Abstract
The prevalence of liver disease, and especially of advanced liver fibrosis, in the German population is poorly defined. The aim of the study was to explore liver enzymes and surrogate scores of hepatic steatosis and advanced hepatic fibrosis in a population‐based cohort study in Germany. In the cross‐sectional population‐based Gutenberg Health study, data of 14,950 participants enrolled between 2007 and 2012 were captured and analyzed. The distribution of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma‐glutamyltransferase (GGT), fatty liver index (FLI), and Fibrosis‐4 (FIB‐4) score, as well as the underlying risk factors, were assessed by regression models. Elevated liver enzymes in this population‐based sample were seen in 19.9% for ALT, 12.8% for AST, and 14% for GGT. Risk factors for liver disease included alcohol use and the presence of the metabolic syndrome, which were both risk factors associated with increased liver enzymes. The FLI suggested that 37.5% of the population exhibited hepatic steatosis and 1.1% of patients exhibited a FIB‐4 above the upper cutoff, while 19.2% were in the intermediate range. Interestingly, advanced fibrosis was significantly more frequent in men compared with women (FIB‐4: 1.5% vs. 0.6% [P < 0.0001]; NFS: 3.6% vs. 1.9% [P < 0.0001]). In addition, age was a relevant risk factor for exhibiting a noninvasive surrogate score suggestive of advanced fibrosis in the current study population. Conclusion: Elevated liver enzymes were seen in almost a fifth of the German population. At the population‐based level, the prevalence of advanced fibrosis was estimated at 1% in Germany.

Abbreviations
- ALT
alanine aminotransferase
- APRI
AST to platelet ratio index
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
95% confidence interval
- FIB‐4
Fibrosis‐4
- FLI
fatty liver index
- GGT
gamma‐glutamyltransferase
- GHS
Gutenberg Health Study
- HbA1C
hemoglobin A1c
- HDL
high‐density lipoprotein
- LDL
low‐density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NFS
NAFLD fibrosis score
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- SHIP
Study of Health in Pomerania
The prevalence of chronic liver disease and cirrhosis in the general population has been increasing over the last decades.( 1 ) The most commonly used tests to screen for liver disease are alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma‐glutamyltransferase (GGT). Despite diagnostic limitations, they are frequently used in patients to check for suspected liver disease, to monitor for safety during pharmacotherapy, or in the context of occupational health surveys. Currently, it is not recommended to screen for liver disease in an asymptomatic, non‐at‐risk population.( 2 ) Therefore, by extrapolating the available data from tertiary care referral cohorts, the true prevalence of liver disease at a population level is overestimated. In the population‐based National Health and Nutrition Examination Survey (NHANES) III, elevated serum aminotransferase activity was reported in 7.9% of the included population sample enrolled between 1988 until 1994 in the United States.( 3 ) A follow‐up study with NHANES data collected between 1999 and 2002 reported an increase of the prevalence of either elevated ALT or AST up to 9.8%.( 4 ) Despite some differences in sample handling between these NHANES analyses, the authors concluded that the rise is mostly likely attributable to an increasing prevalence of nonalcoholic fatty liver disease (NAFLD).( 5 ) In the Germany‐based Study of Health in Pomerania (SHIP), the prevalence of elevated ALT was 15.8% in 4,310 adults in the age group of 20 to 79 years in 1996.( 6 ) Finally, the Italian‐based Dionysus study reported a prevalence of elevated ALT of 17.5% in samples collected between 1991 and 1993 using a cutoff >30 U/L.( 7 ) Overall, these differences in the reported prevalence are likely influenced by the diversity in methodology and study design, but also by cultural and social aspects related to nutrition and alcohol consumption.
More recently, the American Diabetes Association has recommended to screen for nonalcoholic steatohepatitis (NASH) or advanced liver fibrosis when hepatic steatosis or elevated ALT are detected in patients living with type 2 diabetes.( 8 ) This is an important consideration, as the prevalence of NAFLD and NASH are on the rise in Germany( 9 ) and accounts for high health care costs.( 10 ) On the other hand, it has to be considered that normal ALT levels do not exclude fatty liver, and 69% of patients with hepatic steatosis exhibited normal ALT levels.( 11 ) From a clinical perspective, asymptomatic NAFLD is frequently unrecognized,( 12 ) and models of care are urgently needed to address the growing need.( 13 ) A recent analysis from Texas (United States) reported a prevalence of NAFLD of 38% in a colon cancer–screening population that was enrolled between 2015 and 2018.( 14 ) Importantly, the subset of patients with NAFLD who developed advanced fibrosis exhibited an increased overall mortality.( 15 ) To provide a large data set from a cross‐sectional population‐based study in Germany, we report on the prevalence of elevated liver enzymes and surrogate scores of hepatic steatosis and advanced fibrosis in the Gutenberg Health Study (GHS) study.
Patients and Methods
Study Description
We investigated cross‐sectional data of n = 14,950 participants enrolled in the GHS from April 2007 to April 2012. The GHS is a population‐based, prospective, observational, single‐center cohort study in the Rhine‐Main‐Region in western Germany. The GHS has been approved by the local ethics committee and by the local and federal data safety commissioners. Its design has been described in detail.( 16 ) Individuals between the ages of 35 and 74 years were selected randomly from local governmental registries with a sampling procedure that was stratified for sex, residential area (urban vs. rural), and decades of age. All invited participants were offered the opportunity to respond and give a reason for non‐participation.
