Abstract
Cancers are generally recognized as the leading cause of death and a predominant barrier to prolonging life expectancy in both developed and developing countries. Emodin is a typical anthraquinone derivative from various plants that exhibits a wide spectrum of biological activities, such as anticancer, antibacterial, hepatoprotective and anti-inflammatory activities. Much previous preclinical evidence has demonstrated that emodin exhibits reliable effects on several cancer types, including lung cancer, liver cancer, colon cancer, breast cancer, pancreatic cancer, leukemia, cervical cancer, and ovarian cancer, etc. The related molecular mechanisms corresponding to the anticancer activities of emodin are involved in the induction of apoptosis, inhibition of cell proliferation, enhanced reactive oxygen species (ROS) accumulation, and induction of autophagy, etc. In the present review, we summarized the sources, anticancer properties in vitro and in vivo, molecular mechanisms, metabolic transformation and toxicities of emodin. In addition, we also discussed the limitations of the present investigations of emodin against cancers and gave some perspectives for them, which would be beneficial for the further exploration and development of this natural compound as a clinical cancer drug.
Keywords: Emodin, Apoptosis, Metastasis and invasion, Cycle arrest, Drug resistant, Autophagy
Introduction
Currently, epidemiological evidence reveals that the incidence and mortality of cancers are rapidly rising worldwide, and cancers are also generally recognized as the leading causes of death and a predominant barrier to prolonging life expectancy in both developed and developing countries 1, 2. Importantly, it is estimated that there were more than 180,000,000 new cancer cases and 100,000,000 cancer-caused deaths each year nowadays 1,3. Furthermore, it has been reported that regardless of sex, lung cancer is the most common cancer clinically and the primary cause of cancer-related death, followed by breast cancer, colorectal cancer, prostate cancer, stomach cancer, liver cancer, esophageal cancer, cervical cancer, thyroid cancer and bladder cancer, and these ten cancer types account for approximately 65% of newly occurring cancer cases 1, 4. In recent years, although the diagnosis and treatment of cancers have improved greatly, the 5-year survival rate of cancer patients is still relatively poor due to the gradually appearing drug resistance of cancer cells and the excessively high price of anticancer drugs, particularly for developing areas 5,6. In addition to surgery and radiotherapy, chemotherapy remains the predominant treatment for cancer, and the currently available anticancer drugs usually target fast-dividing cells, which could damage both the cancer cells and some normal cells, such as epithelial cells of the digestive tract, marrow cells and hair follicles, leading to serious side effects, including hair loss, myelosuppression, vomiting and diarrhea 5, 7. Therefore, finding more reliable and economical treatment strategies with low toxicity against cancers is urgently needed.
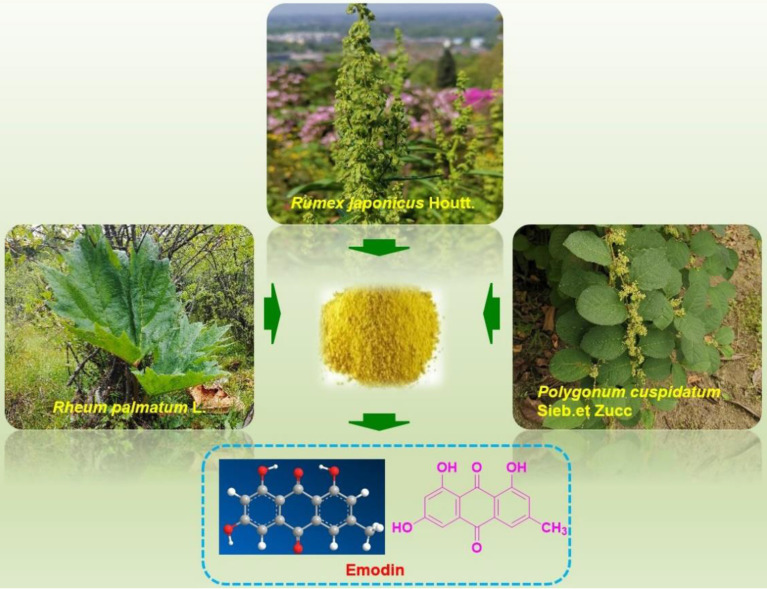
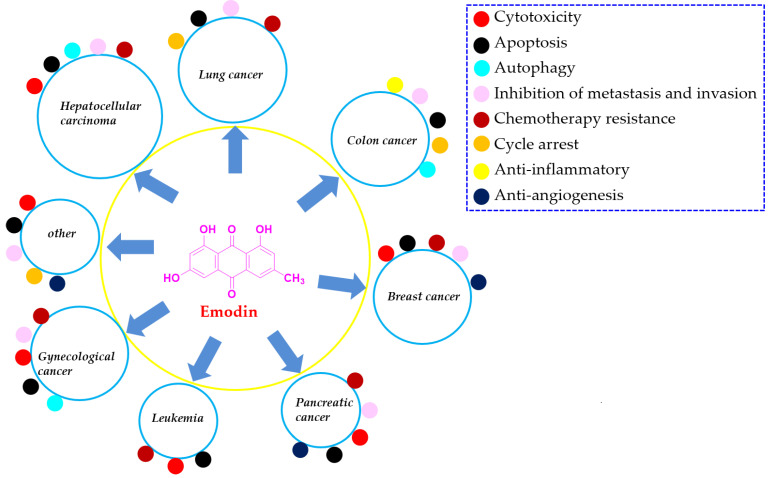
Currently, an increasing number of studies have focused on the possible capacities of natural agents extracted from herbal medicines to prevent or cure cancers and have discovered many anticancer drugs, such as taxol, vincaleukoblastinum, and camptothecin, etc. 5, 8, 9. Emodin (chemical structure shown in Figure 1) is a typical anthraquinone derivative from herbal medicine that exhibits a wide spectrum of biological activities, such as anticancer, antibacterial, hepatoprotective and anti-inflammatory activities, according to previous investigations 10, 11. Much previous preclinical evidence has demonstrated that emodin exhibits reliable effects on several cancer types, including lung cancer, liver cancer, colon cancer, breast cancer, pancreatic cancer, leukemia, cervical cancer, and ovarian cancer 12, 13. The related molecular mechanisms corresponding to the anticancer activities of emodin are involved in the induction of apoptosis, inhibition of cell proliferation, enhanced reactive oxygen species (ROS) accumulation, and induction of autophagy 12, 14-16. In our previous works, we found that emodin exists in Polygonum cuspidatum at a high amount and has interesting effects on anti-methicillin-resistant Staphylococcus aureus (MRSA) 17, 18. Furthermore, we have reported many natural extract/monomer anticancer agents with potential antitumor properties 7, 19-22; as part of our continuing investigations, we also found that emodin has significant anticancer activities against various cancer cell lines and might be a promising compound for development as an effective and economical anticancer drug with low toxicity.
Figure 1.
Polygonum cuspidatum and chemical structure of emodin.
Consequently, in the present review, we summarized the sources, anticancer properties including dose ranges tested in vitro and in vivo, metabolic transformation and toxicities of emodin, which would be beneficial for the further exploration and development of this natural compound as a clinical cancer drug (Table 1).
Table 1.
Antitumor potentials of emodin
| Cancers | Mechanisms | Cell lines/Animals | Dose/Concentration | Potential targets | References | |
|---|---|---|---|---|---|---|
| Up | Down | |||||
| Lung cancer | ||||||
| Cytotoxicity | PTK inhibition | NCI-H1435, NCI-H226, NCI-H460 | 30 μM | HER-2 neu | 39 | |
| Suppression of ERCC1 and Rad 51 via ERK1/2 inactivation | H1650, A549, H520, H1703 | 25-100 μM | ERCC1, Rad51, p-ERK1/2, MKK1/2 | 40 | ||
| Down-regulation of ERCC1 and Rad51 | SK-MES-1, A549 | 40 μM, 70 μM | ERCC1, Rad51 | 41 | ||
| ERCC1 down-regulation and ERK1/2 inactivation | H520, H1703 | 8.1-24.3 μg/mL | ERCC1, p-ERK1/2 | 42 | ||
| Inhibition of ILK expression via increase of phosphorylation of AMPKα & ERK1/2 and suppression of Sp1 and c-Jun. | A549, PC9, H1299, H1650, H1975 | 50 μM | p-AMPKα | ILK, Sp1, c-Jun, p-ERK1/2 | 43 | |
| Inhibition of cell growth and induction of cell cycle arrest at G2/M phase via activation of PPARγ & AMPKα/MEK/ERK, down-regulation of Sp1 and up-regulation of IGFBP1 | A549, H1975 Nude mice (A549) |
50 μM for cell; 25,50 mg/kg for mice |
p-PPARγ, p-AMPKα, MEK, IGFBP1 | Sp1, p-ERK1/2 | 44 | |
| By inhibiting hyaluronan secretion and regulating the expression of cyclin, G1/G0 phase arrest was induced | A549, H520, H1975, H1299, H460 | 30 μM | Cyclin C, Cyclin D, Cyclin E | HAS2, Cyclin A, Cyclin B | 45 | |
| Apoptosis | Emodin-induced cell death is closely associated with the mitochondria- dependent apoptosis | CH27 | 10, 50 μM | c-Caspase 3, c-Caspase 8, c-Caspase 9, Bak, Bax, Cyto C | 46 | |
| Induction of apoptosis via up-regulation of FASL and down-regulation of C-MYC | A549 | 16.85 μg/ml | FASL | c-Myc | 47 | |
| Induction of apoptosis via activation of ER stress and the TRIB3/NF-κB pathway | A549, H1299; BALB/c nu/nu nude mice (A549) | 80 μM for cell; 50 mg/kg | c-Caspase 3, CHOP, TRIB3, GRP78 | 48 | ||
| Induction of mitochondria-dependent apoptosis via activating a ROS-elicited ATM-p53-Bax signaling pathway |
A549 | 50 μM | p-ATM, p53, Bax, Cyct C | Survivin | 15 | |
| Induction of apoptosis via ROS generation and reduced ∆Ψm | A549, H460, CH27, WI38 | 50 μM | c-Caspase 2, c-Caspase 3, c-Caspase 8, c-Caspase 9, Bax, ROS, Cyto C | Bcl-2, p-Akt, p-ERK1/2 | 49 | |
| Induce tumor cell apoptosis | A549 | 9.31 μg/ml | 50 | |||
| Inhibition of MTH1 promotes DNA damage and apoptosis of tumor cells | NCI-H-520, NCI-H-460, A549 | 25, 50, 75 μM | ROS, Cyclin B1, PARP, c-Caspase 3, Bax, | CDK4, Bcl-2, MTH1, CDK2, Cyclin D1, Survivin, VIM | 52 | |
| Inhibition of proliferation of non-small cell lung cancer in vitro and in vivo | A549, H1650, H460, H1975, PC9, H1299 C57 mice (LLC cells) |
20, 40, 60 μM for cell; 25, 50 mg/kg | ROS, Bax, P27, p-AMPK | sPLA2-IIa, NF-κB P65, IKKβ, IκBα, p-mTOR, p-ACC, p-PKM2, p-AKT, Cyclin D1, Cyclin B1, Bcl2 | 53 | |
| Increase ROS, reduce autophagy, induce lung cancer cell apoptosis in vivo and in vitro | LLC cell; ICR mice (urethane-induced lung carcinogenesis) | 20 μM for cell; 10 mg/kg for mice | IFN-γ, IL-12, ROS, P62 | IL-6, TNF-α, TGF-β1, LC3-B | 60 | |
| Autophagy | Induction of autophagy via the mutation independent p53 aggregation. | A549 | 10, 15, 20 μM/70 μM | p53, LC3 | ERCC1, Rad51 | 16,41 |
| Inhibition of metastasis and invasion | Down-regulation of CXCR4 and HER2 | A549 | 100 μM | CXCR4, HER-2 neu | 54 | |
| Inhibition of ATP-induced proliferation and migration by suppression of P2Y receptor and Ca2+ dependent NF-κB pathway | A549 | 1, 5 μM | Bax, Claudin-1, E-cadherin | Bcl-2, Fibronectin, SNAIL, NF-κB p65 | 55 | |
| Suppressing expressions of Twist, SNAIL & Slug, and inhibiting activation of NF-κB | H69, H69AR | 10,20,50 μM | Twist, SNAIL, Slug, NF-κB p65 | 56 | ||
| Chemotherapy resistance | Inhibition of drug efflux enhances cisplatin-induced apoptosis and DNA damage | A549, H460 | 2.5, 5, 10 μM | Pgp | 57 | |
| It synergistically inhibited the proliferation of A549 cells with paclitaxel in vivo and in vitro, and exerted anti-tumor effect | A549; BALB/nude mice (A549) | 10 μM for cell; 50 mg/kg for mice | Bax, c-Caspase 3 | Bcl-2, p-Akt, p-ERK1/2 | 58 | |
| Reverse cisplatin resistance, promote lung cancer cell apoptosis, inhibit cell migration and invasion | A549 | Not mentioned | NF-κB, P-gp, MDR-1, GST | 59 | ||
| Hepatocellular carcinoma | ||||||
| Cycle arrest | G2/M phase arrest of tumor cells | Huh7, Hep3B, HepG2 | 50 μM | Cyclin B, Chk2, Cdk2, P27, CYP1A1, CYP1B1, CHAC1, TIPARP, GDF15, SOS1, RASD1, SLC7A11, CYR61, MRAS, SERPINE1 | Cdc25c, P21, NR1H4, PALMD, TXNIP, IGFBP3, Cyclin A, Cdk1 | 65 |
| Results in G1 phase arrest, increased intracellular ROS level and DNA fragmentation | HepG2 | 30, 60, 90, 120 μM | c-Caspase 8, c-Caspase 9, Cyto C, p53 | Bcl-2, NF-kB p65, p-Caspase 3 | 66 | |
| It can cause G1 phase arrest and cytotoxicity, increase ROS level and inhibit cell glycolysis | HepG2 | 10, 20, 40 μM | PKM2, HK11, LDHA | 67 | ||
| Apoptosis | ROS production is increased, G2/M phase arrest occurs, and the mitochondrial transmembrane potential (∆Ψm) decreases, leading to DNA fragmentation and inducing cell apoptosis | Mahlavu, PLC/PRF/5, HepG2 | Mahlavu (5, 10, 30 μg/ml), PLC/PRF/5 (40, 80, 160 μM), HepG2 (20, 40, 80 μM) | Cyt c, P53, P21, Bax, Cyclin E, c-Caspase 3, c-Caspase 9, c-PARP | Bcl2, Cyclin A, CDK2 | 68-70 |
| Decreased mitochondrial membrane potential (∆Ψm) and induced apoptosis | HepG2 | 50, 100 μM | CypD, Cyt c | p-ERK1/2 | 71 | |
| Induce tumor cell apoptosis and inhibit tumor growth | HepG2, PLC/PRF/5, Hep3B, C3A; Athymic nu/nu female mice (HCCLM3) |
10, 50 μM for cell; 25, 50 mg/kg for mice |
SHP-1, c-Caspase 3; PARP | CD31, p-STAT3, Bcl2, Bcl-xL, survivin, Mcl-1, VEGF, p-JAK2, p-JAK1, p-AKT, p-Src, cyclin D1 | 76 | |
| Inhibit cell viability and promote tumor cell apoptosis through death receptor and mitochondrial pathways | HepG2, HL-7702 | 20, 40, 80 μM | PARP, BAX, Cyt c, Fas, Fas-L, tBid, p-p38 | p-Caspase 3, Bcl2, Bid, p-Caspase 8, p-Akt, p-ERK1/2, p-JNK | 77 | |
| Decrease cell viability and induce apoptosis in vitro. Inhibit tumor growth in vivo, induce apoptosis of tumor cells, improve liver and kidney function of tumor mice | SMMC-7721 male BALB/c-nu nude mice (SMMC-7721) |
25, 50, 100 μM for cell; 25, 50 mg/kg for mice | p-p38, c-Caspase 3, c-Caspase 9 | p-AKT, p-Caspase 9, p-JNK, p-ERK1/2, p-Caspase 3 | 78 | |
| Induce tumor cell apoptosis and inhibit tumor growth | HepG2; BALB/c nude mice (HepG2) | 10, 100 nM for cell; 1, 10 mg/kg for mice | mir-34a | SMAD2, SMAD4, p-VEGFR2, p-AKT, p-ERK1/2 | 79 | |
| Inhibit lipid metabolism of tumor cells, promote apoptosis and inhibit tumor growth | BALB/C mice (Be L-7402) | 20, 40, 80 mg/kg | Bax, c-Caspase 9, c-Ccaspase 3, APAF1, Cyt c, AIF | Bcl2, SREBP1, FASN, ACACA, ACLY, SCD1, SIP, SCAP、Caspase 2 | 80 | |
| It inhibited tumor cell viability, reduced mitochondrial membrane potential, inhibited triglyceride level and fatty acid desaturation, and induced apoptosis | Bel-7402 | 100 μM | c-Caspase 3, c-Caspase 9, APAF1, Cyt c, ENDOG, AIF, Bax | Bcl2, SCD, FASN, ACACA, ACLY, SREBP1 | 81 | |
| Inhibition of metastasis and invasion | Inhibit the migration and invasion of tumor cells and inhibit lung metastasis in vivo | HepG2, Hep3B, PLC/PRF5, HUH7 female Balb/c nude mouse (HCCLM3) |
50 μM for cell; 25, 50 mg/kg for mice |
CXCR4, HER2, NF-kB | 84 | |
| Inhibit tumor cell viability, induce a small amount of apoptosis, inhibit cell migration and invasion | MHCC-97H | 100 μg/kg | p-p38 | p-ERK1/2, p-Akt, MMP-2, MMP-9 | 85 | |
| Chemotherapy resistance | Reversal of cisplatin resistance increases DNA damage | HepG2 | 10 μM | FGFR2, p-ERK1/2, ERCC1 | 89 | |
| Enhanced irradiation induces cytotoxicity G2/M block was induced and apoptosis was induced | HepG2 | 10 μM | c-PARP1 | JMJD1A, HIF-1α, JMJD2B | 90 | |
| It can induce G1 phase arrest and apoptosis, reduce cholesterol synthesis, inhibit tumor growth, and improve Sorafenib resistance | HepG2, Hep3B, Huh7, SK-HEP-1, PLC/PRF5; BALB/c-nude mice (HepG2 or SK-HEP-1) | 20 μM for cell; 10 mg/kg for mice |
c-Caspase 3 | HMGCS1, HMGCR, FDPS, p-AKT, p-4E-BP1, p-STAT3 | 91 | |
| Enhance the toxicity of cisplatin and inhibit the migration and invasion of tumor cells | HepG2 cell | 25, 50 μg/ml | E-cadherin | 92 | ||
| Colon cancer | ||||||
| Cell cycle | Intracellular ROS production and Ca2+ release were induced, and G0/G1 phase arrest was induced in tumor cells | LS1034; Athymic BALB/c nu/nu mice (LS1034) | 10, 20, 30, 40, 50 μM for cell; 40 mg/kg for mice | c-Caspase 3, c-Caspase 9, Bax, AIF, Cyt c | Bcl2 | 96 |
| Apoptosis | Increase intracellular ROS production and induce tumor cell apoptosis | HCT116 | 20, 40, 80 μM | Bax, Cyt c, P53 | Bcl2 | 97 |
| Inducing tumor cell apoptosis through mitochondrial pathway | LOVO | 10, 20, 40 μM | Bax, Cyt c, | Bcl2 | 98 | |
| Increase intracellular ROS level, inhibit tumor cell proliferation and induce apoptosis. | SW480, SW620 | 20, 40, 60, 80 μM | p-P38, P53, Puma | 99 | ||
| Inhibition of fatty acid synthesis of tumor cells plays an anti-proliferation and pro-apoptotic role | HCT116, SW480 | 25 μM | FASN, p-AKT, p-PI3K | 100 | ||
| Regulation of PI3K/AKT pathway induces G2/M cycle arrest and apoptosis of human colon cancer cells | CACO-2 | 15, 30, 60 μM | Bax | Bcl2, p-PI3K, p-Akt | 101 | |
| Induce cell apoptosis, inhibit migration and invasion, inhibit tumor growth, and reverse 5-FU resistance | SW480, SW480/5-Fu BALB/c nude mice (SW480/5-Fu) |
9 μM for cell; 40 mg/kg for mice |
Bax, c-Caspase 3 | Bcl2, p-ERK1/2, p-AKT | 102 | |
| Autophagy | Increase intracellular ROS accumulation, induce cell apoptosis and autophagy | HCT116, LOVO | 20 μM | c-Caspase 9, c-Caspase 3, c-PARP, LC3-2, Beclin 1, LC3-1, Cyt c, Bax | P62, Bcl2 | 103 |
| Inhibition of metastasis and invasion | Inhibit the migration and invasion of tumor cells | DLD-1 | 10, 20, 30, 40 μM | α- ERM pThr567 | PRL-3 | 104 |
| The ROS level in tumor cells was increased, G2/M phase arrest occurred, and the migration and invasion of tumor cells were inhibited | SW480, SW620 | 50 μM | CDH1, EP300 | β-catenin, TCF, LEF, hbp1, PCNA, Cyclin D1, c-Myc, SNAIL, VIM, MMP-2, MMP-9 |
105 | |
| Blocking EMT, and inhibits the invasion and migration of tumor cells in vivo and in vitro | HT29, RKO Balb.c nude mice (RKO) |
5, 10, 20 μM for cell; 40 mg/kg for mice | E-cadherin | VEGF, MMP-7, MMP-9, N-cadherin, SNAIL, N-catenin, TCF4, Cyclin D1, c-Myc | 106 | |
| Inhibit the growth, adhesion and migration of HCT116 cells, and inhibit the growth of xenograft tumor | HCT116; BALB/c nude mice (HCT116 cells) | 15, 30, 60 μg/ml for cell; 20, 40, 80 mg/kg for mice | VEGFR2, p-PI3K, p-AKT | 107 | ||
| Anti-inflammatory | Inhibit intestinal inflammation related to cancer and prevent the occurrence and progression of intestinal tumors | SW620, HCT116 AOM/DSS model mice |
10, 20 ,40 μM for cell; 50 mg/kg for mice | TNFa, IL1a/b, IL6, CCL2, CXCL5, COX-2, iNOS | 108 | |
| Breast cancer | ||||||
| Cytotoxicity | Inhibit the growth of cancer cells, induce the production of lipid droplets, and promote the mature differentiation of BC cells | MDA-MB453, BT-483, MDA-MB231, MCF-7 | 40 μM | HER-2/neu | 111 | |
| Apoptosis | Apoptosis is induced by mitochondrial signaling pathway | BCap-37 | 20, 50 μM | Bax, Cyt-c | Bcl2 | 115 |
| Apoptosis is induced by the destruction of mitochondrial signaling pathways in cells | BCap-37 | 20, 50 μM | P21, P53 | IGF-2 | 116 | |
| Induce DNA breakage and DNA fragmentation, and induce tumor cell apoptosis and cycle arrest through internal and external pathways | MCF-7 | 30 μg/ml | Fasl | Mcl-1, Cyclin D, c-MYC | 117 | |
| Inhibition of ERα pathway and PI3K/Akt pathway inhibited the proliferation of tumor cells and induced apoptosis | MCF-7, MDA-MB-231 | 20, 40 μM | ERα, Cyclin D1, BCL2, p-MAPK, p-AKT | 118 | ||
| Induce growth inhibition and apoptosis of human breast cancer cells | Bcap-37, ZR-75-30 | 10, 40 μM | c-Caspase 3, PARP, p53, Bax | Bcl-2 | 119 | |
| It exerts anti-tumor activity by activating AhR-CYP1A1 signaling pathway | MCF-7 | 25, 50, 100 μM | AHR, CYP1A1 | 120 | ||
| Chemotherapy resistance | Increase tumor sensitivity to paclitaxel and improve tumor drug resistance | MDA-MB-361, MDA-MB-453, BT-483, SKBr-, BT474, MDA-MB-231, MCF-7; Nu/nu mice (MDA-MB-361 or MDA-MB-231) |
20 μM for cells; 40 mg/kg for mice |
HER-2/neu | 113 | |
| Inhibit DNA damage repair and reverse multidrug resistance of tumor cells | MCF-7/Adr MCF-7 |
20 μg/ml | ERCC1 | 123 | ||
| Enhance apoptosis of breast cancer cells, resulting in cell senescence | MCF-7 | 20 μM | P21, P16, P27, ROS | E2F1, NRARP, GSH | 124 | |
| It increased the sensitivity of BC cells to doxorubicin, inhibited cell proliferation and induced DNA damage | MDA-MB-231, MCF-7 | 110 μM | γH2A, P53 | AKT1, XRCC1, PARP1, RAD51 | 125 | |
| Inhibition of metastasis and invasion | Inhibition of tumor cell metastasis by targeting HER-2/ neu | MDA-MB453, MCF-7 | 20 μM | HER-2/neu | 112 | |
| Inhibits the invasion of breast cancer cells in vivo and in vitro | MDA-MB-435s, MDA-MB-468 | 1, 10 μM | P2X7R | 126 | ||
| It can reduce the infiltration of macrophages, reduce the migration of macrophages to tumor environment, inhibit the polarization of macrophages M2, and inhibit the lung metastasis of tumor | 4T1 cell, EO771 BALB/c or C57BL/6 mice (4T1 cell and EO771 cell) |
10, 100 μM fo cells; 40 mg/kg for mice | p-STAT6, C/EBPβ | 129 | ||
| Inhibit the EMT of breast cancer cells and the formation of cancer stem cells, and prevent the recurrence of lung metastasis after breast cancer | EO771, 4T1, MCF7, MDA-MB-231 C57BL/6, BALB/c, NOD-SCID mice (EO771, 4T1, MCF7, MDA-MB-231) |
40 mg/kg | TGF-β1 | 130 | ||
| Inhibit macrophage infiltration and m2-like polarization, block their migration and adhesion to the tumor site, inhibit tumor growth, increase T cell activation, and reduce tumor angiogenesis | 4T1, EO771 C57BL/6 and BALB/c mice (4T1 cells, EO771 cells) |
0-100 μM for cells; 40 mg/kg for mice | iNOS | MMP 2, MMP 9, JMJD3, Arg1, p-STAT6, C/EBPβ, CSF-1, MCP-1, ICAM1, Thy1 | 131 | |
| Inhibit TGF-β and inhibit the EMT and migration of cancer-associated fibroblasts | BT20 | 30 μM | E-cadherin | β-catenin, VIM, MMP-2 | 132 | |
| Inhibit tumor cell migration in vivo and in vitro, and inhibit lung metastasis of breast cancer in nude mice | MDA-MB-231 athymic nude mice (MDA-MB-231) |
10, 20, 40, 80 μM for cells; 40 mg/kg for mice | MMP 2, MMP 9, uPA, uPAR, p38, p-ERK1/2 | 133 | ||
| Inhibit CCL5 secretion of adipocytes, inhibit EMT of tumor cells, inhibit tumor growth and lung liver metastasis | MDA-MB-231, MDA-MB-453 Balb/C nude mice (MDA-MB-231) |
50 μM for cells; 40 mg/kg for mice |
GSK3, E-cadherin | CCL5, p-AKT, β-catenin, vimentin, SNAIL, p-CCR5, MMP2, MMP9 | 134 | |
| Anti-angiogenesis | Tumor cell - induced metastasis and angiogenesis were inhibited in vitro and in vivo | EA.hy 926; NOD/SCID mice/ SD rats (MDA-MB-231) | 10, 20, 40 μM for cells; 40, 80 mg/kg for mice | MMP9, MMP13, p-Runx2, p-VEGFR-2 | 135 | |
