Abstract
Background:
Hospitals have implemented diverse quality improvement (QI) interventions to reduce rates of catheter-associated urinary tract infections (CAUTI). The economic value of these QI interventions is uncertain.
Objective:
To systematically review economic evaluations of QI interventions designed to prevent CAUTI in acute care hospitals.
Methods:
Ovid MEDLINE, Econlit, Centre for Reviews & Dissemination, New York Academy of Medicine’s Grey Literature Report, Worldcat (January 2000 to October 2020), IDWeek conference abstracts, and prior systematic reviews.
We included English-language studies of any design that evaluated organizational or structural changes to prevent CAUTI in acute care hospitals, and reported program and infection-related costs.
Dual reviewers assessed study design, effectiveness, costs, and study quality. For each eligible study, we performed a cost-consequences analysis from the hospital perspective, estimating the incidence rate ratio [IRR] and incremental net cost/savings per hospital over three years. Unadjusted weighted regression analyses tested predictors of these measures, weighted by catheter-days per study.
Results:
Fifteen unique economic evaluations were eligible, encompassing 74 hospitals. Across 12 studies amenable to standardization, QI interventions were associated with a 43% decline in infections (mean IRR 0.57, 95% CI 0.44 to 0.70) and wide ranges of net costs (mean U.S. $52,000, 95% CI -$288,000 to $392,000), relative to usual care.
Conclusions:
QI interventions were associated with large declines in infection rates and net costs to hospitals that varied greatly but that, on average, were not significantly different from zero over three years. Future research should examine specific practices associated with cost savings and clinical effectiveness, and examine whether or not more comprehensive interventions offer hospitals and patients the best value.
Prospero Registration Number:
CRD42015014950
Keywords: catheter-associated urinary tract infection; quality improvement; economic evaluation (cost-effectiveness, return on investment, budget impact analysis, business case analysis, cost-benefit analysis); healthcare associated infection; hospital
Introduction
Health care-associated infections (HCAI) are the most frequently reported patient safety issue in health care delivery worldwide, occurring in seven out of every 100 hospitalized patients in high-income countries.1,2 The financial burden of HCAI is also high at approximately €7 billion in Europe and about $6.5 billion in the United States annually.2 In Europe and the United States, urinary tract infections (UTI) are the most common type of HCAI (36% and 27%, respectively), with approximately 75% of these infections occurring in association with a urinary catheter.1,3 More than 19,000 catheter-associated urinary tract infections (CAUTI) occurred nationwide in the U.S. in 2019 with attributable costs well over $1,000 per CAUTI.4–6
To address this problem in the U.S., the Centers for Medicare & Medicaid Services (CMS), a federal agency within the U.S. Department of Health and Human Services that administers the Medicare federal health insurance program and works with state governments to administer Medicaid,7 implemented several policies that created financial incentives for hospitals to reduce rates of CAUTI. In 2008, CMS implemented the Hospital-Acquired Conditions policy, which requires hospitals to absorb the costs associated with CAUTI and seven other hospital-acquired conditions.8 The Hospital Value-Based Purchasing Program (VBP), implemented in 2012, increases or decreases Medicare payments, the largest payer for health care in the U.S., to hospitals based on performance relative to other hospitals on several quality measures, including CAUTI.9 Most recently, in 2015, CMS implemented the Hospital-Acquired Condition Reduction Program (HAC), which also adjusts payments to hospitals based on quality measures for CAUTI and other hospital-associated infections.10
In response to these policies, U.S. hospitals have implemented various CAUTI-prevention practices, including purchasing antimicrobial catheters and/or changing hospital policies and practices, such as reducing the frequency of catheter placement, assuring proper catheter insertion and maintenance, employing automated reminder and stop order systems, and promptly removing catheters.11–14 CAUTI rates have subsequently decreased over time.15 However, little is known about the economic value of quality improvement (QI) interventions for CAUTI, meaning the associated changes in clinical outcomes relative to the net cost.16 QI initiatives require substantial investments of staff time, supplies, and other economic resources, which together comprise QI program costs.17 As CAUTI rates decline, hospitals avoid the costs associated with treating these infections.
We sought to systematically review economic evaluations of QI interventions for the prevention of CAUTI in the hospital setting, examining both QI program costs and changes in infection-related costs. Our primary research objective was to evaluate whether QI interventions designed to prevent CAUTIs were associated with net cost or savings to hospitals as well as changes in infection rates.
Methods
We conducted a systematic review of original research to assess the clinical and economic outcomes of QI interventions addressing CAUTI in acute care hospitals. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines guided reporting of this systematic review.18 The study protocol is posted on the Prospero registry (CRD42015014950).19
Search Strategy
We developed search terms with the help of a reference librarian and informed by prior literature on economic evaluation (Appendix 1).20 Queried databases included PubMed, CINAHL (The Cumulative Index to Nursing and Allied Health Literature), and the Centre for Reviews & Dissemination Economic Evaluation. We utilized WorldCat and the Grey Literature Report to identify grey literature. We restricted our search to studies published in English between January 2000 and October 2020, since clinical practices and cost structures change over time.
Study Selection
For our qualitative synthesis, we used six inclusion criteria for study selection. Included studies must (1) be original investigations, (2) examine QI interventions designed to prevent CAUTI, (3) include an economic evaluation, (4) involve acute care hospitals, (5) measure or model QI program costs (i.e. the cost of implementing the intervention), and (6) report clinical effectiveness.
We used the definition of a QI intervention by Danz, et al: “an effort to change/improve the clinical structure, process, or outcomes of care by means of an organizational or structural change.”21 We interpreted this definition to include: (1) changes in the protocols and practices that clinicians used to manage catheters, and (2) changes in catheter material and design, because both are organization-level changes that can affect the incidence of CAUTI.22 We did not impose a definition of CAUTI, but relied on study-specified definitions. To capture as many relevant studies as possible, we included diverse clinical evaluation designs and economic evaluation approaches, analytical perspectives, and time horizons. We excluded studies from countries defined as low- to middle-income by the World Bank Country and Lending Groups 2018 classification of economies, as differences in care practices and cost structures produce heterogeneity that prevents meaningful meta-analysis.23
For inclusion in our quantitative analysis, a study must report the following data elements necessary for standardization: (1) estimated catheter days, (2) baseline CAUTI rates, and (3) CAUTI-related costs. This information needed to be reported directly in the study or there needed to be enough information included in the study for the research team to derive it.
Two members of the research team independently reviewed abstracts and full text articles to determine eligibility. We reviewed articles and discussed discrepancies; disagreement was resolved by consensus or through discussion with the wider research team. When we identified eligible economic evaluations, we also obtained and extracted data from any associated publications, i.e., prior publications by the same authors using the same data, typically focused on intervention design or effectiveness.
Data Extraction and Quality Assessment
Two physicians with backgrounds in hospital epidemiology and hospital medicine extracted data regarding CAUTI prevention practices in each QI intervention. Two members of the research team with training in cost-effectiveness analysis extracted economic data. We resolved discrepancies by consensus or through discussion with the larger research team.