Baseline Examination
Baseline‐examination in the study center consisted of evaluation of clinical variables and prevalent classical comorbidities, a computer‐assisted personal interview, and laboratory examinations from a venous blood sample, blood pressure, and anthropometric measurements. In general, all examinations were taken out according to standard operating procedures by certified medical technical assistants.
Anthropometric Data
Weight, height, and waist and hip circumference were measured according to a written, standardized manual. Waist circumference (WC) was measured with a tape measure midway between the lowest rib and the pelvis in position of expiration. A WC of <94 cm in men and <80 cm in women was considered normal.
Presence of Comorbidities
Body mass index (BMI) was calculated as weight (kg)/height (m)2. Obesity was defined as BMI ≥ 30 kg/m2, while overweight was defined as BMI > 25 to <30 kg/m2. Diabetes was defined based on self‐reported physician diagnosis, medication use, fasting plasma glucose ≥126 mg/dL after an overnight fasting of at least 8 hours, or a blood glucose level of ≥200 mg/dL after a fasting period <8 hours. Hemoglobin A1c (HbA1C) values were divided into three groups: low (HbA1C < 5.7%), intermediate (HbA1C 5.7%‐6.5%), and high (HbA1C > 6.5%). HbA1C values > 6.5% were considered increased. Dyslipidemia was defined based on self‐reported physician diagnosis, medication use, or a low‐density lipoprotein (LDL)/high‐density lipoprotein (HDL) ratio of >3.5. Hypertension was defined based on self‐reported physician diagnosis, medication use, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg in the second and third standardized measurement after 8 and 11 minutes of rest. The presence of the metabolic syndrome was defined according to the definitions of the Joint Scientific Statement for Harmonizing the Metabolic Syndrome.( 17 ) A history of liver disease was recorded when self‐reported. Additional testing for specific liver disease, including autoimmune or chronic viral hepatitis, were not available. Smoking was dichotomized into nonsmokers (no nicotine consumption and ex‐nicotine consumption) and smokers (occasional consumption <1 cigarette/day was also included in this group). Daily alcohol consumption was recorded and defined according to the criteria for NAFLD with less than 20 g for women and less than 30 g for men.( 18 ) In addition, a subgroup with high daily consumption was defined as ≥40 g in women and ≥60 g in men.( 19 )
Reference Values for Elevated Liver Enzymes
Upper limit of normal was defined as follows: GGT > 64 U/L in men and >36 U/L in women, ALT > 50 U/L in men and >35 U/L in women, and AST > 35 U/L in men and >31 U/L in women. Surrogate scores of hepatic steatosis (fatty liver index [FLI])( 20 ) and fibrosis (NAFLD fibrosis score [NFS], Fibrosis‐4 [FiB‐4] score [lower cutoff 1.3; upper cutoff 2.67]), and the AST to platelet ratio index [APRI]) were analyzed, and advanced fibrosis was defined according to previously published cutoffs.( 21 , 22 )
Statistical Analysis
Statistical analysis was done with R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables are described by mean values and SD or for skewed distribution by median (interquartile range). Discrete variables are described through relative and absolute frequencies. To analyze the relationship between elevated liver function tests and associated risk factors, separate logistic regression models with liver enzymes (ALT, AST, and GGT) as dichotomous dependent variables were calculated. All P values correspond to two‐tailed tests. As this is an explorative study, no adjustments were made for multiple comparisons. Due to the large number of tests applied in this study, P values must be interpreted with caution and in connection with effect size estimates.
Results
Patient Characteristics and Prevalence of Comorbidities
A total of 14,950 participants with a mean age of 55.0 ± 11.1 years were enrolled in the GHS between 2007 and 2012 and were included for analysis. The overall response rate in the study was 70%. Reasons for refusing to participate included a lack of time, fear of the exams and possible results, privacy concerns, and finally unreported reasons. Both sexes were equally represented, with 50.6% being male and 49.4% female. Most participants were overweight (n = 9.782, 65.4%) with a mean BMI of 27.4 kg/m2 ± 5.02 in the entire study cohort. Demographic data and prevalence of comorbidities are presented in Table 1. The prevalence of metabolic risk factors significantly differed between men and women. Arterial hypertension was present in 54.6% of men and 49.8% of women (P < 0.0001). Likewise, diabetes (11.4% vs. 7.1%, P < 0.0001), obesity (26.3% vs. 24.1%, P < 0.01), and dyslipidemia (43.1% vs. 25.9%, P < 0.0001) were more prevalent in men. In the GHS, the prevalence of metabolic syndrome was 30.5%, with a prevalence of 38.7% in men and 22.2% in women (P < 0.0001). The median HbA1C was 5.5%, and high HbA1C values were present in 8.0% in men and 5.0% in women (P < 0.0001). Dyslipidemia was present in 34.6%, with a mean LDL of 139.0 ± 35.5 mg/dL, HDL of 57.3 ± 15.6 mg/dL, and total cholesterol of 221.0 ± 40.8 mg/dL. Men more frequently showed lower levels of HDL (18.3% vs. 16.8%, P = 0.01) and higher triglycerides levels (31.9% vs. 18.5%, P < 0.0001). Most patients (n = 12.024, 80.6%) were nonsmokers and reported alcohol consumption of less than 20 g/day in women and less than 30 g/day in males (n = 12.658, 84.9%). High alcohol consumption was reported by only 2.9% (n = 434). In 34 persons, data concerning alcohol consumption were not available.