| Inhibit angiogenesis and tumor growth | MDA-MB-231, 4T1; BALB/c NOD-SCID mice; and, BALB/c mice | 5, 10, 20 μM for cells; 10 mg/kg | SerRS, HOXB1, PCK1, UCP1, NCOR2, HDAC3 | VEGFA | 136 | |
| Pancreatic cancer | ||||||
| Cytotoxicity | Promote the demethylation of tumor suppressor genes and inhibit the growth of pancreatic cancer cells | PANC-1 | 10,20,40μM | P16, RASSF1A, ppENK | 5mC, DNMT1, DNMT3a | 145, 146 |
| Inhibit tumor cell growth, angiogenesis and glycolysis, reduce cancer cachexia | AsPC-1, BxPC-3, HPAF-2, MiaPaCa2, Panc-1; Male athymic Balb/c mice (MiaPaCa2) | 100 μM for cells; 50 mg/kg for mice | HIF-1α, Glut1, HK-II, PFK- 1, VEGF, caveolin-1, p-Akt, p-ERK1/2, PHD-2 | 148 | ||
| Apoptosis | It plays anti-tumor proliferative role by inducing apoptosis | Mia Paca-2, BxPC-3, panc -1, L3.6pl | 12.5, 25, 50 μM | PARP | 149 | |
| Induced apoptosis of pancreatic cancer cells and increased sensitivity of pancreatic cancer to gilotrif | PANC-1, BxPC-3 BALB/c nude mice (PANC-1) |
30, 60, 90 μM for cells; 50 mg/kg for mice | c-Caspase 3, bax | p-STAT3, Bcl2, EGFR | 153 | |
| Chemotherapy resistance | Enhanced the antitumor activity of gemcitabine | Mia Paca-2, BxPC-3, panc -1, L3.6pl | 40, 80 μM | c-Caspase 3, PPAR | Survivin, b-catenin | 154 |
| Enhanced the antitumor activity of gemcitabine | SW1990, SW1990/GZ | 20 μM | NF-κB | 155 | ||
| Increased sensitivity of tumor cells to gemcitabine | SW1990; BALB/c female mice (SW1990) | 40 μM for cells; 40 mg/kg for mice | Bax, CytC, c-Caspase 3 | Bcl-2 | 156 | |
| Improve chemotherapy resistance of tumor cells to Gemcitabine | BALB/c female mice (SW1990) | 40 mg/kg for mice | Bax, c-Caspase 9, c-Caspase 3, CytC | p-AKT, Bcl-2, NF-κB p65 | 157 | |
| Enhanced the antitumor activity of gemcitabine | SW1990; Female BALB/c nude mice (SW1990) | 40 μM for cells; 40 mg/kg for mice | XIAP, NF-Κb p65 | 158 | ||
| Enhanced the antitumor activity of gemcitabine | BaLB/c male mice (Panc-1) | 40 mg/kg | c-Caspase 9, c-Caspase 3 | XIAP, NF-κb p65, Survivin | 159 | |
| Increased sensitivity of tumor cells to gemcitabine | SW1990, SW1990/GZ | 10, 20, 40, 80, 160 μM | Bax, Cytc, c-Caspase 9, c-Caspase 3 | MDR-1 (P-gp), NF-κB p65, Bcl-2 | 160 | |
| Increased sensitivity of resistant cells to gemcitabine treatment | Bxpc-3/Gem | 40 μM | MDR-1 (P-gp), NF-κB p65, XIAP, survivin | 161 | ||
| To enhance the therapeutic effect of gemcitabine and improve the drug resistance of tumor cells to gemcitabine | BALB/c mice (PANC‑1) | 40 mg/kg | MDR-1(P-gp), MRP1, MRP5 | 162 | ||
| Reversal of gemcitabine resistance in pancreatic cancer cell lines | pan -1/Gem, MIAPaCa-2/Gem | 40 μM | c-Caspase 3, c-Caspase 9, IκB-α | Survivin, XIAP, NF-κB p65, IKKβ, P-gp | 163 | |
| Inhibition of metastasis and invasion | Inhibit metastasis of pancreatic cancer | SW1990; BALB/c nu/nu mice (SW1990) | 10, 20, 40 μM for cells; 20, 40 mg/kg for mice | c-Caspase-3 | MMP9, NF-κB p65, survivin | 164 |
| Inhibit EMT and invasion of pancreatic cancer cells, and inhibit hepatic metastasis of pancreatic cancer | SW1990; Nude mice (SW1990) | 20, 40 μM for cells; 50 mg/kg for mice | miR-1271, E-cadherin | ZEB1, TWIST1 | 166 | |
| Anti-angiogenesis | Regulating the expression of angiogenesis related factors can promote apoptosis and inhibit angiogenesis | SW1990, Panc-1, ECs Female athymic BALB/c nu/nu mice (Panc-1 cells) |
40 μM for cells; 1 mg/mouse for mice |
NF-κB p65, VEGF, MMP 2, MMP 9, p-eNOS | 167 | |
| Inhibits angiogenesis in pancreatic cancer | SW1990; Female athymic BALB/c nu/nu mice | 20, 40, 80 mg/kg for mice | miR-20b, Smad4, TβRI, TβRII | TGF-β1, Angptl 4, miR-155, miR-210 | 168 | |
| Leukemia | ||||||
| Cytotoxicity | Induction of ROS production, improve the sensitivity of tumor cells to arsenic trioxide | C8166 cells, MT2, II85, LAF, Jurkat | 10 μM | PARP, ROS | Akt, Jun D, JAB 1 | 171 |
| Apoptosis | Apoptosis of HL-60 cells was induced by ROS independent method | HL-60 | 40 μM | Caspase3, PARP, D4-GD1, | Mcl-1, | 172 |
| G0/G1 phase arrest was induced and apoptosis was induced | K562 | 20, 40, 80, 100 μM | c-myc | 173 | ||
| Apoptosis of tumor cells was induced by caspase signaling pathway | K562 | 20, 30, 40 μM | c-Caspase 3, c-Caspase 9, c-Caspase 8 | 174 | ||
| Inhibit the growth of tumor cells in vivo and in vitro and induce their apoptosis | BALB/c nude mice (K562) | 25, 50, 100 mg/kg | Bax | Bcl2 | 175 | |
| Cause tumor regression and induce cell apoptosis | K562; Male BALB/c nude mice (K562) | 25, 50, 100 μM for cells; 20, 50 mg/kg for mice | Bax, c-Caspase 3, c-Caspase 8, c-Caspase 9 | Bcl2 | 176 | |
| Induce G0/G1 phase arrest and apoptosis | U937 | 30, 60, 90 μM | Bax | Bcl2, CPP32 | 177 | |
| Apoptosis of human myeloma cells was significantly induced by inhibition of McL-1 | RPMI8226, U266, IM-9 | 10, 20, 50 μM | c-Caspase 3, c-Caspase 9 | p-JAK2, p-STAT3, Mcl-1, Histone H2 | 178 | |
| Inhibit hL-60 cell proliferation, induce G0/G1 phase arrest, and induce apoptosis | HL-60 | 10, 20, 40 μM | p-AKT, p-IκB-α, p-p65, p-mTOR | 179 | ||
| Decreased cell mitochondrial membrane potential, caused cell G0/G1 phase arrest, induced apoptosis, improved doxorubicin resistance | HL-60 (ADR) | 10, 20, 40 μM | c-Caspase-3 | Bcl-2, c-myc、 | 180 | |
| Induction of tumor cell apoptosis, overcoming all-trans retinoic acid resistance | NB4, MR2, primary AML | 10, 30, 60 μM | c-Caspase 9, c-caspase 3, PARP | Bcl-2, RARα, p-Akt, p-mTOR, 4E-BP1, p70S6K | 181 | |
| Inducing apoptosis of tumor cells in vivo and in vitro | K562; BALB/c nude mice (K562) | 25, 50, 100 μM for cells; 25, 50, 100, 120 mg/kg for mice | PTEN | PI3K, AKT, BCR-ABL | 182 | |
| Decreased cell viability, induced DNA damage, decreased ΔΨm levels, and induced apoptosis through endoplasmic reticulum stress (ER) and mitochondrial pathways | WEHI-3;Male BALB/c mice (WEHI-3) | 25, 50, 100 μM for cells; 5, 10 mg/kg for mice | ROS, c-Caspase 8, c-Caspase 9, Cyt-c, c-Caspase 7, c-Caspase 12, c-Caspase 3, PARP, Apaf-1, AIF, Endo G, GADD153, GRP78, ATF-6α, Bax, Bad | Bcl2, Bcl-xl | 183 | |
| Chemotherapy resistance | Increase the sensitivity of resistant cells to chemotherapeutic drugs | K562/ADM | 6.1, 17.6, 33.2 μM | MDR1, P-gp | 187 | |
| The doxorubicin resistance of K562/ADM cells was reversed | K562/ADM | 50, 100, 200 μM | P-gp | 188 | ||
| Increased cytotoxicity of 3'-azido-3'-deoxythymidine to tumor cells | K562 | 8, 16, 32 μM | EGR1 | β-catenin | 189 | |
| The chemosensitivity of AML cells to ARA-C was increased, and the survival rate of AML transplanted tumor mice was improved. | HL-60/ADR; BALB/C-nude mice (HL-60/H3) |
5, 10 μM for cells; 20, 40 mg/kg for mice | PARP, c-Caspase 9, c-Caspase 3, Bax | Bid, p-Akt, p-mTOR, p-4E-BP1, p-ERK1/2, p-P70S6K, Bcl2 | 190 | |
| Enhanced the sensitivity of drug-resistant cells to imatinib, inhibited cell proliferation and induced cell apoptosis | K562, G01 | 20, 40 μM | c-Caspase-3, c-PARP | p-Bcr-Abl, c-MYC, MCL-1, Bcl-2, p-STAT5, Src, p-Src | 191 | |
| Cervical cancer | ||||||
| Cytotoxicity | It inhibited the proliferation of HeLa cells and reduced the tumor growth of tumor-bearing mice | HeLa; Female old athymic nude mice (HeLa) | 1, 10, 25 μM for cells; 25 mg/kg for mice | p-STAT1, p-STAT2, IFNAR1, p-TYK2 | p-STAT3 | 193 |
| Apoptosis | Inhibits DNA synthesis and induces apoptosis through the mitochondrial pathway | HeLa, Ca Ski, ME-180, Bu 25TK | 25, 50 μM | c-Caspase 3, c-Caspase 9 | 194 | |
| Induce tumor cell apoptosis | HeLa | 40 μM | p-JUN | p-AKT, mTPR, p-PTEN, P-MAPK | 195 | |
| Apoptosis is induced by internal mitochondrial and external death receptor pathways | HeLa | 20, 40, 80 μM | caspase-3, caspase-9, caspase-8, Fas, Fasl, FADD, Cyt-c, Apaf-1 | JAK2, STAT3, Mcl-1 | 196 | |
| Induce apoptosis and autophagy, inhibit cell cycle, inhibit angiogenesis | Hela, JAR, HO-8910 | 5, 10, 15 μM | Atg12-Atg5, Beclin-1, c-Caspase-9, c-Caspase-3 | Cyclin D1, Cyclin E1, VEGF, VEGFR-2, Bcl2, Mcl-1, MAPLC3 | 197 | |
| Autophagy | By increasing the number of lysosome, the number of autophagic vacuoles and the activity of lysosome hydrolase can induce lysosome membrane damage and promote the death of tumor cells | HeLa | 1, 15, 30, 60, 100 μM | Cathepsin D, Cathepsin L | 198 | |
| To improve the toxicity of photodynamic therapy to cervical cancer cells and increase the activity of caspase-3 and autophagy | SiHa, CaSki | 30 μM | c-Caspase 2, ROS, ATF2, AURKA, AURKC, BIRC5, CDK1, CDK7, GSTP1, HDAC4, HIF1A, HSP90AA1, MDM4, MTOR, PARP4, PIK3C2A, PIK3C3, PIK3CA, PLK2, PLK4, RHOA, RHOB, TNKS, TOP2B |
CTSS, ESR1 | 199 | |
| Inhibition of metastasis and invasion | Inhibit the invasion, migration and stem cell characteristics of tumor cells and reverse EMT | SiHa, Hela | 20 μM | Bax | TGFRII, Smad2, Smad3, Smad4, CyclinD1, p21, Pin1, p15, p16, CDK6, p27, SNAIL, Slug, Bcl 2, β-catenin | 200 |
| Ovarian cancer | ||||||
| Cytotoxicity | Induced DNA damage and inhibited cell proliferation | A2780 | 1 μM | 202 | ||
| Inhibit cell viability, and reduce cell viability and colony formation of A2780 cells | A2780 | 20 μM | FOXD3, miR-199a | TGF-β2 | 203 | |
| Apoptosis | Inhibit tumor cell proliferation, induce apoptosis and inhibit invasion | SKOV3, HO8910 | 20, 60 μM | surviving | 204 | |
| Inhibition of metastasis and invasion | Inhibit EMT, migration and invasion of tumor cells, and inhibit metastasis of ovarian cancer | A2780, SK-OV-3 | 20, 40, 80 μM | E-cadherin, Claudin | N-cadherin, vimentin, p-GSK-3β, ILK, β-Catenin, and Slug | 205 |
| Inhibit EMT and invasion of tumor cells | A2780, SK-OV-3 | 10, 20, 40 μM | E-cadherin, keratin | N-cadherin, Vimentfin, MMP 9, MMP 2, ZEB1, p-GSK-3β, β-Catenin | 206 | |
| Inhibit the proliferation, migration and invasion of ovarian cancer cells | A2780, SK-OV-3; Female BALB/C nude mice (SK-OV-3) |
20 μM for cells; 50 mg/kg for mice | E-cadherin | Slug, MMP 9, Vimentin, ILK | 207 | |
| Chemotherapy resistance | Induced apoptosis and increased sensitivity of drug-resistant cells to paclitaxel | A2780 | 10 μM | c-Caspase 3 | P-gp, XIAP, MDR-1, surviving | 208 |
| Increase the sensitivity of drug-resistant cancer cells to cisplatin | COC1 | 50 μM | ROS | MRP1 | 209 | |
| Inhibit the growth of cancer cells and enhance the sensitivity of drug-resistant cells to cisplatin therapy | SKOV3, OVCAR3, MDH2774, and ES2 | 0-50 μM | AURKA | 220 | ||
| Head and neck neoplasm | ||||||
| Cytotoxicity | Induced cell DNA damage | SCC-4 | 25, 50, 100 μM | ATM, ATR, 14-3-3σ, BRCA1, DNA-PK, MGMT | 212 | |
| Inhibit the growth, proliferation and cell division cycle of human oral squamous cell carcinoma cells | Tca8113 | 10, 20, 40, 80 μM | CDK2, Cyclin E, P21 | 216 | ||
| Inhibition of cell cycle markers play an anti-proliferation role | Buccal mucosa of hamsters treated with DMBA | 50 mg/kg | Cyclin D1, PCNA, CDK4, CDK6, survivin | 217 | ||
| Prevention of DMBA - induced hamster buccal pouch carcinogenesis by proapoptotic and antioxidant effects | Buccal mucosa of hamsters treated with DMBA | 50 mg/kg | P53, Bid, Bax, c-Caspase 3, c-Caspase 9 | Bcl-xl | 218 | |
| Apoptosis | Increased ROS level leads to DNA damage, endoplasmic reticulum stress and apoptosis of tumor cells | SCC-4 | 30 μM | ROS, c-Caspase 9, c-Caspase 3, P21, Chk2, Cyto c, AIF, GADD153, GRP78, Bax | Cyclin B1, Cdc2, Bcl2 | 211 |
| Apoptosis was induced by the production of ROS and the decrease of pH | EC-109 | 2.5, 5, 10, 20 μM | ROS | Intracellular PH | 214 | |
| Tumor cell death was induced by apoptosis and necrosis | HSC-3 | 46.3, 92.5, 185 μM | Bax, c-Caspase-9, c-Caspase-3 | Bcl2, p-AKT | 219 | |
| Induce cell cycle arrest and apoptosis | Human nasopharyngeal carcinoma cells (CNE-2Z) | 50 μM | chloride channel | 220 | ||
| Inhibit the proliferation of thyroid papillary carcinoma cells, induce cell cycle arrest and apoptosis | TPC‑1; Balb/c female nude mice (TPC‑1 cells) |
10, 25, 50 μM for cells; 40 mg/kg for mice | p-AMPK, c-Caspase 3, Cyclin D1 | PCNA, p-MEK, p-ERK1/2 | 222 | |
| Inhibition of metastasis and invasion | Inhibit tumor cell migration and invasion | SCC-4 | 15,30μM | TIMP-1 | MMP 2, u-PA, FAK, NF-κB p65, p-AKT, p-P38, p-JNK, p-ERK1/2 | 213 |
| Inhibit EMT of tumor cells and inhibit migration and invasion | FaDu, HEK-293T, OECM-1; Severe combined immunodeficient (SCID) mice | 5 μM for cells; 50 mg/kg for mice | E-cadherin, p-GSK-3β | TEIST1, Vimentin, p-AKT | 215 | |
| Inhibit tumor angiogenesis and lung metastasis | 8505c, SW1736 Balb/c nude mice (SW1736) |
10, 15, 20, 25 μM for cells; 100 mg/kg for mice | TRAF6, HIF1α, VEGF, CD147, MMP 9 | 221 | ||
| Glioma | ||||||
| Inhibition of metastasis and invasion | The invasion of hyaluronic acid (HA) induced glioma cells was inhibited | Hyaluronic acid (HA)-induced invasion of human glioma cells. | 40 μM | MMP2, MMP9, p-FAK, p-ERK1/2, p-Akt, p-PKB, AP-1, NF-kB-p65 | 223 | |
| Inhibit cell migration and intracellular glycolysis | U-87 MG or ΔFBP1 U-87 MG male BALB/c athymic nude mice (U-87 MG or ΔFBP1 U-87 MG) |
20, 40 μM for cells; 40 mg/kg for mice | E-cadherin | FBP1, Vimentin, Fibronectin | 224 | |
| Apoptosis | Apoptosis of glioma stem cells was induced, the invasiveness of glioma stem cells was decreased, and the sensitivity of glioma stem cells to ionizing radiation was increased | X01 and X03, and CSC2 | 5 μM | Hsp90 | b-catenin, p-STAT3, p-Akt, SNAIL, slug, p-EGFR | 225 |
| The proliferation of U251 cells was inhibited and apoptosis and necrosis were induced | U251; BALB/C nude mice (U251) |
10, 20, 40 μM for cells; 20, 40, 80 mg/kg for mice | TNF-α, RIP 1, RIP 3, MLKL, c-Caspase-3 | Caspase 8 | 226 | |
| Neuroblastoma | Inhibit the migration and invasion of tumor cells in vitro | SH-SY5Y | 10, 25 μM | GRB2, RhoA, NF-kB p65, HIF-1a, VEGF, FAK, Ras, COX2, p-p38, p-JNK, MMP2, MMP9, MMP7 | 227 | |
| Trigger caspase cascade signaling pathway and induce tumor cell apoptosis | IMR-32 | 20 μM | Ca2+, ROS, p53, p21, c-Caspase-9, c-Caspase-3 | 228 | ||
| Prostatic cancer | Inhibit the growth of tumor cells and prolong the survival time of tumor mice | LNCaP, PC3, DU-145 Male athymic nude mice (PC3) |
10, 20, 40 μM for cells; 40 mg/kg for mice | PARP | AR, PSA | 229 |
| Inhibit cell proliferation and induce cell apoptosis through mitochondrial pathway | LNCaP, PC-3 | 10, 20, 30, 40 μM | p53, p21, Bax, c-Caspase 3, c-Caspase 9 | Bcl-2, AR, PSA | 230 | |
| Enhance anti-tumor effect of cisplatin in vivo and in vitro and reverse drug resistance | DU-145; BALB/c-nu/nu mice (DU0145) | 50 μM for cells; 50 mg/kg for mice | ROS | MDR1, HIF-1 | 231 | |
| Inhibit tumor growth | LNCaP, PC-3 | 50 μM | ROS, LRP1 | AR | 232 | |
| Inhibit the migration and invasion of tumor cells | DU145 | 100 μM | CXCR4, HER2, NF-Κb p65 | 54 | ||
| Inhibit the growth of tumor cells and induce cell cycle arrest and apoptosis | PC-3 | 10, 20, 40, 60, 80 μM | Notch1 | Jagged1, VEGF, bFGF | 233 | |
| Bladder cancer | Reverse the transformation of cancer epigenetics to normal epigenetics, and inhibit the occurrence of tumors | T24, TSGH8301, MBT24 | 40, 80 μM | H3K27me3 | HBP17, FABP4, pH3Ser10 | 234 |
| Improve cisplatin resistance of tumor cells in vitro and in vivo | T24, J82; BALB/cnu/nu mice (T24) | 20 μM for cells; 50 mg/kg for mice | ROS | MRP1 | 235 | |
| Lymphoma | Mitochondrial apoptosis pathway of Dalton's lymphoma (DL) cells was induced in vivo | Inbred AKR strain mice (DL cells) | 40 mg/kg | H2O2, Bax, Cyto c, SOD2, SOD1 | Bcl2, GPx | 236 |
| It can reduce the survival rate of tumor cells, induce apoptosis and increase the sensitivity of tumor cells to doxorubicin | Raji | 6.25, 12.5, 25, 50 mg/kg | c-Caspase 3, c-Caspase 9, PARP, DNMT3A | p53, UHRF1 | 237 | |
| Inhibit cell proliferation and induce cell apoptosis | SU-DHL4 | 10, 20, 40 μM | p-PI3K, P53, p-AKT | 238 | ||
| Gallbladder carcinoma | Induce apoptosis and improve the sensitivity of tumor cells to chemotherapy | SGC996; BALB/c-nu/nu mice (SGC996) | 50 μM for cells; 50 mg/kg for mice | ROS | GSH, MRP1 | 241 |
| Cisplatin-induced apoptosis of gallbladder carcinoma cells is promoted in a ROS dependent manner | SGC996; BALB/c-nu/nu mice (SGC996) | 50 μM for cells; 50 mg/kg for mice | ROS | surviving | 242 | |
| Osteosarcoma | In vitro and in vivo anti-angiogenesis of osteosarcoma | SOSP-9607, MG63, SAOS-2; Male BALB/c nude mice (SOSP-9607, MG63, SAOS-) | 2.5 μM for cells; 0.3 mg/kg for mice |
SIRT1 | VEGF, H4-K16AC | 243 |
| Reduce the radiation resistance of tumor cells and promote cell apoptosis | MG63 | 15, 30, 45, 60 μM | c-Caspase 3 | Shh, bcl2, Gli 1 | 244 | |
| Apoptosis is induced by mitochondrial pathway and endoplasmic reticulum stress | U2OS | 120 μM | ROS, GRP78, CHOP, c-Caspase 4 | 245 | ||
| Inhibit tumor cell proliferation and synergistic anti-tumor with cisplatin | MG-63 | 10 μM | Nrf2 | 246 | ||
| Skin cancer | Inhibit skin tumor formation in mice | ICR mice skin tumors induced by 7,12-dimethylbenz[a]anthracene as an initiator and 12- O-tetradecanoylphorbol-13-acetate (TPA) | / | 247 | ||
| It induced the disorder of cell redox balance and accelerated cell apoptosis | B16F10; C57BL6J mice (B16F10) | 30 μM for cells; 5 mg/kg for mice | 8-OH-dG, MDA, ROS, c-PARP, Drp1, Bax, | IDH2, p-4EBP1, p-P38, p-ERK1/2, OPA1 | 248 | |
| Inhibit the proliferation and migration of tumor cells and induce G2/M phase cycle arrest | B16-F10; C57BL/6 (B16-F10) | 20, 50 μM for cells; 50 mg/kg for mice | CD155 | 249 | ||
| Inhibit glycolysis of tumor cells and inhibit their proliferation | B16F10 | 4, 8 μM | p-AMPK | P53, AMPKα, ATP | 250 | |
| Inhibit the growth, migration and invasion of melanoma cells | B16F10, A375 | 20, 40, 60 μM | Bax | β-catenin, c-Myc, TCF, Bcl2, MMP2, MMP9 | 251 | |
| Gastric carcinoma | Induction of anoikis, a detachment-initiated apoptosis, in tumor cells, and increases the antitumor effect of arsenic trioxide | SGC-7901 | 5 μM | ROS, c-Caspase 3 | RhoA | 252 |
| The proliferation of SGC-7901 cells was inhibited and apoptosis was induced | SGC-7901 | 15, 30, 45, 60 μM | PRL-3 | 253 | ||
Resources of emodin
Emodin is a time-honored natural compound that was first reported from plants of the Rheum genus by von Warren de la Rue and Hugo Nuller in the year of 1857 23. Emodin, 3-methyl-1,6,8-trihydroxyanthraquinone, was reported to be the most ubiquitous natural anthraquinone in the natural world and can be found in many higher plants, lichens and even fungi 10, 11, 13, 24, 25. Emodin commonly exists abundantly in plants of the Polygonaceae family, such as Polygonum multiflorum, Rheum palmatum, Rheum tanguticum, Rheum officinale, Polygonum Cuspidatum, Rumex japonicus, Rumex hydrolapathum, Rumex scutatus, Rumex confertus, Rumex altissimus, Rumex crispus, Rumex stenophyllus, Rumex arifolius, Rumex patientia, Rumex sanguineus, Rumex brownii, Rumex pulcher, Rumex acetosa, Rumex conglomeratus, Rumex acetosella, Rumex nepalensis, Rumex maritimus, Rumex alpinus, Rumex palustris, and Rumex obtusifolius 11, 24, 26, 27. Additionally, emodin was also reported in plants in several other families, such as Aloe vera (Asphodelaceae), Acorus tatarinowii (Araceae), Cassia obtusifolia (Leguminosae), Cassia occidentalis (Leguminosae), Eriocaulon buergerianum (Eriocaulaceae), Dendrobium thyrsiflorum (Orchidaceae), Fibraurea tinctoria (Aristolochiaceae), Coptis chinensis (Ranunculacea), Scutellaria baicalensis (Lamiaceae), and Isatis indigotica (Brassicaceae) 10,13. Among these herbs and plants, Polygonum cuspidatum might possess the highest content of emodin, with contents ranging from 0.6% to 2.3%, and it is currently the primary resource for the isolation of emodin 28. The extraction of emodin from plants is inefficient and requires a large amount of plant material. Fortunately, the yield of emodin can be greatly increased through synthetic methods. Through biosynthetic pathway, emodin was synthesized by acetyl CoA carboxylase (ACC1), polyketo synthase (PKS), thiesterase (MβL-TE) decarboxylase (DC) and other enzymes, starting from Pyruvate 29. In addition, some efficient chemical synthesis methods also enrich the sources of emodin.
