Abstract
Liver is a major site for glucose metabolism. Patients with type 2 diabetes (T2D) and obesity have increased risk of liver cancer. We explored the association of glycemic burden (GB) and obesity with liver cancer in T2D in the prospective Hong Kong Diabetes Register (1995‐2019). We calculated GB using the area under the curve above hemoglobin A1c (HbA1c) of 5.7% and defined obesity as body mass index (BMI) ≥ 25 kg/m2. We used Cox proportional hazards models to evaluate the association between GB and liver cancer. We included 15,280 patients with at least 10 years of disease duration before liver cancer occurred or censor date, ≥3 years of observation, and ≥5 HbA1c measurements (64% male, age: 58.23 ± 12.47 years, HbA1c: 7.60 ± 1.65%, BMI: 25.58 ± 4.10 kg/m2). We excluded 3 years of HbA1c values before liver cancer to avoid reverse causality. Every 1‐SD increase in GB was associated with an adjusted hazard ratio (aHR) of liver cancer of 1.22 (95% confidence interval [CI]: 1.01‐1.47). The top GB quartile group (range: >2.41) had aHR of 1.78 (1.01‐3.13) versus the lowest quartile group (0‐1.19). The aHRs for each SD increase in GB were 1.34 (1.05, 1.70) in the obese group and 1.12 (0.81‐1.53) in the nonobese group, but no interaction (P interaction = 0.120). When stratified by GB median (1.69 [1.13, 2.43]) and obesity, obese patients with high GB had the highest aHR of 2.51 (1.44‐4.37) for liver cancer versus the nonobese group with low GB, but no interaction (P interaction = 0.071). Subgroup analysis of patients with available hepatitis B surface antigen status (n = 9,248) yielded similar results. Conclusion: Our results emphasized the importance of glycemic and weight control for reducing the risk of liver cancer in T2D.

Abbreviations
- aHR
adjusted hazard ratio
- ALT
alanine aminotransferase
- AUC
area under the curve
- BMI
body mass index
- BP
blood pressure
- CI
confidence interval
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- EMR
electronic medical record
- GB
glycemic burden
- GB_MD
median value of glycemic burden
- GB_SD
SD of glycemic burden
- GB_QA
quartile value of glycemic burden
- HA
Hospital Authority
- HbA1c
hemoglobin A1c
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- HCC
hepatocellular carcinoma
- HDLC
high‐density lipoprotein cholesterol
- HKDR
Hong Kong Diabetes Register
- HR
hazard ratio
- ICD‐9
International Classification of Diseases, Ninth Revision
- IQR
interquartile range
- LDLC
low‐density lipoprotein cholesterol
- LLD
lipid‐lowering drug
- NASH
nonalcoholic steatohepatitis
- OGLD
oral glucose‐lowering drug
- RAS
renin angiotensin system
- T2D
type 2 diabetes
- TG
triglyceride
Liver cancer is the sixth commonest cancer worldwide, and the fourth leading cause of cancer‐related mortality worldwide.( 1 ) Chronic hepatitis B virus (HBV) and hepatitis C virus infections are the leading causes of liver cancer, often preceded by chronic hepatitis and cirrhosis.( 2 , 3 ) Acute and chronic hepatic viral infections are endemic in Asia, where 72% of hepatocellular carcinoma (HCC), the most common type of liver cancer, occurred.( 4 ) Other causes including obesity and alcoholism might contribute to the increasing incidence of liver cancer and related death in Europe and North/Latin America in recent years.( 5 , 6 )
Epidemiological analysis suggested close associations between diabetes and liver cancer.( 7 , 8 ) In a systematic review of 49 case‐control and cohort studies, type 2 diabetes (T2D) was associated with 2.2 fold increased risk of liver cancer with a 95% confidence interval (CI) of 1.7‐3.0.( 9 ) In a meta‐analysis of 10 prospective cohorts, every unit increase in fasting blood glucose was associated with a relative risk of 1.77 (1.46‐2.13) in liver cancer.( 10 ) In 2019, 9.3% of the global population, including 463 million people, had diabetes, with 80% coming from low‐income and middle‐income countries.( 11 ) Taken together, these figures suggested a looming epidemic of diabetes‐associated liver cancer.
Liver plays a pivotal role in glucose homeostasis, including glycogenesis, glycogenolysis, glycolysis, and gluconeogenesis.( 12 ) Proinflammatory and pro‐oxidant effects of hyperglycemia on liver cancer might be amplified by increased release of adipocytokines and inflammatory infiltrates due to excess adiposity.( 13 ) Obesity is associated with many types of cancer, including liver cancer.( 14 ) These adverse internal milieu might cause abnormal cellular signaling and cellular growth, which contribute to increased cancer risk.( 15 ) For liver cancer, most epidemiological analysis only used baseline or mean blood glucose or HbA1c as a predictor. However, these glycemic indexes could not fully reflect the cumulative exposure to hyperglycemia over a long period. These indexes also could not distinguish between the latent effects of glycemic burden (GB) on the risk of liver cancer and the confounding effects of liver cancer on hyperglycemia in T2D.
The Hong Kong Diabetes Register (HKDR) is an ongoing register that was set up in 1995 with comprehensive assessment of risk factors at enrollment, with periodic linkage to hospitalization and death data. Leveraging the long history of HKDR and availability of repeated measures of HbA1c, we hypothesized that GB and obesity increased the risk of liver cancer. To test this hypothesis, we selected a subgroup of patients with at least 10 years of diabetes duration before liver cancer occurred or censor date to explore the risk association of GB and obesity with incident liver cancer after adjusting for metabolic factors and confounders.
