Abstract
The gut microbiota is a well‐known prognostic factor and a modulator of treatment sensitivity in patients with cancers treated with immune checkpoint inhibitors. However, data on hepatocellular carcinoma (HCC) are lacking. This study aimed to evaluate the prognostic role of the gut microbiota and changes produced by immunotherapy on the intestinal environment in patients with cirrhosis and HCC. Eleven patients treated with Tremelimumab and/or Durvalumab were included in the analysis. All study participants underwent gut microbiota profiling, quantification of fecal calprotectin, serum levels of zonulin‐1, lipopolysaccharide binding protein (LBP), and programmed death‐ligand 1 (PD‐L1) at baseline and at each treatment cycle until the third cycle, then every three cycles until treatment discontinuation or last visit. The 6 patients who achieved disease control (DC) showed lower pretreatment fecal calprotectin (median, 12.5; interquartile range [IQR], 5‐29 vs. median, 116; IQR, 59‐129 µg/g; P = 0.047) and PD‐L1 serum levels (median, 0.08; IQR, 0.07‐0.09 vs. median, 1.04; IQR, 0.17‐1.95 ng/mL; P = 0.02) than nonresponders. The relative abundance of Akkermansia (log2 fold change [FC], 2.72; adjusted P [Padj] = 0.012) was increased, whereas that of Enterobacteriaceae (log2 FC, −2.34; Padj = 0.04) was reduced in the DC group. During treatment, fecal calprotectin showed a temporal evolution opposite to the Akkermansia to Enterobacteriaceae ratio and gut microbiota alpha diversity, but similar to zonulin‐1 and LBP. Bifidobacterium had a stable behavior in patients with a long follow‐up, while Akkermansia was more variable. Akkermansia and Bifidobacterium showed similar temporal patterns and causative relationships with Prevotella, Veillonella, Ruminococcus, Roseburia, Lachnospira, Faecalibacterium, and Clostridium. Conclusion: A favorable composition of the gut microbiota and low intestinal inflammation are associated with achieving DC. The intestinal environment changes dynamically during therapy.

Abbreviations
- adj
adjusted
- AE
Akkermansia to Enterobacteriaceae
- DC
disease control
- DTW
dynamic time warping
- FC
fold change
- HCC
hepatocellular carcinoma
- ICI
immune checkpoint inhibitor
- IQR
interquartile range
- LBP
lipopolysaccharide binding protein
- NAFLD
nonalcoholic fatty liver disease
- PD‐L1
anti‐programmed death‐ligand 1
- PT
patient
Immune checkpoint inhibitors (ICIs) are molecules able to enhance the immune system response; they represent the new frontier for treatment of various types of cancer, including hepatocellular carcinoma (HCC).( 1 ) Recent findings suggest that nonalcoholic fatty liver disease (NAFLD) could be associated with immune dysregulation and unfavorable response to ICIs treatment in patients with HCC, probably owing to nonalcoholic steatohepatitis‐related aberrant T‐cell activation and exhaustion.( 2 )
The gut microbiota has been linked to the response to ICIs in patients with solid tumors.( 3 ) Chronic inflammation is a well‐known risk factor for the development of HCC, and has been associated with the gut microbiota profile, intestinal inflammation, and immune system imbalance.( 3 , 4 ) However, studies analyzing the gut microbiota of patients with cirrhosis and HCC treated with ICIs are lacking.
We report a case series of patients with cirrhosis and HCC treated with ICIs, in order to evaluate the prognostic role of the gut microbiota and changes produced by immunotherapy on the intestinal environment.
Patients and Methods
Patients affected by liver cirrhosis and unresectable HCC who were consecutively enrolled in clinical trials D4190C00022 (NCT02519348) and D419CC00002 (NCT03298451, HIMALAYA) 5 at the Fondazione Policlinico Agostino Gemelli, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) in Rome between May 2018 and December 2019 were included in this prospective observational study. Patients received Tremelimumab, an anti‐cytotoxic T‐lymphocyte antigen 4 (CTLA‐4) monoclonal antibody, and/or Durvalumab, an anti‐programmed death‐ligand 1 (PD‐L1) monoclonal antibody, every 4 weeks (one treatment cycle) at different doses and combination schedules, as described in the protocols of the previously mentioned clinical trials. As the interaction between the gut microbiota and ICIs was the target of the study, patients in the Sorafenib arm of trial D419CC00002 were excluded.