QI Intervention, Context, and Clinical Evaluation
For each study, reviewers extracted data related to the nature of the QI intervention, setting, study design and reporting of the clinical evaluation, funding source, and findings. When characterizing infection-prevention practices, we identified practices strongly recommended in a recent evidence review from The Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA).24 We classified QI interventions into three categories: (1) use of silver- and nitrofurazone antibiotic- impregnated antimicrobial catheters, (2) CAUTI-related policies and practices (e.g., readily available supplies for aseptic catheter insertion, use of bladder scanners, and/or unit-specific feedback, among others), and (3) both antimicrobial catheters and CAUTI-related policies and practices.
Contextual variables included academic status (major, minor, non-teaching) and location (urban, suburban/small city, rural). Clinical study designs included randomized controlled trials, non-randomized controlled trials, controlled before-after analyses, uncontrolled before-after analyses, interrupted time series and repeated measures studies, and modeling exercises.25 We assessed the reporting of the clinical evaluation using elements from the Minimum Quality Criteria Set (items 3–7, 10–11, 13), a tool for critically appraising the reporting of QI interventions.26 Funding sources included government, non-profit, commercial, and none. Finally, reviewers extracted rates of CAUTI in the intervention and comparison groups.
Economic Evaluation
Reviewers extracted the evaluation approach (cost analyses such as cost-consequences or business-case analyses vs. cost-effectiveness and related analyses), economic perspective (hospital, health system, payer, society), time horizon, year and currency of cost data, and incremental program and infection-related costs.
Study Quality
Given our primary outcome was economic cost, we used a modified version of the Quality of Health Economics Studies Checklist (mQHES)27,28 to assess whether studies adhered to basic standards for economic evaluations, as done in prior research.29,30 Checklist domains include clarity of study objectives, statement of perspective, quality of variable estimates, and handling of uncertainty and bias. Scores range from 0 (lowest quality) to 115 (highest quality). We did not assess study quality for secondary outcomes, including clinical effectiveness.
Data Standardization
To facilitate comparisons, we used the extracted data from each primary study to perform a cost-consequences analysis from the hospital perspective. A cost-consequences analysis is a type of economic evaluation that reports clinical outcomes and costs as separate measures.31 Our clinical outcome was the incidence rate ratio (IRR), meaning the CAUTI rate in the intervention group divided by the rate in the comparison group. When a primary study did not report an IRR, we calculated it based on data in the paper and associated publications. The economic outcome in our analysis was the incremental net cost of a QI intervention per hospital over three years.
We standardized all costs by converting to 2018 U.S. dollars and scaling all costs to the hospital level over 3 years (e.g., if the study had 3 hospitals and extended for 8 months, we divided by 3, then by 8 and then multiplied by 36). We also converted foreign currencies to U.S. dollars and then inflated the U.S. dollars from the year of the cost data to 2018.
We used two methods to estimate infection-related cost losses/savings. For studies that estimated costs based on published literature, we calculated the change in infection-related cost by multiplying the number of infections added/averted (difference in number of infections per hospital per year between intervention and comparison conditions) by the average cost per CAUTI nationally based on a prior meta-analysis ($1,175 after inflation to 2018 U.S. dollars; $896 in 2012 U.S. dollars).6 When studies reported infection-related costs based on their own local data, we extracted and used those costs. We standardized these costs in the same manner as for program costs: to 2018 U.S. dollars per hospital over three years.
Finally, to yield the incremental net cost, we summed standardized program costs and the change in infection-related costs. For example, if a hospital invested $270,000 in antimicrobial catheters and CAUTI-related costs declined by $200,000, the incremental net cost would be $70,000 (a net loss). If CAUTI-related costs declined by $370,000, the incremental net cost would be -$100,000 (a net savings).
Analysis
We conducted separate unadjusted weighted regression analyses to individually identify factors associated with greater effectiveness (lower IRR) and savings (lower incremental net cost). Factors potentially associated with effectiveness were identified a priori, and included intervention type (antimicrobial, policies and practices, or both), study publication year, program cost per hospital over three years, and the academic status of the hospitals included in the studies. Factors potentially associated with incremental net costs included the same factors as above, with the addition of quality of economic analysis as measured by mQHES score and intervention effectiveness. Analyses were weighted by the estimated number of catheter days analyzed per study. Sensitivity analyses involved jackknife resampling with sequential exclusion of each study to examine if results changed, particularly for larger studies.32
Results
Study Selection
We identified 575 publications and selected 69 for full text review; 14 articles met all eligibility criteria (Figure 1).33–44 One article included two separate interventions which we considered as two studies,38 bringing the total number of studies included in our qualitative analysis to 15.
Figure 1: PRISMA Flow Diagram.
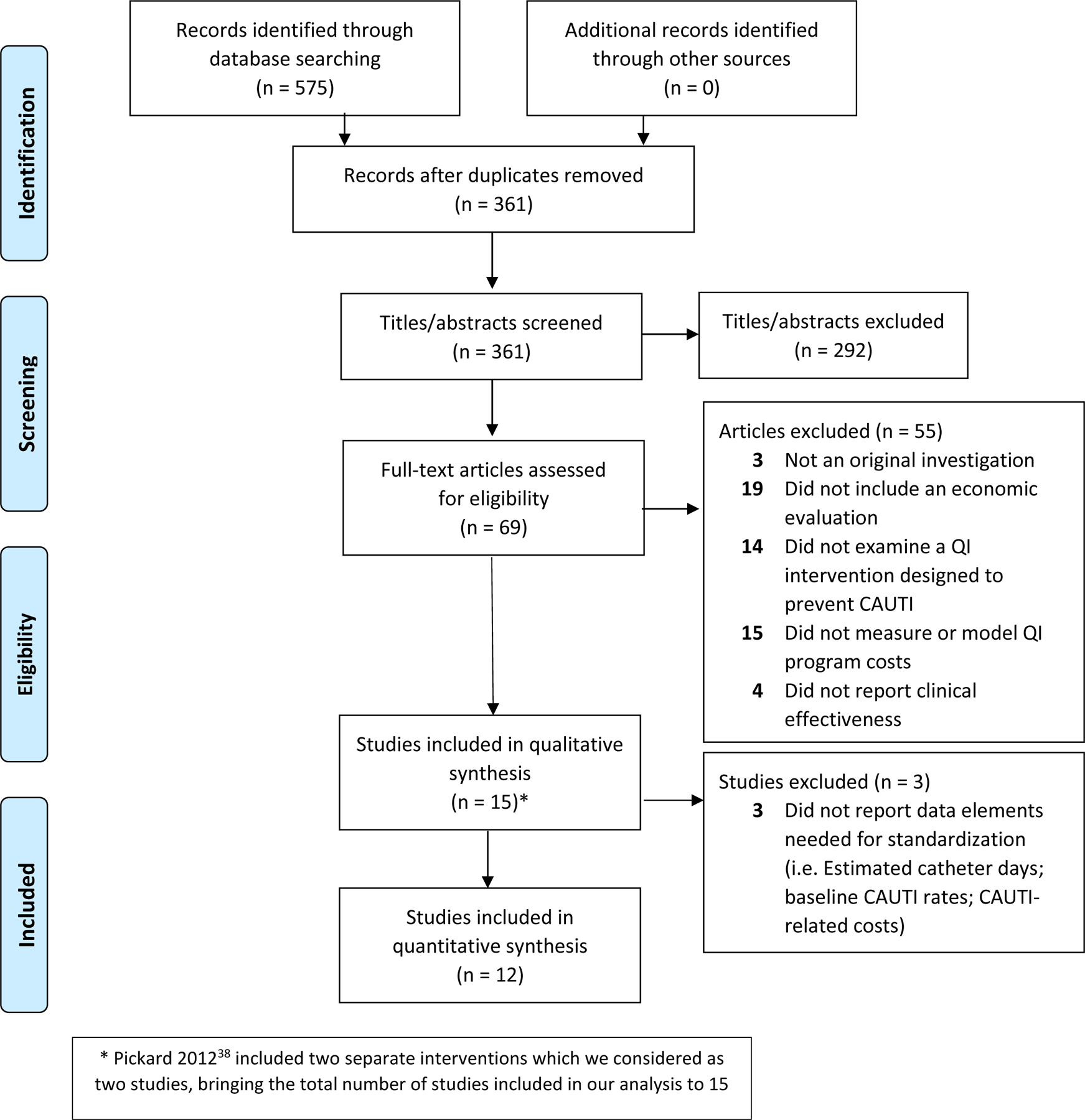
* Pickard 201238 included two separate interventions which we considered as two studies, bringing the total number of studies included in our analysis to 15
Of the 55 articles excluded under full-text review, three were systematic literature reviews and did not include original data. Nineteen articles did not include an economic evaluation, while 14 articles did not examine a QI intervention designed to prevent CAUTI. Thirty-four articles did not measure or model program costs (i.e., implementation costs of the QI intervention). Finally, four articles did not report clinical effectiveness. Queries of grey literature did not identify eligible articles (Figure 1).
