Abstract
Background:
Data on human metapneumovirus (HMPV)-associated severe acute respiratory illness (SARI) are limited in settings with high human immunodeficiency virus (HIV) infection prevalence.
Objectives:
To describe clinical characteristics and seasonality (all sites), and incidence (Soweto only) of HMPV-associated SARI among children and adults.
Study design:
Active, prospective, hospital-based, sentinel surveillance for patients hospitalised with SARI was conducted at four sites in South Africa from February 2009–December 2013. Upper respiratory tract samples were tested by multiplex real-time polymerase chain reaction assays for HMPV and other respiratory viruses. Incidence of hospitalisation, stratified by age and HIV-infection status, was calculated for one hospital with population denominators.
Results:
HMPV was identified in 4.1% of patients enrolled, including 5.6% (593/10503) in children and 1.7% in adults (≥18 years; 119/6934). The majority of adults (84.0%) had an underlying medical condition, including HIV infection in 87/110 (79.1%). HMPV detection occurred perennially with periods of increased detection, which varied from year to year. The incidence of HMPV-associated hospitalisation in Soweto was highest in infants (653.3 per 100,000 person years; 95% confidence interval (CI) 602.2–707.6). The incidence was higher in HIV-infected persons compared to HIV-uninfected persons in age-groups 5–17 years (RR 6.0; 1.1–20.4), 18−44 years (RR 67.6; 38.0–132.6) and 45–64 years (RR 5.3; 3.4–8.3), while not differing in other age-groups.
Conclusions:
The burden of HMPV-associated SARI hospitalisation among adults occurred predominantly in HIV-infected persons. Among children, infants were at highest risk, with similar burden of hospitalisation in HIV-infected and HIV-uninfected children.
Keywords: Human metapneumovirus, HIV, Lower respiratory tract infection
1. Background
Human metapneumovirus (HMPV) has been implicated as an etiologic agent of lower respiratory tract infection (LRTI) among both healthy and immunocompromised individuals across age groups [1]. The burden of disease is greatest in young children with the majority of infections occurring in children without underlying medical conditions [2,3]. Rates of HMPV infection are lower in adults than children, and severe disease occurs predominantly in the immunocompromised and elderly, who are at increased risk of hospitalisation [4–7].
The role of HMPV in low- and middle-income countries is less well described, although it has been identified in respiratory infections among hospitalised children in Asia, Latin America and Africa [8–15]. HMPV has also been associated with LRTI among adults in Egypt and more frequently detected among older children and adults with acute respiratory infection compared to asymptomatic controls in Kenya [16,17].
Studies undertaken prior to availability of antiretroviral therapy (ART) reported that children infected with human immunodeficiency virus (HIV) had a higher incidence of respiratory viral-associated LRTI, as well as poorer outcomes [18,19]. A previous study in ART-naive HIV-infected children, reported a higher incidence of HMPV-associated hospitalisation, longer duration of hospitalisation, higher mortality rate and higher rates of concurrent bacteraemia than in HIV-uninfected children [15]. The role of HMPV in HIV-infected adults and in the era of ART management has largely not been evaluated.
Although the epidemiology of viral-associated acute LRTI has been previously described in both children and adults in South Africa, with HIV-infection shown to be associated with an increased risk of LRTI hospitalisation and death, the epidemiology of HMPV was not specifically described [20,21].
2. Objectives
We aimed to describe the incidence, seasonality and characteristics of HMPV-associated severe acute respiratory illness (SARI) in HIV-infected and HIV-uninfected children and adults in South Africa.
3. Study design
Prospective, hospital-based, sentinel surveillance on SARI was undertaken from February 2009 to December 2013. The three initial surveillance sites included Soweto in Gauteng Province, Bushbuckridge in Mpumalanga Province and Pietermaritzburg in KwaZulu-Natal Province. An additional site, Klerksdorp in North West Province, was included from June 2010 (Supplementary material). The national HIV prevalence estimate among children <5 years was 4.1% in 2009, decreasing to 3.5% by 2013, whereas prevalence among those ≥18 years remained steady across the study period at ∼16.0% [22]. The rollout of ART in the public sector started in 2004 [23].
An episode of SARI was defined as symptom duration ≤7 days and meeting the following age-specific criteria: 2 days to <3 months: physician-diagnosed suspected sepsis or acute LRTI; 3 months to <5 years: physician-diagnosed acute LRTI including bronchiolitis, pneumonia, bronchitis and pleural effusion; ≥5 years: sudden onset of fever (>38 °C) plus cough or sore throat plus shortness of breath or difficulty in breathing. All patients hospitalised for at least one night from Monday to Friday with SARI were eligible for enrolment, except at the Soweto site where enrolment of adult patients was limited to two of every five working days per week due to high patient numbers and limited resources. In 2013 enrolment at this site was further down-scaled (Supplementary material).
Demographics, socioeconomic factors, clinical characteristics and outcome were recorded by parent or patient interview and hospital record review. A nasopharyngeal aspirate (patients <5 years) or nasopharyngeal and throat swab (patients ≥5 years) and whole blood specimen were collected within 24 h of admission. Respiratory specimens, in viral transport media, and blood specimens were stored at 4–8 °C and transported to the National Institute for Communicable Diseases, Johannesburg, South Africa.
Total nucleic acids were extracted from 200 μL of specimen using a MagNA Pure 96 instrument (Roche, Mannheim, Germany) and eluted into 100 μL of elution buffer. Respiratory specimens were tested by multiplex real-time reverse-transcription polymerase chain reaction (PCR) assay for 10 respiratory viruses including HMPV, adenovirus, enterovirus, influenza A and B viruses, respiratory syncytial virus (RSV), parainfluenza virus I−III and rhinovirus [24]. Streptococcus pneumoniae was identified by lytA quantitative real-time PCR detection from whole blood specimens [25]. Determination of HIV infection status was based either on HIV testing undertaken at the discretion of the attending physician or through anonymised linked dried blood spot specimen testing (HIV PCR for patients <18 months and enzyme-linked immunosorbent assay (ELISA) for patients ≥18 months of age) as part of the study.
3.1. Incidence calculations
Incidence estimates were calculated for the Soweto site, the only site for which population denominators were available. The incidence of HMPV-associated SARI hospitalisations (per 100,000 person-years) was estimated using the number of patients testing positive for HMPV, adjusting for days when enrolment did not occur and non-enrolment due to refusal, in the numerator; and the mid-year population for Soweto (Region D) in the denominator (Supplementary material). Incidence estimates were calculated for the period 2009–2012 (2013 was not included as enrolment was down-scaled in this year) and stratified by age group and HIV status. HIV prevalence in the Soweto population was estimated from projections of the Actuarial Society of South Africa’s 2008 AIDS and Demographical model [22]. For patients without an HIV result, we assumed that the HIV prevalence (by age group and year) among those not tested for HIV was the same as those tested. We also conducted a sensitivity analysis assuming all those with unknown HIV status were HIV-uninfected. Confidence intervals for incidence estimates were calculated using the Poisson distribution.