Chemical properties of emodin
The molecular formula of emodin is C15H10O5, and the molecular weight is 270.23. Emodin is commonly orange powder, and the melting point range is 256-257 °C. Emodin contains multiple hydroxyl and carbonyl groups in its structure, which can chelate with metal ions in biological target enzymes to form relatively stable chelates, which is also one of the important reasons for its wide biological activity. Emodin can be used as a reversible binding agent for DNA. Its aromatic ring plane structure can be embedded and superimposed between the double helix base pairs of DNA, combine with DNA, destroy the normal helix structure of DNA, and interfere with the role of DNA binding enzymes such as DNA topoisomerase and DNA polymerase. Simply put, emodin can capture the DNA-topoisomerase II lysable complex and form the emodin-DNA-topoisomerase terpolymer complex to stabilize it and interfere with the reconnection reaction of DNA breakpoints. The interference of emodin with DNA polymerase may not only inhibit the elongation of the DNA chain from 5' to 3' but also prevent DNA error correction by inhibiting the shearing process of mismatched residues (hydrolysis of phosphate diester bond). In conclusion, emodin can inhibit DNA replication and exert pharmacological activity through the chimerism of DNA. At the same time, emodin's DNA-damaging activity also involves electron transfer chemistry, which is consistent with other anthracycline antibiotics. Under the action of cytochrome CYP450 reductase, emodin undergoes one-electron reduction to produce superoxide (O2-), which in turn produces a large number of hydroxyl radicals, causing DNA chain breaks 30. It is well known that the typical anthraquinone tricyclic aromatic structure of emodin results in poor water solubility (0.07 mg/mL) and low bioavailability, which limits its clinical application 31. However, by chemically modifying the structure of emodin and introducing hydrophilic groups such as -OH and -NH-, it is possible to improve its water solubility and bioavailability. In 2004, Teich et al. reported on the synthesis and activity of emodin derivatives and found that emodin derivatives with different amine substitutions at four sites exhibited stronger antitumor fine activity than emodin itself 32. Shao et al. synthesized a series of quaternary ammonium derivatives of emodin by introducing quaternary ammonium salts at sites 6 and 3 of emodin, tested the biological activities of these compounds, and found that the derivatives had strong anti-proliferation ability against the HepG2 GC-823 AGS cell line but low toxicity against the HELF normal cell line 33. A recent study reported that the new semisynthetic anthraquinone derivatives with the NαFmoc-l-Lys and ethynyl group NαFmoc-l-Lys synthesized from emodin significantly increased the inhibition rate of HT-29 and HeLa cells 34. In addition, dosage form modification of emodin by pharmaceutical means can also improve the bioavailability and pharmacological activity of emodin. Di et al. used piperine as a bioenhancer to inhibit the glucuronidation of emodin in the liver and intestine to improve its bioavailability 35. Emodin was modified by arginine-glycine-aspartic acid (RGD) to produce a targeted liposome that can effectively inhibit vasculogenic mimicry (VM) channel formation and metastasis in breast cancer tumors and increase the antitumor effect of emodin 36. A recent study found that a transfer form of nano-emodin, a novel sonar-responsive nanomaterial, was synthesized to enhance the accumulation and penetration of nanoparticles and could be used as an effective intervention for the treatment of head and neck squamous cell carcinoma (HNSCC) 37. In addition, Krajnovi´c et al. used mesoporous silica as a carrier to transport emodin, which can ensure the release of emodin in the extremely acidic environment of the stomach, prevent the photodecomposition of emodin, and improve the anti-proliferation and pro-apoptotic effects of emodin on a variety of tumor cells 38.
Anticancer effects of emodin
Lung cancer
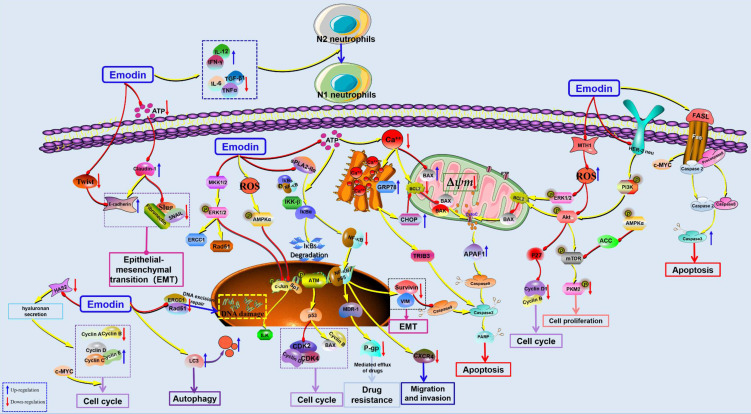
Lung cancer currently ranks as the most diagnosed malignant cancer type clinically and remains the leading cause of cancer-related death 1,2. Currently, increasing scientific evidence has demonstrated that emodin shows potential anticancer effects against lung cancers in vivo and in vitro via inhibition of cell proliferation and metastasis, induction of apoptosis and cell cycle arrest, and increase in ROS, etc. (Figure 2). In 1996, a study by Zhang and Hung indicated that emodin possesses significant anti-proliferative activity and could also reverse the drug resistance of HER-2/neu-overexpressing lung cancer cells to chemotherapeutic drugs (cisplatin, doxorubicin or VP16) via inhibition of the protein tyrosine kinase 39. Later, in 2010, Ko et al. reported that emodin exhibited strong cytotoxicity against lung cancer cell lines via suppression of excision repair cross-complementing gene (ERCC) 1 and Rad 51 40; similarly, He et al. reported that emodin exerted suppressive activity against the proliferation of A549 cells in a concentration-dependent manner, and the possible molecular mechanisms involved the downregulation of ERCC1 and Rad51 41. Another report by Ko et al. suggested that emodin could also strengthen cisplatin-induced cytotoxicity in lung cancer cells via ERCC1 downregulation and ERK1/2 inactivation 42. In addition, Tang et al. revealed that the possible mechanisms regarding the anticancer effects of emodin against lung cancer might involve the inhibition of ILK expression via an increase in the phosphorylation of AMPKα and ERK1/2 and the suppression of Sp1 and c-Jun 43. Recently, a research report by the same research team of Tang et al. demonstrated that emodin could not only inhibit cell growth but also induce cell cycle arrest at G2/M phase in A549 cells, and the potential mechanism is associated with activation of PPARγ and the AMPKα/MEK/ERK signaling pathway, downregulation of Sp1 and upregulation of IGFBP1 44. Li et al. found that emodin can induce G1/G0 phase arrest of lung cancer cells by regulating the secretion of hyaluronic acid 45.
Figure 2.
The potential mechanisms for antitumor effect of emodin against lung cancer.
Furthermore, the induction of apoptosis was also observed as an important reason corresponding to its anticancer effects against lung cancer. In 2001, Lee reported that emodin-induced cell death is closely associated with mitochondria-dependent apoptosis in CH27 cells 46. Later, Li et al. reported that emodin induced A549 cell growth inhibition and apoptosis through extrinsic apoptotic pathways and induction of cell cycle arrest 47. Another research study showed that emodin could reduce the viability of A549 cells in a concentration-dependent manner through the induction of apoptosis via the activation of ER stress and the TRIB3/NF-κB pathway, and the antitumor effect of emodin was confirmed in an A549 tumor-bearing BALB/c nude mouse model in vivo 48. It has been reported that emodin can also induce cancer cell apoptosis by enhancing intracellular ROS levels. Lai et al. revealed that emodin could induce mitochondria-dependent apoptosis in A549 cells by activating the ROS-elicited ATM-p53-Bax signaling pathway 15. Similar to the works of Lai et al., the findings of another study by Su et al. suggested that emodin-induced apoptosis in A549 cells is closely correlated with emodin-mediated ROS generation and reduced ∆Ψm 49. Previous studies have also found that emodin can significantly induce apoptosis of A549 cells 50. The 18 kD human MutT homolog 1 (MTH1) protein, also known as Nudix hydrolase 1 (NUDT1), is a major intracellular pyrophosphatase that prevents oxidative nucleotide precursors from misfitting into genomic DNA, preventing damage and cell death 51. A recent study found that emodin can act as an MTH1 inhibitor to induce ROS production and promote DNA damage and apoptosis of tumor cells 52. Zhang et al. also found that emodin could significantly inhibit the proliferation of NSCLC in vitro and in vivo but had low cytotoxicity to normal lung cell lines. The mechanism was related to the inhibition of mTOR and AKT and the activation of the AMPK pathway 53.
In 2012, He et al. reported that after treatment with emodin, some typical autophagosomes could be observed in A549 cells 41; recently, Haque et al. indicated that emodin-induced autophagy has a close relationship with mutation-independent p53 aggregation 16. Metastasis is undoubtedly one of the worst outcomes of cancer development. In 2012, emodin suppressed CXCL12-induced A549 cell migration and invasion, and the molecular mechanism involved in the downregulation of CXCR4 and HER2 expression 54. In addition, Wang et al. demonstrated that emodin suppressed ATP-induced proliferation and migration by suppressing the P2Y receptor and Ca2+-dependent NF-κB pathway 55. Furthermore, Ying et al. suggested that emodin can inhibit epithelial-mesenchymal transition (EMT), cell proliferation, migration and invasion of lung cancer cells, as well as effectively reverse the resistance of H69AR to Dox. The possible mechanism is involved in suppressing the expression of Twist, Snail & Slug and inhibiting the activation of NF-κB 56. In addition, Peng et al. found that emodin can be used as a chemotherapy sensitization agent to enhance the antitumor effect of cisplatin 57. Similarly, emodin can also increase the antitumor effect of paclitaxel in vivo and in vitro 58. Teng et al. also found that emodin can reverse cisplatin resistance in A549 cells by regulating the NF-κB pathway 59. Li et al. found that emodin can selectively inhibit N2 neutrophils to prevent hypercoagulation and lung carcinogenesis 60. Recently, Zhang et al. reported that emodin could induce apoptosis in NSCLC cells, and the related molecular mechanisms are correlated with the downregulation of the sPLA2-Iia and NF-κB pathways 61.
Hepatocellular carcinoma
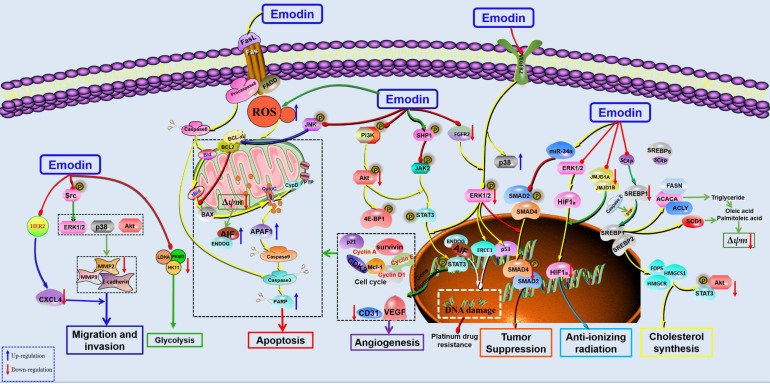
Hepatocellular carcinoma (HCC), one of the world's leading malignancies, accounts for an estimated 800,000 deaths according to a 2017 study 62. Currently, the common treatment methods for liver cancer include surgical resection and radiofrequency ablation 63. Unfortunately, the high recurrence rate and metastatic tendency of liver cancer make these treatments less effective. Although chemical inhibitors or biologic agents are available for clinical use, they are often limited by tolerance and adverse reactions in patients. Therefore, there is an urgent need for safe and effective new drugs to prevent and treat liver cancer 64. HCC is another type of malignant cancer that has been comprehensively investigated with emodin, and previous studies have suggested that emodin possesses remarkable anticancer properties against various subtypes of liver cancers through the induction of apoptosis and cell cycle arrest, inhibition of cancer cell metastasis, and enhancement of DNA damage (Figure 3). Hsu et al. found that emodin induced G2/M phase arrest in HCC cells by upregulating CYP1A1, CYP1B1, GDF15, SERPINE1, SOS1, RASD1, MRAS, Cyclin A, cyclin B, Chk2, Cdk2 and P27 while downregulating NR1H4, PALMD, TXNIP, Cdc25c, and P21 65. Yu et al. also revealed that emodin can induce intracellular ROS accumulation in HCC cells, leading to increased cytochrome C in the cytoplasm and finally resulting in the activation of caspase-8 and caspase-9. In addition, emodin increased p53 protein levels and decreased NF-kB/P65 in HepG2 cells, and emodin directly binds to the BH3 domain of Bcl-2 by forming a hydrogen bond with Ala146 residues 66. In addition, Xing et al. found the cytotoxic effects of emodin against HepG2 cells are related to many endogenous metabolites, such as oxidative stress and amino acid and energy metabolism disorders 67.
Figure 3.
The potential mechanisms for antitumor effect of emodin against HCC.
In 2002, Jing et al. selected 3 hepatoma cell lines, human hepatocellular carcinoma cell lines, Mahlavu, PLC/PRF/5 and HepG2, to investigate the anticancer effects of emodin. The results showed that emodin could effectively induce apoptosis in human hepatoma cell lines. Further studies have found that emodin promotes apoptosis by inducing the production of ROS, which leads to a decrease in mitochondrial transmembrane potential (MMOP) and then activates caspase-9 and caspase-3 to achieve DNA fragmentation. In this study, oxidative stress was also found to play a key role in the proapoptotic effect of emodin 68. Interestingly, similar results were found in the studies of Xion et al. and Dong et al. 69, 70. Zhang et al. also reported that emodin induced mitochondrial dysfunction and apoptosis in HepG2 cells by regulating the mitochondrial matrix protein cyclophilin D (CyPD), providing a new perspective for emodin against liver cancer 71. Signal transducer and activator of transcription (STAT) is a cytoplasmic transcription factor highly expressed in various cancers 72, 73. Studies have found that inhibition of STAT3 can inhibit the proliferation of HCC cells and induce apoptosis 74, 75, suggesting that STAT3 might be a potential target for HCC. Subsequently, emodin showed significant antitumor effects against several HCC cell lines (HepG2, PLC/PRF/5, Hep3B and C3A cells) by inhibiting the activation and nuclear transcription of STAT3. Furthermore, another interesting paper revealed that emodin could inhibit the growth of in situ tumors in the liver and induce apoptosis by suppressing the activation of STAT3. In addition, this study first found that emodin can upregulate SHP-1 in HCC cells to inhibit the JAK/STAT signaling pathway 76. In 2016, emodin was reported to induce apoptosis of HCC cells through death receptor-mediated apoptosis and mitochondrial-dependent apoptosis by inhibiting the PI3K/Akt and MAPK signaling pathways 77. Interestingly, in addition to its antitumor effect against liver cancer, emodin can also improve liver and kidney functions in tumor-bearing mice, suggesting that emodin may improve quality of life in mice implanted with tumors 78. In 2019, emodin was shown to inhibit HCC by inhibiting VEGFR2-Akt-ERK1/2 signaling and upregulating miR-34A 79. Recently, Zhu et al. reported that the anticancer effect of emodin might be related to inhibiting graft tumor lipid metabolism via downregulation of SREBP cleavage-activating protein (SCAP) 80. Another study also found that emodin could inhibit the triglyceride level and fatty acid desaturation of HCC cells and induce apoptosis of HCC cells via regulation of SREBP1 81.
Migration is one of the main reasons leading to poor prognosis of tumor treatment, and chemokine receptors play important roles in tumor migration 82, 83. Manu et al. studied the effects of emodin on the chemokine CXCR4 in HCC cells and found that emodin can suppress CXCL12-induced migration in HCC cells by downregulating CXCR4 84. Using a HCC cell line with high malignant invasion potential, MHCC-97H, Lin et al. found that emodin can inhibit the migration and invasion of HCC cells by inhibiting the expression of matrix metalloproteinases (MMPs)-2 and -9 and regulating the MAPK and PI3K/Akt signaling pathways 85.
The anticancer effects of chemotherapy drugs are related to DNA damage in tumor cells, which is closely related to ERCC1 86, 87. Currently, the fibroblast growth factor receptor (FGFR) 2, a gene upstream of ERCC1, has also attracted much attention in anticancer studies 88. Chen et al. found that emodin can act as a tyrosine kinase inhibitor to promote DNA damage in HepG2 cells. Furthermore, emodin could also downregulate the expression of ERCC1 and p-ERK in HepG2 cells, which could be blocked by knockdown of FGFR2, suggesting that FGFR2 might be a potential target for the antitumor effects of emodin 89. In addition, emodin has been used as a radiosensitization agent in the treatment of HCC. It has been reported that the combination of emodin and radiotherapy can regulate the cycle progression of HCC cells and induce apoptosis, and the mechanism is related to upregulation of cleaved-PARP1 and downregulation of JMJD1A and JMJD2B 90. In addition, emodin can also enhance the anticancer effect of sorafenib on liver cancer cells 91. Similarly, Wang et al. reported that emodin could enhance the effect of oxaliplatin to inhibit the migration and invasion of HepG2 cells by promoting the expression of E-cadherin 92.
Colon cancer
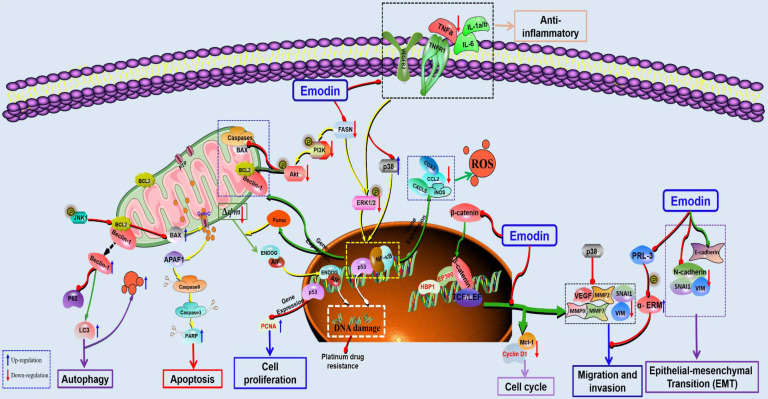
Colorectal cancer (CRC) is another common cancer in China with high mortality and mortality 93-95. Increasing research has indicated that emodin is an effective natural agent for the treatment of CRC with promising prospects (Figure 4). In 2012, a study reported that emodin can induce G0/G1 phase arrest and mitochondria-mediated apoptosis in the colon cancer cell line LS1034 and inhibit the growth of xenograft tumors in nude mice in vivo 96. Another study revealed that the antitumor effect of emodin might be related to mitochondria-mediated apoptosis by regulating oxidative stress and p38/p53/Puma 97-99. Fatty acid synthase (FASN), a key enzyme for fatty acid synthesis, is highly expressed in tumor tissues of CRC patients, so it is considered a promising target for CRC treatment. In 2017, Lee et al. found that emodin had antiproliferation and proapoptotic effects on HCT116 cells by inhibiting intracellular FASN enzyme activity and reducing intracellular free fatty acids 100. The PI3K/Akt pathway is involved in the regulation of cell proliferation, differentiation, apoptosis, glucose transport and other physiological functions and has become a major focus of medical attention. A study in 2018 reported that the antitumor effects of emodin against the CRC cell line Caco-2 might be related to regulating PI3K/Akt 101. In addition, emodin can reverse the drug resistance of 5-FU-resistant CRC by regulating the PI3K/Akt pathway 102. Wang et al. reported that emodin can induce autophagy to promote the apoptosis of CRC cells and emphasized that autophagy is a necessary condition for CRC apoptosis via ROS accumulation 103.