Stool and peripheral venous blood samples were collected at baseline and at each treatment cycle until the third cycle, then every three cycles until treatment discontinuation or the last visit. Gut microbiota analysis and quantification of fecal calprotectin, lipopolysaccharide binding protein (LBP), and zonulin‐1 serum levels were performed at the above time points, as reported.( 4 , 6 ) Commercial enzyme‐linked immunosorbent assays (ELISA) for the detection of serum levels of PD‐L1 (#DB7H10; R&D Systems Inc., Minneapolis, MN) and LBP (human LBP; Hycult Biotech, the Netherlands) were also performed. Clinical variables, adverse events, and reasons for treatment discontinuation were recorded.
Radiological tumor response was assessed every two cycles of treatment using the modified Response Evaluation Criteria in Solid Tumors (mRECIST). Disease control (DC) was defined as evidence of stable disease, partial response, or complete response based on the best radiological response recorded over the entire treatment.
In order to avoid bias in the analysis of the gut microbiota, patients with active alcohol intake and those treated with antibiotics, prebiotics, probiotics, nonabsorbable disaccharides, laxatives, or proton pump inhibitors in the last 3 months before enrollment were excluded. Bacterial DNA extraction, amplification of the 16s ribosomal RNA V3‐V4 region, and sequencing were performed according to the described protocol.( 7 )
Statistical Analysis
Data were reported as median and interquartile range (IQR) for continuous variables or frequency and percentage for categorical ones. For the analysis of gut microbiota, the Chao1 index of alpha diversity was calculated on raw reads. Bray‐Curtis distance was computed after applying the variance‐stabilizing transformation for data normalization, and nonmetric multidimensional scaling (NMDS) plots were used to visualize the results. Baseline inflammatory features and the gut microbiota profile of patients who achieved DC and those who did not were compared using the Wilcoxon test, whereas differential abundance analysis of the gut microbiota at the family and genus levels was performed using DESeq2, as described.( 7 ) To reduce error and uncertainty, log2 fold change (FC) of taxa that were highly variable between samples or had low read counts were subjected to shrinkage.
To explore the dynamics of the gut microbiota during ICIs treatment, variation in relative taxa abundance was analyzed to identify the core microbiota (taxa observed at all time points for at least 6 months/cycles), the persistent microbiota (taxa observed in 20% or more of time points for at least 6 months/cycles, with at least 75% consecutive observations), and the transient microbiota (taxa observed in 50% or more of time points for at least 6 months/cycles, but with at most 74% of consecutive observations).( 8 ) The presence of taxa with nonstationary behavior (i.e., those with mean abundance, standard deviation, variance, covariance, and autocorrelation that change over time) was also assessed as an indirect marker of intermicrobial competition or other factors that may influence the dynamics of the gut microbiota community.
A pairwise modified dynamic time warping (DTW) algorithm was then used to identify sets of taxa with similar patterns over time in.( 8 ) The modified DTW distance ranged from 0 to 1, with values close to 0 showing similar temporal trends; results were plotted as hierarchical trees and heatmaps. The Granger algorithm was adopted to identify causal relationships between taxa that may be involved in gut microbiota temporal changes. Analyses were performed using the R statistics program (version 3.6.2) or the web application “TIME.”( 8 )
Results
During the study period, 25 patients were evaluated for inclusion in the two clinical trials (19 in D4190C00022, 6 in D419CC00002). Nine of these patients did not meet the inclusion criteria, thus 16 were finally randomized; 2 patients in the Sorafenib arm of the D419CC00002 trial were excluded and 3 were unable to provide the baseline fecal sample. Therefore, 11 patients, 9 men and 2 women, were enrolled in this study (Table 1). Median age was 68 years (IQR, 66‐80 years); the most common etiology of cirrhosis was viral (five cases), followed by NAFLD (three cases) and alcohol abuse (three cases). All participants showed good liver function (Child‐Pugh A) and performance status (grade 0 according to the Eastern Cooperative Oncology Group [ECOG] classification). Endoscopic signs of portal hypertension were observed in approximately half of patients.
TABLE 1.