3.2. Analysis of risk factors
Data from HMPV-associated SARI cases enrolled at all sites from 2009 to 2013 were used to estimate prevalence, describe seasonality and evaluate risk factors associated with HMPV-associated SARI. Clinical and epidemiologic characteristics associated with HIV infection were determined separately for children (patients < 18 years) and adults (patients ≥18 years). Proportions were compared using the χ2 and Fisher exact tests, as appropriate, and continuous data were compared using the Wilcoxon rank sum test. Odds ratios with 95% CI were calculated. All tests were two-sided and p < 0.05 was considered significant. Data analysis was performed using STATA version 12.1 (StataCorp, TX, USA).
3.3. Ethical considerations
Approval was obtained from ethics committees of the University of the Witwatersrand (M081042) and University of KwaZulu-Natal (BF 157/08).
4. Results
A total of 17,801 patients were enrolled from all sites during February 2009–December 2013, of which 17,437 (98.0%) were tested for HMPV. HMPV was detected in 712 (4.1%) and the percentage positive varied by age group: 6.2% (389/6324) in infants, 5.7% (113/1989) in 1-year olds, 5.1% (78/1538) in 2–4 year olds, 2.0% (13/652) in 5–17 year olds, 1.8% (81/4573) in 18–44 year olds, 1.5% (28/1881) in 45–64 year olds, and 2.1% (10/480) in those ≥65 years (p < 0.001).
4.1. Incidence in Soweto
The incidence (per 100,000 person years; 95% CI) of hospitalisation for HMPV-associated SARI in Soweto was highest in infants (653.3; 602.2–707.6), with a lower incidence in 1-year olds (170.8; 145.4–199.3) and 2–4 year olds (33.8; 27.4–41.2; Fig. 1). The lowest incidence was found in children aged 5–17 years (1.7; 1.1–2.6). Incidence in adults was similar across age groups (18–44 years: 10.5; 9.3–11.9; 45–64 years: 10.5; 8.5–13.0; ≥65 years: 10.4; 6.9–15.1).
Fig. 1.
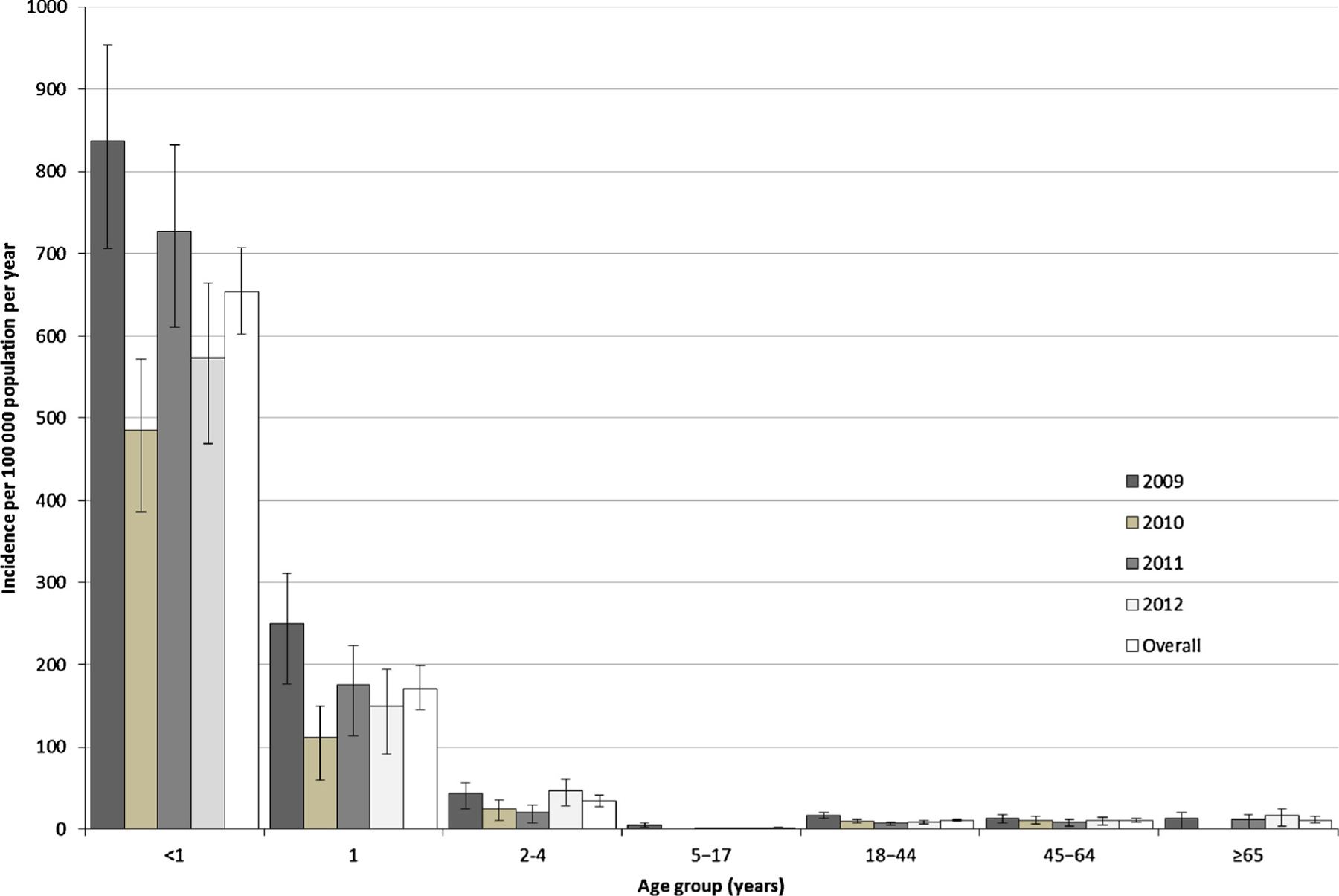
Incidence of hospitalisation (per 100,000 person years) by age group and year for HMPV-associated severe acute respiratory illness in Soweto, South Africa: 2009–2012.
Incidence was similar among HIV-infected and HIV-uninfected children <5 years of age (Table 1). The relative risk for hospitalisation for HMPV-associated SARI among HIV-infected persons was elevated in children 5–17 years (6.0, 95% CI: 1.1–20.4), adults 18–44 years (67.6, 38.0–132.6), and 45–64 years (5.3, 3.4–8.3; Table 1). Incidence estimates obtained from the sensitivity analysis showed similar results (Table 1).
Table 1.