Study Characteristics and Quality Assessment
Definition of CAUTI
Definitions of CAUTI varied across the 15 studies (Table 1). Five studies33,34,38,39,43 utilized surveillance criteria from the United States National Healthcare Safety Network (NHSN) prior to 2015.45 In 2015, the NHSN modified its CAUTI surveillance criteria to improve clinical specificity.45 One study44 used this new surveillance criteria from the NHSN.46 Five studies35–37,42,47 created a custom definition of CAUTI, and three studies40,41,48 did not specify a definition of CAUTI.
Table 1:
Definition of Catheter-Associated Urinary Tract Infection (CAUTI)
| Definition of CAUTI | Pickard 201238 | Rupp 200436 | Lai 200239 | Karchmer 200043 | Saint 200042 | Cartwright 201841 | Pashnik 201744 | Anderson 201133 | van den Broek 201135 | Saint 200537 | Quinn 201540 | Clarke 201334 | Palmer 201947 | Mitchell 201948 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NHSN* Surveillance Criteria before 201556 | X | X | X | X | X | |||||||||
| NHSN Surveillance Criteria 2015 and after45 † | X | |||||||||||||
| Study-specific definition | X | X | X | X | X | |||||||||
| Not specified in study | X | X | X |
United States National Healthcare Safety Network
changes to the NHSN surveillance criteria were made to improve specificity by excluding candiduria and lower bacteriuria levels (i.e., <105 cfu).
QI Interventions
The 15 eligible studies each tested one or more strategies recommended by SHEA and IDSA24,49 to prevent CAUTI in acute care hospitals (Table 2).33–44,47,48 Strategies involving changes in protocols and practices included written criteria for acceptable catheter indications (2 studies),35,40 readily available supplies for aseptic catheter insertion (2 studies),41,48 documentation of catheter indications and care (4 studies),34,35,40,44 CAUTI surveillance using standardized criteria (3 studies),33,35,40 unit-specific feedback (2 studies),40,44 meatal cleaning with antiseptic prior to insertion (1 study),48 use of bladder scanners (3 studies),35,41,44 automated reminders of persistent catheterization (4 studies),34,35,37,40 analysis and reporting of catheter use and adverse events (2 studies),40,44 periodic review of catheter necessity (3 studies),35,40,44 CAUTI-specific nursing-focused education (7 studies),33–35,40,41,44,47 CAUTI-specific physician-focused education (1 study),40 automated catheter stop orders (1 study),35 engagement of hospital leadership (4 studies),33,37,40,44 a multi-disciplinary CAUTI prevention team (4 studies),34,35,40,44 use of a “bladder bundle” (2 studies),33,44 electronic alerts to confirm catheter necessity (2 studies),34,40 and audits on compliance (2 studies).34,44 Changes to protocols and practices that are not explicitly included as part of the current recommended prevention strategies by SHEA or IDSA24,49 but were utilized in the studies included in this review were: use of a CAUTI “toolkit” (1 study),33 use of a Bard Tray (1 study),47 use of physician champions (3 studies),33,40,44 root cause analysis of CAUTI events in real time (2 studies),40,47 routine site visits (1 study),33 patient education (1 study),44 and use of an Advanced Practice Registered Nurse and/or specially trained nurses (1 study).35
Table 2:
Use of strategies designed to prevent Catheter-Associated Urinary Tract Infections (CAUTI) in studies with economic evaluations
| Components of QI Intervention | Pickard 201238 | Rupp 200436 | Lai 200239 | Karchmer 200043 | Saint 200042 | Cartwright 201841 | Pashnik 201744 | Anderson 201133 | †van den Broek 201135 | Saint 200537 | Quinn 201540 | Clarke 201334 | Palmer 201947 | Mitchell 201948,57 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Changes to Hospital CAUTI-Related Policies and Practices | ||||||||||||||
| SHEA/IDSA* Recommended Strategies for CAUTI prevention | ||||||||||||||
| Written criteria for catheter indications | I | I | ||||||||||||
| Supplies for aseptic insertion readily available | I | |||||||||||||
| Documentation of catheter indications and care | I | I | I | I | ||||||||||
| CAUTI surveillance using standardized criteria | I | I | I | |||||||||||
| Unit-specific feedback | I | I | ||||||||||||
| Meatal cleaning with antiseptic prior to insertion | I | |||||||||||||
| SHEA/IDSA Recommended Special Approaches for preventing CAUTI in locations with unacceptably high CAUTI rates | ||||||||||||||
| Use of bladder scanners | I | I | I | |||||||||||
| Automated reminders of persistent catheterization | I | I | I | I | ||||||||||
| Report catheter use and adverse events | I | I | ||||||||||||
| Periodic review of catheter necessity | I | I | I | |||||||||||
| SHEA/IDSA Recommended Implementation Strategies: Engagement, Education, and Execution | ||||||||||||||
| CAUTI-specific nursing-focused education | I | I | I | I | I | I | I | |||||||
| CAUTI-specific physician-focused education | I | |||||||||||||
| Automated catheter stop orders | I | |||||||||||||
| Engagement of hospital leadership | I | I | I | I | ||||||||||
| Multi-disciplinary CAUTI prevention team | I | I | I | I | ||||||||||
| “Bladder bundle”‡ | I | I | ||||||||||||
| Electronic alerts to confirm catheter necessity | I | I | ||||||||||||
| Audits on compliance | I | I | ||||||||||||
| Other Approaches | ||||||||||||||
| CAUTI “toolkit”§ | I | |||||||||||||
| Bard Tray | I | |||||||||||||
| Physician Champions | I | I | I | |||||||||||
| Root cause analysis of CAUTI events in real time | I | I | ||||||||||||
| Routine site visits | I | |||||||||||||
| Patient education | I | |||||||||||||
| Specially trained Advanced Practice nurses | I | |||||||||||||
| Antimicrobial Catheters | ||||||||||||||
| Approaches NOT recommended by SHEA/IDSA as routine part of CAUTI prevention | ||||||||||||||
| Silver alloy-impregnated catheters | I | I | I | I | I | I | I | |||||||
| Nitrofurazone-impregnated catheters | I | |||||||||||||
The Society for Healthcare Epidemiology of America/The Infectious Diseases Society of America
Ten hospitals implemented their own interventions; one or more hospitals implemented the intervention marked in the column.
