidities
At the beginning of interviews, patients described long histories of severe and debilitating pain. Many patients attributed their chronic pain to a specific traumatic event, such as a car accident, which not only caused physical injury, but also psychological suffering and emotional distress. At the time of the traumatic event, they were prescribed opioids. At the direction of their clinician, patients had tried a variety of non-opioid therapies to treat persisting pain with limited success. Barriers to alternative treatment success included lack of efficacy and limited duration of effect. One patient described the limited efficacy of local cortisone injections:
I’ve gotten the [cortisone] shots. Didn’t work. It worked for a week. It’s supposed to last 90 days. (ID07; Male, 50-59 years)
Patients also cited additional barriers that are illustrative of micro-level economic factors, such as the cost of multi-modal treatment or an inability to take time off work to attend physical therapy appointments:
[T]hey want[ed] me to do physical therapy, which I was working [at my job] and I couldn’t… I didn’t have time. (ID06; Female, 60-69years)
As a result, patients reported that pain remained persistent, negatively influencing their quality of life and interfering with their ability to function. While some patients described moderate improvements with non-opioid therapies, many failed to find adequate relief and described being prescribed opioids long-term to improve function and quality of life. One caregiver discussed the positive impact of opioid medication on her husband:
As soon as he [patient’s physician] started the methadone, almost immediately stopped the trips to the emergency room, they stopped… it was like, a night and day. The spikes [in pain] really mellowed out, became more manageable and came less often. And it really, really made a big difference, made a huge difference, good change. (ID20, Male, 60-69 years)
While patients who experienced an overdose reported improved pain and function with opioids, they nonetheless often described persistent, severe pain and unmet medical needs. While some patients felt they had received adequate care, many patients described a variety of concurrent issues, such as prior trauma, stress from chronic pain itself, and other significant health conditions that were not always adequately assessed, treated, or ‘taken seriously.’ Patients described challenges receiving comprehensive care that addressed the biological, psychological and social aspects of their pain:
I knew I was struggling. I didn’t know it was called anxiety and I didn’t know it was called PTSD or anything. And I also didn’t know like my hives had to do with like their medications that they were giving me. So, I was very frustrated. I felt like no one listened and no one wanted to help me. (ID01, Female, 20-29 years)
They often attributed comorbidities, including mental health conditions, to changes in quality of life and function that resulted from chronic pain. For example, one patient reported that “[t]he mental health part is, has been more, as horrendous as the pain…” Another factor that contributed to the micro policy risk environment is the time constraint placed on the clinician-patient interaction. Some patients expressed feeling unheard by their clinicians within the constraints of short appointment windows.
Overall, patients described a social risk environment marked by feelings of frustration, being ignored, and desperation for relief, and a local policy environment where time-constraints in patient-provider interactions provoked feeling like their needs were unmet. Combined with economic factors, feelings of frustration about persistent pain and symptoms from comorbidities were common and constituted a micro risk environment in which patients attempted to manage pain on their own terms.
Limited knowledge about opioid medication safety and effectiveness
Patients’ limited knowledge about opioid medication safety and effectiveness was a byproduct of early phases of the overdose risk environment. Many of the patients we interviewed initiated opioids during an earlier macro policy risk environment when there was a national emphasis on patient satisfaction and treating pain liberally with opioids, a macro policy environment that was bolstered by the aggressive marketing of opioid medications (Dasgupta et al., 2018). As a result, there may have been less attention paid to how to provide the most effective education on medication risks at the micro level of clinical encounters than during later phases of the opioid crisis. The aggressive marketing of opioids as safe and effective may have contributed to a micro social risk environment where education about medication safety may not have been delivered accurately, consistently and specifically in ways that helped patients absorb the information.
Despite being prescribed opioids for many years, some patients who experienced overdoses reported receiving only limited education about the risks associated with opioids or specific instructions on how to safely take their medications. When asked to elaborate on the information they received when they initiated opioid therapy, some patients reported not being aware about potential adverse effects associated with opioids, including risk of physical dependence, addiction or overdose:
[The education I received] was just… “Don’t take it any differently. If you have any problems or questions, give us a call and if you have any side effects, go to a hospital.” If you look at the prescription instructions you get from the pharmacy, they’ll all say the same thing except for the name of the medication…I would not have taken [prescribed] fentanyl if I would have known it was that addictive – that it was 300 times addictive than morphine…No way in hell. (ID09, Male, 40-49years)
In contrast, other patients recalled being counseled about the risks of taking opioid pain medications when initiating chronic opioid therapy:
Interviewer: What kind of conversations would you have about the risks?
Patient: She told me don’t mix with alcohol, heavy equipment driving. Try to take it before bed. Just all the necessary precautions. (ID07, Male, 50-59 years)
However, patients who received information at the time they initiated opioids did not necessarily recall receiving additional information since starting opioid therapy. Other participants could not recall whether they were educated about adverse events associated with opioids.
Interviewer: Did they talk about any of the possible risk of – you mentioned the accidental overdose. Did they talk about the risk of those at all?
Patient: I honestly don’t remember to tell you the truth. (ID20, Male, 60-69 years)
They thought education may have occurred at a time when they were in significant pain and incapable of absorbing and retaining medication safety information.
Interviewer: When you had the knee surgery, did you talk with the doctor or the surgeon about pain expectations or how to take opioid medications or any of the side effects or risk factors that we are associated with it?
Patient: I don’t remember… I don’t recall… It doesn’t stand out in my brain. And again, maybe I forgot because my brain was fuzzy. (ID05, Female, 60-69 years)
Additionally, some patients described being referred to medication package inserts and labels or pharmacists for information about adverse risks and directions for taking medications. Information from these sources was characterized as excessively lengthy, generic and/or unspecific:
Interviewer: Did you ever have conversations with your doctors about like the risks of the medication or like some of the side effects?