Incidence of HMPV-associated severe acute respiratory illness per 100 000 person-years, by age group and HIV infection status in Soweto, South Africa, 2009–2012.
| HIV-infected | HIV-uninfected | Sensitivity analysisb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Age group (years) | HMPV positive | Population | Incidencea (95% CI) | HMPV positive | Population | Incidencea (95% CI) | Relative risk (95% CI) | Incidence HIV-infected (95% CI) | Incidence HIV-uninfected (95% CI) | Relative risk (95% CI) |
| <1 | 27 | 3159 | 854.7 (563.3–1243.5) |
575 | 88988 | 646.2 (594.4–701.2) |
1.3 (0.9–1.9) |
664.8 (411.5–1016.2) |
652.9 (600.9–708.2) |
1.0 (0.6–1.6) |
| 1 | 7 | 4226 | 165.6 (66.6–341.3) |
154 | 90032 | 171.1 (145.1–200.3) |
1.0 (0.4–2.0) |
94.7 (25.8–242.4) |
174.4 (148.2–203.9) |
0.5 (0.1–1.4) |
| 2–4 | 7 | 13286 | 52.7 (21.2–108.6) |
91 | 276949 | 32.9 (26.5–40.3) |
1.6 (0.6–3.4) |
30.1 (8.2–77.1) |
33.9 (27.4–41.5) |
0.9 (0.2–2.3) |
| 5–17 | 3 | 32936 | 9.1 (1.9–26.6) |
19 | 1254118 | 1.5 (0.9–2.4) |
6.0 (1.1–20.4) |
9.1 (1.9–26.6) |
1.5 (0.9–2.4) |
6.0 (1.1–20.4) |
| 18–44 | 240 | 546520 | 43.9 (38.5–49.3) |
12 | 1846142 | 0.7 (0.3–1.1) |
67.6 (38.0–132.6) |
41.0 (35.8–46.7) |
1.5 (1.0–2.2) |
27.0 (18.2–41.6) |
| 45–64 | 39 | 108053 | 36.1 (25.7–49.3) |
50 | 736895 | 6.8 (5.0–9.0) |
5.3 (3.4–8.3) |
34.2 (24.1–47.2) |
7.1 (5.3–9.3) |
4.9 (3.1–7.5) |
| ≥65 | 0 | 3582 | 0 | 28 | 265363 | 10.6 (7.0–15.3) |
– | 0 | 10.6 (7.0–15.3) |
– |
CI – confidence interval.
Assuming HIV prevalence among those not tested for HIV was the same as those who were tested for HIV.
Assuming all those with unknown HIV status were HIV-uninfected.
4.2. Seasonality
HMPV prevalence was 4.9% (179/3624) in 2009, 2.9% (113/3936) in 2010, 3.9% (165/4189) in 2011, 4.6% (178/3862) in 2012 and 4.2% (77/1826) in 2013 (p = 0.747). Although HMPV was detected year-round, some months had higher detection but this varied from year to year. Increased HMPV detection coincided with periods of high influenza detection and followed high RSV detection periods in 2009, 2010 and 2013; however, higher HMPV detection coincided with increased RSV detection in 2011 and 2012 (Fig. 2).
Fig. 2.
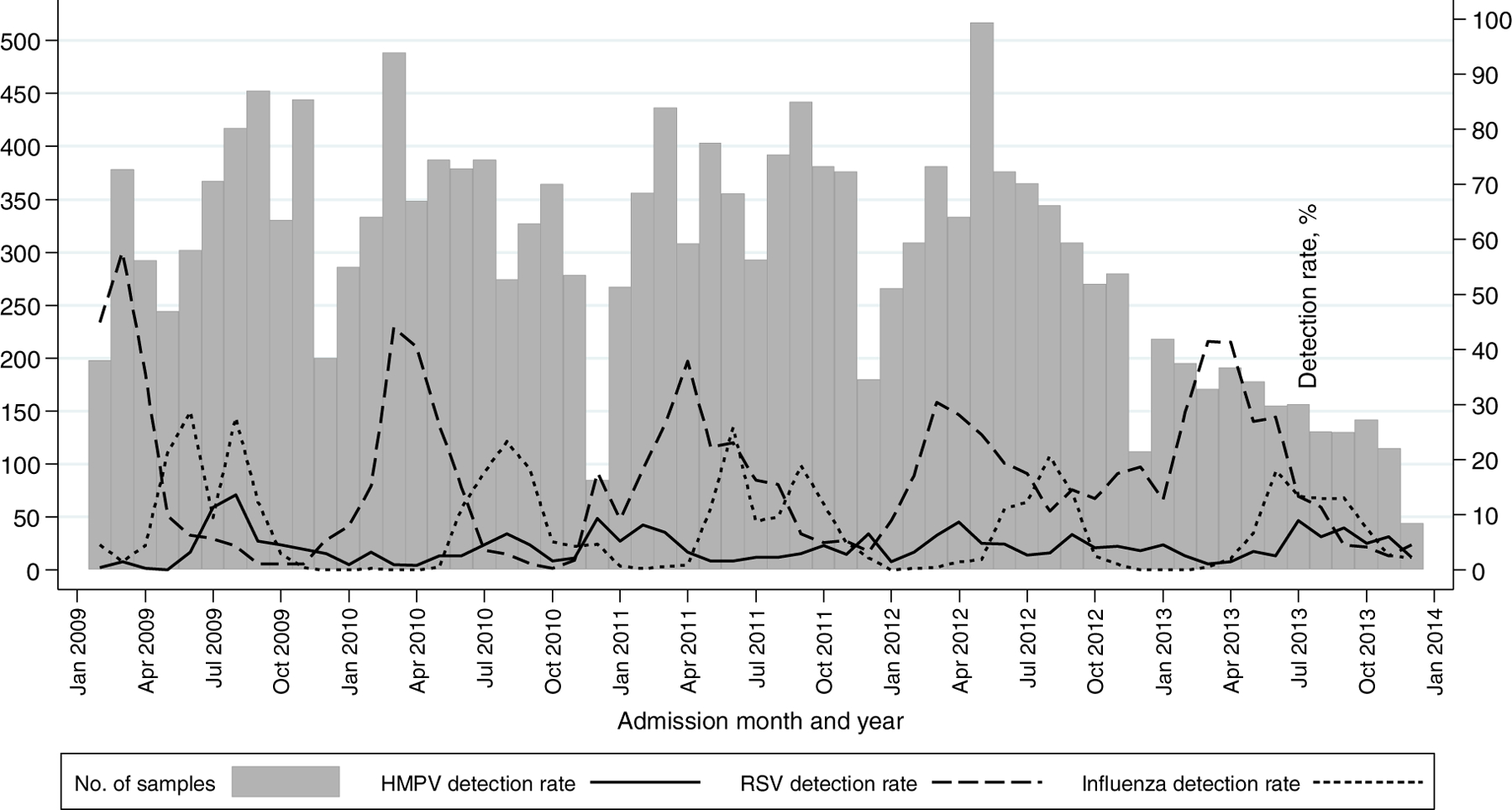
Detection rate of HMPV, RSV and Influenza virus (A and B) among patients hospitalised for severe acute respiratory illness at all sites, by month and year: 2009–2013. Detection rate – number testing positive divided by total number of samples tested per month × 100.
Footnote: In 2013 enrolment at Chris Hani Baragwanath Academic Hospital was down-scaled, with enrolment of paediatric patients limited to two of the five working days and adult patients to one of the five working days. Enrolment in 2013 at the other five hospitals continued as previously, with enrolment from Monday through Friday.
4.3. HMPV-associated SARI in children (<18 years)
Among 593 children with HMPV-associated SARI, the median age was 7.7 months and 9.6% had a documented underlying medical condition. An HIV result was available for 75.2% (446/593), of which 7.8% (35/446) tested HIV-positive. Twenty-three percent (8/35) of these children were documented as being on ART at the time of hospitalisation. HIV-infected cases were significantly older than HIV-uninfected cases. Compared with HIV-uninfected HMPV-positive patients <18 years of age, HIV-infected individuals were 20 times more likely to be in the older age category (5–17 years of age versus <1 year of age; Table 2). HIV-infected and HIV-uninfected children were similar with respect to gender, race, socioeconomic characteristics, duration of symptoms and in-hospital treatment. HIV-infected children had a significantly longer duration of hospitalisation compared to HIV-uninfected children.