Demographic characteristics of the 11 patients included in the study
| ID | Age (Years) | Sex | BMI | Etiology | Esophageal/Gastric Varices | Size (cm, Overall) | Size (cm, Largest Nodule) | Number of Nodules | MVI | Extrahepatic Disease/Type | ECOG | Child‐Pugh | MELD | Treatment Duration (Months) | ICI Type | DC | Reason for Discontinuation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT1 | 59 | Male | 19.7 | HCV | Yes | 7.5 | 5.5 | Multinodular | No | Bone, adrenal | 0 | A | 10 | 6.93 | Tremelimumab plus Durvalumab | No | Progression |
| PT2 | 68 | Male | 32.1 | HBV | 0 | 12 | 6.4 | 3 | No | No | 0 | A | 7 | 4.33 | Durvalumab | Yes | Side effects |
| PT3 | 81 | Male | 22.3 | NASH | Yes | 22.5 | 12 | Multinodular | No | No | 0 | A | 10 | 4.33 | Tremelimumab | No | Progression/sepsis |
| PT4 | 68 | Male | 27.1 | HBV | 0 | 10 | 3.5 | 4 | No | No | 0 | A | 9 | 18.73 | Tremelimumab | Yes | Progression |
| PT5 | 67 | Male | 23 | Alcohol* | 0 | 25 | 9.5 | Multinodular | No | Adrenal | 1 | A | 11 | 8.73 | Tremelimumab plus Durvalumab | No | Progression/decompensation |
| PT6 | 65 | Male | 28.3 | NASH | 0 | 9.7 | 2.7 | Multinodular | No | No | 0 | A | 7 | 18.97 | Durvalumab | Yes | Ongoing |
| PT7 | 79 | Male | 31.22 | NASH, alcohol* | Yes | 13.1 | 9 | 3 | No | Lymph Nodes | 0 | A | 10 | 12.00 | Tremelimumab | Yes | Decompensation |
| PT8 | 81 | Female | 20.4 | HCV | Yes | 16 | 16 | Multinodular | No | No | 0 | A | 9 | 2.13 | Tremelimumab | Yes | Decompensation |
| PT9 | 65 | Female | 20.7 | HCV | 0 | 10.9 | 7.6 | 3 | No | Bone Lymph Nodes | 0 | A | 7 | 5.73 | Tremelimumab plus Durvalumab | No | Progression/decompensation |
| PT10 | 76 | Male | 22.6 | Alcohol* | Yes | 15 | 8 | Multinodular | Yes | Peritoneal | 0 | A | 7 | 3.67 | Durvalumab | No | Progression |
| PT11 | 85 | Male | 23.4 | Alcohol* | 0 | 12 | 12 | 1 | Yes | No | 0 | A | 8 | 5.97 | Durvalumab | Yes | Ongoing |
Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; ICI, immune checkpoint inhibitor; MELD, Model for End‐Stage Liver Disease; MVI, macrovascular invasion; NASH, nonalcoholic steatohepatitis.
Alcohol consumption had been discontinued for at least 3 years before inclusion in the study.
Seven patients presented a multinodular HCC, with a median overall tumor size of 12 cm (IQR, 10.5‐15.5 cm); extrahepatic spread was recognized in 4 patients and macrovascular invasion in 1, whereas 1 patient presented both. Four patients received the combination of Tremelimumab plus Durvalumab; the others received either Durvalumab (4 patients) or Tremelimumab (3 patients) monotherapy.
At the time of the analysis, the median treatment duration was 5.97 months (IQR, 4.33‐10.37 months), and treatment was still ongoing for 2 patients who showed stable disease at cycle 18 and cycle 3. Six patients achieved DC; these subjects showed lower fecal calprotectin concentrations (median, 12.5; IQR, 5‐29 vs. median, 116; IQR, 59‐129 µg/g; P = 0.047) and PD‐L1 serum levels (median, 0.08; IQR, 0.07‐0.09 vs. median, 1.04; IQR, 0.17‐1.95 ng/mL; P = 0.02) at baseline compared with nonresponders, whereas zonulin‐1 (median, 7.22; IQR, 0.63‐9.05 vs. median, 5.13; IQR, 4.79‐9.53 ng/mL; P = 0.75) and LBP (2.17; IQR, 1.77‐2.52 vs. median, 2.22; IQR, 2.12‐2.39 mg/L; P = 0.99) were similar between the two groups. No significant pretreatment difference was observed between responders and nonresponders regarding the Chao1 index of alpha diversity (P = 0.43). Notably, in the DC group, pretreatment relative abundance of Akkermansia (log2FC, 2.72; adjusted P [Padj] = 0.012) was increased whereas Staphylococcus (log2FC, −2.90; Padj = 0.042), Neisseria (log2FC, −3.08; Padj < 0.0001), and Enterobacteriaceae (log2FC, −2.34; Padj = 0.04) were reduced.