TABLE 1.
Demographic Data and Prevalence of Comorbidities
| Parameter | n (%) |
|---|---|
| Male sex | 7,564 (50.6) |
| Age# (range) (years) | 55.0 (25‐74) |
| BMI# (kg/m2) | 26.6 (23.9; 30.0) |
| Obesity | 3,767 (25.2) |
| Type 2 diabetes | 1,385 (9.3) |
| Hypertension | 7,437 (49.8) |
| Dyslipidemia | 5,160 (34.6) |
| Metabolic syndrome | 4,567 (30.5) |
| Chronic heart disease | 2,156 (14.4) |
| Chronic kidney disease | 602 (4) |
| Chronic obstructive pulmonary disease | 740 (5) |
| Cancer | 1,351 (9) |
| Chronic liver disease | 110 (0.7) |
| Smoker | 2,899 (19.4) |
| High alcohol consumption | 434 (2.9) |
Data are expressed as number (percentage) or #median (25th, 75th percentiles). Obesity is defined as BMI > 30 kg/m2. Metabolic syndrome is defined according to the definitions of the Joint Scientific Statement for Harmonizing the Metabolic Syndrome.(15) High alcohol consumption was defined as >60 g/day in men and >40 g/day in women.
Prevalence and Distribution of Elevated Liver Enzymes
Elevated liver enzymes were detected in almost a fifth of all participants and most frequently seen for increased ALT levels (19.9%), whereas increased AST levels were present in 12.8% and elevated GGT levels in 14.0%. Looking at sex‐specific cutoffs, the frequency of ALT elevations was comparable between males and females, whereas increased AST levels were more prevalent in men (14.6% vs. 11.1%, P < 0.0001) and increased GGT was more frequently seen in women (13.4 vs. 14.6, P < 0.05). With increasing age, the prevalence of elevated ALT was not significantly different (<55 vs. ≥55 years) (ALT: 20.3% vs. 19.5%; P = 0.23), but the frequency of AST and GGT above the upper limit of normal was higher in the elderly (<55 vs. ≥55 years) (AST: 11.2% vs. 14.4%, P < 0.001; GGT: 10.7% vs. 17.1%, P < 0.001).
Cofactors of Elevated Liver Enzymes
To define the risk factors that contribute to elevated liver enzymes in this population‐based cohort, exploratory analysis by stepwise logistic regression was performed. Female sex, age, active smoking, any alcohol consumption, high alcohol consumption, and the presence of metabolic syndrome were significantly correlated with elevated ALT, AST, and GGT (Table 2). Interestingly, increased levels of LDL correlated with ALT (odds ratio [OR] 1.411; 95% confidence interval [CI]: 1.286‐1.548; P < 0.0001) and GGT (OR 1.334; CI 1.199‐1.482; P < 0.0001), whereas decreased levels of HDL correlated with elevated ALT (OR 2.290; CI 2.067‐2.535; P < 0.0001), AST (OR 1.444; CI 1.271‐1.636; P < 0.0001), and GGT (OR 1.476; CI 1.301‐1.671; P < 0.0001). High alcohol use exhibited the highest OR for elevated GGT (OR 3.168; CI 2.546‐3.927; P < 0.0001), followed by the presence of metabolic syndrome. Additional effects of the degree of alcohol consumption on ALT, AST, and GGT are explored in the Supporting Tables.
TABLE 2.
Logistic Regression Models Regarding Elevated Liver Function Tests
| Parameter | AST Increase | ALT Increase | GGT Increase |
|---|---|---|---|
| Sex (women) | 0.820; 0.741‐0.907; P < 0.001 | 1.230; 1.130‐1.340; P < 0.0001 | 1.407; 1.272‐1.554; P < 0.0001 |
| Age (10‐year intervals) | 1.051; 1.001‐1.103; P < 0.05 | 0.820; 0.787‐0.855; P < 0.0001 | 1.126; 1.072‐1.183; P < 0.0001 |
| Alcohol consumption | 1.219; 1.084‐1.368; P < 0.001 | 1.296; 1.172‐1.432; P < 0.0001 | 1.699; 1.521‐1.897; P < 0.0001 |
| High alcohol consumption | 2.400; 1.903‐3.007; P < 0.0001 | 1.774; 1.414‐2.211; P < 0.0001 | 3.168; 2.546‐3.927; P < 0.0001 |
| Current smoker | 0.689; 0.598‐0.790; P < 0.0001 | 0.734; 0.656‐0.820; P < 0.0001 | 1.147; 1.012‐1.298: P < 0.05 |
| Metabolic syndrome | 1.526; 1.360‐1.711; P < 0.0001 | 2.044; 1.850‐2.259; P < 0.0001 | 2.284; 2.044‐2.552; P < 0.0001 |
| LDL increase | 0.989; 0.882‐1.107; P = 0.84 | 1.411; 1.286‐1.548; P < 0.0001 | 1.334; 1.199‐1.482; P < 0.0001 |
| HDL decrease | 1.444; 1.271‐1.636; P < 0.0001 | 2.290; 2.067‐2.535; P < 0.0001 | 1.476; 1.301‐1.671; P < 0.0001 |
Data are expressed as OR; 95% CI (n = 14,742). Data are adjusted for sex, age, high alcohol consumption, current smoker, metabolic syndrome, increased LDL, and decreased HDL. Alcohol consumption was defined as an average daily intake of ≥20 g for women and ≥30 g for men; high alcohol consumption was defined as >60 g/day in men and >40 g/day in women.