Table 2.
Comparison of the clinical and epidemiologic characteristics of children (<18 years) hospitalised with HMPV-associated severe acute respiratory illness at four sentinel surveillance sites, South Africa 2009–2013.
| Characteristic | Children (<18years) n = 593 | HIV-infected n = 35 |
HIV-uninfected n = 411 |
HIV status unknown n = 147 | Odds ratioa (95% CI) | P valuea |
|---|---|---|---|---|---|---|
| Site | 0.007 | |||||
| Soweto | 406/593 (68.5) | 14/35 (40.0) | 276/411 (67.2) | 116/147 (78.9) | Reference | |
| Bushbuckridge | 80/593 (13.5) | 11/35 (31.4) | 58/411 (14.1) | 11/147 (7.5) | 3.7 (1.6–8.8) | |
| Pietermaritzburg | 85/593 (14.3) | 8/35 (22.9) | 58/411 (14.1) | 19/147 (12.9) | 2.7 (1.1–6.8) | |
| Klerksdorp | 22/593 (3.7) | 2/35 (5.7) | 19/411 (4.6) | 1/147 (0.7) | 2.1 (0.4–9.9) | |
| Age in months (median, IQR) | 7.7 (3.9–17.1) | 14.0 (5.1–27.9) | 7.4 (3.8–15.7) | 8.0 (3.9–18.0) | 0.006 | |
| Age group | <0.001 | |||||
| <1 year | 389/593 (65.6) | 17/35 (48.6) | 277/411 (67.4) | 95/147 (64.6) | Reference | |
| 1 year | 113/593 (19.1) | 8/35 (22.9) | 77/411 (18.7) | 28/147 (19.0) | 1.7 (0.7–4.1) | |
| 2–4 years | 78/593 (13.2) | 5/35 (14.3) | 53/411 (12.9) | 20/147 (13.6) | 1.5 (0.5–4.4) | |
| 5–17 years | 13/593 (2.2) | 5/35 (14.3) | 4/411 (1.0) | 4/147 (2.7) | 20.4 (4.6–90.0) | |
| Male gender | 328/593 (55.3) | 19/35 (54.3) | 227/411 (55.2) | 82/147 (55.8) | 1.0 (0.5–1.9) | 0.914 |
| Black race | 585/593 (98.7) | 34/35 (97.1) | 407/411 (99.0) | 144/147 (98.0) | 0.3 (0.0–3.1) | 0.337 |
| Brick houseb | 425/590 (72.0) | 25/34 (73.5) | 297/409 (72.6) | 103/147 (70.1) | 1.0 (0.5–2.3) | 0.909 |
| Number of rooms for sleeping | 418/591 (70.7) | 29/35 (82.9) | 288/409 (70.4) | 101/147 (68.7) | 2.0 (0.8–5.0) | 0.118 |
| >1 | ||||||
| Number of people in house >4 | 295/590 (50.0) | 14/35 (40.0) | 207/408 (51.7) | 74/147 (50.3) | 0.6 (0.3–1.3) | 0.223 |
| Duration of symptoms (median, IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | – | 0.316 |
| Underlying illnessc (including HIV infection) | 57/593 (9.6) | – | – | – | – | – |
| Underlying illness (excluding HIV infection) | 23/593 (3.9) | 1/35 (2.9) | 20/411 (4.9) | 2/147 (1.4) | 0.6 (0.1–4.4) | >0.999 |
| In-hospital treatment | ||||||
| Antibiotics prescribed during hospitalisation | 541/585 (92.5) | 33/34 (97.1) | 377/405 (93.1) | 131/146 (89.7) | 2.5 (0.3–18.7) | 0.715 |
| Oxygen therapy | 183/592 (30.9) | 13/35 (37.1) | 138/410 (33.7) | 32/147 (21.8) | 1.2 (0.6–2.4) | 0.676 |
| Outcome | ||||||
| Duration of hospitalization (days, IQR) | 2 (1–5) | 5 (2–8) | 3 (1–5.5) | 2 (1–4) | – | 0.003 |
| Prolonged hospitalisation (>3 days) | 242/589 (41.1) | 22/35 (62.9) | 174/408 (42.7) | 46/146 (31.5) | 2.3 (1.1–4.7) | 0.021 |
| Death | 5/590 (0.8) | 1/34 (2.9) | 2/410 (0.5) | 2/147 (1.4) | 6.2 (0.5–70.7) | 0.213 |
| Co-infections | ||||||
| Viral | ||||||
| Any viral co-infectiond | 333/593 (56.2) | 24/35 (68.6) | 238/411 (57.9) | 71/147 (48.3) | 1.6 (0.8–3.3) | 0.219 |
| Influenza | 14/593 (2.4) | 0/35 (0) | 9/411 (2.2) | 5/147 (3.4) | – | >0.999 |
| RSV | 47/593 (7.9) | 2/35 (5.7) | 35/411 (8.5) | 10/147 (6.8) | 0.7 (0.1–2.8) | 0.756 |
| >1 viral co-infection | 95/593 (16.0) | 8/35 (22.9) | 72/411 (17.5) | 15/147 (10.2) | 1.4 (0.5–3.3) | 0.429 |
| S. pneumoniae - lytA PCRe | 27/370 (7.3) | 3/29 (10.3) | 21/305 (6.9) | 3/36 (8.3) | 1.6 (0.4–5.6) | 0.451 |
Values are number of patients with characteristic/total number with available data (%) unless otherwise indicated.
HIV-infected compared to HIV-uninfected children, excluding those with unknown HIV status.
Compared to house made of iron sheeting or mud.
Asthma, other chronic lung disease, chronic heart disease, liver disease, renal disease, diabetes mellitus, obesity, immunocompromising conditions, neurologic disease, prematurity, kwashiorkor. HIV infection (n = 35), asthma (n = 4), chronic heart disease (n = 3), neurologic disease (n = 3), prematurity (n = 8), kwashiorkor (n = 3), renal disease (n = 1),immunocompromising condition other than HIV (n = 1).
Co-infection with HMPV and ≥1 of the following: adenovirus, enterovirus, influenza A and B viruses, respiratory syncytial virus (RSV), parainfluenza virus type 1, 2 and 3, and rhinovirus. Adenovirus testing was not done Aug–Oct 2009, missing: n = 78.
Available for 370, not done in 223 (38%).
Fifty-six percent (333/593) had co-detection of HMPV plus one or more other respiratory viruses (Fig. 3a). Whole blood lytA PCR was positive in 7.3% (27/370) of those tested. No significant differences were found in the proportion with co-detection of HMPV and other respiratory viruses or HMPV and S. pneumoniae between HIV-infected and HIV-uninfected children (Table 2). Four of the five children who died had co-detection of HMPV and another respiratory virus, including adenovirus (n = 2), parainfluenza type I, III plus rhinovirus (n = 1) and adenovirus plus enterovirus (n = 1). LytA PCR was negative in the three children who died in whom testing was undertaken
Fig. 3.
