Abstract
The value of noninvasive tools in the diagnosis of autoimmune hepatitis (AIH)–related cirrhosis and the prediction of clinical outcomes is largely unknown. We sought to evaluate (1) the utility of liver stiffness measurement (LSM) in the diagnosis of cirrhosis and (2) the performance of the Sixth Baveno Consensus on Portal Hypertension (Baveno VI), expanded Baveno VI, and the ANTICIPATE models in predicting the absence of varices needing treatment (VNT). A multicenter cohort of 132 patients with AIH‐related cirrhosis was retrospectively analyzed. LSM and endoscopies performed at the time of cirrhosis diagnosis were recorded. Most of the patients were female (66%), with a median age of 54 years. Only 33%‐49% of patients had a LSM above the cutoff points described for the diagnosis of AIH‐related cirrhosis (12.5, 14, and 16 kPa). Patients with portal hypertension (PHT) had significantly higher LSM than those without PHT (15.7 vs. 11.7 kPa; P = 0.001), but 39%‐52% of patients with PHT still had LSM below these limits. The time since AIH diagnosis negatively correlated with LSM, with longer time being significantly associated with a lower proportion of patients with LSM above these cutoffs. VNT was present in 12 endoscopies. The use of the Baveno VI, expanded Baveno VI criteria, and the ANTICIPATE model would have saved 46%‐63% of endoscopies, but the latter underpredicted the risk of VNT. Conclusions: LSM cutoff points do not have a good discriminative capacity for the diagnosis of AIH‐related cirrhosis, especially long‐term after treatment initiation. Noninvasive tools are helpful to triage patients for endoscopy.
Abbreviations
- AIH
autoimmune hepatitis
- ALT
alanine aminotransferase
- ANA
antinuclear autoantibodies
- AST
aspartate aminotransferase
- Baveno VI
Sixth Baveno Consensus on Portal Hypertension
- HE
hepatic encephalopathy
- IgG
immunoglobulin G
- IQR
interquartile range
- LSM
liver stiffness measurement
- LSM‐cirrhosis
LSM at diagnosis of cirrhosis
- NPV
negative predictive value
- PBC
primary biliary cholangitis
- SBP
spontaneous bacterial peritonitis
- VNT
varices needing treatment
- VCTE
vibration‐controlled transient elastography
Approximately 30% of patients with autoimmune hepatitis (AIH) present with cirrhosis at diagnosis,( 1 ) and a further 10% develop cirrhosis during follow‐up.( 2 ) Fibrosis progression can occur despite biochemical remission due to the fluctuating course of the disease and the persistence of histological activity in up to 40% of patients.( 3 ) Therefore, patients with AIH require close and life‐long follow‐up.
Similar to other liver diseases, the use of vibration‐controlled transient elastography (VCTE) to determine liver stiffness in patients with AIH has emerged as an objective and robust tool to monitor disease progression and avoid serial biopsies.( 4 ) The results of studies assessing the performance of VCTE in AIH have been confounded by the contribution of inflammation in the interpretation of liver stiffness measurement (LSM), especially during the first 6 months after treatment initiation.( 4 , 5 , 6 , 7 , 8 , 9 ) As a result, it has been difficult to identify consistent cutoff points for the diagnosis of AIH‐related cirrhosis.( 5 , 7 ) Furthermore, these studies have additional limitations: (1) The number of patients with cirrhosis was low, and (2) there was a lack of data on the clinical predictive value of LSM in patients with AIH‐related cirrhosis.
A major event in the natural history of portal hypertension (PHT) in patients with cirrhosis is the development of esophageal varices with the subsequent risk of variceal bleeding. The Sixth Baveno Consensus on Portal Hypertension (Baveno VI) recommended the use of noninvasive tools to rule out the presence of varices with high risk of bleeding (varices needing treatment [VNT]). According to Baveno VI, patients with compensated advanced chronic liver disease with a normal platelet count (>150,000) and LSM < 20 kPa do not need endoscopic surveillance because the predicted risk of having VNT is less than 5% (“safe” risk threshold defined by the Baveno VI consensus). Implementing this recommendation into clinical practice could spare 20%‐40% of surveillance endoscopies.( 10 ) The expanded Baveno VI criteria (platelets > 110,000 and LSM < 25 kPa) have also proven to be safe, sparing a higher number of endoscopies than the original criteria.( 11 ) Nonetheless, it is important to consider that Baveno VI and expanded Baveno VI criteria were derived mostly from patients with chronic viral hepatitis and alcohol‐associated liver disease. Although subsequent studies showed that the same thresholds could be useful in other etiologies,( 12 , 13 ) patients with autoimmune liver disease remained underrepresented and in need of more robust validations.
In an attempt to refine the prediction of clinically significant PHT and VNT using noninvasive tools, Abraldes et al. conducted the ANTICIPATE study,( 14 ) which incorporated a continuous risk prediction model that assigned a probability of VNT to each of the predictor values (platelets and LSM). This provided an individual absolute risk of VNT for each patient. Again, this cohort consisted primarily of patients with chronic viral hepatitis or alcohol‐associated liver disease, and the use of this model in patients with primary biliary cholangitis (PBC) underpredicted the risk of VNT and required recalibration.( 15 )
Given these limitations, and the differences in the natural history of AIH as compared with cholestatic diseases, we designed a large multicenter cohort study to evaluate (1) the utility of LSM in the diagnosis of AIH‐related cirrhosis and the prediction of clinical outcomes, and (2) the performance of the Baveno VI, expanded Baveno VI, and the ANTICIPATE models in predicting the absence of VNT in patients with AIH.