Overall, the gut microbiota composition showed clear fluctuations during the treatment period (Figs. 1 and 2). Calprotectin followed a similar trend to zonulin‐1 and LBP, but showed an opposite trend with respect to the Akkermansia to Enterobacteriaceae (AE) ratio and to the gut microbiota alpha diversity (Fig. 3). In responders or nonresponders who discontinued treatment due to decompensation, side effects, or progression, we observed a drop in the AE ratio at the last follow‐up, whereas a progressive increase was observed in patient (PT)6 and PT11, who were the two responders still on treatment at the end of the study. Interestingly, serum levels of PD‐L1 increased over time in patients who received the anti‐PD‐L1 monoclonal antibody Durvalumab.
FIG. 1.
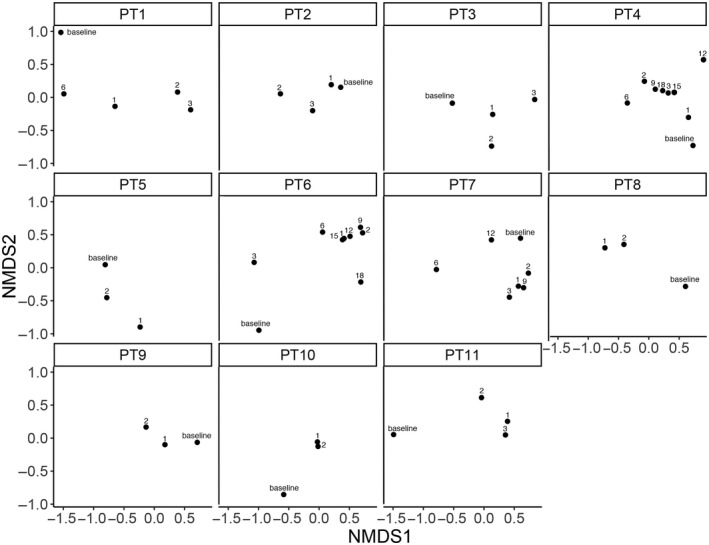
Nonmetric multidimensional scaling (NMDS) ordination plots on Bray‐Curtis distance computed after data normalization using the variance‐stabilizing transformation. Each plot shows treatment time points (indicated by labels) for an individual patient.
FIG. 2.
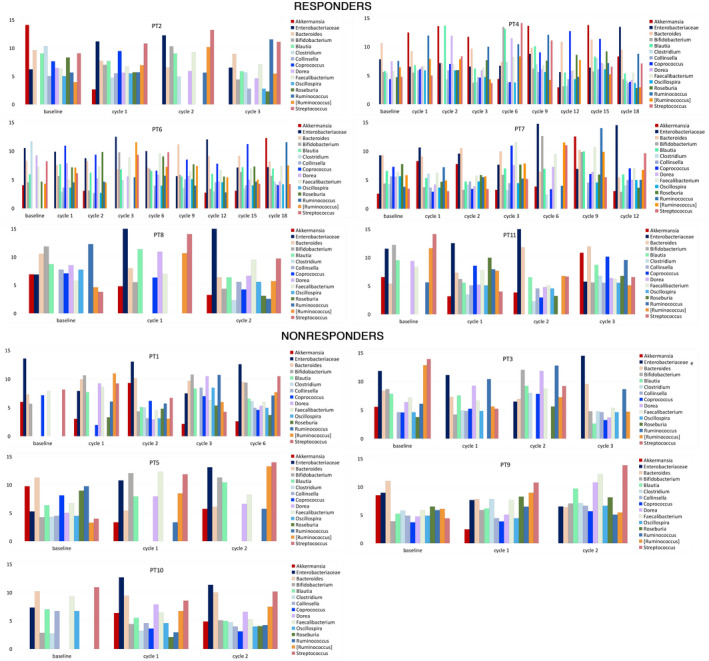
Evolution of gut microbiota composition during treatment. Bar plots show gut microbiota temporal trend; normalized bacterial abundance (variance‐stabilizing transformation) is plotted on the y axis.