Figure 4.
The potential mechanisms for antitumor effect of emodin against colorectal cancer.
Han et al. found that emodin could inhibit the migration and invasion of DLD-1 cells by inhibiting the activity of PRL-3 phosphatase 104. The ability of tumor cells to invade and migrate allows them to separate from the tumor tissue and enter the fluid circulation, which is the main cause of cancer metastasis and the main problem encountered in clinical treatment. Increasing evidence has suggested that epithelial-mesenchymal transformation (EMT) is essential for the invasion and metastasis of cancer cells. EMT can be activated by a variety of signaling pathways, among which Wnt/β-catenin signaling is an important pathway. Previous studies have suggested that emodin can regulate EMT via the Wnt/β-catenin pathway and inhibit the proliferation and growth of CRC cells. Emodin can downregulate key regulatory factors (such as β-catenin and TCF7L2) and their downstream targets (including cyclin D1, c-Myc, snail, vimentin, MMP-2 and MMP-9) in the Wnt signaling pathway to inhibit the migration and growth of the CRC cell lines SW480 and SW620. In addition, ROS play a key role in emodin-mediated downregulation of Wnt signaling 105. Gu et al. found that emodin can inhibit the invasion and migration of CRC cells in vivo and in vitro via downregulation of VEGF, MMP-7 and MMP-9 in tumor cells. Further studies found that emodin inhibited EMT through inactivation of Wnt/β-catenin signaling 106. Dai et al. reported that downregulation of VEGFR2 is also an important mechanism for the inhibitory effects of emodin against adhesion and migration in CRC 107. In addition, Zhang et al. found that anti-inflammation is also important for the antitumor effects of emodin against CRC. Emodin can inhibit the recruitment of inflammatory cells in the tumor microenvironment, such as CD11b+ and F4/80+, reduce cytokines, such as TNFα, IL1α/β, IL6, CCL2, and CXCL5, and inhibit COX-2 and NOS2 to inhibit the active adhesion, migration and invasion of CRC cells 108.
Breast cancer
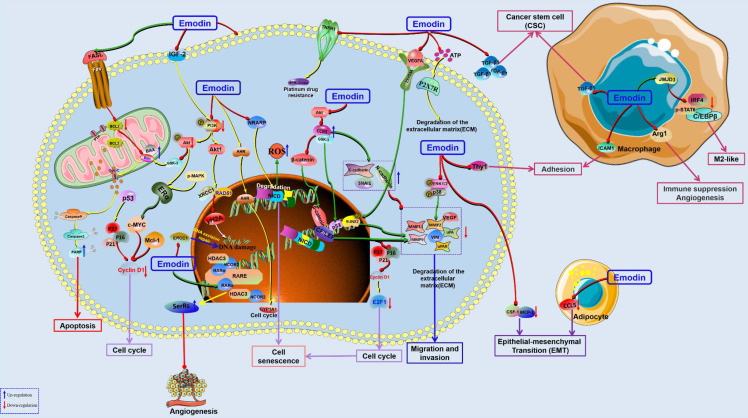
Breast cancer (BC) is one of the most common types of cancer in women and the second leading cause of cancer deaths worldwide 109, 110. Zhang et al. found that emodin could act as a tyrosine kinase inhibitor to inhibit the activity of HER-2/neu tyrosine kinase in MDA-MB453 cells, inhibit the growth of cancer cells, induce the production of lipid droplets, and promote the mature differentiation of BC cells 111. Furthermore, they also found that emodin can inhibit the transformation phenotype and metastatic ability of BC cells with HER-2/neu overexpression 112. In addition, it has been reported that the combination of emodin and paclitaxel can synergistically inhibit the growth and survival of BC cells, increase tumor sensitivity to paclitaxel, and improve tumor drug resistance. The mechanism is related to the reduction of tyrosine phosphorylation of HER-2/neu, suggesting that HER-2/neu inhibition is one of the important approaches of emodin in BC treatment 113. Ueno et al. found that emodin also effectively inhibited the growth of MDA-MB-435 cells with low HER-2/neu expression by inhibiting tyrosine kinase 114. In 2008, Huang et al. found that emodin could inhibit the proliferation of BCAP-37 cells and induce cell apoptosis 115, and subsequent studies revealed that emodin regulated 30 specific genes in BCAP-37 cells using an apoptosis-associated cDNA microarray, especially p53 and IGF-2 116. Li et al. found that emodin inhibited the growth of MCF-7 cells (IC50 = 7.22 μg/mL) and induced obvious apoptotic characteristics such as DNA fragmentation via both endogenous and exogenous apoptosis pathways 117.
Breast cancer is an estrogen-dependent malignant tumor that is closely related to estrogen activity, and estrogen needs to bind specifically with estrogen receptor (ER) to form a hormone-receptor complex to exert its biological effect. ERα is an important type of estrogen receptor in the body that is located in the nucleus and mediates the genotype effect of estrogen, that is, by regulating the transcription of specific target genes to exert a regulatory effect. Sui et al. found that emodin inhibited estrogen-induced proliferation of MCF-7 and MDA-MB-231 cells, promoted apoptosis and arrested the cell cycle in G0/G1 phase by downregulating the expression of cyclin D1 Bcl-2 and ERα proteins 118. Zu et al. also suggested that emodin has good antitumor effects on the BC cell lines BCAP-37 and ZR-75-30 119. Using virtual screening, Zhang et al. found that emodin is an effective aromatic hydrocarbon receptor (AhR) agonist. Subsequent in vitro experiments also found that the expression levels of AhR and cytochrome P450 1A1 (CYP1A1) in MCF-7 cells were significantly upregulated by emodin treatment, suggesting that the antitumor effects of emodin against BC might be related to the AhR-CYP1A1 signaling pathway 120.
Multidrug resistance to chemotherapeutic drugs is the main cause of BC treatment failure, and overexpressed ERCC1, a key protein in nucleotide excision repair (NER), is one of the main causes of drug resistance 121, 122. It was reported that emodin at 10 μg/mL could downregulate ERCC1 and inhibit doxorubicin (DOX)-cisplatin resistance in MCF-7 cells 123. In 2018, emodin (20 μM) was reported to increase the sensitivity of MCF-7 breast cancer cells to chemotherapy and promote 5-FU-induced apoptosis and senescence of BC cells. The mechanism was related to the inhibition of NRARP, and silencing NRARP blocked the effect of emodin on MCF-7 cells 124. DOX is a commonly used chemical drug against BC, but the rapid emergence of drug resistance is a major culprit limiting its clinical use. Li et al. reported that DOX combined with emodin can improve the sensitivity of MDA-MB-231 and MCF-7 cells to chemotherapy, and the mechanism is closely related to increasing γH2A in cancer cells and regulating AKT1-mediated DNA damage 125.
The adenosine 5ʹ-triphosphate (ATP)-gated Ca2+-permeable channel P2X7 receptor (P2X7R) is highly expressed in many tumors and cancer cells and has been found to play an important role in the migration and invasion of metastatic tumor cells. Jelassi et al. reported that emodin could significantly inhibit ATP-induced Ca2+ increase, specifically inhibit P2x7R-mediated currents, and inhibit the invasion ability of cancer cells in vitro, and these effects almost disappeared after P2x7R interference. In addition, emodin can inhibit the invasion of MDA-MB-435S cells overexpressing P2X7R in zebrafish, suggesting that emodin can specifically antagonize P2X7R to inhibit the invasion of BC cells 126. Solid tumor tissue is composed not only of tumor cells but also of a variety of nontumor cells, such as fibroblasts, adipocytes, endothelial pericytes, mesenchymal stem cells (MSCs) and immune cells, which together constitute the tumor microenvironment. The interaction between tumor cells and nontumor cells plays an important role in tumor progression and therapy 127, 128. Macrophages are the most abundant immune cells in primary and metastatic tumor tissues and play an important role in the genesis, development and metastasis of tumors. Tumor cells can recruit macrophages and secrete chemokines and growth factors to induce macrophages to produce an M2-like phenotype. Similarly, macrophages promote tumor growth by promoting angiogenesis, suppressing immune responses, regulating the extracellular matrix and promoting tumor cell migration. In 2014, it was reported that emodin can have an antitumor effect against BC by influencing the physiological activity of macrophages. The results showed that emodin can inhibit primary tumor growth, lung metastasis and lung macrophage infiltration in mice with orthotopic inoculation of 4T1 or EO771 BC cells 129. Recently, it was reported that emodin can inhibit the production of TGF-β1 in BC cells and macrophages, attenuate TGF-β1- or macrophage-induced EMT in BC cells and cancer stem cell (CSC) formation, inhibit the migration and invasion of BC cells, and prevent postoperative lung metastatic recurrence of BC 130. Iwanowycz et al. also suggested that emodin could inhibit tumor growth by inhibiting macrophage infiltration and M2-like polarization while increasing T-cell activation and reducing tumor angiogenesis. However, the tumor suppressive effect of emodin disappeared in tumor-bearing mice with macrophage deficiency. In addition, emodin can inhibit the migration and adhesion of macrophages to the tumor site by inhibiting the secretion of MCP1 and CSF1 and the expression of THY-1 in tumor cells, suggesting that emodin can act on BC cells and macrophages simultaneously, effectively blocking the feedback loop between the two cells and exerting an antitumor effect 131. Fibroblasts are also important parts of the tumor microenvironment, which can promote the remodeling of the extracellular matrix and the production of generated growth factors and cytokines (such as TGF-β), promote the growth and migration of tumor cells, and generate EMT phenotypes. Hsu et al. found that emodin could effectively inhibit EMT and migration of BT20 cells induced by fibroblasts, while emodin pretreatment could also significantly inhibit EMT and migration of BT20 cells induced by TGF-β 132. These results suggest that emodin plays an anticancer role against BC by improving the tumor microenvironment. In addition, Sun et al. also found that emodin could inhibit lung metastasis in MDA-MB-231 xenograft mice. Using the MDA-MB-231 cell model, it was found that the antitumor effects of emodin on BC metastasis were closely related to the downregulation of MMP-2, MMP-9, uPA, and uPAR and the decreased activity of P38 and ERK1/2 133. Triple-negative breast cancer (TNBC) has the lowest survival rate among all BC subtypes due to its strong aggressiveness and metastasis. Previous studies have shown that peritumoral adipose tissue contributes to the invasion and proliferation of TNBC cells, and emodin could downregulate CCL5 and inhibit the growth and invasion of TNBC cell lines MDA-MB-231 and MDA-MB-453 134.
Furthermore, another study reported that emodin inhibited BC cell-induced metastasis and angiogenesis by inhibiting the expression of MMPs and VEGFR-2. In addition, the antitumor effect of emodin against BC may be related to the downregulation of Runx2 transcriptional activity 135. Zou et al. found that emodin increased the expression of SerRS, which is a strong transcriptional inhibitor of VEGFA in TNBC cells. In addition, they identified a direct target of emodin, namely, nuclear receptor corepressor 2 (NCOR2). When NCOR2 binds to emodin, it is released from the SerRS promoter, resulting in activation of SerRS and ultimately inhibition of VEGFA transcription 136. In addition, many studies on BC are not limited to emodin alone but also include berberine, thymoquinone, daunorubicin, curcumin and other small molecule compounds. These combined therapies seem to achieve better antitumor effects, which may become an effective strategy for breast cancer treatment 137-140 (Figure 5).
Figure 5.
The potential mechanisms for antitumor effect of emodin against breast cancer.
Pancreatic cancer
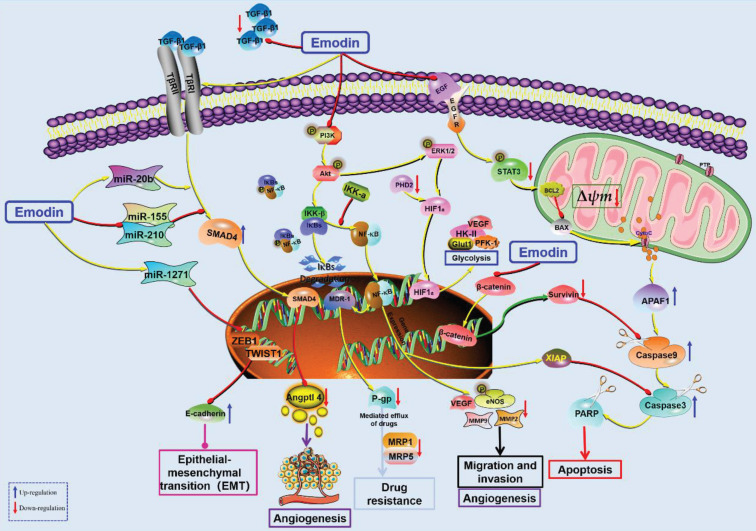
Pancreatic cancer (PC) is a malignant tumor occurring in the pancreatic exocrine gland and is one of the most common malignant tumors worldwide with high mortality 141-144. In addition to surgery, chemotherapy is another predominant way to improve the survival of patients with advanced PC, but drug resistance and side effects limit the clinical efficacy of the currently used chemotherapeutic drugs. Interestingly, an increasing number of studies have suggested that emodin might be a potential new drug for treating PC with less drug resistance and fewer side effects. It has been reported that the antitumor effects of emodin on PC growth may be related to the demethylation of tumor suppressor genes 145, 146. High expression of HIF-1α in tumor cells supports growth, angiogenesis, and high glycolysis, which is also known as the Warburg effect in tumors 147. Emodin can reduce the biosynthesis of HIF-1α in ASPC-1, BXPC-3, HPAF-2, MiaPaCa2, and PANC-1 cells and reduce their gene transcription or protein stability. In addition, the expression of HIF-1α-regulated downstream proteins (Glut1, HK-II, PFK-1, VEGF, caveolin-1, etc.) was also decreased, and emodin inhibited the phosphorylation of Akt and ERK/1/2 and downregulated intracellular signaling to reduce HIF-1α levels and attenuate cancer cachexia in athymic mice carrying cancer cells 148.
In 2008, Cai et al. reported the antitumor effect of emodin against PC, and emodin significantly inhibited the proliferation of four PC cell lines (Mia PacA-2, BXPC-3 panc-1 and L3.6PL) by inducing apoptosis 149. EGFR is overexpressed in 90% of pancreatic tumors, and EGFR-targeting drugs have become a hotspot in recent years. However, the drug resistance of targeted drugs seriously limits their clinical application 150-152. Interestingly, emodin was reported to increase the anti-proliferative effect of an EGFR inhibitor (afatinib) against PC through downregulation of EGFR by promoting STAT3 phosphorylation 153. Survivin, a member of the apoptosis suppressor gene family, is involved in controlling cell division and inhibiting apoptosis and is a target gene of β-catenin/Tcf/Lef. Guo et al. found that emodin enhanced the antitumor effect of gemcitabine against PC by downregulating survivin and β-catenin expression and reversed the drug resistance behavior of drug-resistant cells 154. In 2010, Liu et al. also reported that emodin enhanced the antitumor effect of gemcitabine on PC by downregulating NF-κB 155. Subsequently, they found that the synergistic effect of emodin on gemcitabine was associated with a decrease in Bcl-2/Bax and promotion of Cyt-C release from mitochondria to the cytoplasm 156. In addition, chemotherapeutic resistance to gemcitabine has also been reported to be associated with Akt activation, and gemcitabine combined with emodin increased cell death and mitochondrial fragmentation by inhibiting Akt phosphorylation and increasing the activation of caspase-3 and -9 157. XIAP is another important member of the anti-apoptotic gene family, which is highly expressed in many tumor cells and promotes tumor cell proliferation and anti-apoptosis. Wang et al. found that gemcitabine intervention upregulated XIAP expression in SW1990 cells and PANCC-1 cells, which was associated with the development of clinical drug resistance. Interestingly, the addition of emodin can significantly downregulate XIAP 158, 159. In addition, the combination of emodin can also reverse the resistance of BXPC-3 and SW1990 cells to gemcitabine by reducing the function of multidrug resistance gene-1 (MDR-1), reducing the expression of transmembrane glycoprotein P-gp (which pumps chemotherapeutic drugs out of cells), activating the mitochondrial apoptosis pathway, and reducing the resistance of BXPC-3 and SW1990 cells to gemcitabine 160, 161. Guo et al. reported that emodin can enhance the antitumor effect of gemcitabine (the gold standard chemotherapy drug for PC) by inhibiting MDR1/P glycoprotein, MRP expression, and the IKKβ/NF-κB signaling pathway 162. A recent study also found that emodin reversed gemcitabine resistance in PC cells by inhibiting the IKKβ/NF-κB signaling pathway 163.
In addition, emodin has been reported to have significant anti-proliferation and anti-metastasis effects on PC by downregulating NF-κB DNA-binding activity and survivin and MMP-9 in PC cells and promoting apoptosis 164, 165. Li et al. found that emodin could significantly inhibit the proliferation, migration and invasion of SW1990 in vitro and regulate the expression of nuclear genes encoding EMT-related proteins. It has also been found that emodin can inhibit hepatic metastasis of PC in vivo by inhibiting EMT and invasion of PC based on the ug-regulation of miR-1271 166. Further investigations found that emodin can inhibit tumor angiogenesis in PC and reduce the expression of the angiogenesis-related factors NF-κB, VEGF, MMP-2 and eNOS and the phosphorylation of eNOS 167. In addition, emodin inhibited angiogenesis in tumor tissues by altering the TGF-β/Smad pathway and the activities of angiogenesis-related miR-20b, miR-155 and miR-210 168 (Figure 6).
Figure 6.
The potential mechanisms for antitumor effect of emodin against pancreatic cancer.
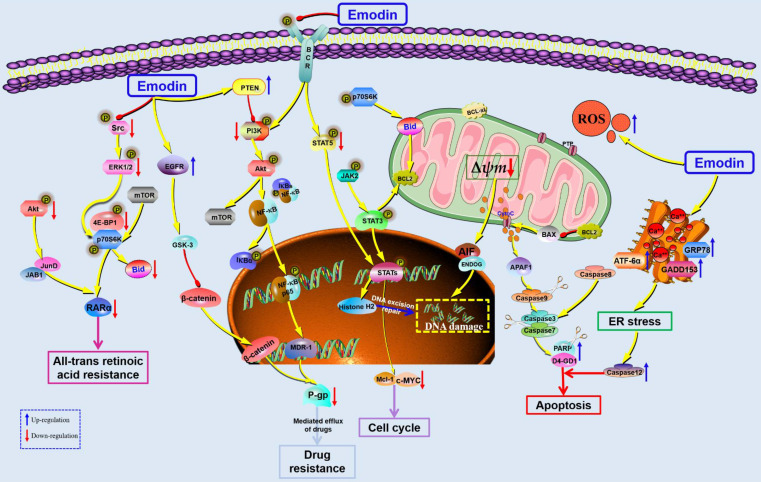
Leukemia
Recently, accumulating studies have shown that emodin has obvious antitumor effects against chronic myelogenous leukemia (CML). CML is a common malignant tumor characterized by abnormal proliferation and accumulation of mature granulocytes 169. It was first discovered in 1973 that CML in more than 90% of patients is caused by a genetic variation called the Philadelphia (Ph) chromosome 170. Brown et al. found that a 100-fold reduction in the dose of Arsenic trioxide (As2O3) in combination with emodin could be highly toxic to APL tumor cells 171. In 2002, Chen et al. studied the anti-CML effect of emodin for the first time and found that emodin exhibited significant cytotoxicity to HL-60 cells, accompanied by the emergence of a DNA ladder. Further studies showed that emodin could induce apoptosis of HL-60 cells by activating the caspase cascade, but this antitumor activity was independent of ROS production 172-176. Emodin also showed antitumor effects in U937 cells via a decrease in Bcl-2/Bax and activation of pro-caspase-3 177. Myeloid cell leukemia 1 (Mcl-1), a signal transducer and activator of transcription 3 (STAT3)-regulated molecule, is necessary for myeloma cell survival. In 2007, Muto et al. found that emodin inhibited the growth of multiple myeloma (MM) cells and induced cell apoptosis by inhibiting Janus-activated kinase 2 (JAK2) activity and phosphorylation of STAT3 and downregulating Mcl-1 178. Zheng et al. confirmed that the anti-proliferation, cell cycle arrest and apoptosis induced by emodin in HL-60 cells were related to the inhibition of the Akt pathway 179. Chen et al. reported that emodin inhibited proliferation and cell colony formation and induced cell apoptosis due to G0/G1 phase arrest in HL-60/ADR cells 180. Importantly, Chen et al. further indicated that emodin, as a novel PI3K/Akt inhibitor, can specifically inhibit the phosphorylation at tSer473 of Akt and Ser2448 of mTOR in acute myeloid leukemia (AML) cells 181. In addition, emodin-induced apoptosis in K562 cells was also associated with the inhibition of PETN/PI3K/Akt and BCR-ABL deletion 182. In addition, endoplasmic reticulum stress (ERS), the caspase cascade and independent mitochondrial pathways are also involved in emodin-induced apoptosis of the leukemia cell line WEHI-3 183.
The 3'-azido-3'-deoxythymidine (AZT) can competitively inhibit the reverse transcription process, inhibit telomerase activity, block telomere elongation, and inhibit the growth of cancer cells 184-186. It has been reported that P-gp is also highly expressed in the tumor tissues of CML patients, leading to drug resistance. Interestingly, emodin combined with AZT can synergistically induce the growth of K562/ADM cells and S-phase cell arrest by downregulating MDR1 and P-gp 187. Furthermore, Min et al. found that emodin may be a potential substrate of P-gp with competitive binding at its R site 188. Another study reported the synergistic effect of emodin on AZT through upregulation of early growth response-1 (EGR1) and inhibition of the Wnt/β-catenin pathway 189. In addition, emodin combined with cytarabine (ARA-C) can significantly inhibit the growth of HL-60/ADR cells in vivo and in vitro through dual targeting of the Akt and ERK pathways 190. A recent study reported that emodin can enhance the sensitivity of K562/G01 cells to imatinib via inhibition of phosphorylation of Bcr-Abl and STAT5 and phosphorylation of Src 191 (Figure 7).
Figure 7.
The potential mechanisms for antitumor effect of emodin against Leukemia.
Gynecological cancer
Cervical cancer
As the second most common malignant tumor in females in the world, cervical cancer seriously threatens the lives and health of women. Human papillomavirus (HPV) infection has been identified as the main cause of cervical cancer, especially HPV16 and HPV18. Type I interferons (IFN-α and IFN-β) play important roles in innate immune responses and can play antiviral, antitumor, antiproliferative and immunomodulatory roles by activating JAK/STAT pathway signals 192. The 26S proteasome is a molecular complex that catalyzes ubiquitination protein degradation and is also a negative regulator of IFN-α/β signal transduction, participating in the negative regulation of JAK/STAT. Inhibition of the 26S proteasome to enhance the immunomodulatory effect of IFN-α/β is a new strategy for the development of antitumor drugs. Emodin was found to be an effective inhibitor of the human 26S proteasome, which increased phosphorylation of STAT1, decreased phosphorylation of STAT3, increased endogenous gene expression stimulated by IFN-α, inhibited the degradation of type I interferon receptor 1 (IFNAR1), and enhanced the anti-proliferation effect of IFN-α on HeLa cervical cancer cells 193. In 2003, Srinivas et al. reported the anti-proliferative effect of emodin on cervical cancer cells via the induction of apoptosis 194. Furthermore, Olsen et al. suggested that the antitumor effect of emodin against cervical cancer might be related to regulation of the PI3K/Akt pathway 195. In 2013, emodin was reported to induce HeLa cell apoptosis through endogenous mitochondrial and exogenous death receptor pathways via regulation of caspase-8 196. Wang et al. found that the antitumor effects of emodin against cervical cancer (HeLa) might be related to many mechanisms, such as apoptosis, autophagy, G0/G1 phase arrest and angiogenesis inhibition 197.