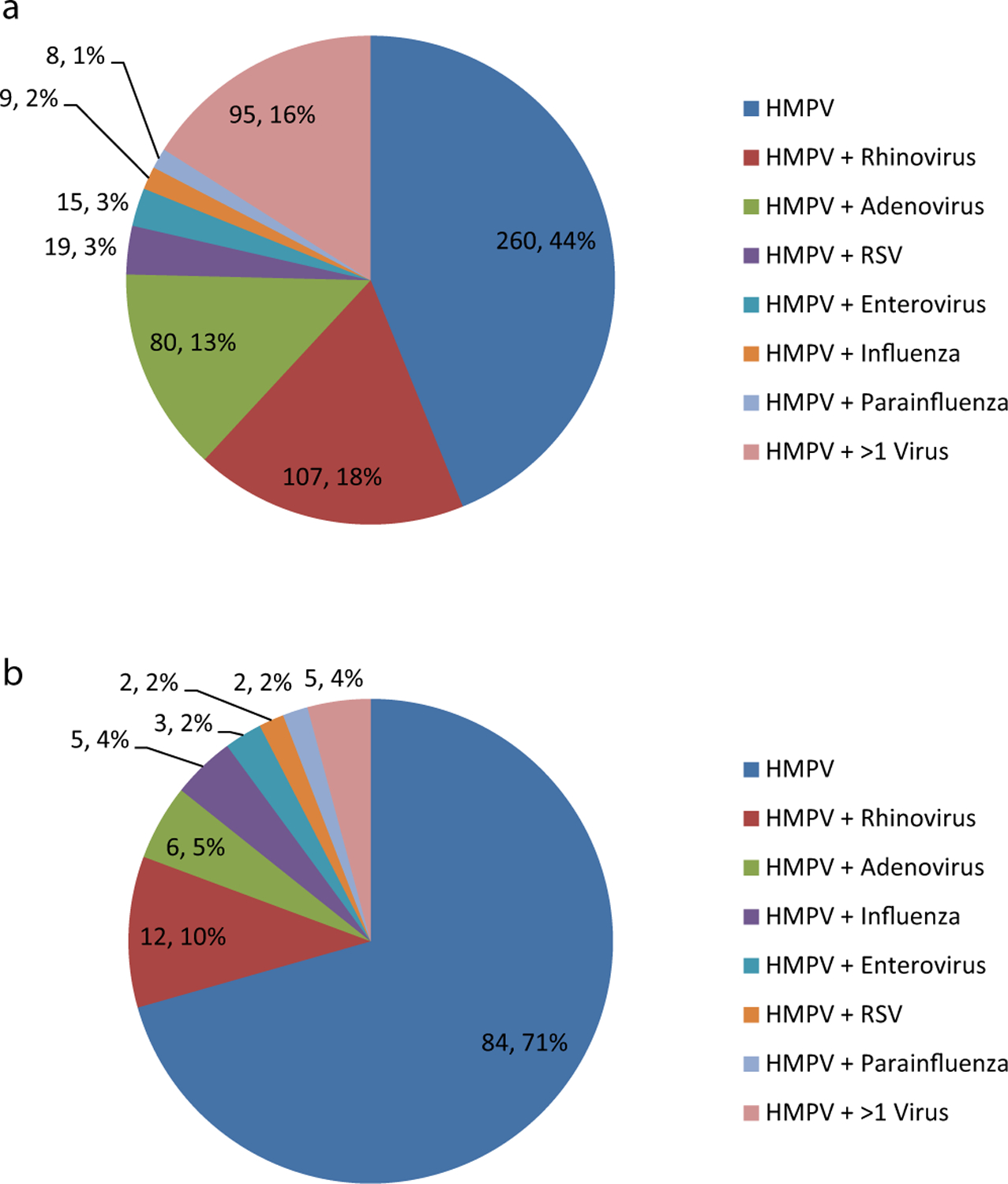
Number and proportion (n; %) of HMPV-positive patients with HMPV detected as the sole virus, co-detection of HMPV and one other respiratory virus (as specified), and co-detection of HMPV and >1 other respiratory virus in (a) children <18 years (b) adults ≥18 years.
Note: Co-detection of HMPV and other respiratory viruses is detailed in the Supplementary material.
4.4. HMPV-associated SARI in adults (≥18 years)
The median age of adults with HMPV-associated SARI was 38.6 years and 84.0% had a documented underlying medical condition. An HIV result was available for 92.4% (110/119) of HMPV-SARI cases, with a HIV-positivity prevalence of 79.1% (87/110), including 23.0% (20/87) who were documented as being on ART at the time of hospitalisation. HIV-infected cases were significantly younger than HIV-uninfected cases. Compared with HIV-uninfected HMPV-positive patients, HIV-infected individuals were 12 times more likely to be in the younger age category (18–44 years of age versus 45–64 years of age; Table 3). HIV-infected adults were less likely than HIV-uninfected adults to live in a brick house and have >1 room used for sleeping, but were otherwise similar with respect to gender, race, socioeconomic characteristics, duration of symptoms, in-hospital treatment and duration of hospitalisation. Twenty-nine percent (35/119) of HMPV-associated SARI cases had one or more other respiratory viruses detected (Fig. 3b). There were no significant differences in the proportion with co-detection of HMPV and other respiratory viruses (Table 3). Of the 116HMPV-SARI adult cases tested by whole blood lytA PCR, 12.1% (n = 14) were positive.
Table 3.
Comparison of the clinical and epidemiologic characteristics of adults (≥18 years) hospitalised with HMPV-associated severe acute respiratory illness at four sentinel surveillance sites, South Africa 2009–2013.