Patients and Methods
Patients
The HKDR, established in 1995 at the Prince of Wales Hospital, consecutively enrolled patients referred to the Diabetes Center for structured assessment of risk factors and complications, as part of a research‐driven quality improvement program. All enrolled patients had laboratory data retrievable from a territory‐wide electronic medical record (EMR) system and were observed until the time of death.( 16 )
For this analysis, we included patients from enrollment in 1995 until December 31, 2019. Due to the long latent period before cancer development, we included patients with at least 10 years of disease duration before liver cancer occurred or censor date with at least five HbA1c( 17 ) values for analysis. Liver cancer was diagnosed using hospitalization record based on International Classification of Diseases, Ninth Revision code (ICD‐9). We excluded patients with non‐Chinese ethnicity and history of any cancer before enrollment. All patients provided written, informed consent for research and publication purposes using anonymized data. The study was approved by the Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee, and with full adherence to the Declaration of Helsinki.
Baseline Measurement
The protocol for comprehensive assessment was adapted from the European DiabCare protocol with all patients attending the Diabetes Center after an overnight fast.( 18 ) Structured record forms were used to document key demographics, vital signs (blood pressure [BP]) and anthropometric measurements (body weight, body height, and waist circumference), medical history, and drug use at the time of assessment. Blood samples were collected to measure HbA1c, plasma glucose, lipid profile (total cholesterol, high‐density lipoprotein cholesterol [HDLC], calculated low‐density lipoprotein cholesterol [LDLC], and triglyceride [TG]), and renal and liver function. We collected a random spot urine sample to measure albumin‐to‐creatinine ratio. Microalbuminuria was defined as 2.5‐30 mg/mmol in women and 3.5‐30 mg/mmol in men, and macroalbuminuria as >30 mg/mmol. Laboratory assays were performed at the Department of Chemical Pathology, Prince of Wales Hospital, with external accreditation programs for all assays. Hypertension was defined by BP ≥ 130/80 mm Hg or use of BP‐lowering drugs at baseline. Obesity was defined as body mass index (BMI) ≥ 25 kg/m2. Retinopathy was assessed and graded by endocrinologists or trained physicians using retinal photography and defined by the presence of dot and blot hemorrhages, hard exudates, cotton wool spots, neovascularization, laser scars, or a history of vitrectomy. Sensory neuropathy was diagnosed by fulfilling two of the three features of (1) reduced sensation to monofilament examination in any part of the sole with normal skin, (2) a score of ≤6/8 (age younger than 65 years old) or ≤4/8 (age older than 65 years old) using a 128‐Hz graduated tuning fork, or (3) abnormal sensation in lower limbs. We also retrieved HBV surface antigen (HBsAg) results from the EMR if available.
Glycated Hemoglobin During Follow‐up
Hong Kong is a cosmopolitan city of 7.5 million people, primarily Southern Chinese. The city adopts a universal health care system. The Hospital Authority (HA) operates a territory‐wide network of clinics and hospitals, which provide 95% of total hospital beds and 80% of the outpatient visits. All clinics and hospitals share a single EMR system including laboratory and hospitalization data. Due to the noncompulsory nature of private insurance, most patients with chronic diseases such as diabetes and cancer were followed up in publicly funded HA clinics/hospitals. In this study, we retrieved all HbA1c values measured during outpatient and inpatient settings from the day of enrollment to the HKDR until the first hospitalization with liver cancer or study censor date of December 31, 2019, whichever came earlier.
Outcome Definition
A team of trained personnel at the HA headquarter routinely verified and coded all hospital discharge principal diagnoses according to the ICD‐9. ICD‐9 codes were used to identify first admission due to liver cancer, defined as first diagnosis of liver cancer (Code 155) for this analysis.
Statistical Analysis
Based on 1% prevalence of liver cancer in patients with T2D with available HBsAg status in the HKDR( 19 ) and a HR of 1.77 per SD increase of fasting plasma glucose associated with liver cancer,( 10 ) a sample size of 15,047 will give the study a power of 0.8 and an alpha value less than 0.05. We included 15,280 patients in the multivariate analysis models. For descriptive analysis, we used mean ± SD or median (interquartile range [IQR]) to describe continuous variables, and number (percentages) to describe categorical variables. Patients with or without liver cancer were compared for baseline demographics and clinical characteristics using the chi‐square test for comparison of categorical variables, Student t test for normally distributed continuous variables, and Wilcoxon rank‐sum test for continuous variables with skewed distribution. Follow‐up time was calculated as the period from HKDR enrollment to the date of first liver cancer diagnosis, death, or censor date of December 31, 2019, whichever came first.
We used trapezoidal integration of the area under the curve (AUC) above the prediabetic glycosylated Hb level (HbA1c > 5.7%) to calculate GB over time for each patient, expressed as AUC_A1c( 20 ) (Supporting Fig. S1A). We calculated the risk associations of GB with liver cancer using 1‐SD increment (GB_SD), quartile (GB_QA), and median values (GB_MD). We selected patients with long‐term (≥3 years) observation to study the latent effect of GB on liver cancer. We used all HbA1c values except those captured within 3 years of the occurrence of liver cancer.
We constructed multivariate Cox regression models to examine the association of GB with incident liver cancer adjusted for confounders at baseline expressed as hazard ratio (HR) with 95% CI. In model 1, we adjusted for age, sex, and disease duration. In model 2, we further adjusted for BMI, use of tobacco and alcohol, HDLC, TG, LDLC, alanine aminotransferase (ALT), bilirubin, alcohol, estimated glomerular filtration rate (eGFR), and albuminuria. In model 3, we further adjusted for use of oral glucose‐lowering drugs (OGLDs), insulin, lipid‐lowering drugs (LLDs), renin angiotensin system (RAS) inhibitors at baseline, history of cardiovascular disease (CVD), and hospitalization with heart failure. Kaplan‐Meier curves were used to assess liver cancer–free probability in patients stratified by GB_MD and obesity with log‐rank test. To assess the effect of competing risk from death on liver cancer, we fitted Fine‐Gray models( 21 ) to estimate subdistribution HRs with death as the competing event. HBV is one of the leading causes of liver cancer.( 2 , 3 ) We compared the clinical profiles of patients in the subcohort with available HBsAg status with the analysis cohort. We repeated all analyses in the subcohort with adjustment for HBsAg status. We used R 4.0.3 to perform the analyses. A P value (two‐sided) less than 0.05 was considered significant.