In 2017, Trybus et al. found that emodin could cause significant changes in the lysosomal compartment of HeLa cells, which manifested as an increase in the number of lysosomes and an increase in autophagic vacuoles, leading to lysosomal membrane damage 198. Furthermore, a recent study reported that emodin is a photosensitizer in combination with photodynamic therapy that significantly increased cytotoxicity to SiHa, CaSki, and HaCaT cells, which was associated with increased ROS production, caspase-3 activity, and autophagy 199. The TGF-β signaling pathway is involved in the growth, development, and differentiation of tumor cells and can also induce epithelial cells to transform into mesenchymal cells, promoting advanced tumor progression. It has been suggested that emodin can regulate TGF-β signaling in SiHa and HeLa cells and affect the growth, migration, and invasion of cervical cancer cells via downregulation of the TGF-β signaling pathway by reducing TGF-β receptor II and Smad4, inhibiting cyclin D1, P21 and Pin1, and downregulating Snail and Slug 200.
Ovarian cancer
Ovarian cancer is another common gynecological cancer worldwide, causing 152,000 cancer-related deaths each year 201. In 2009, Kurokawa et al. reported that emodin enhanced the toxicity of cisplatin against the human ovarian cancer cell line A2780, and this effect of emodin could be eliminated by knocking down hCtr1 202. In 2018, Song et al. reported a novel mechanism of emodin against ovarian cancer by promoting FOXD3 and activating miR-199a in turn, which inhibited TGF-β2 and reduced A2780 cell viability and colony formation 203. Emodin can induce apoptosis and inhibit cell invasion in SKOV3 and HO8910 cells by regulating survival 204. In addition, emodin can inhibit the proliferation, invasion and migration of ovarian cancer cells via suppression of EMT by regulating the ILK/GSK-3β/Slug and GSK-3β/β-catenin/ZEB1 signaling pathways 205, 206. A subsequent study further confirmed the mentioned effects of emodin on ovarian cancer cells without targeted toxicity to hepatocytes, renal cells and cardiomyocytes in nude mice 207.
Ovarian cancer cells, like other malignancies, are resistant to anticancer drugs. Interestingly, emodin can overcome paclitaxel resistance in A2780 cells by downregulating P-gp, XIAP and survival 208. In addition, emodin can also assist in enhancing the anticancer effects of cisplatin by inducing ROS production and downregulating MRP1 209. A recent study found that emodin can be recognized as an inhibitor of Aurora kinase A (AURKA) kinase and induce apoptosis in cisplatin-resistant ovarian cancer cells 210.
Others
Head and neck cancer
Some studies have also reported antitumor effects of emodin on head and neck cancers. Emodin can induce apoptosis and cell cycle arrest in human tongue carcinoma SCC-4 cells via regulation of many proteins, such as P21, Chk2, cyclin B1, CDC2, cyto-C, caspase-3, -9, Bcl-2, Bax, GADD153 and GRP78 211. Chen et al. found that emodin can induce DNA damage in SCC-4 cells by inhibiting DNA damage repair genes, such as ataxia telangiectasia mutated (ATM), ataxia-telangiectasia and Rad3-related (ATR), 14-3-3sigma (14-3-3σ), breast cancer 1 early onset (BRCA1), and DNA-dependent serine/threonine protein kinase (DNA-PK) 212. In addition, another paper by Chen et al. found that emodin could inhibit the migration and invasion of SCC-4 cells by inhibiting MMP-2, U-PA, FAK, NF-κB P65, pAKT, P-P38, P-JNK, P-ERK, and MMP9 and promoting TIMP-1 213.
Similarly, emodin treatment can also induce morphological changes and rapid apoptosis of EC-109 cells by inducing intracellular ROS eruption and significantly reducing pH 214. The ectopic expression of TWIST1 in head and neck squamous cell carcinoma cells triggers EMT and leads to the acquisition of mesenchymal phenotypes of tumor cells, while the increased proportion of CD44-labeled cells in the tumor population can activate tumor initiation. Emodin treatment reduced the tumor-initiating ability of FaDu-pFLAG-TWIST1 cells and inhibited cell migration and invasion by inhibiting the β-catenin and Akt pathways to inhibit TWIST1-induced EMT 215. In another study, emodin significantly inhibited the growth and proliferation behavior of Tca8113 cells. In addition, emodin also caused G0/G1 phase arrest in Tca8113 cells and reduced the expression of CDK2, Cyclin E and P21 216. Furthermore, emodin also inhibited oral tumorigenesis in DMBA-treated hamsters by regulating cell cycle markers such as cyclin D1, PCNA, CDK4, CDK6 and survivin 217, and the proapoptotic and antioxidant effects of emodin also play important roles in this process 218. Moreira et al. reported that emodin plays an antitumor role in oral squamous cell carcinoma cells (HSC-3) by increasing oxidative stress and DNA damage, inhibiting Akt activation, and activating apoptosis and necrosis 219.
A similar anticancer effect of emodin has been observed in human nasopharyngeal carcinoma cells. Emodin can significantly inhibit the growth of CNE-2Z cells and induce their cycle arrest and apoptosis 220.
TNF receptor-associated factor 6 (TRAF6) is closely associated with tumor angiogenesis and metastasis. Emodin can inhibit angiogenesis and metastasis in anaplastic thyroid cancer (ATC) by inhibiting the TRAF6/HIF-1α/VEGF and TRAF6/CD147/MMP9 signaling pathways 221. In addition, it has also been reported that the antitumor effect of emodin on thyroid papillary carcinoma is related to the activation of AMPK and inhibition of the MEK-ERK pathway 222.
Glioma and Neuroblastoma
In 2005, Kim et al. found that emodin can effectively inhibit HA-induced MMP9 secretion and invasion of glioma by inhibiting the activation of FAK, ERK1/2 and Akt/PKB, as well as the transcriptional activities of AP-1 and NF-κB 223. In glioblastoma (GBM) cells treated with ionizing radiation (IR), fructose 1,6-bisphosphatase 1 (FBP1) is downregulated, along with increased glucose uptake and extracellular acidification, indicating increased intracellular glycolysis. At the same time, intracellular Ets1 was overexpressed, suggesting that Ets1 is a transcriptional inhibitor of FBP1. Emodin inhibited the glycolysis rate and IR-induced GBM migration in orthotopic xenograft mice 224. Kim et al. evaluated the effects of emodin on glioma stem cells (GSCs) and found that emodin can significantly inhibit the self-renewal activity of GSCs, partially induce apoptosis, inhibit cell invasion, and increase the sensitivity of GSCs to IR. In addition, emodin can promote EGFR/EGFRvIII degradation by blocking the interaction between EGFR/EGFRvIII and Hsp90 and inhibiting the expression of Notch, β-catenin and STAT3, which leads to the inhibition of GSC stemness 225. Another study reported that emodin can inhibit proliferation and induce apoptosis and necrosis in U251 cells by upregulating TNF-α, RIP1, RIP3 and MLKL levels and activating the TNF-α/RIP1/RIP3 axis 226.
The antitumor effects of emodin have also been observed in neuroblastoma cells. When sh-SY5Y cells were exposed to emodin, cell viability was significantly decreased. In addition, emodin also significantly inhibited the migration and invasion of SH-SY5Y cells by inhibiting GRB2, RhoA, HIF-1A, VEGF, FAK, iNOS, COX2, p-P38, p-C-Jun, MMP2, MMP9 and MMP7 and promoting PKC, PI3K, MEKK3 and NF-κB p65 227. Emodin can induce apoptosis of human neuroblastoma IMR-32 cells by regulating ROS, P53, P21, caspase-3, -9 228.
Prostate cancer and bladder cancer
Prostate cancer is one of the most common malignant tumors of the male genitourinary system. The level of androgen in the body is closely related to the incidence of prostate cancer. Abnormal secretion of androgen is also the main cause of inducing male prostate cancer. Androgen receptor (AR) is involved in the effect of androgen on tumor initiation and plays a major role in the recurrence and outcome of prostate cancer. It has been reported that emodin can directly target AR to inhibit the growth of prostate cancer cells and prolong the survival time of tumor-bearing mice 229. Yu et al. observed that emodin could inhibit the proliferation of prostate cancer cells LNCaP via the mitochondrial apoptosis pathway by decreasing the expression of AR and PSA and increasing the expression of p53 and P21 230. Emodin can also increase the chemotherapy sensitivity of DU-145 cells to cisplatin and inhibit the growth of prostate tumors by inducing ROS production, downregulating MDR1, inhibiting HIF-1, and promoting drug retention 231. Masaldan et al. also confirmed that the inhibitory effect of emodin on the proliferation of LNCaP and PC-3 cells was largely related to inducing high levels of ROS production. In addition, they found that LRP1 also appears to play an important role in emodin's antitumor effects 232. Furthermore, the CXCR4/CXCL12 axis is reported to be involved in promoting tumor invasion and metastasis, and downregulation of CXCR4 is of great significance in inhibiting cancer metastasis. Emodin can inhibit the migration and invasion of DU145 cells by downregulating CXCR4 mRNA and inhibiting NF-κB activation 54. Another study found that emodin upregulated Notch1, promoted Notch1 nuclear transfer and inhibited Jagged1, VEGF and bFGF 233.
Epigenetic studies showed that histone H3K27 trimethylation (H3K27me3) expression was low in human bladder cancer, and histone H3S10 phosphorylation (pH3Ser10) expression was high, while the two epigenetic markers showed opposite expression patterns in normal bladder cancer. Emodin could inhibit pH3Ser10, FABP4 & HBP17 and elevate H3K27me3 234. Chemotherapeutic tolerance is one of the main causes of tumor progression and recurrence of bladder cancer, and ROS are a key factor in the chemical sensitivity of cancer cells. Emodin can effectively enhance the cytotoxicity of cisplatin-treated human bladder cancer cells T24 and J82 by increasing the ROS level through reducing the GSH-cisplatin conjugate. In line with in vitro experiments, emodin/cisplatin therapy also increased xenograft tumor cell apoptosis, and MDR1 was ultimately responsible for these phenomena 235.
Lymphoma
In 2013, emodin reduced the number of living cells in Dalton's lymphoma (DL) and significantly extended the lifespan of DL mice by inducing the production of H2O2 and reciprocal regulation of degradation antioxidant enzymes, inducing the DL cell mitochondrial apoptosis pathway 236. Lin et al. reported that emodin can reduce the survival rate of Raji cells via induction of cell apoptosis by downregulating ubiquitin-like protein containing PHD and RING domains 1 (UHRF1). In addition, they found that emodin could increase the sensitivity of Raji cells to adriamycin 237. To explore the mechanism of emodin in aggressive non-Hodgkin's lymphoma (NHL), Chen et al. predicted the potential anti-NHL targets of emodin with the help of bioinformatics techniques. The results showed that TP53 and PI3K may be important molecules and pathways of emodin in NHL treatment. Interestingly, subsequent experiments confirmed the predicted results that emodin could significantly inhibit the proliferation and induce apoptosis of SU-DHL4 cells, and these pharmacological effects were related to the inhibition of TP53 and phosphorylation of PI3K/Akt 238.
Gallbladder carcinoma
Gallbladder carcinoma (GBC) is one of the most common malignant tumors of the bile duct, ranking 6th among gastrointestinal tumors worldwide 239, 240. However, GBC is resistant to many anticancer drugs, which is the main obstacle to its clinical treatment. Wang et al. found that emodin combined with cisplatin, carboplatin or oxaliplatin significantly enhanced the chemosensitivity of SGC996 cells, which was associated with the inhibition of glutathione levels and MRP1 in SGC996 cells. In addition, emodin combined with cisplatin inhibited tumor growth by increasing apoptosis and downregulating MRP1 241. Another study reported that emodin promotes cisplatin-induced apoptosis of GBC cells by inhibiting survivin 242.
Osteosarcoma
Qu et al. evaluated the effect of emodin on angiogenesis in human osteosarcoma and found that exogenous HMGB1 supplementation positively promoted angiogenesis in nude mouse grafted tumor tissues, as demonstrated by increased VEGF and vWF expression, which was effectively reversed by emodin treatment. In vitro, emodin also significantly inhibited the proliferation of the osteosarcoma cell lines SOSP-9607, MG63 and SAOS-2, decreased HMGB1-induced VEGF production, and increased the expression of SIRT1 and deacetylase activity 243. In another study, they evaluated the inhibitory effect of emodin on the antiradiation ability of MG63 cells. Emodin treatment inhibited cell viability and survival of MG63R cells after irradiation, as well as colony formation, and increased cell apoptosis. Further studies showed that emodin inhibited the expression of Shh and Bcl-2 and the nuclear translocation of Gli1 in MG63R cells after radiation exposure and increased the expression of C-caspase-3 244. Ying et al. found that emodin inhibited human osteosarcoma cells by ROS-independent ER stress 245. Similarly, emodin can enhance the antitumor effect of cisplatin in human osteosarcoma via the Nrf2 pathway 246.
Skin cancer
In 2002, emodin was reported to have a strong inhibitory effect on excessive nitric oxide (NO)-induced skin canceration in mice 247. It has been reported that emodin can increase ROS levels and induce apoptosis in mouse melanoma B16F10 cells 248. Emodin can significantly reduce the expression of CD155 in tumor cells, inhibit the proliferation and migration of tumor cells, and induce cell cycle arrest in the G2/M phase. Similarly, emodin has been observed to inhibit tumor growth and inhibit CD155 expression in B16 melanoma mice 249. Another study found that emodin acted as a mitochondrial decoupler, downregulating ATP levels in tumor cells and inhibiting growth 250. Li et al. found that emodin had significant antiproliferation and proapoptotic activities on B16F10 and A375 melanoma cells with strong metastatic ability and significantly inhibited the migration and invasion of tumor cells 251.
Gastric cancer
In addition, emodin also showed anticancer activity against gastric cancer. Cai et al. found that emodin, as an ROS producer, can combine with arsenic trioxide (ATO) to cause oxidative stress in SGC-7901 cells, promote RhoA inactivation, lead to actin silk breakage, and lead to structural destruction of the site adhesion complex, ultimately leading to anoikis, which can be partially reversed by the antioxidant N-acetylcysteine (NAC) 252. In addition, emodin can inhibit proliferation and induce apoptosis of SGC-7901 cells by downregulating prL-3 253.
Metabolic transformation of emodin
It is of great significance for directional synthesis, structural modification and activity screening of drugs to explore the metabolic or biotransformation process and pathway of drugs in vivo and further confirm the structure of metabolites. The liver is considered to be the main site of metabolism, with over 50% of oral emodin found in bile 254. However, emodin seems to be more important in the gut. A 2010 study found that the maternal level of emodin in blood samples of rats decreased rapidly after intravenous injection of emodin, while the emodin glucuronides omega-hydroxy emodin (ω-OHE, a phase I metabolite) and ω-OHE sulfates/glucuronides immediately appeared. However, after oral administration of emodin, only emodin glucuronides were found in serum, while emodin, ω-OHE and ω-OHE sulfates/glucuronides were not detected, which may be because most emodin will be metabolized in the intestine first after absorption, and a small amount will reach the liver for stage I transformation. Furthermore, the results of this study clearly show that the intrinsic clearance values of emodin are very high, leading to the rate of emodin's glucuronidation being rapid via the liver and intestinal microsomes of male rats. This suggests that the oral bioavailability of emodin parents is almost zero due to rapid and extensive binding metabolism during delivery 255. Glucuronidation metabolism via glucuronidation in the gut appears to be one of the main reasons for the low bioavailability of emodin, and the other important reason is its low solubility. Therefore, reducing emodin glucuronidation and improving its solubility are effective means to improve its bioavailability. In addition, there was a sex difference in the rate of emodin glucuronidation in animals, which was due to the unique expression pattern of UGT2B1 that facilitated this process in male mice. However, because UGT2B1 is not expressed in humans, there may be no sex effect of emodin glucuronidation in humans 256. In addition, the absorption of emodin on Caco-2 cells showed significant concentration dependence. The higher the concentration was, the higher the rate of emodin absorption, indicating that emodin was mainly absorbed by passive diffusion in Caco-2 cells 257.
Toxicity of emodin
Although emodin has excellent performance in the treatment of cancer, the toxicity or adverse reactions caused by long-term use should not be ignored. Radiac Brkanac et al. used human peripheral blood lymphocytes (HPBLs) to study the cytotoxicity, genotoxicity and oxidative stress parameters of emodin and found that emodin could induce cell death and DNA damage at concentrations of 150 μg/mL and 200 μg/mL. At 25 μg/mL, emodin induced an ROS increase, suggesting that emodin has cytotoxicity and genotoxicity against HPBLs, and oxidative stress is involved in its toxic mechanism 258. In addition, studies have reported that emodin can cause hepatotoxicity in rats by activating CYP3A and consuming GSH 259. In an experimental study conducted at the National Institutes of Health over two years, high doses of emodin (280, 830, 2500 ppm) were found to induce zymmbal adenocarcinoma in female F344/N rats but were not carcinogenic in female F344/N rats. Rare renal tubular neoplasms also occur in male B6C3F1 mice. However, low doses of emodin (312, 625, 1250 ppm) showed no carcinogenic effect, suggesting that emodin over a certain dose range has a certain carcinogenic effect, and this effect is different between genders 260. In addition, cytochrome P450 1A2 is involved in the formation of emodin metabolites in the liver 261. Similarly, Wang et al. also found that emodin can induce the expression of P450 1A1 and 1B1 in human lung adenocarcinoma CL5 cells, which may be an important factor affecting the metabolism and toxicity of emodin in vivo 262. Another study also described the embryotoxicity of emodin, with emodin ingestion leading to cell apoptosis and decreased cell proliferation, inhibiting early embryo development to the blastocyst stage. Meanwhile, 25-75 μM emodin could induce apoptosis of blastocyst cells and decrease the success rate of blastocyst implantation. In addition, in vitro treatment with emodin can cause fetal weight loss 263. A study in 2015 also reported the reproductive toxicity of emodin, which significantly inhibited the total motility, forward motility and linear velocity of sperm at levels greater than 100 μM. The mechanism may be related to a reduced intracellular Ca2+ concentration and tyrosine phosphorylation 264. A recent study evaluated the hepatorenal toxicity of emodin in KM mice and found that high doses of emodin (600 mg/kg) for 28 consecutive days produced significant systemic toxicity in mice, accompanied by liver, kidney, gallbladder and spleen injury 265. In conclusion, in addition to the wide range of activities of emodin, we should also pay attention to and correctly understand the toxic reactions caused by different doses of drugs.
Conclusion
In recent decades, an increasing number of pharmacists have suggested that natural products are valuable resources for finding reliable candidate drugs 266,267. In this paper, we summarized the reported references that investigated the natural anthraquinone of emodin with versatile potential in the chemotherapy of various types of cancer, such as lung cancer, liver cancer, colon cancer, breast cancer, and pancreatic cancer. Currently, the continued progress made in various aspects of this natural agent suggests that emodin might be a promising lead compound for development as a new clinical chemotherapy drug for treating various cancers, especially lung cancer, liver cancer, and breast cancer (Figure 8).
Figure 8.
The main molecular mechanism of emodin anticancer action.
However, there are still many areas in both preclinical and clinical investigations of emodin that need to be improved upon in the future. First, most of the presented studies about the antitumor effects of emodin are in vitro cell studies, and the animal evidence is inadequate. Therefore, more work should be devoted to animal studies to evaluate the antitumor effects of emodin in vivo. Second, the available data of this natural compound have primarily focused on preclinical studies but rarely clinical experiments for treating tumors. Therefore, future studies of emodin may devote more work to the study of its therapeutic effects in the clinic. Third, tumors commonly require long-term treatment, so the safety of the candidate drug is very important. However, few investigations of the target organ toxicity of emodin have been carried out thus far. Therefore, more drug safety studies might be encouraged to explore the side effects of emodin. Fourth, the bioavailability of emodin in vivo is also very low, which is an important limitation for the drug development of this monomer. Due to this limitation, chemical structure modification and novel nanodrug delivery systems for emodin might be promising strategies to solve this problem. Lastly, previous references mentioned lots of targets for emodin, however, the real drug targets for emodin are still needed to be further clarified due to most of the reported proteins regulated by emodin might be the signalling molecules instead of the drug targets. In conclusion, this paper provides an updated overview of the current studies regarding the antitumor effects of emodin, which is helpful for the development of this natural monomer as a candidate drug for treating cancers in the clinic.
Acknowledgments
This work was supported by the Project of Sichuan Science and Technology Program (No. 22NSFSC1510, 2019JDRC0074, 2020YFS0523), State Administration of Traditional Chinese Medicine of Sichuan Province of China (No. 2021MS460, No. 2020HJZX001), and the Xinglin scholar discipline promotion talent program of Chengdu University of traditional Chinese medicine (No. BSH2018006).