Patient: [My doctor] told me that, “Everything is fine, follow the instructions; your pharmacist will explain everything to you.” I went to the pharmacist when I would get a new prescription and they would give me very blunt, plain – “Just follow the instructions from the doctor; don’t take too much of this and if you start haring any sort of symptoms, like your mouth or your tongue is swelling or your throat is swelling or you can’t breathe, go to a hospital… But when I opened up a fentanyl box and I read the instructions – I don’t know if you’ve ever seen them or not, but they’re like an encyclopedia. They’re quite long and very thorough in their explanation… and even the paperwork they send with you, on your paperwork when you pick up a prescription, it was very vague too. It was, “Follow the doctor’s instructions and if you have any interactions, go to the emergency room or contact your doctor. ”(ID09, Male, 40-49 years)
Overall, the attempted transmission of knowledge in the clinical encounter context was insufficient to counter a micro level risk environment in which patients who experienced an overdose were generally aware of risks for addiction or overdose, but under the impression that if they loosely followed directions they were at limited risk for an overdose or other adverse events. For example, one patient described combining medication doses to achieve a greater effect. This patient did not think she was ‘taking them wrong’ because she was not taking more than prescribed in total for a single day:
Interviewer: But…you didn’t think [overtaking them] would be an issue?
Patient: Not ever, not ever. [I, I figured if I didn’t take more in a day than I was prescribed it was okay. So instead of spreading the Dilaudid out the way the bottle said, I took half in the morning and half at night. (ID13, Female, 70-79 years)
Limited knowledge about medication safety likely contributed to a micro level risk environment in which overdoses occurred.
Some patients who overdosed also described limited knowledge about the effectiveness of opioid pain medications. One patient who was prescribed opioids after a knee replacement surgery felt frustrated after her pain did not recede in the days following surgery (ID10, Female, 70-79 years). She did not think that the educational courses she took before surgery adequately set realistic expectations about pain and recovery. She recalled nurses telling her after the surgery “Oh, just walk and you’ll be fine” and believed that better education about pain expectations would have prevented her from taking more opioids to find relief.
Restrictive opioid prescribing policies that contributed to feelings of stigma, fear and mistrust that prevented open patient-clinician communication
Patients expressed concerns related to the policy risk environment that contributed to their overdose events. For example, some patients who had been prescribed opioids for several years described experiencing abrupt medication discontinuation, tapers and formula changes, which they ascribed to new and restrictive prescribing policies. These patients described experiencing poorly controlled pain and a diminished quality of life as a result of these changes:
In the beginning years back, they would give me a low dose of Percocet, and that would work wonders. I could sleep through the night, wake up in the morning like. But now, it just hurts. I’m just sick and tired of being in pain… They just stopped giving it [Percocet]… I guess they changed their policy. (ID07; Male, 50-59 years)
But more often than directly impacting their ability to access opioid medications to manage pain, the effects of policies aimed at reducing opioid supply and dosage were less overt or direct. At the macro-level, federal prescribing policies are explicit in advising clinicians to individually assess the benefits and harms of opioids for each patient (Dowell et al., 2016). Nonetheless, some patients believed that policies developed by government agencies stigmatized them as potential ‘drug abusers,’ inducing fear of tapers and fostering a climate of mistrust toward their clinicians. Other patients reported feelings of stigma and shame for taking opioids for the treatment of pain as emerging within the broader social risk environment.
While some patients described benefiting from participating in a pain management program that involved cognitive behavioral and physical therapy, other patients who had experienced an overdose were suspicious of such programs, perceiving that the ‘main objective’ of these programs was to ‘[get] people off of opiates altogether.’ Patients who were adamant they were taking their opioids as prescribed felt stigmatized and ‘lumped’ together with people who deliberately deceived clinicians in order to obtain and misuse opioids:
And so those of us who have not abused our medications are suffering… We don’t doctor shop, we don’t even pharmacy shop; we don’t go to other clinics. We never have. We only have our prescriptions filled at one clinic. So, they’re passing judgment upon us for what other actions have been done. (ID09, Male, 40-49 years)
At the micro-level, this sense of stigmatization increased patients’ vulnerability by undermining their willingness to communicate openly with their clinician.
Macro level policies impacted the social risk environment by complicating interactions between patient and provider by eroding trust and inducing fear. As macro-level restrictive opioid prescribing policies became more prevalent, they influenced patient perceptions by leading them to believe that clinicians were subject to external pressures to taper and discontinue opioid medications. As a result, patients worried that policies and shifting opioid prescribing norms would interfere with their clinicians’ ability to provide the pain treatment they needed. They were concerned that clinicians would indiscriminately change their prescribing to align with policies and guidelines rather than tailor treatment to address their chronic pain. One patient believed that clinicians were applying policies bluntly with little regard for the individual health needs of patients: “They go strictly about protocol. They don’t take in each person as an individual.”
Fear discouraged patients from speaking openly with their clinicians about challenges managing their pain, opioid alternatives, or even, discontinuing opioid therapy. Patients were acutely aware of raising red flags for suspected drug-seeking and risk losing access to medications:
Patient: You don’t want to ask too many questions sometimes because then the doctor will say “Well, that’s not good.” You know, people don’t want to ask things because they’re afraid it will raise a red flag on them for getting the drug… in other words, you don’t want to cause any problems where they might say ‘Well, let’s just take her off that drug.’
Interviewer: And what kind of questions [would you like to] be able to ask…?
Patient: :…[I]s there an alternative? Something that’s like this but is not so ranked high on the list where I have to lock them up in a safe because somebody’s going to take them… the only thing, too, that I’ve always wondered… If I wanted to totally stop, what would I have to do?(ID14, Female, 70-79)
Feeling excluded from contributing to treatment decisions and fearful of losing access to their medication, these patients took measures to prevent the consequences of a taper and corresponding unmet pain needs. For example, some patients who had not been tapered, but were fearful of losing future access to opioids, began stockpiling medications:
Patient: One thing that I did that I hid from my doctor was, I had piled a cache of opioid medication, morphine and oxycodone…
Interviewer: What inspired you to stockpile it? Was that a conscious decision?