Surrogate Scores of Hepatic Steatosis and Advanced Fibrosis
The FLI as surrogate score of hepatic steatosis was positive in 37.5% (n = 5,601) participants. There were significant differences with regard to sex and age. In the subgroup with a positive FLI, men were significantly overrepresented compared with women (49.4% vs. 25.3%, P < 0.0001). Moreover, in the age group above 55, the FLI was more frequently positive (<55 vs. ≥55 years: 30.1% vs. 44.5%; P < 0.0001) (Fig. 1). The likelihood of advanced liver fibrosis was estimated using noninvasive surrogate scores of hepatic fibrosis. In this population‐based analysis, using the respective upper cutoffs, the frequency of elevated age‐adapted NFS was 2.8% (n = 412), 1.1% (n = 162) using the FIB‐4 cutoff of 2.67, and 0.2% (n = 31) using the APRI. A total of 1.1% of patients exhibited a FIB‐4 above the upper cutoff, whereas 19.2% were in the intermediate range. Interestingly, scores indicative of advanced fibrosis were significantly more frequent in men compared with women (NFS: 3.6% vs. 1.9%; P < 0.0001; FIB‐4: 1.5% vs. 0.6%; P < 0.0001). In addition, with increasing age the prevalence scores indicative of advanced fibrosis were more frequent (<55 vs. ≥55 years) (NFS: 0.2% vs. 5.2%; P < 0.0001; FIB‐4: 0.2% vs. 1.9%, P < 0.0001) (Fig. 2). These differences were even more pronounced in the FIB‐4 intermediate range (<55 vs. ≥55 years) (NFS: 5.8% vs. 31.1%, P < 0.0001; FIB‐4: 4.6% vs. 32.9%; P < 0.0001). This was not observed for the APRI score, probably because of the low frequency of high scores in this population (0.2%, n = 31). To define the risk factors that contribute to hepatic steatosis and advanced fibrosis, exploratory analysis by stepwise logistic regression was performed. Male sex, age, high alcohol consumption, presence of metabolic syndrome, elevated LDL levels, and decreased levels of HDL as well as elevated levels of HbA1C were significantly correlated with a positive FLI as surrogate marker of hepatic steatosis. Looking at the liver enzymes, only elevated levels of ALT and GGT, but not AST, were significantly correlated with high FLI (Table 3). Exploring the subgroup of participants with advanced fibrosis, male sex, age, and elevated LDL, HbA1C ,and liver function tests were significantly correlated with advanced fibrosis according to the NFS and FIB‐4 high group (Table 4). The presence of metabolic syndrome correlated only with NFS but not FIB‐4 or APRI. Because of the low frequency of high scores in APRI in this population, analysis was done as a comparison of high (0.2%, n = 31) and intermediate (2.8%, n = 423) group versus the low APRI group. Here, a significant correlation of age, high alcohol consumption, elevated LDL levels, and decreased HDL levels, as well as elevated liver function tests, were seen (Table 4).
FIG. 1.
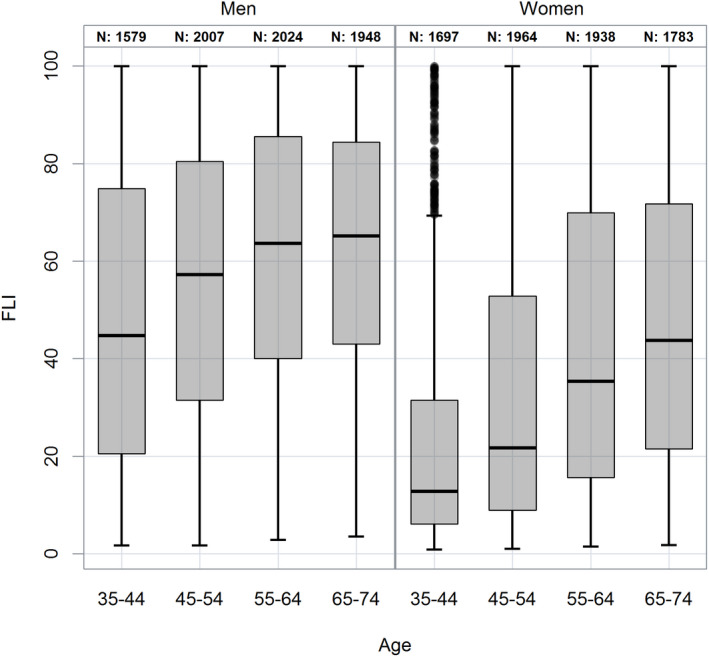
Distribution of the study cohort in the FLI according to sex and age.
FIG. 2.