Results
Supporting Fig. S1B listed the patient‐selection procedures in this study. Between 1995 and 2019, 30,241 patients were enrolled in the HKDR. After excluding those with a history of cancer, 346 patients developed incident liver cancer with an incidence rate of 1.2 per 1,000 patient‐years during a follow‐up period of 288,900 patient‐years. After excluding 13,831 patients with non‐Chinese ethnicity, a history of cancer, disease duration less than 10 years before occurrence of liver cancer or censor date, fewer than five HbA1c measurements, and missing data of covariates for multivariate analysis, 15,280 patients were included for analysis, of whom, 9,014 were tested for HBsAg. Among these 15,280 patients, liver cancer occurred in 117 patients (0.77%) during a median (IQR) follow‐up period of 10.69 (8.39, 15.49) years (183,898 patient‐years) with an incidence rate of 0.64 per 1,000 patient‐years. Among the 9,041 patients with available HBsAg status, liver cancer occurred in 106 patients during a median follow‐up period of 10.55 (8.32, 15.52) years (108,655 patient‐years) with an incidence rate of 0.98 per 1,000 patient‐years. Liver cancer cases were primarily due to HCC, in which only 4 of 117 had cholangiocarcinoma.
Clinical Characteristics
Patients with liver cancer had higher waist‐to‐hip ratio, ALT, and bilirubin than those without liver cancer. They were more likely to be men, and less likely to use OGLDs, LLDs, RAS inhibitors, and insulin at baseline. They were less likely to have a history of CVD at baseline, had a shorter follow‐up duration, and more likely to die. Other metabolic profiles and risk factors were similar between the cancer and noncancer groups (Table 1).
TABLE 1.
Baseline Clinical Characteristics of Patients With Duration of T2D ≥ 10 years and observed for ≥3 years before the occurrence of liver cancer or censor date
| Patients without liver cancer | Patients with liver cancer | P Value | |
|---|---|---|---|
| n | 15,163 | 117 | |
| Age (years) | 58.58 (12.31) | 58.74 (10.57) | 0.887 |
| Men (n, %) | 7678 (50.6) | 73 (62.4) | 0.015 |
| Diabetes duration (years) | 8.52 (7.25) | 7.43 (6.35) | 0.103 |
| Family history of diabetes (n, %) | 7723 (50.9) | 52 (44.4) | 0.192 |
| Use of tobacco (n, %) | 0.345 | ||
| Current | 1,712 (11.3) | 15 (12.8) | |
| Former | 2,813 (18.6) | 27 (23.1) | |
| Nonsmoker | 10,638 (70.2) | 75 (64.1) | |
| Use of alcohol (n, %) | 0.118 | ||
| Former | 1,741 (11.5) | 13 (11.1) | |
| Never | 10,205 (67.3) | 89 (76.1) | |
| Occasional | 2,321 (15.3) | 9 (7.7) | |
| Regular | 896 (5.9) | 6 (5.1) | |
| BMI (kg/m2) | 25.58 (4.11) | 26.18 (3.94) | 0.118 |
| Waist‐to‐hip ratio | 0.91 (0.07) | 0.93 (0.08) | 0.005 |
| Systolic BP (mm Hg) | 134.52 (19.07) | 135.65 (19.09) | 0.522 |
| Diastolic BP (mm Hg) | 76.25 (10.65) | 77.55 (10.67) | 0.189 |
| Comorbidities | |||
| Sensory neuropathy (n, %) | 1,827 (12.0) | 17 (14.5) | 0.498 |
| Retinopathy (n, %) | 4,006 (26.4) | 41 (35.0) | 0.045 |
| History of CVD (n, %) | 3,699 (24.4) | 17 (14.5) | 0.018 |
| History of heart failure (n, %) | 578 (3.8) | 3 (2.6) | 0.645 |
| Laboratory tests | |||
| Fasting plasma glucose (mmol/L) | 7.60 [6.30, 9.40] | 7.65 [6.38, 10.00] | 0.668 |
| HbA1c (%) | 7.57 (1.62) | 7.81 (1.80) | 0.115 |
| Mean of AUC_A1c | 1.85 (1.04) | 2.00 (1.12) | 0.100 |
| Median of AUC_A1c | 1.68 [1.12, 2.41] | 1.90 [1.23, 2.60] | 0.070 |
| Patients in AUC_A1c quartiles (n, %) | 0.215 | ||
| Quartile 1 (AUC_range: <1.19) | 3,841 (25.3) | 25 (21.4) | |