Patient: Yeah, it really was. I was just afraid that there would be times when I would be in more pain and they were willing to pull me through… especially after the pain management program. I didn’t trust my doctor or any of the therapists on the team. (ID21, Male, 50-59 years)
Taking matters into their own hands: engaged in behaviors to manage pain on their own terms
Paradoxically, as opioid prescribing policies became more restrictive, the patients we interviewed, who had all experienced an overdose, engaged in more high-risk behaviors. They perceived that their access to the only remaining source of pain relief in sanctioned medical settings was threatened. Feeling frustrated trying to manage pain within an environment in which their pain treatment needs felt secondary, patients took matters into their own hands when they experienced a distressing event or acute exacerbation of their chronic pain. Patients acted within the macro and micro risk environments by adjusting the way they took medications to achieve greater pain relief, by taking more medications than prescribed, taking them in different intervals than prescribed, mixing them with alcohol and other substances, or using illicitly obtained opioids, ultimately leading to an accidental overdose. For example, the patient who had stockpiled medications in fear of a taper described taking more of these medications to find relief from uncontrolled pain:
Interviewer: What was going on the day that you had the overdose?
Patient: My pain was just out of control… And I just started taking more [not as prescribed] and I finally just passed out and my [home] nurse found me in the morning on the floor. (ID21, Male, 50-59 years)
Some patients were aware of the risks but engaged in risky behaviors anyway because they were desperate for relief. In the face of unrelenting pain, one patient described taking more medications than prescribed and mixing them with alcohol:
I just couldn’t stop the pain, and I just got mad. I was like, goddammit, I’m tired of being in pain. And so, I think the anger and just wanting to be pain-free… At that point, I had taken one [pill]. It didn’t work. So, I took another one. Still didn’t work. So, I took another one… It was excruciating. And then I said, you know what? Then I took a shot of some brandy and I fell asleep. And my wife said I – my breathing got [slow] (ID07; Male, 50-59 years)
Another patient described feeling frustrated by the perception that her clinicians was not taking her pain seriously enough:
I felt like when I would go to the doctor it was like – it was like not even important really. Like it was not – it was like “Okay, take some Benadryl; you’re going to be fine.” And I was like oh my God, I’m covered in hives; like I’m not going to be fine. Like do something. (ID01, Female, 20-29 years)
When her pain became intense, she described feeling like she did not have anyone to turn to for help who would take her pain seriously. Desperate for relief, and lacking adequate knowledge about medication safety, she described mixing tramadol prescribed previously for an unrelated painful condition with diphenhydramine, leading to her overdose:
I was under a lot of stress and I had a lot of hives, so I didn’t know what to do…I was desperate…I didn’t know what else to do with that. I mean I was in a lot of stress and during the time I also wanted to take a nap, but it was really hard when you have hives and you’re scratching and it’s just too much. So, I wanted to sleep, and obviously like all the stuff that I tried in the past wasn’t working. And my provider had [dispensed] me in the past tramadol; so I said, well, I don’t know. I remember it made pain stop so maybe – I don’t know. I just was connecting the thoughts I guess kind-of wrongly, and thought well, maybe Benadryl will make me fall asleep and tramadol will make the pain stop. So I did that… And I was just very sleepy and I passed out. (ID01, Female, 20-29 years)
Some patients unwittingly engaged in risky behaviors they did not perceive as dangerous in order to relieve severe, uncontrolled pain and/or related acute conditions. Others, who were more educated about risks, engaged in these behaviors because they felt desperate about their pain and symptoms.
In summary, patients described shifts in the macro policy and micro social risk environments that contributed to these factors (Figure 1). Together, these results illustrate a complex overdose risk environment in which patients perceived limited options for pain management treatment that were feasible, effective, trusted, and concordant with expectations, a mismatch between medication safety information needs and commonly available sources of information in clinical settings, and a shifting clinical and policy landscape with regards to the role of opioids in the management of chronic pain.
Figure 1.
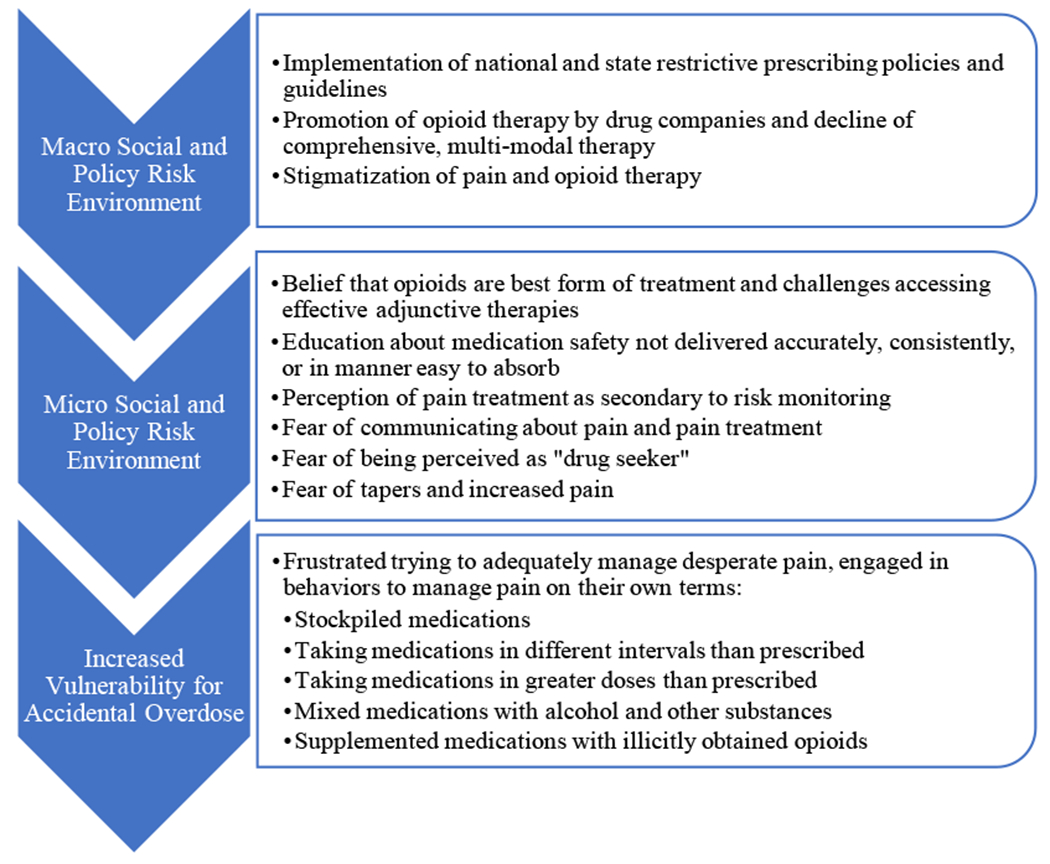
Interaction of Risk Environments Contributing to Accidental Overdose Among Patients Prescribed Opioids
Discussion
Summary of findings
By focusing on the lived experiences of patients prescribed opioids who had an overdose, this study sought to examine the unintended consequences of opioid prescribing policies on patient behaviors and patient-provider interactions that contributed to the risk environment for opioid overdose in the third phase of the opioid epidemic(Ciccarone, 2019). We found that patients largely attributed their overdose events to three frequently coinciding factors: desperation from persistent pain and comorbidities, limited knowledge about opioid medication safety and effectiveness, and restrictive opioid prescribing policies that contributed to feelings of stigma, fear and mistrust and prevented open patient-clinician communication. These findings suggest that macro-level restrictive policies and guidelines, while designed to reduce opioid overdoses, may in fact interact with micro policy and social environments to increase vulnerability to overdoses. (Rhodes, 1995, 2002)