Definition from Pashnick and Anderson 2011.
Definition from Anderson 2011.
Strategies involving changes to catheter equipment included silver-impregnated antimicrobial catheters (7 studies),34,36,38–40,42,43 and antibiotic-impregnated antimicrobial catheters (1 study).38 It should be noted that antimicrobial impregnated catheters are not recommended by SHEA/IDSA as part of routine CAUTI prevention.24,49
Context
Eight of the 15 unique studies were based in the United States,33,34,36,37,39,40,43,44 three in the United Kingdom,38,41,47 one in the Netherlands,35 and one in Australia48 (Table 3). One study had no location because it used a hypothetical cohort to create a decision model.42 Most studies were set at a single hospital, although two studies included 24 hospitals,33,38 two included three hospitals,47,48 one included 10 hospitals,35 and one included two hospitals.41 In total, data came from 74 hospitals. Six studies were based at only major academic institutions,36,37,39,41,43,44 four studies were based at only community hospitals,33,34,40,48 one study was based at both academic and community hospitals,35 two studies were based at National Health Service hospitals in the United Kingdom,38,47 and one study did not state academic status.42 See Appendix 2 for elements from the Minimum Quality Criteria Set and study funding sources.
Table 3:
Summary of economic evaluations for Quality Improvement (QI) interventions designed to prevent Catheter-Associated Urinary Tract Infections (CAUTI) as reported by original study authors
| Author (Year) |
Intervention | Setting | Design & Comparator | Estimated Catheter Days | Baseline CAUTI Rate | Effectiveness | Approach, Perspective, Year of Costs | Program Costs | CAUTI-related Costs | Incremental Net Cost | mQHES |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Changes to Hospital CAUTI-Related Policies and Practices
| |||||||||||
| Palmer 201947 | Catheterization tray, training program | U.K., 3 community hospitals | UCBA, SQ | NR | 13.3% of catheterized patients | IRR = 0.16 | Cost analysis, hospital, 2014 |
£5,440 for 3 hospitals | NR | -£33,000 for 3 hospitals | 87.5 |
|
| |||||||||||
| Mitchell 201948,57 | Routine use of chlorhexidine prior insertion | Australia, 3 community hospitals | Model, SQ | NR | 4.55 / 1000 catheter-days | IRR = 0.06 (95% CI: 0.01 to 0.32) |
Cost analysis, hospital, 2018 |
AUD$89,012 per 100,000 catheter-izations | NR | −AUD$387,909 per 100,000 catheter- izations |
89.5 |
|
| |||||||||||
| Cartwright 201841 | Catheterization tray, training program | U.K., 2 academic hospitals | UCBA, SQ | 14,586 | 102 CAUTI per 2 HPY | IRR = 0.21 | Cost analysis, hospital, 2015 |
−£40,000 per 2 HPY |
−£159,400 per 2 HPY |
NR | 72 |
|
| |||||||||||
| Pashnik 201744 | Nurse-led teaching and evaluation | U.S., 1 academic hospital | UCBA, SQ | 49,359 | 1.3 / 1000 catheter-days | IRR = 0.70 | Cost analysis, hospital, 2016 |
$100,000 PH over 1.75 yr | $6,834 per CAUTI (1o data) | NR | 64.5 |
|
| |||||||||||
| Anderson 201158 | Checklists, audit & feedback for CLABSI, CAUTI, VAP, MRSA | U.S., 24 community hospitals (23 eligible) |
UCBA, SQ |
282,915 | 4.4 / 1000 catheter-days | IRR 0.50 | Cost analysis, hospital, 2009 |
$20,000 to $40,000 PHPY | −$82,722 to −$159,902 PHPY |
−$7.9 to −$15.4 million for 24 hospitals over 5 years | 102 |
|
| |||||||||||
| van den Broek 201135 | Removal reminder, ultrasound | Netherlands, 2 academic & 8 community hospitals | Time series, SQ |
15,820 | 12.6% of catheterized patients |
IRR 1.01 | Cost analysis, hospital, 2008 |
€2,638 per hospital plus −€5.37 per hospitalized patient |
$0 (not effective) |
NR | 101 |
|
| |||||||||||
| Saint 200537 | Physician reminders re: catheters | U.S., 1 academic hospital | CBA, SQ |
NR | NR | Time catheterized: ↓7.6% vs. ↑15.1% | Cost analysis, hospital, 2003 |
$53,200 PHPY | −$53,449 PHPY |
−$249 PHPY |
82 |
|
Antimicrobial Catheters | |||||||||||
| Pickard 201238 | Silver alloy catheter | U.K., 24 academic & community hospitals | RCT, PTFE-coated catheter |
20,596 | 12.6% of catheterized patients |
RD = −0.1%, IRR = 0.99 | Cost-effectiveness, health system, 2007 |
£5.38 per catheterized patient | £548 per CAUTI (1o data) | +£5.05 per catheterized patient | 113 |
|
| |||||||||||
| Nitrofurazone catheter | 19,992 | RD = −2.1%, IRR = 0.84 | £4.19 per catheterized patient | −£7.10 per catheterized patient | |||||||
|
| |||||||||||
| Rupp 200436 | Silver alloy catheter | U.S., 1 academic hospital | UCBA, uncoated catheter | 48,662 | 6.13 / 1000 catheter-days | IRR = 0.43 | Cost analysis, hospital, 2002 |
$64,794 PHPY | −$71,118 to −$549,377 PHPY |
−$5,811 to −$535,452 PHPY |
95.5 |
|
| |||||||||||
| Lai 200239 | Silver alloy catheter | U.S., 1 academic hospital | UCBA, uncoated catheter | 62,855 | 4.9 / 1000 patient-days | IRR = 0.55 | Cost analysis, hospital, 1997 |
$120,000 PHPY | $1,214 per CAUTI (1o data) | −$142,315 PHPY |
97 |
|
| |||||||||||