| Quartile 2 (AUC_range: 1.19‐1.68) | 4,832 (31.9) | 31 (26.5) | |
| Quartile 3 (AUC range: 1.68‐2.41) | 2,771 (18.3) | 24 (20.5) | |
| Quartile 4 (AUC range: >2.41) | 3,719 (24.5) | 37 (31.6) | |
| TG quartile (%) | 0.528 | ||
| Quartile 1 (range: <0.97 mmol/L) | 3,847 (25.4) | 31 (26.5) | |
| Quartile 2 (range: 0.97‐1.35) | 3,882 (25.6) | 35 (29.9) | |
| Quartile 3 (range: 1.35‐2.0) | 4,086 (26.9) | 31 (26.5) | |
| Quartile 4 (≥2.0) | 3,348 (22.1) | 20 (17.1) | |
| Total cholesterol (mmol/L) | 4.85 (1.01) | 4.89 (1.04) | 0.739 |
| HDLC (mmol/L) | 1.28 [1.10, 1.50] | 1.29 [1.01, 1.60] | 0.490 |
| LDLC (mmol/L) | 2.80 (0.96) | 2.76 (1.00) | 0.681 |
| eGFR (ml/min/1.73m2) | 81.39 (22.86) | 82.79 (18.40) | 0.509 |
| Albuminuria (n, %) | 6,044 (39.9) | 46 (39.3) | 0.980 |
| Microalbuminuria (n, %) | 1,850 (12.2) | 9 (7.7) | 0.179 |
| Macroalbuminuria (n, %) | 4,194 (27.7) | 37 (31.6) | 0.395 |
| ALT (mmol/L) | 29.21 (31.35) | 63.82 (61.89) | <0.001 |
| Bilirubin (mmol/L) | 11.07 (5.63) | 14.95 (8.08) | <0.001 |
| Medications | |||
| OGLDs (n, %) | 12,188 (80.4) | 81 (69.2) | 0.004 |
| Insulin (n, %) | 3,122 (20.6) | 35 (29.9) | 0.018 |
| LLDs (n, %) | 5,825 (38.4) | 21 (17.9) | <0.001 |
| BP‐lowering drugs (n, %) | 8,738 (57.7) | 70 (59.8) | 0.705 |
| RAS inhibitors (n, %) | 5,547 (36.6) | 30 (25.6) | 0.019 |
| Outcomes | |||
| Follow‐up duration (years) | 11.91 (5.30) | 10.06 (4.27) | <0.001 |
| Death (n, %) | 2,724 (18.0) | 73 (62.4) | <0.001 |
All data are expressed as mean (SD), median (IQR) or number (%).
AUC_A1c was calculated using trapezoidal integration of the AUC above the prediabetic glucose level (HbA1c > 5.7%) over time for each patient.
Association of Glycemic Burden With Liver cancer
Because liver cancer might affect glycemic level, we excluded HbA1c values measured within 3 years of the occurrence of liver cancer to estimate the latent effects of GB on the risk of liver cancer. Every 1‐unit increase in GB_SD was associated with a HR of 1.21 (1.02‐1.43) after adjustment for age, sex, and disease duration in model 1. The HR increased to 1.30 (1.08‐1.55) after further adjusting for other risk factors, including ALT and bilirubin in model 2. The HR was 1.22 (1.01‐1.47) after additional adjusting for use of medications and comorbidities in model 3 (P trend = 0.029). On quartile analysis, there were linear relationships between GB_QA and incidence of liver cancer in three models (P trend: 0.021 in model 1, 0.004 in model 2, and 0.029 in model 3) (Fig. 1). After controlling for competing risk of death, the HRs of GB_SD with liver cancer remained similar in all three models, although not significant due to the reduced sample size (Supporting Table S1).
FIG. 1.
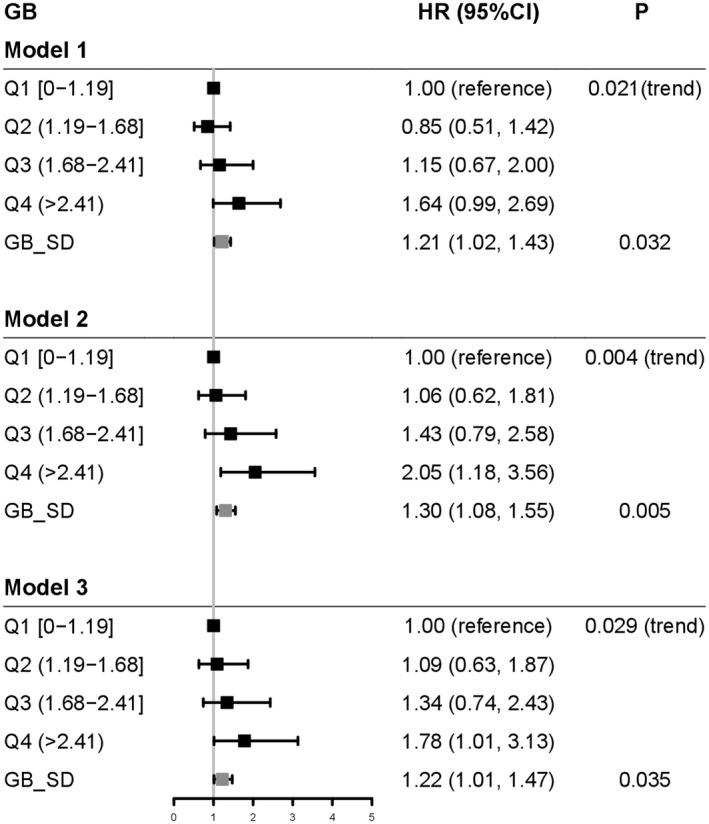
Linear associations of GB with liver cancer in patients with duration of T2D ≥ 10 years and observed for ≥3 years before the occurrence of liver cancer or censor date. Model 1: adjusted for age, sex, and disease duration. Model 2: model 1 plus BMI, use of tobacco and alcohol, HDLC, TG, LDLC, ALT, bilirubin, eGFR, microalbuminuria, and macroalbuminuria. Model 3: models 1 and 2 plus use of OGLDs, insulin, LLDs, RAS inhibitors, and history of CVD and heart failure. Q1 (range: <1.19], Q2 (1.19‐1.68), Q3 (1.68‐2.42), and Q4: (>2.42). GB_SD: 1.05. HRs (95% CIs) are expressed per quartile of GB (GB_QA), using quartile 1 as reference. P for trend across quartiles of the HbA1c burden is shown in Cox regression model.