Patients and Methods
Patients
This was a retrospective, multicenter, cohort study in which all patients with AIH‐related cirrhosis at 11 Spanish centers (Hospital Clínic Barcelona, Hospital Ramón y Cajal, Hospital Universitario Central de Asturias, Hospital Vall d’Hebron, Hospital de Burgos, Hospital Gregorio Marañón, Hospital del Mar, Hospital La Fé de Valencia, Mutua de Terrassa, Hospital Universitario Reina Sofía, and Fundación Hospital Alcorcón) were enrolled between January 1985 and January 2020. Patients were identified by the revision of local databases of patients with AIH.
The inclusion criteria were (1) probable or definite diagnosis of AIH according to the International Autoimmune Hepatitis Group (IAIHG) simplified criteria( 16 ) as recommended by the European Association for the Study of the Liver guidelines( 17 ); (2) cirrhosis defined by histological, clinical (decompensation of liver disease including ascites, hepatic encephalopathy [HE], spontaneous bacterial peritonitis [SBP], and variceal bleeding) and/or ultrasound criteria (surface nodularity, heterogenous echostructure, segmental hypertrophy, or atrophy)( 18 , 19 ); and (3) availability of LSM at the time of cirrhosis diagnosis. The exclusion criteria were (1) the presence of other chronic liver disease (including viral hepatitis, alcohol, and/or nonalcoholic fatty liver disease) and (2) variant forms of AIH (overlap with PBC or primary sclerosing cholangitis).
Data Collection
Demographic and clinical data such as age, sex, autoimmune comorbidities, date of AIH diagnosis, endoscopic results, development and characterization of flares, as well as decompensations were collected. Laboratory data, assessed at the time of AIH diagnosis, the diagnosis of cirrhosis, and the evaluation of Baveno criteria included aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, gamma‐glutamyl transferase, total bilirubin, international normalized ratio, immunoglobulin G (IgG) levels, antinuclear autoantibodies (ANA), anti‐smooth muscle antibody (ASMA), anti‐liver kidney microsome type 1, anti‐liver cytosol 1, anti‐soluble liver antigen, anti‐mitochondrial, and platelet count. The original reports of the liver biopsies performed at diagnosis were also retrospectively analyzed. Hematoxylin and eosin staining was used to determine portal and lobular necrosis and inflammation, type of cell infiltrate, the presence of interface hepatitis, hepatocyte ballooning, and/or emperipolesis. Masson’s trichomic was used to evaluate fibrosis stage.
Immunosuppressive treatment was recorded. Standard treatment was defined as the use of prednisone in monotherapy, prednisone in combination with azathioprine, or azathioprine in monotherapy. Other treatments prescribed to patients due to intolerance or nonresponse were considered as second‐line or third‐line therapies.( 20 ) Remission was defined as ALT and IgG levels within normal limits. Lack of remission within 6 months of treatment initiation was considered as insufficient response.( 20 )
The presence of PHT was determined according to the presence of decompensation (ascites, SBP, HE, or variceal bleeding), esophageal varices, or signs of PHT in the liver ultrasound (collateral circulation, paraumbilical vein recanalization, splenomegaly, or reverse flow in portal vein).
Noninvasive Prediction of Cirrhosis and Clinical Outcomes
LSM at diagnosis of cirrhosis (LSM‐cirrhosis) determined by VCTE (FibroScan) and upper gastrointestinal endoscopy were recorded. LSM was performed by experienced operators following the manufacturer’s recommendations, with the patient in fasting conditions. Only reliable (≥10 valid measurements, ≥60% success rate, and interquartile range [IQR]/median ratio ≤0.30) LSM was included in the analysis. Due to the important role of inflammation in LSM, VCTE performed within 6 months of the diagnosis of AIH was excluded from the analysis. The maximum interval between VCTE and gastrointestinal endoscopy was 1 year, as recommended by the Baveno VI consensus.( 10 ) In patients with ascites, LSM was always performed after the resolution of this complication. Based on the current literature, we evaluated three cutoff points that have proven to be useful in the diagnosis of cirrhosis (14 kPa)( 21 , 22 ) and specifically in the diagnosis of AIH‐related cirrhosis (12.5 kPa( 7 ) and 16 kPa( 5 )).
Finally, we studied whether the Baveno VI criteria (defined as platelets > 150,000 and LSM < 20 kPa) and the Baveno VI expanded criteria (platelets > 110,000 and LSM < 25 kPa) can avoid screening endoscopies in patients with AIH cirrhosis and a low risk of having VNT.( 10 , 11 ) VNT was defined as large varices and/or small varices with high‐risk stigmata. To evaluate the association between platelet count and elastography and the presence of VNT, we evaluated the performance of the model generated in the ANTICIPATE study( 14 ) (probability of VNT = 1/[1 + e‐logit] where logit = −4.458421 + 1.3193115 * ln[SM‐TE in kPa] – 0.016306902 * platelet count [in n × 109/L], capped at 150).
Statistical Analysis
Categorical variables were expressed as number and percentage. Continuous variables were expressed as median and IQR. Differences in proportions were compared by the χ2 test or Fisher’s exact test, as appropriate. Continuous variables were compared by Mann‐Whitney U test non‐parametrical testing. Bivariate correlation between the time after the diagnosis (and starting of treatment) of AIH and LSM was analyzed using the Spearman rank‐order correlation test. The Cochran‐Armitage test of trend was used to determine whether there was a linear trend between time after the diagnosis of AIH (categorized in four periods: <12 months, 12‐36 months, 36‐60 months, and >60 months) and the probability of having a LSM above the cutoff points used to determine the presence of cirrhosis. P < 0.05 was considered statistically significant.