FIG. 3.
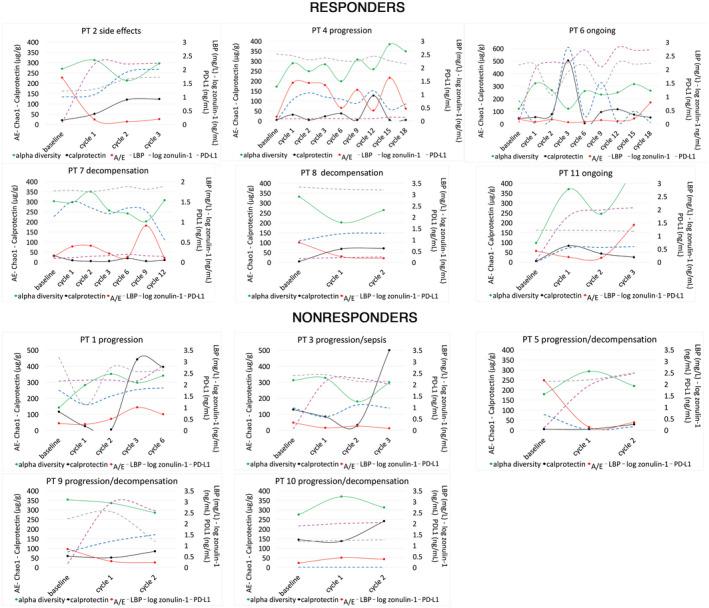
Changes in markers of gut dysbiosis (AE ratio), intestinal inflammation, and permeability (calprotectin, zonulin‐1, LBP) and PD‐L1 serum levels over the treatment period. Abbreviations: AE, Akkermansia to Enterobacteriaceae ratio; LBP, lipopolysaccharide binding protein; PD‐L1, programmed death ligand 1.
To reliably assess the stability of the gut microbiota during treatment with ICIs and the microbial connections possibly linked by causal relationships, only PT1, PT4, PT6, and PT7, who were followed for more than 6 months were considered. As expected in a population of patients with cirrhosis, Enterobacteriaceae was a core family; Akkermansia, on the other hand, showed mainly nonstationary and variable behavior (Supporting Table S1). Bifidobacterium was a core or persistent component in all four participants with the longest duration of treatment. Longitudinal analysis of temporal connections revealed that Enterobacteriaceae followed a temporal pattern similar to that of Methanobacteriaceae and different from that of Bifidobacteriaceae and Verrucomicrobiaceae (Fig. 4; Supporting Table S2). A close causal connection between Enterobacteriaceae and Methanobacteriaceae was generally observed; in addition, changes in Enterobacteriaceae were caused by Bifidobacteriaceae and Verrucomicrobiaceae. Bifidobacterium and Akkermansia belonged to clusters of bacteria with similar temporal patterns and causal relationships, including Prevotella, Veillonella, Ruminococcus, Roseburia, Lachnospira, Faecalibacterium, and Clostridium.
FIG. 4.