Abbreviations
- 14-3-3σ
14-3-3sigma
- AhR
aromatic hydrocarbons receptor
- APL
acute promyelocytic leukemia
- AR
androgen receptor
- ATC
anaplastic thyroid cancer
- ATM
ataxia telangiectasia mutated
- ATO
arsenic trioxide
- ATP
adenosine 5ʹ-triphosphate
- ATR
ataxia-telangiectasia and Rad3-related
- AZT
3'-azido-3'-deoxythymidine
- BC
breast cancer
- BRCA1
breast cancer 1 early onset
- CML
chronic myeloGenous leukemia
- CSC
cancer stem cell
- CyPD
cyclophilin D
- CRC
colorectal cancer
- DL
dalton's lymphoma
- DNA-PK
DNA-dependent serine/threonine protein kinase
- DOX
doxorubicin
- EGR1
early growth response-1
- EMT
epithelial-mesenchymal transformation
- ER
estrogen receptor
- ERCC
excision repair cross-complementing gene
- ERS
endoplasmic reticulum stress
- FASN
fatty acid synthase
- FBP1
fructose 1, 6-Bisphosphatase 1
- FGFR
fibroblast growth factor receptor
- GBC
gallbladder carcinoma
- GBM
glioblastoma
- GSCs
glioma stem cells
- HNSCC
head and neck squamous cell carcinoma
- HCC
hepatocellular carcinoma
- HPV
human papillomavirus
- IR
ionizing radiation
- JAK2
Janus-activated kinase 2
- Mcl-1
myeloid cell Leukemia 1
- MDR-1
multidrug resistance gene-1
- MM
multiple myeloma
- MMOP
mitochondrial transmembrane potential
- MMP
matrix metalloproteinases
- MRSA
methicillin-resistant Staphylococcus aureus
- MSCs
mesenchymal stem cells
- MTH1
MutT homologue 1
- NAC
N-acetylcysteine
- NCOR2
nuclear receptor corepressor 2
- NER
nucleotide excision repair
- NHL
non-Hodgkin's Lymphoma
- NO
nitric oxide
- NUDT1
nudix hydrolase 1
- P2X7R
permeable channel P2X7 receptor
- PC
pancreatic cancer
- Ph
philadelphia
- ROS
reactive oxygen species
- TNBC
triple-negative breast cancer
- UHRF1
ubiquitin-like protein containing PHD and RING domains 1
- VM
vasculogenic Mimicry
- STAT3
signal transducer and activator of transcription 3
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SL, Xu J, Kochanek KD, Arias E. Mortality in the United States. NCHS Data Brief. 2017;328:1–8. [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China. CA Cancer J Clin. 2015;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Sharma B, Kanwar SS, Kumar A. Lead phytochemicals for anticancer drug development. Front Plant Sci. 2016;7:1–13. doi: 10.3389/fpls.2016.01667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madunić J, Madunić IV, Gajski G, Popić J, Garaj-Vrhovac V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018;413:11–22. doi: 10.1016/j.canlet.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Peng W, Wu JG, Jiang YB, Liu YJ, Sun T, Wu N. et al. Antitumor activity of 4-O-(2′′-O-acetyl-6′′-O-p-coumaroyl-β-D-glucopyranosyl)-p-coumaric acid against lung cancers via mitochondrial-mediated apoptosis. Chem Bio Interact. 2015;233:8–13. doi: 10.1016/j.cbi.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 9.Lichota A, Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci. 2018;19:3533. doi: 10.3390/ijms19113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong X, Fu J, Yin X, Cao S, Li X, Lin L. et al. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother Res. 2016;30:1207–1218. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng W, Qin R, Li X, Zhou H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: a review. J Ethnopharmacol. 2013;148:729–745. doi: 10.1016/j.jep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Wei WT, Lin SZ, Liu DL, Wang ZH. The distinct mechanisms of the antitumor activity of emodin in different types of cancer (Review) Oncol Rep. 2013;30:2555–2562. doi: 10.3892/or.2013.2741. [DOI] [PubMed] [Google Scholar]
- 13.Hsu SC, Chung JG. Anticancer potential of emodin. Biomedicine (Taipei) 2012;2:108–116. doi: 10.1016/j.biomed.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivas G, Babykutty S, Sathiadevan PP, Srinivas P. Molecular mechanism of emodin action: transition from laxative ingredient to an antitumor agent. Med Res Rev. 2007;27:591–608. doi: 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]
- 15.Lai JM, Chang JT, Wen CL, Hsu SL. Emodin induces a reactive oxygen species-dependent and ATM-p53-Bax mediated cytotoxicity in lung cancer cells. Eur J Pharmacol. 2009;623:1–3. doi: 10.1016/j.ejphar.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Haque E, Kamil M, Irfan S, Sheikh S, Hasan A, Nazir A. et al. Blocking mutation independent p53 aggregation by emodin modulates autophagic cell death pathway in lung cancer. Int J Biochem Cell Biol. 2018;96:90–95. doi: 10.1016/j.biocel.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Cao F, Peng W, Li X, Liu M, Li B, Qin R. et al. Emodin is identified as the active component of ether extracts from Rhizoma Polygoni Cuspidati for anti-MRSA activity. Can J Physiol Pharmacol. 2015;93:485–493. doi: 10.1139/cjpp-2014-0465. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Peng W, Qin R, Yan Z, Cen Y, Zheng X. et al. The direct anti-MRSA effect of emodin via damaging cell membrane. Appl Microbiol Biotechnol. 2015;99:7699–7709. doi: 10.1007/s00253-015-6657-3. [DOI] [PubMed] [Google Scholar]
- 19.Wu JG, Peng W, Yi J, Wu YB, Chen TQ, Wong KH. et al. Chemical composition, antimicrobial activity against Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the essential oil from Toona sinensis (A. Juss.) Roem. leaves. J Ethnopharmacol. 2014;154:198–205. doi: 10.1016/j.jep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng W, Hu C, Shu Z, Han T, Qin L, Zheng C. Antitumor activity of tatariside F isolated from roots of Fagopyrum tataricum (L.) Gaertn against H22 hepatocellular carcinoma via up-regulation of p53. Phytomedicine. 2015;22:730–736. doi: 10.1016/j.phymed.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Wu LS, Jia M, Chen L, Zhu B, Dong HX, Si JP. et al. Cytotoxic and antifungal constituents isolated from the metabolites of endophytic fungus DO14 from Dendrobium officinale. Molecules. 2015;21:14. doi: 10.3390/molecules21010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Yi J, Wu Y, Chen X, Zeng J, Wu J. et al. 3, 3'-dimethylquercetin inhibits the proliferation of human colon cancer RKO cells through inducing G2/M cell cycle arrest and apoptosis. Anticancer Agents Med Chem. 2018;19:402–409. doi: 10.2174/1871520618666181106120718. [DOI] [PubMed] [Google Scholar]
- 23.Eder R, Widmer C. Untersuchungen über derivate des β-Methylanthrachinons. III. mitteilung. synthese des Frangula-Emodins. Helv. Chim Acta. 1923;6:966–981. [Google Scholar]
- 24.Vasas A, Orbán-Gyapai O, Hohmann J. The genus Rumex: review of traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2015;175:198–228. doi: 10.1016/j.jep.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Liang Y, Yu DZ. Progress in chemical synthesis and structural modification of emodin. Chin J Organ Chem. 2011;31:1324–1333. [Google Scholar]
- 26.Fairbairn JW, ElMuhtadi FJ. Chemotaxonomy of anthraquinones in Rumex. Phytochemistry. 1972;11:263–268. [Google Scholar]
- 27.Zhang WG, Yang SG, Li B, Chen SF. Advance on chemical ingredients of polygonaceae in china. Prog Modern Biomed. 2008;8:393–396. [Google Scholar]
- 28.Cai JZ, Lin CL, Wang XQ, Lin GY. Determination and comparison of emodin in six kinds of Polygonaceae herbs. Chin Arch Trad Chin Med. 2012;30:1372–1374. [Google Scholar]
- 29.Sun L, Liu GY, Li Y, Jiang DY, Guo WF, Zhan RT. Metabolic engineering of Saccharomyces cerevisiae for efficient production of endocrocin and emodin. Meta Eng. 2019;54:212–221. doi: 10.1016/j.ymben.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Guo ZR. The organic Chemistry of Drug Design and Drug Action. Beijing: Chemical Industry Press. 2008. pp.278-279.
- 31.Teng ZH, Zhou SY, Yang RT, Liu XY, Liu RW, Yang X. et al. Quantitation assay for absorption and first-pass metabolism of emodin in isolated rat small intestine using liquid chromatography-tandem mass spectrometry. Biol Pharm Bull. 2007;30:1628–1633. doi: 10.1248/bpb.30.1628. [DOI] [PubMed] [Google Scholar]
- 32.Teich L, Daub KS, Krügel V, Nissler L, Gebhardt R, Eger K. Synthesis and biological evaluation of new derivatives of emodin. Bioorg Med Chem. 2004;12:5961–71. doi: 10.1016/j.bmc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Shao JW, Zhang FS, Bai ZD, Wang CH, Yuan YF, Wang WF. Synthesis and antitumor activity of emodin quatemaiy ammonium salt derivatives. Eur J Med Chem. 2012;56:308–319. doi: 10.1016/j.ejmech.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 34.Gecibesler IH, Disli F, Bayindir S, Toprak M, Tufekci AR, Sahin Yaglıoglu A. et al. The isolation of secondary metabolites from Rheum ribes L. and the synthesis of new semi-synthetic anthraquinones: Isolation, synthesis and biological activity. Food Chem. 2021;42:128378. doi: 10.1016/j.foodchem.2020.128378. [DOI] [PubMed] [Google Scholar]
- 35.Di X, Wang X, Di X, Liu YP. Effect of piperine on the bioavailability and pharmacokinetics of emodin in rats. J Pharm Biomed Anal. 2015;115:144–149. doi: 10.1016/j.jpba.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 36.Fu M, Tang W, Liu JJ, Gong XQ, Kong L, Yao XM. et al. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J Drug Target. 2020;28:245–258. doi: 10.1080/1061186X.2019.1656725. [DOI] [PubMed] [Google Scholar]
- 37.Pourhajibagher M, Etemad-Moghadam S, Alaeddini M, Bahador A. Modulation of the triggered apoptosis by nano emodin transfersome-mediated sonodynamic therapy on head and neck squamous cell carcinoma cell lines. Photodiagn Photodyn Ther. 2021;9:102253. doi: 10.1016/j.pdpdt.2021.102253. [DOI] [PubMed] [Google Scholar]
- 38.Krajnovi´c T, Maksimovi´c-Ivani´c D, Mijatovi´c S, Draˇca D, Wolf K, Edeler D. et al. Drug delivery system for emodin based on mesoporous silica SBA-15. Nanomaterials. 2018;8:322. doi: 10.3390/nano8050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Hung MC. Sensitization of HER-2/neu-overexpressing non-small cell lung cancer cells to chemotherapeutic drugs by tyrosine kinase inhibitor emodin. Oncogene. 1996;12:571–576. [PubMed] [Google Scholar]
- 40.Ko JC, Su YJ, Lin ST, Jhan JY, Ciou SC, Cheng CM. et al. Suppression of ERCC1 and Rad51 expression through ERK1/2 inactivation is essential in emodin-mediated cytotoxicity in human non-small cell lung cancer cells. Biochem Pharmacol. 2010;79:655–664. doi: 10.1016/j.bcp.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 41.He L, Bi JJ, Guo Q, Yu Y, Ye XF. Effects of emodin extracted from Chinese herbs on proliferation of non-small cell lung cancer and underlying mechanisms. Asian Pac J Cancer Prev. 2012;13:1505–1510. doi: 10.7314/apjcp.2012.13.4.1505. [DOI] [PubMed] [Google Scholar]
- 42.Ko JC, Su YJ, Lin ST, Jhan JY, Ciou SC, Cheng CM. et al. Emodin enhances cisplatin-induced cytotoxicity via down-regulation of ERCC1 and inactivation of ERK1/2. Lung Cancer. 2010;69:155–164. doi: 10.1016/j.lungcan.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Tang Q, Zhao SY, Wu JJ, Zheng F, Yang LJ, Hu JH. et al. Inhibition of integrin-linked kinase expression by emodin through crosstalk of AMPKα and ERK1/2 signaling and reciprocal interplay of Sp1 and c-Jun. Cell Signal. 2015;27:1469–1477. doi: 10.1016/j.cellsig.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Tang Q, Wu JJ, Zheng F, Hann SS, Chen YQ. Emodin Increases Expression of Insulin-Like Growth Factor Binding Protein 1 through Activation of MEK/ERK/AMPKα and Interaction of PPARγ and Sp1 in Lung Cancer. Cell Physiol Biochem. 2017;41:339–357. doi: 10.1159/000456281. [DOI] [PubMed] [Google Scholar]
- 45.Li MZ, Jin SB, Cao Y, Xu J, Zhu SD, Li Z. Emodin regulates cell cycle of non-small lung cancer (NSCLC) cells through hyaluronan synthase 2 (HA2)-HA-CD44/receptor for hyaluronic acid-mediated motility (RHAMM) interaction-dependent signaling pathway. Cancer Cell Int. 2021;21:19. doi: 10.1186/s12935-020-01711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HZ. Effects and mechanisms of emodin on cell death in human lung squamous cell carcinoma. Br J Pharmacol. 2001;134:11–20. doi: 10.1038/sj.bjp.0704205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li WY, Ng YF, Zhang H, Guo ZD, Guo DJ, Kwan YW. et al. Emodin elicits cytotoxicity in human lung adenocarcinoma A549 cells through inducing apoptosis. Inflammopharmacology. 2014;22:127–134. doi: 10.1007/s10787-013-0186-4. [DOI] [PubMed] [Google Scholar]
- 48.Su J, Yan Y, Qu J, Xue X, Liu Z, Cai H. Emodin induces apoptosis of lung cancer cells through ER stress and the TRIB3/NF-κB pathway. Oncol Rep. 2017;37:1565–1572. doi: 10.3892/or.2017.5428. [DOI] [PubMed] [Google Scholar]
- 49.Su YT, Chang HL, Shyue SK, Hsu SL. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem Pharmacol. 2005;70:229–241. doi: 10.1016/j.bcp.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, Liu J, Li RL, Zhao R, Zhang MM, Wei SJ. et al. A network pharmacology approach to investigate the anticancer mechanism and potential active ingredients of rheum palmatum L. against lung cancer via induction of apoptosis. Front Pharmacol. 2020;11:528308. doi: 10.3389/fphar.2020.528308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakabeppu Y. Molecular genetics and structural biology of human MutT homolog, MTH1. Mutation research. 2001;477:59–70. doi: 10.1016/s0027-5107(01)00096-3. [DOI] [PubMed] [Google Scholar]
- 52.Wahi D, Soni D, Grover A. A Double-Edged Sword: The anti-Cancer effects of emodin by inhibiting the redox-protective protein MTH1 and augmenting ROS in NSCLC. J Cancer. 2021;12:652–681. doi: 10.7150/jca.41160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang FY, Li RZ, Xu C, Fan XX, Li JX, Meng WY, Emodin induces apoptosis and suppresses non-small-cell lung cancer growth via downregulation of sPLA2-IIa. Phytomedicine. 2021; 153786, doi: 10.1016/j.phymed. 2021. 153786. [DOI] [PubMed]
- 54.Ok S, Kim SM, Kim C, Nam D, Shim BS, Kim SH. et al. Emodin inhibits invasion and migration of prostate and lung cancer cells by downregulating the expression of chemokine receptor CXCR4. Immunopharmacol Immunotoxicol. 2012;34:768–78. doi: 10.3109/08923973.2012.654494. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Li L, Guan RJ, Zhu DN, Song NN, Shen LL. Emodin inhibits ATP-induced proliferation and migration by suppressing P2Y receptors in human lung adenocarcinoma cells. Cell Physiol Biochem. 2017;44:1337–1351. doi: 10.1159/000485495. [DOI] [PubMed] [Google Scholar]
- 56.Yuan Y, Liao QW, Xue MM, Song ZJ, Tong CY, Tao ZG. Emodin: one main ingredient of shufeng jiedu capsule reverses chemoresistance of lung cancer cells through inhibition of EMT. Cell Physiol Biochem. 2017;42:1063–1072. doi: 10.1159/000478754. [DOI] [PubMed] [Google Scholar]
- 57.Peng S, Wang JC, Lu C, Xu ZL, Chai JJ. et al. Emodin enhances cisplatin sensitivity in non-small cell lung cancer through Pgp downregulation. Oncol Lett. 2019;19:230. doi: 10.3892/ol.2021.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen S.F, Zhang Z.Y, Zhang J.L. Emodin enhances antitumor effect of paclitaxel on human non-small-cell lung cancer cells in vitro and in vivo. Drug Des Devel Ther. 2021;13:1145–1153. doi: 10.2147/DDDT.S196319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teng X, Wang YS, Shi YQ, Fan XF, Liu S, Xing Y. et al. The role of emodin on cisplatin resistance reversal of lung adenocarcinoma A549/DDP cell. Anticancer Drugs. 2021;32:939–949. doi: 10.1097/CAD.0000000000001086. [DOI] [PubMed] [Google Scholar]
- 60.Li ZB, Lin YK, Zhang SH, Zhou L, Yan GX, Wang YH. et al. Emodin regulates neutrophil phenotypes to prevent hypercoagulation and lung carcinogenesis. J Transl Med. 2019;17:90. doi: 10.1186/s12967-019-1838-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang FY, Li RZ, Xu G, Fan XX, Li JX, Meng WY. et al. Emodin induces apoptosis and suppresses non-small-cell lung cancer growth via downregulation of sPLA2-Iia. Phytomedicine. 2022;95:153786. doi: 10.1016/j.phymed.2021.153786. [DOI] [PubMed] [Google Scholar]
- 62.Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA. et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balogh J, Victor 3rd D, Asham EH, Burroughs SG, Boktour M, Saharia A. et al. Hepatocellular carcinoma: a review, J. Hepatocell Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakamoto K, Ogawa K, Tohyama T, Ueno Y, Tamura K, Inoue H. et al. Serosal invasion is a strong prognostic factor for hepatocellular carcinoma after hepatectomy. Hepatol Res. 2018;49:419–431. doi: 10.1111/hepr.13285. [DOI] [PubMed] [Google Scholar]
- 65.Hsu CM, Hsu YA, Tsai Y, Shieh FK, Huang SH, Wan L. et al. Emodin inhibits the growth of hepatoma cells: finding the common anti-cancer pathway using Huh7, Hep3B, and HepG2 cells. Biochem Biophys Res Commun. 2010;392:473–478. doi: 10.1016/j.bbrc.2009.10.153. [DOI] [PubMed] [Google Scholar]
- 66.Yu JQ, Bao W, Lei JC. Emodin regulates apoptotic pathway in human liver cancer cells. Phytother Res. 2013;27:251–257. doi: 10.1002/ptr.4703. [DOI] [PubMed] [Google Scholar]
- 67.Xing YX, Li MH, Tao L, Ruan LY, Hong W, Chen C. et al. Anti-cancer effects of emodin on HepG2 cells as revealed by 1H NMR based eetabolic profiling. J Proteome Res. 2018;17:1943–1952. doi: 10.1021/acs.jproteome.8b00029. [DOI] [PubMed] [Google Scholar]
- 68.Jing XB, Ueki N, Cheng JD, Imanishi H, Hada T. Induction of apoptosis in hepatocellular carcinoma cell lines by emodin. Jpn J Cancer Res. 2002;93:874–882. doi: 10.1111/j.1349-7006.2002.tb01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong SM, Zhang JX, Kang W, Wang L, Jing BQ, Zhang ZP. et al. Study on mitochondrial apoptosis induced by emodin in human hepatoma HepG2 cells. Drug Evaluat Res. 2018;41:773–779. [Google Scholar]
- 70.Dong XX, Ni BR, Fu J, Yin XB, You LT, Leng X. et al. Emodin induces apoptosis in human hepatocellular carcinoma HepG2 cells via the mitochondrial caspase dependent pathway. Oncol Rep. 2018;40:1985–1993. doi: 10.3892/or.2018.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L, He D, Li K, Liu HL, Wang BT, Zheng LF. et al. Emodin targets mitochondrial cyclophilin D to induce apoptosis in HepG2 cells. Biomed Pharmacother. 2017;90:222–228. doi: 10.1016/j.biopha.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 72.Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011;7:536–550. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subramaniam A, Shanmugam MK, Perumal E, Li F, Nachiyappan A, Dai X. et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta. 2013;1835:46–60. doi: 10.1016/j.bbcan.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Subramaniam A, Shanmugam MK, Ong TH, Li F, Perumal E, Chen LX. et al. Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3. Br J Pharmacol. 2013;170:807–821. doi: 10.1111/bph.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui YT, Lu P, Song G, Liu Q, Zhu D, Liu XB. Involvement of PI3K/Akt, ERK and p38 signaling pathways in emodin-mediated extrinsic and intrinsic human hepatoblastoma cell apoptosis. Food Chem Toxicol. 2016;92:26–37. doi: 10.1016/j.fct.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 78.Lin WF, Zhong MF, Yin HX, Chen YG, Cao QX, Wang C. et al. Emodin induces hepatocellular carcinoma cell apoptosis through MAPK and PI3K/Akt signaling pathways in vitro and in vivo. Oncol Rep. 2016;36:961–7. doi: 10.3892/or.2016.4861. [DOI] [PubMed] [Google Scholar]
- 79.Bai JG, Wu JF, Tang RF, Sun C, Ji JW, Yin ZL. et al. Emodin, a natural anthraquinone, suppresses liver cancerin vitro and in vivo by regulating VEGFR2 and miR-34a. Invest New Drugs. 2020;38:229–245. doi: 10.1007/s10637-019-00777-5. [DOI] [PubMed] [Google Scholar]
- 80.Zhu Y. Emodin inhibits the growth of transplanted tumor of liver carcinoma by down-regulating SCAP. Master thesis: Hubei University of Traditional Chinese Medicine. 2020.
- 81.Yang N, Li C, Li HL, Liu M, Cai XJ, Cao FJ. et al. Emodin induced SREBP1-dependent and SREBP1-independent apoptosis in hepatocellular carcinoma cells. Front Pharmacol. 2019;10:709. doi: 10.3389/fphar.2019.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 83.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manu KA, Shanmugam MK, Ong TH, Subramaniam A, Siveen KS, Perumal E. et al. Emodin suppresses migration and invasion through the modulation of CXCR4 expression in an orthotopic model of human hepatocellular carcinoma. PLoS One. 2013;8:e57015. doi: 10.1371/journal.pone.0057015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin WF, Zhong MF, Liang SF, Chen YG, Liu D, Yin ZF. et al. Emodin inhibits migration and invasion of MHCC 97H human hepatocellular carcinoma cells. Exp Ther Med. 2016;12:3369–3374. doi: 10.3892/etm.2016.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park CJ, Choi BS. The protein shuffle Sequential interactions among components of the human nucleotide excision repair pathway. FEBS J. 2006;273:1600–1608. doi: 10.1111/j.1742-4658.2006.05189.x. [DOI] [PubMed] [Google Scholar]
- 87.Altaha R, Liang X, Yu JJ, Reed E. Excision repair cross complementing-group 1: gene expression and platinum resistance. Int J Mol Med. 2004;14:959–970. [PubMed] [Google Scholar]
- 88.Qiu H, Yashiro M, Zhang X, Miwa A, Hirakawa K. A FGFR2 inhibitor, Ki23057, enhances the chemosensitivity of drug-resistnt gastric cancer cells. Cancer Lett. 2011;307:47–52. doi: 10.1016/j.canlet.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 89.Chen G, Qiu H, Ke SD, Hu SM, Yu SY, Zou SQ. Emodin regulating excision repair cross-complementation group 1 through fibroblast growth factor receptor 2 signaling. World J Gastroenterol. 2013;19:2481–2491. doi: 10.3748/wjg.v19.i16.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang SY, Heo K, Kim JS, Im JW, Lee SM, Cho M. et al. Emodin attenuates radioresistance induced by hypoxia in HepG2 cells via the enhancement of PARP1 cleavage and inhibition of JMJD2B. Oncol Rep. 2015;33:1691–1698. doi: 10.3892/or.2015.3744. [DOI] [PubMed] [Google Scholar]
- 91.Kim YS, Lee YM, Oh TI, Shin DH, Kim GH, Kan SY. et al. Emodin sensitizes hepatocellular carcinoma cells to the anti-cancer effect of sorafenib through suppression of cholesterol metabolism. Int J Mol Sci. 2018;19:3127. doi: 10.3390/ijms19103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang HL. Experimental study on the effects of emodin and cisplatin on the invasion and migration of HepG2 cells. Master thesis, Changchun University of Traditional Chinese Medicine. 2020.