Based on contingency tables, we calculated the negative predictive value (NPV) and the number of endoscopies that could be saved by using Baveno VI and expanded Baveno VI criteria. The performance of the ANTICIPATE study continuous predictive model was assessed with the c‐statistic (discrimination) and by plotting the predicted versus observed rates of VNT (calibration).( 15 , 23 ) Calibration was assessed with the Z calibration statistic described by Spiegelhalter,( 24 ) with its P value. The closer the value of the Z‐statistic to zero, and the higher its P value, the better the calibration of the model.
Statistical analysis was conducted with Stata version 15.1 (Stata Corp, College Station, TX), SPSS 20.0 (IBM Corp., Armonk, NY), and R Statistical Software (Foundation for Statistical Computing, Vienna, Austria), using the rms package.( 25 )
Ethics
The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in the approval by the Ethics Committee at the Hospital Clinic of Barcelona.
Results
Baseline Characteristics
A total of 132 patients diagnosed with AIH‐related cirrhosis were included in the study. One hundred sixteen (88%) had a definite diagnosis according to the IAIHG simplified criteria, and the remaining 16 (12%) patients did not undergo a liver biopsy, but all of them had a probable diagnosis of AIH, responded to treatment, and achieved biochemical remission. Twenty‐one patients had an acute presentation (16%), 20 (15%) a severe acute AIH, and 91 (68%) had a chronic presentation. As shown in Fig 1, 60 (45%) patients had cirrhosis at the time of the initial diagnosis of AIH, whereas in the remaining 72 (55%) patients, cirrhosis was identified a median of 66 months (IQR 19‐131) after having been diagnosed with AIH. The baseline characteristics of these patients are found in Table 1. Briefly, most of the patients were female (n = 85, 64%), with type 1 AIH (n = 120, 90.9%), and a median age of 54 years (IQR 42‐63) at the time of diagnosis. The median laboratory results at AIH diagnosis were as follows: ALT 298 IU/L (IQR 146‐817), AST 359 IU/L (IQR 143‐835), total bilirubin 1.9 mg/dL (IQR 0.9‐7.3), IgG 20.9 g/L (IQR 16‐29.3), ANA titers 1:160 (IQR 1:50‐1:640), and ASMA titers 1:40 (IQR 0‐1:80).
FIG. 1.
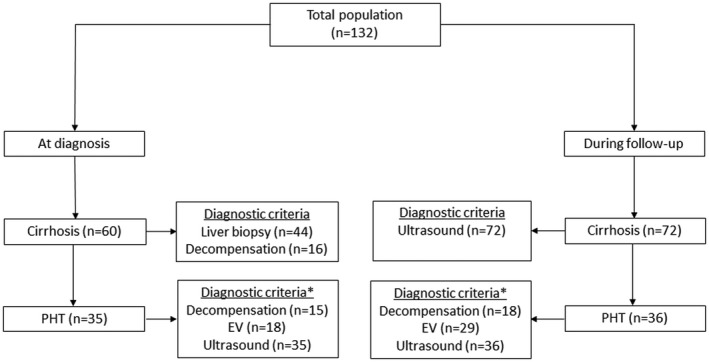
Flowchart of the patients included in the study. Abbreviation: EV, esophageal varices.
TABLE 1.
Clinical Characteristics of Patients at the Time of AIH Diagnosis
| Variable | All (n = 132) | Cirrhosis at Diagnosis of AIH (n = 60) | Cirrhosis During Follow‐up (n = 72) | P Value |
|---|---|---|---|---|
| Age (years) | 54 (42‐63) | 58 (50‐71) | 49 (29‐59) | 0.001 |
| Female (n, %) | 85 (64%) | 40 (67%) | 45 (63%) | 0.377 |
| Autoimmune comorbidity (n, %) | 36 (27%) | 18 (30%) | 18 (25%) | 0.327 |
| Laboratory results at diagnosis | ||||
| AST (U/L) | 359 (143‐835) | 334 (92‐575) | 489 (149‐1,172) | 0.046 |
| ALT (U/L) | 298 (146‐817) | 240 (80‐640) | 367 (202‐1,107) | 0.016 |
| ALP (U/L) | 175 (122‐243) | 171 (124‐238) | 178 (121‐313) | 0.531 |
| GGT (U/L) | 165 (84‐329) | 180 (102‐353) | 148 (69‐293) | 0.196 |
| Bilirubin (mg/dL) | 1.9 (0.9‐7.3) | 2 (1‐6.4) | 1.7 (0.9‐8) | 0.573 |
| IgG (g/dL) | 20.9 (16‐29.3) | 20.7 (16.1‐28) | 21.4 (15.2‐32) | 0.493 |
| ANA (titer) | 160 (50‐640) | 160 (40‐640) | 160 (60‐640) | 0.764 |
| SMA (titer) | 40 (0‐80) | 0 (0‐80) | 40 (0‐160) | 0.236 |
| AMA positivity (n, %) | 19 (14%) | 8 (13%) | 11 (15%) | 0.613 |
| Anti‐LKM positivity (n, %) | 12 (9%) | 4 (7%) | 8 (11%) | 0.362 |
| Anti‐SLA positivity (n, %) | 12 (9%) | 4 (7%) | 8 (11%) | 0.353 |
| Platelet count (×109) | 156 (121‐223) | 150 (113‐210) | 173 (130‐236) | 0.125 |
| INR | 1.1 (1.0‐1.4) | 1.2 (1‐1.4) | 1.1 (1‐1.4) | 0.822 |
| MELD score* | 10 ‐18) | 10 (7‐18) | — | — |
| Fibrosis stage at diagnosis * (n, %) | <0.001 | |||
| F0‐F1 | 19 (16%) | 0 | 17 (30%) | |
| F2 | 9 (8%) | 0 | 9 (15%) | |
| F3 | 44 (38%) | 14 (24%) | 32 (55%) | |
| F4 | 44 (38%) | 44 (76%) | 0 | |
| Decompensation at diagnosis (n, %) | 16 (13%) | 16 (25%) | — | — |
| Simplified score HAI | 8 (6‐8) | 8 (6‐8) | 8 (6‐8) | 0.880 |
| Treatment (n, %) | 0.150 | |||
| Prednisone + azathioprine | 74 (56%) | 34 (57%) | 40 (57%) | |
| Azathioprine in monotherapy | 3 (2%) | 2 (3%) | 1 (1%) | |
| Prednisone in monotherapy | 46 (35%) | 18 (30%) | 28 (40%) | |
| Other † | 9 (7%) | 8 (10%) | 3 (2%) |
Data are expressed as median (IQR).