| Karchmer 200043 | Silver alloy catheter | U.S., 1 academic medical center | Randomized crossover, uncoated catheter | 112,846 | 3.00 / 1000 patient-days | IRR = 0.79 (95% CI: 0.63 to 0.99) | Cost analysis, hospital, 1997 |
$107,225 PHPY | −$121,681 to −$680,518 PHPY |
−$14,456 to −$573,293 PHPY |
103 |
|
| |||||||||||
| Saint 200042 | Silver alloy catheter | Hypothetical cohort, 1 academic hospital | Model, uncoated catheter | 11,410 | 30 / 1000 catheterized patients |
IRR = 0.53 | Cost analysis, payer, 1998 |
$5.30 per catheterized patient | $374–402 + ($2041*0.04) per CAUTI (1o data) | −$4.09 per catheterized patient | 105.5 |
|
Both CAUTI-Related Policies & Practices and Antimicrobial Catheters | |||||||||||
| Quinn 201540 | Silver alloy catheter, nurse-driven protocol | U.S., 1 community hospital | UCBA, SQ |
35,461 | 4.95 / 1000 catheter-days | IRR 0.08 (2012) | Cost analysis, hospital, 2009 |
$75,000 PHPY | $132,000 vs. $6,000 (2012) PHPY | NR | 87 |
|
| |||||||||||
| Clarke 201359 | Silver alloy catheter, reminders, education | U.S, 1 community hospital | UCBA, SQ |
5,821 | 5.2 / 1000 catheter-days | IRR 0.29 | Cost analysis, Hospital, 2008 |
$23,924 PHPY | $1200 to $4700 per CAUTI (literature) | −$6,892 to −$96,772 PHPY |
77 |
Abbreviations: mQHES, Quality of Health Economics Studies Checklist; CAUTI, catheter-associated urinary tract infection; QI, quality improvement; U.S., United States; U.K., United Kingdom; SQ, status quo (usual care); RR, rate ratio; IRR, incidence rate ratio; RD, risk difference; CEA, cost-effectiveness, cost-benefit, and related analyses; RCT, randomized control trial; UCBA, uncontrolled before-after analysis; CBA, controlled before-after analysis; NR, not reported; PTFE, polytetrafluoroethylene; PHPY, per hospital per year
Clinical Evaluation
All 15 unique studies compared QI interventions with usual care (Table 3). Eight studies used an uncontrolled, before-after study design,33,34,36,39–41,44,47 one study used a pretest-posttest design with a nonequivalent control group,37 one study used a time-series analysis,35 one study used a randomized control trial design,38 and one used a randomized crossover design.43 Finally, two of the studies reported a modeling exercise based on a randomized controlled trial.42,48
Cost Evaluation
Most of the 15 unique studies reported cost analyses from the hospital perspective (Table 3).33–37,39–41,43,44,47 One study was a cost analysis from the payer perspective,42 one study was a cost-effectiveness analysis from the health system perspective,38 and one study was a cost-effectiveness analysis from a societal perspective.48
Among the 15 studies, the resources invested in CAUTI prevention and the associated program costs varied. All 15 studies estimated annually recurring program costs (standardized median per hospital $131,000; interquartile range [IQR] 71,000 to 360,000). One study also reported start-up program costs (€2,638 per hospital; 10 hospitals total),35 which included hiring an implementation expert for the intervention. Other studies either reported that start-up costs were zero36,38–44,48 or did not describe start-up costs.33,34,37,47
Study Quality
Cost evaluation methods were of moderate to high quality (defined as mQHES scores greater than 50.1 where 1=lowest quality and 150=highest quality),27 with median mQHES scores of 99.0 (IQR 19 to 104) (Table 3).
Data Standardization
Among the 15 unique studies, three lacked sufficient data to standardize. Two studies did not include estimated catheter days or CAUTI-related costs,47,48 while one study did not include estimated catheter days or baseline CAUTI rates.37 The total number of studies included for standardization was 12 (Appendix 3 and 4).33–44 Among the 12 studies, the median total program cost per hospital over 3 years was $131,000 (IQR 71,000 to 360,000), and the median incremental infection-related cost was -$143,000 (IQR −258,000 to 196,000), relative to usual care.33–44 Based on differences between program and incremental infection-related costs, the median net cost was $9,000 (IQR −65,000 to 142,000).33–44 These estimates are unweighted (Figure 2).
Figure 2: Standardized Quality Improvement (QI) Program, Infection-Related, and Incremental Net Costs of QI Interventions.
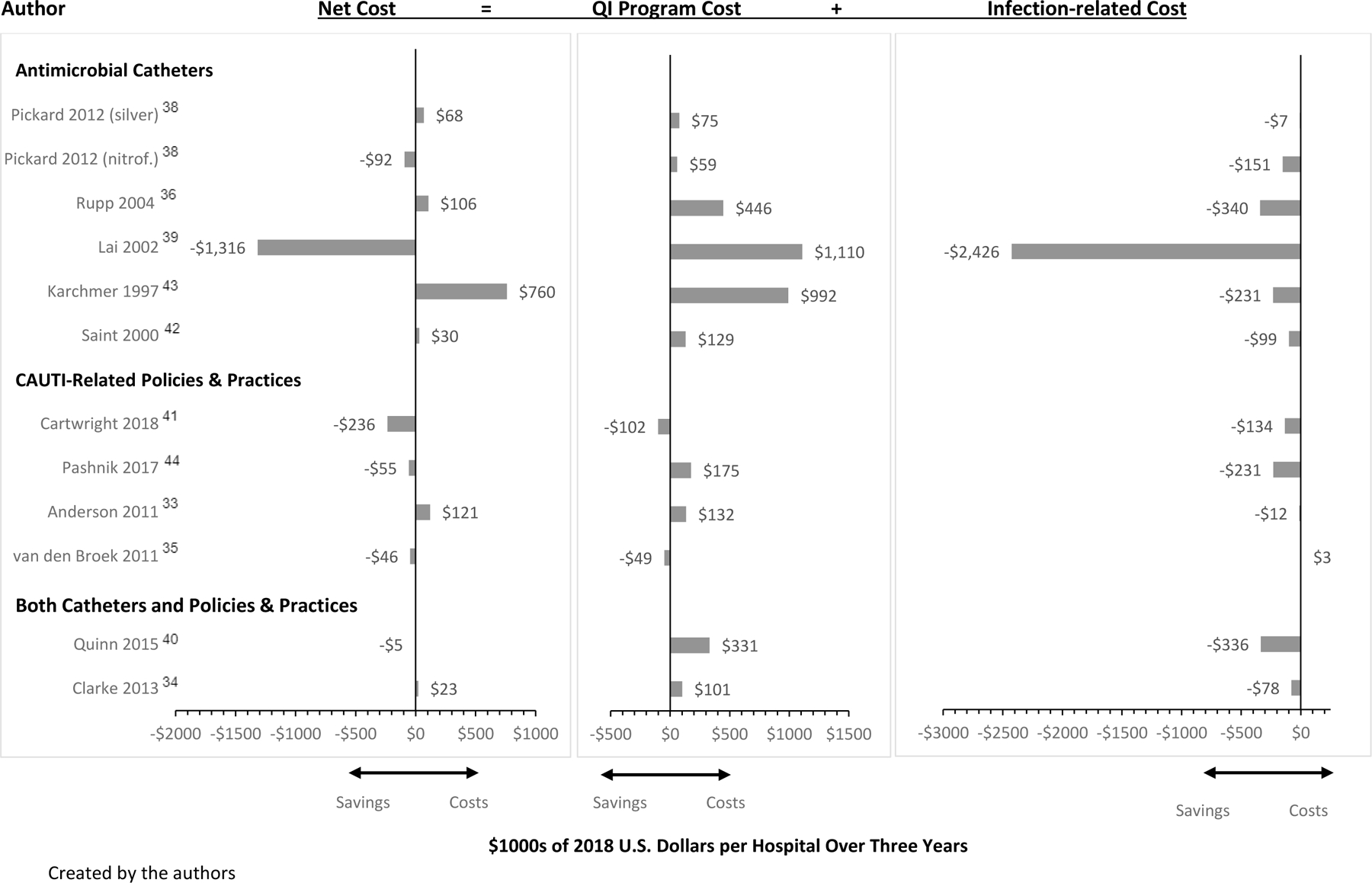
Created by the authors
Two studies in particular varied widely in overall net costs when compared with the other studies included in this analysis,39,43 attributed in part to differences in baseline CAUTI rates and overall intervention effectiveness, but importantly one of the studies39 used a high cost estimate per infection (nearly $4,000 per CAUTI, compared to $1,175 based on a prior meta-analysis).6
Analysis