- 93.Cekaite L, Eide PW, Lind GE, Skotheim RI, Lothe RA. MicroRNAs as growth regulators, their function and biomarker status in colorectal cancer. Oncotarget. 2016;7:6476–6505. doi: 10.18632/oncotarget.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 95.Lin J, Chuang CC, Zuo L. Potential roles of microRNAs and ROS in colorectal cancer: diagnostic biomarkers and therapeutic targets. Oncotarget. 2017;8:17328–17346. doi: 10.18632/oncotarget.14461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH, Yang JS. Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem Toxicol. 2012;50:1271–1278. doi: 10.1016/j.fct.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 97.Xie MJ, Ma YH, Miao L, Wang Y, Wang HZ, Xing YY. et al. Emodin-provoked oxidative stress induces apoptosis in human colon cancer HCT116 cells through a p53-mitochondrial apoptotic pathway. Asian Pac J Cancer Prev. 2014;15:5201–5205. doi: 10.7314/apjcp.2014.15.13.5201. [DOI] [PubMed] [Google Scholar]
- 98.Ma L, Li W. Emodin inhibits LOVO colorectal cancer cell proliferation via the regulation of the Bcl-2/Bax ratio and cytochrome c. Exp Ther Med. 2014;8:1225–1228. doi: 10.3892/etm.2014.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu B, Yuan B, Zhang L, Mu W, Wang C. ROS/p38/p53/Puma signaling pathway is involved in emodin-induced apoptosis of human colorectal cancer cells. Int J Clin Exp Med. 2015;8:15413–15422. [PMC free article] [PubMed] [Google Scholar]
- 100.Lee KH, Lee MS, Cha EY, Sul JY, Lee JS, Kim JS. et al. Inhibitory effect of emodin on fatty acid synthase, colon cancer proliferation and apoptosis. Mol Med Rep. 2017;15:2163–2173. doi: 10.3892/mmr.2017.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma Q, Ding Y, Wu Z, Li Y. Antitumor effects of emodin in Caco-2 human colon carcinoma cells are mediated via apoptosis, cell cycle arrest and downregulation of PI3K/AKT signalling pathway. J BUON. 2018;23:587–591. [PubMed] [Google Scholar]
- 102.Li T, Si W, Zhu J, Yin L, Zhong C. Emodin reverses 5-Fu resistance in human colorectal cancer via downregulation of PI3K/Akt signaling pathway. Am J Transl Res. 2020;12:1851–1861. [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Luo Q, He X, Wei H, Wang T, Shao J. et al. Emodin induces apoptosis of colon cancer cells via induction of autophagy in a ROS-dependent manner. Oncol Res. 2018;26:889–899. doi: 10.3727/096504017X15009419625178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han YM, Lee SK, Jeong DG, Ryu SE, Han DC, Kim DK. et al. Emodin inhibits migration and invasion of DLD-1 (PRL-3) cells via inhibition of PRL-3 phosphatase activity. Bioorg Med Chem Lett. 2012;22:323–326. doi: 10.1016/j.bmcl.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 105.Pooja T, Karunagaran D. Emodin suppresses Wnt signaling in human colorectal cancer cells SW480 and SW620. Eur J Pharmacol. 2014;742:55–64. doi: 10.1016/j.ejphar.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 106.Gu J, Cui CF, Yang L, Wang L, Jiang XH. Emodin inhibits colon cancer cell invasion and migration by suppressing epithelial-mesenchymal transition via the Wnt/b-Catenin pathway. Oncol Res. 2019;27:193–202. doi: 10.3727/096504018X15150662230295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Dai G, Ding K, Cao Q, Xu T, He F, Liu S. et al. Emodin suppresses growth and invasion of colorectal cancer cells by inhibiting VEGFR2. Eur J Pharmacol. 2019;859:172525. doi: 10.1016/j.ejphar.2019.172525. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y, Pu W, Bousquenaud M, Cattin S, Zaric J, Sun LK. et al. Emodin inhibits inflammation, carcinogenesis, and cancer progression in the AOM/DSS model of colitis-associated intestinal tumorigenesis. Front Oncol. 2021;10:564674. doi: 10.3389/fonc.2020.564674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kamath R, Mahajan KS, Ashok L, Sanal TS. A study on risk factors of breast cancer among patients attending the tertiary care hospital, in Udupi district. Indian J Community Med. 2013;38:95–99. doi: 10.4103/0970-0218.112440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Waks AG, Winer EP. Breast cancer treatment: A Review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 111.Zhang L, Chang CJ, Bacus SS, Hung MC. Suppressed transformation and induced differentiation of HER-2/neu-overexpressing breast cancer cells by emodin. Cancer Res. 1995;55:3890–6. [PubMed] [Google Scholar]
- 112.Zhang L, Lau YK, Hong RL, Xi L, Kim DS, Chen CF. et al. Tyrosine kinase inhibitors, emodin and its derivative repress HER-2/neu-induced cellular transformation and metastasis-associated properties. Oncogene. 1998;16:2855–2863. doi: 10.1038/sj.onc.1201813. [DOI] [PubMed] [Google Scholar]
- 113.Zhang L, Lau YK, Xia W, Hortobagyi GN, Hung MC. Tyrosine kinase inhibitor emodin suppresses growth of HER-2/neu-overexpressing breast cancer cells in athymic mice and sensitizes these cells to the inhibitory effect of paclitaxel. Clin Cancer Res. 1999;5:343–353. [PubMed] [Google Scholar]
- 114.Ueno N, Kiyokawa N, Hung M. Growth suppression of low HER-2/neu-expressing breast cancer cell line MDA-MB-435 by tyrosine kinase inhibitor emodin. Oncol Rep. 1996;3:509–511. doi: 10.3892/or.3.3.509. [DOI] [PubMed] [Google Scholar]
- 115.Huang ZW, Chen GC, Shi P. Emodin-induced apoptosis in human breast cancer BCap-37 cells through the mitochondrial signaling pathway. Arch Pharm Res. 2008;31:742–748. doi: 10.1007/s12272-001-1221-6. [DOI] [PubMed] [Google Scholar]
- 116.Huang ZW, Chen GC, Shi P. Effects of emodin on the gene expression profiling of human breast carcinoma cells. Cancer Detect Prev. 2009;32:286–91. doi: 10.1016/j.cdp.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 117.Li WY, Chan RYK, Yu PHF, Chan SH. Emodin induces cytotoxic effect in human breast carcinoma MCF-7 cell through modulating the expression of apoptosis-related genes. Pharm Biol. 2013;51:1175–81. doi: 10.3109/13880209.2013.782322. [DOI] [PubMed] [Google Scholar]
- 118.Sui JQ, Xie KP, Zou W, Xie MJ. Emodin inhibits breast cancer cell proliferation through the ERα-MAPK/Akt-Cyclin D1/Bcl-2 signaling pathway. Asian Pac J Cancer Prev. 2014;15:6247–51. doi: 10.7314/apjcp.2014.15.15.6247. [DOI] [PubMed] [Google Scholar]
- 119.Zu C, Zhang MD, Xue H, Cai XP, Zhao L, He A. et al. Emodin induces apoptosis of human breast cancer cells by modulating the expression of apoptosis-related genes. Oncol Lett. 2015;10:2919–2924. doi: 10.3892/ol.2015.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang N, Wang JW, Sheng A, Huang S, Tang YY, Ma ST. et al. Emodin inhibits the proliferation of MCF-7 human breast cancer cells through activation of aryl hydrocarbon receptor (AhR) Front Pharmacol. 2021;11:622046. doi: 10.3389/fphar.2020.622046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferry KV, Hamilton TC, Johnson SW. Increased nucleotide excision repair in cisplatin-resistant ovarian cancer cells: role of ERCC1-XPF. Biochem Pharmacol. 2000;60:1305–1313. doi: 10.1016/s0006-2952(00)00441-x. [DOI] [PubMed] [Google Scholar]
- 122.Selvakumaran M, Pisarcik DA, Bao R, Yeung AT, Hamilton TC. Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res. 2003;63:1311–1316. [PubMed] [Google Scholar]
- 123.Fu JM, Zhou J, Shi J, Xie JS, Huang L, Yip AYS. et al. Emodin affects ERCC1 expression in breast cancer cells. J Transl Med. 2012;10:S7. doi: 10.1186/1479-5876-10-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zu C, Qin GY, Yang CS, Liu N, He A, Zhang MD. et al. Low dose Emodin induces tumor senescence for boosting breast cancer chemotherapy via silencing NRARP. Biochem Biophys Res Commun. 2018;505:973–978. doi: 10.1016/j.bbrc.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 125.Li B, Zhao X, Zhang L, Cheng W. Emodin interferes with Akt1-mediated DNA damage and decreases resistance of breast cancer cells to doxorubicin. Front Oncol. 2021;10:588533. doi: 10.3389/fonc.2020.588533. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Jelassi B, Anchelin M, Chamouton J, Cayuela ML, Clarysse L, Li JY. et al. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis. 2013;34:1487–1496. doi: 10.1093/carcin/bgt099. [DOI] [PubMed] [Google Scholar]
- 127.Petrulio CA, Kim-Schulze S, Kaufman HL. The tumour microenvironment and implications for cancer immunotherapy. Exp Opin Biol Ther. 2006;6:671–684. doi: 10.1517/14712598.6.7.671. [DOI] [PubMed] [Google Scholar]
- 128.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jia XM, Yu F, Wang JF, Iwanowycz S, Saaoud F, Wang YZ. et al. Emodin suppresses pulmonary metastasis of breast cancer cells accompanied with decreased macrophage recruitment and M2 polarization in the lungs. Breast Cancer Res Treat. 2014;148:291–302. doi: 10.1007/s10549-014-3164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Q, Hodge J, Wang JF, Wang YZ, Wang LM, Singh U. et al. Emodin reduces breast cancer lung metastasis by suppressing macrophage-induced breast cancer cell epithelial-mesenchymal transition and cancer stem cell formation. Theranostics. 2020;10:8365–8381. doi: 10.7150/thno.45395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Iwanowycz S, Wang JF, Hodge J, Wang YZ, Yu F, Fan DP. Emodin inhibits breast cancer growth by blocking the tumor-promoting feedforward loop between cancer cells and macrophages. Mol Cancer Ther. 2016;15:1931–1942. doi: 10.1158/1535-7163.MCT-15-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hsu HC, Liu LC, Wang HY, Hung CM, Lin YC, Ho CT. et al. Stromal fibroblasts from the interface zone of triple negative breast carcinomas induced epithelial-mesenchymal transition and its inhibition by emodin. PLoS One. 2017;12:e0164661. doi: 10.1371/journal.pone.0164661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun Y, Wang XF, Zhou QM, Lu YY, Zhang H, Chen QL. et al. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol Rep. 2015;33:338–346. doi: 10.3892/or.2014.3585. [DOI] [PubMed] [Google Scholar]
- 134.Song XY, Zhou XQ, Qin YN, Yang JF, Wang Y, Sun ZP. et al. Emodin inhibits epithelial-mesenchymal transition and metastasis of triple negative breast cancer via antagonism of CC-chemokine ligand 5 secreted from adipocytes. Int J Mol Med. 2018;42:579–588. doi: 10.3892/ijmm.2018.3638. [DOI] [PubMed] [Google Scholar]
- 135.Ma JC, Lu H, Wang S, Chen B, Liu ZJ, Ke XQ. et al. The anthraquinone derivative Emodin inhibits angiogenesis and metastasis through downregulating Runx2 activity in breast cancer. Int J Oncol. 2015;46:1619–1628. doi: 10.3892/ijo.2015.2888. [DOI] [PubMed] [Google Scholar]
- 136.Zou GY, Zhang XT, Wang L, Li XY, Xie TY, Zhao J. et al. Herb-sourced emodin inhibits angiogenesis of breast cancer by targeting VEGFA transcription. Theranostics. 2020;10:6839–6853. doi: 10.7150/thno.43622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bhattacharjee M, Upadhyay P, Sarker S, Basu A, Das S, Ghosh A. et al. Combinatorial therapy of thymoquinone and emodin synergistically enhances apoptosis, attenuates cell migration and reduces stemness efficiently in breast cancer. Biochim Biophys Acta Gen Subj. 2020;1864:129695. doi: 10.1016/j.bbagen.2020.129695. [DOI] [PubMed] [Google Scholar]
- 138.Fu M, Tang W, Liu JJ, Gong XQ, Kong L, Yao XM. et al. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J Drug Target. 2020;28:245–258. doi: 10.1080/1061186X.2019.1656725. [DOI] [PubMed] [Google Scholar]
- 139.Guo JL, Li WP, Shi HL, Xie XH, Li LS, Tang HL. et al. Synergistic effects of curcumin with emodin against the proliferation and invasion of breast cancer cells through upregulation of miR-34a. Mol Cell Biochem. 2013;382:103–111. doi: 10.1007/s11010-013-1723-6. [DOI] [PubMed] [Google Scholar]
- 140.Ponnusamy L, Kothandan G, Manoharan R. Berberine and Emodin abrogates breast cancer growth and facilitates apoptosis through inactivation of SIK3-induced mTOR and Akt signaling pathway. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165897. doi: 10.1016/j.bbadis.2020.165897. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y, Shi S, Hua J, Xu J, Zhang B, Liu J. et al. Differentiation of solid-pseudopapillary tumors of the pancreas from pancreatic neuroendocrine tumors by using endoscopic ultrasound. Clin Res Hepatol Gastroenterol. 2020;44:947–953. doi: 10.1016/j.clinre.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 142.Moore A, Donahue T. Pancreatic cancer. JAMA. 2019;322:1426. doi: 10.1001/jama.2019.14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fan Z, Luo G, Gong Y, Xu H, Qian Y, Deng S. et al. Prognostic value of the c-reactive protein/Lymphocyte ratio in pancreatic cancer. Ann Surg Oncol. 2020;27:4017–4025. doi: 10.1245/s10434-020-08301-3. [DOI] [PubMed] [Google Scholar]
- 144.Sijde FVD, Vietsch EE, Mustafa DAM, Li Y, van Eijck CHJ. Serum miR-338-3p and miR-199b-5p are associated with the absolute neutrophil count in patients with resectable pancreatic cancer. Clin Chim Acta. 2020;505:183–189. doi: 10.1016/j.cca.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 145.Zhang H, Chen L, Bu HQ, Yu QJ, Jiang DD, Pan FP. et al. Effects of emodin on the demethylation of tumor-suppressor genes in pancreatic cancer PANC-1 cells. Oncol Rep. 2015;33:3015–3023. doi: 10.3892/or.2015.3914. [DOI] [PubMed] [Google Scholar]
- 146.Pan FP, Zhou HK, Bu HQ, Chen ZQ, Zhang H, Xu LP. et al. Emodin enhances the demethylation by 5-Aza-CdR of pancreatic cancer cell tumor-suppressor genes P16, RASSF1A and ppENK. Oncol Rep. 2016;35:1941–1949. doi: 10.3892/or.2016.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 148.Hu L, Cui R, Liu H, Wang F. Emodin and rhein decrease levels of hypoxia-inducible factor-1α in human pancreatic cancer cells and attenuate cancer cachexia in athymic mice carrying these cells. Oncotarget. 2017;8:88008–88020. doi: 10.18632/oncotarget.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cai J, Razzak A, Hering J, Saed A, Babcock TA, Helton S. et al. Feasibility evaluation of emodin (rhubarb extract) as an inhibitor of pancreatic cancer cell proliferation in vitro. JPEN J Parenter Enteral Nutr. 2008;32:190–196. doi: 10.1177/0148607108314371. [DOI] [PubMed] [Google Scholar]
- 150.Li Z, Wang M, Yao X, Luo W, Qu Y, Yu D. et al. Development of a novel EGFR-targeting antibody-drug conjugate for pancreatic cancer therapy. Target Oncol. 2019;14:93–105. doi: 10.1007/s11523-018-0616-8. [DOI] [PubMed] [Google Scholar]
- 151.Wang Q, Yang S, Wang K, Sun SY. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J Hematol Oncol. 2019;121:63. doi: 10.1186/s13045-019-0759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee K, Kim Y, Jung HA, Lee SH, Ahn JS, Ahn MJ. et al. Repeat biopsy procedures and T790M rates after afatinib, gefitinib, or erlotinib therapy in patients with lung cancer. Lung cancer. 2019;130:87–92. doi: 10.1016/j.lungcan.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 153.Wang Z, Chen H, Chen J, Hong Z, Liao Y, Zhang Q. et al. Emodin sensitizes human pancreatic cancer cells to EGFR inhibitor through suppressing Stat3 signaling pathway. Cancer Manag Res. 2019;11:8463–8473. doi: 10.2147/CMAR.S221877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo QQ, Chen Y, Zhang B, Kang MX, Xie QP, Wu YL. Potentiation of the effect of gemcitabine by emodin in pancreatic cancer is associated with survivin inhibition. Biochem Pharmacol. 2009;77:1674–83. doi: 10.1016/j.bcp.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 155.Liu A, Chen H, Tong H, Ye S, Qiu M, Wang Z. et al. Emodin potentiates the antitumor effects of gemcitabine in pancreatic cancer cells via inhibition of nuclear factor-κB. Mol Med Rep. 2011;4:221–227. doi: 10.3892/mmr.2011.414. [DOI] [PubMed] [Google Scholar]
- 156.Chen H, Wei W, Guo Y, Liu A, Tong H, Wang Z. et al. Enhanced effect of gemcitabine by emodin against pancreatic cancer in vivo via cytochrome C-regulated apoptosis. Oncol Rep. 2011;25:1253–1261. doi: 10.3892/or.2011.1174. [DOI] [PubMed] [Google Scholar]
- 157.Wei WT, Chen H, Ni ZL, Liu HB, Tong HF, Fan L. et al. Antitumor and apoptosis-promoting properties of emodin, an anthraquinone derivative from Rheum officinale Baill, against pancreatic cancer in mice via inhibition of Akt activation. Int J Oncol. 2011;39:1381–1390. doi: 10.3892/ijo.2011.1147. [DOI] [PubMed] [Google Scholar]
- 158.Wang ZH, Chen H, Guo HC, Tong HF, Liu JX, Wei WT. et al. Enhanced antitumor efficacy by the combination of emodin and gemcitabine against human pancreatic cancer cells via downregulation of the expression of XIAP in vitro and in vivo. Int J Oncol. 2011;39:1123–1131. doi: 10.3892/ijo.2011.1115. [DOI] [PubMed] [Google Scholar]
- 159.Guo HC, Bu HQ, Luo J, Wei WT, Liu DL, Chen H. et al. Emodin potentiates the antitumor effects of gemcitabine in PANC-1 pancreatic cancer xenograft model in vivo via inhibition of inhibitors of apoptosis. Int J Oncol. 2012;40:1849–1857. doi: 10.3892/ijo.2012.1389. [DOI] [PubMed] [Google Scholar]
- 160.Liu DL, Bu H, Li H, Chen H, Guo HC, Wang ZH. et al. Emodin reverses gemcitabine resistance in pancreatic cancer cells via the mitochondrial apoptosis pathway in vitro. Int J Oncol. 2012;40:1049–1057. doi: 10.3892/ijo.2011.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang W, Chen H, Liu DL, Li H, Luo J, Zhang JH. et al. Emodin sensitizes the gemcitabine-resistant cell line Bxpc-3/Gem to gemcitabine via downregulation of NF-κB and its regulated targets. Int J Oncol. 2013;42:1189–1196. doi: 10.3892/ijo.2013.1839. [DOI] [PubMed] [Google Scholar]
- 162.Guo H, Liu F, Yang S, Xue T. Emodin alleviates gemcitabine resistance in pancreatic cancer by inhibiting MDR1/P-glycoprotein and MRPs expression. Oncol Lett. 2020;20:167. doi: 10.3892/ol.2020.12030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 163.Tong H, Huang Z, Chen H, Zhou B, Liao Y, Wang Z. Emodin reverses gemcitabine resistance of pancreatic cancer cell lines through inhibition of IKKβ/NF-κB signaling pathway. Onco Targets Ther. 2020;13:9839–9848. doi: 10.2147/OTT.S253691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu A, Chen H, Wei W, Ye S, Liao W, Gong J. et al. Antiproliferative and antimetastatic effects of emodin on human pancreatic cancer. Oncol Rep. 2011;26:81–89. doi: 10.3892/or.2011.1257. [DOI] [PubMed] [Google Scholar]
- 165.Liu JX, Zhang JH, Li HH, Lai FJ, Chen KJ, Chen H. et al. Emodin induces Panc-1 cell apoptosis via declining the mitochondrial membrane potential. Oncol Rep. 2012;28:1991–1916. doi: 10.3892/or.2012.2042. [DOI] [PubMed] [Google Scholar]
- 166.Li N, Wang C, Zhang P, You S. Emodin inhibits pancreatic cancer EMT and invasion by up-regulating microRNA-1271. Mol Med Rep. 2018;18:3366–3374. doi: 10.3892/mmr.2018.9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin SZ, Wei WT, Chen H, Chen KJ, Tong HF, Wang ZH. et al. Antitumor activity of emodin against pancreatic cancer depends on its dual role: promotion of apoptosis and suppression of angiogenesis. PLOS one. 2012;7:e42146. doi: 10.1371/journal.pone.0042146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin SZ, Xu JB, Ji X, Chen H, Xu HT, Hu P. et al. Emodin inhibits angiogenesis in pancreatic cancer by regulating the transforming growth factor-β/drosophila mothers against decapentaplegic pathway and angiogenesis-associated microRNAs. Mol Med Rep. 2015;12:5865–5871. doi: 10.3892/mmr.2015.4158. [DOI] [PubMed] [Google Scholar]
- 169.Hoffmann VS, Baccarani M, Hasford J, Lindoerfer D, Burgstaller S, Sertic D. et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015;29:1336–1343. doi: 10.1038/leu.2015.73. [DOI] [PubMed] [Google Scholar]
- 170.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 171.Brown M, Bellon M, Nicot C. Emodin and DHA potently increase arsenic trioxide interferon-alpha-induced cell death of HTLV-I-transformed cells by generation of reactive oxygen species and inhibition of Akt and AP-1. Blood. 2007;109:1653–1659. doi: 10.1182/blood-2006-04-015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY, Ko CH. et al. Emodin induces apoptosis in human promyeloleukemic HL-60 cells accompanied by activation of caspase 3 cascade but independent of reactive oxygen species production. Biochem Pharmacol. 2002;64:1713–1724. doi: 10.1016/s0006-2952(02)01386-2. [DOI] [PubMed] [Google Scholar]
- 173.Zhang XX, Sun YQ, Wei XF, Li HL, Zhang QK. Effects of emodin on proliferation and apoptosis of human chronic myeloid leukemia K562 cells and expression of C-myC gene. Chin J cancer prev treal. 2009;16:1541–1545. [Google Scholar]
- 174.Jin DT, Liu BZ, Liu C, Wang CG, Wang C, Wang DS. et al. Experimental study of emodin induced apoptosis of leukemia K562 cells. J Chongqing Med Univ. 2009;34:822–825. [Google Scholar]
- 175.Wang CG, Liu BZ, Jin DT, Wang C, Wu Y, Zhu D. et al. Inhibitory effect of emodin on the subcutaneously transplanted tumor of human K562cell in nude mice. Chin Hosp Pharm J. 2010;30:179–182. [Google Scholar]
- 176.Wang CG, Yang JQ, Liu BZ, Jin DT, Wang C, Zhong L. et al. Anti-tumor activity of emodin against human chronic myelocytic leukemia K562 cell lines in vitro and in vivo. Eur J Pharmacol. 2010;627:33–41. doi: 10.1016/j.ejphar.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 177.Lian XL, Hu JD, Zheng ZH, Chen YY, Zheng HY. Emodin induced apoptosis of leukemia U937 cells and its mechanism. Chin Pharmacol Bull. 2007;23:1312–6. [Google Scholar]
- 178.Muto A, Hori M, Sasaki Y, Saitoh A, Yasuda I, Maekawa T. et al. Emodin has a cytotoxic activity against human multiple myeloma as a Janus-activated kinase 2 inhibitor. Mol Cancer Ther. 2007;6:987–994. doi: 10.1158/1535-7163.MCT-06-0605. [DOI] [PubMed] [Google Scholar]
- 179.Zheng HY, Hu JD, Zheng ZH, Huang LY, Chen YY, Zheng J. et al. Emodin induces leukemic HL-60 cells apoptosis probably by inhibiting Akt signal pathway. Acta Pharm Sin. 2007;42:1142–1146. [PubMed] [Google Scholar]
- 180.Chen YY, Zheng HY, Hu JD, Zheng ZH, Zheng J, Lian XL. et al. Inhibitory effects of emodin on drug-resistant HL-60/ADR cell proliferation and its induction of apoptosis. J Exp Hemat. 2007;15:955–960. [PubMed] [Google Scholar]
- 181.Chen Y, Li J, Hu J, Zheng J, Zheng Z, Liu T. et al. Emodin enhances ATRA-induced differentiation and induces apoptosis in acute myeloid leukemia cells. Int J Oncol. 2014;45:2076–2084. doi: 10.3892/ijo.2014.2610. [DOI] [PubMed] [Google Scholar]