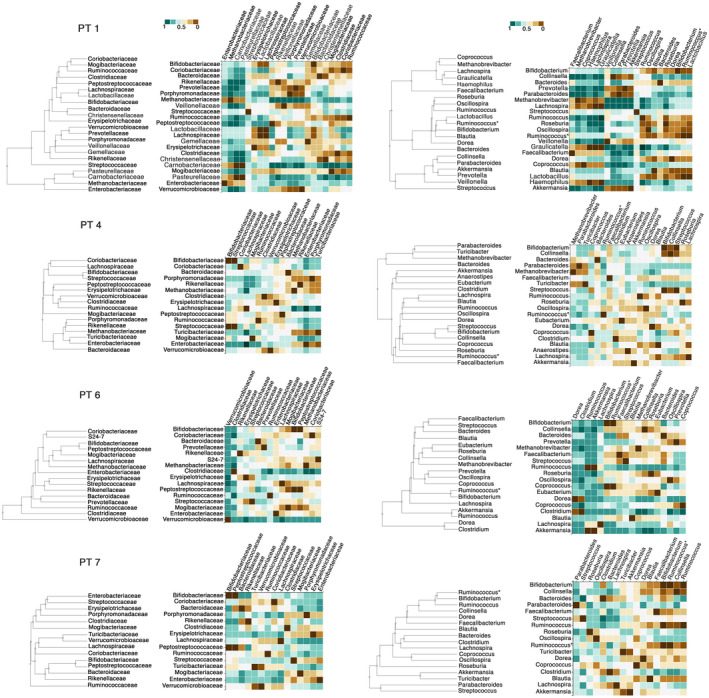
Similarities of temporal patterns of gut microbiota families (left side) and genera (right side) of PT1, PT4, PT6, and PT7. Hierarchical clustering and heatmaps based on pairwise modified dynamic time warping (DTW) distance show bacteria with a similar temporal variation (DTW near 0, brown color) or different temporal trends (DTW near 1, green color). Ruminococcus is Firmicutes ; Clostridia; Clostridiales; Ruminococcaceae; Ruminococcus. Ruminococcus* is Firmicutes ; Clostridia; Clostridiales; Lachnospiraceae; Ruminococcus.
Discussion
ICIs represent a new treatment option for a large number of solid tumors, with several data pointing to a role for the gut microbiota in the modulation of response. Our study reports the first evidence supporting a prognostic role of intestinal inflammation‐associated parameters, such as calprotectin, in patients with liver cirrhosis and HCC receiving ICIs, and demonstrates that the gut microbiota changes dynamically during the treatment period. These modifications are mainly represented by fluctuations in the ratio of beneficial to harmful bacteria, and are related to changes in intestinal inflammation and permeability.
In our case series, pretreatment abundance of Akkermansia and low fecal calprotectin concentration were associated with DC. This confirms the association of Akkermansia with a better response reported in patients with other solid tumors treated with ICIs, and is consistent with the anti‐inflammatory properties of Akkermansia.( 9 ) In agreement with these findings, patients who achieved DC had a lower fecal calprotectin concentration at baseline, which is indicative of a lower degree of intestinal inflammation. Persistent gut‐derived inflammation in patients with cirrhosis leads to exhaustion and paralysis of the immune system, and is an additional factor triggering tumor immune escape.( 10 ) DC was also associated with low baseline serum levels of PD‐L1, as already reported for other cancers,( 11 ) as an expression of a milder suppression of the immune response.
During treatment, the gut microbiota changed in all study participants, and the AE ratio, which we used as a marker of dysbiosis, showed an inverse trend compared with fecal calprotectin, markers of intestinal permeability and bacterial translocation. Furthermore, AE decreased at the time of treatment discontinuation, as if an unfavorable shift in the composition of the gut microbiota was associated with a loss of response to immunotherapy or unfavorable clinical events, such as decompensation. Although the exact immunomodulatory effects of Akkermansia muciniphila are still unclear and probably correlated with its anti‐inflammatory action and protective role on the intestinal barrier, it has been shown that its supplementation can enhance the effects of ICIs therapy.( 12 ) This can be of paramount importance in some categories of patients, such as those with NAFLD; indeed, a recent study showed a suboptimal response to ICIs in patients with NAFLD‐related HCC,( 2 ) in whom a profound derangement of the gut microbiota was demonstrated.( 4 ) Therefore, we think that the role of Akkermansia administration as adjuvant therapy to enhance the response to ICIs in patients with HCC deserves further evaluation in future studies.
Our longitudinal analysis also highlighted that Bifidobacterium was a core element in patients with the longest follow‐up, whereas Akkermansia had a more variable behavior. Akkermansia and Bifidobacterium are usually depleted in patients with cirrhosis and HCC.( 4 ) In addition to Akkermansia, Bifidobacterium can enhance the effects of ICIs exerting a protective effect on the liver and reinforcing the intestinal barrier.( 9 ) The temporal and causal connections between Akkermansia, Bifidobacterium, and other members of the gut microbiota, such as Roseburia, Lachnospira, Ruminococcus, and Faecalibacterium, could be explained by cross‐feeding relationships through the production and use of short chain fatty acids (SCFAs), which are key regulators of the immune system function and have been associated with increased likelihood of response during treatment with ICIs.( 13 ) In contrast, the connections of Enterobacteriaceae with Methanobacteriaceae could be explained by the implementation of resistance mechanisms based on bacterial metabolism (e.g., bile salt hydrolysis, iron metabolism).