Calculated only in patients with cirrhosis at diagnosis.
According to liver biopsy (available in 116 patients at diagnosis of AIH).
Other treatments included no treatment (n = 7), ursodeoxycholic acid (n = 1), and budesonide in combination with azathioprine (n = 1).
Abbreviations: ALP, alkaline phosphatase; AMA, anti‐mitochondrial antibody; ANA, antinuclear antibodies; anti‐LKM, anti‐liver kidney microsome type 1; anti‐SLA, anti‐soluble liver antigen; GGT, gamma‐glutamyltransferase; HAI, histological activity index; MELD: Model for End‐Stage Liver Disease; SMA, smooth muscle antibodies.
One hundred sixteen patients underwent a liver biopsy at diagnosis. Eighty‐eight (76%) had advanced fibrosis (METAVIR fibrosis stage F3 or F4). Among the 16 patients without liver biopsy, 3 had clinical evidence of cirrhosis and PHT (esophageal varices and collateral circulation). Patients with cirrhosis at the time of the diagnosis of AIH were significantly older (58 vs. 49 years; P = 0.001) and had lower AST (334 vs. 489; P = 0.046) and ALT (240 vs. 367; P = 0.016) levels.
Seventy‐four patients (56%) received combined therapy with prednisone and azathioprine from diagnosis, and 46 (35%) were given prednisone in monotherapy. One hundred nine patients (83%) achieved disease remission. Thirty patients (23%) required second‐line or third‐line therapies due to intolerance to standard treatment (n = 3) or insufficient response (n = 27). Insufficient responders were younger (45 vs. 55 years; P = 0.010) compared with those who achieved remission. There were no other differences in clinical, biochemical, or histological characteristics between responders and patients with insufficient response (Supporting Table S1).
Thirty‐five patients (27%) had signs of PHT at the time of the initial diagnosis of AIH, and 15 (11%) were already decompensated (13 had ascites, 6 had HE, 3 had SBP, and 1 had variceal bleeding). During a median follow‐up of 127 months (IQR 61‐189), 36 (27%) additional patients developed features of PHT; 18 patients (15%) were decompensated (15 had ascites, 3 had HE, 3 had SBP, and 1 had variceal bleeding); 6 patients (5%) underwent liver transplantation; and 8 (6%) patients died (4 due to solid organ neoplasms including 1 hepatocellular carcinoma, 3 due to infection, and in the remaining patient the cause of death was unknown).
LSM for Diagnosis of AIH‐Related Cirrhosis and PHT
To avoid the impact of liver inflammation on liver stiffness in the first months of AIH treatment,( 5 ) only 107 patients in whom LSM‐cirrhosis had been performed ≥6 months after the diagnosis of AIH were included in this analysis.( 5 ) The median LSM value was 12.6 kPa (IQR: 8.5‐20.4). Fifty‐two (49%) patients had a LSM ≥ 12.5 kPa, whereas in 44 (41%) patients the LSM was ≥14 kPa, and in 37 (33%) it was ≥16 kPa, which are the cutoff points described for the diagnosis of AIH‐related cirrhosis (5‐7) (Table 2). Seventy‐six patients (71%) were in remission (normal ALT and IgG levels) at the time of LSM‐cirrhosis. As indicated in Table 2, there were no significant differences in the number of patients, with or without disease remission, with LSM values higher than these cutoff points.
TABLE 2.
Patients With LSM Above the Defined Cutoff Point for the Diagnosis of Cirrhosis
| Cutoff | All (n = 107) | Response to Therapy* | PHT | ||||
|---|---|---|---|---|---|---|---|
| Remission (n = 76) | Not in Remission (n = 31) | P Value | No (n = 53) | Yes (n = 54) | P Value | ||
| ≥12.5 kPa | 52 (49%) | 33 (43%) | 19 (61%) | 0.131 | 19 (36%) | 33 (61%) | 0.012 |
| ≥14 kPa | 44 (41%) | 28 (37%) | 16 (52%) | 0.196 | 13 (24%) | 31 (57%) | 0.001 |
| ≥16 kPa | 37 (33%) | 22 (29%) | 13 (42%) | 0.256 | 9 (17%) | 26 (48%) | 0.001 |
Analysis based on 107 patients with a LSM performed 6 months after starting immunosuppressive treatment; 76 were in biochemical remission.
Thirty‐two of the 44 patients with cirrhosis diagnosed by liver biopsy had a LSM performed ≥6 months after the diagnosis of AIH. The median LSM in this subgroup of patients was 13.7 kPa (IQR 10.2‐20.1). Similar to what was observed in the whole cohort, among the patients with cirrhosis in the liver biopsy, 16 (50%) had a LSM ≥ 12.5 kPa, 14 (44%) ≥14 kPa, and 11 (34%) ≥16 kPa.