This study breaks new ground by drawing on the lived experience of patients to elucidate behaviors and experiences that preceded overdose events and to situate them in the evolving risk environment (Rhodes, 1995). Many of the patients we interviewed reported initiating opioid therapy years prior in earlier risk environments, characterized by indiscriminate opioid prescribing, few effective alternatives and poorly managed pain. Liberal, and in some cases inappropriate, prescribing put many patients at increased risk for dependence, addiction and overdose (Dasgupta et al., 2018). Many patients we interviewed also described receiving inadequate information about the potential for addiction, a byproduct of being prescribed them in an macro policy and social environment in which opioid medications were being aggressively marketed as safe and with little potential for addiction (Van Zee, 2009). At the time, pain treatment policies emphasized patient satisfaction, and as a result, patients may have been more likely to communicate with their clinicians when experiencing acute episodes of pain.
With shifts in the macro policy, the micro social environments in which patients and clinicians discussed medication safety and pain treatment also changed. Polices were implemented to protect patients by preventing exposure, addiction and overdose, but led to interactions between macro and micro risk environments that may have, paradoxically, put some patients at increased risk for overdose. During later phases of the opioid overdose epidemic, patients described being afraid to communicate openly about pain and medications because of raising concerns about drug-seeking and tapers. This shift in the micro social environment precipitated changes in individual patients’ medication taking behaviors and increased vulnerability to overdose events.
Implications for Pain Management
The Risk Environment Framework advocates for the creation of “enabling environments” for harm reduction that address and remove systemic factors of risk (Rhodes, 2002). Recognizing the broader social and policy contexts in which overdoses occur, there are a variety of potential implications derived from our findings that can create enabling environments to prevent overdose.
In the restrictive opioid policy context, some patients mistrusted recommendations for adjunctive non-opioid treatment as an attempt to replace opioid pain medications. Providers and programs may need to explicitly address these concerns by reassuring patients that they are prioritizing access to evidence-based comprehensive pain management treatment. While some patients we interviewed reported being offered non-opioid pain treatments, others described a reluctance to engage in discussion of these due to mistrust. Some also described systemic and individual barriers to following clinicians’ recommendations, such as the need to take time off work and mobility limitations, reflective of the economic and physical domains of risk environment framework, that prevented physical therapy attendance. Minimizing such barriers to comprehensive pain management programs and adjunctive, non-opioid pain management modalities may help improve pain and foster enabling environments to prevent overdoses associated with persistent pain. In addition, supporting wider implementation of psychological interventions, such as acceptance and commitment therapy and cognitive behavioral therapy, may reduce pain catastrophizing and improve functioning and quality of life (Smeets et al., 2006; Vowles & McCracken, 2008).
Some patients attributed behaviors that led to overdose to feelings of desperation from persistent pain and contributing comorbidities, both physical and psychological in nature. Some patients may benefit from more intensive support and validation during episodes of increased pain and distress. Pain validation includes listening and paying close attention to patients experience of pain, reflecting back to patients what they have said to convey understanding, and expressing that the patient’s experience is understandable and legitimate, even if there is disagreement about the patient’s perspective (Edmond & Keefe, 2015). Treating patients as unique individuals may improve patients’ emotional state and health behaviors (Edmond & Keefe, 2015), and improve outcomes (Hadjistavropoulos et al., 2011). Further, ensuring that the language used in patient education materials, policies, and clinical guidelines is validating and conveys support may also be important. While these communication strategies represent high quality patient-centered care, it is unknown whether they also prevent overdose outcomes.
Additionally, increased screening and treatment for co-occurring mental health conditions or traumatic events related to patients’ chronic pain could help prevent accidental opioid overdose. Indeed, there is increasing recognition of the impacts of lifetime trauma on one’s overall health (Machtinger et al., 2015) and on chronic pain in particular (Driscoll et al., 2020). Trauma-informed approaches to care may contribute to an enabling environment for risk reduction by allowing clinicians to better contextualize patients’ experiences of pain (Hales et al., 2018) and interact with patients in a way that promotes feelings of safety, trustworthiness, choice, collaboration and empowerment (Driscoll et al., 2020). Implementing this approach could in turn improve patient-provider communication and mutual understanding and reduce barriers to effective treatment of pain and related comorbidities (Driscoll et al., 2020), creating an environment that supports risk reduction.