| Characteristic | Adults (≥18years) n = 119 |
HIV-infected n = 87 |
HIV-uninfected n = 23 |
HIV status unknown n = 9 |
Odds ratioa (95% CI) | p valuea |
|---|---|---|---|---|---|---|
| Site | ||||||
| Soweto | 94/119 (79.0) | 65/87 (74.7) | 23/23 (100) | 6/9 (66.7) | – | 0.065 |
| Bushbuckridge | 15/119 (12.6) | 13/87 (14.9) | 0 | 2/9 (22.2) | ||
| Pietermaritzburg | 5/119 (4.2) | 4/87 (4.6) | 0 | 1/9 (11.1) | ||
| Klerksdorp | 5/119 (4.2) | 5/87 (5.8) | 0 | 0 | ||
| Age in years (median, IQR) | 38.6 (31.3–53.1) | 35.5 (29.3–43.0) | 60.2 (46.8–70.9) | 33.4 (32.5–46.3) | <0.001 | |
| Age group | <0.001 | |||||
| 18–44 years | 81/119 (68.0) | 71/87 (81.6) | 4/23 (17.4) | 6/9 (66.7) | 12.2 (3.0–50.4) | |
| 45–64 years | 28/119 (23.5) | 16/87 (18.4) | 11/23 (47.8) | 1/9 (11.1) | Reference | |
| ≥65 years | 10/119 (8.4) | 0 | 8/23 (34.8) | 2/9 (22.2) | – | |
| Male gender | 37/119 (31.1) | 24/87 (27.6) | 10/23 (43.5) | 3/9 (33.3) | 0.5 (0.2–1.3) | 0.142 |
| Black race | 115/119 (96.6) | 85/87 (97.7) | 21/23 (91.3) | 9/9 (100) | 4.0 (0.5–31.3) | 0.192 |
| Brick houseb | 87/119 (73.1) | 58/87 (66.7) | 22/23 (95.7) | 7/9 (77.8) | 0.1 (0.0–0.8) | 0.004 |
| Number of rooms for sleeping >1 | 76/119 (63.9) | 49/87 (56.3) | 21/23 (91.3) | 6/9 (66.7) | 0.1 (0.0–0.6) | 0.001 |
| Number of people in house >4 | 46/119 (38.7) | 34/87 (39.1) | 8/23 (34.8) | 4/9 (44) | 1.2 (0.5–3.2) | 0.706 |
| Duration of symptoms (median, IQR) | 3 (2–5) | 3 (2–5) | 4 (3–5) | 5 (3–6) | – | 0.380 |
| Underlying illnessc (including HIV infection) | 100/119 (84.0) | – | – | – | – | – |
| Underlying illness (excluding HIV infection) | 22/119 (18.5) | 9/87 (10.3) | 10/23 (43.5) | 3/9 (33.3) | 0.2 (0.1–0.5) | <0.001 |
| In-hospital treatment | ||||||
| Antibiotics prescribed during hospitalisation | 112/115 (97.4) | 83/85 (97.7) | 20/21 (95.2) | 9/9 (100) | 2.1 (0.2–24.0) | 0.488 |
| Oxygen therapy | 52/119 (43.7) | 37/87 (42.5) | 11/23 (47.8) | 4/9 (44.4) | 0.8 (0.3–2.0) | 0.649 |
| Outcome | ||||||
| Duration of hospitalisation (days, IQR) | 6 (4–8) | 6 (4–8) | 5 (4–7) | 4 (4–7) | – | 0.654 |
| Prolonged hospitalisation (>3 days) | 91/118 (77.1) | 66/86 (76.7) | 18/23 (78.2) | 7/9 (78) | 0.9 (0.3–2.8) | >0.999 |
| Death | 9/119 (7.6) | 4/87 (4.6) | 3/23 (13.0) | 2/9 (22.2) | 0.3 (0.1–1.6) | 0.158 |
| Co-infections | ||||||
| Viral | ||||||
| Any viral co-infectiond | 35/119 (29.4) | 27/87 (31.0) | 5/23 (21.7) | 3/9 (33.3) | 1.6 (0.5–4.9) | 0.449 |
| Influenza | 6/119 (5.0) | 5/87 (5.8) | 1/23 (4.4) | 0 | 1.3 (0.1–12.2) | >0.999 |
| RSV | 3/119 (2.5) | 3/87 (3.5) | 0/23 (0) | 0 | – | >0.999 |
| >1 viral co-infection | 5/119 (4.2) | 5/87 (5.7) | 0/23 (0) | 0 | – | 0.582 |
| S. pneumoniae - Lyt A PCRe | 14/116 (12.1) | 11/85 (12.9) | 1/22 (4.6) | 2/9 (22.2) | 3.1 (0.4–26.1) | 0.453 |
Values are number of patients with characteristic/total number with available data (%) unless otherwise indicated.
HIV-infected compared to HIV-uninfected adults, excluding those with unknown HIV status.
Compared to house made of iron sheeting or mud.
Asthma, other chronic lung disease, chronic heart disease, liver disease, renal disease, diabetes mellitus, obesity, immunocompromising conditions, neurologic disease, prematurity, pregnancy. HIV infection (n = 87), asthma (n = 5), other chronic lung disease (n = 4), chronic heart disease (n = 4), diabetes mellitus (n = 5), obesity (n = 1), neurologic disease (n = 2), pregnancy (n = 2).
Co-infection with HMPV and ≥1 of the following: adenovirus, enterovirus, influenza A and B viruses, respiratory syncytial virus (RSV), parainfluenza virus type 1, 2 and 3, and rhinovirus. Adenovirus testing was not done Aug–Oct 2009, missing: n = 14.
Available for 116, not done in 3 (3%).
Four HMPV-associated adult deaths (44.4%) had at least one other respiratory virus identified (RSV, n = 1; adenovirus, n = 2; and parainfluenza type II, n = 1). LytA PCR was positive among four of eight patients who died, two of whom also had other respiratory virus co-infections (RSV and parainfluenza type II, respectively). HMPV-positive cases who died were more likely to have blood lytA PCR positivity (50.0%) than those who survived (9.3%, p = 0.007).
5. Discussion
We observed a higher incidence of HMPV-associated SARI hospitalisation in HIV-infected compared to HIV-uninfected adults in Soweto. The majority of adults had an underlying medical condition, with HIV infection being the most common condition identified, in 79% of cases tested for HIV. The study corroborates previous studies in which the incidence of HMPV-associated LRTI hospitalisation was highest in infants [1,3]. The majority of childhood cases were otherwise healthy, with HIV-infection being the most common underlying medical condition, in 8% of cases tested for HIV.
Previously published results of the SARI surveillance programme in South Africa showed that respiratory viruses were detected in a high proportion (78%) of LRTI cases among children <5 years of age, with rhinovirus (37%), RSV (26%) and adenovirus (26%) being most commonly identified [21]. Among older children and adults a respiratory virus, most commonly rhinovirus, adenovirus and influenza, was detected in 32% of LRTI cases [20]. We have now described the epidemiology of HMPV-associated LRTI among patients enrolled into this surveillance programme. Among childhood HMPV-associated SARI, HIV-infected children were older and had a longer duration of hospitalisation compared to HIV-uninfected children, as previously described [15]. The incidence of HMPV-associated SARI hospitalisation in our study was similar among HIV-infected and HIV–uninfected children <5 years in Soweto. The incidence among HIV-infected children was lower than that previously described in the same setting prior to the era of ART management of HIV-infected children, where HIV-infected children had a 5-fold higher incidence than HIV-uninfected children [15]. This suggests that the increase in ART coverage of HIV-infected children since 2004 may have decreased the incidence of HMPV-associated SARI among these children in our setting.
HIV infection is an important risk factor for pneumonia among adults, who commonly present with co-infections and comorbidity, but the role of respiratory viruses in HIV-infected adults is not well documented [26]. In Canada, viruses accounted for 64% of acute respiratory illness in HIV-infected adult outpatients, with HMPV the second most isolated pathogen, highlighting the potential importance of this pathogen in this group of patients [27]. There are no incidence data available for adult HMPV-associated SARI prior to the rollout of ART in South Africa so we were unable to determine whether there has been a decrease in incidence as a result of improved access to ART, as has been shown for pneumonia [28]. Hospitalised patients with HMPV infection in high-income settings were found to be mainly elderly and had underlying chronic heart and lung disease, [4,29,30] which was similar to that observed among HIV-uninfected adults in our study, among whom the median age was 60 years and 43% had an underlying medical condition.
Although the seasonality of HMPV infection is not fully characterized globally, other studies suggest higher incidence during the winter and spring months. Season-on-season variation has also been shown [2,3,10]. A study from Brazil showed similar HMPV activity as observed by us, with perennial circulation of HMPV-associated with small peaks in winter months for some years and in spring months for others, and generally coinciding with the latter half of RSV epidemics [10].