Fifty‐four of the 71 patients with PHT underwent LSM ≥ 6 months after the diagnosis of AIH. The median LSM in these patients was significantly higher as compared to patients without PHT (15.7 kPa vs. 11.7 kPa; P = 0.001). The proportion of patients with a LSM above the cutoff points for the diagnosis of AIH‐related cirrhosis was significantly higher in patients with PHT as compared to those without PHT. However, 39%‐52% of patients with PHT had a LSM below these limits (Table 2). Only 23 patients (43%) with PHT had a LSM > 20 kPa—the cutoff used in the Baveno VI criteria to predict the risk of having VNT. There were no significant differences in the proportion of patients with LSM above the cutoff points for the diagnosis of cirrhosis between patients with PHT in remission and those with PHT without remission (Supporting Table S2)
The impact of the time elapsed between the diagnosis of AIH (the time of starting immunosuppressive treatment) and LSM on the capacity of LSM to detect cirrhosis was then analyzed. As shown in Fig. 2A, there was a modest, albeit statistically significant, negative correlation between time and LSM performance (rs = −0.338; 95% CI: −0.487 to −0.169; P < 0.001). A Cochran‐Armitage test of trend was run to determine whether there was a linear trend between time and the proportion of patients with LSM above the cutoffs for the diagnosis of AIH‐related cirrhosis. According to the distribution by quartiles, the time since the diagnosis of AIH was divided into four periods: <12 months, 12‐36 months, 36‐60 months, and >60 months. The proportion of patients with LSM‐cirrhosis above the evaluated cutoff points is shown in Fig. 2B. The Cochran‐Armitage test showed a statistically significant linear trend, with longer time being associated with a lower proportion of patients with LSM ≥ 12.5 kPa, ≥14 kPa, and ≥16 kPa. Similar results were obtained when analyzing only the patients with PHT (Fig. 2C). Altogether, these results suggest that LSM‐cirrhosis is lower with time after treatment initiation; therefore the probability of misdiagnosing patients as not having AIH‐related cirrhosis increases with time.
FIG. 2.
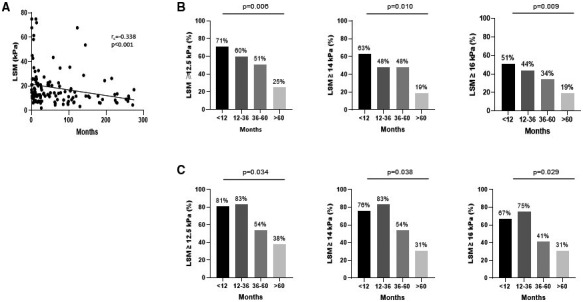
Impact of time between the initiation of immunosuppressive treatment and LSM on the ability of LSM to detect cirrhosis in patients with AIH. (A) Correlation between time (in months) and LSM (kPa). Bivariate correlation was analyzed using the Spearman rank‐order correlation test. (B) Proportion of patients with cirrhosis and LSM ≥ 12.5 kPa, 14 kPa, and 16 kPa according to time after treatment initiation (divided into four periods: ≤12 months, 12‐36 months, 36‐60 months, and ≥60 months). (C) Same as (B) but analyzing only patients with portal hypertension.
Performance of the Baveno VI, Expanded Baveno VI, and ANTICIPATE Criteria
Ninety‐two of the 132 patients were selected for this analysis, which included 110 surveillance endoscopies. The reasons for excluding the remaining patients are depicted in Supporting Fig. S1 (lack of endoscopy or LSM, decompensation, and/or primary prophylaxis). The characteristics of these patients are given in Supporting Table S3. The median AST and ALT levels at the time of endoscopy were 38 IU/mL and 32 IU/mL, respectively, and 71% of patients were in remission. Esophageal varices were present in 47 endoscopies (43%), and in 12, these were considered as VNT.
Individual platelet counts and LSM were associated with the risk of having VNT (Fig. 3A,B). Although only one (8%) patient with VNT had a platelet count > 150,000, 53 (54%) of the patients without VNT had a platelet count above that cutoff point (P = 0.003). Three (25%) and 6 (50%) of the patients with VNT had a LSM < 20 kPa or <25 kPa, respectively. These numbers are lower than those of patients without VNT (73 [75%] and 79 [81%] had a LSM < 20 kPa or <25 kPa, respectively; P = 0.001 and 0.017).
FIG. 3.
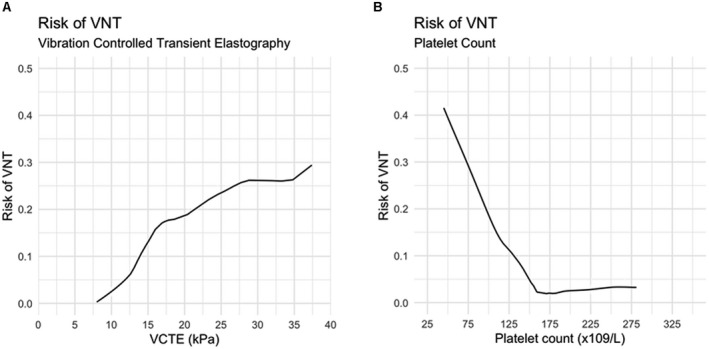
Association between VCTE values and platelet count and the risk of VNT in patients with cirrhosis related to AIH. These exploratory plots were constructed with non‐parametric local regression (locally weighted least squares).
Both the Baveno VI and expanded Baveno VI criteria had a NPV of 100%, but the use of the expanded Baveno VI criteria for triaging patients for endoscopy would have saved more procedures (63% as compared with 46% when the Baveno VI criteria were used). The use of the ANTICIPATE continuous prediction model with a risk threshold of 5% was also associated with a NPV of 100% and would have spared 63% of endoscopies. The c‐statistic of the model was 0.86, indicating excellent discrimination. However, the model underpredicted the risk of VNT when the patients had an observed risk of between 5% and 20% (Fig. 4A). The analysis excluding patients with a LSM < 10 kPa obtained the same results (data not shown). Next, the ANTICIPATE model recalibrated for patients with PBC was evaluated. As shown in Fig. 4B, this model showed good agreement between the predicted and observed probabilities of VNT in patients with AIH‐related cirrhosis. Using a 5% risk threshold of VNT, the corrected model would have saved 54% of the endoscopies, with a NPV of 100%. The performance of the models is found in Table 3.