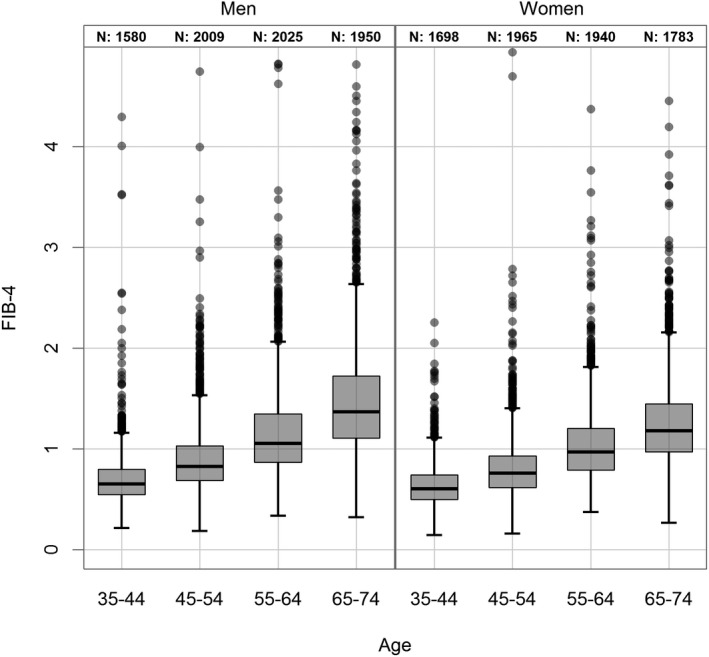
Distribution of the study population in the FIB‐4 index according to sex and age.
TABLE 3.
Logistic Regression Models Regarding FLI
| Parameter | FLI (High) Versus the Rest |
|---|---|
| Sex (women) | 0.309; 0.284‐0.337; P < 0.0001 |
| Age (10‐year intervals) | 1.167; 1.121‐1.215; P < 0.0001 |
| Alcohol consumption | 1.075; 0.937‐1.233; P = 0.30 |
| High alcohol consumption | 1.358; 1.066‐1.729; P < 0.05 |
| Current smoker | 0.915; 0.824‐1.017; P = 0.099 |
| Metabolic syndrome | 3.855; 3.511‐4.235; P < 0.0001 |
| LDL increase | 1.238; 1.130‐1.355; P < 0.0001 |
| HDL decrease | 5.491; 4.933‐6.118; P < 0.0001 |
| HbA1C increase | 1.836; 1.507‐2.242; P < 0.0001 |
| AST increase | 0.945; 0.824‐1.084; P = 0.42 |
| ALT increase | 2.750; 2.453‐3.084; P < 0.0001 |
| GGT increase | 3.683; 3.251‐4.177; P < 0.0001 |
| ALP increase | 1.980; 1.483‐2.652; P < 0.0001 |
Data are expressed as OR; 95% CI (n = 14,736). Data are adjusted for all listed parameters.
Alcohol consumption was defined as an average daily intake of ≥20 g for women and ≥30 g for men; high alcohol consumption was defined as >60 g/day in men and >40 g/day in women.
Abbreviation: ALP, alkaline phosphatase.
TABLE 4.
Logistic Regression Models Regarding Noninvasive Surrogate Scores of Hepatic Fibrosis
| Parameter | NFS | FIB‐4 | APRI |
|---|---|---|---|
| Sex (women) | 0.641; 0.501‐0.816; P < 0.001 | 0.394; 0.264‐0.579; P < 0.0001 | 0.886; 0.713‐1.100; P = 0.27 |
| Age (10‐year intervals) | 4.720; 3.885‐5.793; P < 0.0001 | 3.263; 2.594‐4.168; P < 0.0001 | 1.444; 1.295‐1.611; P < 0.0001 |
| Alcohol consumption | 1.031; 0.736‐1.428; P = 0.86 | 0.921; 0.540‐1.521; P = 0.75 | 0.891; 0.600‐1.301; P = 0.56 |
| High alcohol consumption | 1.047; 0.508‐1.783 P = 0.87 | 1.284; 0.673‐2.307; P = 0.42 | 1.913; 1.252‐2.850; P < 0.01 |
| Current smoker | 0.769; 0.508‐1.129; P = 0.19 | 1.071; 0.606‐1.794; P = 0.8 | 1.043; 0.785‐1.369; P = 0.77 |
| Metabolic syndrome | 2.108; 1.597‐2.797; P < 0.0001 | 1.064; 0.716‐1.576; P = 0.76 | 1.072; 0.832‐1.378; P = 0.59 |
| LDL increase | 0.312; 0.213‐0.44; P < 0.0001 | 0.350; 0.194‐0.590; P < 0.001 | 0.588; 0.455‐0.754; P < 0.0001 |
| HDL decrease | 1.714; 1.291‐2.267; P < 0.001 | 1.223; 0.740‐1.973; P = 0.42 | 1.410; 1.077‐1.837; P < 0.05 |
| HbA1C increase | 4.901; 3.706‐6.471; P < 0.0001 | 0.393; 0.184‐0.773; P < 0.05 | 0.757; 0.502‐1.119; P = 0.17 |
| AST increase | 2.641; 1.902‐3.651; P < 0.0001 | 17.178; 11.369‐26.185; P < 0.0001 | — |
| ALT increase | 0.512; 0.358‐0.724; P < 0.001 | 0.574; 0.376‐0.874; P < 0.01 | 11.324; 8.865‐14.562; P < 0.0001 |
| GGT increase | 1.592; 1.191‐2.115; P < 0.01 | 2.466; 1.664‐3.648; P < 0.0001 | 2.975; 2.381‐3.718; P < 0.0001 |
| ALP increase | 1.267; 0.675‐2.251; P = 0.44 | 1.491; 0.670‐3.027; P = 0.3 | 1.482; 0.955‐2.243; P = 0.07 |
Data are expressed as OR; 95% CI (n = 14,742). NFS and FIB‐4 are comparisons of high scores versus the rest. APRI is a comparison of high and intermediate scores versus the rest. Data are adjusted for all listed parameters. Alcohol consumption was defined as an average daily intake of ≥20 g for women and ≥30 g for men; high alcohol consumption was defined >60 g/day in men and >40 g/day in women.