Newer diagnostics often lead to frequent detection of more than one virus in a respiratory sample. Our surveillance showed co-detection of HMPV and another respiratory virus in 56% of children and 29% of adults, but this did not differ significantly between HIV-infected and HIV-uninfected persons, as was shown in Kenya [17]. Co-infections have been associated with an increased risk of more severe disease in some studies, although results have been conflicting for RSV/HMPV dual infection, both common infections in young children [31–34]. We were not powered to assess whether HMPV and RSV co-detection was associated with more severe disease. Multiplex PCR diagnostic methods allow sensitive detection of respiratory viruses but the extent to which specific viruses contribute towards disease is not always clear, as viruses have been detected in the airways of asymptomatic persons and are not necessarily associated with disease. We did not evaluate healthy controls as a comparison group, which limited our ability to attribute the aetiology of SARI to HMPV among patients in whom HMPV was detected. HMPV has, however, been shown to be more prevalent among case patients with acute respiratory illness than asymptomatic controls in studies in both adults and children [17,31,35]. While HMPV seems likely to be a significant respiratory pathogen capable of causing disease, the proportion of SARI caused by HMPV is lower than the proportion in which HMPV is detected. Our incidence estimates of HMPV-associated SARI may thus overestimate the true incidence of disease. Co-detection of other respiratory pathogens also limits out ability to attribute SARI solely to HMPV. However, co-detection does not always mean co-infection as, some of the viruses co-detected with HMPV in our study, for example adenovirus, rhinovirus and enterovirus, have been found to have high detection rates in asymptomatic individuals [17,31].
Viral-bacterial co-infections are important in the pathogenesis of viral-associated LRTI in children [36]. We showed co-detection of HMPV and S. pneumoniae in 7% of children and 12% of adults, but did not find a significant difference in HIV-infected compared to HIV-uninfected adults or children, although numbers were small. In adults, co-detection with S. pneumoniae was associated with more severe disease, as has been shown previously for HMPV [16]. Although the utility of whole blood LytA positivity for attributing causality of pneumonia to S. pneumoniae is controversial, some studies have shown this to be specific and more sensitive than blood culture for attributing causality to S. pneumoniae [37]. We did not investigate all concurrent bacterial aetiology, only S. pneumoniae, and this was not available on all patients.
There were some other limitations to our study. Our case definition for patients ≥5 years included sudden onset of fever, however, respiratory infections may not always present with fever and we may have underestimated the burden of disease in these age groups by excluding afebrile patients. Incidence estimates could only be calculated at one site which may not be representative of other regions of South Africa. HIV population denominators were based on the ASSA model predictions, which if overestimating the number of HIV-infected persons could lead to an underestimate of the incidence in this group, and vice versa. HIV status was not available for all patients (25% of children and 8% of adults) and exclusion of those with unknown HIV status in the risk factor analyses is a limitation of the study.
The present study contributes to understanding the incidence of HMPV associated severe respiratory illness across different age-groups in a setting with a high prevalence of HIV infection. Despite the roll-out of antiretroviral treatment of HIV-infected individuals, the burden of HMPV-associated SARI hospitalisation among adults occurred predominantly in HIV-infected individuals. Infants, however, had the highest incidence of HMPV associated hospitalization, with similar rates burden observed between HIV-infected and HIV-uninfected children. Although attempts are being made to develop effective preventive and therapeutic interventions against HMPV infection, including vaccines and monoclonal antibodies, current treatment options are limited and not specific to HMPV. Timely initiation of ART in HIV-infected persons could, however, possibly reduce HMPV-associated morbidity and mortality.
Supplementary Material
Funding
This study received funding from the National Institute for Communicable Diseases of the National Health Laboratory Service and was supported in part by funds from the United States Centers for Disease Control and Prevention (CDC), Atlanta, Georgia Preparedness and Response to Avian and Pandemic Influenza in South Africa (Cooperative Agreement Number: U51/IP000155–04). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC. The funders had no role in study design, implementation, manuscript writing or the decision to submit for publication. The corresponding author had full access to all the data in the study and takes final responsibility for the decision to submit for publication.
Abbreviations:
- ART
antiretroviral therapy
- CHBAH
Chris Hani Baragwanath Academic Hospital
- CI
confidence interval
- ELISA
enzyme-linked immunosorbent assay
- HIV
human immunodeficiency virus
- HMPV
human metapneumovirus
- LRTI
lower respiratory tract infection
- OR
odds ratio
- PCR
polymerase chain reaction
- RR
relative risk
- RSV
respiratory syncytial virus
- SARI
severe acute respiratory illness
Footnotes
Competing interests
Halima Dawood - honoraria from MSD, Novartis and GSK, South Africa; travel grant from Novartis.
Michelle Groome - personal fees from GlaxoSmithKline and Sanofi Pasteur outside the submitted work.
Anne von Gottberg - grant funds from Pfizer.
Shabir Madhi - grants and personal fees from GSK, grants and personal fees from Pfizer, grants from Novartis, grants and personal fees from Sanofi Pasteur, outside the submitted work.
The other authors do not declare any conflict of interests.
Ethical approval
Approval for the study was obtained from ethics committees of the University of the Witwatersrand (M081042) and University of KwaZulu-Natal (BF 157/08). CDC human subjects review deemed this surveillance activity not research and relied on approval from the local ethics committees.