This study suffers from the limitations of case series because it consists mainly of a small number of patients included, due to restrictive selection criteria; furthermore, we cannot exclude the influence of comorbidities on the baseline gut microbiota composition. However, to our knowledge, this is the first Western study focusing on the prognostic role of gut microbiota, intestinal permeability, and inflammation in patients with cirrhosis and HCC receiving ICIs and on their longitudinal evolution. A previous study analyzed the gut microbiota of 8 Asian patients with HCC treated with anti‐PD‐L1 monoclonal antibodies, and demonstrated that Akkermansia among other bacteria was associated with treatment response.( 14 ) However, the study population was not well characterized, and the paper did not report how many patients were affected by liver cirrhosis. In addition, differences in lifestyle and etiology of liver disease make it difficult to apply those findings to Western patients.
In conclusion, cirrhotic patients with HCC who respond to ICIs appear to have a favorable composition of the gut microbiota and a low degree of intestinal inflammation. Dynamic fluctuations in the ratio between beneficial and harmful bacteria are related to changes in intestinal inflammation and permeability during treatment. Our data should prompt further studies in larger cohorts, as precise characterization and modulation of the intestinal environment could allow to identify a subgroup of patients with high probability of a response to ICIs therapy.
Supporting information
Table S1
Table S2
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Hiam‐Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer 2021;21:345‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti‐tumour surveillance in immunotherapy‐treated HCC. Nature 2021;592:450‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daillère R, Routy B, Goubet A‐G, Cogdill A, Ferrere G, Alves‐Costa Silva C, et al. Elucidating the gut microbiota composition and the bioactivity of immunostimulatory commensals for the optimization of immune checkpoint inhibitors. OncoImmunology 2020;9:1794423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 2019;69:107‐120. [DOI] [PubMed] [Google Scholar]
- 5. Abou‐Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open‐label, multicenter study of tremelimumab (T) and durvalumab (D) as first‐line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol 2022;40:379‐379. [Google Scholar]
- 6. Addolorato G, Ponziani FR, Dionisi T, Mosoni C, Vassallo GA, Sestito L, et al. Gut microbiota compositional and functional fingerprint in patients with alcohol use disorder and alcohol‐associated liver disease. Liver Int 2020;40:878–888. [DOI] [PubMed] [Google Scholar]
- 7. Ponziani FR, Putignani L, Paroni Sterbini F, Petito V, Picca A, Del Chierico F, et al. Influence of hepatitis C virus eradication with direct‐acting antivirals on the gut microbiota in patients with cirrhosis. Aliment Pharmacol Ther 2018;48:1301‐1311. [DOI] [PubMed] [Google Scholar]
- 8. Baksi KD, Kuntal BK, Mande SS. “TIME”: a web application for obtaining insights into microbial ecology using longitudinal microbiome data. Front Microbiol 2018;9:36. Erratum in: Front Microbiol 2020;11:605295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yi M, Yu S, Qin S, Liu Q, Xu H, Zhao W, et al. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J Hematol Oncol 2018;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ponziani FR, Nicoletti A, Gasbarrini A, Pompili M. Diagnostic and therapeutic potential of the gut microbiota in patients with early hepatocellular carcinoma. Ther Adv Med Oncol 2019;11:1758835919848184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei W, Xu B, Wang Y, Wu C, Jiang J, Wu C. Prognostic significance of circulating soluble programmed death ligand‐1 in patients with solid tumors: a meta‐analysis. Medicine (Baltimore) 2018;97:e9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science 2018;359:91‐97. [DOI] [PubMed] [Google Scholar]
- 13. Malczewski AB, Navarro S, Coward JI, Ketheesan N. Microbiome‐derived metabolome as a potential predictor of response to cancer immunotherapy. J Immunother Cancer 2020;8:e001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng YI, Wang T, Tu X, Huang Y, Zhang H, Tan DI, et al. Gut microbiome affects the response to anti‐PD‐1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer 2019;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