Abbreviation: ALP, alkaline phosphatase.
Discussion
In the current study we explored the prevalence of elevated ALT, AST, and GGT and surrogate scores of hepatic steatosis and advanced fibrosis in a large, cross‐sectional population cohort in Germany using individual patient data from 14,950 participants enrolled between 2007 and 2012. Recently, an increasing prevalence of liver disease has been predicted based on sedentary lifestyle and obesity, which promote the development of hepatic steatosis. In addition, the consumption of alcoholic beverages, a well‐defined risk factor for hepatic steatosis and liver disease, is highly prevalent in Europe and the United States. The prevalence of the metabolic syndrome in this study cohort was 30.3%, with a median BMI of 26.6 kg/m² (CI: 23.9, 30.0) and a prevalence of type 2 diabetes of 9.3%. These data are comparable to the German SHIP study, which recruited 4,308 participants between 1997 and 2001 and observed a median BMI of 26.7 kg/m² (CI: 26.6, 26.7) and a rate of the metabolic syndrome according the modified National Cholesterol Education Program’s Adult Treatment Panel III criteria of 23.8% (women 18.6%, men 29.1%).( 23 )
Obesity and overweight are established risk factors to develop NAFLD and advanced fibrosis.( 21 , 24 ) The prevalence of metabolic risk factors significantly differed between men and women. Men were more frequently affected by arterial hypertension, diabetes, obesity, and dyslipidemia compared with women. Smoking as a risk factor for cardiovascular disease was more prevalent in men in this cohort. In addition, alcohol consumption was more frequent in men compared with women.
We observed elevated ALT levels in almost a fifth of the German study population. Data from a systematic review of 37 included studies published between 2000 and 2014 showed a wide range of prevalence of abnormal liver enzymes in the general population (10.0%‐21.7%).( 25 ) In the United States, an evaluation of about 15,000 individuals showed elevated ALT and GGT in approximately 13% of each.( 26 ) A direct comparison between these studies is difficult, related to different definitions of the upper limit of normal. In the study by Ruhl et al., abnormal ALT was defined as >30 U/L in men or >19 U/L in women, and abnormal GGT was defined as >51 U/L in men or >33 U/L in women.( 26 ) The current analysis used a reference value for ALT of >50 U/L in men and >35 U/L in women.
In the literature, an increasing burden of liver disease has been reported and predicted. Analyses derived from the US‐NHANES indicated an increase in the frequency of liver enzyme elevations from the period of 1988‐1994 (7.9%)( 3 ), and 9.8%( 4 ) in the 1999‐2002 period. This is likely related to the increasing prevalence of NAFLD. Accordingly, data from the past decades have also shown a marked increase in the prevalence of NAFLD from 15% in 2005 to 25% in 2010.( 27 )
In the current analysis, elevated GGT showed the strongest association with the presence of the metabolic syndrome, while the OR for elevated ALT was higher in obese participants. This is of interest, as GGT has previously been shown to be associated with an increase in cardiovascular risk.( 28 ) This is in line with observations that patients with NASH exhibited a high Framingham Risk Score, which predicts the 10‐year risk for coronary heart disease.( 29 )
Another important aspect to consider is that ALT levels can decrease with age( 30 ) and the presence of cirrhosis. In our analysis, we did not observe a difference in ALT based on an age cutoff of 55 years. On the other hand, the prevalence of advanced fibrosis based on the FIB‐4 and the NFS was more frequent in the population above 55 years. Previous studies have shown an effect of age on FIB‐4 results and performance.( 31 )
Another important finding in this population‐based study was that 37.5% of the entire population exhibited a positive FLI, suggestive of hepatic steatosis. Steatosis was more frequent in men compared with women, in parallel with the higher prevalence of the metabolic syndrome in men compared with women.
The prevalence of advanced fibrosis at the population level was about 1% according to the FIB‐4, and 110 patients (0.7%) reported a history of chronic liver disease. We observed an influence of sex and age on the frequency of elevated liver enzymes and advanced fibrosis. This is in line with studies that used histology to define NAFLD and identified age as an important risk factor of advanced fibrosis.( 21 ) Importantly, the positive predictive value of the NFS declines above the age of 65 years; therefore, we applied age‐specific cutoffs.( 32 ) Using the NFS, the predicted prevalence of advanced fibrosis increased to 2.8% in the entire study population. Importantly, this subgroup of the population exhibits an increased liver‐specific and overall mortality,( 15 ) and an increased risk of cardiovascular disease.( 26 ) In contrast, the effect of ALT on overall mortality is less clear. While the relative risk of liver‐associated mortality of NAFLD is increased approximately 8‐fold with ALT elevations, the all‐cause mortality cannot be reliably predicted by ALT elevation.( 33 ) Likewise, elevated ALT levels have a low negative predictive value for advanced liver disease.( 34 ) The frequency of participants positive for the high‐risk group of advanced liver disease in this population‐based study was between 1% and 2.5%. It is important to note that these surrogate scores have not been validated in this context of use, and thus are likely prone to misclassification based on the low pretest probability of advanced fibrosis in the general population. On the other hand, these data help to provide solid estimates on the number of patients who will be detected in patient and referral pathways that are currently being developed to screen for liver disease. One aspect we observed in this analysis was the impact of sex on risk factors of liver disease. Males had a higher rate of self‐reported alcohol consumption and overweight. These data support that risk‐based preventive measures could be useful to address liver disease.