Conference presentation
These data were presented at the 9th Respiratory Syncytial Virus Symposium, 9–13 November 2014, Stellenbosch, South Africa.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2015.06.089
References
- [1].Kahn JS, Epidemiology of human metapneumovirus, Clin. Microbiol. Rev 19 (3) (2006. Jul) 546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Caracciolo S, Minini C, Colombrita D, Rossi D, Miglietti N, Vettore E, et al. , Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease: virologic and clinical features, Pediatr. Infect Dis. J 27 (May (5)) (2008) 406–412. [DOI] [PubMed] [Google Scholar]
- [3].Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, et al. , Burden of human metapneumovirus infection in young children, N. Engl. J. Med 368 (February (7)) (2013) 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hamelin ME, Cote S, Laforge J, Lampron N, Bourbeau J, Weiss K, et al. , Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease, Clin. Infect Dis 41 (August (4)) (2005) 498–502. [DOI] [PubMed] [Google Scholar]
- [5].Walsh EE, Peterson DR, Falsey AR, Human metapneumovirus infections in adults: another piece of the puzzle, Arch. Internal Med 168 (December (22)) (2008) 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Cote S, et al. , Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups, J. Infect. Dis 186 (November (9)) (2002) 1330–1334. [DOI] [PubMed] [Google Scholar]
- [7].Falsey AR, Walsh EE, Viral pneumonia in older adults, Clin. Infect. Dis 42 (February (4)) (2006) 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ali A, Khowaja AR, Bashir MZ, Aziz F, Mustafa S, Zaidi A, Role of human metapneumovirus, influenza a virus and respiratory syncytial virus in causing who-defined severe pneumonia in children in a developing country, PloS One 8 (9) (2013) e74756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Banerjee S, Bharaj P, Sullender W, Kabra SK, Broor S, Human metapneumovirus infections among children with acute respiratory infections seen in a large referral hospital in India, J. Clin. Virol 38 (January (1)) (2007) 70–72. [DOI] [PubMed] [Google Scholar]
- [10].Oliveira DB, Durigon EL, Carvalho AC, Leal AL, Souza TS, Thomazelli LM, et al. , Epidemiology and genetic variability of human metapneumovirus during a 4-year-long study in Southeastern Brazil, J. Med. Virol 81 (May (5)) (2009) 915–921. [DOI] [PubMed] [Google Scholar]
- [11].Berkley JA, Munywoki P, Ngama M, Kazungu S, Abwao J, Bett A, et al. , Viral etiology of severe pneumonia among Kenyan infants and children, JAMA 303 (May (20)) (2010) 2051–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].O’Callaghan-Gordo C, Diez-Padrisa N, Abacassamo F, Perez-Brena P, Casas I, Alonso PL, et al. , Viral acute respiratory infections among infants visited in a rural hospital of southern Mozambique, Trop. Med. Int. Health 16 (September (9)) (2011) 1054–1060. [DOI] [PubMed] [Google Scholar]
- [13].Madhi SA, Ludewick H, Abed Y, Klugman KP, Boivin G, Human metapneumovirus-associated lower respiratory tract infections among hospitalized human immunodeficiency virus type 1 (HIV-1)-infected and HIV-1-uninfected African infants, Clin. Infect. Dis 37 (December (12)) (2003) 1705–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].IJpma FF, Beekhuis D, Cotton MF, Pieper CH, Kimpen JL, van den Hoogen BG, et al. , Human metapneumovirus infection in hospital referred South African children, J. Med. Virol 73 (July (3)) (2004) 486–493. [DOI] [PubMed] [Google Scholar]
- [15].Madhi SA, Ludewick H, Kuwanda L, van Niekerk N, Cutland C, Klugman KP, Seasonality, incidence, and repeat human metapneumovirus lower respiratory tract infections in an area with a high prevalence of human immunodeficiency virus type-1 infection, Pediatr Infect Dis J 26 (August (8)) (2007) 693–699. [DOI] [PubMed] [Google Scholar]
- [16].El Sayed Zaki M, Raafat D, El-Metaal AA, Ismail M, Study of human metapneumovirus-associated lower respiratory tract infections in Egyptian adults, Microbiol. Immunol 53 (Novemmber (11)) (2009) 603–608. [DOI] [PubMed] [Google Scholar]
- [17].Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, Audi A, et al. , Etiology and Incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010, PloS One 7 (8) (2012) e43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, et al. , Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study, Lancet 369 (April (9571)) (2007) 1440–1451. [DOI] [PubMed] [Google Scholar]
- [19].Madhi SA, Schoub B, Simmank K, Blackburn N, Klugman KP, Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1, J. Pediatr 137 (July (1)) (2000) 78–84. [DOI] [PubMed] [Google Scholar]
- [20].Cohen C, Walaza S, Moyes J, et al. , Epidemiology of severe acute respiratory illness (SARI) among adults and children aged >/=5 years in a high HIV-prevalence setting, 2009–2012, PloS One 10 (2) (2015) e0117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cohen C, Walaza S, Moyes J, et al. , Epidemiology of viral-associated acute lower respiratory tract infection among children <5 years of age in a high HIV prevalence setting: South Africa, 2009–2012, Pediatr Infect. Dis. J 34 (1) (2015) 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Actuarial Society of Soth Africa (ASSA). AIDS and Demographic Model 2008. Available at: http://aids.actuarialsociety.org.za/ASSA2008-Model-3480htm. Accessed 2 June 2014.
- [23].Johnson L, Access to antiretroviral treatment in South Africa, 2004–2011, Southern Afr. J. HIV Med 13 (2012) 22–27. [Google Scholar]
- [24].Pretorius MA, Madhi SA, Cohen C, Naidoo D, Groome M, Moyes J, et al. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness–South Africa, 2009–2010. J Infect Dis 2012. Dec 15;206 Suppl 1:S159–65. [DOI] [PubMed] [Google Scholar]
- [25].Carvalho Mda G, Tondella ML, McCaustland K, Weidlich L, McGee L, Mayer LW, et al. , Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA, J Clin Microbiol 45 (August (8)) (2007) 2460–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zar HJ, Madhi SA, Aston SJ, Gordon SB, Pneumonia in low and middle income countries: progress and challenges, Thorax 68 (November (11)) (2013) 1052–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Klein MB, Yang H, DelBalso L, Carbonneau J, Frost E, Boivin G, Viral pathogens including human metapneumovirus are the primary cause of febrile respiratory illness in HIV-infected adults receiving antiretroviral therapy, J. Infect. Dis 201 (January (2)) (2010. Jan 15) 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Iwuji CC, Mayanja BN, Weiss HA, Atuhumuza E, Hughes P, Maher D, et al. , Morbidity in HIV-1-infected individuals before and after the introduction of antiretroviral therapy: a longitudinal study of a population-based cohort in Uganda, HIV Med 12 (October (9)) (2011) 553–561. [DOI] [PubMed] [Google Scholar]
- [29].Falsey AR, Erdman D, Anderson LJ, Walsh EE, Human metapneumovirus infections in young and elderly adults, J. Infect. Dis 187 (March (5)) (2003) 785–790. [DOI] [PubMed] [Google Scholar]
- [30].Johnstone J, Majumdar SR, Fox JD, Marrie TJ, Human metapneumovirus pneumonia in adults: results of a prospective study, Clin. Infect. Dis 46 (February (4)) (2008) 571–574. [DOI] [PubMed] [Google Scholar]
- [31].Rhedin S, Lindstrand A, Rotzen-Ostlund M, Tolfvenstam T, Ohrmalm L, Rinder MR, et al. , Clinical utility of PCR for common viruses in acute respiratory illness, Pediatrics 133 (March (3)) (2014) e538–e545. [DOI] [PubMed] [Google Scholar]
- [32].Papenburg J, Boivin G, The distinguishing features of human metapneumovirus and respiratory syncytial virus, Rev. Med. Virol 20 (July (4)) (2010) 245–260. [DOI] [PubMed] [Google Scholar]
- [33].Wolf DG, Greenberg D, Kalkstein D, Shemer-Avni Y, Givon-Lavi N, Saleh N, et al. , Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children, Pediatr. Infect. Dis J 25 (April (4)) (2006) 320–324. [DOI] [PubMed] [Google Scholar]
- [34].Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, et al. , Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis, J. Infect. Dis 191 (February (3)) (2005) 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Madhi SA, Ludewick H, Kuwanda L, Van Niekerk N, Cutland C, Little T, et al. , Pneumococcal coinfection with human metapneumovirus, J. Infect. Dis 193 (May (9)) (2006) 1236–1243. [DOI] [PubMed] [Google Scholar]
- [36].Azzari C, Cortimiglia M, Moriondo M, et al. , Pneumococcal DNA is not detectable in the blood of healthy carrier children by real-time PCR targeting the lytA gene, J. Med. Microbiol 60 (Pt 6) (2011) 710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pretorius M, Tempia S, Walaza S, Cohen A, Moyes J, Hellferscee O, et al. , Association of influenza and other respiratory viruses with severe acute respiratory infection and influenza-like illness, relative to healthy controls in South Africa, Options Conf (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