FIG. 4.
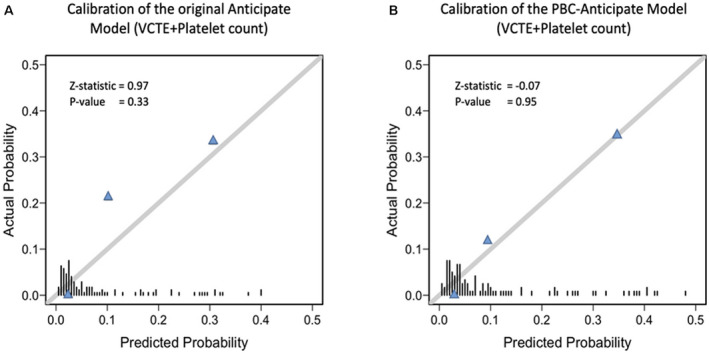
Performance (in terms of calibration) of the original ANTICIPATE model and the ANTICIPATE‐PBC model in predicting VNT. (A) Calibration plot of the original ANTICIPATE model. This model underpredicted the risk of VNT in the segment of patients with a risk of between 5% and 20%. (B) Calibration plot of the ANTICIPATE‐PBC model, which shows excellent agreement between the predicted and observed probabilities of VNT. The bars over the x‐axis show the distribution of the patients according to predicted risks. Z‐value represents the Spiegelhalter calibration( 20 ) with its P value. The closer the value of the Z‐statistic to zero, and the higher its P value, the better the calibration of the model.
TABLE 3.
Performance of the Different Criteria to Rule Out the Presence of VNT
| Criteria | Spared Endoscopies | Sensitivity | Specificity | +LR | −LR | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Baveno VI | 45 (46%) | 1.0 (0.73‐1.0) | 0.54 (0.44‐0.74) | 2.18 (1.76‐2.70) | 0 | 0.19 (0.16‐0.23) | 1.0 |
| Expanded Baveno VI | 62 (63%) | 1.0 (0.74‐1.0) | 0.63 (0.53‐0.73) | 2.72 (2.10‐3.53) | 0 | 0.23 (0.19‐0.28) | 1.0 |
| ANTICIPATE* | 62 (63%) | 1.0 (0.74‐1.0) | 0.63 (0.53‐0.73) | 2.72 (2.10‐3.53) | 0 | 0.23 (0.19‐0.28) | 1.0 |
| ANTICIPATE‐PBC* | 53 (54%) | 1.0 (0.74‐1.0) | 0.54 (0.44‐0.64) | 2.18 (1.76‐2.70) | 0 | 0.21 (0.18‐0.25) | 1.0 |
Risk threshold of 5%.
Abbreviations: +LR, positive likelihood ratio; −LR, negative likelihood ratio; PPV, positive predictive value.
Discussion
On analyzing a large cohort of patients with AIH‐related cirrhosis, it was found that a significant proportion of patients with cirrhosis (including those with histological evidence of cirrhosis at the diagnosis of AIH) had low LSM. In fact, these figures were lower than the recommended cutoff points for the diagnosis of cirrhosis in general (14 kPa),( 21 , 22 ) and specifically for the diagnosis of AIH‐related cirrhosis (12.5 kPa and 16 kPa).( 5 , 7 , 26 ) More importantly, up to 52% of patients with evidence of PHT (decompensation of liver disease, esophageal or gastric varices, or the presence of collaterals) had a LSM below these cutoffs. These findings have significant implications because current clinical practice guidelines recommend the use of LSM to monitor fibrosis progression in patients with AIH.( 26 ) This proposal was based on the study by Hartl et al.,( 5 ) showing a significant correlation between fibrosis stage and LSM when VCTE was performed 6 months after starting immunosuppressive treatment to avoid the effect of liver inflammation on LSM. However, the number of patients with cirrhosis included in this study was very low, and therefore might not be representative of what occurs in clinical practice.
As shown by Hartl et al.,( 5 ) time is of paramount importance. The current study found that the time between starting immunosuppression and LSM significantly affected the ability of LSM to predict cirrhosis and PHT. Although 51%‐71% of patients with cirrhosis and a VCTE performed within the first 12 months of treatment initiation had a LSM above the established cutoff points for the detection of cirrhosis, only 19%‐25% had a LSM above these cutoffs when it was performed ≥60 months after starting treatment. Similar results were obtained when analyzing patients with cirrhosis and PHT. Altogether, these results indicate that the LSM is not accurate to predict AIH‐cirrhosis (with or without PHT) in the long term. Interestingly, these findings are similar to what has been described in patients with hepatitis C (HCV)–related cirrhosis treated with direct‐acting antivirals, in which cirrhosis and PHT persist despite a decrease in LSM. Indeed, Lens et al.( 27 ) found that viral eradication was associated with a significant decrease in LSM in patients with HCV‐related cirrhosis and clinically significant PHT (CSPH). Nevertheless, 96 months after viral eradication, 30% of the patients with a LSM < 13.6 kPa still presented CSPH determined by a hepatic venous gradient pressure ≥10 mm Hg.( 27 , 28 )
Of course, it cannot be excluded that this observation was due to a potential regression of fibrosis or even cirrhosis in successfully treated patients with AIH.( 29 , 30 , 31 ) However, in the authors’ opinion, the most plausible explanation is that the decrease in necro‐inflammation together with liver remodeling or fibrosis reabsorption that occurs in the context of immunosuppressive treatment may impair adequate fibrosis estimation with noninvasive techniques.