Limitations of the study include the cross‐sectional design; therefore, no causal relation can be shown. Participants were invited to the GHS, and the overall response rate was 70%. No data on nonresponders are available, and factors that introduce potential bias are related to differences in health‐seeking behavior or education. Importantly, this response rate is comparable to other population‐based studies that have been conducted in Germany, such as the SHIP study.( 35 ) In addition, the current analysis is limited to the lack of imaging modalities to stage liver disease. Although ultrasound‐based modalities have a higher accuracy compared with the blood‐based makers,( 22 ) the FLI, FIB‐4, and NFS have been extensively studied and are well‐validated.( 36 ) Unfortunately, patented multivariate tests such as FibroTest, Enhanced Liver Fibrosis, or Fibrometer were not available for comparison. Another limitation is that the degree of alcohol consumption was self‐reported, leaving room for underreporting. Also, we had only limited, self‐reported information about the presence of pre‐existing liver disease, and no additional laboratory testing for liver disease was available. This prospectively defined study describes the prevalence of elevated liver enzymes, hepatic steatosis, and advanced fibrosis in Germany.
In summary, in a representative sample of the German population aged 35 through 75 years, the prevalence of fatty liver disease is high, while the prevalence of advanced fibrosis ranges between 1% and 2.5% depending on the surrogate score used. Among the risk factors that contribute to elevated liver enzymes, we identified age, sex, and high alcohol consumption as relevant contributors. These data inform on the burden of liver disease at a population‐based level and could be used for risk‐based counseling and prevention to address the increasing prevalence of chronic liver disease.
Supporting information
Supplementary Material
Supported by the Center for Translational Vascular Biology, Stiftung Rheinland‐Pfalz für Innovation (AZ 961‐386261/733), and Wissen Schafft Zukunft.
Potential conflict of interest: J.M.S. consults for and received grants from Gilead Sciences, Boehringer Ingelheim, and Siemens Healthcare. He consults for BMS, Boehringer Ingelheim, Echosens, Genfit, Intercept Pharmaceuticals, Madrigal, Nordic Bioscience, Merk, Novartis, Pfizer, Roche, and Sanofi. He is a Speaker Honorarium from Falk Foundation. P.G. consults for and advises Novartis. P.S.W. consults for, advises, and received grants from Boehringer Ingelheim. P.R.G. consults for Novartis.
References
- 1. Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 2020;72:1605‐1616. [DOI] [PubMed] [Google Scholar]
- 2. Schattenberg JM, Anstee QM, Caussy C, Bugianesi E, Popovic B. Differences between current clinical guidelines for screening, diagnosis and management of nonalcoholic fatty liver disease and real‐world practice: a targeted literature review. Expert Rev Gastroenterol Hepatol 2021;15:1253‐1266. [DOI] [PubMed] [Google Scholar]
- 3. Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003;98:960‐967. [DOI] [PubMed] [Google Scholar]
- 4. Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999‐2002. Am J Gastroenterol 2006;101:76‐82. [DOI] [PubMed] [Google Scholar]
- 5. Flores YN, Yee HF Jr, Leng M, Escarce JJ, Bastani R, Salmeron J, et al. Risk factors for chronic liver disease in Blacks, Mexican Americans, and Whites in the United States: results from NHANES IV, 1999–2004. Am J Gastroenterol 2008;103:2231‐2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumeister SE, Volzke H, Marschall P, John U, Schmidt CO, Flessa S, et al. Impact of fatty liver disease on health care utilization and costs in a general population: a 5‐year observation. Gastroenterology 2008;134:85‐94. [DOI] [PubMed] [Google Scholar]
- 7. Bellentani S, Tiribelli C. The spectrum of liver disease in the general population: lesson from the Dionysos study. J Hepatol 2001;35:531‐537. [DOI] [PubMed] [Google Scholar]
- 8. American DA. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes‐2019. Diabetes Care 2019;42:S34‐S45. [DOI] [PubMed] [Google Scholar]
- 9. Estes C, Anstee QM, Arias‐Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016‐2030. J Hepatol 2018;69:896‐904. [DOI] [PubMed] [Google Scholar]
- 10. Schattenberg JM, Lazarus JV, Newsome PN, Serfaty L, Aghemo A, Augustin S, et al. Disease burden and economic impact of diagnosed non‐alcoholic steatohepatitis in five European countries in 2018: a cost‐of‐illness analysis. Liver Int 2021;41:1227‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kotronen A, Juurinen L, Hakkarainen A, Westerbacka J, Corner A, Bergholm R, et al. Liver fat is increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese nondiabetic subjects. Diabetes Care 2008;31:165‐169. [DOI] [PubMed] [Google Scholar]
- 12. Lazarus JV, Colombo M, Cortez‐Pinto H, Huang T‐K, Miller V, Ninburg M, et al. NAFLD—sounding the alarm on a silent epidemic. Nat Rev Gastroenterol Hepatol 2020;17:377‐379. [DOI] [PubMed] [Google Scholar]