Implications for Knowledge about Medication Safety and Effectiveness
In our study, some patients who experienced an overdose relied on package inserts and pharmacy documents for information about medication safety but found these difficult to understand or lacking in specificity. Other patients reported limited education from their personal clinician about how to take their medications, which may have been worsened by mistrust related to the shifting macro policy environment. Some patients exhibited knowledge gaps about what to do in an acute pain flare up or had inappropriate expectations for the resolution of pain. Both chronic pain and chronic opioid therapy are associated with reduced spatial memory and impaired performance in working memory (Schiltenwolf et al., 2014). While the patients in our study who reported little or no education may not have received it, it is also possible that information was not provided in an appropriate format, at an adequate frequency, or in the appropriate manner to meet patients’ cognitive needs. Patients may also have needed different guidance and instruction from clinicians than they received, such as information about self-management techniques and when and how emergency care should be sought (Institute of Medicine Committee on Advancing Pain Research & Education, 2011). Further efforts to improve written materials that accompany prescriptions and to effectively communicate overdose risk may be indicated, and clinical teams may need to consider who is best to communicate key information to patients, in what format, and how often.
Another approach that has been proposed to guide clinicians is the “benefit-to-harm framework,” which advocates for more transparent communication about medical decisions involving opioids and invites shared decision-making about pain treatment (Nicolaidis, 2011). This approach emphasizes patient-centered care and ‘focuses decisions and discussions on judging the treatment, not the patient’ (Nicolaidis, 2011). Adopting this framework may help clinicians address stigma, fear and mistrust fostered in the current macro policy and social environments by minimizing the perceived deviance of people taking opioids (Treloar et al., 2016). Our results also suggest that patients interpreted the conditions that led to their overdose events differently from common narratives that patients intentional misuse opioids for euphoric effects. Encouraging clinicians to understand the factors that contributed to overdose events from patients’ perspectives may help them work together with patients to develop strategies to prevent recurrent events.
Implications for Policies and Guidelines
With the introduction of more restrictive opioid prescribing policies in 2015-2016, domains within both macro and micro levels of the risk environment shifted for patients. Clinicians report that reducing dosage and limiting opioid prescribing has become a major emphasis in pain treatment (Webster et al., 2019), and that they are increasingly being asked to play the role of police officer and judge, prescribing opioids within a law enforcement framework (Nicolaidis, 2011). An ethnography of clinicians who provided care to patients living with chronic pain found that they were fearful of losing their license for perceived inappropriate prescribing, and as a result, felt that their primary responsibility of providing treatment and care had shifted to policing their patients and limiting their risk of malpractice (Webster et al., 2019). While policies may prevent the initiation of opioids, research shows that these risk mitigation strategies may have the unintended effect of perpetuating ‘a climate of distrust and stigmatization without correcting systemic factors that may have placed patients and others at risk in the first place’ (Ho, 2017).
Restrictive prescribing policies may exacerbate stigma for people seeking treatment for chronic pain in the wider macro social environment and become problematic when patients are actively discriminated against (Tsai et al., 2019). But policies are also problematic when patients’ vague or incomplete perceptions of policies lead them to internalize stigma and adopt maladaptive behaviors to prevent discriminatory behavior (Tsai et al., 2019). In the case of our study, many patients did not describe direct changes to their medications, but instead described evolving clinical environments where they were reluctant to engage with clinicians regarding challenging medical needs out of fear of being tapered should they ask questions or request treatment that affirmed stereotypes of the ‘drug abuser.’ Subsequently, patients changed the way they communicated with clinicians and took their medication to manage their pain.
Consistent with our study findings, Antoniou et. al, found that changes in opioid-related policies had unintentional consequences for people prescribed opioids (Antoniou et al., 2019). They found that policies had the effect of increasing stigma, undermining patient autonomy, and intensifying structural vulnerabilities with negative repercussion for patients seeking help (Antoniou et al., 2019). To counteract stigma that is enacted at a macro level through policies and guidelines, they advocate for greater inclusion of people taking opioids in the formulation and implementation of opioid policies.
Limitations
This study describes the perceptions of patients prescribed opioids who experienced an opioid overdose that was documented in the medical record and survived, and thus, is not generalizable to pain management or overdose experiences of all patients in the health systems we studied. Furthermore, we only interviewed patients who were willing and able to discuss their experience. Our purposive sampling approach specifically identified individuals with an adverse event from opioids after a major shift in the opioid policy landscape to learn from their experiences. Thus, this study does not represent the perspectives of those patients who had positive outcomes and benefited from policies and clinical practices and lacks the perspective of clinicians. Patients may not have recalled the series of events that directly preceded an opioid overdose or remembered them differently after they experienced an opioid overdose. In a limited number of interviews, caregivers and family members were able to provide supplemental information, but we found that some information about the events leading up overdose events were missing from patients’ descriptions and was discordant with the information in the medical record. Finally, this study used a sampling approach and qualitative methodology designed to uncover and document remaining problems from the perspective of patients, and thus was not designed to identify how many individuals may have benefited from pain management, educational, and policy interventions implemented in these health systems that were designed to address the opioid crisis.
Future Research
Given the possible paradoxical effects of restrictive prescribing policies on opioid overdose events (Foglia, 2019; Glod, 2017; Yuanhong Lai et al., 2019), future research should systematically examine intended and unintended effects of policies on the patient-clinician relationship and overdose outcomes. Consistent with the Risk Enviroment Framework that aims to create “enabling environments” for the reduction of drug related harm, future studies should also identify and evaluate the effectiveness of interventions specifically designed to remove “micro-barriers” to overdose (Moore, 2004). Future epidemiologic investigations could include assessing the net benefits and harms of changes in pain management approaches, educational approaches, and policy interventions, and how anticipated changes impact risk behavior. Given patients’ perspectives that stigma may have contributed to overdose events, future research should evaluate ways to reduce stigma toward individuals prescribed opioids for pain.