Association of Glycemic Burden With Liver Cancer in Obese and Nonobese Patients
We divided patients into obese and nonobese groups (Table 2), stratified by BMI ≥ 25 kg/m2. In the nonobese group, GB_SD was not associated with liver cancer in all three models (Table 2). In the obese group, every increment of GB_SD was associated with a HR of 1.33 (1.07‐1.64) of liver cancer adjusted for age, sex, and disease duration in model 1. The respective HRs were 1.40 (1.11‐1.76) in model 2 and 1.34 (1.05‐1.70) in model 3. There was no interaction between obesity and GB on the risk of liver cancer (P interaction = 0.12).
TABLE 2.
Association of GB Expressed as 1‐SD Increment With Liver Cancer in Obese and Nonobese Patients With Duration of T2D ≥ 10 Years and Observed for ≥3 Years Before the Occurrence of Liver Cancer or Censor Date
| Liver Cancer | Event/Total | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| 117 of 15,280 | Model 1 | Model 2 | Model 3 | ||||
| Nonobesity (BMI < 25 kg/m2) | 45 of 7,374 | 1.04 (0.78, 1.39) | 0.786 | 1.18 (0.87, 1.60) | 0.297 | 1.12 (0.81, 1.53) | 0.498 |
| Obesity (BMI ≥ 25 kg/m2) | 72 of 7,906 | 1.33 (1.07, 1.64) | 0.010 | 1.40 (1.11, 1.76) | 0.005 | 1.34 (1.05, 1.70) | 0.018 |
| Interaction | 0.316 | 0.090 | 0.120 | ||||
Model 1: adjusted for age, sex, and disease duration. Model 2: model 1 plus BMI, use of tobacco and alcohol, HDLC, TG, LDLC, ALT, bilirubin, eGFR, microalbuminuria, and macroalbuminuria. Model 3: models 1 and 2 plus use of OGLDs, insulin, LLDs, RAS inhibitors, and history of CVD and heart failure. HRs (95% CIs) are expressed per 1‐SD increment (GB_SD).
Liver Cancer–Free Probability and Association of Glycemic Burden and Obesity With Liver Cancer
Using the median value of GB (GB_MD, 1.69 [1.13, 2.43]), we stratified patients into four subgroups: low GB_MD & nonobesity, high GB_MD & nonobesity, low GB_MD & obesity, and high GB_MD & obesity. The high GB_MD & obesity group had the lowest liver cancer–free probability (Fig. 2A). In these three models, using the low GB_MD & nonobesity as the referent group, the HRs increased linearly in the three groups, stratified by GB_MD and BMI. In model 3, the HR increased marginally to 1.05 in the high GB_MD & nonobesity group, but increased to 1.58 in the low GB_MD & obesity group, and 2.58 in the high GB_MD & obesity group (Fig. 2B), but no interaction (P interaction = 0.071).
FIG. 2.
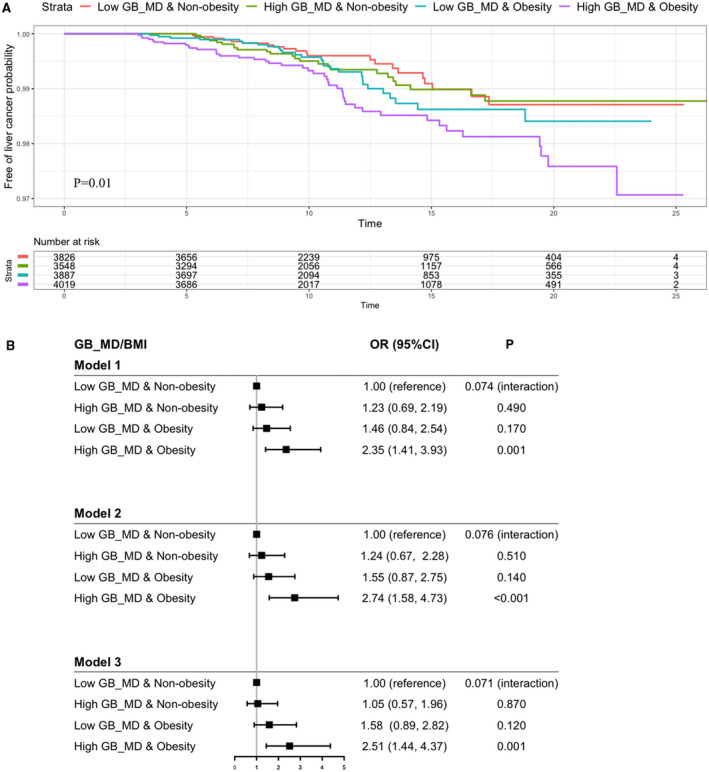
(A) Liver cancer–free probability in patients stratified by GB_MD and obesity (BMI ≥ 25 kg/m2) in patients with duration of T2D ≥ 10 years and observed for ≥3 years before the occurrence of liver cancer or censor date. (B) Risk association of liver cancer in patients with duration of T2D ≥ 10 years and observed for ≥3 years, stratified by GB_MD and obesity (BMI ≥ 25 kg/m2). Model 1: adjusted for age, sex, and disease duration. Model 2: model 1 plus use of tobacco and alcohol, HDLC, TG, LDLC, ALT, bilirubin, eGFR, microalbuminuria, and macroalbuminuria. Model 3: models 1 and 2 plus use of OGLDs, insulin, LLDs, RAS inhibitors, and history of CVD and heart failure. HRs are expressed in these four groups stratified by GB_MD and BMI (≥25 kg/m2) using low GB_MD plus nonobesity as reference. P for interaction between GB_MD and obesity in Cox regression model is shown in (B). P values were estimated by log‐rank test in (A). Abbreviation: OR, odds ratio.