- 13. Lazarus JV, Anstee QM, Hagström H, Cusi K, Cortez‐Pinto H, Mark HE, et al. Defining comprehensive models of care for NAFLD. Nat Rev Gastroenterol Hepatol 2021;18:717‐729. 10.1038/s41575-021-00477-7 [DOI] [PubMed] [Google Scholar]
- 14. Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM, et al. Prospective evaluation of the prevalence of non‐alcoholic fatty liver disease and steatohepatitis in a large middle‐aged US cohort. J Hepatol 2021;75:284‐291. [DOI] [PubMed] [Google Scholar]
- 15. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology 2020;158:1611‐1625.e1612. [DOI] [PubMed] [Google Scholar]
- 16. Wild PS, Zeller T, Beutel M, Blettner M, Dugi KA, Lackner KJ, et al. Die Gutenberg Gesundheitsstudie. Bundesgesundheitsblatt ‐ Gesundheitsforschung ‐ Gesundheitsschutz 2012;55:824‐830 10.1007/s00103-012-1502-7 [DOI] [PubMed] [Google Scholar]
- 17. Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 18. Baumeister SE, Alte D, Meyer C, John U. Health risk drinking and problematic consumption of alcohol in Pomerania: comparative analysis of the Study of Health in Pomerania (SHIP) compared with the Federal German Health and Examination Survey in 1998. Gesundheitswesen 2005;67:39‐47. [DOI] [PubMed] [Google Scholar]
- 19. Burger M, Bronstrup A, Pietrzik K. Derivation of tolerable upper alcohol intake levels in Germany: a systematic review of risks and benefits of moderate alcohol consumption. Prev Med 2004;39:111‐127. [DOI] [PubMed] [Google Scholar]
- 20. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Labenz C, Huber Y, Kalliga E, Nagel M, Ruckes C, Straub BK, et al. Predictors of advanced fibrosis in non‐cirrhotic non‐alcoholic fatty liver disease in Germany. Aliment Pharmacol Ther 2018;48:1109‐1116. [DOI] [PubMed] [Google Scholar]
- 22. Michel M, Schattenberg JM. Liver‐specific diagnostic for non‐alcoholic fatty liver disease (NAFLD)—time to replace liver biopsy? Z Gastroenterol 2020;58:1233‐1240. [DOI] [PubMed] [Google Scholar]
- 23. Schipf S, Alte D, Völzke H, Friedrich N, Haring R, Lohmann T, et al. Prävalenz des Metabolischen Syndroms in Deutschland: Ergebnisse der Study of Health in Pomerania (SHIP). Diabetologie Und Stoffwechsel ‐ DIABETOL STOFFWECHS 2010;5:161‐168. [Google Scholar]
- 24. Wong RJ, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross‐sectional analysis of 2011‐2014 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2017;46:974‐980. [DOI] [PubMed] [Google Scholar]
- 25. Radcke S, Dillon JF, Murray AL. A systematic review of the prevalence of mildly abnormal liver function tests and associated health outcomes. Eur J Gastroenterol Hepatol 2015;27:1‐7. [DOI] [PubMed] [Google Scholar]
- 26. Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma‐glutamyltransferase and mortality in the United States population. Gastroenterology 2009;136:477‐485.e411. [DOI] [PubMed] [Google Scholar]
- 27. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 28. Haring R, Wallaschofski H, Nauck M, Dörr M, Baumeister SE, Völzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma‐glutamyl transpeptidase levels. Hepatology 2009;50:1403‐1411. 10.1002/hep.23135 [DOI] [PubMed] [Google Scholar]
- 29. Labenz C, Prochaska JH, Huber Y, Nagel M, Straub BK, Wild P, et al. Cardiovascular risk categories in patients with nonalcoholic fatty liver disease and the role of low‐density lipoprotein cholesterol. Hepatol Commun 2019;3:1472‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Claus M, Antoni C, Hofmann B. Factors associated with elevated alanine aminotransferase in employees of a German chemical company: results of a large cross‐sectional study. BMC Gastroenterol 2021;21:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ishiba H, Sumida Y, Tanaka S, Yoneda M, Hyogo H, Ono M, et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi‐center study. J Gastroenterol 2018;53:1216‐1224. [DOI] [PubMed] [Google Scholar]
- 32. McPherson S, Hardy T, Dufour J‐F, Petta S, Romero‐Gomez M, Allison M, et al. Age as a confounding factor for the accurate non‐invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver‐related mortality in non‐alcoholic fatty liver disease. J Hepatol 2008;49:608‐612. [DOI] [PubMed] [Google Scholar]
- 34. Wong V W‐S, Wong G L‐H, Tsang S W‐C, Hui AY, Chan A W‐H, Choi P C‐L, et al. Metabolic and histological features of non‐alcoholic fatty liver disease patients with different serum alanine aminotransferase levels. Aliment Pharmacol Ther 2009;29:387‐396. [DOI] [PubMed] [Google Scholar]
- 35. Völzke H, Ittermann T, Schmidt CO, Baumeister SE, Schipf S, Alte D, et al. Prävalenztrend lebensstilabhängiger Risikofaktoren. Dtsch Arztebl Int 2015;112:185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, et al. Nonalcoholic fatty liver disease in diabetes. Part I: Epidemiology and Diagnosis. Diabetes Metab J 2019;43:31‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