Using unadjusted regression analysis weighted by the estimated number of catheter days analyzed per study, we found that the mean IRR among the 12 studies was 0.57 (95% CI: 0.44 to 0.70), reflecting a 43% decline in infections relative to usual care. Utilizing both types of infection prevention practices at the same time (antimicrobial catheters and CAUTI prevention-related policies and practices) was associated with a statistically significant 89% decline in infections relative to usual care (IRR: 0.11; 95% CI: −0.06 to 0.27; p=0.03), based on two studies. It should be noted that the null value of the confidence interval for a ratio is one, which means that in this analysis, the null hypothesis can be rejected.50
The mean incremental net cost over three years was $52,000 per hospital, but estimates could be as high as saving $288,000 or spending $392,000 (95% CI: -$288,000 to $392,000). Implementing both antimicrobial catheters and CAUTI prevention-related policies and practices was associated with nonsignificant net costs of -$31,000 over three years (95% CI: -$1,708,000 to $1,647,000; p=0.97) when compared with usual care, and this was not significantly different from the net cost observed in studies comparing antimicrobial catheters with usual care ($30,000; 95% CI -$752,000 to $812,000; p=0.97 for comparison of net costs).
We saw no differences in effectiveness or net costs according to QI program costs, publication year, or hospital academic status. We also saw no difference in net costs according to clinical effectiveness or the quality of the economic evaluation (Appendix 5).
These results were robust to sequential exclusion of each study, irrespective of study size, with one exception. This study utilized both antimicrobial catheters and CAUTI-related policies and practices in its intervention and exhibited an unusually large reduction in infections, with an IRR of 0.08.40 After excluding this study, the use of both intervention types was no longer associated with effectiveness.
Discussion
On the basis of 15 unique economic evaluations encompassing 74 hospitals, our systematic review and weighted regression analysis showed that QI interventions aimed at reducing rates of CAUTI in acute care hospitals yield highly variable net costs to hospitals with, on average, an insignificant net cost of $52,000 per hospital, even when the QI interventions are clinically effective. This cost assessment does not take into consideration potential penalties or incentives for hospitals with higher or lower CAUTI rates via the Medicare financial incentive programs.9,10 Among studies including an economic evaluation, we found that overall, QI interventions involving one or more strategies recommended by SHEA and IDSA24,49 were associated with a 43% decline in infections.
To our knowledge, there have been no previous systematic reviews examining the economic implications of CAUTI-related QI interventions, meaning the associated changes in clinical outcomes relative to net cost. A 2013 systematic review and meta-analysis by Zimlichman et al. estimated the cost to hospitals of treating a CAUTI event to be $896 (95% CI: $603 to $1,189) in 2012 U.S. dollars.6 Our analysis balanced the savings from preventing these events against the investments in QI interventions, which we found had a median program cost of $131,000 per year.
Over the last decade, hospitals in the U.S. have come under increasing pressure from policymakers to devote resources to the reduction of hospital-acquired infections, including CAUTI. The Centers for Medicare & Medicaid Services, the federal agency administering Medicare and assisting the states with administering local Medicaid programs, implemented several policies that created financial incentives for hospitals to reduce rates of CAUTI. These included requiring hospitals to absorb the costs associated with CAUTI and adjusting payments to hospitals based on quality measures for CAUTI and other hospital-acquired infections.8–10 In response to these policies, U.S. hospitals have implemented various CAUTI-prevention practices, and CAUTI rates have subsequently decreased over time.11,12,15 The studies evaluated in this review report similar degrees of effectiveness in reducing rates of CAUTI compared with prior reviews of CAUTI-related QI interventions.14,51–53 Although CAUTI rates have been declining, we found no evidence that net costs or clinical effectiveness differed in recent studies as compared with earlier studies in our study publication year analysis.
Our results suggest that effective interventions are on average a good value for hospitals, despite the initial investment required. With an average 43% reduction in infections and a net cost of $52,000 over three years, some hospitals may find that the program cost of implementation is fully offset or nearly so by the cost of infections prevented. However, financial outcomes varied greatly in this analysis. Notably, our results also suggest that investing more money in a CAUTI-reduction program is not necessarily helpful, as higher program costs did not consistently lead to better outcomes clinically.