Despite the limited applicability of LSM to predict cirrhosis and PHT in patients with AIH, the performance of the Baveno VI and expanded Baveno VI criteria in predicting the absence of VNT was excellent with a NPV of 100%, and its use would have reduced the need for surveillance endoscopies by between 43% and 64%, respectively. Use of the ANTICIPATE model would also have spared 64% with a NPV of 100%. The advantage of the latter model is that it provides continuous predictions according to LSM and platelet counts.( 14 , 15 ) Nevertheless, the association between LSM and platelet count and the risk of VNT was different in AIH‐related cirrhosis compared with that observed in other etiologies such as viral‐related or alcohol‐related cirrhosis. Similar to what was observed in patients with PBC,( 15 ) in the current study patients with AIH had a higher risk of VNT than that predicted with the ANTICIPATE model. This probably reflects the fact that several patients with AIH‐related cirrhosis have low posttreatment LSM while still having PHT. In addition, it cannot be completely ruled out that this was due to the presence of other causes of PHT located at presinusoidal levels (and not captured by LSM), such as inflammation within the portal tracts. In fact, the use of the ANTICIPATE model corrected for patients with PBC,( 15 ) in which the presinusoidal component of PHT has been well studied,( 32 , 33 ) calibrated well (with good agreement between predicted and observed probabilities of VNT), and was able to accurately predict the risk of VNT in patients with AIH‐related cirrhosis.
We should acknowledge that this study has limitations. First, it is a retrospective study; therefore, liver biopsies and endoscopies were not available in all patients. Second, it lacked follow‐up biopsies, and in patients developing cirrhosis during the follow‐up the diagnosis was based on ultrasound criteria, which are not the gold standard. However, several studies have shown a good performance of liver ultrasound in the detection of cirrhosis with a sensitivity ranging between 54% and 84%, and more importantly, a specificity for the detection of cirrhosis of between 78% and 100%.( 34 , 35 , 36 , 37 ) Likewise, the results were very similar to those obtained in patients with PHT and histologically proven cirrhosis. Third, the retrospective nature of the study might have introduced a selection bias, as the results of LSM could have influenced the decision of performing (or not performing) gastrointestinal endoscopy. Nonetheless, 73% of the endoscopies available were performed in patients with LSM < 20 kPa. Finally, the number of patients with VNT was low, and this might affect the precision of the noninvasive tools evaluated in this study. Despite these limitations, the study provides important information and validates the use of noninvasive tools to rule out the presence of high‐risk varices in patients with AIH‐related cirrhosis.
In conclusion, the current LSM cutoff points do not have good discriminative capacity for the diagnosis of AIH‐related cirrhosis with/without PHT, especially long‐term after treatment initiation. Therefore, LSM should be interpreted with caution and always alongside other criteria for the diagnosis of cirrhosis. Nonetheless, noninvasive tools (Baveno VI, expanded Baveno VI criteria, and ANTICIPATE‐PBC continuous model) to triage patients for endoscopy are useful in patients with AIH‐related cirrhosis. Multicenter, prospective studies are needed to corroborate these results.
Supporting information
Supplementary Material
Supported by Clínic‐La Pedrera (Resident Award) and Instituto de Salud Carlos III (PI17/00955).
Potential conflict of interest: A.P. consults for Intercept Pharmaceuticals and Genfit. M.S. advsies Astellas and Novartis. M.G.R. is on the speakers’ bureau of and received grants from Gilead and AbbVie. C.A.N. is on the speakers’ bureau of and received grants from Intercept. M.R.B. is on the speakers’ bureau of and received grants from Gilead.
References
- 1. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol 2015;63:971‐1004. [DOI] [PubMed] [Google Scholar]
- 2. Werner M, Prytz H, Ohlsson B, Almer S, Bjornsson E, Bergquist A, et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol 2008;43:1232‐1240. [DOI] [PubMed] [Google Scholar]
- 3. Dhaliwal HK, Hoeroldt BS, Dube AK, McFarlane E, Underwood JCE, Karajeh MA, et al. Long‐term prognostic significance of persisting histological activity despite biochemical remission in autoimmune hepatitis. Am J Gastroenterol 2015;110:993‐999. [DOI] [PubMed] [Google Scholar]
- 4. Hartl J, Ehlken H, Sebode M, Peiseler M, Krech T, Zenouzi R, et al. Usefulness of biochemical remission and transient elastography in monitoring disease course in autoimmune hepatitis. J Hepatol 2018;68:754‐763. [DOI] [PubMed] [Google Scholar]
- 5. Hartl J, Denzer U, Ehlken H, Zenouzi R, Peiseler M, Sebode M, et al. Transient elastography in autoimmune hepatitis: timing determines the impact of inflammation and fibrosis. J Hepatol 2016;65:769‐775. [DOI] [PubMed] [Google Scholar]
- 6. Guo L, Zheng L, Hu L, Zhou H, Yu L, Liang W. Transient elastography (FibroScan) performs better than non‐invasive markers in assessing liver fibrosis and cirrhosis in autoimmune hepatitis patients. Med Sci Monit 2017;23:5106‐5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu Q, Sheng L, Bao H, Chen X, Guo C, Li H, et al. Evaluation of transient elastography in assessing liver fibrosis in patients with autoimmune hepatitis. J Gastroenterol Hepatol 2017;32:639‐644. [DOI] [PubMed] [Google Scholar]