Subanalysis in Patients With Available HBsAg Status
Compared with the analysis cohort, patients in the subcohort with available HBsAg status were more likely to be men, and used insulin and OGLDs at baseline. They had higher albuminuria, ALT, death rate, and less likely to use RAS inhibitors. Other metabolic profiles and risk factors were similar between the two cohorts (Supporting Table S1). We repeated these analyses in the subcohort with available HBsAg status. In this cohort, the HRs of GB_SD with liver cancer remained unchanged with adjustment of HBsAg status (Supporting Table S3). The quartile analysis of GB_SD with liver cancer showed increasing HRs after adjusting for HBsAg status (Supporting Table S4). The high GB_MD & obesity group had the highest HR (2.85 [1.55, 5.27]) for liver cancer, compared with the low GB_MD & nonobesity group, but no interaction between GB and obesity (Supporting Table S5). The adjusted HRs of GB_SD with liver cancer were similar between HBsAg carriers and noncarriers (Supporting Table S6). After excluding patients with a history of HBV, the HRs of GB_SD with liver cancer remained unchanged (Supporting Table S7).
Discussion
In this prospective analysis, we included 15,280 Chinese patients with T2D for at least 10 years, observed for at least 3 years before liver cancer occurred or censor date. The incidence rate was 0.64 per 1,000 patient‐years. In this analysis, we leveraged the long follow‐up duration of the HKDR to investigate the latent effect of GB and obesity on liver cancer risk. We excluded HbA1c values measured within 3 years before first hospitalization with liver cancer to minimize the possibility of reverse causality. We found a linear relationship between quartiles of GB and liver cancer, in which the top quartile GB_QA group had a HR of 1.78 compared with the lowest quartile group. When stratified by the median value of GB and obesity, the high GB_MD & obesity group had the highest HR of 2.51, compared with the low GB_MD group & nonobesity group, but formal testing for interaction was not significant (P interaction = 0.071). In our sensitivity analysis, there was no competing risk of death in the association between GB and liver cancer. On subgroup analysis in patients with available HBsAg status, the associations of GB with liver cancer remained unchanged, suggesting that this association was independent of chronic HBV infection.
Most of the reports on risk association between diabetes and liver cancer came from population‐based cohorts. Compared to people without diabetes, diabetes was associated with liver cancer with HRs of 1.54 and 1.78 in Korean( 7 ) and Chinese populations, respectively.( 22 , 23 , 24 ) In these studies, a single plasma glucose or HbA1c at baseline was used as the risk predictor. However, the effect of cumulative exposure to hyperglycemia in patients with T2D cannot be reflected by a one‐time plasma glucose level or HbA1c. In this report of long‐term hyperglycemia and liver cancer risk, we used serial HbA1c values to estimate GB further analyzed by SD increment, quartile range, and median values, and found consistent and independent risk association between GB and liver cancer, which were primarily due to HCC.
Experimentally, hyperglycemia might activate multiple pathways, notably the insulin growth factor‐1. The latter increased the risk of liver cancer by activating the progression of cell cycle and inhibiting apoptosis, leading to the dysregulation of cellular proliferation and survival.( 25 , 26 ) In both randomized clinical trials (e.g., United Kingdom Prospective Diabetes Study, Steno 2 Study) and cohort analyses,( 27 , 28 , 29 ) researchers had reported the latent effects of poor glycemic control on microvascular and macrovascular complications. They also reported the delayed benefits of optimizing glycemic control in reducing long‐term complications.( 30 , 31 ) In this context, our findings suggested that optimizing glycemic control might reduce cancer risk especially in patients with long disease duration, such as those with young onset diabetes.( 32 )
Obesity was often used to explain the frequent co‐occurrence of diabetes and cancer.( 33 )
In patients without T2D, researchers had reported a higher risk of liver cancer in obese than nonobese subjects.( 34 , 35 ) In a Korean population–based study, there was a linear association between the risk of all‐site cancer and fasting plasma glucose in nonobese, overweight, and obese subjects.( 36 ) Obesity‐associated nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) were known risk factors for liver cancer.( 37 ) In subjects with NASH, release of free radicals from lipid peroxide might cause genetic mutation to increase the risk of liver cancer.( 38 ) Moreover, release of proinflammatory cytokines from adipose tissue, including tumor necrosis factor‐alpha, interleukin‐6 and leptin, could cause hepatic inflammation with increased risk of cirrhosis and liver cancer.( 39 )
Nonobese patients with type 1 diabetes also had a higher risk of liver cancer than control subjects, supporting the importance of hyperglycemia in cancer development.( 40 ) In the Swedish Obesity Subject Study, researchers recently reported a HR of 0.63 for all‐site cancer in obese patients with diabetes who underwent metabolic surgery versus those with conventional obesity management.( 41 ) In a separate analysis of the HKDR, both glycemic variability and obesity were associated with all cancer events and related death.( 42 ) In this analysis, each increment of GB_SD was associated with a nonsignificant HR of 1.12 for liver cancer in nonobese patients, which increased to a significant HR of 1.34 in obese patients, but there was no significant interaction. When further stratified by GB_MD and obesity, the high GB_MD & obese group had the highest HRs of 2.51 for liver cancer, compared with the low GB_MD & nonobesity group, but the interaction was also not significant. The nonsignificant interaction between GB and obesity might be due to a lack of statistical power with a small sample size. It also might be due to no significant modification effect of obesity on liver cancer as well as sampling variation. Nevertheless, our results supported the importance of optimizing glycemic control and body weight to reduce the risk of liver cancer in patients with T2D.
Liver plays an important role in balancing between glucose production and glycogen storage through glycogenesis, glycogenolysis, glycolysis, and gluconeogenesis.( 43 ) It is also the major site of synthesis and breakdown of regulatory enzymes and transcription factors in glucose metabolism.( 12 ) Thus, liver dysfunction due to cancer might impair glucose metabolism, leading to hyperglycemia.( 44 ) By excluding the HBA1c values in the 3 years before occurrence of liver cancer, we have minimized the confounding effects of subclinical liver cancer on GB with reverse causality.