A sub-analysis based on two of the studies suggested that implementing a multifaceted CAUTI-prevention strategy utilizing both antimicrobial catheters and CAUTI-related policies and practices may be better at reducing infection rates than either strategy alone, and not more costly. Caution should be used in interpreting this result, however, as it is driven largely by one single-center study where multiple policies and practices were changed in addition to the use of antimicrobial catheters.40 It is not possible to discern from this study which intervention had the largest effect on CAUTI reduction. Furthermore, there is significant debate over the role and value of antimicrobial-impregnated urinary catheters in CAUTI prevention, with multiple conflicting studies. A 2014 Cochrane review did not find clear evidence of the benefit of these types of catheters,54 and the 2014 SHEA/IDSA guidelines for CAUTI prevention explicitly advise against routine use of antimicrobial-impregnated catheters.24
Limitations
Our analysis has several limitations. A limited number of studies have examined the cost of QI interventions related to CAUTI, and most of these used weak uncontrolled before-after designs. Additionally, we used estimates from a systematic review and meta-analysis6 to estimate the infection related costs in the majority of included studies. There may be methodologic limitations to the cost analyses in both the primary studies that we included in our review and in the primary studies included in the meta-analysis of CAUTI-related costs, including inadequate adjustments, residual confounding, and time dependent bias, which can overestimate the costs associated with nosocomial infections.55 This may potentially make these interventions more costly to hospitals than they appear. Additionally, the QI interventions in the included studies had heterogeneous components and were highly complex, limiting our ability to identify the specific elements driving intervention effectiveness and cost savings. Economic evaluations have not addressed some of the most evidence-based and widely used QI strategies for CAUTI; therefore, further research is needed. Finally, the definitions of CAUTI varied across included studies which may affect CAUTI detection rates but would be less likely to bias estimates of intervention effectiveness since the CAUTI definition is applied equally to the intervention group and the control group. Jackknife resampling with sequential exclusion of each study in our sensitivity analyses, including those studies with unspecified definitions of CAUTI, revealed that results did not change. Additionally, the IRR facilitates standardization across disparate studies as a ratio of the rate of infection in the intervention group to that in the control group, and it provides a unit-free proportion that ranges from 0 to 1. Using the IRR facilitates standardization but does not eliminate the problem of different studies using different definitions, since a given intervention might be particularly effective in a subset of CAUTI cases that are not counted using a particular CAUTI definition, for example. Despite these limitations, our findings reflect 74 sites and thousands of catheter-days, and the changes in CAUTI rates we observed are consistent with prior reviews.14,51–53
Conclusions
QI interventions that involved antimicrobial catheters and/or changes to CAUTI-related policies and practices were associated with declines in infection rates and net costs to hospitals that varied greatly but that, on average, were not significantly different from zero over three years. Future research should seek to tease out specific practices associated with cost savings and clinical effectiveness, and examine whether or not more comprehensive QI interventions offer hospitals and patients the best value.
Supplementary Material
Appendix 1: Literature Search Strategy
Appendix 2: Study Quality and Funding Sources
Appendix 3: Methods for Standardizing Costs
Appendix 5: Results of Weighted Regression: Associations between Intervention and Setting Characteristics and Incidence Rate Ratio or Incremental Net Cost per Hospital over Three Years
Appendix 4: Standardized Costs per Hospital
Funding Source:
Agency for Healthcare Research and Quality (R01 HS22644–01)
Footnotes
Competing Interests: None declared
References
- 1.World Health Organization. Report on the burden of endemic health care-associated infection worldwide 2011; http://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf?sequence=1. Accessed August 2021.
- 2.World Health Organization. Health care-associated infections FACT SHEET 2015; https://www.who.int/gpsc/country_work/gpsc_ccisc_fact_sheet_en.pdf. Accessed August 2021.
- 3.Centers for Disease Control and Prevention. Healthcare-associated Infections: Catheter-associated Urinary Tract Infections (CAUTI) 2015; https://www.cdc.gov/hai/ca_uti/uti.html. Accessed August 2021.
- 4.Centers for Disease Control and Prevention. Catheter-Associated Urinary Tract Infections 2020; https://arpsp.cdc.gov/profile/infections/CAUTI. Accessed August 2021.
- 5.Hollenbeak CS, Schilling AL. The attributable cost of catheter-associated urinary tract infections in the United States: A systematic review. American Journal of Infection Control 2018;46(7):751–757. [DOI] [PubMed] [Google Scholar]
- 6.Zimlichman E, Henderson D, Tamir O, et al. Health care–associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA internal medicine 2013;173(22):2039–2046. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare and Medicaid Services CMS.gov. 2021; https://www.cms.gov/. Accessed September 2021.
- 8.Saint S, Meddings JA, Calfee D, Kowalski CP, Krein SL. Catheter-associated urinary tract infection and the Medicare rule changes. Annals of internal medicine 2009;150(12):877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services. Hospital Value-Based Purchasing 2019; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Hospital-Value-Based-Purchasing-.html. Accessed May, 2019.
- 10.Centers for Medicare and Medicaid Services. Hospital‐Acquired Condition Reduction Program 2016; https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/hac-reduction-program.html. Accessed March, 2019. [DOI] [PubMed]
- 11.Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Committee HICPA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infection Control & Hospital Epidemiology 2010;31(4):319–326. [DOI] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality. Toolkit for Reducing Catheter-Associated Urinary Tract Infections in Hospital Units: Implementation Guide http://www.ahrq.gov/professionals/quality-patient-safety/hais/cauti-tools/impl-guide/index.html. Accessed Marcg, 2019.
- 13.Meddings J, Rogers MA, Macy M, Saint SJCID. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients 2010;51(5):550–560. [DOI] [PubMed] [Google Scholar]
- 14.Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ quality & safety 2014;23(4):277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality. AHRQ National Scorecard on Hospital-Acquired Conditions http://www.ahrq.gov/professionals/quality-patient-safety/pfp/index.html.
- 16.Porter ME. What is value in health care. N Engl J Med 2010;363(26):2477–2481. [DOI] [PubMed] [Google Scholar]
- 17.Nuckols TK, Escarce JJ, Asch SMJTMQ. The effects of quality of care on costs: a conceptual framework 2013;91(2):316–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 19.Nuckols T, Maglione M, Shekelle P, Morton S, Escarce J, Keeler E. Systematic review of cost outcomes of quality improvement. PROSPERO 2015.
- 20.Glanville J, Kaunelis D, Mensinkai S. How well do search filters perform in identifying economic evaluations in MEDLINE and EMBASE. International journal of technology assessment in health care 2009;25(4):522–529. [DOI] [PubMed] [Google Scholar]
- 21.Danz MS, Rubenstein LV, Hempel S, et al. Identifying quality improvement intervention evaluations: is consensus achievable? BMJ Quality & Safety 2010;19(4):279–283. [DOI] [PubMed] [Google Scholar]
- 22.Newman DK. The indwelling urinary catheter: principles for best practice. Journal of Wound Ostomy & Continence Nursing 2007;34(6):655–661. [DOI] [PubMed] [Google Scholar]
- 23.Goeree R, Burke N, O’Reilly D, Manca A, Blackhouse G, Tarride J-E. Transferability of economic evaluations: approaches and factors to consider when using results from one geographic area for another. Current medical research and opinion 2007;23(4):671–682. [DOI] [PubMed] [Google Scholar]
- 24.Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infection Control & Hospital Epidemiology 2014;35(5):464–479. [DOI] [PubMed] [Google Scholar]
- 25.Cochrane Consumers & Communication Review Group Study Design Guide For Review Authors. The Cochrane Collaboration June 2013:1–56. http://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/uploads/Study_design_guide2013.pdf.
- 26.Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ quality & safety 2015;24(12):796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiou C-F, Hay JW, Wallace JF, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Medical care 2003:32–44. [DOI] [PubMed]
- 28.Walker DG, Wilson RF, Sharma R, et al. Best practices for conducting economic evaluations in health care: a systematic review of quality assessment tools 2012. [PubMed]
- 29.Nuckols TK, Keeler E, Morton S, et al. Economic evaluation of quality improvement interventions designed to prevent hospital readmission: a systematic review and meta-analysis. JAMA internal medicine 2017;177(7):975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuckols TK, Keeler E, Morton SC, et al. Economic evaluation of quality improvement interventions for bloodstream infections related to central catheters: a systematic review 2016;176(12):1843–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Advisors OJNIfH, Excellence C. Community engagement: approaches to improve health and reduce health inequalities: cost-consequence analysis 2016.