- 182.Wang CG, Zhong L, Liu YL, Shi XJ, Shi LQ, Zeng L. et al. Emodin exerts an antiapoptotic effect on human chronic myelocytic leukemia K562 cell lines by targeting the PTEN/PI3K-Akt signaling pathway and deleting BCR-ABL. Integr Cancer Ther. 2017;16:526–539. doi: 10.1177/1534735416664784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chang YC, Lai TY, Yu CS, Chen HY, Yang JS, Chueh FS, Emodin induces apoptotic death in murine myelomonocytic leukemia WEHI-3 cells in vitro and enhances phagocytosis in leukemia mice in vivo. Evid Based Compl Alt Med. 2011. 523596. [DOI] [PMC free article] [PubMed]
- 184.Falchetti A, Franchi A, Bordi C, Mavilia C, Masi L, Cioppi F. et al. Azidothymidine induces apoptosis and inhibits cell growth and telomerase activity of human parathyroid cancer cells in culture. J Bone Miner Res. 2005;20:410–418. doi: 10.1359/JBMR.041123. [DOI] [PubMed] [Google Scholar]
- 185.Humer J, Ferko B, Waltenberger A, Rapberger R, Pehamberger H, Muster T. Azidothymidine inhibits melanoma cell growth in vitro and in vivo. Melanoma Res. 2008;18:314–321. doi: 10.1097/CMR.0b013e32830aaaa6. [DOI] [PubMed] [Google Scholar]
- 186.Olivero OA, Tejera AM, Fernandez JJ, Taylor BJ, Das S, Divi RL. et al. Zidovudine induces S-phase arrest and cell cycle gene expression changes in human cells. Mutagenesis. 2005;20:139–146. doi: 10.1093/mutage/gei019. [DOI] [PubMed] [Google Scholar]
- 187.Chen P, Liu Y, Sun Y, Chen C, Qi Y, Zhang Y. AZT and emodin exhibit synergistic growth-inhibitory effects on K562/ADM cells by inducing S phase cell cycle arrest and suppressing MDR1 mRNA/P-gp protein expression. Pharm Biol. 2013;51:1586–1591. doi: 10.3109/13880209.2013.803257. [DOI] [PubMed] [Google Scholar]
- 188.Min H, Niu M, Zhang W, Yan J, Li J, Tan X. et al. Emodin reverses leukemia multidrug resistance by competitive inhibition and downregulation of P-glycoprotein. PloS one. 2017;12:e0187971. doi: 10.1371/journal.pone.0187971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ma W, Liu F, Yuan L, Zhao C, Chen C. Emodin and AZT synergistically inhibit the proliferation and induce the apoptosis of leukemia K562 cells through the EGR1 and the Wnt/β-catenin pathway. Oncol Rep. 2020;43:260–269. doi: 10.3892/or.2019.7408. [DOI] [PubMed] [Google Scholar]
- 190.Chen Y, Gan D, Huang Q, Luo X, Lin D, Hu J. Emodin and its combination with cytarabine induce apoptosis in resistant acute myeloid leukemia cells in vitro and in vivo. Cell Physiol Biochem. 2018;48:2061–2073. doi: 10.1159/000492544. [DOI] [PubMed] [Google Scholar]
- 191.Wang XY, Sun GB, Wang YJ, Yan F. Emodin inhibits resistance to imatinib by downregulation of Bcr-Abl and STAT5 and allosteric inhibition in chronic myeloid leukemia cells. Biol Pharm Bull. 2020;43:1526–1533. doi: 10.1248/bpb.b20-00325. [DOI] [PubMed] [Google Scholar]
- 192.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR. et al. Interferons at age 50: past, current and future impact on biomedicine. Nature reviews. Drug discovery. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.He Y, Huang J, Wang P, Shen X, Li S, Yang L. et al. Emodin potentiates the antiproliferative effect of interferon α/β by activation of JAK/STAT pathway signaling through inhibition of the 26S proteasome. Oncotarget. 2016;7:4664–4679. doi: 10.18632/oncotarget.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Srinivas G, Anto RJ, Srinivas P, Vidhyalakshmi S, Senan VP, Karunagaran D. Emodin induces apoptosis of human cervical cancer cells through poly (ADP-ribose) polymerase cleavage and activation of caspase-9. Eur J Pharmacol. 2003;473:117–125. doi: 10.1016/s0014-2999(03)01976-9. [DOI] [PubMed] [Google Scholar]
- 195.Olsen BB, Bjørling-Poulsen M, Guerra B. Emodin negatively affects the phosphoinositide 3-kinase/Akt signalling pathway: a study on its mechanism of action. Int J Biochem Cell Biol. 2007;39:227–237. doi: 10.1016/j.biocel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 196.Wang YX, Yu H, Zhang YY, Liu YQ, Ge X, Wu XK. Emodin induces apoptosis of human cervical cancer hela cells via intrinsic mitochondrial and extrinsic death receptor pathway. Cancer cell Int. 2013;13:71. doi: 10.1186/1475-2867-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang Y, Yu H, Zhang J, Ge, X Gao J, Zhang Y. et al. Anti-tumor effect of emodin on gynecological cancer cells. Cell Oncol (Dordr) 2015;38:353–63. doi: 10.1007/s13402-015-0234-8. [DOI] [PubMed] [Google Scholar]
- 198.Trybus W, Król T, Trybus E, Kopacz-Bednarska A, Król G, Karpowicz E. Changes in the lysosomal system of cervical cancer cells induced by emodin action. Anticancer Res. 2017;37:6087–6096. doi: 10.21873/anticanres.12057. [DOI] [PubMed] [Google Scholar]
- 199.Galiardi-Campoy AEB, Machado FC, Carvalho T, Tedesco AC, Rahal P, Calmon MF. Effects of photodynamic therapy mediated by emodin in cervical carcinoma cells. Photodiagnosis Photodyn Ther. 2021;35:102394. doi: 10.1016/j.pdpdt.2021.102394. [DOI] [PubMed] [Google Scholar]
- 200.Thacker PC, Karunagaran D. Curcumin and emodin down-regulate TGF-β signaling pathway in human cervical cancer cells. PloS one. 2015;10:e0120045. doi: 10.1371/journal.pone.0120045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9–32. doi: 10.20892/j.issn.2095-3941.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kurokawa T, He G, Siddik ZH. Protein kinase inhibitors emodin and dichlororibofuranosylbenzimidazole modulate the cellular accumulation and cytotoxicity of cisplatin in a schedule-dependent manner. Cancer Chemother Pharmacol. 2010;65:427–36. doi: 10.1007/s00280-009-1045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Song K, Lv T, Chen Y, Diao Y, Yao Q, Wang Y. Emodin inhibits TGF-β2 by activating the FOXD3/miR-199a axis in ovarian cancer cells in vitro. Oncology reports. 2018;39:2063–2070. doi: 10.3892/or.2018.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xue H, Chen Y, Cai X, Zhao L, He A, Guo K. et al. The combined effect of survivin-targeted shRNA and emodin on the proliferation and invasion of ovarian cancer cells. Anticancer Drugs. 2013;24:937–944. doi: 10.1097/CAD.0b013e328364efe0. [DOI] [PubMed] [Google Scholar]
- 205.Lu JJ, Xu Y, Wei X, Zhao Z, Xue J, Liu PS. Emodin inhibits the epithelial to mesenchymal transition of epithelial ovarian cancer cells via ILK/GSK-3 β/Slug signaling pathway. Biomed Res Int. 2016. 6253280. [DOI] [PMC free article] [PubMed]
- 206.Hu C, Dong TT, Li R, Lu JJ, Wei X, Liu PS. Emodin inhibits epithelial to mesenchymal transition in epithelial ovarian cancer cells by regulation of GSK-3β/β-catenin/ZEB1 signaling pathway. Oncol Rep. 2016;35:2027–34. doi: 10.3892/or.2016.4591. [DOI] [PubMed] [Google Scholar]
- 207.Lu JJ, Xu Y, Zhao Z, Ke XN, Wei X, Kang J. et al. Emodin suppresses proliferation, migration and invasion in ovarian cancer cells by down regulating ILK in vitro and in vivo. Onco Targets Ther. 2017;10:3579–3589. doi: 10.2147/OTT.S138217. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 208.Li J, Liu P, Mao H, Wang A, Zhang X. Emodin sensitizes paclitaxel-resistant human ovarian cancer cells to paclitaxel-induced apoptosis in vitro. Oncology reports. 2009;21:1605–1610. [PubMed] [Google Scholar]
- 209.Ma J, Yang J, Wang C, Zhang N, Dong Y, Wang CJ. et al. The combined effect of survivin-targeted shRNA and emodin on the proliferation and invasion of ovarian cancer cells. Anticancer Drugs. 2013;24:937–44. doi: 10.1097/CAD.0b013e328364efe0. [DOI] [PubMed] [Google Scholar]
- 210.Wu FL, Chu PY, Chen GY, Wang K, Hsu WY, Ahmed A. et al. Natural anthraquinone compound emodin as a novel inhibitor of aurora A kinase: A pilot study. Chem Biol Drug Des. 2021 doi: 10.1111/cbdd.13938. Doi: 10.1111/cbdd.13938. [DOI] [PubMed] [Google Scholar]
- 211.Lin SY, Lai WW, Ho CC, Yu FS, Chen GW, Yang JS. et al. Emodin induces apoptosis of human tongue squamous cancer SCC-4 cells through reactive oxygen species and mitochondria-dependent pathways. Anticancer Res. 2009;29:327–35. [PubMed] [Google Scholar]
- 212.Chen YY, Chang SY, Lin JG, Yang JS, Ma YS, Liao CL. et al. Emodin, sloe-emodin and rhein induced DNA damage and inhibited DNA repair gene expression in SCC-4 human tongue cancer cells. Anticancer Res. 2010;30:945–51. [PubMed] [Google Scholar]
- 213.Chen YY, Chiang SY, Lin JG, Ma YS, Liao CL, Weng SW. et al. Emodin, aloe-emodin and rhein inhibit migration and invasion in human tongue cancer SCC-4 cells through the inhibition of gene expression of matrix metalloproteinase-9. Int J Oncol. 2010;36:1113–20. doi: 10.3892/ijo_00000593. [DOI] [PubMed] [Google Scholar]
- 214.Wang QJ, Cai XB, Liu MH, Hu H, Tan XJ, Jing XB. Apoptosis induced by emodin is associated with alterations of intracellular acidification and reactive oxygen species in EC-109 cells. Biochem Cell Biol. 2010;88:767–74. doi: 10.1139/O10-020. [DOI] [PubMed] [Google Scholar]
- 215.Way TD, Huang JT, Chou CH, Huang CH, Yang MH, Ho CT. Emodin represses TWIST1-induced epithelial mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the b-catenin and Akt pathways. Eur J Cancer. 2014;50:366–78. doi: 10.1016/j.ejca.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 216.Zhang KL, Jiao LL, Zhu YJ, Wu F, Li JP, Yu ZH. Effect of emodin on proliferation and cell cycle progression of human oral squamous cell carcinoma Tca8113. J South Med Univ. 2015;35:665–670. [PubMed] [Google Scholar]
- 217.Manimaran A, Buddhan R, Manoharan S. Emodin downregulates cell proliferation markers during dmba induced oral carcinogenesis in golden Syrian hamsters. Afr J Tradit Complement Altern Med. 2017;14:83–91. doi: 10.21010/ajtcam.v14i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Manimaran A, Manoharan S. Tumor Preventive efficacy of emodin in 7,12-Dimethylbenz[a]Anthracene-induced oral carcinogenesis: a histopathological and biochemical Approach. Pathol Oncol Res. 2018;24:19–29. doi: 10.1007/s12253-017-0205-7. [DOI] [PubMed] [Google Scholar]
- 219.Moreira TF, Sorbo JM, Souza FO, Fernandes BC, Ocampos F, de Oliveira D. et al. Emodin, physcion, and crude extract of rhamnus sphaerosperma var. pubescens induce mixed cell death, increase in oxidativestress, DNA damage, and inhibition of AKT in cervical and oral squamous carcinoma cell lines. Oxid Med Cell Longev. 2018;2018:2390234. doi: 10.1155/2018/2390234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ma LS, Yang YP, Yin ZZ, Liu M, Wang LW, Chen LX. et al. Emodin suppresses the nasopharyngeal carcinoma cells by targeting the chloride channels. Biomed Pharmacother. 2017;90:615–625. doi: 10.1016/j.biopha.2017.03.088. [DOI] [PubMed] [Google Scholar]
- 221.Shi GH, Zhou L. Emodin suppresses angiogenesis and metastasis in anaplastic thyroid cancer by affecting TRAF6-mediated pathways in vivo and in vitro. Mol Med Rep. 2018;18:5191–5197. doi: 10.3892/mmr.2018.9510. [DOI] [PubMed] [Google Scholar]
- 222.Li WL, Wang D, Li MJ, Li BY. Emodin inhibits the proliferation of papillary thyroid carcinoma by activating AMPK. Exp Ther Med. 2021;22:1075. doi: 10.3892/etm.2021.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim MS, Park MJ, Kim SJ, Lee CH, Yoo H, Shin SH. et al. Emodin suppresses hyaluronic acid-induced MMP-9 secretion and invasion of glioma cells. Int J Oncol. 2005;27:839–46. [PubMed] [Google Scholar]
- 224.Son S, Lee S, Kim H, Kang H, Jeon J, Jo S. et al. Decreased FBP1 expression rewires metabolic processes affecting aggressiveness of glioblastoma. Oncogene. 2020;39:36–49. doi: 10.1038/s41388-019-0974-4. [DOI] [PubMed] [Google Scholar]
- 225.Kim J, Lee JS, Jung J, Lim I, Lee JY, Park MJ. Emodin suppresses maintenance of stemness by augmenting proteosomal degradation of epidermal growth factor receptor/epidermal growth factor receptor variant III in glioma stem cells. Stem Cells Dev. 2015;24:284–95. doi: 10.1089/scd.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhou JB, Li GH, Han GK, Feng S, Liu YH, Chen J. et al. Emodin induced necroptosis in the glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway. Invest New Drugs. 2020;38:50–59. doi: 10.1007/s10637-019-00764-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lu HF, Lai KC, Hsu SC, Lin HJ, Kuo CL, Liao CL. et al. Involvement of matrix metalloproteinases on the inhibition of cells invasion and migration by emodin in human neuroblastoma SH-SY5Y cells. Neurochem Res. 2009;34:1575–83. doi: 10.1007/s11064-009-9946-3. [DOI] [PubMed] [Google Scholar]
- 228.Lu HF, Lai KC, Hsu SC, Lin HJ, Kuo CL, Liao CL. et al. Characterization of apoptosis induced by emodin and related regulatory mechanisms in human neuroblastoma cells. Neurochem Res. 2009;34:1575–83. doi: 10.1007/s11064-009-9946-3. [DOI] [PubMed] [Google Scholar]
- 229.Cha TL, Qiu L, Hen CT, Wen Y, Hung MC. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005;65:2287–95. doi: 10.1158/0008-5472.CAN-04-3250. [DOI] [PubMed] [Google Scholar]
- 230.Yu CX, Zhang XQ, Kang LD, Zhang PJ, Chen WW, Liu WW. et al. Emodin induces apoptosis in human prostate cancer cell LNCaP. Asian J Androl. 2008;10:625–34. doi: 10.1111/j.1745-7262.2008.00397.x. [DOI] [PubMed] [Google Scholar]
- 231.Huang XZ, Wang J, Huang C, Chen YY, Shi GY, Hu QS. et al. Emodin enhances cytotoxicity of chemotherapeutic drugs in prostate cancer cells: the mechanisms involve ROS-mediated suppression of multidrug resistance and hypoxia inducible factor-1. Cancer Biol Ther. 2008;7:468–75. doi: 10.4161/cbt.7.3.5457. [DOI] [PubMed] [Google Scholar]
- 232.Masaldan S, Iyer VV. Exploration of effects of emodin in selected cancer cell lines: enhanced growth inhibition by ascorbic acid and regulation of LRP1 and AR under hypoxia-like conditions. J Appl Toxicol. 2014;34:95–104. doi: 10.1002/jat.2838. [DOI] [PubMed] [Google Scholar]
- 233.Deng G, Ju X, Meng Q, Yu ZJ, Ma LB. Emodin inhibits the proliferation of PC3 prostate cancer cells in vitro via the Notch signaling pathway. Mol Med Rep. 2015;12:4427–4433. doi: 10.3892/mmr.2015.3923. [DOI] [PubMed] [Google Scholar]
- 234.Cha TL, Chuang MJ, Tang SH, Wu ST, Sun KH, Chen TT. et al. Emodin modulates epigenetic modifications and suppresses bladder carcinoma cell growth. Mol Carcinog. 2015;54:167–77. doi: 10.1002/mc.22084. [DOI] [PubMed] [Google Scholar]
- 235.Li XX, Wang HL, Wang J, Chen YY, Yin XB, Shi GY. et al. Emodin enhances cisplatin-induced cytotoxicity in human bladder cancer cells through ROS elevation and MRP1 downregulation. BMC Cancer. 2016;16:578. doi: 10.1186/s12885-016-2640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Singh KB, Trigun SK. Apoptosis of Dalton's lymphoma due to in vivo treatment with emodin is associated with modulations of hydrogen peroxide metabolizing antioxidant enzymes. Cell Biochem Biophys. 2013;67:439–49. doi: 10.1007/s12013-011-9305-2. [DOI] [PubMed] [Google Scholar]
- 237.Lin Y, Chen WM, Wang ZH, Cai PW. Emodin promotes the arrest of human lymphoma Raji cell proliferation through the UHRF1-DNMT3A-∆Np73 pathways. Mol Med Rep. 2017;16:6544–6551. doi: 10.3892/mmr.2017.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chen YX, Mei XQ, Gan DH, Wu ZJ, Cao YQ, Lin MH. et al. Integration of bioinformatics and experiments to identify TP53 as a potential target in Emodin inhibiting diffuse large B cell lymphoma. Biomed Pharmacother. 2018;107:226–233. doi: 10.1016/j.biopha.2018.07.168. [DOI] [PubMed] [Google Scholar]
- 239.Bizama C, Garcia P. Targeting specific molecular pathways holds promise for advanced gallbladder cancer therapy. Cancer Treat. 2015;41:222–234. doi: 10.1016/j.ctrv.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 240.Kam AE, Masood A, Shroff RT. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol Hepatol. 2021;6:956–969. doi: 10.1016/S2468-1253(21)00171-0. [DOI] [PubMed] [Google Scholar]
- 241.Wang WI, Sun YP, Huang XZ, He M, Chen YY, Shi GY. et al. Emodin enhances sensitivity of gallbladder cancer cells to platinum drugs via glutathion depletion and MRP1 downregulation. Biochem Pharmacol. 2010;79:1134–1140. doi: 10.1016/j.bcp.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 242.Wang W, Sun YP, Li XX, Li H, Chen YY, Tian Y. et al. Emodin potentiates the anticancer effect of cisplatin on gallbladder cancer cells through the generation of reactive oxygen species and the inhibition of survivin expression. Oncol Rep. 2011;26:1143–1148. doi: 10.3892/or.2011.1390. [DOI] [PubMed] [Google Scholar]
- 243.Qu W, Wang YF, Wu QN, Liu JJ, Hao DJ. Emodin inhibits HMGB1-induced tumor angiogenesis in human osteosarcoma by regulating SIRT1. Int J Clin Exp Med. 2015;8:15054–1564. [PMC free article] [PubMed] [Google Scholar]
- 244.Qu W, Wang YF, Wu QN, Hao DJ, Li DC. Emodin impairs radioresistance of human osteosarcoma cells by suppressing sonic hedgehog signaling. Med Sci Monit. 2017;5:5767–5773. doi: 10.12659/MSM.907453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ying JH, Xu H, Wu D, Wu XG. Emodin induces apoptosis of human osteosarcoma cells via mitochondria- and endoplasmic reticulum stress-related pathways. Int J Clin Exp Pathol. 2015;8:12837–44. [PMC free article] [PubMed] [Google Scholar]
- 246.Yan L, Hu R, Tu S, Cheng WJ, Zheng Q, Wang JW. et al. Emodin mitigates the oxidative stress induced by cisplatin in osteosarcoma MG63 cells. Oncol Lett. 2016;12:1981–1985. doi: 10.3892/ol.2016.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Koyama J, Morita I, Tagahara K, Nobukuni Y, Mukainaka T, Kuchide M. et al. Chemopreventive effects of emodin and cassiamin B in mouse skin carcinogenesis. Cancer Lett. 2002;182:135–9. doi: 10.1016/s0304-3835(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 248.Ku HJ, Kwon OS, Kang BS, Lee DS, Lee HS, Park JW. IDH2 knockdown sensitizes tumor cells to emodin cytotoxicity in vitro and in vivo. Free Radic Res. 2016;50:1089–1097. doi: 10.1080/10715762.2016.1178739. [DOI] [PubMed] [Google Scholar]
- 249.Fang L, Zhao F, Iwanowycz S, Wang JF, Yin S, Wang YZ. et al. Anticancer activity of emodin is associated with downregulation of CD155. Int Immunopharmacol. 2019;75:105763. doi: 10.1016/j.intimp.2019.105763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sugiyama Y, Shudo T, Hosokawa S, Watanabe A, Nakano M, Kakizuka A. Emodin, as a mitochondrial uncoupler, induces strong decreases in adenosine triphosphate (ATP) levels and proliferation of B16F10 cells, owing to their poor glycolytic reserve. Genes Cells. 2019;24:569–584. doi: 10.1111/gtc.12712. [DOI] [PubMed] [Google Scholar]
- 251.Liu C, Chen L, Wang WC, Qin DK, Jia CL, Yuan MJ. et al. Emodin suppresses the migration and invasion of melanoma cells. Biol Pharm Bull. 2021;44:771–779. doi: 10.1248/bpb.b20-00807. [DOI] [PubMed] [Google Scholar]
- 252.Cai J, Niu X, Chen YY, Hu QS, Shi GY, Wu HC. et al. Emodin-induced generation of reactive oxygen species inhibits RhoA activation to sensitize gastric carcinoma cells to anoikis. Neoplasia. 2008;10:41–51. doi: 10.1593/neo.07754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sun ZH, Bu P. Downregulation of phosphatase of regenerating liver-3 is involved in the inhibition of proliferation and apoptosis induced by emodin in the SGC-7901 human gastric carcinoma cell line. Exp Ther Med. 2012;3:1077–1081. doi: 10.3892/etm.2012.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bachmann M, Schlatter C. Metabolism of [14C] emodin in the rat. Xenobiotica. 1981;11:217–25. doi: 10.3109/00498258109045294. [DOI] [PubMed] [Google Scholar]
- 255.Shia CS, Hou YC, Tsai SY, Huieh PH, Leu YL, Chao PD. Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats. J Pharm Sci. 2010;99:2185–95. doi: 10.1002/jps.21978. [DOI] [PubMed] [Google Scholar]
- 256.Liu W, Tang L, Ye L, Cai Z, Xia BJ, Zhang JJ. et al. Species and gender differences affect the metabolism of emodin via glucuronidation. AAPS J. 2010;12:424–36. doi: 10.1208/s12248-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liu W, Feng Q, Li Y, Ye Ling, Hu Ming, Liu ZQ. Coupling of UDP-glucuronosyltransferases and multidrug resistance-associated proteins is responsible for the intestinal disposition and poor bioavailability of emodin. Toxicol Appl Pharmacol. 2012;265:316–24. doi: 10.1016/j.taap.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 258.Radiac Brkanac S, Geric M, Gajski G, Vujcic V, Garaj-Vrhovac V, Kremer D. et al. Toxicity and antioxidant capacity of Frangula alnus Mill. bark and its active component emodin. Regul Toxicol Pharmacol. 2015;73:923–929. doi: 10.1016/j.yrtph.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 259.Jiang LL, Jiang Y, Zhao DS, Fan YX, Yu Q, Li P. et al. CYP3A activation and glutathione depletion aggravate emodin-induced liver injury. Chem Res Toxicol. 2018;31:1052–1060. doi: 10.1021/acs.chemrestox.8b00117. [DOI] [PubMed] [Google Scholar]
- 260.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of EMODIN (CAS NO.518-82-1) Feed Studies in F344/N Rats and B6C3F1 Mice. Natl Toxicol Program Tech Rep Ser. 2001;493:1–278. [PubMed] [Google Scholar]
- 261.Mueller SO, Stopper H, Dekant W. Biotransformation of the anthraquinones emodin and chrysophanol by cytochrome P450 enzymes. Bioactivation to genotoxic metabolites. Drug Metab Dispos. 1998;26:540–546. [PubMed] [Google Scholar]
- 262.Wang HW, Chen TL, Yang PC, Ueng TH. Induction of cytochromes P450 1A1 and 1B1 by emodin in human lung adenocarcinoma cell line CL5. Drug Metab Dispos. 2001;29:1229–1235. [PubMed] [Google Scholar]
- 263.Chang MH, Huang FJ, Chan WH. Emodin induces embryonic toxicity in mouse blastocysts through apoptosis. Toxicology. 2012;299:25–32. doi: 10.1016/j.tox.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 264.Luo T, Li N, He YQ, Weng SQ, Wang T, Zou QX. et al. Emodin inhibits human sperm functions by reducing sperm [Ca2+]i and tyrosine phosphorylation. Reprod Toxicol. 2015;51:14–21. doi: 10.1016/j.reprotox.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 265.Huo GT, Wang YN, Lv JJ, Yang YW, Lin Z, Qu Z. et al. Chronic toxicity study of emodin monomer in Kunming mice by ig administration. Drug Evalu Res. 2021;44:1425–1433. [Google Scholar]
- 266.Peng W, Chen Y, Tumilty S, Liu L, Luo L, Yin H. et al. Paeoniflorin is a promising natural monomer for neurodegenerative diseases via modulation of Ca2+ and ROS homeostasis. Curr Opin Pharmacol. 2022;62:97–102. doi: 10.1016/j.coph.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 267.Li R, Zhang Q, Liu J, Sun J, He L, Duan H. et al. Hydroxy-α-sanshool possesses protective potentials on H2O2 stimulated PC12 cells by suppression of oxidative stress induced apoptosis through regulation of PI3K/Akt signal pathway. Oxid Med Cell Longev. 2020;2020:3481758. doi: 10.1155/2020/3481758. [DOI] [PMC free article] [PubMed] [Google Scholar]