Changes in clinical practices and policies recommended here may help modify the factors that constitute risk environments, which increased vulnerability to the overdose events we studied. In addition, it is important to note that a majority of clinicians who treat chronic pain experience difficulties when caring for patients with chronic non-cancer pain, leading to burnout and emotional exhaustion (Kroll et al., 2016; Matthias et al., 2010). Improving support and training for clinicians may improve the patient-clinician relationship and improve patient care and, thus, potentially, overdose outcomes (Matthias et al., 2010).
In conclusion, for a subgroup of patients prescribed opioids, perceptions about restrictive prescribing policies may produce risk environments that lead to unintended consequences and deleterious effects. Policies concerned with opioid supply restrictions alone may be insufficient to address the opioid crisis, as persistent pain and a need for increased knowledge about medication safety persist. To address these unmet needs, there has been a recent push to better understand the effects of stigma and the necessity for better pain treatment (Volkow, 2020). Until these other contributors to the risk environment are addressed and enabling environments for harm reduction are improved, behaviors that result in accidental overdose may continue.
Funding
Research reported in this publication was supported by the National Institute On Drug Abuse of the National Institutes of Health under award number R01DA042059. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix 1:
International Classification of Diseases, Tenth Revision (ICD-10) Codes Used to Identify Opioid Overdoses
| DX | Description |
|---|---|
| T40.0X1 | Poisoning by opium, accidental (unintentional) |
| T40.0X2 | Poisoning by opium, intentional self-harm |
| T40.0X3 | Poisoning by opium, assault |
| T40.0X4 | Poisoning by opium, undetermined intent |
| T40.1X1 | Poisoning by heroin, accidental (unintentional) |
| T40.1X2 | Poisoning by heroin, intentional self-harm |
| T40.1X3 | Poisoning by heroin, assault |
| T40.1X4 | Poisoning by heroin, undetermined intent |
| T40.2X1 | Poisoning by other opioids, accidental (unintentional) |
| T40.2X2 | Poisoning by other opioids, intentional self-harm |
| T40.2X3 | Poisoning by other opioids, assault |
| T40.2X4 | Poisoning by other opioids, undetermined intent |
| T40.3X1 | Poisoning by methadone, accidental (unintentional) |
| T40.3X2 | Poisoning by methadone, intentional self-harm |
| T40.3X3 | Poisoning by methadone, assault |
| T40.3X4 | Poisoning by methadone, undetermined intent |
| T40.4X1 | Poisoning by other synthetic narcotics, accidental (unintentional) |
| T40.4X2 | Poisoning by other synthetic narcotics, intentional self-harm |
| T40.4X3 | Poisoning by other synthetic narcotics, assault |
| T40.4X4 | Poisoning by other synthetic narcotics, undetermined intent |
| T40.6X1 | Poisoning by unspecified narcotics, accidental (unintentional) |
| T40.6X2 | Poisoning by unspecified narcotics, intentional self-harm |
| T40.6X3 | Poisoning by unspecified narcotics, assault |
| T40.6X4 | Poisoning by unspecified narcotics, undetermined intent |
| T50.9X1 | Poisoning by unspecified drugs, accidental (unintentional) |
| T50.9X2 | Poisoning by unspecified drugs, intentional self-harm |
| T50.9X3 | Poisoning by unspecified drugs, assault |
| T50.9X4 | Poisoning by unspecified drugs, undetermined intent |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Interest
Dr. Ingrid Binswanger receives royalties from UpToDate but does not have any additional conflict of interest. All remaining authors declare that they do not have a conflict of interest.
References
- Antoniou T, Ala-Leppilampi K, Shearer D, Parsons JA, Tadrous M, & Gomes T (2019). “Like being put on an ice floe and shoved away”: A qualitative study of the impacts of opioid-related policy changes on people who take opioids. International Journal of Drug Policy, 66, 15–22. 10.1016/i.drugpo.2019.01.015 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, & National Center for Health Statistics. (April 29, 2020). Drug overdose mortality by state. Retrieved July 7, 2020 from https://www.cdc.gov/nchs/pressroom/sosmap/drug_poisoning_mortality/drug_poisoning.htm [PubMed]
- Cho JY, & Lee E-H (2014). Reducing confusion about grounded theory and qualitative content analysis: Similarities and differences. The qualitative report, 19(32), 1–20. [Google Scholar]
- Ciccarone D (2019). The triple wave epidemic: Supply and demand drivers of the us opioid overdose crisis. International Journal of Drug Policy, 71, 183–188. 10.1016/i.drugpo.2019.0L010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colorado Department of Health Care Policy and Financing. (September 2018). Opioid use in Colorado: Colorado medicaid addresses addiction. Retrieved June 12, 2020 from https://www.colorado.gov/pacific/sites/default/files/Opioid%20Use%20In%20Colorado%20August%202018.pdf
- Dasgupta N, Beletsky L, & Ciccarone D (2018). Opioid crisis: No easy fix to its social and economic determinants. American Journal of Public Health, 108(2), 182–186. 10.2105/AJPH2017.3Q4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, & Chou R (2016). CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR: Recommendations and Reports, 65(1), 1–49. 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- Driscoll M, Adams L, & Satchell J (2020). Optimizing care using a trauma-informed approach. Practical Pain Management, 20(3), 29–31. [Google Scholar]
- Drug Enforcement Administration, D.o.J. (2014). Schedules of controlled substances: Rescheduling of hydrocodone combination products from schedule iii to schedule ii. Final rule. Federal Register, 79(163), 49661–49682. [PubMed] [Google Scholar]