- 32.Friedl H, Stampfer EJWSSRO . Jackknife resampling 2014.
- 33.Anderson DJ, Miller BA, Chen LF, et al. The network approach for prevention of healthcare-associated infections: long-term effect of participation in the Duke Infection Control Outreach Network. Infection Control & Hospital Epidemiology 2011;32(4):315–322. [DOI] [PubMed] [Google Scholar]
- 34.Clarke K, Tong D, Pan Y, et al. Reduction in catheter-associated urinary tract infections by bundling interventions. International Journal for Quality in Health Care 2013;25(1):43–49. [DOI] [PubMed] [Google Scholar]
- 35.van den Broek PJ, Wille JC, van Benthem BH, Perenboom RJ, van den Akker-van Marle ME, Niel-Weise BS. Urethral catheters: can we reduce use? BMC Urol 2011;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rupp ME, Fitzgerald T, Marion N, et al. Effect of silver-coated urinary catheters: efficacy, cost-effectiveness, and antimicrobial resistance. American journal of infection control 2004;32(8):445–450. [DOI] [PubMed] [Google Scholar]
- 37.Saint S, Kaufman SR, Thompson M, Rogers MA, Chenoweth CE. A reminder reduces urinary catheterization in hospitalized patients. The Joint Commission Journal on Quality and Patient Safety 2005;31(8):455–462. [DOI] [PubMed] [Google Scholar]
- 38.Pickard R, Lam T, Maclennan G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial-and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technology Assessment 2012. [DOI] [PubMed]
- 39.Lai KK, Fontecchio SA. Use of silver-hydrogel urinary catheters on the incidence of catheter-associated urinary tract infections in hospitalized patients. American Journal of Infection Control 2002;30(4):221–225. [DOI] [PubMed] [Google Scholar]
- 40.Quinn P Chasing zero: a nurse-driven process for catheter-associated urinary tract infection reduction in a community hospital. Nursing Economics 2015;33(6):320. [PubMed] [Google Scholar]
- 41.Cartwright A Reducing catheter-associated urinary tract infections: standardising practice. British Journal of Nursing 2018;27(1):7–12. [DOI] [PubMed] [Google Scholar]
- 42.Saint S, Veenstra DL, Sullivan SD, Chenoweth C, Fendrick AM. The potential clinical and economic benefits of silver alloy urinary catheters in preventing urinary tract infection. Archives of Internal Medicine 2000;160(17):2670–2675. [DOI] [PubMed] [Google Scholar]
- 43.Karchmer TB, Giannetta ET, Muto CA, Strain BA, Farr BM. A randomized crossover study of silver-coated urinary catheters in hospitalized patients. Archives of Internal Medicine 2000;160(21):3294–3298. [DOI] [PubMed] [Google Scholar]
- 44.Pashnik B, Creta A, Alberti L. Effectiveness of a Nurse-Led Initiative, Peer-to-Peer Teaching, on Organizational CAUTI Rates and Related Costs. Journal of Nursing Care Quality 2017;32(4):324–330. [DOI] [PubMed] [Google Scholar]
- 45.Fakih MG, Groves C, Bufalino A, Sturm LK, Hendrich ALJICHE. Definitional change in NHSN CAUTI was associated with an increase in CLABSI events: evaluation of a large health system 2017;38(6):685–689. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control Prevention. Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [CAUTI] and Non-Catheter-Associated Urinary Tract Infection [UTI]) Events 2020; https://www.cdc.gov/nhsn/pdfs/pscManual/7pscCauticurrent.pdf. Accessed January, 2020.
- 47.Palmer S, Dixon R. Reducing catheter-associated urinary tract infections through best practice: Sherwood Forest Hospitals’ experience. British Journal of Nursing 2019;28(1):11–15. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell BG, Fasugba O, Cheng AC, et al. Chlorhexidine versus saline in reducing the risk of catheter associated urinary tract infection: a cost-effectiveness analysis. International journal of nursing studies 2019;97:1–6. [DOI] [PubMed] [Google Scholar]
- 49.Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: an updated critical analysis of the evidence for patient safety practices. Evidence report/technology assessment 2013(211):1. [PMC free article] [PubMed] [Google Scholar]
- 50.Tan SH, Tan SBJPoSH. The correct interpretation of confidence intervals 2010;19(3):276–278. [Google Scholar]
- 51.Mauger B, Marbella A, Pines E, Chopra R, Black ER, Aronson N. Implementing quality improvement strategies to reduce healthcare-associated infections: A systematic review. American journal of infection control 2014;42(10):S274–S283. [DOI] [PubMed] [Google Scholar]
- 52.Durant DJ. Nurse-driven protocols and the prevention of catheter-associated urinary tract infections: a systematic review. American Journal of Infection Control 2017;45(12):1331–1341. [DOI] [PubMed] [Google Scholar]
- 53.Beattie M, Taylor J. Silver alloy vs. uncoated urinary catheters: a systematic review of the literature. Journal of clinical nursing 2011;20(15‐16):2098–2108. [DOI] [PubMed] [Google Scholar]
- 54.Lam TB, Omar MI, Fisher E, Gillies K, MacLennan SJCdosr. Types of indwelling urethral catheters for short‐term catheterisation in hospitalised adults 2014(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barnett AG, Beyersmann J, Allignol A, Rosenthal VD, Graves N, Wolkewitz MJVih. The time-dependent bias and its effect on extra length of stay due to nosocomial infection 2011;14(2):381–386. [DOI] [PubMed] [Google Scholar]
- 56.Control CfD, Prevention. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) and other urinary system infection [USI]) events. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nhsn/PDFs/pscManual/7pscCAUTIcurrentpdf. 2015. [Google Scholar]
- 57.Mitchell BG, Fasugba O, Gardner A, et al. Reducing catheter-associated urinary tract infections in hospitals: study protocol for a multi-site randomised controlled study 2017;7(11):e018871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson DJ, Miller BA, Chen LF, et al. The network approach for prevention of healthcare-associated infections: long-term effect of participation in the Duke Infection Control Outreach Network. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America 2011;32(4):315–322. [DOI] [PubMed] [Google Scholar]
- 59.Clarke K, Tong D, Pan Y, et al. Reduction in catheter-associated urinary tract infections by bundling interventions. International journal for quality in health care : journal of the International Society for Quality in Health Care / ISQua 2013;25(1):43–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Literature Search Strategy
Appendix 2: Study Quality and Funding Sources
Appendix 3: Methods for Standardizing Costs
Appendix 5: Results of Weighted Regression: Associations between Intervention and Setting Characteristics and Incidence Rate Ratio or Incremental Net Cost per Hospital over Three Years
Appendix 4: Standardized Costs per Hospital