Most patients with acute HBV infection recovered with the disappearance of HBsAg.( 45 ) Some might become chronic HBsAg carriers, defined by the presence of HBsAg for more than 6 months.( 45 ) These carriers might have a greater than 100‐fold increased risk of developing liver cancer than non‐HBsAg carriers.( 46 ) HBsAg was not routinely tested in the HKDR enrollees, although other researchers had reported higher frequency of chronic HBV infection in patients with T2D than control subjects.( 47 ) To preserve statistical power, we adjusted for ALT and bilirubin, but not HBsAg, in the multivariate models in the analysis cohort. In the subanalysis including nearly 60% of patients tested for HBsAg, adjustment for HBsAg did not alter the HRs in the three models. After excluding patients with a history of HBV‐induced hepatitis as indicated by HBsAg status, the risk association of GB with liver cancer remained constant. Some workers had reported similar risk estimate of T2D for liver cancer after adjustment for HBsAg status.( 35 ) While others reported similar( 48 , 49 ) risk of T2D‐associated liver cancer between HBsAg carriers and noncarriers.
Our study has several limitations. The diagnosis of liver cancer was based on the first hospitalization record in the EMR. Thus, patients with subclinical liver cancer or diagnosed in the private sector might have been missed. Due to unmeasured confounders for drug use in observational studies, we only adjusted for drug use at baseline. Despite the relatively small number of liver cancer, we had sufficient power to test our hypothesis regarding the association between GB and cancer risk using multivariate analyses.
In summary, in this long‐term prospective study of patients with T2D for at least 10 years, both obesity and GB were associated with increased risk of liver cancer without significant interactions. Optimizing glycemic control and body weight will be important to reduce the risk of liver cancer, especially in young patients who face a long disease duration and high‐risk patients with NAFLD, NASH, chronic HBV infection, and alcohol‐associated liver disease.
Supporting information
Fig S1
Table S1‐S7
Acknowledgment
The authors thank all clinical and supporting staff, including doctors, nurses and health care assistants, in implementing the data‐driven quality‐improvement program and establishing the HKDR.
Supported by the Chinese University of Hong Kong Direct Grant.
Potential conflict of interest: J.C. received research grants, honorarium, and speakers’ fees from Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Celltrion, Hua Medicine, Lee Powder, Eli Lilly, Merck Sharpe Dohme, Merck Serono, Pfizer, Sanofi, Servier, and Viatris Pharmaceutical. E.C. received grants from Lee Powder and Sanofi. A.P.S.K. received research grants and/or speaker honoraria from Abbott, Astra Zeneca, Eli‐Lilly, Merck Serono, Nestle, and Novo Nordisk. R.C.W.M. reported receiving grants and/or honoraria for consultancy or giving lectures from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, Tricia Inc., Pfizer and Takeda.
Data Availability Statement
The data supporting the findings of this study are available upon reasonable request from the corresponding author (J.C.N.C.).
References
- 1. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson AB, et al. Annual report to the nation on the status of cancer, 1975–2014. Featuring survival. J Natl Cancer Inst 2017;109:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maucort‐Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer 2018;142:2471‐2477. [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Yang H, Iloeje UH, You S, Lu S, Wang Li–Yu, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver‐related death. Gastroenterology 2010;138:1747‐1754. [DOI] [PubMed] [Google Scholar]
- 4. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol 2020;72:250‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hashim D, Boffetta P, La Vecchia C, Rota M, Bertuccio P, Malvezzi M, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol 2016;27:926‐933. [DOI] [PubMed] [Google Scholar]
- 6. Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362:k2817. 10.1136/bmj.k2817. PMID: 30021785; PMCID: PMC6050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005;293:194‐202. [DOI] [PubMed] [Google Scholar]
- 8. El‐Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004;126:460‐468. [DOI] [PubMed] [Google Scholar]
- 9. Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta‐analysis. Diabetes Metab Res Rev 2012;28:109‐122. [DOI] [PubMed] [Google Scholar]
- 10. Han H, Zhang T, Jin Z, Guo H, Wei X, Liu Y, et al. Blood glucose concentration and risk of liver cancer: systematic review and meta‐analysis of prospective studies. Oncotarget 2017;8:50164‐50173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, Bommer C, et al. Global and regional estimates and projections of diabetes‐related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2020;162:108072‐108080. [DOI] [PubMed] [Google Scholar]
- 12. Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver‐centric perspective. Exp Mol Med 2016;48:218‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab 2000;11:327‐332. [DOI] [PubMed] [Google Scholar]
- 14. Calle EE, Thun MJ. Obesity and cancer. Oncogene 2004;23:6365‐6378. [DOI] [PubMed] [Google Scholar]
- 15. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010;49:1603‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan JCN, Lim L‐L, Luk AOY, Ozaki R, Kong APS, Ma RCW, et al. From Hong Kong Diabetes Register to JADE Program to RAMP‐DM for data‐driven actions. Diabetes Care 2019;42:2022‐2031. [DOI] [PubMed] [Google Scholar]
- 17. Li S, Nemeth I, Donnelly L, Hapca S, Zhou K, Pearson ER. Visit‐to‐visit HbA(1c) variability is associated with cardiovascular disease and microvascular complications in patients with newly diagnosed type 2 diabetes. Diabetes Care 2020;43:426‐432. [DOI] [PubMed] [Google Scholar]