- Edmond SN, & Keefe FJ (2015). Validating pain communication: Current state of the science. Pain, 156(2), 215–219. 10.1097/0Li.pain.000046030L182Q7.c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo S, & Kyngas H (2008). The qualitative content analysis process. Journal of Advanced Nursing, 62(1), 107–115. 10.1111/j.1365-2648.2007.04569.x [DOI] [PubMed] [Google Scholar]
- Foglia MB (2019). When people you love are the unintended consequences of opioid policy. Health Affairs, 38(12), 2105–2108. 10.1377/hlthaff.2019.00697 [DOI] [PubMed] [Google Scholar]
- Foley KM (1985). The treatment of cancer pain. New England Journal of Medicine, 313(2), 84–95. [DOI] [PubMed] [Google Scholar]
- Friese S (2019). Qualitative data analysis with ATLAS. Ti. SAGE Publications Limited. [Google Scholar]
- Gale NK, Heath G, Cameron E, Rashid S, & Redwood S (2013). Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Medical Research Methodology, 13(1), 117. 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A (2010). The pastoral clinic : Addiction and dispossession along the rio grande. University of California Press. [Google Scholar]
- Glanz JM, Narwaney KJ, Mueller SR, Gardner EM, Calcaterra SL, Xu S, Breslin K, & Binswanger IA (2018). Prediction model for two-year risk of opioid overdose among patients prescribed chronic opioid therapy. Journal of General Internal Medicine, 33(10), 1646–1653. 10.1007/s11606-017-4288-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glod SA (2017). The other victims of the opioid epidemic. New England Journal of Medicine, 376(22), 2101–2102. 10.1056/NEJMp1702188 [DOI] [PubMed] [Google Scholar]
- Green TC, Grau LE, Blinnikova KN, Torban M, Krupitsky E, Ilyuk R, Kozlov A, & Heimer R (2009). Social and structural aspects of the overdose risk environment in st. Petersburg, russia. International Journal on Drug Policy, 20(3), 270–276. https://doi.org/S0955-3959(08)00166-7 [pii] 10.1016/j.drugpo.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjistavropoulos T, Craig KD, Duck S, Cano A, Goubert L, Jackson PL, Mogil JS, Rainville P, Sullivan MJL, Williams ACC, Vervoort T, & Fitzgerald TD (2011). A biopsychosocial formulation of pain communication. Psychological Bulletin, 137(6), 910–939. 10.1037/a0023876 [DOI] [PubMed] [Google Scholar]
- Hales TW, Green SA, Bissonette S, Warden A, Diebold J, Koury SP, & Nochajski TH (2018). Trauma-informed care outcome study. Research on Social Work Practice, 29(5), 529–539. 10.1177/1049731518766618 [DOI] [Google Scholar]
- Hartung DM, Kim H, Ahmed SM, Middleton L, Keast S, Deyo RA, Zhang K, & McConnell KJ (2017). Effect of a high dosage opioid prior authorization policy on prescription opioid use, misuse, and overdose outcomes. Substance Abuse, 39(2), 239–246. 10.1080/08897077.2017.1389798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Professionals for Patients in Pain. (March 6, 2019). Letter to the CDC HP3 march 6, 2019 https://docs.google.com/document/d/1RzQDSppUKhjiAsEmhW2WbTXlP5V8vJ4M_vBPQLKhK_8/edit
- Ho A (2017). Reconciling patient safety and epistemic humility: An ethical use of opioid treatment plans. Hastings Center Report, 47(3), 34–35. 10.1002/hast.703 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Advancing Pain Research, C., & Education. (2011). The national academies collection: Reports funded by national institutes of health (Relieving pain in america: A blueprint for transforming prevention, care, education, and research. National Academies Press (US) Copyright © 2011, National Academy of Sciences. 10.17226/13172 [DOI] [Google Scholar]
- Jones MR, Viswanath O, Peck J, Kaye AD, Gill JS, & Simopoulos TT (2018). A brief history of the opioid epidemic and strategies for pain medicine. Pain and therapy, 7(1), 13–21. 10.1007/s40122-018-0097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz SG (2016). Turning the tide or riptide? The changing opioid epidemic. Substance Abuse, 1–6. 10.1080/08897077.2016.1261070 [DOI] [PubMed] [Google Scholar]
- Koester S, Mueller SR, Raville L, Langegger S, & Binswanger IA (2017). Why are some people who have received overdose education and naloxone reticent to call emergency medical services in the event of overdose? The International journal on drug policy, 48, 115–124. 10.1016/i.drugpo.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll HR, Macaulay T, & Jesse M (2016). A preliminary survey examining predictors of burnout in pain medicine physicians in the United States. Pain Physician, 19(5), E689–696. [PubMed] [Google Scholar]
- Machtinger EL, Cuca YP, Khanna N, Rose CD, & Kimberg LS (2015). From treatment to healing: The promise of trauma-informed primary care. Women’s Health Issues, 25(3), 193–197. 10.1016/i.whi.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Mars SG, Fessel JN, Bourgois P, Montero F, Karandinos G, & Ciccarone D (2015). Heroin-related overdose: The unexplored influences of markets, marketing and source-types in the United States. Social Science and Medicine, 140, 44–53. 10.1016/i.socscimed.2015.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias MS, Parpart AL, Nyland KA, Huffman MA, Stubbs DL, Sargent C, & Bair MJ (2010). The patient-provider relationship in chronic pain care: Providers’ perspectives. Pain Medicine, 11(11), 1688–1697. 10.1111/j.1526-4637.2010.00980.x [DOI] [PubMed] [Google Scholar]
- McLean K (2016). “There’s nothing here”: Deindustrialization as risk environment for overdose. International Journal on Drug Policy, 29, 19–26. 10.1016/i.drugpo.2016.01.009 [DOI] [PubMed] [Google Scholar]
- Moore D (2004). Governing street-based injecting drug users: A critique of heroin overdose prevention in australia. Social Science and Medicine, 59(7), 1547–1557. 10.1016/j.socscimed.2004.01.029 S0277953604000346 [pii] [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. (April 2020). Colorado: Opioid-involved deaths and related harms. Retrieved June 10, 2020 from https://www.drugabuse.gov/drugs-abuse/opioids/opioid-summaries-by-state/colorado-opioid-summary#:~:text=In%20Colorado%2C%20564%20opioid%2Dinvolved,9.5)%20were%20reported%20in%202018.