- 8. Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, et al. Cancer Immunotherapy with anti‐CTLA‐4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis 2016;10:395‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahmud N, Doshi SD, Forde KA, Khungar V. Transient elastography reliably estimates liver fibrosis in autoimmune hepatitis. Clin Exp Hepatol 2019;5:244‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Franchis R, Abraldes JG, Bajaj J, Berzigotti A, Bosch J, Burroughs AK, et al. Expanding consensus in portal hypertension Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;743‐752. [DOI] [PubMed] [Google Scholar]
- 11. Augustin S, Pons M, Maurice JB, Bureau C, Stefanescu H, Ney M, et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology 2017;66:1980‐1988. [DOI] [PubMed] [Google Scholar]
- 12. Maurice JB, Brodkin E, Arnold F, Navaratnam A, Paine H, Khawar S, et al. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J Hepatol 2016;65:899‐905. [DOI] [PubMed] [Google Scholar]
- 13. Moctezuma‐Velazquez C, Abraldes JG. Non‐invasive diagnosis of esophageal varices after Baveno VI. Turkish J Gastroenterol 2017;28:159‐165. [DOI] [PubMed] [Google Scholar]
- 14. Abraldes JG, Bureau C, Stefanescu H, Augustin S, Ney M, Blasco H, et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: the “Anticipate” study. Hepatology 2016;64:2173‐2184. [DOI] [PubMed] [Google Scholar]
- 15. Moctezuma‐Velazquez C, Saffioti F, Tasayco‐Huaman S, Casu S, Mason A, Roccarina D, et al. Non‐invasive prediction of high‐risk varices in patients with primary biliary cholangitis and primary sclerosing cholangitis. Am J Gastroenterol 2019;114:446‐452. [DOI] [PubMed] [Google Scholar]
- 16. Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169‐176. [DOI] [PubMed] [Google Scholar]
- 17. Association E. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol 2015;63:97‐1004. [DOI] [PubMed] [Google Scholar]
- 18. Abraldes JG, Araujo IK, Turón F, Berzigotti A. Diagnosing and monitoring cirrhosis: liver biopsy, hepatic venous pressure gradient and elastography. Gastroenterol Hepatol 2012;35:488‐495. [DOI] [PubMed] [Google Scholar]
- 19. Kennedy P, Bane O, Hectors SJ, Fischman A, Schiano T, Lewis S, et al. Noninvasive imaging assessment of portal hypertension. Abdom Radiol 2020;45:3473‐3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lohse AW, Sebode M, Jørgensen MH, Ytting H, Karlsen TH, Kelly D, et al. Second‐line and third‐line therapy for autoimmune hepatitis: a position statement from the European Reference Network on Hepatological Diseases and the International Autoimmune Hepatitis Group. J Hepatol 2020;73:1496‐1506. [DOI] [PubMed] [Google Scholar]
- 21. Friedrich–Rust M, Ong M, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta‐analysis. Gastroenterology 2008;134:960‐974.e8. [DOI] [PubMed] [Google Scholar]
- 22. Coco B, Oliveri F, Colombatto P, Ciccorossi P, Sacco R, Bonino F, et al. Monitoring liver stiffness: a new tool to measure liver fibrosis during therapy. Hepatology 2005;42:435A. [Google Scholar]
- 23. Steyerberg EW, Eijkemans MJC, Harrell FE, Habbema JDF. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Mak 2001;21:45‐56. [DOI] [PubMed] [Google Scholar]
- 24. Spiegelhalter DJ. Probabilistic prediction in patient management and clinical trials. Stat Med 1986;5:421‐433. [DOI] [PubMed] [Google Scholar]
- 25. Harrell FE rms: Regression Modeling Strategies. R package version 5.1‐0. 2017. Available from https://cran.r‐project.org/package5rms. Accessed April 7, 2020.
- 26. Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the study of liver diseases. Hepatology 2020;72:671‐722. [DOI] [PubMed] [Google Scholar]
- 27. Lens S, Alvarado‐Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J, et al. Effects of all‐oral anti‐viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus‐associated cirrhosis. Gastroenterology 2017;153:1273‐1283.e1. [DOI] [PubMed] [Google Scholar]
- 28. Lens S, Baiges A, Alvarado E, LLop E, Martinez J, Fortea JI, et al. Clinical outcome and hemodynamic changes following HCV eradication with oral antiviral therapy in patients with clinically significant portal hypertension. J Hepatol 2020;73:1415‐1424. [DOI] [PubMed] [Google Scholar]
- 29. Dufour JF, DeLellis R, Kaplan MM. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med 1997;127:981‐985. [DOI] [PubMed] [Google Scholar]
- 30. Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol 2004;40: 646‐652. [DOI] [PubMed] [Google Scholar]
- 31. Aj C. Review article: the prevention and reversal of hepatic fibrosis in autoimmune hepatitis. Aliment Pharmacol Ther 2014;39:385‐406. [DOI] [PubMed] [Google Scholar]
- 32. Navasa M, Parés A, Bruguera M, Caballería J, Bosch J, Rodés J. Portal hypertension in primary biliary cirrhosis. Relationship with histological features. J Hepatol 1987;5:292‐298. [DOI] [PubMed] [Google Scholar]
- 33. Lebrec D, Sicot C, Degott C, Benhamou JP. Portal hypertension and primary biliary cirrhosis. Digestion 1976;14:220‐226. [DOI] [PubMed] [Google Scholar]
- 34. Lurie Y, Webb M, Cytter‐Kuint R, Shteingart S, Lederkremer GZ. Non‐invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol 2015;21:11567‐11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saverymuttu SH, Joseph AEA, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J 1986;292:13‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harbin WP, Robert NJ, Ferrucci JT. Diagnosis of cirrhosis based on regional changes in hepatic morphology. A radiological and pathological analysis. Radiology 1980;135:273‐283. [DOI] [PubMed] [Google Scholar]
- 37. Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D'Errico A, et al. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol 1997;27:979‐985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