- 18. Piwernetz K, Home PD, Snorgaard O, Antsiferov M, Staehr‐Johansen K, Krans M. Monitoring the targets of the St Vincent Declaration and the implementation of quality management in diabetes care: the DIABCARE initiative. The DIABCARE Monitoring Group of the St Vincent Declaration Steering Committee. Diabet Med 1993;10:371‐377. [DOI] [PubMed] [Google Scholar]
- 19. Yang X, Wang Y, Luk AOY, So WY, Ma RCW, Kong APS, et al. Enhancers and attenuators of risk associations of chronic hepatitis B virus infection with hepatocellular carcinoma in type 2 diabetes. Endocr Relat Cancer 2013;20:161‐171. [DOI] [PubMed] [Google Scholar]
- 20. Nichols GA, Rosales AG, Perrin NA, Fortmann SP. The association between different A1C‐based measures of glycemia and risk of cardiovascular disease hospitalization. Diabetes Care 2014;37:167‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496‐509. [Google Scholar]
- 22. Yang WS, Shu XO, Gao J, Li HL, Cai H, Yang G, et al. Prospective evaluation of type 2 diabetes mellitus on the risk of primary liver cancer in Chinese men and women. Ann Oncol 2013;24:1679‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lo S‐F, Chang S‐N, Muo C‐H, Chen S‐Y, Liao F‐Y, Dee S‐W, et al. Modest increase in risk of specific types of cancer types in type 2 diabetes mellitus patients. Int J Cancer 2013;132:182‐188. [DOI] [PubMed] [Google Scholar]
- 24. Pang Y, Kartsonaki C, Turnbull I, Guo YU, Clarke R, Chen Y, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology 2018;68:1308‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adachi Y, Nojima M, Mori M, Matsunaga Y, Akutsu N, Sasaki S, et al. Insulin‐like growth factor‐related components and the risk of liver cancer in a nested case‐control study. Tumour Biol 2016;37:15125‐15132. [DOI] [PubMed] [Google Scholar]
- 26. Adamek A, Kasprzak A. Insulin‐like growth factor (IGF) system in liver diseases. Int J Mol Sci 2018;19:11‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lam E, Batty GD, Huxley RR, Martiniuk A, Barzi F, Lam TH, et al. Associations of diabetes mellitus with site‐specific cancer mortality in the Asia‐Pacific region. Ann Oncol 2011;22:730‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause‐specific death. N Engl J Med 2011;364:829‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan Y, Wei S, Zhang W, Yang J, Yang J, Yan L. Type 2 diabetes mellitus increases the risk of hepatocellular carcinoma in subjects with chronic hepatitis B virus infection: a meta‐analysis and systematic review. Cancer Manag Res 2019;11:705‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodríguez‐Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes 2016;9:504‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stolar M. Glycemic control and complications in type 2 diabetes mellitus. Am J Med 2010;123:S3‐S11. [DOI] [PubMed] [Google Scholar]
- 32. Ke C, Stukel TA, Shah BR, Lau E, Ma RC, So W‐Y, et al. Age at diagnosis, glycemic trajectories, and responses to oral glucose‐lowering drugs in type 2 diabetes in Hong Kong: a population‐based observational study. PLoS Medicine 2020;17:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer‐related mortality. Physiol Rev 2015;95:727‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625‐1638. [DOI] [PubMed] [Google Scholar]
- 35. Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, Fraumeni JF, et al. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 2001;12:13‐21. [DOI] [PubMed] [Google Scholar]
- 36. Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol 2005;23:4742‐4754. [DOI] [PubMed] [Google Scholar]
- 37. Yu J, Shen J, Sun TT, Zhang X, Wong N. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Semin Cancer Biol 2013;23:483‐491. [DOI] [PubMed] [Google Scholar]
- 38. Caldwell SH, Crespo DM, Kang HS, Al‐Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology 2004;127:S97‐S103. [DOI] [PubMed] [Google Scholar]
- 39. Papa S, Bubici C, Zazzeroni F, Franzoso G. Mechanisms of liver disease: cross‐talk between the NF‐kappaB and JNK pathways. Biol Chem 2009;390:965‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carstensen B, Read SH, Friis S, Sund R, Keskimäki I, Svensson A‐M, et al. Cancer incidence in persons with type 1 diabetes: a five‐country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia 2016;59:980‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sjöholm K, Carlsson LMS, Svensson P‐A, Andersson‐Assarsson JC, Kristensson F, Jacobson P, et al. Association of bariatric surgery with cancer incidence in patients with obesity and diabetes: long‐term results from the Swedish obese subjects study. Diabetes Care 2021:dc211335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mao D, Lau ESH, Wu H, Yang A, Shi M, Fan B, Tam CHT, et al. Risk associations of long‐term HbA1c variability and obesity on cancer events and cancer‐specific death in 15,286 patients with diabetes—a prospective cohort study. The Lancet Regional Western Pacific 2022;18:100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr 1999;19:379‐406. [DOI] [PubMed] [Google Scholar]
- 44. Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med 2007;120:829‐834. [DOI] [PubMed] [Google Scholar]
- 45. Terrault NA, Lok ASF, McMahon BJ, Chang K‐M, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arbuthnot P, Kew M. Hepatitis B virus and hepatocellular carcinoma. Int J Exp Pathol 2001;82:77‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu J, Hou X, Tu H, Tang Z, Xiang Y, Bao Y, et al. Chronic hepatitis B virus infection status is more prevalent in patients with type 2 diabetes. J Diabetes Investig 2017;8:619‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Han H, Deng H, Han T, Zhao H, Hou F, Qi X. Association between hepatocellular carcinoma and type 2 diabetes mellitus in Chinese hepatitis B virus cirrhosis patients: a case‐control study. Med Sci Monit 2017;23:3324‐3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li X, Wang X, Gao P. Diabetes mellitus and risk of hepatocellular carcinoma. Biomed Res Int 2017;2017:5202684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S7
Data Availability Statement
The data supporting the findings of this study are available upon reasonable request from the corresponding author (J.C.N.C.).