- Nicolaidis C (2011). Police officer, deal-maker, or healthcare provider? Moving to a patient-centered framework for chronic opioid management. Pain medicine (Malden, Mass.), 12(6), 890–897. 10.llll/i.1526-4637.2011.01117.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy RK, & Foley KM (1986). Chronic use of opioid analgesics in non-malignant pain: Report of 38 cases. Pain, 25(2), 171–186. [DOI] [PubMed] [Google Scholar]
- Raji MA, Kuo YF, Adhikari D, Baillargeon J, & Goodwin JS (2018). Decline in opioid prescribing after federal rescheduling of hydrocodone products. Pharmacoepidemiology and Drug Safety, 27(5), 513–519. 10.10Q2/pds.4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Razaghi n., Abdolrahimi m, & Salsali m. (2015). Memo and memoing in qualitative research: A narrative review
 . jqr.ir, 4(2), 206–217. http://jqr.kmu.ac.ir/article-1-413-en.html [Google Scholar]
. jqr.ir, 4(2), 206–217. http://jqr.kmu.ac.ir/article-1-413-en.html [Google Scholar] - Rhodes T (1995). Theorizing and researching ‘risk’: Notes on the social relations of risk in heroin users’ lifestyles. AIDS: Safety, sexuality and risk, 125–143. [Google Scholar]
- Rhodes T (2002). The ‘risk environment’: A framework for understanding and reducing drug-related harm. International journal of drug policy, 13(2), 85–94. [Google Scholar]
- Rhodes T (2009). Risk environments and drug harms: A social science for harm reduction approach. International Journal on Drug Policy, 20(3), 193–201. https://doi.org/S0955-3959(08)00203-X [pii] 10.1016/j.drugpo.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Rhodes T, Singer M, Bourgois P, Friedman SR, & Strathdee SA (2005). The social structural production of hiv risk among injecting drug users. Social Science and Medicine, 61(5), 1026–1044. 10.1016/j.socscimed.2004.12.024 [DOI] [PubMed] [Google Scholar]
- Rhodes T, Stimson GV, Moore D, & Bourgois P (2010). Qualitative social research in addictions publishing: Creating an enabling journal environment. International Journal of Drug Policy, 21(6), 441–444. https://doi.org/DOI 10.1016/j.drugpo.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich RLC Jr. (2020). Prescribing opioids for chronic pain: Unintended consequences of the 2016 CDC guideline. American Family Physician, 101(8), 458–459. [PubMed] [Google Scholar]
- Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, Burroughs H, & Jinks C (2018). Saturation in qualitative research: Exploring its conceptualization and operationalization. Quality & quantity, 52(4), 1893–1907. 10.1007/s11135-017-0574-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltenwolf M, Akbar M, Hug A, Pfüller U, Gantz S, Neubauer E, Flor H, & Wang H (2014). Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain Physician, 17(1), 9–20. [PubMed] [Google Scholar]
- Smeets RJEM, Vlaeyen JWS, Kester ADM, & Knottnerus JA (2006). Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. The Journal of Pain, 7(4), 261–271. 10.1016/j.jpain.2005.10.011 [DOI] [PubMed] [Google Scholar]
- State member board briefs. (2014). Journal of Medical Regulation, 100(4), 32–34. 10.30770/2572-1852-100.4.32 [DOI] [Google Scholar]
- Substance use-disorder prevention that promotes opioid recovery and treatment (support) for patients and communities act (public law 115-271) (2018).
- Tompkins DA, Hobelmann JG, & Compton P (2017). Providing chronic pain management in the “fifth vital sign” era: Historical and treatment perspectives on a modern-day medical dilemma. Drug and Alcohol Dependence, 173 Suppl 1(Suppl 1), S11–S21. 10.1016/i.drugalcdep.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar C, Rance J, Yates K, & Mao L (2016). Trust and people who inject drugs: The perspectives of clients and staff of needle syringe programs. International Journal on Drug Policy, 27, 138–145. 10.1016/j.drugpo.2015.08.018 [DOI] [PubMed] [Google Scholar]
- Tsai AC, Kiang MV, Barnett ML, Beletsky L, Keyes KM, McGinty EE, Smith LR, Strathdee SA, Wakeman SE, & Venkataramani AS (2019). Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Medicine, 16(11), e1002969. 10.1371/iournal.pmed.1002969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zee A (2009). The promotion and marketing of oxycontin: Commercial triumph, public health tragedy. American Journal of Public Health, 99(2), 221–227. 10.2105/AJPH.2007.131714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND (2020). Stigma and the toll of addiction. New England Journal of Medicine, 382(14), 1289–1290. 10.1056/NEJMp1917360 [DOI] [PubMed] [Google Scholar]
- Vowles KE, & McCracken LM (2008). Acceptance and values-based action in chronic pain: A study of treatment effectiveness and process. Journal of Consulting and Clinical Psychology, 76(3), 397–407. 10.1037/0022-0Q6x.76.3.397 [DOI] [PubMed] [Google Scholar]
- Ward DJ, Furber C, Tierney S, & Swallow V (2013). Using framework analysis in nursing research: A worked example. Journal of Advanced Nursing, 69(11), 2423–2431. 10.1111/ian.12127 [DOI] [PubMed] [Google Scholar]
- Webster F, Rice K, Katz J, Bhattacharyya O, Dale C, & Upshur R (2019). An ethnography of chronic pain management in primary care: The social organization of physicians’ work in the midst of the opioid crisis. PloS One, 14(5), e0215148. 10.1371/iournal.pone.0215148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M (October 31, 2018). Colorado medicaid to further tighten opioid policies. Retrieved June 12, 2020 from https://www.colorado.gov/pacific/hcpf/news/colorado-medicaid-further-tighten-opioid-policies
- Yuanhong Lai A, Smith KC, Vernick JS, Davis CS, Caleb Alexander G, & Rutkow L (2019). Perceived unintended consequences of prescription drug monitoring programs. Substance Use and Misuse, 54(2), 345–349. 10.1080/10826084.2018.1491052 [DOI] [PubMed] [Google Scholar]


